Abstract
Adrenarche is the maturational increase in adrenal androgen production that normally begins in early childhood. It results from changes in the secretory response to adrenocorticotropin (ACTH) that are best indexed by dehydroepiandrosterone sulfate (DHEAS) rise. These changes are related to the development of the zona reticularis (ZR) and its unique gene/enzyme expression pattern of low 3ß-hydroxysteroid dehydrogenase type 2 with high cytochrome b5A, sulfotransferase 2A1, and 17ß-hydroxysteroid dehydrogenase type 5. Recently 11-ketotestosterone was identified as an important bioactive adrenarchal androgen. Birth weight, body growth, obesity, and prolactin are related to ZR development. Adrenarchal androgens normally contribute to the onset of sexual pubic hair (pubarche) and sebaceous and apocrine gland development. Premature adrenarche causes ≥90% of premature pubarche (PP). Its cause is unknown. Affected children have a significantly increased growth rate with proportionate bone age advancement that typically does not compromise growth potential. Serum DHEAS and testosterone levels increase to levels normal for early female puberty. It is associated with mildly increased risks for obesity, insulin resistance, and possibly mood disorder and polycystic ovary syndrome. Between 5% and 10% of PP is due to virilizing disorders, which are usually characterized by more rapid advancement of pubarche and compromise of adult height potential than premature adrenarche. Most cases are due to nonclassic congenital adrenal hyperplasia. Algorithms are presented for the differential diagnosis of PP. This review highlights recent advances in molecular genetic and developmental biologic understanding of ZR development and insights into adrenarche emanating from mass spectrometric steroid assays.
Keywords: adrenal androgens, adrenarche, polycystic ovary syndrome, pubarche, steroidogenic enzyme expression, zona reticularis
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS
Adrenarche results from changes in the secretory response to ACTH, best indexed by a rise in serum dehydroepiandrosterone sulfate above that of preschool children.
Adrenarche is related to the development of the zona reticularis and its unique pattern of steroidogenic enzyme expression.
11-ketotestosterone has been recently recognized as an adrenarchal androgen that contributes significantly to serum androgenic bioactivity.
Adrenarchal androgens normally contribute to the onset of pubic hair (pubarche) and sebaceous and apocrine gland development.
Premature adrenarche is the most common cause of premature pubarche.
Premature adrenarche usually seems to be an extreme variation of normal, but it confers a modest risk for obesity and insulin resistance and possibly mood disorder and hyperandrogenism.
Premature adrenarche may be mimicked during the early stages of hyperandrogenic disorders, the most common of which is nonclassic congenital adrenal hyperplasia, which accounts for about 5% of premature pubarche.
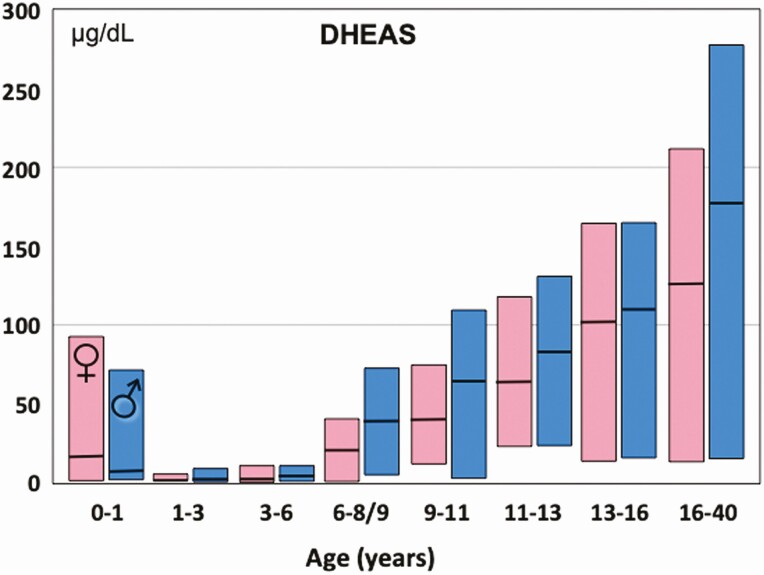
Adrenarche is the term for the maturational rise in adrenal androgen secretion that normally begins in early childhood. This “puberty of the adrenal gland” is independent of true (central/complete/gonadotropin-dependent) puberty: it begins before true puberty and maximizes in the late teenage years (1). It is indexed by a rise in the blood level of dehydroepiandrosterone sulfate (DHEAS) (Fig. 1) (1,2).
Figure 1.
Plasma DHEAS median and normal range during healthy childhood. DHEAS determined by LCMSMS. Concentrations fall during the neonatal period due to waning function of the fetal zone of the adrenal cortex. They begin to rise again starting at 3 to 6 years of age. Note: 6- to 8-year-old girls were studied (rather than 6- to 9-years-old, as in boys) to minimize the contribution of true puberty to the findings. Nevertheless, boys had significantly higher levels at most ages from 6 years onward, a trend consistent with most data. These results correlate closely with, but are lower than, those obtained by a standard direct assay of DHEAS in serum: DHEAS by radioimmunoassay = 1.8 (DHEAS by LCMSMS) – 8.4 (personal communication with AE Kulle, May 26, 2017). To convert DHEAS in µg/dL to µmol/L, multiply by 0.0271.
Graphed from data of Kulle et al (2).
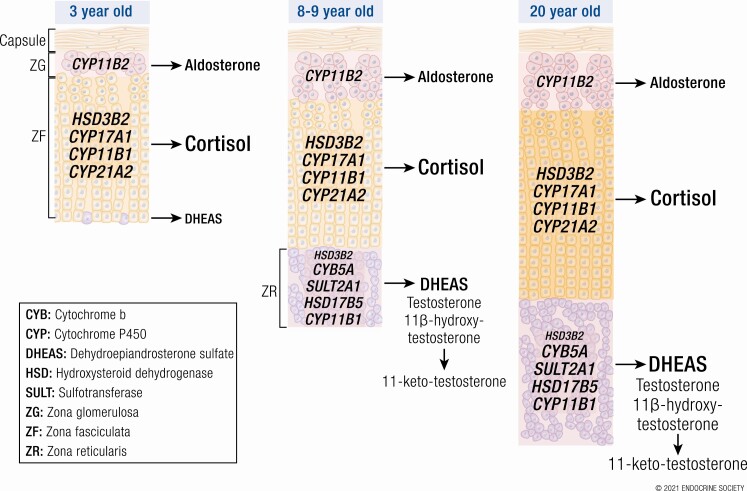
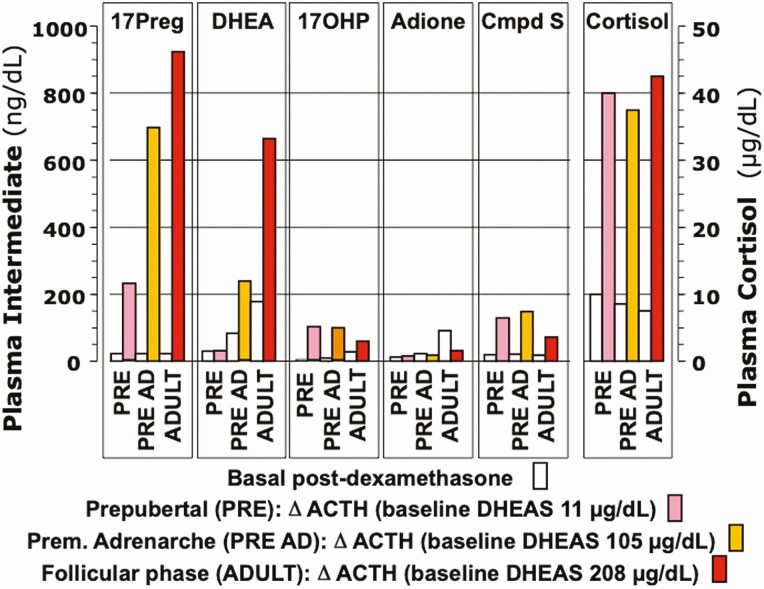
By 2000, when premature adrenarche (PreAd) was last reviewed in Endocrine Reviews (1), it was recognized that (1) adrenarche is characterized by a change in the pattern of the adrenocortical steroid response to adrenocorticotropin (ACTH) due to apparent increases in 17,20-lyase and sulfotransferase activity and a decrease in 3ß-hydroxysteroid dehydrogenase (3ßHSD) activity (Fig. 2) (1,3), (2) adrenarche was related to the growth and development of the adrenal zona reticularis (ZR) and its pattern of enzyme expression (1,4,5), and (3) adrenal androgen secretions are ACTH-dependent, and, thus, readily glucocorticoid-suppressible (1,6). The ZR becomes discernable as foci in the central adrenal cortex at 3 to 5 years of age (1,4,7), during which time a small increase in DHEAS occurs (8). At approximately 5 years of age, the ZR begins to develop into a continuous zone (4), after which the adrenarchal increase in DHEAS production becomes increasingly clear.
Figure 2.
Changing rapid steroid secretory response to ACTH stimulation across adrenarche in females. Note increased responses of the Δ 5-3ß-hydroxysteroids 17-hydroxypregnenolone and DHEA through maturational stages, in parallel with baseline DHEAS. Serum androstenedione and its Δ 4-3-ketosteroid precursor 17-hydroxyprogesterone (17OHP) rise to a lesser extent. Cortisol responses remain unchanged. Basal levels were obtained early morning after overnight dexamethasone suppression. Post-ACTH levels obtained 30 min post-ACTH1-24 administered intravenously at 8 am. Prepubertal children were 2 to 12 years old; adrenarchal girls had PreAd; adults were normal volunteers in early follicular phase of menstrual cycle. For conversion factors to nmol/L, see Table 1.
Data are from Rich et al (3). Figure adapted from Rosenfield RL, Cooke DW, Radovick S. Puberty and its disorders in the female. In: Sperling M, Majzoub JA, Menon RK, Stratakis CA, eds. Pediatric Endocrinology. 5th ed. Elsevier; 2021: 528-626. Copyright Elsevier 2021. Abbreviations: 11-deoxycortisol, Cmpd S; androstenedione, adione.
During mid-childhood prior to puberty, as a consequence of a changing pattern of steroidogenesis in response to ACTH and the related development of the ZR, the baseline pattern of adrenal steroid levels changes in a unique way (Table 1) (9). In the preadrenarchal child, from mid-infancy through age 5 years, adrenal androgen levels are very low. Thereafter, as serum DHEAS, dehydroepiandrosterone (DHEA), and their Δ 5-3ß-hydroxysteroid precursors rise in parallel, testosterone, androstenedione, and their Δ 4-3-ketosteroid precursors rise to a lesser extent, and serum cortisol levels remain constant.
Table 1.
Representative reference ranges for DHEAS, testosterone, and precursors in healthy girls, adults, and premature adrenarche: standard 2020 specialty laboratory reference radioimmunoassay methodology
| DHEAS (µg/dL) | DHEA (ng/dL) | Androstenedione (ng/dL) | Testosterone (ng/dL) | 17PREG (ng/dL) | 17OHPa (ng/dL) | 11-Deoxy-cortisol (ng/dL) | Cortisol (µg/dL) | |
|---|---|---|---|---|---|---|---|---|
| Before ACTH (8:00 am) | ||||||||
| Children, 1-5 years old | 5-35 | 20-130 | 10-50 | <20 | 10-105 | 5-115 | 20-160 | 3-20 |
| Children, 6-10 years old | 10-115 | 20-345 | 10-75 | <20 | 10-200 | 5-115 | 20-160 | 3-20 |
| Premature adrenarche | 40-130 | 50-600 | 20-75 | 10-35 | 20-350 | 5-200 | 20-160 | 3-20 |
| Early pubertal girls | 35-130 | 40-600 | 40-175 | 10-35 | 35-350 | 15-200 | 20-160 | 3-20 |
| Adult females, follicular phase | 75-255 | 100-850 | 60-200 | 20-60 | 55-360 | 15-200 | 20-160 | 3-20 |
| After ACTH1-24 (30-60 min after ≥ 10 µg/m2 IV) | ||||||||
| Children, 1-5 years old | 5-35 | 25-100 | 15-70 | <20 | 45-350 | 50-270 | 95-300 | 17-45 |
| Children, 6-10 years old | 10-115 | 70-320 | 25-100 | <20 | 60-650 | 85-270 | 95-300 | 17-45 |
| Premature adrenarche | 40-130 | 80-725 | 25-100 | 10-35 | 80-750 | 85-270 | 95-300 | 17-45 |
| Early pubertal girls | 35-130 | 70-725 | 55-230 | 10-35 | 150-750 | 90-270 | 95-300 | 17-45 |
| Adult females, follicular phase | 75-255 | 250-1470 | 60-250 | 20-60 | 150-1070 | 35-270 | 95-300 | 17-45 |
| Conversion multipliers to SI units | 0.0271 µmol/L | 0.0347 nmol/L | 0.0349 nmol/L | 0.0347 nmol/L | 0.0316 nmol/L | 0.0303 nmol/L | 0.0289 nmol/L | 0.0276 µmol/L |
Ranges for radioimmunoassay after preparatory chromatography, except for direct immunoassay of cortisol and DHEAS. Ranges differ to varying extent among laboratories. DHEAS values by LCMSMS average about 45% lower.
a 17-Hydroxyprogesterone (17OHP) early and mid-follicular phase baseline levels >140 ng/dL are found in normal women who are heterozygous for 21-hydroxylase deficiency, and they often have responses to ACTH greater than those shown. 17OHP begins rising in the preovulatory phase and peaks as high as 400 ng/dL in the luteal phase of the cycle.
Modified from Rosenfield RL. Identifying children at risk of polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:787-796.
Abbreviations: DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulfate; 17PREG, 17-hydroxypregnenolone.
The developmental biology and significance of adrenarche are among the great mysteries of endocrinology. The current review will update knowledge of the molecular genetic and biochemical basis of adrenarche, adrenarche’s contribution to serum androgenic activity, and clinically relevant aspects of normal and PreAd.
Normal Adrenarche Physiology: Adrenocortical Hormone Biosynthesis and Metabolism Overview
Endocrine Gland Hormone Biosynthesis Overview
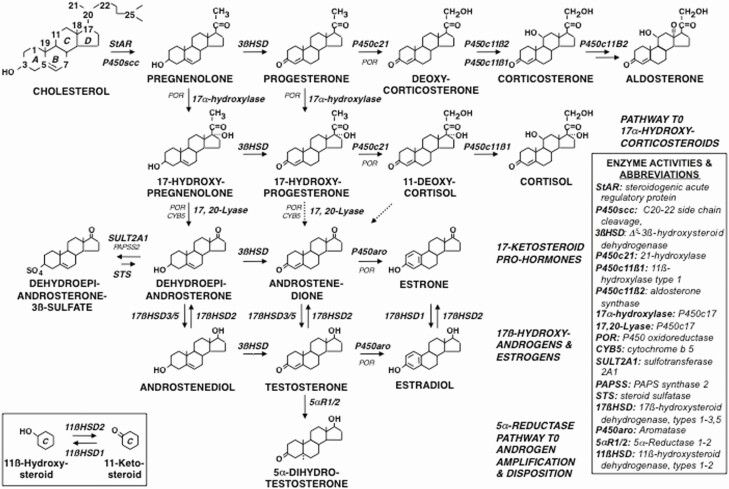
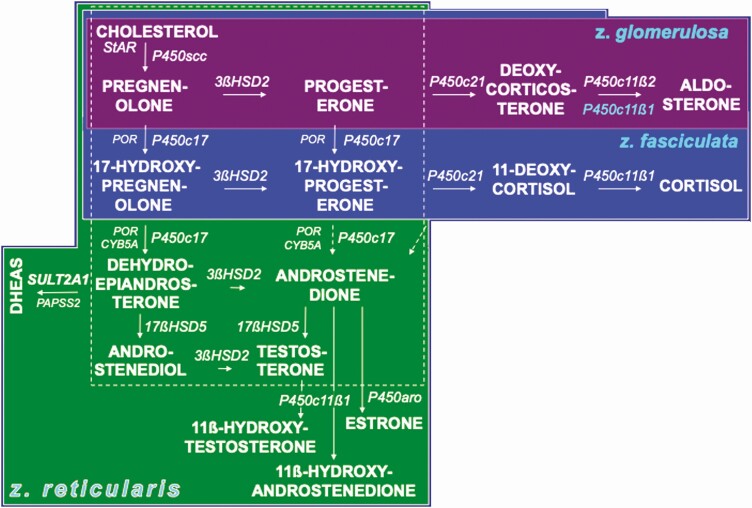
Figure 3 shows the major pathways for steroid biosynthesis—utilized in specific patterns by the adrenocortical zones and each gonad (10,11). All adrenocortical zones form the C21-Δ 5-3ß-hydroxysteroid precursor pregnenolone and express Δ 5-3ß-hydroxysteroid dehydrogenase (encoded by HSD3B2) activity to form steroids with the Δ 4-3-ketosteroid configuration (5,11). Each adrenocortical zone additionally forms characteristic secretions (Fig. 4).
Figure 3.
Major pathways of steroid hormone formation. Cholesterol carbon atoms are designated by conventional numbers and rings by conventional letters. The flow of steroidogenesis is generally downward and to the right. The top row is the pathway to progesterone and mineralocorticoids; the second row, the pathway to glucocorticoids; the third row, the 17-ketosteroids; the fourth row, 17ß-hydroxysteroids; and the bottom row, the 5α-reductase pathway to amplification and disposition of androgen. The dotted 17,20-lyase pathways are probably minor. The steroidogenic enzymes are italicized. Steroids before 3ßHSD action have the Δ 5-3ß-hydroxysteroid configuration, those formed by 3ßHSD are Δ 4-3-ketosteroids. Inset: 11ß-hydroxysteroid dehydrogenase interconversions of 11-oxysteroids, which occur in peripheral tissues. Abbreviations for enzymes are indicated in the side panel in approximate order of appearance.
Modified from Rosenfield et al (10).
Figure 4.
Organization of the adrenocortical zones. The area within the dotted square contains the core steroidogenic activities common to zona reticularis, ovarian theca cells, and testicular Leydig cells (although the latter express 17ßHSD type 3 rather than 17ßHSD5). The left column shows the Δ 5-3ß-hydroxysteroid pathway and the columns to its right shows the Δ 4-3-ketosteroid pathway. The adrenal zones are color-coded to facilitate visualizing overlapping and unique steps in the adrenal zones: the top row shows the zona glomerulosa pathway to mineralocorticoids culminating in aldosterone; the second row shows the zona fasciculata pathway to cortisol. The third row shows the zona reticularis steps to DHEAS and other 17-ketosteroids. The steroidogenic and accessory enzymes are italicized and abbreviated as in Figure 3 side panel. P450c11ß1 is expressed only in the zona fasciculata (P450c11ß1 blue lettering on mineralocorticoid pathway indicates the zona fasciculata formation of corticosterone, not shown) and zona reticularis. 3ßHSD2 expression is lower in the zona reticularis than other zones. Dotted P450c17 17,20-lyase pathways for Δ4-3-ketosteroids are relatively minor.
Modified with permission from Rosenfield RL. Identifying children at risk of polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:787-796.
The zona glomerulosa (ZG), the outer adrenocortical layer, forms mineralocorticoids. It expresses both 21-hydroxylase (P450c21) and its unique 11ß-hydroxylase (type 2, P450c11ß2) that sequentially forms corticosterone, 18-hydroxycorticosterone (not shown), and aldosterone.
The middle adrenocortical zona fasciculata (ZF) expresses P450c17 (CYP17A1), the 17α-hydroxylase activity of which is necessary for glucocorticoid and sex hormone biosynthesis. The ZF expresses P450c21 (CYP21A2) and P450c11ß1 (CYP11B1) to form corticosterone in the17-deoxycorticoid/mineralocorticoid path and cortisol in the 17α-hydroxycorticoid/glucocorticoid path.
The innermost adrenocortical ZR, ovarian theca cells, and testicular Leydig cells form 17-ketosteroids (DHEA > androstenedione) primarily via 17-hydroxypregnenolone by the 17,20-lyase activity of P450c17, which requires both the electron transfer enzymes P450 oxidoreductase and cytochrome b5 (CYB5) as cofactors and is under posttranslational regulation (12,13). Estrone is formed from androstenedione in the weakly aromatase (P450aro, CYP19A1)-expressing ZR (14,15) and in Leydig cells (16). ZR also expresses CYP11B1 (17).
17ß-Hydroxysteroids are potent sex steroids. 17ß-Hydroxysteroid dehydrogenase (17ßHSD) type 5 (17ßHSD5) is required to form testosterone from 17-ketosteroids in ZR and ovarian theca (11). This enzyme is structurally aldo-ketoreductase AKR1C3 (HSD17B5/AKR1C3). Leydig cells instead express 17ßHSD type 3 (HSD17B3) to form testosterone more efficiently (11). Estradiol is formed in ovarian granulosa cells mostly from androstenedione by the successive actions of 17ßHSD type 1 and aromatase (18). Leydig cell aromatase preferentially forms estradiol from testosterone (16) and also converts androstenedione to estrone, from which Sertoli cell 17ßHSD type 1 forms estradiol (19).
Relation of Zona Reticularis Enzyme Expression to Adrenarchal Steroid Secretion
Adults’ ACTH-stimulated adrenal secretory pattern corresponds well to the unique ZR pattern of steroidogenic enzyme expression (3,15,20).
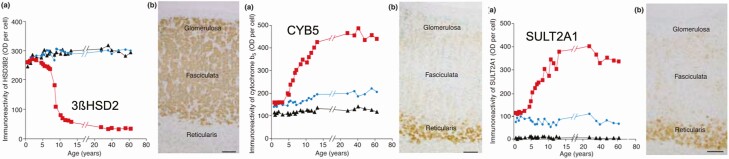
Low ZR HSD3B2/3ßHSD2 gene and enzyme expression (Fig. 5A) is the major factor underlying ZR preferential formation of Δ 5-3ß-hydroxysteroid precursors (eg, 17-hydroxypregnenolone) over their respective Δ 4-3-ketosteroid products (eg, cortisol) (5,17,21). Then preferential ZR expression of CYB5 (Fig. 5B) enhances P450c17’s 17,20-lyase already preferential conversion of 17-hydroxypregnenolone to the 17-ketosteroid DHEA (5,21). Subsequently, preferential ZR expression of sulfotransferase (SULT2A1) (Fig. 5C) sulfonates DHEA to DHEAS. DHEAS is the major circulating adrenal 17-ketosteroid secretion because within the ZR it acts to trap DHEA, shifting the reaction equilibrium in its favor (22). Liquid chromatography-mass spectrometric assay (LCMSMS) of microdissected adrenal zones indicates that 80% of DHEAS and androstenediol-sulfate arise in ZR, while pregnenolone sulfate and 17-hydroxypregnenolone sulfate arise equally in ZR and ZG (20).
Figure 5.
Age-related changes in immunoreactivity per cell of 3ßHSD2, CYPB5, and SULT2A1 in each adrenocortical zone. (a) age-related changes in immunohistochemical staining optical density/cell, (b) immunohistochemistry at 8 to 9 years of age. 3ßHSD2 (left panel) immunoreactivity is strong in the cytoplasm of adrenocortical cells of the z. glomerulosa, the z. fasciculata, and the ZR from age 7 months to 3 years. However, after age 3, 3ßHSD2 expression begins to fall in the ZR, whereas its expression remains relatively constant in the z. glomerulosa and z. fasciculata. 3ßHSD2 immunohistochemical staining at 9 years of age is marked in the z. glomerulosa and fasciculata but is low in ZR. Immunoreactivity of CYB5 (middle panel) is weakly detected in cytoplasm of adrenocortical cells in the z. glomerulosa, z. fasciculata, and ZR from ages 7 months to 3 years; its immunoreactivity becomes more pronounced in the developing z. reticularis thereafter until it reaches a plateau after age 13, while CYB5 immunoreactivity remains relatively low in the other zones. Immunohistochemical staining at 8.4 years was strong in the zona reticularis, relatively weak in the other zones. SULT2A1 (right panel) immunoreactivity followed a similar pattern to CYB5 in the zona fasciculata and zona reticularis but was very low in zona glomerulosa. SULT2A1 immunohistochemical staining at 8.4 years was strong in the ZR. Key: z. glomerulosa, black triangles; z. fasciculata, blue circles; z. reticularis, red squares. Scale bar = 100 μm.
Reprinted from Rainey et al (10) with permission from Elsevier.
Adrenal testosterone secretion seems attributable to enhanced ZR expression of HSD17B5/17ßHSD5 (17,23). ZR P450c11ß1 further converts testosterone and androstenedione to 11ß-hydroxyandrostenedione and 11ß-hydroxytestosterone (Fig. 3, inset; Fig. 4) (17).
Blood production rates (the sum of secretion and peripheral conversion of secreted precursors) of androgens in normal mid-follicular phase females are approximately 7 mg/day each of DHEA and DHEAS [serum DHEAS is higher than serum DHEA because of its extremely slow metabolic clearance rate (24)], 3.4 mg/day androstenedione, 0.5 mg/day androstenediol, and 0.23 mg testosterone (24,25). Adrenocortical secretion, directly or indirectly via peripheral conversion, accounts for most of this DHEAS production and approximately half that of the others in follicular phase women (25). Negligible amounts of estrogen (estrone > estradiol) are normally secreted by the adrenals (15).
Androgen Peripheral Interconversion
In normal women, while approximately half of androstenedione is secreted by the adrenals, half by the ovaries, peripheral conversion of serum androstenedione rather than secretion, accounts for approximately half of serum testosterone (24,26-28). This androstenedione-testosterone conversion is mediated by 17ßHSD5 (11) and occurs partly in the liver and partly in extrasplanchnic tissues (26,29).
Circulating DHEA undergoes hepatic conversion to serum DHEAS, androstenedione, and testosterone (25,30). In contrast, DHEAS contributes minimally to blood DHEA (31), contrary to early research (32); peripheral steroid sulfatase activity is normally low although ubiquitous (31,33-35). Low sulfatase activity contributes to serum DHEAS’s 2-day effective half-life (36), as does interconversion with androstenediol-sulfate and entero-hepatic sulfation of DHEA (37-39).
Secreted 11ß-hydroxyandrogens are converted by 11ß-hydroxysteroid dehydrogenase type 2 (11ßHSD2), primarily in the kidney, to 11-ketoandrostenedione and 11-ketotestosterone, by 11ßHSD2 (HSD11B2) (Fig. 3, inset) (40). These 11-ketosteroids are back-converted, mainly in liver and lung and less so in adipose tissue, by 11ßHSD1 (HSD11B1) to their 11ß-hydroxysteroid form. These conversions are essentially activity-neutral, unlike the primary role of 11ßHSD2 in inactivating corticoids (11,41).
Androgen Amplification
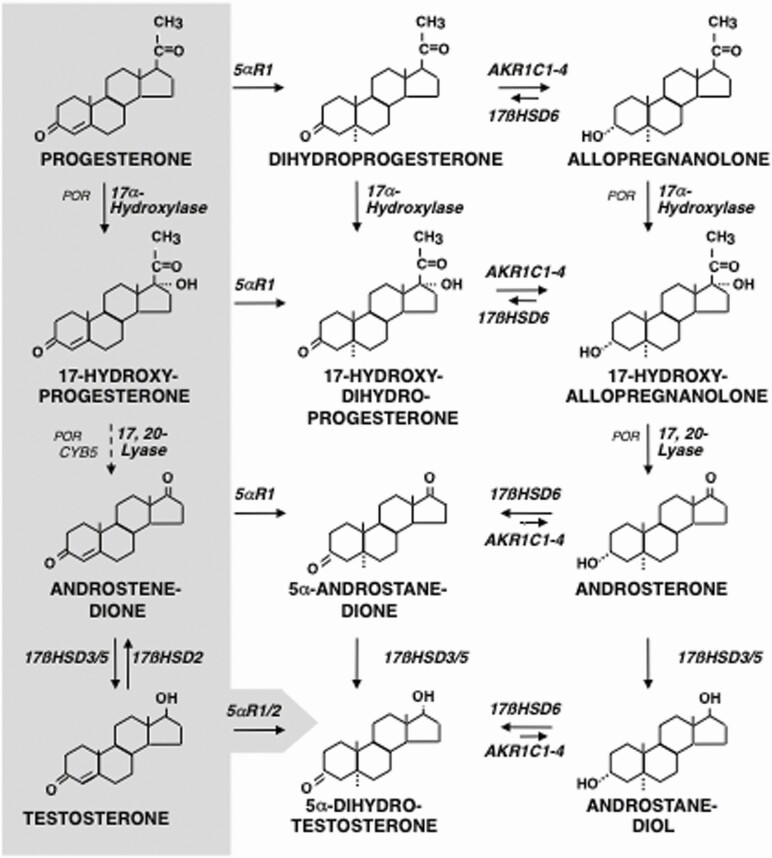
Androgen action is augmented by “intracrine” metabolism of secreted precursors within target cells. 5α-Dihydrotestosterone (DHT), the most potent natural androgen, is formed from testosterone by 5α-reductase type 2 (SDR5A2) (Fig. 3) within classic androgen targets, eg, the genital tract, genital skin, and beard dermal papillae. In nongenital skin and sebaceous cells, and also liver, 5α-reductase type 1 (5αR1) mediates DHT formation (11,42). Minor intracrine “backdoor” pathways to DHT (eg, androstenedione → androstanedione → DHT) (Fig. 6) are important in castration-resistant cancer and fetal male differentiation (11,33), as discussed in the following section.
Figure 6.
Disposition pathways of steroid metabolism and the alternative pathway to DHT. Hepatic 5α-reduction, predominantly by the type 1 isoform, is a first step on the path towards excretion of Δ 4-3-ketosteroids as glucuronides and sulfates. The endocrine biosynthetic pathway from progesterone through testosterone (Fig. 3) is shown on the gray background to the left. The disposition pathways culminate in the formation of androsterone (and etiocholanolone via a parallel 5ß-reduced pathway) as water-soluble conjugates. Key fetal tissues express versions of this pathway that permit genital tissue to form DHT from androsterone, rather than testosterone; this constitutes the “backdoor” pathway to DHT. 17-Hydroxyallopregnanolone conversion to androsterone does not require CYB5, and both reductive and oxidative 3αHSD activities of AKR1C2/4 and 17ßHSD6 (retinol dehydrogenase/3α-hydroxysteroid epimerase) are required for this pathway.
Based on Miller and Auchus (10), Flück et al (54), and Janner et al (167).
Intracrine metabolism is also a consideration in assessing DHEAS bioactivity. Although DHEAS cannot activate the androgen receptor, androgen-responsive skin expresses all the necessary enzyme activities to convert DHEAS to DHT, particularly the sebaceous gland, which has high 3ßHSD activity (43,44). Whether this pathway is sufficiently robust to mediate adrenarchal manifestations is unknown, but DHEAS levels are related to adult cystic acne (45).
Steroid Disposition and the Alternative Pathway to DHT
Androgen disposition for excretion begins with hepatic metabolism to 17-ketosteroids as sulfate and glucuronide conjugates (46). Urinary DHEAS originates from both serum DHEAS and from DHEA via hepatic SULTA1 action (11). The main urinary 17-ketosteroids, however, are androsterone and its 5ß-isomer etiocholanolone. They arise primarily from DHEA (47) after hepatic 3ßHSD1 conversion to androstenedione (Fig. 3) and from secreted androstenedione, but also from C21-precursors. Processing of androstenedione proceeds via 5αR1 (Fig. 6) (46) (or 5ß-reduction via AKR1D1, in a parallel pathway not shown (48)). Then hepatic 3α-reduction, primarily by AKR1C2 and AKR1C4 (11), forms androsterone (or etiocholanolone).
Most testosterone glucuronide, the major urinary testosterone metabolite, is formed in the liver from DHEA and androstenedione without ever reaching the peripheral circulation as unconjugated testosterone (49). Therefore, the urinary output of testosterone glucuronide poorly reflects androgen status (50). The DHT metabolite androstanediol glucuronide is likewise mainly formed in the liver (51).
Progesterone hepatic disposition is primarily along the 5ß-reduced pathway through pregnanolone (the 5ß-isomer of allopregnanolone (Fig. 6), not shown), which undergoes 20α-hydroxysteroid dehydrogenation and glucuronidation or sulfation to form pregnanediol conjugates (not shown) (11,52,53). During pregnancy, as placental progesterone production markedly increases, the “excess” progesterone [and 17-hydroxyprogesterone (17OHP) in parallel] are predominantly metabolized instead by hepatic 5αR1 and AKR1C2/4 to allopregnanolone (and 17-hydroxyallopregnanolone) (54). When these metabolites circulate through the fetus’ huge fetal adrenal, P450c17 17α-hydroxylation and 17,20-lyation form 17-hydroxyallopregnanolone and/or androsterone (Fig. 6) (11,54-56). Androsterone serves as a DHT precursor in the genital tubercle, which expresses the enzymes necessary to form DHT (Fig. 6) (56). This “backdoor” pathway to DHT, which does not require testosterone as an intermediate, contributes to the differentiation of normal male genitalia and to congenital virilization of females in states of high 17OHP production, eg, some forms of congenital adrenal hyperplasia (CAH) (56-58).
Bioactivity of Adrenal Androgens
Androgenic Biopotency of Adrenarchal Steroids
The absence of a consistent relationship of testosterone levels to pubarche, as with hirsutism and castration-resistant cancer activity, has raised the possibility of other circulating bioactive androgens. Although testosterone accounts for serum androgenic bioactivity in adult women (59-61), current bioassay insensitivity may obscure differences at low androgen levels (60).
11-Oxyandrogens were originally thought to arise as inactive metabolites of cortisol and corticosterone (62). After adrenal secretion of unconjugated 11ß-hydroxyandrostenedione and 11ß-hydroxytestosterone and the presence of their 11-keto-analogues were identified by LCMSMS, their bioactivity was addressed (15).
Table 2 displays the biopotency of adrenarchal androgens in a cell line bioassay system that stably expresses an androgen-responsive mouse mammary tumor virus-luciferase androgen receptor reporter gene and is sensitive to physiologic concentrations of androgens (ie, about 1.0 nM) (15). Of the secreted adrenal C19 steroids, only testosterone normally circulates in concentrations that exceed 50% of its maximally effective concentration for androgen receptor activation (Table 2). 11ß-Hydroxytestosterone, with a serum concentration about one third that of testosterone, is 22% to 30% as potent in activating the androgen receptor.
Table 2.
Androgenic activity 11-oxysteroid derivatives of testosterone and androstenedione
| Steroid | Adult female average serum levela ng/dL | Adult female average serum levela nmol/L | EC50b nmol/L | Potency relative to testosteroneb (%) | Maximal fold-induction luciferase (at concentration) |
|---|---|---|---|---|---|
| Testosterone | 32 | 1.1 | 0.52 | 100 | 16 (at 10 nmol/L) |
| 11KT | 21 | 0.7 | 2.7 | 33 (101) | 16 (at 30 nmol/L) |
| 11OHT | 11 | 0.4 | 2.3 | 22 (30) | 12 (at 30 nmol/L) |
| Androstenedione | 89 | 3.1 | 87 | 4.0 | 8 (at 1000 nmol/L) |
| 11KA | 15 | 0.5 | 469 | 0.3 | 10 (at 1000 nmol/L) |
| 11OHA | 117 | 3.9 | - | <0.3 | 1 (at 1000 nmol/L) |
Data from Rege et al (15).
Abbreviation: EC50, half maximal effective concentration
a From Davio et al (99).
b Relative potency determined from the concentration of a steroid that exerts the same effect as an EC50 concentration of testosterone. Extrapolated from data in Figure 1 of Rege et al (15). Potency data in parentheses indicates estimates of relative agonist activity obtained at 1.0 nM by Storbeck et al (63).
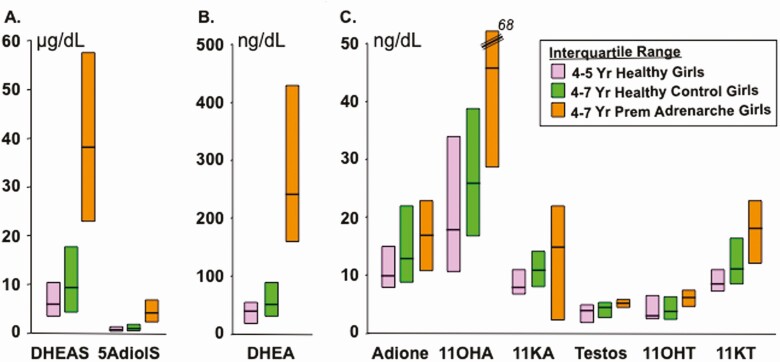
Among peripherally formed adrenarchal androgens, 11-ketotestosterone has a biopotency 33% to 101% that of testosterone (15,63). Its serum concentration is sufficient in women (Table 2) and adrenarchal girls (Fig. 7) (64) to rival testosterone in contributing to serum androgenic bioactivity. Androstenedione biopotency may contribute slightly, but its 11-oxy-derivatives do not (Table 2).
Figure 7.
Peripheral serum concentrations (median and interquartile range) of adrenarchal C19 steroids in girls with and without PreAd. Samples obtained during clinic hours; subjects predominantly non-Hispanic White; PreAd group had Tanner pubic hair stage 2. Steroids all assayed by LCMSMS. All the differences between the steroids in PreAd and the age-matched control girls were statistically significant except for androstenedione. Note that each panel shows steroids on a different scale. Conversion multiplier for androstenediol sulfate (5AdioS) from µg/dL to µmol/L: 0.0270. Conversion multipliers from ng/dL to nmol/L for unconjugated steroids not listed in Table 1: 11β-hydroxyandrostenedione (11OHA) 0.0331; 11-ketotestosterone (11KT) 0.0331; 1-ketoandrostenedione (11KA) 0.0331; 11β-hydroxytestosterone (11OHT) 0.0328. For comparison, normal median (interquartile range) values for the 11-oxyandrogens in reproductive-age women are 11-hydroxyandrostenedione 117 (86-151), 11-ketoandrostenedione 15 (12-21), 11-hydroxytestosterone 11 (7-16), and 11-ketotestosterone 21 (16-32) ng/dL (99). The 11-oxyandrogen values of men this age are minimally higher (99).
Graphed from data of Rege et al (54).
DHT is about 2-fold more potent than testosterone in activating the androgen receptor (61,63,65). Thus, at an average normal adult female serum concentration of 10 ng/dL (0.35 nmol/L), it seems to contribute significantly to serum androgenic bioactivity in adult women. DHT levels in children <10 year old average ≤3 ng/dL (≤0.1 mol/L); LCMSMS data in PreAd are unreported (66). 11-Keto-DHT equals DHT in biopotency (63), but its serum concentration is normally extremely low (67); nevertheless, it has intracrine relevance to castration-resistant cancer (65).
Some caveats about extrapolating from in vitro to in vivo biopotency. For one, not all bioassay systems assess bioactivity at physiologic serum levels, and assessment of endocrine potency at concentrations substantially above this (eg, 100-300 nM) yields potency overestimates (Table 2) (61,64,65). For another, androgen receptor bioassay systems are artificial and have not considered intracrine steroid metabolism activating or inactivating androgens in specific target tissues (68). This may account for potent androgens differing widely in the extent to which they activate or suppress specific androgen receptor-responsive genes within a prostate cancer cell bioassay system: testosterone frequently, and often 11KDHT, is more effective than DHT in regulating many individual androgen-responsive genes (65). Furthermore, none of these estimates has yet considered the potential role of 11-oxyandrogen binding to sex hormone-binding globulin, an important determinant of the bioavailability of serum androgens (69,70).
Bone and Brain Actions of Adrenarchal Steroids
In contrast to estrogen, which accelerates epiphyseal chondrocyte maturation and senescence, leading to epiphyseal fusion, nonaromatizable androgen stimulates longitudinal epiphyseal growth (71). The DHT analogue oxandrolone in low dose is well-documented to be growth stimulatory with minimal, if any, virilization (72). In bone cells, DHEA, after metabolism to DHT, appears to stimulate osteoblast proliferation via interleukin-6 inhibition and insulin-like growth factor 1 (IGF-1) stimulation (73).
DHEAS and its precursor, pregnenolone sulfate, as well as allopregnanolone, have direct neuroactive nongenomic effects in rat model systems (74,75). A transport system has been identified in humans and rats that is capable of actively transporting these sulfates across the blood-brain barrier (76). DHEAS can also be formed from DHEA by brain hydroxysteroid sulfotransferases (76). DHEA can enter the brain by passive diffusion across the blood brain barrier (74). In addition, the rodent brain expresses P450c17 during early development; if this were demonstrable in humans, which is thus far controversial, this would signify that DHEA (and DHEAS) are formed within the brain during development (74,75).
DHEAS and pregnenolone sulfate are potentially neuroactive as ligands of plasma membrane glutamate and gamma-aminobutyric acid receptors, which modulate neurotransmitter signaling. Neuroactive steroid effects include neurite growth promotion and modulation of neuroplasticity in model systems (74,75,77,78).
It has been proposed that the prepubertal adrenarchal rise in DHEAS and DHEA plays a role in modulating early brain development, perhaps by promoting brain plasticity (79). In humans DHEAS and DHEA show correlations to childhood cortical development (positive) and depression (inverse) (79). The association of adrenarchal changes with the emergence of sexually dimorphic sexual attraction, stress-adaptive, and social maturational behavior during middle childhood, prior to true puberty, has led to the suggestion that adrenarchal steroids play a role in activating these behaviors (80-82). Such human studies, while intriguing, are associative, and their physiologic relevance to the human brain is unclear.
Development and Growth of the Zona Reticularis
Adrenal androgen production is almost unique to humans and some primates (83-88). Adrenocortical development has primarily been studied in mice, although only the spiny mouse develops a clear ZR (88,89).
Mouse subcapsular adrenocortical stem and progenitor cell differentiation and formation of the ZG is critically dependent on as yet incompletely understand interactions involving capsular differentiation and transcription factors, such as Wilms tumor protein homolog 1, the WNT and sonic hedgehop signaling pathways, and steroidogenic factor-1 (90). Androgen maintains the stem/progenitor pool through activation of WNT signaling (91). Mature ZG cells, indicated by expression of Cyp11b2 (aldosterone synthase), proliferate centrally to form the ZF cells of the definitive (adult) adrenal cortex as they turn off WNT signaling due to ACTH-induced activation of protein kinase A signaling and switch to expressing Cyp11b1 (90). The pool of ZG cells is meanwhile replenished by induction interactions at the ZG-capsular interface (90,92).
Until recently, there has been reason to question the relevance of mouse zonation studies to human ZR development. Mice do not develop a ZR; instead, their inner cortical zone (“x-zone”) is transient and does not express Cyp17, and therefore, it cannot secrete cortisol or androgens (91,93). Furthermore, mice also have a ZG-independent mechanism for ZF formation (92).
However, it has recently proved possible to induce formation of a mouse ZR with expression of some of the steroidogenic genes characteristic of human ZR cells (eg, Cyp17a1, Cyb5a, and sulfotransferase) from zona glomeruosa precursors in the mouse in a state of protein kinase A signaling activation with low androgen (via WNT repression) signaling (88,91). In a related development, it has been shown that forced expression of human DENND.V2 (differentially expressed in normal and neoplastic development, variant 2), a stimulator of androgen formation that is overexpressed in polycystic ovary syndrome (PCOS), induces adrenocortical Cyp17a1 mRNA expression in transgenic mice (94).
The fetal zone of the human is the inner adrenocortical zone and constitutes >80% of the adrenal gland from midgestation: at birth its relative size is >10- to 20 fold greater than the adult adrenal gland, and it accounts for the >10-fold higher DHEAS secretion of the fetal than the adult adrenal (95). It regresses completely over the first several months of life.
The ZR develops in the site of the former fetal zone from an unrelated cell population at 3 to 5 years of age (4). The ZR grows as precursor cells from the ZF migrate centripetally and proliferate (7). Concurrently, ZR 3ßHSD2 expression falls, and CYB5 and SULT2A1 expression rises from about 5 years (Fig. 5) (21) and 17ßHSD5 expression increases from age 9 years (7). Following the onset of puberty, the female ZR initially expands more quickly than that of the male, but no sex difference in ZR size remains at maturity around 20 years (7).
The ZR partially involutes with aging (“andropause”) as cell senescence and apoptosis predominate over cell proliferation. After 40 years of age, the ZR regresses and production of DHEAS, DHEAS precursors, androstenedione, and testosterone decreases (96,97). However, the ZF expands (98), and 11-oxyandrogen levels change very little with aging (99).
Regulation of Zona Reticularis Growth, Development, and Function
ACTH signaling is necessary for ZR development. Congenital ACTH resistance, due to disruption of the ACTH-melanocortin 2 receptor signaling pathway, prevents adrenal zonation beyond the ZG (100) and severely attenuates adrenarche (101).
ACTH sufficiency also is critical for the maintenance and function of the ZR. ACTH deficiency reverses adrenarchal steroid secretion (6,102). Notably, DHEA and DHEAS are more sensitive than cortisol levels to ACTH withdrawal and recover more slowly than cortisol when suppression is removed (6,103). High cortisol levels at the ZF-ZR border resulting from centripetal ZF blood flow (104) have been hypothesized to support the functional conversion of ZF to ZR cells at this location (7); they also seem likely to maintain the ZR’s functional integrity.
ACTH effects on adrenal androgen secretion are modulated by diverse signaling networks (13). Amplifiers of ACTH action include insulin and IGF-1, which at physiologic concentrations stimulate expression of adrenal P450c17 and 3ßHSD2 (105-108). Leptin up-regulates 17,20-lyase activity in adrenocortical carcinoma cells (109). Modulators of the androgenic response to ACTH include a stimulatory isoform of DENND1A (DENN/MADD domain-containing protein 1A; DENND1A.V2) that is overexpressed in PCOS theca cells and reported to be expressed in ZR cells. DENND1A.V2 overexpression upregulates androgen formation from P450scc onward, and its constitutive overexpression may plausibly account for half of PCOS (18,110). Interleukin 6, which stimulates ACTH secretion, also directly stimulates production of all adrenal steroid classes independently of ACTH and is strongly expressed throughout all zones of the adrenal cortex (111,112). Bone morphogenetic protein type 4 is inhibitory (13,113).
The other factors that control the growth and development of an ACTH-responsive ZR are less clear (3). Prolactin is a candidate factor for modulating ZR development and function. Adrenarche is severely attenuated in congenital pituitary disorders in which prolactin and growth hormone deficiency occur without ACTH deficiency (114,115). Hyperprolactinemia in mature women is accompanied by hyperandrogenemia, which is ACTH-dependent (116).
An adrenal growth theory of ZR development postulates that ACTH stimulation of adrenal growth, coupled with product inhibition of 3ßHSD2 activity by cortisol (117,118), contributes to the adrenarchal rise in DHEA (104,118,119). This adrenal growth theory posits that children’s growth demands for increasing cortisol production lead to compensatory homeostatic resetting of ACTH. This leads to increasing intra-adrenal cortisol concentrations, which then inhibit 3ßHSD2; this, in turn, leads to the need for more ACTH to compensate for the hindrance to ZF cortisol synthesis, while diverting steroidogenesis toward DHEA as ACTH also stimulates ZR growth and 17,20-lyase activity. Growth demands for frequent resetting of this cycle throughout childhood are proposed to promote ZR growth and DHEA production while cortisol production is maintained stable relative to body size. High fatty acid levels also may inhibit 3ßHSD activity (120), which suggests a potential direct nutritional influence on this process.
Body growth, particularly obesity commencing with early childhood rapid weight gain, is associated with increased DHEAS levels, independently of birth weight, in normal children (121-126). Insulin, IGF-1, and leptin have all been suggested as determinants of the relationship (107,109,122).
Birth weight, unlike postnatal growth, is inversely associated with DHEAS levels independently of adrenarchal weight: among healthy children, infants born small for gestational age have increased serum DHEAS at 8 years of age, particularly after rapid weight gain in infancy (123), and children born large for gestational age have lower levels at 5 to 8 years (127). Similar observations were made in a study of urinary steroid metabolites in another cohort of healthy children; urinary glucocorticoids paralleled the 17-ketosteroids (128).
Ovarian function affects ZR growth and DHEAS levels, through unclear mechanisms. Puberty is associated with earlier expansion of the female than the male ZR (7). Ovariectomy precipitates an early decline in DHEAS levels that is unrelated to estrogenic status (129). Paradoxically, ovarian failure is associated with an earlier rise in DHEAS levels (although later pubarche) (130). Men’s serum DHEAS levels are about 50% higher than women’s (2,131), which seems explicable by higher testicular than ovarian secretion of precursor DHEA (132), contrary to indications of testicular DHEAS secretion from older methodology (133-135).
Clinical Manifestations of Adrenarche
Androgens are a prerequisite for the growth and development of the pilosebaceous unit (PSU) in “sexual” areas of skin (42,136). As DHEAS rises >40 to 50 µg/dL (1.08-1.35 µmol/L), adrenarchal androgens suffice to successively initiate sebaceous gland development, apocrine gland development, and pubarche in young children. The PSU consists of a prepubertal vellus follicle in which the hair and sebaceous components are virtually invisible to the naked eye. In acne-prone skin areas, androgen causes the prepubertal vellus follicle to develop into a sebaceous gland. The PSUs of sexual hair areas respond to increasing androgen levels by increasingly producing a thicker (terminal) hair follicle. Estrogens also seem to directly promote the growth of sexual hair to a small extent (42,136).
Normally, adrenarchal androgen action on sebaceous glands is first manifest clinically as microcomedonal acne, which is the basis of the change in facial complexion that occurs in mid-childhood. Adrenarchal androgen action on apocrine glands is manifest as the development of adult-type axillary body odor.
DHEAS plays an important role within the adrenal cortex: it protects against adrenal hyperandrogenism (22). As discovered in apparent sulfotransferase deficiency (22), without SULT2A1 activity to convert DHEA to DHEAS, increased intra-adrenal DHEA is available as a substrate for excessive androstenedione and testosterone production.
Whether adrenarche plays other roles in normal childhood development is unclear. Adrenarchal androgens may play a role in advancing the onset of puberty. Levels 1 to 2 years before true puberty begins correlate with the onset of puberty (137), an earlier age of menarche independent of IGF-1, and body mass index (BMI) (138). Prepubertal adrenarchal androgen output is not normally related to mid-childhood linear growth velocity (139) but is an independent predictor of radial diaphyseal cortical bone strength at puberty (140,141).
Associational studies suggest that DHEAS elevation in obesity exerts a protective effect on plasma lipids (126). Adrenarchal steroids may also play a role in childhood neurobiologic development (see previous section on bioactivity of adrenal androgens).
Table 3 summarizes this discussion.
Table 3.
Normal adrenarche summary
| • Adrenarche is the maturational increase in adrenal androgen secretion that begins in mid-childhood, best indexed by a rise in DHEAS. • Adrenarche represents a change in the pattern of adrenal biosynthetic and secretory response to ACTH. • These changes correspond to the growth and development of the zona reticularis and its unique pattern of steroidogenic enzyme expression (low 3ßHSD2; high CYB5, SULT2A1, and 17ßHSD5), which promotes DHEA, DHEAS, and, to a lesser extent, testosterone formation. • 11ß-hydroxytestosterone is formed in the zona reticularis and is metabolized peripherally to 11-ketotestosterone: together they rival testosterone as significant bioactive androgens. • ACTH regulates adrenal androgen secretion. • ACTH signaling is necessary but insufficient for zona reticularis development and maintenance. • The factor(s) responsible for zona reticularis growth and development are incompletely understood, but may include prolactin, nutrition, and growth. • Adrenarchal androgens contribute to the growth and development of sebaceous and apocrine glands and pubic hair independently of gonadotropins. • DHEAS plays an intra-adrenal inhibitory role in the formation of more potent adrenal androgens, and along with other adrenarchal steroids may have other unique functions (eg, neurobiologic). |
Premature Adrenarche
Definitions: Pubarche, Premature Pubarche, and Premature Adrenarche
Pubarche is the term applied to the onset of sexual hair development. The designation is best reserved to designate children with Tanner stage 3 pubic hair. Tanner stage 2 (presexual) pubic hair, which is intermediate between fine, light, and straight vellus hair and sexual hair, is an unreliable sign of androgen excess because it can be mimicked by hypertrichosis (see following discussion).
Sexual pubic hair (Tanner stage 3) grows longer, darker, and curlier than vellus hair and is photographable with flat lighting (142). It typically appears first on the labia majora, then rises usually onto the pubis before the axillae, but sometimes vice versa.
Pubarche is a clinical sign of androgen action. Pubarche follows the attainment over time of a threshold level of androgen that is usually in the pubertal range but differs considerably among individuals, seemingly due to individual differences in the sensitivity of hair follicles to androgen, analogous to the situation among women with respect to their PSU responses to hyperandrogenemia (143). Pubarche ordinarily occurs approximately coincident with or shortly after clinical evidence of the true puberty but is occasionally the first sign of it in girls and boys (144) (see later section on differential diagnosis of premature pubarche).
Premature pubarche (PP) is present when pubarche develops before 8 year of age in girls and before 9 years in boys (1,145). Tanner stage 3 pubic hair appears in less than 5% of healthy American 8- and 9-year-old girls and boys, respectively, independent of ethnicity, according to the 1988-1994 National Health and Nutrition Examination Survey III (146). Within these limits, it occurred significantly earlier in non-overweight non-Hispanic Black and Mexican-American than non-Hispanic White girls, and it was significantly more prevalent in overweight/obese than in non-overweight 8- to 10-year-old girls. However, it is a common finding in pediatric practice (147). PP onset is after 4 years of age in 90% of cases (148).
Idiopathic PP is diagnosed in children with PP but no evidence of puberty or biochemical evidence of PreAd or any other hyperandrogenic condition (1). DHEAS, testosterone, androstenedione, and 8 am 17OHP levels are normal for preschool children. This seems analogous to idiopathic hirsutism (143), for which it is a risk factor in some (149). Premature genital hair that develops in early infancy is a special case: in most cases, DHEAS levels are slightly increased for age but not to the extent of older children with PreAd; it often remits (148,150,151). Some cases are related to intense diaper rash (152); although this has been attributed to prevention cream treatment, it may be due to inflammatory hyperemia (153).
Premature adrenarche is an idiopathic condition. It is diagnosed when slowly progressive, isolated (ie, no other evidence of true puberty) PP and/or related clinical manifestation of adrenarche (eg, microcomedonal acne or increased body odor) is associated with adrenarchal levels of serum androgens (Table 1) without evidence of an underlying virilizing disorder (1,145,154,155).
Premature Adrenarche Clinical Picture
Biochemical characteristics
The customary discriminant androgen level is a serum DHEAS 40 or 50 to 130 µg/dL (1.08/1.36-3.5 µmol/L) by radioimmunoassay (Table 1). This is above that of children <6 years of age and similar to that of early pubertal girls of a similar pubic hair stage. This elevated DHEAS is associated with significant, small increases in serum total and/or free testosterone and androstenedione (124,156). Sex hormone binding globulin is also significantly low (60,156).
Normally, both DHEAS and testosterone levels in mid-childhood overlap with the normal range of both preschool children and early pubertal girls (Table 1). Thus, the diagnosis of PreAd is often made in children who have DHEAS levels that, while indicative of adrenarche, are ordinarily not responsible for pubic hair. These differences may arise from individual differences in response to androgens (70,143) or from unmeasured serum androgens.
The possibility that elevated biologically active androgens other than free testosterone circulate in PreAd has long been suspected but remains unclear (60,156). Recently, it was discovered by Rege et al that serum 11-ketotestosterone levels were elevated 3.5-fold higher than testosterone in over half of girls with early PreAd (Fig. 7) (64). Their supplementary analysis indicated that the statistical difference for 11-ketotestosterone levels between PreAd and control girls applied only to the obese PreAd cases, although it was not attributable to BMI Z-score per se. It is possible that adipose tissue assumes increased significance as a determinant of 11-ketotestosterone levels in hyperinsulinemic obesity since adipose tissue expresses both 11ßHSD1 (157) and 17ßHSD5 (158), and 17ßHSD5 expression is upregulated by insulin (159). Considering that 11-ketotestosterone appears to have substantial biopotency (Table 2), it contributes as much or more to androgen action as testosterone in these girls. Nevertheless, 11-oxyandrogens are, as for testosterone and androstenedione, less diagnostically discriminant (sensitivity 67%-81%) than DHEAS or DHEA (sensitivity 100%) in distinguishing PreAd from normal (Fig. 7) (64).
The response to ACTH1-24 is characteristic (Fig. 1, Table 1) and is indicative of the underlying low 3ßHSD2 activity and high 17,20-lyase activity of the ZR. Serum DHEA and precursors generally parallel the baseline changes in DHEAS, with 17-hydroxypregnenolone and DHEA rises predominating and increasing into the early pubertal female range, as do 17OHP and testosterone (Table 1, Fig. 2) (3,9). Androstenedione responses change little over the prepubertal; the pubertal rise in serum androstenedione is predominantly of ovarian origin. Peripheral serum DHEAS responses are typically undetectable in the 60 min sampling period.
An ACTH stimulation test performed in otherwise healthy PreAd boys with high-normal DHEAS levels showed that DHEA and/or 17-hydroxypregnenolone responses were significantly increased over prepubarchal controls (160). Such dissociation between DHEAS and DHEA levels has not been explicable by genetic differences in SULT2A1 genotype (161), and the phenotype is inconsistent with sulfatase deficiency (162).
Some cases have very high baseline or post-ACTH adrenal C19-steroid levels that approach those of nonclassic 3ßHSD2 deficiency (163). These have sometimes been termed “exaggerated adrenarche” (164,165), but since this term has been applied so diversely as to sow confusion (166), they are here designated “atypical PreAd.”
Recently, the PreAd urinary steroid metabolite profile by LCMSMS was interpreted as increased 17ß-hydroxysteroid dehydrogenase activity in the alternative pathway (167). PreAd patients were documented to have significantly increased urinary output (vs age-matched controls) of the 17-ketosteroid conjugates of androsterone + etiocholanolone (1645 vs 457 nmol/24 h), while cortisol and cortisone 17-hydroxy-C21 tetrahydro precursors of these 17-ketosteroids [tetrahydrocortisone (THE) + (tetrahydrocortisol) THF + 5α-THF] were not increased. This yielded a significantly decreased ratio of cortisol to 17-ketosteroid metabolites, leading the investigators to suggest increased 17ßHSD activity in the alternative pathway. However, the relevance of this ratio is uncertain since it does not reflect immediate precursor-product relationships and these metabolites only account for about half of pertinent metabolites (C. Flück, personal communication 11/30/2020). Also, without evidence that DHT production is increased, the increased 17-ketosteroid output may be confined to inactive conjugated metabolites.
Steroid assay methodology: current vs emerging
The specificity of direct radioimmunoassay of serum unconjugated sex steroids in children and women is generally suspect due to unanticipated cross-reactions of unidentified steroids (168,169), although a few have been found to be accurate when tested against generally more specific methods (36,170). For this reason, the current standard for sex steroid assays is postchromatographic radioimmunoassay or an assay comparably specific.
On the other hand, the current and historical (nearly 50 yr) standard for serum DHEAS is direct radioimmunoassay. Structurally related steroids like androsterone-3ß-sulfate cross-react in these assays (3), but this overestimation of DHEAS levels has been of no diagnostic importance. However, these DHEAS levels are nearly 1.8-fold higher than the level determined by highly specific and sensitive LCMSMS assays (compare DHEAS in Table 1 with those in Figure 1).
LCMSMS has emerged as a highly accurate, specific and increasingly commercially feasible technique for assaying several steroids in small quantities of serum. LCMSMS methods are nearly 10-fold more sensitive than radioimmunoassays (64), which has made possible better definition of the lower limits of childhood sex steroid concentrations. Thus, LCMSMS indicates that serum DHT hovers about the limit of detection of 3 ng/dL in normal children <10 years of age (66), far lower than was previously estimated (171). Differences among LCMSMS results for testosterone and androstenedione in healthy children make it difficult to determine the extent to which systematic differences exist among these methods or whether there are systematic differences between LCMSMS methods and standard radioimmunoassay reference data (64,66,172). Comparison of LCMSMS methods for children’s C21 steroid levels showed no significant differences between results of LCMSMS and postextraction/chromatography radioimmunoassay although the results by LCMSMS averaged 88% (range 63%-100%) of radioimmunoassay results (173). There is currently insufficient data to reach a conclusion about the extent to which LCMSMS methodology will necessitate substantial redefinition of the expected normal ranges for young children’s sex steroid levels, but some redefinition will be necessary.
A case in point: a recent report compared LCMSMS-assayed androgens in 3- to 8/9-year-old girl/boy controls, PP, and PreAd (n = 9-11), defining DHEAS > 50 µg/dL (1.36 µmol/L) as PreAd (174). Controls were significantly younger than other cohorts, and axillary odor was similarly prevalent in PP and PreAd. DHEAS interquartile ranges by group were respectively 10 to 12, 36 to 46, and 65 to 110 µg/dL. However, 11-oxy-androgens were similarly elevated in PP and PreAd. The data suggest that PreAd should have been defined at an LCMSMS-specific DHEAS level <50 µg/dL and that the PP group was actually a mild PreAd group. Thus, while 11-oxyandrogens may prove to be elevated in idiopathic PP, these data do not clearly demonstrate it.
Clinical characteristics
PP is usually caused by PreAd. It occurs 5 to 9 times more commonly in girls than boys (148,175,176). There are few reports in Asian populations (177,178). Less common, but most worrisome, PP may be the first sign of a serious virilizing disorder (179). There is a broad spectrum of severity of the clinical manifestations in PreAd.
PreAd signs were 5-fold more common in girls than boys, in a Finnish survey of 6.6- to 8.9-year-olds, despite similarly adrenarchal serum DHEAS ≥ 37 µg/dL (≥1.0 µmol/L) (175). Those with any PreAd signs (pubarche 0%-1.1%; others 10%-22.1%) had significantly higher DHEAS and body fat than those without. This sexual dimorphism might be explained by sex-dependent differences in peripheral androgen metabolism or action that are modified by body fat (175).
The PP of PreAd is slowly progressive. Adult-type body odor, oily hair or skin, and acne, predominantly microcomedonal (180), are frequently noted. These are the first PreAd manifestations in over half of cases, particularly if sought (155,175), and occur at significantly lower DHEAS levels than pubarche (155); their normal ages of onset remain to be established.
Stature and bone age (BA) are slightly but significantly increased in PreAd, but they are usually within the broad limits of normal (124,181-184). About one third of cases have average BA and stature. Another third have 1- to 2-year BA advancement and proportionately above-average stature (181). Bone mineral density is slightly increased independently of BA (185).
About one third have BA advancement ≥2 years (124,181), and in 10% of this group, BA is advanced ≥3 years (181). Children in the top tertile for BA were significantly taller [mean 1.7 SD score (SDS)] than those in the lowest tertile and had a significantly higher BMI (1.69 vs 1.07 SDS) and higher DHEAS (86 vs 63 µg/dL). They also had a significantly lower height potential (~3.8 cm) than expected from parental heights.
Onset of puberty or menarche averages 1 to 2 years early in PreAd girls (1,186,187); this is associated with a less vigorous pubertal growth spurt and attainment of normal height potential slightly earlier than their peers (1,183,186,188). This is a normal-variant (“advanced”) growth pattern (189) that seems partly attributable to the slight elevation of androgen levels and partly associated with obesity (124); some have found it related to low birth size (186,187). In overweight girls, slowly progressive central precocity may develop simultaneously with PreAd (190).
Early childhood obesity is frequently associated with and is a determinant of the serum DHEAS level (2,124,175,191,192). Insulin resistance was originally defined definitively in an overweight-obese cohort with acanthosis nigricans (193). Subsequently, evidence of insulin resistance, usually indicated by hyperinsulinemia (absolute or relative to glucose) has been found in most studies. As tabulated by Idkowiak (166), it was reported in 8 of 10 PreAd populations of Western girls of diverse ethnicity and 2 of 3 studies of Western boys. In 3 of these studies, increased plasma IGF-1 was also reported. Insulin resistance is usually obesity-related. It has been disproportionate to obesity in some studies (193-195), but in others it is similar to that of weight-matched controls (191,196). In the Catalan cohort (see following discussion), it is associated with increased central fat but not generalized obesity (1). Other components of the metabolic syndrome have been found less frequently in PreAd (197): dyslipidemia of various sorts (1,198-200) and hypertension (198,199).
In 1998, Ibanez et al identified the association of fetal growth retardation with functional ovarian hyperandrogenism (FOH) and hyperinsulinemia in their Catalan cohort of PreAd girls (201). Furthermore, they reported that the severity of growth retardation was least in those with PP alone (birthweight SDS −0.8 vs controls) and increased successively in those with PP + FOH (−1.4) and PP + FOH + insulin resistance (−2.4). This PP cohort was far more likely to develop anovulatory symptoms (ie, PCOS) in late puberty than controls (1,202,203). Ibanez et al proposed that intrauterine growth restriction indexed a common fetal origin for these disorders (204) and that when it is followed by early childhood central adiposity, it may be linked through insulin resistance to cardiovascular risk as well as PCOS (201,203,205,206). An alternate possible mechanism is that low birth weight is an indicator of fetal stress, which programs insulin resistance by activating the limbic-hypothalamic-pituitary-adrenal system to cause fetal glucocorticoid excess (207).
Low birth weight, independent of gestational age, has been found to be related to adrenarchal DHEAS level and/or PP in several non-Catalan PreAd populations (123,148,208-210). However, the relationship was not found in several other PreAd cohorts (182,186,192,211-213).
Increased PCOS prevalence by NIH criteria was not a feature in follow-up studies by others (149,211). However, significantly increased hirsutism score (prevalence about 25%) and significant mild hyperandrogenemia were found, along with increased ovarian volume in one of these studies. Together with the functional adrenal hyperandrogenism of PCOS bearing a strong resemblance to an exaggeration of adrenarche, these findings add to the evidence that a PreAd-PCOS relationship is plausible (1,9,18,166). Conversely, PCOS daughters followed longitudinally did not develop PP although peripubertally they developed significantly increased DHEAS without DHEA hyperresponsiveness to ACTH (214).
A Finnish follow-up of 30 PreAd girls until 18 years old found that obesity, metabolic syndrome, IGF-1, and DHEAS levels normalized compared to controls (215,216). However, the PreAd group developed significant central adiposity, insulin resistance, and increased metabolic syndrome scores; metabolic syndrome developed in those who remained or became obese (215). Other series had similar findings regarding decreasing adiposity (183,188,211). A Greek follow-up of 21 former PreAd girls at 21 years found them to be nonobese but similarly to have significant insulin resistance without increased prevalence of metabolic syndrome (149).
Early sexual maturation has been hypothesized to be disadvantageous to psychosocial development (217). On average, PreAd and control girls aged 6 to 8 years were found to have similar intelligence and executive functioning scores, but those with lower executive function were at risk for emotional and behavioral problems as a result of early maturation (218). Quality of life of Finnish PreAd girls and boys followed at 12 years was similar to controls, although overweight girls had lower quality of life than normal-weight girls independently of PreAd (219). On the other hand, the Greek PreAd girls followed into early adulthood have been found to have significantly increased psychological test scores for anxiety, depression, and eating disorders, resembling a matched and other PCOS cohorts (149,220-222). In PCOS, these symptoms have been attributed to poor self-esteem (222) and distress with body appearance (223).
Etiology
The etiology of PreAd is unknown. Most cases accord with the traditional concept that it is an extreme variant of normal development (ie, “normal adrenarche occurring early”). It is commonly assumed that the process is caused by premature development of the adrenal ZR (see previous discussion of normal adrenarche). If this were correct, PreAd would have no long-term consequences. However, follow-up studies suggest that PreAd may sometimes be a risk factor for insulin resistance and, in the Catalan population, PCOS. Fetal programming has been suggested to underlie epigenetic change as the mechanism for PreAd (1,224), but there is scanty evidence to support this theory.
Associations of PreAd with temporal lobe lesions (160) and poliomyelitic scoliosis (225) have led to speculation that ZR growth or function are regulated by neural pathways. Heterozygosity for gene variants associated with CAH may be a risk factor for PreAd (226,227).
Heritable influences contribute to the DHEAS serum level (228,229). Estimates of heritability range from 26% to 66%, varying with sex (female > male) and ethnicity (non-Hispanic > Mexican American). Genomewide association studies have identified several single nucleotide polymorphisms associated with DHEAS levels, 2 of which were in SULT2A1, but these accounted for only 4% to 7% of the genetic variance (229), and there is no evidence of causality (161). Polymorphic variations in androgen receptor cytosine-adenine-guanine repeat length (230,231), aromatase, and the ACTH receptor MCR2 (232) have been reported in PP or PreAd, but their relationship to functionally significant alterations in hormone actions remains speculative.
Differential Diagnosis of Isolated Premature Pubarche
Other than PreAd and idiopathic PP, several causes of isolated PP (ie, PP in the absence of evidence of true sexual precocity) must be considered, the most serious being virilizing disorders, of which NCCAH accounts for the vast majority. These are summarized in Table 4.
Table 4.
Causes of isolated premature pubarche
| • Premature adrenarche |
| • Idiopathic premature pubarche |
| • Hypertrichosis |
| • Central precocious puberty |
| • Exogenous androgenic and anabolic steroids |
| • Virilizing disorders |
| 1. Dexamethasone-suppressible disorders |
| a. Virilizing congenital adrenal hyperplasia |
| (1) 21-hydroxylase deficiency, classic and nonclassic |
| (2) 11ß-hydroxylase deficiency, classic and nonclassic |
| (3) 3ß-hydroxysteroid dehydrogenase deficiency, classic and nonclassic |
| b. Rare congenital disorders of adrenal steroid metabolism |
| (1) Cortisone reductase deficiency (and apparent CRD) |
| (2) Apparent sulfotransferase deficiency |
| c. Peripheral androgen metabolic disorders |
| (1) Portosystemic shunting |
| 2. Dexamethasone-resistant androgen and glucocorticoid excess |
| a. Endogenous Cushing’s syndrome |
| b. Glucocorticoid resistance |
| 3. Dexamethasone-resistant androgen excess without glucocorticoid excess |
| a. Virilizing tumor |
| b. Gonadal hyperandrogenism |
| (1) Gonadotropin-independent precocious puberty |
| (2) Human chorionic gonadotropin-secreting germ cell tumors |
| (3) Familial isolated luteinizing hormone excess |
Exclusions
Hypertrichosis
PP must be distinguished from hypertrichosis, a generalized excess of vellus body hair (70,136). Hypertrichosis may mimic Tanner stage 2 hair by presenting with fine hair on the genital area that resembles forearm hair. It is not caused by excess androgen, although hyperandrogenemia may aggravate it. It is most commonly familial or caused by medications (eg, phenytoin, cyclosporine); metabolic disorders are rare causes (eg, hypothyroidism, anorexia nervosa, porphyria).
Central precocious puberty
Breast development in girls or bilateral testes enlargement in boys before or coincident with pubarche indicates the onset of true (gonadotropin-dependent) precocious puberty. However, pubarche is occasionally the first sign of true puberty in girls and boys (144); such girls have significantly higher DHEAS levels than girls with thelarche at the onset of puberty (144). Central precocious puberty can be ruled out in such children by a few month’s observation or by determining 8:00 am serum luteinizing hormone (LH) to be <0.15 mIU/mL (233).
Exogenous androgen exposure
PP can result from passive transfer of androgens in topical preparations used by caretakers (152,234). This is increasingly common as topical androgens are used for hormone replacement therapy or enhancement of sexual or muscular function by adults of both sexes. Anabolic steroids, such as those used for therapeutic purposes like growth promotion or abused by athletes, are well known to have weak androgenic effects capable of causing PP and clitoromegaly (235). They are not detected by routine androgen assays but require special LCMSMS assays (236).
Virilizing Disorders
Premature pubarche may be the initial manifestation of a virilizing disorder. These are unusual, accounting for 5% to 10% of PP, but their potential harm requires vigilance. Severe hyperandrogenism in children is indicated by frank virilization and rapid progression. Mildly virilizing disorders, on the other hand, closely mimic PreAd (see previous section on differential diagnosis of isolated PP).
Virilizing disorders are here categorized as to whether they are dexamethasone-suppressible (ie, ACTH-dependent) or dexamethasone-resistant (Table 4), and the order corresponds approximately to that encountered the recommended work-up (see next section).
Virilizing congenital adrenal hyperplasia
CAH is a term historically applied to disorders that arise from specific autosomal recessive defects of enzymes involved in adrenal corticosteroid biosynthesis Those that cause virilization are ACTH-dependent and reversible by glucocorticoid replacement therapy. NCCAH due to 21-hydroxylase deficiency accounts for the great majority of such cases, and together in boys with the less common, simple virilizing classic CAH, causes most virilizing disorders responsible for PP (179,237).
Deficiency of 21-hydroxylase (due to inactivating CYP21A2 variants).
21-Hydroxylase deficiency causes most CAH/NCCAH. The world-wide prevalence of CAH is 1:10 000 to 1:20 000, and that of NCCAH is 1:200 to 1:1 000 (237). NCCAH is more prevalent in certain inbred populations (eg, Ashkenazi Jews) and less prevalent in Blacks (0.9%) (238). Its worldwide prevalence in hyperandrogenic women averages 4.2% (238); meager data suggest a much higher prevalence in Asian children (239).
Although hyperandrogenism is significantly more severe in NCCAH than in PreAd, no clinical features reliably distinguish these entities. Likewise, there is considerable overlap in BA and serum DHEAS, testosterone, and androstenedione levels (179,238,240,241), so 17OHP determination is critical for diagnosis (238,242). NCCAH cases usually have normal cortisol reserve (243) and adult stature (244).
Classic virilizing CAH in its most common and severe salt-wasting form presents neonatally. In the simple virilizing form, the classic genital ambiguity of girls sometimes escapes notice in infancy and, then like boys, PP and growth acceleration by 4 years of age are the first clear manifestations (237). Inadequate corticosteroid therapy leads to progressive hyperandrogenism.
NCCAH results from mutations that cause mild hyperandrogenism rather than frank virilization. Affected girls lack the genital ambiguity of classic CAH. NCCAH may first present with PP in mid-childhood, later with a PCOS-like picture, or remain asymptomatic (“cryptic”) and be discovered during family studies (238). Slowly progressive PP or asymptomatic NCCAH cases may not require glucocorticoid treatment (242,244). BMI is usually normal (245), in contrast to most PreAd.
Deficiency of 11ß-hydroxylase type 1 (due to CYP11B1 inactivating variants).
CYP11B1 mutations classically presents with hypertension and virilization (including genital ambiguity in girls), but its nonclassic form occasionally presents from mid-childhood onward with mild hyperandrogenism (246-248). The hypertension and hypokalemia expected from deoxycorticosterone excess are not necessarily present.
Deficiency of 3ßHSD2 (due to HSD3B2 inactivating variants).
HSD3B2 mutations classically presents in infancy with genital ambiguity (in both sexes, because hepatic 3ßHSD1 converts Δ 5-3ß-hydroxysteroids to androstenedione and thus permits testosterone and DHT formation (11)) and symptoms of cortisol and aldosterone deficiency. The nonclassic form occasionally presents later with mild symptoms such as PP or adolescent hirsutism (163,249). The diagnosis of this disorder presents a particular diagnostic quandary in atypical PreAd because it is characterized by marked elevation of DHEAS and its Δ 5-3ß-precursors; 17-hydroxypregnenolone elevation of >11SD post-ACTH is the best discriminant (163).
Rare congenital disorders of adrenal steroid metabolism
Rare congenital steroid metabolism disorders other than CAH may cause virilization.
Cortisone reductase deficiency (CRD) causes excessively rapid cortisol turnover due to failure to regenerate cortisol from cortisone in peripheral tissues; this leads to a compensatory increase in ACTH production and mild to moderate adrenal hyperandrogenism. CRD is caused by variants of 11ßHSD1 itself (due to dominant negative HSD11B1 mutations), which is functionally the major cortisone reductase (250). “Apparent CRD” is more common and is due to autosomal recessive variants of hexose-6-phosphate dehydrogenase (H6PDH), a cofactor for HSD11B1 (251). The serum steroid pattern is not well-characterized, but a disproportionate elevation of androstenedione rather than DHEAS seems likely (252). The characteristic laboratory features are elevated urinary corticoids with a low ratio of cortisol to cortisone tetrahydro metabolites (THF + 5α-THF/THE) (46,251).
Apparent DHEA sulfotransferase deficiency is due to autosomal recessive, inactivating mutations in the gene encoding the sulfate donor to sulfotransferases (PAPSS2), causing defective DHEA sulfation (22,35). DHEAS levels are uniformly undetectable to low. The index case (22) had variably elevated serum DHEA, androstenedione, and testosterone. Corticosteroid secretion is not affected. All cases have spondyloepimetaphyseal dysplasia and most lack androgen excess. The index case indicates the crucial role of DHEA sulfation as a gatekeeper to human androgen synthesis and demonstrates that the role of DHEA sulfation in adrenal function is to prevent unconjugated DHEA from becoming available for conversion to active androgens.
Peripheral androgen metabolic disorders
PP has been reported in the setting of congenital portosystemic shunting (253). Serum DHEA, androstenedione, and/or testosterone levels were high, while DHEAS was normal and became low. This was attributed to decreased hepatic sulfation of DHEA, permitting it to become available as a hepatic substrate for testosterone synthesis; thus, it appears to be a form of acquired sulfotransferase deficiency. Other endocrine effects of portosystemic shunting include hyperinsulinemia and thyroxine-binding globulin deficiency.
Dexamethasone-resistant androgen and glucocorticoid excess
Glucocorticoid and androgen excess persist after glucocorticoid suppression of ACTH in endogenous Cushing syndrome of diverse causes and glucocorticoid resistance. Cushing syndrome is an unusual cause of childhood virilization (254). Attenuation of linear growth is typical of childhood glucocorticoid excess (255) but may be masked by the growth spurt of virilization (256). The serum steroid pattern is uncharacteristic for PreAd, with androstenedione elevation disproportionate to DHEAS elevation (179).
Glucocorticoid resistance results from rare autosomal dominant, inactivating mutations in the glucocorticoid receptor (257). Cushingoid signs are absent despite elevated 24-h urine free cortisol. ACTH overproduction that is compensatory for resistance to cortisol negative feedback causes hyperandrogenism. The serum steroid pattern is not well-characterized: cortisol hyperresponsiveness and androstenedione elevation disproportionate to DHEAS is expected (252). A recent claim that this disorder commonly causes PreAd is based on unsubstantiated methodology (258).
Dexamethasone-resistant androgen excess without glucocorticoid excess
Isolated androgen excess that is not suppressible by glucocorticoid administration is of neoplastic or gonadal origin.
Virilizing tumors are rare causes of PP (259). Ovarian tumors are the most rare (260-262); children with 45,X/46,XY mosaicism are at high risk (263). Testicular tumors typically cause asymmetrical scrotal enlargement. Adrenal rest tumors complicating CAH are the most common of the testicular tumors of boys (264-266), and 11-oxyandrogens are specific markers for these (264). Adrenocortical carcinoma may be accompanied by testicular enlargement (266). These tumors are typified by a disproportionately elevated DHEAS level in ~90%, but DHEAS elevation is not specific for them (267). The abnormal differentiation that underlies tumor formation typically leads to abnormal patterns of steroid secretion, with androstenedione and/or 17OHP elevation (261,268,269).
Rare types of gonadal precocious puberty cause testosterone-predominant puberty independent of regulation by the normal hypothalamic-pituitary axis. These occur predominantly in boys, who characteristically have small testes.
Gonadotropin-independent precocious puberty.
Autosomal dominant mutations cause constitutive LH receptor signaling that produces Leydig cell function independent of pituitary gonadotropins (270). The rare peripheral precocity of McCune-Albright syndrome in boys is accompanied by macroorchidism (271,272).
Human chorionic gonadotropin-secreting germ cell tumors.
These have not been reported to virilize girls, perhaps because of a limited capacity of theca cells to produce sufficient testosterone over a short period of time (273). These may occur in the gonads or diverse extragonadal sites, most often liver (hepatoblastoma), mediastinum, and intracranial. Serum human chorionic gonadotropin and alpha-fetoprotein are useful markers. They can be difficult to localize (274).
Familial isolated luteinizing hormone excess. Familial isolated LH excess caused mild virilization with isolated PP in a 1.5-year-old boy and his 4-year-old sister (275). This 1980 report uniquely reported PP in a girl with isolated LH excess that seemed autonomous in its resistance to estrogen suppression. Serum DHEAS was adrenarchal; testosterone was inappropriately high.
Diagnostic Evaluation of Premature Pubarche
While a good argument can be made for an initial period of watchful waiting in many PP cases (276,277), methodology is now widely available for the consulting endocrinologist to diagnose virilizing disorders early by beginning evaluation with a baseline hormone determination in addition to history, examination, and BA determination.
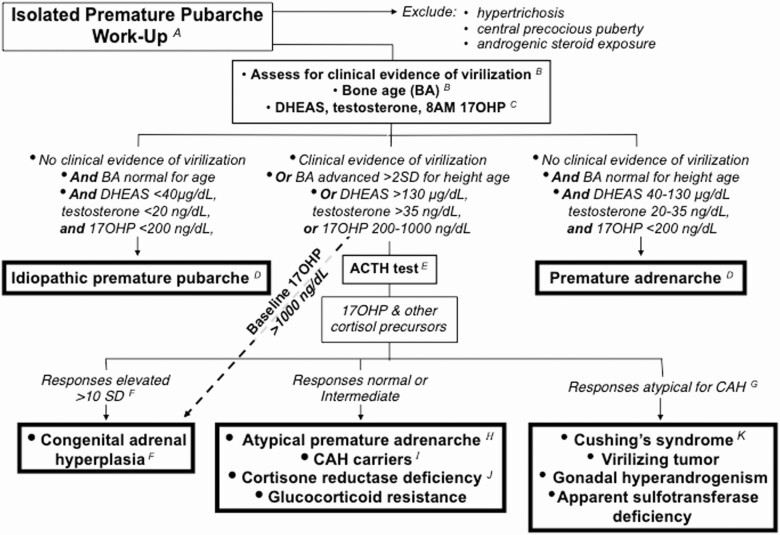
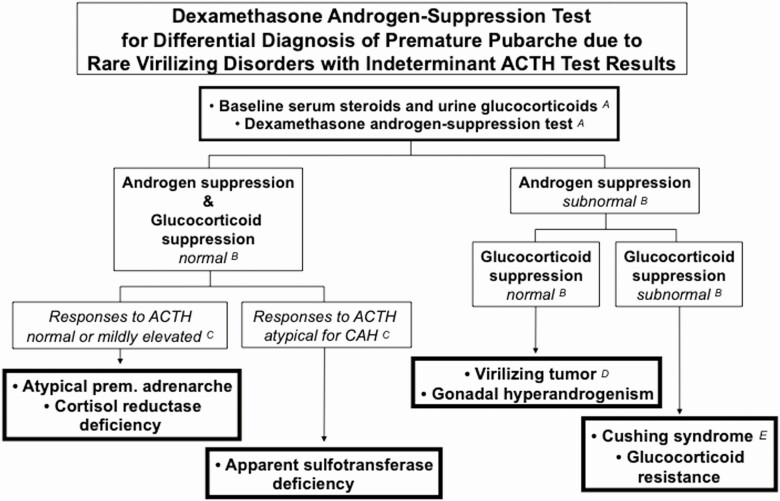
This suggested diagnostic approach to isolated PP (Figs. 8 and 9) is of low intensity for the great majority of cases and is predicated on the low frequency of virilizing causes: in a series of 267 children in whom serum DHEAS, testosterone, and androstenedione were obtained for diverse reasons, androgen excess explained 95: their diagnoses were PreAd (n = 86), classic CAH or NCCAH (n = 6), virilizing adrenocortical carcinoma (n = 1), and Cushing’s disease (n = 1) (179). Thus, over 90% of children presenting with signs of androgen excess can be expected to have PreAd. ACTH and dexamethasone androgen-suppression tests are reserved for those with unusually elevated baseline serum androgen or 17OHP levels or other evidence suggesting a virilizing disorder (Figs. 8 and 9). Clinical follow-up is an integral part of this diagnosis and management strategy.
Figure 8.
A work-up to screen for virilizing disorders as a cause of isolated premature pubarche. Premature adrenarche accounts for the great majority of isolated premature pubarche. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency is the most common virilizing cause. (A) History and examination can usually exclude hypertrichosis, central precocious puberty, and exposure to androgenic/anabolic steroids. Hypertrichosis must be excluded by inspection to avoid fruitlessly pursing an endocrine cause for a cosmetic condition. Since pubarche may be the first sign of gonadotropin-dependent precocity in both girls and boys, in the absence of breast development or testicular enlargement, gonadotropin determination may be advisable (see text). Anabolic or androgenic steroid use by the child or a caretaker can usually be ruled out by a careful history; otherwise, special LCMSMS urine studies are required. (B) The earliest manifestations of virilization may be acceleration of height velocity followed by BA becoming disproportionately advanced for height. (C) A baseline serum DHEAS or testosterone much above the usual adrenarchal range (130 µg/dL and 35 ng/dL, respectively) or 8 am 17OHP ≥ 200 ng/mL (6.0 nmol/L) indicates increased risk for a virilizing disorder. (This 17OHP level has a ≥95% sensitivity and specificity for CAH/NCCAH among premature pubarche patients when obtained by 8:00 am). However, normal baseline levels do not necessarily exclude 21-hydroxylase deficiency or more rare forms of CAH. (D) Idiopathic premature pubarche is diagnosed when BA is normal for chronologic age and androgen and 17OHP levels are preadrenarchal with no clinical evidence of virilization. PreAd is characterized by a slightly advanced BA that is normal for height age and androgen and 17OHP levels appropriate for an early pubertal girl with no clinical evidence of virilization. (E) Cosyntropin (ACTH1-24) 0.250 mg is infused IV over 1 min intravenously after obtaining baseline steroids; peak serum steroid responses occur at 60 min. (F) The typical response in untreated CAH, whether classic or nonclassic, is that the steroid immediately prior to the enzyme block is extremely elevated (>10 SD above average), and steroids earlier in the biosynthetic pathway are successively less elevated the further removed they are from the block. For example, in 21-hydroxylase deficiency, 17OHP is >1000 ng/dL (30 nmol/L) and androstenedione and testosterone are successively less elevated; in 3ßHSD2 deficiency, 17OHP and DHEA responses are >4000 ng/dL (120 nmol/L) and 17OHP, androstenedione, and testosterone are mild-moderately elevated. 21-Hydroxylase deficiency is responsible for the vast majority of CAH, so many practitioners check only 17OHP responses, but we advise checking all the pertinent steroid intermediates from 17-hydroxypregnenolone to cortisol and to androstenedione through testosterone to potentially detect unusual disorders with a single ACTH test. (G) An example of a pattern that is atypical for any type of CAH would be androstenedione or testosterone levels greater than those of 17OHP. (H) Atypical premature adrenarche is the most common cause of baseline DHEAS and stimulated DHEA or 17-hydroxypregnenolone levels that approach those of 3ßHSD2 deficiency. It is a diagnosis of exclusion (see Fig. 9). (I) Carriers for 21-hydroxylase deficiency have 17OHP responses to ACTH that are >200 to 1000 ng/dL (6-30 nmol/L). Other forms of CAH may also have 17OHP responses in this range. (J) Normal or elevated cortisol levels may be found in endogenous Cushing’s syndrome, glucocorticoid resistance, and cortisone reductase deficiency (or apparent CRD). A DAST may be indicated for diagnosis (Fig. 9). (K) C19 steroid patterns atypical for CAH may arise from Cushing’s syndrome (androstenedione disproportionately elevated), virilizing tumors (very high DHEAS, etc.), male gonadal hyperandrogenism (testosterone elevated with pubertal steroid pattern), and apparent sulfotransferase deficiency (low DHEAS and mild DHEA hyperresponsiveness).
Figure 9.
Dexamethasone androgen-suppression test for rare virilizing disorders causing premature pubarche with responses to ACTH that are indeterminate (normal, elevated, or atypical for CAH) (see Fig. 8). (A) First, an early morning blood sample for testosterone, cortisol, and DHEAS and any other suspect steroid and a` 24-h urine for glucocorticoids (ie, urine free cortisol, 17alpha-hydroxycorticosteroids and/or cortisol and cortisone metabolites for quantitative steroid profiling) are obtained. Then the DAST is begun: dexamethasone, 1 mg/m2/day is given in 3 to 4 divided doses daily for 4 days, and then serum cortisol and androgens are measured on the morning of the fifth day after a final dexamethasone dose. On days 2 and 4 another 24-hr urine for glucocorticoids may be collected. If Cushing’s disease is clinically suspect, one may then begin high-dose dexamethasone (2.5 mg/m2 every 6 h) on days 2 to 4. (B) Normal androgen and glucocorticoid suppression is indicated in young children when serum testosterone falls to <10 ng/dL, DHEAS to <40 ug/dL, and cortisol to <1 µg/dL (28 nmol/L). (C) Normal androgen and glucocorticoid suppression is found in atypical premature adrenarche and in cortisol reductase deficiency or apparent CRD. Among those with responses to ACTH atypical for CAH (Fig. 8), those with apparent sulfotransferase deficiency suppress normally as well. (D) Poor androgen suppression with normal glucocorticoid suppression is characteristic of virilizing tumors and gonadal hyperandrogenism. Testosterone-secreting tumors are usually distinguishable by an abnormal pattern of testosterone precursors. Male hyperandrogenism (eg, male-limited gonadotropin-independent sexual precocity) is characterized by a male pubertal steroid pattern. (E) The elevated androgens and glucocorticoids of endogenous Cushing’s syndrome and glucocorticoid resistance are not normally suppressible by low-dose dexamethasone (ie, DAST), but those of Cushing’s disease are suppressible by high-dose dexamethasone. Assay of serum dexamethasone can be added to assess the possibility of test noncompliance if cortisol suppression is subnormal.
Frankly virilizing disorders are suggested by the rapid progression of sexual hair development, topical treatment-resistant acne, growth spurt and increasing musculature with reciprocal loss of body fat (278), lowering of voice, and clitoromegaly (with genital ambiguity when virilization commences in early fetal life). Frank virilization causes progressive BA advancement disproportionate to linear growth within months. These children have an increased growth rate and become tall during early childhood, but they undergo premature epiphyseal fusion, resulting in short stature if the underlying disorder goes untreated for long. A serum testosterone level above that of normal females should be considered virilizing in a young child.
Mildly virilizing disorders, on the other hand, closely mimic PreAd because androgen levels are only slightly elevated, so they are more slowly progressive. In such cases, advancement of pubarche, growth, and BA become apparent slowly.
History and physical examination
The history should include birth weight, history of androgen exposure, growth records, rapidity of progression, and other evidence of puberty or virilization. The family history should cover possible similar conditions, metabolic syndrome, hirsutism, and menstrual irregularity. Accurate height measurements are critical to establish growth velocity. Documenting the stage and amount of sexual hair and acne, breasts, and external genital is essential. In infants, diaper rash and its treatment should be documented.
Bone age
BA is determined from a left wrist-hand anterior-posterior radiograph (279,280). An accurate BA reading is essential to diagnosis (145,154). It is crucial to the diagnosis and management of PP that the BA be interpreted by a pediatric endocrinologist or pediatric radiologist trained and experienced in this technique. BA is defined as the chronologic age for which the BA would be average (279). BA advancement is early evidence of a hyperandrogenic condition unless it is very mild (eg, PreAd, some NCCAH) or of very recent onset. The BA of young children normally averages within 33% of chronologic age (eg, BA up to 4 years for a 3-year-old child, 8 years for a 6 year old) (279) .
BA advancement for chronologic age but normal for height age (within 20%) indicates normal growth potential (281). Height age is defined as the chronologic age for which the child’s height would be average. Once children are ≥6 to 7 years old, they can be followed for disproportionate BA advancement using the Bayley-Pinneau adult height prediction tables (281,282). BA excessive for height age or a deteriorating predicted adult height that is or becomes subnormal for parental heights (283) is characteristic of virilization. However, BA may be normal early in the course of rapid virilization.
Hormonal measurements
Assays for testosterone and its precursors should only be ordered and interpreted by a physician who has access to a specialty laboratory with specialized steroid methodology, such as by post-chromatographic radioimmunoassay or LCMSMS (see previous section on steroid assay methodology: current vs emerging) (168). Determination of serum DHEAS, total testosterone, and 8:00 am 17OHP suffice for initial endocrine screening for childhood hyperandrogemia.
DHEAS and testosterone.
DHEAS and DHEA are the most discriminant steroids for detecting PreAd (Fig. 7) (64), and DHEAS is generally of more diagnostic utility than DHEA because of its lesser episodic and diurnal changes (284). Children with PreAd may have early pubertal DHEAS and testosterone levels (Table 1) (179). However, typical adrenarchal levels do not rule out NCCAH (179,182,241).
Androstenedione is also measured by some experts. However, there is no evidence that it is cost-effective early in the investigation. Here it is reserved for the work-up of patients with atypical features who require ACTH testing.
17-hydroxyprogesterone (8:00 AM).
Guidelines recommend obtaining serum 17OHP by 8:00 am as a cost-effective screening test for 21-hydroxylase deficiency (242). Early-morning timing is critical because 17OHP levels peak before 8:00 am and fall rapidly during the course of the day, paralleling cortisol. Results are interpreted as follows:
Baseline serum 17OHP < 200 ng/dL (3.5-6.0 nmol/L) by 8:00 am excludes CAH with approximately 95% specificity and sensitivity, according to several sizable series using LCMSMS or radioimmunoassay (238,241,285).
Baseline 17OHP 200 to 1000 ng/dL (6-30 nmol/L) is consistent with CAH or a carrier state. An ACTH stimulation test is recommended for diagnosis (see following section on ACTH stimulation test).
Baseline 17OHP > 1000 ng/dL (45 nmol/L) is diagnostic of 21-hydroxylase deficiency CAH/NCCAH (Fig. 8), with the rare exception of some virilizing neoplasms. An ACTH test is not necessary (242).
ACTH stimulation test.
ACTH testing is indicated in patients whose baseline data are atypical for PreAd and in whom the baseline 17OHP is nondiagnostic or sampling for 17OHP cannot be obtained by 8:00 am (Fig. 8) (242). It is advisable from an economy of time and effort perspective to test pre- and post-ACTH for 17OHP, 17-hydroxypregnenolone, 11-deoxycortisol, cortisol, DHEA, and androstenedione to detect rare types of CAH and other virilizing disorders. Typical normal responses are shown in Table 2. A post-ACTH serum 17OHP peak >1000 ng/dL (30 nmol/L) is definitive for diagnosing 21-hydroxylase deficiency (238,242,286).
The baseline (pre-ACTH) pattern of serum steroid intermediates may offer diagnostic clues to virilizing disorders other than NCCAH. Virilizing tumors can manifest atypical patterns of 17OHP and androstenedione elevations (268). 17OHP levels are mildly to moderately elevated in 3ßHSD2 (163) and P450c11ß1 deficiency (246). Baseline DHEAS > 700 mcg/dL (19 micromol/L) suggests adrenocortical carcinoma or nonclassic 3ßHSD2 deficiency (249) or adrenal virilizing tumor, although DHEAS is often normal in 3ßHSD2 deficiency.
Genotyping is not generally recommended for NCCAH diagnosis because of the complexity of the CYP21A2 locus (238,242). Genotyping is advisable only when hormonal tests are equivocal or suspect (287), an ACTH test cannot be performed, or for genetic counseling (242).
Dexamethasone androgen-suppression test.
If the work-up to this point is unremarkable and responses to ACTH are normal or minimally elevated, there is <1% to 2% possibility of a rare, mildly virilizing disorder (166). If the evidence suggests a frankly virilizing tumor, prompt imaging is advisable. Otherwise, The further approach to the laboratory work-up of PP is discretionary. It is reasonable to choose to defer further testing at this point in favor of careful follow-up monitoring if no supportive clinical or familial evidence of a virilizing disorder has emerged and the family is agreeable to this approach (145). If concerns remain, we favor the dexamethasone androgen-suppression test (DAST) as the next diagnostic step in children with indeterminate responses to expeditiously reach a definitive diagnosis.
Collection of a baseline overnight or 24-h urine sample for urine free cortisol and LCMSMS determination of cortisol and cortisone metabolites is an important prelude to dexamethasone-suppression testing. Low ratios of these metabolites (THF + 5α-THF/THE) characterizes cortisol reductase deficiency and apparent CRD (46,251).
The DAST modifies the dexamethasone suppression (Liddle) test used to discriminate Cushing’s syndrome from ordinary obesity (288) by prolonging the “low” dexamethasone dose from 2 to 4 days so as to clearly suppress serum DHEAS (36). The DAST protocol is given in Fig. 9. Normal suppression indicates an ACTH-dependent origin of androgen excess. Otherwise, circulating androgens and cortisol remaining at the end of this test have either a dexamethasone-resistant adrenal or extra-adrenal origin (or indicate noncompliance with the dexamethasone regime).
Post-DAST DHEAS, androgens and steroid intermediates fall to normal preadrenarchal concentrations in PreAd. The hyperandrogenism of NCCAH meets normal DAST androgen suppression criteria, but requires 1 to 2 weeks to suppress completely. This seems partly due to the long serum half-life of serum DHEAS and partly to slow resolution of adrenocortical hyperplasia.
These principles allow dissecting the alternative possible etiologies of childhood hyperandrogenism with the DAST (Fig. 9). If the ACTH test has indicated NCCAH, replacement glucocorticoid therapy will confirm the diagnosis by correcting the abnormalities (242). In patients with virilizing tumors, DAST will reveal that 1 or more androgen and/or steroid intermediate levels remain above the benchmark norms in the presence of normal cortisol suppression. On rare occasions DAST will reveal a virilizing tumor that has mimicked the high 17OHP of NCCAH but has a pattern of steroid intermediates otherwise uncharacteristic of any type of CAH (268). In endogenous Cushing syndrome or glucocorticoid resistance, neither androgens nor glucocorticoids suppress normally.
Imaging.
Ultrasonography is the initial radiographic study of choice to screen for the location of a pelvic, scrotal, or adrenal neoplasm (289). However, the ultrasonographic detection of small adrenal neoplasms is highly dependent on the expertise of the ultrasonographer. On rare occasions, ultrasonography has even been insensitive in detecting an ovarian tumor in adults (290). Computed tomography (267) and magnetic resonance imaging (291) permit the best visualization and more detailed assessment of tumors.
Management of Premature Adrenarche
After diagnosing PreAd, it is important to re-examine the patient at 6 and 12 months to be sure that progression of symptoms proves slow and height prediction remains stable. Thereafter, reassessment for the possibility of an overlooked, very mild virilizing disorder can be at longer intervals.
PreAd requires no specific endocrine treatment. Deodorants (paraben-free) can be used for excess body odor, and pubic hairs may be shaved or clipped if the girls find them embarrassing.
Patients and parents should be informed that idiopathic PP is ordinarily an extreme variation of normal due to inordinate sensitivity of the hair follicles to normal trace amounts of male hormone or that idiopathic PreAd is a normal incomplete form of puberty that is occurring early and is occasionally followed by a propensity to gain excessive weight and develop menstrual irregularity. In either case, true puberty has not begun, so menses and increased sexuality are not imminent (although in the case of PreAd, they may occur earlier than average). Therefore, it is advisable for the family to be conscious of maintaining healthy attitudes about these early body changes, lifestyle, and body weight. Associated obesity, particularly if the child has elements of metabolic syndrome, are an indication for counseling about a diet and exercise program for the child, with active participation by parents. The discussion of PreAd is summarized in Table 5.
Table 5.
Premature adrenarche summary
| • Premature adrenarche is the most common cause of the isolated premature onset of pubic hair development (before 8 years in girls, before 9 years in boys). • It is a very mildly hyperandrogenic condition that is slowly progressive and is often (or alternatively) associated with mild acne and increased axillary body odor. • It is more common in girls, which may reflect a sexual dimorphism in androgen metabolism or action. • The treatment is symptomatic with anticipatory guidance. • Although it typically seems to be a variant of normal development, premature adrenarche is frequently associated with a mildly advanced growth pattern, obesity, and insulin resistance and possibly an increased risk of mood disorder and hyperandrogenism. • The adult outcome of premature adrenarche remains to be well-defined. • Premature adrenarche is characterized by a distinctive serum steroid pattern in which serum DHEAS is typically 40 to 130 µg/dL (1.08-3.5 µmol/L), as determined by direct radioimmunoassay, and testosterone and its precursors are no greater than normal for early female puberty • Clinical steroid assay methodology is advancing: LCMSMS, due to its higher specificity, results in serum DHEAS levels nearly half those by direct radioimmunoassay, and customary norms for other sex steroids may well change in the near future. • The differential diagnosis includes idiopatnic premature pubarche, hypertrichosis, topical androgen exposure, true precocious puberty, nonclassic 21-hydroxylase deficiency congenital adrenal hyperplasia and other more rare virilizing disorders, including neoplasia, that collectively cause 5% to 10% of cases. • An algorithmic approach to the differential approach is suggested for the consulting endocrinologist in which further testing for virilizing disorders is advised for cases with atypical advancement of BA or atypically increased baseline serum DHEAS, testosterone, or 8 am 17-hydroxyprogesterone. |
Future Research Directions
A model system for understanding ZR development and its regulation is needed: recent research gives hope for a mouse ZR model. The increasing availability of high-sensitivity/throughput LCMSMS as the standard for steroid identification and quantitation urgently necessitates cross-laboratory harmonization of LCMSMS assays and the establishment of assay-appropriate norms.
11ß-Hydroxy- and 11-ketotestosterone have emerged as significant bioactive androgens of adrenarchal origin. It will be important to learn how obesity affects their levels and how plasma sex hormone-binding globulin affects their bioavailability. This may expand our understanding of idiopathic PP, idiopathic hirsutism, and the apparent variable response of the pilosebaceous unit to androgen excess. It remains to be proven whether the backdoor pathway for DHT formation contributes to PreAd. Association studies suggest that PreAd steroids may play a role in brain development and psychosocial disorders; mechanistic research is necessary. There is a need for prospective long-term follow-up studies and family studies of PreAd patients to better understand the basis of the relationship of PreAd to congenital factors, including heredity and birth weight, and the extent to which this mild childhood hyperandrogenism, often associated with obesity, and insulin resistance influences outcomes. Advances in molecular genetics and epigenetics can be expected to follow and enable us to advance understanding of PreAd.
Acknowledgments
I am grateful to the unknown reviewers for their critical comments and suggestions and to Walter L Miller, MD, for his assistance with some steroidogenic fine points.
Financial Support: The author’s research in this field was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement (U54-041859) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, and RR-00055 and UL1RR024999 from the National Center for Research Resources.
Glossary
Abbreviations
- AKR
aldo-ketoreductase
- BMI
body mass index
- DHEA
dehydroepiandrosterone
- DHEAS
DHEA-3-sulfate
- DHT
5α-dihydrotestostererone
- 17OHP
17-hydroxyprogesterone
- HSD
hydroxysteroid dehydrogenase
- LCMSMS
liquid chromatography-tandem mass spectrometry
- NCCAH
nonclassic congenital adrenal hyperplasia
- PSU
pilosebaceous unit
- PCOS
polycystic ovary syndrome
- PreAd
premature adrenarche
- PP
premature pubarche
- ZF
zona fasciculata
- ZG
zona glomerulosa
- ZR
zona reticularis.
Additional Information
Disclosures: No financial conflicts of interest to disclose.
References
- 1. Ibáñez L, Dimartino-Nardi J, Potau N, Saenger P. Premature adrenarche: normal variant or forerunner of adult disease? Endocr Rev. 2000;21(6):671-696. [DOI] [PubMed] [Google Scholar]
- 2. Kulle AE, Reinehr T, Simic-Schleicher G, Hornig NC, Holterhus PM. Determination of 17OHPreg and DHEAS by LC-MS/MS: impact of age, sex, pubertal stage, and BMI on the Δ5 steroid pathway. J Clin Endocrinol Metab. 2017;102(1):232-241. [DOI] [PubMed] [Google Scholar]
- 3. Rich BH, Rosenfield RL, Lucky AW, Helke JC, Otto P. Adrenarche: changing adrenal response to adrenocorticotropin. J Clin Endocrinol Metab. 1981;52(6):1129-1134. [DOI] [PubMed] [Google Scholar]
- 4. Dhom G. The prepuberal and puberal growth of the adrenal (adrenarche). Beitr Pathol. 1973;150(4):357-377. [DOI] [PubMed] [Google Scholar]
- 5. Suzuki T, Sasano H, Takeyama J, et al. . Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf). 2000;53(6):739-747. [DOI] [PubMed] [Google Scholar]
- 6. Cutler GB Jr, Davis SE, Johnsonbaugh RE, Loriaux DL. Dissociation of cortisol and adrenal androgen secretion in patients with secondary adrenal insufficiency. J Clin Endocrinol Metab. 1979;49(4):604-609. [DOI] [PubMed] [Google Scholar]
- 7. Hui XG, Akahira J, Suzuki T, et al. . Development of the human adrenal zona reticularis: morphometric and immunohistochemical studies from birth to adolescence. J Endocrinol. 2009;203(2):241-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim JH, Lee YA, Lim YH, et al. . Changes in adrenal androgens and steroidogenic enzyme activities from ages 2, 4, to 6 years: a prospective cohort study. J Clin Endocrinol Metab. 2020;105(10):dgaa498. [DOI] [PubMed] [Google Scholar]
- 9. Rosenfield RL. Clinical review: Identifying children at risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92(3):787-796. [DOI] [PubMed] [Google Scholar]
- 10. Rosenfield RL, Lucky AW, Allen TD. The diagnosis and management of intersex. Curr Probl Pediatr. 1980;10(7):1-66. [DOI] [PubMed] [Google Scholar]
- 11. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller WL, Tee MK. The post-translational regulation of 17,20 lyase activity. Mol Cell Endocrinol. 2015;408:99-106. [DOI] [PubMed] [Google Scholar]
- 13. Udhane SS, Flück CE. Regulation of human (adrenal) androgen biosynthesis: new insights from novel throughput technology studies. Biochem Pharmacol. 2016;102:20-33. [DOI] [PubMed] [Google Scholar]
- 14. Moreau F, Mittre H, Benhaim A, et al. . Aromatase expression in the normal human adult adrenal and in adrenocortical tumors: biochemical, immunohistochemical, and molecular studies. Eur J Endocrinol. 2009;160(1):93-99. [DOI] [PubMed] [Google Scholar]
- 15. Rege J, Nakamura Y, Satoh F, et al. . Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J Clin Endocrinol Metab. 2013;98(3):1182-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocr Rev. 2001;22(3):289-318. [DOI] [PubMed] [Google Scholar]
- 17. Rege J, Nakamura Y, Wang T, Merchen TD, Sasano H, Rainey WE. Transcriptome profiling reveals differentially expressed transcripts between the human adrenal zona fasciculata and zona reticularis. J Clin Endocrinol Metab. 2014;99(3):E518-E527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heinosalo T, Saarinen N, Poutanen M. Role of hydroxysteroid (17beta) dehydrogenase type 1 in reproductive tissues and hormone-dependent diseases. Mol Cell Endocrinol. 2019;489:9-31. [DOI] [PubMed] [Google Scholar]
- 20. Rege J, Nanba AT, Auchus RJ, et al. . Adrenocorticotropin acutely regulates pregnenolone sulfate production by the human adrenal in vivo and in vitro. J Clin Endocrinol Metab. 2018;103(1):320-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends Endocrinol Metab. 2002;13(6):234-239. [DOI] [PubMed] [Google Scholar]
- 22. Noordam C, Dhir V, McNelis JC, et al. . Inactivating PAPSS2 mutations in a patient with premature pubarche. N Engl J Med. 2009;360(22):2310-2318. [DOI] [PubMed] [Google Scholar]
- 23. Nakamura Y, Hornsby PJ, Casson P, et al. . Type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J Clin Endocrinol Metab. 2009;94(6):2192-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenfield RL. Role of androgens in growth and development of the fetus, child, and adolescent. Adv Pediatr. 1972;19:171-213. [Google Scholar]
- 25. Kirschner MA, Sinhamahapatra S, Zucker IR, Loriaux L, Nieschiag E. The production, origin and role of dehydroepiandrosterone and delta 5-androstenediol as androgen prehormones in hirsute women. J Clin Endocrinol Metab. 1973;37(2):183-189. [DOI] [PubMed] [Google Scholar]
- 26. Horton R, Tait JF. Androstenedione production and interconversion rates measured in peripheral blood and studies on the possible site of its conversion to testosterone. J Clin Invest. 1966;45(3):301-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bardin CW, Lipsett MB. Testosterone and androstenedione blood production rates in normal women and women with idiopathic hirsutism or polycystic ovaries. J Clin Invest. 1967;46(5):891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bordini B, Rosenfield RL. Normal pubertal development: Part I: the endocrine basis of puberty. Pediatr Rev. 2011;32(6):223-229. [DOI] [PubMed] [Google Scholar]
- 29. Rivarola MA, Singleton RT, Migeon CJ. Splanchnic extraction and interconversion of testosterone and androstenedione in man. J Clin Invest. 1967;46(12):2095-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arlt W, Callies F, van Vlijmen JC, et al. . Dehydroepiandrosterone replacement in women with adrenal insufficiency. N Engl J Med. 1999;341(14):1013-1020. [DOI] [PubMed] [Google Scholar]
- 31. Hammer F, Subtil S, Lux P, et al. . No evidence for hepatic conversion of dehydroepiandrosterone (DHEA) sulfate to DHEA: in vivo and in vitro studies. J Clin Endocrinol Metab. 2005;90(6):3600-3605. [DOI] [PubMed] [Google Scholar]
- 32. Longcope C. Dehydroepiandrosterone metabolism. J Endocrinol. 1996;150(Suppl):S125-S127. [PubMed] [Google Scholar]
- 33. Schiffer L, Arlt W, Storbeck KH. Intracrine androgen biosynthesis, metabolism and action revisited. Mol Cell Endocrinol. 2018;465:4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kríz L, Bicíková M, Hampl R. Roles of steroid sulfatase in brain and other tissues. Physiol Res. 2008;57(5):657-668. [DOI] [PubMed] [Google Scholar]
- 35. Oostdijk W, Idkowiak J, Mueller JW, et al. . PAPSS2 deficiency causes androgen excess via impaired DHEA sulfation: in vitro and in vivo studies in a family harboring two novel PAPSS2 mutations. J Clin Endocrinol Metab. 2015;100(4):E672-E680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosenfield RL, Mortensen M, Wroblewski K, Littlejohn E, Ehrmann DA. Determination of the source of androgen excess in functionally atypical polycystic ovary syndrome by a short dexamethasone androgen-suppression test and a low-dose ACTH test. Hum Reprod. 2011;26(11):3138-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sandberg E, Gurpide E, Lieberman S. Quantitative studies on the metabolism of dehydroisoandrosterone sulfate. Biochemistry. 1964;3:1256-1267. [DOI] [PubMed] [Google Scholar]
- 38. Abraham GE. Ovarian and adrenal contribution to peripheral androgens during the menstrual cycle. J Clin Endocrinol Metab. 1974;39(2):340-346. [DOI] [PubMed] [Google Scholar]
- 39. Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA. The regulation of steroid action by sulfation and desulfation. Endocr Rev. 2015;36(5):526-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Turcu AF, Rege J, Auchus RJ, Rainey WE. 11-Oxygenated androgens in health and disease. Nat Rev Endocrinol. 2020;16(5):284-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Diederich S, Quinkler M, Burkhardt P, Grossmann C, Bähr V, Oelkers W. 11Beta-hydroxysteroid-dehydrogenase isoforms: tissue distribution and implications for clinical medicine. Eur J Clin Invest. 2000;30(Suppl 3):21-27. [DOI] [PubMed] [Google Scholar]
- 42. Zouboulis CC, Chen WC, Thornton MJ, Qin K, Rosenfield R. Sexual hormones in human skin. Horm Metab Res. 2007;39(2):85-95. [DOI] [PubMed] [Google Scholar]
- 43. Hay JB, Hodgins MB. Metabolism of androgens in vitro by human facial and axillary skin. J Endocrinol. 1973;59(3):475-486. [DOI] [PubMed] [Google Scholar]
- 44. Hay JB, Hodgins MB. Distribution of androgen metabolizing enzymes in isolated tissues of human forehead and axillary skin. J Endocrinol. 1978;79(1):29-39. [DOI] [PubMed] [Google Scholar]
- 45. Marynick SP, Chakmakjian ZH, McCaffree DL, Herndon JH Jr. Androgen excess in cystic acne. N Engl J Med. 1983;308(17):981-986. [DOI] [PubMed] [Google Scholar]
- 46. Storbeck KH, Schiffer L, Baranowski ES, et al. . Steroid metabolome analysis in disorders of adrenal steroid biosynthesis and metabolism. Endocr Rev. 2019;40(6):1605-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dehennin L, Ferry M, Lafarge P, Pérès G, Lafarge JP. Oral administration of dehydroepiandrosterone to healthy men: alteration of the urinary androgen profile and consequences for the detection of abuse in sport by gas chromatography-mass spectrometry. Steroids. 1998;63(2):80-87. [DOI] [PubMed] [Google Scholar]
- 48. Rižner TL, Penning TM. Role of aldo-keto reductase family 1 (AKR1) enzymes in human steroid metabolism. Steroids. 2014;79:49-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Korenman SG, Lipsett MB. Direct peripheral conversion of dehydroepiandrosterone to testosterone glucuronoside. Steroids. 1965;85:509-517. [DOI] [PubMed] [Google Scholar]
- 50. Camacho AM, Migeon CJ. Testosterone excretion and production rate in normal adults and in patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1966;26(8):893-896. [DOI] [PubMed] [Google Scholar]
- 51. Rittmaster RS, Zwicker H, Thompson DL, Konok G, Norman RW. Androstanediol glucuronide production in human liver, prostate, and skin. Evidence for the importance of the liver in 5 alpha-reduced androgen metabolism. J Clin Endocrinol Metab. 1993;76(4):977-982. [DOI] [PubMed] [Google Scholar]
- 52. Ryan KJ, Hopper BR. Placental biosynthesis and metabolism of steroid hormones in primates. Contrib Primatol. 1974;3:258-283. [PubMed] [Google Scholar]
- 53. Auchus RJ. Steroid degrdation and secretion. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction. 1. 4th ed. Academic Press; 2015:304-312. [Google Scholar]
- 54. Flück CE, Meyer-Böni M, Pandey AV, et al. . Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. Am J Hum Genet. 2011;89(2):201-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. O’Shaughnessy PJ, Antignac JP, Le Bizec B, et al. . Alternative (backdoor) androgen production and masculinization in the human fetus. PloS Biol. 2019;17(2):e3000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Reisch N, Taylor AE, Nogueira EF, et al. . Alternative pathway androgen biosynthesis and human fetal female virilization. Proc Natl Acad Sci U S A. 2019;116(44):22294-22299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kamrath C, Hochberg Z, Hartmann MF, Remer T, Wudy SA. Increased activation of the alternative “backdoor” pathway in patients with 21-hydroxylase deficiency: evidence from urinary steroid hormone analysis. J Clin Endocrinol Metab. 2012;97(3):E367-E375. [DOI] [PubMed] [Google Scholar]
- 58. Auchus RJ, Miller WL. Congenital adrenal hyperplasia: more dogma bites the dust. J Clin Endocrinol Metab. 2012;97(3):772-775. [DOI] [PubMed] [Google Scholar]
- 59. Chen J, Sowers MR, Moran FM, et al. . Circulating bioactive androgens in midlife women. J Clin Endocrinol Metab. 2006;91(11):4387-4394. [DOI] [PubMed] [Google Scholar]
- 60. Liimatta J, Laakso S, Utriainen P, et al. . Serum androgen bioactivity is low in children with premature adrenarche. Pediatr Res. 2014;75(5):645-650. [DOI] [PubMed] [Google Scholar]
- 61. Campana C, Rege J, Turcu AF, et al. . Development of a novel cell based androgen screening model. J Steroid Biochem Mol Biol. 2016;156:17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Turcu AF, Nanba AT, Auchus RJ. The rise, fall, and resurrection of 11-oxygenated androgens in human physiology and disease. Horm Res Paediatr. 2018;89(5):284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Storbeck KH, Bloem LM, Africander D, Schloms L, Swart P, Swart AC. 11β-Hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: a putative role in castration resistant prostate cancer? Mol Cell Endocrinol. 2013;377(1-2):135-146. [DOI] [PubMed] [Google Scholar]
- 64. Rege J, Turcu AF, Kasa-Vubu JZ, et al. . 11-Ketotestosterone is the dominant circulating bioactive androgen during normal and premature adrenarche. J Clin Endocrinol Metab. 2018;103(12):4589-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pretorius E, Africander DJ, Vlok M, Perkins MS, Quanson J, Storbeck KH. 11-ketotestosterone and 11-ketodihydrotestosterone in castration resistant prostate cancer: potent androgens which can no longer be ignored. PLoS One. 2016;11(7):e0159867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kulle AE, Riepe FG, Melchior D, Hiort O, Holterhus PM. A novel ultrapressure liquid chromatography tandem mass spectrometry method for the simultaneous determination of androstenedione, testosterone, and dihydrotestosterone in pediatric blood samples: age- and sex-specific reference data. J Clin Endocrinol Metab. 2010;95(5):2399-2409. [DOI] [PubMed] [Google Scholar]
- 67. Häkkinen MR, Murtola T, Voutilainen R, et al. . Simultaneous analysis by LC-MS/MS of 22 ketosteroids with hydroxylamine derivatization and underivatized estradiol from human plasma, serum and prostate tissue. J Pharm Biomed Anal. 2019;164:642-652. [DOI] [PubMed] [Google Scholar]
- 68. Nimrod A, Rosenfield RL, Otto P. Relationship of androgen action to androgen metabolism in isolated rat granulosa cells. J Steroid Biochem. 1980;13(9):1015-1019. [DOI] [PubMed] [Google Scholar]
- 69. Laurent MR, Helsen C, Antonio L, et al. . Effects of sex hormone-binding globulin (SHBG) on androgen bioactivity in vitro. Mol Cell Endocrinol. 2016;437:280-291. [DOI] [PubMed] [Google Scholar]
- 70. Martin KA, Anderson RR, Chang RJ, et al. . Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(4):1-25. [DOI] [PubMed] [Google Scholar]
- 71. Nilsson O, Marino R, De Luca F, Phillip M, Baron J. Endocrine regulation of the growth plate. Horm Res. 2005;64(4):157-165. [DOI] [PubMed] [Google Scholar]
- 72. Gravholt CH, Andersen NH, Conway GS, et al. ; International Turner Syndrome Consensus Group . Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol. 2017;177(3):G1-G70. [DOI] [PubMed] [Google Scholar]
- 73. Zhou S, Glowacki J. Dehydroepiandrosterone and Bone. Vitam Horm. 2018;108:251-271. [DOI] [PubMed] [Google Scholar]
- 74. Schverer M, Lanfumey L, Baulieu EE, Froger N, Villey I. Neurosteroids: non-genomic pathways in neuroplasticity and involvement in neurological diseases. Pharmacol Ther. 2018;191:190-206. [DOI] [PubMed] [Google Scholar]
- 75. Mellon SH. Neurosteroid regulation of central nervous system development. Pharmacol Ther. 2007;116(1):107-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Grube M, Hagen P, Jedlitschky G. Neurosteroid transport in the brain: role of ABC and SLC transporters. Front Pharmacol. 2018;9:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Paul SM, Purdy RH. Neuroactive steroids. Faseb J. 1992;6(6):2311-2322. [PubMed] [Google Scholar]
- 78. Harteneck C. Pregnenolone sulfate: from steroid metabolite to TRP channel ligand. Molecules. 2013;18(10):12012-12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Greaves RF, Wudy SA, Badoer E, et al. . A tale of two steroids: the importance of the androgens DHEA and DHEAS for early neurodevelopment. J Steroid Biochem Mol Biol. 2019;188:77-85. [DOI] [PubMed] [Google Scholar]
- 80. Herdt G, McClintock M. The magical age of 10. Arch Sex Behav. 2000;29(6):587-606. [DOI] [PubMed] [Google Scholar]
- 81. Campbell BC. Adrenarche and middle childhood. Hum Nat. 2011;22(3):327-349. [DOI] [PubMed] [Google Scholar]
- 82. Del Giudice M. Sex, attachment, and the development of reproductive strategies. Behav Brain Sci. 2009;32(1):1-21; discussion 21. [DOI] [PubMed] [Google Scholar]
- 83. Nguyen AD, Mapes SM, Corbin CJ, Conley AJ. Morphological adrenarche in rhesus macaques: development of the zona reticularis is concurrent with fetal zone regression in the early neonatal period. J Endocrinol. 2008;199(3):367-378. [DOI] [PubMed] [Google Scholar]
- 84. Nguyen AD, Corbin CJ, Pattison JC, Bird IM, Conley AJ. The developmental increase in adrenocortical 17,20-lyase activity (biochemical adrenarche) is driven primarily by increasing cytochrome b5 in neonatal rhesus macaques. Endocrinology. 2009;150(4):1748-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nakamura Y, Gang HX, Suzuki T, Sasano H, Rainey WE. Adrenal changes associated with adrenarche. Rev Endocr Metab Disord. 2009;10(1):19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Conley AJ, Bernstein RM, Nguyen AD. Adrenarche in nonhuman primates: the evidence for it and the need to redefine it. J Endocrinol. 2012;214(2):121-131. [DOI] [PubMed] [Google Scholar]
- 87. Campbell B. DHEAS and human development: an evolutionary perspective. Front Endocrinol (Lausanne). 2020;11:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dumontet T, Martinez A. Adrenal androgens, adrenarche, and zona reticularis: a human affair? Mol Cell Endocrinol. 2021;528:111239. [DOI] [PubMed] [Google Scholar]
- 89. Quinn TA, Ratnayake U, Dickinson H, Castillo-Melendez M, Walker DW. The feto-placental unit, and potential roles of dehydroepiandrosterone (DHEA) in prenatal and postnatal brain development: A re-examination using the spiny mouse. J Steroid Biochem Mol Biol. 2016;160:204-213. [DOI] [PubMed] [Google Scholar]
- 90. Finco I, Mohan DR, Hammer GD, Lerario AM. Regulation of stem and progenitor cells in the adrenal cortex. Curr Opin Endocr Metab Res. 2019;8:66-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dumontet T, Sahut-Barnola I, Septier A, et al. . PKA signaling drives reticularis differentiation and sexually dimorphic adrenal cortex renewal. JCI Insight. 2018;3(2):e98394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Freedman BD, Kempna PB, Carlone DL, et al. . Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev Cell. 2013;26(6):666-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hershkovitz L, Beuschlein F, Klammer S, Krup M, Weinstein Y. Adrenal 20alpha-hydroxysteroid dehydrogenase in the mouse catabolizes progesterone and 11-deoxycorticosterone and is restricted to the X-zone. Endocrinology. 2007;148(3):976-988. [DOI] [PubMed] [Google Scholar]
- 94. Teves ME, Modi BP, Kulkarni R, et al. . Human DENND1A.V2 drives Cyp17a1 expression and androgen production in mouse ovaries and adrenals. Int J Mol Sci. 2020;21(7):2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997;18(3):378-403. [DOI] [PubMed] [Google Scholar]
- 96. Nanba AT, Rege J, Ren J, Auchus RJ, Rainey WE, Turcu AF. 11-oxygenated C19 steroids do not decline with age in women. J Clin Endocrinol Metab. 2019;104(7):2615-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nunes-Souza E, Silveira ME, Mendes MC, et al. . From adrenarche to aging of adrenal zona reticularis: precocious female adrenopause onset. Endocr Connect. 2020;9(12):1212-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tezuka Y, Atsumi N, Blinder AR, et al. . The age-dependent changes of the human adrenal cortical zones are not congruent. J Clin Endocrinol Metab. Published online February 2021. doi:10.1210/clinem/dgab007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Davio A, Woolcock H, Nanba AT, et al. . Sex differences in 11-oxygenated androgen patterns across adulthood. J Clin Endocrinol Metab. 2020;105(8):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Novoselova TV, Hussain M, King PJ, et al. . MRAP deficiency impairs adrenal progenitor cell differentiation and gland zonation. FASEB J. 2018;32(11):6186-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Weber A, Clark AJ, Perry LA, Honour JW, Savage MO. Diminished adrenal androgen secretion in familial glucocorticoid deficiency implicates a significant role for ACTH in the induction of adrenarche. Clin Endocrinol (Oxf). 1997;46(4):431-437. [DOI] [PubMed] [Google Scholar]
- 102. Korth-Schutz S, Levine LS, New MI. Dehydroepiandrosterone sulfate (DS) levels, a rapid test for abnormal adrenal androgen secretion. J Clin Endocrinol Metab. 1976;42(6):1005-1013. [DOI] [PubMed] [Google Scholar]
- 103. Brigell DF, Fang VS, Rosenfield RL. Recovery of responses to ovine corticotropin-releasing hormone after withdrawal of a short course of glucocorticoid. J Clin Endocrinol Metab. 1992;74(5):1036-1039. [DOI] [PubMed] [Google Scholar]
- 104. Anderson DC. The adrenal androgen-stimulating hormone does not exist. Lancet. 1980;2(8192):454-456. [DOI] [PubMed] [Google Scholar]
- 105. Endoh A, Kristiansen SB, Casson PR, Buster JE, Hornsby PJ. The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3 beta-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab. 1996;81(10):3558-3565. [DOI] [PubMed] [Google Scholar]
- 106. l’Allemand D, Penhoat A, Lebrethon MC, et al. . Insulin-like growth factors enhance steroidogenic enzyme and corticotropin receptor messenger ribonucleic acid levels and corticotropin steroidogenic responsiveness in cultured human adrenocortical cells. J Clin Endocrinol Metab. 1996;81(11):3892-3897. [DOI] [PubMed] [Google Scholar]
- 107. Baquedano MS, Berensztein E, Saraco N, et al. . Expression of the IGF system in human adrenal tissues from early infancy to late puberty: implications for the development of adrenarche. Pediatr Res. 2005;58(3):451-458. [DOI] [PubMed] [Google Scholar]
- 108. Kristiansen SB, Endoh A, Casson PR, Buster JE, Hornsby PJ. Induction of steroidogenic enzyme genes by insulin and IGF-I in cultured adult human adrenocortical cells. Steroids. 1997;62(2):258-265. [DOI] [PubMed] [Google Scholar]
- 109. Biason-Lauber A, Zachmann M, Schoenle EJ. Effect of leptin on CYP17 enzymatic activities in human adrenal cells: new insight in the onset of adrenarche. Endocrinology. 2000;141(4):1446-1454. [DOI] [PubMed] [Google Scholar]
- 110. Rosenfield RL. Current concepts of polycystic ovary syndrome pathogenesis. Curr Opin Pediatr. 2020;32(5):698-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev. 1998;19(2):101-143. [DOI] [PubMed] [Google Scholar]
- 112. Päth G, Bornstein SR, Ehrhart-Bornstein M, Scherbaum WA. Interleukin-6 and the interleukin-6 receptor in the human adrenal gland: expression and effects on steroidogenesis. J Clin Endocrinol Metab. 1997;82(7):2343-2349. [DOI] [PubMed] [Google Scholar]
- 113. Rege J, Nishimoto HK, Nishimoto K, Rodgers RJ, Auchus RJ, Rainey WE. Bone morphogenetic protein-4 (BMP4): a paracrine regulator of human adrenal C19 steroid synthesis. Endocrinology. 2015;156(7):2530-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Taha D, Mullis PE, Ibáñez L, de Zegher F. Absent or delayed adrenarche in Pit-1/POU1F1 deficiency. Horm Res. 2005;64(4):175-179. [DOI] [PubMed] [Google Scholar]
- 115. Van Hulle S, Craen M, Callewaert B, et al. . Delayed adrenarche may be an additional feature of immunoglobulin super family member 1 deficiency syndrome. J Clin Res Pediatr Endocrinol. 2016;8(1):86-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Glickman SP, Rosenfield RL, Bergenstal RM, Helke J. Multiple androgenic abnormalities, including elevated free testosterone, in hyperprolactinemic women. J Clin Endocrinol Metab. 1982;55(2):251-257. [DOI] [PubMed] [Google Scholar]
- 117. Byrne GC, Perry YS, Winter JS. Kinetic analysis of adrenal 3 beta-hydroxysteroid dehydrogenase activity during human development. J Clin Endocrinol Metab. 1985;60(5):934-939. [DOI] [PubMed] [Google Scholar]
- 118. Topor LS, Asai M, Dunn J, Majzoub JA. Cortisol stimulates secretion of dehydroepiandrosterone in human adrenocortical cells through inhibition of 3betaHSD2. J Clin Endocrinol Metab. 2011;96(1):E31-E39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Majzoub JA, Topor LS. A new model for adrenarche: inhibition of 3beta-hydroxysteroid dehydrogenase type 2 by intra-adrenal cortisol. Horm Res Paediatr. 2018;89(5):311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bellanger S, Battista MC, Fink GD, Baillargeon JP. Saturated fatty acid exposure induces androgen overproduction in bovine adrenal cells. Steroids. 2012;77(4):347-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Smith CP, Dunger DB, Williams AJ, et al. . Relationship between insulin, insulin-like growth factor I, and dehydroepiandrosterone sulfate concentrations during childhood, puberty, and adult life. J Clin Endocrinol Metab. 1989;68(5):932-937. [DOI] [PubMed] [Google Scholar]
- 122. Guercio G, Rivarola MA, Chaler E, Maceiras M, Belgorosky A. Relationship between the growth hormone/insulin-like growth factor-I axis, insulin sensitivity, and adrenal androgens in normal prepubertal and pubertal girls. J Clin Endocrinol Metab. 2003;88(3):1389-1393. [DOI] [PubMed] [Google Scholar]
- 123. Ong KK, Potau N, Petry CJ, et al. ; Avon Longitudinal Study of Parents and Children Study Team . Opposing influences of prenatal and postnatal weight gain on adrenarche in normal boys and girls. J Clin Endocrinol Metab. 2004;89(6):2647-2651. [DOI] [PubMed] [Google Scholar]
- 124. Sopher AB, Jean AM, Zwany SK, et al. . Bone age advancement in prepubertal children with obesity and premature adrenarche: possible potentiating factors. Obesity (Silver Spring). 2011;19(6):1259-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Corvalán C, Uauy R, Mericq V. Obesity is positively associated with dehydroepiandrosterone sulfate concentrations at 7 y in Chilean children of normal birth weight. Am J Clin Nutr. 2013;97(2):318-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Mäntyselkä A, Lindi V, Viitasalo A, et al. . Associations of dehydroepiandrosterone sulfate with cardiometabolic risk factors in prepubertal children. J Clin Endocrinol Metab. 2018;103(7):2592-2600. [DOI] [PubMed] [Google Scholar]
- 127. Nordman H, Voutilainen R, Antikainen L, Jaaskelainen J. Prepubertal children born large for gestational age have lower serum DHEAS concentrations than those with a lower birth weight. Pediatr Res. 2018;82(2):285-289. [DOI] [PubMed] [Google Scholar]
- 128. Clark PM, Hindmarsh PC, Shiell AW, Law CM, Honour JW, Barker DJ. Size at birth and adrenocortical function in childhood. Clin Endocrinol (Oxf). 1996;45(6):721-726. [DOI] [PubMed] [Google Scholar]
- 129. Cumming DC, Rebar RW, Hopper BR, Yen SS. Evidence for an influence of the ovary on circulating dehydroepiandrosterone sulfate levels. J Clin Endocrinol Metab. 1982;54(5):1069-1071. [DOI] [PubMed] [Google Scholar]
- 130. Martin DD, Schweizer R, Schwarze CP, Elmlinger MW, Ranke MB, Binder G. The early dehydroepiandrosterone sulfate rise of adrenarche and the delay of pubarche indicate primary ovarian failure in Turner syndrome. J Clin Endocrinol Metab. 2004;89(3):1164-1168. [DOI] [PubMed] [Google Scholar]
- 131. Rege J, Karashima S, Lerario AM, et al. . Age-dependent increases in adrenal cytochrome b5 and serum 5-androstenediol-3-sulfate. J Clin Endocrinol Metab. 2016;101(12):4585-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Forti G, Toscano V, Casilli D, et al. . Spermatic and peripheral venous plasma concentrations of testosterone, 17-hydroxyprogesterone, androstenedione, dehydroepiandrosterone, delta 5-androstene-3 beta,17 beta-diol, dihydrotestosterone, 5 alpha-androstane-3 alpha,17 beta-diol, 5 alpha-androstane-3 beta,17 beta-diol, and estradiol in boys with idiopathic varicocele in different stages of puberty. J Clin Endocrinol Metab. 1985;61(2):322-327. [DOI] [PubMed] [Google Scholar]
- 133. Chapdelaine A, MacDonald PC, Gonzalez O, Gurpide E, Vande Wiele RL, Lieberman S. Studies on the secretion and interconversion of the androgens. IV. Quantitative results in a normal man whose gonadal and adrenal function were altered experimentally. J Clin Endocrinol Metab. 1965;25(12):1569-1579. [DOI] [PubMed] [Google Scholar]
- 134. Laatikainen T, Laitinen EA, Vihko R. Secretion of free and sulfate-conjugated neutral steroids by the human testis: effect of administration of human chorionic gonadotropin. J Clin Endocrinol Metab. 1971;32(1):59-64. [DOI] [PubMed] [Google Scholar]
- 135. Lindsay J, Wang LL, Li Y, Zhou SF. Structure, function and polymorphism of human cytosolic sulfotransferases. Curr Drug Metab. 2008;9(2):99-105. [DOI] [PubMed] [Google Scholar]
- 136. Deplewski D, Rosenfield RL. Role of hormones in pilosebaceous unit development. Endocr Rev. 2000;21(4):363-392. [DOI] [PubMed] [Google Scholar]
- 137. Remer T, Shi L, Buyken AE, Maser-Gluth C, Hartmann MF, Wudy SA. Prepubertal adrenarchal androgens and animal protein intake independently and differentially influence pubertal timing. J Clin Endocrinol Metab. 2010;95(6):3002-3009. [DOI] [PubMed] [Google Scholar]
- 138. Thankamony A, Ong KK, Ahmed ML, Ness AR, Holly JM, Dunger DB. Higher levels of IGF-I and adrenal androgens at age 8 years are associated with earlier age at menarche in girls. J Clin Endocrinol Metab. 2012;97(5):E786-E790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Remer T, Manz F. The midgrowth spurt in healthy children is not caused by adrenarche. J Clin Endocrinol Metab. 2001;86(9):4183-4186. [DOI] [PubMed] [Google Scholar]
- 140. Remer T, Boye KR, Hartmann M, et al. . Adrenarche and bone modeling and remodeling at the proximal radius: weak androgens make stronger cortical bone in healthy children. J Bone Miner Res. 2003;18(8):1539-1546. [DOI] [PubMed] [Google Scholar]
- 141. Remer T, Manz F, Hartmann MF, Schoenau E, Wudy SA. Prepubertal healthy children’s urinary androstenediol predicts diaphyseal bone strength in late puberty. J Clin Endocrinol Metab. 2009;94(2):575-578. [DOI] [PubMed] [Google Scholar]
- 142. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Reingold SB, Rosenfield RL. The relationship of mild hirsutism or acne in women to androgens. Arch Dermatol. 1987;123(2):209-212. [PubMed] [Google Scholar]
- 144. Biro FM, Huang B, Daniels SR, Lucky AW. Pubarche as well as thelarche may be a marker for the onset of puberty. J Pediatr Adolesc Gynecol. 2008;21(6):323-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Witchel SF, Pinto B, Burghard AC, Oberfield SE. Update on adrenarche. Curr Opin Pediatr. 2020;32(4):574-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Rosenfield RL, Lipton RB, Drum ML. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics. 2009;123(1):84-88. [DOI] [PubMed] [Google Scholar]
- 147. Herman-Giddens ME, Slora EJ, Wasserman RC, et al. . Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99(4):505-512. [DOI] [PubMed] [Google Scholar]
- 148. Charkaluk ML, Trivin C, Brauner R. Premature pubarche as an indicator of how body weight influences the onset of adrenarche. Eur J Pediatr. 2004;163(2):89-93. [DOI] [PubMed] [Google Scholar]
- 149. Livadas S, Bothou C, Kanaka-Gantenbein C, et al. . Unfavorable hormonal and psychologic profile in adult women with a history of premature adrenarche and pubarche, compared to women with polycystic ovary syndrome. Horm Metab Res. 2020;52(3):179-185. [DOI] [PubMed] [Google Scholar]
- 150. Nebesio TD, Eugster EA. Pubic hair of infancy: endocrinopathy or enigma? Pediatrics. 2006;117(3):951-954. [DOI] [PubMed] [Google Scholar]
- 151. Kaplowitz P, Soldin SJ. Steroid profiles in serum by liquid chromatography-tandem mass spectrometry in infants with genital hair. J Pediatr Endocrinol Metab. 2007;20(5):597-605. [DOI] [PubMed] [Google Scholar]
- 152. Ramos CO, Macedo DB, Bachega TASS, et al. . Premature pubarche due to exogenous testosterone gel or intense diaper rash prevention cream use: a case series. Horm Res Paediatr. 2019;91(6):411-415. [DOI] [PubMed] [Google Scholar]
- 153. Lachgar S, Charveron M, Gall Y, Bonafe JL. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br J Dermatol. 1998;138(3):407-411. [DOI] [PubMed] [Google Scholar]
- 154. Rosenfield RL. Premature adrenarche. In: Post TW, ed. UpToDate. 2018. https://www.uptodate.com/contents/premature-adrenarche [Google Scholar]
- 155. Utriainen P, Voutilainen R, Jääskeläinen J. Continuum of phenotypes and sympathoadrenal function in premature adrenarche. Eur J Endocrinol. 2009;160(4):657-665. [DOI] [PubMed] [Google Scholar]
- 156. Rosenfield RL. Plasma 17-ketosteroids and 17-beta hydroxysteroids in girls with premature development of sexual hair. J Pediatr. 1971;79(2):260-266. [DOI] [PubMed] [Google Scholar]
- 157. Svendsen PF, Madsbad S, Nilas L, Paulsen SK, Pedersen SB. Expression of 11beta-hydroxysteroid dehydrogenase 1 and 2 in subcutaneous adipose tissue of lean and obese women with and without polycystic ovary syndrome. Int J Obes (Lond). 2009;33(11):1249-1256. [DOI] [PubMed] [Google Scholar]
- 158. O’Reilly MW, Kempegowda P, Walsh M, et al. . AKR1C3-mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(9):3327-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Du X, Rosenfield RL, Qin K. KLF15 Is a transcriptional regulator of the human 17beta-hydroxysteroid dehydrogenase type 5 gene. A potential link between regulation of testosterone production and fat stores in women. J Clin Endocrinol Metab. 2009;94(7):2594-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Rosenfield RL, Rich BH, Lucky AW. Adrenarche as a cause of benign pseudopuberty in boys. J Pediatr. 1982;101(6):1005-1009. [DOI] [PubMed] [Google Scholar]
- 161. Haring R, Wallaschofski H, Teumer A, et al. . A SULT2A1 genetic variant identified by GWAS as associated with low serum DHEAS does not impact on the actual DHEA/DHEAS ratio. J Mol Endocrinol. 2013;50(1):73-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Idkowiak J, Taylor AE, Subtil S, et al. . Steroid sulfatase deficiency and androgen activation before and after puberty. J Clin Endocrinol Metab. 2016;101(6):2545-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Lutfallah C, Wang W, Mason JI, et al. . Newly proposed hormonal criteria via genotypic proof for type II 3beta-hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab. 2002;87(6):2611-2622. [DOI] [PubMed] [Google Scholar]
- 164. Likitmaskul S, Cowell CT, Donaghue K, et al. . ‘Exaggerated adrenarche’ in children presenting with premature adrenarche. Clin Endocrinol (Oxf). 1995;42(3):265-272. [DOI] [PubMed] [Google Scholar]
- 165. Lazar L, Kauli R, Bruchis C, Nordenberg J, Galatzer A, Pertzelan A. Early polycystic ovary-like syndrome in girls with central precocious puberty and exaggerated adrenal response. Eur J Endocrinol. 1995;133(4):403-406. [DOI] [PubMed] [Google Scholar]
- 166. Idkowiak J, Lavery GG, Dhir V, et al. . Premature adrenarche: novel lessons from early onset androgen excess. Eur J Endocrinol. 2011;165(2):189-207. [DOI] [PubMed] [Google Scholar]
- 167. Janner M, Sommer G, Groessl M, Flück CE. Premature adrenarche in girls is characterized by enhanced 17,20-lyase and 17β-hydroxysteroid dehydrogenase activities. J Clin Endocrinol Metab. 2020;105(12):1-13. [DOI] [PubMed] [Google Scholar]
- 168. Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92(2):405-413. [DOI] [PubMed] [Google Scholar]
- 169. Rosner W, Hankinson SE, Sluss PM, Vesper HW, Wierman ME. Challenges to the measurement of estradiol: an endocrine society position statement. J Clin Endocrinol Metab. 2013;98(4):1376-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Yi X, Leung EKY, Bridgman R, Koo S, Yeo KJ. High-sensitivity micro LC-MS/MS assay for serum estradiol without derivatization. J Appl Lab Med. 2016;1(1):14-24. [DOI] [PubMed] [Google Scholar]
- 171. Voutilainen R, Perheentupa J, Apter D. Benign premature adrenarche: clinical features and serum steroid levels. Acta Paediatr Scand. 1983;72(5):707-711. [DOI] [PubMed] [Google Scholar]
- 172. Kushnir MM, Blamires T, Rockwood AL, et al. . Liquid chromatography-tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clin Chem. 2010;56(7):1138-1147. [DOI] [PubMed] [Google Scholar]
- 173. Kulle AE, Welzel M, Holterhus PM, Riepe FG. Implementation of a liquid chromatography tandem mass spectrometry assay for eight adrenal C-21 steroids and pediatric reference data. Horm Res Paediatr. 2013;79(1):22-31. [DOI] [PubMed] [Google Scholar]
- 174. Wise-Oringer BK, Burghard AC, O’Day P, et al. . The unique role of 11-oxygenated C19 steroids in both premature adrenarche and premature pubarche. Horm Res Paediatr. 2020;93(7-8):460-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Mäntyselkä A, Jääskeläinen J, Lindi V, et al. . The presentation of adrenarche is sexually dimorphic and modified by body adiposity. J Clin Endocrinol Metab. 2014;99(10):3889-3894. [DOI] [PubMed] [Google Scholar]
- 176. Silverman SH, Migeon C, Rosemberg E, Wilkins L. Precocious growth of sexual hair without other secondary sexual development: premature pubarche, a constitutional variation of adolescence. Pediatrics. 1952;10(4):426-432. [PubMed] [Google Scholar]
- 177. Jiang YZ, Zhu M, Xiong F, Deng LL, Luo YH. Dehydroepiandrosterone sulfate and insulin of prepubertal girls born small for gestational age. Zhonghua Er Ke Za Zhi. 2006;44(1):37-40. [PubMed] [Google Scholar]
- 178. Kim SH, Moon JY, Sasano H, Choi MH, Park MJ. Body fat mass is associated with ratio of steroid metabolites reflecting 17,20-lyase activity in prepubertal girls. J Clin Endocrinol Metab. 2016;101(12):4653-4660. [DOI] [PubMed] [Google Scholar]
- 179. Idkowiak J, Elhassan YS, Mannion P, et al. . Causes, patterns and severity of androgen excess in 487 consecutively recruited pre- and post-pubertal children. Eur J Endocrinol. 2019;180(3):213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Eichenfield LF, Krakowski AC, Piggott C, et al. ; American Acne and Rosacea Society . Evidence-based recommendations for the diagnosis and treatment of pediatric acne. Pediatrics. 2013;131(Suppl 3):S163-S186. [DOI] [PubMed] [Google Scholar]
- 181. DeSalvo DJ, Mehra R, Vaidyanathan P, Kaplowitz PB. In children with premature adrenarche, bone age advancement by 2 or more years is common and generally benign. J Pediatr Endocrinol Metab. 2013;26(3-4):215-221. [DOI] [PubMed] [Google Scholar]
- 182. von Oettingen J, Sola Pou J, Levitsky LL, Misra M. Clinical presentation of children with premature adrenarche. Clin Pediatr (Phila). 2012;51(12):1140-1149. [DOI] [PubMed] [Google Scholar]
- 183. Oron T, Lebenthal Y, de Vries L, Yackobovitch-Gavan M, Phillip M, Lazar L. Interrelationship of extent of precocious adrenarche in appropriate for gestational age girls with clinical outcome. J Pediatr. 2012;160(2):308-313. [DOI] [PubMed] [Google Scholar]
- 184. Voutilainen R, Jääskeläinen J. Premature adrenarche: etiology, clinical findings, and consequences. J Steroid Biochem Mol Biol. 2015;145:226-236. [DOI] [PubMed] [Google Scholar]
- 185. Sopher AB, Thornton JC, Silfen ME, et al. . Prepubertal girls with premature adrenarche have greater bone mineral content and density than controls. J Clin Endocrinol Metab. 2001;86(11):5269-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Liimatta J, Utriainen P, Voutilainen R, Jääskeläinen J. Girls with a history of premature adrenarche have advanced growth and pubertal development at the age of 12 years. Front Endocrinol (Lausanne). 2017;8:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Ibáñez L, Jiménez R, de Zegher F. Early puberty-menarche after precocious pubarche: relation to prenatal growth. Pediatrics. 2006;117(1):117-121. [DOI] [PubMed] [Google Scholar]
- 188. de Ferran K, Paiva IA, Garcia Ldos S, Gama Mde P, Guimarães MM. Isolated premature pubarche: report of anthropometric and metabolic profile of a Brazilian cohort of girls. Horm Res Paediatr. 2011;75(5):367-373. [DOI] [PubMed] [Google Scholar]
- 189. Rosenfield RL. Essentials of growth diagnosis. Endocrinol Metab Clin North Am. 1996;25(3):743-758. [DOI] [PubMed] [Google Scholar]
- 190. Bordini BD, Littlejohn EE, Rosenfield RL. LH dynamics in overweight girls with premature adrenarche and slowly progressive sexual precocity. Intl J Pediatr Endocrinol. 2010;2010:724696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Utriainen P, Jääskeläinen J, Romppanen J, Voutilainen R. Childhood metabolic syndrome and its components in premature adrenarche. J Clin Endocrinol Metab. 2007;92(11):4282-4285. [DOI] [PubMed] [Google Scholar]
- 192. Marakaki C, Karapanou O, Gryparis A, Hochberg Z, Chrousos G, Papadimitriou A. Early adiposity rebound and premature adrenarche. J Pediatr. 2017;186:72-77. [DOI] [PubMed] [Google Scholar]
- 193. Oppenheimer E, Linder B, DiMartino-Nardi J. Decreased insulin sensitivity in prepubertal girls with premature adrenarche and acanthosis nigricans. J Clin Endocrinol Metab. 1995;80(2):614-618. [DOI] [PubMed] [Google Scholar]
- 194. Denburg MR, Silfen ME, Manibo AM, et al. . Insulin sensitivity and the insulin-like growth factor system in prepubertal boys with premature adrenarche. J Clin Endocrinol Metab. 2002;87(12):5604-5609. [DOI] [PubMed] [Google Scholar]
- 195. Teixeira RJ, Ginzbarg D, Rodrigues Freitas J, Fucks G, Silva CM, Bordallo MA. Serum leptin levels in premature pubarche and prepubertal girls with and without obesity. J Pediatr Endocrinol Metab. 2004;17(10):1393-1398. [DOI] [PubMed] [Google Scholar]
- 196. Williams KM, Oberfield SE, Zhang C, McMahon DJ, Sopher AB. The relationship of metabolic syndrome and body composition in children with premature adrenarche: is it age related? Horm Res Paediatr. 2015;84(6):401-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Utriainen P, Laakso S, Liimatta J, Jääskeläinen J, Voutilainen R. Premature adrenarche: a common condition with variable presentation. Horm Res Paediatr. 2015;83(4):221-231. [DOI] [PubMed] [Google Scholar]
- 198. Mathew RP, Najjar JL, Lorenz RA, Mayes DE, Russell WE. Premature pubarche in girls is associated with functional adrenal but not ovarian hyperandrogenism. J Pediatr. 2002;141(1):91-98. [DOI] [PubMed] [Google Scholar]
- 199. Güven A, Cinaz P, Bideci A. Is premature adrenarche a risk factor for atherogenesis? Pediatr Int. 2005;47(1):20-25. [DOI] [PubMed] [Google Scholar]
- 200. Andiran N, Yordam N. Lipoprotein(a) levels in girls with premature adrenarche. J Paediatr Child Health. 2008;44(3):138-142. [DOI] [PubMed] [Google Scholar]
- 201. Ibáñez L, Potau N, Francois I, de Zegher F. Precocious pubarche, hyperinsulinism, and ovarian hyperandrogenism in girls: relation to reduced fetal growth. J Clin Endocrinol Metab. 1998;83(10):3558-3562. [DOI] [PubMed] [Google Scholar]
- 202. Ibáñez L, de Zegher F, Potau N. Anovulation after precocious pubarche: early markers and time course in adolescence. J Clin Endocrinol Metab. 1999;84(8):2691-2695. [DOI] [PubMed] [Google Scholar]
- 203. Ibáñez L, Valls C, Potau N, Marcos MV, de Zegher F. Polycystic ovary syndrome after precocious pubarche: ontogeny of the low-birthweight effect. Clin Endocrinol (Oxf). 2001;55(5):667-672. [DOI] [PubMed] [Google Scholar]
- 204. Barker DJ, Eriksson JG, Forsén T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31(6):1235-1239. [DOI] [PubMed] [Google Scholar]
- 205. Francois I, de Zegher F. Adrenarche and fetal growth. Pediatr Res. 1997;41(3):440-442. [DOI] [PubMed] [Google Scholar]
- 206. de Zegher F, López-Bermejo A, Ibáñez L. Central obesity, faster maturation, and ‘PCOS’ in girls. Trends Endocrinol Metab. 2018;29(12):815-818. [DOI] [PubMed] [Google Scholar]
- 207. Buhl CS, Stødkilde-Jørgensen H, Videbech P, et al. . Escitalopram ameliorates hypercortisolemia and insulin resistance in low birth weight men with limbic brain alterations. J Clin Endocrinol Metab. 2018;103(1):115-124. [DOI] [PubMed] [Google Scholar]
- 208. Ghirri P, Bernardini M, Vuerich M, et al. . Adrenarche, pubertal development, age at menarche and final height of full-term, born small for gestational age (SGA) girls. Gynecol Endocrinol. 2001;15(2):91-97. [DOI] [PubMed] [Google Scholar]
- 209. Veening MA, van Weissenbruch MM, Roord JJ, de Delemarre-van Waal HA. Pubertal development in children born small for gestational age. J Pediatr Endocrinol Metab. 2004;17(11):1497-1505. [DOI] [PubMed] [Google Scholar]
- 210. Neville KA, Walker JL. Precocious pubarche is associated with SGA, prematurity, weight gain, and obesity. Arch Dis Child. 2005;90(3):258-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Meas T, Chevenne D, Thibaud E, et al. . Endocrine consequences of premature pubarche in post-pubertal Caucasian girls. Clin Endocrinol (Oxf). 2002;57(1):101-106. [DOI] [PubMed] [Google Scholar]
- 212. Boonstra VH, Mulder PG, de Jong FH, Hokken-Koelega AC. Serum dehydroepiandrosterone sulfate levels and pubarche in short children born small for gestational age before and during growth hormone treatment. J Clin Endocrinol Metab. 2004;89(2):712-717. [DOI] [PubMed] [Google Scholar]
- 213. Paterson WF, Ahmed SF, Bath L, et al. . Exaggerated adrenarche in a cohort of Scottish children: clinical features and biochemistry. Clin Endocrinol (Oxf). 2010;72(4):496-501. [DOI] [PubMed] [Google Scholar]
- 214. Maliqueo M, Sir-Petermann T, Pérez V, et al. . Adrenal function during childhood and puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94(9):3282-3288. [DOI] [PubMed] [Google Scholar]
- 215. Liimatta J, Utriainen P, Laitinen T, Voutilainen R, Jääskeläinen J. Cardiometabolic risk profile among young adult females with a history of premature adrenarche. J Endocr Soc. 2019;3(10):1771-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Liimatta J, Utriainen P, Voutilainen R, Jääskeläinen J. Trajectories of growth and serum DHEAS and IGF-1 concentrations in girls with a history of premature adrenarche: attenuation of the phenotype by adulthood. Front Endocrinol (Lausanne). 2018;9:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Zehr JL, Culbert KM, Sisk CL, Klump KL. An association of early puberty with disordered eating and anxiety in a population of undergraduate women and men. Horm Behav. 2007;52(4):427-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Sontag-Padilla LM, Dorn LD, Tissot A, Susman EJ, Beers SR, Rose SR. Executive functioning, cortisol reactivity, and symptoms of psychopathology in girls with premature adrenarche. Dev Psychopathol. 2012;24(1):211-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Liimatta J, Sintonen H, Utriainen P, Voutilainen R, Jääskeläinen J. Children with a history of premature adrenarche have good health-related quality of life at the age of 12 years. Horm Res Paediatr. 2018;89(3):184-188. [DOI] [PubMed] [Google Scholar]
- 220. Dokras A, Stener-Victorin E, Yildiz BO, et al. . Androgen Excess- Polycystic Ovary Syndrome Society: position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil Steril. 2018;109(5):888-899. [DOI] [PubMed] [Google Scholar]
- 221. Greenwood EA, Yaffe K, Wellons MF, Cedars MI, Huddleston HG. Depression over the lifespan in a population-based cohort of women with polycystic ovary syndrome: longitudinal analysis. J Clin Endocrinol Metab. 2019;104(7):2809-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Tay CT, Teede HJ, Hill B, Loxton D, Joham AE. Increased prevalence of eating disorders, low self-esteem, and psychological distress in women with polycystic ovary syndrome: a community-based cohort study. Fertil Steril. 2019;112(2):353-361. [DOI] [PubMed] [Google Scholar]
- 223. Alur-Gupta S, Chemerinski A, Liu C, et al. . Body-image distress is increased in women with polycystic ovary syndrome and mediates depression and anxiety. Fertil Steril. 2019;112(5):930-938.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Baquedano MS, Belgorosky A. Human adrenal cortex: epigenetics and postnatal functional zonation. Horm Res Paediatr. 2018;89(5):331-340. [DOI] [PubMed] [Google Scholar]
- 225. Rappaport R, Forest MG, Bayard F, Duval-Beaupere G, Blizzard RM, Migeon CJ. Plasma androgens and LH in scoliotic patients with premature pubarche. J Clin Endocrinol Metab. 1974;38(3):401-406. [DOI] [PubMed] [Google Scholar]
- 226. Witchel SF, Lee PA, Suda-Hartman M, Hoffman EP. Hyperandrogenism and manifesting heterozygotes for 21-hydroxylase deficiency. Biochem Mol Med. 1997;62(2):151-158. [DOI] [PubMed] [Google Scholar]
- 227. Nayak S, Lee PA, Witchel SF. Variants of the type II 3beta-hydroxysteroid dehydrogenase gene in children with premature pubic hair and hyperandrogenic adolescents. Mol Genet Metab. 1998;64(3):184-192. [DOI] [PubMed] [Google Scholar]
- 228. An P, Rice T, Gagnon J, et al. . Race differences in the pattern of familial aggregation for dehydroepiandrosterone sulfate and its responsiveness to training in the HERITAGE Family Study. Metabolism. 2001;50(8):916-920. [DOI] [PubMed] [Google Scholar]
- 229. Goodarzi MO, Carmina E, Azziz R. DHEA, DHEAS and PCOS. J Steroid Biochem Mol Biol. 2015;145:213-225. [DOI] [PubMed] [Google Scholar]
- 230. Vottero A, Capelletti M, Giuliodori S, et al. . Decreased androgen receptor gene methylation in premature pubarche: a novel pathogenetic mechanism? J Clin Endocrinol Metab. 2006;91(3):968-972. [DOI] [PubMed] [Google Scholar]
- 231. Lappalainen S, Utriainen P, Kuulasmaa T, Voutilainen R, Jääskeläinen J. Androgen receptor gene CAG repeat polymorphism and X-chromosome inactivation in children with premature adrenarche. J Clin Endocrinol Metab. 2008;93(4):1304-1309. [DOI] [PubMed] [Google Scholar]
- 232. Williams RM, Ward CE, Hughes IA. Premature adrenarche. Arch Dis Child. 2012;97(3):250-254. [DOI] [PubMed] [Google Scholar]
- 233. Rosenfield RL, Bordini B, Yu C. Comparison of detection of normal puberty in girls by a hormonal sleep test and a gonadotropin-releasing hormone agonist test. J Clin Endocrinol Metab. 2013;98(4):1591-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Kunz GJ, Klein KO, Clemons RD, Gottschalk ME, Jones KL. Virilization of young children after topical androgen use by their parents. Pediatrics. 2004;114(1):282-284. [DOI] [PubMed] [Google Scholar]
- 235. Klein KO, Rosenfield RL, Santen RJ, et al. . Estrogen replacement in turner syndrome: literature review and practical considerations. J Clin Endocrinol Metab. 2018;103(5):1790-1803. [DOI] [PubMed] [Google Scholar]
- 236. Börjesson A, Lehtihet M, Andersson A, et al. . Studies of athlete biological passport biomarkers and clinical parameters in male and female users of anabolic androgenic steroids and other doping agents. Drug Test Anal. 2020;12(4):514-523. [DOI] [PubMed] [Google Scholar]
- 237. Merke DP, Auchus RJ. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. N Engl J Med. 2020;383(13):1248-1261. [DOI] [PubMed] [Google Scholar]
- 238. Carmina E, Dewailly D, Escobar-Morreale HF, et al. . Non-classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency revisited: an update with a special focus on adolescent and adult women. Hum Reprod Update. 2017;23(5):580-599. [DOI] [PubMed] [Google Scholar]
- 239. Jaruratanasirikul S, Thaiwong M. Precocious pubarche in Thai children. J Med Assoc Thai. 2012;95(11):1404-1410. [PubMed] [Google Scholar]
- 240. Binay C, Simsek E, Cilingir O, Yuksel Z, Kutlay O, Artan S. Prevalence of nonclassic congenital adrenal hyperplasia in Turkish children presenting with premature pubarche, hirsutism, or oligomenorrhoea. Int J Endocrinol. 2014;2014:768506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Armengaud JB, Charkaluk ML, Trivin C, et al. . Precocious pubarche: distinguishing late-onset congenital adrenal hyperplasia from premature adrenarche. J Clin Endocrinol Metab. 2009;94(8):2835-2840. [DOI] [PubMed] [Google Scholar]
- 242. Speiser PW, Arlt W, Auchus RJ, et al. . Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103(11):4043-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Ghizzoni L, Cappa M, Vottero A, et al. . Relationship of CYP21A2 genotype and serum 17-hydroxyprogesterone and cortisol levels in a large cohort of Italian children with premature pubarche. Eur J Endocrinol. 2011;165(2):307-314. [DOI] [PubMed] [Google Scholar]
- 244. Wasniewska MG, Morabito LA, Baronio F, et al. ; Adrenal Diseases Working Group of the Italian Society for Pediatric Endocrinology and Diabetology . Growth trajectory and adult height in children with nonclassical congenital adrenal hyperplasia. Horm Res Paediatr. 2020;93(3):173-181. [DOI] [PubMed] [Google Scholar]
- 245. de Vries L, Lebenthal Y, Phillip M, Shalitin S, Tenenbaum A, Bello R. Obesity and cardiometabolic risk factors in children and young adults with non-classical 21-hydroxylase deficiency. Front Endocrinol (Lausanne). 2019;10:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Joehrer K, Geley S, Strasser-Wozak EM, et al. . CYP11B1 mutations causing non-classic adrenal hyperplasia due to 11 beta-hydroxylase deficiency. Hum Mol Genet. 1997;6(11):1829-1834. [DOI] [PubMed] [Google Scholar]
- 247. Tonetto-Fernandes V, Lemos-Marini SH, Kuperman H, Ribeiro-Neto LM, Verreschi IT, Kater CE. Serum 21-deoxycortisol, 17-hydroxyprogesterone, and 11-deoxycortisol in classic congenital adrenal hyperplasia: clinical and hormonal correlations and identification of patients with 11beta-hydroxylase deficiency among a large group with alleged 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2006;91(6):2179-2184. [DOI] [PubMed] [Google Scholar]
- 248. Reisch N, Högler W, Parajes S, et al. . A diagnosis not to be missed: nonclassic steroid 11β-hydroxylase deficiency presenting with premature adrenarche and hirsutism. J Clin Endocrinol Metab. 2013;98(10):E1620-E1625. [DOI] [PubMed] [Google Scholar]
- 249. Rosenfield RL, Rich BH, Wolfsdorf JI, et al. . Pubertal presentation of congenital delta 5-3 beta-hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab. 1980;51(2):345-353. [DOI] [PubMed] [Google Scholar]
- 250. Lawson AJ, Walker EA, Lavery GG, et al. . Cortisone-reductase deficiency associated with heterozygous mutations in 11beta-hydroxysteroid dehydrogenase type 1. Proc Natl Acad Sci U S A. 2011;108(10):4111-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Lavery GG, Idkowiak J, Sherlock M, et al. . Novel H6PDH mutations in two girls with premature adrenarche: ‘apparent’ and ‘true’ CRD can be differentiated by urinary steroid profiling. Eur J Endocrinol. 2013;168(2):K19-K26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Rosenfield RL, Grossman BJ, Ozoa N. Plasma 17-ketosteroids and testosterone in prepubertal children before and after ACTH administration. J Clin Endocrinol Metab. 1971;33(2):249-253. [DOI] [PubMed] [Google Scholar]
- 253. Bas S, Guran T, Atay Z, et al. . Premature pubarche, hyperinsulinemia and hypothyroxinemia: novel manifestations of congenital portosystemic shunts (Abernethy malformation) in children. Horm Res Paediatr. 2015;83(4):282-287. [DOI] [PubMed] [Google Scholar]
- 254. Stratakis CA. An update on Cushing syndrome in pediatrics. Ann Endocrinol (Paris). 2018;79(3):125-131. [DOI] [PubMed] [Google Scholar]
- 255. Minnetti M, Caiulo S, Ferrigno R, et al. . Abnormal linear growth in paediatric adrenal diseases: pathogenesis, prevalence and management. Clin Endocrinol (Oxf). 2020;92(2):98-108. [DOI] [PubMed] [Google Scholar]
- 256. Shahidi NT, Crigler JF Jr. Evaluation of growth and of endocrine systems in testosterone-corticosteroid-treated patients with aplastic anemia. J Pediatr. 1967;70(2):233-242. [DOI] [PubMed] [Google Scholar]
- 257. Nicolaides NC, Charmandari E. Chrousos syndrome: from molecular pathogenesis to therapeutic management. Eur J Clin Invest. 2015;45(5):504-514. [DOI] [PubMed] [Google Scholar]
- 258. Rosenfield RL.Letter to the editor: “glucocorticoid resistance in premature adrenarche and PCOS: from childhood to adulthood.” J Endocr Soc. 2020;4(1):bvaa11. [DOI] [PMC free article] [PubMed]
- 259. Ghazi AA, Mofid D, Salehian MT, et al. . Functioning adrenocortical tumors in children-secretory behavior. J Clin Res Pediatr Endocrinol. 2013;5(1):27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Harris AC, Wakely PE Jr, Kaplowitz PB, Lovinger RD. Steroid cell tumor of the ovary in a child. Arch Pathol Lab Med. 1991;115(2):150-154. [PubMed] [Google Scholar]
- 261. Arhan E, Cetinkaya E, Aycan Z, Aslan AT, Yücel H, Vidinlisan S. A very rare cause of virilization in childhood: ovarian Leydig cell tumor. J Pediatr Endocrinol Metab. 2008;21(2):181-183. [DOI] [PubMed] [Google Scholar]
- 262. Kalfa N, Méduri G, Philibert P, et al. . Unusual virilization in girls with juvenile granulosa cell tumors of the ovary is related to intratumoral aromatase deficiency. Horm Res Paediatr. 2010;74(2):83-91. [DOI] [PubMed] [Google Scholar]
- 263. Coyle D, Kutasy B, Han Suyin K, et al. . Gonadoblastoma in patients with 45,X/46,XY mosaicism: a 16-year experience. J Pediatr Urol. 2016;12(5):283.e1-283.e7. [DOI] [PubMed] [Google Scholar]
- 264. Turcu AF, Mallappa A, Elman MS, et al. . 11-oxygenated androgens are biomarkers of adrenal volume and testicular adrenal rest tumors in 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2017;102(8):2701-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Olivier P, Simoneau-Roy J, Francoeur D, et al. . Leydig cell tumors in children: contrasting clinical, hormonal, anatomical, and molecular characteristics in boys and girls. J Pediatr. 2012;161(6):1147-1152. [DOI] [PubMed] [Google Scholar]
- 266. Korwutthikulrangsri M, Wattanatranon D, Teeraratkul S, Wijarn P, Mahachoklertwattana P, Poomthavorn P. Testicular enlargement in a pre-pubertal boy with adrenocortical tumour. Paediatr Int Child Health. 2018;38(1):66-68. [DOI] [PubMed] [Google Scholar]
- 267. Pinto EM, Zambetti GP, Rodriguez-Galindo C. Pediatric adrenocortical tumours. Best Pract Res Clin Endocrinol Metab. 2020;34(3):101448. [DOI] [PubMed] [Google Scholar]
- 268. Rosenfield RL, Cohen RM, Talerman A. Lipid cell tumor of the ovary in reference to adult-onset congenital adrenal hyperplasia and polycystic ovary syndrome. A case report. J Reprod Med. 1987;32(5):363-369. [PubMed] [Google Scholar]
- 269. Idkowiak J, Malunowicz EM, Dhir V, et al. . Concomitant mutations in the P450 oxidoreductase and androgen receptor genes presenting with 46,XY disordered sex development and androgenization at adrenarche. J Clin Endocrinol Metab. 2010;95(7):3418-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Kremer H, Mariman E, Otten BJ, et al. . Cosegregation of missense mutations of the luteinizing hormone receptor gene with familial male-limited precocious puberty. Hum Mol Genet. 1993;2(11):1779-1783. [DOI] [PubMed] [Google Scholar]
- 271. De Luca F, Mitchell V, Wasniewska M, et al. . Regulation of spermatogenesis in McCune-Albright syndrome: lessons from a 15-year follow-up. Eur J Endocrinol. 2008;158(6):921-927. [DOI] [PubMed] [Google Scholar]
- 272. Boyce AM, Chong WH, Shawker TH, et al. . Characterization and management of testicular pathology in McCune-Albright syndrome. J Clin Endocrinol Metab. 2012;97(9):E1782-E1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Englund AT, Geffner ME, Nagel RA, Lippe BM, Braunstein GD. Pediatric germ cell and human chorionic gonadotropin-producing tumors. Clinical and laboratory features. Am J Dis Child. 1991;145(11):1294-1297. [DOI] [PubMed] [Google Scholar]
- 274. Arya VB, Davies JH. Idiopathic gonadotropin-independent precocious puberty: is regular surveillance required? J Pediatr Endocrinol Metab. 2019;32(4):403-407. [DOI] [PubMed] [Google Scholar]
- 275. Rosenfeld RG, Reitz RE, King AB, Hintz RL. Familial precocious puberty associated with isolated elevation of luteinizing hormone. N Engl J Med. 1980;303(15):859-862. [DOI] [PubMed] [Google Scholar]
- 276. Kaplowitz P, Bloch C; Section on Endocrinology American Academy of Pediatrics. Evaluation and referral of children with signs of early puberty. Pediatrics. 2016;137(1):e20153732. [DOI] [PubMed] [Google Scholar]
- 277. Kaplowitz PB. For premature thelarche and premature adrenarche, the case for waiting before testing. Horm Res Paediatr. Published online December 2020. doi:10.1159/000512764 [DOI] [PubMed] [Google Scholar]
- 278. Herbst KL, Bhasin S. Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7(3):271-277. [DOI] [PubMed] [Google Scholar]
- 279. Gruelich WW, Pyle SI.. Radiographic Atlas of Skeletal Development of the Hand and Wrist. Stanford Univ Press; 1959. [Google Scholar]
- 280. Tanner J, Oshman D, Bahhage F, Healy M. Tanner-Whitehouse bone age reference values for North American children. J Pediatr. 1997;131(1 Pt 1):34-40. [DOI] [PubMed] [Google Scholar]
- 281. Kreiter M, Burstein S, Rosenfield RL, et al. . Preserving adult height potential in girls with idiopathic true precocious puberty. J Pediatr. 1990;117(3):364-370. [DOI] [PubMed] [Google Scholar]
- 282. Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J Pediatr. 1952;40(4):423-441. [DOI] [PubMed] [Google Scholar]
- 283. Tanner JM, Goldstein H, Whitehouse RH. Standards for children’s height at ages 2-9 years allowing for heights of parents. Arch Dis Child. 1970;45(244):755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Rosenfeld RS, Rosenberg BJ, Fukushima DK, Hellman L. 24-Hour secretory pattern of dehydroisoandrosterone and dehydroisoandrosterone sulfate. J Clin Endocrinol Metab. 1975;40(5):850-855. [DOI] [PubMed] [Google Scholar]
- 285. Chesover AD, Millar H, Sepiashvili L, Adeli K, Palmert MR, Hamilton J. Screening for nonclassic congenital adrenal hyperplasia in the era of liquid chromatography-tandem mass spectrometry. J Endocr Soc. 2020;4(2):bvz030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Bidet M, Bellanné-Chantelot C, Galand-Portier MB, et al. . Clinical and molecular characterization of a cohort of 161 unrelated women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency and 330 family members. J Clin Endocrinol Metab. 2009;94(5):1570-1578. [DOI] [PubMed] [Google Scholar]
- 287. Ambroziak U, Kępczyńska-Nyk A, Kuryłowicz A, et al. . The diagnosis of nonclassic congenital adrenal hyperplasia due to 21-hydroxylase deficiency, based on serum basal or post-ACTH stimulation 17-hydroxyprogesterone, can lead to false-positive diagnosis. Clin Endocrinol (Oxf). 2016;84(1):23-29. [DOI] [PubMed] [Google Scholar]
- 288. Nieman LK, Biller BM, Findling JW, et al. . The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Shah RU, Lawrence C, Fickenscher KA, Shao L, Lowe LH. Imaging of pediatric pelvic neoplasms. Radiol Clin North Am. 2011;49(4):729-48, vi. [DOI] [PubMed] [Google Scholar]
- 290. Pascale MM, Pugeat M, Roberts M, et al. . Androgen suppressive effect of GnRH agonist in ovarian hyperthecosis and virilizing tumours. Clin Endocrinol (Oxf). 1994;41(5):571-576. [DOI] [PubMed] [Google Scholar]
- 291. Sangüesa C, Veiga D, Llavador M, Serrano A. Testicular tumours in children: an approach to diagnosis and management with pathologic correlation. Insights Imaging. 2020;11(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]