Abstract
Purpose
Nonadherence is a leading cause of death-censored allograft loss in kidney transplant recipients. Strong associations have tied tacrolimus intrapatient variability (IPV) to degree of nonadherence and high tacrolimus IPV to clinical endpoints such as rejection and allograft loss. Nonadherence is a dynamic, complex problem best targeted by multidimensional interventions, including mobile health (mHealth) technologies.
Methods
This was a secondary planned analysis of a 12-month, parallel, 2-arm, semiblind, 1:1 randomized controlled trial involving 136 adult kidney transplant recipients. The primary aims of the TRANSAFE Rx study were to assess the efficacy of a pharmacist-led, mHealth-based intervention in improving medication safety and health outcomes for kidney transplant recipients as compared to usual care.
Results
Patients were randomized equally to 68 patients per arm. The intervention arm demonstrated a statistically significant decrease in tacrolimus IPV over time as compared to the control arm (P = 0.0133). When analyzing a clinical goal of tacrolimus IPV of less than 30%, the 2 groups were comparable at baseline (P = 0.765), but significantly more patients in the intervention group met this criterion at month 12 (P = 0.033). In multivariable modeling, variables that independently impacted tacrolimus IPV included time, treatment effect, age, and warm ischemic time.
Conclusion
This secondary planned analysis of an mHealth-based, pharmacist-led intervention demonstrated an association between the active intervention in the trial and improved tacrolimus IPV. Further prospective studies are required to confirm the mutability of tacrolimus IPV and impact of reducing tacrolimus IPV on long-term clinical outcomes.
Keywords: adherence, kidney transplant, mHealth, tacrolimus, telemedicine
Key Points.
A multimodal mobile health system targeting medication safety can improve medication nonadherence.
Our study represents only the second interventional study to demonstrate an impact on tacrolimus intrapatient variability, a surrogate endpoint for nonadherence.
Tacrolimus intrapatient variability has been highly associated with acute rejection and allograft loss, indicating that it is a surrogate endpoint worthy of consideration in future prospective studies.
In the most recent Scientific Registry of Transplant Recipients (SRTR) report, 90% of patients with new kidney transplants received an immunosuppressant regimen based on the calcineurin inhibitor tacrolimus.1 Having tacrolimus as the primary maintenance immunosuppressant has vastly reduced rejection rates over the past 2 decades.2 However, tacrolimus does not come without limitations, including posttransplant diabetes mellitus (PTDM), nephrotoxicity, patient intolerabilities, adverse drug reactions, and a need for strict adherence to the regimen. Additionally, some clinicians question the amount of time and effort required to maintain patients within a narrow goal therapeutic range when there has been little evidence to correlate whole blood tacrolimus trough concentration (Cmin) ranges of tacrolimus and efficacy.3,4 While whole blood tacrolimus Cmin values have been poorly associated with long-term area under the curve (AUC) and efficacy, associations between increased intrapatient variability (IPV) and rejection or graft loss have been increasingly demonstrated.3-6 Populations of highly adherent kidney transplant recipients have been demonstrated to have IPVs as narrow as 15%; however, IPVs of 30% or greater have been associated with inferior graft outcomes, likely indicating patients with clinical nonadherence.6,7
Nonadherence, which is a causative factor in at least 47% of allograft losses by rejection, has proven to be a difficult foe due to its myriad causes, including socioeconomic factors, healthcare organization barriers, disease chronicity–related factors, therapy-related factors (adverse events and medication errors), and patient-related factors (healthcare beliefs, work schedule, etc).8,9 It is apparent that generalized interventions are less effective than personalized interventions and that unidimensional interventions are less effective than multidimensional interventions.8 In the digital age, multidimensional interventions to combat nonadherence increasingly rely on mobile technology, especially because of the ability to monitor chronic disease management, adverse reactions and drug toxicities, and medication adherence via electronic medication dispensers or self-report and to personalize communication with providers, all through a single medium.10-14 Reese and colleagues10 demonstrated significant improvement in medication adherence as the multimodality of the intervention increased. However, the method of adherence capture (electronic pill bottles) does not translate well into clinical care as a monitoring modality, and they did not assess the comparability of electronic monitoring to any widely used community-monitoring methodology. McGillicuddy and colleagues15 performed an exploratory secondary planned analysis of a randomized controlled trial (RCT) involving a comprehensive mobile health (mHealth) intervention targeted to improve adherence in a demonstrably nonadherent population to evaluate its impact on tacrolimus IPV. They found that, despite similar baseline IPV, patients in their intervention saw significant improvement in their 12-month IPVs and that a significantly larger proportion of intervention vs control patients achieved an IPV less than 40%.
We conducted a prospective, RCT that tested an mHealth-based, pharmacist-led telehealth intervention (TRANSAFE Rx study) with the primary objective of improving medication safety. Within the study, we planned a secondary analysis to identify the impact of the intervention on tacrolimus IPV as compared to the control group. We theorized that better control of medication errors and adverse events would improve patient nonadherence. Here we report the results of the secondary analysis.
Materials and methods
Study design.
TRANSAFE Rx was a 12-month, parallel, 2-arm, semiblind, 1:1 RCT involving 136 adult kidney transplant recipients. Details on the study rationale and design have previously been published (ClinicalTrials.gov identifier, NCT03247322).16
Study population.
Adult kidney recipients between 6 and 36 months after transplantation were eligible for the study. Patients were excluded if they were multiorgan recipients; incapable of measuring their own blood pressure and blood glucose (if applicable); incapable of self-administering medications; incapable of speaking, hearing, and reading English; or incapable of utilizing the mHealth app following sufficient training.
Study objectives.
The primary aims of the TRANSAFE Rx study were to assess the efficacy of a pharmacist-led, mHealth-based intervention on improving medication safety and health outcomes in kidney transplant recipients as compared to usual care. The primary outcome of the TRANSAFE Rx study was the incidence and severity of medication errors and adverse events, as compared between the intervention and control arms. Here we report a secondary planned analysis of the impact of the intervention on tacrolimus IPV as compared to usual care.
Study intervention.
Patients randomized to the intervention arm were provided the same usual care as the control cohort. In addition, this group received clinical pharmacist–led supplemental medication therapy monitoring and management, utilizing a smartphone-enabled mHealth app, integrated with risk-driven televisits and home-based blood pressure and blood glucose monitoring (when applicable). The mHealth app, developed by our group, provided patients with an accurate list of their medication regimen that was automatically updated from the electronic medical record (EMR), timely medication reminders, automated messages triggered by missed doses or scheduled health monitoring, personalized value-based messages to encourage continued involvement, and blood pressure and blood glucose trends (when applicable). Monthly and patient-initiated surveys regarding the frequency and severity of common side effects were delivered through the app. The intervention included dedicated clinical transplant pharmacist telemonitoring of medications, tacrolimus IPV, reported adverse effects, medical appointment adherence, and weekly blood pressure and glucose readings (if applicable). The clinical transplant pharmacist was notified of any medication changes (from the transplant team or outside providers) and transitions of care (hospitalization or emergency room visits at any hospital) by patient self-report or via new medications reported in the EMR and was automatically notified of critical blood pressure or blood glucose values. The pharmacist responded to alerts through communication with the patient and care team. Televisits enabled the pharmacist to conduct medication reviews to identify any medication safety issues, ensure accurate medications through transitions of care, screen for drug interactions, and provide recommendations to the patient. One dedicated clinical transplant pharmacist provided all care to the intervention arm. Full details regarding the development and validation of the mHealth app and dashboard are published elsewhere.16
Statistical analysis.
The analysis utilized the intent-to-treat methodology. Data are reported using percentages for nominal and ordinal variables and were compared using Fisher’s exact test or Pearson’s χ 2 test as appropriate. For continuous variables with a normal distribution, results are reported as mean and SD with statistical comparison performed using Student’s t test for 2 independent samples. For non–normally distributed variables, results are reported as median and interquartile range, with statistical comparison conducted using the Mann-Whitney U test. Tacrolimus IPV as measured through the coefficient of variation (CV) ([mean/SD] ⋅ 100) was assessed at monthly intervals for each patient (12-month rolling average), starting at the time of randomization and continuing for 12 months. Rolling averages were calculated and estimated on a monthly interval. Each month, 1 additional month of tacrolimus levels was added to the CV calculation and the levels from the previous month were removed. We used a generalized linear mixed model (GLMM) with Gaussian distribution to account for correlation of repeated measures and the nonindependence of measures over the course of time, with coefficients estimated using generalized estimating equations (GEE). A 2-sided P value of <0.05 was considered statistically significant. All data were analyzed using SAS 9.4 (SAS Institute, Cary, NC).
Study variables.
Variables included within the GLMM included time (months post randomization), the treatment effect per month post randomization, age (years), female gender, African American race, years on dialysis before transplantation, calculated panel reactive antibody (a measure of the degree of sensitization to human antibodies), human leukocyte antigen (HLA) mismatches, cold ischemic time (the time the organ spent in cold storage between procurement from the donor and being taken out to be transplanted in the recipient), warm ischemic time (the time the organ spent at normal temperatures during the act of sewing it into the recipient and reperfusing it with blood), rabbit anti-thymocyte globulin (rATG) induction, delayed graft function (need for dialysis within a week of transplantation), donor seropositivity and recipient seronegativity for cytomegalovirus (CMV), donation after cardiac death, kidney donor risk index (estimation of the risk of graft failure due to donor factors), and history of diabetes.
Results
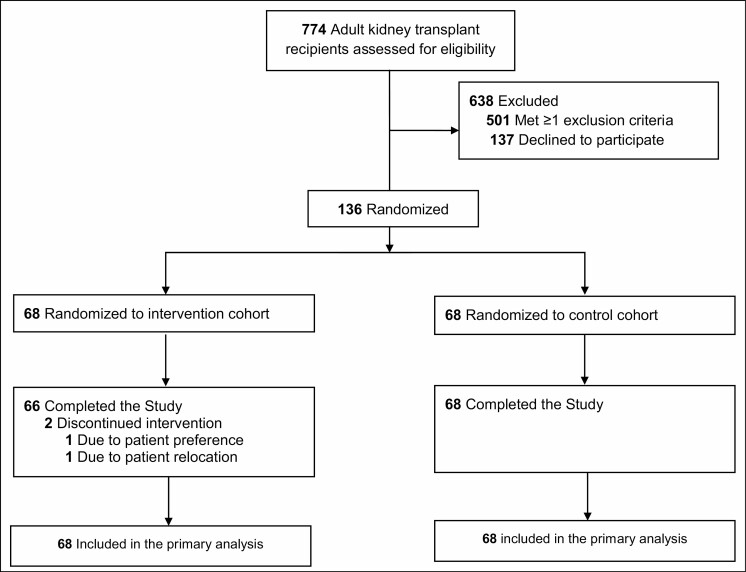
Of the 774 patients undergoing kidney transplantation assessed for eligibility between October 2017 and January 2019, 273 were approached and 136 agreed to participate, provided informed consent, and were randomized evenly to the intervention and control cohorts (Figure 1). Two patients withdrew from the study intervention arm before completing the study, for a 98.5% retention rate; both patients are included in this intent-to-treat analysis. For the entire cohort, the mean age was 50.7 years, 56.6% of patients were male, and 64.0% of patients were black. The primary etiologies of end-stage renal disease were diabetes and hypertension, followed by polycystic kidney disease and lupus. When comparing the treatment arms, baseline characteristics were similar (Table 1). There were more patients in the control group with a history of diabetes, along with a larger proportion of kidneys transplanted after cardiac death of the donor, while there were more patients in the intervention group who experienced delayed graft function (Table 1).
Figure 1.
Randomization and patient flow in the TRANSAFE Rx study.
Table 1.
Baseline Characteristics
| Characteristic | Control (n = 68)a | Intervention (n = 68)a |
|---|---|---|
| Age, mean (SD), years | 51.2 (13.7) | 50.2 (12.3) |
| Male | 42 (61.8) | 35 (51.5) |
| Female | 26 (38.2) | 33 (48.5) |
| White | 19 (27.9) | 27 (39.7) |
| Black | 47 (69.1) | 40 (58.8) |
| Hispanic | 2 (2.9) | 1 (1.5) |
| History of diabetes | 35 (51.5) | 19 (27.9) |
| History of hypertension | 61 (91.0) | 63 (92.6) |
| cPRA at transplant, mean (SD) | 49.7 (41.0) | 50.0 (38.6) |
| HLA mismatch, median (IQR) | 4 (4–5) | 5 (3–5) |
| Cold ischemic time, mean (SD), hours | 17.5 (8.0) | 16.4 (8.0) |
| Warm ischemic time, mean (SD), minutes | 38.0 (15.8) | 38.1 (11.9) |
| Basiliximab induction | 26 (38.2) | 25 (36.8) |
| Thymoglobulin induction | 42 (61.8) | 43 (63.2) |
| Delayed graft function | 8 (12.7) | 18 (26.5) |
| Time post transplantation, mean (SD), years | 1.2 (0.8) | 1.2 (0.7) |
| CMV serostatus (donor/recipient) | ||
| Neg/Neg | 7 (10.3) | 5 (7.4) |
| Neg/Pos | 22 (32.4) | 13 (19.1) |
| Pos/Pos | 30 (44.1) | 29 (42.6) |
| Pos/Neg | 8 (11.8) | 18 (26.5) |
| Unknown | 1 (1.5) | 3 (4.4) |
| Donor age, mean (SD), years | 37.3 (14.5) | 32.9 (11.9) |
| Donor gender | ||
| Male | 47 (69.1) | 41 (60.3) |
| Female | 20 (29.4) | 27 (39.7) |
| Missing | 1 (1.5) | 0 (0.0) |
| Living donor | 7 (10.3) | 6 (8.8) |
| DCD donor | 11 (18.3) | 5 (8.2) |
| KDRI in deceased donor, mean (SD), % | 46.2 (24.8) | 37.1 (23.4) |
Abbreviations: CMV, cytomegalovirus; cPRA, calculated panel reactive antibody; DCD, donation after cardiac death; HLA, human leukocyte antigen; IQR, interquartile range. KDRI, Kidney Donor Risk Index; Neg, negative; Pos, positive.
aAll data are number (percentage) of patients unless indicated otherwise.
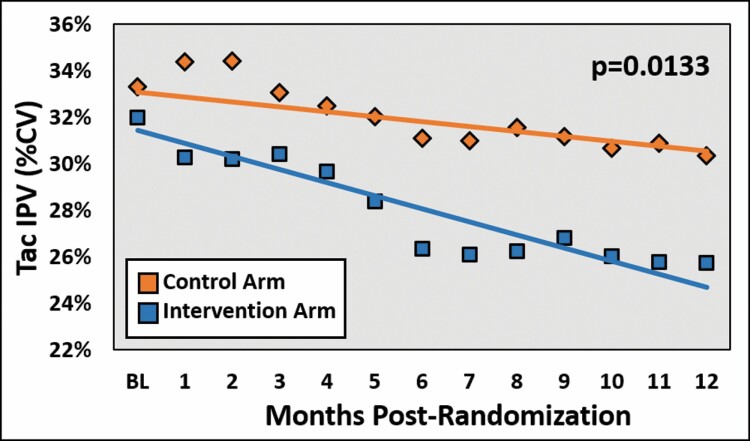
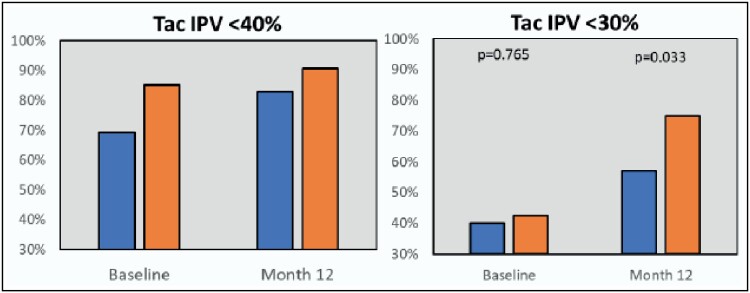
The mean tacrolimus IPV for each treatment arm was assessed on a monthly interval using a 12-month rolling period, and the change in slope was compared between the groups. As displayed in Figure 2, the intervention arm demonstrated a statistically significant decrease in tacrolimus IPV over time as compared to the control arm (P = 0.0133). When assessed by cutpoint, a higher proportion of patients in the control group had a tacrolimus IPV below 40% at baseline (P = 0.017), while the 2 groups were comparable at 12 months (P = 0.224). When analyzing a tighter goal of <30%, the 2 groups were comparable at baseline (P = 0.765), but significantly more patients in the intervention group met this criterion at month 12 as compared to the control group (P = 0.033; Figure 3). In multivariable modeling, variables that independently impacted tacrolimus IPV included time post randomization (–0.25; 95% confidence interval [CI], –0.38 to –0.11; P = 0.0003), treatment effect (–0.25 [95% CI, –0.44 to –0.05], P = 0.0133), age (–0.24 [95% CI, –0.39 to –0.08], P = 0.0026), and warm ischemic time (0.11 [95% CI, 0.06 to 0.30], P = 0.0416).
Figure 2.
Mean tacrolimus intrapatient variability from baseline to 12 months post randomization, comparing the treatment and control arms. Tac IPV indicates tacrolimus intrapatient variability; CV, coefficient of variation.
Figure 3.
Proportion of patients achieving tacrolimus intrapatient variability of <30% and <40% at baseline and at end of study, comparing the control and treatment arms. The blue bars for each category represent the control group, while the orange bars represent the intervention group. Tac IPV indicates tacrolimus intrapatient variability.
The vast majority of patients in the intervention arm completed a poststudy satisfaction survey (62 [91%]) that included a multiple-choice question with 5 answers (2 answers demonstrating dissatisfaction or worsening, 1 answer indicating a neutral opinion or no change, and 2 answers demonstrating satisfaction or improvement). In terms of the app itself, 93% of respondents indicated that they were either satisfied (n = 15 [24%]) or very satisfied (n = 43 [69%]) with the ease of use of the app. There were also favorable responses to questions on how well the intervention worked with the patient’s personal health situation (n = 19 [31%]“satisfied”; n = 39 [63%] “very satisfied”) and the impact the intervention had on communication with the patient’s medical providers (n = 17 [27%] “no change”; n = 22 [36%] “better”; n = 23 [37%] “very much better”), the patient’s perceived involvement in medical decisions (n = 18 [29%] “no change”; n = 18 [29%] “better”; n = 26 [42%] “very much better”), and the patient remembering to take medications (n = 2 [3%] “worse”; n = 13 [21%] “no change”; n = 19 [31%] “better”; n = 28 [45%] “very much better”).
Discussion
This secondary planned analysis of an mHealth-based, pharmacist-led intervention demonstrated an association between the active intervention in the trial and improving tacrolimus IPV.
We showed a strong association between the intervention and the change in slope in tacrolimus IPV over time as well as an increased proportion of patients falling within a clinical goal for IPV of <30%. The treatment effect was demonstrated to be independently associated with tacrolimus IPV in multivariable modeling, along with already known impactful variables such as time from transplantation and age. Tacrolimus IPV becomes less variable over time with fewer medication adjustments typically occurring, while patients also demonstrate better medication adherence as they advance farther away from the age range of 18 to 25 years. Warm ischemic time was also identified as independently associated with IPV, but we have a harder time theorizing the reason for this. While various methods of measuring tacrolimus IPV have been utilized in research studies, higher tacrolimus IPV has consistently been associated with increased rates of rejection and allograft loss.6 Although these data are well recognized, the ability for interventions to impact tacrolimus IPV is less prevalent. With the commonly held belief that tacrolimus IPV is strongly associated with medication adherence, researchers have sought to improve tacrolimus IPV by switching patients from twice-daily tacrolimus to once-daily extended-release tacrolimus, a well-known strategy for improving adherence to a number of medications.17 This strategy has met with varied success, with Wu and colleagues18 demonstrating improved achievement of a clinical goal tacrolimus IPV after conversion to a once-daily formulation (3.1% vs 17.4%, P < 0.01), whereas Stifft and colleagues19 did not see a clinically significant change in tacrolimus IPV after conversion to a once-daily formulation.
In an RCT of a technological intervention with a primary aim of improving adherence in a nonadherent population of individuals receiving kidney transplants, McGillicuddy and colleagues15 were able to demonstrate a significant reduction in 12-month IPV (estimated difference between the arms of 0.48% per month, 5.8% greater reduction over the 12-month follow-up period, P = 0.046) and improvement in the proportion of patients achieving a clinical goal of a tacrolimus IPV of <40% (80% vs 70%, P = 0.001) in the intervention arm as compared to the control arm. We believe this is the only other interventional clinical trial to demonstrate the ability to significantly improve tacrolimus IPV. In this previous trial, the multidimensional intervention included an electronic medication tray with reminder capabilities, a Bluetooth-enabled blood pressure monitor, and a smartphone app. While the aim of our study was not specifically to improve adherence, tacrolimus IPV and medical clinic visit adherence were part of the assessment, in addition to other factors that impact adherence, such as side effects and transitions of care. Our intervention was similar to that of McGillicuddy and colleagues in terms of it being a multidimensional mHealth system; however, we did not specifically target nonadherent patients. Our mHealth system was also developed with the goal of fitting seamlessly into any patient’s lifestyle and therefore relied on patient-reported adherence instead of an electronic medication tray, which is burdensome and difficult to accommodate for patients who work and/or travel. We believe the excellent response rate for the end-of-study survey and favorable responses to questions on ease of use and perceived utility are representative of our attempts to fit the app seamlessly into patient lifestyle and improve the patient-provider relationship.
Our study and this analysis had several limitations. We did not use an attention control in the control arm to minimize the risk of bias from increased attention and its impact on adherence or other factors that might impact tacrolimus IPV, such as quicker and easier access to a healthcare professional in situations where patients might have lost access to their medications. Because of this, it is impossible to determine whether singular components of the intervention or the intervention as a whole impacted tacrolimus IPV. It is worth noting, however, that the purpose of the mHealth intervention and pharmacist focused on the care of these patients was to increase attention to patients, identify medication-related problems before they resulted in clinical sequelae, and improve communication between healthcare providers and reduce errors during transitions of care. Our study also included patients who were more than 6 months from transplantation and so cannot represent accurately what impact our intervention would have, if any, on tacrolimus IPV in patients within the early timeframe. Within 6 months of transplantation, however, patients receive significantly more attention from healthcare providers, which gradually reduces over time and is likely a key factor impacting increasing nonadherence rates with increasing time post transplantation. The time window we studied is also more suited to identifying patients with high tacrolimus IPV due to nonadherence, because patients are less likely to have other causative factors such as frequent dose changes, interacting medications, and hospitalizations. Additionally, our study did not specifically target tacrolimus IPV as a primary endpoint, and therefore any associations seen in this secondary planned analysis are hypothesis generating and cannot be taken as proof that the intervention can improve tacrolimus IPV in a pragmatic cohort. We also do not present long-term outcomes to demonstrate that an improvement in tacrolimus IPV results in reduced rates of deleterious clinical endpoints. There is sufficient evidence, however, that high tacrolimus IPV, as an indicator of nonadherence, is highly associated with acute rejection and increased risk of allograft loss. Thus, tacrolimus IPV is a surrogate endpoint worthy of future study.
Conclusion
In conclusion, we present the impact of an mHealth-based, pharmacist-led intervention on tacrolimus IPV and demonstrate a strong independent association between the intervention and improved tacrolimus IPV. We believe we are only the second group to see such an effect based on an interventional study. Because of the consistent association between high tacrolimus IPV and deleterious allograft outcomes, our results indicate that the use of multidimensional technologies may lead to improved clinical outcomes. Further prospective studies are required to confirm the mutability of tacrolimus IPV and impact of reducing tacrolimus IPV on long-term clinical outcomes.
Disclosures
Funding for this work was provided by the Agency for Healthcare Research and Quality (grant R18HS023754). The authors have declared no potential conflicts of interest.
References
- 1. Hart A, Smith JM, Skeans MA, et al. . OPTN/SRTR 2017 Annual Data Report: Kidney. Am J Transplant. 2019;19Suppl 2:19-123. [DOI] [PubMed] [Google Scholar]
- 2. Shrestha BM. Two decades of tacrolimus in renal transplant: basic science and clinical evidences. Exp Clin Transplant. 2017;15(1):1-9. [DOI] [PubMed] [Google Scholar]
- 3. Andrews LM, Li Y, De Winter BCM, et al. . Pharmacokinetic considerations related to therapeutic drug monitoring of tacrolimus in kidney transplant patients. Expert Opin Drug Metab Toxicol. 2017;13(12):1225-1236. [DOI] [PubMed] [Google Scholar]
- 4. Laskow DA, Vincenti F, Neylan JF, Mendez R, Matas AJ. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: a report of the United States Multicenter FK506 Kidney Transplant Group. Transplantation. 1996;62(7):900-905. [DOI] [PubMed] [Google Scholar]
- 5. Fleming JN, Weimert NA. Novel strategies for immune monitoring in kidney transplant recipients. Adv Chronic Kidney Dis. 2010;17(5):e63-e77. [DOI] [PubMed] [Google Scholar]
- 6. Gonzales HM, McGillicuddy JW, Rohan V, et al. . A comprehensive review of the impact of tacrolimus intrapatient variability on clinical outcomes in kidney transplantation. Am J Transplant. 2020;20(8):1969-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leino AD, King EC, Jiang W, et al. . Assessment of tacrolimus intrapatient variability in stable adherent transplant recipients: establishing baseline values. Am J Transplant. 2019;19(5):1410-1420. [DOI] [PubMed] [Google Scholar]
- 8. Low JK, Williams A, Manias E, Crawford K. Interventions to improve medication adherence in adult kidney transplant recipients: a systematic review. Nephrol Dial Transplant. 2015;30(5):752-761. [DOI] [PubMed] [Google Scholar]
- 9. Sellarés J, de Freitas DG, Mengel M, et al. . Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12(2):388-399. [DOI] [PubMed] [Google Scholar]
- 10. Reese PP, Bloom RD, Trofe-Clark J, et al. . Automated reminders and physician notification to promote immunosuppression adherence among kidney transplant recipients: a randomized trial. Am J Kidney Dis. 2017;69(3):400-409. [DOI] [PubMed] [Google Scholar]
- 11. McGillicuddy JW, Gregoski MJ, Weiland AK, et al. . Mobile health medication adherence and blood pressure control in renal transplant recipients: a proof-of-concept randomized controlled trial. JMIR Res Protoc. 2013;2(2):e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taber DJ, Pilch NA, McGillicuddy JW, Mardis C, Treiber F, Fleming JN. Using informatics and mobile health to improve medication safety monitoring in kidney transplant recipients. Am J Health-Syst Pharm. 2019;76(15):1143-1149. [DOI] [PubMed] [Google Scholar]
- 13. Fleming JN, Taber DJ, McElligott J, McGillicuddy JW, Treiber F. Mobile health in solid organ transplant: the time is now. Am J Transplant. 2017;17(9):2263-2276. [DOI] [PubMed] [Google Scholar]
- 14. Levine D, Torabi J, Choinski K, Rocca JP, Graham JA. Transplant surgery enters a new era: increasing immunosuppressive medication adherence through mobile apps and smart watches. Am J Surg. 2019;218(1):18-20. [DOI] [PubMed] [Google Scholar]
- 15. McGillicuddy JW, Chandler JL, Sox LR, Taber DJ. Exploratory analysis of the impact of an mHealth medication adherence intervention on tacrolimus trough concentration variability: post hoc results of a randomized controlled trial. Ann Pharmacother. 2020;54(12):1185-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fleming JN, Treiber F, McGillicuddy J, Gebregziabher M, Taber DJ. Improving transplant medication safety through a pharmacist-empowered, patient-centered, mHealth-based intervention: TRANSAFE Rx study protocol. JMIR Res Protoc. 2018;7(3):e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15(6):e22-e33. [PubMed] [Google Scholar]
- 18. Wu MJ, Cheng CY, Chen CH, et al. . Lower variability of tacrolimus trough concentration after conversion from prograf to advagraf in stable kidney transplant recipients. Transplantation. 2011;92(6):648-652. [DOI] [PubMed] [Google Scholar]
- 19. Stifft F, Stolk LM, Undre N, van Hooff JP, Christiaans MH. Lower variability in 24-hour exposure during once-daily compared to twice-daily tacrolimus formulation in kidney transplantation. Transplantation. 2014;97(7):775-780. [DOI] [PubMed] [Google Scholar]