Abstract
Clinical and experimental studies show that hypertension induces intracerebral hemorrhages (ICH), including cerebral microhemorrhages in the aged brain, which contribute to the pathogenesis of vascular cognitive impairment (VCI). Previous studies showed that aging increased oxidative stress-mediated activation of matrix metalloproteinases (MMPs) that importantly contributes to the pathogenesis of ICHs. In particular, oxidative stress has been implicated in activation of MMP-9, which is known to be involved in the degradation of the extracellular matrix and cleavage of collagen IV, a key constituent of the basal membrane of cerebral vessels. To determine the role of MMP-9 activation in the genesis of ICHs, we induced hypertension in 20-month-old MMP-9 null and age-matched control mice by angiotensin II and L-NAME treatment. Contrary to our hypothesis, MMP-9 deficiency did not delay the onset or incidence of neurological consequences of hypertension-induced ICHs. Our results indicate that MMP-9 activation does not play a role in the age-related exacerbation of hypertension-induced ICH.
Keywords: Microbleed, Artery, Arteriole, Cerebral microhemorrhage, Stroke, Oxidative stress, Aging
Introduction
Stroke is the second most common cause of death in the European Union, the fourth leading cause of death in the USA, and one of the leading causes of long-term disability in both continents [1, 2]. Intracerebral hemorrhages (ICH) account for approximately 10–15% of all strokes, and mortality rates range from 35 to 52% [3, 4]. Significant advances in magnetic resonance imaging (MRI) techniques (including T2* gradient-recall echo, susceptibility-weighted imaging MRI sequences) have allowed the detection of previously undetectable small ICH, termed cerebral microhemorrhages, in elderly patients [5]. Cerebral microhemorrhages are small (< 5 mm) vascular lesions associated with rupture of small intracerebral vessels, which contribute to cognitive decline [6, 7].
Advanced age and hypertension are the primary risk factors for the development of both larger ICH [8–10] and cerebral microhemorrhages [5, 11]. Incidence of ICH increases in persons older than 55 years and doubles with each decade until age 80 years. The prevalence of cerebral microhemorrhages also significantly increases with advanced age, from ~ 6.5% in persons aged 45 to 50 years to ~ 35 to 50% or more in older adults [11]. Recent data from rodent models extend the clinical observations, showing that aging and high blood pressure synergistically interact to exacerbate the genesis of ICHs [12]. Preclinical studies suggest that in addition to stiffening of the conduit arteries [13–17] and increased penetration of high pressure waves into the vulnerable distal portion of the cerebral microcirculation [18–29], aging likely promotes the development of ICHs and cerebral microhemorrhages by exacerbating vascular oxidative stress and activation of matrix metalloproteinases (MMPs), which compromise the structural integrity of the cerebral vasculature [12]. Yet, the role of specific MMPs in increased susceptibility of the aged cerebral vasculature to rupture remains elusive.
MMP-9, also known as type IV collagenase or gelatinase B, is a collagenase enzyme involved in the degradation of the extracellular matrix [30, 31]. Aging associates with increased MMP-9 expression in many tissues, including the heart [32, 33] and the human aorta [34]. Increased MMP-9 activation has been causally linked to the genesis of ICH in various experimental murine models, including ICH associated with chronic hypertension and cerebral amyloid angiopathy [35–38]. Importantly, MMP-9 deletion and inhibition have been shown to confer protective effects in a range of animal models of cardiovascular disease [30].
The present study was designed to test the hypothesis that MMP-9 contributes to the development of hypertension-induced ICH in aging mice. To test this hypothesis, we induced hypertension in aged mice with genetic depletion of MMP-9 and respective controls (by treatment with angiotensin II [Ang-II] and the NO synthesis inhibitor L-NAME) and compared the incidence of neurological manifestations of ICH. Our previous studies demonstrate that the approach used in the present study, longitudinal analysis of hypertension-induced changes in the mouse neuroscore, closely predict the incidence of histologically verified ICH in the mouse brain [12, 39, 40]. In aging, the activity of the vascular renin-angiotensin system is elevated. Moreover, hypertension in older adults can be successfully treated with angiotensin converting enzyme inhibitors and angiotensin II receptor blockers. Thus, aged mice with Ang-II-induced hypertension is a clinically highly relevant animal model to investigate hypertension-related cerebrovascular alterations in the context of aging [21]. Previous studies by the Heistad laboratory [35, 41] and subsequent investigations by our investigative team [12, 39] showed that co-administration of L-NAME and Ang-II results in an ~ 15-mmHg additional increase in blood pressure, which associates with a significant increase in the incidence of ICH in the presence of underlying microvascular fragility.
Methods
Experimental animals
Male MMP-9 null mice were used from a breeding colony that originated with mice generated by Zena Werb’s laboratory and backcrossed by Lynn Matrisian’s laboratory [42, 43]. Male C57BL/6 J mice purchased from Jackson Laboratories were used as the control group (n = 17).
Animals were identically housed in the Rodent Barrier Facility at OUHSC under specific pathogen-free barrier conditions, on a 12-h light/12-h dark cycle, with access to standard rodent chow (Purina Mills, Richmond, IN) and water ad libitum. Animals were randomized to groups, and investigators were blinded to group throughout the protocol.
Induction of spontaneous ICH
To study the effects of MMP-9 on hypertension-induced intracerebral hemorrhages, we used a previously well-characterized mouse model [12, 35, 41]. Briefly, in 20-month-old male MMP-9 deficient mice (n = 22) and respective age-matched control mice (n = 17), hypertension was induced by a combination treatment with ω-nitro-L-arginine-methyl ether (L-NAME, 100 mg/kg/day, in drinking water) and administration of angiotensin II (Ang-II; s.c. via osmotic mini-pumps [Alzet Model 2006, 0.15 µl/h, 42 days; Durect Co, Cupertino, CA]). Pumps were filled either with saline vehicle or solutions of angiotensin II (Sigma Chemical Co., St. Louis, Missouri, USA) delivered subcutaneously at 1 µg/min/kg of angiotensin II, thus generating two experimental groups: (1) wild type control + Ang-II + L-NAME and (2) MMP9−/− + Ang-II + L-NAME. Pumps were placed into the subcutaneous space of isoflurane anesthetized mice through a small incision in the back of the neck that was closed with surgical sutures. All incision sites healed rapidly without the need for additional medication. Since aging is associated with increased activity of the vascular renin-angiotensin system and Ang-II-dependent hypertension is common among older individuals [44], Ang-II-dependent hypertension is a clinically highly relevant model to study aging-related cerebrovascular alterations [21].
Blood pressure of the animals was recorded before the treatment and every second day during the treatment period using a tail-cuff blood pressure machine (CODA Non-Invasive Blood Pressure System, Kent Scientific Co., Torrington, CT), as described [12, 19, 21]. Each experimental group was closely monitored, and mice were sacrificed upon the occurrence of clinical signs of intracerebral hemorrhages.
All procedures were approved by the Institutional Animal Use and Care Committees of the University of Oklahoma Health Sciences Center.
Standardized neurological examination of mice
To assess the occurrence of clinical features of hemorrhages, daily neurological examination was performed by assessing each animal’s spontaneous activity, symmetry in the movement of the four limbs, forelimb outstretching, climbing ability, body proprioception, response to vibrissae touch, and gait coordination. Each examined animal was provided with a daily score calculated by the summation of all individual test scores. When a consistent decline in the neurological score was observed or on day 11 of the study, mice were euthanized by CO2 asphyxiation.
Statistical analysis
Cumulative incidence of neurological signs of ICH was evaluated using a Kaplan–Meier test, and the difference among groups was analyzed by log-rank test (Mantel-Cox). A p value less than 0.05 was considered statistically significant.
Results
Incidence of neurological signs of hypertension-induced ICHs in MMP-9 null mice
Treatment with Ang-II plus L-NAME resulted in comparable increases in blood pressure both in MMP-9 null (149 ± 6 mmHg) and age-matched control wild-type mice (150 ± 5 mmHg).
We found that during the experimental period, 35% of control mice and 77% of MMP-9 null mice developed clinically manifest signs of hypertension-induced ICH, as assessed by neurological examination. The cumulative distribution curves for time-to-event in the two groups were statistically different (log rank [Mantel-Cox] test; χ2 = 5.701; P = 0.017).
Discussion
This is the first study to demonstrate that genetic MMP-9 deficiency does not ameliorate the incidence of neurological manifestations of hypertension-induced ICHs in aged mice (Fig. 1).
Fig. 1.
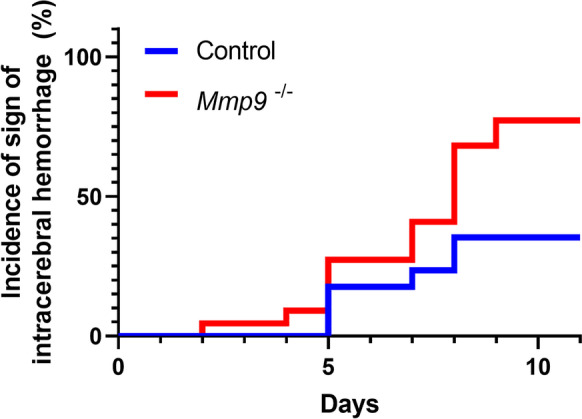
Genetic MMP-9 deficiency does not ameliorate, rather increases, the incidence of hypertension-induced intracerebral hemorrhages in aged mice. Shown are cumulative incidence curves for neurological signs of hypertension-induced intracerebral hemorrhages in 20-month-old control (n = 17) and age-matched MMP-9 null mice (n = 22). In MMP-9 deficient mice, the incidence of ICHs was higher to that in control mice after induction of hypertension (log-rank test; Mantel-Cox; p = 0.017)
Several lines of evidence suggest that both aging and hypertension upregulate MMP-9 in the vasculature. First, increased expression of MMP-9 was reported in aorta samples derived from older adults [34]. Older patients with small vessel vascular dementia also present with elevated levels of MMP-9 in the cerebrospinal fluid [45]. Interestingly, no increase in MMP-9 expression was found in smooth muscle cells derived from aged mice [46]. Second, Ang-II-induced hypertension in mice also increases MMP-9 activity in the cerebral vasculature due to increased levels of oxidative stress, which could potentially be linked to the genesis of ICHs [35, 41]. Finally, MMP-9 is upregulated in the brain after development of ICH, which has been linked to disruption of the blood brain barrier [47–51].
Contrary to the prediction based on our hypothesis, our findings show that genetic depletion of MMP-9 does not prevent/delay neurological manifestations of hypertension-induced ICHs in aged mice. These results are consistent with the concept that the cellular and molecular mechanisms responsible for increased susceptibility of aged cerebral microvessels to rupture are not dependent on the presence of MMP-9 and likely involve other MMPs. Genetic MMP-9 deficiency was also reported to enhance, rather than attenuate, collagenase-induced ICHs, brain injury, and mortality in mice [52]. This observation is also in line with our findings and refutes the idea that increased MMP-9 is a cause for increased incidence and mortality of ICH in aging.
In addition to MMP-9, a wide range of other MMPs are expressed in the cerebral arteries and the brain, which likely play complex roles in the pathogenesis of ICHs. They are known to degrade collagen and elastin and other components of the basal lamina and extracellular matrix, compromising the structural integrity of the cerebral vasculature. Cerebral arteries express MMP-1 (collagens, types I, II, and III), MMP-2 (collagens I, II, III, IV, VII, X), MMP-3 (collagens II, IV, IX, X, X), MMP-8 (collagens I, II, III, VII, VIII, X), MMP-12 (elastin, fibronectin, collagen IV), and MMP-13 (collagens I, II, III, IV, IX, X, XIV). Previous studies demonstrate that aging is associated with MMP-2 expression in the human aorta [34, 53], mammary artery [54], and aortas of non-human primates [55] and rodent models [56–58]. MMP-3 and MMP-12 are also upregulated in the cardiovascular system [59] and the brain [60] of aged mice. MMP-3 has also been linked to aging-induced vascular remodeling in humans [61]. Aging is also associated with the accumulation of senescent cells in the cerebral circulation [62]. Senescent cells can affect the surrounding tissue microenvironment, one of these effects is the senescence-associated secretory phenotype (SASP), characterized by up-regulation and local the release of elastase and various MMPs (including MMP-1, MMP-3 and MMP-13), whose proteolytic activity can lead to focal weakening of the vascular wall, potentially creating loci of least resistance, promoting the genesis of ICHs and cerebral microhemorrhages. Further, in response to ICH, several MMPs were reported to be upregulated in the brain, which likely plays an important role in blood–brain barrier dysfunction, and thereby affect the extent of the neuronal damage and survival [52, 63, 64]. At present, it is unclear how age-related changes in MMP expression alter susceptibility of cerebral vessels to pressure-induced rupture. There is strong evidence that in MMP-9 null mice, the expression of other MMPs is dysregulated [52], which may overcompensate the loss of MMP-9, altering microvascular fragility and influencing bleeding and brain injury. In MMP-9 null mice, increased expression of MMP-2, MMP-3, MMP-8, and/or MMP-13 as well as TIMP-1 has been documented in various tissues, including cardiomyocyte s [65, 66].
Previously, advanced aging was found to exacerbate hypertension-induced generation of reactive oxygen species (ROS) in cerebral vessels, suggesting that redox-sensitive MMP activation [35, 67] may potentially contribute to the observed phenotype. This concept is supported by the observations that increased hypertension-related MMP activation in the aged cerebral vasculature can be attenuated by antioxidative treatments [12]. Increased hypertension-induced oxidative stress in aged arteries in murine models has been attributed to increased activation/expression of NAD(P)H oxidases (NOX enzymes), increased ROS production by mitochondrial sources, and age-related impairment of Nrf2-dependent cellular antioxidant defense pathways [12, 68–70]. Growing evidence suggests that inhibition of ROS generation by these sources can prevent/delay development of ICHs in models of aging, hypertension, [12, 35] and even Alzheimer's disease [71].
The present study has important limitations. Several animal models of hypertension have been used to investigate the effects of high blood pressure on the brain [72–79]. Although none of the aforementioned models can fully recapitulate the hemodynamic alterations, vascular pathologies, and long-tern neurological consequences associated with chronic presence of “essential” hypertension in humans, these experimental models have proved to be valuable tools to elucidate the potential mechanisms underlying the susceptibility of the brain to hypertension-induced injury. The mouse model used in the present study (Ang-II plus L-NAME to induce hypertension), along with similar murine models, has been specifically developed to study the pathogenesis of hypertension-induced ICHs [35, 41, 80]. It is an advantage of the model that due to the “aggressive” regimen of hypertension induction, the development of ICHs can be studied in a short time window. Although in the present study histological evaluation of ICHs has not been performed, previous studies demonstrated that the incidence of the neurological manifestations of ICHs closely correlates with the susceptibility of the cerebral microvasculature to rupture and the actual ICH burden 12, 40, 39].
In conclusion, our results do not support a key role for MMP-9 in the pathogenesis of hypertension-induced ICHs in the aged mouse brain. Future studies should determine the role of specific MMPs in the genesis of ICHs using selective pharmacological inhibitors and inducible knockout mouse models. The causal link between increased oxidative stress, MMP activation and genesis of ICHs should also be determined.
Funding
This work was supported by grants from the American Heart Association, the Oklahoma Center for the Advancement of Science and Technology, the National Institute on Aging (R01-AG055395, R01-AG047879; R01-AG038747; R01-AG072295, K01AG073614), the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS100782), the National Cancer Institute (NCI;1R01CA255840), the Oklahoma Shared Clinical and Translational Resources (OSCTR) program funded by the National Institute of General Medical Sciences (U54GM104938, to AY), the Presbyterian Health Foundation, and the NKFIH (Nemzeti Szivlabor). The authors acknowledge the support from the NIA-funded Geroscience Training Program in Oklahoma (T32AG052363), the Oklahoma Nathan Shock Center (P30AG050911), and the Cellular and Molecular GeroScience CoBRE (1P20GM125528, sub#5337).
Declarations
Disclaimer
The funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Competing interests
Dr. Anna Csiszar serves as Associate Editor for The Journal of Gerontology, Series A: Biological Sciences and Medical Sciences and GeroScience. Dr. Andriy Yabluchanskiy serves as Guest Editor for The American Journal of Physiology-Heart and Circulatory Physiology. Dr. Zoltan Ungvari serves as Editor-in-Chief for GeroScience and as Consulting Editor for The American Journal of Physiology-Heart and Circulatory Physiology.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Stefano Tarantini, Andriy Yabluchanskiy, Anna Csiszar, and Zoltan Ungvari contributed equally to this work.
References
- 1.Wafa HA, Wolfe CDA, Emmett E, Roth GA, Johnson CO, Wang Y. Burden of stroke in Europe: thirty-year projections of incidence, prevalence, deaths, and disability-adjusted life years. Stroke. 2020;51:2418–2427. doi: 10.1161/STROKEAHA.120.029606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for disease control and prevention prevalence and most common causes of disability among adults–United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421–426. [PubMed] [Google Scholar]
- 3.Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, Mayberg M, Morgenstern L, Ogilvy CS, Vespa P, Zuccarello M, American Heart Association/American Stroke Association Stroke C. American Heart Association/American Stroke Association High Blood Pressure Research C. Quality of C and Outcomes in Research Interdisciplinary Working G Guidelines for the management of spontaneous intracerebral hemorrhage in adults 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 2007;116:e391–413. doi: 10.1161/CIRCULATIONAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 4.Hostettler IC, Seiffge DJ, Werring DJ. Intracerebral hemorrhage: an update on diagnosis and treatment. Expert Rev Neurother. 2019;19:679–694. doi: 10.1080/14737175.2019.1623671. [DOI] [PubMed] [Google Scholar]
- 5.Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A, Prodan CI. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol. 2017;312:H1128–H1143. doi: 10.1152/ajpheart.00780.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akoudad S, Wolters FJ, Viswanathan A, de Bruijn RF, van der Lugt A, Hofman A, Koudstaal PJ, Ikram MA, Vernooij MW. Association of Cerebral Microbleeds With Cognitive Decline and Dementia. JAMA Neurol. 2016;73:934–943. doi: 10.1001/jamaneurol.2016.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poels MM, Ikram MA, van der Lugt A, Hofman A, Niessen WJ, Krestin GP, Breteler MM, Vernooij MW. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. 2012;78:326–333. doi: 10.1212/WNL.0b013e3182452928. [DOI] [PubMed] [Google Scholar]
- 8.Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 9.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 10.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 11.Poels MM, Ikram MA, van der Lugt A, Hofman A, Krestin GP, Breteler MM, Vernooij MW. Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study. Stroke. 2011;42:656–661. doi: 10.1161/STROKEAHA.110.607184. [DOI] [PubMed] [Google Scholar]
- 12.Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. 2015;14:400–408. doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babici D, Kudej RK, McNulty T, Zhang J, Oydanich M, Berkman T, Nishimura K, Bishop SP, Vatner DE, Vatner SF. Mechanisms of increased vascular stiffness down the aortic tree in aging, premenopausal female monkeys. Am J Physiol Heart Circ Physiol. 2020;319:H222–H234. doi: 10.1152/ajpheart.00153.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DuPont JJ, Kim SK, Kenney RM, Jaffe IZ. Sex differences in the time course and mechanisms of vascular and cardiac aging in mice: role of the smooth muscle cell mineralocorticoid receptor. Am J Physiol Heart Circ Physiol. 2021;320:H169–H180. doi: 10.1152/ajpheart.00262.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toth L, Czigler A, Szarka N, Toth P. The role of transient receptor potential channels in cerebral myogenic autoregulation in hypertension and aging. Am J Physiol Heart Circ Physiol. 2020;319:H159–H161. doi: 10.1152/ajpheart.00403.2020. [DOI] [PubMed] [Google Scholar]
- 16.Charlton PH, Mariscal Harana J, Vennin S, Li Y, Chowienczyk P, Alastruey J. Modeling arterial pulse waves in healthy aging: a database for in silico evaluation of hemodynamics and pulse wave indexes. Am J Physiol Heart Circ Physiol. 2019;317:H1062–H1085. doi: 10.1152/ajpheart.00218.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pagoulatou SZ, Bikia V, Trachet B, Papaioannou TG, Protogerou AD, Stergiopulos N. On the importance of the nonuniform aortic stiffening in the hemodynamics of physiological aging. Am J Physiol Heart Circ Physiol. 2019;317:H1125–H1133. doi: 10.1152/ajpheart.00193.2019. [DOI] [PubMed] [Google Scholar]
- 18.Springo Z, Toth P, Tarantini S, Ashpole NM, Tucsek Z, Sonntag WE, Csiszar A, Koller A, Ungvari ZI. Aging impairs myogenic adaptation to pulsatile pressure in mouse cerebral arteries. J Cereb Blood Flow Metab. 2015;35:527–530. doi: 10.1038/jcbfm.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toth P, Csiszar A, Tucsek Z, Sosnowska D, Gautam T, Koller A, Schwartzman ML, Sonntag WE, Ungvari Z. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol. 2013;305:H1698–H1708. doi: 10.1152/ajpheart.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–H20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–1742. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34:1887–1897. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim DH, Choi JH, Moon JS, Kim HJ, Cha JK. Association between the severity of cerebral small vessel disease, pulsatility of cerebral arteries, and brachial ankle pulse wave velocity in patients with lacunar infarction. Eur Neurol. 2010;64:247–252. doi: 10.1159/000319923. [DOI] [PubMed] [Google Scholar]
- 24.Mackey RH, Sutton-Tyrrell K, Vaitkevicius PV, Sakkinen PA, Lyles MF, Spurgeon HA, Lakatta EG, Kuller LH. Correlates of aortic stiffness in elderly individuals: a subgroup of the Cardiovascular Health Study. Am J Hypertens. 2002;15:16–23. doi: 10.1016/s0895-7061(01)02228-2. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the age, gene/environment susceptibility–Reykjavik study. Brain. 2011;134:3398–3407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Sullivan C, Duggan J, Lyons S, Thornton J, Lee M, O'Brien E. Hypertensive target-organ damage in the very elderly. Hypertension. 2003;42:130–135. doi: 10.1161/01.HYP.0000084050.73533.C5. [DOI] [PubMed] [Google Scholar]
- 27.Seo WK, Lee JM, Park MH, Park KW, Lee DH. Cerebral microbleeds are independently associated with arterial stiffness in stroke patients. Cerebrovasc Dis. 2008;26:618–623. doi: 10.1159/000166837. [DOI] [PubMed] [Google Scholar]
- 28.Shimoyama T, Iguchi Y, Kimura K, Mitsumura H, Sengoku R, Kono Y, Morita M, Mochio S. Stroke patients with cerebral microbleeds on MRI scans have arteriolosclerosis as well as systemic atherosclerosis. Hypertens Res. 2012;35:975–979. doi: 10.1038/hr.2012.84. [DOI] [PubMed] [Google Scholar]
- 29.Thorin-Trescases N, de Montgolfier O, Pincon A, Raignault A, Caland L, Labbe P, Thorin E. Impact of pulse pressure on cerebrovascular events leading to age-related cognitive decline. Am J Physiol Heart Circ Physiol. 2018;314:H1214–H1224. doi: 10.1152/ajpheart.00637.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML. Matrix metalloproteinase-9: many shades of function in cardiovascular disease. Physiology (Bethesda) 2013;28:391–403. doi: 10.1152/physiol.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaminski AR, Moore ET, Daseke MJ, 2nd, Valerio FM, Flynn ER, Lindsey ML. The compendium of matrix metalloproteinase expression in the left ventricle of mice following myocardial infarction. Am J Physiol Heart Circ Physiol. 2020;318:H706–H714. doi: 10.1152/ajpheart.00679.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yabluchanskiy A, Ma Y, Chiao YA, Lopez EF, Voorhees AP, Toba H, Hall ME, Han HC, Lindsey ML, Jin YF. Cardiac aging is initiated by matrix metalloproteinase-9-mediated endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2014;306:H1398–H1407. doi: 10.1152/ajpheart.00090.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma Y, Chiao YA, Clark R, Flynn ER, Yabluchanskiy A, Ghasemi O, Zouein F, Lindsey ML, Jin YF. Deriving a cardiac ageing signature to reveal MMP-9-dependent inflammatory signalling in senescence. Cardiovasc Res. 2015;106:421–431. doi: 10.1093/cvr/cvv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007;50:219–227. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- 35.Wakisaka Y, Chu Y, Miller JD, Rosenberg GA, Heistad DD. Critical role for copper/zinc-superoxide dismutase in preventing spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. Stroke. 2010;41:790–797. doi: 10.1161/STROKEAHA.109.569616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee CZ, Xue Z, Zhu Y, Yang GY, Young WL. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracerebral hemorrhage. Stroke. 2007;38:2563–2568. doi: 10.1161/STROKEAHA.106.481515. [DOI] [PubMed] [Google Scholar]
- 37.Lee JM, Yin KJ, Hsin I, Chen S, Fryer JD, Holtzman DM, Hsu CY, Xu J. Matrix metalloproteinase-9 and spontaneous hemorrhage in an animal model of cerebral amyloid angiopathy. Ann Neurol. 2003;54:379–382. doi: 10.1002/ana.10671. [DOI] [PubMed] [Google Scholar]
- 38.Zhao L, Arbel-Ornath M, Wang X, Betensky RA, Greenberg SM, Frosch MP, Bacskai BJ. Matrix metalloproteinase 9-mediated intracerebral hemorrhage induced by cerebral amyloid angiopathy. Neurobiol Aging. 2015;36:2963–2971. doi: 10.1016/j.neurobiolaging.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, Ungvari Z. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell. 2017;16:469–479. doi: 10.1111/acel.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nyul-Toth A, Tarantini S, Kiss T, Toth P, Galvan V, Tarantini A, Yabluchanskiy A, Csiszar A and Ungvari Z. Increases in hypertension-induced cerebral microhemorrhages exacerbate gait dysfunction in a mouse model of Alzheimer's disease. Geroscience. 2020;42(6):1685–98. 10.1007/s11357-020-00256-3 [DOI] [PMC free article] [PubMed]
- 41.Wakisaka Y, Chu Y, Miller JD, Rosenberg GA, Heistad DD. Spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. J Cereb Blood Flow Metab. 2010;30:56–69. doi: 10.1038/jcbfm.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, McClister DM, Jr, Su H, Gannon J, MacGillivray C, Lee RT, Sinusas AJ, Spinale FG. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;290:H232–H239. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]
- 43.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M, Khazan B, Lakatta EG. Central arterial aging and angiotensin II signaling. Curr Hypertens Rev. 2010;6:266–281. doi: 10.2174/157340210793611668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adair JC, Charlie J, Dencoff JE, Kaye JA, Quinn JF, Camicioli RM, Stetler-Stevenson WG, Rosenberg GA. Measurement of gelatinase B (MMP-9) in the cerebrospinal fluid of patients with vascular dementia and Alzheimer disease. Stroke. 2004;35:e159–e162. doi: 10.1161/01.STR.0000127420.10990.76. [DOI] [PubMed] [Google Scholar]
- 46.Moon SK, Cha BY, Lee YC, Nam KS, Runge MS, Patterson C, Kim CH. Age-related changes in matrix metalloproteinase-9 regulation in cultured mouse aortic smooth muscle cells. Exp Gerontol. 2004;39:123–131. doi: 10.1016/j.exger.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 47.Abilleira S, Montaner J, Molina CA, Monasterio J, Castillo J, Alvarez-Sabin J. Matrix metalloproteinase-9 concentration after spontaneous intracerebral hemorrhage. J Neurosurg. 2003;99:65–70. doi: 10.3171/jns.2003.99.1.0065. [DOI] [PubMed] [Google Scholar]
- 48.Alvarez-Sabin J, Delgado P, Abilleira S, Molina CA, Arenillas J, Ribo M, Santamarina E, Quintana M, Monasterio J, Montaner J. Temporal profile of matrix metalloproteinases and their inhibitors after spontaneous intracerebral hemorrhage: relationship to clinical and radiological outcome. Stroke. 2004;35:1316–1322. doi: 10.1161/01.STR.0000126827.69286.90. [DOI] [PubMed] [Google Scholar]
- 49.Gasche Y, Copin JC, Sugawara T, Fujimura M, Chan PH. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:1393–1400. doi: 10.1097/00004647-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Kawakita K, Kawai N, Kuroda Y, Yasashita S, Nagao S. Expression of matrix metalloproteinase-9 in thrombin-induced brain edema formation in rats. J Stroke Cerebrovasc Dis. 2006;15:88–95. doi: 10.1016/j.jstrokecerebrovasdis.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Yamani MH, Starling RC, Cook DJ, Tuzcu EM, Abdo A, Paul P, Powell K, Ratliff NB, Yu Y, McCarthy PM, Young JB. Donor spontaneous intracerebral hemorrhage is associated with systemic activation of matrix metalloproteinase-2 and matrix metalloproteinase-9 and subsequent development of coronary vasculopathy in the heart transplant recipient. Circulation. 2003;108:1724–1728. doi: 10.1161/01.CIR.0000087604.27270.5B. [DOI] [PubMed] [Google Scholar]
- 52.Tang J, Liu J, Zhou C, Alexander JS, Nanda A, Granger DN, Zhang JH. Mmp-9 deficiency enhances collagenase-induced intracerebral hemorrhage and brain injury in mutant mice. J Cereb Blood Flow Metab. 2004;24:1133–1145. doi: 10.1097/01.WCB.0000135593.05952.DE. [DOI] [PubMed] [Google Scholar]
- 53.McNulty M, Spiers P, McGovern E, Feely J. Aging is associated with increased matrix metalloproteinase-2 activity in the human aorta. Am J Hypertens. 2005;18:504–509. doi: 10.1016/j.amjhyper.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 54.Chung AW, Booth AD, Rose C, Thompson CR, Levin A, van Breemen C. Increased matrix metalloproteinase 2 activity in the human internal mammary artery is associated with ageing, hypertension, diabetes and kidney dysfunction. J Vasc Res. 2008;45:357–362. doi: 10.1159/000119755. [DOI] [PubMed] [Google Scholar]
- 55.Wang M, Takagi G, Asai K, Resuello RG, Natividad FF, Vatner DE, Vatner SF, Lakatta EG. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension. 2003;41:1308–1316. doi: 10.1161/01.HYP.0000073843.56046.45. [DOI] [PubMed] [Google Scholar]
- 56.Li Z, Froehlich J, Galis ZS, Lakatta EG. Increased expression of matrix metalloproteinase-2 in the thickened intima of aged rats. Hypertension. 1999;33:116–123. doi: 10.1161/01.hyp.33.1.116. [DOI] [PubMed] [Google Scholar]
- 57.Wang M, Lakatta EG. Altered regulation of matrix metalloproteinase-2 in aortic remodeling during aging. Hypertension. 2002;39:865–873. doi: 10.1161/01.hyp.0000014506.13322.66. [DOI] [PubMed] [Google Scholar]
- 58.Spiers JP, Kelso EJ, Siah WF, Edge G, Song G, McDermott BJ, Hennessy M. Alterations in vascular matrix metalloproteinase due to ageing and chronic hypertension: effects of endothelin receptor blockade. J Hypertens. 2005;23:1717–1724. doi: 10.1097/01.hjh.0000176787.04753.ee. [DOI] [PubMed] [Google Scholar]
- 59.Geng X, Hwang J, Ye J, Shih H, Coulter B, Naudin C, Jun K, Sievers R, Yeghiazarians Y, Lee RJ, Boyle AJ. Aging is protective against pressure overload cardiomyopathy via adaptive extracellular matrix remodeling. Am J Cardiovasc Dis. 2017;7:72–82. [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Y, Zhang M, Hao W, Mihaljevic I, Liu X, Xie K, Walter S, Fassbender K. Matrix metalloproteinase-12 contributes to neuroinflammation in the aged brain. Neurobiol Aging. 2013;34:1231–1239. doi: 10.1016/j.neurobiolaging.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 61.Medley TL, Kingwell BA, Gatzka CD, Pillay P, Cole TJ. Matrix metalloproteinase-3 genotype contributes to age-related aortic stiffening through modulation of gene and protein expression. Circ Res. 2003;92:1254–1261. doi: 10.1161/01.RES.0000076891.24317.CA. [DOI] [PubMed] [Google Scholar]
- 62.Kiss T, Nyul-Toth A, Balasubramanian P, Tarantini S, Ahire C, DelFavero J, Yabluchanskiy A, Csipo T, Farkas E, Wiley G, Garman L, Csiszar A, Ungvari Z. Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience. 2020;42:429–444. doi: 10.1007/s11357-020-00177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu F, Kamada H, Niizuma K, Endo H, Chan PH. Induction of mmp-9 expression and endothelial injury by oxidative stress after spinal cord injury. J Neurotrauma. 2008;25:184–195. doi: 10.1089/neu.2007.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grossetete M, Rosenberg GA. Matrix metalloproteinase inhibition facilitates cell death in intracerebral hemorrhage in mouse. J Cereb Blood Flow Metab. 2008;28:752–763. doi: 10.1038/sj.jcbfm.9600572. [DOI] [PubMed] [Google Scholar]
- 65.Chiao YA, Ramirez TA, Zamilpa R, Okoronkwo SM, Dai Q, Zhang J, Jin YF, Lindsey ML. Matrix metalloproteinase-9 deletion attenuates myocardial fibrosis and diastolic dysfunction in ageing mice. Cardiovasc Res. 2012;96:444–455. doi: 10.1093/cvr/cvs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ali MM, Mahmoud AM, Le Master E, Levitan I, Phillips SA. Role of matrix metalloproteinases and histone deacetylase in oxidative stress-induced degradation of the endothelial glycocalyx. Am J Physiol Heart Circ Physiol. 2019;316:H647–H663. doi: 10.1152/ajpheart.00090.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, Lakatta E, Csiszar A. Age-associated vascular oxidative stress, Nrf2 dysfunction and NF-kB activation in the non-human primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–75. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301:H363–72. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Springo Z, Tarantini S, Toth P, Tucsek Z, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. J Gerontol A Biol Sci Med Sci. 2015;70:1355–1359. doi: 10.1093/gerona/glu244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han BH, Zhou ML, Johnson AW, Singh I, Liao F, Vellimana AK, Nelson JW, Milner E, Cirrito JR, Basak J, Yoo M, Dietrich HH, Holtzman DM, Zipfel GJ. Contribution of reactive oxygen species to cerebral amyloid angiopathy, vasomotor dysfunction, and microhemorrhage in aged Tg2576 mice. Proc Natl Acad Sci U S A. 2015;112:E881–E890. doi: 10.1073/pnas.1414930112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Santisteban MM, Ahn SJ, Lane D, Faraco G, Garcia-Bonilla L, Racchumi G, Poon C, Schaeffer S, Segarra SG, Korbelin J, Anrather J, Iadecola C. Endothelium-macrophage crosstalk mediates blood-brain barrier dysfunction in hypertension. Hypertension. 2020;76:795–807. doi: 10.1161/HYPERTENSIONAHA.120.15581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wiesmann M, Roelofs M, van der Lugt R, Heerschap A, Kiliaan AJ and Claassen JA. Angiotensin II, hypertension, and angiotensin II receptor antagonism: roles in the behavioural and brain pathology of a mouse model of Alzheimer's disease. J Cereb Blood Flow Metab. 2017;37(7):2396–413. 10.1177/0271678X16667364 [DOI] [PMC free article] [PubMed]
- 74.Passos GF, Kilday K, Gillen DL, Cribbs DH, Vasilevko V. Experimental hypertension increases spontaneous intracerebral hemorrhages in a mouse model of cerebral amyloidosis. J Cereb Blood Flow Metab. 2016;36:399–404. doi: 10.1177/0271678X15606720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poulet R, Gentile MT, Vecchione C, Distaso M, Aretini A, Fratta L, Russo G, Echart C, Maffei A, De Simoni MG, Lembo G. Acute hypertension induces oxidative stress in brain tissues. J Cereb Blood Flow Metab. 2006;26:253–262. doi: 10.1038/sj.jcbfm.9600188. [DOI] [PubMed] [Google Scholar]
- 76.Bailey EL, Wardlaw JM, Graham D, Dominiczak AF, Sudlow CL, Smith C. Cerebral small vessel endothelial structural changes predate hypertension in stroke-prone spontaneously hypertensive rats: a blinded, controlled immunohistochemical study of 5- to 21-week-old rats. Neuropathol Appl Neurobiol. 2011;37:711–726. doi: 10.1111/j.1365-2990.2011.01170.x. [DOI] [PubMed] [Google Scholar]
- 77.Mayhan WG, Faraci FM, Heistad DD. Mechanisms of protection of the blood-brain barrier during acute hypertension in chronically hypertensive rats. Hypertension. 1987;9:III101–5. doi: 10.1161/01.hyp.9.6_pt_2.iii101. [DOI] [PubMed] [Google Scholar]
- 78.Barry DI, Strandgaard S, Graham DI, Braendstrup O, Svendsen UG, Vorstrup S, Hemmingsen R, Bolwig TG. Cerebral blood flow in rats with renal and spontaneous hypertension: resetting of the lower limit of autoregulation. J Cereb Blood Flow Metab. 1982;2:347–353. doi: 10.1038/jcbfm.1982.35. [DOI] [PubMed] [Google Scholar]
- 79.Mueller SM, Heistad DD. Effect of chronic hypertension on the blood-brain barrier. Hypertension. 1980;2:809–812. doi: 10.1161/01.hyp.2.6.809. [DOI] [PubMed] [Google Scholar]
- 80.Wakisaka Y, Miller JD, Chu Y, Baumbach GL, Wilson S, Faraci FM, Sigmund CD, Heistad DD. Oxidative stress through activation of NAD(P)H oxidase in hypertensive mice with spontaneous intracranial hemorrhage. J Cereb Blood Flow Metab. 2008;28:1175–1185. doi: 10.1038/jcbfm.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]


