Abstract
The overarching mission of the Einstein-Nathan Shock Center (E-NSC) is to make scientific discoveries in geroscience, leveraging on the expertise in our center in 6 out of the 7 pillars of aging, and to translate their effects towards drug discovery. The relevance of this basic biology of aging discoveries to humans will be confirmed through the unique gero-human resource at E-NSC. This is achieved through services provided by E-NSC, connectivity among its members, attracting worldwide investigators, and providing them with the opportunities to become future leaders. The two central components of the E-NSC are (a) cutting-edge research programs and (b) unique E-NSC research support cores. E-NSC scientists lead NIH-supported cutting-edge research programs that integrate key hallmarks of aging including proteostasis/autophagy, metabolism/inflammaging, genetic/epigenetics, stem cells/regeneration, and translational aging/longevity. Since the inception of the E-NSC, the well-integrated, collaborative, and innovative nature of the multiple supporting state-of-the-art E-NSC research cores form the bedrock of research success at the E-NSC. The three state-of-the-art E-NSC research cores, (i) Proteostasis of Aging Core (PAC), (ii) the Health Span Core (HSC), and (iii) the Human Multi-Omics Core (HMOC), have allowed impressive expansion of translational biological research programs. Expansion was facilitated through the wealth of data coming from genomics/proteomics and metabolomic analysis on human longevity studies, due to access to a variety of biological samples from elderly subjects in clinical trials with aging-targeting drugs, and new drug design services via the PAC to target the hallmarks of aging.
Keywords: Health span, Metabolism, Parabiosis, Proteostasis, Autophagy, Proteomics, Genomics
Introduction: the theme of E-NSC
Key to the strategic vision for aging research at Einstein was the founding of the Institute for Aging Research (IAR) in 2002. Since then, the Institute grew significantly in the number of scientists and funding, with approximately 30% of Einstein’s investigators having a role in the Institute. The scientific goal of the Einstein Nathan Shock Center (E-NSC) is to provide services and create opportunities for researchers in and outside the field of biology of aging, who aim to discover mechanisms of aging and longevity with the goal to design and implement strategies to delay aging in humans. Our E-NSC, with its multi-dimensional geroscience approach, has the following goals: (i) to advance research programs in the areas defined as hallmarks of aging and in the emerging area of the integrated physiology of aging, and (ii) to facilitate career development of the future leaders in aging research. We have remarkable leadership, membership, services, and overall expertise in these areas, and we view ourselves as an exemplary “catalyzing” center. Because the hallmarks of aging accelerate each other, and because targeting of one hallmark influences the others, we have initiated and maintained major efforts to provide core services and stimulate collaborations to capture the totality of biology of aging. Furthermore, we have successfully and substantially bridged animal and human studies on aging and continue to expand upon these bidirectional interactions through the integration of the Cores with the translational Gero-Human Resource.
At the IAR, we have created a unique environment by building a programmatic approach to aging research. Funding of several program projects (P01, U01-2 grants) that support interactions between basic and translational scientists has contributed to enhancing the scientific productivity of the IAR. The experience and success of the P01 Cores greatly facilitated development of the Research Resource Cores of the E-NSC. Another component that contributes to the enriching environment of the E-NSC is the Paul Glenn Center for the Biology of Human Aging (E-Glenn), which supports research projects with specific emphasis on human aging. The unique goals of the E-Glenn are to fund projects that come out of E-NSC biological efforts which aim to (i) discover genetic and biological mechanisms that protect against human aging and age-related diseases, (ii) test the efficacy of first-generation aging-targeting interventions in human aging, and (iii) develop novel preventive and therapeutic interventions to combat cellular aging in humans. Some of those discoveries and resources are services provided in our Cores.
Key to the E-NSC mission is our commitment to train the next generation of geroscientists. In this regard, our training grant (T32) has already been funded for four cycles and its success is based on the high quality of research in geroscience exemplified by E-NSC investigators/mentors and their trainees, resulting in a rich preparatory mentoring environment. We also run the Graduate course on the Biology of Aging, which is unique in our geographical area, and provides in-depth analyses and discussions of the different aspects of the biology of aging, building from changes at the molecular and cellular level to analysis of the consequences at the organismal level. Importantly, this course is a requirement for all T32 graduate students and post-doctoral fellows and is strongly recommended for Pilot and Feasibility award recipients who typically are young investigators and would benefit from the course. Other E-NSC members as well as young trainees from neighboring institutions who may benefit from a part, or the whole of the course, are also welcomed to participate.
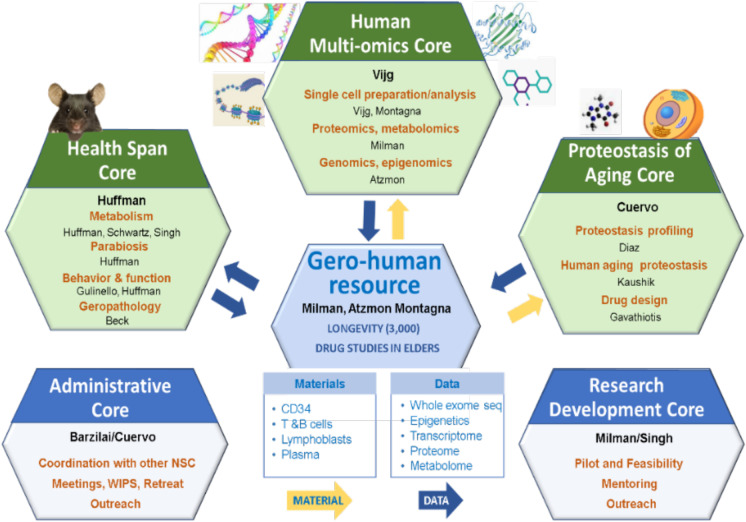
The three E-NSC Research Resource Cores (Fig. 1) focus on the development of innovative methodologies and procedures, not offered as services elsewhere. This allows each Core to shine as a unique resource for the aging research community and is the main reason behind the higher than predicted utilization. In fact, the Cores have provided help to 167 investigators within the last 4 years. Additionally, in the face of challenges to validation of published studies, it is even more important to provide investigators with unbiased (blinded) high-quality services that enable standardization of procedures and use of internal quality controls that are not likely to be challenged.
Fig. 1.
Cartoon depicting the integration between the unique resources and the specialized core services offered by the E-NSC
In the following sections, we describe newly launched technologies emanating from our three Research Resource Cores, which offer approaches to study novel selective forms of autophagy, a drug development service, approaches to assess health span measures such as mobility, cognition, and geropathology, and access to/assistance with analysis and interpretation of omics data from human studies. Not described here, but also central to the E-NSC mission, are the Research Development Core (RDC) and the Administrative Core (ADM) that contribute to the enrichment program provided via the assistance of personnel from the Research Resource Cores (Fig. 1). These cores coordinate efforts to provide a rich mentoring environment, excellent sources of advice, and opportunities to incentivize collaboration within Einstein and with both national and international research groups.
Research Cores
Proteostasis of Aging Core (PAC)
Core contacts:
Ana Maria Cuervo: ana-maria.cuervo@einsteinmed.org
Susmita Kaushik: susmita.kaushik@einsteinmed.org
Evripidis Gavathiotis: evripidis.gavathiotis@einsteinmed.org
Loss of protein homeostasis (proteostasis) is a common feature in most cells and tissues from old organisms and has been recognized as one of the pillars of aging [60]. Alterations in proteostasis have been described in multiple age-related disorders and are tightly linked to additional mechanisms of aging such as molecular damage, cellular response to stress, metabolism, inflammation, and altered stem cell function [45]. Genetic interventions in invertebrates and mammals now support the beneficial effects of modulating components of the intracellular pathways that maintain chaperone function/proteolytic systems and proteostasis on extension of life and health span [45]. Translation of these findings into humans will require (a) characterization of changes in proteostasis in old organisms, (b) an understanding of the molecular mechanisms behind proteostasis loss during aging, and (c) extensive testing of the effect of chemical and natural compounds on the proteostasis machinery and their development into drugs in the future (Fig. 2). The Proteostasis of Aging Core (PAC) was created in response to these needs of the aging research community and, during its 12 years in operation, has been providing reliable and highly validated services, state-of-the-art methodology, expertise, and a cutting-edge knowledge base related to proteostasis to both internal and external E-NSC members and the aging research groups worldwide.
Fig. 2.
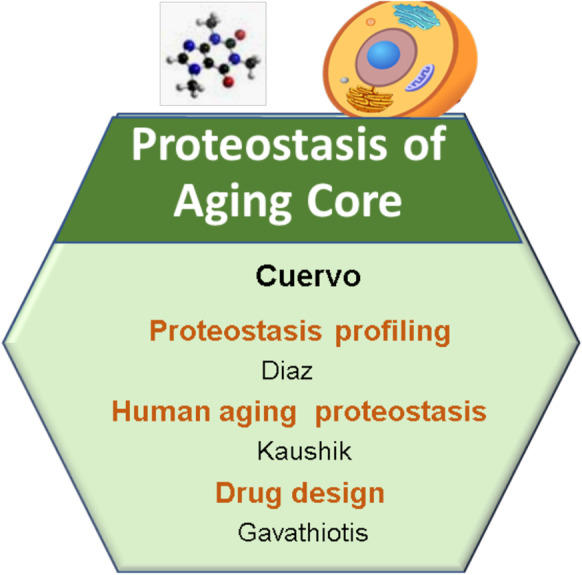
Cartoon depicting the structure of the Proteostasis of Aging Core (PAC) at the Einstein-Nathan Shock Center (E-NSC)
Services provided
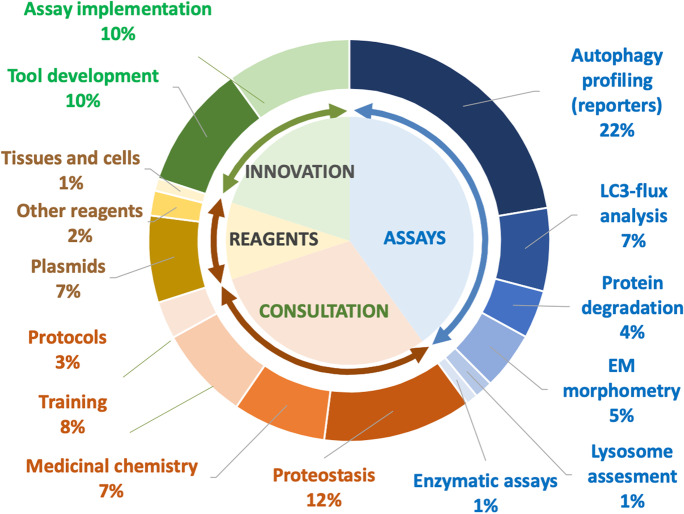
The PAC activities are divided into four groups: (a) assays performed at the Core (40%), (b) use of experimental tools and reagents (10%), (c) consultations and training (30%), and (d) innovation (20%) (Fig. 3). We next describe resources and procedures offered and the mechanisms in place to monitor use/performance and to accommodate demand.
Fig. 3.
Cartoon depicting the diverse services offered by the Proteostasis of Aging Core (PAC) of the Einstein-Nathan Shock Center (E-NSC)
Assays
The PAC offers six different types of assays related to the study of cellular proteostasis that, due to space limitations, are only briefly discussed. Additional details and fee structure, including discounts and charges can be found on https://www.einsteinmed.org/centers/aging/centers-of-excellence/nathan-shock/proteostatis-of-aging-core/.
Autophagy profiling
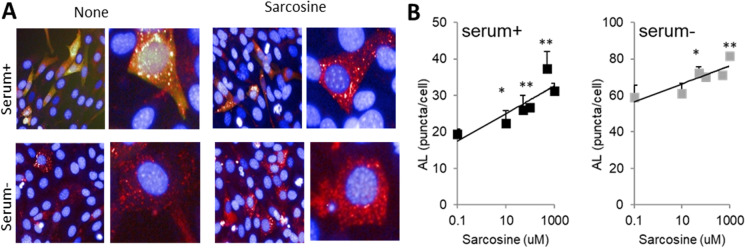
Abundant evidence links autophagy activity to aging and longevity. Autophagy is required to attain maximal longevity in long-lived invertebrate mutants in worms [32, 43, 65]. In fact, overexpression of autophagy components increases life and health span in both invertebrates and mice [38, 73, 80]. Additional evidence of this association comes from studies showing autophagy malfunction in multiple tissues from an older organism [76, 78], and that autophagy blockade leads to phenotypes of accelerated aging [33, 47, 69, 78]. In addition, autophagy is also often the target of pathogenic proteins that cause disease. Hence, accurate assessment of autophagy is central to understanding the contribution of proteostasis failure to onset of aging. To this end, measurement of steady-state levels of autophagic markers only provides limited information, making it necessary to use functional autophagy activity assays [50]. Functional reporters require specific instrumentation (for photo-switching) and complex analysis, making them inaccessible for most investigators. The PAC is the only Core worldwide that performs comprehensive functional analysis of the three main types of autophagy in mammals, by leveraging tools developed and implemented by the Core Director’s group [13, 51]. The Core adapted the latest available generation of autophagy reporters to high content microscopy (HCM) and multi-well plate format to allow testing of multiple replicates and conditions. As an example, the comprehensive nature of the autophagic assays offered by the core was critical in identifying a novel macroautophagy activator, sarcosine, whose circulating levels decrease with age, and can be induced by anti-aging interventions, e.g., dietary restriction (Fig. 4) [88]. Similar approaches helped demonstrate that disulfiram, a drug that reduces metabolic disfunction in middle-aged mice [9], or lonafarnib, a farnesyltransferase inhibitor that reduces tau pathology in mice [39], mediates these benefits by activating three distinct types of autophagy in mammalian cells.
-
a
Macroautophagy is measured using the mcherry-GFP-LC3 reporter that enables identification of autophagosomes as yellow puncta where the red and green fluorophores colocalize and autolysosomes are red-only puncta [50] (Fig. 4). The PAC generated the reporter on a lentiviral backbone that has been dispatched to laboratories worldwide. In addition, the PAC has developed a modified version of an immunoblot-based LC3 flux assay that provides information on LC3 synthesis, degradation, and overall flow [83]. Depending on the user group’s expertise with small protein immunoblots, the Core provides the pertinent antibody and protocol or performs the analysis for the investigator.
-
b
Chaperone-mediated autophagy (CMA): The PAC has implemented for HCM, photo-switchable reporters for CMA, generated by the Cuervo lab. In these reporters, the CMA-targeting motif recognized by hsc70 for lysosomal targeting is in frame with PS-CFPs1 or PS-Dendra1 fluorescent proteins that change from green to blue or green to red, respectively, upon exposure to a 405-nm LED light [51]. This color switch allows for pulse and chase-type experiments to measure CMA dynamics in response to diverse test conditions.
-
c
Endosomal microautophagy (eMI): The PAC has adapted to HCM, the eMI reporter developed by the Cuervo lab [13]. This reporter uses the N-terminus and C-terminus halves of the Venus fluorescent protein tagged with the KFERQ-motif, which is recognized by hsc70 for eMI targeting. Only when both parts of the protein coincide in the confined space of multivesicular bodies in late endosomes will they fluoresce, allowing measurement of eMI as the number of fluorescent puncta per cell, and eMI degradation as the increase in puncta in the presence of inhibitor of acid proteolysis, leupeptin.
-
d
Other autophagy and proteolysis variants: The PAC has also been established for HCM, a mitophagy reporter, mitokeima, based on dual signals in mitochondria and the quenching of the green signal upon delivery to an acidic compartment [44]. In addition, the PAC is working to implement reporters for ER-phagy and lipophagy in the new award period. The Degron reporter was also adapted to HCM for analysis of ubiquitin/proteasome degradation calculated as the lactacystin-induced gain in fluorescence intensity. For this reporter assay, UPS-resistant degron (FS) serves as the negative control [26].
Fig. 4.
Effect of sarcosine on macroautophagy. Mouse fibroblasts expressing mCherry-GFP-LC3 were exposed to the indicated concentrations of sarcosine for 16 h in the absence or presence of serum. A Representative images (500 μM sarcosine). B Quantification of autophagic flux (number of autophagosomes matured into autolysosomes) at indicated sarcosine concentrations. Values are mean + s.e.m. n = 6 *P < 0.05, **P < 0.01. (Image from [88]
Protein turnover
Changes in rates of protein synthesis and degradation are a good indicator of cellular proteostasis and the function of the proteolytic systems responsible for quality control [46, 50]. Measurement of protein turnover requires radiolabeled amino acids and large equipment, in particular, the scintillation counter, not typically available in investigator laboratories. The PAC can measure protein synthesis as the incorporation of 3H-valine into proteins (acid precipitable 3H) [2], and degradation rates of short (30-min pulse) and long-lived protein s (48-h pulse) by quantifying acid precipitable radioactivity (protein) transformed into acid-soluble radioactivity (peptide and amino acids) [46]. Pharmacological inhibitors for lysosomes (leupeptin), macroautophagy (3-methyladenine), or proteasomes (lactacystin) allow determination of their contribution to total proteolysis [50]. Examples of the use of this methodology to successfully differentiate blockage from enhanced autophagy can be found in the following publications [8, 20, 24, 39, 71, 74, 88, 92]:
Electron microscopy and morphometry
This service combines consulting time regarding experimental design at the beginning of the experiment and assistance with analysis of results (Fig. 3). However, the experimental procedures are performed by the Einstein Analytical Image Core facilities in the case of internal E-NSC investigators, or their respective institutional electron microscopy services. The investigators submit the electron micrographs of their samples to the Core, and the technician and director provide either annotations of several of the images with examples of different autophagic profiles and instructions on how to perform area and volume quantification (60% of cases) or a complete analysis of the whole set of micrographs (40% of cases). Investigators request this service to complement results with other procedures, for example, functional analysis.
Lysosomal function
The PAC also offers comprehensive testing of lysosomal function through LysoTracker labeling to determine changes in size, number, and acidification of lysosomes and endosomes. In addition, the PAC offers assessments of a panel of lysosomal markers to determine levels and maturation states of cathepsins B, D, and L, which are proteases inside lysosomes and levels of lysosomal membrane proteins, LAMP1, LAMP2A, and LAMP2B, as well as levels of two V-ATPase proton pump subunits. The PAC also provides a “lysosome-antibody sampler” with 2–5 ul of each antibody, 7 ul of a lysosomal extract as a positive control, and instructions for sample preparation and analysis. Since immunoblots are routine procedures in wet labs, the PAC typically provides the kit but will also perform the assay in < 10% of the cases.
Enzymatic assays
PAC performs or offers reagents for measurement of activity of lysosomal proteolytic enzymes, cathepsins, assayed by colorimetry or fluorometry to track degradation of a peptide substrate at acidic pH. The PAC will also determine proteasome catalytic activities in tissue homogenates and cell lysates by standard fluorometric procedures using amino-methyl-coumarin (AMC)-conjugated substrates.
Reagents and samples
The PAC offers the following reagents: (a) plasmids (or lentiviral particles) for expression of each of the autophagy reporters (see above), components of the autophagy machinery (i.e., LAMP2A), and validated shRNA probes for depletion of key autophagy components (i.e., Atg7, LAMP2A, VPS4A/B); (b) antibodies as individual antibodies or the sampler kit reported above; (c) dyes for lysosomes (Lysotracker and Lysosensor) and mitochondria (MitoTracker) highlighted by direct fluorescence; (d) tissues from the collection of different-aged mice used for age-related studies in the Cuervo lab. The PAC technician participates in dissections of these animals obtained from the NIA colony and collects, stores, and catalogs any tissue not used in the experiment; and (e) cells such as IMR-90 cells at different passage and skin ear fibroblasts from mice of different ages relevant for aging research.
Consulting and training
Consulting activities are divided between the Core Director, Dr. Ana Maria Cuervo, the Core Proteostasis Consultant, Dr. Susmita Kaushik, and the Core Technician, depending on the nature of the consultations. Advice in experimental planning and design, complex data interpretation, and follow-up studies are provided by the Core Director and/or Core Proteostasis Consultant (70% by e-mail communication and 30% in person). The Proteostasis Consultant also advises on technical aspects of preparation of samples, controls, general protocols, and interpretation of routine experiments. The Technician assists with standard troubleshooting and routine questions. The PAC also includes consulting with the PAC Drug Design Consultant Dr. Evripidis Gavathiotis who assists with the experimental design of studies using interventions with chemical compounds, queries related to controls and data quality, recommendations for compound synthesis and validation, interpretation of results, and follow-up efforts to develop compounds as drugs, i.e., standard medicinal chemistry design, advice on resources for pharmacokinetic, and toxicity studies. Training is also provided on a one-to-one basis by the Core Technician after initial discussions with the Core Director have established a clear series of training objectives. Finally, the PAC often presents school-wide half-day workshops on methods for autophagy analysis and webinars for the assessment of proteostasis.
Health Span Core (HSC)
Core contacts:
Derek Huffman PhD: derek.huffman@einsteinmed.org
Gary J. Schwartz, PhD: gary.schwartz@einsteinmed.org
Rajat Singh, MD, MBBS: rajat.singh@einsteinmed.org
Maria Gulinello, PhD: maria.gulinello@einsteinmed.org
Amanda Beck, DVM: amanda.beck@einsteinmed.org
The HSC provides unique and sophisticated whole-animal physiologic measurements, specialized surgical procedures, and advisement in evaluating healthy aging phenotypes to investigators. As Core development has been a major point of emphasis, the HSC has worked to increase the range of capabilities in order to maximize Core use and meet investigator needs in the field with rigorous and innovative techniques. The HSC has long-standing expertise in performing sophisticated in vivo metabolic phenotyping studies, hormonal assays, specialized surgeries, and parabiosis services, by providing both surgical services and a related heterochronic parabiosis tissue repository to the research community (Fig. 5).
Fig. 5.
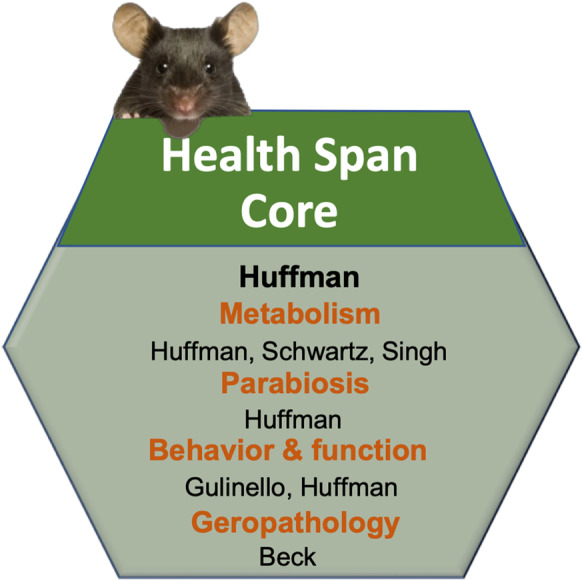
Cartoon depicting the structure of the Health Span Core (HSC) at the Einstein-Nathan Shock Center (E-NSC)
Services provided
The activities of the HSC are divided into three groups: (a) experimental assays (70%), (b) consulting and training (15%), and (c) innovation (15%). We describe resources and procedures offered by the HSC including specialized expertise in place to ensure appropriate oversight of our extensive offerings.
Assays
The HSC offers a broad array of assays involving specialized surgeries, tissue resources, and health span assessments in aging rodent models. Below provides a brief overview of these services. For a more comprehensive list of procedures, including discounts and charges, please visit https://www.einsteinmed.org/centers/aging/centers-of-excellence/nathan-shock/health-span-core/.
A. Parabiosis
The concept of cell autonomous and cell non-autonomous contributors to cellular and tissue aging has gained traction in recent years, and this notion has been buoyed in part via heterochronic parabiosis studies [12, 16, 17, 25, 40, 59, 67, 70, 75, 81, 87]. Despite its obvious utility and appeal, barriers prevent the implementation of this approach in many laboratories. Thus, we leveraged our surgical expertise in the HSC to address an important gap in the field by establishing parabiosis as a Core service. The surgery is performed similarly as described with slight modifications [34–37]. Our standard paradigm involves generating isochronic (Young-Young, Old-Old) and heterochronic (Young-Old) pairs for investigators utilizing C57BL/6 mouse model from the NIA colony [67]. We typically perform the surgical union in 4–5-month-old young mice, an age when long bones are nearly developed, and in 18–19-month-old mice. Beyond our standard scheme, inclusion of transgenic mice in the design, particularly pairing knockout mice to controls, allows investigators to go beyond descriptive studies to employ an approach, we refer to as “parabiotic rescue,” to isolate the effects of candidate geronic factors or pathways involved in the transposition of the aged phenotype. We also perform sham control pairings in which strain, age, and sex-matched animals undergo a similar surgical procedure and post-operative care as parabionts, but animals are not attached to control for potential effects of co-housing. Animals are then typically remained joined for ~ 8 weeks and tissues from C57BL/6 male and female isochronic and heterochronic mice, respectively, including heart, liver, skeletal muscle, kidney, gut adipose tissue, and specific brain regions are archived in a tissue bank that is available to investigators upon request [49, 57, 67, 91].
In addition to standard unions between wild-type mice, “custom” parabiosis studies are also available to outside investigators. Upon appropriate Institutional approvals, the HSC can accept mice after release from quarantine from approved animal facilities for requested studies. Such efforts are carefully coordinated in advance with HSC availability to ensure studies are performed in a time-sensitive manner. Parabiosis and post-operative care are then performed by HSC staff, and tissues are collected and processed as requested and shipped to the investigator.
B. In vivo metabolism
Insulin resistance is typical of mammalian aging, and has been associated with other co-morbidities, justifying the need for HSC services to assess glucose metabolism, and facilitate discovery of interventions that improve insulin action. The clamp technique, in combination with glucose tracer methodology, was developed in rats at Einstein by Barzilai and others [4–7, 15, 19, 22, 22, 23, 23, 30, 30, 31, 31, 62, 68], and is considered the “gold standard” for assessment of insulin sensitivity. Whole-body insulin action is routinely determined in mice and rats with similar plasma insulin levels and characterized in major insulin-responsive organs such as muscle, liver, and adipose tissue. For some investigators, access to blood sampling alone via an indwelling line is an important service. Once body weight returns to within 3% of pre-surgical weight, clamps are performed in awake, unstressed, and chronically catheterized rodents. Insulin sensitivity in hepatic and peripheral tissues is determined in the context of euglycemia via glucose infusion in presence of basal or similar physiological increases in plasma insulin. This procedure lends itself to co-infusion of other peptides, or performance of hyperglycemic clamps to establish insulin secretion, and to studies utilizing hypothalamic or intracerebroventricular (ICV) probes [14, 41, 63]. Typical clamp results provided to the investigator include glucose infusion rate (GIR), hepatic glucose production (HGP), and peripheral glucose uptake (Rd). Plasma concentrations of insulin, glucose, free fatty acids, and glucose-specific activity are obtained regularly during studies. These assays are made possible by expert technicians highly skilled in microvascular surgery and stereotactic placement of cannulas targeting the CNS in young and aged rodents. Furthermore, because clamps are a terminal procedure, some investigators may elect instead for glucose or insulin tolerance tests to enable metabolic testing for longitudinal studies, and these services are provided as well.
C. Energetics
In vivo assessment of body composition and energy balance is performed with guidance from Dr. Gary Schwartz, who is the Director of the Diabetes Research Center Animal Physiology Core and has successfully assisted with multiple investigators [1, 10, 11, 48, 79],van [85, 95]. Body composition can be assessed by quantitative magnetic resonance (qMR, Echo 3-in-1 Composition Analyzer, Echo Medical Systems, Houston, TX) in rats or mice for determining lean body mass (LBM) and fat mass (FM) without sedation. Body fat distribution can be measured longitudinally and non-invasively by microCT or directly determined at sacrifice by weighing individual fat depots. Data provided to investigators includes qMR, body mass, LBM, and FM, and for microCT, relative fat volume (e.g., subcutaneous vs. visceral fat). Energy metabolism, feeding behavior, and locomotion studies are performed in Oxymax CLAMS systems for rats and mice, respectively [21, 64, 88]. Data generated include energy expenditure (EE; absolute and LBM adjusted), substrate utilization, activity levels, and feeding behavior. EE is determined from O2 consumption and CO2 production using the equation of Lusk [61], and substrate utilization is calculated as the respiratory exchange ratio (RER; VCO2/VO2). Data provided to investigators include EE, RER, activity, and food intake during light, dark, and total over 24 h, as well as fasting and re-fed challenges upon request.
D. Behavioral phenotyping
Cognitive and behavioral assessments are performed with close advisement from Dr. Maria Gulinello, who directs the Einstein Neuroscience Behavioral Core, and oversees training of the HSC technical staff [18, 28, 29, 58, 84, 86, 89]. A representative number of tests are described here, but the HSC offers several other additional tests upon request. For instance, locomotor activity, anxiety, and exploration are performed by the Open Field test. Cognitive function is assessed by Object Recognition (OR) and Object Placement (OP) tests. Spatial working memory is assessed by spontaneous alternation during the Y-maze test. Depressive-like behavior is determined by the Porsolt forced swim test. Anxiety is assessed by the Zero Maze or Elevated Plus Maze. Startle and Pre-pulse Inhibition are employed to assess acoustic startle reflex, for which decrements have been shown in aging.
E. Physical function
Coordination, strength, stamina, and health status are comprehensively evaluated in mice using a battery of assays. Balance beam is used to assess gross motor coordination. Mice are first familiarized with walking across a 4-ft plank prior to being tested for their ability to cross 4-ft-long round beams of increasing difficulty (1″ easy; 0.75″ medium, 0.5″ difficult) with light and food cues as motivation to cross. The number of slips while crossing the beam are counted and recorded [21, 64]. Rotarod is available as an alternative assay. In addition, fine motor coordination is assessed by the tape removal test. Exercise capacity is determined by a single maximal forced exercise test to voluntary fatigue on a motorized treadmill (Exer 3/6, Columbus Instruments) as described [42]. Mice are first familiarized to the treadmill for three non-consecutive days and then challenged with a graduated fatigue test that is customized for a given strain and age. Grip strength is assessed by either wire hang or tail-suspension grip test. Frailty is assessed using a 31-point mouse clinical frailty index as previously described [90].
F. Geropathology and histopathology
Geropathology and histopathology of multiple tissues, resulting in a composite lesion score (CSL) for each examined tissue, is offered to investigators in conjunction with the Einstein Histopathology Core led by Dr. Amanda Beck, a board-certified veterinary pathologist, using the Geropathology Grading Platform (GGP). The GGP has already been demonstrated to detect marked age-sensitive increases in the CSL for heart, lungs, kidney, and liver among other tissues [53–55, 82].
Human Multi-Omics Core (HMOC)
Core contacts:
Jan Vijg, PhD: jan.vijg@einsteinmed.org
Alex Maslov, MD: alex.maslov@einsteinmed.org
Sofiya Milman, MD, MS: sofiya.milman@einsteinmed.org
A large part of the rationale for the HMOC is dictated by three major questions in the research base of Einstein’s Institute of Aging: (a) Are the mechanisms for longevity discovered in animal models relevant to humans? (b) What are the proteomic fingerprints of chronological aging? and (c) Can we capture changes in the biology of aging in humans treated with aging-targeting interventions? Resolving these three questions requires extensive multi-omics analyses; thus, the HMOC assists in the design, performance, and data analyses necessary for carrying out such studies. To facilitate answering these research questions, we utilize our two unique human longevity cohorts, (i) Longevity Genes Project (LGP), a cross-sectional study of more than 600 centenarian families who are generally free of age-related diseases and controls without familial longevity [3], and (ii) LonGenity, a longitudinal study of over 1200 subjects, half of who are offspring of centenarians and are enriched with longevity genes [27]. Furthermore, we are expanding and diversifying our human resources with another cohort of 1000 centenarians over the next 2 years to include the most robust collection and resource for translational longevity. Additionally, a younger cohort was established more recently, LifeLong, which banks samples from individuals age 20–59 and serves as a repository of young control samples. To provide these services, the HMOC does not perform actual experimentation and data collection because the high cost of such services would limit us to very few users and would not be cost-effective. Instead, we provide advice, share data, and provide samples from our large cohort repositories of human age– and longevity-related samples.
Multi-omics as a discipline to advance the study of healthy aging and longevity is entirely based on physiological longevity control systems, including a series of molecular and cellular defense and stress response systems that are generally upregulated in organisms with longer life spans, e.g., extremely long-lived species, long-lived strains of the same species, organisms subjected to dietary restriction or dampened insulin/IGF-1 or mTOR signaling, and humans at extremely old age (i.e., centenarians and super-centenarians) [66, 93, 94]. One of the major questions in the basic biology of aging is how these systems are regulated and why they eventually break down, resulting in the bewildering series of structural and functional changes that increase disease incidence and eventually bring life to a close. During the past few years, it has become clear that high-throughput, global analysis systems are critically important to unravel this network of pro-longevity systems. Currently, most of these large-scale biology platforms are based on next-generation sequencing (NGS) and offered as Core services at major biomedical research centers or are available commercially. Einstein has a particularly well-developed Core infrastructure in genomics and its sub-disciplines of epigenomics and transcriptomics. While access to NGS-based assays is available at many places, there is a serious lack of understanding as to the design and data analysis of genome-wide studies of aging and longevity. For example, to most investigators in the field, it is often not clear that due to limitations in coverage and annotation only a very small fraction of the many whole-genome sequences of a species can, in fact, be used to address specific comparative genomics of aging questions. In addition, even if both genetics Core services and technological know-how are available, data resources relevant for an aging or longevity study are typically lacking. Hence, the HMOC fills a gap in our current technology base for aging and longevity research by providing expert advice, technical support for special applications, and dedicated NGS-based Core services, including data analysis for a variety of high-throughput genomics applications, as well as primary cell and data resources (Fig. 6). It helps to greatly increase the efficiency of high-throughput molecular studies in aging and longevity research and could become instrumental in the integration of data across “omics” levels to gain an understanding of the functional variation in the genome and its impact on aging and disease.
Fig. 6.
Cartoon depicting the structure of the Human Multi-Omics Core (HMOC) at the Einstein-Nathan Shock Center (E-NSC)
Design of services
A. Human cell isolation and repository
Since 2016, our Einstein Glenn Center has funded the collection of live cells, DNA and RNA from the Ashkenazi Jewish (AJ) aging, and extreme longevity cohorts. This biorepository is maintained by the Department of Genetics’ Molecular Cytogenetics Core. The Core established protocols for the isolation of CD34 + as well as T and B-lymphocytes from fresh peripheral blood and banks lineage negative cells. In addition to enriched blood cell subtypes, this biobank stores peripheral mononuclear cells (PBMCs) from a subset of the cohort. All cell samples are cryopreserved in liquid nitrogen and can be accessed for experimental usage at any time upon approval by an internal committee. For all samples, genomic DNA and RNA is isolated on request and stored at − 80 °C. For selected blood samples, we establish Epstein-Barr Virus-transformed B lymphoblastoid cell lines (EBV-LCLs). The biobank currently houses samples collected from 1194 individuals ranging from 20 to 109 years of age. We provide cells, DNA or RNA for studies in young and old subjects, centenarians, and offspring with or without specific functional genotypes.
B. Shared use of multi-omics data on aging and longevity
A service proven to be extremely popular in the past is the in silico testing of candidate genes identified, for example, in invertebrate organisms, using our existing multi-omics data sets on human cohorts, including centenarians. We specifically focus on genetic associations of protective genomic factors with exceptional longevity in humans and resistance to age-related diseases in our human centenarian cohorts. It is critically important to test if the multiple conserved longevity pathways identified in model organisms are also active in human aging. In addition to the previously available SNP-based GWAS data, we now also have whole-exome sequences from our centenarian cohorts and can test for enrichment of rare variants in centenarians rather than common variants.
C. Transcriptomics in geroscience
The Einstein Glenn Center provides funding to conduct studies, the resources of which will be made available via the E-NSC HMOC. The overall goal is to implement well-designed human clinical studies to establish proof-of-concept and mechanism(s) of action of novel therapeutic agents, and to provide a framework for their subsequent development for therapeutic use in counteracting aging and age-related disease. To date, we have studied resveratrol [72], metformin [52], acarbose, and exercise, obtaining physiological/clinical, transcriptomic, and some metabolomic data. RNA analysis of these samples shows minimal degradation, as measured by RNA Integrity Number (RIN > 7), when processed for library preparation and sequenced on the Illumina HiSeq 2500 using a paired-end 101 BP dual indexing protocol [72]. Raw sequence reads were preprocessed using WASP 3.0, an in-house pipeline, for quality control, trimming adapter sequences, etc. Transcriptome files and remnant samples are stored for additional analysis. We can establish upstream regulators of important pathways and identify specific transcripts in those pathways changing in human tissues.
D. Proteomics resources
Proteomics data for 1030 human older subjects from the LonGenity study is available for query through the HMOC. The proteomic data can be queried in association with offspring-control status, genotypes, and aging phenotypes, as the LonGenity cohort undergoes deep phenotyping with annual neurocognitive and functional assessments, collection of medical history, brain MRIs, cardiac CTs, and measures of autophagic flux [27, 56, 77],Zhang, Aleksic, et al. 2020). Thus, this study design allows not only correlation of protein levels with age, but also with genetic predisposition to longevity and healthy aging. This resource can and has been utilized to confirm the relevance of findings from in vitro and animal models of human aging. The Core curates the proteomic data, updates it regularly, and provides researchers with data from aging humans for their proteins of interest. As additional proteomic data for new subjects and longitudinal measurements is generated, it will be made available for Core users. Over the next 5 years, we plan to obtain additional proteomic measurements for at least 1400 subjects, 1000 of whom will be aged 100 and older.
Acknowledgements
The Nathan Shock Center of Excellence for the basic Biology of Aging (NIH NIA P30AG038072), the Einstein-Paul Glenn Foundation for Medical Research Center for the Biology of Human Aging
Author contribution
AMC, NB, DMH, JV, SM, and RS contributed to this manuscript.
Funding
The Einstein-Nathan Shock Center of Excellence for the basic Biology of Aging is supported by the NIH NIA grant P30AG038072.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
AMC is co-founder and board member of Selphagy Therapeutics (now a program of Life Biosciences LLC, MA) and consults for Generian Pharmaceuticals, Inc, Seed Therapeutics, Inc. and Cognition Therapeutics, Inc. NB, JV, DMH, SM, and RS have nothing to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aja S, Sahandy S, Ladenheim EE, Schwartz GJ, Moran TH. Intracerebroventricular CART peptide reduces food intake and alters motor behavior at a hindbrain site. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1862–R1867. doi: 10.1152/ajpregu.2001.281.6.R1862. [DOI] [PubMed] [Google Scholar]
- 2.Auteri JS, Okada A, Bochaki V, Dice JF. Regulation of intracellular protein degradation in IMR- 90 human diploid fibroblasts. J Cell Physiol. 1983;115:159–166. doi: 10.1002/jcp.1041150210. [DOI] [PubMed] [Google Scholar]
- 3.Barzilai N, Atzmon G, Schechter C, Schaefer EJ, Cupples AL, Lipton R, Cheng S, Shuldiner AR. Unique lipoprotein phenotype and genotype associated with exceptional longevity. JAMA. 2003;290:2030–2040. doi: 10.1001/jama.290.15.2030. [DOI] [PubMed] [Google Scholar]
- 4.Barzilai N, Banerjee S, Hawkins M, Chen W, Rossetti L. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J Clin Invest. 1998;101:1353–1361. doi: 10.1172/JCI485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barzilai N, Rossetti L. Relationship between changes in body composition and insulin responsiveness in models of the aging rat. Am J Physiol. 1995;269:E591–E597. doi: 10.1152/ajpendo.1995.269.3.E591. [DOI] [PubMed] [Google Scholar]
- 6.Barzilai N, Rossetti L. Age-related changes in body composition are associated with hepatic insulin resistance in conscious rats. Am J Physiol. 1996;270:E930–E936. doi: 10.1152/ajpendo.1996.270.6.E930. [DOI] [PubMed] [Google Scholar]
- 7.Barzilai N, Wang J, Massilon D, Vuguin P, Hawkins M, Rossetti L. Leptin selectively decreases visceral adiposity and enhances insulin action. J Clin Invest. 1997;100:3105–3110. doi: 10.1172/JCI119865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bejarano EJT, Murray X, Wang O, Pampliega DM, Yin B, Patel A, Yuste A, Wolkoff W and Cuervo AM. ‘Defective recruitment of motor proteins to autophagic compartments contributes to autophagic failure in aging’, Aging Cell. 2018;17(1):E12692. [DOI] [PMC free article] [PubMed]
- 9.Bernier M, Mitchell SJ, Wahl D, Diaz A, Singh A, Seo W, Wang M, Ali A, Kaiser T, Price NL, Aon MA, Kim EY, Petr MA, Cai H, Warren A, Di Germanio C, Di Francesco A, Fishbein K, Guiterrez V, Harney D, Koay YC, Mach J, Enamorado IN, Pulpitel T, Wang Y, Zhang J, Zhang L, Spencer RG, Becker KG, Egan JM, Lakatta EG, O'Sullivan J, Larance M, LeCouteur DG, Cogger VC, Gao B, Fernandez-Hernando C, Cuervo AM, de Cabo R. Disulfiram treatment normalizes body weight in obese mice. Cell Metab. 2020;32:203–14.e4. doi: 10.1016/j.cmet.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bi S, Ladenheim EE, Schwartz GJ, Moran TH. A role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R254–R260. doi: 10.1152/ajpregu.2001.281.1.R254. [DOI] [PubMed] [Google Scholar]
- 11.Blouet C, Ono H, Schwartz GJ. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab. 2008;8:459–467. doi: 10.1016/j.cmet.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 13.Caballero BY, Wang A, Diaz I, Tasset YR, Juste B, Stiller EM, Mandelkow E, Mandelkow and Cuervo AM. ‘Interplay of pathogenic forms of human tau with different autophagic pathways’, 2018;17(1):E12692. [DOI] [PMC free article] [PubMed]
- 14.Carey M, Lontchi-Yimagou E, Mitchell W, Reda S, Zhang K, Kehlenbrink S, Koppaka S, Maginley SR, Aleksic S, Bhansali S, Huffman DM, Hawkins M. Central KATP channels modulate glucose effectiveness in humans and rodents. Diabetes. 2020;69:1140–1148. doi: 10.2337/db19-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cases JA, Barzilai N. The regulation of body fat distribution and the modulation of insulin action. Int J Obes Relat Metab Disord. 2000;24(Suppl 4):S63–S66. doi: 10.1038/sj.ijo.0801508. [DOI] [PubMed] [Google Scholar]
- 16.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 17.Conboy MJ, Conboy IM, Rando TA. Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell. 2013;12:525–530. doi: 10.1111/acel.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai M, Reznik SE, Spray DC, Weiss LM, Tanowitz HB, Gulinello M, Desruisseaux MS. Persistent cognitive and motor deficits after successful antimalarial treatment in murine cerebral malaria. Microbes Infect. 2010;12:1198–1207. doi: 10.1016/j.micinf.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Einstein FH, Fishman S, Bauman J, Thompson R, Atzmon G, Barzilai N, Muzumdar RH. Activation of a nutrient-sensor, contributes to the insulin resistance and inflammatory state of aging: primary role for the hexosamine biosynthetic pathway in the biological phenotype of aging. FASEB J. 2008;22:3450–3457. doi: 10.1096/fj.08-109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esteban-Martinez L, Sierra-Filardi E, McGreal RS, Salazar-Roa M, Marino G, Seco E, Durand S, Enot D, Grana O, Malumbres M, Cvekl A, Cuervo AM, Kroemer G, Boya P. Programmed mitophagy is essential for the glycolytic switch during cell differentiation. EMBO J. 2017;36:1688–1706. doi: 10.15252/embj.201695916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farias Quipildor GE, Mao K, Hu Z, Novaj A, Cui MH, Gulinello M, Branch CA, Gubbi S, Patel K, Moellering DR, Tarantini S, Kiss T, Yabluchanskiy A, Ungvari Z, Sonntag WE, Huffman DM. Central IGF-1 protects against features of cognitive and sensorimotor decline with aging in male mice. Geroscience. 2019;41:185–208. doi: 10.1007/s11357-019-00065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, Scherer P, Rossetti L, Barzilai N. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002;51:2951–2958. doi: 10.2337/diabetes.51.10.2951. [DOI] [PubMed] [Google Scholar]
- 23.Gabriely I, Ma XH, Yang XM, Rossetti L, Barzilai N. Leptin resistance during aging is independent of fat mass. Diabetes. 2002;51:1016–1021. doi: 10.2337/diabetes.51.4.1016. [DOI] [PubMed] [Google Scholar]
- 24.Gong Z, Tasset I, Diaz A, Anguiano J, Tas E, Cui L, Kuliawat R, Liu H, Kuhn B, Cuervo AM, Muzumdar R. Humanin is an endogenous activator of chaperone-mediated autophagy. J Cell Biol. 2018;217:635–647. doi: 10.1083/jcb.201606095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gontier G, Iyer M, Shea JM, Bieri G, Wheatley EG, Ramalho-Santos M, Villeda SA. Tet2 rescues age-related regenerative decline and enhances cognitive function in the adult mouse brain. Cell Rep. 2018;22:1974–1981. doi: 10.1016/j.celrep.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greussing R, Unterluggauer H, Koziel R, Maier AB and P J-D. ‘Monitoring of ubiquitin-proteasome activity in living cells using a Degron (dgn)-destabilized green fluorescent protein (GFP)-based reporter protein’, J Vis Exp. 2012 [DOI] [PMC free article] [PubMed]
- 27.Gubbi S, Schwartz E, Crandall J, Verghese J, Holtzer R, Atzmon G, Braunstein R, Barzilai N, Milman S. Effect of exceptional parental longevity and lifestyle factors on prevalence of cardiovascular disease in offspring. Am J Cardiol. 2017;120:2170–2175. doi: 10.1016/j.amjcard.2017.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulinello M, Acquarone M, Kim JH, Spray DC, Barbosa HS, Sellers R, Tanowitz HB, Weiss LM. Acquired infection with Toxoplasma gondii in adult mice results in sensorimotor deficits but normal cognitive behavior despite widespread brain pathology. Microbes Infect. 2010;12:528–537. doi: 10.1016/j.micinf.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gulinello M, Chen F, Dobrenis K. 'Early deficits in motor coordination and cognitive dysfunction in a mouse model of the neurodegenerative lysosomal storage disorder. Sandhoff disease', Behav Brain Res. 2008;193:315–319. doi: 10.1016/j.bbr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta G, Cases JA, She L, Ma XH, Yang XM, Hu M, Wu J, Rossetti L, Barzilai N. Ability of insulin to modulate hepatic glucose production in aging rats is impaired by fat accumulation. Am J Physiol Endocrinol Metab. 2000;278:E985–E991. doi: 10.1152/ajpendo.2000.278.6.E985. [DOI] [PubMed] [Google Scholar]
- 31.Gupta G, She L, Ma XH, Yang XM, Hu M, Cases JA, Vuguin P, Rossetti L, Barzilai N. Aging does not contribute to the decline in insulin action on storage of muscle glycogen in rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R111–R117. doi: 10.1152/ajpregu.2000.278.1.R111. [DOI] [PubMed] [Google Scholar]
- 32.Hansen M, Chandra A, Mitic LL, Onken B, Driscoll M, Kenyon C. 'A role for autophagy in the extension of lifespan by dietary restriction in C. elegans.'. PLoS Genet. 2008;4:e24. doi: 10.1371/journal.pgen.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 34.Harris RB. Parabiosis between db/db and ob/ob or db/+ mice. Endocrinology. 1999;140:138–145. doi: 10.1210/endo.140.1.6449. [DOI] [PubMed] [Google Scholar]
- 35.Harris RB. Contribution made by parabiosis to the understanding of energy balance regulation. Biochim Biophys Acta. 2013;1832:1449–1455. doi: 10.1016/j.bbadis.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris RB, Hervey E, Hervey GR, Tobin G. Body composition of lean and obese Zucker rats in parabiosis. Int J Obes. 1987;11:275–283. [PubMed] [Google Scholar]
- 37.Harris RB, Martin RJ. Specific depletion of body fat in parabiotic partners of tube-fed obese rats. Am J Physiol. 1984;247:R380–R386. doi: 10.1152/ajpregu.1984.247.2.R380. [DOI] [PubMed] [Google Scholar]
- 38.Hars ES, Qi H, Ryazanov AG, Jin S, Cai L, Hu C, Liu LF. Autophagy regulates ageing in C. elegans. Autophagy. 2007;3:93–95. doi: 10.4161/auto.3636. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez I, Luna G, Rauch JN, Reis SA, Giroux M, Karch CM, Boctor D, Sibih YE, Storm NJ, Diaz A, Kaushik S, Zekanowski C, Kang AA, Hinman CR, Cerovac V, Guzman E, Zhou H, Haggarty SJ, Goate AM, Fisher SK, Cuervo AM and Kosik KS. ‘A farnesyltransferase inhibitor activates lysosomes and reduces tau pathology in mice with tauopathy’, Sci Transl Med. 2019;11. [DOI] [PMC free article] [PubMed]
- 40.Huang Q, Ning Y, Liu D, Zhang Y, Li D, Zhang Y, Yin Z, Fu B, Cai G, Sun X, Chen X. A young blood environment decreases aging of senile mice kidneys. J Gerontol A Biol Sci Med Sci. 2018;73:421–428. doi: 10.1093/gerona/glx183. [DOI] [PubMed] [Google Scholar]
- 41.Huffman DM, Farias Quipildor G, Mao K, Zhang X, Wan J, Apontes P, Cohen P, Barzilai N. Central insulin-like growth factor-1 (IGF-1) restores whole-body insulin action in a model of age-related insulin resistance and IGF-1 decline. Aging Cell. 2016;15:181–186. doi: 10.1111/acel.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huffman DM, Moellering DR, Grizzle WE, Stockard CR, Johnson MS, Nagy TR. Effect of exercise and calorie restriction on biomarkers of aging in mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1618–R1627. doi: 10.1152/ajpregu.00890.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jia K, Levine B. Autophagy is required for dietary restriction-mediated life span extension in C. elegans. Autophagy. 2007;3:597–599. doi: 10.4161/auto.4989. [DOI] [PubMed] [Google Scholar]
- 44.Katayama H, Kogure T, Mizushima N, Yoshimori T, Miyawaki A. A sensitive and quantitative technique for detecting autophagic events based on lysosomal delivery. Chem Biol. 2011;18:1042–1052. doi: 10.1016/j.chembiol.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Kaushik S, Cuervo AM. Proteostasis and aging. Nat Med. 2015;21:1406–1415. doi: 10.1038/nm.4001. [DOI] [PubMed] [Google Scholar]
- 46.Kaushik S, Cuervo AM. Methods to monitor chaperone-mediated autophagy. Methods Enzymol. 2009;452:297–324. doi: 10.1016/S0076-6879(08)03619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaushik S, Rodriguez-Navarro JA, Arias E, Kiffin R, Sahu S, Schwartz GJ, Cuervo AM, Singh R. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metab. 2011;14:173–183. doi: 10.1016/j.cmet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiss T, Tarantini S, Csipo T, Balasubramanian P, Nyul-Toth A, Yabluchanskiy A, Wren JD, Garman L, Huffman DM, Csiszar A, Ungvari Z. Circulating anti-geronic factors from heterochonic parabionts promote vascular rejuvenation in aged mice: transcriptional footprint of mitochondrial protection, attenuation of oxidative stress, and rescue of endothelial function by young blood. Geroscience. 2020;42:727–748. doi: 10.1007/s11357-020-00180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klionsky DJ et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8(4):445–544. [DOI] [PMC free article] [PubMed]
- 51.Koga H, Martinez-Vicente M, Macian F, Verkhusha VV, Cuervo AM. A photoconvertible fluorescent reporter to track chaperone-mediated autophagy. Nat Commun. 2011;2:386. doi: 10.1038/ncomms1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kulkarni AS, Brutsaert EF, Anghel V, Zhang K, Bloomgarden N, Pollak M, Mar JC, Hawkins M, Crandall JP and Barzilai N. ‘Metformin regulates metabolic and nonmetabolic pathways in skeletal muscle and subcutaneous adipose tissues of older adults’. Aging Cell. 2018;17(2):E12723. [DOI] [PMC free article] [PubMed]
- 53.Ladiges W. The emerging role of geropathology in preclinical aging studies. Pathobiol Aging Age Relat Dis. 2017;7:1304005. doi: 10.1080/20010001.2017.1304005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ladiges W. Pathobiology of aging and age-related diseases is the official journal of the Geropathology Research Network. Pathobiol Aging Age Relat Dis. 2019;9:1593786. doi: 10.1080/20010001.2019.1593786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ladiges W, Snyder JM, Wilkinson E, Imai DM, Snider T, Ge X, Ciol M, Pettan-Brewer C, Pillai SPS, Morton J, Quarles E, Rabinovitch P, Niedernhofer L, Liggitt D. A new preclinical paradigm for testing anti-aging therapeutics. J Gerontol A Biol Sci Med Sci. 2017;72:760–762. doi: 10.1093/gerona/glx019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lehallier B, Gate D, Schaum N, Nanasi T, Lee SE, Yousef H, Moran Losada P, Berdnik D, Keller A, Verghese J, Sathyan S, Franceschi C, Milman S, Barzilai N, Wyss-Coray T. Undulating changes in human plasma proteome profiles across the lifespan. Nat Med. 2019;25:1843–1850. doi: 10.1038/s41591-019-0673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lei C, Colangelo D, Patil P, Li V, Ngo K, Wang D, Dong Q, Yousefzadeh MJ, Lin H, Lee J, Kang J, Sowa G, Wyss-Coray T, Niedernhofer LJ, Robbins PD, Huffman DM, Vo N. Influences of circulatory factors on intervertebral disc aging phenotype. Aging (Albany NY) 2020;12:12285–12304. doi: 10.18632/aging.103421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y, Abdourahman A, Tamm JA, Pehrson AL, Sanchez C, Gulinello M. Reversal of age-associated cognitive deficits is accompanied by increased plasticity-related gene expression after chronic antidepressant administration in middle-aged mice. Pharmacol Biochem Behav. 2015;135:70–82. doi: 10.1016/j.pbb.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 59.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall'Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lusk, G. The elements of the science of nutrition. Nutrition Bulletin. 1976;3(6):401.
- 62.Ma XH, Muzumdar R, Yang XM, Gabriely I, Berger R, Barzilai N. Aging is associated with resistance to effects of leptin on fat distribution and insulin action. J Gerontol A Biol Sci Med Sci. 2002;57:B225–B231. doi: 10.1093/gerona/57.6.b225. [DOI] [PubMed] [Google Scholar]
- 63.Mann SN, Hadad N, Holte NM, Rothman AR, Sathiaseelan R, Mondal SA, Agbaga MP, Unnikrishnan A, Subramaniam M, Hawse J, Huffman DM, Freeman WM and Stout MB. ‘Health benefits attributed to 17alpha-estradiol, a lifespan-extending compound, are mediated through estrogen receptor alpha’. Elife. 2020;9:E59616. [DOI] [PMC free article] [PubMed]
- 64.Mao K, Quipildor GF, Tabrizian T, Novaj A, Guan F, Walters RO, Delahaye F, Hubbard GB, Ikeno Y, Ejima K, Li P, Allison DB, Salimi-Moosavi H, Beltran PJ, Cohen P, Barzilai N, Huffman DM. Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nat Commun. 2018;9:2394. doi: 10.1038/s41467-018-04805-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melendez A, Talloczy Z, Scaman M, Eskelinen EL, Hall DH, Levine B. Essential role of autophagy genes in dauer development and lifespan extension in C. elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- 66.Milman S, Barzilai N. Dissecting the mechanisms underlying unusually successful human health span and life span. Cold Spring Harb Perspect Med. 2015;6:a025098. doi: 10.1101/cshperspect.a025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morrison EJ, Champagne DP, Dzieciatkowska M, Nemkov T, Zimring JC, Hansen KC, Guan F, Huffman DM, Santambrogio L, and D'Alessandro A. ‘Parabiosis incompletely reverses aging-induced metabolic changes and oxidant stress in mouse red blood cells’. Nutrients. 2019;11(6):1337. [DOI] [PMC free article] [PubMed]
- 68.Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, Budagov T, Cui L, Einstein FH, Poduval A, Hwang D, Barzilai N, Cohen P. Humanin: a novel central regulator of peripheral insulin action. PLoS ONE. 2009;4:e6334. doi: 10.1371/journal.pone.0006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 70.Ozek C, Krolewski RC, Buchanan SM, Rubin LL. Growth differentiation factor 11 treatment leads to neuronal and vascular improvements in the hippocampus of aged mice. Sci Rep. 2018;8:17293. doi: 10.1038/s41598-018-35716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pajares M, Rojo AI, Arias E, Diaz A, Cuervo AM, and Cuadrado A. 'Transcription factor NRF2 modulates chaperonemediated autophagy through the regulation of LAMP2A'. Autophagy. 2018;14(8):1310–1322. [DOI] [PMC free article] [PubMed]
- 72.Pollack RM, Barzilai N, Anghel V, Kulkarni AS, Golden A, O'Broin P, Sinclair DA, Bonkowski MS, Coleville AJ, Powell D, Kim S, Moaddel R, Stein D, Zhang K, Hawkins M, Crandall JP. Resveratrol improves vascular function and mitochondrial number but not glucose metabolism in older adults. J Gerontol A Biol Sci Med Sci. 2017;72:1703–1709. doi: 10.1093/gerona/glx041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pyo JO, Yoo SM, Ahn HH, Nah J, Hong SH, Kam TI, Jung S, Jung YK. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat Commun. 2013;4:2300. doi: 10.1038/ncomms3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raz Y, Guerrero-Ros I, Maier A, Slagboom PE, Atzmon G, Barzilai N, Macian F. Activation-induced autophagy is preserved in CD4+ T-cells in familial longevity. J Gerontol A Biol Sci Med Sci. 2017;72:1201–1206. doi: 10.1093/gerona/glx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rebo J, Mehdipour M, Gathwala R, Causey K, Liu Y, Conboy MJ, Conboy IM. A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood. Nat Commun. 2016;7:13363. doi: 10.1038/ncomms13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 77.Sathyan S, Ayers E, Gao T, Weiss EF, Milman S, Verghese J, Barzilai N. Plasma proteomic profile of age, health span, and all-cause mortality in older adults. Aging Cell. 2020;19:e13250. doi: 10.1111/acel.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schneider JL, Cuervo AM. Liver autophagy: much more than just taking out the trash. Nat Rev Gastroenterol Hepatol. 2014;11:187–200. doi: 10.1038/nrgastro.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwartz GJ, Moran TH. Leptin and neuropeptide y have opposing modulatory effects on nucleus of the solitary tract neurophysiological responses to gastric loads: implications for the control of food intake. Endocrinology. 2002;143:3779–3784. doi: 10.1210/en.2002-220352. [DOI] [PubMed] [Google Scholar]
- 80.Simonsen A, Cumming RC, Brech A, Isakson P, Schubert DR, Finley KD. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4:176–184. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- 81.Smith LK, He Y, Park JS, Bieri G, Snethlage CE, Lin K, Gontier G, Wabl R, Plambeck KE, Udeochu J, Wheatley EG, Bouchard J, Eggel A, Narasimha R, Grant JL, Luo J, Wyss-Coray T, Villeda SA. beta2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med. 2015;21:932–937. doi: 10.1038/nm.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Snyder JM, Snider TA, Ciol MA, Wilkinson JE, Imai DM, Casey KM, Vilches-Moure JG, Pettan-Brewer C, Pillai PS, Carrasco SE, Salimi S and Ladiges W. ‘Validation of a geropathology grading system for aging mouse studies’. Geroscience. 2019;41(4):455–465. [DOI] [PMC free article] [PubMed]
- 83.Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- 84.Tome WA, Gokhan S, Gulinello ME, Brodin NP, Heard J, Mehler MF, Guha C. Hippocampal-dependent neurocognitive impairment following cranial irradiation observed in pre-clinical models: current knowledge and possible future directions. Br J Radiol. 2016;89:20150762. doi: 10.1259/bjr.20150762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, Elmquist J, Lowell BB, Barsh GS, de Luca C, Myers MG, Jr, Schwartz GJ, Chua SC., Jr Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vijayanathan V, Gulinello M, Ali N, Cole PD. Persistent cognitive deficits, induced by intrathecal methotrexate, are associated with elevated CSF concentrations of excitotoxic glutamate analogs and can be reversed by an NMDA antagonist. Behav Brain Res. 2011;225:491–497. doi: 10.1016/j.bbr.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 87.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walters RO, Arias E, Diaz A, Burgos ES, Guan F, Tiano S, Mao K, Green CL, Qiu Y, Shah H, Wang D, Hudgins AD, Tabrizian T, Tosti V, Shechter D, Fontana L, Kurland IJ, Barzilai N, Cuervo AM, Promislow DEL, Huffman DM. Sarcosine is uniquely modulated by aging and dietary restriction in rodents and humans. Cell Rep. 2018;25:663–76 e6. doi: 10.1016/j.celrep.2018.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wen J, Maxwell RR, Wolf AJ, Spira M, Gulinello ME, Cole PD. Methotrexate causes persistent deficits in memory and executive function in a juvenile animal model. Neuropharmacology. 2018;139:76–84. doi: 10.1016/j.neuropharm.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Whitehead JC, Hildebrand BA, Sun M, Rockwood MR, Rose RA, Rockwood K, Howlett SE. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2014;69:621–632. doi: 10.1093/gerona/glt136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yousefzadeh MJ, Wilkinson JE, Hughes B, Gadela N, Ladiges WC, Vo N, Niedernhofer LJ, Huffman DM, Robbins PD. Heterochronic parabiosis regulates the extent of cellular senescence in multiple tissues. Geroscience. 2020;42:951–961. doi: 10.1007/s11357-020-00185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang J, Johnson JL, He J, Napolitano G, Ramadass M, Rocca C, Kiosses WB, Bucci C, Xin Q, Gavathiotis E, Cuervo AM, Cherqui S, and Catz SD. ‘Cystinosin, the small GTPase Rab11, and the Rab7 effector RILP regulate intracellular trafficking of the chaperone-mediated autophagy receptor LAMP2A’. J Biol Chem. 2017;292(25):10328–10346. [DOI] [PMC free article] [PubMed]
- 93.Zhang WB, Aleksic S, Gao T, Weiss EF, Demetriou E, Verghese J, Holtzer R, Barzilai N and Milman S. ‘Insulinlike growth factor-1 and IGF binding proteins predict all-cause mortality and morbidity in older adults’. Cells. 2020;9(6):1368. [DOI] [PMC free article] [PubMed]
- 94.Zhang ZD, Milman S, Lin JR, Wierbowski S, Yu H, Barzilai N, Gorbunova V, Ladiges WC, Niedernhofer LJ, Suh Y, Robbins PD, Vijg J. Genetics of extreme human longevity to guide drug discovery for healthy ageing. Nat Metab. 2020;2:663–672. doi: 10.1038/s42255-020-0247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zizola CF, Schwartz GJ, Vogel S. Cellular retinol-binding protein type III is a PPARgamma target gene and plays a role in lipid metabolism. Am J Physiol Endocrinol Metab. 2008;295:E1358–E1368. doi: 10.1152/ajpendo.90464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.