Abstract
As the molecular mechanisms of biological aging become better understood, there is growing interest in identifying interventions that target those mechanisms to promote extended health and longevity. The budding yeast Saccharomyces cerevisiae has served as a premier model organism for identifying genetic and molecular factors that modulate cellular aging and is a powerful system in which to evaluate candidate longevity interventions. Here we screened a collection of natural products and natural product mixtures for effects on the growth rate, mTOR-mediated growth inhibition, and replicative lifespan. No mTOR inhibitory activity was detected, but several of the treatments affected growth rate and lifespan. The strongest lifespan shortening effects were observed for green tea extract and berberine. The most robust lifespan extension was detected from an extract of Pterocarpus marsupium and another mixture containing Pterocarpus marsupium extract. These findings illustrate the utility of the yeast system for longevity intervention discovery and identify Pterocarpus marsupium extract as a potentially fruitful longevity intervention for testing in higher eukaryotes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-021-00418-x.
Keywords: Lifespan-extending interventions, mTOR inhibitors, Natural products, Saccharomyces cerevisiae
Introduction
The field of geroscience has yielded important mechanistic insights into biological aging processes and their connection to disease [1], with invertebrate model organisms at the forefront of these discoveries [2]. Several evolutionarily conserved molecular “hallmarks” or “pillars” of aging appear to play a causal role in the cellular, tissue, and functional declines that accompany old age [3, 4]. As our understanding of aging biology evolves, a growing emphasis is now being placed on identifying interventions that target these hallmarks of aging [5]. Such interventions have the potential to delay the onset and progression of multiple age-related diseases simultaneously, with the goal of significantly extending both lifespan and healthspan.
The budding yeast Saccharomyces cerevisiae has been used extensively to characterize genetic and molecular mechanisms of cellular aging [6, 7]. Replicative lifespan (RLS) in yeast is defined as the number of daughter cells produced by a mother cell prior to irreversible cell cycle arrest [8]. Numerous studies over the past two decades indicate that many of the genetic and environmental determinants of aging in multicellular eukaryotes are shared with yeast during replicative aging. For example, studies in budding yeast contributed substantially to our understanding of the mechanisms by which caloric restriction [9, 10], sirtuins [11, 12], and mechanistic target of rapamycin (mTOR) [13, 14] signaling impact aging, and how conserved molecular processes including mitochondrial function [15–20], pH homeostasis [21–25], proteasome activity [26, 27], autophagy [28, 29], and genome instability [30, 31] all play important roles during yeast replicative aging.
Identifying pharmacological interventions that directly target the molecular mechanisms of biological aging and thereby increase healthspan and lifespan is a rapidly growing area of research [32]. The nematode Caenorhabditis elegans is used effectively in this regard, as evidenced by the fact that more than 50% of the experiments contained in the DrugAge database are performed in this organism [33, 34]. Budding yeast is the fourth most utilized model system (3.5%) in DrugAge, behind both fruit flies (26.2%) and mice (6%). While this frequency does not specify the magnitude or direction of effect on lifespan, it does provide an indication of the relative use of each model system for intervention studies within the field. Despite the fact that lifespan studies in yeast are less expensive and less time-consuming than studies in mice, or even fruit flies, the utility of this system as a discovery platform for pro-longevity interventions has lagged.
We recently described a system for identifying candidate pro-longevity compounds in yeast through analysis of high-resolution growth kinetics [35]. This system specifically detects compounds with either direct or indirect mTOR inhibitory effects by quantifying differential effects on growth rate in different genetic backgrounds. This system also successfully discriminates between allosteric rapamycin-like inhibitors and ATP-competitive catalytic inhibitors. From a small unbiased natural product screen [35], we identified caffeine as an mTOR inhibitor. This may explain its lifespan-extending properties in yeast [36] and worms [37, 38]. Here we describe lifespan and outgrowth analyses of additional natural products from a collection of natural product compounds, extracts, and mixtures. While no new mTOR inhibitory compounds were identified, we did observe significant lifespan extension from an extract of Pterocarpus marsupium, a tree found in Southeast Asia with a long history of use in Ayurvedic medicine.
Results
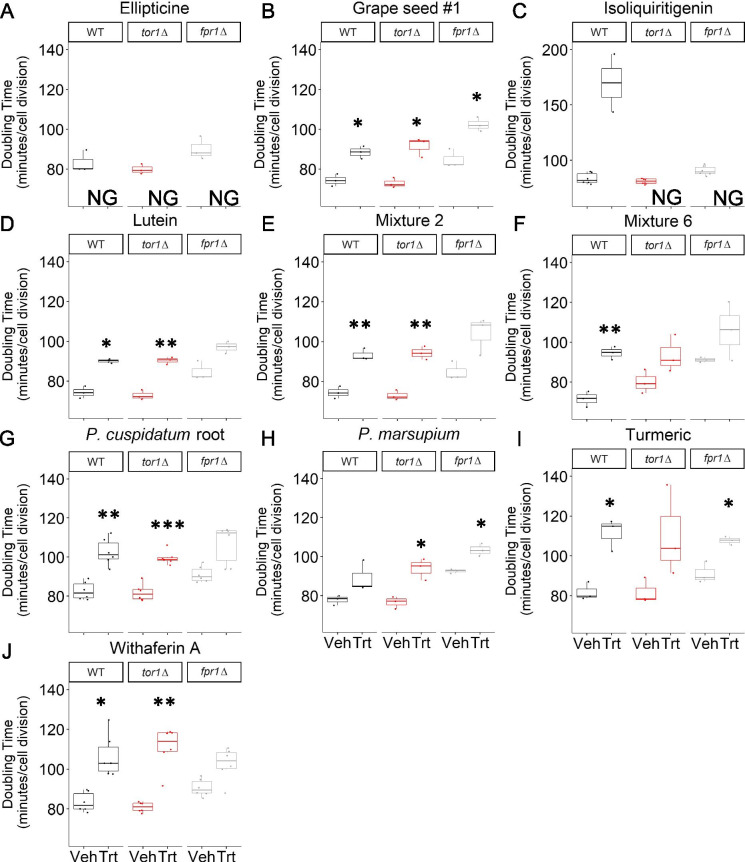
A screen of 42 natural product compounds, extracts (Table 1), and mixtures (Table 2) was conducted to understand their impact on growth, mTOR inhibition, and lifespan in yeast. Several of the tested compounds or mixture components were previously reported to impact lifespan in different organisms (Table 1). All compounds and mixtures were initially tested at a concentration of 100 μg/mL in a rich growth medium (YPD) (see Materials and Methods). The results of the growth analyses are summarized in Table 3 with outgrowth curves and doubling time analysis provided in Supplemental Fig. 1. None of the compounds or mixtures tested showed evidence for particular mTOR inhibitory activity in the initial screen, which is determined by greater growth inhibition in a tor1Δ strain relative to wild type and fpr1Δ [35]. Several compounds, extracts, and mixtures significantly inhibited growth in at least one strain, including grape seed (Indena), lutein, mixture 2, mixture 6, Polygonum cuspidatum root, Pterocarpus marsupium, turmeric, and withaferin A (Fig. 1). While not every strain reached statistically significant growth inhibition with these treatments, all strains showed a trend toward increased doubling time. Two compounds, ellipticine and isoliquiritigenin, had particularly pronounced effects on growth at 100 μg/mL. All of the cultures treated with ellipticine failed to achieve an OD of 0.3 after 20 h of growth and therefore fell below our threshold for doubling time quantitation (see Methods). For isoliquiritigenin, all strains were also severely growth inhibited at this concentration, with most cultures falling below our threshold for quantitative doubling time calculation and those that did grow to OD > 0.3 barely achieving this threshold, indicating severe inhibition (Fig. 1). To better understand the growth inhibitory effect of isoliquiritigenin, we performed a follow-up dose response (Table 4). Gradual growth inhibition was observed with increasing concentration of isoliquiritigenin from 1 to 50 μg/mL without evidence for mTOR inhibitory effects (Supplemental Fig. 1).
Table 1.
Natural product compounds and extracts used in this study along with reported lifespan extension. For compound
source information, see the “Methods” section
| Compound/extract | Lifespan extension |
|---|---|
| Allantoin | Worms [39] |
| Alpha-lipoic acid | Worms [40, 41], flies [42] |
| Ashwagandha root | Worms [43] |
| Berberine | Mice [44], flies [45] |
| Broccoli concentrate | - |
| Cardamonin | - |
| Celastrol | Worms [46] |
| Choline bitartrate | - |
| Coumaric Acid | - |
| Curcumin phytosome complex | - |
| Dong Quai | - |
| Ellipticine | - |
| Ginseng | - |
| Ginsenoside Rc | Worms [47] |
| Glucosamine | Mice and worms [48] |
| Grape seed #1 | - |
| Grape seed #2 | - |
| Green tea | Worms [49], flies [50, 51], mice – midlife effect in females [52] |
| Hesperidin | Yeast [53] |
| Hydroxytyrosol | - |
| Isoliquiritigenin | - |
| Licorice root | Worms [54] |
| Lutein | Flies [55] |
| Lycopene | - |
| Milk thistle | Worms [56] |
| N-acetyl L-cysteine | Worms [57], flies [58, 59], mice [60] |
| Olive fruit | - |
| Polygonum cuspidatum root | - |
| Pterocarpus marsupium | - |
| Quercetin | Worms [61, 62], flies [63] |
| Rutin | Flies [64], mice [65] |
| Turmeric | Flies [66] |
| Tyrosol | Worms [67] |
| Umbelliferone | - |
| Verbascoside | - |
| Withaferin A | Mice [68], flies [69] |
Table 2.
Natural product mixtures used in this study. Composition of mixtures is shown by weight. All mixtures provided by USANA Health Science Inc
| Mixture | Description |
|---|---|
| 1 | 40.4% Pterocarpus marsupium extract, 34.6% alpha-lipoic acid, 23.1% quercetin, 1.9% olive fruit extract |
| 2 | 22.7% alpha-lipoic acid, 19.2% hesperidin, 14.1% resveratrol, 12.1% curcumin phytosome complex, 11.7% green tea extract, 10.6% quercetin, 7.1% rutin, 2.5% olive fruit extract |
| 3 | 87.8% choline bitartrate, 4.5% coenzyme Q10, 4.2% lutein, 3.5% lycopene |
| 4 | 30.1% choline bitartrate, 19.0% milk thistle extract, 17.8% N-acetyl L-cysteine, 16.8% alpha-lipoic acid, 5.7% broccoli concentrate, 3.6% green tea extract, 3.4% turmeric extract, 1.9% biotin, 1.7% olive fruit extract |
| 5 | 86.1% glucosamine, 13.9% turmeric extract |
| 6 | 60% grape seed extract #1, 40% grape seed extract #2 |
Table 3.
Growth and mTOR inhibitor screen of natural product compounds, extracts, and mixtures. Doubling time (DT) (minutes/cell division), standard error of the mean (SEM), percent change compared to experiment-matched vehicle control, and number of biological replicate cultures tested (n) for wild type (WT), tor1Δ, and fpr1Δ cells. Interventions tested at 100 µg/mL
| Treatment | WT | tor1Δ | fpr1Δ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DT (SEM) | % change | n | DT (SEM) | % change | n | DT (SEM) | % change | n | |
| 1% DMSO | 79.5 (1.0) | - | 29 | 79.6 (0.8) | - | 29 | 89.8 (0.7) | - | 29 |
| 2% DMSO | 85.4 (1.6) | - | 6 | 84.2 (2.2) | - | 6 | 92.3 (3.1) | - | 6 |
| 0.27 N NaOH | 74.5 (0.04) | - | 3 | 77.4 (0.2) | - | 3 | 85.8 (1.7) | - | 3 |
| Compounds and extracts | |||||||||
| Allantoin | 81.7 (1.2) | − 2.0 | 6 | 80.0 (1.0) | − 1.1 | 6 | 87.7 (2.2) | − 3.1 | 6 |
| Ashwagandha root | 82.5 (1.1) | − 0.9 | 3 | 80.4 (1.1) | 0.6 | 3 | 87.9 (1.6) | − 2.4 | 3 |
| Cardamonin† | 78.7 (2.0) | − 7.9 | 6 | 84.4 (1.1) | 0.3 | 6 | 96.4 (1.1) | 4.4 | 6 |
| Choline bitartrate | 82.0 (2.5) | 14.8 | 3 | 81.2 (1.8) | 1.6 | 3 | 89.4 (1.6) | − 1.9 | 3 |
| Coumaric acid | 82.2 (1.3) | − 1.4 | 6 | 81.0 (1.3) | 0.1 | 6 | 89.3 (2.5) | − 1.4 | 6 |
| Curcumin phytosome complex | 75.8 (0.9) | 2.0 | 3 | 71.0 (1.5) | − 2.6 | 3 | 89.8 (0.7) | 6.0 | 3 |
| Dong Quai | 79.9 (1.6) | − 4.2 | 6 | 81.0 (1.2) | 0.1 | 6 | 87.7 (2.3) | − 3.2 | 6 |
| Ellipticine | NG** | - | 3 | NG** | - | 3 | NG** | - | 3 |
| Ginseng | 83.4 (2.6) | 0.04 | 6 | 81.9 (1.7) | 1.2 | 6 | 89.3 (3.0) | − 1.4 | 6 |
| Ginsenoside Rc | 83.4 (3.7) | 0.2 | 3 | 80.1 (2.3) | 0.2 | 3 | 90.5 (2.4) | 0.5 | 3 |
| Grape seed #1 | 88.4 (1.8) | 18.9* | 3 | 91.6 (2.8) | 25.6* | 3 | 102.4 (2.1) | 20.8* | 3 |
| Grape seed #2 | 99.0 (5.6) | 38.5 | 3 | 77.3 (2.6) | − 3.3 | 3 | 88.2 (2.0) | − 3.2 | 3 |
| Green tea | 91.6 (0.2) | 11.9 | 3 | 92.9 (1.8) | 13.5 | 3 | 103.3 (1.6) | 13.2 | 3 |
| Hesperidin# | 76.5 (0.9) | 2.7 | 3 | 76.6 (0.2) | − 1.0 | 3 | 80.7 (1.2) | − 6.0 | 3 |
| Hydroxytyrosol | 83.3 (2.3) | − 0.1 | 6 | 82.5 (1.8) | 2.0 | 6 | 90.9 (2.7) | 0.4 | 6 |
| Isoliquiritigenin†† | 169.9 (26.2) | 103.7 | 2 | NG** | - | 6 | NG** | - | 6 |
| Licorice root | 83.8 (2.2) | 1.4 | 6 | 83.6 (2.9) | 2.2 | 6 | 89.9 (3.0) | − 1.4 | 6 |
| Lutein | 90.2 (0.6) | 21.3* | 3 | 90.4 (1.0) | 23.9* | 3 | 97.1 (1.8) | 14.5 | 3 |
| Milk thistle | 85.4 (1.5) | 3.3 | 6 | 85.8 (2.9) | 4.8 | 6 | 94.7 (3.0) | 3.9 | 6 |
| N-Acetyl l-cysteine | 82.0 (1.2) | − 0.9 | 6 | 81.0 (1.3) | − 1.0 | 6 | 89.0 (2.3) | − 2.3 | 6 |
| Olive fruit | 84.6 (1.4) | 2.3 | 6 | 84.4 (2.0) | 3.1 | 6 | 92.1 (3.1) | 1.1 | 6 |
| Polygonum cuspidatum root | 102.6 (2.8) | 24.0* | 6 | 99.6 (1.4) | 21.7* | 6 | 106.6 (4.1) | 17.0 | 6 |
| Pterocarpus marsupium | 89.1 (4.6) | 14.5 | 3 | 93.9 (3.2) | 22.6* | 3 | 103.3 (1.9) | 11.6* | 3 |
| Rutin | 85.6 (1.2) | 3.5 | 6 | 83.7 (1.5) | 2.3 | 6 | 92.8 (2.8) | 1.8 | 6 |
| Turmeric | 111.5 (4.6) | 36.1* | 3 | 110 (13.2) | 34.9 | 3 | 107.7 (1.2) | 18.0* | 3 |
| Tyrosol | 82.0 (2.0) | − 1.5 | 3 | 79.5 (0.3) | − 0.5 | 3 | 88.3 (0.8) | − 1.9 | 3 |
| Umbelliferone | 86.5 (1.3) | 3.7 | 6 | 83.7 (0.5) | 3.5 | 6 | 91.9 (2.8) | 1.5 | 6 |
| Verbascoside | 84.2 (2.2) | 1.2 | 3 | 82.6 (1.1) | 3.3 | 3 | 91.2 (3.7) | 1.3 | 3 |
| Withaferin A | 106.6 (4.4) | 27.9* | 6 | 110.9 (4.3) | 37.0* | 6 | 102.6 (3.4) | 13.3 | 6 |
| Mixtures | |||||||||
| 1 | 95.1 (4.8) | 22.2 | 3 | 103.9 (8.7) | 35.6 | 3 | 110.2 (13.4) | 19.0 | 3 |
| 2 | 93.3 (1.7) | 25.4* | 3 | 94.3 (1.9) | 29.3* | 3 | 104 (5.5) | 22.7 | 3 |
| 3 | 88.4 (0.7) | 23.8 | 3 | 88.2 (1.2) | 10.4 | 3 | 94.2 (0.8) | 3.3 | 3 |
| 4 | 77.9 (7.5) | 9.0 | 3 | 91.2 (2.9) | 14.1 | 3 | 83.1 (4.1) | − 8.9 | 3 |
| 5 | 81.8 (1.7) | 10.0 | 3 | 76.5 (1.7) | 4.9 | 3 | 81.3 (1.0) | − 4.1 | 3 |
| 6 | 94.6 (1.9) | 32.4* | 3 | 93.5 (5.4) | 17.0 | 3 | 105.8 (8.5) | 16.0 | 3 |
*p < 0.05, compared to vehicle, Welch’s (unequal variance) t-test
**Doubling time not calculated due to minimal growth over the time course of the experiment, see Methods
#0.27 N NaOH vehicle
†2% DMSO vehicle
††Doubling time for four of six tested WT cultures could not be calculated
Fig. 1.
Doubling times for compounds, extracts, and mixtures that significantly inhibit growth. Inflection doubling time (DT) of WT, tor1Δ, and fpr1Δ microcultures. Number of replicates n = 3 for panels A, B, D–F, H, and I. Number of replicates n = 6 for panels C, G, and J. Welch’s T-test with Bonferroni correction used to compare treatment (Trt) to vehicle (Veh) (*p < 0.05, **p < 0.01, ***p < 0.001). NG denotes no growth (see Methods for more details)
Table 4.
Dose response for selected natural product compounds. Doubling time (DT) (minutes/cell division), standard error of the mean (SEM), percent change, and number of biological replicate cultures tested (n) for wild type (WT), tor1Δ, and fpr1Δ cells
| Treatment | WT | tor1Δ | fpr1Δ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DT (SEM) | % change | n | DT (SEM) | % change | n | DT (SEM) | % change | n | |
| 1% DMSO | 80.0 (2.8) | - | 3 | 81.1 (1.8) | - | 3 | 85.9 (1.3) | - | 3 |
| Berberine 1 µg/mL | 81.2 (1.2) | 1.5 | 3 | 85.2 (0.4) | 5.1 | 3 | 89.5 (0.3) | 4.2 | 3 |
| Berberine 10 µg/mL | 93.7 (1.4) | 17.0 | 3 | 88.3 (3.2) | 9.0 | 3 | 98.1 (4.8) | 14.2 | 3 |
| Berberine 50 µg/mL | 146.3 (4.2) | 82.8* | 3 | 135.7 (1.0) | 67.4* | 3 | 143.1 (0.9) | 66.7* | 3 |
| 1% DMSO | 83.1 (1.6) | - | 3 | 82.3 (0.7) | - | 3 | 92.0 (1.4) | - | 3 |
| Celastrol 5 µM | 86.0 (0.8) | 3.4 | 3 | 85.8 (1.1) | 4.3 | 3 | 92.4 (0.7) | 0.4 | 3 |
| Celastrol 10 µM | 92.7 (2.1) | 11.5 | 3 | 92.4 (1.2) | 12.3* | 3 | 102.0 (0.8) | 10.9* | 3 |
| Celastrol 100 µM | 94.2 (2.8) | 13.4 | 3 | 92.5 (4.2) | 12.4 | 3 | 100.8 (2.4) | 9.5 | 3 |
| 1% DMSO | 81.6 (0.8) | - | 3 | 79.1 (1.4) | - | 3 | 90.7 (1.6) | - | 3 |
| Isoliquiritigenin 1 µg/mL | 79.3 (1.2) | -2.8 | 3 | 77.7 (1.2) | -1.8 | 3 | 87.1 (1.1) | -4.0 | 3 |
| Isoliquiritigenin 10 µg/mL | 82.4 (2.1) | 1.0 | 3 | 81.3 (1.1) | 2.8 | 3 | 92.4 (2.5) | 2.0 | 3 |
| Isoliquiritigenin 50 µg/mL | 98.7 (2.9) | 21.0 | 3 | 99.0 (1.7) | 25.2* | 3 | 113.6 (2.5) | 25.3* | 3 |
*p < 0.05, compared to 1% DMSO, Welch’s (unequal variance) t-test
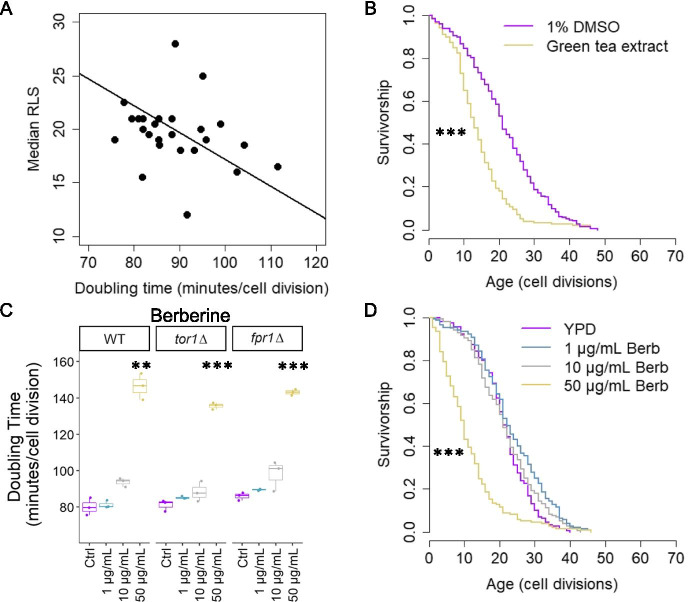
Twenty-four compounds and mixtures were selected from our initial screen and a prior study [35] for RLS analysis in wild-type cells (Supplemental Fig. 1 and Table 5). Across all of the compounds and mixtures tested, no significant correlation was observed between doubling time and lifespan (Fig. 2A, standard major axis regression; adjusted R-squared = 0.06, p-value = 0.24). Most substances had no significant effect on lifespan, including alpha-lipoic acid, glucosamine, quercetin, and Polygonum cuspidatum root extract, which is 50% resveratrol (w/w) (Supplemental Fig. 1). Significant shortening of lifespan was observed for green tea extract (p-value < 0.001, Wilcoxon rank-sum test, Bonferroni corrected) (Fig. 2B). Turmeric extract (raw p-value = 0.009, Bonferroni-corrected p-value = 0.208, Wilcoxon rank-sum test) and mixture 5 (raw p-value = 0.042, Bonferroni-corrected p-value = 0.997, Wilcoxon rank-sum test) significantly shortened lifespan when p-values were uncorrected, but these did not reach significance after Bonferroni correction (Supplemental Fig. 1). Mixture 5 is 13.9% w/w turmeric and one of only two mixtures tested that contains turmeric (the other, mixture 4, contains only 3.4% turmeric w/w) (Table 2).
Table 5.
Median RLS (95% confidence intervals), number of cells dissected (n), percent change, and p-value (Wilcoxon rank-sum test, Bonferroni-corrected p-value for multiple testing and uncorrected p-value in parentheses) of natural product compounds, extracts, and mixtures compared to vehicle (1% DMSO) control. All interventions tested at 100 µg/mL
| Treatment | n | Median RLS (95% CI) | % change | Bonferroni-corrected p-value (uncorrected) |
|---|---|---|---|---|
| 1% DMSO (vehicle control) | 198 | 21 (20–24) | 0.0 | - |
| Compounds and extracts | ||||
| Alpha-lipoic acid | 40 | 21 (19–26) | 0.0 | 1 (0.859) |
| Broccoli concentrate | 80 | 19.5 (16–24) | − 7.2 | 1 (0.342) |
| Choline bitartrate | 160 | 21 (19–23) | 0.0 | 1 (0.820) |
| Curcumin phytosome complex | 40 | 19 (17–24) | − 9.5 | 1 (0.480) |
| Glucosamine HCl | 116 | 21 (19–25) | 0.0 | 1 (0.815) |
| Grape seed #1 | 118 | 19.5 (18–24) | − 7.2 | 1 (0.913) |
| Grape seed #2 | 80 | 20.5 (19–24) | − 2.4 | 1 (0.945) |
| Green tea | 160 | 12 (11–14) | − 42.9 | < 0.001 (< 0.001) |
| Lutein | 39 | 18 (16–28) | − 14.3 | 1 (0.984) |
| Lycopene | 118 | 19 (17–22) | − 9.5 | 1 (0.096) |
| Milk thistle | 40 | 19 (15–26) | − 9.5 | 1 (0.540) |
| N-acetyl l-cysteine | 39 | 20 (17–27) | − 4.8 | 1 (0.664) |
| Olive fruit | 80 | 20.5 (19–26) | − 2.4 | 1 (0.722) |
| Polygonum cuspidatum root | 40 | 16 (13–22) | − 23.8 | 1 (0.073) |
| Pterocarpus marsupium | 104 | 28 (25–31) | 33.3 | 0.001 (< 0.001) |
| Quercetin | 40 | 18.5 (16–27) | − 11.9 | 1 (0.836) |
| Rutin | 40 | 18.5 (15–26) | − 11.9 | 1 (0.309) |
| Turmeric | 40 | 16.5 (14–22) | − 21.4 | 0.208 (0.009) |
| Mixtures | ||||
| 1 | 80 | 25 (22–27) | 19.0 | 0.219 (0.009) |
| 2 | 40 | 18 (14–21) | − 14.3 | 1 (0.056) |
| 3 | 80 | 21 (18–23) | 0.0 | 1 (0.609) |
| 4 | 40 | 22.5 (20–26) | 7.1 | 1 (0.883) |
| 5 | 40 | 15.5 (13–21) | − 26.2 | 0.997 (0.042) |
| 6 | 40 | 20 (16–24) | − 4.8 | 1 (0.377) |
Fig. 2.
Doubling time and lifespan of natural product-treated yeast with outgrowth kinetics and Kaplan–Meier curves for lifespan decreasing treatments. A Relationship between doubling time (minutes/cell division) and median RLS for natural product-treated yeast (standard major axis regression, adjusted R-squared = 0.06, p-value = 0.24). B Green tea extract (GTE) decreases RLS (Wilcoxon rank-sum test, Bonferroni corrected (*p < 0.05, **p < 0.01, ***p < 0.001)). C Berberine is a general inhibitor of yeast growth (Welch’s T-test, Bonferroni corrected). D Berberine RLS dose response (Wilcoxon rank-sum test, Bonferroni corrected). For number of cells or cultures tested and p-values, see Tables 5 and 6
Berberine, in particular, has been described as a potential geroprotective compound with mTOR inhibitory properties [70]. In a previous growth screen, we failed to detect an mTOR inhibitory effect in cells treated with 100 μg/mL berberine [35]. However, the severe growth inhibition at 100 μg/mL suggested that this concentration is above the toxic threshold for this compound. We therefore performed a dose–response RLS experiment and follow-up mTOR activity screen for concentrations of berberine ranging from 1–50 μg/mL (Fig. 2C–D and Tables 4 and 6). We also performed dose–response mTOR activity and RLS experiments for 5–100 μM celastrol, an activator of the heat shock response and putative healthspan intervention [71–73]. We resolved no significant RLS effects with any celastrol dose, but concentrations ≥ 10 μM weakly inhibited growth in all strains (Tables 4 and 6 and Supplemental Figs. 1 and 2). The significant growth inhibitory effect of berberine seen at 100 μg/mL was also observed in all strains at 50 μg/mL (p-value ≤ 0.01, Welch’s t-test, Bonferroni corrected) with less pronounced effects on growth at lower concentrations (Fig. 2C and Table 4). RLS was significantly shortened at 50 μg/mL berberine (p-value < 0.001, Wilcoxon rank-sum test), but no difference was detected at either 10 μg/mL or 1 μg/mL compared to YPD control (berberine suspended in sterile H2O instead of DMSO for RLS) (Fig. 2D and Table 6).
Table 6.
Median RLS (95% confidence intervals), number of cells dissected (n), percent change, and p-value (Wilcoxon rank-sum test, Bonferroni-corrected for multiple testing within each dose response) of natural products compared to vehicle control. Note: separate control used for each compound. Berberine suspended in H2O and thus compared to YPD
| Treatment | Concentration | n | Median RLS (95% CI) | % change | Bonferroni-corrected p-value |
|---|---|---|---|---|---|
| YPD | NA | 160 | 21.5 (20–23) | - | - |
| Berberine | 1 µg/mL | 155 | 22 (21–25) | 2.3 | 0.173 |
| Berberine | 10 µg/mL | 160 | 21 (20–23) | − 2.3 | 1 |
| Berberine | 50 µg/mL | 157 | 10 (9–11) | − 53.5 | < 0.001 |
| DMSO | 1% | 60 | 23.5 (21–30) | - | - |
| Celastrol | 5 µM | 60 | 24.5 (22–29) | 4.3 | 1 |
| Celastrol | 10 µM | 60 | 25 (23–29) | 6.4 | 1 |
| Celastrol | 100 µM | 60 | 23 (18–28) | − 2.1 | 0.841 |
Only one treatment, Pterocarpus marsupium extract (PME), significantly extended lifespan (p-value < 0.001, Wilcoxon rank-sum test, Bonferroni corrected) (Table 5 and Fig. 3). Mixture 1 significantly extended lifespan when p-values were uncorrected but did not reach significance after Bonferroni correction (raw p-value = 0.009, Bonferroni-corrected p-value = 0.219, Wilcoxon rank-sum test) (Table 5 and Supplemental Fig. 1). Interestingly, mixture 1 is the only mixture to contain PME as its primary constituent, along with lesser amounts of alpha-lipoic acid, quercetin, and olive fruit extract (Table 2). This suggests that the lifespan extension from mixture 1 may be related to the presence of PME in the mixture.
Fig. 3.
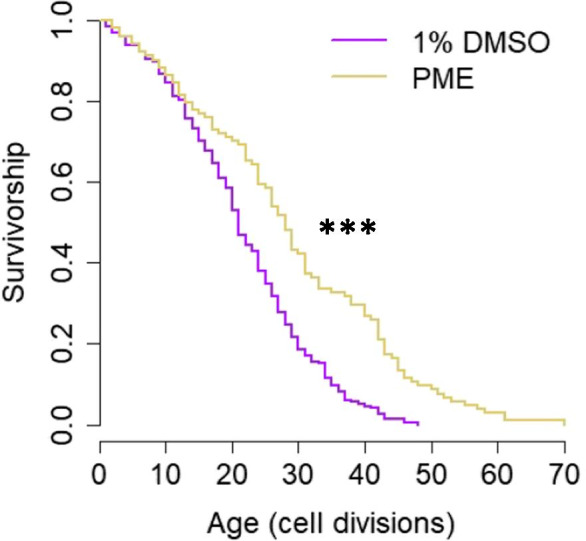
Pterocarpus marsupium extract (PME) extends replicative lifespan. 100 μg/mL PME (n = 104) extends lifespan compared to 1% DMSO vehicle control (n = 198) (p < 0.001, Wilcoxon rank-sum test, Bonferroni corrected)
Discussion
From a screen of 42 natural products and natural product mixtures, we identified PME as a novel pro-longevity intervention in yeast. PME is an extract from the plant Pterocarpus marsupium with well-known antidiabetic properties [74–76]. PME contains several components, presumably one or more of which contributes to its positive effects on RLS. Common components of PME include pterostilbene, epicatechin, pterosupin, marsupin, liquiritigenin, along with a variety of other molecules present in lower abundance [77–79].
Two of the most abundant PME constituents, pterostilbene and epicatechin, are suggested to promote longevity [80, 81]. These major components were quantified in our extract using HPLC, and our extract contained 5.00–5.50% pterostilbene w/w and 0.01–1.00% epicatechin w/w. We could find no published data supporting lifespan extension from pterostilbene. Epicatechin, on the other hand, has been reported to increase lifespan in fruit flies [82]; however, other studies failed to detect significant lifespan extension from epicatechin in worms or flies [63, 83, 84]. It will be of interest in future studies to determine which of the various components of PME contribute to the RLS extension observed here, as well as their mechanisms of action.
It is unexpected that none of the compounds or mixtures tested showed consistent evidence for mTOR inhibitory activity, as several have previously been reported to inhibit mTOR signaling. These include both resveratrol [85, 86] and berberine [87–89]. While the Bioscreen C MBR assay is extremely sensitive, there are several potential explanations for the lack of mTOR inhibition observed from the compounds and mixtures in this study. For example, some pharmaceutical compounds that act as ATP-competitive mTOR inhibitors are less effective in yeast relative to mammalian cells [35, 90]. Likewise, compounds that affect mTOR signaling indirectly in mammalian cells, via upstream regulators of mTOR or through other components of the mTOR signaling network such as AMP kinase, may not have identical effects in yeast. For example, the tuberous sclerosis complex proteins TSC1 and TSC2 act as key upstream regulators of mTOR in mammals but do not have clear orthologs in either S. cerevisiae or C. elegans [91–93]. Thus, compounds affecting mTOR activity via interactions with TSC1/2 in mammalian cells would not be predicted to have these effects in budding yeast.
Some compounds previously reported to extend lifespan in various model systems did not show lifespan-extending effects in our screen and, in some cases, shortened lifespan. There are many potential explanations for this, including the possibility that lifespan extension would have been observed at concentrations other than those tested here. Resveratrol was initially reported to extend RLS in yeast by more than 70% in another strain background [94]. However, we failed to observe any effect on lifespan in the BY4742 strain background with a Polygonum cuspidatum root extract containing 50% w/w resveratrol, which is consistent with prior data using a pure resveratrol preparation [95]. Importantly, while resveratrol has continued to be widely studied for a variety of health effects in both mice and people, it does not appear to extend mouse lifespan [96]. Interestingly, in our mTOR inhibitor screen, Polygonum cuspidatum root extract behaved as a general growth inhibitor. A previous study by our group showed no effect of pure resveratrol on yeast growth [35], suggesting that the growth inhibitory effects of Polygonum cuspidatum root extract are independent of, or at least not solely mediated by, resveratrol.
Berberine was recently reported to attenuate senescence in fibroblasts, as well as extend lifespan in middle-aged mice and in yeast [44]. However, using similar doses of berberine, we failed to resolve a lifespan-extending effect and instead observed significant growth inhibition and shortening of RLS. A key difference between the two studies is the RLS assay method; manual dissection of yeast on solid media was used in our study as opposed to a microfluidic-based approach where yeast is submerged in liquid media. While it could be argued that effects in human cells and mice are likely to be more translationally relevant, the strong dose-dependent toxicity of berberine seen in our study suggests that caution is warranted when considering its untested and unregulated use by people, especially over months or years.
Green tea has long been touted as a health and longevity-promoting beverage [97–99]. In yeast, however, it shortens lifespan, at least at the concentrations tested here. One reported mechanism for green tea’s health benefits is by altering the gut and oral microbiomes [100, 101], and S. cerevisiae and related fungal species are present in the human microbiome [102]. For interventions that improve human health by modifying the microbiome, it is not obvious what effects to expect on individual microbes from among the impacted community. It could be that the set of interventions that negatively impact yeast lifespan are enriched for interventions that could improve human health by remodeling the microbiome. More broadly, novel antifungal compounds identified as part of screening for lifespan-extending interventions may have clinical utility outside of geroscience.
Using combined growth and lifespan analysis in yeast, we identified a lifespan-extending natural product, Pterocarpus marsupium extract. Our approach sets the foundation for future genetic and biochemical studies to characterize the mechanistic basis for lifespan extension from PME and provides an incentive to test PME in invertebrate and mammalian model systems. Overall, our study demonstrates the utility of using yeast as a rapid and inexpensive screening tool to identify interventions of interest for geroscience and medicine.
Materials and methods
Yeast strains and culture conditions
All yeast strains used were in the BY4742 genetic background (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) and have been previously described [35] except for the celastrol RLS dose response which used the BY4741 genetic background (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) [103]. For overnight culture and growth analysis, YPD (1% w/v Bacto™ yeast extract (BD), 2% w/v Bacto™ peptone (BD), 2% w/v dextrose) media was used. Yeast was cultured at 30 °C for all experiments.
Outgrowth analysis
All compounds and mixtures were initially screened at a concentration of 100 μg/mL in DMSO, except for hesperidin which was suspended in 0.027 N NaOH. Analysis of maximal growth rate in yeast strains was performed to identify growth-inhibiting interventions using a Bioscreen C MBR (Growth Curves USA, Piscataway NJ, USA) as previously described [35, 104–106]. Raw optical density data were smoothed using the R package “smooth.spline.” Doubling times were calculated using the inflection method—identifying the maximum semi-log slope along growth curves within the optical density range linearly correlated with a number of yeast cells—with the online web tool Yeast Outgrowth Data Analyzer (YODA) [107]. All experiments were repeated with at least three biological replicates. The number of biological replicates is indicated in each figure caption. Doubling time was calculated for all cultures where OD ≥ 0.3 after 20-h growth. Otherwise, culture was listed as no growth (NG). In one case (isoliquiritigenin), growth was below this threshold in most cultures and near the threshold in the remaining cultures and is also reported as NG. Interventions were classified as mTOR inhibitors based on preferential growth inhibition in the tor1Δ strain, relative to WT and fpr1Δ strains, as previously described [35]. Welch’s (unequal variance) t-test with Bonferroni multiple testing corrections was used to assess differences relative to experiment-matched vehicle control cultures using R. We applied multiple testing correction using the p.adjust function.
Replicative lifespan analysis
A modified RLS protocol for treatments was developed based on previously described methods [108, 109]. Briefly, cells were grown on freshly prepared YPD plates at 30 °C until single colonies were visible. Cells were selected from a single colony and lightly patched onto YPD plates supplemented with the designated treatment or vehicle overnight. In the morning, founder cells were aligned and selected as newborn daughter cells using a micromanipulator. Cells were monitored for cell divisions every 90–120 min, and subsequent budded daughter cells were separated and removed as they formed. The process continued until cells stopped dividing. Replicative lifespan was calculated as the number of times each mother cell divided before it underwent permanent cell cycle arrest. Plates were kept in the refrigerator at 4 °C overnight. Plates were kept wrapped in tinfoil except while being dissected to avoid potential light sensitivity of any compounds. All experimenters were blinded to the identity of any of the treatments at the time of dissection. Cells were dissected in groups of 20 and 39–198 cells dissected for each intervention tested. A linear model comparing doubling time and median RLS of treated yeast constructed using the standard major axis regression (SMA) option in the lmodel2 package in R.
Intervention preparation and suppliers
All compounds and mixtures were suspended in DMSO, except hesperidin which was suspended in 0.027 N NaOH. Berberine was purchased from Sigma-Aldrich (St. Louis MO, USA). Celastrol was purchased from Cayman Chemical (Ann Arbor MI, USA). All other compounds and mixtures were provided by USANA Health Sciences, Inc. (Salt Lake City UT, USA). The composition of the mixtures tested is provided in Table 2.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This work was supported by the University of Washington Nathan Shock Center of Excellence in the Basic Biology of Aging Invertebrate Longevity and Healthspan Core (NIH P30AG013280) and a grant to MK from USANA Health Sciences. M.B.L. was supported by the National Institutes of Health (NIH) Alzheimer’s Disease Training Program (NIH T32 AG052354), the Howard Hughes Medical Institute (HHMI) Gilliam Fellowship for Advanced Study, the NIH Cellular and Molecular Biology training grant (NIH T32 GM727039), and the University of Washington Graduate Opportunities and Minority Achievement Program (UW GO-MAP) Bank of America Fellowship. M.G.K. was supported by NIH award R01AG056359 and the Biological Mechanisms of Healthy Aging Training Program (NIH T32 AG066574). D.P. was supported in part by NIH award R01AG049494.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mitchell B. Lee and Michael G. Kiflezghi contributed equally to this work
Change history
3/23/2022
A Correction to this paper has been published: 10.1007/s11357-022-00544-0
References
- 1.Sierra F, Kohanski R. Geroscience and the trans-NIH Geroscience Interest Group. Geroscience. 2017;39:1–5. doi: 10.1007/s11357-016-9954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee MB, Kaeberlein M. Translational geroscience: from invertebrate models to companion animal and human interventions Translational Medicine of. Aging. 2018;2:15–29. doi: 10.1016/j.tma.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks aging cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy BK, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaeberlein M. Translational geroscience: a new paradigm for 21st century medicine Translational Medicine of Aging. 2017;1:1–4 10.1016/j.tma.2017.09.004 [DOI] [PMC free article] [PubMed]
- 6.Kaeberlein M. Lessons on longevity from budding yeast. Nature. 2010;464:513–519. doi: 10.1038/nature08981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longo VD, Shadel GS, Kaeberlein M, Kennedy B. Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metab. 2012;16:18–31. doi: 10.1016/j.cmet.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mortimer RK, Johnston JR. Lessons on longevity from budding yeast. Nature. 1959;183:1751–1752. doi: 10.1038/1831751a0. [DOI] [PubMed] [Google Scholar]
- 9.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289(5487):2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 10.Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. Faseb J. 2000;14:2135–2137. doi: 10.1096/fj.00-0242fje. [DOI] [PubMed] [Google Scholar]
- 11.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13(19):2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2(9):E296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292(5515):288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 14.Kaeberlein M, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310(5751):1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 15.Kirchman PA, Kim S, Lai CY, Jazwinski SM. Interorganelle signaling is a determinant of longevity in Saccharomyces cerevisiae. Genetics. 1999;152:179–190. doi: 10.1093/genetics/152.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai CY, Jaruga E, Borghouts C, Jazwinski SM. A mutation in the ATP2 gene abrogates the age asymmetry between mother and daughter cells of the yeast Saccharomyces cerevisiae. Genetics. 2002;162(1):73–87. doi: 10.1093/genetics/162.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miceli MV, Jiang JC, Tiwari A, Rodriguez-Quinones JF, Jazwinski SM. Loss of mitochondrial membrane potential triggers the retrograde response extending yeast replicative lifespan. Front Genet. 2011;2:102. doi: 10.3389/fgene.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veatch JR, McMurray MA, Nelson ZW, Gottschling DE. Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell. 2009;137(7):1247–1258 S0092 8674(09)00402 4. doi: 10.1016/j.cell.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes CE, Coody TK, Jeong MY, Berg JA, Winge DR, Hughes AL. Cysteine toxicity drives age-related mitochondrial decline by altering iron homeostasis. Cell. 2020;180(296–310):e218. doi: 10.1016/j.cell.2019.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delaney JR, et al. Stress profiling of longevity mutants identifies Afg3 as a mitochondrial determinant of cytoplasmic mRNA translation and aging. Aging Cell. 2013;12(1):156–166. doi: 10.1111/acel.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes AL, Gottschling DE. An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast. Nature. 2012;492:261–265. doi: 10.1038/nature11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson KA, Hughes AL, Gottschling DE. Mother-daughter asymmetry of pH underlies aging and rejuvenation in yeast eLife. 2014;3:e03504. 10.7554/eLife.03504. [DOI] [PMC free article] [PubMed]
- 23.Chen KL, et al. Loss of vacuolar acidity results in iron-sulfur cluster defects and divergent homeostatic responses during aging in Saccharomyces cerevisiae. Geroscience. 2020 doi: 10.1007/s11357-020-00159-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murakami C, et al. pH neutralization protects against reduction in replicative lifespan following chronological aging in yeast. Cell Cycle. 2012;11(16):3087–3096. doi: 10.4161/cc.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mouton SN et al. (2020) A physicochemical perspective of aging from single-cell analysis of pH, macromolecular and organellar crowding in yeast eLife 9. 10.7554/eLife.54707 [DOI] [PMC free article] [PubMed]
- 26.Kruegel U, et al. Elevated proteasome capacity extends replicative lifespan in Saccharomyces cerevisiae. PLoS Genet. 2011;7:e1002253. doi: 10.1371/journal.pgen.1002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao Y, et al. Proteasomes, Sir2, and Hxk2 form an interconnected aging network that impinges on the AMPK/Snf1-regulated transcriptional repressor Mig1. PLoS Genet. 2015;11(1):e1004968. doi: 10.1371/journal.pgen.1004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Z et al. (2018) Ssd1 and Gcn2 suppress global translation efficiency in replicatively aged yeast while their activation extends lifespan eLife 7 10.7554/eLife.35551 [DOI] [PMC free article] [PubMed]
- 29.Aris JP, et al. Autophagy and leucine promote chronological longevity and respiration proficiency during calorie restriction in yeast. Exp Gerontol. 2013;48:1107–1119. doi: 10.1016/j.exger.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinclair DA, Guarente L. Extrachromosomal rDNA circles--a cause of aging in yeast. Cell. 1997;91(7):1033–1042. doi: 10.1016/S0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 31.Crane MM et al. (2019) DNA damage checkpoint activation impairs chromatin homeostasis and promotes mitotic catastrophe during aging eLife 8. 10.7554/eLife.50778 [DOI] [PMC free article] [PubMed]
- 32.Myers A, Lithgow GJ. Drugs that target aging: how do we discover them? Expert Opin Drug Discov. 2019;14(6):541–548. doi: 10.1080/17460441.2019.1597049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barardo D, et al. The DrugAge database of aging-related drugs. Aging Cell. 2017;16:594–597. doi: 10.1111/acel.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tacutu R, et al. Human ageing genomic resources: new and updated databases. Nucleic Acids Res. 2018;46:D1083–D1090. doi: 10.1093/nar/gkx1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee MB, et al. A system to identify inhibitors of mTOR signaling using high-resolution growth analysis in Saccharomyces cerevisiae. Geroscience. 2017;39:419–428. doi: 10.1007/s11357-017-9988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wanke V, Cameroni E, Uotila A, Piccolis M, Urban J, Loewith R, De Virgilio C. Caffeine extends yeast lifespan by targeting TORC1. Mol Microbiol. 2008;69(1):277–285. doi: 10.1111/j.1365-2958.2008.06292.x. [DOI] [PubMed] [Google Scholar]
- 37.Sutphin GL, Bishop E, Yanos ME, Moller RM, Kaeberlein M. Caffeine extends life span, improves healthspan, and delays age-associated pathology in Caenorhabditis elegans. Longev Healthspan. 2012;1:9. 10.1186/2046-2395-1-9 [DOI] [PMC free article] [PubMed]
- 38.Bridi JC, Barros AG, Sampaio LR, Ferreira JC, Antunes Soares FA, Romano-Silva MA. Lifespan extension induced by caffeine in Caenorhabditis elegans is partially dependent on adenosine signaling. Front Aging Neurosci. 2015;7:220. doi: 10.3389/fnagi.2015.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calvert S, Tacutu R, Sharifi S, Teixeira R, Ghosh P, de Magalhaes JP. A networkpharmacology approach reveals new candidate caloric restriction mimetics in C. elegans. Aging Cell. 2016;15:256–266. doi: 10.1111/acel.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown MK, Evans JL, Luo Y. Beneficial effects of natural antioxidants EGCG andalpha-lipoic acid on life span and age-dependent behavioral declines in Caenorhabditis elegans. Pharmacol Biochem Behav. 2006;85:620–628. doi: 10.1016/j.pbb.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 41.Benedetti MG, et al. Compounds that confer thermal stress resistance and extendedlifespan. Exp Gerontol. 2008;43:882–891. doi: 10.1016/j.exger.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bauer JH, Goupil S, Garber GB, Helfand SL. An accelerated assay for theidentification of lifespan-extending interventions in Drosophila melanogaster. Proc Natl Acad Sci USA. 2004;101:12980–12985. doi: 10.1073/pnas.0403493101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar R, Gupta K, Saharia K, Pradhan D, Subramaniam JR. Withania somnifera rootextract extends lifespan of Caenorhabditis elegans. Ann Neurosci. 2013;20:13–16. doi: 10.5214/ans.0972.7531.200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dang Y, et al. Berberine ameliorates cellular senescence and extends the lifespan of mice via regulating p16 and cyclin protein expression. Aging Cell. 2020;19(1):e13060. doi: 10.1111/acel.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Navrotskaya VV, Oxenkrug G, Vorobyova LI, Summergrad P. Berberine Prolongs Life Span andStimulates Locomotor Activity of Drosophila melanogaster. Am J Plant Sci. 2012;3:1037–1040. doi: 10.4236/ajps.2012.327123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jung S-K, Aleman-Meza B, Riepe C, Zhong W. QuantWorm: A Comprehensive SoftwarePackage for Caenorhabditis elegans Phenotypic Assays. PLOS ONE. 2014;9:e84830. 10.1371/journal.pone.0084830. [DOI] [PMC free article] [PubMed]
- 47.Lee J-H, et al. Effects of ginsenosides, active ingredients of Panax ginseng, ondevelopment, growth, and life span of Caenorhabditis elegans. Biol Pharm Bull. 2007;30:2126–2134. doi: 10.1248/bpb.30.2126. [DOI] [PubMed] [Google Scholar]
- 48.Weimer S, et al. D-Glucosamine supplementation extends life span of nematodesand of ageing mice. Nat Commun. 2014;5:1–12. doi: 10.1038/ncomms4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abbas S, Wink M. Epigallocatechin gallate from green tea (Camellia sinensis) increases lifespan and stress resistance in Caenorhabditis elegans. Planta Medica. 2009;75:216. doi: 10.1055/s-0028-1088378. [DOI] [PubMed] [Google Scholar]
- 50.Lopez T, Schriner SE, Okoro M, Lu D, Chiang BT, Huey J, Jafari M. Green teapolyphenols extend the lifespan of male drosophila melanogaster while impairingreproductive fitness. J Med Food. 2014;17:1314–1321. doi: 10.1089/jmf.2013.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner AE, et al. Epigallocatechin gallate affects glucose metabolism andincreases fitness and lifespan in Drosophila melanogaster. Oncotarget. 2015;6:30568. doi: 10.18632/oncotarget.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strong R, et al. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of geneticallyheterogeneous mice. The journals of gerontology Series A, Biological sciencesand medical sciences. 2013;68:6–16. doi: 10.1093/gerona/gls070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun K, Xiang L, Ishihara S, Matsuura A, Sakagami Y, Qi J. Anti-aging effects of hesperidin on Saccharomyces cerevisiae via inhibition of reactive oxygen species and UTH1 gene expression. Bioscience, Biotechnology, and Biochemistry. 2012;1202232809–1202232809 [DOI] [PubMed]
- 54.Reigada I, Moliner C, Valero MS, Weinkove D, Langa E, Gómez Rincón C. Antioxidantand antiaging effects of licorice on the Caenorhabditis elegans model. JMed Food. 2019;23:72–78. doi: 10.1089/jmf.2019.0081. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Z, Han S, Wang H, Wang T. Lutein extends the lifespan of Drosophila melanogaster. Arch Gerontol Geriatr. 2014;58:153–159. doi: 10.1016/j.archger.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 56.Kumar J, Park KC, Awasthi A, Prasad B. Silymarin extends lifespan and reduces proteotoxicity in C. elegans Alzheimer's model. CNS & Neurological DisordersDrug Targets. 2015;14:295–302. doi: 10.2174/1871527314666150116110212. [DOI] [PubMed] [Google Scholar]
- 57.Oh S-I, Park J-K, Park S-K. Lifespan extension and increased resistance to environmental stressors by N-acetyl-L-cysteine in Caenorhabditis elegans. Clinics. 2015;70:380–386. doi: 10.6061/clinics/2015(05)13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaposhnikov MV, Zemskaya NV, Koval LA, Schegoleva EV, Zhavoronkov A, Moskalev AA. Effects of N-acetyl-L-cysteine on lifespan, locomotor activity andstress-resistance of 3 Drosophila species with different lifespans. Aging. 2018;10:2428–2458. doi: 10.18632/aging.101561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brack C, Bechter-Thuring E, Labuhn M. N-acetylcysteine slows down ageing and increases the life span of Drosophila melanogaster. Cell Mol Life Sci. 1997;53:960–966. doi: 10.1007/PL00013199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Flurkey K, Astle CM, Harrison DE. Life extension by diet restriction andN-acetyl-L-cysteine in genetically heterogeneous mice. The Journals ofGerontology Series A, Biological Sciences and Medical Sciences. 2010;65:1275–1284. doi: 10.1093/gerona/glq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kampkötter A, Timpel C, Zurawski RF, Ruhl S, Chovolou Y, Proksch P, Wätjen W. Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2008;149:314–323. doi: 10.1016/j.cbpb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Pietsch K, Saul N, Menzel R, Stürzenbaum SR, Steinberg CE. Quercetin mediatedlifespan extension in Caenorhabditis elegans is modulated by age-1, daf-2, sek-1 and unc-43. Biogerontology. 2009;10:565–578. doi: 10.1007/s10522-008-9199-6. [DOI] [PubMed] [Google Scholar]
- 63.Proshkina E, Lashmanova E, Dobrovolskaya E, Zemskaya N, Kudryavtseva A, Shaposhnikov M, Moskalev A (2016) Geroprotective and radioprotective activity of quercetin, (-)-epicatechin, and ibuprofen in Drosophila melanogaster Front Pharmacol 7:505. 10.3389/fphar.2016.00505 [DOI] [PMC free article] [PubMed]
- 64.Chattopadhyay D, Chitnis A, Talekar A, Mulay P, Makkar M, James J, Thirumurugan K. Hormetic efficacy of rutin to promote longevity in Drosophila melanogaster. Biogerontology. 2017;18:397–411. doi: 10.1007/s10522-017-9700-1. [DOI] [PubMed] [Google Scholar]
- 65.Li S, Li J, Pan R, Cheng J, Cui Q, Chen J, Yuan Z. Sodium rutin extends lifespan and health span in mice including positive impacts on liver health. Br J Pharmacol. 2021. 10.1111/bph.15410. [DOI] [PubMed]
- 66.Rawal S, Singh P, Gupta A, Mohanty S. Dietary intake of Curcuma longa and Emblica officinalis increases life span in Drosophila melanogaster. BioMed Res Int. 2014. [DOI] [PMC free article] [PubMed]
- 67.Cañuelo A, Gilbert-López B, Pacheco-Liñán P, Martínez-Lara E, Siles E, Miranda-VizueteA, Tyrosol, a main phenol present in extra virgin olive oil, increaseslifespan and stress resistance in Caenorhabditis elegans. Mech Ageing Dev. 2012;133:563–574. doi: 10.1016/j.mad.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 68.PatelP Julien J-P, Kriz J. Early-Stage treatment with withaferin a reduces levels of misfolded superoxide dismutase 1 and extends lifespan in a mouse model of amyotrophic lateral sclerosis. Neurotherapeutics. 2015;12:217–233. doi: 10.1007/s13311-014-0311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koval L, Zemskaya N, Aliper A, Zhavoronkov A, Moskalev A. Evaluation of thegeroprotective effects of withaferin A in Drosophila melanogaster. Aging. 2021;13:1817–41. 10.18632/aging.202572. [DOI] [PMC free article] [PubMed]
- 70.McCubrey JA, et al. Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs. Aging (Albany NY) 2017;9(6):1477–1536. doi: 10.18632/aging.101250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Westerheide SD, et al. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–56060. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- 72.Kiaei M, Kipiani K, Petri S, Chen J, Calingasan NY, Beal MF. Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:246–254. doi: 10.1159/000090364. [DOI] [PubMed] [Google Scholar]
- 73.Chellappa K, Perron IJ, Naidoo N, Baur JA. The leptin sensitizer celastrol reduces age-associated obesity and modulates behavioral rhythms. Aging Cell. 2019;18:e12874. doi: 10.1111/acel.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahmad F, Khalid P, Khan MM, Chaubey M, Rastogi AK, Kidwai JR. Hypoglycemie activity of Pterocarpus marsupium wood. J Ethnopharmacol. 1991;35:71–75. doi: 10.1016/0378-8741(91)90134-Y. [DOI] [PubMed] [Google Scholar]
- 75.Maruthupandian A, Mohan V (2011) Antidiabetic, antihyperlipidaemic and antioxidant activity of Pterocarpus marsupium Roxb. in alloxan induced diabetic rats Int J Pharm Tech Res 3:1681–1687
- 76.Halagappa K, Girish HN, Srinivasan BP. The study of aqueous extract of Pterocarpus marsupium Roxb. on cytokine TNF-α in type 2 diabetic rats. Indian J Pharmacol. 2010;42(6):392–396. doi: 10.4103/0253-7613.71922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maurya R, Singh R, Deepak M, Handa SS, Yadav PP, Mishra PK. Constituents of Pterocarpus marsupium: an ayurvedic crude drug. Phytochemistry. 2004;65:915–920. doi: 10.1016/j.phytochem.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 78.Devgun M, Nanda A, Ansari SH (2009) Pterocarpus marsupium roxb. - a comprehensive review Phcog Rev 3:359–363
- 79.Tiwari M, Sharma M, Khare HN. Chemical constituents and medicinal uses of Pterocarpus marsupium roxb. Flora Fauna. 2015;21:55–59. [Google Scholar]
- 80.Li YR, Li S, Lin CC. Effect of resveratrol and pterostilbene on aging and longevity. BioFactors. 2018;44:69–82. doi: 10.1002/biof.1400. [DOI] [PubMed] [Google Scholar]
- 81.Si H, Lai CQ, Liu D (2019) Dietary epicatechin, a novel anti-aging bioactive small molecule Current medicinal chemistry. 10.2174/0929867327666191230104958 [DOI] [PubMed]
- 82.Si H, et al. Dietary epicatechin promotes survival of obese diabetic mice and Drosophila melanogaster. J Nutr. 2011;141:1095–1100. doi: 10.3945/jn.110.134270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bartholome A, Kampkotter A, Tanner S, Sies H, Klotz LO. Epigallocatechin gallate-induced modulation of FoxO signaling in mammalian cells and C. elegans: FoxO stimulation is masked via PI3K/Akt activation by hydrogen peroxide formed in cell culture. Arch Biochem Biophys. 2010;501(1):58–64. doi: 10.1016/j.abb.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 84.Massie HR, Aiello VR, Williams TR. Inhibition of iron absorption prolongs the life span of Drosophila. Mech Ageing Dev. 1993;67:227–237. doi: 10.1016/0047-6374(93)90001-8. [DOI] [PubMed] [Google Scholar]
- 85.Liu M, et al. Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J Biol Chem. 2010;285:36387–36394. doi: 10.1074/jbc.M110.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Demidenko ZN, Blagosklonny MV. At concentrations that inhibit mTOR, resveratrol suppresses cellular senescence. Cell Cycle. 2009;8(12):1901–1904. doi: 10.4161/cc.8.12.8810. [DOI] [PubMed] [Google Scholar]
- 87.Mao L, et al. Berberine decelerates glucose metabolism via suppression of mTOR-dependent HIF-1α protein synthesis in colon cancer cells. Oncol Rep. 2018;39(5):2436–2442. doi: 10.3892/or.2018.6318. [DOI] [PubMed] [Google Scholar]
- 88.Qin H, Dan M, Sha S, Shanshan F, Lin W, Ming D. ERK-dependent mTOR pathway is involved in berberine-induced autophagy in hepatic steatosis. J Mol Endocrinol. 2016;57:251–260. doi: 10.1530/JME-16-0139. [DOI] [PubMed] [Google Scholar]
- 89.Wang N, Feng Y, Zhu M, Tsang C, Man K, Tong Y, Tsao S. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: the cellular mechanism. J Cell Biochem. 2010;111:1426–1436. doi: 10.1002/jcb.22869. [DOI] [PubMed] [Google Scholar]
- 90.Wu T-J, Wang X, Zhang Y, Meng L, Kerrigan JE, Burley SK, Zheng XFS. Identification of a non-gatekeeper hot spot for drug-resistant mutations in mTOR kinase. Cell Rep. 2015;11:446–459. doi: 10.1016/j.celrep.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakashima A, Tamanoi F (2010) Conservation of the Tsc/Rheb/TORC1/S6K/S6 signaling in fission yeast Enzymes 28:167–187. 10.1016/S1874-6047(10)28008-3 [DOI] [PMC free article] [PubMed]
- 92.Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412(2):179–190. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bar DZ, Charar C, Dorfman J, Yadid T, Tafforeau L, Lafontaine DLJ, Gruenbaum Y. Cell size and fat content of dietary-restricted Caenorhabditis elegans are regulated by ATX-2, an mTOR repressor. Proc Natil Acad Sci USA. 2016;113(32):E4620–E4629. doi: 10.1073/pnas.1512156113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Howitz KT, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 95.Kaeberlein M, et al. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 96.Miller RA, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66(2):191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang L, Jie G, Zhang J, Zhao B. Significant longevity-extending effects of EGCG on Caenorhabditis elegans under stress. Free Radic Biol Med. 2009;46:414–421. doi: 10.1016/j.freeradbiomed.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 98.Basu A, Lucas EA. Mechanisms and effects of green tea on cardiovascular health. Nutr Rev. 2007;65:361–375. doi: 10.1111/j.1753-4887.2007.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 99.Yang CS, Wang X. Green tea and cancer prevention. Nutr Cancer. 2010;62(2):931–937. doi: 10.1080/01635581.2010.509536. [DOI] [PubMed] [Google Scholar]
- 100.Yuan X, et al. Green tea liquid consumption alters the human intestinal and oral microbiome. Mol Nutr Food Res. 2018;62:1800178. doi: 10.1002/mnfr.201800178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen T, et al. Green tea polyphenols modify the gut microbiome in db/db mice as co-abundance groups correlating with the blood glucose lowering effect. Mol Nutr Food Res. 2019;63:1801064. doi: 10.1002/mnfr.201801064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nash AK, et al. The gut mycobiome of the human microbiome project healthy cohort. Microbiome. 2017;5:153. doi: 10.1186/s40168-017-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, Boeke JD. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14(2):115–132. doi: 10.1002/(SICI)1097-0061(19980130)14:2<115::AID-YEA204>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 104.Murakami CJ, Burtner CR, Kennedy BK, Kaeberlein M. A method for high-throughput quantitative analysis of yeast chronological life span. J Gerontol A Biol Sci Med Sci. 2008;63:113–121. doi: 10.1093/gerona/63.2.113. [DOI] [PubMed] [Google Scholar]
- 105.Burtner CR, Murakami CJ, Kennedy BK, Kaeberlein M. A molecular mechanism of chronological aging in yeast. Cell Cycle. 2009;8:1256–1270. doi: 10.4161/cc.8.8.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murakami CJ, Wall V, Basisty N, Kaeberlein M. Composition and acidification of the culture medium influences chronological aging similarly in vineyard and laboratory yeast. PLoS ONE. 2011;6:e24530. doi: 10.1371/journal.pone.0024530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Olsen B, Murakami CJ, Kaeberlein M (2010) YODA: software to facilitate high-throughput analysis of chronological life span, growth rate, and survival in budding yeast BMC bioinformatics 11:141. 10.1186/1471-2105-11-141 [DOI] [PMC free article] [PubMed]
- 108.Steffen KK, Kennedy BK, Kaeberlein M. Measuring replicative life span in the budding yeast Journal of visualized experiments. JoVE. 2009;25(28):1209. doi: 10.3791/1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beaupere C, et al. Genetic screen identifies adaptive aneuploidy as a key mediator of ER stress resistance in yeast. Proc Natl Acad Sci U S A. 2018;115:9586–9591. doi: 10.1073/pnas.1804264115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.