Abstract
This study was conducted to investigate the effects of combined supplementation of sodium humate (HNa) and glutamine (Gln) on growth performance, diarrhea incidence, serum parameters, intestinal microbiome, and metabolites of weaned calves. In Exp. 1, 40 calves were randomly assigned to four treatments: 1) NC (negative control, basal diet), 2) 1% H+1% G (basal diet extra orally gavaged with 1 g of HNa and 1 g of Gln daily), 3) 3% H+1% G (basal diet extra orally gavaged with 3 g of HNa and 1 g of Gln daily), and 4) 5% H+1% G (basal diet extra orally gavaged with 5 g of HNa and 1 g of Gln daily). The HNa and Gln were together mixed with 100 mL of milk replacer (51 to 58 d of age) or water (59 to 72 d of age) and orally administrated to each calf from a bottle before morning feeding. In a 21-d trial, calves on the 5% HNa+1% Gln group had higher (P < 0.05) average daily gain (ADG) and lower (P < 0.05) diarrhea incidence than those in the control group. In Exp. 2, 20 calves were randomly assigned to two treatments fed with a basal diet and a basal diet supplemented with 100 mL of 5% HNa+1% Gln. In a 21-d trial, calves supplemented with HNa and Gln had higher (P < 0.05) ADG, IgG concentration and glutathione peroxidase (GSH-Px), and total antioxidant capacity (T-AOC) activities in the serum, but lower (P < 0.05) diarrhea incidence, as well as serum diamine oxidase (DAO), D-isomer of lactic acid (D-lac), tumor necrosis factor-α (TNF-α), and malondialdehyde (MDA) concentrations compared with control group. Results of intestinal microbiota indicated that supplementation with HNa and Gln significantly increased (P < 0.05) the abundance of intestinal beneficial microbiota. Moreover, supplementation with HNa and Gln altered 18 metabolites and enriched 6 Kyoto Encyclopedia of Genes and Genomes pathways in weaned calves. In conclusion, combined supplementation with HNa and Gln could decrease diarrhea incidence of weaned calves via altering intestinal microbial ecology and metabolism profile.
Keywords: dairy calf, diarrhea incidence, glutamine, intestinal microbiota, metabolomics, sodium humate
Introduction
Weaning is one of the most stressful periods in calf life, which can cause gastrointestinal tract dysfunction and diarrhea (Slanzon et al., 2019). High diarrhea incidence in weaned calves is the main cause of growth retardation and death, which seriously affects the welfare of calves and causes serious economic losses in the dairy industry (Pempek et al., 2019). Antibiotics have long been acted in calves to promote growth and prevent diarrhea. However, the antibiotic resistance and antimicrobial residues have accelerated the seeking for novel alternatives to antibiotics (Ranjbar and Farahani. 2019). Previous studies indicated that intestinal microbiota plays an essential role in intestinal morphology, nutrient absorption, immunity response, and host health (Zou et al., 2021). Meanwhile, intestinal microbiota participates in many metabolic activities of the host, such as amino acid and vitamin synthesis, and lipid and bile acid metabolism (Chen et al., 2020). Studies have shown that weaning can significantly alter intestinal microbiota and metabolism in pigs, resulting in diarrhea and growth retardation (Zhang et al., 2020).
Humic acids (HAs), derived from the decomposition and transformation of decaying organic matter in the soil, are natural organic bioactive agents. As a type of HAs, sodium humate (HNa) is rich in active groups such as phenolic-hydroxyl, carboxyl, sulfhydryl, and carbonyl (Klučáková, 2018), and its antimicrobial, antioxidant, anti-inflammatory, and antidiarrheal activities were reported (Písaříková et al., 2010). Furthermore, the HNa showed therapeutic effects on dyspepsia, diarrhea, and acute intoxication in animals (Zhao et al., 2015). The phenolic-hydroxyl and carboxyl groups may contribute to its biological activity, and the sulfhydryl and carbonyl are responsible for its antidiarrheal function (Proidakov, 2009; Szot et al., 2019). Wang et al. (2020) confirmed the beneficial effects of HNa supplementation in finishing pigs. The growth-promotion efficacy of HNa was also confirmed in broilers (Taklimi et al., 2012). Glutamine (Gln) is a major fuel source for rapidly dividing cells including enterocytes, macrophages, and lymphocytes in the intestine, indicating that Gln could maintain intestinal integrity and prevent bacterial translocation (Cruzat et al., 2018). The beneficial effects of Gln supplementation on improving growth performance, repairing intestinal epithelium, enhancing nutrient utilization, activating the immune system, and modulating intestinal microbiota have been observed in rats (Ren et al., 2014), broilers (Hu et al., 2016), weaning piglets (He et al., 2019), and calves (Nemati et al., 2018). Furthermore, our previous study found that combined supplementation with HNa and Gln effectively decreased diarrhea incidence in weaned calves (Wang et al., 2021). Based on the studies above, we hypothesized that HNa and Gln combined supplementation might have therapeutic potential on calf diarrhea by modulating intestinal microbiota and metabolites in weaned calves. In the present study, intestinal microbiota sequencing and fecal metabolomics were integrated to investigate the beneficial effects of combined supplementation of HNa and Gln on weaned calves.
Materials and Methods
The experimental protocol was approved by the Ethics Committee of Northeast Agricultural University (Harbin, China). The study was conducted at Harbin Modern Farming (Harbin, China).
Preparation of sodium humate and glutamine
HNa (purity, 75%) was provided by the Institute of Coal Chemistry, Chinese Academy of Sciences (Shan Xi, China). It consists of 75% HA (dry basis), 20.52% burning residue (dry basis), 14.22% water (air dry basis), and 4.48% water soluble substances (dry basis) according to analysis report of the product. Glutamine (purity, 99.9%) was purchased from Sigma-Aldrich (Wisconsin).
Animals, diets, and experimental design (Exp. 1)
A total of 40 female Holstein calves at 51 ± 3 d of age with similar body weight (66.82 ± 4.31 kg) were fed 5 liters of milk replacer until weaning (58 d of age), twice daily at 8:30 am and 16:30 pm. All the calves were housed in individual pens covered with straw and surrounded by an iron fence. The milk replacer used in this study contained lactose ≥40%, CP ≥ 22%, crude fat ≥ 19%, water ≤ 4.0%, ash ≤ 8.0%, and fiber ≤ 0.3%. All calves had free access to water and starter (basal diet) during the entire experimental period. The ingredients and chemical composition of the starter are shown in Table 1.
Table 1.
Ingredients and chemical composition of the starter concentrate diet (basal diet, Exp. 1 and Exp. 2)
| Ingredient1 | Content (%) |
|---|---|
| Corn | 40.74 |
| Soybean meal | 35.00 |
| Wheat bran | 2.80 |
| Cottonseed meal | 6.80 |
| Molasses | 4.00 |
| Wheat middlings | 7.80 |
| CaCO3 | 1.63 |
| Soybean oil | 0.80 |
| NaCl | 0.10 |
| CaHPO3 | 0.10 |
| MgO | 0.07 |
| Selenium yeast | 0.02 |
| Premix2 | 0.14 |
| Chemical composition | |
| DM | 87.78 |
| EE | 4.35 |
| CP | 23.56 |
| ADF | 5.71 |
| NDF | 9.82 |
| Ash | 3.73 |
| Ca | 0.81 |
| Phosphorus | 0.49 |
1The content is on an as-fed basis.
2The premix provided the following per kg of diet: Fe 206.74 mg, Cu3 3.49 mg, Zn 108.81 mg, I 0.60 mg, Se 0.44 mg, Mn 79.99 mg, Co 0.36 mg, vitamin A 9,000 U, vitamin D 24,000 U, vitamin E 47.22 mg.
All of the calves were randomly assigned to four treatments (n = 10): 1) NC (negative control, basal diet), 2) 1% H+1% G (basal diet extra orally gavaged with 1 g of HNa and 1 g of Gln daily), 3) 3% H+1% G (basal diet extra orally gavaged with 3 g of HNa and 1 g of Gln daily), and 4) 5% H+1% G (basal diet extra orally gavaged with 5 g of HNa and 1 g of Gln daily). The HNa and Gln were together mixed with 100 mL of milk replacer (51 to 58 d of age) or water (59 to 72 d of age) and orally administrated to each calf from a bottle before morning feeding. The trial lasted for 21 d.
Animals, diets, and experimental design (Exp. 2)
A total of 20 female Holstein calves at 51 ± 3 d of age with similar body weight (69.37 ± 6.28 kg) were randomly assigned to two treatments (n = 10): 1) NC (negative control, basal diet) and 2) H+G (basal diet extra orally gavaged with 5 g of HNa and 1 g of Gln daily). The HNa and Gln were together mixed with 100 mL of milk replacer (51 to 58 d of age) or water (59 to 72 d of age) and orally administrated to each calf from a bottle before morning feeding. The HNa concentration applied in Exp. 2 was based on the results of Exp. 1, which suggested that 5% HNa+1% Gln resulted in lower fecal score, diarrhea incidence, and higher ADG. The trial lasted for 21 d.
Growth performance, fecal score, and diarrhea incidence (Exp. 1 and Exp. 2)
Feed intake of per calf was daily recorded to calculate average daily feed intake (ADFI). Body weight of each calf was recorded on day 1, and day 21 of the experiment to calculate the average daily gain (ADG) and ratio of gain to feed (G:F).
The fecal scores were monitored daily before the morning feeding according to the method of Renaud et al. (2019). Fresh feces were scored by consistency: 0 = firm, 1 = loose or moderate consistency, 2 = very loose or mild diarrhea, and 3 = watery or profuse diarrhea. Diarrhea was defined as fecal scores ≥2 occurring for 2 or more consecutive days. The diarrhea incidence was calculated according to Renaud et al. (2019). Diarrhea incidence (%) = Number of diarrheal calves × Diarrhea days/ (Number of calves × Test days) × 100.
Serum parameters analysis (Exp.2)
Blood samples were obtained from the jugular vein of each calf at 73 d of age (before the morning feed). Samples were collected in 10-mL vacuum tubes and then centrifuged for 15 min at 3,000 × g, 4 °C. The serum supernatants were collected and stored at −20 °C for further analysis. IgG, IgM, IgA, IL-6, tumor necrosis factor-α (TNF-α), diamine oxidase (DAO), and D-isomer of lactic acid (D-lac) were detected using ELISA kits (Jingmei Biotechnology Co., Ltd, Jiangsu, China), and glutathione peroxidase (GSH-Px), total superoxide dismutase (T-SOD), total antioxidant capacity (T-AOC) activities, and malondialdehyde (MDA) concentration were measured by colorimetric assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
DNA extraction, 16S rDNA amplification, and high-throughput sequencing (Exp. 2)
At 73 d of age, fresh fecal samples were directly collected from the rectum of the individual calf before the morning feeding, and then immediately frozen in liquid nitrogen until microbiota analysis. Total genome DNA from each digested sample was extracted using cetyltriethylammnonium bromide (CTAB) method, and then the integrity of extracted DNAs was evaluated by 1% agarose gel. The V3–V4 regions of the bacterial 16S rDNA gene were amplified by specific primers: 341F (5′-CCTACGGGNGGCWGCAG-3′) and 806R (5′-GGACTACHVGGGTATCTAAT-3′) with the following procedures: initial denaturation at 98 °C for 1 min, followed by 30 cycles of denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s, and elongation at 72 °C for 60 s, finally 72 °C for 5 min. PCR products were detected by 1% agarose gel and purified with AxyPrepDNA Gel Extraction Kit (Axygen Biosciences, Union City, CA). The library was constructed using the TruSeq DNA PCR-Free Sample Preparation Kit (Thermo Scientific, Waltham, MA). The constructed library was quantified by Qubit 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. After the library was qualified, it was sequenced by Illumina HiSeq (Illumina, San Diego).
Paired-end reads from the original DNA fragments were merged using FLASH, and the sequences analysis was performed by UPARSE software package using the UPARSE-OTU and UPARSE-OTUref algorithms. In-house Perl scripts were used to analyze alpha (within samples) and beta (among samples) diversity, and PCoA (principal coordinates analysis) of weighted unifrac was generated in R project Vegan package (version 2.5.3). Sequences with ≥97% similarity were assigned to the same OTUs. The statistical significance of comparison in bacterial community composition between the two groups was assessed using Student’s t-test, and STAMP software was utilized to confirm differences in the abundances of individual taxonomy between the two groups.
LC–MS/MS analysis and data processing (Exp. 2)
Each fecal sample (50 mg) was added to 200 μL of H2O, homogenized, and vortexed for 60 s. Then, 800 μL methanol/acetonitrile solution (1:1, v/v) were added to homogenized solution for metabolite extraction. The mixture was centrifuged for 15 min (14,000 × g, 4 °C). The supernatant was used for LC–MS/MS analysis.
LC–MS/MS analyses were performed using an UHPLC (1290 Infinity LC, Agilent Technologies) coupled to a quadrupole time-of-flight (TripleTOF 6600, AB Sciex, USA) in Shanghai Applied Protein Technology Co., Ltd. For HILIC separation, samples were analyzed using a 2.1 mm×100 mm ACQUITY UPLC BEH Amide 1.7 μm, 2.1 mm × 100 mm column (waters, Milford). The collected data were used to identify the structure of metabolites using self-built MetDDA and LipDDA methods (Shanghai Applied Protein Technology Co. Ltd). The original data were converted into mzXML format by ProteoWizard MSConvert, and then the XCMS program was used for peak alignment, retention time correction, and peak area extraction. In the extracted ion features, only the variables having more than 50% of the nonzero measurement values in at least one group were kept. The metabolite structure identification was based on accurate mass matching (<25 ppm) and secondary spectrum matching methods and search of the laboratory’s self-built commercial database (Shanghai Applied Protein Technology Co. Ltd).
After normalized to total peak intensity, the processed data were analyzed by R package (ropls), where it was subjected to multivariate data analysis, including partial least-squares discriminant analysis (PLS-DA) and orthogonal partial least-squares discriminant analysis (OPLS-DA). The 7-fold cross-validation and response permutation testing was used to evaluate the robustness of the model. The variable importance in the projection (VIP) value of each variable in the OPLS-DA model was calculated to indicate its contribution to the classification. Metabolites with the VIP value > 1 were further applied to Student’s t-test at univariate level to measure the significance of each metabolite, the P values < 0.05 were considered as statistically significant. The differential metabolites were further to cluster analysis and metabolic pathway analysis by Kyoto Encyclopedia of Genes and Genomes (KEGG) (http://www.genome.jp/kegg). Fisher’s Exact Test was used to analyze and calculate the significance level of the enrichment pathway.
Statistical analysis
Individual calves served as the experimental unit. For the growth performance in Exp. 1, data were analyzed by one-way ANOVA using SPSS 20.0 software (SPSS Inc., IBM, Chicago). The differences among treatments were evaluated using Turkey’s test. For the growth performance, fecal score, and serum cytokines concentration and antioxidant capacity in Exp. 2, data were analyzed using the independent sample t-test of the SPSS. Results are presented as mean ± standard error of the mean (SEM) except for the diarrhea incidence. Differences were considered significant at P < 0.05. The correlation analysis among intestinal microbiota, metabolites, ADG, fecal score, and serum parameters was estimated by Spearman’s correlation coefficient. Correlations were considered significantly different at r > 0.50 or r < −0.50, P < 0.05.
Results
Growth performance and diarrhea incidence
As shown in Table 2 (Exp. 1), the initial BW, final BW, ADFI, and G:F of calves were similar among treatments (P > 0.05). However, the ADG treatment of calves in 3% H+G and 5% H+G was higher (P < 0.05) than NC group. The decreased fecal scores and diarrhea incidence were significantly associated with the HNa and Gln supplementation. The diarrhea incidence of calves in the NC, 1% H+G, 3% H+G, and 5% H+G group was 25.81%, 17.08%, 21.42%, and 15.41%, separately.
Table 2.
Effects of sodium humate and glutamine combined supplementation on the growth performance and diarrhea incidence of calves1 (Exp. 1)
| Item2 | Experimental treatments | P-value | |||
|---|---|---|---|---|---|
| NC | 1% H+1% G | 3% H+1% G | 5% H+1% G | ||
| Initial BW, kg(51 d) | 67.70 ± 2.01 | 66.43 ± 1.30 | 65.13 ± 1.82 | 68.05 ± 1.01 | 0.430 |
| Final BW, kg (72 d) |
88.00 ± 2.29 | 90.43 ± 1.15 | 90.82 ± 1.81 | 93.90 ± 1.69 | 0.092 |
| ADG, kg | 0.96 ± 0.04b | 1.14 ± 0.04ab | 1.22 ± 0.06a | 1.23 ± 0.08a | 0.025 |
| ADFI, kg | 2.29 ± 0.04 | 2.49 ± 0.05 | 2.61 ± 0.06 | 2.58 ± 0.15 | 0.062 |
| G:F | 0.42 ± 0.02 | 0.45 ± 0.02 | 0.46 ± 0.02 | 0.48 ± 0.04 | 0.479 |
| Fecal score | 2.1 ± 0.12a | 1.5 ± 0.14b | 1.6 ± 0.11b | 1.4 ± 0.07b | 0.001 |
| Diarrhea incidence, % | 25.81 | 17.08 | 21.42 | 15.41 |
a,bMeans within a row with different letters differ significantly (P < 0.05).
1Data are the mean of 10 replicates of 1 calf per treatment.
2BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; G:F, ratio of gain to feed.
Data were shown as means ± SEM (n = 10).
As shown in Table 3 (Exp. 2), compared with NC group, the calves in H+G group have greater final BW (P = 0.026) and ADG (P = 0.003). No significant differences of G:F and ADFI were observed among treatments. In addition, the calves in H+G group had lower fecal scores (P = 0.001) and diarrhea incidence than calves in NC group.
Table 3.
Effects of sodium humate and glutamine combined supplementation on the growth performance and diarrhea incidence of calves1 (Exp. 2)
| Item2 | Experimental treatments | P-value | |
|---|---|---|---|
| NC | H+G | ||
| InitialBW, kg(51 d) | 68.50 ± 1.96 | 70.25 ± 2.07 | 0.548 |
| Final BW, kg (72 d) | 91.17 ± 2.71b | 101.38 ± 3.00a | 0.026 |
| ADG, kg | 1.08 ± 0.06b | 1.48 ± 0.06a | 0.003 |
| ADFI, kg | 1.98 ± 0.15 | 2.37 ± 0.23 | 0.182 |
| G:F | 0.58 ± 0.08 | 0.68 ± 0.08 | 0.384 |
| Fecal score | 2.0 ± 0.15a | 1.4 ± 0.17b | 0.001 |
| Diarrhea incidence, % | 27.16 | 15.69 |
a,bMeans within a row with different letters differ significantly (P < 0.05).
1Data are the mean of 10 replicates of 1 calf per treatment.
2BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; G:F, ratio of gain to feed. NC, negative control (basal diet); H+G, basal diet+5% HNa and 1% Gln.
Data were shown as means ± SEM (n = 10).
Serum parameters
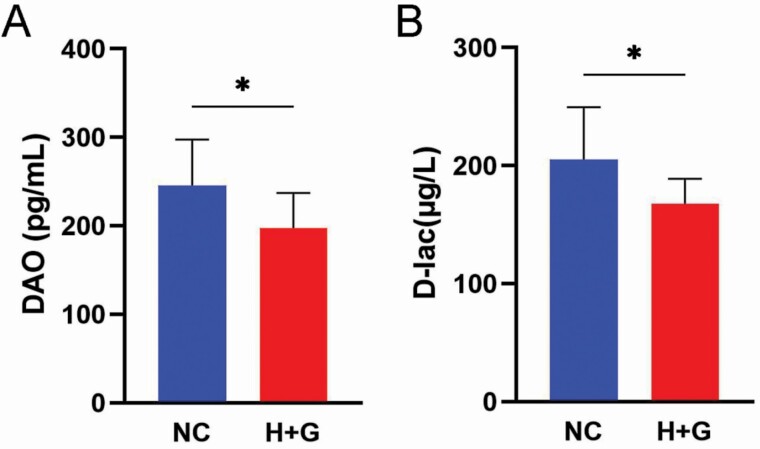
As shown in Figure 1, the inclusion of HNa and Gln decreased serum concentration of DAO and D-lac compared with NC group (P < 0.05). As shown in Table 4, compared with NC group, HNa and Gln inclusion increased the IgG concentration (P = 0.018), as well as activities of GSH-Px (P = 0.003) and T-AOC (P = 0.005) in the serum of calves. Furthermore, lower concentrations of serum TNF-α (P = 0.047) and MDA (P = 0.002) were observed in the H+G group than the NC one. There was no significant difference among treatments in serum IgA, IgM, IL-6, and T-SOD concentration (P > 0.05).
Figure 1.
Effects of sodium humate and glutamine combined supplementation on the serum concentration of diamine oxidase (DAO) and D-isomer of lactic acid (D-lac) of weaned calves. Data were shown as means ± SEM (n = 10). NC, negative control (basal diet); H+G, basal diet+5% HNa and 1% Gln. *P < 0.05.
Table 4.
Effects of sodium humate and glutamine combined supplementation on the concentrations of cytokines and antioxidant capacity of calves in the serum1 (Exp. 2)
| Item2 | Experimental treatments | P-value | |
|---|---|---|---|
| NC | H+G | ||
| IgA, µg/mL | 78.32 ± 4.61 | 89.51 ± 3.62 | 0.073 |
| IgG, µg/mL | 1083.57 ± 46.06b | 1245.86 ± 41.61a | 0.018 |
| IgM, µg/mL | 69.33 ± 6.79 | 74.24 ± 7.50 | 0.295 |
| IL-6, ng/L | 8.64 ± 0.51 | 8.17 ± 0.62 | 0.569 |
| TNF-α, ng/L | 211.22 ± 8.34a | 186.46 ± 8.10b | 0.047 |
| GSH-Px, U/L | 128.65 ± 7.00b | 167.48 ± 9.15a | 0.003 |
| T-SOD, pg/mL | 59.64 ± 7.76 | 66.52 ± 4.70 | 0.458 |
| T-AOC, U/mL | 6.58 ± 0.27b | 7.94 ± 0.33a | 0.005 |
| MDA, mmol/mL | 3.08 ± 0.43a | 2.29 ± 0.51b | 0.002 |
a,bMeans within a row with different letters differ significantly (P < 0.05).
1Data are the mean of 10 replicates of 1 calf per treatment.
2Ig, immunoglobulin; IL-6, interleukin6; TNF-α, tumor necrosis factor-α; GSH-Px, glutathione peroxidase; T-SOD, total superoxide dismutase; T-AOC, total antioxidant capacity; MDA, malondialdehyde. NC, negative control (basal diet); H+G, basal diet+5% HNa and 1% Gln.
Data were shown as means ± SEM (n = 10).
Intestinal microbial diversity and community
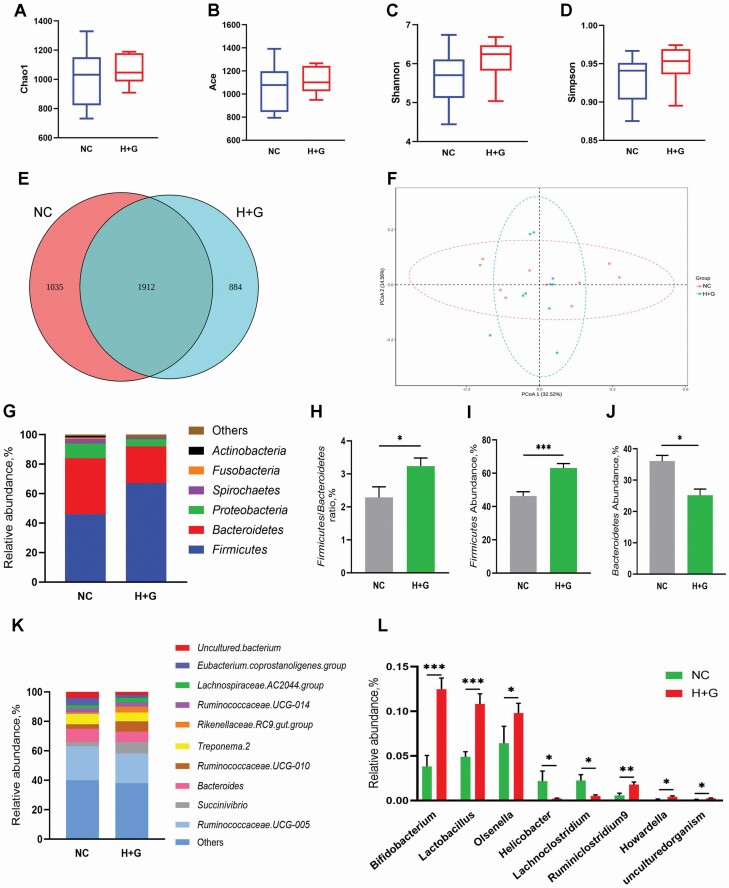
The Chao1, Ace, Shannon, and Simpson indexes associated with bacterial richness and diversity were similar among groups (Figure 2A–D). In the microbiome study, 5,116,836 effective tags were acquired after filtering the data quality, with an average number of 255,842 tags per sample. Based on the 97% identity level, these sequences were decomposed into 1,912 operational taxonomic units (OTUs), while 1,035 and 884 specific OTUs were observed in NC and H+G groups, respectively (Figure 2E). The principal coordinate analysis (PCoA) plots showed an overlap of partial samples between two groups (Figure 2F).
Figure 2.
Effects of sodium humate and glutamine combined supplementation on intestinal microbiota of weaned calves. The Chao 1, Ace, Shannon, and Simpson indexes of α-diversity analysis in the OTU level among two groups (A–D). Venn diagram of the operational taxonomic unit (OTU) distribution shows unique and shared OTUs between the two groups (E). β-Diversity principal coordinate analysis (PCoA) was performed to calculate the different intestinal microbiota structures among the two groups (F). Bar graphs represent relative abundance of gut bacteria at the phylum level (G). The ratio of Firmicutes to Bacteroidetes (H). The relative abundance of Firmicutes (I) and Bacteroidetes (J). Bar graphs represent relative abundance of gut bacteria at the genus level (K). The relative abundance of gut bacteria with significant differences in the genus level (L). Data were shown as means ± SEM (n = 10). NC, negative control (basal diet); H+G, basal diet+5% HNa and 1% Gln. *P < 0.05, **P < 0.01, ***P < 0.001 vs. the NC group.
At the phylum level (Figure 2G), Firmicutes, Bacteroidetes, Proteobacteria, Spirochaetes, Fusobacteria, and Actinobacteria were the predominant bacteria in these two groups. Compared with NC group, HNa and Gln supplementation significantly increased (P < 0.05) the ratio of Firmicutes to Bacteroidetes (Figure 2H) and the relative abundance (P < 0.001) of Firmicutes (Figure 2I), but decreased (P < 0.05) the relative abundance of Bacteroidetes (Figure 2J).
At the genus level (Figure 2K), Ruminococcaceae.UCG-005, Succinivibrio, Bacteroides, Treponema.2, Rikenellaceae.RC9.gut.group, Ruminococcaceae.UCG-014, Ruminococcaceae.UCG-010, uncultured bacterium, Lachnospiraceae.AC2044.group, and Eubacterium.coprostanoligenes.group were the most predominant genera in all the samples. Compared with NC group (Figure 2L), H+G group had higher relative abundance of Bifidobacterium (P < 0.001), Lactobacillus (P < 0.001), Olsenella (P < 0.05), Ruminiclostridium 9 (P < 0.01), Howardella (P < 0.05), and uncultured organism (P < 0.05), but relative lower abundance of Helicobacter (P < 0.05) and Lachnoclostridium (P < 0.05).
Analysis of metabolic profiling in weaned calves
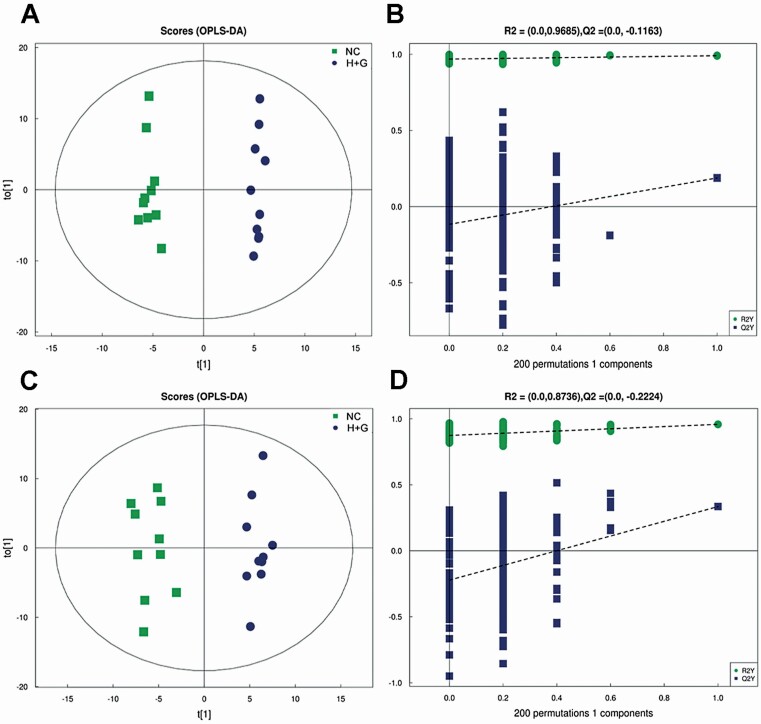
There were overall 289 and 199 metabolites shown in the feces of weaned calves under the positive and negative mode, respectively. The results of fecal metabolic profiling showed clear segregation in the positive ion mode (R2 = 0.968, Q2 =–0.116; Figure 3A and B) and negative ion mode (Figure 3C and D) (R2 = 0.873, Q2 = −0.222), indicating the significant differences in the fecal metabolism among the two groups.
Figure 3.
Effects of sodium humate and glutamine combined supplementation on fecal metabolic profiles of weaned calves. Orthotopic partial least-squares discriminant analysis (OPLS-DA) scores plot among the two groups for serum metabolites in positive (A) and negative (C) ion mode. R2 and Q2 represent the interpretability and predictability of models in positive (B) and negative ion mode (D), respectively. NC, negative control (basal diet); H+G, basal diet+5% HNa and 1% Gln.
Metabolites with VIP values >1.0 and P-value < 0.05 were considered significantly change. As shown in Table 5, a total of 18 (10 positive ion mode and 8 negative ion mode) significantly changed metabolites in fecal samples of weaned calves were detected among two groups according to multivariate statistical analysis. Additionally, there were 16 (1-palmitoylglycerol, terbutaline, 3-aminobutanoic acid, Leu-Ala, Thr-Arg, nitrosobenzene, 1-palmitoyl-sn-glycero-3-phosphocholine, Tyr-Met, N6-methyladenine, oleic acid, sebacic acid, 13(S)-HODE, D-mannose, nname,cis-9,10-epoxystearic acid, cis-9-palmitoleic acid, and L-malic acid) significantly upregulated metabolites and 2 (2-methylbenzoic acid and N-acetyl-d-lactosamine) downregulated metabolites in the H+G group compared with NC group (P < 0.05).
Table 5.
Effects of sodium humate and glutamine combined supplementation on fecal metabolites of weaned calves (Exp. 2)
| Adduct1 | Metabolite | VIP2 | FC3 | P-value | m/z4 | Rt (s)5 | Trend |
|---|---|---|---|---|---|---|---|
| (M+H-H2O)+ | 1-Palmitoylglycerol | 2.5 | 2.43 | 0.007 | 313.27 | 72.26 | ↑ |
| (M+CH3CN+Na)+ | Terbutaline | 1.07 | 1.67 | 0.007 | 289.15 | 195.19 | ↑ |
| (M+H)+ | 3-Aminobutanoic acid | 2.38 | 3.07 | 0.01 | 104.07 | 45.25 | ↑ |
| (M+CH3CN+H)+ | Leu-Ala | 1.01 | 3.12 | 0.012 | 244.16 | 254.23 | ↑ |
| (M+H)+ | Thr-Arg | 2.01 | 2.01 | 0.019 | 276.16 | 398.92 | ↑ |
| (M+NH4)+ | Nitrosobenzene | 1.83 | 1.3 | 0.02 | 125.07 | 67.04 | ↑ |
| (M+H)+ | 1-Palmitoyl-sn-glycero-3-phosphocholine | 4.94 | 1.67 | 0.037 | 496.34 | 190.05 | ↑ |
| (M+H-H2O)+ | Tyr-Met | 1.2 | 1.41 | 0.044 | 295.11 | 129.32 | ↑ |
| (M+H-H2O)+ | N-Acetyl-d-lactosamine | 1.16 | 0.54 | 0.045 | 366.14 | 364.75 | ↓ |
| (M+H)+ | N6-Methyladenine | 1.28 | 1.55 | 0.05 | 150.08 | 126.98 | ↑ |
| (M-H) − | Oleic acid | 31.47 | 1.56 | 0.001 | 281.25 | 37.72 | ↑ |
| (M-H) − | 2-Methylbenzoic acid | 1.04 | 0.74 | 0.005 | 135.05 | 155.13 | ↓ |
| (M-H) − | Sebacic acid | 1.23 | 2.03 | 0.008 | 201.11 | 321.5 | ↑ |
| (M-H) − | 13(S)-HODE | 1.92 | 1.39 | 0.016 | 295.23 | 37.64 | ↑ |
| (M+CH3COO) − | d-Mannose | 3.22 | 1.58 | 0.018 | 239.08 | 369.75 | ↑ |
| (M-H) − | Nname,cis-9,10-epoxystearic acid | 7.68 | 2.07 | 0.018 | 297.24 | 37.53 | ↑ |
| (M-H) − | cis-9-Palmitoleic acid | 5.98 | 1.43 | 0.032 | 253.22 | 37.69 | ↑ |
| (M-H) − | l-Malic acid | 1.55 | 1.62 | 0.045 | 133.01 | 405.99 | ↑ |
Difference metabolites identified by positive and negative ion mode (multidimensional statistical analysis of VIP > 1 and univariate statistical analysis of P-value < 0.05), the H+G group vs. the NC group.
1Adduct, adduct ion information of the compound;
2VIP, variable importance in the projection;
3FC, Fold change;
4 m/z, mass-to-charge ratio;
5Rt(s), retention time; ↑, the compound is upregulated; ↓, the compound is downregulated.
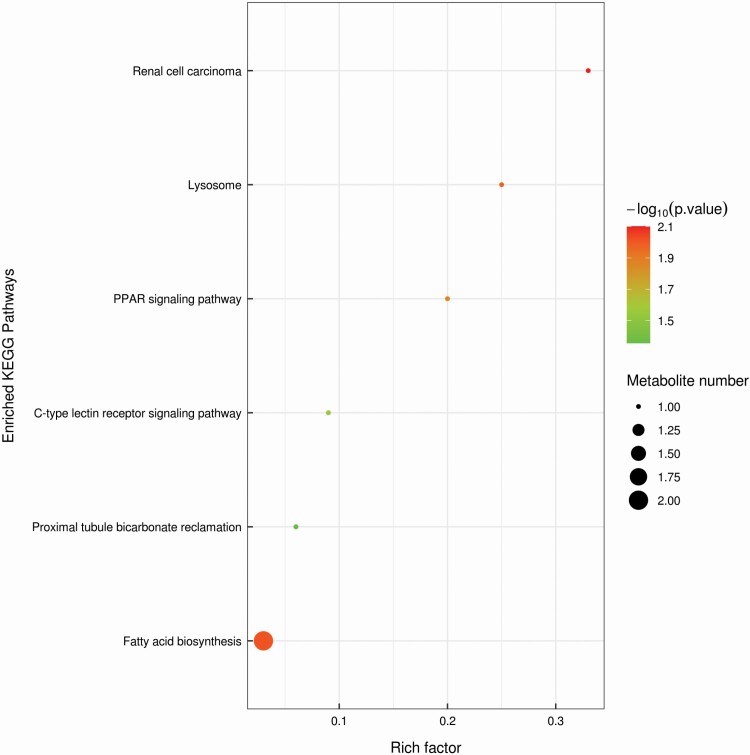
KEGG enrichment analysis was further performed to find the underlying enriched pathway of the changed metabolites. The data of pathway analysis reflected that there were six significant enriched pathways of metabolites in weaned calves supplemented with HNa and Gln, which included: 1) fatty acid biosynthesis, 2) proximal tubule bicarbonate reclamation, 3) C-type lectin receptor signaling pathway, 4) PPAR signaling pathway, 5) lysosome, and 6) renal cell carcinoma (Figure 4).
Figure 4.
Enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment of fecal differential metabolites. The color of the point was P-value, and the redder, the more significant enrichment. The size of the spot represented the number of different metabolites enriched.
Correlation analysis
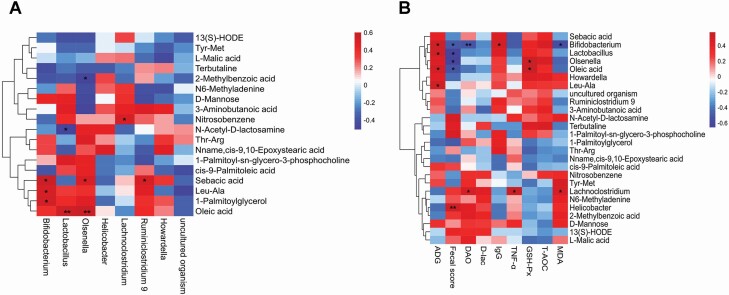
Additionally, spearman’s correlation analysis was conducted to further investigate the correlation of the altered intestinal microbiota and metabolites in weaned calves. In detail, as shown in Figure 5A, Bifidobacterium was positively correlated (r > 0.52, P < 0.05) with Leu-Ala, Sebacic acid, and 1-palmitoylglycerol. Lactobacillus was positively correlated (r = 0.56, P < 0.01) with oleic acid, and negatively correlated (r = -0.50, P = 0.04) with N-acetyl-d-lactosamine. Olsenella was positively correlated (r > 0.51, P < 0.01) with oleic acid, sebacic acid, and negatively correlated (r = -0.50, P = 0.02) with 2-methylbenzoic acid. Lachnoclostridium was positively correlated (r = 0.52, P = 0.01) with nitrosobenzene. Ruminiclostridium 9 was positively correlated (r = 0.53, P = 0.01) with sebacic acid.
Figure 5.
Heatmap of Sperman’s correlation analysis between significantly different intestinal microbiota at the genus level and differential metabolites (A), ADG, fecal score, and serum parameters (B). The significant correlations (r > 0.50 or r < −0.50, P < 0.05) were shown in the correlation heatmaps. The intensity of the colors represented the degree of correlation (red, positive correlation; blue, negative correlation). Significant correlations were marked by *P < 0.05, **P < 0.01.
The correlation of altered intestinal microbiota, metabolites and ADG, fecal score, and serum parameters in weaned calves are shown in Figure 5B, Bifidobacterium, Lactobacillus, Leu-Ala, and Oleic acid was positively correlated (r > 0.51, P < 0.02) with ADG. Helicobacter was positively correlated (r = 0.53, P < 0.01) with fecal score, and Bifidobacterium, Lactobacillus, Olsenella, and oleic acid was negatively correlated (r <–0.50, P < 0.04) with fecal score. Lachnoclostridium was positively correlated (r = 0.50, P = 0.01) with DAO and Bifidobacterium was negatively correlated (r = −0.51, P < 0.01) with DAO. Bifidobacterium was positively correlated (r = 0.51, P = 0.02) with IgG. Lachnoclostridium was positively correlated (r = 0.56, P = 0.01) with TNF-α. Olsenella and oleic acid was positively correlated (r > 0.57, P < 0.03) with GSH-Px. Lachnoclostridium was positively correlated (r = 0.50, P = 0.02) with MDA and Bifidobacterium was negatively correlated (r = −0.55, P = 0.01) with MDA.
Discussion
Diarrhea, one of the most prevalent diseases of weaned calves, could result in growth retardation, feed utilization reduced, and mortality increased (Slanzon et al., 2019). In the present study, the ADG of weaned calves was significantly improved by HNa and Gln combined supplementation. Additionally, the diarrhea incidence and fecal score of weaned calves were also dramatically decreased. In our previous study, we confirmed that inclusion with 5% HNa and Gln decreased the fecal score and diarrhea incidence of weaned calves (Wang et al., 2021). Moreover, the previous studies also reported that dietary supplementation of HNa and Gln decreased the diarrhea incidence of piglets and growth-retarded yaks (Kaevska et al., 2016; Ma et al., 2021). Calve diarrhea induced by weaning is associated with impaired intestinal epithelial barrier (Rice et al., 2019). Diamine oxidase and D-isomer of lactic acid can be used as useful biomarkers for monitoring the integrity of intestinal mucosa barrier (Peng et al., 2004; Wu et al., 2018). In the present study, weaned calves supplemented with HNa and Gln had a significantly lower serum DAO and D-lac concentration than control group. Similar with our results, inclusion with HAs decreased serum D-lac concentration in rats (Yasar et al., 2002). Dietary Gln supplementation also showed beneficial effects on intestinal barrier function in animals (Wang et al., 2015; Xue et al., 2018; Ma et al., 2021).
Some investigators have indicated that HNa or Gln could boost immunity by improving host antioxidant function and immunoglobulin concentration (Tohid et al., 2010; Trckova et al., 2017; Yan et al., 2020). Weaning is one of the most severe early-life stresses for calves which could induce inflammatory response, oxidative stress, intestinal dysfunction, and diarrhea. In our study, we found that calves in NC group had higher diarrhea incidence and concentration of TNF-α and MDA in the serum. In contrast, these indicators were lower in H+G group. Additionally, serum concentration of IgG, GSH-Px, and T-AOC was increased, which indicated that HNa and Gln may alleviate diarrhea caused by weaning via improving the antioxidant and anti-inflammatory capacity of calves. Consistent with our results, previous research found that dietary supplementation with HAs improved serum activities of T-SOD and GSH-Px, but decreased serum MDA level in juvenile broilers (Mao, 2019). Rensburg (2015) indicated that HAs could inhibit the releasing of pro-inflammatory cytokines by inhibiting the activity of classical inflammatory pathways. Moreover, the anti-inflammatory and immunomodulatory activities of Gln have been widely reported. For example, Zhou et al. (2012) indicated that intravenously administered Gln increased the concentration of serum IgA and IgG, and intestinal mucosal sIgA in early-weaned calves. Ma et al. (2021) demonstrated that dietary supplementation with Gln significantly decreased the mRNA expression of IL-1β and TNF-α in growth-retarded yaks.
It is well known that intestinal microbiota plays an important role in nutrient utilization, intestinal morphology and immunity (Guevarra et al., 2018; He et al., 2020; Kim et al., 2021). To clarify the mechanism of beneficial effects of HNa and Gln supplementation in weaned calves, the 16S rDNA sequencing and untargeted LC−MS metabolomics analysis were performed. From the results of phylum analysis, we found that the intestinal microbiota was dominated by Firmicutes and Bacteroidetes, and HNa and Gln significantly increased the abundance of Firmicute but decreased the abundance of Bacteroidetes of weaned calves, which is consistent with previous studies conducted by Zhang et al. (2020). Firmicutes is considered as one of the producers of short-chain fatty acid, and more efficient in promoting nutrition absorption than Bacteroidetes (Li et al., 2021). At the genus level, weaned calves supplemented with HNa and Gln had higher abundance of Bifidobacterium, Lactobacillus, Olsenella, Ruminiclostridium 9, Howardella, and uncultured organism when compared with NC group. It is well known, Bifidobacterium and Lactobacillus are probiotics, which have excellent efficacy in reducing gastrointestinal infections (Vemuri et al., 2018; He et al., 2020). The results of the present study indicated that supplemented with HNa and Gln could increase the abundance of probiotics in intestinal microbiota. In addition, spearman’s correlation analysis also indicated that Bifidobacterium, Lactobacillus, and Olsenella were positively correlated with IgG, ADG, and GSH-Px, while negatively correlated with MDA, DAO, and fecal score, respectively. This observation might explain that HNa and Gln inclusion could improve growth performance, anti-inflammatory, antioxidative status and alleviate diarrhea of weaned calves via increasing the abundance of intestinal beneficial microbiota.
Feces metabolites may also reflect the physiological status of calves (Lei et al., 2019; Ma et al., 2020). Currently, LC–MS-based metabolomics analyses are being increasingly performed to explore the alteration of metabolites (Flint et al., 2012). The results showed that the levels of fatty acid metabolites (oleic acid, sebacic acid, 1-palmitoylglycerol, Nname,cis-9,10-epoxystearic acid, and cis-9-palmitoleic acid), amino acid metabolites (3-aminobutanoic acid, Leu-Ala, Thr-Arg, and Tyr-Met), and carbohydrates metabolites (D-mannose) were significantly upregulated by HNa and Gln supplementation. Furthermore, the results of KEGG enrichments suggested that weaned calves supplemented with HNa and Gln primarily upregulated the fatty acid biosynthesis pathway. Surprisingly, Spearman’s correlation analysis found that upregulated metabolites were positively correlated with increased beneficial intestinal microbiota. For example, Bifidobacterium was positively correlated with Leu-Ala and sebacic acid, Lactobacillus was positively correlated with 3-aminobutanoic acid and oleic acid, Olsenella was positively correlated with 1-palmitoylglycerol and oleic acid. Taken together, the decreased diarrhea incidence observed in this study may be attributed to the positive role of HNa and Gln on the intestinal microbiota and metabolites in weaned claves.
An interesting result obtained by Spearman’s correlation analysis was that oleic acid, sebacic acid, and 1-palmitoylglycerol was negatively correlated with the fecal score, while Bifidobacterium, Lactobacillus, Olsenella, Ruminiclostridium 9, and Howardella were positively correlated with oleic acid, sebacic acid, and 1-palmitoylglycerol, which indicated that decreased diarrhea incidence is closely related to increased abundance of beneficial intestinal microbiota and altered metabolites. This might provide a new evidence for the mechanism of decreased diarrhea of weaned calves supplemented with HNa and Gln.
In conclusion, weaned calves supplemented with HNa and Gln have a higher ADG, antioxidant status as well as lower diarrhea incidence. Moreover, analysis of intestinal microbiota and metabolic profile revealed that weaned calves supplemented with HNa and Gln increased the relative abundance of intestinal beneficial microbiota and enriched many lipid metabolites and KEGG pathway of fatty acid biosynthesis. These findings could provide useful information for developing an effective and safe alternative for antibiotics to prevent and cure calve diarrhea in the calf industry.
Acknowledgment
This work was supported by the National Key Research and Development Program of China (2016YFD051310).
Glossary
Abbreviations
- ADFI
average daily feed intake
- ADG
average daily gain
- BW
body weight
- CTAB
cetyltriethylammnonium bromide
- DAO
diamine oxidase
- D-lac
D-isomer of lactic acid
- G:F
gain:feed
- Gln
glutamine
- GSH-Px
glutathione peroxidase
- H+G
5% HNa+1% Gln
- HNa
sodium humate
- HA
humic acid
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- IL-6
interleukin-6
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LC–MS
liquid chromatograph–mass spectrometer
- MDA
malondialdehyde
- NC
negative control
- OPLS-DA
orthotopic partial least-squares discriminant analysis
- OTUs
operational taxonomic units
- PCoA
principal coordinate analysis
- PLS-DA
partial least-squares discriminant analysis
- T-AOC
total antioxidant capacity
- TNF-α
tumour necrosis factor-α
- T-SOD
total superoxide dismutase
- VIP
variable importance in the projection
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Chen, Y., Wang J., Yu L., Xu T., and Zhu N.. . 2020. Microbiota and metabolome responses in the cecum and serum of broiler chickens fed with plant essential oils or virginiamycin. Sci. Rep. 10:5382. doi: 10.1038/s41598-020-60135-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzat, V., Macedo Rogero M., Noel Keane K., Curi R., and Newsholme P.. . 2018. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. 10:1564. doi: 10.3390/nu10111564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint, H. J., Scott K. P., Duncan S. H., Louis P., and Forano E.. . 2012. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 3:289–306. doi: 10.4161/gmic.19897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevarra, R. B., Hong S. H., Cho J. H., Kim B. R., Shin J., Lee J. H., Kang B. N., Kim Y. H., Wattanaphansak S., Isaacson R. E., . et al. 2018. The dynamics of the piglet gut microbiome during the weaning transition in association with health and nutrition. J. Anim. Sci. Biotechnol. 9:54. doi: 10.1186/s40104-018-0269-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y., Fan X., Liu N., Song Q., Kou J., Shi Y., Luo X., Dai Z., Yang Y., Wu Z., . et al. 2019. l-Glutamine represses the unfolded protein response in the small intestine of weanling piglets. J. Nutr. 149:1904–1910. doi: 10.1093/jn/nxz155 [DOI] [PubMed] [Google Scholar]
- He, Y., Jinno C., Kim K., Wu Z., Tan B., Li X., Whelan R., and Liu Y.. . 2020. Dietary Bacillus spp. enhanced growth and disease resistance of weaned pigs by modulating intestinal microbiota and systemic immunity. J. Anim. Sci. Biotechnol. 11:101. doi: 10.1186/s40104-020-00498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, H., Bai X., Shah A. A., Wen A. Y., Hua J. L., and Che C. Y.. . 2016. Dietary supplementation with glutamine and γ-aminobutyric acid improves growth performance and serum parameters in 22- to 35-day-old broilers exposed to hot environment. J. Anim. Physiol. Anim. Nutr. 100:361–370. doi: 10.1111/jpn.12346 [DOI] [PubMed] [Google Scholar]
- Kaevska, M., Lorencova A., Videnska P., Sedlar K., Provaznik I., and Trckova M.. . 2016. Effect of sodium humate and zinc oxide used in prophylaxis of post-weaning diarrhoea on faecal microbiota composition in weaned piglets. Vet. Medic. 61:328–336. doi: 10.17221/54/2016-VETMED [DOI] [Google Scholar]
- Kim, H. S., Whon T. W., Sung H., Jeong Y. S., Jung E. S., Shin N. R., Hyun D. W., Kim P. S., Lee J. Y., Lee C. H., . et al. 2021. Longitudinal evaluation of fecal microbiota transplantation for ameliorating calf diarrhea and improving growth performance. Nat. Commun. 12:161. doi: 10.1038/s41467-020-20389-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klučáková, M. 2018. Size and charge evaluation of standard humic and fulvic acids as crucial factors to determine their environmental behavior and impact. Front. Chem. 6:235. doi: 10.3389/fchem.2018.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, M., Menon R., Manteiga S., Alden N., Hunt C., and Alaniz R. C.. . 2019. Environmental chemical diethylhexyl phthalate alters intestinal microbiota community structure and metabolite profile in mice. MSystems. 4:e719–e724. doi: 10.1128/mSystems.00724-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, A., Yang Y., Qin S., Lv S., Jin T., Li K., Han Z., and Li Y.. . 2021. Microbiome analysis reveals gut microbiota alteration of early-weaned Yimeng black goats with the effect of milk replacer and age. Microb. Cell Fact. 20:78. doi: 10.1186/s12934-021-01568-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, B. H., Mei X. R., Lei C. W., Li C., Gao Y. F., and Kong L. H.. . 2020. Enrofloxacin shifts intestinal microbiota and metabolic profiling and hinders recovery from Salmonella enterica subsp. enterica serovar typhimurium infection in neonatal chickens. MSphere. 5:e00725–20. doi: 10.1128/mSphere.00725-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J., Shah A. M., Wang Z. S., Hu R., Zou H. W., Wang X. Y., Zhao S. N., and Kong X. Y.. . 2021. Dietary supplementation with glutamine improves gastrointestinal barrier function and promotes compensatory growth of growth-retarded yaks. Animal 15:100108. doi: 10.1016/j.animal.2020.100108 [DOI] [PubMed] [Google Scholar]
- Mao, Y. 2019. Modulation of the growth performance, meat composition, oxidative status, and immunity of broilers by dietary fulvic acids. Poult. Sci. 98:4509–4513. doi: 10.3382/ps/pez281 [DOI] [PubMed] [Google Scholar]
- Nemati, M., Menatian S., Joz Ghasemi S., Hooshmandfar R., Taheri M., and Saifi T.. . 2018. Effect of protected-glutamine supplementation on performance, milk composition and some blood metabolites in fresh Holstein cows. Iran. J. Vet. Res. 19:225–228 [PMC free article] [PubMed] [Google Scholar]
- Pempek, J. A., Watkins L. R., Bruner C. E., and Habing G. G.. . 2019. A multisite, randomized field trial to evaluate the influence of lactoferrin on the morbidity and mortality of dairy calves with diarrhea. J. Dairy Sci. 102:9259–9267. doi: 10.3168/jds.2019-16476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, X., Yan H., You Z., Wang P., and Wang S.. . 2004. Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns 30:135–139. doi: 10.1016/j.burns.2003.09.032 [DOI] [PubMed] [Google Scholar]
- Písaříková, B., Zralý Z., and Herzig I.. . 2010. The effect of dietary sodium humate supplementation on nutrient digestibility in growing pigs. Acta Vet. Brno. 79:349–353. doi: 10.2754/avb201079030349 [DOI] [Google Scholar]
- Proidakov, A. G. 2009. Humic acids from mechanically treated coals: a review. Solid Fuel Chem. 43:9–14. doi: 10.3103/s0361521909010030 [DOI] [Google Scholar]
- Ranjbar, R., and Farahani A.. . 2019. Shigella: antibiotic-resistance mechanisms and new horizons for treatment. Infect. Drug Resist. 12:3137–3167. doi: 10.2147/IDR.S219755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, W., Duan J., Yin J., Liu G., Cao Z., Xiong X., Chen S., Li T., Yin Y., Hou Y., . et al. 2014. Dietary L-glutamine supplementation modulates microbial community and activates innate immunity in the mouse intestine. Amino Acids 46:2403–2413. doi: 10.1007/s00726-014-1793-0 [DOI] [PubMed] [Google Scholar]
- Renaud, D., Shock D., and Roche S. M.. . 2019. Evaluation of Saccharomyces cerevisiae boulardii CNCM I-1079 fed before weaning on health and growth of male dairy calves. Appl. Anim. Sci. 35:570–576. doi: 10.15232/aas.2019-01889 [DOI] [Google Scholar]
- Rensburg, C. E. J. 2015. The antiinflammatory properties of humic substances: a mini review. Phytother. Res. 29:791–795. doi: 10.1002/ptr.5319 [DOI] [PubMed] [Google Scholar]
- Rice, E. M., Aragona K. M., Moreland S. C., and Erickson P. S.. . 2019. Supplementation of sodium butyrate to postweaned heifer diets: effects on growth performance, nutrient digestibility, and health. J. Dairy Sci. 102:3121–3130. doi: 10.3168/jds.2018-15525 [DOI] [PubMed] [Google Scholar]
- Slanzon, G. S., Toledo A. F., Silva A. P., Coelho M. G., da Silva M. D., Cezar A. M., and Bittar C. M. M.. . 2019. Red propolis as an additive for preweaned dairy calves: effect on growth performance, health, and selected blood parameters. J. Dairy Sci. 102:8952–8962. doi: 10.3168/jds.2019-16646 [DOI] [PubMed] [Google Scholar]
- Szot, K., Góralczyk K., Michalska M., Veryho N., Chojnowski J., Ponikowska I., and Rość D.. . 2019. The effects of humic water on endothelial cells under hyperglycemic conditions: inflammation-associated parameters. Environ. Geochem. Health. 41:1577–1582. doi: 10.1007/s10653-018-0238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taklimi, S. M. S. M., Ghahri H., and Isakan M. A.. . 2012. Influence of different levels of humic acid and esterified glucomannan on growth performance and intestinal morphology of broiler chickens. Agric. Sci. 03:663–668. doi: 10.4236/as.2012.35080 [DOI] [Google Scholar]
- Tohid, T., Hasan G., and Alireza T.. . 2010. Efficacy of mannanoligosaccharides and humate on immune response to Avian influenza (H9) disease vaccination in broiler chickens. Vet. Res. Commun. 34:709–717. doi: 10.1007/s11259-010-9444-8 [DOI] [PubMed] [Google Scholar]
- Trckova, M., Lorencova A., Babak V., Neca J., and Ciganek M.. . 2017. Effects of sodium humate and zinc oxide used in prophylaxis of post-weaning diarrhoea on the health, oxidative stress status and fatty acid profile in weaned piglets. Vet. Medic. 62:16–28. doi: 10.17221/70/2016-VETMED [DOI] [Google Scholar]
- Vemuri, R., Shinde T., Gundamaraju R., Gondalia S., Karpe A. V., and Beale D. L.. . 2018. Lactobacillus acidophilus DDS-1 modulates the gut microbiota and improves metabolic profiles in aging mice. Nutrients. 10:1255. doi: 10.3390/nu10091255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D., Du Y., Wang S., You Z., and Liu Y.. . 2021. Effects of sodium humate and glutamine combined supplementation on growth performance, diarrhea incidence, blood parameters, and intestinal microflora of weaned calves. Anim. Sci. J. 92:e13584. doi: 10.1111/asj.13584 [DOI] [PubMed] [Google Scholar]
- Wang, Q., Ying J. F., Zou P., Zhou Y. H., Wang B. K., and Yu D. Y.. . 2020. Effects of dietary supplementation of humic acid sodium and zinc oxide on growth performance, immune status and antioxidant capacity of weaned piglets. Animals. 10:2104. doi: 10.3390/ani10112104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., Zhang C., Wu G., Sun Y., Wang B., He B., Dai Z., and Wu Z.. . 2015. Glutamine enhances tight junction protein expression and modulates corticotropin-releasing factor signaling in the jejunum of weanling piglets. J. Nutr. 145: 25–31. doi: 10.3945/jn.114.202515 [DOI] [PubMed] [Google Scholar]
- Wu, Q. J., Liu N., Wu X. H., Wang G. Y., and Lin L.. . 2018. Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poult. Sci. 97:2675–2683. doi: 10.3382/ps/pey123 [DOI] [PubMed] [Google Scholar]
- Xue, G. D., Barekatain R., Wu S. B., Choct M., and Swick R. A.. . 2018. Dietary L-glutamine supplementation improves growth performance, gut morphology, and serum biochemical indices of broiler chickens during necrotic enteritis challenge. Poult. Sci. 97:1334–1341. doi: 10.3382/ps/pex444 [DOI] [PubMed] [Google Scholar]
- Yan, Y., Xu B., Yin B., Xu X., Niu Y., Tang Y., Wang X., Xie C., Yang T., Zhou S., . et al. 2020. Modulation of gut microbial community and metabolism by dietary glycyl-glutamine supplementation may favor weaning transition in piglets. Front. Microbiol. 10:3125. doi: 10.3389/fmicb.2019.03125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasar, S., Gokcimen A., Altuntas I., Yonden Z., and Petekkaya E.. . 2002. Performance and ileal histomorphology of rats treated with humic acid preparations. J. Anim. Physiol. Anim. Nutr. (Berl). 86:257–264. doi: 10.1046/j.1439-0396.2002.00383.x [DOI] [PubMed] [Google Scholar]
- Zhang, W., Bao C., Wang J., Zang J., and Cao Y.. . 2020. Administration of Saccharomyces boulardii mafic-1701 improves feed conversion ratio, promotes antioxidant capacity, alleviates intestinal inflammation and modulates gut microbiota in weaned piglets. J. Anim. Sci. Biotechnol. 11:112. doi: 10.1186/s40104-020-00516-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y., Paderu P., Delmas G., Dolgov E., Lee M. H., Senter M., Park S., Leivers S., and Perlin D. S.. . 2015. Carbohydrate-derived fulvic acid is a highly promising topical agent to enhance healing of wounds infected with drug-resistant pathogens. J. Trauma Acute Care Surg. 79(4 Suppl. 2):S121–S129. doi: 10.1097/TA.0000000000000737 [DOI] [PubMed] [Google Scholar]
- Zhou, Y., Zhang P., Deng G., Liu X., and Lu D.. . 2012. Improvements of immune status, intestinal integrity and gain performance in the early-weaned calves parenterally supplemented with L-alanyl-L-glutamine dipeptide. Vet. Immunol. Immunopathol. 145:134–142. doi: 10.1016/j.vetimm.2011.10.020 [DOI] [PubMed] [Google Scholar]
- Zou, T., Yang J., Guo X., He Q., Wang Z., and You J.. . 2021. Dietary seaweed-derived polysaccharides improve growth performance of weaned pigs through maintaining intestinal barrier function and modulating gut microbial populations. J. Anim. Sci. Biotechnol. 12:28. doi: 10.1186/s40104-021-00552-8 [DOI] [PMC free article] [PubMed] [Google Scholar]