Abstract
Alzheimer’s disease (AD) is a significant burden for human health that is increasing in prevalence as the global population ages. There is growing recognition that current preclinical models of AD are insufficient to recapitulate key aspects of the disease. Laboratory models for AD include mice, which do not naturally develop AD-like pathology during aging, and laboratory Beagle dogs, which do not share the human environment. In contrast, the companion dog shares the human environment and presents a genetically heterogeneous population of animals that might spontaneously develop age-associated AD-like pathology and cognitive dysfunction. Here, we quantitatively measured amyloid beta (Aβ42 or Abeta-42) levels in three areas of the companion dog brain (prefrontal cortex, temporal cortex, hippocampus/entorhinal cortex) and cerebrospinal fluid (CSF) using a newly developed Luminex assay. We found significant positive correlations between Aβ42 and age in all three brain regions. Brain Aβ42 abundance in all three brain regions was also correlated with Canine Cognitive Dysfunction Scale score in a multivariate analysis. This latter effect remained significant when correcting for age, except in the temporal cortex. There was no correlation between Aβ42 in CSF and cognitive scores; however, we found a significant positive correlation between Aβ42 in CSF and body weight, as well as a significant negative correlation between Aβ42 in CSF and age. Our results support the suitability of the companion dog as a model for AD and illustrate the utility of veterinary biobanking to make biospecimens available to researchers for analysis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-021-00422-1.
Keywords: Dogs, Canine Cognitive Dysfunction, Abeta-42, Luminex, Alzheimer’s disease, Neurodegeneration, Age-related disease, Tissue banking
Introduction
Alzheimer’s disease (AD) is the leading cause of dementia among the elderly and is an urgent national and international research priority [1, 2]. There is a growing consensus that biological aging underlies this relationship and that the known “hallmarks” of aging [3] either contribute causally to AD or, at a minimum, create a permissive physiology that is necessary for AD onset and progression [4, 5]. Neuropathologically, AD is characterized by the aggregation of protein deposits in various parts of the cerebrum, including the prefrontal cortex, temporal cortex, hippocampus, and entorhinal cortex. These aggregates notably consist of amyloid beta (Aβ42) senile plaques and hyperphosphorylated Tau (pTau) neurofibrillary tangles, both of which are accompanied by neuronal death, loss of synaptic connections in the brain, and cerebral atrophy. Ultimately, this results in the development and progression of clinical dementia. The underlying pathological processes leading to these outcomes are complex and not completely understood. While there are several medical interventions available that may slow progression of the disease, it remains incurable, debilitating, and terminal.
A major limitation to AD research is a lack of animal models that spontaneously develop AD-like pathology and also adequately reflect the genetic and environmental complexity of humans. For example, mice do not naturally develop age-related AD-like pathology, and consequently, all currently used mouse models have been genetically engineered to specifically develop various aspects of AD-like pathology [6, 7]. Laboratory Beagle dogs do spontaneously develop AD-like pathology as they age [8]; however, they are kept in a laboratory setting that fails to model the environmental variation experienced by people. Similarly, both AD-model mouse strains and laboratory Beagle dogs are relatively genetically inbred and fail to capture the impact of genetic diversity on AD progression and clinical presentation.
In this context, the privately owned companion dog offers an opportunity to overcome these limitations. Companion dogs are genetically heterogeneous, with a powerful and well-understood genetic architecture, consisting of more than 300 relatively inbred, genetically isolated purebred breeds along with a rich population of genetically diverse mixed breed dogs [9]. Companion dogs also share the complex human environment, including exposure to many potential risk factors of AD. Dogs also develop many of the same age-related pathologies as people, but they age more rapidly than humans [10–12]. This accelerated aging rate makes it possible to rapidly understand the impact of specific genetic and environmental factors on age-related disease, including age-associated neurodegenerative disease.
Just like in humans, cognitive decline is considered to be a natural part of the biological aging process in dogs, but also like in humans, a subset of dogs will develop dementia in old age, which is referred to as Canine Cognitive Dysfunction (CCD) [13]. CCD is a syndrome that mirrors many of the clinical features of human AD, including progressive loss of cognitive function such as decreased ability to learn, increased anxiety, loss of normal sleep patterns, and aimless wandering. It can be reliably diagnosed using the owner- or veterinarian-administered Canine Cognitive Dysfunction Rating Scale (CCDRS) [14], which is based on a validated questionnaire that assesses the dog’s cognitive function using 13 variables scored on a scale of 1–5 points each, leading to CCD scores that can range from 13 to 65 points. CCD scores of 50 points and above are indicative of a diagnosis of CCD.
Molecular features of Alzheimer’s disease in humans that have been associated with CCD in dogs include brain Aβ42 deposition, which accumulates in diffuse plaques and in the cerebral vasculature, as well as hyperphosphorylated Tau (pTau) found both diffusely and in neurofibrillary tangles [15–17]. The canine amyloid precursor protein (APP) is ~ 98% similar to human APP, and the canine Aβ42 peptide is identical to human Aβ42. The mechanism of amyloid beta accumulation, oligomerization, and deposition is also thought to be similar, if not identical, in dogs and people [17, 18].
Several studies have looked at Aβ42 and/or pTau histopathology in dog brains using immunohistochemistry (IHC), including studies correlating Aβ42 pathology with age [10, 16, 19] and clinical CCD [11, 12, 20], pTau presence with clinical CCD [21] or age [10, 16], and at least one study correlating Aβ42 and pTau presence with age and body size [16]. While there is research into the correlation between Aβ42 levels and cognitive function in laboratory beagles [22], to our knowledge nobody has as of yet measured Aβ42 in companion dog brains in a quantitative manner and correlated these measurements with cognitive function. Here, we used a newly developed Luminex assay [23, 24] to quantitatively measure Aβ42 in brains and cerebrospinal fluid (CSF) from privately owned companion dogs with known CCD scores and report statistically significant correlations between cognitive phenotype and Aβ42 abundance in these brains.
Methods
Dog brain samples, CSF, and cognitive scores and other phenotype information were provided by the Canine Brain and Tissue Bank (CBTB) of the Senior Family Dog Project at ELTE in Budapest, Hungary (https://familydogproject.elte.hu/canine-brain-and-tissue-bank-for-researchers/) [25]. Samples were obtained within 4 h post mortem from privately owned companion dogs that had been euthanized for medical reasons and whose brains and CSF were then frozen and stored at − 80 °C. All the dissections and samplings were performed at ELTE by a veterinary anatomist (KC). Samples were shipped to the University of Washington on dry ice. Sampled regions analyzed here consisted of prefrontal cortex (gyrus proreus), temporal cortex (gyrus ectosylvius medius), and entorhinal cortex/hippocampus (at the level of the distal part of cornu temporale ventriculi lateralis) as shown in Fig. 1, as well as CSF. Owners had previously provided informed consent to donate their dogs’ bodies upon death and were asked to fill in the Canine Cognitive Dysfunction Rating Scale questionnaire [14] translated into Hungarian, reflecting the most recent known cognitive status of their dogs. The absence of any disease that would be transmissible through brain tissue or any medical status that would foreclose the donation was affirmed in the consent form by the veterinarian who had performed the euthanasia.
Fig. 1.
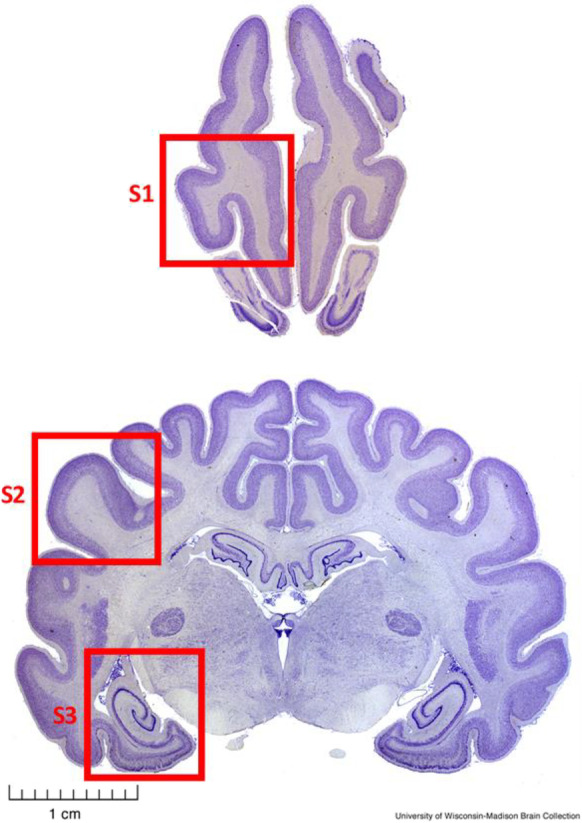
Transverse sections of a fixed dog brain illustrating the sampled brain regions. S1, Prefrontal cortex (prorean gyrus); S2, Temporal cortex (middle ectosylvian gyrus); S3, Hippocampus and entorhinal cortex. Pictures from University of Wisconsin-Madison Brain Collection (http://www.brainmuseum.org/Specimens/carnivora/basenji, sections #440 and #1440)
Tissue from dog brain samples was first homogenized in chilled RIPA buffer (1% NP-40, 0.5% sodium deoxycholate, 150 mM sodium chloride, 50 mM Tris hydrochloride, 0.5 mM magnesium sulfate—all from Sigma-Aldrich; St. Louis, MO) that was supplemented with Complete Mini protease inhibitor (Sigma-Aldrich; St. Louis, MO). Samples were then centrifuged for 45 min at 21,000 × g and 4 °C. Supernatants containing the RIPA-soluble fraction were removed and stored. The remaining pellets were washed with RIPA buffer and centrifuged a second time. The RIPA buffer containing supernatant was then combined with the first RIPA buffer containing supernatant, aliquoted, and stored at − 80 °C. The combined RIPA buffer supernatant contained soluble proteins. The pellets were then resuspended in 150 µL of chilled 5 M guanidine-hydrochloride (Gu-HCl) buffer that also contained Complete Mini protease inhibitor. Resuspended pellets were then sonicated on ice (10 pulses). Sonicated samples were then centrifuged 30 min at 21,000 × g and 4 °C. After centrifugation, the supernatants were transferred into fresh tubes, aliquoted, and stored at − 80 °C. The Gu-HCl-soluble supernatant contains aggregates of proteins like Aβ42. Total protein content of all Gu-HCl supernatants was determined using a BCA kit (Pierce; Rockford, IL) together with colorimetric detection (absorbance at 562 nm) in a plate reader. Brain region and CSF Aβ42 concentrations were then measured using a previously described Luminex assay [23], resulting in quantitative measurements of Aβ42 protein levels for all available samples. For dog brain tissue, we always used 200 ng of Gu-HCl-soluble total protein per well, and for CSF samples, we always used 50 µL of CSF per well. All samples were tested in duplicate.
Data were stored and managed in Microsoft Excel and analyzed in R [26]. We used multiple linear regression on raw and log transformed data in R to simultaneously analyze the influence of measured Aβ42 levels in all three brain regions and CSF on cognitive scores, as well as Wilcoxon and Spearman tests and linear regression models to test for individual correlations in our data. A p-value (P) of 0.05 or less was considered statistically significant for all statistical tests.
Results
We obtained data and/or samples from a total of n = 45 dogs that had been euthanized at the CBTB between May of 2017 and June of 2019. This included brain samples from n = 33 dogs, CSF samples from n = 27 dogs, and CCD scores from n = 17 dogs. N = 14 dogs had both brains and CCD scores available, n = 11 dogs had both CSF and CCD scores available, and n = 10 dogs had all three available. CCD scores in all dogs ranged from 26 to 56, with a median of 40. CCD scores in dogs with available brain samples ranged from 32 to 50, with a median of 39. Ages in all dogs ranged from 1 to 18 years, with a median of 13 years; ages in dogs with available brain samples ranged from 1 to 17 years, with a median of 12 years. Apart from CCD scores and Aβ42 measurements where available, phenotypic data included sex, breed, age, and body weight at necropsy for all 45 dogs. The demographic and measured data for all 45 dogs are provided in Supplemental Table 1.
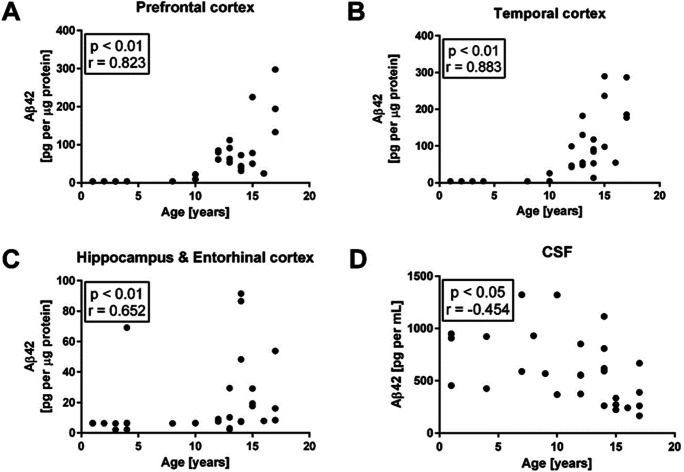
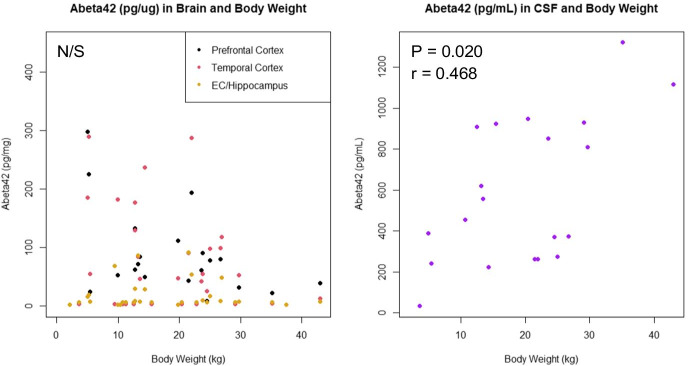
There was a significant positive correlation between Aβ42 levels and age in all three brain regions studied (prefrontal cortex: P = 3E-9, Spearman’s rho = 0.823; temporal cortex: P = 5E-12, Spearman’s rho = 0.883; hippocampus/entorhinal cortex: P = 3E-5, Spearman’s rho = 0.652), and a significant negative correlation between Aβ42 and age in CSF samples (P = 0.02, Spearman’s rho = − 0.454), as shown in Fig. 2. When performing linear regression on age and raw versus log transformed Aβ42 data, log transformed data yielded both higher R2 and lower P values for all three brain regions, but not for CSF, indicating an exponential increase in Aβ42 levels in the brain with age (R2 = 0.46 vs. 0.84 for frontal, 0.50 vs. 0.82 for temporal, and 0.07 vs. 0.18 for hippocampus/entorhinal cortex; P = 1.3E-5 vs. 1.3E-13 for frontal, 2.5E-6 vs. 2.4E-13 for temporal, and 0.084 vs. 0.0095 for hippocampus/entorhinal cortex). Interestingly, we found no significant correlation between Aβ42 levels in the brain and the dogs’ body weight; however, there was a significant positive correlation between Aβ42 levels in CSF and body weight (Fig. 3).
Fig. 2.
Quantitative measurements of Aβ42 levels by Luminex and dog age in all three brain regions and in CSF. Significant positive correlations exist between brain Aβ42 levels and age in all three sampled regions, and a significant negative correlation exists in CSF. Statistics as displayed are based on Spearman tests
Fig. 3.
Quantitative measurements of Aβ42 levels by Luminex and body weight in kilograms. No significant correlation was found for Aβ42 and body weight in any of the three brain regions; however, a significant positive correlation was found in CSF. Statistics are based on Spearman tests
When comparing Aβ42 levels by sex, we found a significant difference between males and females in the prefrontal cortex from dogs aged 10 years and older, with males having significantly lower levels than females (P = 0.012, W = 83, Wilcoxon rank-sum two-sided test — see Fig. 4 and Supplemental Fig. 1).
Fig. 4.
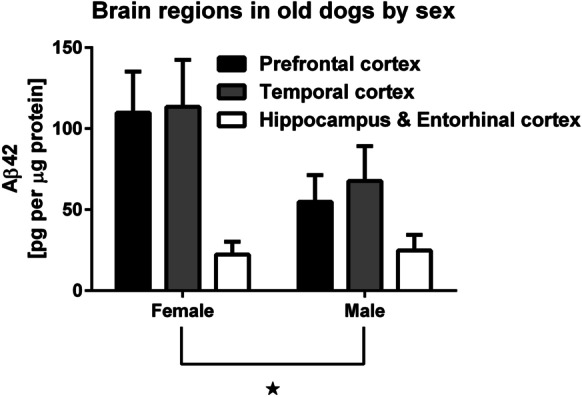
Aβ42 levels in all three brain regions by sex in dogs aged 5 years and older. There is a significant sex difference in prefrontal cortex, with females having higher levels than males (P = 0.012, Wilcoxon rank-sum two-sided test). There was no significant age difference between the sexes (P = 0.64, Wilcoxon rank-sum two-sided test)
CCD scores were not correlated with age (P = 0.351, Spearman’s rho = 0.117) or body weight (P = 0.307, Spearman’s rho = 0.154). When considering the impact of Aβ42 levels in all three brain regions and CSF, as well as age, on CCD scores in general linear models, we found significant effects of Aβ42 levels on CCD scores in all three brain regions, but not in CSF. When including age as a covariate, this effect remained significant in the temporal cortex and the entorhinal cortex/hippocampus, but not in the prefrontal cortex. There was no significant correlation between Aβ42 levels in CSF and any of the three brain regions in a general linear model. Results for the linear models are provided in Supplemental Table 2 and Supplemental Fig. 2.
Discussion
Here, we describe a study using a recently developed Luminex assay that allows us to quantitatively measure Aβ42 in various parts of the companion dog brain and CSF. We show that the measures we obtained in brains correlate with cognitive function as measured by an owner-completed questionnaire using the Canine Cognitive Dysfunction Scale (CCDS). This scale has been shown to have an overall 98.9% diagnostic accuracy, with a 77.8% positive predictive value, a 99.3% negative predictive value, and a high re-test reliability over 2 months [14]. The CCDS has also been cross-validated against the CAnine DEmentia Scale (CADES), which is the other commonly used scale for clinical CCD assessments [27].
In human patients, it is well established that Aβ42 and other AD-related pathologies emerge in the brain years or even decades before clinical AD symptoms manifest [28]. Here, we describe a correlation between brain Aβ42 levels and age in dogs, which appears to progress exponentially, as reported previously [29]. Additionally, we also show a correlation between cognitive function and Aβ42 levels in multiple brain regions in a cohort of companion dogs. It is noteworthy that this correlation exists even among dogs with cognitive function scores that did not meet the diagnostic threshold (score > 50) for CCD. This is encouraging in that it may mirror a similar trajectory in the aging dog, where increased Aβ42 levels in the brain appear to be associated with cognitive impairment that might have progressed to clinical CCD had the dogs not been euthanized for other reasons.
We did not find a correlation between brain Aβ42 levels and body weight; however, we did find such a correlation between CSF Aβ42 levels and body weight. This is interesting in that larger dogs generally live less long than smaller dogs and could thus be expected to develop CCD and associated pathology earlier than small dogs, as happens with various other age-related diseases [30–32]. However, this is not the case for CCD, which appears to not affect large dogs at earlier ages than small dogs [33]. It may be that body condition rather than measured weight may be a better predictor; however, our data unfortunately did not include this information.
The current National Institute on Aging–Alzheimer’s Association (NIA–AA) consensus criteria for the neuropathologic diagnosis of AD are based on histologic characterization of the anatomic distribution of amyloid plaques and pTau-containing neurofibrillary tangles (NFTs) [34–36]. These criteria are based on distribution of amyloid plaques and NFTs, and on cortical density of neuritic plaques. They are not quantitative measures but rather ordered rankings, which limits their utility in studies that aim to correlate Aβ42 pathology and cognitive function. To overcome this limitation, we developed a Luminex-based assay for quantification of Aβ42 in human brain autopsy tissue [24], and we have further established parameters for this assay that allow quantification of Aβ42 in mutation-based animal models of AD and in natural models like non-human primates and dogs [23]. The data set from our current study in dogs constitutes one of the first applications of our Luminex assay to probe the quantitative relationship between brain Aβ42 and cognitive changes.
Both the Dog Aging Project and the Senior Family Dog Project aim to leverage privately owned companion dogs as models for aging and age-related disease in humans. One aspect of being able to investigate normative and/or pathological aging in dogs is the availability of biospecimens from various organs for research, which should also include clinical and demographic information for these animals. Both the existing Canine Brain and Tissue Bank (CBTB) at ELTE and the future Dog Aging Project Biobank at Cornell University address this emerging need and will be useful to conduct larger-scale studies in the future as more specimens become available.
In summary, to our knowledge, this is the first study that quantitatively measures Aβ42 in companion dog brains and CSF and correlates these findings with measured cognitive scores. Taken together, our data support the notion that CCD in companion dogs models several key aspects of human AD, underscoring the suitability and potential usefulness of companion dogs as a model for both observational and interventional studies of AD. Our results also confirm previous findings on behavioral and neuropathological markers associated with age-related cognitive dysfunction in laboratory Beagles [37, 38] and show that these findings likely also apply to companion dogs, which are genetically diverse and share the human environment and its various risk factors [39]. In particular, companion dogs model aspects of the disease that currently cannot be adequately studied in a laboratory setting, because companion dogs share much of the human environment and present a genetically diverse, heterogeneous population. This illustrates that they are a potentially valuable model that could be used not only for descriptive studies of risk factors, behavior, and neuropathology, but also to study possible interventions aimed at preventing or treating Alzheimer-like pathology that have considerable translational potential for human health [40, 41].
Limitations of the present study include the relatively low number of samples, as well as the absence of dogs with CCD scores that are diagnostic for CCD in the available tissue samples. Nevertheless, the fact that we were able to find a significant correlation between CCD scores below the diagnostic threshold for CCD and Aβ42 levels in all three brain regions examined is encouraging and will be useful as a foundation to conduct additional research into this question as more samples become available in the future. In particular, we plan to also investigate Tau and pTau levels in these same dog brains and correlate them with CCD scores, and we will also conduct immunohistochemical and neuropathological analyses to further explore the correlation between cognitive function/CCD, Aβ42, and Tau/pTau levels and pathology in the aged companion dog brain.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank study participants, their dogs, and community veterinarians for their important contributions.
Funding
This work was supported by NIH grant 3U19AG057377-02S3 to DP. The Dog Aging Project is supported by U19 grant AG057377 from the National Institute on Aging, a part of the National Institutes of Health, and by private donations. The Senior Family Dog Project receives funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Grant Agreement No. 680040).
Data availability
The full data used for this analysis are provided in Supplemental Table 1.
Declarations
Conflict of interest
The authors declare no competing interests.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Enikő Kubinyi and Matt Kaeberlein jointly supervised this work.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps (including brain maps) and institutional affiliations.
References
- 1.Khachaturian ZS, Khachaturian AS, Thies W. The draft "National Plan" to address Alzheimer's disease - National Alzheimer's Project Act (NAPA) Alzheimers Dement. 2012;8(3):234–236. doi: 10.1016/j.jalz.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Hebert LE, et al. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Otin C, et al. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaeberlein M, Galvan V. Rapamycin and Alzheimer's disease: time for a clinical trial? Sci Transl Med. 2019;11(476):eaar4289. doi: 10.1126/scitranslmed.aar4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaeberlein M. Time for a new strategy in the war on Alzheimer’s disease. Public Policy Aging Rep. 2019;29(4):119–122. doi: 10.1093/ppar/prz020. [DOI] [Google Scholar]
- 6.Drummond E, Wisniewski T. Alzheimer's disease: experimental models and reality. Acta Neuropathol. 2017;133(2):155–175. doi: 10.1007/s00401-016-1662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jankowsky JL, Zheng H. Practical considerations for choosing a mouse model of Alzheimer's disease. Mol Neurodegener. 2017;12(1):89. doi: 10.1186/s13024-017-0231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotman CW, Head E. The canine (dog) model of human aging and disease: dietary, environmental and immunotherapy approaches. J Alzheimers Dis. 2008;15(4):685–707. doi: 10.3233/JAD-2008-15413. [DOI] [PubMed] [Google Scholar]
- 9.Plassais J, et al. Whole genome sequencing of canids reveals genomic regions under selection and variants influencing morphology. Nat Commun. 2019;10(1):1489. doi: 10.1038/s41467-019-09373-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schutt T, et al. Dogs with cognitive dysfunction as a spontaneous model for early Alzheimer's disease: a translational study of neuropathological and inflammatory markers. J Alzheimers Dis. 2016;52(2):433–449. doi: 10.3233/JAD-151085. [DOI] [PubMed] [Google Scholar]
- 11.Colle MA, et al. Vascular and parenchymal Abeta deposition in the aging dog: correlation with behavior. Neurobiol Aging. 2000;21(5):695–704. doi: 10.1016/S0197-4580(00)00113-5. [DOI] [PubMed] [Google Scholar]
- 12.Torp R, Head E, Cotman CW. Ultrastructural analyses of beta-amyloid in the aged dog brain: neuronal beta-amyloid is localized to the plasma membrane. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(5):801–810. doi: 10.1016/S0278-5846(00)00107-X. [DOI] [PubMed] [Google Scholar]
- 13.Dewey CW, et al. Canine Cognitive Dysfunction: pathophysiology, diagnosis, and treatment. Vet Clin North Am Small Anim Pract. 2019;49(3):477–499. doi: 10.1016/j.cvsm.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Salvin HE, et al. The canine cognitive dysfunction rating scale (CCDR): a data-driven and ecologically relevant assessment tool. Vet J. 2011;188(3):331–336. doi: 10.1016/j.tvjl.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Head E. A canine model of human aging and Alzheimer's disease. Biochim Biophys Acta. 2013;1832(9):1384–1389. doi: 10.1016/j.bbadis.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt F, et al. Detection and quantification of beta-amyloid, pyroglutamyl Abeta, and Tau in aged canines. J Neuropathol Exp Neurol. 2015;74(9):912–923. doi: 10.1097/NEN.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 17.Johnstone EM, et al. Conservation of the sequence of the Alzheimer's disease amyloid peptide in dog, polar bear and five other mammals by cross-species polymerase chain reaction analysis. Brain Res Mol Brain Res. 1991;10(4):299–305. doi: 10.1016/0169-328X(91)90088-F. [DOI] [PubMed] [Google Scholar]
- 18.Selkoe DJ, et al. Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer's disease. Science. 1987;235(4791):873–877. doi: 10.1126/science.3544219. [DOI] [PubMed] [Google Scholar]
- 19.Satou T, et al. The progression of beta-amyloid deposition in the frontal cortex of the aged canine. Brain Res. 1997;774(1–2):35–43. doi: 10.1016/S0006-8993(97)81684-8. [DOI] [PubMed] [Google Scholar]
- 20.Pugliese M, et al. Canine cognitive deficit correlates with diffuse plaque maturation and S100beta (-) astrocytosis but not with insulin cerebrospinal fluid level. Acta Neuropathol. 2006;111(6):519–528. doi: 10.1007/s00401-006-0052-1. [DOI] [PubMed] [Google Scholar]
- 21.Yu CH, et al. Histopathological and immunohistochemical comparison of the brain of human patients with Alzheimer's disease and the brain of aged dogs with cognitive dysfunction. J Comp Pathol. 2011;145(1):45–58. doi: 10.1016/j.jcpa.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Head E, et al. A two-year study with fibrillar beta-amyloid (Abeta) immunization in aged canines: effects on cognitive function and brain Abeta. J Neurosci. 2008;28(14):3555–3566. doi: 10.1523/JNEUROSCI.0208-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urfer SR, et al. Cross species application of quantitative neuropathology assays developed for clinical Alzheimer's disease samples. Pathobiol Aging Age Relat Dis. 2019;9(1):1657768. doi: 10.1080/20010001.2019.1657768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keene CD, et al. Luminex-based quantification of Alzheimer's disease neuropathologic change in formalin-fixed post-mortem human brain tissue. Lab Invest. 2019;99(7):1056–1067. doi: 10.1038/s41374-018-0165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandor S, et al. Man's best friend in life and death: scientific perspectives and challenges of dog brain banking. GeroScience. 2021. 10.1007/s11357-021-00373-7 [DOI] [PMC free article] [PubMed]
- 26.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. https://www.R-project.org/. Accessed 8/5/2021.
- 27.Madari A, et al. Assessment of severity and progression of canine cognitive dysfunction syndrome using the CAnine DEmentia Scale (CADES) Appl Anim Behav Sci. 2015;171(10):138–145. doi: 10.1016/j.applanim.2015.08.034. [DOI] [Google Scholar]
- 28.Jansen WJ, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313(19):1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Head E, et al. Region-specific age at onset of beta-amyloid in dogs. Neurobiol Aging. 2000;21(1):89–96. doi: 10.1016/S0197-4580(00)00093-2. [DOI] [PubMed] [Google Scholar]
- 30.Urfer SR, et al. Lifespan of companion dogs seen in three independent primary care veterinary clinics in the United States. Canine Med Genet. 2020;7:7. doi: 10.1186/s40575-020-00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galis F, et al. Do large dogs die young? J Exp Zool B Mol Dev Evol. 2007;308(2):119–126. doi: 10.1002/jez.b.21116. [DOI] [PubMed] [Google Scholar]
- 32.Kraus C, Pavard S, Promislow DE. The size-life span trade-off decomposed: why large dogs die young. Am Nat. 2013;181(4):492–505. doi: 10.1086/669665. [DOI] [PubMed] [Google Scholar]
- 33.Watowich MM, et al. Age influences domestic dog cognitive performance independent of average breed lifespan. Anim Cogn. 2020;23(4):795–805. doi: 10.1007/s10071-020-01385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montine TJ, et al. Recommendations of the Alzheimer's disease-related dementias conference. Neurology. 2014;83(9):851–860. doi: 10.1212/WNL.0000000000000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montine TJ, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123(1):1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hyman BT, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8(1):1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Head E. Neurobiology of the aging dog. Age (Dordr) 2011;33(3):485–496. doi: 10.1007/s11357-010-9183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torp R, et al. Ultrastructural evidence of fibrillar beta-amyloid associated with neuronal membranes in behaviorally characterized aged dog brains. Neuroscience. 2000;96(3):495–506. doi: 10.1016/S0306-4522(99)00568-0. [DOI] [PubMed] [Google Scholar]
- 39.Kaeberlein M, Creevy KE, Promislow DE. The dog aging project: translational geroscience in companion animals. Mamm Genome. 2016;27(7–8):279–288. doi: 10.1007/s00335-016-9638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin SB, Dowling AL, Head E. Therapeutic interventions targeting Beta amyloid pathogenesis in an aging dog model. Curr Neuropharmacol. 2011;9(4):651–661. doi: 10.2174/157015911798376217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barone E, et al. Biliverdin reductase-A: a novel drug target for atorvastatin in a dog pre-clinical model of Alzheimer disease. J Neurochem. 2012;120(1):135–146. doi: 10.1111/j.1471-4159.2011.07538.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full data used for this analysis are provided in Supplemental Table 1.