Abstract
Background and Objectives
Frailty describes an increased vulnerability to adverse events such as disease or injury. Combating this state remains a major challenge for geriatric research. By exploring how and why frailty changes throughout later life we will be better positioned to improve ways of identifying and treating those at high risk.
Research Design and Methods
We systematically reviewed publications that captured rate of frailty progression over time and established any associated risk or protective factors that affected this progression. We included longitudinal observational studies which quantified frailty trajectories in adults aged 50+ using any validated continuous frailty measurement tool.
Results
After screening 8,318 publications, 25 met our criteria. Findings show that despite a great degree of heterogeneity in the literature, progression of frailty is unquestionably affected by numerous risk and protective factors, with particular influence exhibited by social demographics, brain pathology, and physical comorbidities.
Discussion and Implications
Findings that the gradient of frailty progression is affected by various influencing factors are valuable to clinicians and policymakers as they will help identify those at highest frailty risk and inform prevention strategies. However, the heterogeneous methodological approaches of the publications included in this review highlight the need for consensus within the field to promote more coordinated research. Improved consistency of methods will enable further data synthesis and facilitate a greater understanding of the shape of frailty over time and the influencing factors contributing to change, the results of which could have crucial implications for frailty risk reduction.
Keywords: Frail, Trajectory, Rate of change, Risk factors
Defining frailty proves highly challenging and while a universally accepted definition remains elusive, it is generally accepted to describe an age-related vulnerability which increases an individual’s susceptibility to injury, disability, hospitalization, and mortality (Iwasaki et al., 2018). Although this syndrome is greatly associated with the aging process, it is not an inevitable part of it (Ahmed, Mandel, & Fain, 2007). Hence, the study of frailty change becomes imperative to understand why certain individuals become increasingly frail at a quicker rate than others do. With an improved understanding of this process comes the enhanced ability to identify and treat those at greatest risk of decline.
Currently, there is no gold standard frailty measurement tool (Aguayo et al., 2017); however, several measures have been devised and widely utilized in the field. The frailty index (FI) conceptualizes frailty as an accumulation of deficits across multiple body systems (e.g., physical, social, cognitive) and calculates an individual’s total number of deficits to quantify frailty on a continuous scale from 0 to 1 (Marshall, Nazroo, Tampubolon, & Vanhoutte, 2015; Mitnitski, Mogilner, & Rockwood, 2001; Rockwood & Mitnitski, 2007; Searle, Mitnitski, Gahbauer, Gill, & Rockwood, 2008). Importantly, the researcher chooses the deficits included in the FI and accordingly the composition of each FI differs between different studies. Provided the deficits included in the composition of an FI follow the established criteria proposed by Searle et al. (2008), FI scores have been shown to be reliable across studies (Mitnitski et al., 2001; Rockwood & Mitnitski, 2007). Other frailty measures have taken a different approach by focusing on markers that predict the emergence of physical frailty. By far the most utilized of this method is the Fried Phenotype, which measures frailty according to five criteria thought to reflect the affected systems of frailty: weight loss; exhaustion; weakness; slowness while walking; and low levels of physical activity (Fried et al., 2001). This method typically measures frailty as a categorical variable meaning that individuals are deemed either Non-Frail, Pre-Frail, or Frail. However, variations of this method allow scores to be used on a continuous scale of affected dimensions from 1 to 5. A further development of this yields a continuous composite score by standardizing raw dimension scores into z-scores and averaging these to create a composite measure of frailty (Buchman, Wilson, Bienias, & Bennett, 2009).
Previous research has largely focused on categorizing individuals into discrete frailty states rather than considering frailty as a continuum (Kojima, Taniguchi, Iliffe, Jivraj, & Walters, 2019). Research has also primarily been confined to cross-sectional studies (Stenholm et al., 2018). While these studies can be informative, they make the examination of frailty changes over time much more difficult as they are restricted to one time point and they only reveal an individual’s overall status (Non-Frail, Pre-Frail, or Frail), rather than a more precise measurement (Buchman et al., 2009). Understanding how frailty changes over time by synthesizing current longitudinal findings is a crucial step in identifying the most harmful and most promising frailty trajectories. Improved understanding of these trajectories will help to inform future interventions that aim to put individuals on a frailty path which is less detrimental to their overall health (Chamberlain et al., 2016). Furthermore, identifying the factors that can affect the nature of frailty trajectories is paramount, as these will inform clinical care strategies. For instance, particular lifestyle factors may influence the progression of frailty over time and subsequently represent potentially modifiable areas for prevention and treatment strategies to focus on.
A previous review considered continuously measured frailty trajectories: O’Caoimh et al. (2018) conducted a systematic review on frailty trajectories and transitions exclusively sourced from publications released by the European Joint Action Member States. Three publications were identified, among which a high level of heterogeneity was found in the type of frailty change reported, follow-up length, and their choice of sampling approach. O’Caoimh et al. (2018) suggested that widening the search to publications from countries across the world would allow better insight into the topic. Accordingly, this systematic review considers observational longitudinal publications published around the world, which have measured frailty as a continuous variable over time and subsequently explored frailty trajectories. In this systematic review, we aim to highlight the field’s current limitations and summarize our understanding of how frailty progresses over time and how the rate of frailty change can be influenced by certain factors. By doing so we aim to inform future researchers and policymakers about the way in which frailty can manifest in certain groups, information which will be useful for creating effective intervention strategies.
Research Design and Methods
This systematic review followed standard guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher, Liberati, Tetzlaff, & Altman, 2009). A protocol was created and published on the International Prospective Register of Systematic Review, reference; CRD42019126334 (accessible at https://edin.ac/2zeie9a).
Rationale for Eligibility Criteria
Inclusion/exclusion criteria were defined by following the PICOS format, a framework used to develop a structured response to a health care question (Higgins & Green, 2008). Due to higher prevalence of frailty in older age we focused on adults 50 years and older (Ahmed et al., 2007). Frailty measures were only included if they had been widely used and validated. Only measures that quantified frailty as a continuous variable were included as this allowed rate of change to be assessed. Some scales, although categorical by design, are used in a continuous fashion. In these cases, it was decided that these publications should be included. Additionally, intervention studies were excluded because this review was not focused on the effect of an intervention on frailty trajectories, simply the effect of aging. Full eligibility criteria were as follows: Publications were included if: (a) they contained any validated tool which measured frailty as a continuous variable, (b) frailty trajectories were quantified over more than two time points, (c) they contained human participants aged 50 years old or older at baseline, and (d) it was an observational study. Publications were excluded if: (a) the publication was any of the following: intervention studies, letters, editorials, systematic reviews, meta-analyses, viewpoints, comments, books, abstracts, dissertations, and (b) the full text was not written in English.
Identification of Publications
The scientific literature was systematically searched using the databases MEDLINE, EMBASE, and CINAHL. Each database was searched using headings and free text. The searches were not limited by publication date and included all publications up to March 30, 2020. For a link to the search strategy, see Supplementary Text S1. Reference lists of included studies were also hand-searched and screened for any further publications.
Publication Selection
M. Welstead and N. D. Jenkins independently screened titles and abstracts of the publications retrieved in the searches. At each stage of screening and quality assessment, disputes were discussed and resolved by a third independent reviewer (G. Muniz-Terrera) with expertise in frailty trajectories. For publications which potentially met the eligibility criteria, full texts were reviewed by M. Welstead and N. D. Jenkins and for those included, the quality and risk of bias of the publications were assessed using the Newcastle–Ottawa scale (NOS) for cohort studies (Wells et al., 2011). Consistent with previous literature (Kojima et al., 2019), publications scoring 5 or less were excluded.
Data Extraction
Data were extracted from all included publications including: author, journal, year of publication, title, contact details, country, study type, cohort, population, baseline age, baseline and end of follow-up sample size, time points/follow-up time, study objectives, frailty measure, statistical methodology, covariates, findings, and conclusions. Data were extracted by M. Welstead, with N. D. Jenkins undertaking a 20% sample extraction to verify similar findings.
Results
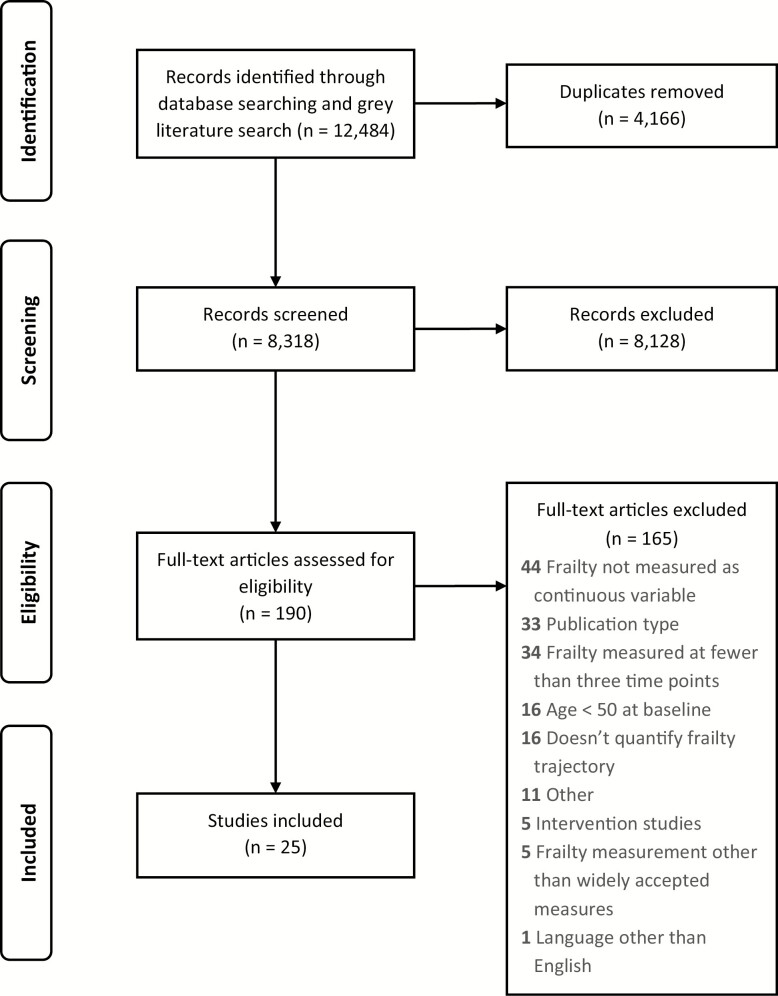
A total 12,484 publications were initially identified. After removing duplicates, 8,318 publications remained, the titles and abstracts of which were read and screened. Subsequently, the full text of 190 publications was screened. Of these, 25 were selected that met the eligibility criteria. No further publications were found from hand-searching reference lists. Figure 1 shows a PRISMA flow diagram illustrating this process and detailing the reasons for exclusion. Of the publications included, data were collected in the United States (n = 9) (Buchman, Boyle, Wilson, Tang, & Bennett, 2007; Buchman et al., 2009, 2014; Buchman, Yu, Wilson, Schneider, & Bennett, 2013; Chen, Mair, Bao, & Yang, 2015; Liu, Han, Gahbauer, Allore, & Gill, 2018; Lohman, Mezuk, & Dumenci, 2017; Peek, Howrey, Ternent, Ray, & Ottenbacher, 2012; Yang & Lee, 2010), United Kingdom (n = 3) (Marshall et al., 2015; Rogers et al., 2017; Stow, Matthews, & Hanratty, 2018), Canada (n = 3) (Gajic-Veljanoski et al., 2018; Li, Papaioannou, Thabane, Cheng, & Adachi, 2016; Mitnitski, Song, & Rockwood, 2012), the Netherlands (n = 1) (Hoogendijk et al., 2018), and across Europe (n = 3) (Stolz, Mayerl, Rásky, & Freidl, 2018; Stolz, Mayerl, Waxenegger, Rásky, & Freidl, 2017; Walkden et al., 2018). Data quality was assessed by the NOS (Wells et al., 2011) found that publications ranged in their scores from 7 to 9 (mean [SD] = 8.2 [0.6]). Table 1 provides a summary of all included publications.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram showing the pathway of systematically reviewing frailty trajectories.
Table 1.
Summary of Included Publications Which Assess Rate of Frailty Change Over Time
| Author (year), country | Frailty measurement tool | Baseline n | Years follow-up | Time points |
|---|---|---|---|---|
| Aguayo et al. (2019), United Kingdom | Frailty index (36 items) | 5,377 | 10 | 6 |
| Buchman et al. (2014), United States | Composite Fried criteria | 2,167 | 15 | Up to 15 |
| Buchman et al. (2013), United States | Composite Fried criteria | 791 | Until death | Up to 14 |
| Buchman et al. (2007), United States | Composite Fried criteria | 823 | 8 | Up to 8 |
| Buchman et al. (2009), United States | Composite Fried criteria | 832 | 8 | Up to 8 |
| Chen aet al. (2015), United States | Frailty index (30 items) | 10,312 | 12 | Up to 7 |
| Gajic-Veljanoski et al. (2018), Canada | Frailty index (30 items) | 7,753 | 10 | 3 |
| Hoogendijk et al. (2018), The Netherlands | Frailty index (32 items) | 1,659 | 17 | 6 |
| Li et al. (2016), Canada | Frailty index (34 items) | 3,985 | 3 | 4 |
| Liu et al. (2018), United States | Continuous Fried criteria (0–5) | 690 | 9 | Up to 6 |
| Lohman et al. (2017), United States | Frailty index (30 items) | 13,495 | 8 | 5 |
| Machado-Fragua et al. (2019), The Netherlands | Frailty index (32 items) | 644 | 13 | 5 |
| Marshall et al. (2015), United Kingdom | Frailty index (60 items) | 11,220 | 12 | 5 |
| Mitnitski et al. (2012), Canada | Frailty index (31 items) | 4,330 | 6 | 7 |
| Peek et al. (2012), United States | Continuous Fried criteria (0–4) | 2,061 | 11 | 5 |
| Rogers et al. (2017), United Kingdom | Frailty index (56 items) | 8,649 | Mean of 10 | Up to 6 |
| Rogers and Fancourt (2020), United Kingdom | Frailty index (56 items) | 4,575 | 10 | 6 |
| Stephan et al. (2020), Germany | Frailty index (50 items) | 632 | 9 | 3 |
| Stolz et al. (2017), Europe | Frailty index (40 items) | 20,965 | 9 | 4 |
| Stolz et al. (2018), Europe | Frailty index (40 items) | 21,044 | 12 | 5 |
| Stolz et al. (2019), Europe | Frailty index (50 items) | 4,514 | 12 | 6 |
| Stow et al. (2018), United Kingdom | Frailty index (36 items) | 13,149 | 1 | Up to 12 |
| Thibeau et al. (2019), Canada | Frailty index (33 items) | 2,512 | 8 | 3 |
| Walkden et al. (2018), Europe | Frailty index (60 items) | 95,635 | 9 | 5 |
| Yang and Lee (2010), United States | Frailty index (30 items) | 84,878 | Various | 5 |
Studies Using a Method Based on the FI
The vast majority of publications exploring continuously measured frailty change over time used the FI (n = 13). However, within this selection of publications, choice of statistical analytic method varied. Publications either employed generalized estimating equations (GEEs), variations of random effects models, or mixture modeling methodologies to estimate frailty trajectories. GEE is a methodology that extends generalized linear models to correlated measures over time and estimates the population change over time (Muthén & Shedden, 1999). Random effects models (and latent growth curve models) estimate the average change over time and the heterogeneity of individual trajectories about this average (Laird & Ware, 1982). Mixture models can be understood as extensions of these models to identify subgroups of individuals with similar developmental trajectories (Liang & Zeger, 1986).
Generalized estimating equations
Four publications utilized GEE as a model for exploring the average trajectories of frailty over time. Hoogendijk et al. (2018) tracked FI change in the Longitudinal Aging Study Amsterdam (LASA) over a 17-year period with follow-ups every 3 years. Overall mean FI increased from baseline to end of follow-up (mean FI 0.17–0.39), showing a statistically significant increase in FI over time (0.05, p < .001) when adjusted for sex and baseline age. A quadratic term was added to the model to test for nonlinearity; however, this was not statistically significant. Machado-Fragua et al. (2019) also explored frailty trajectories of 644 participants of the LASA study, finding that FI increased linearly (mean FI 0.13–0.17) after 13 years’ follow-up. Li et al. (2016) studied 3,985 women aged 55 and older from the Global Longitudinal Study of Osteoporosis in Women 3-Year Hamilton Cohort (GLOW) to explore changes in FI after a major osteoporotic fracture. It was found that average change in FI progressed linearly over 3 years post-baseline (scores at each respective yearly follow-up = 0.24, 0.29, 0.31, 0.34). Lastly, Gajic-Veljanoski et al. (2018) followed community-dwelling adults aged 50 and older from the Canadian Multicentre Osteoporosis Study (CaMos) with follow-up at 5 and 10 years. Overall frailty as assessed by an FI was found to increase over the 5-year follow-up (mean increase of 0.03), but subsequently decrease the following 5 years (mean decrease of 0.02), thus exhibiting an unexpected nonlinear trajectory.
Random effect approaches
Ten publications used random-effect approaches that considered within-participant rate of frailty change. Yang and Lee (2010) collated various birth cohorts from the Health and Retirement Survey (HRS). Using growth curve models to estimate age trajectories of an FI, linear and quadratic age coefficients showed statistical significance (p < .001), indicating an increase in average FI (average increase 0.05) with further increases for every additional 10 years of age (0.01 with every 10 years). Using the same HRS cohort, Lohman et al. (2017) followed 13,495 participants over five waves between 2004 and 2012. Latent growth models estimated the number of average increases in deficits for each wave. A model adjusted for age, race, gender, marital status, household income, and smoking found a positive slope of frailty deficit accumulation over time by showing that on average 0.56 deficits (95% confidence interval [CI]: 0.49–0.63) were accumulated at each wave. Due to their FI consisting of 30 items, this equates to an average FI increase of 0.02 (0.56 ÷ 30) at each time point, building upon the baseline average FI of 0.18 (5.26 ÷ 30). Chen et al. (2015) also used the HRS in the United States to examine frailty trajectories stratifying the sample by race. Overall, growth curve analysis showed linear FI increases in the three race groups over a 12-year follow-up, although not significantly so for the Hispanic group. A quadratic effect was also statistically significant for all groups.
Stolz et al. (2017) used the Survey of Health Aging and Retirement in Europe (SHARE) data set to assess the impact of occupational class and wealth on the FI trajectories of 24,383 participants over a period of 9 years. By utilizing a quadratic growth model, it was revealed that FI trajectories increased nonlinearly (0.01, p < .001). Walkden et al. (2018) also used the SHARE data set with random effects multilevel models to determine FI trajectories, finding linear but not quadratic increases in frailty over 9 years in both a migrant and nonmigrant group. Thibeau, McDermott, McFall, Rockwood, and Dixon (2019) used a latent growth model to explore the frailty trajectory of 632 participants in the Victoria Longitudinal Study. Findings showed a significant increase in frailty scores over 8 years’ follow-up (p < .01), and significant variability in the patterns of decline (p < .01). Various publications used the English Longitudinal Study of Aging (ELSA) to explore frailty trajectories. Marshall et al. (2015) used growth curve modeling across five waves of ELSA, finding an acceleration in frailty over time (p < .001). Rogers and Fancourt (2020) calculated the trajectory of frailty progression over 10 years in the ELSA study, finding a linear rate of progression in FI. Rogers et al. (2017) also analyzed frailty trajectories using the same cohort, and reported a significant quadratic term of the estimated mean trajectory (p < .001). Finally, using linear mixed-effects models, Aguayo et al. (2019) found that among 5,377 ELSA participants, frailty had a significant linear increase (0.002 in FI with each year of age) and they also found a small quadratic effect.
Mixture models
Mixture models were employed by two of the publications to identify latent subpopulations of FI trajectories. Stow et al. (2018) found that in an English cohort of individuals attending their general practitioner (GP), a quadratic mixed model was the best fit. Using electronic GP records to create an FI, over 1 year with monthly intervals, FI progressed significantly over the year (mean of 0.25 at baseline with a linear increase of 0.002 per month). An added quadratic term improved the model but only showed a small additive effect per month. Latent growth mixture models allowed the identification of three distinct frailty trajectories: rapidly rising (0.02 FI increase per month, 2.2% of sample), moderately increasing (0.01 FI increase per month, 21.2% of sample), and stable (0.001 FI increase per month, 76.6% of sample).
Stephan et al. (2020) explored frailty trajectories of five adjacent birth cohorts from the Cooperative Health Research in the Region of Augsburg cohort study. Using generalized linear mixed models, findings showed that although frailty levels were higher in more recent cohorts (>1933), FI showed a consistent increase with age in all cohorts. Stolz et al. (2018) used SHARE over a 12-year period with five waves of FI data. Despite a high attrition rate whereby more than half of the participants dropped out of the study, linear mixed models, which assumed missing data at random, were able to provide good estimates of frailty trajectories. Findings showed a great deal of heterogeneity within individual-level trajectories but an overall nonlinear increase in FI scores with particularly heightened increases from 70 years and older. It was noted that trajectories of the participants who dropped out (n = 12,381) were steeper than those who completed the final wave of follow-up (n = 8,663). Consequently, the effect of higher attrition at each follow-up wave meant that subsequent waves became increasingly selective toward those with a more gradual incline in FI and accordingly may have underestimated the trend of frailty change. Stolz, Mayerl, and Freidl (2019) followed their 2018 study by using mixed location-scale models to model frailty trajectories of 4,514 participants in the SHARE data set. This approach is an extension of a mixed effects model that permits the explicit modeling of the variance terms and showed linear frailty growth increased over a 10-year period. However, findings also showed that within-person deviations from the trajectories increased with age, meaning that over time, the rates of up and down fluctuations from an individual’s frailty trajectory also increased.
Other models
Finally, of those publications using the FI, Mitnitski et al. (2012) used the Canadian National Population Health Survey, with Poisson distributions which can calculate the number of accumulations in FI over time. Analyses found that on average FI rates increased linearly at each of the waves of data collection; however, individual rates of change were subject to much heterogeneity in their level of decline, with some even showing improvements over time.
Publications Using a Measurement Based on the Fried Phenotype
Publications measuring rate of change with a continuous (n = 2) or composite modification (n = 4) of the Fried Phenotype (Fried et al., 2001) were less common but still provided a valuable insight into rate of frailty change. As with the FI, differences in statistical analyses existed between publications.
GEEs and Random Effects Models
Buchman and colleagues used data from the Religious Orders Study (ROS) and the Memory and Aging Project (MAP) in several publications where a frailty measure similar to Fried’s criteria was employed. This included grip strength, timed walk, body composition, and fatigue. A composite score was calculated from these factors by converting them into z-scores using the means and standard deviations of scores at baseline. Similar trends showing gradual increases in frailty were found; however, these varied slightly depending on the cohorts and statistical analyses used. Buchman et al. (2007) followed 823 participants of the MAP study every year for up to 8 years. Using ordinary least squares regression, the rate of frailty change on the composite scale over the follow-up period was calculated for each participant. Initial scores ranged from −1.73 to 1.92, and average rate of change was found to increase at 0.09 (±0.30) units per year. This publication was followed up by Buchman et al. (2009), who used the same participants and used GEE to show similar findings that frailty composite scores were found to significantly increase with each annual time point (0.08, p < .001). Importantly, this publication also compared the difference in rate of change estimations when looked at cross-sectionally versus longitudinally. Findings showed that the cross-sectional effect of frailty across age substantially underestimated the actual rate of change, with the effect in the longitudinal models two and a half times higher. Buchman et al. (2013) followed 791 participants of the MAP and ROS studies every year for up to 14 years (mean = 6.4, SD = 2.8) to explore frailty rate of change. Using a linear mixed-effect model, this time frailty was found to increase each year (mean of 0.12 units per year). A follow-up from this group reinforced these findings with Buchman et al. (2014) using a sample of 2,167 participants in the same cohorts. Using bivariate random coefficient models to estimate the rate of change across multiple observations, frailty was shown to increase each year (0.09 units per year).
Mixture models
Peek et al. (2012) used a sample of 2,061 Mexican Americans aged 65 and older within five waves of the Hispanic Established Populations for the Epidemiologic Study of the Elderly. Over the course of 12 years, rate of frailty change was assessed using a continuously measured modification of the Fried Phenotype (Fried et al., 2001). Using trajectory mixture modeling, three distinct frailty trajectories were identified: a consistently low group, a progressive moderate group, and a progressive high group. Despite the lack of reported coefficients for each slope, the three trajectories were reported to differ, significantly so between the progressive moderate and progressive high group (p = .01). Liu et al. (2018) also explored distinct trajectories of frailty, this time in relation to overlap between cognition and frailty. Using the Yale Precipitating Events Project, a continuous modification of the Fried Phenotype was used to quantify frailty every 18 months for 9 years. In this instance, a mixed modeling approach allowed for the identification of four distinct joint trajectories into which individuals fell: those with no cognitive or frailty decline (27.8%); a slow cognitive decline with a progressive frailty (45.5%); a quick cognitive decline with a progressive frailty (20.2%); and an accelerated cognitive and frailty decline (6.5%).
Handling of missing data
The majority of publications either did not report a method of handling missing data, or used a method involving the exclusion of participants who had a certain level of missing data throughout the follow-up period. Other publications dealt with missing data with a variety of statistical analysis methods including multiple and mean imputation and dummy variable adjustment that made the assumption that data were missing at random. See Tables 2 and 3 for details.
Table 2.
Summary of the Statistical Models Used in Included Publications Using the Frailty Index (FI)
| Type of analysis | Publication | Statistical method as reported in publication | Time metric | Covariates explored | Results (rate of change) | Treatment of missing data |
|---|---|---|---|---|---|---|
| Generalized estimating equations | Gajic-Veljanoski et al. (2018) | Multivariate generalized estimating equations | Time | Osteoporotic fractures, obesity, physical activity | Linear then nonlinear | Multiple imputation and scenario analyses |
| Hoogendijk et al. (2018) | Generalized estimating equations | Time | Age | Linear | Excluded those with >20% missing data | |
| Li et al. (2016) | Generalized estimating equations | Time | Osteoporotic fractures | Linear | Multiple imputation | |
| Machado-Fragua et al. (2019) | Multivariable generalized estimating equations | Time | Vitamin K | Linear | Excluded those with missing values | |
| Random effect approaches | Aguayo et al. (2019) | Linear mixed-effects models | Age | Diabetes, hemoglobin A1C, fasting plasma glucose | Linear and nonlinear | Multiple imputation |
| Chen et al. (2015) | Growth curve analysis | Time | Hours of caregiving undertaken, race | Linear | Mean imputation | |
| Lohman et al. (2017) | Latent growth curve modeling | Time | Depression, falls, nursing home admissions | Linear | Secondary missing data analysis | |
| Marshall et al. (2015) | Multilevel growth curve models | Age | Gender, wealth | Linear and nonlinear | Excluded those with <30 FI items | |
| Rogers et al. (2017) | Multilevel growth curve models | Age | Age, physical activity | Nonlinear | Excluded those with <30 FI items | |
| Rogers and Fancourt (2020) | Multilevel growth curve models | Age | Cultural engagement | Linear | Excluded those with missing values | |
| Stolz et al. (2017) | Growth curve models | Time | Country | Nonlinear | Excluded those with >5% missing data | |
| Thibeau et al. (2019) | Latent growth modeling | Age | Sex, executive function, processing speed | Linear | Excluded participants without enough data | |
| Walkden et al. (2018) | Random effects multilevel models | Age | Migrant status | Linear | Coded as missing at random where >20% missing data | |
| Yang and Lee (2010) | Growth curve models | Age | Age | Linear and nonlinear | Excluded those with <25 FI items | |
| Mixed models | Stephan et al. (2020) | Generalized linear mixed models | Age | Birth cohort membership | Linear | Excluded those with >20% missing data |
| Stolz et al. (2018) | Linear mixed model | Age | Education, effect of dropping out of study | Nonlinear | Compared data missing at random and not at random | |
| Stolz et al. (2019) | Mixed-effects regression models | Age | Location, education, sex | Linear | Not reported | |
| Stow et al. (2018) | Latent growth mixture models | Time to death | Age, mortality | Nonlinear | Not reported | |
| Other models | Mitnitski et al. (2012) | Poisson approximation model | Time | Age | Linear | Not reported |
Table 3.
Summary of the Statistical Models Used in Included Publications Using the Fried Criteria
| Type of analysis | Publication | Statistical method as reported in publication | Time metric | Covariates explored | Results (rate of change) | Treatment of missing data |
|---|---|---|---|---|---|---|
| Generalized estimating equations | Buchman et al. (2009) | Generalized estimating equations | Time | Mortality | Linear | Excluded those without valid follow-up data |
| Random effects models | Buchman et al. (2007) | Linear fixed-effects model | Time | Brain pathology | Linear | Excluded those without valid follow-up data |
| Buchman et al. (2013) | Linear fixed-effects model | Time | Brain pathology | Linear | Excluded those without valid follow-up data | |
| Buchman et al. (2014) | Bivariate random coefficient models | Time | Brain pathology | Linear | Excluded those without valid follow-up data | |
| Mixed models | Liu et al. (2018) | Group-based mixed modeling approach | Time | Mini-Mental State Examination scores | Linear | Not reported, low rate of attrition |
| Peek et al. (2012) | Trajectory mixed models | Time | Age, education, wealth, social support | Linear | Not reported |
Risk and Protective Factors
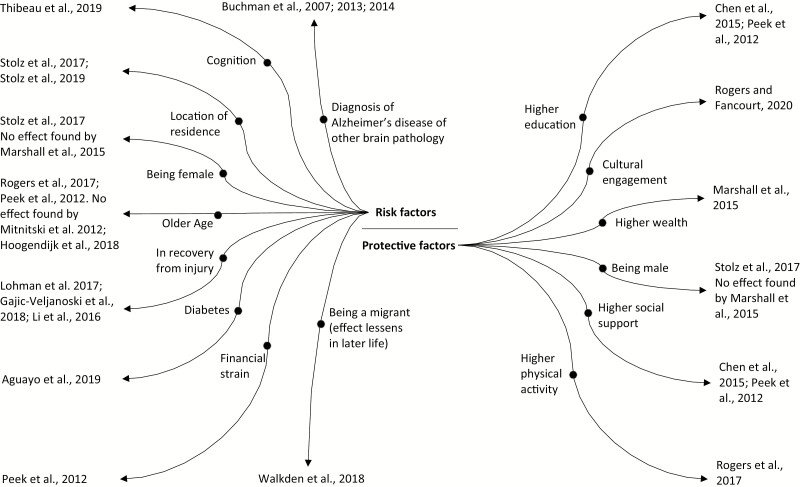
All of the included publications discussed rate of change and factors associated with it. However, the risk and protective factors associated with rate of change varied across publications. Significant factors are summarized in Figure 2.
Figure 2.
Diagram summarizing the risk and protective factors associated with rate of frailty change.
Age
Age is a factor which was frequently found to be associated with frailty level and change, although the direction of the association varied by publication. Whereas Rogers et al. (2017) showed that the gradient of frailty trajectory was found to differ by age group in older adults, others reported either the opposite or null effects. Mitnitski et al. (2012) reported that slopes of frailty change did not differ according to age and Hoogendijk et al. (2018) found that despite absolute change in FI being higher for those who were older at baseline, the rate of increase across the follow-up was similar between a 65–75 and 75+ group, indicating that rate of change is relatively stable across age groups. Peek et al. (2012), who used mixture models to find three distinct frailty trajectories, found that age was a significant factor in determining membership in a moderate and high progressive trajectory, suggesting that older age not only increases risk of frailty progression but also follows a separate trajectory to younger age groups. By comparing different birth cohorts, Stephan et al. (2020) showed that frailty levels can differ depending on when you were born; however, the actual rate of frailty change over time is not significantly affected.
Gender
A further example of the disparities in findings is shown in the effect gender can have on rate of frailty change. While Stolz et al. (2017) found that women accumulated health deficits at a quicker rate than men, Marshall et al. (2015) found that although women had higher frailty scores than men at each time point, their slopes were consistent across the follow-up period, indicating that these gender disparities did not widen over time.
Brain pathology
Three publications from the same research group explored the rate of frailty change in relation to brain pathology. Buchman et al. (2007) found that rate of frailty change was associated with incident Alzheimer’s disease, showing that every 0.1 increase per year in frailty score equated to a 12% increased risk of developing Alzheimer’s disease. Furthermore, Buchman et al. (2013) showed that more than 8% of the variance of a steeper decline in frailty was explained by several brain pathologies including microinfarcts, Alzheimer’s disease, Lewy body disease, and nigral neuronal loss, indicating that brain pathologies are associated with steeper declines of frailty in older age. Finally, Buchman et al. (2014) showed that brain pathology showed independent associations with frailty change and also with cognitive change over their follow-up period.
Comorbidities and injury
Surprisingly, few longitudinal publications have focused on the associations between frailty rate of change and disease, illness, or injury. Aguayo et al. (2019) showed that in a fully adjusted model, the presence of baseline diabetes was associated with significantly higher FI levels over time and that this difference stayed consistent over time. Risk of injury was also associated with rate of frailty change. Lohman et al. (2017) found that per-unit increase in frailty deficit accumulation at each wave the likelihood of suffering from a serious fall increased by 52% (odds ratio [OR] = 1.52, CI: 1.12–2.08). Related to these findings were two publications suggesting that rate of frailty change is particularly high for those recovering from osteoporotic fractures (Gajic-Veljanoski et al., 2018; Li et al., 2016).
Socioeconomic factors
Two studies found a protective effect of education on rate of frailty change (Chen et al., 2015; Peek et al., 2012). Peek et al. (2012) identified three distinct frailty trajectories and found that higher education related to a lower chance of membership to the high frailty trajectory group (−0.19, p < .01). Similarly, financial factors were found to affect trajectories, with the same publication showing that financial strain was significantly related to frailty increase in the low and moderate groups, but not in the high group (low; 0.24, p < .05, moderate; 0.06, p < .01). Marshall et al. (2015) reinforced these findings with results showing that poorer individuals have steeper slopes than those in a wealthier category. Finally, social support networks were suggested to affect frailty change. Findings by Chen et al. (2015) suggested that caring for grandchildren is associated with less of a decline in frailty even after accounting for a healthy older adult effect, although reverse causality cannot be ruled out. Peek et al. (2012), however, only found that social support influenced one out of their three distinct trajectories of frailty progression (a progressive moderate trajectory).
Location
Country of residence also seemed to have some influence on frailty change, possibly due to differences in socioeconomics between countries. Stolz et al. (2017) showed that when quadratic growth curve models were stratified by country, FI trajectories were steeper for those living in Southern European countries than countries further north. Stolz et al. (2019) reinforced these findings using mixed-effects location-scale regression models to show that frailty levels in Europe were lowest in Switzerland and highest in Spain. While no other studies explored these geographical differences, Walkden et al. (2018) explored differences in migrants versus nonmigrants and found that at 50 years old, migrants have higher levels on the FI compared with nonmigrants (0.15 vs 0.14, p < .001). However, over time migrants accumulate deficits at a slower rate than nonmigrants, until there is no significant difference between groups in those 80–90 years old. This convergence effect remained even after adjustment for numerous confounders.
Physical activity
Rogers et al. (2017) found that for those who engaged in vigorous physical activity, frailty progression was significantly slowed in all age groups, indicating that lifestyle factors such as physical activity may be able to improve frailty trajectories, but that these changes must be substantial. However, discussion within this publication points out that they cannot prove causality, so it may be that increases in frailty decrease the level of physical activity. It is noted that future research should aim to address this issue of reverse causality.
Cognition
Although the study of “cognitive frailty” has been gaining traction in the frailty literature, we only found one publication which explored the association between cognitive ability and frailty trajectories. Thibeau et al. (2019) used latent growth models to show that a steeper increase in FI was associated with a more rapid decline in executive functions. A similar effect was found for processing speed in females but not in males. The direction of this relationship remains unclear.
Cultural engagement
Rogers and Fancourt (2020) investigated the association between frailty and the engagement in cultural activities such as attending the theater, cinema, or a museum on a regular basis. A fully adjusted multilevel growth model showed FI trajectories for those with varying degrees of cultural engagement. Findings showed a dose–response relationship between older adults who had higher levels of cultural engagement (every few months or more) and lower risks of developing frailty and a slower progression of frailty over a 10-year follow-up.
Diet
Diet is a factor somewhat underrepresented in the longitudinal frailty literature. Only one publication addressed the effect that an element of diet can have on frailty rate of change. Machado-Fragua et al. (2019) investigated the association between frailty and vitamin K, a group of vitamins obtained from animal foods to aid the body in several essential processes. Findings showed that although higher baseline vitamin K was associated with a higher level of frailty, it was not associated with rate of change over time as FI levels increased consistently across all groups.
Discussion and Implications
Overall, our findings show a heterogeneous field of research with frailty trajectories measured in diverse ways, statistical analyses differing, and inconsistency in the reporting of findings. Despite this lack of consistency, in general, trajectories show a gradual worsening in frailty over time. Most publications reported linear trajectories; however, several also found quadratic changes which suggest a variation in rate of change over time, while some publications (Gajic-Veljanoski et al., 2018; Mitnitski et al., 2012; Rogers et al., 2017) did report small improvements in frailty over time for some participants. These improvements could be explained by the different rates of attrition of frailer individuals at follow-up visits across publications, potentially resulting in a healthy survivor effect.
Numerous risk factors were investigated in the publications, showing somewhat inconsistent findings. In particular the effect of age and gender on frailty trajectories remains unclear and warrants further study as current findings show conflicting results. Inconsistent findings regarding the effect of age could be attributed to the differential impact of a healthy survivor effect across samples; gender differences across publications might be explained by the combination of a longer life expectancy of women and differences in sample compositions. A common theme of the publications was that of socioeconomic status and its relationship to frailty. In general it was found that those who are less affluent, with lower education, and lower levels of social support, tend to follow a steeper frailty trajectory (Marshall et al., 2015; Stolz et al., 2017; Walkden et al., 2018). In particular, the effect of social support on frailty change was reported as a significant factor, with the potential to provide protective effects against a rapid decline trajectory (Chen et al., 2015; Peek et al., 2012). This highlights a major public health priority that should be explored further; if those with low socioeconomic status are at greater risk of a steep frailty decline, then interventions can potentially target these populations with greater effect. Injury (Gajic-Veljanoski et al., 2018) and brain pathology (Buchman et al., 2007, 2009, 2013, 2014) also seem to contribute to the gradient of frailty slope. Again, these findings have important implications by showing the potential for targeted interventions, such as lifestyle changes or earlier screening for brain health abnormalities, to mitigate harmful frailty trajectories in those at highest risk. Several publications were able to identify latent subpopulations with differing frailty trajectories. These findings indicate that frailty progression affects individuals differently according to a number of factors. This area of research is crucial as it allows us to explore why certain individuals have similar trajectories and potentially allows for interventions to be tailored to those at highest risk of membership to a steep decline trajectory. Accordingly, further longitudinal research should continue to explore why trajectories differ across individuals. For instance, future studies could investigate the differences in frailty trajectories according to certain hormones, inflammatory biomarkers, genetics, or even personality traits. Additionally, it will be important to understand how these risk factors interact with one another; for instance, future research may focus on uncovering mediating effects of particular factors on the rate of frailty change. There is significant potential for this line of research as it could help inform future intervention strategies and guide policy for those at highest risk of frailty. With a robust research base in frailty risk and prevention there will be the potential to improve our ability to accurately identify individuals who are at greatest risk of frailty. This paves the way for intervention strategies to be designed and tested to ultimately prevent or even reverse frailty progression. For instance, the research included in this review suggests several potential modifiable protective factors such as increased physical activity (Rogers et al., 2017), social support (Chen et al., 2015; Peek et al., 2012), and cultural engagement (Rogers & Fancourt, 2020). Accordingly, clinical care may adapt to target those with a high risk of rapid frailty progression, and provide increased support and education related to physical activity.
There were several limitations which make the results less generalizable. While the types of populations included were varied, developing countries with lower income, education, and health care were somewhat underrepresented. Also, our search only found publications that considered community-based populations with no publications looking at frailty in care homes or in clinical care. The lack of inclusion of these populations makes overall conclusions less generalizable as these individuals are likely to be the most frail (Gajic-Veljanoski et al., 2018). Our collective knowledge of frailty progression will be enhanced if future research improves the generalizability of study populations by including less-represented populations. Additionally, we did not search gray literature, ongoing/unpublished studies, or publications written in a language other than English, all of which may have provided additional information. Due to time restraints, primarily one researcher undertook data extraction with the second researcher undertaking a 20% sample extraction; however, ideally both researchers should complete the entire extraction independently to ensure validity. A further limitation, which affects most longitudinal research, is the high incidence of sample attrition. The publications included in this review were no exception; for instance, Hoogendijk et al. (2018) saw a reduction in participants from 1,659 at baseline to 297 at 17-year follow-up. Several publications discussed their sample attrition rates and their method of dealing with missing data through the use of imputation or techniques which account for missing data such as maximum likelihood estimation (Lohman et al., 2017). These methods require assumptions to be made about the reasons for which data were missing. However, these assumptions were rarely explained or justified. Although longitudinal models have been shown to remain effective even with high sample attrition (Stolz et al., 2018), handling missing data in a justifiable manner should be prioritized in future research to reduce bias toward those who remain in the study and likely have lower levels of frailty. Without dealing with this issue and considering the condition of those who drop out of the study, we may be underestimating the levels of frailty in the general population.
An additional limitation that hampered our ability to synthesize published research is the diversity of factors investigated in relation to frailty trajectories. To enhance opportunities to evaluate consistency and reproducibility of results and perform evidence synthesis, future research should aim to estimate within-person frailty changes in a coordinated manner with higher consistency in choice of analytical models and variable coding. This will generate knowledge that will permit the comparison of trajectories of reference persons with identical age at baseline, gender, and education.
In the context of frailty trajectories, the use of continuous frailty measures rather than categorical comes with the distinct advantage of being able to assess minor but significant changes. This was demonstrated in many of the included publications whereby the changes recorded from baseline to follow-up would not have been considered significant had they been measured by a categorical measure. The identification of these subtle temporal changes and examination of factors associated with these changes is essential to enhance our knowledge of how frailty can be prevented or slowed down. However, the inclusion of any continuously quantified frailty measurement also has limitations due to the discrepancies between how frailty is conceptualized and the lack of a gold standard definition (Levers, Estabrooks, & Ross Kerr, 2006). The different methods are assumed to be measuring the same construct when they may in fact be tapping into different domains. Following a template set by Gale, Westbury, and Cooper (2018), utilizing both categorical and continuous measures of frailty may be useful in the future to compare these differing methods. It has been suggested that these measures should be used to complement rather than oppose each other to build a better picture of frailty (Cesari, Gambassi, van Kan, & Vellas, 2013). By comparing these methods it may help to bridge the gap and work toward establishing a gold standard of frailty measurement which allows results to be compared in an accurate and replicable fashion (Aguayo et al., 2017). However, as it stands, without a defined framework of frailty measurement and analysis, research will continue to be inconsistent and incomparable.
Given the high rates of frailty in older adults and the detrimental effects it can have on overall health and mortality, studying the way frailty progresses is crucial. Overall, our findings show a heterogeneous field of research with vastly different methods for measuring, analyzing, and reporting data, hampering our ability to undertake a meta-analysis. Much of our discussion emphasizes the necessity for a more consistent approach to longitudinal research. While progress has been made on the definition of frailty with senior researchers reaching a consensus on some aspects of a frailty definition (Morley et al., 2013), it may be necessary to undertake a coordinated methodological approach to longitudinal frailty research. By using similar approaches and statistical methods, it will be more likely that a meta-analysis can be undertaken, and a more precise understanding of our current research can be obtained.
Despite these mixed results, our overall findings help to elucidate the progressive nature of frailty and highlight the disparity in how it affects separate groups of individuals in different ways. In particular socioeconomic factors, social support, physical activity, and brain pathologies seem to provide some determination of how an individual’s frailty will progress over time, a finding which has important implications for public health policy as well as individuals and their caregivers. A major issue with drawing firm conclusions is the differences in how frailty is conceptualized and measured. Consequently, future research needs to provide a more consistent method to frailty measurement, a first step in establishing this consistency may be by incorporating multiple frailty measures within the same studies in order to contrast findings. Researchers should also focus on longitudinal studies which explore the risk factors associated with frailty trajectories as these are likely to have implications for how frailty can be prevented and treated in the future.
Funding
This work was supported by the University of Edinburgh and will be included in a PhD thesis by M. Welstead. A PhD scholarship was awarded by Age UK as part of the Disconnected Mind project to M. Welstead. The Alzheimer Scotland Dementia Research Centre which is funded by Alzheimer Scotland funds T. Russ. G. Muniz-Terrera is funded by a NIH/NIA program project grant (P01AG043362; 2013–2018).
Supplementary Material
Acknowledgments
Thanks to Donna Watson from the University of Edinburgh who advised on the suitability of our search terms.
Conflict of Interest
None reported.
References
- Aguayo, G. A., Donneau, A. F., Vaillant, M. T., Schritz, A., Franco, O. H., Stranges, S.,...Witte, D. R. (2017). Agreement between 35 published frailty scores in the general population. American Journal of Epidemiology, 186(4), 420–434. doi: 10.1093/aje/kwx061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo, G. A., Hulman, A., Vaillant, M. T., Donneau, A. F., Schritz, A., Stranges, S.,...Witte, D. R. (2019). Prospective association among diabetes diagnosis, HbA1c, glycemia, and frailty trajectories in an elderly population. Diabetes Care, 42(10), 1903–1911. doi: 10.2337/dc19-0497 [DOI] [PubMed] [Google Scholar]
- Ahmed, N., Mandel, R., & Fain, M. J. (2007). Frailty: An emerging geriatric syndrome. The American Journal of Medicine, 120(9), 748–753. doi: 10.1016/j.amjmed.2006.10.018 [DOI] [PubMed] [Google Scholar]
- Buchman, A. S., Boyle, P. A., Wilson, R. S., Tang, Y., & Bennett, D. A. (2007). Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosomatic Medicine, 69(5), 483–489. doi: 10.1097/psy.0b013e318068de1d [DOI] [PubMed] [Google Scholar]
- Buchman, A. S., Wilson, R. S., Bienias, J. L., & Bennett, D. A. (2009). Change in frailty and risk of death in older persons. Experimental Aging Research, 35(1), 61–82. doi: 10.1080/03610730802545051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman, A. S., Yu, L., Wilson, R. S., Boyle, P. A., Schneider, J. A., & Bennett, D. A. (2014). Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 69(12), 1536–1544. doi: 10.1093/gerona/glu117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman, A. S., Yu, L., Wilson, R. S., Schneider, J. A., & Bennett, D. A. (2013). Association of brain pathology with the progression of frailty in older adults. Neurology, 80(22), 2055–2061. doi: 10.1212/WNL.0b013e318294b462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari, M., Gambassi, G., van Kan, G. A., & Vellas, B. (2013). The frailty phenotype and the frailty index: Different instruments for different purposes. Age and Ageing, 43(1), 10–12. doi: 10.1093/ageing/aft160 [DOI] [PubMed] [Google Scholar]
- Chamberlain, A. M., St Sauver, J. L., Jacobson, D. J., Manemann, S. M., Fan, C., Roger, V. L.,...Finney Rutten, L. J. (2016). Social and behavioural factors associated with frailty trajectories in a population-based cohort of older adults. BMJ Open, 6(5), e011410. doi: 10.1136/bmjopen-2016-011410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, F., Mair, C. A., Bao, L., & Yang, Y. C. (2015). Race/ethnic differentials in the health consequences of caring for grandchildren for grandparents. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 70(5), 793–803. doi: 10.1093/geronb/gbu160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried, L. P., Tangen, C. M., Walston, J., Newman, A. B., Hirsch, C., Gottdiener, J.,...McBurnie, M. A.; Cardiovascular Health Study Collaborative Research Group . (2001). Frailty in older adults: Evidence for a phenotype. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 56(3), M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- Gajic-Veljanoski, O., Papaioannou, A., Kennedy, C., Ioannidis, G., Berger, C., Wong, A. K. O.,...Adachi, J. D. (2018). Osteoporotic fractures and obesity affect frailty progression: A longitudinal analysis of the Canadian multicentre osteoporosis study. BMC Geriatrics, 18(1), 4. doi: 10.1186/s12877-017-0692-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale, C. R., Westbury, L., & Cooper, C. (2018). Social isolation and loneliness as risk factors for the progression of frailty: The English Longitudinal Study of Ageing. Age and Ageing, 47(3), 392–397. doi: 10.1093/ageing/afx188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. T., & Green, S. (Eds.). (2008). Cochrane handbook for systematic reviews of interventions. Chichester, UK: John Wiley & Sons, Ltd. [Google Scholar]
- Hoogendijk, E. O., Rockwood, K., Theou, O., Armstrong, J. J., Onwuteaka-Philipsen, B. D., Deeg, D. J. H., & Huisman, M. (2018). Tracking changes in frailty throughout later life: Results from a 17-year longitudinal study in the Netherlands. Age and Ageing, 47(5), 727–733. doi: 10.1093/ageing/afy081 [DOI] [PubMed] [Google Scholar]
- Iwasaki, M., Yoshihara, A., Sato, N., Sato, M., Minagawa, K., Shimada, M.,...Miyazaki, H. (2018). A 5-year longitudinal study of association of maximum bite force with development of frailty in community-dwelling older adults. Journal of Oral Rehabilitation, 45(1), 17–24. doi: 10.1111/joor.12578 [DOI] [PubMed] [Google Scholar]
- Kojima, G., Taniguchi, Y., Iliffe, S., Jivraj, S., & Walters, K. (2019). Transitions between frailty states among community-dwelling older people: A systematic review and meta-analysis. Ageing Research Reviews, 50, 81–88. doi: 10.1016/j.arr.2019.01.010 [DOI] [PubMed] [Google Scholar]
- Laird, N. M., & Ware, J. H. (1982). Random-effects models for longitudinal data. Biometrics, 38(4), 963–974. [PubMed] [Google Scholar]
- Levers, M. J., Estabrooks, C. A., & Ross Kerr, J. C. (2006). Factors contributing to frailty: Literature review. Journal of Advanced Nursing, 56(3), 282–291. doi: 10.1111/j.1365-2648.2006.04021.x [DOI] [PubMed] [Google Scholar]
- Li, G., Papaioannou, A., Thabane, L., Cheng, J., & Adachi, J. D. (2016). Frailty change and major osteoporotic fracture in the elderly: Data from the global longitudinal study of osteoporosis in women 3-year Hamilton cohort. Journal of Bone and Mineral Research, 31(4), 718–724. doi: 10.1002/jbmr.2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, K.-Y., & Zeger, S. L. (1986). Longitudinal data analysis using generalized linear models. Biometrika, 73(1), 13–22. doi: 10.1093/biomet/73.1.13 [DOI] [Google Scholar]
- Liu, Z., Han, L., Gahbauer, E. A., Allore, H. G., & Gill, T. M. (2018). Joint trajectories of cognition and frailty and associated burden of patient-reported outcomes. Journal of the American Medical Directors Association, 19(4), 304–309.e2. doi: 10.1016/j.jamda.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman, M. C., Mezuk, B., & Dumenci, L. (2017). Depression and frailty: Concurrent risks for adverse health outcomes. Aging & Mental Health, 21(4), 399–408. doi: 10.1080/13607863.2015.1102199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Fragua, M. D., Hoogendijk, E. O., Struijk, E. A., Rodriguez-Artalejo, F., Lopez-Garcia, E., Beulens, J. W., & van Ballegooijen, A. J. (2019). High dephospho-uncarboxylated matrix Gla protein concentrations, a plasma biomarker of vitamin K, in relation to frailty: The Longitudinal Aging Study Amsterdam. European Journal of Nutrition. Advance online publication. doi: 10.1007/s00394-019-01984-9 [DOI] [PubMed] [Google Scholar]
- Marshall, A., Nazroo, J., Tampubolon, G., & Vanhoutte, B. (2015). Cohort differences in the levels and trajectories of frailty among older people in England. Journal of Epidemiology and Community Health, 69(4), 316–321. doi: 10.1136/jech-2014-204655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitnitski, A. B., Mogilner, A. J., & Rockwood, K. (2001). Accumulation of deficits as a proxy measure of aging. TheScientificWorldJournal, 1, 323–336. doi: 10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitnitski, A., Song, X., & Rockwood, K. (2012). Trajectories of changes over twelve years in the health status of Canadians from late middle age. Experimental Gerontology, 47(12), 893–899. doi: 10.1016/j.exger.2012.06.015 [DOI] [PubMed] [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G.; PRISMA Group . (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- Morley, J. E., Vellas, B., van Kan, G. A., Anker, S. D., Bauer, J. M., Bernabei, R.,...Walston, J. (2013). Frailty consensus: A call to action. Journal of the American Medical Directors Association, 14(6), 392–397. doi: 10.1016/j.jamda.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén, B., & Shedden, K. (1999). Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics, 55(2), 463–469. doi: 10.1111/j.0006-341x.1999.00463.x [DOI] [PubMed] [Google Scholar]
- O’Caoimh, R., Galluzzo, L., Rodríguez-Laso, Á., Van der Heyden, J., Ranhoff, A. H., Carcaillon-Bentata, L.,...Liew, A.; Work Package 5 of the Joint Action ADVANTAGE . (2018). Transitions and trajectories in frailty states over time: A systematic review of the European Joint Action ADVANTAGE. Annali dell’Istituto Superiore di Sanita, 54(3), 246–252. doi: 10.4415/ANN_18_03_12 [DOI] [PubMed] [Google Scholar]
- Peek, M. K., Howrey, B. T., Ternent, R. S., Ray, L. A., & Ottenbacher, K. J. (2012). Social support, stressors, and frailty among older Mexican American adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 67(6), 755–764. doi: 10.1093/geronb/gbs081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood, K., & Mitnitski, A. (2007). Frailty in relation to the accumulation of deficits. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 62(7), 722–727. doi: 10.1093/gerona/62.7.722 [DOI] [PubMed] [Google Scholar]
- Rogers, N. T., & Fancourt, D. (2020). Cultural engagement is a risk-reducing factor for frailty incidence and progression. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 75(3), 571–576. doi: 10.1093/geronb/gbz004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, N. T., Marshall, A., Roberts, C. H., Demakakos, P., Steptoe, A., & Scholes, S. (2017). Physical activity and trajectories of frailty among older adults: Evidence from the English Longitudinal Study of Ageing. PLoS One, 12(2), e0170878. doi: 10.1371/journal.pone.0170878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle, S. D., Mitnitski, A., Gahbauer, E. A., Gill, T. M., & Rockwood, K. (2008). A standard procedure for creating a frailty index. BMC Geriatrics, 8, 24. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenholm, S., Ferrucci, L., Vahtera, J., Hoogendijk, E. O., Huisman, M., Pentti, J., . . . Kivimäki, M. (2018). Natural course of frailty components in people who develop frailty syndrome: Evidence from two cohort studies. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 74(5), 667–674. doi: 10.1093/gerona/gly132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan, A. J., Strobl, R., Schwettmann, L., Meisinger, C., Ladwig, K. H., Linkohr, B.,...Grill, E. (2020). The times we are born into and our lifestyle choices determine our health trajectories in older age—Results from the KORA-Age study. Preventive Medicine, 133, 106025. doi: 10.1016/j.ypmed.2020.106025 [DOI] [PubMed] [Google Scholar]
- Stolz, E., Mayerl, H., & Freidl, W. (2019). Fluctuations in frailty among older adults. Age and Ageing, 48(4), 547–552. doi: 10.1093/ageing/afz040 [DOI] [PubMed] [Google Scholar]
- Stolz, E., Mayerl, H., Rásky, É., & Freidl, W. (2018). Does sample attrition affect the assessment of frailty trajectories among older adults? A joint model approach. Gerontology, 64(5), 430–439. doi: 10.1159/000489335 [DOI] [PubMed] [Google Scholar]
- Stolz, E., Mayerl, H., Waxenegger, A., Rásky, É., & Freidl, W. (2017). Impact of socioeconomic position on frailty trajectories in 10 European countries: Evidence from the survey of health, ageing and retirement in Europe (2004–2013). Journal of Epidemiology and Community Health, 71(1), 73–80. doi: 10.1136/jech-2016-207712 [DOI] [PubMed] [Google Scholar]
- Stow, D., Matthews, F. E., & Hanratty, B. (2018). Frailty trajectories to identify end of life: A longitudinal population-based study. BMC Medicine, 16(1), 171. doi: 10.1186/s12916-018-1148-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibeau, S., McDermott, K., McFall, G. P., Rockwood, K., & Dixon, R. A. (2019). Frailty effects on non-demented cognitive trajectories are moderated by sex and Alzheimer’s genetic risk. Alzheimer’s Research & Therapy, 11(1), 55. doi: 10.1186/s13195-019-0509-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkden, G. J., Anderson, E. L., Vink, M. P., Tilling, K., Howe, L. D., & Ben-Shlomo, Y. (2018). Frailty in older-age European migrants: Cross-sectional and longitudinal analyses of the Survey of Health, Aging and Retirement in Europe (SHARE). Social Science & Medicine (1982), 213, 1–11. doi: 10.1016/j.socscimed.2018.07.033 [DOI] [PubMed] [Google Scholar]
- Wells, G. A., Shea, B., O’Connell, D., Peterson, J., Welch, V., Losos, M., & Tugwell, P. (2011). The Newcastle–Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, ON, Canada: Ottawa Hospital Research Institute. Retrieved from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- Yang, Y., & Lee, L. C. (2010). Dynamics and heterogeneity in the process of human frailty and aging: Evidence from the US older adult population. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65(2), 246–255. doi: 10.1093/geronb/gbp102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.