Abstract
Purpose:
To summarize key findings from a systematic review of the effectiveness and safety of transepithelial corneal crosslinking (CXL) compared with versus epithelium-off CXL for progressive keratoconus.
Design:
Cochrane systematic review
Methods:
We included in our review only randomized controlled trials (RCTs) in which transepithelial and epithelium-off CXL had been compared among participants with progressive keratoconus. The primary outcome was keratoconus stabilization based on post-operative maximum keratometry (Kmax). We adhered to Cochrane methods for trial selection, data extraction, risk of bias evaluation, and data synthesis.
Results:
13 RCTs with 567 participants (661 eyes) were included; 11 studies compared non-iontophoresis-assisted transepithelial with epithelium-off CXL. Keratoconus stabilization was described as an outcome in 2 studies. The estimated difference in Kmax means (MD) from meta-analysis of 177 eyes in 5 RCTs indicated that there was no difference between intervention groups in Kmax at 12 months or later (MD 0.99 D; 95% CI −0.11 to 2.09). Meta-analysis of keratometry and visual acuity outcomes at 12 months or longer after surgery from 2 studies that had compared transepithelial CXL using iontophoresis provided no conclusive evidence of an advantage over epithelium-off CXL.
Conclusions:
Lack of precision due to small sample sizes, indeterminate risk of bias due to inadequate reporting, and inconsistency in how outcomes were measured and reported among studies make it difficult to state with confidence whether transepithelial CXL confers an advantage over epithelium-off CXL for patients with progressive keratoconus with respect to stabilization of keratoconus, visual acuity, or patient-reported outcomes based on available data.
Table of Contents Statement
Based on a systematic review and meta-analysis, it remains unknown whether transepithelial corneal crosslinking (CXL) confers an advantage over epithelium-off CXL for patients with progressive keratoconus with respect to keratoconus stabilization, visual acuity, or patient-reported outcomes. Methods of defining, assessing, and reporting keratoconus progression and peri-, intra-, and postoperative care should be standardized to increase precision of estimates for outcomes and to allow meaningful comparisons of CXL methods.
INTRODUCTION
Keratoconus can cause loss of uncorrected and best-corrected distance visual acuity (UDVA and CDVA, respectively) through corneal perforation, ectasia of the central or paracentral cornea or corneal scarring, all of which can lead to irregular astigmatism. Keratoconus is bilateral but often asymmetric in severity.1 The condition may be influenced by environmental factors but is often described as the most common corneal dystrophy. The prevalence in the Netherlands was recently estimated to be 1:375 (265 cases per 100,000, 95% confidence interval [CI]: 260 to 270),2 which is six times higher than the previous estimate of 1:2000 from a 48-year clinical and epidemiologic study of keratoconus in residents of Olmsted County, Minnesota.3 The annual incidence of keratoconus of 1:7500 (13.3 cases per 100 000, 95% CI: 11.6 to 15.2) also was five to 10 times higher than previous population studies reported.2 The reason for increased prevalence and incidence is likely improved detection with increased use of corneal imaging.
Multiple topographic and tomographic imaging modalities and diagnostic indices exist. There is, however, no universal method to diagnose keratoconus nor are there standardized criteria to judge progressive keratoconus. Newer Scheimpflug-based tomography helps distinguish subclinical keratoconus from normal corneas by interpretation and display of change in either anterior and posterior elevations, location of and corneal pachymetry at the thinnest point, pachymetric progression, and maximum keratometry (Kmax for “maximum K” to denote the maximum curvature power of the whole anterior corneal surface; also known as maximum cone apex curvature).4 Increase in Kmax by 1 diopter (D) or more remains the most frequently reported index of disease progression,5–8 more so than worsening of refractive or corneal astigmatism or change in UDVA or CDVA9,10 or worsening of corneal topographical indices other than Kmax.
Corneal collagen crosslinking (CXL) using ultraviolet A (UVA) light applied to the cornea is the only treatment shown to slow progression of keratoconus. Theoretically CXL slows keratoconus progression by strengthening and stabilizing the collagen lamellae, resulting in corneal mechanical stiffening. CXL may improve refractive error by reducing the irregular astigmatism caused by corneal biochemical instability11 and preventing further corneal steepening. The original technique involves application of UVA light to de-epithelialized cornea to which riboflavin, a photosensitizer, is added topically before and during irradiation. Transepithelial CXL is a newer alternative with many variants in which the epithelium is not denuded, offering the putative advantages of faster healing, less patient discomfort, faster visual rehabilitation, and less risk of corneal haze. An increasing number of studies comparing transepithelial and epithelium-off CXL are being undertaken to determine which technique better achieves the stated goal of CXL, which is to halt or slow progression of keratoconus.
Because estimation of the rate of turnover of collagen and the extracellular matrix of the corneal stroma may require years or decades, long-term follow-up after CXL is essential to determine longevity of effects and to identify long-term complications.7,9,10, 12–15 The main objective of this summary of our Cochrane systematic review16 is to report the comparative safety and effectiveness of transepithelial CXL compared with epithelium-off CXL based on the best currently available evidence.
METHODS
Adhering to the methods in the Cochrane Handbook for Systematic Reviews of Interventions, we included randomized controlled trials (RCTs) in our review.17 Methods are summarized below; details are given in the full Cochrane systematic review.16 Eligible trials compared transepithelial CXL with epithelium-off CXL for treatment of progressive keratoconus. We excluded studies of participants with corneal ectasia of other types (e.g. status post laser in-situ keratomileusis). We excluded studies that enrolled participants under age 14 (US Food and Drug Administration approval for epithelium-off CXL is for patients 14 years of age and older). Trials that included adjunctive therapy or modification of either technique (such as iontophoresis or chemical enhancers to improve stromal absorption of riboflavin) were eligible. While the irradiance in standard epithelium-off CXL has been 3 mW/cm2 for 30 minutes, trials with use of all irradiance levels and duration were included.
Search methods
To identify RCTs potentially eligible for this review (and also controlled clinical trials in case we found no or few RCTs), we searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register), Ovid MEDLINE, OvidMEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE, EMBASE, PubMed, Latin American and Caribbean Health Sciences Literature Database (LILACS), the metaRegister of Controlled Trials (mRCT) (www.controlledtrials.com), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not impose any date or language restrictions in the electronic search for trials. We also used the Science Citation Index Expanded database to identify additional studies that had cited trials included in this review. We last searched the electronic databases on January 15, 2020. The Cochrane systematic review provides our detailed search strategies for the electronic databases.16 We also searched the references cited by included studies for trials not identified by any automated search.
Trial Selection
Pairs of authors independently reviewed titles and abstracts resulting from the search to identify citations referring to trials that definitely or possibly were eligible based on our inclusion criteria. The final eligibility decision was based on independent review by two authors of the full text of articles; disagreements were resolved by discussion. After ascertaining that more than one RCT was eligible for inclusion, we elected to include only RCTs in this review.
Outcomes of Interest
The primary outcome for this review was stabilization of keratoconus progression as indicated by Kmax, a measurement of corneal curvature used to assess keratoconus progression. The outcome time points of primary interest were 12 and 24 months after corneal CXL; however, participants in included trials rarely had been followed for one year or longer. Therefore, we also considered outcomes after shorter follow-up periods. We analyzed Kmax both as a continuous outcome (change in Kmax from baseline) and as a dichotomous outcome (proportion of participants whose Kmax decreased by at least 2 diopters (indicating arrest or slowing of disease progression), proportion of eyes whose Kmax increased by at least 2 diopters from baseline, and proportion of eyes whose Kmax remained stable18 vs increased by > 1 D,5–8 used to denote progression. We considered the following secondary outcomes at 12 months or more after CXL, based on measurements made at the longest follow-up time: the mean change in CDVA from baseline recorded and analyzed as the number of letters read correctly on a chart with a logarithm of the minimum angle of resolution (logMAR) scale19; the proportion of participants who gained 10 or more logMAR letters from baseline, equivalent to 2 lines (0.2) on a logMAR scale; the proportion who lost 10 or more logMAR letters from baseline; vision-related quality of life as assessed by a validated questionnaire (e.g., the National Eye Institute Visual Function Questionnaire); the proportion who experienced adverse events; and direct and indirect costs of either type of surgery.
Data Collection and Assessment of Trials for Risk of Bias
Two review authors (SN, ICK) independently assessed the included studies for potential sources of bias according to Cochrane guidelines.17 The same authors independently extracted data from the included studies regarding trial characteristics, methods, participants, interventions, outcomes, and funding sources and recorded data on standard forms. One author (SN) entered the data using the Review Manager software,20 and another author (ICK) verified the data entered. Participant and surgery characteristics were compared among the trials to judge clinical and methodologic heterogeneity.
Included studies were evaluated for risk of selection, performance, outcome detection, attrition, selective reporting of outcomes, and other bias based on the Cochrane Risk of Bias tool. We judged each study to have been at “low risk,” high risk,” or “unclear risk” (whenever the information provided was insufficient to make an assessment) of bias of each type. Reasons were documented for these assessments; discrepancies were resolved through discussion. We attempted to contact study investigators by electronic mail when information in publications was insufficient for us to assess bias or when information required for data extraction was not reported clearly.
Data Synthesis and Analysis
Data synthesis and analysis were performed following Cochrane methods. We determined whether the design of each included trial specified intervention on one or both eyes from each participant and whether investigators randomized at the participant or at the eye level. We excluded studies with a paired-eyes design from our sensitivity analysis because of possible intra-person correlation of outcomes in the two eyes of a participant. We used the difference in means (“mean difference”) to compare interventions with respect to continuous outcomes, including change in Kmax, change in CDVA, and change in participant questionnaire responses regarding subjective visual function parameters. We separately analyzed studies by length of follow-up (< 12 months and ≧ 12 months).
We calculated summary risk ratios (RRs) with 95% confidence intervals (CIs) to compare interventions with respect to dichotomous outcomes for which sufficient data were available from the included trials. Such outcomes included the proportion of eyes whose Kmax gained or lost 2 diopters, the proportion of eyes whose Kmax remained stable, the proportion of eyes that gained or lost 10 or more logMAR letters, and the proportion of eyes with adverse outcomes.
We calculated the I2 statistic (%) to determine the proportion of variation in outcomes due to heterogeneity; we considered a value above 60% to suggest substantial statistical heterogeneity. We performed separate analyses by methods of outcome assessment to investigate observed heterogeneity.
We classified studies based on techniques within transepithelial CXL, and we compared epithelium-off CXL with transepithelial CXL separately with and without use of iontophoresis (i.e. transepithelial CXL versus epithelium-off CXL, and transepithelial CXL using iontophoresis versus epithelium-off CXL). We performed meta-analysis when clinical and methodological heterogeneity was minimal. To combine the outcome data from included trials in a meta-analysis to estimate the overall effect of epithelium-off CXL relative to transepithelial CXL, we used a random-effects model; when fewer than three studies were included in an analysis, we used a fixed-effect model. We explored comparisons between transepithelial and epithelium-off CXL techniques within subgroups to explain observed heterogeneity whenever sufficient data were available. Whenever the quality of the available data from a study prevented meaningful analysis, we omitted the study from quantitative analyses and reported the data in a narrative format when appropriate.
We assessed the certainty of evidence for each outcome according to the GRADE approach. We began our assessment by judging the randomized design of each included study to confer a high certainty of evidence and downgraded certainty to moderate, low, or very low when there was evidence of high risk of bias, inconsistency, indirectness, or imprecision.
RESULTS
The electronic searches yielded 3364 records, of which we screened 2785 after removing duplicates (Figure 1). We obtained and screened the full-text reports of 57 citations that were potentially relevant. We included 13 RCTs, published in 22 reports, in this review. Our searches of other sources did not identify any other potentially eligible trials. Seven studies were parallel-group RCTs in which one eye of each participant was treated, five studies were RCTs in which both eyes of some or all participants were assigned to the same intervention, and one study was an RCT with a paired-eyes design.21,22 In the trial with paired-eyes design, both eyes of participants had progressive keratoconus; one eye was randomly assigned to transepithelial CXL and the other eye was assigned to epithelium-off CXL. We analyzed data from the studies that had assessed outcomes in both eyes separately without taking into account intra-person correlation.
Figure 1.
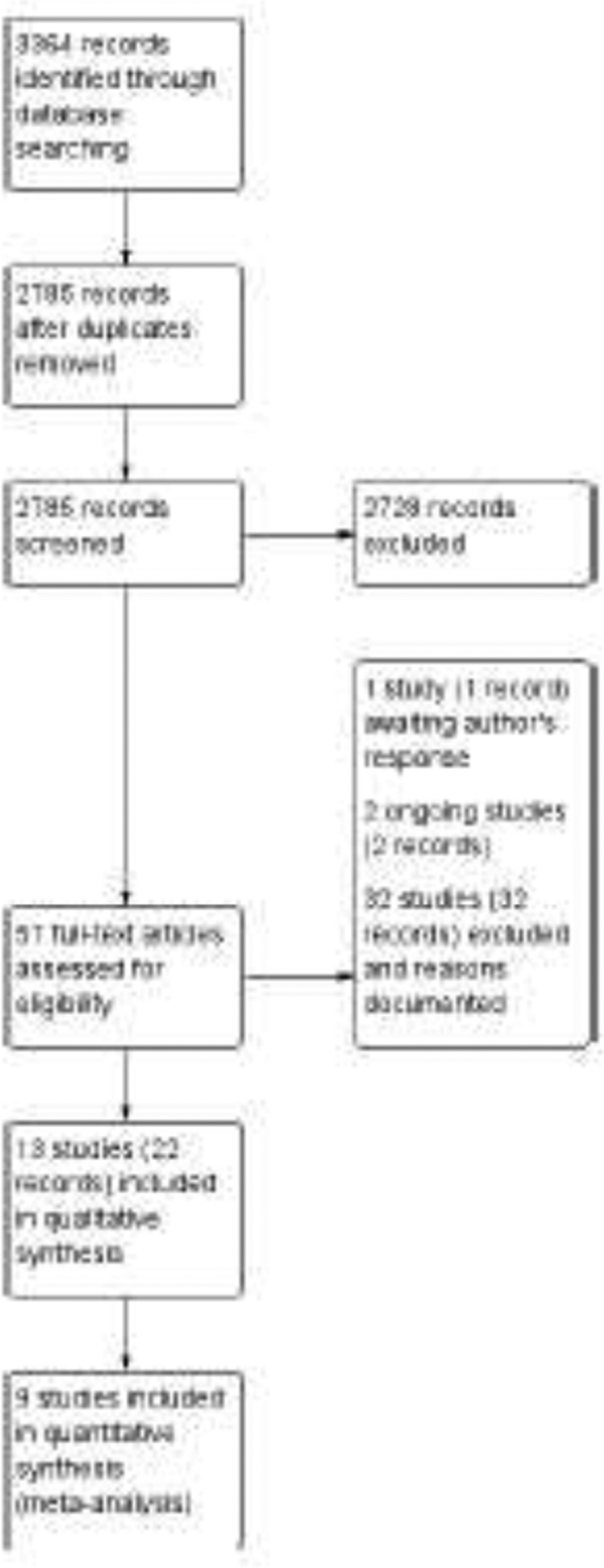
Identification and selection of randomized trials that had compared transepithelial corneal crosslinking (with or without iontophoresis) with epithelium-off corneal crosslinking.
Description of Included Studies
The 13 included studies were conducted in Europe (7), the Middle East (3), India (1), Russia (1) and Turkey (1). The studies reported data from a total of 661 eyes of 567 participants with progressive keratoconus; 345 eyes underwent transepithelial CXL and 316 underwent epithelium-off CXL. Of the eyes that underwent transepithelial CXL, 108 had iontophoresis-assisted transepithelial CXL. Clinical and methodological heterogeneity based on participant characteristics, topographic/tomographic devices, and descriptions of surgical technique provided in the trials was significant (Table 1—Summary of Studies by Characteristics of Participants and of Surgery). The definition of progressive keratoconus and methods by which to assess it at trial entry varied across trials.
Table 1.
Summary of Studies by Characteristics of Participants and of Surgery
| Study/year | Country | Number of parti-cipants/eyes | Definition of Progressive Keratoconus | Keratometric Device | Iontophoresis Assisted | Baseline Equivalence | Concentration of riboflavin pretreatment before transepithelial CXL until saturation | Topical instillation of riboflavin 0.1% drops during transepithelial treatment | Postoperative regimen after either type of CXL (exception noted) | Diameter for epithelium-off treatment (mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Acar 2014 | Turkey | 13/13 eyes | “increase in the steepest keratometry of 1.0 D or more in a 1-year period, 0.50 D increase in manifest refraction spherical equivalent, 1.00 D increase in manifest cylinder, or need for new contact lens fitting more than once in 2 years” | (not reported) | no | (not reported) | riboflavin 0.1%+ + dextran 15% + trometalol + EDTA | Q 5 minutes | Only for epithelium-off CXL: contact lens and netilmicin antibiotic drops | 7–9 |
| Al Fayez 2015 | Saudi Arabia | 70/70 eyes | “increase in the maximum K value or manifest astigmatism ≥1 D within the previous year based on repeated corneal topography” | Scheimpflug (Pentacam) | no | comparable | 0.10% | No | “fluorometholone for 2 weeks,” started post re-epithelialization in epithelium-off arm | 9 |
| Al Zubi 2019 | Jordan | 80/80 eyes | “greater than 0.5D rise in six months or greater than 1 D rise in steep K/12months” | Scheimpflug (Pentacam) | no | Unclear/contra-dictory between text and table | 0.10% | Q 3 minutes | Fluorometholone QID taper over 4 weeks | 7 |
| Bikbova 2016 | Russia | 119/149 eyes | “increase in steepest keratometry value by≥ 1.0 diopter (D) or manifest cylinder, or ≥ 0.5 D in manifest spherical equivalent refraction by repeated keratotopography ODP-scans ARK-1000.” | Placido disc (OPD Nidek) | yes | comparable | 0.10% | (constant exposure for iontophoresis arm) | “corticosteroid drops”× 2 weeks | 9 |
| Cifariello 2018 | Italy | 32/40 eyes | “Progression of keratoconus was documented through a clinical and instrumental (topographic, pachymetric, or aberrometric) worsening in the previous 6 months of observation.” | Placido disc/Scheimpflug (CSO Eye-top) and Scheimpflug (Pentacam) | no | no differences in baseline ocular surface disease index; statistical comparisons between other preoperative outcomes were not reported; mean age was significantly greater in transepithelial CXL group | riboflavin 0.1%+ dextran 15% + trometalol + EDTA | Q 5 minutes | transepithelial CXL arm: tobramycin QID × 1 week; no steroid Epithelium-off CXL dexamethasone 0.1% QID × 2 weeks | 9 |
| Lombardo 2016 | Italy | 25/34 eyes | “if there was an increase of at least 1 D in the Kmax derived by computerized Placido disc corneal topography over the 12 months preceding the operation” | Placido disc (Visante) | yes | Comparable baseline age, Kmax, preoperative endothelial count, central corneal thickness | riboflavin 0.1%+ + trometalol + EDTA | (constant exposure for iontophoresis arm) | fluorometholone BID starting week 1 after procedure thru 3 weeks | 10 |
| Mastropasqua 2013 | Italy | 35/40 eyes | “mean central K-reading change of >=1.5 diopters observed in 3 consecutive topographies during the preceding 6 months, or a mean central corneal thickness decrease of >=5% in 3 consecutive examinations performed in the previous 6 months” | Scheimpflug (Pentacam) | no | (Not reported) | riboflavin 0.1%+ + dextran 15% + trometalol + EDTA | 0.1% Q 3 minutes | (Not reported) | (not reported) |
| Nawaz 2015 | India | 40/40 eyes | “documented keratoconus progression on topography over at least 1-year follow-up” | Scanning slit (Orbscan II) | no | Comparable baseline age, gender, Kmax, preoperative endothelial cell count, visual acuity, keratometric astigmatism | 0.10% | riboflavin 0.10% + dextran 20%, with proparacaine, Q 5 minutes | Prednisolone acetate 1% QID and taper × 4 weeks | 7 |
| Razmjoo 2014 | Iran | 22/44 eyes | “in past 12 months, an increase in maximum keratometry (K) of 1.00 diopter (D), an increase of refractive astigmatism of 1 D of or increase of refractive error of 0.5 D” | Scheimpflug (Pentacam) | no | Comparable best corrected visual acuity topographic indices, and corneal density | 0.10% | Q 2–3 minutes | betamethasone q3 hrs × 4 weeks | 9 |
| Rossi 2015 | Italy | 20/20 patients | “progressive keratoconus with a documented clinical and instrumental (topographic, pachymetric, or aberrometric) worsening in the previous 6 months of observation” | Scheimpflug (Pentacam) | no | UDVA and CDVA were higher in the
epithelium-off CXL group No significant differences in age, pachymetry, and keratometry |
riboflavin 0.1%+ dextran 15% + trometalol + EDTA | dexamethasone QID and taper × 2 weeks | 9 | |
| Rossi 2018 | Italy | 30/30 eyes | “documented clinical and instrumental (topographic, pachymetric, or aberrometric) worsening in the prior 6 months” | Placido disc/Scheimpflug (CSO Eye-top) | Yes (one of three arms) | “homogeneous for sex and age” | riboflavin 0.1%+ trometalol + EDTA for iontophoresis arm; riboflavin 0.1%+dextran 15% + EDTA for non-iontophoresis arm | (constant exposure for iontophoresis arm) | dexamethasone QID and taper × 2 weeks | 9 |
| Soeters 2015 | the Netherlands | 61/61 eyes | “increase in Kmax, Ksteep, mean keratometry, and/r topographic cylinder value by ≥0.5 D over the previous 6–12 months” | Scheimpflug (Pentacam) | no | comparable except for “lower spherical equivalent and logMAR UCDA in the transepithelial group” | riboflavin 0.1%+ dextran 15% + EDTA | Fluorometholone BID × 2 weeks starting 1 week after procedure | 9 | |
| Stojanovic 2014 | Norway | 20/40 eyes | “increase of astigmatism or myopia by 1 D or increase in average Sim K by 1.50 D.” | Placido disc (OPD-II, Nidek) | no | Unclear but no difference at any follow up through 12 months in topographic features, visual acuity, refraction, Kmax | 0.50% | No | Dexamethasone 0.1% QID × 1 week | 8 |
Kmax = steepest keratometry (i.e., maximum keratometry, apical keratometry, maximum cone apex curvature)
Ksteep = steep stimulated keratometry (i.e., steepest central simulated keratometry; K2)
EDTA = ethylene diamine-tetraacetic acid
D = diopter
No trial was judged to have low risk of bias across all criteria (Figure 2). We judged most trials (8 of 13) to have had unclear or high risk of bias in at least 5 of 7 domains assessed. Performance bias (failure to mask participants and personnel other than surgeons to the surgical approach assigned) was the bias domain for which we judged the largest number of trials to have been at high risk of bias.
Figure 2.
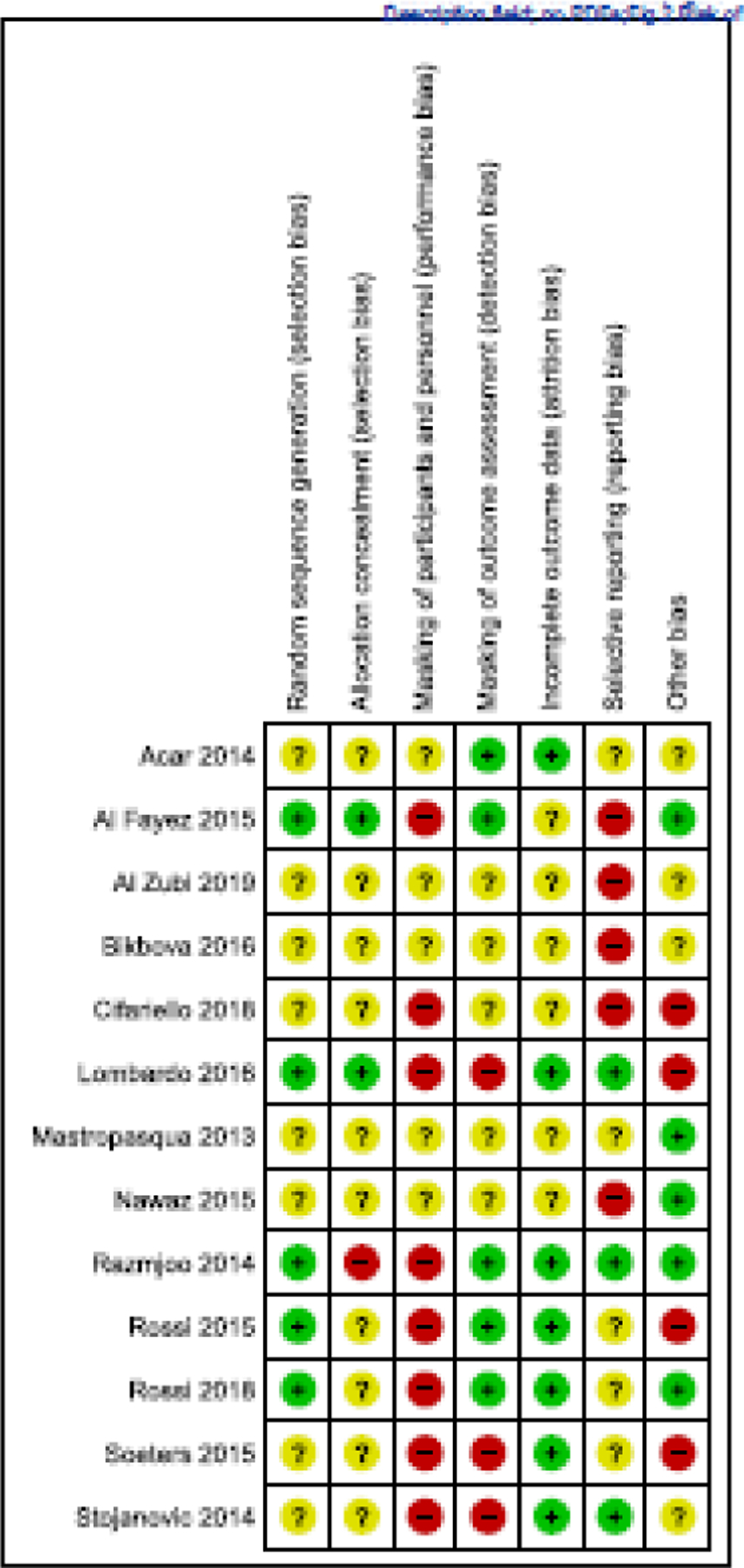
Authors’ assessments of risk of bias in individual trials by domain. Green, low risk of bias; yellow, unclear/unknown risk of bias; red, high risk of bias.
Because of implicit heterogeneity, transepithelial CXL trials were divided into the three trials that had compared the outcomes from those that had used iontophoresis-assisted transepithelial CXL23–30 and the eleven without iontophoresis assistance.14,15,21,22,30–39 Rossi et al.30 was a three-arm trial and contributed data to both groups of trials.
Agents employed in transepithelial CXL to enhance riboflavin penetration included pretreatment with frequent topical benzalkonium chloride (BAK) 0.02% for 30 minutes32 as well as BAK 0.005%-preserved proparacaine 0.5%, and BAK 0.005%-preserved gentamycin 0.3% for 5 minutes before saturation and concomitant use of BAK-preserved proparacaine 0.5% along with riboflavin every 30 seconds until saturation was confirmed at the slit lamp.21,22 In the latter trial, investigators used a Merocel sponge to produce microabrasions of the superficial epithelial layers caused by friction upon blinking by the patient so as to enhance riboflavin uptake.21,22 Several trials utilized a combination drug formulated specifically for transepithelial procedures that consisted of riboflavin 0.1% plus dextran 15% enhanced with amino acid Tris (hydroxymethyl) aminomethane (trometamol) and ethylenediaminetetraacetic acid (EDTA) prior to irradiation. One study described transepithelial CXL of the central 3 mm of the cornea, leaving the epithelium intact in the 3-mm wide ring surrounding this zone.38
Riboflavin (0.1% if specified) was instilled every 2–5 minutes during the 30-minute irradiation with a power of 3 mW/cm2 (corresponding to a dose of 5.4 J/cm2) in both transepithelial (without iontophoresis) and epithelium-off CXL groups except in one study32 in which riboflavin was not instilled during irradiation of the transepithelial group. Postoperative topical steroid drops were instilled for 1–4 weeks after surgery; postoperative regimens included dexamethasone 0.1%, fluorometholone 0.1%, prednisolone acetate 1%, and betamethasone (percentage unspecified).
Two iontophoresis-assisted transepithelial studies utilized riboflavin 0.1% with trometalol and EDTA, without dextran.25–30 One trial described irradiation with an intensity of 10 mW/cm2 for 9 minutes,25–29 the other for 10 minutes.30
Epithelium-off protocols were more uniform. Most investigators followed the “Dresden” protocol, which utilizes UVA-light diodes (370 nm) at a 1-cm distance for 30 minutes using 3 mW/cm2 irradiance. As with the transepithelial studies, different cross-linking devices were used. Pre-irradiation riboflavin 0.1% and dextran 20% were instilled in all eyes undergoing epithelium-off procedures except for one trial that used 0.5% riboflavin without dextran.21,22 During irradiation, the concentration of riboflavin instilled was 0.1% except for one trial that had used 0.025%.25–29 Postoperative topical steroid drop regimens varied.
Stabilization of Progressive Keratoconus: Change in Kmax, Final Value of Kmax, or Proportion with Specified Change in Kmax
Except for two studies that looked at histological changes following CXL,31,36 all studies examined keratometry outcomes. Eight of the remaining 11 studies provided quantifiable keratometric data for meta-analysis at the following endpoints: 6 months,37,38 12 months,14,15,21,22,30,39 and 24 months.25–29,34,35 The estimated effects on all keratometry outcomes, whether comparing mean difference or risk ratios between transepithelial and epithelium-off CXL, were of very low certainty because of imprecision, risk of performance and other biases, and inconsistency among studies. Two studies (one comparing epithelium-off CXL with transepithelial CXL, the other comparing epithelium-off CXL with iontophoresis-assisted transepithelial CXL) reported the numbers or proportions of eyes with decreases or increases of ≥ 2 D in Kmax. Because of imprecision, high risk of performance and other biases, and inconsistency among studies, the effects of CXL on decrease or increase of ≥ 2 D again were estimated with very low certainty. Three studies reported data on progression of keratoconus; data were analyzable for two, one from transepithelial CXL vs. epithelium-off CXL and one from iontophoresis-assisted transepithelial CXL vs. epithelium-off CXL. Because keratometry was the outcome measured and reported most often and is used to monitor keratoconus progression and response to CXL, we have focused our analyses on keratometry outcomes.
Comparison 1: Transepithelial CXL versus Epithelium-off CXL
Of 11 studies that had compared outcomes amongst participants assigned to transepithelial CXL vs. epithelium-off CXL, three studies had reported data for a follow-up period of 6 months and 8 studies for a follow-up period of 12 months. One trial of the 8 had compared three arms—transepithelial, iontophoresis-assisted transepithelial, and epithelium-off CXL.30 In three studies, both eyes of all or some participants were included. In these studies, participants were randomized, but the eye was used as unit of analysis without taking into account that the data were clustered within participants (unit-of-analysis error). We analyzed the data as reported. Therefore, confidence intervals were wider than they would have been if the potential within-person correlation was accounted for.
Seven RCTs reported keratometry data suitable for meta-analysis, 2 with 6-month follow-up and 5 with follow-up of 12 months or more. These data were reported as either mean change in Kmax from baseline to 12 months or mean Kmax at 6 or 12 months after surgery. Regardless of endpoint, the confidence intervals were consistent with no difference between the two interventions. For the outcome of mean change in Kmax at 12 months or later, the mean difference between interventions for 177 eyes was 0.99 D (95% CI, −0.11 to 2.09) with moderate statistical heterogeneity (I2=41%) (Figure 3). This analysis included the study with the paired-eye design with 40 eyes.21,22 When this study21,22 was excluded to examine how much of the heterogeneity was accounted for by this study, heterogeneity remained moderate (I2=41%), and the confidence interval remained consistent with no difference.
Figure 3.
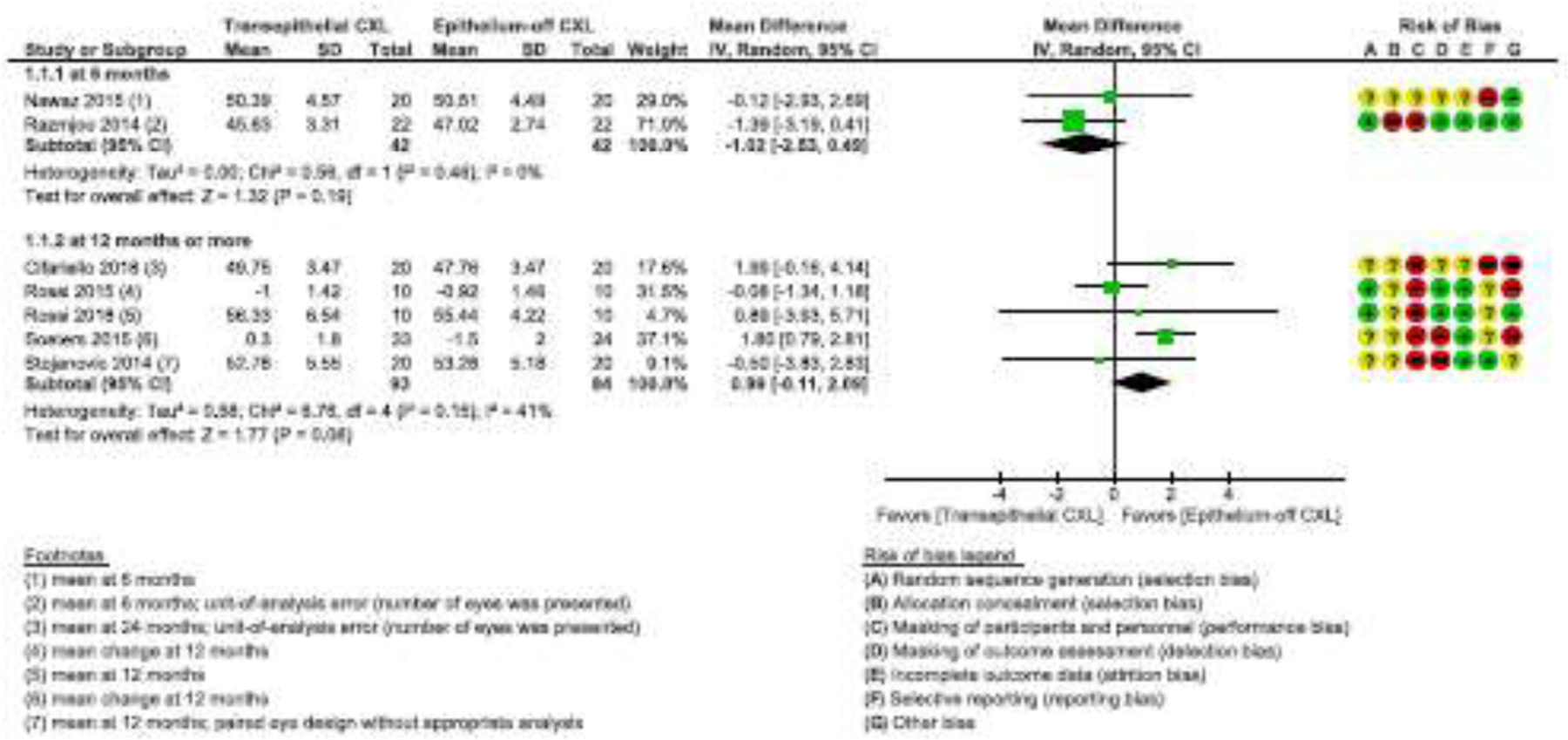
Forest plot showing mean change in Kmax from baseline or final value in Kmax with 95% confidence interval (CI).
Neither method of reporting keratometry outcomes, whether mean final Kmax or mean change in Kmax from baseline, showed a difference between transepithelial and epithelium-off CXL. From three RCTs that reported mean final Kmax,21,22,30,34,35 the estimated mean difference was 1.21 D (95% CI, −0.48 to 2.90; I2=0). In two RCTs that reported mean change in Kmax from baseline,14,15,39 the mean difference was 0.90 D (95% CI, −0.94 to 2.74; I2=81%). Two additional trials reported mean final Kmax of 84 eyes measured 6 months after surgery.37,38 The confidence interval was consistent with no difference between interventions, with mean difference of −1.02 D (95% CI, −2.53 to 0.49).
Two studies reported data regarding progression of keratoconus (increase of 1 D > in Kmax).14,15,32 We did not combine their data because considerable heterogeneity was detected (I2 = 92%). The estimated risk ratio for stable keratoconus was 0.45 (95% CI 0.31 to 0.65; 70 eyes) at 36 months after surgery in one study32 and 0.81 (95% CI 0.68 to 0.96; 61 eyes) at 12 months or longer after surgery in another study,14,15 suggesting that epithelium-off CXL may have a small effect on stabilizing keratoconus compared to transepithelial CXL. However, the certainty of evidence is very low due to high risk of bias, imprecision, and unexplained heterogeneity.
Comparison 2: Transepithelial CXL using iontophoresis versus Epithelium-off CXL
Three studies had compared outcomes of transepithelial CXL using iontophoresis with those of epithelium-off CXL; all had reported outcomes based on measurements made 12–24 months postoperatively. Two studies had included both eyes of some participants; as neither trial considered intra-person correlation, the estimated confidence interval was wider than it would have been if we had been able to correct for correlation. Two studies (51 eyes) reported quantitative data to permit analysis of change in Kmax from baseline to 12 months or longer after surgery.25–30 One study favored epithelium-off CXL, but the other favored transepithelial CXL. Because we detected substantial heterogeneity (I2=68%), we did not estimate an overall effect.
One study reported data for decrease or increase in Kmax by ≥ 2 D.25–29 Twenty-four months after surgery, one of 20 eyes in the epithelium-off CXL group and none of 11 eyes in the transepithelial CXL with iontophoresis group had experienced a 2-D or larger decrease and no eye in either arm had a 2-D or larger increase. This study also reported that in none of 20 eyes in the transepithelial CXL with iontophoresis arm versus in 2 (18%) of 11 eyes in the epithelium-off CXL arm had progression of keratoconus been observed.
Secondary outcomes
Six studies (two of which had compared iontophoresis-assisted transepithelial CXL vs. epithelium-off CXL) reported change in logMAR CDVA in 178 eyes from baseline to 12 months or beyond. The confidence interval was consistent with no change or a clinically insignificant difference of 2 or fewer letters. In response to our inquiry, an investigator of one of the 6 studies provided data on the proportion of participants with gain or loss of ≥10 logMAR letters (0.2 logMAR) from baseline.25–29 In this trial of 34 eyes, by 24 months 5 (23%) of 22 eyes in the iontophoresis-assisted transepithelial-CXL arm had gained ≥ 10 letters vs. 1 (8%) of 12 eyes in the epithelium-off CXL arm. No eye in either arm had lost ≥10 logMAR letters from baseline. The evidence to support the overall estimates was very low for the 6 trials because of imprecision, high risk of performance and other biases, and unexplained heterogeneity amongst trials comparing transepithelial CXL with epithelium-off CXL from which data were reported.
The estimated overall mean change in logMAR CDVA from baseline to 6 months or 12 months or later showed no difference between either type of transepithelial CXL and epithelium-off CXL, with a confidence interval consistent with no or only a trivial difference (2 letters or fewer of a line on a Snellen visual acuity chart). We judged the certainty of evidence as very low because of imprecision, high risk of performance and other biases, and unexplained heterogeneity (I2 = 70%) amongst trials comparing transepithelial CXL with epithelium-off CXL, which precluded pooling of data.
Only one group of investigators had assessed any patient-reported outcomes; they compared the responses of 40 participants to the Ocular Surface Disease Index after CXL.34,35 Mean scores at 1 month after surgery favored transepithelial CXL over epithelium-off CXL [mean difference −2.30 (95% CI −3.62 to −0.98; n = 40), but the evidence for this effect was low because of imprecision and risk of bias. No study reported costs of any intervention.
Adverse outcomes
Adverse outcomes were reported from 11 studies with clinical outcomes. Eye pain experienced in the early postoperative period had been noted in an unspecified number of eyes following epithelium-off CXL. Corneal haze or scarring was reported in 8/106 eyes after epithelium-off CXL and in none of 115 eyes after transepithelial CXL (without iontophoresis) in 4 studies that had compared these two techniques. We judged the evidence to be of moderate certainty that eyes that had undergone epithelium-off CXL had experienced corneal haze or scarring more often than eyes after transepithelial CXL. Herpetic keratitis, sterile infiltrate, and epithelial defect were observed in one eye each in the epithelium-off arm of 1 trial.14,15
Corneal haze or scarring was reported in 6 of 85 eyes in epithelium-off CXL arms of 2 studies in contrast with none of 98 eyes undergoing iontophoresis-assisted transepithelial CXL. The certainty of evidence was judged as low because of high risk of bias and imprecision from small sample size and wide confidence intervals.
DISCUSSION
In this review of 13 randomized controlled trials in which a total of 661 eyes of 567 participants with progressive keratoconus were analyzed, we compared the effectiveness of transepithelial CXL, with or without iontophoresis, relative to epithelium-off CXL to stabilize progressive keratoconus. To our knowledge, our systematic review and meta-analysis includes the largest group of RCTs reviewed to date, from which we attempted to estimate quantitative differences in effects between interventions on keratometric indicators of progression of keratoconus, assessed as mean change in Kmax, final Kmax, and proportions experiencing a ≥ 2 D change in Kmax; on CDVA, assessed as mean change in logMAR units or change of ≥ 10 logMAR letters; and on adverse outcomes based on data reported from the trials. From the 8 studies with quantitative data suitable for meta-analysis, we found no evidence of any important difference between interventions with respect to mean change in Kmax from baseline or final Kmax measurements at either 6 months (included because of paucity of randomized controlled trials) or ≥ 12 months after surgery. Very low certainty of evidence suggested that there may be little to no difference between interventions regarding progression of keratoconus. Change in CDVA from baseline to 6 or ≥ 12 months after each type of surgery was no more than a few letters, i.e., less than one line on a Snellen visual acuity chart. No study reported loss of 10 letters (0.2 logMAR units) of CDVA following surgery. Moderate evidence was found for a difference between surgical strategies that favored epithelium-off CXL regarding observation of post-operative corneal haze or scarring; however, no assessment has been made of the visual significance of the corneal status to participants. It is important to note, however, that transepithelial techniques described in studies in this review varied widely (there was substantial heterogeneity even for the two iontophoresis-assisted studies) and will continue to evolve.
The thirteen included studies had clinical and methodological diversity such as outcome definitions, methods of measurements, and risk of bias. Inadequate sample sizes and high risk of bias among studies downgraded the certainty of the body of evidence to low or very low for most outcomes. The largest trial included in the review enrolled 119 participants (149 eyes); the remaining trials enrolled many fewer participants. Subgroup analyses to account for differences in types of participants, types of pharmacotherapies, and power and timing of UV light exposure were not feasible because of small samples sizes. Six (55%) of 13 studies failed to provide a detailed definition of progressive keratoconus as used in their studies. Most commonly, they did not specify the period of time prior to CXL during which progression was noted or did not provide any numerical value (keratometry, astigmatism, or refractive error) by which to define progression of keratoconus. Adoption of a standard definition of progressive keratoconus would enable comparison of baseline characteristics of patients and eyes. In addition, agreement on core keratometric outcome measures, a standard definition of progression of keratoconus, and assessment of systematic differences in readings from different measurement devices would improve the methodologic rigor of future trials designed to compared CXL methods. Moreover, standardization of perioperative, intraoperative, and postoperative patient care protocols would allow valid comparisons of techniques. In only one study of 13 had subjective visual function been assessed; we found no report of intervention costs or cost effectiveness from any of the trials. We recommend that future randomized trials of CXL surgery investigate subjective visual function and the cost of interventions.
Three smaller systematic reviews have examined the same question as this review,40–42 but the meta-analyses included fewer studies, some of which were included in our systematic review, and others which were not RCTs42; did not separate transepithelial CXL studies by whether they were assisted by iontophoresis40–42; included studies of eyes with all types of corneal ectasia, not restricted to progressive keratoconus41; and included one study of pediatric CXL.40 None provided convincing evidence of the superiority of one of the three CXL methods with respect to Kmax or visual acuity. Similar to our results, their reviews showed that transepithelial CXL techniques may be associated with less than 0.1 logMAR improvement in visual acuity,41,42 i.e., equivalent to less than one line on a Snellen visual acuity chart.
Results of studies with 6 months of follow-up or less may not be relevant from both a clinical and patient perspective than results from studies with 12 or more months of follow-up, because a longer time is needed for corneal remodeling after kerato-refractive procedures.43 Kmax usually increases in the first few months after epithelium-off CXL,11 then decreases, and that reduction in Kmax can continue past the first year.7 Longer follow-up also is important because the rate of progression of keratoconus is not constant over time. Patients can show stable keratometry values at the 1-year follow-up visit and progression 2 years after transepithelial CXL.12 Because stability at 1-year may be part of the natural disease course and not related to CXL at all, studies with longer follow-up are essential to document the long-term effects of CXL on patients with keratoconus, most of whom will live for decades after surgery.
Study design and implementation deficiencies and lack of information about them were limitations of many of the studies included in this systematic review. Eleven (85%) RCTs had at least one domain that was judged as having unclear (indeterminate) risk of bias because insufficient information was provided for us to make an informed judgment. Risk of bias was unclear or high for most domains for most studies. Eight (62%) studies were judged as having high risk of performance and detection biases. The most common bias was performance bias, not unexpected because it would have been difficult to mask study personnel and participants given the nature of interventions. It is likely participants and study personnel had been able to tell which eyes had undergone epithelium-off CXL because of post-procedural pain. In addition, study personnel would have been able to discern the intervention when assessing participants’ eyes at the slit-lamp. The second most common bias was selective reporting of outcomes; 4 (31%) studies were judged to have been at high risk of selective outcome reporting. All studies reportedly had recorded keratometry outcomes and visual acuity, key outcomes for clinical practice and for this systematic review, but few provided data in a form suitable for inclusion in meta-analysis.
For several studies, the report authors failed to specify outcome measures in study protocols or in their description of methodology or embedded them in the Results section, or they failed to report key outcomes or reported outcomes incompletely without indicators of precision such as standard deviations or depicted them graphically without stating numerical values. Another issue was use of inappropriate methods of data analysis when both eyes of participants were included in a study and analyzed without consideration of the likely correlation of outcomes between the two eyes of a participant. Five RCTs in which both eyes of some or all participants were assigned to the same interventions and one RCT with a paired-eyes design did not describe or refer to appropriate analytical methods to handle intra-participant correlations. Our estimates of confidence intervals for outcomes from those studies were thus affected because we chose to analyze these data as reported rather than exclude them. Methodologic rigor in trial design, conduct, and reporting, as recommended in the CONSORT statement, would increase certainty of evidence and enable better estimates of intervention effects.
Baseline characteristics of participants and eyes, when reported, varied among the studies; variations in baseline Kmax measurements may have been due in part to differences in measurement devices. Topography/tomography units were based on different technologies: Placido disc (CSO Eye-top30; Visante25–29; OPD Nidek21–24), scanning-slit (Orbscan II37), and Scheimpflug (CSO Eye-top,30 Pentacam14,15,34,35,38,39). Increase in Kmax by ≥1 D or more remains the most frequently reported index of disease progression,5–8 However, this 1D threshold falls in the variability (“noise”) of topographic and tomographic measurements, especially in a keratoconus cohort, making it difficult to distinguish between measurement imprecision and true corneal change.44 Moreover, the threshold change in Kmax used to define progression likely should be stratified by preoperative keratoconus severity, with one group of investigators showing significant progression occurring with 0.67 D increase in Kmax in eyes with Kmax < 49.0 D vs. a 1.42 D increase in eyes with Kmax ≥ 49.0 when using single measurements.45 While a 2 diopter change in Kmax to indicate arrest or slowing of keratoconus progression may be reasonable and conservative, this threshold also may need to be modified based on preoperative Kmax. Therefore, lack of a standard definition of progressive keratoconus (at baseline and after treatment) and different measurement devices,46 day-to-day44 and intraday47 variability of measurements in keratoconic eyes (with lesser known variability in crosslinked eyes), and diverse perioperative, intraoperative, and postoperative patient care protocols increase heterogeneity and limit applicability of evidence.
A robust outcome measure that the ophthalmologist can use to monitor keratoconus progression and response to therapy reliably does not yet exist. Although often used as a proxy for the relative efficacy of CXL, Kmax may not represent a reliable biomarker of keratoconus progression as its change often does not correlate with changes in visual acuity or manifest refraction.6,46,48 Arrest of progression of keratoconus, not reduction in Kmax, should be the primary outcome of interest in comparative effectiveness trials in which one approach to CXL is evaluated relative to another.
Few trials had reported adverse outcomes or mentioned monitoring for specific adverse outcomes. Corneal haze and temporary postoperative pain were the adverse outcomes reported. Research is required to document the risk profile of individual CXL protocols in conjunction with evaluating effectiveness regarding both clinical outcomes and outcomes of visual significance to patients.
In conclusion, CXL is the only method shown to slow progression of keratoconus. Transepithelial CXL is intrinsically desirable to avert complications from denuding the epithelium, but transepithelial CXL techniques must overcome the barrier the epithelium poses to riboflavin penetration, to UVA (which the epithelium can reflect), and to oxygen availability. However, because of lack of precision, frequent indeterminate or high risk of bias, and inconsistency in methods and outcomes among studies included in this systematic review, it remains to be shown whether transepithelial CXL, or any other approach, confers an advantage over epithelium-off CXL for patients with progressive keratoconus with respect to progression of keratoconus, visual acuity outcomes, and patient-reported visual function. At this time, transepithelial techniques vary widely. With improved trial design methodology and better means of uptake of UVA by the corneal stroma, estimates for outcomes will become more precise, which, in turn, will guide the development of more uniform, effective transepithelial techniques. Until a better biomarker is found, the relative change in Kmax is a reasonable proxy for stabilization of progressive keratoconus. Because Kmax has been measured using a wide variety of topographic and tomographic devices, reliance on final Kmax value as an outcome measure is inherently problematic. Investigators in future studies should correlate clinical assessments of corneal haze, which was observed after epithelium-off CXL but not after trans-epithelial CXL, and other adverse outcomes with assessments of its visual significance to the patient. Perioperative, intraoperative, and postoperative protocols require further investigation and standardization where possible to promote corneal wound healing. Iontophoresis, chemical adjuvants, and mechanical injury to the epithelium help increase penetration of riboflavin through intact epithelium, but the most effective method is not known. The current dose of UVA energy and/or length of irradiation (both of which affect radiant exposure in J/cm2) may be insufficient for transepithelial CXL to be optimally effective. Finally, costs of CXL and subjective visual function and visual anomalies, such as photophobia, difficulty driving at night, difficulty reading, diplopia, and fluctuation in vision reported by patients after CXL, should be documented in future studies.
Funding/Support
Ms. Ng and Dr. Hawkins receive salary support from the Cochrane Eyes and Vision U.S. Project co-operative agreement with the National Eye Institute, National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, Maryland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
No financial disclosures
This article is based on a Cochrane Review published in the Cochrane Database of Systematic Reviews (CDSR) 2021, Issue 3, DOI: 10.1002/14651858. CD013512.pub2. (see www.cochranelibrary.com for information). Cochrane Reviews are updated as new evidence emerges and in response to feedback; the CDSR should be consulted for the most recent version of the review.
REFERENCES
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319. [DOI] [PubMed] [Google Scholar]
- 2.Godefrooij DA, de Wit GA, Uiterwaal CS, Imhof SM, Wisse RP. Age-specific incidence and prevalence of keratoconus: a nationwide registration study. Am J Ophthalmol. 2017;175:169–172. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101(3):267–273. [DOI] [PubMed] [Google Scholar]
- 4.Cavas-Martínez F, De la Cruz Sánchez E, Nieto Martínez J, Fernández Cañavate FJ, Fernández-Pacheco DG. Corneal topography in keratoconus: state of the art. Eye and Vision. 2016;3:5–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am J Ophthalmol. 2010;149(4):585–593. [DOI] [PubMed] [Google Scholar]
- 6.Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37(1):149–160. [DOI] [PubMed] [Google Scholar]
- 7.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen cross-linking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. 2008;34(3):796–801. [DOI] [PubMed] [Google Scholar]
- 8.Wittig-Silva C, Whiting M, Lamoureux E, Lindsay RG, Sullivan LJ, Snibson GR. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus: preliminary results. J Refract Surg. 2008;24(7):S720–S725. [DOI] [PubMed] [Google Scholar]
- 9.O’Brart DP, Patel P, Lascaratos G, et al. Corneal cross-linking to halt the progression of keratoconus and corneal ectasia: seven-year follow-up. Am J Ophthalmol. 2015;160(6):1154–1163. [DOI] [PubMed] [Google Scholar]
- 10.Poli M, Lefevre A, Auxenfans C, Burillon C. Corneal collagen cross-linking for the treatment of progressive corneal ectasia: 6-Year prospective outcome in a French population. Am J Ophthalmol. 2015;160(4):654–662. [DOI] [PubMed] [Google Scholar]
- 11.Hersh PS, Stulting RD, Muller D, Durrie DS, Rajpal RK, United States Crosslinking Study Group. United States Multicenter Clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmology. 2017;124(12):1878. Erratum in: Ophthalmology 2017; 1124(1879):1259–1878. Erratum in: Ophthalmology 2017; 1124(1879):1270. [DOI] [PubMed] [Google Scholar]
- 12.Caporossi A, Mazzotta C, Paradiso AL, Baiocchi S, Marigliani D, Caporossi T. Transepithelial corneal collagen crosslinking for progressive keratoconus: 24-month clinical results. J Cataract Refract Surg. 2013;39:1157–1163. [DOI] [PubMed] [Google Scholar]
- 13.Shalchi Z, Wang X, Nanavaty MA. Safety and efficacy of epithelium removal and transepithelial corneal collagen crosslinking for keratoconus. Eye. 2015;29:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soeters N, Wisse RP, Godefrooij DA, Imhof SM, Tahzib NG. Transepithelial versus epithelium-off corneal cross-linking for the treatment of progressive keratoconus: a randomized controlled trial. Am J Ophthalmol. 2015;159(5):821–828. [DOI] [PubMed] [Google Scholar]
- 15.Soeters N Standard versus transepithelial corneal crosslinking. Available at https://clinicaltrials.gov/ct2/show/NCT02349165. Accessed March 21, 2021.
- 16.Ng SM, Ren M, Lindsley KB, Hawkins BS, Kuo IC. Transepithelial versus epithelium-off corneal crosslinking for progressive keratoconus. Cochrane Database of Syst Rev. 2021, Issue 3. Art. No.: CD013512. DOI: 10.1002/14651858.CD013512.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017) Available at www.training.cochrane.org/handbook. Accessed March 21, 2021. [Google Scholar]
- 18.Asri D, Touboul D, Fournié P, et al. Corneal collagen crosslinking in progressive keratoconus: multicenter results from the French national reference center for keratoconus. J Cataract Refract Surg. 2011;37(12):2137–2143. [DOI] [PubMed] [Google Scholar]
- 19.Early Treatment Diabetic Retinopathy Study (ETDRS) research group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103(12):1796–806. [PubMed] [Google Scholar]
- 20.Review Manager 5 (RevMan 5) [computer program]. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
- 21.Stojanovic A, Zhou W, Utheim TP. Corneal collagen cross-linking with and without epithelial removal: a contralateral study with 0.5% hypotonic riboflavin solution. Biomed Res Int. 2014;2014:619398–619398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stojanovic A Transepithelial corneal collagen cross-linking (CXL) in treatment of keratoconus. Available at https://clinicaltrials.gov/ct2/show/NCT01181219. Accessed March 21, 2021.
- 23.Bikbova G, Bikbov M. Standard corneal collagen crosslinking versus transepithelial iontophoresis-assisted corneal crosslinking, 24 months follow-up: randomized control trial. Acta Ophthalmol (Copenh). 2016;94(7):e600–e606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bikbov M. Standard corneal collagen crosslinking versus transepithelial corneal crosslinking by Iontophoresis of Riboflavin. Available at https://clinicaltrials.gov/ct2/show/NCT02456961. Accessed March 21, 2021. [DOI] [PMC free article] [PubMed]
- 25.Lombardo M, Serrao S, Raffa P, Rosati M, Lombardo G. Novel technique of transepithelial corneal cross-linking using iontophoresis in progressive keratoconus. J Ophthalmol. 2016;2016:7472542–7472542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lombardo M, Giannini D, Lombardo G, Serrao S. Randomized controlled trial comparing transepithelial corneal cross-linking using iontophoresis with the Dresden protocol in progressive keratoconus. Ophthalmology. 2017;124(6):804–812. [DOI] [PubMed] [Google Scholar]
- 27.Lombardo M, Serrao S, Lombardo G, Schiano-Lomoriello D. Two-year outcomes of a randomized controlled trial of transepithelial corneal crosslinking with iontophoresis for keratoconus. J Cataract Refract Surg. 2019;45(7):992–1000. [DOI] [PubMed] [Google Scholar]
- 28.Serrao S, Lombardo G, Giannini D, Lombardo M. Corneal topography and aberrometry changes one-year after transepithelial corneal cross-linking using iontophoresis versus standard corneal cross-linking. Invest Ophthalmol Vis Sci; 2017;58:3504. [Google Scholar]
- 29.Lombardo M Transepithelial corneal cross-linking using iontophoresis. Available at https://clinicaltrials.gov/ct2/show/NCT02117999. Accessed March 21, 2021. [DOI] [PMC free article] [PubMed]
- 30.Rossi S, Santamaria C, Boccia R, et al. Standard, transepithelial and iontophoresis corneal cross-linking: clinical analysis of three surgical techniques. Int Ophthalmol. 2018;38(6):2585–2592. [DOI] [PubMed] [Google Scholar]
- 31.Acar BT, Utine CA, Ozturk V, Acar S, Ciftci F. Can the effect of transepithelial corneal collagen cross-linking be improved by increasing the duration of topical riboflavin application? An in vivo confocal microscopy study. Eye Contact Lens. 2014;40(4):207–212. [DOI] [PubMed] [Google Scholar]
- 32.Al Fayez MF, Alfayez S, Alfayez Y. Transepithelial Versus Epithelium-Off Corneal Collagen Cross-Linking for Progressive Keratoconus: a Prospective Randomized Controlled Trial. Cornea. 2015;34 Suppl 10:S53–S56. [DOI] [PubMed] [Google Scholar]
- 33.Al Zubi K, Albakar Y, Nasser R. Transepithelial versus epithelium off crosslinking for treating keratoconus among jordanians. Open Ophthalmology Journal. 2019;13(1):8–14. [Google Scholar]
- 34.Cifariello F, Minicucci M, Di Renzo F, et al. Epi-off versus epi-on corneal collagen cross-linking in keratoconus patients: a comparative study through 2-year follow-up. Journal of Ophthalmology. 2018;2018:4947983–4947983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costagliola C Epi off versus epi on corneal collagen cross-linking in keratoconus patients. Available at https://clinicaltrials.gov/ct2/show/NCT03598634. Accessed March 21, 2021. [DOI] [PMC free article] [PubMed]
- 36.Mastropasqua L, Nubile M, Lanzini M, et al. Morphological modification of the cornea after standard and transepithelial corneal cross-linking as imaged by anterior segment optical coherence tomography and laser scanning in vivo confocal microscopy. Cornea. 2013;32(6):855–861. [DOI] [PubMed] [Google Scholar]
- 37.Nawaz S, Gupta S, Gogia V, Sasikala NK, Panda A. Trans-epithelial versus conventional corneal collagen crosslinking: A randomized trial in keratoconus. Oman J Ophthalmol. 2015;8(1):9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Razmjoo H, Rahimi B, Kharraji M, Koosha N, Peyman A. Corneal haze and visual outcome after collagen crosslinking for keratoconus: A comparison between total epithelium off and partial epithelial removal methods. Adv Biomed Res. 2014;3:221–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossi S, Orrico A, Santamaria C, et al. Standard versus trans-epithelial collagen cross-linking in keratoconus patients suitable for standard collagen cross-linking. Clin Ophthalmol. 2015;9:503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen D, Song B, Li Q, et al. Comparison of epithelium-off versus transepithelial corneal collagen cross-linking for keratoconus: a systematic review and meta-analysis. Cornea. 2018;37(8):1018–1024. [DOI] [PubMed] [Google Scholar]
- 41.Kobashi H, Rong SS, Ciolino JB. Transepithelial versus epithelium-off corneal crosslinking for corneal ectasia. J Cataract Refract Surg. 2018;44:1507–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li W, Wang B. Efficacy and safety of transepithelial corneal collagen crosslinking surgery versus standard corneal collagen crosslinking surgery for keratoconus: a meta-analysis of randomized controlled trials. BMC Ophthalmol. 2017;17(1):262–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clearfield E, Hawkins BS, Kuo IC. Conjunctival autograft versus amniotic membrane transplantation for treatment of pterygium: findings from a Cochrane Systematic Review. Am J Ophthalmol. 2017;182:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunner M, Czanner G, Vinciguerra R, Romano V, Ahmad S, Batterbury M, Britten C, Willoughby CE, Kaye SB. Improving precision for detecting change in the shape of the cornea in patients with keratoconus, Sci Rep. 2018;8(1):12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gustafsson I, Bergström A, Cardiakides A, Ivarsen A, Hjortdal JØ, The inter-day repeatability of parameters for the assessment of progressive disease in subjects with less advanced keratoconus, Am J Ophthalmol. 2021; doi: 10.1016/j.ajo.2020.12.028. [DOI] [PubMed] [Google Scholar]
- 46.Gomes JA, Tan D, Rapuano CJ, et al. , group of panelists for the global Delphi panel of keratoconus and ectatic diseases. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359–369. [DOI] [PubMed] [Google Scholar]
- 47.Gustafsson I, Bergström A, Myers AC, Ivarsen A, Hjortdal J. Association between keratoconus disease severity and repeatability in measurements of parameters for the assessment of progressive disease. PLoS One. 2020; 15(2):e0228992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Randleman JB, Khandelwal SS, Hafezi F. Corneal crosslinking. Surv Ophthalmol. 2015;60(6):509–523. [DOI] [PubMed] [Google Scholar]


