Abstract
Reproductive hormones play a crucial role in the growth and maintenance of the mammalian skeleton. Indeed, the biological significance for this hormonal regulation of skeletal homeostasis is best illustrated by common clinical reproductive disorders, such as primary ovarian insufficiency, hypothalamic amenorrhea, congenital hypogonadotropic hypogonadism, and early menopause, which contribute to the clinical burden of low bone mineral density and increased risk for fragility fracture. Emerging evidence relating to traditional reproductive hormones and the recent discovery of newer reproductive neuropeptides and hormones has deepened our understanding of the interaction between bone and the reproductive system. In this review, we provide a contemporary summary of the literature examining the relationship between bone biology and reproductive signals that extend beyond estrogens and androgens, and include kisspeptin, gonadotropin-releasing hormone, follicle-stimulating hormone, luteinizing hormone, prolactin, progesterone, inhibin, activin, and relaxin. A comprehensive and up-to-date review of the recent basic and clinical research advances is essential given the prevalence of clinical reproductive disorders, the emerging roles of upstream reproductive hormones in bone physiology, as well as the urgent need to develop novel safe and effective therapies for bone fragility in a rapidly aging population.
Keywords: bone, kisspeptin, GnRH, FSH, LH, prolactin, progesterone, inhibin, activin, relaxin
Graphical Abstract
Graphical Abstract.
Essential Points.
Skeletal homeostasis is regulated by reproductive hormones and their fluctuations throughout life
Prevalent reproductive disorders, including premature ovarian insufficiency, hypothalamic amenorrhea and hyperprolactinemia, are common causes of low bone mass
While it is traditionally held that gonadal sex steroids play a major role in skeletal homeostasis, research has advanced into the influence of other reproductive hormones on bone physiology
Skeletal homeostasis in mammals is tightly regulated by the process of bone remodeling, which preserves optimal bone mass and strength, thereby preventing fractures during normal physical activity. During bone remodeling, bone mass is maintained by a tight balance between osteoclastic bone resorption and osteoblastic bone formation. Furthermore, this bone remodeling is a considerably energy-demanding process as clearly demonstrated in rodents studies (1). Indeed, from an evolutionary perspective, during the physiological response to starvation, skeletal integrity as well as reproduction may be relinquished (2), whereas conversely food consumption is regarded as a positive stimulus both for bone (3) and reproduction (4). How bone remodeling is coupled specifically to energy metabolism has been the focus of several seminal studies revealing the importance of leptin in the interplay between bone and the central nervous system (5-7). Beyond leptin, other factors linked with nutrient intake and energy metabolism are suggested to regulate bone remodeling, including gastrointestinal hormones such as glucagon-like peptide 1, glucagon-like peptide 2, and glucose-dependent insulinotropic polypeptide (8, 9), as well as adipose-tissue factors such as adiponectin (10).
In skeletal diseases, bone remodeling is frequently disrupted. Osteoporosis, the most prevalent metabolic bone disease, is characterized by a deficit in osteoblastic bone formation relative to osteoclastic bone resorption, resulting in loss of bone mass and microarchitectural deterioration, and resultant susceptibility to fractures (11, 12). Postmenopausal bone loss is a central risk factor for developing osteoporosis (13, 14). The higher risk and prevalence of fractures results in disability, reduced quality of life, and increased mortality (15). Notably, the increasing incidence of osteoporosis (16) and health care costs associated with an aging society (13), accentuates the need to better understand bone physiology and the pathogenesis of bone loss.
It is well recognized that skeletal homeostasis depends on the traditional hormonal mediators of calcium and phosphate homeostasis, such as parathyroid hormone (PTH), and vitamin D (17). Interestingly, coexpression of vitamin D receptor and vitamin D metabolizing enzymes in animal and human testis has been reported (18, 19), suggesting that vitamin D could be considered a partly gonadal-derived factor, which influences bone. Moreover, while vitamin D may be formed and secreted from the testes, small animal and human studies reveal that active vitamin D promotes testosterone and sperm production, highlighting that vitamin D also acts indirectly on bone by promoting testosterone production in the testes (20). In addition, numerous other circulating factors with different primary roles are important in bone physiology, in particular reproductive hormones. Indeed, the importance of the crosstalk between reproductive hormones and bone is clearly illustrated by common reproductive disorders, which contribute to the clinical burden of low bone mineral density (BMD), such as primary ovarian insufficiency (21-25), hypothalamic amenorrhea (26-28), congenital hypogonadotropic hypogonadism (29-32), pregnancy and lactation-associated osteoporosis (33-38) and hyperprolactinemia (39-44) (summarized in Table 1).
Table 1.
Effects of reproductive disorders on bone
| Reproductive disorder | Effects on bone |
|---|---|
| Premature ovarian insufficiency (POI) | • Low bone mass due to insufficient peak bone mass accrual (depending on onset) and increased bone remodeling (predominately bone resorption) secondary to estrogen deficiency (21) |
| • Bone loss greater in trabecular than cortical bone (22) | |
| • Prevalence of osteoporosis of 8% to 14% (23) | |
| • Almost 50% of patients have significantly reduced BMD within 1.5 years of POI diagnosis (24) | |
| • 2% to 3% lower BMD at lumbar, femoral neck, and hip compared to normally menstruating women (25) | |
| Functional hypothalamic amenorrhea (FHA) | • Bone loss related to duration of amenorrhea and degree of estrogen deficiency (26) |
| • Prevalence of low BMD in female athletes with FHA or oligomenorrhea estimated up to 15.9% (27) | |
| • Average reduction in lumbar spine BMD of 15% by 3 y compared to normally menstruating women (26) | |
| • Significant fracture risk, including stress fractures (28) | |
| Congenital hypogonadotropic hypogonadism (CHH) | • Chronic gonadal steroid deficiency associated with reduced peak bone mass in early adulthood and accelerated bone loss (29) |
| • Prevalence of low BMD almost 45% of untreated young men with CHH (30) | |
| • BMD improves during gonadal sex steroid replacement, especially in skeletally immature men (31), but does not fully reverse skeletal abnormalities (32) | |
| Pregnancy and lactation-associated osteoporosis | • Mechanisms include negative calcium balance and lactational estrogen deficiency (33), along with additional hormonal changes predisposing to ligament laxity (34) |
| • Associated with fractures during late pregnancy or postpartum (35) | |
| • Frequently manifests as severe back pain (36, 37) | |
| • Risk of recurrence in subsequent pregnancies (38) | |
| Hyperprolactinemia | • Decreased bone density due to direct and indirect (via hypogonadism) effects of prolactin on bone physiology (39) |
| • Associated with early alterations in bone turnover markers that precede BMD changes (40) | |
| • Bone mass diminished predominantly in trabecular rather than cortical bone (41) | |
| • Bone loss more marked when hyperprolactinemia develops at a younger age, which restricts peak bone mass acquisition (42) | |
| • High risk of radiological vertebral fractures in men and women with PRL-secreting pituitary adenomas, averaging 32% to 37% (43, 44) |
Abbreviations: BMD, bone mineral density; PRL, prolactin.
It was traditionally understood that the skeletal consequences of these disorders were primarily attributable to the effects of sex steroids (the gonadal reproductive hormones, estrogen and testosterone). However, a decline in bone density during the perimenopausal transition frequently occurs despite unchanged circulating estrogen levels (45). Similarly, in rats, ovariectomy plus hypophysectomy results in less bone loss than ovariectomy alone, underscoring a role for upstream reproductive hormones (46, 47). Finally, while the prevalence of fractures in women with PRL-secreting pituitary adenomas is high, this risk has been observed to be similar between amenorrheic and eugonadal premenopausal women, suggesting that hyperprolactinemia directly causes bone loss regardless of associated hypogonadism (44). Taken together, these data reveal that additional hormonal factors contribute to the changes in bone mass in these reproductive disorders, rather than solely caused by changes in sex steroids. To this end, there is accumulating evidence identifying the importance of reproductive hormones of the hypothalamic-pituitary-gonadal (HPG) axis beyond estrogens and androgens in modulating key processes in skeletal homeostasis.
Notably, there are numerous existing reviews that comprehensively examine the effects of gonadal reproductive hormones (principally estrogen and androgens) on bone (48-52). Moreover, adrenal androgens (such as dehydroepiandrosterone, dehydroepiandrosterone sulfate, and androstenedione), which act as precursors for peripheral conversion to more potent androgens and estrogens, also have established effects on bone homeostasis (53-58).
In this review, we provide a contemporary summary of the literature examining the relationship between bone and reproductive signals beyond these estrogens and androgens, including the recent basic (in vitro and in vivo) and clinical research advances, and the new players in the field. While several of the discussed hormones merit a review on their own, we have endeavored to combine them into a single atlas to provide an overall view of the relationship between reproductive hormones with bone beyond estrogens and androgens. A comprehensive and up-to-date review is essential given the prevalence of clinical reproductive disorders, the emerging roles of reproductive hormones beyond estrogens and androgens on bone physiology, as well as the urgent need to develop novel, safe, and effective therapies for low bone mass to prevent bone fractures in an aging population.
Materials and Methods
We performed a literature review and identified pertinent publications by a series of PubMed searches for English-language articles. The search terms were (“kisspeptin” OR “KISS1” OR “gonadotropin-releasing hormone” OR “GnRH” OR “luteinizing hormone” OR “LH” OR “follicle-stimulating hormone” OR “FSH” OR “prolactin” OR “PRL” OR “progesterone” OR “inhibin” OR “activin” OR “relaxin”) AND (“osteocyte” OR “osteoblast” OR “osteoclast” OR “skeletal/skeleton” OR “bone” AND (“metabolism” OR “physiology” OR “structure” OR “remodeling” OR “modeling” OR “homeostasis” OR “tissue”) OR “bone mineral density” OR “osteoporosis” OR “fracture”). Relevant data were subsequently extracted from the identified publications, and secondary data sources identified therein. To ensure the inclusion of the most current data available, searches were performed up until October 21, 2020.
The Hypothalamic-Pituitary-Gonadal Axis: A Historical Perspective and Overview
Reproduction is governed by the HPG axis, which acts as a classical negative feedback loop to regulate gonadal function. Indeed, it was Geoffrey Harris’ 1955 monograph “Neural Control of the Pituitary Gland” that first posited that the secretion of pituitary gonadotropins was controlled by chemical substances of hypothalamic origin released into the hypophysial (pituitary) portal circulation (59). Consequently, this conceptual groundwork led to the isolation and sequencing of gonadotropin-releasing hormone (GnRH) by the laboratories of Schally (60) and Guillemin (61) in 1971, and their award of the 1977 Nobel Prize in Physiology or Medicine. For decades GnRH would be considered the key determinant of reproductive function.
However, in 2003 two independent groups published reports in short succession revealing that humans with inactivating mutations of the kisspeptin receptor (GPR54/KISS1R) resulted in failed puberty and resultant infertility (62, 63). These landmark findings in reproductive biology were followed by a series of studies demonstrating that kisspeptin (encoded by Kiss1/KISS1) administration acted as a potent stimulator of gonadotropin release in animals (64-67) and humans (68-70), whereas pretreatment with a GnRH antagonist abolished this stimulatory effect of kisspeptin (71).
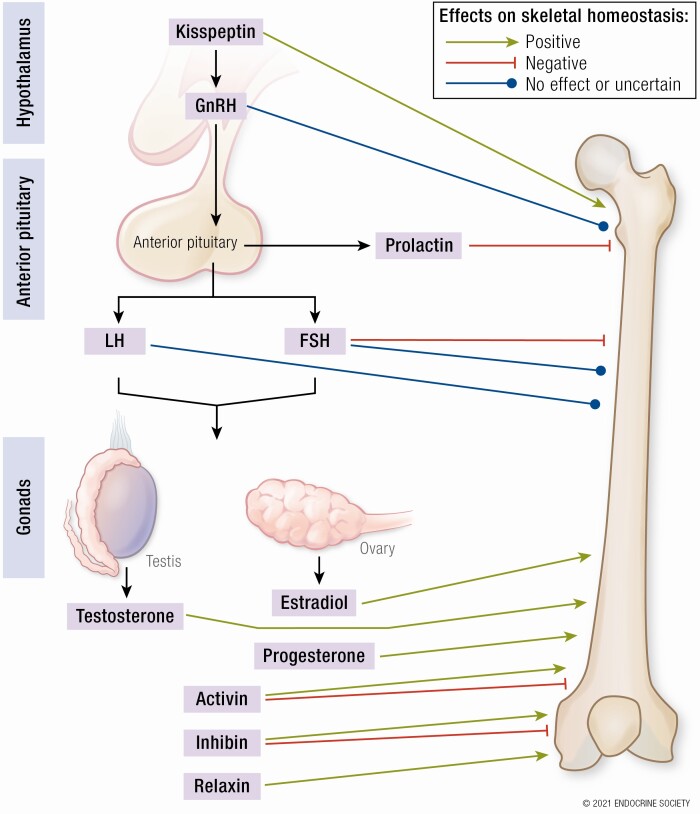
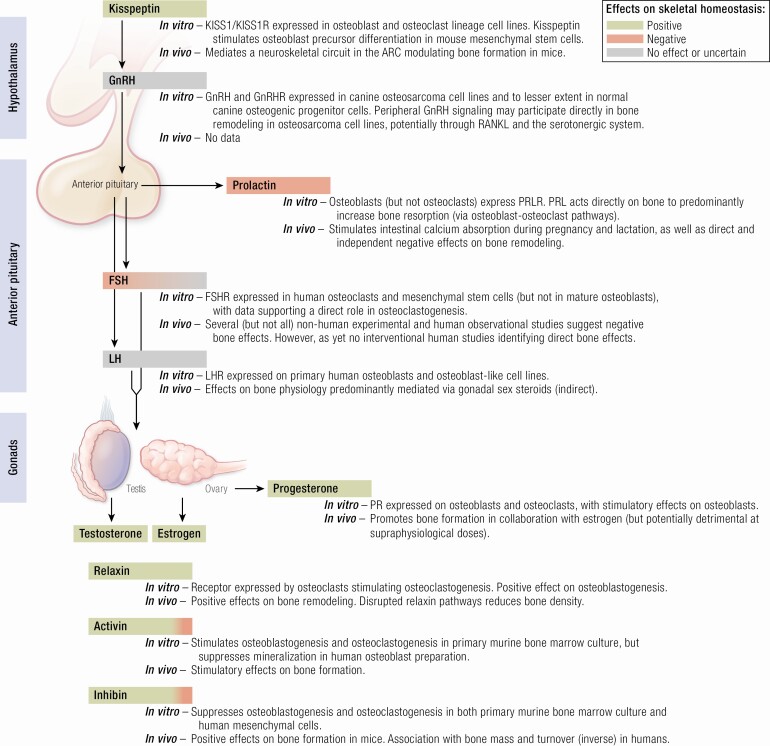
In light of such fundamental discoveries, the historical understanding of the hormonal cascade that controls the HPG axis was revised (Figure 1): Kisspeptin sits at the apex of the reproductive axis and is secreted by hypothalamic KISS1 neurons. Kisspeptin activates kisspeptin receptors expressed on GnRH neurons to secrete GnRH into the local hypophyseal-portal circulation in a pulsatile manner. Subsequently, GnRH is responsible for stimulating the biosynthesis and secretion of gonadotropins (luteinizing hormone [LH] and follicle-stimulating hormone [FSH]) from the anterior pituitary gland, which circulate systemically to reach the gonads to promote gamete maturation and the release of sex steroids (estradiol, testosterone, and progesterone). In addition to these established members, gonadal-derived activin and inhibin are closely related reproductive hormones with diametrically opposing biological effects: Activin enhances FSH secretion, whereas conversely inhibin inhibits FSH secretion.
Figure 1.
Schematic of the hypothalamic-pituitary-gonadal reproductive axis and summary of the direct effects on bone. Green shading denotes predominantly positive effect and red shading predominantly negative effects on skeletal homeostasis. Gray shading denotes no direct or uncertain overall effects on skeletal homeostasis. ARC, hypothalamic arcuate nucleus; FSH, follicle-stimulating hormone; FSHR, follicle-stimulating hormone receptor; GnRH, gonadotropin-releasing hormone; GnRHR, gonadotropin-releasing hormone receptor; KISS1, kisspeptin gene; KISS1R, kisspeptin receptor gene; LH, luteinizing hormone; LHR, luteinizing hormone receptor; PR, progesterone receptor; PRLR, prolactin receptor.
Bone Modeling and Remodeling: A Brief Overview
Bone modeling is a bone maintenance mechanism mediated by osteoclastic bone resorption followed by osteoblastic bone formation, which are coupled in time and space. Under steady state, there is a balance between bone resorption and bone formation and thus stable bone mass. Osteoclastic and osteoblastic functions are regulated by circulating extrinsic factors or locally by osteocytes. These are outlined below for context (and reviewed comprehensively in [72]).
Osteocytes comprise more than 90% of bone cells and are the longest-lived bone cell, surviving for several decades, compared with days or weeks for osteoclasts and several months for osteoblasts (73). They are derived from a subpopulation of mature osteoblasts that during the bone formation phase become embedded within mineralized bone matrix and function as the primary skeletal mechanosensors (74). RNA-sequencing analysis over the course of osteoblast to osteocyte transition using an osteoblast-like murine cell line has revealed significant changes in gene expression, with these changes associated with notable epigenic modifications to histones H3 and H4 (75). Importantly, these alterations are likely to be influential in determining the osteocyte phenotype (75). Functionally, osteocytes are predominantly responsible for modulating bone remodeling by regulating osteoclast and osteoblast differentiation through the release of several specific molecules, including sclerostin, receptor activator of nuclear factor-κB ligand (RANKL), osteoprotegerin (OPG), and dickkopf-1 (73, 76).
Osteoclasts are multinucleated cells responsible for bone resorption and are derived from hematopoietic mononuclear cells of the monocyte/macrophage lineage (77) and have recently been demonstrated to recycle via daughter cells known as osteomorphs (78). Differentiation of the precursor cells into osteoclasts is primarily regulated by macrophage colony-stimulating factor (M-CSF) and RANKL. M-CSF acts on osteoclasts through its receptor c-FMS to induce the proliferation and survival of osteoclast precursor cells through the activation of extracellularly regulated kinase (ERK) and protein kinase B (79). By comparison, RANKL binds to its receptor, RANK, leading to the recruitment of adaptor molecules, such as tumor necrosis factor receptor-associated factor 6, which subsequently leads to the activation of mitogen-activated protein kinases (MAPKs), and the transcription factors nuclear factor-κB (NF-κB) and activator protein-1 (80). Activated nuclear factor-κB induces the nuclear factor of activated T-cells cytoplasmic 1 (NFATc1), a major osteoclastogenesis regulator (80). Meanwhile, depending on their state of differentiation, osteoblasts (discussed later) transiently release RANKL and OPG. OPG functions as a decoy receptor for RANKL, to prevent the activation of RANK and thereby inhibit osteoclast recruitment (81). In addition, osteocyte apoptosis with secondary necrosis results in release of damage-associated molecular patterns that induces osteoclastogenesis and bone loss (82, 83).
Osteoblasts are mononucleated cells that are derived from bone marrow skeletal stem cells (also known as stromal or mesenchymal stem cells). The commitment of mesenchymal stem cells toward the osteoprogenitor lineage is regulated by a network of pro-osteogenic mediators, including bone morphogenetic proteins (BMPs) and members of the Wingless (Wnt) family (84). In addition, other important genes involved in this osteoblast differentiation include runt-related transcription factors 2 (Runx2), distal-less homeobox (Dlx5), and osterix (Osx) (84). During bone formation, mature osteoblasts synthesize and secrete bone matrix proteins, type 1 collagen and several noncollagen proteins such as osteocalcin (OC), osteopontin, and bone sialoprotein (84). Osteoblasts therefore mediate bone formation through the deposition of unmineralized osteoid matrix and its subsequent mineralization (85).
Each of these cell types is regulated by a variety of endocrine factors. In addition to established hormonal mediators, such as PTH, vitamin D, calcitonin, thyroid hormone, growth hormone, insulin-like growth factor 1, and glucocorticoids (72, 86, 87), a wealth of emerging studies highlights the importance of reproductive hormones, as detailed in the present review. Therefore, we have considered the key components of the HPG axis (starting from the top) beyond estrogens and androgens, with regards to in vitro as well as nonhuman/human in vivo perspectives to ensure clarity and full appreciation of the important differences between these methodologies and species, and the implications of their findings.
Kisspeptin
Kisspeptin refers to a family of structurally related endogenous peptides encoded by the human KISS1 gene (nonhuman Kiss1 gene). Diverging in amino acid length, they are the proteolytic products of a common 145-amino acid precursor protein (88, 89). Four circulating fragments have been identified in the human circulation: kisspeptin-54, -14, -13 and -10 (with suffix denoting the number of amino acids) (89). All have a common RF-amide C terminus, which acts as the endogenous ligand for a G-protein–coupled receptor, the kisspeptin receptor (KISS1R/Kiss1r) (90). While the KISS1 gene was initially identified for its ability to reduce the metastatic potential of malignant melanoma cells (91), subsequent studies illustrated the indispensable influence of kisspeptin-signaling in pubertal progression and fertility (62, 63, 92). Interest has further accelerated in response to contemporary work highlighting a more expansive role for kisspeptin in the control of human reproductive behavior, mood, and emotions (93-96).
From a skeletal perspective, loss of function in the kisspeptin-signaling pathway in humans is associated with delayed skeletal maturation (62), whereas conversely activating mutations in KISS1R produces a phenotype of accelerated growth and skeletal maturation due to central precocious puberty (97). However, in both cases the direct contribution of kisspeptin on the skeleton cannot be adequately assessed because of the respective large decreases and increases in gonadal sex steroids resulting from these mutations.
In humans, notable peripheral KISS1R expression has been detected in the heart, kidney, lung, pancreas, placenta, small intestine, spleen, stomach, testis, and thymus (88, 89, 98). Within the hypothalamus, the distribution of kisspeptin-expressing neurons varies in a species-dependent manner. In humans, 2 principal populations exist: (1) the infundibular nucleus, and (2) the rostral preoptic area (99, 100). In rodents, Kiss1 exists predominantly in 2 distinct populations: (1) the arcuate nucleus (equivalent to the human infundibular nucleus), and (2) the anteroventral periventricular nucleus (71, 101, 102). The detection of Kiss1 signaling in bone cell lines (103) may imply its putative role in bone physiology as detailed in the following section.
In Vitro Studies
KISS1 messenger RNA (mRNA) and protein are both strongly expressed in immortalized human fetal osteoblastic cells transformed by expression of SV40 large T antigen (hFOB1.19) (103). By contrast, KISS1 mRNA and protein expression are moderate, weak, and almost lost in the osteosarcoma cell lines U-2 OS, Saos-2, and MG-63, respectively (103). However, interestingly this did not match human osteosarcoma specimens in which 20 of the 44 specimens exhibited strong KISS1 expression by immunohistochemistry, positively correlating with earlier distant metastasis compared with KISS1-negative patients (103). Consistent with this, the KISS1R protein product is highly expressed in MG-63 osteoblast-like osteosarcoma cells (104). Furthermore, Kiss1r expression has also been variably detected in normal canine osteoprogenitor cells (ie, committed but not differentiated osteoblastic cells) (105) and human osteoprogenitor and skeletal stem cells (106). Further work is required to determine the precise expression pattern of KISS1 and its receptor in healthy mature primary bone cells.
Building on these observations, a recent study provided the first data for the direct role of Kiss1 in osteoblast differentiation. In C3H10T/2 mouse mesenchymal stem cells, kisspeptin-10 dose-dependently induced the expression of osteoblastic marker genes including Dlx5, Runx2, and alkaline phosphatase (ALP) (107). Given that BMP-2 stimulates bone formation by regulating the transcription of these osteogenic genes (108, 109), the investigators subsequently demonstrated that kisspeptin-10 increased BMP-2 gene and protein expression, via the transcriptional factor NFATc4 (107). Conversely, in Kiss1r null cells, osteoblast differentiation was suppressed (107). Hence, these data illustrate that in C3H10T/2 cells kisspeptin-10 (acting via Kiss1r) stimulates osteoblast differentiation through NFATc4-mediated BMP-2 expression and activation (107), which suggests a potential osteoanabolic role for kisspeptin in bone physiology.
In addition to BMP-2, kisspeptin signaling also regulates the expression of BMP-7 (another osteogenic gene) through the cooperative effect of the transcription factors NFATc2 and Sp1 in the embryonic kidney (110). Notably, Kiss1r deletion resulted in decreased BMP-7 expression and abnormal kidney branching morphogenesis and glomerular development in vivo and in explanted kidneys in vitro (110). Similarly, the mutual interaction of Kiss1, estrogen, and BMP-4 has also been identified to regulate GnRH production in mouse hypothalamic GT1-7 cells (111). Critically, whether the interaction between kisspeptin and BMP-4 or -7 affects skeletal morphogenesis remains to be elucidated.
KISS1R has also been detected in osteoclast cell lines differentiated in vitro from CD14-selected monocytes (112), suggesting that KISS1 signaling may have direct roles not only in osteoblast but also osteoclast physiology, again highlighting the need for the assessment of KISS1/KISS1R expression in mature primary bone cells. Whether KISS1 and its receptor are expressed in osteocytes and influence secretion of factors involved in remodeling (such as sclerostin) remains unknown and will no doubt be a focus of future investigation.
In Vivo Nonhuman Studies
A contemporary pivotal murine study using a combination of different genetic models and stereotaxic surgery demonstrated that deleting estrogen receptor alpha (ERα) signaling in the hypothalamic arcuate nucleus resulted in a significant increase in bone mass without changes to food intake (113). In this study, the effect was sex-specific (occurring in female mice only) with an impressive increase in trabecular bone mass of approximately 700% with an average 80% increase in bone volume over total volume (113). Moreover, an increase in trabecular number and thickness as well as increased overall mechanical strength of long bones was observed, in the absence of significant changes in several measured circulating hormones including leptin, thyroxine, LH, FSH, testosterone, and estrogen (113). Mechanistically, these changes were accompanied by a significant increase in bone formation rate and mineralized surface, indicating enhanced osteoblastic functions (113). Transcriptional profiling demonstrated upregulation of BMP signaling and osteoblast differentiation (113). Remarkably, ablation of arcuate ERα after ovariectomy resulted in a 50% increase in bone density, indicating that even in the absence of gonadal hormones, the brain circuit remains partially intact (113) and suggesting a possible therapeutic avenue for postmenopausal loss of bone mass. In addition, loss of ERα specifically in Kiss1-expressing arcuate cells recapitulated this bone phenotype, defining central Kiss1 signaling as a key mediator in ER-neuroskeletal circuit (113). Importantly, arcuate Kiss1 neurons are well established as major neurons involved in coordinating energy states with reproduction (114). Therefore, it is interesting to speculate that these Kiss1 neurons are part of a wider system that controls energy-demanding bone remodeling to maintain reproduction.
Based on the earlier described KISS1/KISS1R expression in vitro data (Figure 1), whether peripheral kisspeptin signaling may also have direct beneficial roles in bone physiology in vivo remains to be seen. Along these lines, future investigations should seek to determine the skeletal consequences of conditional deletion of KISS1/KISS1R in bone cells as well as the effects of peripheral kisspeptin administration to examine this further and potentially reveal new therapeutic avenues.
Gonadotropin-Releasing Hormone
The decapeptide GnRH is produced by neurosecretory GnRH neurons within the preoptic area and mediobasal hypothalamus and is released in synchronized pulses into the local hypophysial-portal circulation (115). Thereafter, it binds to its high affinity 7-transmembrane G-protein–coupled receptor, GnRH receptor (GnRHR), expressed at the cell surface of gonadotropin cells of the anterior pituitary gland, and signals through a Gq/11-dependent intracellular pathway to control both the biosynthesis and secretion of the 2 gonadotropins (LH and FSH) (116).
In many vertebrates, 3 forms of GnRH (GnRH I, II, and III) have been identified, although only 2 exist in reptiles, birds, and mammals (117). Correspondingly, 3 cognate receptor subtypes (types I, II, and III) are present in amphibians, whereas in mammals only type I and type II are found (117). Indeed, GnRH II is widely distributed in the nervous system, and has been detected in normal and cancerous human tissues, including breast, endometrium, ovary, and prostate (118), as well as bone marrow (119). Based on the latter, its potential involvement in bone physiology has been examined as reviewed in the following section.
In Vitro Studies
While data from primary cell culture are lacking, a recent study provided potential evidence for direct effects of GnRH on osteoblast-like cells. Both in canine osteosarcoma cell lines (COS, POS, HMPOS, D17, and C4) and to a lesser extent in normal canine osteogenic progenitor cells, GnRH and GnRHR expression were observed (105). Furthermore, using the tumor cell line COS, detectable concentrations of GnRH were identified using radioimmunoassay (105), suggesting that these osteoblast-like cells can secrete GnRH in measurable amounts. Critically, as these are osteosarcoma cells, this may reflect epigenetic changes. Remarkably, exogenous kisspeptin-10 applied to COS cells stimulated GnRH secretion 4- to 5-fold (105), therefore recapitulating within these cells the normal functional relationship observed in the hypothalamic component of the HPG axis. In these studies, GnRH (and KISS1) treatment increased both COS proliferation and the expression of the bone remodeling ligand RANKL (but not OPG expression), effects that were blocked by treatment with a GnRHR inhibitor (105). GnRH and kisspeptin-10 treatment also increased the expression of the serotonin receptor htr2a 2- to 8-fold (105). Interestingly, the serotonergic system has been reported to regulate bone mass via osteoblast recruitment and proliferation (120) and suggests that GnRH and kisspeptin may exert osteoblastic proproliferative effects in these osteosarcoma cells. These results, although based on nonhealthy (osteosarcoma) cell lines, provide an interesting insight into a possible role for GnRH on bone remodeling, potentially through interplay with the serotonergic system (see Figure 1). Owing to the short half-life of GnRH of 2 to 6 minutes in vivo caused by high renal clearance and proteolytic degradation (121), there unfortunately remains a paucity of data examining the direct influence of GnRH on skeletal metabolism using in vivo models. However, cell-specific gene targeting may provide some answers, for instance by generating a GnRHR-floxed mouse with osteoblast-specific deletion of the GnRHR to examine the direct effect of GnRH on bone. Moreover, it is unknown whether GnRHR is expressed in osteocytes, which warrants further investigation.
In Vivo Human Studies
Given that GnRH is released into the local hypophysial-portal circulation and has a very short half-life as mentioned earlier, circulating levels cannot currently be analyzed in peripheral blood, making clinical studies challenging. Conversely, a plethora of studies have examined the effect of GnRH agonists on BMD during therapeutic use as they suppress gonadal function and cause hypogonadotropic hypogonadism. For instance, androgen-deprivation therapy remains the backbone of management for patients with prostate cancer, with GnRH agonists as the most widely used first-line treatment (122). In a study of 47 men with advanced or recurrent prostate cancer and no bone metastases, treatment with the GnRH agonist leuprolide was associated with a 2% to 3% reduction in lumbar spine, trochanteric, and total hip BMD, as well as a decrease in trabecular BMD by 8.5% after treatment for 48 weeks (123). In keeping with these data, most studies report a 2% to 3% decrease per year in BMD of spine and hip during initial GnRH agonist treatment (124). These significant changes in BMD result in a clinically relevant increased fracture risk. In men surviving at least 5 years after a prostate cancer diagnosis, 19.4% of those who received androgen-deprivation therapy (orchidectomy or GnRH agonist) experienced a fracture (most commonly the femoral neck, rib, spine, and hand), compared with 12.6% of those who did not receive androgen-deprivation therapy (P < .001) (125). Moreover, the risk of fracture increased with the number of doses administered during the first year after diagnosis (125). It is significant to note that novel GnRH antagonists (such as degarelix) are increasingly available for the treatment of advanced prostate cancer (126). Compared with GnRH agonists, they produce rapid and sustained suppression of testosterone without eliciting an initial testosterone surge (127). However, whereas the skeletal consequences associated with GnRH agonists are now well characterized, less is known regarding the bone effects of GnRH antagonists in patients with prostate cancer. In fact, a recent meta-analysis of randomized controlled trials in patients with metastatic disease reported that GnRH antagonists use was associated with fewer musculoskeletal events (relative risk 0.76, including fractures) compared with GnRH agonists (128). However, as the authors rightly conclude, given the low number of musculoskeletal events and fractures observed, caution should be applied when interpreting these findings (128). Therefore, further clinical trials examining BMD and fracture risk are warranted before drawing definitive conclusions regarding the effects of GnRH agonists vs antagonists on bone.
In addition to prostate cancer, GnRH agonists are also commonly employed as a treatment strategy to suppress ovarian function in the management both of endometriosis (129) and premenopausal/perimenopausal women with breast cancer (130). In an analysis of 50 women with endometriosis, treatment with leuprolide administered for 24 weeks resulted in –4.9% and –3.4% reductions in BMD following 6 months of treatment and at 12 months post treatment, respectively (131). Similar reductions in BMD have also been observed in women with breast cancer. In a small, multicenter study of premenopausal women with breast cancer, 2 years of goserelin alone caused a mean 5% loss of bone density, whereas a combination with tamoxifen resulted in a lesser decline of –1.4% (132). It is notable that only partial recovery from bone loss was observed on cessation of goserelin treatment alone at 1 year (132). Given these findings, the effects of concomitant treatment with bisphosphonates on preventing bone loss associated with GnRH agonists was examined. In the ABCSG-12 study, after 3 years of treatment in premenopausal women with endocrine-responsive breast cancer, endocrine therapy alone (goserelin and anastrozole or goserelin and tamoxifen) resulted in a significant reduction of BMD of the lumbar spine (–11.3%) and trochanter (–7.3%) (133). Again, only partial recovery was observed 2 years after completing treatment (133). By comparison, patients who also received zoledronic acid had stable BMD at 3 years and increased BMD at 5 years (133), suggesting that concurrent bisphosphonate treatment has the potential to attenuate bone loss associated with GnRH agonists in this patient group.
Taken together, these studies indicate that bone remodeling is affected by GnRH agonist therapy (and probably GnRH antagonists), resulting in bone loss. In addition, from a mechanistic standpoint, GnRH agonist treatment of premenopausal women with endometriosis has been observed to result in osteocyte apoptosis in human bone, providing a further cellular mechanism for the increased bone fragility associated with these agents (134). However, although these results do not necessarily indicate an absence of the direct effect from GnRH on bone physiology per se, the observed deleterious effects on the skeleton most likely result predominantly from suppressing the release of subsequent downstream reproductive hormones, which have more established effects on bone.
Follicle-Stimulating Hormone
FSH is synthesized by gonadotrope cells in the anterior pituitary and plays a key role in mammalian reproduction during puberty and gamete production in adulthood. In women, FSH is responsible for follicular development and estrogen production (135), whereas in men FSH predominantly regulates testicular development and spermatogenesis (136).
FSH is a glycoprotein dimer consisting of an alpha (α) and beta (β) subunit. Whereas the α subunit is common to thyroid stimulating hormone (TSH), human chorionic gonadotropin (hCG), and LH, the β subunit is unique to FSH. This allows binding to its cognate receptor, the FSH receptor (FSHR), which belongs to the family of G-protein–coupled receptors (137). In addition to the canonical Gsα/3′,5′-cyclic adenosine 5′-monophosphate (cAMP)/protein kinase signaling pathway, it is now clear that FSHR activation triggers numerous other intracellular signaling pathways to elicit its biological actions (138).
Whereas FSHR was traditionally accepted to be exclusively localized in the gonads (137), recent studies have identified its expression in a variety of healthy extragonadal tissues, including the placenta, umbilical cord vessels, uterus, liver, and bone (139). Hence, interest in the putative direct actions of FSH on bone has flourished, particularly in view of early observations that a decline in bone density occurs during the perimenopausal transition when circulating FSH levels are markedly raised despite preserved estrogen levels (45).
In Vitro Studies
FSHR mRNA has been detected in murine and human osteoclasts and marrow skeletal stem cells, but not in mature osteoblasts or fibroblasts (140). Consistent with this, FSH (but not LH) stimulates osteoclastogenesis from human mononuclear cell precursors in a dose-dependent manner (140). Moreover, FSH stimulates the expression of the differentiation marker tartrate-resistant acid phosphatase, but does not affect precursor proliferation, indicating that FSH may preferentially influence the differentiation rather than the proliferation of osteoclast precursors (140). Indeed, FSH upregulates 3 established osteoclastogenic pathways by enhancing the phosphorylation of Erk1/2, IκBα, and protein kinase B to simulate osteoclast formation (140).
Furthermore, FSH induces the expression of RANK on CD14+ human peripheral blood mononuclear cells (141). It is interesting to note that this occurs in a biphasic manner, such that FSH at a concentration of 10 mIU/mL (ie, similar to circulating follicular-phase FSH levels of women during reproductive years) or 100 mIU/mL (ie, FSH levels after menopause) had no significant influence on RANK expression (141). By comparison, at 50 IU/mL (ie, typical during perimenopause), FSH significantly increased RANK expression (141), tentatively providing further evidence for the role of FSH as a stimulus for osteoclast differentiation, particularly during the perimenopausal transition.
In addition to directly enhancing osteoclastogenesis as described earlier, FSH has also been implicated in modulating the activity of several proinflammatory cytokines involved in regulating osteoclastic bone resorption. Murine bone marrow cultures exposed to recombinant FSH stimulated tumor necrosis factor α production, resulting in an increase in the osteoclast precursor pool (142). In addition, isolated mononuclear cells from 36 premenopausal women incubated with exogenous FSH induced the mononuclear cells to secrete interleukin (IL)-1β (143).
In murine calvarial organ cultures using ex vivo calcein labeling, FSH did not increase calcein-labeled surface area, whereas BMP-2 as a positive control increased it by 6-fold (140). Mechanistically, this is consistent with absent FSHR on mature osteoblasts, suggesting that FSH modulates predominantly osteoclastic rather than osteoblastic activity. Moreover, FSHR in osteocytes remains to be examined.
In Vivo Nonhuman Studies
To examine the influence of FSH on bone, the in vivo effects of deleting FSH or its receptor have been examined in mice. In FSHR null females, whereas areal and volumetric BMD at both trabecular and cortical sites were indistinguishable between the mutant and ovariectomized controls, the latter group demonstrated a 15% reduction in lumbar spine areal BMD by 8 weeks compared to the mutant mice (140). Furthermore, despite severe hypogonadism (as evidenced by atrophic ovaries and thread-like uteri), FSHβ-deficient homozygous female mice did not lose bone, with both areal and volumetric BMD increased (140). Comparatively, FSHβ-deficient heterozygous females were eugonadal (as evidenced by normal ovaries and uteri) and fertile (with a 50% reduction in FSH levels), which was associated with an increase in spinal and femoral areal BMD (140). Although it may be tempting to speculate that FSH action is required for hypogonadal bone loss, it is worth bearing in mind that in this study, circulating levels of LH, estrogen, and testosterone were not reported. This is particularly relevant given that it is known that LH and testosterone both become elevated in FSHβ and FSHR null mice (144-146), which makes a definite conclusion about the direct role of FSH from these experiments somewhat uncertain. Therefore, to fully establish that complete loss of FSH signaling in FSHβ and FSHR null mice protects from bone loss (despite severe hypogonadism), ovariectomy in the mutant mice (thus eliminating gonadal sex steroids including androgens) could have been useful in this regard (147), an experiment that was performed solely in the control mice in this study (140). Possible experimental alternatives include genetic or chemical approaches to inhibit androgen secretion or gene expression in the ovaries.
To examine the effect of blocking FSH on ovariectomy-induced bone loss in mice, a 13-amino-acid-long peptide polyclonal antibody that is directed to the receptor-binding domain of the β subunit of FSH has been generated (148). The FSH antibody abolished FSH-induced osteoclast formation in vitro (148). Moreover, when administered to ovariectomized mice, it was effective in attenuating bone loss by stimulating bone formation and inhibiting bone resorption, suggesting a possible therapeutic avenue (148).
In contrast to the aforementioned studies, further experimental data reveal that a direct role for FSH in bone remains unclear. By age 3 months, a 4.9% and 5.6% reduction in femoral and lumbar spine BMD, respectively, was observed in FSHR null mice (149). It is striking that these deleterious effects were rescued by allogenic ovarian transplantation, which increased circulating estradiol levels, and reduced LH and testosterone (149), suggesting that downstream ovarian function is potentially more important for age-dependent bone loss in this model. Moreover, bilateral ovariectomy decreased elevated testosterone levels in FSHR null mice and reduced BMD to levels comparable with ovariectomized wild-type controls (149). Hence, to investigate whether elevated ovarian androgens contribute to the skeletal responses to ovariectomy, FSHR null mice were treated with the androgen receptor antagonist flutamide and the aromatase inhibitor letrozole (149). Notably, both resulted in bone volume reductions in these mice, suggesting that ovarian androgens as well as estrogens affect skeletal homeostasis independent of the action of FSH (149).
In keeping with these data, the effects of elevated FSH on bone mass and structure have been studied using transgenic female mice expressing human FSH (150), an experimental paradigm that results in increasing circulating FSH levels with age (151). In this study, increased FSH induced bone formation and increased bone mass, an effect that was observed to be dependent on ovarian function but independent of GnRH or LH activity (150). Moreover, further experiments reveal that estrogen deficiency is the dominant factor impairing bone loss in ovariectomized Wistar rats (152). Here, while FSH and LH were observed to modulate bone loss, changes in estrogen had a more powerful influence (152). Taken together, while a range of studies suggest a role for FSH in bone physiology, the reported effects may be (in part) mediated via gonadal pathways. An additional limitation of the reported experiments is that they were limited to female (not male) mice models, and so studies in male mice would be useful to examine for possible sexual dimorphisms. Approaches including FSHR-floxed mice with osteoclast-specific deletion of the FSHR may be helpful to definitively determine the physiological role of FSH in bone and reconcile these findings.
In Vivo Human Studies
Numerous observational studies report an association between increasing serum FSH levels and bone loss. The multisite, longitudinal Study of Women’s Health Across the Nation examined 2375 premenopausal and early perimenopausal women of African American, White, Japanese, and Chinese background (153). In these premenopausal and early menopausal women, higher FSH concentrations (but not other serum reproductive hormone levels) were associated with higher concentrations of the bone turnover markers urinary N-terminal telopeptide of type I collagen (NTX) and serum OC, before as well as after adjusting for covariates (including body mass index [BMI], smoking status, physical activity, and dietary intake variables, such as alcohol and calcium) (153). These observational findings suggest a possible relationship between increased levels of FSH and premenopausal increased in bone turnover. In a further observational study of 2311 premenopausal and early perimenopausal women, statistical modeling using the baseline FSH values and subsequent follow-up FSH levels predicted the 4-year BMD reduction after adjusting for various factors (such as ethnicity and baseline age) (154). In this cohort, spinal and hip BMD reduction during the menopausal transition was strongly associated with the initial FSH and follow-up FSH levels, but not with estradiol levels (154).
Given the putative relationship between both BMD and bone turnover with serum FSH in postmenopausal women, the influence of harboring certain polymorphisms in the FSH/FSHR system has been evaluated. A total of 289 postmenopausal women were genotyped for the single-nucleotide polymorphism rs6166 in exon 10 of the FSHR gene (155). In this observational study, AA rs6166 women demonstrated lower BMD at the femoral neck and total body, along with higher serum levels of ALP and C-terminal telopeptide of type 1 collagen (CTx), compared with GG rs6166 women (155). Moreover, the prevalence of osteoporosis was significantly higher in AA rs6166, an effect that was shown however to be independent of circulating levels of FSH or estrogen (155). By comparison, in the largest meta-analysis of genome-wide association studies for BMD involving 32 961 individuals of European and East Asian ancestry, 56 genome-wide loci were associated with BMD of the lumbar spine and/or femoral neck, and 14 of those were also associated with fracture risk in a case-control meta-analysis involving 31 016 fracture cases and 102 444 controls without fractures (156). This large genomics study did not identify any FSH-related signal, including in the FSHR gene. Importantly, future studies employing mendelian randomization would be informative to further interrogate a possible causal relationship between FSH and bone.
In addition to bone loss associated with the menopausal transition, the bone effects of elevated levels of FSH observed in secondary amenorrhea in women of reproductive age has been investigated. In a small observational study of 22 amenorrhoeic and 12 eumenorrheic women younger than 40 years, amenorrhoeic women had lower lumbar BMD (although no difference in femoral neck BMD) than eumenorrheic women (157). The amenorrhoeic women were then separated into 2 groups according to their FSH levels: hypergonadotropic amenorrhea (ie, FSH > 40 IU/L) and hypogonadotropic amenorrhea (ie, FSH ≤ 40 IU/L) (157). The hypergonadotropic women displayed a greater reduction in BMD than the hypogonadotropic women (157). Consistent with this, only FSH had a negative correlation with lumbar spine BMD in the hypergonadotropic group, whereas there was no correlation between FSH levels, BMI, age, or duration of amenorrhea in the hypogonadotropic group (157). Importantly, the analysis was not adjusted for age, despite the hypergonadotropic women being 7.6 years older than the hypogonadotropic women (37.43 vs 29.8 years) (157), which may in part explain some of the differences in observed BMD between the groups.
By comparison, other observational studies do not reveal an association between FSH and bone. In a study involving 137 middle-aged infertile men (due to spermatogenic failure) and 70 aged-matched healthy men with normal fertility, 15 years after infertility workup there was no difference in BMD between the 2 groups, despite a significantly higher median FSH value (9.8 vs 3.7 IU/L) (158). Importantly, total testosterone and estradiol were similar between the 2 groups at follow-up (158). Indeed, neither the baseline nor follow-up FSH levels exhibited a significant correlation with axial, femoral, or total body BMD, indicating that infertile (but eugonadal) men with high FSH levels do not have lower BMD (158). In a similar cohort involving 307 men with idiopathic infertility and 28 men with Klinefelter syndrome, serum FSH levels did not exhibit significant correlation with BMD, nor with the RANKL/OPG ratio, OPG, PTH, or OC (159). Interestingly, FSH was inversely correlated with serum levels of soluble RANKL in both cohorts of men, an effect that remained significant after adjustment for relevant nonhormonal confounders (age, body fat percentage, and smoking in the idiopathic infertility cohort, compared with age adjustment only in the Klinefelter syndrome cohort) and serum estradiol (159). Critically, while numerous observational studies suggest an association between FSH and bone, only interventional studies are able to detect a reliable direct cause-and-effect relationship between FSH and bone turnover. Indeed, in a seminal prospective study involving 21 postmenopausal women treated with the GnRH agonist leuprolide and 20 control women receiving placebo injections, both groups concurrently received the aromatase inhibitor letrozole to eliminate variations in endogenous estrogen levels (160). At 3.5 months, in response to GnRH agonist-induced suppression, serum FSH fell by 86% (into the premenopausal range), but did not change significantly in the control women (160). Notably, in the women receiving the GnRH agonist, suppression of FSH release resulted in larger increases in bone resorption markers than in controls (160). Furthermore, although there was also a small decrease in testosterone in the women administered GnRH agonist (21% reduction in an already low postmenopausal testosterone), it is unlikely to have masked any significant positive effects of FSH reduction on bone resorption. Taken together, this experimental model provides direct evidence suggesting that FSH does not modulate bone resorption markers in a postmenopausal woman.
In keeping with this interventional study, further experimental evidence in humans also suggests that FSH does not exert independent effects on bone physiology. In a study involving 29 infertile women undergoing in vitro fertilization, administration of the GnRH analogue leuprolide was followed by stimulation with recombinant FSH (rFSH) (161). In response to leuprolide-induced suppression of serum FSH and estrogen levels, bone turnover markers increased as indicated by a significant rise in serum β-CTX (161). Moreover, 3 days after the first dose of rFSH, despite serum FSH values above the reference range for the early follicular phase (with estradiol maintained in the reference range), no significant change in serum β-CTX was observed (161). By comparison, serum β-CTX was lower and FSH and estradiol levels higher 10 days after the first administration of rFSH (161). Therefore, in this experimental model, short-term administration of rFSH did not exert any significant change in the serum levels of bone turnover markers, which instead exhibited significant correlation with serum estradiol levels.
Given the experimental data examining the influence of FSH on bone turnover both in premenopausal and postmenopausal women, it is interesting to consider its effects in men. In a randomized controlled trial involving eugonadal men, participants were treated with a monthly GnRH agonist (goserelin) and topical testosterone and compared with a control group receiving placebo (162). Importantly, participants in the intervention group were individually matched with participants in the control group to ensure the mean serum testosterone and estradiol levels achieved during the treatment period did not differ between groups, therefore eliminating the confounding influence of gonadal sex steroids (162). Following 16 weeks of treatment, serum FSH fell by 60% in the intervention group and 2% in the control group (162). Despite the substantial suppression of FSH levels in the intervention group, serum levels of biochemical markers of bone resorption (serum NTX and CTX) and bone formation (serum osteocalcin) did not change (162), suggesting that in the eugonadal range, FSH does not affect bone turnover in men.To this end, the putative role of FSH as a direct modulator of bone physiology (see Figure 1) remains a controversial area (147). In vitro and in vivo animal studies have reported conflicting results regarding a direct role for FSH in the modulation of bone turnover. Given some of the inconsistencies in the literature, in vivo conditional deletion studies would be important to reconcile some of these discrepancies. In addition, while these assessments of bone turnover markers in shorter-term interventional studies are highly informative, longer-term (> 6 months) studies in women and men with comprehensive additional bone assessments could be illuminating. Ultimately, although several (but not all) observational studies in humans have produced results suggestive of FSH effects on bone turnover, the nature of these studies does not prove causality. Indeed, to date, no interventional study in humans has detected a direct cause-and-effect relationship between FSH and bone turnover.
Luteinizing Hormone
The glycoprotein hormone LH is a heterodimer consisting of a noncovalently associated α subunit common to several peptide hormones (including FSH and TSH) and a specific β subunit conferring biological specificity (163). Belonging to the cystine knot superfamily (164), LH is secreted by the anterior pituitary gland at the time of pubertal onset to promote the maturation of the reproductive system in males and females and thereafter the secretion of the gonadal reproductive hormones. LH signals through a 7-transmembrane domain G-protein–coupled receptor, the LH receptor (LHR) (165), which is expressed in skin, mammary gland, placenta, uterus, urinary bladder, prostate, adrenals, as well as osteoblast cell lines (166, 167).
Furthermore, hCG is the placental homologue of LH and an additional ligand of the LHR (168), which represents the principal circulating gonadotropin during pregnancy. Importantly, the established skeletal changes seen in puberty, pregnancy, and menopause (169) (ie, during periods of amplified levels of LH/hCG), may functionally imply that LH and hCG may participate directly in bone physiology as discussed in the following sections.
In Vitro Studies
Unlike FSH, there are fewer data about the effects of LH on bone. The presence of the LHR in extracts of primary human osteoblasts and osteoblast-like cell lines (mC3Ts-E1, MG63, and SAOS2) has been identified by Western blotting, immunolocalization, and reverse transcriptase–polymerase chain reaction (167). However, stimulation of osteoblastic LHR with hCG did not increase downstream cAMP or ERK phosphorylation, raising the possibility that osteoblasts may actually express either low receptor numbers or nonfunctional receptors (167). In this study, the presence of LHR in osteoclasts and osteocytes was not reported.
By contrast, other investigators have observed that human osteoblasts treated with a urine-derived formulation of hCG resulted in osteoblast proliferation as indicated by elevated ALP activity and increased matrix metalloproteinase 2 expression (170). In fact, although hCG alone was capable of stimulating an increase in ALP activity, cotreatment with hCG and calcitriol resulted in a 5-fold increase in ALP (170). In addition, in organ cultures of Ca45-labeled murine calvaria treatment with urinary hCG resulted in a dose-dependent release of Ca45 into the medium, suggesting a modest stimulation of osteoclastic bone resorption (170). Curiously, repeating these experiments with recombinant hCG (rather than urinary-derived hCG), resulted in no change in osteoblast activity, which might indicate the presence of contaminating agents in urine-derived hCG, an effect shown to be accounted for by the presence of epidermal growth factor (170). Taken together, these data do not robustly support that hCG influences human osteoblasts, given there was no response when nonurine-derived hCG was used. Moreover, whether LH/hCG directly influences osteoclast or osteocyte biology remains to be answered.
In Vivo Nonhuman Studies
To explore the putative role of LH/hCG on the skeleton in vivo, an LH receptor null mutant mouse model and a murine transgenic model overexpressing both hCG subunits (hCG αβ+) have been generated (167). Ablation of LHR resulted in a 43% reduction in femoral BMD by age 5 months, and histomorphometric analysis revealed reduction in cancellous bone volume, trabecular width, and number (167). Interestingly, 6-month-old male hCG αβ+ mice had comparable BMD to wild-types, and female hCG αβ+ mice exhibited approximately 30% increases both in tibial and femoral BMD, suggesting the presence of sexual dimorphism (167). It is interesting to speculate as to whether the increase in BMD represented a consequence of reduced bone resorption and/or heightened bone formation, as well as a possible synergistic effect between additional ovarian factors and increased levels of hCG. Hence, to determine if the observed increase in BMD was a direct effect of raised serum hCG, or an indirect ovarian effect, female hCG αβ+ and wild-type mice were bilaterally ovariectomized at age 3 weeks, which resulted in 36% and 33% reductions in femoral and tibial BMD, respectively (167). Collectively, these data support the role of the ovary in precipitating the increases in bone volume observed in hCG-overexpressing mice, thus confirming an indirect effect of LH on the skeleton (167). It is important to note that to date the in vitro and in vivo studies have involved the application of hCG (rather than LH itself because of LH’s much shorter half-life) since both hormones signal through the same receptor. An interesting future area of study would be to investigate whether hCG and recombinant LH have differential effects on bone physiology.
In Vivo Human Studies
The relationship between the level of serum LH and cytokines associated with skeletal homeostasis has been investigated in 694 healthy Chinese women (171). After adjusting for age, BMI, and estradiol levels, serum LH showed no significant correlation with serum cytokine levels in premenopausal women, but exhibited a significant positive correlation with OPG and transforming growth factor (TGF)-β2 in perimenopausal women (171). Additionally, in postmenopausal women, LH levels also showed a positive correlation with OPG levels, but not with TGF-β2 (171). These observations provide potential mechanistic insight into bone physiology during perimenopausal and postmenopausal periods (ie, at times of amplified LH levels with concentrations up to 10 times those found premenopause) although association does not indicate causality.
Nonetheless consistent with these findings, LH has also been shown to be positively correlated with levels of bone turnover indicators in the same cohort of Chinese women (172). Notably, while the influence of FSH was approximately 7 to 20 times greater, LH was observed to explain 2.1% and 1.1% of the changes in bone formation markers bone-specific ALP and OC, respectively, but had no apparent effect on bone resorption markers (172).
Whether these changes in cytokines and bone turnover markers exert or represent a deleterious influence on BMD has been the focus of a plethora of studies. In a cross-sectional study by the same group, in healthy Chinese women (aged 20-82 years), serum LH negatively correlated with BMD at all skeletal sites and for each 10 IU/L increase in LH levels, BMD decreased by 4.4%, 2.8%, 3.6%, and 2.4% at the posterior-anterior spine, lateral spine, total hip, and radius/ultradistal, respectively (173). This finding was also observed in a later study in Mongolian women. In 260 women (aged 50.1 ± 4.4 years), serum LH was found to be higher in women with low BMD compared to women with normal BMD (174). It is notable that BMD was measured in the forearm and tibia, rather than more commonly assessed in the hip and spine (174). By contrast, additional studies have found no association between serum LH levels and BMD. In an analysis of 36 ovulatory women (aged 20-50 years) from the United States, whereas serum FSH concentration was inversely related to BMD measures, LH was not (143). Similarly, no difference in serum LH levels was detected in 73 postmenopausal Turkish women with low vs normal femoral and lumbar BMD (175). Given the previous studies, it is possible that the latter 2 studies failed to detect an association because of their small sample sizes.
Studies have also examined the relationship between LH and BMD in men. In community-dwelling older men, a significant inverse association between longitudinal change in hip BMD and serum LH (unlike testosterone and estrogen) has been observed in both univariate and multivariate analyses, suggesting that higher LH levels at baseline was associated with greater bone loss at the hip over the subsequent 5 years (176). Correspondingly, higher LH levels correlated with increased hip and nonvertebral fractures in univariate models, an effect that did not however remain significant after adjustment for age, BMI, smoking status, physical activity, and comorbidity (176).
A recent study assessed the association between reproductive hormones and the incidence of fractures in 3307 community-dwelling Australian older men (aged 76.8 ± 3.5 years) over a median follow-up period of 10.6 years (177). Men who experienced any incident fracture had a higher LH and lower baseline testosterone than men who did not experience a fracture (177). However, once adjusted for age, medical comorbidities, and frailty, a U-shaped association between plasma testosterone and fracture was apparent, whereas there was no association with LH (177). This suggests that whereas LH stimulates testicular testosterone production, circulating testosterone (but not LH) appeared to determine fracture risk. However, the analysis was based on a single baseline blood sample rather than serial hormones measurements over time.
Taken together, the relationship between serum LH levels and BMD has been inconsistent across different populations. It is important to recognize that because these are observational studies, causality cannot be established especially in the absence of conclusive mechanistic in vitro data. In addition, larger prospective studies are necessary to elucidate the relationship between LH and BMD in greater detail and to isolate the effects mediated directly by LH. Considering the data from in vitro and in vivo experiments collectively, it is likely that LH exerts an indirect effect on bone and its effects are mediated predominantly via the downstream gonadal sex steroids (see Figure 1).
Prolactin
PRL is a peptide hormone present in all vertebrates, with the mature peptide composed of 199 amino acids (178). It is synthesized and secreted by lactotroph cells of the anterior pituitary gland, and exerts its biological actions by binding to its receptor, the PRL receptor (PRLR), a type 1 cytokine receptor (179).
Beyond the pituitary, PRL is produced by many other cells and tissues, including several brain regions, lacrimal and sweat glands, thymus, lymph nodes, breast, spleen, skin, myometrium, decidual cells of the placenta, and bone marrow (180). In the majority of these regions, the physiological role of prolactin remains to be determined. Evidence suggests that PRL participates in excess of 300 identified biological processes in various vertebrates, including lactation, reproduction, metabolism, osmoregulation, behavior, growth and development, and immune regulation and protection (181). However, patients with PRL-secreting pituitary adenomas typically present with symptoms attributable to a space-occupying lesion, HPG axis dysfunction, and/or galactorrhea (182), rather than impairments of the aforementioned functions suggesting a lesser relevant role.
In animals and humans, circulating PRL is significantly elevated during pregnancy and lactation, along with other corresponding changes in circulating estrogens and progesterone amongst others. Notably, these physiological states are recognized to induce a maternal bone-resorptive state to provide the necessary calcium for fetal and neonatal skeletal growth and development (183). As such, the potential role of PRL in bone physiology has been the focus of several studies.
In Vitro Studies
Osteoblasts express PRLR (184), providing early evidence that PRL may play a physiological role in bone physiology. In certain osteoblastic cell lines, such as human osteosarcoma cells, the expression of PRLR mRNA is strongly influenced by the presence of osteotropic factors, such as the physiological concentration of 1–25-(OH)2 vitamin D3 (185). In contrast to osteoblastic cells, evidence from rats reveals that osteoclasts and osteocytes do not express PRLR (186).
PRL is able to indirectly regulate osteoclastic activity through osteoblastic cells. Indeed, the mRNA expression of osteoblast-derived osteoclast-regulating factors has been studied using rat osteoblast-like UMR106 cells treated with PRL in various concentrations (187). mRNA expression of MCP-1 and Cox-2 were upregulated approximately 2- to 3-fold in the presence of 200 to 500 ng/mL PRL (187). Interestingly, only higher PRL concentrations of 500 ng/mL (ie, comparable to the average suckling-induced PRL surge) upregulated TNF-α and IL-1 by approximately 3-fold and approximately 2-fold, respectively, whereas M-CSF and IL-6 mRNA expressions were unaffected by 100 to 500 ng/mL PRL (187). In addition to activating osteoclastic cells by osteoblast-secreted cytokines, PRL has also been observed to upregulate the expression of ephrin-B1 approximately 2-fold after exposure to 300 ng/mL PRL (187). This latter finding suggests that PRL can also facilitate osteoblast-osteoclast communication through direct cell-cell contact using the ephrin system in vitro (187). Moreover, the MG-63 cell line, exposed to sustained pathological concentrations of PRL (up to ~1000 ng/mL), increased the RANKL/OPG ratio, triggering an increase in osteoclastic bone resorption (39). In addition to the effects of PRL-induced activation of osteoclasts through osteoblast-secreted factors, PRL has also been observed to suppress osteoblast formation itself. In the MG-63 cell line, treatment with PRL led to lower expression of ALP and OC mRNA and a decrease in ALP activity (39). Collectively, these data reveal that hyperprolactinemia may act directly on bone to stimulate bone turnover, resulting in increased bone resorption rather than formation through osteoblast-related pathways.
In Vivo Nonhuman Studies
In keeping with in vitro models, in vivo animal experiments reveal a prominent role for PRL in calcium and bone homeostasis. Studies in rats during pregnancy and lactation revealed that PRL stimulates intestinal calcium absorption (188). In fact, long-term exposure to PRL (over a period of several days) in pregnancy and lactation induced specific changes in duodenal cells by increasing the expression of genes related to transcellular transport (such as TRPV5/6 and calbindin-D9k) and paracellular transport (such as claudin-3), thereby increasing calcium absorption (188). Remarkably, during suckling the transient PRL surge increased calcium absorption within 30 minutes to match the calcium loss in milk. This effect of enhanced transcellular and paracellular calcium transport is mediated by phosphoinositide 3-kinase, protein kinase C, and RhoA-associated coiled-coil–forming kinase pathways (188). Taken together, these data reveal that PRL acts in part as a calcium-regulating hormone by stimulating intestinal calcium absorption during pregnancy, lactation, and related suckling.
Congruous to its influence on intestinal calcium absorption, PRL also has demonstrable direct effects on bone. PRLR gene–deficient mice exhibited delayed calvarial ossification (in 18.5-day-old embryos) (184). In addition, adult PRLR gene–deficient mice demonstrated a significant decrease in trabecular and cortical mineral apposition rates in the long bones (tibia and femora), along with a 60% decrease in bone formation rate, but no significant changes in the number of osteoclasts (184). Collectively, this suggests a role in bone formation with limited effects on osteoclastic bone resorption. Notably, this model also resulted in major changes in the levels of calciotropic and reproductive hormones including elevated serum PTH levels and reduced serum estradiol and progesterone (184). Hence, in addition to direct effects from PRL on bone, some of these observed effects may be attributable to secondary hormonal changes.
Indeed, the direct effects of prolactin on bone physiology are frequently difficult to dissect because of the complex hormonal changes from hyperprolactinemia-induced hypogonadism in vivo (189, 190). To overcome this, anterior pituitary allografts have been transplanted under the renal capsule of recipient female rats with or without ovariectomy (39). Within 15 days of transplantation, continuous PRL secretion from the ectopic pituitary glands resulted in PRL levels of 91 ng/mL (equivalent to pregnancy levels in rats), but without increasing other pituitary hormones because of the absence of upstream stimulatory hypothalamic signals (39). Whereas femoral BMD and bone mineral content were unaffected, histomorphometric studies indicated enhanced bone resorption with decreases in bone volume and trabecular number, whereas trabecular separation, and the osteoblast and osteoclast surfaces were increased (39). Interestingly, the presence of high physiological PRL levels (ie, 90-100 ng/mL) may have resulted in extra calcium from enhanced intestinal calcium absorption, therefore contributing to the observed increase in the mineralization process, which in turn preserved the BMD. Crucially, estrogen supplementation did not restore the effect of estrogen deficiency in the pituitary allograft plus ovariectomy rats, suggesting estrogen-independent effects of PRL (39).
When taking into consideration the data both from in vitro and in vivo experiments, it appears that PRL exerts direct effects on bone remodeling such that during periods of hyperprolactinemia, bone remodeling is stimulated with increased bone resorption and possibly decreased bone formation. Although the aforementioned data suggest direct effects, the presence of other reproductive hormone–dependent effects is plausible. Future studies employing techniques such as cell-specific gene targeting (eg, for the osteoblastic PRLR) may help delineate the precise direct effects of PRL further.
In Vivo Human Studies
The relationship between hyperprolactinemia and bone loss secondary to increased bone resorption has been the focus of a plethora of human studies. In a small study of 20 hyperprolactinemic men, although the majority had low BMD, 4 patients were found to have normal BMD both at the lumbar spine and femoral neck (40). Serum OC levels were lower, whereas urinary NTX levels were higher than the reference range in all the hyperprolactinemic men (40), suggesting that alterations in bone turnover may occur even before changes in BMD become apparent in men with hyperprolactinemia. In a subsequent analysis, a significant negative correlation was found between serum OC and PRL levels and disease duration, and a significant positive correlation between the urinary NTX and PRL levels and disease duration (42). Patients with hyperprolactinemia exhibit decreased bone mass at skeletal sites enriched with trabecular (such as the spine and hip) rather than cortical bone (such as the distal radius) (22, 41, 191). Consistent with this, additional studies of patients with hyperprolactinemia have demonstrated that vertebral BMD decreases by 20% to 30%, while forearm BMD decreases by 2.5% to 10% (192). In addition, the deleterious effects on skeletal health are more marked when hyperprolactinemia develops at a younger age, owing to the decreased peak bone mass. In keeping with this, in a study comparing 20 patients with hyperprolactinemia with disease onset during adolescence and 20 patients with disease onset during adulthood, BMD was significantly lower in younger compared to older adult patients both at the lumbar spine and femoral neck, highlighting the important clinical need to address hyperprolactinemia in adolescence to ensure optimal peak bone mass acquisition (42).
The prevalence of skeletal fractures in patients with hyperprolactinemia has been investigated in a number of studies. In a series of 86 patients with prolactinomas, the excess fracture risk before diagnosis was observed to be 60% higher compared with healthy controls (193). Furthermore, in a study of 32 men with prolactinomas (10 with microadenomas and 22 with macroadenomas), vertebral fractures were identified in 37% of the men, compared with 8% of age-matched healthy controls (43). In fact, bone fractures occurred more frequently in those patients with a longer duration of disease and in patients with untreated hyperprolactinemia (43). Interestingly, the prevalence of vertebral fractures was not different between eugonadal and hypogonadal patients, nor was there a difference in serum testosterone between fracture and nonfracture groups (43), suggesting a gonadal-independent effect on fracture risk.
Consistent with the high prevalence of radiological vertebral fractures in men with prolactinomas, parallel studies in women reveal a similar susceptibility to skeletal fracture. In a cross-sectional study of 78 women with prolactinomas, vertebral fractures occurred in 32% of patients, compared with 13% of age-matched healthy controls (44). Patients with fracture were older, had lower BMD, longer duration of disease, higher serum PRL, and lower insulin-like growth factor 1 levels compared to women without bone fracture (44). Similar to men, bone fractures occurred more frequently in women with untreated hyperprolactinemia compared with patients treated with dopamine agonists (44).
Adequate treatment of hyperprolactinemia and resultant hypogonadism rescues bone loss; however, recovery of BMD is only partial. In a study of 20 hyperprolactinemic men, despite restoration of testosterone and suppression of PRL, serum OC levels were normalized, whereas neither urinary NTX levels nor BMD values were normalized after 18 months of treatment with dopaminergic agents (40). Importantly, despite correction of serum PRL levels after 6 to 12 months of medical treatment, the improvement in BMD both at 12 and 24 months of treatment remained reduced in patients with disease onset during adolescence than onset during adulthood (42).
Taken together, these studies demonstrate direct and indirect roles for PRL in bone physiology. However, from a clinical perspective, there remain many unknowns. First, although restoration of normal PRL and gonadal function improves BMD, this is not always associated with complete normalization of BMD, emphasizing the clinical importance of early treatment of hyperprolactinemia. Second, the effect of treatments for hyperprolactinemia on BMD and fracture risk has limited data as yet (40, 42). Third, most clinical evidence for a negative effect of hyperprolactinemia on bone is derived from patients with prolactinomas, despite medication-induced hyperprolactinemia representing the most frequent cause of nonphysiological hyperprolactinemia (194), principally from neuroleptics and antipsychotic agents (195). However, there are some studies of medication-induced hyperprolactinemia implicating it in reduced bone density and increased fracture risk (196, 197). Further research in this area is warranted to inform more robust clinical guidelines regarding the use of these medications from a bone health perspective (198). Finally, many data in humans have been derived from small, heterogeneous, and cross-sectional or retrospective studies, which are likely to affect the generalizability of the results. To improve the granularity of the data, larger, multicenter and prospective studies are warranted, although the importance of PRL in overall bone physiology remains unquestionable (see Figure 1).
Progesterone
Most of the focus on gonadal reproductive hormones involves estrogens and androgens. However, progesterone is another critical gonadal hormone acting in tandem with estrogens as a requisite hormone for maintaining fertility (199). Progesterone is produced in the gonads and adrenal glands of both sexes. As with other gonadal steroids, progesterone is synthesized from pregnenolone, which itself is derived from cholesterol (200).
In Vitro Studies
Within the bone microenvironment, osteoblasts and osteoclasts both express the progesterone receptor (PR) (201, 202). Furthermore, PR expression on osteoblasts can be stimulated by estrogen, thus it is possible that some of the effects on bone physiology attributed to estrogen may be mediated in part via enhanced progesterone signaling (201, 203, 204).
While estrogen’s role in decreasing bone resorption is an important factor for maintaining bone health, there is evidence that progesterone contributes to bone formation through its actions on osteoblastic cells. Low physiologic doses of progesterone increased osteoblastic production of TGF-β1, TGF-β2, and TGF-β3 mRNA (205) and bone-specific ALP (205). Importantly, this effect was seen independently of pretreatment or cotreatment with estrogen (206). Progesterone also regulates the function of metalloproteinases in cultures of human osteoblast-like cells, which may have local effects on osteoblastogenesis or matrix remodeling (205).
The effects of progesterone on osteoclasts and osteocytes have not been well explored to date, although PR has been identified on osteoclasts (202) and chondrocytes (207).
In Vivo Nonhuman Studies
To address whether PR signaling is requisite for normal bone growth and turnover, global knockout (PRKO) mice with deletions both in PR A and B isoforms have been studied. Histomorphometric and microcomputed tomography analyses demonstrated normal longitudinal bone growth at the tibia (208, 209); however, total trabecular and cortical bone mass were increased at other skeletal sites such as the humerus and distal femur (209, 210), suggesting that at certain sites PR signaling attenuates the accumulation of cortical and trabecular bone mass during periods of rapid bone growth, such as adolescence. In general, PRKO mice do not typically exhibit alterations in circulating levels of gonadal sex steroids; however, loss of PR signaling is known to affect other upstream hormones including PRL and LH (199). Furthermore, male PRKO mice display significantly lower FSH levels, whereas female PR knockout mice have higher inhibin A levels (210). Therefore, it is important to consider these additional hormonal consequences of PR deletion and their confounding impact on bone physiology.
In Vivo Human Studies
Postmenopausal estrogen deficiency is a major risk factor for osteoporosis; however, it is now established that estrogen and progesterone work in tandem (206). Data from the Women’s Health Initiative demonstrate that estrogen plus progesterone (compared to placebo) taken for an average of 5.6 years reduces the risk of fractures in postmenopausal women (hazard ratio, 0.76 overall) (211). A more recent meta-analysis of randomized controlled trials of more than 1000 menopausal women reported that estrogen plus progesterone therapy resulted in a superior increase in lumbar BMD compared to estrogen therapy alone (212), highlighting an additive action for progesterone.
During states of estrogen deficiency such as amenorrhea, and surgical and physiological menopause, low progesterone is almost indistinguishable temporally from low estrogen levels, making it difficult to isolate its effects. However, progesterone deficiency also occurs silently in conditions of subclinical ovulatory disturbance (SOD) whereby ovulation is disturbed with shorter luteal phases but normal cycle length and preserved estrogen levels (213). SOD therefore provides a useful context for the study of progesterone effects on female bone (214). Premenopausal women with lower BMD exhibit significantly lower progesterone levels despite regular cycles and frequently normal estrogen levels (213). Furthermore, studies of healthy premenopausal women have found levels of bone formation and resorption markers change across the menstrual cycle with increased markers of bone formation and higher osteoblastic activity occurring during the (progesterone-rich) luteal phase. However, most of these studies have not been able to differentiate ovulatory from anovulatory cycles, which limits the interpretation of their findings.
Interventional studies assessing the use of cyclical oral progesterone to provide luteal phase support in SOD have demonstrated some benefits in lumbar BMD after 1 year (cyclic medroxyprogesterone plus calcium +1.7% vs placebo plus calcium –0.7%) at doses that do not suppress endogenous estrogen production (215). While cyclical progesterone provides an interesting and potentially novel therapeutic option for women at risk of bone-related complications of SOD, caution should be applied given the highly heterogeneous nature of patients presenting with SOD with variable energy expenditure, physical activity, and diet (213). Further interventional studies in this area may be useful in elucidating the role and therapeutic potential of progesterone in premenopausal bone health. These would be particularly useful given that by contrast to the aforementioned preclinical and interventional studies identifying positive effects, depot medroxyprogesterone acetate at contraceptive doses has consistently been shown to result in reversible BMD loss in longitudinal studies (216, 217) and possible increased fracture risk in observational studies (218, 219). However, these findings likely represent the effects of supraphysiological progestin levels that decrease levels of associated beneficial hormones (particularly estrogen).
The importance of progesterone in male physiology is less well studied; however, progesterone is known to suppress LH and testosterone in men although there is also evidence that progesterone therapy markedly increased BMD in a trial involving 23 steroid-dependent asthmatic men (220). However, further studies in this area are required to categorically isolate the effects of progesterone on bone physiology from associated hormonal confounders, potentially through the development of PR-floxed mice with osteoblast-specific deletion of the PR.
Relaxin
Relaxin (RLN) is a member of the insulin-like peptide superfamily (221), which consists of 7 peptides of high structural similarity. During pregnancy, it is produced by the corpus luteum and placenta, hence it is traditionally recognized as a pregnancy hormone with significant roles in promoting cervical softening and elongation of the pubic symphysis to facilitate birth during the peripartum (222). There are 7 established relaxin family peptides (RXFP), including relaxin (RLN)1, RLN2, RLN3, and insulin-like peptide (INSL)3, INSL4, INSL5, and INSL6 (223). Relaxin mediates its actions by binding to and activating the G-protein–coupled RXFP receptors with RLN1, RLN2, and RLN3 the ligands for RXFP1, RXFP2 and RXFP3, respectively. In addition, INSL3 signals through RXFP2.
In Vitro Studies
Expression of RXFP1 mRNA and transcripts have been detected in primary cell cultures of human osteoclasts by reverse transcriptase–polymerase chain reaction and immunofluorescence analyses (224). In the same study, relaxin was shown to induce the differentiation of peripheral blood mononuclear cells into mature osteoclasts, providing early evidence for the role of relaxin in osteoclastogenesis (224). Furthermore, relaxin itself is not produced by human osteoclasts (225), signifying that it acts on osteoclasts as a circulating endocrine factor, and not as an autocrine/paracrine mediator.
Relaxin induces the expression of classical stimulators of osteoclastogenesis implicated in the differentiation, survival, and activation of osteoclasts, including RANK, NF-κB, NFATc1, and tartrate-resistant acid phosphatase (225). Notably, relaxin induces the expression of the cysteine protease CTSK, a key enzyme produced by mature osteoclasts and involved in the resorption of the organic matrix of bone (225).
In addition to the data underscoring the role of relaxin as an osteoclast-activating factor increasing bone resorption, in vitro studies have examined its effect on osteoblastic cells. Treatment of the mouse calvarial osteoblast cell line MC3T3-E1 with recombinant human RLN1 increased ERK1/2 phosphorylation and increased the expression of Runx2 and ALP while inhibiting OPG and RANKL expression (226). As a consequence of long-term exposure, relaxin inhibited collagen synthesis and enhanced matrix metalloproteinase activity (226).
Relaxin has been shown to synergistically augment BMP-2–induced osteoblast differentiation and bone formation in vitro by upregulation of Runx2 expression and activity (227). In fact, a recent study examined the therapeutic application of relaxin as an enhancer of BMP-2 for bone regeneration, identifying that a combination of relaxin and BMP-2 significantly reduced the BMP-2 dose required to regenerate an equivalent amount of bone in rats (228).
RXFP2 mRNA expression and protein have been detected in the osteosarcoma cell line MG-63 and also in primary osteoblast cell culture (229). Interestingly, while RXFP2 expression in human osteoblasts is approximately 20 times lower than that of PTH receptor type 1, the level of expression in these cells is 20% higher than that seen in the testis (the primary site of INSL3 [which signals through RXFP2] production) (229). Human osteoblasts respond in a dose- and time-dependent manner to INSL3 with respect to cAMP production and proliferation (229). Mechanistically, in cultured osteoblast progenitor cells, INSL3 treatment significantly induced ALP activity (230). Recent data also demonstrate that the INSL3/RXFP2 system acts on the MAPK cascade and stimulates the transcription of important genes of osteoblast maturation/differentiation and osteoclastogenesis (230).
Finally, expression both of Rxfp1 and Rxfp2 has been detected in the osteocytes lining the trabecular formations of developing mouse calvarial bones (226). However, unlike the more defined roles in osteoblast and osteoclast biology, no effect of relaxin on osteocytes has been reported to date (231).
In Vivo Nonhuman Studies
Building on the in vitro data highlighting the role for INSL3 in bone physiology, RXFP2-deficient mice have been generated. Bone histomorphometric and microcomputed tomography analyses at the lumbar and femoral sites revealed diminished bone mass and altered trabecular organization (229). Furthermore, the mineralizing bone was reduced, resulting in a lower bone formation rate (229). In addition, the number of osteoclasts was maintained, but the osteoclast surface was reduced, signifying impaired osteoclast differentiation (229). Collectively, these findings suggest that the low bone mass in mutant mice is attributable to impaired bone formation and a negative balance between bone formation and resorption.
A recent study generated gene knockouts for RLN1 by CRISPR-Cas9 technology (232). Newborn mutant pups exhibited small skeletal size at birth when compared with wild-type littermates, whereas adult mutant mice (age 12 weeks) grew normally and showed normal bone density (232). This may imply that RLN1 is involved predominantly in prenatal bone development and has less of an effect on bone remodeling in postnatal bone.
Given its influence in stimulating local angiogenesis, vasculogenesis, and osteogenesis, further animal experiments have investigated the potential therapeutic benefit of relaxin in accelerating bone fracture healing. Relaxin treatment did not accelerate closure of calvarial defects in mice, despite administering physiological to supraphysiological range doses to different mouse ages (3-4 and 13-14 months) and allowing for different investigational durations (233). On the other hand, relaxin appeared to enhance trabecular bone growth in an uninjured control bone (femur) (233). This may suggest an enhancing effect of relaxin on bone formation. Future studies are warranted using different experimental models of bone fracture and different species. However, to provide definitive data for the role of relaxin in bone physiology (see Figure 1), studies involving osteoblast-specific deletion of the relaxin receptor expressed on osteoblasts (induced in adulthood) are necessary, although the human study discussed later provides promising (although not osteoblast-specific) data.
In Vivo Human Studies
Building on the data illustrating that disruption of RXFP2 signaling is associated with reduced bone mass in mice, a similar phenotype has been identified in humans. In a study of 25 young men (age 27-41 years) harboring a T222P mutation in the RXFP2 gene, 64% were found to have significantly reduced bone density, despite normal levels of testosterone and gonadal function and no other causes for reduced bone mass (229). Of significance, BMD levels of the femoral neck and lumbar spine revealed osteopenia in 10 participants and osteoporosis in 6 participants (229). Hence, this study provides an interesting link between the RXFP2/INSL3 hormonal system and bone mass. Future clinical studies may provide further insight into the phenotype in carriers of pathogenic variants in FXFP2 that may advance our understanding of the effects of this signaling-system on bone, such as by assessments of bone turnover markers and dynamic histomorphometry. Whether the relaxin-bone pathway can be manipulated for therapeutic purposes in humans remains to be explored.
Activin and Inhibin
Activin and inhibin are structurally related regulatory glycoprotein hormones with diametrically opposing biological effects on reproductive function. Activin, a member of the TFGβ superfamily, consists of 3 homodimer protein complexes linked by disulfide bridges: β Aβ A (activin A), β Aβ B (activin AB), and β Bβ B (activin B) (234). Inhibin, also belonging to the TFGβ superfamily, consists of 2 heterodimeric protein complexes: αβ A (inhibin A) and αβ B (inhibin B) (235). Inhibins are endogenous antagonists of activin-signaling governing pituitary FSH secretion and normal gonadal function.
Similar to the other TFGβ superfamily members, activin signals are transmitted through 2 types of transmembrane serine/threonine kinase receptors: activin type I receptors (ie, ACVR1, ACVR1B, and ACVR1C) and type II receptors (ie, ACVR2A and ACVR2B). Initially, activins bind to a type II receptor, which results in the recruitment, phosphorylation, and activation of type I receptor (236). Inhibin antagonizes activin signaling through displacement of activin by binding to type II receptors through its β subunits, without leading to phosphorylation of type I receptors (237, 238).
Activins are produced in many organs, including the gonads, pituitary gland, and placenta (239, 240). In men, inhibin is secreted primarily from the Sertoli cells, whereas in women, it is produced more widely including by the granulosa and theca cells of the ovary, as well as the pituitary gland and placenta (241). Outside of reproduction, both hormones have been shown to participate in a broad range of biological processes, including regulation of energy metabolism, inflammation, and skeletal homeostasis (242). Regarding the latter, abnormal activin signaling is implicated in certain skeletal disorders. In particular, mutations in the ACVR1 gene causes fibrodysplasia ossificans progressiva, a rare disorder characterized by abnormal development of bone in skeletal muscle and connective tissue (termed heterotopic ossification) (243, 244), further illustrating that activin signaling plays a role in skeletal homeostasis.
In Vitro Studies
Using fetal-rat calvarial osteoblastic cultures, recombinant human activin A stimulated cell proliferation and both collagen and noncollagen protein synthesis (245). In primary murine bone marrow cultures, activin has a stimulatory effect both on osteoblasts and osteoclasts, whereas inhibin exhibited opposite effects (246). The stimulatory effect of locally produced activin on osteoblast and osteoclast differentiation does not, however, override the suppressive effects of inhibin produced by the gonads (246). That the suppressive effect of inhibin is maintained in the presence of activin or BMP (246) suggests the presence of a distinct inhibin-specific receptor. In addition, ACVR2A and ACVR2B have also been detected within murine cortical and trabecular bone osteocytes (247).
Consistent with observations in murine cells, both inhibin A and inhibin B suppress osteoblastogenesis and osteoclastogenesis in human skeletal and hematopoietic progenitor cells (248). Collectively, these data reveal that inhibins have direct negative effects on osteoblast and osteoclast differentiation and these effects are consistent in human and murine in vitro models.
In stark contrast to activin stimulatory effects in murine bone marrow cultures discussed above (246), in several human osteoblast models, activin treatment dose-dependently inhibits matrix protein production and suppresses in vitro mineralization through autocrine signaling (249). This may reflect species differences or differences in the in vitro test system.
In Vivo Nonhuman Studies
A number of studies demonstrate stimulatory effects of activin on bone formation in vivo. In neonatal rat calvaria, daily periosteal injections of activin promoted bone formation in a dose- and time-dependent manner (250). Furthermore, the effects of activin (administered intramuscularly 3 times a week for 12 weeks) in aged ovariectomized rats has been examined. In this study, activin markedly increased lumbar vertebral bone mass, mechanical strength, and compression strength of the vertebral body 1.5-fold (251). Moreover, activin did not affect the urinary excretion of deoxypyridinoline, suggesting that activin enhanced bone formation rather than inhibited bone resorption (251). In keeping with these data, using a rat fibula fracture model, activin injected daily into the fracture site enhanced callus formation in a dose-dependent manner (252). In fact 3 weeks of treatment increased the mechanical strength of the healed fractured bone, and histological analysis revealed that activin promoted bone formation (252). Collectively, these in vivo studies support a stimulatory role for activin on osteoblast activity.
In a transgenic mouse model engineered to overexpress liver-derived human inhibin A, the resultant continuous inhibin A exposure led to increased total BMD, bone volume, and increased biomechanical properties at the proximal tibia (253). In fact, inhibin A also prevented gonadectomy-induced loss of BMD and bone volume and strength both at the spine and proximal tibia in male mice (253), suggesting in this model that inhibin may have an even larger influence than sex steroids in regulating bone mass. Also, inhibin A increased mineral apposition rate, and serum OC levels in vivo and osteoblastogenesis in ex vivo cultures without affecting osteoclast number or activity (253). Taken together, these data suggest that the effects from inhibin A are mediated through bone formation rather than effects on osteoclasts. Whether the increase in bone formation without overt change in osteoclast activity reflects the uncoupling of bone remodeling remains to be investigated. These results seemingly contradict the aforementioned suppressive effects of inhibin A in vitro (246, 248), which may imply that the endocrine effects of continuous exposure in vivo may override the direct suppressive effects of inhibin A treatment on osteoblastogenesis in vitro. Further studies are required for clarification.
In Vivo Human Studies
In a cross-sectional, age-stratified study of 188 premenopausal and postmenopausal women, serum inhibin A and inhibin B levels were found to negatively correlate with bone formation (ie, serum ALP) and to a lesser extent resorption markers (ie, serum CTx and 24-hour urinary levels of pyridinoline and deoxypyridinoline of type I collagen) in premenopausal women and women of perimenopausal age (45-54 years) (248). In postmenopausal women, inhibin A (but not inhibin B) negatively correlated with bone formation markers (248). Furthermore, using multivariate analyses, premenopausal serum inhibin A levels exhibited a stronger correlation with bone formation and resorption markers compared with either FSH or estradiol (248), suggesting circulating inhibin A levels may be used as a clinical biomarker of high bone turnover and bone loss before detectable changes in estrogen levels.
Consistent with these data, in a study of 87 regularly menstruating women aged 35 to 50 years, decreased inhibin B levels were highly related to bone resorption, as indicated by increased excretion of urinary NTX (254). Furthermore, multivariate regression analysis revealed that serum inhibin B levels were also an independent contributor of lumbar bone BMD (254), providing further evidence for the role of inhibin in bone physiology.
Taken together, these data demonstrate the positive bone effects of activin and a crucial role for inhibins in bone physiology, seemingly independent of other reproductive hormones (see Figure 1). Indeed, these data suggest a role for the early decrease in inhibins as the result of early ovarian failure, as a mechanism driving the observed perimenopausal-accelerated bone turnover that precedes overt decreases in circulating estrogens. Based on these in vitro and in vivo findings, inhibin may therefore serve as a novel biomarker of bone loss in women with early follicular-phase blood collection likely to be most useful (255) and warrants further study in larger cohorts. Moreover, unlike inhibin, activin has been less studied in human bone physiology, hence underscoring the need for further studies in humans—particularly so given that activin signaling has been proposed as a novel and emerging therapeutic target for osteoporosis (256-258) that requires further validation in human studies.
Conclusion
Skeletal homeostasis and the process of bone remodeling depends on the tightly regulated coupling of bone resorption and bone formation. While it is traditionally accepted that gonadal sex steroids play the key role in bone physiology, recent advances have demonstrated an important role of other reproductive hormones in bone physiology that is independent of their role in reproduction, as discussed in this review (see Figure 1). Our aim has been to present an overview of the literature into a single comprehensive assessment of the current state of the field. Kisspeptin, the master regulator of the HPG axis, influences osteoblast differentiation, with recent data indicating its role as a key mediator in a neuroskeletal circuit in the arcuate nucleus controlling bone formation. Although limited data exist regarding the direct effects of GnRH, emerging results from canine models reveal its participation in bone remodeling, potentially through interplay with the serotonergic system (although this is based on osteosarcoma cell lines that are not representative of normal bone cells). While numerous observational studies in humans have produced results suggestive of direct FSH effects on bone, no human interventional study has detected a direct cause-and-effect relationship between FSH and bone turnover. LH is likely to have minimal influence on skeletal homeostasis, with its effects predominantly occurring via gonadal sex steroid changes. The direct influence of PRL to stimulate bone turnover, with a greater involvement in bone resorption than formation, is now well established and included in hyperprolactinemia-management decision making. Finally, the roles for RLN, activin, and inhibin in bone physiology are increasingly recognized.
Crucially, many questions remain unanswered with a significant amount of knowledge gained purely from in vitro models relying on cell lines or transformed cells. Despite these valuable studies, very little is known regarding the effects of reproductive hormones on osteocyte biology. Furthermore, the complex interrelationship between the HPG hormones themselves makes isolating their independent effects difficult experimentally, although these have been successfully overcome by several studies mentioned in the present review. Indeed, despite the multiplicity of in vitro studies, clear mechanisms and pathway data remain to be fully defined and replicated. In an effort to circumvent the inherent limitations associated with in vitro experiments, further in vivo studies are necessary to establish the effects of reproductive hormones on the bone microenvironment and downstream signaling and to examine for sexual dimorphisms, as well as additional studies in nonmurine animals to clarify species differences. Notably, in vivo studies frequently examine global knockout or transgenic mice resulting in long-term developmental consequences and systemic sequelae that may confound interpretation of the effects of reproductive hormones on bone. Finally, it is also important to recognize that a significant proportion of human data comes from observational studies that simply report associations and are limited in numbers, therefore causality cannot be fully inferred.
An important way to address these fundamental problems will be the development of in vivo models with inducible cell-specific deletion of key receptors in bone cells to evaluate the roles of reproductive hormones signaling pathways in bone. This has the ability to determine whether certain reproductive hormones other than estrogen and testosterone have physiologically important direct roles in the skeleton, with important therapeutic implications.
In summary, while the study of several reproductive hormones remains in its infancy and many mechanistic details remain to be identified, accumulating evidence highlights a wide range of reproductive hormones beyond estrogens and androgens as key components of the physiological endocrine inventory that regulates skeletal homeostasis. To this end, these data emphasize the existing and emerging therapeutic possibilities of related manipulations of the HPG system and its constituents for the prevention and treatment of metabolic bone diseases.
Acknowledgments
This article presents independent research funded by the Medical Research Council (MRC) and supported by the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre and NIHR Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the MRC, the NIHR, or the Department of Health.
Financial Support: E.G.M. and L.Y. are funded by MRC Clinical Research Training Fellowships (Nos. MR/T006242/1 and MR/R000484/1), W.S.D. is funded by an NIHR Research Professorship (NIHR No. RP-2014-05-001), and A.N.C. is supported by the NHS.
Glossary
Abbreviations
- ALP
alkaline phosphatase
- BMD
bone mineral density
- BMI
body mass index
- BMP
bone morphogenetic proteins
- cAMP
3′,5′-cyclic adenosine 5′-monophosphate
- Ctx
C-terminal telopeptide of type 1 collagen
- Dlx5
distal-less homeobox
- ERα/β
estrogen receptor alpha/beta
- ERK
extracellularly regulated kinase
- FSH
follicle-stimulating hormone
- FSHR
follicle-stimulating hormone receptor
- GnRH
gonadotropin-releasing hormone
- GnRHR
gonadotropin-releasing hormone receptor
- hCG
human chorionic gonadotropin
- HPG
hypothalamic-pituitary-gonadal
- IL
interleukin
- INSL
insulin-like peptide
- KISS1
kisspeptin gene
- LH
luteinizing hormone
- LHR
luteinizing hormone receptor
- MAPKs
mitogen-activated protein kinases
- M-CSF
macrophage colony-stimulating factor
- mRNA
messenger RNA
- NF-κB
nuclear factor-κB
- NFATc1-4
nuclear factor of activated T-cells, cytoplasmic 1-4
- NTX
N-terminal telopeptide of type I collagen
- OC
osteocalcin
- OPG
osteoprotegerin
- Osx
osterix
- PR
progesterone receptor
- PRKO mice
global knockout mice with deletions in PR A and B isoforms
- PRL
prolactin
- PRLR
prolactin receptor
- PTH
parathyroid hormone
- RANKL
receptor activator of nuclear factor-κB ligand
- rFSH
recombinant follicle-stimulating hormone
- RLN
relaxin
- RXFP
relaxin family peptides
- Runx2
runt-related transcription factors 2
- SOD
subclinical ovulatory disturbance
- TGF
transforming growth factor
- TSH
thyroid stimulating hormone
Additional Information
Disclosures: E.G.M., L.Y., M.F.N., M.K., and A.N.C. have nothing to disclose. W.S.D. has undertaken consultancy work for Myovant Sciences Ltd and KaNDy Therapeutics.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Riddle RC, Clemens TL. Bone cell bioenergetics and skeletal energy homeostasis. Physiol Rev. 2017;97(2):667-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dwyer DS, Horton RY, Aamodt EJ. Role of the evolutionarily conserved starvation response in anorexia nervosa. Mol Psychiatry. 2011;16(6):595-603. [DOI] [PubMed] [Google Scholar]
- 3. Willems HME, van den Heuvel EGHM, Schoemaker RJW, Klein-Nulend J, Bakker AD. Diet and exercise: a match made in bone. Curr Osteoporos Rep. 2017;15(6):555-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Silvestris E, Lovero D, Palmirotta R. Nutrition and female fertility: an interdependent correlation. Front Endocrinol (Lausanne). 2019;10:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305-317. [DOI] [PubMed] [Google Scholar]
- 6. Elefteriou F, Ahn JD, Takeda S, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514-520. [DOI] [PubMed] [Google Scholar]
- 7. Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122(5):803-815. [DOI] [PubMed] [Google Scholar]
- 8. Hansen MSS, Tencerova M, Frølich J, Kassem M, Frost M. Effects of gastric inhibitory polypeptide, glucagon-like peptide-1 and glucagon-like peptide-1 receptor agonists on bone cell metabolism. Basic Clin Pharmacol Toxicol. 2018;122(1):25-37. [DOI] [PubMed] [Google Scholar]
- 9. Schiellerup SP, Skov-Jeppesen K, Windeløv JA, et al. Gut hormones and their effect on bone metabolism. potential drug therapies in future osteoporosis treatment. Front Endocrinol (Lausanne). 2019;10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dimitri P, Rosen C. The central nervous system and bone metabolism: an evolving story. Calcif Tissue Int. 2017;100(5):476-485. [DOI] [PubMed] [Google Scholar]
- 11. Kanis JA, Melton LJ III, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9(8):1137-1141. [DOI] [PubMed] [Google Scholar]
- 12. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393(10169):364-376. [DOI] [PubMed] [Google Scholar]
- 13. Tu KN, Lie JD, Wan CKV, et al. Osteoporosis: a review of treatment options. P T. 2018;43(2):92-104. [PMC free article] [PubMed] [Google Scholar]
- 14. Eastell R, O’Neill TW, Hofbauer LC, et al. Postmenopausal osteoporosis. Nat Rev Dis Primers. 2016;2:16069. [DOI] [PubMed] [Google Scholar]
- 15. Sattui SE, Saag KG. Fracture mortality: associations with epidemiology and osteoporosis treatment. Nat Rev Endocrinol. 2014;10(10):592-602. [DOI] [PubMed] [Google Scholar]
- 16. Clynes MA, Harvey NC, Curtis EM, Fuggle NR, Dennison EM, Cooper C. The epidemiology of osteoporosis. Br Med Bull. 2020;133(1):105-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Civitelli R, Ziambaras K. Calcium and phosphate homeostasis: concerted interplay of new regulators. J Endocrinol Invest. 2011;34(7 Suppl):3-7. [PubMed] [Google Scholar]
- 18. De Toni L, De Filippis V, Tescari S, et al. Uncarboxylated osteocalcin stimulates 25-hydroxy vitamin D production in Leydig cell line through a GPRC6a-dependent pathway. Endocrinology. 2014;155(11):4266-4274. [DOI] [PubMed] [Google Scholar]
- 19. de Angelis C, Galdiero M, Pivonello C, et al. The role of vitamin D in male fertility: a focus on the testis. Rev Endocr Metab Disord. 2017;18(3):285-305. [DOI] [PubMed] [Google Scholar]
- 20. Lorenzen M, Boisen IM, Mortensen LJ, Lanske B, Juul A, Blomberg Jensen M. Reproductive endocrinology of vitamin D. Mol Cell Endocrinol. 2017;453:103-112. [DOI] [PubMed] [Google Scholar]
- 21. Panay N, Anderson RA, Nappi RE, et al. Premature ovarian insufficiency: an International Menopause Society white paper. Climacteric. 2020;23(5):426-446. [DOI] [PubMed] [Google Scholar]
- 22. Cann CE, Martin MC, Genant HK, Jaffe RB. Decreased spinal mineral content in amenorrheic women. JAMA. 1984;251(5):626-629. [PubMed] [Google Scholar]
- 23. Hamoda H; British Menopause Society and Women’s Health Concern . The British Menopause Society and Women’s Health Concern recommendations on the management of women with premature ovarian insufficiency. Post Reprod Health. 2017;23(1):22-35. [DOI] [PubMed] [Google Scholar]
- 24. Anasti JN, Kalantaridou SN, Kimzey LM, Defensor RA, Nelson LM. Bone loss in young women with karyotypically normal spontaneous premature ovarian failure. Obstet Gynecol. 1998;91(1):12-15. [DOI] [PubMed] [Google Scholar]
- 25. Popat VB, Calis KA, Vanderhoof VH, et al. Bone mineral density in estrogen-deficient young women. J Clin Endocrinol Metab. 2009;94(7):2277-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davies MC, Hall ML, Jacobs HS. Bone mineral loss in young women with amenorrhoea. BMJ. 1990;301(6755):790-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gibbs JC, Williams NI, De Souza MJ. Prevalence of individual and combined components of the female athlete triad. Med Sci Sports Exerc. 2013;45(5):985-996. [DOI] [PubMed] [Google Scholar]
- 28. Warren MP, Brooks-Gunn J, Hamilton LH, Warren LF, Hamilton WG. Scoliosis and fractures in young ballet dancers. Relation to delayed menarche and secondary amenorrhea. N Engl J Med. 1986;314(21):1348-1353. [DOI] [PubMed] [Google Scholar]
- 29. Laitinen EM, Hero M, Vaaralahti K, Tommiska J, Raivio T. Bone mineral density, body composition and bone turnover in patients with congenital hypogonadotropic hypogonadism. Int J Androl. 2012;35(4):534-540. [DOI] [PubMed] [Google Scholar]
- 30. Li CX, Tang ST, Zhang Q. Changes in bone mineral density and metabolic parameters after pulsatile gonadorelin treatment in young men with hypogonadotropic hypogonadism. Int J Endocrinol. 2015;2015:324524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Finkelstein JS, Klibanski A, Neer RM, et al. Increases in bone density during treatment of men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1989;69(4):776-783. [DOI] [PubMed] [Google Scholar]
- 32. Boehm U, Bouloux PM, Dattani MT, et al. Expert consensus document: European consensus statement on congenital hypogonadotropic hypogonadism—pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2015;11(9):547-564. [DOI] [PubMed] [Google Scholar]
- 33. Møller UK, Streym SV, Mosekilde L, Rejnmark L. Changes in bone mineral density and body composition during pregnancy and postpartum. A controlled cohort study. Osteoporos Int. 2012;23(4):1213-1223. [DOI] [PubMed] [Google Scholar]
- 34. Clemetson IA, Popp A, Lippuner K, Ballmer F, Anderson SE. Postpartum osteoporosis associated with proximal tibial stress fracture. Skeletal Radiol. 2004;33(2):96-98. [DOI] [PubMed] [Google Scholar]
- 35. Dunne F, Walters B, Marshall T, Heath DA. Pregnancy associated osteoporosis. Clin Endocrinol (Oxf). 1993;39(4):487-490. [DOI] [PubMed] [Google Scholar]
- 36. Yun KY, Han SE, Kim SC, Joo JK, Lee KS. Pregnancy-related osteoporosis and spinal fractures. Obstet Gynecol Sci. 2017;60(1):133-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ofluoglu O, Ofluoglu D. A case report: pregnancy-induced severe osteoporosis with eight vertebral fractures. Rheumatol Int. 2008;29(2):197-201. [DOI] [PubMed] [Google Scholar]
- 38. O’Sullivan SM, Grey AB, Singh R, Reid IR. Bisphosphonates in pregnancy and lactation-associated osteoporosis. Osteoporos Int. 2006;17(7):1008-1012. [DOI] [PubMed] [Google Scholar]
- 39. Seriwatanachai D, Thongchote K, Charoenphandhu N, et al. Prolactin directly enhances bone turnover by raising osteoblast-expressed receptor activator of nuclear factor κB ligand/osteoprotegerin ratio. Bone. 2008;42(3):535-546. [DOI] [PubMed] [Google Scholar]
- 40. Di Somma C, Colao A, Di Sarno A, et al. Bone marker and bone density responses to dopamine agonist therapy in hyperprolactinemic males. J Clin Endocrinol Metab. 1998;83(3):807-813. [DOI] [PubMed] [Google Scholar]
- 41. Schlechte J, el-Khoury G, Kathol M, Walkner L. Forearm and vertebral bone mineral in treated and untreated hyperprolactinemic amenorrhea. J Clin Endocrinol Metab. 1987;64(5):1021-1026. [DOI] [PubMed] [Google Scholar]
- 42. Colao A, Di Somma C, Loche S, et al. Prolactinomas in adolescents: persistent bone loss after 2 years of prolactin normalization. Clin Endocrinol (Oxf). 2000;52(3):319-327. [DOI] [PubMed] [Google Scholar]
- 43. Mazziotti G, Porcelli T, Mormando M, et al. Vertebral fractures in males with prolactinoma. Endocrine. 2011;39(3):288-293. [DOI] [PubMed] [Google Scholar]
- 44. Mazziotti G, Mancini T, Mormando M, et al. High prevalence of radiological vertebral fractures in women with prolactin-secreting pituitary adenomas. Pituitary. 2011;14(4):299-306. [DOI] [PubMed] [Google Scholar]
- 45. Ebeling PR, Atley LM, Guthrie JR, et al. Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab. 1996;81(9):3366-3371. [DOI] [PubMed] [Google Scholar]
- 46. Yeh JK, Chen MM, Aloia JF. Ovariectomy-induced high turnover in cortical bone is dependent on pituitary hormone in rats. Bone. 1996;18(5):443-450. [DOI] [PubMed] [Google Scholar]
- 47. Yeh JK, Chen MM, Aloia JF. Effects of 17 beta-estradiol administration on cortical and cancellous bone of ovariectomized rats with and without hypophysectomy. Bone. 1997;20(5):413-420. [DOI] [PubMed] [Google Scholar]
- 48. Riggs BL, Khosla S, Melton LJ III. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23(3):279-302. [DOI] [PubMed] [Google Scholar]
- 49. Khosla S. New insights into androgen and estrogen receptor regulation of the male skeleton. J Bone Miner Res. 2015;30(7):1134-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mohamad NV, Soelaiman IN, Chin KY. A concise review of testosterone and bone health. Clin Interv Aging. 2016;11:1317-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Almeida M, Laurent MR, Dubois V, et al. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev. 2017;97(1):135-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Khosla S, Monroe DG. Regulation of bone metabolism by sex steroids. Cold Spring Harb Perspect Med. 2018;8(1):a031211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kasperk CH, Wakley GK, Hierl T, Ziegler R. Gonadal and adrenal androgens are potent regulators of human bone cell metabolism in vitro. J Bone Miner Res. 1997;12(3):464-471. [DOI] [PubMed] [Google Scholar]
- 54. Scheven BA, Milne JS. Dehydroepiandrosterone (DHEA) and DHEA-S interact with 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) to stimulate human osteoblastic cell differentiation. Life Sci. 1998;62(1):59-68. [DOI] [PubMed] [Google Scholar]
- 55. Wang L, Wang YD, Wang WJ, Zhu Y, Li DJ. Dehydroepiandrosterone improves murine osteoblast growth and bone tissue morphometry via mitogen-activated protein kinase signaling pathway independent of either androgen receptor or estrogen receptor. J Mol Endocrinol. 2007;38(4):467-479. [DOI] [PubMed] [Google Scholar]
- 56. Qiu X, Gui Y, Xu Y, Li D, Wang L. DHEA promotes osteoblast differentiation by regulating the expression of osteoblast-related genes and Foxp3+ regulatory T cells. Biosci Trends. 2015;9(5):307-314. [DOI] [PubMed] [Google Scholar]
- 57. Lin H, Li L, Wang Q, Wang Y, Wang J, Long X. A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA supplementation of bone mineral density in healthy adults. Gynecol Endocrinol. 2019;35(11):924-931. [DOI] [PubMed] [Google Scholar]
- 58. Kirby DJ, Buchalter DB, Anil U, Leucht P. DHEA in bone: the role in osteoporosis and fracture healing. Arch Osteoporos. 2020;15(1):84. [DOI] [PubMed] [Google Scholar]
- 59. Harris GW. Neural Control of the Pituitary Gland. Edward Arnold Publishers Ltd; 1955. [Google Scholar]
- 60. Matsuo H, Baba Y, Nair RM, Arimura A, Schally AV. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Commun. 1971;43(6):1334-1339. [DOI] [PubMed] [Google Scholar]
- 61. Amoss M, Burgus R, Blackwell R, Vale W, Fellows R, Guillemin R. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochem Biophys Res Commun. 1971;44(1):205-210. [DOI] [PubMed] [Google Scholar]
- 62. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972-10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614-1627. [DOI] [PubMed] [Google Scholar]
- 64. Thompson EL, Patterson M, Murphy KG, et al. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16(10):850-858. [DOI] [PubMed] [Google Scholar]
- 65. Messager S, Chatzidaki EE, Ma D, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102(5):1761-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF Jr, Plant TM. Continuous human metastin 45-54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology. 2006;147(5):2122-2126. [DOI] [PubMed] [Google Scholar]
- 67. Comninos AN, Anastasovska J, Sahuri-Arisoylu M, et al. Kisspeptin signaling in the amygdala modulates reproductive hormone secretion. Brain Struct Funct. 2016;221(4):2035-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dhillo WS, Chaudhri OB, Patterson M, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90(12):6609-6615. [DOI] [PubMed] [Google Scholar]
- 69. Dhillo WS, Chaudhri OB, Thompson EL, et al. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab. 2007;92(10):3958-3966. [DOI] [PubMed] [Google Scholar]
- 70. Narayanaswamy S, Jayasena CN, Ng N, et al. Subcutaneous infusion of kisspeptin-54 stimulates gonadotrophin release in women and the response correlates with basal oestradiol levels. Clin Endocrinol (Oxf). 2016;84(6):939-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073-4077. [DOI] [PubMed] [Google Scholar]
- 72. Kenkre JS, Bassett J. The bone remodelling cycle. Ann Clin Biochem. 2018;55(3):308-327. [DOI] [PubMed] [Google Scholar]
- 73. Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dallas SL, Bonewald LF. Dynamics of the transition from osteoblast to osteocyte. Ann N Y Acad Sci. 2010;1192:437-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. St John HC, Bishop KA, Meyer MB, et al. The osteoblast to osteocyte transition: epigenetic changes and response to the vitamin D3 hormone. Mol Endocrinol. 2014;28(7):1150-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen H, Senda T, Kubo KY. The osteocyte plays multiple roles in bone remodeling and mineral homeostasis. Med Mol Morphol. 2015;48(2):61-68. [DOI] [PubMed] [Google Scholar]
- 77. Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504-1508. [DOI] [PubMed] [Google Scholar]
- 78. McDonald MM, Khoo WH, Ng PY, et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell. 2021;184(7):1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kim JH, Kim N. Signaling pathways in osteoclast differentiation. Chonnam Med J. 2016;52(1):12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Park JH, Lee NK, Lee SY. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol Cells. 2017;40(10):706-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wright HL, McCarthy HS, Middleton J, Marshall MJ. RANK, RANKL and osteoprotegerin in bone biology and disease. Curr Rev Musculoskelet Med. 2009;2(1):56-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bellido T. Osteocyte-driven bone remodeling. Calcif Tissue Int. 2014;94(1):25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Andreev D, Liu M, Weidner D, et al. Osteocyte necrosis triggers osteoclast-mediated bone loss through macrophage-inducible C-type lectin. J Clin Invest. 2020;130(9):4811-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int. 2015;2015:421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Neve A, Corrado A, Cantatore FP. Osteoblast physiology in normal and pathological conditions. Cell Tissue Res. 2011;343(2):289-302. [DOI] [PubMed] [Google Scholar]
- 86. Leitch VD, Bassett JHD, Williams GR. Role of thyroid hormones in craniofacial development. Nat Rev Endocrinol. 2020;16(3):147-164. [DOI] [PubMed] [Google Scholar]
- 87. Bassett JH, Williams GR. Role of thyroid hormones in skeletal development and bone maintenance. Endocr Rev. 2016;37(2):135-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ohtaki T, Shintani Y, Honda S, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411(6837):613-617. [DOI] [PubMed] [Google Scholar]
- 89. Kotani M, Detheux M, Vandenbogaerde A, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276(37):34631-34636. [DOI] [PubMed] [Google Scholar]
- 90. Lee DK, Nguyen T, O’Neill GP, et al. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446(1):103-107. [DOI] [PubMed] [Google Scholar]
- 91. Lee JH, Miele ME, Hicks DJ, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88(23):1731-1737. [DOI] [PubMed] [Google Scholar]
- 92. Topaloglu AK, Tello JA, Kotan LD, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366(7):629-635. [DOI] [PubMed] [Google Scholar]
- 93. Comninos AN, Wall MB, Demetriou L, et al. Kisspeptin modulates sexual and emotional brain processing in humans. J Clin Invest. 2017;127(2):709-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Comninos AN, Demetriou L, Wall MB, et al. Modulations of human resting brain connectivity by kisspeptin enhance sexual and emotional functions. JCI Insight. 2018;3(20):e121958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yang L, Demetriou L, Wall MB, et al. Kisspeptin enhances brain responses to olfactory and visual cues of attraction in men. JCI Insight. 2020;5(3):e133633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yang L, Demetriou L, Wall MB, et al. The effects of kisspeptin on brain response to food images and psychometric parameters of appetite in healthy men. J Clin Endocrinol Metab. 2021;106(4):e1837-e1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Teles MG, Bianco SD, Brito VN, et al. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;358(7):709-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Muir AI, Chamberlain L, Elshourbagy NA, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276(31):28969-28975. [DOI] [PubMed] [Google Scholar]
- 99. Hrabovszky E, Ciofi P, Vida B, et al. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010;31(11):1984-1998. [DOI] [PubMed] [Google Scholar]
- 100. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92(7):2744-2750. [DOI] [PubMed] [Google Scholar]
- 101. Clarkson J, d’Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol. 2009;21(8):673-682. [DOI] [PubMed] [Google Scholar]
- 102. Mikkelsen JD, Simonneaux V. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides. 2009;30(1):26-33. [DOI] [PubMed] [Google Scholar]
- 103. Wang FS, Chen H, Wu ZY, Lin JH. KISS1 expression in osteosarcoma: high in Chinese clinical cases, but lower in cell lines. Asian Pac J Cancer Prev. 2011;12(12):3229-3234. [PubMed] [Google Scholar]
- 104. Olbrich T, Ziegler E, Türk G, Schubert A, Emons G, Gründker C. Kisspeptin-10 inhibits bone-directed migration of GPR54-positive breast cancer cells: evidence for a dose-window effect. Gynecol Oncol. 2010;119(3):571-578. [DOI] [PubMed] [Google Scholar]
- 105. Weinman MA, Fischer JA, Jacobs DC, Goodall CP, Bracha S, Chappell PE. Autocrine production of reproductive axis neuropeptides affects proliferation of canine osteosarcoma in vitro. BMC Cancer. 2019;19(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dotterweich J, Tower RJ, Brandl A, et al. The KISS1 receptor as an in vivo microenvironment imaging biomarker of multiple myeloma bone disease. PLoS One. 2016;11(5):e0155087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Son HE, Kim KM, Kim EJ, Jang WG. Kisspeptin-10 (KP-10) stimulates osteoblast differentiation through GPR54-mediated regulation of BMP2 expression and activation. Sci Rep. 2018;8(1):2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Javed A, Bae JS, Afzal F, et al. Structural coupling of Smad and Runx2 for execution of the BMP2 osteogenic signal. J Biol Chem. 2008;283(13):8412-8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jang WG, Kim EJ, Lee KN, Son HJ, Koh JT. AMP-activated protein kinase (AMPK) positively regulates osteoblast differentiation via induction of Dlx5-dependent Runx2 expression in MC3T3E1 cells. Biochem Biophys Res Commun. 2011;404(4):1004-1009. [DOI] [PubMed] [Google Scholar]
- 110. Yi T, Tan K, Cho SG, et al. Regulation of embryonic kidney branching morphogenesis and glomerular development by KISS1 receptor (Gpr54) through NFAT2- and Sp1-mediated Bmp7 expression. J Biol Chem. 2010;285(23):17811-17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Terasaka T, Otsuka F, Tsukamoto N, et al. Mutual interaction of kisspeptin, estrogen and bone morphogenetic protein-4 activity in GnRH regulation by GT1-7 cells. Mol Cell Endocrinol. 2013;381(1-2):8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Knowles HJ, Cleton-Jansen AM, Korsching E, Athanasou NA. Hypoxia-inducible factor regulates osteoclast-mediated bone resorption: role of angiopoietin-like 4. FASEB J. 2010;24(12):4648-4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Herber CB, Krause WC, Wang L, et al. Estrogen signaling in arcuate Kiss1 neurons suppresses a sex-dependent female circuit promoting dense strong bones. Nat Commun. 2019;10(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rønnekleiv OK, Qiu J, Kelly MJ. Arcuate kisspeptin neurons coordinate reproductive activities with metabolism. Semin Reprod Med. 2019;37(3):131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Herbison AE. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol. 2016;12(8):452-466. [DOI] [PubMed] [Google Scholar]
- 116. Stojilkovic SS, Reinhart J, Catt KJ. Gonadotropin-releasing hormone receptors: structure and signal transduction pathways. Endocr Rev. 1994;15(4):462-499. [DOI] [PubMed] [Google Scholar]
- 117. Millar RP. GnRH II and type II GnRH receptors. Trends Endocrinol Metab. 2003;14(1):35-43. [DOI] [PubMed] [Google Scholar]
- 118. Cheung LW, Wong AS. Gonadotropin-releasing hormone: GnRH receptor signaling in extrapituitary tissues. FEBS J. 2008;275(22):5479-5495. [DOI] [PubMed] [Google Scholar]
- 119. White RB, Eisen JA, Kasten TL, Fernald RD. Second gene for gonadotropin-releasing hormone in humans. Proc Natl Acad Sci U S A. 1998;95(1):305-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Collet C, Schiltz C, Geoffroy V, Maroteaux L, Launay JM, de Vernejoul MC. The serotonin 5-HT2B receptor controls bone mass via osteoblast recruitment and proliferation. FASEB J. 2008;22(2):418-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Penchala SC, Miller MR, Pal A, et al. A biomimetic approach for enhancing the in vivo half-life of peptides. Nat Chem Biol. 2015;11(10):793-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Atchia SK, Wallis CJD, Fleshner N, Toren P. Switching from a gonadotropin-releasing hormone (GnRH) agonist to a GnRH antagonist in prostate cancer patients: a systematic review and meta-analysis. Can Urol Assoc J. 2020;14(2):36-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345(13):948-955. [DOI] [PubMed] [Google Scholar]
- 124. Smith MR. Treatment-related osteoporosis in men with prostate cancer. Clin Cancer Res. 2006;12(20 Pt 2):6315s-6319s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154-164. [DOI] [PubMed] [Google Scholar]
- 126. Kunath F, Borgmann H, Blümle A, et al. Gonadotropin-releasing hormone antagonists versus standard androgen suppression therapy for advanced prostate cancer A systematic review with meta-analysis. BMJ Open. 2015;5(11):e008217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rosario DJ, Davey P, Green J, et al. The role of gonadotrophin-releasing hormone antagonists in the treatment of patients with advanced hormone-dependent prostate cancer in the UK. World J Urol. 2016;34(12):1601-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Abufaraj M, Iwata T, Kimura S, et al. Differential impact of gonadotropin-releasing hormone antagonist versus agonist on clinical safety and oncologic outcomes on patients with metastatic prostate cancer: a meta-analysis of randomized controlled trials. Eur Urol. 2021;79(1):44-53. [DOI] [PubMed] [Google Scholar]
- 129. Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382(13):1244-1256. [DOI] [PubMed] [Google Scholar]
- 130. Huerta-Reyes M, Maya-Núñez G, Pérez-Solis MA, et al. Treatment of breast cancer with gonadotropin-releasing hormone analogs. Front Oncol. 2019;9:943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Matsuo H. Prediction of the change in bone mineral density induced by gonadotropin-releasing hormone agonist treatment for endometriosis. Fertil Steril. 2004;81(1):149-153. [DOI] [PubMed] [Google Scholar]
- 132. Sverrisdóttir A, Fornander T, Jacobsson H, von Schoultz E, Rutqvist LE. Bone mineral density among premenopausal women with early breast cancer in a randomized trial of adjuvant endocrine therapy. J Clin Oncol. 2004;22(18):3694-3699. [DOI] [PubMed] [Google Scholar]
- 133. Gnant M, Mlineritsch B, Luschin-Ebengreuth G, et al. ; Austrian Breast and Colorectal Cancer Study Group (ABCSG) . Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008;9(9):840-849. [DOI] [PubMed] [Google Scholar]
- 134. Tomkinson A, Reeve J, Shaw RW, Noble BS. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab. 1997;82(9):3128-3135. [DOI] [PubMed] [Google Scholar]
- 135. Raju GAR, Chavan R, Deenadayal M, et al. Luteinizing hormone and follicle stimulating hormone synergy: a review of role in controlled ovarian hyper-stimulation. J Hum Reprod Sci. 2013;6(4):227-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Huhtaniemi I. A short evolutionary history of FSH-stimulated spermatogenesis. Hormones (Athens). 2015;14(4):468-478. [DOI] [PubMed] [Google Scholar]
- 137. Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18(6):739-773. [DOI] [PubMed] [Google Scholar]
- 138. Casarini L, Crépieux P. Molecular mechanisms of action of FSH. Front Endocrinol (Lausanne). 2019;10:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Chrusciel M, Ponikwicka-Tyszko D, Wolczynski S, Huhtaniemi I, Rahman NA. Extragonadal FSHR expression and function—is it real? Front Endocrinol (Lausanne). 2019;10:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sun L, Peng Y, Sharrow AC, et al. FSH directly regulates bone mass. Cell. 2006;125(2):247-260. [DOI] [PubMed] [Google Scholar]
- 141. Cannon JG, Kraj B, Sloan G. Follicle-stimulating hormone promotes RANK expression on human monocytes. Cytokine. 2011;53(2):141-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Iqbal J, Sun L, Kumar TR, Blair HC, Zaidi M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc Natl Acad Sci U S A. 2006;103(40):14925-14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Cannon JG, Cortez-Cooper M, Meaders E, et al. Follicle-stimulating hormone, interleukin-1, and bone density in adult women. Am J Physiol Regul Integr Comp Physiol. 2010;298(3):R790-R798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Danilovich N, Babu PS, Xing W, Gerdes M, Krishnamurthy H, Sairam MR. Estrogen deficiency, obesity, and skeletal abnormalities in follicle-stimulating hormone receptor knockout (FORKO) female mice. Endocrinology. 2000;141(11):4295-4308. [DOI] [PubMed] [Google Scholar]
- 145. Balla A, Danilovich N, Yang Y, Sairam MR. Dynamics of ovarian development in the FORKO immature mouse: structural and functional implications for ovarian reserve. Biol Reprod. 2003;69(4):1281-1293. [DOI] [PubMed] [Google Scholar]
- 146. Abel MH, Huhtaniemi I, Pakarinen P, Kumar TR, Charlton HM. Age-related uterine and ovarian hypertrophy in FSH receptor knockout and FSHbeta subunit knockout mice. Reproduction. 2003;125(2):165-173. [DOI] [PubMed] [Google Scholar]
- 147. Khosla S. Estrogen versus FSH effects on bone metabolism: evidence from interventional human studies. Endocrinology. 2020;161(8):bqaa111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Ji Y, Liu P, Yuen T, et al. Epitope-specific monoclonal antibodies to FSHβ increase bone mass. Proc Natl Acad Sci U S A. 2018;115(9):2192-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Gao J, Tiwari-Pandey R, Samadfam R, et al. Altered ovarian function affects skeletal homeostasis independent of the action of follicle-stimulating hormone. Endocrinology. 2007;148(6):2613-2621. [DOI] [PubMed] [Google Scholar]
- 150. Allan CM, Kalak R, Dunstan CR, et al. Follicle-stimulating hormone increases bone mass in female mice. Proc Natl Acad Sci U S A. 2010;107(52):22629-22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. McTavish KJ, Jimenez M, Walters KA, et al. Rising follicle-stimulating hormone levels with age accelerate female reproductive failure. Endocrinology. 2007;148(9):4432-4439. [DOI] [PubMed] [Google Scholar]
- 152. Rouach V, Katzburg S, Koch Y, Stern N, Somjen D. Bone loss in ovariectomized rats: dominant role for estrogen but apparently not for FSH. J Cell Biochem. 2011;112(1):128-137. [DOI] [PubMed] [Google Scholar]
- 153. Sowers MR, Greendale GA, Bondarenko I, et al. Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos Int. 2003;14(3):191-197. [DOI] [PubMed] [Google Scholar]
- 154. Sowers MR, Jannausch M, McConnell D, et al. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006;91(4):1261-1267. [DOI] [PubMed] [Google Scholar]
- 155. Rendina D, Gianfrancesco F, De Filippo G, et al. FSHR gene polymorphisms influence bone mineral density and bone turnover in postmenopausal women. Eur J Endocrinol. 2010;163(1):165-172. [DOI] [PubMed] [Google Scholar]
- 156. Estrada K, Styrkarsdottir U, Evangelou E, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44(5):491-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Devleta B, Adem B, Senada S. Hypergonadotropic amenorrhea and bone density: new approach to an old problem. J Bone Miner Metab. 2004;22(4):360-364. [DOI] [PubMed] [Google Scholar]
- 158. Antonio L, Priskorn L, Olesen IA, Petersen JH, Vanderschueren D, Jørgensen N. High serum FSH is not a risk factor for low bone mineral density in infertile men. Bone. 2020;136:115366. [DOI] [PubMed] [Google Scholar]
- 159. Juel Mortensen L, Lorenzen M, Jørgensen N, et al. Possible link between FSH and RANKL release from adipocytes in men with impaired gonadal function including Klinefelter syndrome. Bone. 2019;123:103-114. [DOI] [PubMed] [Google Scholar]
- 160. Drake MT, McCready LK, Hoey KA, Atkinson EJ, Khosla S. Effects of suppression of follicle-stimulating hormone secretion on bone resorption markers in postmenopausal women. J Clin Endocrinol Metab. 2010;95(11):5063-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Omodei U, Mazziotti G, Donarini G, et al. Effects of recombinant follicle-stimulating hormone on bone turnover markers in infertile women undergoing in vitro fertilization procedure. J Clin Endocrinol Metab. 2013;98(1):330-336. [DOI] [PubMed] [Google Scholar]
- 162. Uihlein AV, Finkelstein JS, Lee H, Leder BZ. FSH suppression does not affect bone turnover in eugonadal men. J Clin Endocrinol Metab. 2014;99(7):2510-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Choi J, Smitz J. Luteinizing hormone and human chorionic gonadotropin: origins of difference. Mol Cell Endocrinol. 2014;383(1-2):203-213. [DOI] [PubMed] [Google Scholar]
- 164. Vitt UA, Hsu SY, Hsueh AJ. Evolution and classification of cystine knot-containing hormones and related extracellular signaling molecules. Mol Endocrinol. 2001;15(5):681-694. [DOI] [PubMed] [Google Scholar]
- 165. Dufau ML. The luteinizing hormone receptor. Annu Rev Physiol. 1998;60:461-496. [DOI] [PubMed] [Google Scholar]
- 166. Ziecik AJ, Kaczmarek MM, Blitek A, Kowalczyk AE, Li X, Rahman NA. Novel biological and possible applicable roles of LH/hCG receptor. Mol Cell Endocrinol. 2007;269(1-2):51-60. [DOI] [PubMed] [Google Scholar]
- 167. Yarram SJ, Perry MJ, Christopher TJ, et al. Luteinizing hormone receptor knockout (LuRKO) mice and transgenic human chorionic gonadotropin (hCG)-overexpressing mice (hCG alphabeta+) have bone phenotypes. Endocrinology. 2003;144(8):3555-3564. [DOI] [PubMed] [Google Scholar]
- 168. Riccetti L, Yvinec R, Klett D, et al. Human luteinizing hormone and chorionic gonadotropin display biased agonism at the LH and LH/CG receptors. Sci Rep. 2017;7(1):940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Farr JN, Khosla S. Skeletal changes through the lifespan—from growth to senescence. Nat Rev Endocrinol. 2015;11(9):513-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Mansell JP, Bailey AJ, Yarram SJ. Could bone tissue be a target for luteinizing hormone/chorionic gonadotropin? Mol Cell Endocrinol. 2007;269(1-2):99-106. [DOI] [PubMed] [Google Scholar]
- 171. Wu XY, Wu XP, Luo XH, et al. The relationship between the levels of gonadotropic hormones and OPG, leptin, TGF-β1 and TGF-β2 in Chinese adult women. Clin Chim Acta. 2010;411(17-18):1296-1305. [DOI] [PubMed] [Google Scholar]
- 172. Wu XY, Wu XP, Xie H, et al. Age-related changes in biochemical markers of bone turnover and gonadotropin levels and their relationship among Chinese adult women. Osteoporos Int. 2010;21(2):275-285. [DOI] [PubMed] [Google Scholar]
- 173. Xu ZR, Wang AH, Wu XP, et al. Relationship of age-related concentrations of serum FSH and LH with bone mineral density, prevalence of osteoporosis in native Chinese women. Clin Chim Acta. 2009;400(1-2):8-13. [DOI] [PubMed] [Google Scholar]
- 174. Lkhagvasuren U, Jav S, Zagdsuren B. Correlation between reproductive hormonal level and osteoporosis among women in Mongolia. Cent Asian J Glob Health. 2015;4(2):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Seven A, Yuksel B, Kabil Kucur S, et al. The evaluation of hormonal and psychological parameters that affect bone mineral density in postmenopausal women. Eur Rev Med Pharmacol Sci. 2016;20(1):20-25. [PubMed] [Google Scholar]
- 176. Hsu B, Cumming RG, Seibel MJ, et al. Reproductive hormones and longitudinal change in bone mineral density and incident fracture risk in older men: the Concord Health and Aging in Men Project. J Bone Miner Res. 2015;30(9):1701-1708. [DOI] [PubMed] [Google Scholar]
- 177. Yeap BB, Alfonso H, Chubb SAP, et al. U-shaped association of plasma testosterone, and no association of plasma estradiol, with incidence of fractures in men. J Clin Endocrinol Metab. 2020;105(5):1489-1500. [DOI] [PubMed] [Google Scholar]
- 178. Cooke NE, Coit D, Shine J, Baxter JD, Martial JA. Human prolactin. cDNA structural analysis and evolutionary comparisons. J Biol Chem. 1981;256(8):4007-4016. [PubMed] [Google Scholar]
- 179. Brooks CL. Molecular mechanisms of prolactin and its receptor. Endocr Rev. 2012;33(4):504-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr Rev. 1996;17(6):639-669. [DOI] [PubMed] [Google Scholar]
- 181. Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19(3):225-268. [DOI] [PubMed] [Google Scholar]
- 182. Vroonen L, Daly AF, Beckers A. Epidemiology and management challenges in prolactinomas. Neuroendocrinology. 2019;109(1):20-27. [DOI] [PubMed] [Google Scholar]
- 183. Kovacs CS. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiol Rev. 2016;96(2):449-547. [DOI] [PubMed] [Google Scholar]
- 184. Clément-Lacroix P, Ormandy C, Lepescheux L, et al. Osteoblasts are a new target for prolactin: analysis of bone formation in prolactin receptor knockout mice. Endocrinology. 1999;140(1):96-105. [DOI] [PubMed] [Google Scholar]
- 185. Bataille-Simoneau N, Gerland K, Chappard D, Basle MF, Mercier L. Expression of prolactin receptors in human osteosarcoma cells. Biochem Biophys Res Commun. 1996;229(1):323-328. [DOI] [PubMed] [Google Scholar]
- 186. Charoenphandhu N, Tudpor K, Thongchote K, Saengamnart W, Puntheeranurak S, Krishnamra N. High-calcium diet modulates effects of long-term prolactin exposure on the cortical bone calcium content in ovariectomized rats. Am J Physiol Endocrinol Metab. 2007;292(2):E443-E452. [DOI] [PubMed] [Google Scholar]
- 187. Wongdee K, Tulalamba W, Thongbunchoo J, Krishnamra N, Charoenphandhu N. Prolactin alters the mRNA expression of osteoblast-derived osteoclastogenic factors in osteoblast-like UMR106 cells. Mol Cell Biochem. 2011;349(1-2):195-204. [DOI] [PubMed] [Google Scholar]
- 188. Charoenphandhu N, Nakkrasae LI, Kraidith K, et al. Two-step stimulation of intestinal Ca2+ absorption during lactation by long-term prolactin exposure and suckling-induced prolactin surge. Am J Physiol Endocrinol Metab. 2009;297(3):E609-E619. [DOI] [PubMed] [Google Scholar]
- 189. Wang C, Hsueh AJ, Erickson GF. Prolactin inhibition of estrogen production by cultured rat granulosa cells. Mol Cell Endocrinol. 1980;20(2):135-144. [DOI] [PubMed] [Google Scholar]
- 190. Wang C, Chan V. Divergent effects of prolactin on estrogen and progesterone production by granulosa cells of rat graafian follicles. Endocrinology. 1982;110(4):1085-1093. [DOI] [PubMed] [Google Scholar]
- 191. Koppelman MC, Kurtz DW, Morrish KA, et al. Vertebral body bone mineral content in hyperprolactinemic women. J Clin Endocrinol Metab. 1984;59(6):1050-1053. [DOI] [PubMed] [Google Scholar]
- 192. Shibli-Rahhal A, Schlechte J. The effects of hyperprolactinemia on bone and fat. Pituitary. 2009;12(2):96-104. [DOI] [PubMed] [Google Scholar]
- 193. Vestergaard P, Jørgensen JOL, Hagen C, et al. Fracture risk is increased in patients with GH deficiency or untreated prolactinomas—a case-control study. Clin Endocrinol (Oxf). 2002;56(2):159-167. [DOI] [PubMed] [Google Scholar]
- 194. Vilar L, Vilar CF, Lyra R, Freitas MDC. Pitfalls in the diagnostic evaluation of hyperprolactinemia. Neuroendocrinology. 2019;109(1):7-19. [DOI] [PubMed] [Google Scholar]
- 195. Melmed S, Casanueva FF, Hoffman AR, et al. ; Endocrine Society . Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273-288. [DOI] [PubMed] [Google Scholar]
- 196. Takahashi T, Uchida H, John M, et al. The impact of prolactin-raising antipsychotics on bone mineral density in patients with schizophrenia: findings from a longitudinal observational cohort. Schizophr Res. 2013;147(2-3):383-386. [DOI] [PubMed] [Google Scholar]
- 197. De Hert M, Detraux J, Stubbs B. Relationship between antipsychotic medication, serum prolactin levels and osteoporosis/osteoporotic fractures in patients with schizophrenia: a critical literature review. Expert Opin Drug Saf. 2016;15(6):809-823. [DOI] [PubMed] [Google Scholar]
- 198. Grigg J, Worsley R, Thew C, Gurvich C, Thomas N, Kulkarni J. Antipsychotic-induced hyperprolactinemia: synthesis of world-wide guidelines and integrated recommendations for assessment, management and future research. Psychopharmacology (Berl). 2017;234(22):3279-3297. [DOI] [PubMed] [Google Scholar]
- 199. Nicks KM, Fowler TW, Gaddy D. Reproductive hormones and bone. Curr Osteoporos Rep. 2010;8(2):60-67. [DOI] [PubMed] [Google Scholar]
- 200. Schiffer L, Barnard L, Baranowski ES, et al. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: a comprehensive review. J Steroid Biochem Mol Biol. 2019;194:105439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. MacNamara P, O’Shaughnessy C, Manduca P, Loughrey HC. Progesterone receptors are expressed in human osteoblast-like cell lines and in primary human osteoblast cultures. Calcif Tissue Int. 1995;57(6):436-441. [DOI] [PubMed] [Google Scholar]
- 202. Pensler JM, Langman CB, Radosevich JA, et al. Sex steroid hormone receptors in normal and dysplastic bone disorders in children. J Bone Miner Res. 1990;5(5):493-498. [DOI] [PubMed] [Google Scholar]
- 203. Eriksen EF, Colvard DS, Berg NJ, et al. Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 1988;241(4861):84-86. [DOI] [PubMed] [Google Scholar]
- 204. Wei LL, Leach MW, Miner RS, Demers LM. Evidence for progesterone receptors in human osteoblast-like cells. Biochem Biophys Res Commun. 1993;195(2):525-532. [DOI] [PubMed] [Google Scholar]
- 205. Luo XH, Liao EY, Su X. Progesterone upregulates TGF-b isoforms (b1, b2, and b3) expression in normal human osteoblast-like cells. Calcif Tissue Int. 2002;71(4):329-334. [DOI] [PubMed] [Google Scholar]
- 206. Seifert-Klauss V, Prior JC. Progesterone and bone: actions promoting bone health in women. J Osteoporos. 2010;2010:845180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Dai C, Jia J, Kot A, et al. Selective inhibition of progesterone receptor in osteochondral progenitor cells, but not in mature chondrocytes, modulated subchondral bone structures. Bone. 2020;132:115196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Chappell PE, Lydon JP, Conneely OM, O’Malley BW, Levine JE. Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology. 1997;138(10):4147-4152. [DOI] [PubMed] [Google Scholar]
- 209. Rickard DJ, Iwaniec UT, Evans G, et al. Bone growth and turnover in progesterone receptor knockout mice. Endocrinology. 2008;149(5):2383-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Yao W, Dai W, Shahnazari M, et al. Inhibition of the progesterone nuclear receptor during the bone linear growth phase increases peak bone mass in female mice. PLoS One. 2010;5(7):e11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Cauley JA, Robbins J, Chen Z, et al. ; Women’s Health Initiative Investigators . Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290(13):1729-1738. [DOI] [PubMed] [Google Scholar]
- 212. Prior JC, Seifert-Klauss VR, Giustini D, Adachi JD, Kalyan S, Goshtasebi A. Estrogen-progestin therapy causes a greater increase in spinal bone mineral density than estrogen therapy—a systematic review and meta-analysis of controlled trials with direct randomization. J Musculoskelet Neuronal Interact. 2017;17(3):146-154. [PMC free article] [PubMed] [Google Scholar]
- 213. Prior JC. Progesterone for the prevention and treatment of osteoporosis in women. Climacteric. 2018;21(4):366-374. [DOI] [PubMed] [Google Scholar]
- 214. Danni L, Hitchcock CL, Barr SI, Yu Tricia, Prior JC. Negatrive spinal bone mineral density changes and subclinical ovulatory disturbances - a prospective data in healthy premenopausal women with regular menstrual cycles. Epidemiol Rev. 2014;36:137-147. [DOI] [PubMed] [Google Scholar]
- 215. Prior JC, Vigna YM, Barr SI, Rexworthy C, Lentle BC. Cyclic medroxyprogesterone treatment increases bone density: a controlled trial in active women with menstrual cycle disturbances. Am J Med. 1994;96(6):521-530. [DOI] [PubMed] [Google Scholar]
- 216. Clark MK, Sowers M, Levy B, Nichols S. Bone mineral density loss and recovery during 48 months in first-time users of depot medroxyprogesterone acetate. Fertil Steril. 2006;86(5):1466-1474. [DOI] [PubMed] [Google Scholar]
- 217. Harel Z, Johnson CC, Gold MA, et al. Recovery of bone mineral density in adolescents following the use of depot medroxyprogesterone acetate contraceptive injections. Contraception. 2010;81(4):281-291. [DOI] [PubMed] [Google Scholar]
- 218. Meier C, Brauchli YB, Jick SS, Kraenzlin ME, Meier CR. Use of depot medroxyprogesterone acetate and fracture risk. J Clin Endocrinol Metab. 2010;95(11):4909-4916. [DOI] [PubMed] [Google Scholar]
- 219. Lanza LL, McQuay LJ, Rothman KJ, et al. Use of depot medroxyprogesterone acetate contraception and incidence of bone fracture. Obstet Gynecol. 2013;121(3):593-600. [DOI] [PubMed] [Google Scholar]
- 220. Grecu EO, Weinshelbaum A, Simmons R. Effective therapy of glucocorticoid-induced osteoporosis with medroxyprogesterone acetate. Calcif Tissue Int. 1990;46(5):294-299. [DOI] [PubMed] [Google Scholar]
- 221. Bathgate RA, Ivell R, Sanborn BM, Sherwood OD, Summers RJ. Receptors for relaxin family peptides. Ann N Y Acad Sci. 2005;1041:61-76. [DOI] [PubMed] [Google Scholar]
- 222. Sherwood OD. Relaxin’s physiological roles and other diverse actions. Endocr Rev. 2004;25(2):205-234. [DOI] [PubMed] [Google Scholar]
- 223. Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ. Relaxin family peptides and their receptors. Physiol Rev. 2013;93(1):405-480. [DOI] [PubMed] [Google Scholar]
- 224. Facciolli A, Ferlin A, Gianesello L, Pepe A, Foresta C. Role of relaxin in human osteoclastogenesis. Ann N Y Acad Sci. 2009;1160:221-225. [DOI] [PubMed] [Google Scholar]
- 225. Ferlin A, Pepe A, Facciolli A, Gianesello L, Foresta C. Relaxin stimulates osteoclast differentiation and activation. Bone. 2010;46(2):504-513. [DOI] [PubMed] [Google Scholar]
- 226. Duarte C, Kobayashi Y, Kawamoto T, Moriyama K. RELAXIN enhances differentiation and matrix mineralization through Relaxin/insulin-like family peptide receptor 2 (Rxfp2) in MC3T3-E1 cells in vitro. Bone. 2014;65:92-101. [DOI] [PubMed] [Google Scholar]
- 227. Moon JS, Kim SH, Oh SH, et al. Relaxin augments BMP-2-induced osteoblast differentiation and bone formation. J Bone Miner Res. 2014;29(7):1586-1596. [DOI] [PubMed] [Google Scholar]
- 228. Injamuri S, Rahaman MN, Shen Y, Huang YW. Relaxin enhances bone regeneration with BMP-2-loaded hydroxyapatite microspheres. J Biomed Mater Res A. 2020;108(5):1231-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Ferlin A, Pepe A, Gianesello L, et al. Mutations in the insulin-like factor 3 receptor are associated with osteoporosis. J Bone Miner Res. 2008;23(5):683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Ferlin A, Perilli L, Gianesello L, Taglialavoro G, Foresta C. Profiling insulin like factor 3 (INSL3) signaling in human osteoblasts. PLoS One. 2011;6(12):e29733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Ferlin A, De Toni L, Sandri M, Foresta C. Relaxin and insulin-like peptide 3 in the musculoskeletal system: from bench to bedside. Br J Pharmacol. 2017;174(10):1015-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Bai M, Han Y, Wu Y, et al. Targeted genetic screening in mice through haploid embryonic stem cells identifies critical genes in bone development. PLoS Biol. 2019;17(7):e3000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Conrad KP, Phillips EG, Jiron J, et al. Potential therapeutic use of relaxin in accelerating closure of cranial bone defects in mice. Physiol Rep. 2019;7(11):e14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Matzuk MM, Kumar TR, Shou W, et al. Transgenic models to study the roles of inhibins and activins in reproduction, oncogenesis, and development. Recent Prog Horm Res. 1996;51:123-154; discussion 155-157. [PubMed] [Google Scholar]
- 235. Vale W, Rivier C, Hsueh A, et al. Chemical and biological characterization of the inhibin family of protein hormones. Recent Prog Horm Res. 1988;44:1-34. [DOI] [PubMed] [Google Scholar]
- 236. Dupont J, McNeilly J, Vaiman A, Canepa S, Combarnous Y, Taragnat C. Activin signaling pathways in ovine pituitary and LβT2 gonadotrope cells. Biol Reprod. 2003;68(5):1877-1887. [DOI] [PubMed] [Google Scholar]
- 237. Martens JW, de Winter JP, Timmerman MA, et al. Inhibin interferes with activin signaling at the level of the activin receptor complex in Chinese hamster ovary cells. Endocrinology. 1997;138(7):2928-2936. [DOI] [PubMed] [Google Scholar]
- 238. Lewis KA, Gray PC, Blount AL, et al. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature. 2000;404(6776):411-414. [DOI] [PubMed] [Google Scholar]
- 239. Uccella S, La Rosa S, Genasetti A, Capella C. Localization of inhibin/activin subunits in normal pituitary and in pituitary adenomas. Pituitary. 2000;3(3):131-139. [DOI] [PubMed] [Google Scholar]
- 240. Debieve F, Pampfer S, Thomas K. Inhibin and activin production and subunit expression in human placental cells cultured in vitro. Mol Hum Reprod. 2000;6(8):743-749. [DOI] [PubMed] [Google Scholar]
- 241. Luisi S, Florio P, Reis FM, Petraglia F. Inhibins in female and male reproductive physiology: role in gametogenesis, conception, implantation and early pregnancy. Hum Reprod Update. 2005;11(2):123-135. [DOI] [PubMed] [Google Scholar]
- 242. Namwanje M, Brown CW. Activins and inhibins: roles in development, physiology, and disease. Cold Spring Harb Perspect Biol. 2016;8(7):a021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Kaplan FS, Le Merrer M, Glaser DL, et al. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol. 2008;22(1):191-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Pignolo RJ, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva: clinical and genetic aspects. Orphanet J Rare Dis. 2011;6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Centrella M, McCarthy TL, Canalis E. Activin-A binding and biochemical effects in osteoblast-enriched cultures from fetal-rat parietal bone. Mol Cell Biol. 1991;11(1):250-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Gaddy-Kurten D, Coker JK, Abe E, Jilka RL, Manolagas SC. Inhibin suppresses and activin stimulates osteoblastogenesis and osteoclastogenesis in murine bone marrow cultures. Endocrinology. 2002;143(1):74-83. [DOI] [PubMed] [Google Scholar]
- 247. Goh BC, Singhal V, Herrera AJ, et al. Activin receptor type 2A (ACVR2A) functions directly in osteoblasts as a negative regulator of bone mass. J Biol Chem. 2017;292(33):13809-13822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Perrien DS, Achenbach SJ, Bledsoe SE, et al. Bone turnover across the menopause transition: correlations with inhibins and follicle-stimulating hormone. J Clin Endocrinol Metab. 2006;91(5):1848-1854. [DOI] [PubMed] [Google Scholar]
- 249. Eijken M, Swagemakers S, Koedam M, et al. The activin A-follistatin system: potent regulator of human extracellular matrix mineralization. FASEB J. 2007;21(11):2949-2960. [DOI] [PubMed] [Google Scholar]
- 250. Oue Y, Kanatani H, Kiyoki M, Eto Y, Ogata E, Matsumoto T. Effect of local injection of activin A on bone formation in newborn rats. Bone. 1994;15(3):361-366. [DOI] [PubMed] [Google Scholar]
- 251. Sakai R, Fujita S, Horie T, et al. Activin increases bone mass and mechanical strength of lumbar vertebrae in aged ovariectomized rats. Bone. 2000;27(1):91-96. [DOI] [PubMed] [Google Scholar]
- 252. Sakai R, Miwa K, Eto Y. Local administration of activin promotes fracture healing in the rat fibula fracture model. Bone. 1999;25(2):191-196. [DOI] [PubMed] [Google Scholar]
- 253. Perrien DS, Akel NS, Edwards PK, et al. Inhibin A is an endocrine stimulator of bone mass and strength. Endocrinology. 2007;148(4):1654-1665. [DOI] [PubMed] [Google Scholar]
- 254. Vural F, Vural B, Yucesoy I, Badur S. Ovarian aging and bone metabolism in menstruating women aged 35-50 years. Maturitas. 2005;52(2):147-153. [DOI] [PubMed] [Google Scholar]
- 255. Welt CK, McNicholl DJ, Taylor AE, Hall JE. Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab. 1999;84(1):105-111. [DOI] [PubMed] [Google Scholar]
- 256. Lotinun S, Pearsall RS, Horne WC, Baron R. Activin receptor signaling: a potential therapeutic target for osteoporosis. Curr Mol Pharmacol. 2012;5(2):195-204. [DOI] [PubMed] [Google Scholar]
- 257. Deal C. Potential new drug targets for osteoporosis. Nat Clin Pract Rheumatol. 2009;5(1):20-27. [DOI] [PubMed] [Google Scholar]
- 258. Pearsall RS, Canalis E, Cornwall-Brady M, et al. A soluble activin type IIA receptor induces bone formation and improves skeletal integrity. Proc Natl Acad Sci U S A. 2008;105(19):7082-7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.