Abstract
BACKGROUND:
Osteoporosis is a systemic bone disease characterized by decreased bone density and deterioration of bone microstructure, leading to an increased probability of fragility fractures. Once segmental bone defect occurs, it is easy to cause delayed union and nonunion.
METHODS:
The aim of this study is to investigate the efficacy of extracorporeal shock wave (ESW) and teriparatide-loaded hydrogel (T-Gel) combined strategy on the cell activity and differentiation of osteoporosis derived bone marrow mesenchymal stem cells (OP-BMSCs) in vitro and bone regeneration in osteoporotic segmental bone defects in vivo.
RESULTS:
In vitro, the strategy of combining ESW and T-Gel significantly enhanced OP-BMSCs proliferation, survival, migration, and osteogenic differentiation by up-regulating the alkaline phosphatase activity, mineralization, and expression of runt-related transcription factor-2, type I collagen, osteocalcin, and osteopontin. In the segmental bone defect models of osteoporotic rabbits, Micro-CT evaluation and histological observation demonstrated this ESW-combined with T-Gel injection significantly induced bone healing by enhancing the osteogenic activity of the local microenvironment in osteoporotic defects.
CONCLUSION:
In conclusion, ESW-combined with T-Gel injection could regulate the poor osteogenic microenvironment in osteoporotic defects and show potential for enhancing fragility fractures healing.
Keywords: Osteoporosis, Extracorporeal shock wave, Hydrogel, Teriparatide, Bone generation
Introduction
Osteoporosis is a systemic bone disease characterized by decreased bone density and deterioration of bone microstructure, leading to an increased probability of fragility fractures [1]. The osteoporotic microenvironment lacks bone marrow mesenchymal stem cells (BMSCs). Moreover, osteoporosis derived BMSCs (OP-BMSCs) generally lack osteogenic differentiation activity, as well as low osteoblast capacity and overactive osteoclasts, which ultimately leads to insufficient bone formation and excessive bone loss [2]. With the aging of the population, the prevalence of osteoporosis and the incidence of fragility fractures are increasing in recent years. In addition, the treatment of critical bone defects caused by high-energy trauma, infection, and tumor resection is one of the most challenging clinical issues for orthopedic surgeons, especially in severe osteoporosis patients with poor osteogenic ability [3]. Although bone tissue itself has a good self-healing capacity, it may be still insufficient under some special pathologic states such as critical bone defects in osteoporosis [4]. Once critical bone defects occur in severe osteoporosis, it is easy to cause delayed union, thus resulting in serious complications such as nonunion, bone atrophy, bone deformities, etc. [5, 6]. Therefore, effective strategies to promote critical bone defects healing in osteoporosis is of high clinical significance to address these problems.
Extracorporeal shock wave (ESW) is a special sound wave, which can accelerate the wave by applying a high voltage within a few nanoseconds, and then suddenly release it to generate huge energy [7]. ESW acting on human tissues can produce cavitation effects, increase the expression of growth factors such as transforming growth factor-β (TGF-β), insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF), and induce differentiation of BMSCs towards osteoprogenitor cells to repair the injured tissue [8, 9]. In addition, when the ESW propagates from the skin to the fracture site, the physical stimulation of the shock wave causes microfractures at the fracture ends, thereby inducing the formation of tiny bone callus, promoting the healing of the fracture [10, 11]. Therefore, ESW therapy is a safe and effective alternative strategy to deal with delay-union or nonunion of long bone fractures. However, for some cases of osteoporotic nonunion, shock wave therapy has not yet achieved a satisfactory outcome [12].
At present, the combination of bisphosphonates, estrogen, calcitonin, parathyroid hormone (PTH), and other anti-osteoporosis drugs after orthopedic surgery has been used to promote bone regeneration in patients with osteoporosis [13]. These systemic administration for osteoporosis are often difficult to take effect at the bone defect site to promote local bone regeneration, but always bring numerous adverse events [14]. On the contrary, as a new strategy for managing osteoporosis and osteoporotic bone defects, local controlled-release therapeutic agents with decreased side effects, has begun to catch people’s attention [15, 16]. Teriparatide, also known as 1–34 human PTH, is currently the only anabolic drug approved by the Food and Drug Administration (FDA) for the treatment of osteoporosis. Daily subcutaneous injection of teriparatide 20 μg can significantly increase bone density and decrease the risk of vertebral and non-vertebral fractures in postmenopausal osteoporosis [17, 18]. However, systemic administration of teriparatide brings some unwanted side effects such as headache and nausea [19], and limited improvement on local bone regeneration in defects [20]. These limitations highlight the great need for developing local drug delivery strategies to achieve satisfactory therapeutic efficacy for osteoporotic bone defects.
In this study, Poloxamer 407 hydrogel was used to encapsulate teriparatide to construct an injectable local drug sustained-release system. And then, a synergistic strategy of combining ESW with local injection of teriparatide-loaded hydrogel was applied to enhance segmental bone defects healing and prevent nonunion occurrence in osteoporotic rabbit models. To the best of our knowledge, this is the first attempt to investigate the efficacy of non-invasive therapy combination of ESW and teriparatide-loaded hydrogel in the case of segmental bone defects in osteoporosis, thus, achieving optimal bone repair results and providing a novel strategy for the clinical prevention of bone nonunion.
Materials and methods
Materials
Low Glucose Dulbecco's Modified Eagle's Medium (LG-DMEM), streptomycin–penicillin, fetal bovine serum, and 0.25% trypsin EDTA were supplied by Gibco (Grand Island, NY, USA). Poloxamer 407 purchased from Bayee Chemical Co., Ltd. (Hangzhou, China). Teriparatide powder was purchased from Hangzhou Peptide Biochemical Technology Co., Ltd (Hangzhou, China). Cell Counting Kit-8 (CCK-8) assay and Calcein-AM/Propidium Iodide (PI), Alkaline Phosphatase (ALP) Kit, BCA Protein Assay Kit, and RIPA Lysis Buffer were supplied by Beyotime Biotechnology (Shanghai, China). Medium for osteogenic differentiation of rabbit BMSCs and Alizarin red dye were purchased from Hyclone (Logan, UT, USA). Phosphate buffer (PBS), 4% paraformaldehyde, and Triton X-100 were supplied by Solarbio (Beijing, China). Calcein was supplied by Sigma-Aldrich (St. Louis, MO, USA). Eastep Super Total RNA Extraction Kit was purchased from Promega (Shanghai, China) and Perfect Real Time reagent kit was supplied by Takara Bio (Dalian, China). Hematoxylin eosin (H&E) dye was supplied by Thermo Fisher Scientific (Shanghai, China). Primary antibodies, runt-related transcription factor-2 (Runx-2), type I collagen (Col-1), osteocalcin (OCN), and osteopontin (OPN) were purchased from Abcam (Cambridge, UK), and secondaries antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). Estrogen electrochemical immunoassay kit was purchased from Roche (Mannheim, Germany).
Teriparatide-loaded hydrogel preparation
To prepare the injectable hydrogel with drug loading function, Poloxamer 407 powder was mixed with 0.01 M PBS (pH = 7.4) at a ratio of 25%:75% (w/w). The powder was completely dissolved at 4 °C overnight and transparent solution was formed. The solution was subsequently warmed to 37 °C to change poloxamer 407 from solution into gelation. Rheological measurement of the hydrogel was carried out from 0 °C to 40 °C through a rheometer (Malvern, UK). In addition, the morphology of the hydrogel was observed by a scanning electron microscope (SEM, JSM-6700F, JEOL, Tokyo, Japan) after freeze-drying.
To prepare the teriparatide-loaded hydrogel, teriparatide powder was added into the Poloxamer 407 solution and well-mixed. With the increase of temperature, teriparatide-loaded hydrogels were obtained, and the final concentration of teriparatide in hydrogel was 10–7 M, which was referred to the previous study [21] and its release profiles in the Poloxamer 407 hydrogel. To evaluate the release profiles of teriparatide in the hydrogel, 1 ml teriparatide-loaded hydrogel was prepared and immersed in PBS at 37 °C to detect the release behavior. At predetermined time points, the PBS was collected and replaced by fresh PBS with the same volume. The concentration of released teriparatide was evaluated by high-performance liquid chromatography (HPLC) at 210 nm.
Osteoporosis model establishment and OP-BMSCs extraction
All experimental protocols involving animals and their care were conducted in conformity with NIIH guidelines (NIH Pub. No.85–23, revised 1996) and approved by the Animal Care and Use Ethics Committee of Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine (approval no. SHJT-MRJ-2020–087). New Zealand white rabbits (n = 43, female, five-month-old) were supplied by the Experimental Animal Center of Shanghai Jiaotong University School of Medicine, among them, 40 rabbits were used to establish osteoporosis models by bilateral ovariectomy (OVX), and the remaining three accepted sham surgery as control, according to previous study [15]. Ten months after the OVX operation, the concentration of serum estrogen was tested by an Estrogen electrochemical immunoassay kit according to the manufacturer's protocols. Subsequently, three rabbits in OVX group and sham operation group were sacrificed respectively. The samples of distal radius were collected for Micro-CT examination and quantitative morphometric analysis to confirm the osteoporosis model established successfully.
Once the osteoporosis model was established successfully, OP-BMSCs were extracted and amplified according to the method previously reported [15]. Briefly, Long bones of rabbit limbs were obtained under aseptic conditions. The medullary cavity was opened by bone saw, and then the medullary cavity was repeatedly washed with LG-DMEM by a syringe. After centrifugation at 1000 r/min for 10 min, the supernatant was discarded, and the cells were mixed evenly by LG-DMEM containing 10% FBS (v/v) and 1% penicillin and streptomycin at the atmosphere of 37 °C and 5% CO2. After 24 h of incubation, the non-adherent cells were removed. When cells grew to about 80%–90% confluence, the adherent cells were digested with 0.25% (w/v) trypsin/EDTA at 37 °C for 3 min and passaged. The 3th generation of OP-BMSCs was prepared for further in vitro studies.
OP-BMSCs treated with ESW
The OP-BMSCs, at a density of 1.0 × 106 cells/ml, were suspended in the 15 ml sterile centrifuge tube, and the tube was immersed in the distilled water chamber as the conductive medium of the ESW (PiezoClast EMS, Richard Wolf GmbH, Germany). The parameters of ESW treatment for cell experiments are as follows: 500 pulses, 0.1 mJ/mm2, 4 Hz [9, 22]. After pretreatment with ESW, OP-BMSCs were applied in the related groups for in vitro investigation. Cell experiments were divided into the following four groups, namely, OP-BMSCs without ESW treatment (abbreviated as Con group), OP-BMSCs pretreated with ESW (abbreviated as ESW group), OP-BMSCs co-cultured with teriparatide-loaded hydrogel (abbreviated as T-Gel group), and OP-BMSCs pretreated with ESW and then co-cultured with teriparatide-loaded hydrogel (abbreviated as ESW@T-Gel group), respectively.
Cell viability study
To investigate the effect of ESW and teriparatide-loaded hydrogel on cell viability of OP-BMSCs, CCK-8, Calcein-AM/PI staining, and Transwell test were conducted. OP-BMSCs were seeded in 48-well culture plates at the density of 1 × 104/well. After 1, 4, and 7 days of culture under different conditions, the proliferation of OP-BMSCs was detected by a CCK-8 kit. Briefly, 10% CCK-8 solution was added to each sample after changing the fresh medium. Subsequently, 100 μl solution was extracted from each sample and transferred into a 96-well plate followed by incubated at 37 °C for 2 h. The absorbance of the samples was measured at 450 nm by a Microplate Reader (Multiskan EX, Thermo Fisher Scientific, Waltham, MA, USA).
To research the cell viability of OP-BMSCs treated with different treatments, Calcein-AM/PI staining was conducted after 3 days of incubation according to the manufacturer's instructions. The fluorescent images were observed and recorded by a confocal laser scanning microscope (CLSM, FV1000, Olympus, Tokyo, Japan). The cell survival rate was expressed in the proportion of living cells, which was calculated by Image J software.
In addition, transwell test was conducted to investigate the migration capability of OP-BMSCs in different groups. In brief, OP-BMSCs were seeded in the upper chamber at the density of 1 × 105/well and incubated over 12 h. Subsequently, the samples were treated with 95% ethanol for 15 min, and then stained with 0.2% crystal violet for another 15 min, and then observed through an optical microscope (DSX 500, Olympus, Japan).
Osteogenic differentiation of OP- BMSCs
To detect the efficacy of ESW and teriparatide-loaded hydrogel on osteogenic differentiation of OP-BMSCs, cells were seeded in 12-well plates at the density of 2 × 105/well. After 24 h, the LG-DMEM was replaced by osteogenic induction medium to induce OP-BMSCs osteogenic differentiation for 14 days. The activity of ALP was evaluated by an ALP Assay Kit in the light of the manual. ALP activity was represented by ALP level normalized to the total protein. Moreover, 0.1% alizarin red dye was applied to stain the samples for 30 min at room temperature to study the mineralization of OP-BMSCs. After gross observation, 10% cetylpyridinium chloride was added into the wells to dissolve the mineralized nodules for 30 min, followed by semi-quantitative analysis through a Microplate Reader at 562 nm.
Real-time quantitative PCR (RT-qPCR)
To detect the gene expression of Runx-2, Col-1, OCN, and OPN in the OP-BMSCs at 14 days after osteogenic induction and bone tissue, RT-qPCR was conducted. Primers sequences are summarized in Table 1. The RT-qPCR was conducted through a LightCycler 480 (Roche Diagnostics, Basel, Switzerland) using SYBR Premix Ex Taq Kit according to the manufacturer's instructions. The relative expressions of mRNA were normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and calculated by the 2−ΔΔCt method.
Table 1.
Primer sequences of genes
| Gene | Oligonucleotide Primers (5′-3′) |
|---|---|
| Runx-2 |
F: 5′-ACTACCAGCCACCGAGACCA-3′ R: 5′-ACTGCTTGCAGCCTTAAATGACTCT-3′ |
| Col-1 |
F: 5′-TCCGGCTCCTGCTCCTCTTA-3′ R: 5′-GGCCAGTGTCTCCCTTG-3′ |
| OCN |
F: 5′-AGCCACCGAGACACCATGAGA-3′ R: 5′-AGCCACCGAGACACCATGAGA-3′ |
| OPN |
F: 5′-GCTAAACCCTGACCCATCT-3′ F: 5′-CGTCGGATTCATTGGAGT-3′ |
| GAPDH |
F: 5′-CAATGACCCCTTCATTGACC-3′ R: 5′-TGGACTCCACGACGTACTCA-3′ |
Animals surgical procedures
The segmental radial bone defect models were prepared under general anesthesia by 3% (w/v) pentobarbital at the dose of 50 mg/kg. After disinfecting the skin of the left upper limb, the lateral incision of radius side was selected to expose the tissue layer by layer. A15-mm critical bone defects in the lower radius was prepared by a bone drill, and then the periosteum near both ends of the defects was excised to avoid ossification. Then, the defect was rinsed, and the muscle and skin tissues were sutured layer by layer. After operation, penicillin (1.5 mg/kg) was injected intramuscularly for 3 consecutive days to prevent infection.
Thirty-two osteoporotic rabbits were divided into four groups (n = 8) randomly, namely, without treatment (abbreviated as Con group), treated with ESW (abbreviated as ESW group), injected with 1 ml teriparatide-loaded hydrogel (abbreviated as T-Gel group), and treated with ESW followed by injecting 1 ml teriparatide-loaded hydrogel. ESW treatment and percutaneous injection of teriparatide-loaded hydrogel started 1 week after surgery. For ESW treatment, the probe was placed in bone defects through the skin, and the parameters in vivo were 2000 pulses, 0.1 mJ/mm2, 4 Hz, once a week. Percutaneous injection of teriparatide-loaded hydrogel was biweekly, after receiving ESW therapy. Twelve weeks after the preparation of radius bone defects, the animals were sacrificed by overdose 3% pentobarbital at the dose of 120 mg/kg.
Calcein fluorescent double labeling
Animals were given intramuscular injection with 8 mg/kg calcein 14 days and 4 days respectively before sacrifice. After fixation, the radius samples were embedded in methyl methacrylate and sliced to 40-μm-thick hard tissue sections. The new bone labeled fluorescence was observed by a CLSM. The fluorescence area was quantified by Image J software, and the rate of new bone formation was represented by mineral apposition rate (MAR, μm/day) [23].
Micro-CT
To assess bone formation, the Micro-CT scanning was performed. Briefly, radius samples were assessed with a Micro-CT scanner (Skyscan 1076, Kontich, Belgium) in high-resolution scanning mode a voxel size of 18 mm. Subsequently, quantitative morphometric analysis of bone volume/tissue volume ratio (BV/TV, %) of the columnar-shaped defect region was calculated by micro-CT auxiliary software (NRecon version 1.6.6).
Histological evaluation
After fixation by 4% paraformaldehyde, radius samples were decalcified by decalcification method in the 10% EDTA + ATM solution for 4 weeks. Paraffin sections were prepared for H&E staining and immunofluorescence staining (e.g., Runx-2, Col-1, OCN, and OPN) according to the manufacturer's instructions. Briefly, about 5-μm-thick slides were prepared and blocked with 3% BSA in PBS containing 0.2% Triton X-100 for 1 h. Then, the sections were incubated with primary antibodies, 1:200 Runx-2 anti-mouse polyclonal antibody plus 1:250 Col-1 anti-rabbit monoclonal antibody, 1:150 OCN anti-mouse polyclonal antibody plus 1:200 OPN anti-rabbit polyclonal antibody at 4 °C overnight. After washing with PBS for 3 times, the sections were then incubated with 1:600 Cy3-conjugated goat anti-rabbit or 1:800 goat anti-mouse IgG DyLight 488-conjugated secondary antibodies for 1 h at room temperature. The nuclei were stained with DAPI (1:600). The fluorescence images were observed by CLSM and represented by the average fluorescence intensity of each field at the same condition.
Statistical analysis
All data were expressed as means ± standard deviation, and the statistical analysis were analyzed via Student’s t-test or one-way analysis of variance (ANOVA) by Tukey’s post-hoc analysis (SPSS Inc., Chicago, IL, USA). p < 0.05 was considered to be statistically significant. All experiments were repeated at least 3 times independently.
Results and discussion
Thermosensitive hydrogel preparation and characterization
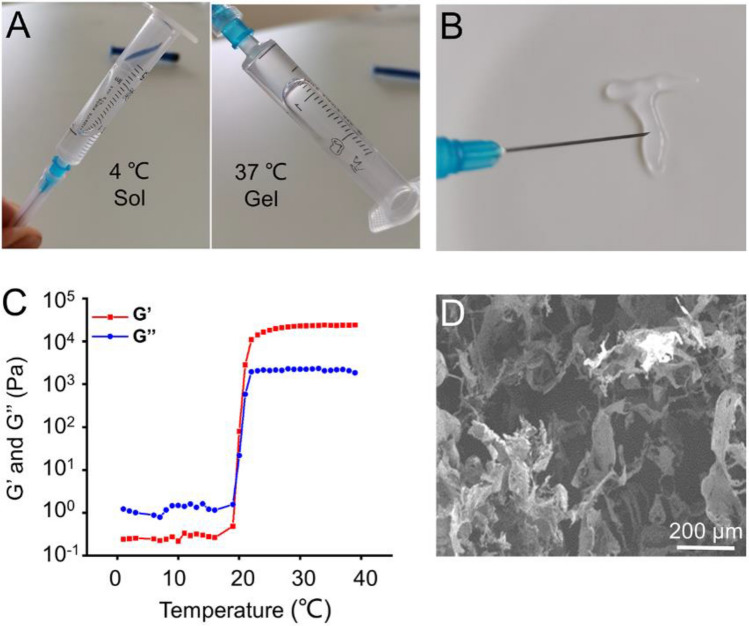
Poloxamer 407 is a thermosensitive material with characteristics of reversible thermo-responsive nature, drug delivery for sustained release, hypotoxicity and high bioavailability and so on, which has been used widely in the food and pharmaceutical industry [24]. Poloxamer 407, α-hydro-ω-hydroxypoly(oxyethylene)poly (oxypropylene)poly- (oxyethylene)block copolymer, is a kind of polymer which is sensitive to temperature change. That is, when the ambient temperature is lower than the critical transition temperature, the polymer exhibits solution state, and the semisolid gel state will appear when the temperature is higher than the critical transition temperature. The formation mechanism of polymer is mainly the reversible change of polymer dispersion or conformation under the action of external temperature, which leads to the change of physical state of polymer, that is, the transformation from solution state to semisolid gel state [25]. As shown in Fig. 1A, the hydrogel underwent a solution-gelation transformation as temperature rose from 4 to 37 °C. The most attractive feature of Poloxamer 407 is its reversible thermo-responsive property, allowing it to undergo gelation near body temperature (∼37 °C) and remain at the site of implantation as a continuous drug delivery device. In low temperature conditions (< 15–25 °C depending on the polymer weight), Poloxamer 407 exists in the form of solution, during which it can be loaded with therapeutics for later release from its gel state [25]. This reversible thermo-responsive nature also enables the hydrogel injectable property. As shown in Fig. 1B, the Poloxamer 407 solution was extruded through a syringe onto a hot plate (37 °C), and it can form a gel state rapidly. The rheological behavior of the thermosensitive hydrogel indicated that both the storage modulus (G′) and loss modulus (G′′) of the hydrogel rapidly increased after 21.2 °C, suggesting that the temperature of the hydrogel to sol–gel transition was 21.2 °C (Fig. 1C). Microstructure of the hydrogel was observed by SEM, which indicated the diameter of the porous structure was about 100− 200 μm (Fig. 1D).
Fig. 1.
A The optical images of the gelation progress. B The injectable and thermosensitive hydrogel rapidly form gel when injected into the hot plate at 37 °C. C Storage modulus (G′) and loss modulus (G′′) of hydrogel as a function of temperature. D Morphologies of hydrogel observed by SEM
This sustained-release performance and injectability provide the possibility for the targeted injection of sustained-release drug systems in vivo to achieve local drug delivery [25]. Profiles of teriparatide released from hydrogel were illustrated in Fig. 2. The release rate of teriparatide in the first three days was slightly faster, and the curves gradually decreased in the following days, indicating a controlled and sustained drug-release rate. On the 14th day, the total proportion of released teriparatide increased to 74.63 ± 2.71%, after which the amount of released teriparatide did not increase significantly. This is probably because the hydrogel has completely degraded in 14 days. In the initial release, drug diffusion likely plays a leading role as the hydrogel has not yet been significantly degraded [26]. A slightly fast release profile initially may be owing to the absorption of the drugs on the hydrogel surface by electrostatic interaction and pore size of 100–200 μm increasing the permeability to teriparatide [27, 28]. Subsequently, the degradation of hydrogel may show a major role in the release process. The slow degradation of hydrogel enables teriparatide to be released in a sustained manner [29]. Our results indicated that the teriparatide-loaded Poloxamer 407 hydrogel could sustained release teriparatide continuously over 14 days, thus providing an excellent local drug delivery system for enhancing bone regeneration.
Fig. 2.
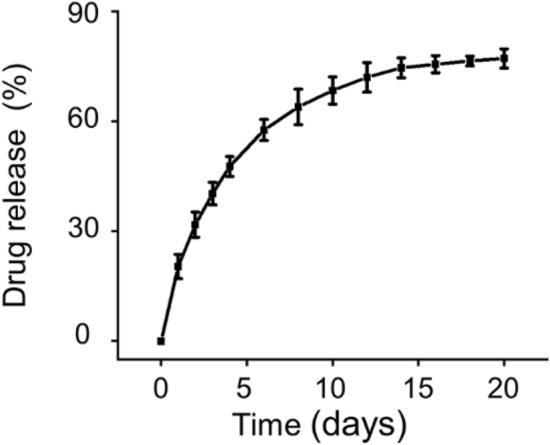
Release profiles of teriparatide in hydrogels
ESW-combined teriparatide-loaded hydrogel promotes OP-BMSCs proliferation, viability and migration
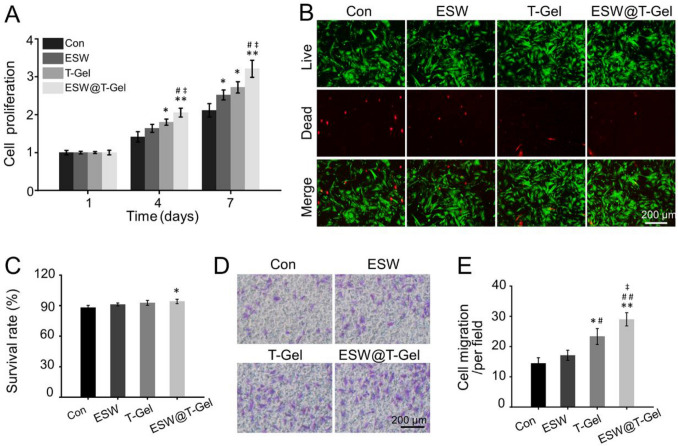
The proliferation, viability, and migration ability of BMSCs in specific microenvironments are crucial for bone generation. As illustrated in Fig. 3A, on the 4th day, the cell proliferation in the T-Gel group was better than that in the Con group (p < 0.05). After 7 days of culture, the proliferation of ESW, T-Gel, and ESW@T-Gel groups were 1.19-fold, 1.29-fold, and 1.52-fold that of Con group, respectively. In addition, no matter on the 4th day or the 7th day, the cell proliferation in the ESW@T-Gel group was always better than that in the Con, ESW, and T-Gel groups (p < 0.05). To observe the viability of OP-BMSCs, Calcein-AM/PI staining was conducted after 3 days of incubation (Fig. 3B). Fluorescence images showed that although most OP-BMSCs in each group were green-stained live cells, the Con group had the most red-stained dead cells and the ESW@T-Gel group had the least red-stained dead cells. Furthermore, the survival rates of OP-BMSCs in the Con, ESW, T-Gel, and ESW@T-Gel groups were 88.21 ± 2.03%, 91.22 ± 1.35%, 92.77 ± 2.42%, 94.18 ± 2.16%, separately, and there was a significant difference between the Con group and ESW@T-Gel group (Fig. 3C). In addition, the migration ability of OP-BMSCs after different treatments was investigated by Transwell test (Fig. 3D). Compared with the Con group and ESW group, the number of migrating cells in the T-Gel group and ESW@T-Gel group was significantly higher, especially in the ESW@T-Gel group (Fig. 3E). These results demonstrated that OP-BMSCs treated with ESW and co-cultured with the teriparatide-loaded hydrogel can significantly increase proliferation, cell survival, and migration ability in vitro.
Fig. 3.
A Effects of different treatment groups on cell proliferation. B Calcein-AM/PI staining indicated the live cells were stained with green and the dead cells were stained with red. C Quantitative analysis of cell survival rate by Calcein AM/PI staining. D The images of migration cells were stained with crystal violet by Transwell test. E Quantitative statistics of the number of migrating cells (*p < 0.05, **p < 0.01 compared with Con group; #p < 0.05, ##p < 0.01 compared with ESW group; ‡p < 0.05 compared with T-Gel group)
ESW-combined teriparatide-loaded hydrogel induces OP-BMSCs osteogenic differentiation
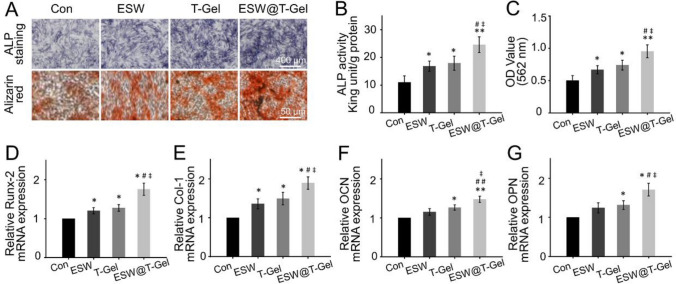
In addition to the proliferation, cell survival, and migration of osteoblast-related cells, osteogenic differentiation of OP-BMSCs is a crucial event for bone healing. Due to the self-proliferation and osteogenic differentiation potential, BMSCs have been widely applied in bone tissue engineering. However, how to enhance the osteogenic ability of OP-BMSCs in osteoporotic bone defects is still a clinical challenge [30]. Herein, we attempted to use the strategy of combination ESW and teriparatide-loaded hydrogel to induce OP-BMSCs osteogenic differentiation. After 14 days of osteogenic induction, the ALP activity evaluation and alizarin red staining were conducted to detect the osteogenic differentiation of OP-BMSCs (Fig. 4A). ALP activity of the ESW, T-Gel, and ESW@T-Gel groups was significantly higher than that of the Con group (Fig. 4B). Simultaneously, the ALP activity of ESW@T-Gel group was also increased by 1.46-fold and 1.37-fold than that of ESW group (p < 0.05) and T-Gel group (p < 0.05), respectively. High expression of ALP activity is an early marker of osteoblasts differentiation and maturation. In addition, alizarin red staining was carried out to identify deposited calcium nodules, which is an important feature of mineralization. Semi-quantitative analysis of alizarin red staining further confirmed that this change was consistent with the results of ALP activity detection (Fig. 4C).
Fig. 4.
A Images of ALP staining and alizarin red staining after 14 days of osteogenic induction. B statistical analysis of ALP activity. C Semi-quantitative analysis of alizarin red staining. D–G The mRNA expression levels of osteogenic-related genes, including Runx-2, Col-1, OCN, and OPN in OP-BMSCs after osteogenic induction for 14 days (*p < 0.05, **p < 0.01 compared with Con group; #p < 0.05, ##p < 0.01 compared with ESW group; ‡p < 0.05 compared with T-Gel group)
Subsequently, RT-qPCR assay was conducted to study the critical markers of osteogenic differentiation at gene level. As exhibited in Fig. 4D–G, compared with the Con group, OP-BMSCs treated with ESW indicated higher expression levels of Runx-2 and Col-1, however, in the T-Gel group and ESW@T-Gel group, all detected genes, including Runx-2, Col-1, OCN, and OPN, were elevated significantly. In addition, compared with ESW treatment or co-cultured with teriparatide-loaded hydrogel alone, the results of combination ESW and teriparatide-loaded hydrogel achieved an enhanced efficacy on osteogenic differentiation (p < 0.05). The activation of Runx-2 is a crucial event in the early osteogenic differentiation, and Runx-2 can enhance the expression of OCN and OPN, subsequently [15, 16]. Col-1, OCN, and OPN, which synthesized and secreted by osteoblasts, usually indicate the late stage of osteogenesis and maturation [31]. Enhancing expression of these genes demonstrated that the OP-BMSCs treated with ESW and teriparatide-loaded hydrogel have the tendency of osteogenic differentiation, suggesting the combination strategy has potential application in the repair of osteoporotic bone defects.
Establishment of osteoporosis model
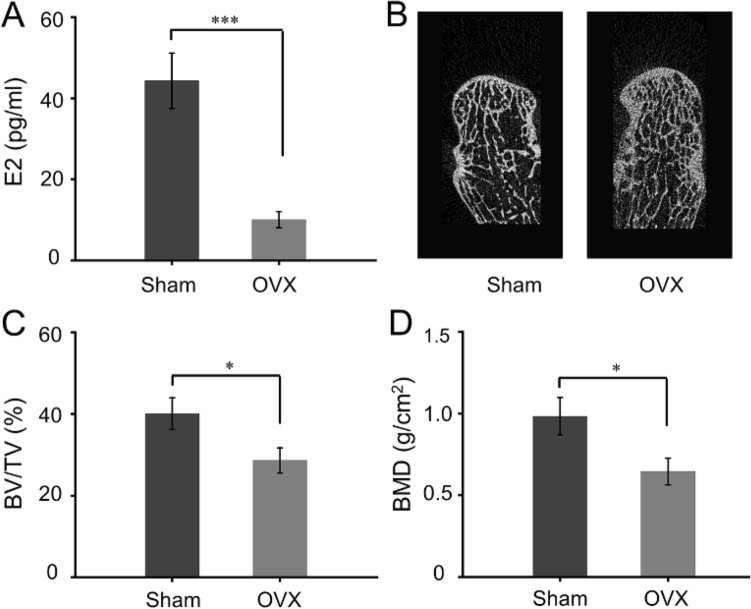
Postmenopausal estrogen deficiency enhances the bone resorption and weakens the property of osteoblast. This imbalance between insufficient bone formation and excessive bone resorption resulting in osteoporosis by reducing BV/TV and bone mineral density (BMD) [32]. Ten months after bilateral OVX, serum estrogen level in Sham group and OVX group was 44.31 ± 6.81 pg/ml and 10.06 ± 2.02 pg/ml, respectively, which indicated a significant difference between the groups (Fig. 5A). Furthermore, Micro-CT scanned radius samples to evaluate the microstructure and bone mass. Reconstructed images displayed trabecular bone became thinner and sparse in OVX group (Fig. 5B). In addition, the quantitative analysis of Micro-CT revealed that the BV/TV and BMD in the OVX group were significantly decreased compared to Sham group (p < 0.05). Based on the above results, it can be proved that the osteoporosis model was successfully prepared 10 months after bilateral OVX.
Fig. 5.
A Serum estrogen (E2) levels at 10 months after OVX. B Reconstruction images of distal radius at 10 months after OVX. C Quantitative analysis of BV/TV according to Micro-CT examination. D Quantitative analysis of BMD according to Micro-CT examination (*p < 0.05, ***p < 0.001 compared with Sham group)
ESW-combined teriparatide-loaded hydrogel promotes bone formation in vivo
The repair of critical bone defects is still a great challenge in clinical, especially for patients with osteoporosis. The declined number and function of endogenous BMSCs in the osteoporotic microenvironment often leads to the fracture difficult to heal, eventually leading to nonunion and disability [33]. In our in vitro detection, the results successfully proved that the combination of ESW and teriparatide-loaded hydrogel could induce OP-BMSCs proliferation, viability, migration, and osteogenic differentiation. Herein, we investigate the bone regeneration effectiveness of ESW treatment and local injection of teriparatide-loaded hydrogel in segmental bone defects in osteoporosis.
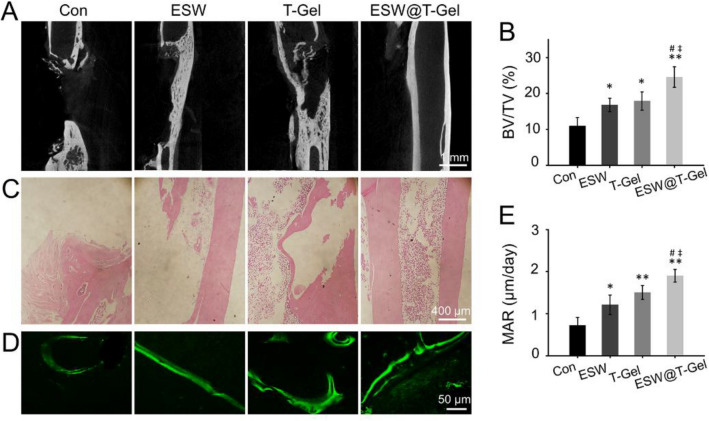
Three months after bone defects preparation, Micro-CT examination was conducted to research the therapeutic efficacy of bone repair. As illustrated in Fig. 6A, there was no bridging callus formation, the medullary cavity of the fractured end had been sealed by dense hardened bone, and the segmental nonunion was obvious in the Con group. Partial regeneration in the defects was observed after ESW treatment alone, but the bone formation was still limited. In the T-Gel group, the imaging pictures indicated that there was continuous regeneration of cortical bone on one side, but incomplete cortical regeneration on the other side. However, the bone defects in the ESW@T-Gel group was completely regenerated and the medullary cavity was recanalized. Quantitative morphometric analysis according to Micro CT exhibited that the BV/TV values of the Con, ESW, T-Gel, and ESW@T-Gel groups were 10.71 ± 2.23%, 16.19 ± 2.30%, 18.59 ± 2.81%, and 24.43 ± 2.15%, respectively (Fig. 6B).
Fig. 6.
A Tomographic images of radius defects by Micro-CT. B Quantitative analysis of BV/TV (%) according to Micro-CT at 12 weeks after treatment. C H and E staining to observe bone regeneration in segmental bone defects. D Representative images of calcein fluorescence double-labeling of regenerated bone in defects. E Quantitative statistics of MAR by calcein fluorescence double-labeling (*p < 0.05, **p < 0.01 compared with Con group; #p < 0.05 compared with ESW group; ‡p < 0.05 compared with T-Gel group)
Histological observation of H and E staining indicated that the bone defects in the Con group were filled with fibrous tissue, as well as sclerotic bone formed and medullary cavity closed. The bilateral cortical bone in the ESW@T-Gel group regenerated completely to achieve a continuous medullary cavity structure, which was similar to the original state (Fig. 6C). These results revealed that ESW treatment or local injection of teriparatide-loaded hydrogel can improve the healing of segmental bone defects, and the combination strategy acquired a more satisfactory repair. Calcein fluorescent double-labeling was also used to evaluate the bone formation rate (Fig. 6D). The results showed that the MAR of ESW, T-Gel, and ESW@T-Gel groups was 1.21 ± 0.23 μm/day, 1.50 ± 0.16 μm/day, and 1.90 ± 0.15 μm/day, which indicated a significant faster new bone formation rate than the Con group (0.72 ± 0.19 μm/day, p < 0.05) (Fig. 6E).
In vivo osteogenesis-related markers expression
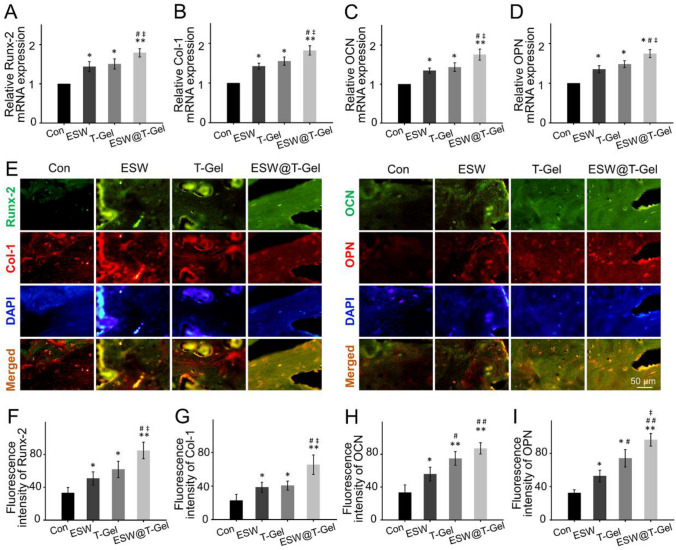
To research the in vivo mechanism of ESW treatment and local injection of teriparatide-loaded hydrogel promoting bone healing, regenerated bone tissue undergone RT-qPCR detection and immunofluorescence staining of osteogenesis-related markers, e.g., Runx-2, Col-1, OCN, and OPN. These results demonstrated ESW treatment and local injection of teriparatide-loaded hydrogel significant activated the expression of osteogenic related genes, especially in the ESW@T-Gel group (Fig. 7A). For immunofluorescence staining, the Con groups gave out weak fluorescence, ESW group and T-Gel group indicated moderate intensity fluorescence, and ESW@T-Gel group delivered the strongest fluorescence (Fig. 7E). Quantitative analysis revealed that the fluorescence intensity of Runx-2, Col-1, OCN, and OPN in newly formed bone matrix at ESW, T-Gel, and ESW@T-Gel groups was significantly higher than that of the Con group (p < 0.05), indicating that endogenous BMSCs in these groups were undergoing a better osteogenic differentiation for enhancing bone healing, especially in the ESW@T-Gel group (Fig. 7F–I).
Fig. 7.
A–D The mRNA expression levels of osteogenic-related genes, including Runx-2, Col-1, OCN, and OPN in regenerated bone tissue. E–I Immunofluorescence staining and quantitative analysis of Runx-2, Col-1, OCN, and OPN in regenerated bone tissue (*p < 0.05, **p < 0.01 compared with Con group; #p < 0.05, ##p < 0.01 compared with ESW group; ‡p < 0.05 compared with T-Gel group)
Osteoporosis patients with low bone regeneration ability are often difficult to self-healing after the occurrence of critical bone defects, thus resulting in nonunion and disability [33]. This requires the introduction of other factors to enhance the osteogenic differentiation of osteoblast-related cells to induce bone regeneration [34]. In this study, we taken the combination of ESW treatment and local injection of teriparatide-loaded hydrogel to induce OP-BMSCs osteogenic differentiation and improve bone healing successfully in segmental bone defects in osteoporosis. ESW is a high-energy pressure wave generated by the sudden release of energy, which has the characteristics of instantaneous pressure increase and high-speed conduction [7]. Since the 1990s, clinicians start to use ESW to the deal with some orthopedic diseases and demonstrated that ESW has the effect of stimulating bone formation, and has achieved satisfactory effects in enhancing healing and reducing delay-union or nonunion of fractures [35, 36]. In this study, the OP-BMSCs pretreated with ESW in vitro following the parameters of 500 pulses, 0.1 mJ/mm2, 4 Hz, had a positive effect on proliferation on day 7 and osteogenic differentiation, without affecting cell viability, which is consistent with what is reported previously [22]. Previous studies have shown that ESW promoted bone healing by up-regulating the expression of osteogenesis related growth factors, as well as extracellular signal-regulated kinase, p38 kinase signal, and Wnt/β-catenin signal [9, 37, 38]. Furthermore, ESW has been shown to elicit membrane perturbation, as well as Ras activation, resulting in the induction of nuclear osteogenic transcription factor activation, expression of Col 1 and OCN, and thus enhance terminal calcium nodule formation [39]. ESW also activates the genes expression of BMP, OCN, ALP, TGF-β1, and IGF, which induce the growth and differentiation of BMSCs towards osteoprogenitor cells [40]. Herein, our study not only found that ESW can promote BMSCs proliferation and osteogenesis in vitro, but also promote the healing of segmental bone defects in vivo to a certain extent by up regulating osteogenic markers, e.g., Runx-2, Col-1, OCN, and OPN.
Considering that ESW alone may be difficult to obtain satisfactory results for critical bone defects in severe osteoporosis. Therefore, additional treatments need to be added to optimize the outcome. Teriparatide, the only anabolic drug approved by FDA to treat osteoporosis, was revealed can significantly increase BMD and decrease the risk of vertebral and non-vertebral fractures in postmenopausal osteoporosis [17, 18]. However, systemic administration of teriparatide brings limited improvement on local bone regeneration in defects [20]. The limitation highlights the great need for local drug delivery strategies to achieve satisfactory therapeutic effects for osteoporotic bone defects. Wang et al. prepared a photothermally triggered biomimetic drug delivery of teriparatide for enhancing osteoporotic bone defects healing successfully [21]. However, considering the complexity of the preparation of the intelligent responsive release system and the difficulty of clinical transformation, we used commercial Poloxamer 407 hydrogel as the delivery system of teriparatide in this study. When OP-BMSCs co-cultured with teriparatide-loaded hydrogel, their proliferation, migration, and osteogenic differentiation ability were significantly improved, even this enhancement was more obvious than ESW treatment alone. There have been various promising strategies for bone regeneration of critical bone defects, but there is not good therapy available for clinical transformation and practical application [41, 42]. In this study, commercial Poloxamer 407 hydrogel, teriparatide, and ESW been widely studied and applied in clinical practice, respectively. Therefore, this new technology is more feasible in ethical approval for clinical application. The strategy of combination ESW with teriparatide-loaded hydrogel can more optimally promote OP-BMSCs proliferation, survival, migration, and osteogenic differentiation in vitro, and achieve complete bone regeneration and medullary cavity recanalization in segmental bone defects in osteoporosis, thus, providing a novel strategy for the clinical prevention of bone nonunion.
Conclusion
To address the challenges in the treatment of segmental bone defects in osteoporosis, we developed a novel therapeutic strategy, combined ESW treatment with local injection teriparatide-loaded hydrogel. The superior efficacy of this therapy was systematically proved by its biological functions of promoting OP-BMSCs proliferation, viability, migration, and osteogenic differentiation in vitro, and significantly improving the healing of segmental bone defects in vivo, thus providing a new strategy for the treatment of bone defects that can be used clinically for osteoporosis.
Acknowledgements
This study has been supported by Health Science and Technology Plan Project of Zhejiang Province (Nos. 2021KY495 and 2021KY506).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
All animal procedures were performed in accordance with the guidelines for Care and Use of Laboratory Animal Experience and approved by the approved by the Animal Care and Use Ethics Committee of Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine (approval no. SHJT-MRJ-2020–087).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qi Chen, Chen Xia, and Binbin Shi have contribute equally to this work.
References
- 1.Porwal K, Pal S, Bhagwati S, Siddiqi MI, Chattopadhyay N. Therapeutic potential of phosphodiesterase inhibitors in the treatment of osteoporosis: Scopes for therapeutic repurposing and discovery of new oral osteoanabolic drugs. Eur J Pharmacol. 2021;899:174015. doi: 10.1016/j.ejphar.2021.174015. [DOI] [PubMed] [Google Scholar]
- 2.Hampson G, Elder GJ, Cohen-Solal M, Abrahamsen B. A review and perspective on the assessment, management and prevention of fragility fractures in patients with osteoporosis and chronic kidney disease. Endocrine. 2021;73:509–29. [DOI] [PMC free article] [PubMed]
- 3.Zhang C, Zhang T, Geng T, Wang X, Lin K, Wang P. Dental implants loaded with bioactive agents promote osseointegration in osteoporosis: a review. Front Bioeng Biotechnol. 2021;9:591796. doi: 10.3389/fbioe.2021.591796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russow G, Jahn D, Appelt J, Märdian S, Tsitsilonis S, Keller J. Anabolic therapies in osteoporosis and bone regeneration. Int J Mol Sci. 2019;20(1):83. doi: 10.3390/ijms20010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottai V, Dell'Osso G, Celli F, Bugelli G, Cazzella N, Cei E, et al. Total hip replacement in osteoarthritis: the role of bone metabolism and its complications. Clin Cases Miner Bone Metab. 2015;12:247–250. doi: 10.11138/ccmbm/2015.12.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandi ML. Healing of the bone with anti-fracture drugs. Expert Opin Pharmacother. 2013;14(11):1441–1447. doi: 10.1517/14656566.2013.801959. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi M, Chijimatsu R, Yoshikawa H, Yoshida K. Extracorporeal shock wave therapy accelerates endochondral ossification and fracture healing in a rat femur delayed-union model. Biochem Biophys Res Commun. 2020;530:632–637. doi: 10.1016/j.bbrc.2020.07.084. [DOI] [PubMed] [Google Scholar]
- 8.Wang CJ, Chen HS, Chen CE, Yang KD. Treatment of nonunions of long bone fractures with shock waves. Clin Orthop Relat Res. 2001;387:95–101. doi: 10.1097/00003086-200106000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Xu J, Huang Z, Yu M, Zhang Y, Chen H, et al. An innovative approach for enhancing bone defect healing using plga scaffolds seeded with extracorporeal-shock-wave-treated bone marrow mesenchymal stem cells (BMSCs) Sci Rep. 2017;7:44130. doi: 10.1038/srep44130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gollwitzer H, Gloeck T, Roessner M, Langer R, Horn C, Gerdesmeyer L, et al. Radial extracorporeal shock wave therapy (rESWT) induces new bone formation in vivo: results of an animal study in rabbits. Ultrasound Med Biol. 2013;39:126–133. doi: 10.1016/j.ultrasmedbio.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Zhang C, Huang H, Yang L, Duan X. Extracorporeal shock wave therapy for pain relief after arthroscopic treatment of osteochondral lesions of talus. J Foot Ankle Surg. 2020;59:190–194. doi: 10.1053/j.jfas.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Willems A, van der Jagt OP, Meuffels DE. Extracorporeal shock wave treatment for delayed union and nonunion fractures: a systematic review. J Orthop Trauma. 2019;33:97–103. doi: 10.1097/BOT.0000000000001361. [DOI] [PubMed] [Google Scholar]
- 13.Black DM, Rosen CJ. Clinical Practice. Postmenopausal Osteoporosis. N Engl J Med. 2016;374:254–62. [DOI] [PubMed]
- 14.Zeng J, Guo J, Sun Z, Deng F, Ning C, Xie Y. Osteoblastic and anti-osteoclastic activities of strontium-substituted silicocarnotite ceramics: In vitro and in vivo studies. Bioact Mater. 2020;5:435–446. doi: 10.1016/j.bioactmat.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai H, Cui Y, Wang C, Wang Z, Luo W, Liu Y, et al. 3D printed porous biomimetic composition sustained release zoledronate to promote osteointegration of osteoporotic defects. Mater Des. 2020;189:108513. doi: 10.1016/j.matdes.2020.108513. [DOI] [Google Scholar]
- 16.Bai H, Zhao Y, Wang C, Wang Z, Wang J, Liu H, et al. Enhanced osseointegration of three-dimensional supramolecular bioactive interface through osteoporotic microenvironment regulation. Theranostics. 2020;10:4779–4794. doi: 10.7150/thno.43736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Napoli N, Langdahl BL, Ljunggren O, Lespessailles E, Kapetanos G, Kocjan T, et al. Effects of teriparatide in patients with osteoporosis in clinical practice: 42-month results during and after discontinuation of treatment from the european extended forsteo (r) observational study (ExFOS) Calcif Tissue Int. 2018;103:359–371. doi: 10.1007/s00223-018-0437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Q, Guo M, Ma X, Pu Y, Long Y, Xu Y. Adherence to teriparatide treatment and risk of fracture: a systematic review and meta-analysis. Horm Metab Res. 2019;51:785–791. doi: 10.1055/a-1062-9447. [DOI] [PubMed] [Google Scholar]
- 19.Tarantino U, Iolascon G, Cianferotti L, Masi L, Marcucci G, Giusti F, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18:S3–S36. doi: 10.1007/s10195-017-0474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stancoven BW, Lee J, Dixon DR, McPherson JC 3rd, Bisch FC, Wikesjö UM, et al. Effect of bone morphogenetic protein-2, demineralized bone matrix and systemic parathyroid hormone (1–34) on local bone formation in a rat calvaria critical-size defect model. J Periodontal Res. 2013;48:243–51. [DOI] [PubMed]
- 21.Wang X, Guo W, Li L, Yu F, Li J, Liu L, et al. Photothermally triggered biomimetic drug delivery of Teriparatide via reduced graphene oxide loaded chitosan hydrogel for osteoporotic bone regeneration. Chem Eng J. 2021;413:127413. doi: 10.1016/j.cej.2020.127413. [DOI] [Google Scholar]
- 22.Alshihri A, Niu W, Kämmerer PW, Al-Askar M, Yamashita A, Kurisawa M, et al. The effects of shock wave stimulation of mesenchymal stem cells on proliferation, migration, and differentiation in an injectable gelatin matrix for osteogenic regeneration shockwave effect on mesenchymal stem cells in hydrogel. J Tissue Eng Regen Med. 2020;14:1630–1640. doi: 10.1002/term.3126. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Li Z, Jiang Y, Liu H, Feng Y, Wang Z, et al. Bioinspired mineral hydrogels as nanocomposite scaffolds for the promotion of osteogenic marker expression and the induction of bone regeneration in osteoporosis. Acta Biomater. 2020;113:614–626. doi: 10.1016/j.actbio.2020.06.024. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh HY, Lin WY, Lee AL, Li YC, Chen YJ, Chen KC, et al. Hyaluronic acid on the urokinase sustained release with a hydrogel system composed of poloxamer 407: HA/P407 hydrogel system for drug delivery. PLoS One. 2020;15:e0227784. doi: 10.1371/journal.pone.0227784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beard MC, Cobb LH, Grant CS, Varadarajan A, Henry T, Swanson EA, et al. Autoclaving of poloxamer 407 hydrogel and its use as a drug delivery vehicle. J Biomed Mater Res B Appl Biomater. 2021;109:338–347. doi: 10.1002/jbm.b.34703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarafder S, Bose S. Polycaprolactone-coated 3D printed tricalcium phosphate scaffolds for bone tissue engineering: in vitro alendronate release behavior and local delivery effect on in vivo osteogenesis. ACS Appl Mater Interfaces. 2014;6:9955–9965. doi: 10.1021/am501048n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Figueroa-Pizano MD, Velaz I, Penas FJ, Zavala-Rivera P, Rosas-Durazo AJ, Maldonado-Arce AD, et al. Effect of freeze-thawing conditions for preparation of chitosan-poly (vinyl alcohol) hydrogels and drug release studies. Carbohydr Polym. 2018;195:476–485. doi: 10.1016/j.carbpol.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Gao Y, Chen Y, Ji X, He X, Yin Q, Zhang Z, et al. Controlled intracellular release of doxorubicin in multidrug-resistant cancer cells by tuning the shell-pore sizes of mesoporous silica nanoparticles. ACS Nano. 2011;5:9788–9798. doi: 10.1021/nn2033105. [DOI] [PubMed] [Google Scholar]
- 29.Posadowska U, Parizek M, Filova E, Wlodarczyk-Biegun M, Kamperman M, Bacakova L, et al. Injectable nanoparticle-loaded hydrogel system for local delivery of sodium alendronate. Int J Pharm. 2015;485:31–40. doi: 10.1016/j.ijpharm.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Guo Y, Ren L, Xie K, Wang L, Yu B, Jiang W, et al. Functionalized TiCu/Ti-Cu-N-coated 3D-printed porous Ti6Al4V scaffold promotes bone regeneration through BMSC recruitment. Adv Mater Interfaces. 2020;7:1901632. doi: 10.1002/admi.201901632. [DOI] [Google Scholar]
- 31.Rashdan NA, Sim AM, Cui L, Phadwal K, Roberts FL, Carter R, et al. Osteocalcin regulates arterial calcification via altered wnt signaling and glucose metabolism. J Bone Miner Res. 2020;35:357–367. doi: 10.1002/jbmr.3888. [DOI] [PubMed] [Google Scholar]
- 32.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Zhou K, Xiao F, Huang Z, Xu J, Chen G, et al. Identification of circRNA-associated ceRNA network in BMSCs of OVX models for postmenopausal osteoporosis. Sci Rep. 2020;10:10896. doi: 10.1038/s41598-020-67750-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song JE, Tian J, Kook YJ, Thangavelu M, Choi JH, Khang G. A BMSCs-laden quercetin/duck's feet collagen/hydroxyapatite sponge for enhanced bone regeneration. J Biomed Mater Res A. 2020;108:784–794. doi: 10.1002/jbm.a.36857. [DOI] [PubMed] [Google Scholar]
- 35.Ozkan E, Bereket MC, Onger ME, Polat AV. The effect of unfocused extracorporeal shock wave therapy on bone defect healing in diabetics. J Craniofac Surg. 2018;29:1081–1086. doi: 10.1097/SCS.0000000000004303. [DOI] [PubMed] [Google Scholar]
- 36.Silveira A, Koenig JB, Arroyo LG, Trout D, Moens NM, LaMarre J, et al. Effects of unfocused extracorporeal shock wave therapy on healing of wounds of the distal portion of the forelimb in horses. Am J Vet Res. 2010;71:229–234. doi: 10.2460/ajvr.71.2.229. [DOI] [PubMed] [Google Scholar]
- 37.Xu JK, Chen HJ, Li XD, Huang ZL, Xu H, Yang HL, et al. Optimal intensity shock wave promotes the adhesion and migration of rat osteoblasts via integrin beta1-mediated expression of phosphorylated focal adhesion kinase. J Biol Chem. 2012;287:26200–26212. doi: 10.1074/jbc.M112.349811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suhr F, Delhasse Y, Bungartz G, Schmidt A, Pfannkuche K, Bloch W. Cell biological effects of mechanical stimulations generated by focused extracorporeal shock wave applications on cultured human bone marrow stromal cells. Stem Cell Res. 2013;11:951–964. doi: 10.1016/j.scr.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Wang FS, Wang CJ, Huang HJ, Chung H, Chen RF, Yang KD. Physical shock wave mediates membrane hyperpolarization and ras activation for osteogenesis in human bone marrow stromal cells. Biochem Bioph Res Commun. 2001;287:648–655. doi: 10.1006/bbrc.2001.5654. [DOI] [PubMed] [Google Scholar]
- 40.Wang CJ, Yang KD, Ko JY, Huang CC, Huang HY, Wang FS. The effects of shockwave on bone healing and systemic concentrations of nitric oxide (NO), TGF-beta 1, VEGF and BMP-2 in long bone non-unions. Nitric Oxide. 2009;20:298–303. doi: 10.1016/j.niox.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Lu J, Cheng C, He YS, Lyu C, Wang Y, Yu J, et al. Multilayered graphene hydrogel membranes for guided bone regeneration. Adv Mater. 2016;28:4025–4031. doi: 10.1002/adma.201505375. [DOI] [PubMed] [Google Scholar]
- 42.Huang L, Zhang J, Hu J, Zhao T, Gu Z. Biomimetic gelatin methacrylate/nano fish bone hybrid hydrogel for bone regeneration via osteoimmunomodulation. ACS Biomater Sci Eng. 2020;6:3270–3274. doi: 10.1021/acsbiomaterials.0c00443. [DOI] [PubMed] [Google Scholar]