Abstract
Introduction
Intraventricular hemorrhage (IVH) is a common cause of morbidity and mortality in preterm neonates. IVH leads to complications such as posthemorrhagic hydrocephalus (PHH), which commonly occurs in neonates with a more severe degree of IVH. Hence, we aimed to evaluate the characteristics and outcomes of PHH in neonates with IVH.
Methods
We performed a systematic review of cases reported from January 1978 to December 2020 through the PubMed database, using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the keywords ‘intraventricular hemorrhage,’ ‘cerebral intraventricular hemorrhage,’ and ‘newborn.’ A total of 79 articles were considered for analysis, and data on neonatal and maternal characteristics and outcomes were collected. The analysis was performed by using the χ2 test, Wilcoxon rank-sum test, and multivariate logistic regression model.
Results
We analyzed a total of 101 IVH cases, 54.5% were male and 62.4% preterm. Thirteen point nine percent (13.9%) presented with grade I, 35.6% grade II, and grade III respectively, and 8% grade IV IVH. Among the 59 (58.4%) neonates with PHH, 33.6% had resolved PHH and 24.8% had unresolved. In adjusted regression analysis, we found that neonates with resolved PHH have lower odds of having neurodevelopmental delay (OR:0.15, 95%CI:0.03-0.74; p=0.02) and death (OR:0.9;95%CI:0.01-0.99; p=0.049) as compared to unresolved PHH.
Conclusion
Our study showed that neonates with resolved PHH have a statistically significant lower risk of neurodevelopmental delay (NDD) and mortality. Future studies should be planned to evaluate the role of treatment and its effect on outcomes in IVH neonates with PHH as a complication.
Keywords: intraventricular hemorrhage, neonates, newborn, hydrocephalus, preterm
Introduction
Intraventricular hemorrhage (IVH) remains a significant cause of morbidity and mortality in newborns, particularly in preterm infants [1]. IVH in preterm infants occurs due to the rupture of fragile blood vessels within the germinal matrix of the developing brain into the ventricular system and fluctuations in the cerebral blood flow. IVH is described in four grades: (1) Bleeding in a small area of the ventricles, (2) Bleeding inside the ventricles, (3) Ventricles are enlarged by the blood, and (4) Bleeding into the brain tissues around the ventricles, of which grade IV is the most severe form of bleeding [2]. Despite the considerable prevalence of IVH in preterm babies, this phenomenon can also occur in term newborns and is accompanied by different etiologies, clinical manifestations, and outcomes [3]. Around 90% of cases of IVH occur within the first three days of life and 20-40% extend during the first week of life. Approximately 60% of premature infants with grade III and IV IVH will incur neurocognitive problems such as motor dysfunction, coordination problems, cerebral palsy, language and learning disabilities, attention deficit-hyperactivity disorder, behavioral issues, and social-emotional difficulties.
IVH leads to complications such as posthemorrhagic ventricular dilatation (PHVD), with or without post-hemorrhagic hydrocephalus (PHH), and periventricular hemorrhagic infarction (PHI) [1]. PHH is a serious complication that occurs in up to 35% of preterm neonates with IVH [4]. It occurs more frequently in neonates with more severe degrees of IVH and can lead to long-term neurological impairment and increased mortality. Treatment modalities for PHH include serial lumbar punctures, ventricular taps, insertion of external ventricular drainage, or placement of ventriculoperitoneal (VP) shunt [4]. Neonates with VP shunt are prone to develop shunt infections, malfunctions, and have worse neurodevelopmental outcomes [5]. Despite many treatment options, there is still no consensus regarding the management of PHH. There is limited literature of case reports and case series on the outcomes of PHH in neonates with IVH. Hence, we sought to perform a systematic review to evaluate the epidemiologic characteristics of neonates with IVH, with a primary focus on the characteristics and outcomes of PHH among neonates with IVH.
Materials and methods
We performed a systematic review of IVH cases reported in both preterm and term neonates and followed the predesigned Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol and standard for reporting the systematic review to the best extent of our possibilities [6].
Aims
The primary aim of the study was to compare the outcomes, including neurodevelopmental delay and death, in neonates with resolved PHH vs. unresolved PHH. The secondary aim was to evaluate the epidemiological characteristics of neonates with IVH and PHH.
The neurodevelopmental delay includes all patients with neurological impairment like gross and fine motor skills, language, and cognitive skills.
Search strategy and eligibility criteria
A comprehensive search was conducted using the PubMed database. We included case reports, case illustrations, letters reporting human cases, and case series from January 1978 to December 2020 by using the keywords ‘intraventricular hemorrhage,’ ‘cerebral intraventricular hemorrhage,’ ‘newborn,’ ‘neonate.’ Manual checks of the reference list were performed. Any articles that were written in the English language and describing details of IVH in term or preterm neonates were included. After the initial search, all titles and abstracts were reviewed. Finally, each article that met the criteria underwent a full-text review. Animal studies, non-English studies, and non-full text studies were excluded.
Selection of studies and data collection
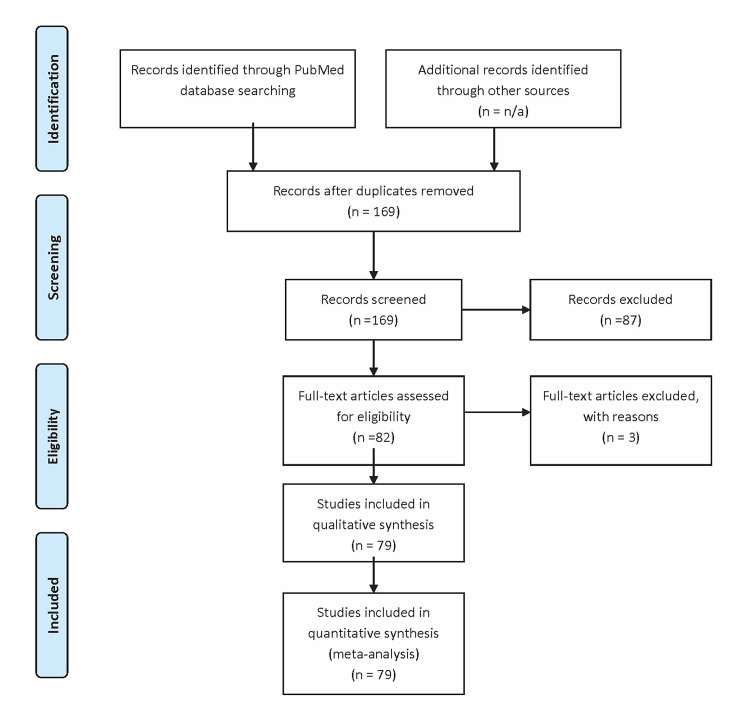
By using this search strategy, a total of 169 articles were identified and screened. After initial screening, 87 articles were excluded, as the onset of IVH was more than 28 days after birth, there was incomplete information on demographics or IVH onset, or they were not well-defined or were difficult to comprehend. Two physicians (CP and PM) independently reviewed all 82 articles, and they were further verified by a third independent reviewer (UP). Any conflict was resolved by consensus. Three articles were excluded, as they did not contain information regarding gestational age. This left us with 79 case reports and case series consisting of 101 cases that were considered for qualitative and quantitative analysis. Figure 1 depicts a flow chart of the literature search and selection process, and Table 1 outlines the studies considered for analysis with age and gender.
Table 1. Distribution of 101 intraventricular hemorrhage cases based on age and gender.
| Author; Year | Number of Cases | Gestational Age (weeks) | Gender |
| Akisu et al.; 2003 [7] | 3 | 38 | M |
| 30 | F | ||
| 28 | M | ||
| Alhifany et al.;2017 [8] | 1 | 24 | F |
| Alvarado & Rodriguez; 2017 [9] | 1 | 27 | Not given |
| Arai et al.; 2018 [10] | 1 | 30 | M |
| Arvanitis et al.; 1996 [11] | 1 | 36 | F |
| Benvenisti et al.; 2015 [12] | 1 | 38 | F |
| Bhattacharya et al.; 2018 [13] | 1 | 37 | M |
| Biermayr et al.; 2016 [14] | 1 | 30 | F |
| Bilguvar et al.; 2009 [15] | 2 | 24 | F |
| 24 | M | ||
| Borenstein-Levin et al.; 2014 [16] | 2 | 29 | M |
| 24 | M | ||
| Brown et al.; 1994 [17] | 2 | 28 | M |
| 26 | M | ||
| Castro Conde et al.; 2005 [18] | 1 | 37 | M |
| Cheng et al.; 2006 [19] | 1 | 38 | M |
| Chun et al.; 2011 [20] | 1 | 26 | F |
| Counsell et al.; 1999 [21] | 1 | 28 | F |
| Duijvestijn et al.; 2003 [22] | 1 | 40 | M |
| Eller & Pasternak; 1985 [23] | 4 | 31 | M |
| 27 | M | ||
| 32 | M | ||
| 30 | M | ||
| Fawer & Levene; 1982 [24] | 2 | 30 | M |
| 30 | M | ||
| Fawer & Dubowitz; 1983 [25] | 1 | 30 | M |
| Friese et al.; 2003 [26] | 1 | 42 | M |
| Fusch et al.; 1997 [27] | 1 | 36 | Not given |
| Golinko et al.; 2018 [28] | 1 | 23 | Not given |
| Hanigan et al.; 1990 [29] | 1 | 39 | F |
| Hashimoto et al.; 1997 [30] | 1 | 36 | M |
| Heafner et al.; 1985 [31] | 1 | 38 | F |
| Heck et al.; 20021 [32] | 1 | 36 | F |
| Heineiking et al.; 2003 [33] | 1 | 39 | M |
| Hentschel et al.; 1993 [34] | 1 | 28 | F |
| Hevner et al.; 1997 [35] | 1 | 28 | M |
| Hill et al.; 1984 [36] | 1 | 38 | Not given |
| Jacob et al.; 2017 [37] | 1 | 35 | F |
| Kamikawa et al.; 2001 [38] | 2 | 36 | F |
| 29 | M | ||
| Katumba et al.; 2005 [39] | 1 | 25 | Not given |
| Khatri et al.; 2018 [40] | 1 | 38 | F |
| Kimura et al.; 2018 [41] | 1 | 24 | M |
| Ko et al.; 2010 [42] | 1 | 30 | Not given |
| Koehne et al.; 2006 [43] | 1 | 27 | M |
| Krueger et al.; 2008 [44] | 1 | 28 | Not given |
| Kumar et al.; 2013 [45] | 1 | 41 | F |
| Mathews et al.; 2017 [46] | 1 | 38 | M |
| Mays et al.; 1995 [47] | 3 | 24 | M |
| 27 | F | ||
| 24 | F | ||
| Ment et al.; 1984 [48] | 2 | 26 | F |
| 28 | M | ||
| Mohila et al.; 2010 [49] | 2 | 33 | F |
| 33 | F | ||
| Nakano et al.; 2007 [50] | 1 | 24 | F |
| Negishi et al.; 1989 [51] | 1 | 38 | M |
| Nelson et al.; 1986 [52] | 1 | 36 | M |
| Pasternak & Volpe; 1979 [53] | 1 | 35 | F |
| Prats et al.; 2001 [54] | 1 | 29 | F |
| Preub et al.; 2015 [55] | 1 | 29 | M |
| Ramenghi et al.; 2002 [56] | 1 | 33 | M |
| Ritschl et al.; 1987 [57] | 1 | 30 | M |
| Scher et al.; 1982 [58] | 5 | 39 | F |
| 42 | M | ||
| 39 | M | ||
| 40 | M | ||
| 40 | F | ||
| Seeburg et al.; 2014 [59] | 2 | 24 | Not given |
| 37 | Not given | ||
| Shackleford et al.; 1984 [60] | 1 | 28 | Not given |
| Sharma et al.; 2012 [61] | 1 | 28 | M |
| Shirane et al.; 1986 [62] | 1 | 38 | F |
| Sobolewska et al.; 2018 [63] | 1 | 41 | M |
| Suksumek et al.; 2013 [64] | 1 | 39 | M |
| Szpecht et al.; 2016 [65] | 2 | 39 | M |
| 39 | M | ||
| Tajdar et al.; 2018 [66] | 1 | 34 | M |
| Tan et al.; 1998 [67] | 1 | 38 | M |
| Van Raay et al.; 2009 [68] | 1 | 38 | F |
| Voutsinas et al.; 1991 [69] | 1 | 38 | M |
| Weinschenk et al.; 2001 [70] | 1 | 27 | M |
| Whitelaw et al.; 1984 [71] | 1 | 37 | F |
| Mitchell et al.; 1980 [72] | 1 | 38 | F |
| Timothy et al.; 1979 [73] | 1 | 41 | F |
| Wehberg et al.; 1991 [74] | 1 | 38 | M |
| Ma et al.; 2017 [75] | 1 | 38 | M |
| Abel et al.; 2003 [76] | 1 | 38 | F |
| De Vries et al.; 2000 [77] | 3 | 28 | F |
| 27 | M | ||
| 40 | M | ||
| Filippi et al; 2004 [78] | 1 | 25 | F |
| Ho et al.; 1987 [79] | 1 | 30 | M |
| Molnar et al.; 2012 [80] | 1 | 28 | M |
| Shah et al.; 2010 [81] | 1 | 24 | Not given |
| Tancabelic et al.; 2004 [82] | 1 | 36 | M |
| Yang et al.; 1999 [83] | 1 | 38 | F |
| Yu et al.; 1994 [84] | 1 | 26 | M |
| Upma et al.; 2016 [85] | 1 | 38 | M |
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the literature search and selection process of intraventricular hemorrhage in neonates.
All eligible studies were reviewed using a standardized web-based form to collect information. All data were summarized, which included neonatal characteristics such as gestational age, birth weight, sex, IVH onset, IVH grade, associated complications like seizures, respiratory distress syndrome, cardiovascular or neurological diseases, presence of PHH, resolved or treated by serial lumbar puncture or permanent ventriculoperitoneal shunt, and maternal characteristics such as maternal age, mode of delivery and maternal steroid use. Data on outcomes of neurodevelopmental delay and mortality were collected.
Statistical analysis
A Microsoft Excel sheet (Microsoft Corporation, Redmond, WA) was used to collect the data of 101 patients, and the data were analyzed using SAS 9.4 (SAS Institute Inc., Cary, NC). We calculated frequency, percentage, and median and standard error for the characteristics of the patients with IVH and PHH. Univariate analysis of categorical data was performed using the chi-square test and differences in the median were evaluated by the Wilcoxon-Mann-Whitney test. Multivariable logistic regression analysis was performed to evaluate the association between outcomes and PHH. Odds ratio, 95% confidence interval, and goodness of fit (c-value) were calculated, and p<0.05 was considered significant. Models were adjusted with gestational age (term vs. preterm), sex, IVH onset, and IVH laterality. We have not adjusted models with maternal characteristics due to a lack of evidence from previous research to establish a relationship between neonatal mortality and neurodevelopmental delay (NDD) with post-PHH resolution. Due to insufficient data on the method of resolution (without intervention, serial lumbar puncture, and VP shunt), we have not included them in the regression analysis.
Results
Neonatal and maternal characteristics of IVH
We included a total of 101 individual cases of IVH in newborns, of which 17 (16.8%) had unilateral and 65 (64.4%) had bilateral IVH. Fifty-five (54.5%) were male and 35 (34.6%) female. Of the 101 neonates, 14 (13.9%) presented with grade I, 36 (35.6%) with grade II, 36 (35.6%) grade III, and eight (8%) grade with grade IV IVH. Imaging studies, such as head ultrasound, were performed in 69 (68.3%), MRI brain in 20 (20%), and CT scan in 37 (36.6%). Fifty-nine (58.4%) neonates developed PHH, which was resolved in 34 (33.6%) and unresolved in 25 (24.8%). Twenty-three (22.7%) underwent serial lumbar puncture (LP) and a permanent VP shunt was placed in 37 (36.6%). NDD occurred in 34 (33.7%) and 17 (16.8%) died from complications of IVH (Table 2).
Table 2. Maternal and clinical characteristics of neonates with intraventricular hemorrhage (IVH).
*IVH Grade: 1-Bleeding is confined to the germinal matrix; 2-Germinal matrix hemorrhagic and IVH occupies <50% of the lateral ventricle volume; 3-Germinal matrix hemorrhagic and IVH occupies more than 50% of the lateral ventricle volume and is associated with acute ventricular distension; 4-Periventricular hemorrhagic infarction; Hemorrhagic infarction in periventricular white matter ipsilateral to large IVH. # Neurodevelopmental delay includes all patients with neurological impairment like gross and fine motor skills, language, and cognitive skills.
| Variables | Intraventricular Hemorrhage N=101 (%) |
| Intraventricular Hemorrhage (IVH) | |
| Unilateral | 17 (16.8%) |
| Bilateral | 65 (64.4%) |
| Laterality Unknown | 19 (18.8%) |
| Post Hemorrhagic Hydrocephalus (PHH) | |
| Yes | 59 (58.4%) |
| No | 42 (41.6%) |
| PHH Resolved | |
| Yes | 34 (33.6%) |
| No | 25 (24.8%) |
| Neonatal Characteristics | |
| Birth Weight (Grams) | |
| Median (IQR) | 1728 (2189) |
| Gender | |
| Male | 55 (54.5%) |
| Female | 35 (34.6%) |
| Gestational Age | |
| Preterm | 63 (62.4%) |
| Term | 38 (37.6%) |
| Ultrasound | |
| Yes | 69 (68.3%) |
| No | 32 (31.7%) |
| Magnetic Resonance Imaging (MRI) | |
| Yes | 20 (20%) |
| No | 81 (80%) |
| Computed Tomography (CT) Scan | |
| Yes | 37 (36.6%) |
| No | 64 (63.4%) |
| IVH Grade* | |
| 1 | 14 (13.9%) |
| 2 | 36 (35.6%) |
| 3 | 36 (35.6%) |
| 4 | 8 (8%) |
| IVH Onset (days from birth) | |
| Median (IQR) | 3 (5) |
| Serial Lumbar Puncture | |
| Yes | 23 (22.7%) |
| No | 76 (75.2%) |
| Ventriculoperitoneal (VP) Shunt | |
| Yes | 37 (36.6%) |
| No | 62 (61.4%) |
| Associated Symptoms | |
| Seizures | 6 (6%) |
| Respiratory Distress Syndrome (RDS) | 24 (23.7%) |
| CVS | 4 (3.9%) |
| CNS | 3 (2.9%) |
| Others | 1 (1%) |
| Maternal Characteristics | |
| Maternal Age (Years) | |
| Median (IQR) | 32 (7) |
| Gravida | |
| Primigravida | 19 (18.8%) |
| Multigravida | 26 (25.7%) |
| Mode of Delivery | |
| Normal Vaginal Delivery | 46 (45.5%) |
| Cesarean Section | 34 (33.7%) |
| Outcomes | |
| Outcomes | |
| Neurodevelopmental Delay# | 34 (33.7%) |
| Death | 17 (16.8%) |
Maternal and neonatal characteristics of posthemorrhagic hydrocephalus (PHH) amongst IVH
Among the 59 (58.4%) neonates who developed PHH, it was resolved in 34 (33.6%) and unresolved in 25 (24.8%). We found a high percentage of males with resolved PHH compared to unresolved PHH (59% vs. 28%; p=0.002). There is no significant difference between the median time of IVH onset for patients with resolved PHH (3±5 days from birth) compared to unresolved PHH (2±5 days from birth; p=0.42). The median birth weight of neonates with resolved PHH was not statistically different from unresolved PHH (1691±1200 vs. 1364±1400; p=0.53). Out of the 59 patients who developed PHH, five (8.5%) had grade I IVH, 26 (44.1%) grade II, 25 (42.4%) grade III, and three (5.1%) with grade IV. A higher frequency of grade III IVH is found in neonates with unresolved PHH compared to those with resolved PHH (60% vs. 29%; p=0.001) (Table 3).
Table 3. Maternal and neonatal characteristics and outcomes of post-hemorrhagic hydrocephalus (PHH) amongst intraventricular hemorrhage (IVH).
Please note all percentages are column percent to compare resolved PHH and unresolved PHH with other variables. *IVH Grade: 1-Bleeding is confined to the germinal matrix; 2-Germinal matrix hemorrhagic and IVH occupies <50% of the lateral ventricle volume; 3- Germinal matrix hemorrhagic and IVH occupies more than 50% of the lateral ventricle volume and is associated with acute ventricular distension; 4-Periventricular hemorrhagic infarction; Hemorrhagic infarction in periventricular white matter ipsilateral to large IVH; # Neurodevelopmental delay includes all patients with neurological impairment like gross and fine motor skills, language, and cognitive skills.
| Variables | Resolved PHH N= 34 (%) | Unresolved PHH N =25 (%) | p-value |
| Intraventricular hemorrhage (IVH) | |||
| Unilateral | 0.37 | ||
| Bilateral | 8 (23%) | 3 (12%) | |
| Laterality Unknown | 21 (62%) | 19 (76%) | |
| 5 (15%) | 3 (12%) | ||
| Neonatal Characteristics | |||
| Birth weight (Grams) | 0.53 | ||
| Median ± IQR | 1691±1200 | 1364±1400 | |
| Gender | 0.002 | ||
| Male | 20 (59%) | 7 (28%) | |
| Female | 10 (29.4%) | 11 (44%) | |
| Gestational age | 0.07 | ||
| Term | 11 (32.3%) | 6 (24%) | |
| Preterm | 23 (67.6%) | 19 (76%) | |
| Ultrasound | 0.69 | ||
| Yes | 25 (73.5%) | 17 (68%) | |
| No | 9 (26.5%) | 8 (32%) | |
| Magnetic Resonance Imaging (MRI) | 0.85 | ||
| Yes | 7 (20.6%) | 4 (16%) | |
| No | 27 (79.4%) | 21 (84%) | |
| Computed Tomography (CT) Scan | 0.81 | ||
| Yes | 11 (32%) | 10 (40%) | |
| No | 23 (67.6%) | 15 (60%) | |
| IVH Grade* | 0.001 | ||
| 1 | 3 (9%) | 2 (8%) | |
| 2 | 19 (56%) | 7 (28%) | |
| 3 | 10 (29%) | 15 (60%) | |
| 4 | 2 (6%) | 1 (4%) | |
| IVH Onset (days from birth) | 0.42 | ||
| Median ± IQR | 3±5 | 2±5 | |
| Serial Lumbar Puncture | <0.001 | ||
| Yes | 17 (50%) | 4 (16%) | |
| No | 17 (50%) | 21 (84%) | |
| Permanent Ventriculoperitoneal (VP) Shunt | <0.001 | ||
| Yes | 21 (62%) | 16 (64%) | |
| No | 13 (38%) | 9 (36%) | |
| Associated Symptoms | 0.007 | ||
| Seizures | 2 (6%) | 4 (16%) | |
| RDS | 12 (35%) | 12 (48%) | |
| CVS | 3 (9%) | 1 (4%) | |
| CNS | 0 (0%) | 3 (12%) | |
| Others | 0 (0%) | 1 (4%) | |
| Maternal Characteristics | |||
| Maternal Age (Years) | 0.96 | ||
| Median ± IQR | 29±9 | 30±13 | |
| Gravida | 0.69 | ||
| Primigravida | 6 (18%) | 5 (20%) | |
| Multigravida | 6 (18%) | 7 (28%) | |
| Mode of Delivery | 0.016 | ||
| Normal Vaginal Delivery | 10 (29.4%) | 13 (52%) | |
| Cesarean Section | 11 (32.3%) | 7 (28%) | |
| Outcomes | |||
| Outcomes | 0.0001 | ||
| Neurodevelopmental Delay# | 8 (23%) | 12 (48%) | |
| Death | 1 (3%) | 9 (36%) | |
In our patients, cranial ultrasound was performed in 42 (71.2%), CT head in 21 (35.6%), and MRI brain in 11 (18.6%). There was no statistically significant association between imaging studies and PHH. We found that unresolved PHH has a higher frequency of seizures, respiratory distress syndrome (RDS), and CNS-related symptoms compared to resolved PHH (16% vs. 6%, 48% vs. 35%, 12% vs 0%; p=0.007), respectively. Furthermore, patients with resolved PHH have higher utilization of serial LP compared to unresolved PHH (50% vs. 16%; p<0.001) (Table 3).
Considering the maternal characteristics evaluated in our study; there was no significant difference between median maternal age for neonates with resolved PHH and unresolved PHH (29±9 vs. 30±13; p=0.96). There was a high frequency of normal vaginal delivery in neonates with unresolved PHH compared to resolved PHH (52% vs. 29.4%; p=0.016). Eleven out of 59(18.6%) of the mothers were primigravida and 13/59 (22%) were multigravida (Table 3).
Univariate analysis of outcomes
We found that neonates with resolved PHH have a lower prevalence of neurodevelopmental delay (NDD) (23% vs. 48%; p=0.0001) and death compared to unresolved PHH (3% vs. 36%; p=0.0001), respectively (Table 3).
Regression analysis
In our adjusted multivariable logistic regression model, we found that neonates with resolved PHH have lower odds of having neurodevelopmental delay (OR:0.15, 95%CI:0.03-0.74; p=0.02) and death (OR:0.9;95%CI:0.01-0.99; p=0.049) compared to unresolved PHH. c-values for the models of neurodevelopmental delay and death were 0.81 and 0.80, respectively, which is >0.7, indicating a good model fit (Table 4).
Table 4. Regression analysis of the outcomes of resolved-post hemorrhagic hydrocephalus (PHH) amongst neonates with intraventricular hemorrhage (IVH).
| Model 1 Neurodevelopmental delay | Model 2 Death | ||||||||
| Odds Ratio (OR) | Confidence Interval (CI) | p-value | Odds Ratio (OR) | Confidence Interval (CI) | p-value | ||||
| LL | UL | LL | UL | ||||||
| Unresolved PHH | Reference | ||||||||
| Resolved PHH | 0.15 | 0.03 | 0.74 | 0.02 | 0.9 | 0.01 | 0.99 | 0.049 | |
| Gestational age | |||||||||
| Term | Reference | ||||||||
| Preterm | 0.25 | 0.03 | 2.02 | 0.192 | 0.67 | 0.061 | 7.16 | 0.738 | |
| Gender | |||||||||
| Female | Reference | ||||||||
| Male | 1.16 | 0.33 | 4.04 | 0.812 | 0.82 | 0.19 | 3.35 | 0.777 | |
| IVH | |||||||||
| Unilateral | Reference | ||||||||
| Bilateral | 0.56 | 0.11 | 2.75 | 0.476 | 1.42 | 0.17 | 11.9 | 0.748 | |
| IVH onset | 1.02 | 0.87 | 1.91 | 0.839 | 0.9 | 0.731 | 1.11 | 0.335 | |
Discussion
The immaturity of the neonatal central nervous system (CNS) results in its vulnerability to injury, particularly when associated with prematurity. The extent of CNS injury can range from IVH (bleeding into the germinal matrix), intraparenchymal hemorrhage (bleeding within the substance of the brain), and white matter injury, including periventricular leukomalacia. The risk of these injuries decreases with increased gestational age, suggesting fragility and immaturity of the premature neonatal brain [1]. Despite the dramatically decreased prevalence of IVH in both term and preterm neonates over the last three to four decades, neonates with severe forms of IVH (grades III and IV) remain at higher risk of developing neurocognitive problems [86-88]. It has been estimated that 60% of premature infants with grade III and IV IVH will incur neurocognitive problems such as developmental delay, cerebral palsy, learning, and intellectual disabilities [2].
In our systematic review, we noted that 59/101 (58.4%) cases with IVH developed PHH. Prematurity was associated with a higher percentage of developing PHH compared to term gestation (17/59, 28.8%). This discrepancy can be attributed to increased maturity of the brain in term neonates and attenuation of underlying risk factors associated with prematurity [3]. Of the 59 patients, resolution of PHH was noted in 34 neonates (57.6%), whereas 25 (42.4%) had unresolved PHH. Furthermore, we found that neonates with resolved PHH have lower odds of developing NDD and death compared to unresolved PHH. This was consistent with findings described by de Vries et al., who noted that neonates with IVH without ventricular dilation had a decreased risk of neurodevelopmental disabilities when compared to those with ventricular dilation, PHH, or IPH [89]. Szpecth et al. in a retrospective analysis identified that approximately 60% of premature infants with grade III and IV IVH with PHH incur neurocognitive problems [2]. We believe that the lower incidence of developing NDD and death in neonates with resolved PHH may be possible as they had a less severe disease when compared to those with unresolved PHH. Secondly, patients with resolved PHH had higher utilization of treatment procedures.
Severe IVH predisposes to ventricular dilation and PHH warranting intermittent spinal or ventricular taps and possible permanent VP shunt placement [86]. There is no general agreement regarding the best treatment option for PHH, and previous literature suggests that there are no standard guidelines that determine when to implant a VP shunt [4]. In our study sample, neonates with resolved PHH had higher utilization of serial LP compared to unresolved PHH (50% vs. 16%, p<0.001), and there was a higher percentage of VP shunt placement in unresolved PHH (62% vs. 64%, p<0.001). The findings reported in our study were similar to those reported by de Vries and Groenendaal et al. and Hong Shen-Lee et al. [5,89]. Published literature recommends that the utilization of these procedures be reserved for symptomatic neonates and long-term neurodevelopmental outcomes of neonates with PHH and permanent shunt have not been systematically determined [4].
Strength and limitations of the study
The major strength of our study is that it analyzed only individual case reports and precisely evaluated the outcomes, including neurodevelopmental delay and death in neonates with PHH. However, our study has some limitations. First, the sample size is small because of the rarity of IVH and PHH in neonates and the strict exclusion criteria, excluding other prospective studies that did not have individual patient data available. Although this was done to avoid duplicate patients and maintain the quality of the article, it further reduced the number of patients in the analysis. Second, there was insufficient data on treatment utilized for PHH in neonates with IVH and the treatment options represent the preferences of the physicians, so we cannot evaluate the role of independent intervention methods in resolving PHH. Nevertheless, given the limited availability of accurate information on this disease, this study shows a relatively large number of patients. Future studies should emphasize the role of early resolution of PHH, the cut-off time to wait before choosing a specific intervention, and the type of intervention to resolve PHH amongst neonates with IVH.
Conclusions
Our study results show that neonates with resolved PHH have a significantly lower risk of neurodevelopmental delay and mortality. Furthermore, neonates with unresolved PHH have a higher prevalence of seizures, respiratory distress syndrome (RDS), and CNS-related symptoms compared to resolved PHH. In conclusion, our findings highlight the importance of periodic reviews to evaluate the efficacy and safety of neuroprotective strategies designed to mitigate the complications associated with IVH in neonates.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Trends in hospitalization of preterm infants with intraventricular hemorrhage and hydrocephalus in the United States, 2000-2010. Christian EA, Jin D, Attenello F, et al. Fluids Barriers CNS. 2015;12:0. doi: 10.3171/2015.7.PEDS15140. [DOI] [PubMed] [Google Scholar]
- 2.Neurodevelopmental outcomes of extremely low-gestational-age neonates with low-grade periventricular-intraventricular hemorrhage. Payne AH, Hintz SR, Hibbs AM, Walsh MC, Vohr BR, Bann CM, Wilson-Costello DE. JAMA Pediatr. 2013;167:451–459. doi: 10.1001/jamapediatrics.2013.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Intraventricular hemorrhage in term neonates: sources, severity and outcome. Afsharkhas L, Khalessi N, Karimi Panah M. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4577696/ Iran J Child Neurol. 2015;9:34–39. [PMC free article] [PubMed] [Google Scholar]
- 4.Hydrocephalus after intraventricular hemorrhage in preterm and low-birth weight infants: analysis of associated risk factors for ventriculoperitoneal shunting. Kazan S, Güra A, Uçar T, Korkmaz E, Ongun H, Akyuz M. Surg Neurol. 2005;64 Suppl 2:0–81. doi: 10.1016/j.surneu.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Posthemorrhagic hydrocephalus in newborns: clinical characteristics and role of ventriculoperitoneal shunts. Lee IC, Lee HS, Su PH, Liao WJ, Hu JM, Chen JY. Pediatr Neonatol. 2009;50:26–32. doi: 10.1016/S1875-9572(09)60026-7. [DOI] [PubMed] [Google Scholar]
- 6.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Moher D, Liberati A, Tetzlaff J, Altman DG. PLoS Med. 2009;6:0. [PMC free article] [PubMed] [Google Scholar]
- 7.Intraventricular administration of recombinant tissue plasminogen activator for intraventricular hemorrhage in the newborn. Akisu M, Yalaz M, Arslanoglu S, Kultursay N. Neurosurg Rev. 2003;26:266–268. doi: 10.1007/s10143-003-0282-9. [DOI] [PubMed] [Google Scholar]
- 8.Premature labor and neonatal septicemia caused by Capnocytophaga ochracea. Alhifany AA, Almangour TA, Tabb DE, Levine DH. Am J Case Rep. 2017;18:674–676. doi: 10.12659/AJCR.903824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Periorbital pallor post application of mydriatic in infants with hydrocephalus associated to systemic effects [Article in Spanish] Alvarado Socarras JL, Rodríguez SC. Rev Chil Pediatr. 2017;88:280–284. doi: 10.4067/S0370-41062017000200014. [DOI] [PubMed] [Google Scholar]
- 10.A case of neonatal lupus erythematosus in a very low-birth-weight infant that suffered intraventricular hemorrhage at birth. Arai H, Nakajima H, Ogino N, et al. Mod Rheumatol. 2018;28:721–723. doi: 10.3109/14397595.2016.1153181. [DOI] [PubMed] [Google Scholar]
- 11.Angiodysgenetic necrotizing encephalopathy or diffuse meningocerebral angiomatosis. Arvanitis DL, Apostolidou IA, Routsis PV, Biskini EI, Kalpoyannis NS. Pediatr Neurol. 1996;14:155–157. doi: 10.1016/0887-8994(96)00004-5. [DOI] [PubMed] [Google Scholar]
- 12."Growing" cerebellum in an infant after shunt insertion. Benvenisti H, Bassan H, Shiran S, Constantini S, Roth J. Pediatr Neurol. 2015;52:222–225. doi: 10.1016/j.pediatrneurol.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Intraventricular haemorrhage and obstructive hydrocephalus in a term neonate: an uncommon presentation of haemophilia B. Bhattacharya D, Sharawat IK, Saini L. BMJ Case Rep. 2018;2018:0–225341. doi: 10.1136/bcr-2018-225341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Case report of a central venous access device-associated thrombosis with aortic embolism in a preterm infant. Biermayr M, Brunner B, Maurer K, Trawoeger R, Kiechl-Kohlendorfer U, Neubauer V. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5012094/ BMC Pediatr. 2016;16:154. doi: 10.1186/s12887-016-0691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.COL4A1 mutation in preterm intraventricular hemorrhage. Bilguvar K, DiLuna ML, Bizzarro MJ, et al. J Pediatr. 2009;155:743–745. doi: 10.1016/j.jpeds.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Post-hemorrhagic hydrocephalus and diabetes insipidus in preterm infants. Borenstein-Levin L, Koren I, Kugelman A, Bader D, Toropine A, Riskin A. J Pediatr Endocrinol Metab. 2014;27:1261–1263. doi: 10.1515/jpem-2014-0098. [DOI] [PubMed] [Google Scholar]
- 17.Real-time ultrasonography of arterial IVH in preterm infants. Brown WD, Gerfen GW, Vachon LA, Nelson MD. Pediatr Neurol. 19941;11:325–327. doi: 10.1016/0887-8994(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 18.CNS siderosis and dandy-walker variant after neonatal alloimmune thrombocytopenia. Castro Conde JR, Martínez ED, Rodríguez RC, Rodríguez De Hoyos AL. Pediatr Neurol. 2005;32:346–349. doi: 10.1016/j.pediatrneurol.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Probable intrafamilial transmission of coxsackievirus b3 with vertical transmission, severe early-onset neonatal hepatitis, and prolonged viral RNA shedding. Cheng LL, Ng PC, Chan PK, Wong HL, Cheng FW, Tang JW. Pediatrics. 2006;118:0–33. doi: 10.1542/peds.2006-0554. [DOI] [PubMed] [Google Scholar]
- 20.Neuroendoscopic fenestration of the foramen of Monro without septostomy for unilateral hydrocephalus following neonatal intraventricular hemorrhage. Chun HJ, Lee Y, Park HK, Kim YS. Childs Nerv Syst. 2011;27:473–478. doi: 10.1007/s00381-010-1272-1. [DOI] [PubMed] [Google Scholar]
- 21.Periventricular haemorrhagic infarct in a preterm neonate. Counsell SJ, Maalouf EF, Rutherford MA, Edwards AD. Eur J Paediatr Neurol. 1999;3:25–27. doi: 10.1053/ejpn.1999.0175. [DOI] [PubMed] [Google Scholar]
- 22.Neonatal intraventricular haemorrhage associated with maternal use of paroxetine. Duijvestijn YC, Kalmeijer MD, Passier AL, Dahlem P, Smiers F. Br J Clin Pharmacol. 2003;56:581–582. doi: 10.1046/j.1365-2125.2003.01906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isolated ventricles following intraventricular hemorrhage. Eller TW, Pasternak JF. J Neurosurg. 1985;62:357–362. doi: 10.3171/jns.1985.62.3.0357. [DOI] [PubMed] [Google Scholar]
- 24.Elusive blood clots and fluctuating ventricular dilatation after neonatal intraventricular haemorrhage. Fawer CL, Levene MI. Arch Dis Child. 1982;57:158–160. doi: 10.1136/adc.57.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Intraventricular haemorrhage in a preterm neonate: discordance between clinical course and ultrasound scan. Fawer CL, Levene MJ, Dubowitz LM. Neuropediatrics. 1983;14:242–244. doi: 10.1055/s-2008-1059587. [DOI] [PubMed] [Google Scholar]
- 26.Isolated internal cerebral venous thrombosis in a neonate with increased lipoprotein (a) level: diagnostic and therapeutic considerations. Friese S, Müller-Hansen I, Schöning M, Nowak-Göttl U, Küker W. Neuropediatrics. 2003;34:36–39. doi: 10.1055/s-2003-38615. [DOI] [PubMed] [Google Scholar]
- 27.Perinatal ultrasonography and magnetic resonance imaging findings in congenital hydrocephalus associated with fetal intraventricular hemorrhage. Fusch C, Ozdoba C, Kuhn P, et al. Am J Obstet Gynecol. 1997;177:512–518. doi: 10.1016/s0002-9378(97)70138-8. [DOI] [PubMed] [Google Scholar]
- 28.Surgical management of craniosynostosis in the setting of a ventricular shunt: a case series and treatment algorithm. Golinko MS, Atwood DN, Ocal E. Childs Nerv Syst. 2018;34:517–525. doi: 10.1007/s00381-017-3648-y. [DOI] [PubMed] [Google Scholar]
- 29.Spontaneous regression of giant arteriovenous fistulae during the perinatal period. Case report. Hanigan WC, Brady T, Medlock M, Smith EB. J Neurosurg. 1990;73:954–957. doi: 10.3171/jns.1990.73.6.0954. [DOI] [PubMed] [Google Scholar]
- 30.Fetal cavernous angioma — case report. Hashimoto H, Sakaki T, Ishida Y, Shimokawara T. Neurol Med Chir (Tokyo) 1997;37:346–349. doi: 10.2176/nmc.37.346. [DOI] [PubMed] [Google Scholar]
- 31.Intraventricular hemorrhage in a term neonate secondary to a third ventricular AVM. Case report. Heafner MD, Duncan CC, Kier EL, Ment LR, Scott DT, Kolaski R, Sorgen C. J Neurosurg. 1985;63:640–643. doi: 10.3171/jns.1985.63.4.0640. [DOI] [PubMed] [Google Scholar]
- 32.Choroid plexus arteriovenous malformation presenting with intraventricular hemorrhage. Heck DV, Gailloud P, Cohen HL, Clatterbuck RE, Tamargo R, Avellino AM, Murphy KP. J Pediatr. 2002;141:710–711. doi: 10.1067/mpd.2002.129033. [DOI] [PubMed] [Google Scholar]
- 33.Intraventricular hemorrhage in a full-term neonate associated with sinus venous thrombosis and homozygosity for the plasminogen activator inhibitor-1 4G/4G polymorphism. Heineking B, Riebel T, Scheer I, Kulozik A, Hoehn T, Bührer C. Pediatr Int. 2003;45:93–96. doi: 10.1046/j.1442-200x.2003.01658.x. [DOI] [PubMed] [Google Scholar]
- 34.Ureaplasma ureulyticum in the cerebrospinal fluid of a premature infant. Hentschel J, Abele-Horn M, Peters J. Acta Paediatr. 1993;82:690–693. doi: 10.1111/j.1651-2227.1993.tb18042.x. [DOI] [PubMed] [Google Scholar]
- 35.November 1996 — premature baby with lethargy and coma. Hevner R, Sobel RA. Brain Pathol. 1997;7:839–840. doi: 10.1111/j.1750-3639.1997.tb01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Congenital hydrocephalus secondary to intra-uterine germinal matrix/intraventricular haemorrhage. Hill A, Rozdilsky B. Dev Med Child Neurol. 1984;26:524–527. doi: 10.1111/j.1469-8749.1984.tb04483.x. [DOI] [PubMed] [Google Scholar]
- 37.Blunt prenatal trauma resulting in fetal epidural or subdural hematoma: case report and systematic review of the literature. Joseph JR, Smith BW, Garton HJ. J Neurosurg Pediatr. 2017;19:32–37. doi: 10.3171/2016.7.PEDS16282. [DOI] [PubMed] [Google Scholar]
- 38.Intraventricular hemorrhage in neonates: endoscopic findings and treatment by the use of our newly developed Yamadori-type 8 ventriculoscope. Kamikawa S, Inui A, Kobayashi N, Tamaki N, Yamadori T. Minim Invasive Neurosurg. 2001;44:74–78. doi: 10.1055/s-2001-16001. [DOI] [PubMed] [Google Scholar]
- 39.Pulmonary tuberculosis and extreme prematurity. Katumba-Lunyenya J, Joss V, Latham P, Abbatuan C. Arch Dis Child Fetal Neonatal Ed. 2005;90:0–83. doi: 10.1136/adc.2004.056549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conservative management of neonatal cerebral sinovenous thrombosis with coexisting thrombophilia. Khatri V, Chan AK, Stein N, Paes B, Bhatt M. Blood Coagul Fibrinolysis. 2018;29:399–403. doi: 10.1097/MBC.0000000000000731. [DOI] [PubMed] [Google Scholar]
- 41.Primary cutaneous aspergillosis caused by Aspergillus tamarii in a premature infant with extremely low birthweight: a case report with short review. Kimura H, Mitsuto I, Taguchi R, Anzawa K, Mochizuki T. J Dermatol. 2018;45:622–625. doi: 10.1111/1346-8138.14263. [DOI] [PubMed] [Google Scholar]
- 42.An intracranial aneurysm and dural arteriovenous fistula in a newborn. Ko A, Filardi T, Giussani C, Ghodke R, Browd SR. Pediatr Neurosurg. 2010;46:450–456. doi: 10.1159/000323420. [DOI] [PubMed] [Google Scholar]
- 43.Genetic deafness in a preterm infant with a critical postnatal course. Koehne PS, Hüseman D, Walch E, et al. Pediatr Crit Care Med. 2006;7:270–272. doi: 10.1097/01.PCC.0000216679.47571.DA. [DOI] [PubMed] [Google Scholar]
- 44.Neonatal heart rate variability and intraventricular hemorrhage: a case study. Krueger CA, Gyland EA, Theriaque D. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3676181/ Pediatr Nurs. 2008;34:401–404. [PMC free article] [PubMed] [Google Scholar]
- 45.A novel mutation in the SerpinC1 gene presenting as unprovoked neonatal cerebral sinus venous thrombosis in a kindred. Kumar R, Moharir M, Yau I, Williams S. Pediatr Blood Cancer. 2013;60:133–136. doi: 10.1002/pbc.24302. [DOI] [PubMed] [Google Scholar]
- 46.Heart failure in the new born; vein of Galen aneurysmal malformation. Mathews AZ, Ibhanesebhor S, Richens T, Manjunatha CM. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3597353/ BMJ Case Rep. 2013;2013:0. doi: 10.1136/bcr.03.2012.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Acute appendicitis in pregnancy and the occurrence of major intraventricular hemorrhage and periventricular leukomalacia. Mays J, Verma U, Klein S, Tejani N. Obstet Gynecol. 1995;86:650–652. doi: 10.1016/0029-7844(95)00211-9. [DOI] [PubMed] [Google Scholar]
- 48.Delayed hemorrhagic infarction. A cause of late neonatal germinal matrix and intraventricular hemorrhage. Ment LR, Ehrenkranz RA, Duncan CC, Lange RC. Arch Neurol. 1984;41:1036–1039. doi: 10.1001/archneur.1984.04050210034010. [DOI] [PubMed] [Google Scholar]
- 49.Cerebellar loss and brain-stem atrophy associated with neonatal alloimmune thrombocytopenia in a discordant twin. Mohila CA, Kubicka ZJ, Ornvold KT, Harris BT. Pediatr Dev Pathol. 2010;13:55–62. doi: 10.2350/08-11-0562.1. [DOI] [PubMed] [Google Scholar]
- 50.Staged operations for posthemorrhagic hydrocephalus in extremely low-birth-weight infants with preceding stoma creation after bowel perforation: surgical strategy. Nakano S, Sugimoto T, Kawasoe T, et al. Childs Nerv Syst. 2007;23:459–463. doi: 10.1007/s00381-006-0237-x. [DOI] [PubMed] [Google Scholar]
- 51.Nonsurgical management of epidural hematoma in neonates. Negishi H, Lee Y, Itoh K, et al. Pediatr Neurol. 1989;5:253–256. doi: 10.1016/0887-8994(89)90086-6. [DOI] [PubMed] [Google Scholar]
- 52.Divergent-convergent eye movements and transient eyelid opening associated with an EEG burst-suppression pattern. Nelson KR, Brenner RP, Carlow TJ. https://pubmed.ncbi.nlm.nih.gov/2939113/ J Clin Neuroophthalmol. 1986;6:43–46. doi: 10.3109/01658108608997324. [DOI] [PubMed] [Google Scholar]
- 53.Full recovery from prolonged brainstem failure following intraventricular hemorrhage. Pasternak JF, Volpe JJ. J Pediatr. 19791;95:1046–1049. doi: 10.1016/s0022-3476(79)80307-8. [DOI] [PubMed] [Google Scholar]
- 54.Multilocular hydrocephalus: ultrasound studies of origin and development. Prats JM, López-Heredia J, Gener B, Freijo MM, Garaizar C. Pediatr Neurol. 20011;24:149–151. doi: 10.1016/s0887-8994(00)00246-0. [DOI] [PubMed] [Google Scholar]
- 55.Retroclival arachnoid cyst in a preterm infant after ventriculitis and intraventricular hemorrhage—a case report. Preuß M, Thome U, Kluge J, Hirsch FW, Viehweger A, Nestler U. Childs Nerv Syst. 2015;31:347–350. doi: 10.1007/s00381-014-2518-0. [DOI] [PubMed] [Google Scholar]
- 56.Thrombophilia and fetal germinal matrix-intraventricular hemorrhage: does it matter? Ramenghi LA, Fumagalli M, Righini A, Triulzi F, Kustermann A, Mosca F. Ultrasound Obstet Gynecol. 2005;26:574–576. doi: 10.1002/uog.2586. [DOI] [PubMed] [Google Scholar]
- 57.Endoscopic evacuation of an intracerebral and intraventricular haemorrhage. Ritschl E, Auer LM. Arch Dis Child. 1987;62:1163–1165. doi: 10.1136/adc.62.11.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Intraventricular hemorrhage in the full-term neonate. Scher MS, Wright FS, Lockman LA, Thompson TR. Arch Neurol. 1982;39:769–772. doi: 10.1001/archneur.1982.00510240031008. [DOI] [PubMed] [Google Scholar]
- 59.Secondary pediatric encephalocele after ventriculosubgaleal shunting for posthemorrhagic hydrocephalus. Seeburg D, Ahn E, Huisman T. Neuropediatrics. 2014;45:252–255. doi: 10.1055/s-0033-1363298. [DOI] [PubMed] [Google Scholar]
- 60.A potential mechanism of pathogenesis for early posthemorrhagic hydrocephalus in the premature newborn. Shackelford D, Volpe J. https://pediatrics.aappublications.org/content/73/1/19. Pediatrics. 1984;73:19–21. [PubMed] [Google Scholar]
- 61.Vitreous hemorrhage in a premature infant with patent hyaloid artery and increased intracranial pressure. Sharma V, Biswas S. https://pubmed.ncbi.nlm.nih.gov/22420619/ J Pediatr Ophthalmol Strabismus. 2012;49:0–4. doi: 10.3928/01913913-20120306-02. [DOI] [PubMed] [Google Scholar]
- 62.Bilateral symmetrical middle cranial fossa arachnoid cysts in a neonate. Shirane R, Tanaka T, Andoh A, Suzuki J. Surg Neurol. 19861;26:395–398. doi: 10.1016/0090-3019(86)90144-8. [DOI] [PubMed] [Google Scholar]
- 63.Intraventricular haemorrhage as the first manifestation of congenital Cytomegalovirus infection. Sobolewska-Pilarczyk M, Pawlak-Osinska K, Drewa S, Smok B, Pawlowska M. Indian J Med Microbiol. 2018;36:279–281. doi: 10.4103/ijmm.IJMM_18_11. [DOI] [PubMed] [Google Scholar]
- 64.Intraventricular hemorrhage and multiple intracranial cysts associated with congenital cytomegalovirus infection. Suksumek N, Scott JN, Chadha R, Yusuf K. J Clin Microbiol. 2013;51:2466–2468. doi: 10.1128/JCM.00842-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Intraventricular hemorrhage in neonates born before 32 weeks of gestation—retrospective analysis of risk factors. Szpecht D, Szymankiewicz M, Nowak I, Gadzinowski J. Childs Nerv Syst. 2016;32:1399–1404. doi: 10.1007/s00381-016-3127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heterozygous FGA p.Asp473Ter (fibrinogen Nieuwegein) presenting as antepartum cerebral thrombosis. Tajdar M, Orlando C, Casini A, et al. Thromb Res. 2018;163:185–189. doi: 10.1016/j.thromres.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 67.Ruptured fusiform cerebral aneurysm in a neonate. Tan MP, McConachie NS, Vloeberghs M. Childs Nerv Syst. 1998;14:467–469. doi: 10.1007/s003810050263. [DOI] [PubMed] [Google Scholar]
- 68.Neonatal ruptured intracranial aneurysms: case report and literature review. Van Raay Y, Darteyre S, Di Rocco F, et al. Childs Nerv Syst. 2009;25:1025–1033. doi: 10.1007/s00381-009-0871-1. [DOI] [PubMed] [Google Scholar]
- 69.Venous sinus thrombosis as a cause of parenchymal and intraventricular hemorrhage in the full-term neonate. Voutsinas L, Gorey MT, Gould R, Black KS, Scuderi DM, Hyman RA. Clin Imaging. 19911;15:273–275. doi: 10.1016/0899-7071(91)90117-e. [DOI] [PubMed] [Google Scholar]
- 70.Combination thrombolytic and anticoagulant therapy for bilateral renal vein thrombosis in a premature infant. Weinschenk N, Pelidis M, Fiascone J. Am J Perinatol. 2001;18:293–297. doi: 10.1055/s-2001-16993. [DOI] [PubMed] [Google Scholar]
- 71.Factor V deficiency and antenatal intraventricular haemorrhage. Whitelaw A, Haines ME, Bolsover W, Harris E. Arch Dis Child. 1984;59:997–999. doi: 10.1136/adc.59.10.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cerebral intraventricular hemorrhages in infants: a widening age spectrum. Mitchell W, O’Tuama L. https://pubmed.ncbi.nlm.nih.gov/7355033/ Pediatrics. 1980;65:35–39. [PubMed] [Google Scholar]
- 73.Neonatal intraventricular hemorrhage due to an intracranial arteriovenous malformation: a case report. Schum TR, Meyer GA, Grausz JP, Glaspey JC. https://pediatrics.aappublications.org/content/64/2/242.short. Pediatrics. 19791;64:242–244. [PubMed] [Google Scholar]
- 74.Intraventricular hemorrhage in the full-term neonate associated with abdominal compression. Wehberg K, Vincent M, Garrison B, Dilustro JF, Frank LM. https://pediatrics.aappublications.org/content/89/2/327.short. Pediatrics. 1992;89:327–329. [PubMed] [Google Scholar]
- 75.Massive neonatal intracranial hemorrhage caused by bromadiolone. A case report. Ma M, Zhang M, Tang X, Li Z. Medicine (Baltimore) 2017;96:8506. doi: 10.1097/MD.0000000000008506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cervical spinal cord hemorrhage secondary to neonatal alloimmune thrombocytopenia. Abel M, Bona M, Zawodniak L, Sultan R, Masterson M. https://journals.lww.com/jpho-online/Abstract/2003/04000/Cervical_Spinal_Cord_Hemorrhage_Secondary_to.17.aspx. J Pediatr Hematol Oncol. 2003;25:340–342. doi: 10.1097/00043426-200304000-00017. [DOI] [PubMed] [Google Scholar]
- 77.Unilateral parenchymal haemorrhagic infarction in the preterm infant. de Vries LS, Roelants-van Rijn AM, Rademaker KJ, Van Haastert IC, Beek FJ, Groenendaal F. Eur J Paediatr Neurol. 2001;5:139–149. doi: 10.1053/ejpn.2001.0494. [DOI] [PubMed] [Google Scholar]
- 78.Hypernatraemia induced by sodium polystyrene sulphonate (Kayexalate) in two extremely low birth weight newborns. Filippi L, Cecchi A, Dani C, Bertini G, Pezzati M, Rubaltelli FF. Paediatr Anaesth. 2004;14:271–275. doi: 10.1046/j.1460-9592.2003.01210.x. [DOI] [PubMed] [Google Scholar]
- 79.A patient with partial duplication 2q and partial deficiency 11q. Ho CK, Henderson KC, Bowyer FP, Eilers KB, Andrews LG. Am J Med Genet. 1987;28:575–579. doi: 10.1002/ajmg.1320280305. [DOI] [PubMed] [Google Scholar]
- 80.Transient diabetes insipidus in a very-low-birthweight preterm infant with intraventricular haemorrhage. Molnar Z, Sotiridou E, Dixon H, Ogilvy-Stuart A. Acta Paediatr Oslo Nor. 1992;101:389–390. doi: 10.1111/j.1651-2227.2012.02756.x. [DOI] [PubMed] [Google Scholar]
- 81.Use of a robotic sampler (PIPER) for evaluation of particulate matter exposure and eczema in preschoolers. Shah L, Mainelis G, Ramagopal M, Black K, Shalat SL. Int J Environ Res Public Health. 2016;13:242. doi: 10.3390/ijerph13020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Management of coagulopathy with recombinant factor VIIa in a neonate with echovirus type 7. Tancabelic J, Haun SE. Pediatr Blood Cancer. 2004;43:170–176. doi: 10.1002/pbc.20078. [DOI] [PubMed] [Google Scholar]
- 83.A huge cystic hygroma of the neck associated with intraventricular haemorrhage in a term neonate. Yang AD, Chang YL, Chaou WT. Acta Paediatr. 1999;88:344–346. doi: 10.1080/08035259950170169. [DOI] [PubMed] [Google Scholar]
- 84.Avoidance of red blood cell transfusion in an extremely preterm infant given recombinant human erythropoietin therapy. Yu VY, Bacsain MB. J Paediatr Child Health. 1994;30:360–362. doi: 10.1111/j.1440-1754.1994.tb00663.x. [DOI] [PubMed] [Google Scholar]
- 85.Intraventricular hemorrhage in full term newborn: a rare phenomenon. Suneja U, Gadiparthi R. https://www.researchgate.net/publication/308134168_Intraventricular_Hemorrhage_in_Full_Term_Newborn_A_Rare_Phenomenon Arch Med. 2016;8 [Google Scholar]
- 86.Institute of Medicine. Washington (DC): National Academies Press (US); 2007. Preterm Birth: Causes, Consequences, and Prevention. [PubMed] [Google Scholar]
- 87.Intraventricular hemorrhage in a full-term neonate associated with sinus venous thrombosis and homozygosity for the plasminogen activator inhibitor-1 4G/4G polymorphism. Heineking B, Riebel T, Scheer I, Kulozik A, Hoehn T, Bührer C. Pediatr Int. 2003;45:93–96. doi: 10.1046/j.1442-200x.2003.01658.x. [DOI] [PubMed] [Google Scholar]
- 88.Early and late complications of germinal matrix-intraventricular haemorrhage in the preterm infant: what is new? Brouwer AJ, Groenendaal F, Benders MJ, de Vries LS. Neonatology. 2014;106:296–303. doi: 10.1159/000365127. [DOI] [PubMed] [Google Scholar]
- 89.Neuroimaging in the preterm infant. de Vries LS, Groenendaal F. Ment Retard Dev Disabil Res Rev. 2002;8:273–280. doi: 10.1002/mrdd.10050. [DOI] [PubMed] [Google Scholar]