Abstract
Background
Antimicrobial resistance is a serious global health concern that emphasizes completing treatment course. Recently, the effectiveness of short versus longer antibiotic courses has been questioned. This study investigated the duration of prescribed antibiotics, their effectiveness, and associated risk of infection-related complications.
Methods
Clinical Practice Research Datalink identified 4 million acute infection episodes prescribed an antibiotic in primary care between January 2014—June 2014, England. Prescriptions were categorized by duration. Risk of infection-related hospitalizations within 30 days was modelled overall and by infection type. Risk was assessed immediately after or within 30 days follow-up to measure confounders given similar and varying exposure, respectively. An interaction term with follow-up time assessed whether hazard ratios (HRs) remained parallel with different antibiotic durations.
Results
The duration of antibiotic courses increased over the study period (5.2–19.1%); 6–7 days were most common (66.9%). Most infection-related hospitalizations occurred with prescriptions of 8–15 days (0.21%), accompanied by greater risk of infection-related complications compared to patients who received a short prescription (HR: 1.75 [95% CI: 1.54–2.00]). Comparing HRs in the first 5 days versus remaining follow-up showed longer antibiotic courses were no more effective than shorter courses (1.02 [95% CI: 0.90–1.16] and 0.92 [95% CI: 0.75–1.12]). No variation by infection-type was observed.
Conclusions
Equal effectiveness was found between shorter and longer antibiotic courses and the reduction of infection-related hospitalizations. Stewardship programs should recommend shorter courses of antibiotics for acute infections. Further research is required for treating patients with a complex medical history.
Summary
Prescribing of longer courses increased over the study period. The majority of hospitalizations occurred for patients receiving longer courses. Risk of developing a complication (immediate vs remaining follow-up) found longer courses were no more effective than shorter courses.
Keywords: antibiotics, antibiotic duration, antimicrobial resistance, infection complication
Resistant bacteria are developing worldwide, causing a serious and concerning threat to global health. The rise in resistance is associated with overuse and misuse of antibiotics, as well as the lack of new drug development. Primary care accounts for 71.4% of all antibiotic prescriptions in the UK [1]. Uncertainty in the distinction between bacterial or viral infections has led to a large variability in the propensity to prescribe an antibiotic for various infectious conditions [2].
Antibiotic susceptibility testing is used to determine the appropriateness of particular antibiotics to bacterial infections and whether bacteria are displaying some resistance to treatment. Microbiology data in combination with clinical review are used to develop best practice recommendations for the treatment of bacterial infections by outlining when it is appropriate to treat an infection with an antibiotic, the specific antibiotic that should be prescribed, as well as the recommended dose and duration of the antibiotic course that is effective. However, recent evidence suggests that regular updates to guidelines have little effect on reducing antibiotic prescribing [2] and a substantial amount of antibiotics prescribed still deviate from recommended guidelines [3]. When the recommended antibiotic type is prescribed in the UK, antibiotic courses tend to be slightly longer than what is recommended [4]. This is probably, in part, because it was taught that to prevent reinfection and reduce resistance it is necessary for patients to complete the entire course of antibiotics even when symptoms have surpassed [5], and that prolonged therapy was needed to avoid treatment failure, suggesting that shorter antibiotic courses were perceived as an inferior treatment to longer courses [6]. These ideas were implemented decades ago when there was little concern about antibiotic overuse and, although over time shorter courses have shown to be comparable in efficacy to longer therapies [5, 7–11], variability in the duration of prescribed antibiotics remains. Additional evidence suggests that prescribers feel more comfortable selecting a middle-range duration, or in some cases the longest duration specified, even if the intention of the specified range is that the shortest duration is adequate for most patients [12]. Further research is needed to determine the most effective strategies for optimizing duration of antibiotic treatment for individual patients, taking patient characteristics into consideration when identifying the patients who fail to recover on shorter courses of antibiotics.
To date, many studies have observed similar effectiveness of shorter courses compared to longer courses of antibiotics in terms of clinical success, but many of these studies focus on one single infection or prescribing overall and review primary or secondary care data in isolation. The current study used a national database of electronic health records linked to hospital admissions in England to further understand the risks or benefits of prescribing different antibiotic durations for multiple infectious conditions in association with the risk of an infection-related complication, such as a hospitalization in the 30 days following.
METHODS
Database
A population-based cohort study was conducted using data from the Clinical Practice Research Databank (CPRD) GOLD containing longitudinal, anonymized, patient-level electronic health records (EHRs) from general practices in England [13, 14]. These records include clinical diagnoses, medication prescribed, vaccination history, diagnostic testing, lifestyle information, clinical referrals, as well as patient demographics. Patient-level data were linked to hospital episode statistics (HES), containing information on the date and the diagnostic code of any hospital admission. Patient-level socioeconomic information was available through linkage of the postcode of a patient’s residence to the Index of Multiple Deprivation (IMD) [15], and aggregated into quintiles for the current analysis. Prescriptions for systemic antibiotics were classified using the British National Formulary (BNF).
Study Population
The start of follow-up was 1 year after the start of practice data collection or date of patients’ registration at the practice, whichever date came last (except newborn babies where follow-up started at registration). The primary study population consisted of all patients prescribed a systemic antibiotic in their general practice between January 2000 and June 2014. Only events with a clinical record of upper respiratory tract infection (URTI, including unspecified URTI, tracheitis, laryngitis, common cold, cough, sore throat, and tonsillitis), lower respiratory tract infection (LRTI, including unspecified LRTI, unspecified chest infections and bronchitis, excluding chronic obstructive pulmonary disease and pneumonia), or urinary tract infections (UTI) were included, as these are common infections recorded in primary care [2, 3]. The full code lists are available at clinicalcodes.org.
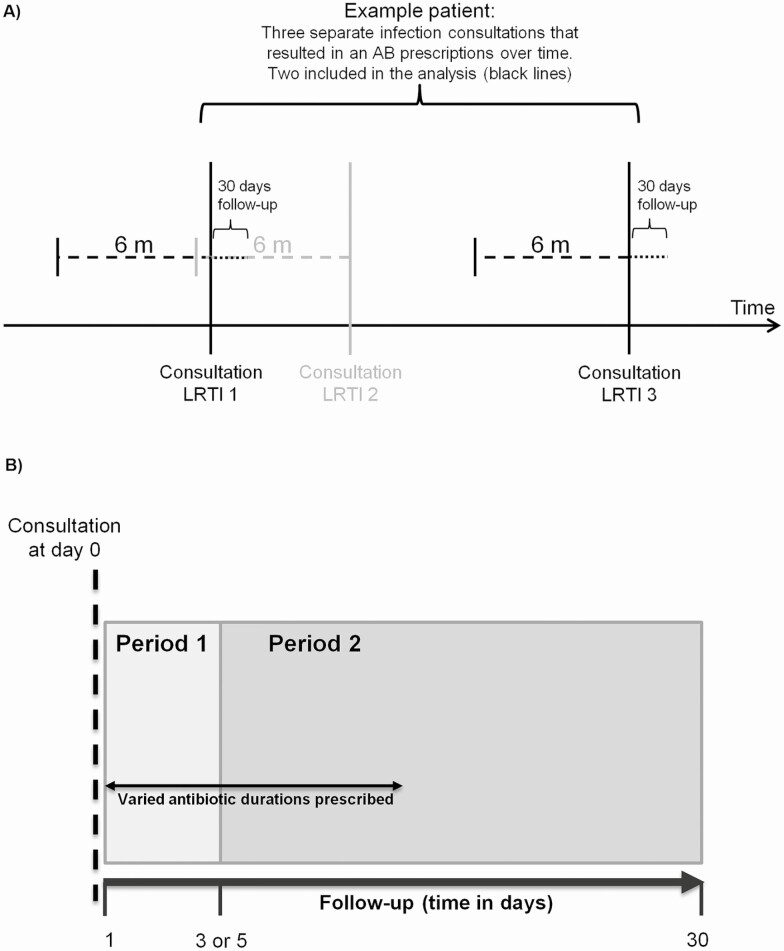
Observations with a record of the same infection in the 6 months before the consultation date were excluded to ensure analysis of acute infections only (Figure 1A). The prescribed duration of the antibiotic prescription was estimated based on the number of tablets divided by the prescribed daily dose. Prescriptions with duration of use less than 5 days for respiratory tract infections (RTIs) or less than 3 days for UTI (due to low frequency) and those over 15 days of use (more likely due to chronic use) were excluded from the analysis (see Supplementary Table 1). Antibiotic duration was categorized depending on infection into 5, 6–7, or 8–15 days for overall and RTIs or 3, 4–5, or 6–15 days for UTI analysis. The shortest course was selected as a reference category because all patients were exposed to antibiotics for this period. The outcome of interest was an infection-related complication recorded as a hospital admission with an infection-related ICD-10 code that occurred in the 30 days follow-up after the consultation. The hospital admissions for infection-related complications were based on the primary admission diagnosis (ICD-10 codes; Supplementary Table 2).
Figure 1.
(A) Study design of an antibiotic (AB) exposure measurement (no history of infection and antibiotic prescribing in 6 months before) and follow-up for infection-related hospitalizations (in 30 days after). (B) Study design showing the initial consultation at day 0 and the overall follow-up period of 1–30 days. The follow-up is split into 2 time periods: period 1 (1–3 or 1–5 days) and period 2 (4–30 or 6–30 days) for UTI and RTIs, respectively. Varied antibiotic duration represent the different number of days each antibiotic course is prescribed (3–15 days or 5–15 days for UTI or RTIs, respectively). Abbreviations: AB, antibiotic; RTIs, respiratory tract infections; UTI, urinary tract infections.
Statistical Analysis
Descriptive statistics were used to assess what antibiotic was most commonly prescribed by indication and course duration. Two different sets of statistical analyses were conducted using time-to-event Cox models: The first set of analyses compared the incidence of infection-related complications immediately following the antibiotic prescriptions (Period One: 3- or 5- days depending on infection) between differently prescribed durations. Any differences in the hazard ratios (HRs) in the first few days may be attributed to differences in infection severity or underlying cofounding as all comparison groups for antibiotic duration were exposed to the same antibiotic duration in this period (Figure 1B). The second set of analyses evaluated whether the incidence rates for infection-related complications changed differently in the second time period, testing whether rates remained parallel over time or diverged/converged between the different duration groups. If a shorter antibiotic duration would be less effective than longer durations, the rate of outcomes would increase with shorter courses relative to the rate with longer course durations. A test for proportionality was conducted in the Cox proportional hazards regression (ie, the interaction between HRs and follow-up time) to assess whether the HRs remained parallel over time between different antibiotic durations.
Statistical adjustments were made for the following variables: age, sex, comorbidity using the Charlson comorbidity score, IMD quintiles, BMI, smoking history, ethnicity, and calendar year, as well as the number of non-antibiotic prescriptions prescribed, if the patient received an influenza vaccination, had a hospital referral or hospital admission in the previous 12 months, and the region of their practice: North-England, South-England, the Midlands, or London. Charlson comorbidity scores were categorized: No comorbidity (score = 0), low (score = 1–2), moderate (score = 3–4), high (score = 5–6) and very high (score ≥7). As the relationship between predictor and outcome were nonlinear for age and the year of the consultation, these were fitted using the restricted cubic spline (rcs) function of the 'rms package' in rstudio (Regression Modeling Strategies; R version 3.6.0). Models were also adjusted with a missing indicator for missing body mass index (BMI), smoking, and IMD values. As a patient could have appeared in the analysis for multiple antibiotic prescriptions over the study period (although none of the follow-up periods would overlap), a sensitivity analysis was conducted selecting one observation at random for each patient.
RESULTS
Over 4 million acute infection-related consultations were identified in primary care resulting in an antibiotic prescription for URTIs (59.1%), LRTIs (24.6%) and UTIs (16.3%). The baseline characteristics, stratified by infection can be seen in Table 1. A repeat antibiotic prescription within 30 days follow-up was most common for UTI infections, but a general practice (GP) recorded infection-related complication or HES recorded hospital admission was more common for antibiotic courses of 6–7 or 8–14 days. No difference was observed between increasing antibiotic duration with the severity of patient’s comorbidity or the overall time to event (Supplementary Table 3). The most commonly prescribed antibiotic course overall was for 6 or 7 days (62.43%), overall and by infection. The trend to prescribe a longer course of increased by year: 6–7 days (19.1%), 8–14 days (5.2%), but decreased for shorter (24.2%) antibiotic courses (Supplementary Figure 1).
Table 1.
Characteristics of Study Population Stratified by Infection
| URTI | LRTI | UTI | ||
|---|---|---|---|---|
| n (%) | 2530425 (59.1) | 1050785 (24.6) | 699495 (16.3) | |
| Age (mean (SD)) | 37.02 (24.95) | 49.12 (24.87) | 51.30 (23.01) | |
| Female (%) | 1464913 (57.9) | 577579 (55.0) | 613462 (87.7) | |
| BMI (%) | Normal [Ref] | 514688 (20.3) | 229280 (21.8) | 209398 (29.9) |
| Low | 235579 (9.3) | 77215 (7.3) | 47273 (6.8) | |
| Obese | 706414 (27.9) | 358558 (34.1) | 221514 (31.7) | |
| Missing | 1073744 (42.4) | 385732 (36.7) | 221310 (31.6) | |
| Smoking history (%) | Nonsmoking [Ref] | 855283 (33.8) | 335339 (31.9) | 322865 (46.2) |
| Past smoker | 355041 (14.0) | 203645 (19.4) | 120426 (17.2) | |
| Current smoker | 425724 (16.8) | 243500 (23.2) | 118338 (16.9) | |
| Missing | 894377 (35.3) | 268301 (25.5) | 137866 (19.7) | |
| Charlson comorbidity (%) | No comorbidity [Ref] | 1712133 (67.7) | 567206 (54.0) | 426884 (61.0) |
| Low (score 1–2) | 677773 (26.8) | 376502 (35.8) | 203143 (29.0) | |
| Moderate (score 3–4) | 107698 (4.3) | 81205 (7.7) | 51639 (7.4) | |
| High (score 5–6) | 24068 (1.0) | 18894 (1.8) | 12985 (1.9) | |
| Very high (score ≥7) | 8753 (0.3) | 6978 (0.7) | 4844 (0.7) | |
| IMD quintiles (%) | 1 (Least deprived) [Ref] | 436830 (17.3) | 229541 (21.8) | 48197 (6.9) |
| 2 | 431611 (17.1) | 230698 (22.0) | 45984 (6.6) | |
| 3 | 374723 (14.8) | 200366 (19.1) | 38650 (5.5) | |
| 4 | 356267 (14.1) | 197461 (18.8) | 35372 (5.1) | |
| 5 (Most deprived) | 291057 (11.5) | 180462 (17.2) | 28218 (4.0) | |
| Missing IMD Information | 639937 (25.3) | 12257 (1.2) | 503074 (71.9) | |
| Count AB Rxa (mean (SD)) | 0.89 (0.7–1.0) | 0.80 (0.7–0.9) | 1.03 (0.9–1.1) | |
| Count other Rxa (mean (SD)) | 16.77 (34.03) | 28.02 (44.94) | 28.01 (47.87) | |
| Repeat AB Rx (%) | 311015 (12.3) | 178079 (16.9) | 143955 (20.6) | |
| Repeat AB Rx in days (mean (SD)) ¥ | 13.55 (8.03) | 12.80 (5.8) | 11.90 (8.25) | |
| GP recorded hospitalization (%) | 3042 (0.1) | 2573 (0.2) | 518 (0.1) | |
| GP recorded poor outcome in days (mean (SD)) ¥ | 8.93 (7.80) | 9.48 (7.67) | 12.20 (8.77) | |
| HES recorded infection-related hospitalization (%) | 3489 (0.1) | 2672 (0.3) | 411 (0.1) | |
| HES recorded hospitalization in days (mean (SD))¥ | 7.71 (7.7) | 7.55 (7.47) | 10.29 (8.57) | |
| Ethnicity (%) | White [Ref] | 751106 (29.7) | 357263 (34.0) | 194890 (27.9) |
| Non-white | 583743 (23.1) | 39846 (3.8) | 374937 (53.6) | |
| Unknown | 1195576 (47.2) | 653676 (62.2) | 129668 (18.5) | |
| Region (%) | London [Ref] | 324250 (12.8) | 88602 (8.4) | 71740 (10.3) |
| North of England | 568425 (22.5) | 281875 (26.8) | 152706 (21.8) | |
| Midlands | 728129 (28.8) | 326271 (31.1) | 222606 (31.8) | |
| South of England | 909621 (35.9) | 354037 (33.7) | 252443 (36.1) | |
| Influenza Vac (%) | 594412 (23.5) | 393449 (37.4) | 237403 (33.9) | |
| Hospital referral—outpatient (%) | 1351709 (53.4) | 599001 (57.0) | 436897 (62.5) | |
| Hospital admission—inpatienta (%) | 44846 (1.8) | 26571 (2.5) | 19883 (2.8) | |
| Length of antibiotic course in days (mean (SD)) | 6.70 (1.39) | 6.65 (1.20) | 5.19 (1.87) | |
| Categorized antibiotic course (%) | 5 days [Ref] | 659787 (26.1) | 243370 (23.2) | … |
| 6–7 days | 1679347 (66.4) | 762739 (72.6) | … | |
| >8 days | 191291 (7.6) | 44676 (4.3) | … | |
| Categorized antibiotic course (%) | 3 days [Ref] | … | … | 219674 (31.4) |
| 4–5 days | … | … | 225228 (32.2) | |
| >6 days | … | … | 254593 (36.4) |
aAccumulative in the 12 months before the consultation date. ¥ mean (sd) for event only.
Abbreviations: AB, antibiotic; BMI, body mass index; HES, hospital episode statistics; IMD, index of multiple deprivation
Rx, prescription.
[Ref] indicates the reference category used as a reference for each of the statistical models.
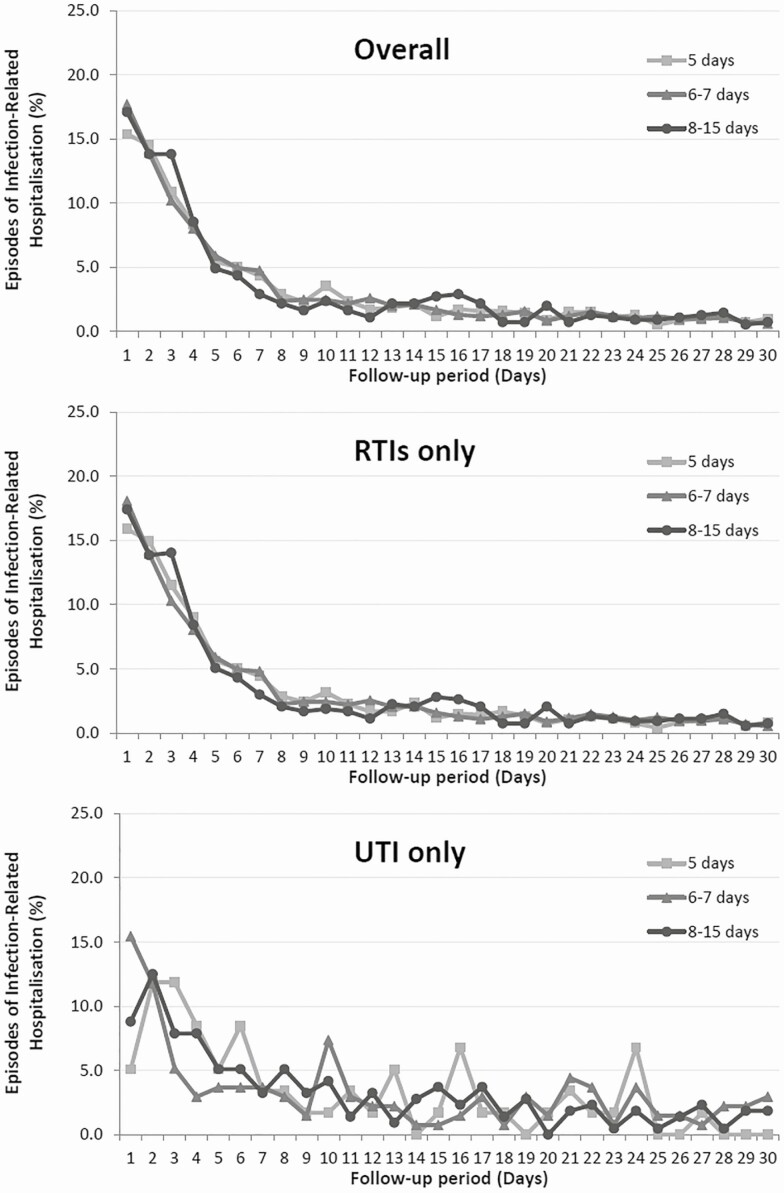
Regardless of the antibiotic duration prescribed, patients were most likely to suffer an infection-related hospitalization in the first week following the consultations (Figure 2). The overall incidence rate of infection-related hospitalizations was 0.15%, where the majority of cases occurred in patients who received an antibiotic course of 8–15 days overall and stratified by infection type (Table 2), where the greatest rate of hospitalizations was for LRTIs (0.39%).
Figure 2.
Episodes of an infection-related hospitalization (%) overall in the follow-up period of 30 days; stratified by the antibiotic course prescribed (5 days, 6–7 days, 8–14 days).
Table 2.
Frequency of Infection-Related Hospitalizations in 30 Days Following Antibiotic Course Overall and Stratified by Infection Type and Duration of Antibiotic Prescription
| 5 Days | 6–7 Days | 8–15 Days | |||||
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||||
| Overall | no case | 1346674 | (99.90) | 2667672 | (99.83) | 258839 | (99.79) |
| case | 1385 | (0.10) | 4637 | (0.17) | 550 | (0.21) | |
| URTI | no case | 659033 | (99.89) | 1676970 | (99.86) | 190933 | (99.81) |
| case | 754 | (0.11) | 2377 | (0.14) | 358 | (0.19) | |
| LRTI | no case | 242934 | (99.82) | 760679 | (99.73) | 44500 | (99.61) |
| case | 436 | (0.18) | 2060 | (0.27) | 174 | (0.39) | |
| 3 Days | 4–5 Days | 6–15 Days | |||||
| n (%) | n (%) | n (%) | |||||
| UTI | no case | 219615 | (99.97) | 225092 | (99.94) | 254377 | (99.92) |
| case | 59 | (0.03) | 136 | (0.06) | 216 | (0.08) | |
Abbreviations: LRTI, lower respiratory tract infections; URTI, upper respiratory tract infections; UTI, urinary tract infections.
The most commonly prescribed antibiotic was amoxicillin for RTIs. Interestingly, the dose increased with duration from 5 to 6 or 7 days. However, the most commonly prescribed antibiotic for 8–15 days was phenoxymethylpenicillin (penicillin V) or doxycycline and frequently issued as an oral suspension. Trimethoprim was most commonly prescribed for UTIs (Supplementary Table 4).
Examining events that occurred in the first few days of follow-up, patients who received longer antibiotic prescription durations had greater risk of developing an infection-related complication compared to patients who received the shortest prescription (HR: 1.40 [CI: 1.29–1.52] and HR: 1.74 [CI: 1.52–1.99], respectively) (Table 3). This effect was also true when stratified by infection. Patients with increased comorbidity were also more likely to experience an infection-related hospitalization in the first 5 days of the treatment overall (Charlson Moderate HR: 1.42 [CI: 1.26–1.60]; High HR: 1.61 [CI: 1.35–1.92], Very High HR: 1.91 [CI: 1.49–2.44]; Supplementary Table 5). This increased risk of hospitalization with increasing comorbidity severity was also true for each infection and was greatest for UTIs (Charlson Moderate HR: 2.59 [CI: 1.45–4.63]), suggesting some difference in underlying patient health and choice of treatment strategy. Patients with a low BMI, currently smoking, or who had an outpatient’s referral or hospital admission in the previous 12 months were also at greater risk of complication, whereas being female or having had an influenza vaccination in the previous 12 months was protective (Supplementary Table 5).
Table 3.
Age-Sex Adjusted and Fully Adjusted Hazard Ratios (HR) of Infection-Related Hospitalization for Patients With Event Within Time Period One (Immediately Follow-up), or Time Period Two (Remaining Follow-up Period) Following Antibiotic Treatment, Stratified by Duration of Antibiotic Course and Infection
| Time Period One | Time Period Two | ||||
|---|---|---|---|---|---|
| 5 Days Follow-up | 6–30 Days Follow-up | ||||
| AB Duration | Age Sex Adjusted | Fully Adjusted | Age Sex Adjusted | Fully Adjusted | |
| Overall | 5 Days | [Ref] | [Ref] | [Ref] | [Ref] |
| 6–7 Days | 1.67 (1.54–1.81) | 1.40 (1.29–1.52) | 1.57 (1.43–1.72) | 1.32 (1.20–1.45) | |
| 8–15 Days | 2.27 (2.00–2.60) | 1.74 (1.52–1.99) | 1.92 (1.65–2.24) | 1.60 (1.37–1.87) | |
| URTI | 5 Days | [Ref] | [Ref] | [Ref] | [Ref] |
| 6–7 Days | 1.23 (1.10–1.37) | 1.34 (1.20–1.50) | 1.19 (1.06–1.35) | 1.16 (1.02–1.32) | |
| 8–15 Days | 1.88 (1.60–2.22) | 1.75 (1.48–2.06) | 1.53 (1.26–1.87) | 1.48 (1.21–1.80) | |
| LRTI | 5 Days | [Ref] | [Ref] | [Ref] | [Ref] |
| 6–7 Days | 1.38 (1.21–1.58) | 1.35 (1.18–1.55) | 1.51 (1.29–1.77) | 1.49 (1.27–1.76) | |
| 8–14 Days | 1.77 (1.40–2.24) | 1.54 (1.22–1.96) | 2.10 (1.61–2.73) | 1.85 (1.42–2.42) | |
| 3 Days Follow-up | 4–30 Days Follow-up | ||||
| AB Duration | Age Sex Adjusted | Fully Adjusted | Age Sex Adjusted | Fully Adjusted | |
| UTI | 3 Days | [Ref] | [Ref] | [Ref] | [Ref] |
| 4–5 Days | 1.74 (0.99–3.07) | 1.46 (0.82–2.62) | 1.53 (1.06–2.21) | 1.57 (0.64–2.95) | |
| 6–15 Days | 1.87 (1.07–3.24) | 1.56 (0.88–2.76) | 2.01 (1.41–2.85) | 2.05 (1.43–2.95) | |
Abbreviations: LRTI, lower respiratory tract infections; URTI, upper respiratory tract infections; UTI, urinary tract infections.
In the second analysis, examining events that occurred in the second period of follow-up, patients who received longer prescriptions also had a greater risk of developing an infection-related complication compared to patients who received a short prescription (HR: 1.32 [95% CI: 1.20–1.45] and HR: 1.60 [95% CI: 1.37–1.87], respectively) (Table 3). All other predictors in the fully-adjusted model for Time Period Two followed a similar pattern to the analysis in Time Period One (Supplementary Table 5).
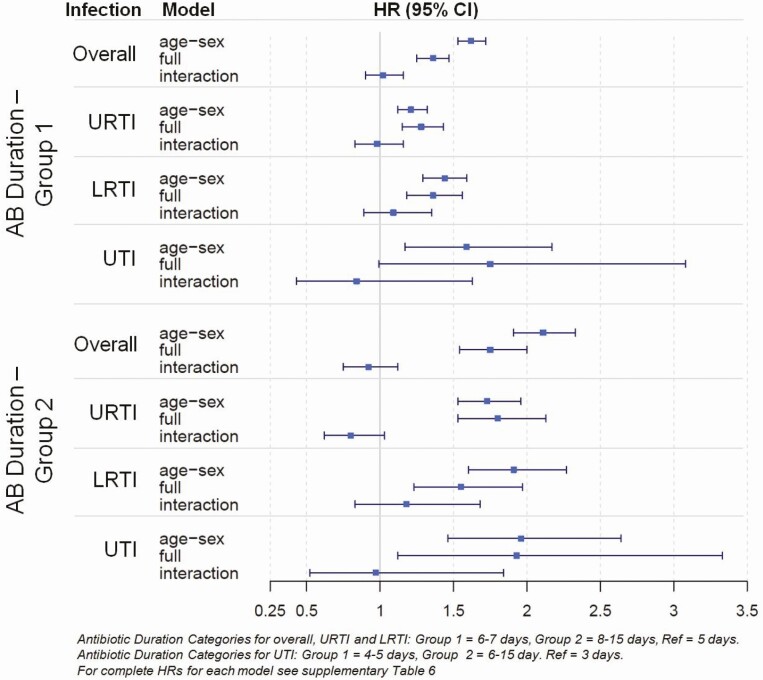
The final analysis compared the rate of infection-related complications in the immediate follow-up (Time Period One) to a longer follow-up (Time Period Two) by the duration of the antibiotic course using an interaction term in the model. This analysis found that the effect of longer antibiotic courses was no longer significant with the consideration of time, suggesting the longer antibiotic courses were no more effective than the shorter course (Figure 3).
Figure 3.
Hazard Ratios (HR) and 95% confidence intervals (CI) for an infection-related hospitalization following different antibiotic (AB) treatment durations for overall infections and stratified by infection. Antibiotic duration categories for overall, URTI and LRTI: group 1 = 6-7 days, group 2 = 8-15 days, Ref = 5 days. Antibiotic duration categories for UTI: group 1 = 4-5 days, group 2 = 6-15 day. Ref = 3 days. For complete HRs for each model see Supplementary Table 6.
As multiple records for the same patient could be included throughout the study period (excluding consultations in the 6 months before and 30 days follow-up) a sensitivity analysis was performed, selecting one observation for each patient at random (Supplementary Table 7). The results from this analysis were similar to the main analysis.
DISCUSSION
The current analysis investigated the relationship of differently prescribed durations of antibiotics and infection-related hospitalizations within the 30 days following the initial consultation. The trend to prescribe longer courses increased and prescribing of shorter courses decreased over the study period, with the most commonly prescribed duration of 6 or 7 days for RTIs. The majority of infection-related hospitalizations occurred for patients receiving longer courses. Overall, patients with antibiotic prescriptions for 6–7 days or 8–14 days had greater risk of developing an infection-related complication in the first few days compared to patients that received a 5 day prescription. When looking in the immediate follow-up period (a treatment period all patients were exposed to), patients with poorer health were also more likely to suffer an infection-related hospitalization, suggesting there was some underlying confounding at baseline. When comparing the HRs in the first few days with the rest of follow-up, it was observed that longer antibiotic courses were no more effective than shorter courses.
The findings in this study further support previous research that found there is no additional benefit in prescribing longer antibiotic courses compared to shorter antibiotic courses when treating a variety of acute infectious conditions [5, 7, 9, 11]. However, in contrast to some research where there is a trend to prescribe shorter courses over time; the current study demonstrated an increased trend to prescribe longer courses of antibiotics over the study period. This discrepancy may be present for a number of reasons. First, it was observed that when longer durations of antibiotics were prescribed for each infectious condition, there was a tendency to prescribe a slightly higher dose of amoxicillin for 6 or 7 days, however prescribing for 8 and 15 days saw alternative treatments or amoxicillin issued as an oral suspension. This may be related to the severity of the patients symptoms on presentation, a high assessment score (eg, FeverPain score); both of which we were unavailable for this analysis, as well as a fear of undertreating an infection [12]. A patients’ age may also effect prescribing decisions, for example, children may be treated with more caution by receiving longer antibiotic courses than adults and treatment as an oral suspension. A recent cross-sectional study using a similar patient population in the UK found a high proportion of antibiotics prescribed that were longer than the recommended guidelines [4], emphasizing the tendency to prescribe on the edge of caution. Although there was no clear difference in prescribing for <16 year olds, in their analysis they did observe a tendency for longer prescriptions issued among younger patients for acute cough and bronchitis, similar to our findings for URTIs. Additionally, the oral suspensions prescribed for longer durations were of a lower dose, supporting the notion that these prescriptions were for more vulnerable patients, such as children or the elderly/frail because of difficulties swallowing tablets and their reduced weight-to-dose ratio.
The current study did observe underlying confounding when looking at the risk of a poor outcome in the first 5 days follow-up (when all comparison groups had the same exposure). It is well known that patients at a higher risk of a complication following an acute infection are often the elderly, patients with impaired immune function and multimorbidity; so it was not surprising to see the incidence increase from 0.12% to 0.76% with an increasing Charlson score. Furthermore, the majority of events occurring within 7 days follow-up, suggesting very unwell patient are admitted before completion of their antibiotic course.
Current recommendations do not inform the prescriber of the best treatment for reoccurring infections (except for UTIs), nor do they consider a patient’s historic antibiotic exposure, or patients that have had repeated-intermittent antibiotic prescriptions, all of which may affect the success of the subsequent antibiotic treatment. The prescriber is left to prescribe empirically, given the patients history with limited trial data to inform guidance for complex prior exposures. A recent epidemiological study observed reduced effectiveness of antibiotic treatment for patients with prior frequent intermittent antibiotic use compared to occasional users, suggesting that frequent use reduces antibiotic effectiveness and may drive individualized resistance, increasing the likelihood of treatment failure in the future [16]. Further research is needed to determine the most effective strategies for optimizing duration of antibiotic treatment for individual patients, taking a combination of patient characteristics, clinical presentation, symptom severity, and prior use into consideration when identifying the patients who are more at risk of failing to recover on shorter antibiotic courses [17].
There are several limitations and strengths in this study. The exclusion of consultations in the previous 6 months may have removed some infection-related consultations from the analysis, introducing a risk of bias, but this exclusion ensured analysis of acute infections only. Modelling any infection-related hospital admission may have included unrelated hospital episodes as an event, although unlikely as most events occurred within 7 days. As the rate of infection-related hospitalization was low for each infection type, it was not possible to run the time-to-event analysis for individual antibiotics. There is a need for this information as no studies have evaluated this [18]. Future studies could investigate the effect of broad spectrum versus narrow spectrum antibiotics by duration in relation to poor outcomes. For observations with missing data, complete case analysis was performed. There was substantially high missingness for smoking history and BMI. Imputation of these variables is discouraged, as these data are not missing at random. However, previous work conducted on a similar population found little difference between complete case analysis and using multiple imputations for these predictors [2] so models were adjusted with a missing indicator. The study period was limited to 2014 and prescribing duration may have changed since. The current analysis is strengthened by the fact that it statistically tested the risk of infection-related hospitalizations that occurred immediately with longer follow-ups, stratified by the duration of the antibiotic course. This analysis was highly valuable as it prevented the false reporting that “the longer duration of an antibiotic course the greater the risk of a poor outcome”; instead it is clear that longer antibiotic courses were no more effective than shorter courses.
In conclusion, there was a trend to prescribe longer courses of antibiotic whilst prescribing of shorter courses decreased; however, longer antibiotic courses were found to be no more effective than a shorter course at preventing an infection-related hospitalization in the following 30 days overall and for respiratory tract infections. Antibiotic stewardship programs should focus on changing the longstanding belief that shorter courses are inferior to long courses of antibiotics when treating an acute infection.
Nonstandard Abbreviations. BMI, body mass index; BNF, British National Formulary; EHR, electronic health records; HES, hospital episode statistics; HR, hazard ratio; IMD, Index of Multiple Deprivation; LRTI, lower respiratory tract infection; rcs, restricted cubic spline; RTI, respiratory tract infections; URTI, upper respiratory tract infection; UTI, urinary tract infections.
Supplementary Material
Notes
Acknowledgments. This study is based on data from the Clinical Practice Research Datalink obtained under licence from the UK Medicines and Healthcare products Regulatory Agency (MHRA). The data is provided by patients and collected by the NHS as part of their care and support. Hospital Episode Data (HES) Copyright (2020) are reused with the permission of The Health and Social Care Information Centre. All rights reserved. The interpretation and conclusions contained in this study are those of the authors alone, and not necessarily those of the MHRA, NHSA, NHS, or the Department of Health. The study protocol was approved by CPRD’s Independent Scientific Advisory Committee (ISAC) (reference: 16_241RMnA). We acknowledge all the data providers who make anonymized data available for research.
Financial support. This work was supported by Connected Health Cities (CHC) and Health Data Research UK North (as part of the BetterRx project [https://www.hdruk.ac.uk/projects/better-care-northern-partnership-better-antibiotic-prescribing-in-frail-elderly-people-with-polypharmacy/]). CHC is a Northern Health Science Alliance led programme funded by the Department of Health and delivered by a consortium of academic and NHS organizations across the north of England.
Transparency declarations. None to declare.
Potential conflicts of interest. The authors express no conflict of interest. All authors have completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and have nothing to disclose.
REFERENCES
- 1. Public-Health-England. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR). 2018:42. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/936199/ESPAUR_Report_2019-20.pdf
- 2. Palin V, Mölter A, Belmonte M, et al. Antibiotic prescribing for common infections in UK general practice: variability and drivers. J Antimicrob Chemother 2019; 74:2440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nowakowska M, van Staa T, Mölter A, et al. Antibiotic choice in UK general practice: rates and drivers of potentially inappropriate antibiotic prescribing. J Antimicrob Chemother 2019; 74:3371–8. [DOI] [PubMed] [Google Scholar]
- 4. Pouwels KB, Hopkins S, Llewelyn MJ, Walker AS, McNulty CA, Robotham JV. Duration of antibiotic treatment for common infections in English primary care: cross sectional analysis and comparison with guidelines. BMJ 2019; 364:l440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spellberg B. The New Antibiotic Mantra-“Shorter Is Better”. JAMA Intern Med 2016; 176:1254–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Llewelyn MJ, Fitzpatrick JM, Darwin E, et al. The antibiotic course has had its day. BMJ 2017; 358:j3418. [DOI] [PubMed] [Google Scholar]
- 7. el Moussaoui R, de Borgie CA, van den Broek P, et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ 2006; 332:1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uranga A, España PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med 2016; 176:1257–65. [DOI] [PubMed] [Google Scholar]
- 9. Royer S, DeMerle KM, Dickson RP, Prescott HC. Shorter versus longer courses of antibiotics for infection in hospitalized patients: a systematic review and meta-analysis. J Hosp Med 2018; 13:336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin E, Hohl P, Guggi T, Kayser FH, Fernex M. Short course single daily ceftriaxone monotherapy for acute bacterial meningitis in children: results of a Swiss multicenter study. Part I: Clinical results. Infection 1990; 18: 70–7. [DOI] [PubMed] [Google Scholar]
- 11. Dawson-Hahn EE, Mickan S, Onakpoya I, et al. Short-course versus long-course oral antibiotic treatment for infections treated in outpatient settings: a review of systematic reviews. Fam Pract 2017; 34:511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Macheda G, Dyar OJ, Luc A, et al. ; ESGAP and SPILF . Are infection specialists recommending short antibiotic treatment durations? An ESCMID international cross-sectional survey. J Antimicrob Chemother 2018; 73:1084–90. [DOI] [PubMed] [Google Scholar]
- 13. Williams T, van Staa T, Puri S, Eaton S. Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther Adv Drug Saf 2012; 3:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herrett E, Gallagher AM, Bhaskaran K, et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015; 44:827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Statistics. English indices of deprivation 2015. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/464430/English_Index_of_Multiple_Deprivation_2015_-_Guidance.pdf.
- 16. van Staa TP, Palin V, Li Y, et al. The effectiveness of frequent antibiotic use in reducing the risk of infection-related hospital admissions: results from two large population-based cohorts. BMC Med 2020; 18:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malotaux J, Wiersinga WJ, Geerlings SE. [Optimal duration of antibiotic therapy: there is no ‘one size fits all’]. Ned Tijdschr Geneeskd 2017; 161:D1891. [PubMed] [Google Scholar]
- 18. Lopez-Alcalde J, Rodriguez-Barrientos R, Redondo-Sanchez J, et al. Short-course versus long-course therapy of the same antibiotic for community-acquired pneumonia in adolescent and adult outpatients. Cochrane Database Syst Rev 2018; 9:CD009070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.