Abstract
Following stroke, attenuation of detrimental inflammatory pathways might be a promising strategy to improve long-term outcome. In particular, cascades driven by pro-inflammatory chemokines interact with neurotransmitter systems such as the GABAergic system. This crosstalk might be of relevance for mechanisms of neuronal plasticity, however, detailed studies are lacking. The purpose of this study was to determine if treatment with 1,1′-[1,4-phenylenebis(methylene)]bis[1,4,8,11-tetraazacyclotetradecane] (AMD3100), an antagonist to the C-X-C chemokine receptor type 4 (CXCR4) and partial allosteric agonist to CXCR7 (AMD3100) alone or in combination with C-X3-C chemokine receptor type 1 (CX3CR1) deficiency, affect the expression of GABAA subunits and glutamate decarboxylase (GAD) isoforms. Heterozygous, CX3CR1-deficient mice and wild-type littermates were subjected to photothrombosis (PT). Treatment with AMD3100 (0.5 mg/kg twice daily i.p.) was administered starting from day 2 after induction of PT until day 14 after the insult. At this time point, GABAA receptor subunits (α3, β3, δ), GAD65 and GAD67, and CXCR4 were analyzed from the peri-infarct tissue and homotypic brain regions of the contralateral hemisphere by quantitative real-time PCR and Western Blot. Fourteen days after PT, CX3CR1 deficiency resulted in a significant decrease of the three GABAA receptor subunits in both the lesioned and the contralateral hemisphere compared to sham-operated mice. Treatment with AMD3100 promoted the down-regulation of GABAA subunits and GAD67 in the ipsilateral peri-infarct area, while the β3 subunit and the GAD isoforms were up-regulated in homotypic regions of the contralateral cortex. Changes in GABAA receptor subunits and GABA synthesis suggest that the CXCR4/7 and CX3CR1 signaling pathways are involved in the regulation of GABAergic neurotransmission in the post-ischemic brain.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12035-021-02510-x.
Keywords: Stroke recovery, Neuronal plasticity, GABA, GABAA, Inhibition, CX3CR1, CXCR4, Glutamate decarboxylase, AMD3100
Background
Stroke remains the leading cause of disability worldwide [1]. Neurological deficits are the result of lost neuronal function and network integrity due to acute tissue demise but also are perpetuated by delayed neuronal dysfunction and delayed neuronal cell death. Neuronal damage and tissue demise in many cases cannot be avoided [2, 3] due to an exaggerated inflammatory response, even though patients have been treated with thrombolytics in the acute phase after stroke onset [4]. Indeed, inflammatory cascades, an increased astroglial and oligodendrocyte reactivity may dampen potential regenerative mechanisms to enhance plasticity mechanisms such as neural circuit remodeling [5, 6]. Hence, the brain recruits repair mechanisms to induce plastic changes such as axonal growth, synaptic remodeling, re-myelination, cortical reorganization, and re-wiring of neural networks to healthy intact tissue [7].
GABAA receptors are involved in neuronal plasticity mechanisms during the recovery phase after stroke. These pentameric ion channels consist of various combinations of 19 different subunits encoded by distinct genes, thus representing high heterogeneity in their functional assembly [8, 9]. Immunohistochemical and autoradiographic studies indicated an overall reduction of functional subunits in both the lesioned and intact hemisphere and a divergent role of the synaptic variant α3 subunit [10, 11]. Following photothrombosis, electrophysiological studies coupled with knockout experiments on the neuronal excitability in the peri-infarct area have demonstrated increased tonic inhibition mediated partially by α5- and δ-containing receptors. Pharmacological antagonism on these receptors resulted in improved outcome [12]. On the other hand, a down-regulation of α4 and δ subunits in cortical layers 2 and 3 in the peri-infarct area after transient middle cerebral artery occlusion (tMCAO) resulted in decreased tonic inhibition. This was associated with improved motor performance but increased susceptibility to epileptic seizures [13].
An increased synaptic inhibition is observed specifically in cortical layer 5 through up-regulation of α1-containing receptors, pharmacological agonism of which improved outcome after stroke [14]. Importantly, changes in the amount of functional α1-subunits do not necessarily correlate with respective mRNA level [15]. The β3 subunits might also be responsive to ischemia [16]. In addition to its eminent role in the regulation of plasticity mechanisms, experimental evidence emerged revealing γ-aminobutyric acid (GABA) signaling as a potential “cross-talker” between inflammatory and plasticity processes [17–19].
Modulation of chemokine pathways has emerged as a promising field promoting recovery after stroke. Regarding the CXCL12/CXCR4/CXCR7 pathway, we have shown that administration of AMD3100, an antagonist of CXCR4 receptor and a partial or allosteric agonist of CXCR7, improved functional outcome in rats subjected to tMCAO [20]. Similar effects have been confirmed in mice subjected to PT [21]. Effects were attributed to suppressed immune and microglial response in the ischemic territory [20, 21]. In addition, the CX3CL1/CX3CR1 chemokine pathway is involved in stroke pathology. Several studies have demonstrated that knockout mice, both in the cx3cr1 and the cx3cl1 locus, exhibit decreased brain injury and mortality rates [22–24]. Exogenous administration of CX3CL1 to wild-type mice reduced the ischemic damage following MCAO. In contrast, administration of the ligand to cx3cl1−/− mice increased the infarct size after the insult [22]. We have shown that CX3CR1 deficiency alters the morphology of microglia after tMCAO, ultimately affecting the microglial response but does not affect infarct size after tMCAO [25].
It is important to understand the mechanisms involved in enhancing plasticity in the post-stroke brain that promote functional outcome after stroke [5]. In this regard, the interplay between chemokine pathways and the GABAergic neurotransmission system has not been addressed. Here, we have investigated the effects of AMD3100 treatment and CX3CR1 deficiency on GABAA subunits in the post-ischemic brain with emphasis on the cortical regions ipsilateral and contralateral to the lesioned hemisphere. We chose to investigate the expression of the α3 subunit, a synaptic variant which has been demonstrated to be relevant to stroke studies regarding its expression in cortical regions, homotopic to the infarct area [10]. In addition, we investigated the potential regulation of the δ subunit as a representative variant of tonic inhibition [8, 12] and the β3 subunit as a representative variant of overall receptor assembly and inhibitory efficacy [26]. In addition, the role of GAD67 and GAD65, enzyme isoforms responsible for converting glutamate to GABA, were investigated as key elements of the GABAergic neurotransmission [27, 28].
Methods
Experimental Design
Studies are a continuation of previous investigations showing beneficial effects of AMD3100 following experimental stroke induced by photothrombosis (PT) without affecting infarct size [21]. All animal experiments were approved by the Malmö/Lund ethical committee. Animals were housed in a controlled environment with a 12:12 h light cycle beginning at 07:00 with room temperature maintained at 22 °C and with ad libitum access to food and water.
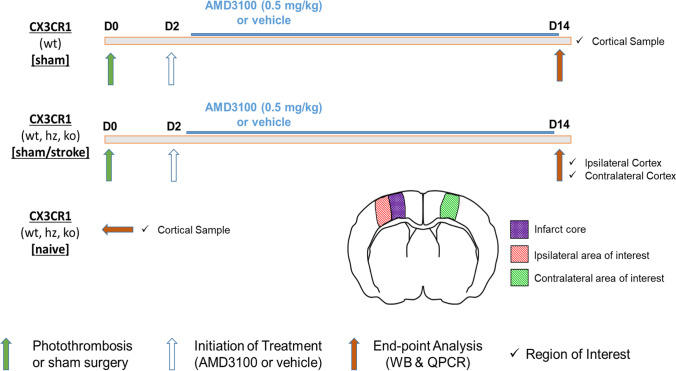
As shown in Fig. 1, wild-type [referred to as (wt) mice], CX3CR1 heterozygous [referred to as (hz) mice], and CX3CR1-deficient [referred to as (ko) mice] mice were subjected to permanent focal ischemia induced by photothrombosis (PT). From day 2 to day 14 after induction of stroke/sham surgery, animals were treated with either AMD3100 (every 12 h, 0.5 mg/kg body weight) or saline vehicle (vh). Treatments were administered by intraperitoneal injections twice daily. Naïve animals from all three genotypes were used as controls. On day 14, animals were sacrificed, brains were snap-frozen, stored at – 80 °C, and subsequently, tissue samples from the peri-infarct area and homotypic areas from the non-lesioned hemisphere were used for endpoint analyses (Fig. 1).
Fig. 1.
Experimental design. Endpoint analysis included quantification of protein levels by Western Blot and quantification of transcript levels by quantitative polymerase chain reaction. Sham-operated wild-type mice were used to study AMD3100 treatment effects in physiological conditions. Naïve animals did not undergo surgery
Animals
To study the effect of the CX3CR1 genotype, transgenic C57BL/6 mice, which carried GFP at the gene locus, under the control of the CX3CR1 promoter, were used [29]. Animals were obtained by own breeding. In total, 64 mice were used in which the experimental groups along with their respective treatment consisted of the following: 3 sham/vh mice consisting of 1 wt, 1 Hz and 1 ko mouse, 4 PT/vh-wt mice, 10 PT/vh-hz mice, 5 PT/vh-ko, 5 PT/AMD-wt, 5 PT/AMD-hz, and 5 PT/AMD-ko mice. Fourteen naïve mice were used of which 5 were wt, 4 were hz and 5 were ko on the CX3CR1 locus. In addition, male C57BL/6 mice were used (28 to 35 g, aged 12 to 16 weeks; Charles River, Sulzfeld, Germany) to investigate the effect of AMD3100 treatment. Thirteen animals underwent sham surgery, 7 of which were treated with saline (sham/vh; n = 7) and 6 of which were treated with AMD3100 (sham/AMD; n = 6).
Photothrombosis
Photothrombosis was performed as previously described [21]. In brief, animals were initially anesthetized with isoflurane (4 l/min) and placed in a stereotactic frame. During surgery, the animals were spontaneously breathing through a facemask delivering 1.7 L/min of isoflurane in a gas mixture of 30% O2 and 70% N2O. The surgery started with a sagittal skin incision made on the scalp to expose the skull. Subcutaneous connective tissue was removed and the skullbone was dried. Thereafter, the photosensitive dye Rose Bengal (concentration 10 mg/ml) was injected intraperitoneally. Five minutes after injection, cold light was applied through a round aperture with a diameter of 2.5 mm through the intact skull at the following coordinates (related to bregma): anterior–posterior 1.5 mm and medio-lateral 1 mm. Illumination was carried out for 20 min at an intensity of 3050 K/4D using a Schott cold light source (Schott KL 1500lcd, Mainz, Germany). Following illumination, the scalp incision was sutured, and the mice were transferred to their home cages. Animals subjected to sham surgery were injected intraperitoneally with saline solution. During surgery, the body temperature was monitored through a rectal thermistor probe connected to a heating pad maintaining body temperature between 36.3 and 37.2 °C (Linton Instrumentation, Norfolk, UK). The breathing of the animal was also monitored to adjust anesthesia.
Genotyping
Genomic DNA was extracted from ear punches. Extraction and PCR were performed using the KAPA Mouse Genotyping Kit (Wilmington, MA, USA) according to the manufacturer’s protocol. Primers CX3CR1_3945 [TTCACGTTCGGTCTGGTGGG], CX3CR1_39456 [GGTTCCTAGTGGAGCTAGGG], and CX3CR1_3947 [GATCACTCTCGGCATGGACG] were used in a final concentration of 500 nΜ. Amplification was carried out in a MJ Mini Personal Thermal Cycler (BioRad, Hercules, USA). Amplified fragments were separated by agarose gel electrophoresis, and results were visualized using a ChemiDoc™ MP system (BioRad).
Western blot
Tissue samples from snap-frozen brains (isopentane – 70 °C) were microdissected from the peri-infarct area/ipsilateral cortex and the homotypic region of the contralateral hemisphere. Samples were homogenized in lysis buffer (20 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 1 mM β-glycerolphosphate, 1 mM sodium orthovanadate (Na3VO4), 1 mM phenylmethylsulfonyl fluoride (PMSF), and cOmplete™ Protease Inhibitor Cocktail (Sigma-Aldrich) by sonication and centrifuged at 14.000 RPM for 20 min at 4 °C. The supernatant was collected and stored at – 80 °C.
As indicated in respective figure legends, twenty, thirty, or sixty micrograms of proteins were separated in Mini-Protean® TGX™ and Mini-Protean® TGX Stain-Free™ Gels (Bio-Rad, Hercules, USA) and casted gels. Thereafter, proteins were electro-blotted using a Trans-blot® Turbo™ (Bio-Rad, Hercules, USA) system. After blocking in 5% non-fat dry milk (in TBST), membranes were incubated with primary antibodies including a mouse monoclonal anti-glutamic acid decarboxylase 67 (GAD67) (1:5000; Merck Millipore, Temecula, USA), a rabbit polyclonal anti-GABAA α3 Receptor (1:500; Alomone Labs, Jerusalem, Israel), a rabbit polyclonal anti-GABAA δ Receptor (1:2000, Novus Biologicals, Colorado, USA), a rabbit polyclonal anti-GABAA β3 Receptor (1:2000, Novus Biologicals, Colorado, USA), a rabbit polyclonal anti-CXCR4 (1:1000, Abcam, ab2074, Cambridge, UK), and a rabbit polyclonal anti-GAD65 (1:1000, Novus Biologicals, Colorado, USA) antibody. For the secondary antibody incubation, anti-mouse horse radish peroxidase (HRP) antibody (1:10.000, Sigma-Aldrich, Deisenhofen, Germany) or anti-rabbit HRP antibody (1:12.000; Sigma-Aldrich, Deisenhofen, Germany) were used, respectively. For loading normalization, HRP-conjugated β-actin antibody was used (1:75.000, Sigma-Aldrich, Deisenhofen, Germany). Membranes were exposed on a ChemiDoc™ MP system (BioRad) using a chemiluminescence kit (Merck Millipore, Billerica, MA, USA). Densitometry analysis was conducted using ImageJ software [30].
Quantitative Real-Time PCR
Extraction of RNA from all tissue brain samples was done with RNeasy® Mini Kit (Qiagen, Hilden, Germany, RefID 74,104) according to the protocol of the manufacturer’s instructions. Dithiothreitol (DTT) was used as a reducing reagent for the isolation of RNA. RNA samples were analyzed for their purity using the NanoDrop (Thermo Scientific, Wilmington, USA). cDNA synthesis was done using iScript cDNA Synthesis Kit (Cat# 1,708,891, Bio-Rad, Hercules, USA) in a MJ Mini Personal Thermal Cycler (Bio-Rad, Hercules, USA) according to the instructions of the manufacturer. Nine-hundred nanograms from each sample were used as template for cDNA synthesis. Samples were diluted to a final concentration of 10 ng/μL.
Quantitative PCR was carried out using the CFX Connect™ Real-Time System (Bio-Rad, Hercules, USA) with SsoAdvanced Universal SYBR® Green Supermix (Cat# 1,725,271, Bio-Rad, Hercules, USA). Sample reaction volume was set to 10 μL. Gene-specific primers were used for the detection of GABAA receptor α3 subunit, δ subunit, β3 subunit, and GAD67 transcripts (online resource). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) transcript levels were used as the reference gene for normalization. Ten nanograms were used from each sample for every respective reaction.
Transcript levels were amplified by applying the following PCR protocol parameters: 35 cycles consisting of an initial step at 95 °C for 30 s, cycle denaturation at 95 °C for 5 s, and 57 °C (α3, δ, β3, GAPDH), 56 °C (CXCR4), or 58 °C (GAD67) annealing/extension stage for 30 s. External standard curves were used for the estimation of the reaction amplification efficiency using a pool of all the samples and consecutive dilutions of 1, 1/2, 1/5, 1/10, 1/20, and 1/50 of the initial pooled sample. Ct values were plotted against the log10 of these dilutions for the calculation of the slope. Concentration of primers specific to GABAA subunits and CXCR4 was set to 200 nM and 300 nM for GAD67 transcripts. Primer concentration for GAPDH reactions was set to 500 nM.
Efficiency of each primer pair was calculated from the slope using the formula [E = − 1 + 10−1/slope] [31]. All reaction efficiencies varied between 99 and 107%. Ct values were converted to fluorescence values based on the exponential amplification formula [Rn = R0 (1 + E) ^n], where E is the efficiency of the reaction, n is the Ct value, Rn is the threshold fluorescence value from which the Ct value is derived, and R0 is the target initial fluorescence value of the sample, corresponding to cDNA levels [32]. Gene expression data was calculated as the ratio of target gene transcript levels to GAPDH transcript levels and are expressed as means ± standard error of the mean (S.E.M.).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8. Expression levels of genes within each hemisphere were evaluated by one-way analysis of variance (ANOVA) and post hoc multiple comparisons were performed with Tukey’s test (significance level 0.05, confidence interval 0.95). For the comparison of the expression levels between the ipsilateral and the contralateral cortex, two-way analysis of variance (ANOVA) was used along with post hoc Sidak’s multiple comparison test. Western blot results were evaluated either by Students t test (significance level 0.05, confidence interval 0.95) or ANOVA and post hoc Tukey’s test. All data are expressed as the mean ± S.E.M.
Results
Effect of CX3CR1 Genotype on GABAA Subunits and GAD67 and GAD65 Expression
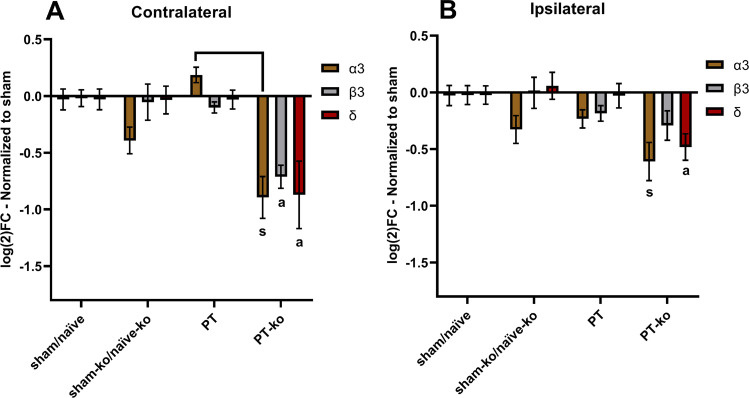
In order to identify alterations in the gene expression of various GABAA subunits in the post-ischemic brain, mRNA levels were quantified in the peri-infarct area, the homotypic region of the contralateral cortex, and respective regions in sham-operated and naïve mice (Fig. 1). Cx3cr1 knockout mice subjected to PT showed a decrease in the mRNA levels of the GABAA subunits α3, β3, and δ in the ipsi- and contralateral hemisphere compared to sham/naïve mice of both genotypes (Fig. 2). This decrease was more profound on the contralateral non-lesioned cortex with knockout mice subjected to PT exhibiting significantly lower mRNA levels of all three GABAA subunits compared to wt/hz littermates after PT (Fig. 2). In both hemispheres, mRNA levels of the GABAA δ subunit in cx3cr1 knockout mice subjected to PT were significantly lower compared to all experimental groups (Fig. 2).
Fig. 2.
Expression of GABAA subunits in CX3CR1 deficient mice. A Effect of receptor deficiency on mRNA levels of the contralateral cortex and B ipsilateral cortex. All data depicted are normalized to expression levels of sham/naïve animals and shown as a log2 fold-change. All animals corresponding to the experimental groups received i.p. saline injections except naïve animals. Bars represent the mean ± S.E.M. [contralateral: sham/naïve n = 18 (8 sham-wt, 1 sham-hz, 5 naïve-wt, 4 naïve-hz), sham-ko/naïve-ko n = 6 (1 sham-ko, 5 naïve-ko), PT n = 13 (4 wt, 9 Hz), PT-ko n = 5; ipsilateral: sham/naïve n = 18 (8 sham-wt, 1 sham-hz, 5 naïve-wt, 4 naïve-hz), sham-ko/naïve-ko n = 6 (1 sham-ko, 5 naïve-ko), PT n = 14 (4 wt, 10 Hz), PT-ko n = 5]. CX3CR1-deficient mice subjected to PT exhibited significantly lower mRNA levels in all GABAA subunits compared to sham/naïve levels [s: (p < 0.01)]. Subunits labelled with [a] exhibit significant difference compared to all groups. Additional brackets indicate significant differences between respective groups. [contralateral α3: PT-ko vs PT (p < 0.0001); contralateral β3: PT-ko vs sham-ko/naïve-ko & PT-ko vs PT (p < 0.005); contralateral δ: PT-ko vs PT (p = 0.007) & PT-ko vs sham-ko/naïve-ko (p < 0.022); ipsilateral α3: PT-ko vs sham/naïve (p = 0.009); ipsilateral δ: PT-ko vs PT (p = 0.001) & PT-ko vs sham-ko/naïve-ko (p = 0.01)]
Throughout all experimental groups, no difference was observed for the β3 between the peri-infarct area and corresponding regions from sham/naïve mice (Fig. 2). In addition, we did not find differences between wt and hz mice in regard to all three subunits and experimental conditions. Thus, these mice were pooled for the analysis. Finally, no significant differences were found between naïve knockout mice and knockout mice subjected to sham surgery compared to wt/hz littermates (Fig. 2). Together, these experiments support the idea that cx3cr1 deletion contributes in the regulation of GABAA receptor subunits synthesis in the recovery phase of stroke.
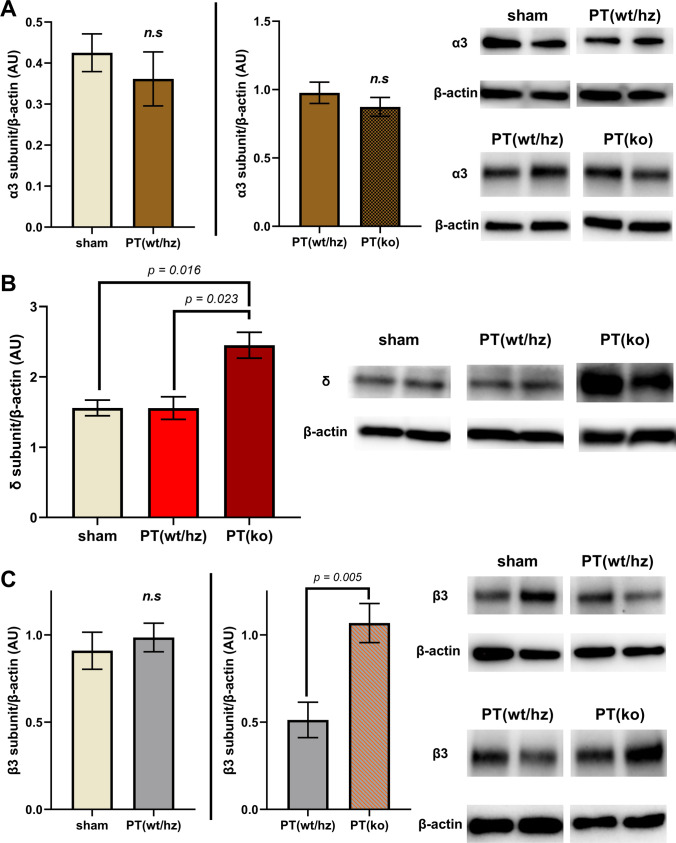
Transcriptional data prompted us to determine protein levels of the three GABAA subunits in the contralateral cortex of wild-type/heterozygous and cx3cr1 knockout mice. As shown in Fig. 3, no difference was observed between the experimental groups regarding the synaptic GABAA α3 subunit. In addition, we also did not find differences in the levels of the α3 subunit comparing sham-operated/naïve animals with wt/hz mice subjected to PT in the contralateral cortex. In contrast to the transcriptional data, we observed increased protein levels of the δ and β3 subunit in knockout mice both compared to wt/hz littermates subjected to PT (Fig. 3). No differences were found between naïve/sham-operated mice and mice subjected to PT (Fig. 3). These findings indicate that the CX3CR1 pathway may regulate the transcription of GABAA subunit as well as it may modulate the composition of GABAA receptors on the post-translational level.
Fig. 3.
Effect of CX3CR1 deficiency on protein levels of GABAΑ subunits on day 14 after stroke. Protein levels of GABAA α3, δ subunits in the contralateral cortex of sham and stroke mice. Specific bands for α3 subunit (55 kDa), δ subunit (52 kDa), b3 subunit (55 kDa), and β-actin were quantified and presented as the percentage ratio of β-actin (AU) as mean ± S.E.M. Protein sample amount used for the α3 subunit was 30 μg, 60 μg for the δ subunit, and 20 μg for the β3 subunit. Representative band images used in each separate graph were taken from the same membrane. Each graph represents a separate Western Blot experiment. All animals corresponding to the experimental groups received i.p. saline injections. [contralateral α3: sham n = 3 (2 wt, 1 Hz) vs PT(wt/hz) n = 4 (2 wt, 2 Hz), PT(wt/hz) n = 6 (4 wt, 2 Hz) vs PT(ko) n = 5. No statistical differences were observed using Student’s test; (sham vs PT(wt/hz) p = 0.50, PT(wt/hz) vs PT(ko), p = 0.35)]. [contralateral δ: sham n = 3 (2 wt, 1 Hz), PT(wt/hz) n = 5 (3 wt, 2 Hz), PT(ko) n = 5. Statistical differences were observed using ANOVA (p = 0.005) and post hoc Tukey’s multiple comparison test; sham vs PT(wt/hz) p > 0.999]. [contralateral β3: sham n = 5 (2 wt, 3 Hz) vs PT(wt/hz) n = 6 (3 wt, 3 Hz), PT(wt/hz) n = 6 (3 wt, 3 Hz) vs PT(ko) n = 5. Statistical differences were evaluated by Student’s test. sham vs PT(wt/hz) p > 0.58]
Moreover, CX3CR1 deficiency did not affect the transcriptional and posttranscriptional regulation of GAD67 (data not shown); no difference in GAD65 protein levels was found comparing CX3CR1-deficient mice with wt/hz littermates (online resource).
Effect of AMD3100 Treatment on GABAA Subunit Expression
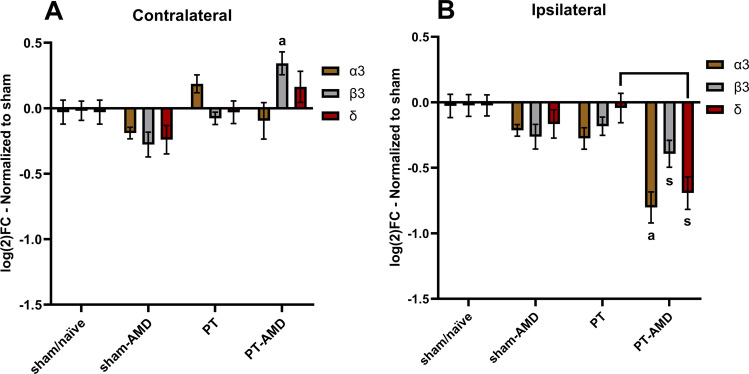
As previously been reported, the CXCL12/CXCR4 pathway directly affects neuronal function [33]. Here, we evaluated if treatment with the CXCR4 antagonist AMD3100 affected the composition of GABAA receptors. Upon treatment, we found an up-regulation of the β3 subunit in the contralateral cortex (Fig. 4A) and a down-regulation of all three subunits in the peri-infarct area (Fig. 4B). In specific, treated mice subjected to PT exhibited significantly lower transcript levels of the GABAΑ α3 and δ subunits in the peri-infarct area, both compared to sham/naïve mice and untreated mice subjected to PT. In addition, these experiments showed no differences in transcript quantities of untreated mice 14 days after PT compared to sham/naïve animals (Fig. 4). Together, these results point towards a down-regulation of GABAA receptors in the ipsilateral and an up-regulation in the contralateral hemisphere after PT upon treatment with AMD3100, respectively.
Fig. 4.
Effect of AMD3100 treatment on the expression of GABAA subunits on day 14 after stroke. mRNA levels of GABAA subunits in the contralateral (A) and in the ipsilateral (B) cortex. All data are normalized to expression levels of sham/naïve animals and shown as a log2 fold-change. Bars represent the mean ± S.E.M. Animals not treated with AMD3100 received i.p. saline injections. [contralateral: sham/naïve n = 18 (8 sham-wt, 1 sham-hz, 5 naïve-wt, 4 naïve-hz), sham/AMD n = 6 (all wt), PT n = 13 (4 wt, 9 Hz), PT/AMD n = 10 (5 wt, 5 Hz); ipsilateral: sham/naïve n = 18 (8 sham-wt, 1 sham-hz, 5 naïve-wt, 4 naïve-hz), sham/AMD n = 6 (all wt), PT n = 14 (4 wt, 10 Hz), PT/AMD n = 10 (5 wt, 5 Hz)]. Mice subjected to PT and treated with AMD3100 exhibited significantly lower mRNA levels in all GABAA subunits compared to sham/naïve levels in the ipsilateral hemisphere (p < 0.05). Subunits labeled with [a] exhibit significant difference compared to all groups. Additional brackets indicate significant differences between respective groups. [contralateral β3: PT-AMD vs all groups (p < 0.005), ipsilateral α3: PT-AMD vs sham-AMD (p < 0.015) & PT-AMD vs PT (p = 0.004), ipsilateral δ: PT-AMD vs PT (p = 0.001)
Expression of CXCR4 in the Post-Ischemic Brain
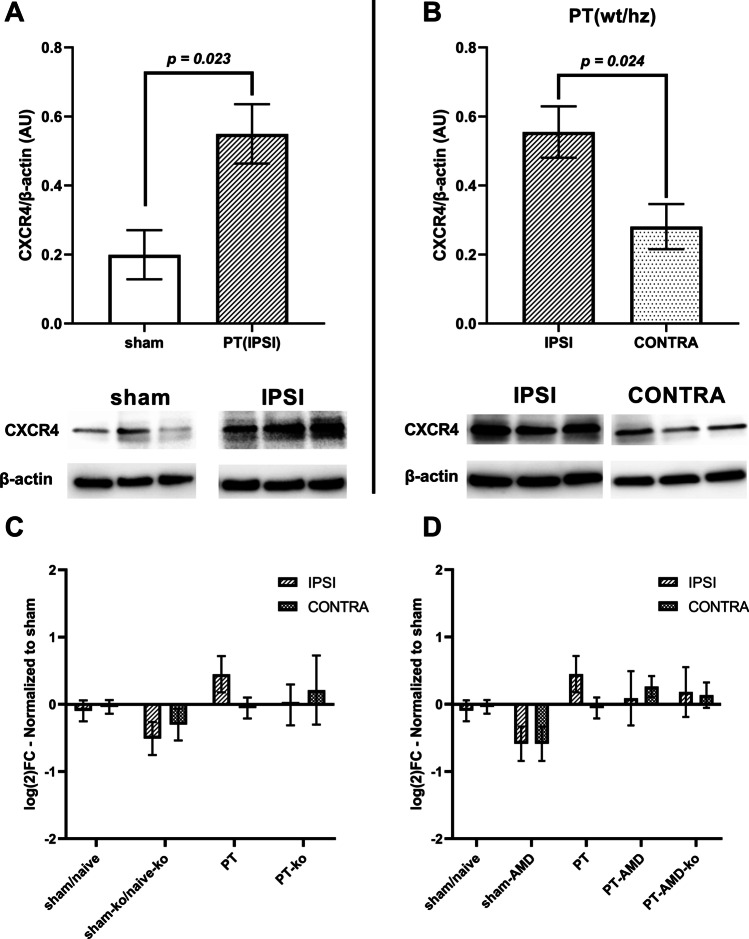
Gene expression and protein levels of CXCR4 were determined between the ipsi- and contralateral hemisphere following PT. As shown in Fig. 5A, we observed increased CXCR4 protein levels in the peri-infarct area of mice subjected to PT, 14 days after stroke, compared to sham-operated mice. In addition, CXCR4 protein levels in the ipsilateral cortex were significantly higher compared to the contralateral intact cortex, in wt/hz mice subjected to PT (Fig. 5B). Expression of CXCR4 in healthy cortical tissue of the non-lesioned hemisphere, even to a lesser extent, confirms the presence of CXCR4 in the contralateral cortex.
Fig. 5.
Levels of CXCR4 in the post-ischemic brain. Comparison of protein levels of CXCR4 between the cortex of sham mice and the ipsilateral cortex of stroke mice (A) and between the contralateral and the ipsilateral cortex (B) after PT. Twenty micrograms of sample protein for used for CXCR4. [sham n = 4 (2 wt, 2 Hz) vs PT-IPSI n = 7 (4 wt, 3 Hz), PT-IPSI n = 6 (4 wt, 2 Hz) vs PT-CONTRA n = 5 (2 wt, 3 Hz)]. Effect of CX3CR1 deficiency on transcript levels of CXCR4 (C) and effect of AMD3100 treatment on transcript levels of CXCR4 (D). Data are normalized to expression levels of sham/naïve animals and shown as a log2 fold-change. Bars represent the mean ± S.E.M. Animals not treated with AMD3100 received i.p. saline injections. Naïve animals did not receive any injections. [contralateral: sham/naïve n = 11 (1 sham-wt, 1 sham-hz, 5 naïve-wt, 4 naïve-hz), sham-ko/naïve-ko n = 6 (1 sham-ko, 5 naïve-ko), sham/AMD n = 6 (all wt), PT n = 12 (4 wt, 8 Hz), PT-ko n = 5, PT-AMD n = 5 (all wt), PT-AMD-ko n = 5]. [ipsilateral: sham/naïve n = 11 (1 sham-wt, 1 sham-hz, 5 naïve-wt, 4 naïve-hz), sham-ko/naïve-ko n = 6 (1 sham-ko, 5 naïve-ko), sham/AMD n = 6 (all wt), PT n = 14 (4 wt, 10 Hz), PT-ko n = 5, PT-AMD n = 5 (all wt), PT-AMD-ko n = 5]. No significant differences were neither observed by post hoc Tukey’s multiple comparisons following One-way ANOVA in respect to each hemisphere nor by Sidak’s multiple comparisons following two-way ANOVA in respect to the two hemispheres
As such, we decided to investigate possible alterations of CXCR4 transcript levels across all the experimental groups. No significant differences were observed between any groups, both in regard toCX3CR1 deficiency and AMD3100 treatment (Fig. 5C, D). These experiments confirm the presence of the receptor in cells located in regions contralateral to the site of injury.
Effect of AMD3100 Treatment on GAD67 and GAD65 Expression
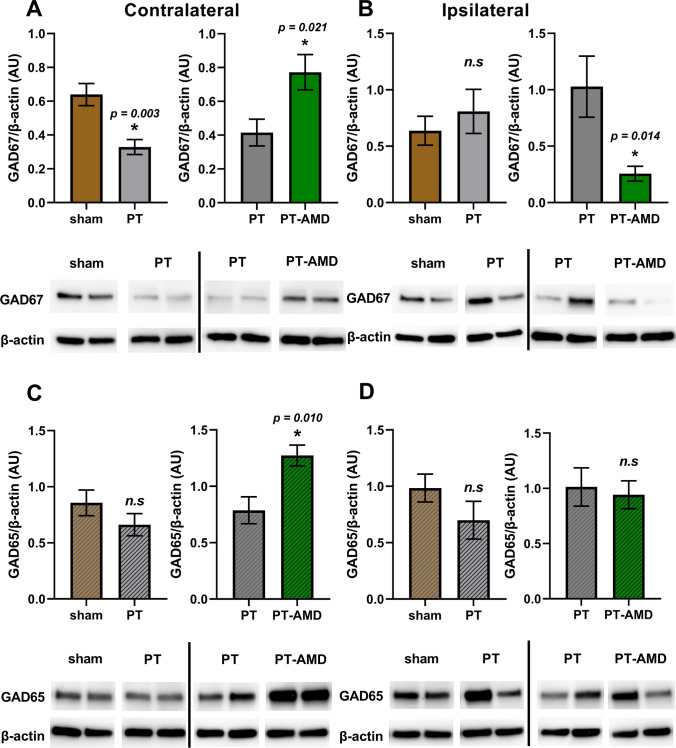
Likewise, we observed an opposite effect of AMD3100 on the protein levels of the GABA synthesis regulatory enzyme GAD67 in the peri-infarct area and contralateral cortex (Fig. 6). Western blot experiments were separated in a way to investigate two conditions at a time. In particular, we found similar levels of GAD67 in the ipsilateral peri-infarct area of vehicle treated-mice subjected to PT (0.81 ± 0.2, n = 6) compared to sham-operated mice (0.64 ± 0.13, n = 5) (Fig. 6B). In contrast, levels were decreased in the contralateral cortex (0.33 ± 0.04, n = 6) compared to sham operated mice (0.64 ± 0.07, n = 5) (Fig. 5A). Treatment with AMD3100 significantly reduced the levels of GAD67 in the peri-infarct area (0.27 ± 0.07, n = 6) compared to vehicle-treated mice (1.03 ± 0.27, n = 5) (Fig. 6B). Levels of GAD67 were significantly higher in AMD-treated mice in the contralateral cortex (0.77 ± 0.17, n = 5) compared to vehicle-treated animals (0.42 ± 0.08, n = 6) after PT (Fig. 6A). Regulation of GAD67 was purely post-transcriptional, since mRNA levels of GAD67 transcripts were similar between the experimental groups (data not shown).
Fig. 6.
Effect of AMD3100 treatment on levels of GAD67 and GAD65 on day 14 after stroke. Levels of GAD67 and GAD65 in the contralateral (A, C) and ipsilateral (B, D) cortex of mice subjected to PT and treated with AMD3100 (AMD) i.p injections for 12 days starting on day 2 after PT. Non-treated mice received saline i.p. injections. Specific bands for GAD67 (67 kDa) and GAD65 (65 kDa) and β-actin were quantified and presented as the percentage ratio of β-actin (AU) as mean ± S.E.M. Twenty micrograms of protein were used for both GAD67 and GAD65. Representative band images used in each separate graph were taken from the same membrane. Each graph represents a separate Western Blot experiment. Statistical differences were evaluated by Student’s test. [contralateral GAD67: sham n = 5 (3 wt, 2 Hz), PT n = 6 (3 wt, 3 Hz), PT-AMD n = 5 (4 wt, 1 Hz); ipsilateral GAD67: sham n = 5 (3 wt, 2 Hz), PT n = 6 (4 wt, 2 Hz), PT-AMD n = 5 (3 wt, 2 Hz)]. [contralateral GAD65: sham n = 5 (3 wt, 2 Hz) vs PT n = 6 (3 wt, 3 Hz), PT n = 5 (1 wt, 4 Hz) vs PT-AMD n = 6 (3 wt, 3 Hz); ipsilateral GAD65: sham n = 5 (3 wt, 2 Hz) vs PT n = 6 (4 wt, 2 Hz), PT n = 5 (4 wt, 1 Hz) vs PT-AMD n = 6 (4 wt, 2 Hz)]
Regarding the protein levels of GAD65, similar levels were found in the ipsilateral peri-infarct area of vehicle treated-mice subjected to PT (0.70 ± 0.17, n = 6) compared to sham-operated mice (0.98 ± 0.12, n = 5) (Fig. 6D). Similarly, we found no significant differences in levels of GAD65 in the contralateral cortex (0.66 ± 0.10, n = 6) compared to sham-operated mice (0.86 ± 0.11, n = 5) (Fig. 6C). Contrary to the effect of AMD3100 on GAD67 levels in the peri-infarct area, treatment did not change GAD65 levels in AMD-treated mice (0.94 ± 0.13, n = 6) compared to vehicle-treated animals (1.01 ± 0.17, n = 5) subjected to PT (Fig. 6D). However, in parallel to the up-regulation of GAD67 in the contralateral cortex due to treatment with AMD3100, we also observed significantly higher levels of GAD65 in the contralateral cortex of AMD-treated mice (1.27 ± 0.09, n = 6) compared to vehicle-treated mice (0.79 ± 0.12, n = 5) after PT (Fig. 6C).
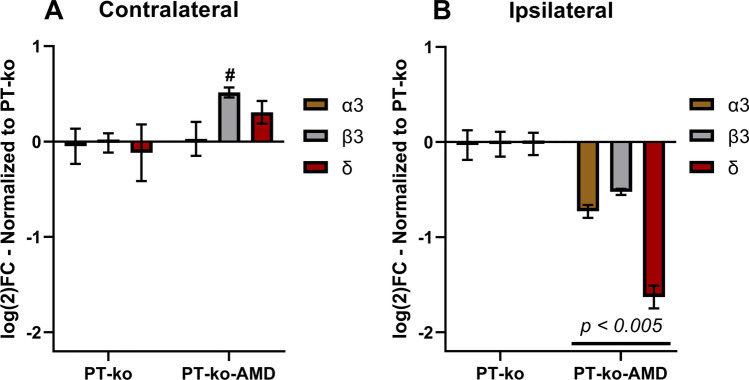
In order to investigate the effect of the treatment together with CX3CR1 deficiency in the post-ischemic brain, expression of gene transcripts for the α3, β3, and δ subunits were analyzed in the ipsilateral peri-infarct area and the contralateral cortex (Fig. 7). Compared to vehicle-treated mice, the expression of all three subunits was significantly reduced in the peri-infarct area following treatment with AMD3100. The opposite effect was found in the contralateral hemisphere showing a significant higher expression of the β3 subunit. The other two subunits remained unchanged compared to levels found in vehicle treated cx3cr1 knockout mice. These results point towards an additive effect of AMD3100 treatment on the down-regulation of the three GABAA subunits in the ischemic hemisphere of cx3cr1 knockout mice.
Fig. 7.
Effect of AMD3100 treatment on post-ischemic CX3CR1-deficient mouse brain. mRNA levels of GABAA subunits in the contralateral (A) and ipsilateral (B) cortex from mice deficient for CX3CR1 and subjected to PT. All data are normalized to expression levels of PT-ko mice and shown as a log2 fold-change. Deficient mice subjected to PT and treated with AMD3100 exhibited significantly lower mRNA levels in all GABAA subunits in the ipsilateral cortex compared to untreated (saline) deficient mice (p < 0.005). [contralateral: PT-ko n = 5, PT-AMD-ko n = 5; ipsilateral: PT-ko n = 5, PT-AMD-ko n = 5]. Additional symbols indicate significant differences between respective groups. [#: PT-ko vs PT-AMD-ko (p = 0.001)]. Statistical differences were evaluated by Student’s test
A more comprehensive view on differences in GABAA receptor subunit expression in the lesioned ipsilateral and the non-lesioned contralateral hemisphere after PT is shown in the online resource. Based on this compilation, a significant difference of mRNA levels of the synaptic α3 subunit between the contralateral and ipsilateral cortex on day 14 after PT and in respective regions of mice subjected to PT and treated with AMD3100 becomes evident (Online resource 1). Moreover, compiled data again show the general down-regulation of GABAA receptor subunit transcripts in cx3cr1 knockout mice and additional down-regulation by treatment with AMD3100. This additive effect of the two experimental conditions seems to be especially high on the down-regulation of GABAA δ subunit transcripts in the peri-infarct area (online resource). Treatment also had an opposite effect of receptor subunits expression in the ipsi- and contralateral hemisphere with higher expression levels in the contralateral cortex.
Discussion
The present study has been conducted based on previous studies providing evidence for an interaction between the CXCL12/CXCR4/7 and the fractalkine/CX3CR1 chemokine pathways with GABAergic neurotransmission. Here, we investigated if CX3CR1 deficiency and treatment with AMD3100, an antagonist on the CXCR4 and partial allosteric agonist on the CXCR7 affects the expression of the GABAA receptor subunits α3, β3, and δ and the synthesis of GABA by regulation of the glutamic acid decarboxylase isoforms 67 and 65. Our results indicate that CX3CR1 deficiency alters the expression of GABAA subunits in the post-ischemic brain. In addition, treatment with AMD3100 decreased the expression of receptor subunits in the ipsilateral peri-infarct area, while an increased expression was found in the non-lesioned contralateral hemisphere.
GABAA Receptor Composition and GAD67/65 Regulation After PT: Effects of AMD3100 Treatment
Scientific and clinical evidence point towards an excitatory/inhibitory (E/I) imbalance between the lesioned and contralateral hemisphere in the post-ischemic brain [34, 35]. It is characterized by an increased activity (excitation, E) in the contralateral hemisphere exerting inhibition towards the lesioned hemisphere. This model of interhemispheric imbalance is believed to impair neuronal plasticity mechanisms required to remodel neuronal connections and circuits [36]. Structural units to maintain inhibition are respective synapses containing GABAA receptors and GAD isoforms as key mediators of both fast-paced and long-term inhibition. Receptor subunits are also characterized by several modes of regulation, including transcriptional control, post-transcriptional, and post-translational modifications [37–39]. As such, GABAA receptor assembly dynamics can be modulated on multiple levels.
Previous studies have associated either pharmacologically induced reduction of tonic inhibition or spontaneously reduced tonic inhibition in the peri-infarct area with improved functional outcome [12, 13]. Here, we found that AMD3100 reduces the expression of the δ-subunit, involved in tonic inhibition, in the ipsilateral peri-infarct area. This effect could complement attenuated inflammatory cascades achieved by the treatment, ultimately resulting in an improved outcome [20].
The β3 subunit has been proposed to possess a vital role in mediating inhibitory transmission through its assembly with the majority of functional receptors, synaptic or extra-synaptic [26]. For this reason, changes in the levels of the β3 subunit could reflect changes in the overall quantity of functional receptors or total inhibitory efficiency. Furthermore, different expression levels of the β3 subunit between the two hemispheres could indicate transcriptional control as a downstream effect of CXCR4 signaling. In addition, we observed an asymmetry in the expression of the synaptic α3 subunit of vehicle-treated mice subjected to PT (online resource). Differential expression in the ipsi- and contralateral hemisphere might be interpreted as an innate attempt to lower the inhibitory tonus in the peri-infarct area and to promote an increased inhibition in the contralateral cortex.
Following stroke, an accumulation of CXCR4 positive cells, including neurons, is observed in the peri-infarct area accompanied with increased levels of CXCL12, the endogenous ligand of the CXCR4 [20, 40, 41]. In contrast, these changes are not found in homotypic regions of the contralateral cortex. Similar to the results of this study, the higher amount of CXCR4 receptors present in the peri-infarct area, 2 weeks after PT, points towards an accumulation of CXCR4 positive cells. Therefore, differential patterns of CXCR4-expressing cells along with different levels of CXCL12 throughout the ischemic brain might explain different compositions of GABAA receptors in different regions and could also explain the different effect of the treatment on the expression profile of GABAA receptor subunits.
The presence of CXCR4+ cells in the ipsilateral non-lesioned cortex, both during the acute and subacute phase [21, 40, 41], indicates the existence of viable targets for AMD3100 during the first weeks after an ischemic insult. Recent studies have extensively delineated the composition of immune CXCR4+ cells that accumulate in the area in and adjacent to the infarct core during the acute phase and that CXCR4 can be used as marker to distinguish microglia from infiltrating monocytes in the ischemic territory [42]. Specifically, it was shown that monocytes greatly populate the peri-infarct area at day 3 after PT. The majority of immune cells from day 8 onwards were Iba+ microglia negative for CXCR4 [39]. As such, the mode of action of AMD3100 probably involves two levels of interaction. First, it modulates the inflammatory response in the acute phase which in turn affects the primary accumulation of immune cells. Subsequently, it regulates mechanisms of neuronal plasticity remodeling in the recovery phase through its interaction with CXCR4+ cells. Such cells could potentially be neurons, microglia, or astrocytes of the glial scar that constitutively or transiently express CXCR4 [20].
However, it still remains to be determined how the chemokine receptor interacts mechanistically with GABAA receptors. The fact that we observed no difference in transcript amounts of GABAA receptor subunits between sham/naïve and PT animals 14 days after stroke may indicate a stabilization of the inhibitory tonus in the peri-infarct area which partially is mediated by CXCR4. Similar transcript levels of CXCR4 observed both 2 weeks after PT with and without AMD3100 treatment might correspond to a stable CXCR4 expression in neurons. In addition, we found no changes in transcriptional activity of GABAA subunit genes in the lesioned ipsilateral hemisphere, ultimately retaining a stable GABAA receptor assembly. Therefore, effects of AMD3100 on the expression of GABAA receptor subunits most likely seem an indirect effect possible through regulation of the fractalkine/CX3CR1 pathway [43].
Differential patterns of the CXCR4 expression on GABAergic interneurons [40, 41] in the ipsilateral and contralateral hemisphere may also have an effect on GAD67 levels. In vitro studies have demonstrated that activation of the CXCR4 signaling through CXCL12 induces the expression of GAD67 in embryonic hippocampal neurons [44]. In line with these data, we found that treatment with AMD3100 resulted in a decrease of GAD67 levels in the peri-infarct area. Moreover, treatment with AMD3100 could possible prevent the establishment of the GABAergic phenotype in migrating neural precursor cells or residing viable neurons in the peri-infarct area, ultimately suppressing the formation of GABAergic interneurons [40, 45]. This effect was not found in the contralateral cortex, possibly due to a different mechanisms of action between healthy tissue and tissue adjacent to the infarct core.
An endogenous down-regulation of GAD67 in the contralateral cortex after PT might be achieved as a result of the interhemispheric imbalance [36]. Increased neuronal activity is observed in the homotopic motor regions of the contralateral hemisphere, reflected by reduced GAD67 levels. Attenuated inhibition due to treatment with AMD3100 elevates neuronal activity in the lesioned hemisphere and thereby might contribute to re-establishes an equilibrium between the two hemispheres. This process includes the normalization of GAD67 levels and the regulation of the β3 subunit in the contralateral hemisphere. In addition, up-regulation of GAD65 in the contralateral cortex by treatment with AMD3100 indicates an increased inhibitory tonus.
Cross-Talk Between the CX3CR1 Pathway and the GABAergic System
Several studies have focused on the involvement of the CX3CL1/CX3CR1 pathway in stroke pathophysiology, presenting ambiguous conclusions on whether activation of the chemokine cascade possesses a neuroprotective or detrimental role [22, 23, 25]. Our results show that CX3CR1 deficiency alters the expression of GABAA subunits in the ipsilateral peri-infarct area and contralateral cortex. In addition, GABAA receptor regulation by the CX3CR1 pathway may include post-translational modifications which may affect membrane stability, assembly dynamics, and degradation rate [37–39]. Interestingly, activation of the pathway in cells of the rat midbrain raphe nuclei increased inhibitory postsynaptic currents (IPSC) in serotoninergic neurons [46]. Susceptibility to inhibitory currents might have been related to an increase in the number of post-synaptic receptors, a theory that could explain the alterations of GABAA subunits seen in our study. Under physiological conditions, the absence of CX3CR1 may promote a compensatory increase of GABAA receptors preserving a sufficient inhibitory tonus in the contralateral hemisphere.
Moreover, administration of CX3CL1 to cortical tissue derived from patients with mesial temporal lobe epilepsy, a pathological condition associated with impaired GABAA inhibitory signaling, attenuated use-dependent decrease (rundown) of GABA-evoked currents suggesting GABAA receptor desensitization or subunit alterations by the CX3CR1 chemokine pathway [47]. It should also not been neglected that alterations of GABA-currents and synaptic modifications might have been induced by microglia [48]. Therefore, further studies are required to elucidate the role of microglia in the regulation of GABAA receptor subunits in cx3cr1 knockout mice and respective wild-type littermates.
Conclusion
Our study shows that treatment with the CXCR4 antagonist AMD3100 modulates the expression of GABAA receptor subunits and regulates the level of GAD65 and 67 in the post-ischemic brain. In addition, we also found that mice deficient for CX3CR1 have altered expression levels of GABAA receptor subunits. Together, results indicate that the CXCR4/CX3CR1 pathways interact with components of GABAergic neurotransmission enhancing mechanisms of neuronal plasticity required to regain lost neurological function.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Ilknur Özen for assistance with laboratory methods.
Abbreviations
- AMD3100
1,1′-[1,4-Phenylenebis(methylene)]bis[1,4,8,11-tetraazacyclotetradecane]
- CX3CL1
C-X3-C Chemokine ligand 1
- CX3CR1
C-X3-C chemokine receptor type 1
- CXCL12
C-X-C Chemokine ligand 12
- CXCR4
C-X-C chemokine receptor type 4
- CXCR7
C-X-C chemokine receptor type 7
- E/I
Excitatory/inhibitory
- GABA
γ-Aminobutyric acid
- GAD67
Glutamate decarboxylase 67
- GAD65
Glutamate decarboxylase 65
- IPSC
Inhibitory postsynaptic currents
- PT
Photothrombosis
- tMCAO
Transient middle cerebral artery occlusion
Author Contribution
GM – Tissue extraction, protein and RNA analyses, wrote first draft of the manuscript.
HLW – Mouse surgery, tissue acquisition.
ARPA – Tissue acquisition, pilot gene and protein analyses.
TW – Conceived the study, data evaluation and interpretation.
DT – Data evaluation and interpretation, brain tissue acquisition.
KR – Conceived and supervised the study, manuscript revision, data evaluation and interpretation.
All authors read and approved the final manuscript.
Funding
Open access funding provided by Lund University. This work was supported by the Swedish Brain Fund, the Alborada Trust, the Crafoord Foundation, the Swedish Research Council, Sveriges Stroke Riksförbundet, the Hans-Gabriel and Alice Trolle Wachtmeister Foundation, Sparbankstiftelsen Färs & Frosta and a grant from the region Skåne (ALF).
Data Availability
All the datasets and materials supporting the conclusions of this article are presented in the manuscript and its online resource information file.
Declarations
Ethics Approval and Consent to Participate
All studies including animals have been approved by Malmö-Lund ethical committees (permit Numbers M20/12, M24/12, M26/12, M50/2015 and 5.8.18–06067 /2020).
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/10/2021
Funding note Open access funding provided by Lund University is added.
References
- 1.Kuklina EV, Tong X, George MG, Bansil P. Epidemiology and prevention of stroke: a worldwide perspective. Expert Rev Neurother. 2012;12(2):199–208. doi: 10.1586/ern.11.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uyttenboogaart M, De Keyser J, Luijckx GJ. Thrombolysis for acute ischemic stroke. Curr Top Med Chem. 2009;9(14):1285–1290. doi: 10.2174/156802609789869655. [DOI] [PubMed] [Google Scholar]
- 3.Woodruff TM, Thundyil J, Tang SC, Sobey CG, Taylor SM, Arumugam TV. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol Neurodegener. 2011;6(1):11. doi: 10.1186/1750-1326-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad M, Graham SH. Inflammation after stroke: mechanisms and therapeutic approaches. Transl Stroke Res. 2010;1(2):74–84. doi: 10.1007/s12975-010-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caleo M. Rehabilitation and plasticity following stroke: insights from rodent models. Neuroscience. 2015;311:180–194. doi: 10.1016/j.neuroscience.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Fang M, Zhong L, Jin X, Cui R, Yang W, Gao S, et al. Effect of inflammation on the process of stroke rehabilitation and poststroke depression. Front Psychiatry. 2019;10:184. doi: 10.3389/fpsyt.2019.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 8.Fritschy JM, Panzanelli P. GABAA receptors and plasticity of inhibitory neurotransmission in the central nervous system. Eur J Neurosci. 2014;39(11):1845–1865. doi: 10.1111/ejn.12534. [DOI] [PubMed] [Google Scholar]
- 9.Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, et al. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50(2):291–314. [PubMed] [Google Scholar]
- 10.Redecker C, Wang W, Fritschy JM, Witte OW. Widespread and long-lasting alterations in GABA(A)-receptor subtypes after focal cortical infarcts in rats: mediation by NMDA-dependent processes. J Cereb Blood Flow Metab. 2002;22(12):1463–1475. doi: 10.1097/01.WCB.0000034149.72481.BD. [DOI] [PubMed] [Google Scholar]
- 11.Que M, Witte OW, Neumann-Haefelin T, Schiene K, Schroeter M, Zilles K. Changes in GABAA and GABAB receptor binding following cortical photothrombosis: a quantitative receptor autoradiographic study. Neuroscience. 1999;93(4):1233–1240. doi: 10.1016/s0306-4522(99)00197-9. [DOI] [PubMed] [Google Scholar]
- 12.Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468(7321):305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaenisch N, Liebmann L, Guenther M, Hubner CA, Frahm C, Witte OW. Reduced tonic inhibition after stroke promotes motor performance and epileptic seizures. Sci Rep. 2016;6:26173. doi: 10.1038/srep26173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiu T, Farzampour Z, Paz JT, Wang EH, Badgely C, Olson A, et al. Enhanced phasic GABA inhibition during the repair phase of stroke: a novel therapeutic target. Brain. 2016;139(Pt 2):468–480. doi: 10.1093/brain/awv360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kharlamov EA, Downey KL, Jukkola PI, Grayson DR, Kelly KM. Expression of GABAA receptor α1 subunit mRNA and protein in rat neocortex following photothrombotic infarction. Brain Res. 2008;1210:29–38. doi: 10.1016/j.brainres.2008.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mele M, Ribeiro L, Inacio AR, Wieloch T, Duarte CB. GABA(A) receptor dephosphorylation followed by internalization is coupled to neuronal death in in vitro ischemia. Neurobiol Dis. 2014;65:220–232. doi: 10.1016/j.nbd.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Jin Z, Mendu SK, Birnir B. GABA is an effective immunomodulatory molecule. Amino Acids. 2013;45(1):87–94. doi: 10.1007/s00726-011-1193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee M, Schwab C, Mcgeer PL. Astrocytes are GABAergic cells that modulate microglial activity. Glia. 2011;59(1):152–165. doi: 10.1002/glia.21087. [DOI] [PubMed] [Google Scholar]
- 19.Velez-Fort M, Audinat E, Angulo MC. Central role of GABA in neuron-glia interactions. Neuroscientist. 2012;18(3):237–250. doi: 10.1177/1073858411403317. [DOI] [PubMed] [Google Scholar]
- 20.Ruscher K, Kuric E, Liu Y, Walter HL, Issazadeh-Navikas S, Englund E, et al. Inhibition of CXCL12 signaling attenuates the postischemic immune response and improves functional recovery after stroke. J Cereb Blood Flow Metab. 2013;33(8):1225–1234. doi: 10.1038/jcbfm.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walter HL, van der Maten G, Antunes AR, Wieloch T, Ruscher K. Treatment with AMD3100 attenuates the microglial response and improves outcome after experimental stroke. J Neuroinflammation. 2015;12:24. doi: 10.1186/s12974-014-0232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cipriani R, Villa P, Chece G, Lauro C, Paladini A, Micotti E, et al. CX3CL1 is neuroprotective in permanent focal cerebral ischemia in rodents. J Neurosci. 2011;31(45):16327–16335. doi: 10.1523/JNEUROSCI.3611-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denes A, Ferenczi S, Halasz J, Kornyei Z, Kovacs KJ. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab. 2008;28(10):1707–1721. doi: 10.1038/jcbfm.2008.64. [DOI] [PubMed] [Google Scholar]
- 24.Soriano SG, Amaravadi LS, Wang YF, Zhou H, Yu GX, Tonra JR, et al. Mice deficient in fractalkine are less susceptible to cerebral ischemia-reperfusion injury. J Neuroimmunol. 2002;125(1):59–65. doi: 10.1016/s0165-5728(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 25.van der Maten G, Henck V, Wieloch T, Ruscher K. CX3C chemokine receptor 1 deficiency modulates microglia morphology but does not affect lesion size and short-term deficits after experimental stroke. BMC Neurosci. 2017;18(1):11. doi: 10.1186/s12868-016-0325-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen QA, Nicoll RA. The GABAA receptor beta subunit is required for inhibitory transmission. Neuron. 2018;98(4):718–25.e3. doi: 10.1016/j.neuron.2018.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji FY, et al. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem Biophys Res Commun. 1996;229(3):891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- 28.Kanaani J, Kolibachuk J, Martinez H, Baekkeskov S. Two distinct mechanisms target GAD67 to vesicular pathways and presynaptic clusters. J Cell Biol. 2010;190(5):911–925. doi: 10.1083/jcb.200912101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20(11):4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45-e [DOI] [PMC free article] [PubMed]
- 32.Tichopad A, Dilger M, Schwarz G, Pfaffl MW (2003) Standardized determination of real‐time PCR efficiency from a single reaction set‐up. Nucleic Acids Research 31(20):e122-e [DOI] [PMC free article] [PubMed]
- 33.Heinisch S, Kirby LG. SDF-1alpha/CXCL12 enhances GABA and glutamate synaptic activity at serotonin neurons in the rat dorsal raphe nucleus. Neuropharmacology. 2010;58(2):501–514. doi: 10.1016/j.neuropharm.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carmichael ST. Brain excitability in stroke: the yin and yang of stroke progression. Arch Neurol. 2012;69(2):161–167. doi: 10.1001/archneurol.2011.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joy MT, Carmichael ST. Encouraging an excitable brain state: mechanisms of brain repair in stroke. Nat Rev Neurosci. 2021;22(1):38–53. doi: 10.1038/s41583-020-00396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boddington LJ, Reynolds JNJ. Targeting interhemispheric inhibition with neuromodulation to enhance stroke rehabilitation. Brain Stimul. 2017;10(2):214–222. doi: 10.1016/j.brs.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Steiger JL, Russek SJ. GABAA receptors: building the bridge between subunit mRNAs, their promoters, and cognate transcription factors. Pharmacol Ther. 2004;101(3):259–281. doi: 10.1016/j.pharmthera.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura Y, Darnieder LM, Deeb TZ, Moss SJ. Regulation of GABAARs by phosphorylation. Adv Pharmacol. 2015;72:97–146. doi: 10.1016/bs.apha.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh H, Auguadri L, Battaglia S, Simone Thirouin Z, Zemoura K, Messner S, et al. Several posttranslational modifications act in concert to regulate gephyrin scaffolding and GABAergic transmission. Nat Commun. 2016;7:13365. doi: 10.1038/ncomms13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, et al. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002;22(14):5865. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schonemeier B, Schulz S, Hoellt V, Stumm R. Enhanced expression of the CXCl12/SDF-1 chemokine receptor CXCR7 after cerebral ischemia in the rat brain. J Neuroimmunol. 2008;198(1–2):39–45. doi: 10.1016/j.jneuroim.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Werner Y, Mass E, Ashok Kumar P, Ulas T, Handler K, Horne A, et al. Cxcr4 distinguishes HSC-derived monocytes from microglia and reveals monocyte immune responses to experimental stroke. Nat Neurosci. 2020;23(3):351–362. doi: 10.1038/s41593-020-0585-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cook A, Hippensteel R, Shimizu S, Nicolai J, Fatatis A, Meucci O. Interactions between chemokines: regulation of fractalkine/{CX}3CL1 homeostasis by {SDF}/{CXCL}12 in cortical neurons. J Biol Chem. 2010;285:10563–10571. doi: 10.1074/jbc.M109.035477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo Y, Lathia J, Mughal M, Mattson MP. SDF1alpha/CXCR4 signaling, via ERKs and the transcription factor Egr1, induces expression of a 67-kDa form of glutamic acid decarboxylase in embryonic hippocampal neurons. J Biol Chem. 2008;283(36):24789–24800. doi: 10.1074/jbc.M800649200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Blanco GD, Lei Z, Xu ZC. Increased GAD expression in the striatum after transient cerebral ischemia. Mol Cell Neurosci. 2010;45(4):370–377. doi: 10.1016/j.mcn.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heinisch S, Kirby LG. Fractalkine/CX3CL1 enhances GABA synaptic activity at serotonin neurons in the rat dorsal raphe nucleus. Neuroscience. 2009;164(3):1210–1223. doi: 10.1016/j.neuroscience.2009.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roseti C, Fucile S, Lauro C, Martinello K, Bertollini C, Esposito V, et al. Fractalkine/CX3CL1 modulates GABAA currents in human temporal lobe epilepsy. Epilepsia. 2013;54(10):1834–1844. doi: 10.1111/epi.12354. [DOI] [PubMed] [Google Scholar]
- 48.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the datasets and materials supporting the conclusions of this article are presented in the manuscript and its online resource information file.