Abstract
Background
B-cell depleting agents are FDA approved for the treatment of RRMS (ocrelizumab (OCR) and ofatumumab (OFA)) and PPMS (OCR). In the case of OCR, prior studies have raised concerns about patients’ ability to form antibodies in response to various antigens, especially SARS-CoV-2. In addition, emerging data have shown an attenuated humoral response to vaccines against SARS-CoV-2. The objective of this study is to determine whether b-cell depleters or sphingosine 1-phosphate (S1P) modulators attenuate the antibody response to various SARS-CoV-2 vaccines in patients with MS as compared with other MS disease modifying therapies (DMTs).
Methods
This is a case-control study looking at the odds of developing antibodies to three SARS-CoV-2 vaccines (Pfizer-BioNTech, Moderna, and Johnson & Johnson) in patients treated with b-cell depleters or S1P modulators versus other disease modifying therapies. Patients were recruited at the Comprehensive MS Center at Methodist Hospitals. Patients who did not have a prior COVID-19 infection and received one of the three vaccines were tested for antibodies against the SARS-CoV-2 spike protein (Labcorp, semi-quantitative total antibody) at least two weeks following the final dose of the vaccine. Groups (B-cell, S1P modulators, other DMT, and no DMT) were compared on antibody level. The main outcome was whether or not a humoral response was detected by antibody testing. Dichotomous antibody response was tested using logistic regression models, and the quantitative response was tested using ANCOVA adjusted for covariates (age, sex, race, MS type, disease duration, vaccine, and lymphocyte count). P-values <0.05 were considered significant.
Results
Sixty-seven patients were enrolled in the study, with 17 on OCR, 3 on OFA, 12 on S1P modulators, 29 on other DMT, and 6 not currently on any DMT. Patients who received OCR or OFA had decreased odds of forming antibodies (OR 0.031, p < 0.001, 95% CI (0.003–0.268)). Patients who received S1P modulators did not have decreased odds of forming antibodies (OR 0.413, p = 0.413, 95% CI (0.28–21.7). However, when analyzing the antibody response as a continuous variable, patients on S1P modulators showed lower absolute levels of antibodies (p = 0.024).
Conclusions
Patients who received B-cell depleters within the prior 6 months of SARS-CoV-2 vaccination had decreased odds of developing antibodies compared with other DMTs. In line with other similar research, this suggests that b-cell depleters attenuate the antibody response to SARS-CoV-2 vaccines. Although S1P modulators had an attenuation of the absolute antibody level, the odds of being negative did not differ from those on other DMTs.
Keywords: OCR, ocrelizumab; OFA, ofatumumab; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; MS, multiple sclerosis; DMT, disease modifying therapy; FTY, fingolimod; S1P, sphingosine-1-phosphate; J&J, Johnson & Johnson; RR, relapse-remitting; SP, secodnary progressive; PP, primary progressive
Keywords: Ocrelizumab, COVID-19, SARS-CoV-2, Disease modifying therapies, Vaccine
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), remains an epidemiological challenge. With the advent of vaccines for SARS-CoV-2, many questions have emerged regarding effectiveness in immunocompromised and immunomodulated patients, including those with multiple sclerosis (MS) on disease modifying therapies (DMTs).
B-cell lymphocyte depleters, such as ocrelizumab (OCR) and ofatumumab (OFA), may interfere in the process of antibody production through inhibition of memory b-cells and to a lesser extent, plasmablasts (Krumbholz et al., 2012). A prospective study showed that in the case of OCR, patients were able to mount a relatively attenuated humoral response to various antigens (Bar-Or et al., 2020). Fingolimod (FTY), which is a sphingosine-=1-phosphate (S1P) modulator has been shown to attenuate humoral immunity by inhibition of germinal center reactions in lymphoid tissues in mice (Han et al., 2004). In humans, a study showed FTY-treated patients had decreased response rates to vaccines as compared with those on placebo, although the majority of patients on FTY had a response (Bingham et al., 2010).
Several studies have demonstrated attenuation of the immune response following infection with SARS-CoV-2 following exposure to OCR as demonstrated by low or absent antibody levels (Conte, 2021, 2020; Lucchini et al., 2020; Sormani et al., 2021; Thornton and Harel, 2020; Zabalza et al., 2021; Zhao et al., 2020). In addition, emerging research has demonstrated variable attenuation of the vaccine response to various DMTs following COVID-19 vaccination. Achiron et al. (2021), demonstrated attenuation by OCR and FTY in response to the Pfizer-BioNTech (Pfizer) vaccine. Bigaut et al. (2021), also found an attenuated humoral response with OCR and S1P modulators, with exposure to both the Pfizer and Moderna vaccines. Guerrieri et al. (2021), found a marked attenuation with OCR (only 37.5% of patients had a response) whereas FTY had a more robust response (62.5%). Novak, et al., found a decreased humoral response to OCR (Novak et al., 2021).
This study aims to add to the literature about the humoral response following vaccination with the Pfizer, Moderna, and Johnson & Johnson (J&J) vaccines in patients with MS on various DMTs, with a focus on B-cell depleters (OCR and OFA) and S1P modulators (FTY, siponimod, and ozanimod).
2. Methods
2.1. Study participants
Patients with MS were recruited at the Comprehensive MS Center at Methodist Hospitals. Patients without a self-reported or documented history of prior COVID-19, who also reported that they had completed a full course of SARS-CoV-2 vaccination, were recruited. Antibody testing was conducted at least two weeks following vaccination.
2.2. SARS-CoV-2 vaccinations
Patients received two intramuscular doses of the Pfizer or Moderna vaccines, or one intramuscular dose of the J&J vaccine.
2.3. Antibody testing
The immunoassay for the detection of IgG antibodies in the patients was performed using the Labcorp anti-SARS-CoV-2 semi-quantitative IgG ECLIA assay against the spike protein receptor domain, which ranged from <0.4 to >250 U/ml. Patients were tested at least 2 weeks following the final dose of the vaccination. Titers <0.8 U/ml were considered negative.
2.4. Data collection
The study was approved by the institutional review board at Methodist Hospitals. Individual, deidentified participant data is available on request.
2.5. Statistical analysis
Analyses were conducted using SPSS Statistics 26.0 (IBM Corp., Armonk, NY). Based on DMT status, the following groups were compared: B-cell, S1P, other, no medication. Due to non-normality of the data as determined by Shapiro-Wilks test, all quantitative antibody analyses were compared using ranked data. First, binary logistic regression analysis was used to determine odds of antibody positive status between groups. Then, ranked ANOVA and ANCOVA tests were conducted on continuous data, with age, sex, race, MS type, disease duration, vaccine, and lymphocyte count as covariates. Using post-hoc 2-way contrasts between medication groups, testing was undertaken to see if pairs differed on vaccine response. In sub-analyses within patients on OCR, logistic and linear regression analyses adjusted for age, sex, race, MS type, disease duration, vaccine, and lymphocyte count were used to investigate whether vaccine response was dependent on the time between OCR infusion and vaccine date. A two-sided p-value of <0.05 was considered statistically significant.
3. Results
3.1. Demographic characteristics
Baseline characteristics are reported in Table 1 . Sixty-seven patients were recruited, with 20 patients on B-cell depleters (17 on OCR, and 3 on OFA), 12 on S1P modulators (8 on siponimod, 3 on FTY, and 1 on ozanimod), 29 on other DMT (13 on teriflunomide, 4 on oral cladribine, 3 on diroximel fumarate, 2 on alemtuzumab, 2 on dimethyl fumarate, 2 on interferon beta-1b, 2 on natalizumab, and 1 on interferon beta-1a), and 6 on no DMT. The majority of patients were female (77.6%). The majority of patients received the Pfizer vaccine (70.1%).
Table 1.
Baseline characteristics.
|
DMT Groups |
|||||
|---|---|---|---|---|---|
| All pts | Bcell | S1P | Other | no DMT | |
| n | 67 | 20 | 12 | 29 | 6 |
| MS Type - n (%) | |||||
| RR | 55 (82.1%) | 19 (95%) | 4 (33.3%) | 28 (69.6%) | 4 (66.7) |
| SP | 10 (14.9%) | 0 | 8 (66.7%) | 1 (3.4%) | 1 (16.7) |
| PP | 2 (3%) | 1 (5%) | 0 | 0 | 1 (16.7) |
| Sex - n (%) female | 52 (77.6%) | 16 (80%) | 10 (83.3%) | 22 (75.9%) | 4 (66.7%) |
| Age - mean (SD) | 52.8 (10.7) | 46.3 (10.5) | 59.3 (8.8) | 52.7 (9.3) | 4 (66.7%) |
| Disease duration, y - mean (SD) | 16.6 (8.9) | 10 (9.9) | 16.7 (6.9) | 18.5 (5.5) | 20.2 (7.8) |
| AB level (raw) - mean, median (SD) IQR | 147.8, 250 (117.3) 2.4–250 | 44.8, 0.8 (89.9) 0.4–45.2 | 106.5, 49.3 (115.9) 3.2–250 | 221.7, 250 (74.0) 250–250 | 216.9, 250 (80.8) 200–250 |
| AB Level (log) - mean, median (SD) IQR | 3.6, 5.5 (2.6) 0.8–5.5 | 1.1, −0.2 (2.5) −0.9–3.8 | 3.1, 3.6 (2.4) 1.1–5.5 | 5.1, 5.5 (1.2) 5.5–5.5 | 5.3, 5.5 (0.6) 5.1–5.5 |
| AB positive (n,%) | 57 (85.1%) | 11 (55%) | 11 (91.7%) | 29 (100%) | 6 (100%) |
| Lymphocyte > 1.1 | 35 (53.8%) | 13 (65%) | 3 (25%) | 15 (51.7%) | 4 (66.7%) |
| IgG >=700 - n (%) | – | 19 (95%) | – | – | – |
| IgM >=40 - n (%) | – | 13 (65%) | – | – | – |
| IgA >=70 - n (%) | – | 16 (80%) | – | – | – |
| Time from last infusion to vaccine, months - mean, median (SD) IQR | – | 3.5, 3.0 (1.5) 2.75–5.0 | – | – | – |
| Vaccine - n (%) | |||||
| Pfizer | 47 (70.1%) | 13 (65%) | 8 (66.7%) | 26 (79.3%) | 3 (50%) |
| Moderna | 19 (28.4%) | 7 (35%) | 3 (25%) | 6 (20.7%) | 3 (50%) |
| J&J | 1 (1.5%) | 0 | 1 (8.3%) | 0 | 0 |
3.2. SARS-CoV-2 antibody status
Overall, 57 (85.1%) of patients in this cohort had positive antibodies to SARS-CoV-2, as determined at a threshold of 0.80 (U/ml). Eleven out of 20 patients on b-cell depleters developed antibodies (55%), 11 out of 12 patients on S1P modulators developed antibodies (91.7%), and all patients on other and no DMT developed antibodies (p < 0.001).
Patients on B-cell depleters had decreased odds of forming antibodies compared to other DMTs (OR 0.031, p < 0.001, (95% CI 0.003, 0.268). S1P showed no difference with forming antibodies compared to other DMTs (OR 2.47, p = 0.413, (95% CI 0.28, 21.7).
3.3. Level of SARS-CoV-2 antibody response
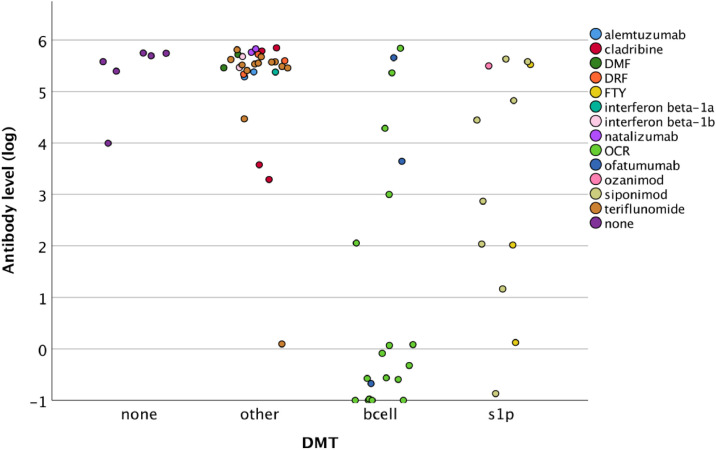
Ranked ANOVA tested showed a significant difference in the level of antibody response across groups (Fig. 1 , p < 0.001, partial η2=0.45). Post-hoc analyses revealed that b-cell depleters had lower levels of antibodies compared to S1P (p = 0.003), no DMT (p < 0.001), or other DMTs (p < 0.001). Other DMT groups did not differ significantly.
Fig. 1.
Scatterplot depicting, by group and DMT, anti-body level (log-transformed).
Ranked ANCOVA models adjusted for covariates showed similar results. DMT groups differed significantly (p < 0.001), with post-hoc contrasts revealing that patients with MS on b-cell depleters had significantly lower antibody levels compared to S1P (p = 0.013), no DMT (p < 0.001), other DMT's (p < 0.001), or any group (p < 0.001). Patients on S1P modulators also had significantly lower vaccine response than patients on other DMT's (p = 0.024). This indicates that even after adjusting for characteristics such as disease duration and vaccine, patients with MS on S1P modulators and especially b-cell depleters had an attenuated vaccine response when considering continuous antibody levels.
3.4. Effects on the time between OCR infusion and vaccine administration
There was a slight but non-significant effect of longer time between OCR infusion and vaccine administration and SARS-CoV-2 antibody level (Spearman r = 0.401, p = 0.111) or immune status (binary logistic regression, OR 1.69, p = 0.170 (95% CI 0.80 – 3.56).
3.5. Effect of lymphocyte levels on antibody response
There was a weakly positive but nonsignificant correlation for lymphocyte levels for S1P modulators (Spearman r = 0.206, p = 0.521) influencing antibody levels.
3.6. Effects of immunoglobulin level on B-cell response
Three patients on OCR had low IgG levels and five had low IgM levels. One of the patients with low IgG levels had an attenuated response while two of the patients with low IgM levels had an attenuated response. All of the OFA patients had normal immunoglobulin levels. There was a weakly positive but nonsignificant correlation for immunoglobulin levels (Spearman r = 0.179, p = 0.463) influencing antibody levels.
4. Discussion
As of the date of writing, vaccination is ongoing to protect against COVID-19. Based on data surrounding post-infection antibody levels, and emerging data surrounding vaccine responses, it is concerning that patients on b-cell depleting therapies may have a differential response to the SARS-CoV-2 vaccines (Achiron et al., 2021; Bigaut et al., 2021; Conte, 2021; Guerrieri et al., 2021; Wallach and Picone, 2021; Zhao et al., 2020). Indeed, in the VELOCE trial, OCR attenuated the humoral response to several antigens although patients were still able to mount an immune response (Bar-Or et al., 2020). The present study has shown that b-cell depleters significantly attenuated the response to SARS-CoV-2 vaccines as compared to other DMTs.
As opposed to other data, I did not find significant attenuation by S1P modulators when compared to other DMTs when analyzed as a binary variable. However, when the antibody levels were analyzed as a continuous variable, there were lower absolute values of antibodies. However, the majority of the S1P patients remained positive. This differs from the findings by Achiron, et al., perhaps due to differences in sensitivity of the assays used (Achiron et al., 2021). Further, I included all S1P modulators in the data analysis, and the majority of patients were on siponimod. Therefore, a differential response between the various S1P modulators cannot be excluded, although I believe based on the similar mechanisms of action, this is unlikely.
It should be noted that there is no consensus on what antibody level denotes adaptive immunity. Further, this data does not reflect on the T-cell response. I continue to recommend all my patients, regardless of DMT, get vaccinated. Further, I am recommending patients who are on B-cell depleters or S1P modulators to have a booster.
This study is limited by a relatively small sample size, especially amongst certain individual DMTs. I also relied on patient's self-reporting prior COVID-19 infection and vaccination status. Therefore, it is possible that some patients had a previous asymptomatic infection or even falsely reported that they had received a vaccination.
CRediT authorship contribution statement
William L. Conte: Conceptualization, Methodology, Data curation, Writing – original draft.
Declaration of Competing Interest
Research funding from Novartis unrelated to the current study. Consultant fees from Bayer, Biogen, Bristol Meyers Squib, Genentech, Novartis, and Sanofi Genzyme. Speaking fees from AbbVie, Alexion, Biogen, Bristol Meyers Squib, EMD Serono, Genentech, Janssen, Novartis, and Sanofi Genzyme.
Acknowledgements
Special thanks to Jesper Hagemeier for help with statistics.
References
- Achiron, A., Mandel, M., Dreyer-Alster, S., Harari, G., Magalashvili, D., Sonis, P., Dolev, M., Menascu, S., Flechter, S., Falb, R., Gurevich, M., 2021. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies: 10.1177/17562864211012835 14. 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed]
- Bar-Or A., Calkwood J.C., Chognot C., Evershed J., Fox E.J., Herman A., Manfrini M., McNamara J., Robertson D.S., Stokmaier D., Wendt J.K., Winthrop K.L., Traboulsee A. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95:e1999–e2008. doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigaut K., Kremer L., Fleury M., Lanotte L., Collongues N., de Seze J. Impact of disease-modifying treatments on humoral response after COVID-19 vaccination: a mirror of the response after SARS-CoV-2 infection. Rev. Neurol. 2021 doi: 10.1016/j.neurol.2021.05.001. (Paris) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham C.O., Looney R.J., Deodhar A., Halsey N., Greenwald M., Codding C., Trzaskoma B., Martin F., Agarwal S., Kelman A. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. 2010;62:64–74. doi: 10.1002/art.25034. [DOI] [PubMed] [Google Scholar]
- Conte W.L. Attenuation of antibody response to SARS-CoV-2 infection in patients with multiple sclerosis on ocrelizumab: a case-control study. Mult. Scler. Relat. Disord. 2021;52 doi: 10.1016/J.MSARD.2021.103014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte W.L. Attenuation of antibody response to SARS-CoV-2 in a patient on ocrelizumab with hypogammaglobulinemia. Mult. Scler. Relat. Disord. 2020 doi: 10.1016/j.msard.2020.102315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrieri S., Lazzarin S., Zanetta C., Nozzolillo A., Filippi M., Moiola L. Serological response to SARS-CoV-2 vaccination in multiple sclerosis patients treated with fingolimod or ocrelizumab: an initial real-life experience. J. Neurol. 2021;1:1–5. doi: 10.1007/S00415-021-10663-X. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Zhang X., Wang G., Guan H., Garcia G., Li P., Feng L., Zheng B. FTY720 suppresses humoral immunity by inhibiting germinal center reaction. Blood. 2004;104:4129–4133. doi: 10.1182/BLOOD-2004-06-2075. [DOI] [PubMed] [Google Scholar]
- Krumbholz M., Derfuss T., Hohlfeld R., Meinl E. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat. Rev. Neurol. 2012;811(8):613–623. doi: 10.1038/nrneurol.2012.203. 2012. [DOI] [PubMed] [Google Scholar]
- Lucchini M., Bianco A., Del Giacomo P., De Fino C., Nociti V., Mirabella M. Is serological response to SARS-CoV-2 preserved in MS patients on ocrelizumab treatment? A case report. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak F., Nilsson A.C., Nielsen C., Holm D.K., Østergaard K., Bystrup A., Byg K.-.E., Johansen I.S., Mittl K., Rowles W., Mcpolin K., Spencer C., Sagan S., Gerungan C., Wilson M.R., Zamvil S.S., Bove R., Sabatino J.J., Sejbaek T. Humoral immune response following SARS-CoV-2 mRNA vaccination concomitant to anti-CD20 therapy in multiple sclerosis. Mult. Scler. Relat. Disord. 2021;0 doi: 10.1016/J.MSARD.2021.103251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani, M.P., Schiavetti, I., Landi, D., Carmisciano, L., Rossi, N.De, Cordioli, C., Moiola, L., Radaelli, M., Immovilli, P., Capobianco, M., Morra, V.B., Trojano, M., Tedeschi, G., Comi, G., Battaglia, M.A., Patti, F., Fragoso, Y.D., Sen, S., Siva, A., Furlan, R., Salvetti, M., 2021. SARS-CoV-2 serology after COVID-19 in multiple sclerosis: an international cohort study: 10.1177/13524585211035318. 10.1177/13524585211035318. [DOI] [PubMed]
- Thornton J.R., Harel A. Negative SARS-CoV-2 antibody testing following COVID-19 infection in Two MS patients treated with ocrelizumab. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach A.I., Picone M.A. The presence of SARS-CoV2 antibodies in MS patients. Mult. Scler. Relat. Disord. 2021 doi: 10.1016/j.msard.2021.102793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabalza A., Cárdenas-Robledo S., Tagliani P., Arrambide G., Otero-Romero S., Carbonell-Mirabent P., Rodriguez-Barranco M., Rodríguez-Acevedo B., Restrepo Vera J.L., Resina-Salles M., Midaglia L., Vidal-Jordana A., Río J., Galan I., Castillo J., Cobo-Calvo Á., Comabella M., Nos C., Sastre-Garriga J., Tintore M., Montalban X. COVID-19 in multiple sclerosis patients: susceptibility, severity risk factors and serological response. Eur. J. Neurol. 2021;00:ene.14690. doi: 10.1111/ene.14690. [DOI] [PubMed] [Google Scholar]
- Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., Wang X., Yuan J., Li T., Li J., Qian S., Hong C., Wang F., Liu Y., Wang Z., He Q., Li Z., He B., Zhang T., Fu Y., Ge S., Liu L., Zhang J., Xia N., Zhang Z. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin. Infect. Dis. 2020;71:2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]