Structured Abstract:
Objective:
We sought to compare overall survival (OS) and disease control for patients with localized pancreatic adenocarcinoma (PDAC) treated with ablative dose radiotherapy (A-RT) vs. resection.
Summary Background Data:
Locoregional treatment for PDAC includes resection when possible or palliative RT. A-RT may offer durable tumor control and encouraging survival.
Methods:
This was a single-institution retrospective analysis of patients with PDAC treated with induction chemotherapy followed by A-RT (≥ 98Gy biologically effective dose (BED) using 15–25 fractions in 3–4.5 Gy/fraction) or pancreatectomy.
Results:
One hundred and four patients received A-RT (49.8%) and 105 (50.2%) underwent resection. Patients receiving A-RT had larger median tumor size after induction chemotherapy [3.2 cm (undetectable-10.9) vs. 2.6 cm (undetectable-10.7), P<0.001], and were more likely to have celiac or hepatic artery encasement (48.1% vs. 11.4%, P<0.001), or superior mesenteric artery encasement (43.3% vs. 9.5%, P<0.001); however, there was no difference in the degree of SMV/PV involvement (P=0.123). There was no difference in locoregional recurrence/progression at 18-months between A-RT and resection; cumulative incidence was 16% (95% CI 10%−24%) vs. 21% (95% CI 14%−30%), respectively (P=0.252). However, patients receiving A-RT had a 19% higher 18-month cumulative incidence of distant recurrence/progression (58% [95% CI 48%−67%] vs. 30% [95% CI 30%−49%], P=0.004). Median OS from completion of chemotherapy was 20.1 months for A-RT patients (95% C.I. 16.4–23.1 mo.) vs. 32.9 months (95% C.I. 29.7–42.3 mo.) for resected patients (P<0.001).
Conclusions:
Ablative radiation is a promising new treatment option for PDAC, offering locoregional disease control similar to that associated with resection and encouraging survival.
Mini-Abstract:
This was a retrospective analysis of patients with pancreatic ductal adenocarcinoma (PDAC) treated with induction chemotherapy followed by ablative dose radiotherapy (A-RT) or pancreatectomy. Locoregional disease control was comparable, with a cumulative incidence of locoregional recurrence/progression of 16% vs. 21%, for A-RT and resection, respectively (P =0.252). Median OS from completion of chemotherapy was 20.1 months for A-RT patients (95% C.I. 16.4–23.1 mo.) vs. 32.9 months (95% C.I. 29.7–42.3 mo.) for resected patients (P<0.001).
Introduction:
Pancreatic cancer is the 3rd leading cause of cancer-related death and carries a dismal 5-year overall survival (OS) of ~10%1. In 2020 alone, it is estimated that there will be 57,600 new cases diagnosed in the United States. Induction chemotherapy is recommended as the initial treatment for patients with borderline resectable (BR-) and locally advanced pancreatic ductal adenocarcinoma (LA-PDAC). However, resectability rates after induction chemotherapy are reported as low as 25% for patients with LA-PDAC, and there is wide-ranging post-resection survival2–8. For patients with unresectable disease, 5-year survival is <10% and treatment options are limited2. The optimal induction strategy is still not known, with early-phase clinical trials and retrospective series demonstrating variable results9,10.
Radiation has been recommended in unresectable PDAC for locoregional control or to palliate symptoms. However to date, neither conventionally fractionated radiation therapy (CFRT) to 50.4–54 Gy, nor hypofractionated stereotactic body radiation therapy (SBRT) in 5-fractions at similar biologically effective doses (BEDs) has been shown to improve overall survival compared to systemic chemotherapy alone11–16. Moreover, the delivery of high doses of radiation has traditionally been limited by the risk of gastrointestinal (GI) toxicity to the surrounding luminal organs17.
However, improvements in survival may be possible if higher BEDs of radiation can be safely delivered to pancreatic tumors18. At Memorial Sloan Kettering Cancer Center (MSKCC), high-dose ablative radiation of ≥ 98Gy BED is being used for the treatment of locally advanced PDAC, with respiratory gating, image guidance, and adaptive planning in order to protect luminal organs while delivering ablative doses of radiation.
In this study, we sought to evaluate post-treatment survival, disease control, and safety in patients with pancreas-limited PDAC treated with induction chemotherapy followed by ablative radiotherapy (A-RT) compared to a contemporaneous cohort of resected patients.
Methods:
Cohort Selection
This study was a retrospective analysis of a prospectively maintained institutional database of patients with PDAC and was approved by the institutional review board (#17–603). We identified patients who were diagnosed with biopsy-proven PDAC between 2014–2018, evaluated by a multi-disciplinary tumor board, and treated with induction chemotherapy with either FOLFIRINOX/mFOLFIRINOX or gemcitabine plus nanoparticle-albumin-bound (nab-) paclitaxel (Figure 1), followed by either curative-intent pancreatectomy or A-RT as definitive locoregional control. Patients were included in the analysis cohort if they had American Joint Committee on Cancer (AJCC) 8th edition clinical stage T4 tumors (involvement of the celiac artery [CA], hepatic artery [HA], or superior mesenteric artery [SMA]) or involvement of the gastroduodenal artery (GDA), splenic artery/vein, superior mesenteric vein (SMV), or portal vein (PV) on baseline/pre-treatment diagnostic imaging. At MSKCC, we consider any involvement of the previously mentioned vascular structures as indicative of aggressive disease biology. Our criteria to recommend induction chemotherapy are similar to the Americas Hepato-Pancreato-Biliary Association (AHPBA)/Society of Surgical Oncology (SSO)/Society for Surgery of the Alimentary Tract (SSAT) definition of borderline and locally advanced PDAC19. Patients were excluded from both cohorts if they had extrapancreatic disease (M1) prior to locoregional therapy, a concurrent malignancy, or tumors that lacked vascular involvement and received induction chemotherapy due to poor fitness, pancreatitis, or comorbidities.
Figure 1.
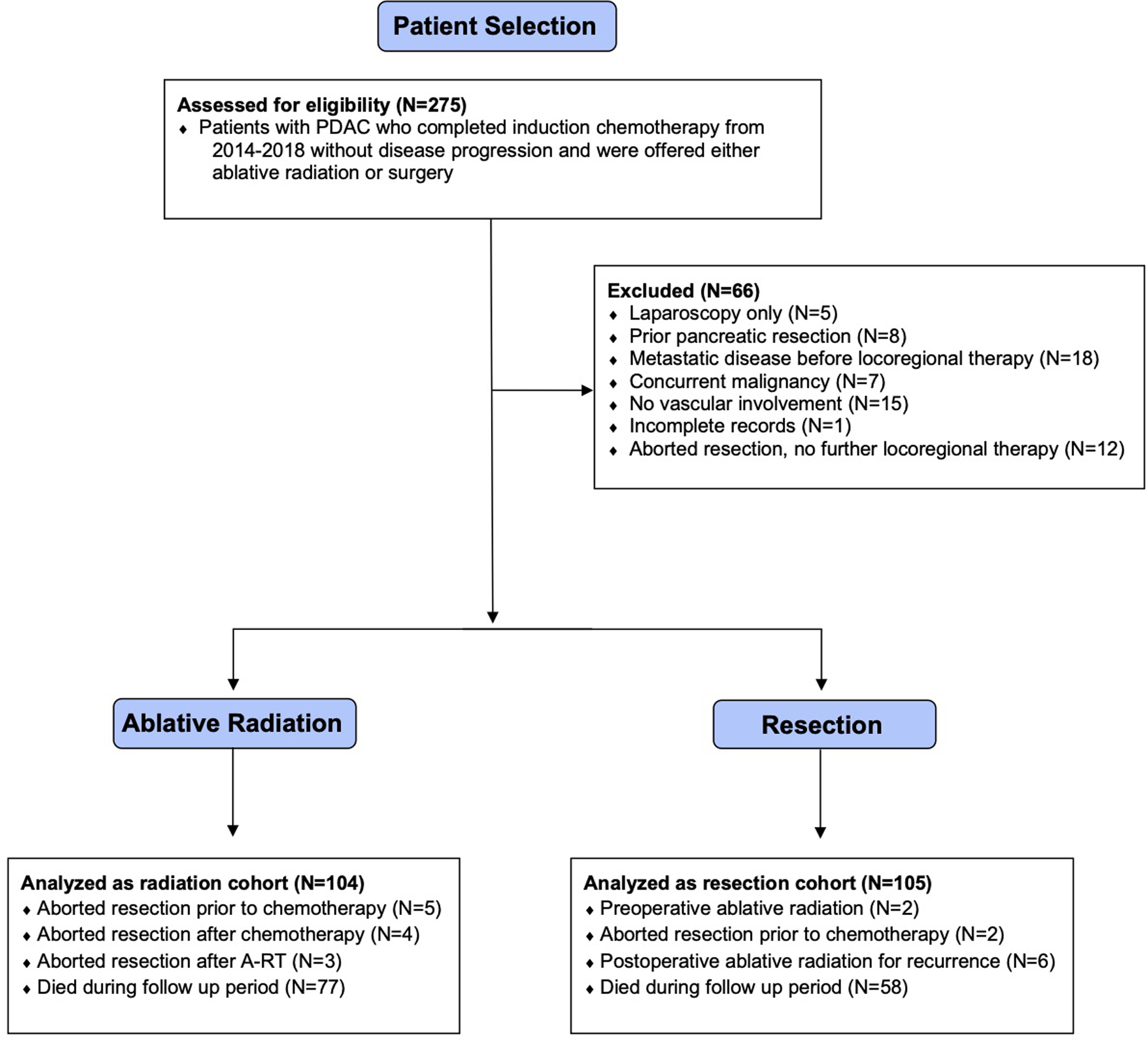
CONSORT diagram for cohort selection.
Ablative Radiation Technique
The background, rationale, and techniques required to administer A-RT in the treatment of LA-PDAC have been described in previous reports, and A-RT has been offered at MSKCC since 201620–22. Briefly, one of two ablative hypofractionated regimens, 75 Gy in 25 fractions (BED10 = 97.5 Gy) or 67.5 Gy in 15 fractions (BED10 = 97.88 Gy), are used based on the distance to the luminal GI tract and delivered with daily conebeam CT guidance and motion management. Patients with ≥ 5cm length of duodenal abutment by the pancreatic tumor are not eligible to receive this modality. The BED is calculated using the linear quadratic formula and is based upon the dose per fraction, total dose, and the response characteristics of the tumor to radiation (defined by the α/β ratio; BED10 assumes an α/β = 10).
Definitions
Vascular involvement was categorized as abutment (≤180°), encasement (>180°), or contour deformity (in the case of SMV/PV involvement) and patients were defined as resectable, borderline resectable (BR), or locally advanced (LA) based on baseline pre-treatment imaging using the AHPBA/SSO/SSAT definition19. An R1 resection was defined as tumor ≤1mm from the following surgical margins: uncinate, pancreatic neck/transection, bile duct, proximal (gastric or duodenal), distal (duodenal or jejunal), PV/SMV groove, or SMA.
For patients who received A-RT, local progression was defined according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria as a 20% increase in the pancreatic tumor measured along the same axis (and a minimum of 5mm increase from pre-radiation baseline), the development of new target regional lymphadenopathy (short axis ≥1.5cm, heterogeneity, or central necrosis), or “unequivocal progression” in the context of a poorly measured tumor or an increase of <20% (such as new vascular thrombosis or gastric outlet obstruction)23,24. For resected patients, local recurrence was defined as the first radiographic appearance of tumor after R0/R1 resection. The development of distant metastases was dated to the first radiographic appearance in both cohorts. Lymph node metastases were determined based on FDG-PET avidity, lymph node biopsy, or the above-mentioned RECIST 1.1 criteria for pathological lymph node involvement.
A modified age-adjusted Charlson Comorbidity Index (CCI) was calculated based on the ICD-9-CM adaptation by Deyo et al., excluding weighted scores for cancer diagnoses25. Ninety-day perioperative complications were graded according to the Clavien-Dindo classification26. Toxicities associated with radiation and post-radiation events were graded according to the Common Terminology Criteria for Adverse Events (CTACE) version 4.0 criteria.
Statistical Analysis
Continuous variables were described with median and range and compared using Wilcoxon Rank sum test. Categorical variables were described with count and percentage and compared using Fischer’s exact test. Overall survival (OS) was defined as time from the completion of induction chemotherapy until death or last follow-up, which was chosen to account for immortal time bias, as patients whose disease progressed during systemic chemotherapy would be excluded from this study. Recurrence/progression-free survival (RFS), locoregional recurrence/progression-free survival (LRFS), and distant recurrence/progression-free survival (DRFS) were all measured from the completion of induction chemotherapy. RFS was calculated as time to first recurrence or progression of any kind with death without recurrence or progression as a competing risk. LRFS was calculated as time to locoregional recurrence or progression with competing risks of death without recurrence or progression or DR before LR. DRFS was calculated as time to distant recurrence or progression with competing risks of death without recurrence or progression or LR before DR. OS was analyzed using the Kaplan-Meier method and the log-rank test was used to assess differences between treatment groups. Cox proportional hazards models were used to measure the association of demographic and clinical features with mortality. RFS, LRFS, DRFS were analyzed using competing risk methods and Fine and Gray test was used to assess differences between treatment groups. A P value of <0.05 was considered statistically significant. Data were analyzed using SAS Version 9.4 (SAS Institute, Cary, North Carolina).
Matching
Anticipating both demographic and tumor-related differences between treatment cohorts, we attempted propensity score matching on several variables including: age, gender, diabetes, coronary artery disease, body mass index (BMI), lymphadenopathy after completion of chemotherapy, pre- and post-chemotherapy tumor size, carbohydrate antigen 19–9 (CA 19–9) and degree of arterial and venous involvement. When matched on these variables, there was limited overlap in propensity score between groups and matching was not possible (Supplementary Figure 1). Thus, we performed direct comparisons between cohorts.
Results:
Between 2014–2018, 209 patients were eligible for analysis, of which 104 (49.8%) received A-RT and 105 (50.2%) underwent curative-intent resection. There were no differences in age, gender, or body mass index (BMI) between cohorts (Table 1). There was no difference in the induction chemotherapy regimen administered, with the majority of patients receiving FOLFIRINOX/mFOLFIRINOX and the remainder receiving gemcitabine plus nab-paclitaxel (P=0.096) (Table 1). Patients who underwent resection were more likely to have a CCI of 0 (71.4% vs. 57.7%, P<0.001), as well as pancreatic head tumors (82.9% vs. 61.5%, P<0.001), whereas patients who received A-RT had higher ECOG scores, although this difference was not statistically significant (ECOG=2+, 12.5% vs. 6.0%, P=0.148). Patients receiving A-RT had larger median tumor size both before (3.7 cm [range 1.2–7.4 cm], vs. 3.1 cm [1.5–7.8 cm], P<0.001) and after induction chemotherapy (3.2 cm [undetectable-10.9 cm] vs. 2.6 cm [undetectable-10.7 cm], P<0.001), as well as higher median CA 19–9 levels both before (220.5 u/mL [range 0–9,635 u/mL] vs. 114 u/mL [0–3,959 u/mL], P=0.035) and after induction chemotherapy (76 u/mL [0–3,507 u/mL] vs. 31 u/mL [0–3,415u/mL], P=0.006). Patients who received A-RT were more likely to have CA/HA encasement (48.1% vs. 11.4%, P<0.001), and SMA encasement (43.3% vs. 9.5%, P<0.001); however, there was no difference in the degree of SMV/PV involvement (P=0.123). Median follow-up time from diagnosis was 33.6 months for patients receiving A-RT and 36.0 months for resected patients, whereas follow-up from the completion of chemotherapy was 27.7 months for patients receiving A-RT and 30.4 months for resected patients.
Table 1.
Demographic, Treatment-Related, and Radiographic Features
| Variable | Ablative Radiation (N=104) | Resection (N=105) | P-Value |
|---|---|---|---|
| Age, years (median, range) | 67.3 (42.4–90.6) | 68.7 (39.1–83.6) | 0.860 |
| Female Sex, N (%) | 52 (50) | 56 (53) | 0.679 |
| BMI, kg/m2 (median, range) | 24.6 (16–42) | 26.1 (18–42) | 0.206 |
| CCI, N (%) | <0.001 | ||
| 0 | 60 (58) | 75 (71) | |
| 1 | 38 (37) | 14 (13) | |
| 2+ | 6 (6) | 16 (15) | |
| ECOG, N (%) | 0.148 | ||
| 0,1 | 91 (88) | 94 (94) | |
| 2,3 | 13 (12) | 6 (6) | |
| CA 19–9 pre-induction therapy, u/mL (median, range) | 220.5 (0–9,635) | 114 (0–3,959) | 0.035 |
| CA 19–9 post-induction therapy, u/mL (median, range) | 76 (0–3,507) | 31 (0–3,415) | 0.006 |
| Tumor size pre-induction therapy, cm (median, range) | 3.7 (1.2–7.4) | 3.1 (1.5–7.8) | <0.001 |
| Tumor size post-induction therapy, cm (median, range) | 3.2 (0.0–10.9) | 2.6 (0.0–10.7) | <0.001 |
| Tumor location, N (%) | <0.001 | ||
| Head | 64 (62) | 87 (83) | |
| Body/tail | 40 (38) | 17 (16) | |
| Head and body/tail | 0 (0) | 1 (1) | |
| Induction chemotherapy regimen, N (%) | 0.096 | ||
| FOLFIRINOX/mFOLFIRINOX | 65 (63) | 77 (76) | |
| Gemcitabine and nab-paclitaxel | 36 (34) | 22 (21) | |
| Unknown/other | 3 (3) | 3 (3) | |
| Celiac or hepatic artery involvement | <0.001 | ||
| None | 46 (44) | 82 (78) | |
| Abutment | 8 (8) | 11 (11) | |
| Encasement | 50 (48) | 12 (11) | |
| Superior mesenteric artery involvement | <0.001 | ||
| None | 41 (39) | 73 (70) | |
| Abutment | 18 (17) | 22 (21) | |
| Encasement | 45 (43) | 10 (9) | |
| Superior mesenteric vein or portal vein involvement | <0.123 | ||
| None | 16 (15) | 19 (18) | |
| Abutment | 38 (37) | 50 (48) | |
| Encasement or contour deformity | 50 (48) | 36 (34) | |
| AHPBA/SSO/SSAT resectability classification | <0.001 | ||
| Resectablea | 2 (2) | 4 (4) | |
| Borderline Resectable | 29 (28) | 81 (77) | |
| Locally Advanced | 73 (70) | 20 (19) | |
| Follow-up time from diagnosis, months (median, range) | 33.6 (6.7–54.9) | 36.0 (5.7–168) | N/A |
| Follow-up time from completion of chemotherapy, months (median, range) | 27.7 (4.3–49.6) | 30.4 (2.3–166) | N/A |
| Time from diagnosis to completion of chemotherapy, months, (median, range) | 4.6 (0.5–14.1) | 3.9 (0.7–17.1) | N/A |
GDA abutment, and splenic arterial/venous involvement are considered resectable disease by this definition
AHPBA = Americas Hepato-Pancreato-Biliary Association, BMI = body mass index, CA 19–9 = carbohydrate antigen 19–9, CCI = Charlson Comorbidity Index, ECOG = Eastern Conference Oncology Group, SSO = Society of Surgical Oncology, SSAT = Society for Surgery of the Alimentary Tract
Twenty-six patients (25.0%) had a toxicity or complication that was potentially attributable to or associated with their radiation treatment (Table 2). Patients may be listed as having had simultaneously occurring toxicities in multiple organ systems; 6 patients (5.8%) experienced GI bleeding, 3 (2.9%) experienced duodenal stenosis, 4 (3.8%) experienced new biliary obstruction, and 3 (2.9%) experienced a vertebral body fracture. Eleven patients (10.6%) developed complications associated with a preexisting biliary stent, including cholangitis, in-stent stenosis, stent migration, or the need for reintervention. In the A-RT cohort, there were five 90-day mortalities (4.8%); 3 patients developed distant metastases within 90-days, and the death of all 5 was cancer-related. Three patients (2.9%) had an aborted resection after A-RT, and one patient went on to receive a pancreaticoduodenectomy at an outside facility.
Table 2.
Toxicities and complications attributed to or associated with ablative radiation
| Ablative Radiation | N (%) | Resection | N (%) |
|---|---|---|---|
| Patients with any toxicity/complicationa | 26 (25.0) | Patients with any complication | 59 (56.2) |
| Summary of toxicities/complications b | Worst complication grade d | ||
| Dyspepsia | 1 (1.0) | Grade 1 (no intervention) | 8 (7.6) |
| Gastric or duodenal ulcer | 1 (1.0) | Grade 2 (pharmacologic intervention only) | 21 (20.0) |
| Vertebral body fracture | 3 (2.9) | Grade 3 (intervention requiring anesthesia) | 22 (21.0) |
| Upper gastrointestinal bleeding | 6 (5.8) | Grade 4 (requiring ICU care) | 6 (5.7) |
| Duodenal stenosis | 3 (2.9) | Grade 5 (death within 90-days) | 2 (1.9) |
| Biliary obstruction | 4 (3.8) | ||
| Complication with biliary stent | 11(10.6) | ||
| Death within 90-daysc | 5 (4.8) |
ICU = intensive care unit
Patients may have more than one toxicity or complication listed
Radiation Therapy Oncology Group (RTOG) and European Organization for Research and Treatment of Cancer (EORTC) criteria
None directly attributed to ablative radiation
Clavien-Dindo classification
In the resected cohort, the majority of patients had AJCC 8th Ed. ypT2 tumors (52/105, 49.5%), 30 (30.5%) received standard-dose preoperative radiation (36–50.4 Gy), and 2 (1.9%) received ablative-doses of preoperative radiation (Supplementary Table 1). Thirty-one patients (29.5%) required a vascular repair, resection, or reconstruction, the majority of which were venous repairs (23/31, 74.2%). Fifty patients (47.6%) had lymph node metastases on pathology and 40 (38.1%) had an R1 resection. Fifty-nine patients (56.2%) had at least one 90-day complication and 30 (28.6%) had a Grade 3 or greater complication (requiring an intervention under anesthesia according to the Clavien-Dindo classification26) (Table 2). There were two 90-day mortalities (1.9%): one patient developed distant metastases within 90-days and this patient died from sepsis of unknown etiology, while the second death was attributed to both pulmonary embolism as well as hepatic arterial hemorrhage.
Patients receiving A-RT had a 20% higher 18-month cumulative incidence of any recurrence/progression (70% [95% CI 61%−78%] vs. 50% [95% CI 40–60%], (P<0.001)) compared to resection (Figure 2). This difference was due to a 19% higher 18-month cumulative incidence of distant recurrence/progression (58% [95% CI 48%−67%] vs. 30% [95% CI 30%−49%], P=0.004). There was no difference in the rate of locoregional recurrence/progression at 18-months between A-RT and resection with a cumulative incidence of 16% (95% CI 10%−24%) vs. 21% (95% CI 14%−30%), respectively (P=0.252) (Figure 3a).
Figure 2.
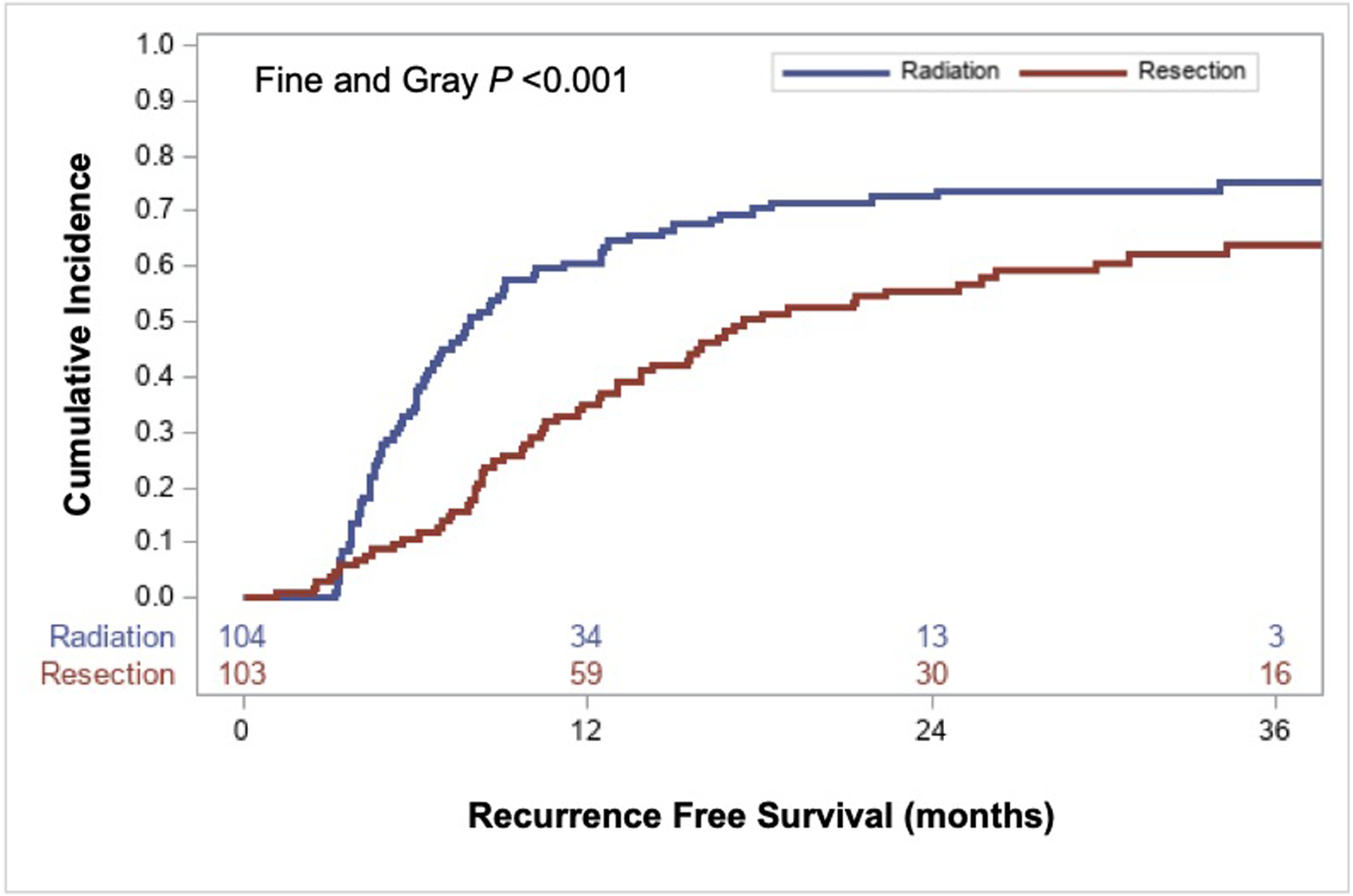
Competing risk analysis demonstrating the cumulative incidence of any recurrence/progression (locoregional or distant) with death without recurrence as a competing risk.
Figure 3.

Competing risk analyses demonstrating (a) time to first locoregional recurrence/progression with death without recurrence/progression and distant recurrence/progression before locoregional recurrence/progression as competing risks and (b) time to first distant recurrence/progression with death without recurrence/progression and locoregional recurrence/progression as competing risks.
There was a 12.8-month difference in the median OS between patients who underwent radiation vs. surgery (P<0.001). OS was 20.1 months for patients who received A-RT (95% CI 16.4–23.1 mo.) and 32.9 months for patients who underwent resection (95% CI 29.7–42.3 mo.) (Figure 4). One- and 2-year survival for patients treated with RT and resection were 76% vs. 83% and 38% vs. 62%, respectively. We performed a sensitivity analysis excluding patients who received preoperative radiation (N=32) and there was no major difference in OS or competing risk analyses evaluating recurrence/progression (Supplementary Figure 1 and 2).
Figure 4.
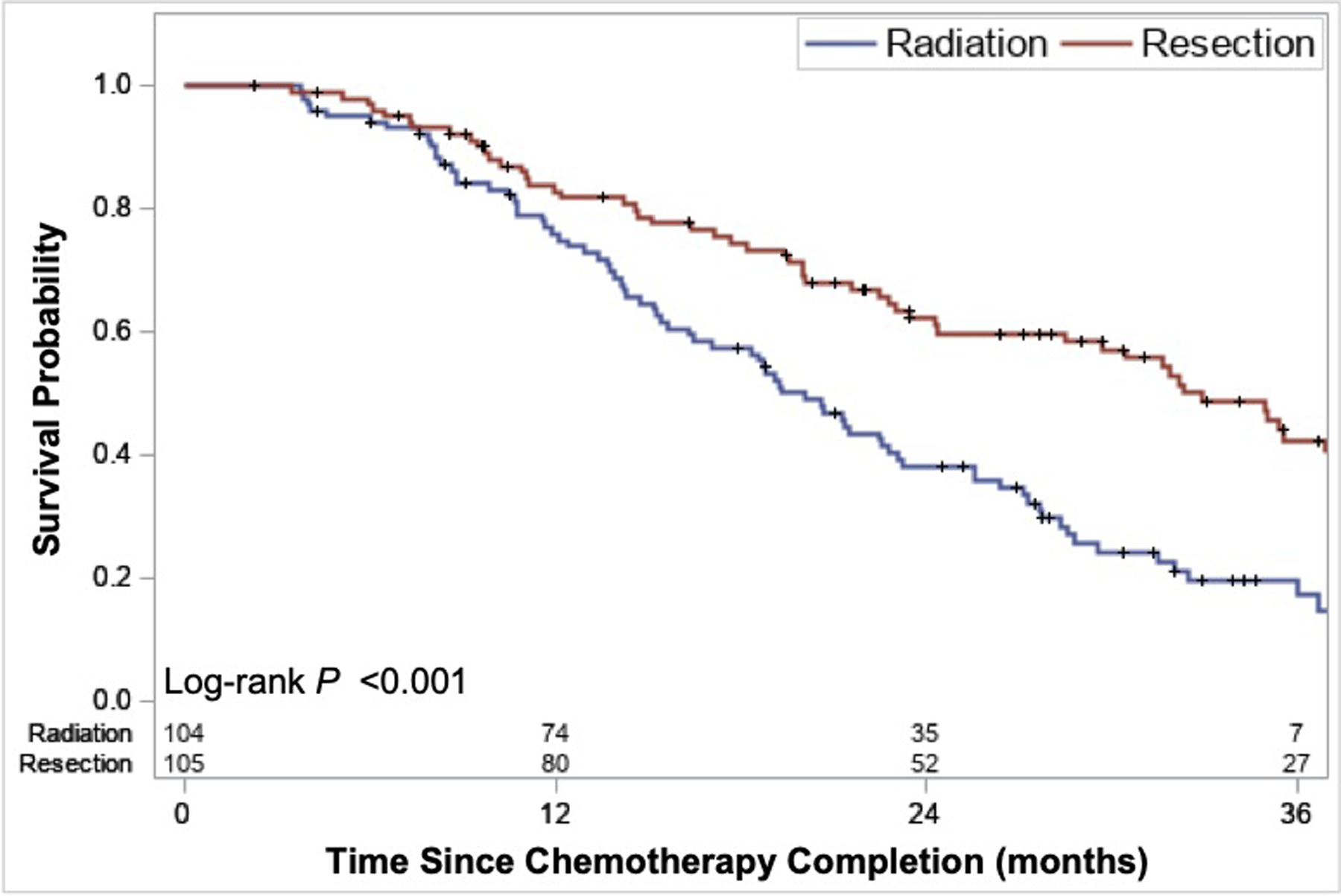
Overall survival comparing patients who underwent resection vs. ablative radiation
On univariable analysis, an ECOG score of ≥ 2 (HR 3.5 [95% CI 2.1–5.9], P<0.001), CA encasement (HR 1.6 [95% CI 1.1–2.3], P =0.020), and SMA encasement (HR 1.7 [95% CI 1.2–2.6], P=0.005) were all associated with worse survival (Supplementary Table 2). SMV and PV abutment or encasement/contour deformity, age gender, BMI, CA 19–9 pre- and post-induction chemotherapy, and tumor size pre- and post-induction chemotherapy were not associated with survival. Given the aforementioned differences between cohorts, a multivariable adjusted model was not performed.
Discussion:
In this study, we report a similar rate of locoregional control for patients with PDAC and vascular involvement when treated by A-RT compared to resection. At 18-months, 84% of patients who received A-RT had durable locoregional tumor control, compared to 79% of resected patients. OS was inferior in the A-RT group, and while this survival difference cannot be fully explained in a retrospective design, this may be due to selection bias, as A-RT patients had a higher rate of distant metastasis, more comorbidities, higher CA 19–9 values, larger tumors, and a greater degree of arterial involvement. There may also be inherent benefit to operative resection and removal of the primary tumor. A-RT had a major adverse event rate of 25.0%, which was similar to surgery; 28.6% of patients experienced grade 3 or worse complications after pancreatectomy. Patients receiving A-RT had encouraging survival, with a median OS of 20.1 months from the completion of induction chemotherapy, and 24.7 months from diagnosis, with a 1- and 2-year survival of 76% and 38%, respectively.
To date, radiotherapy in the treatment of PDAC has produced mixed results, likely due to the RT dose and strategy employed14,15,27. The LAP07 trial randomized 269 patients with LA-PDAC who did not progress after 4 months of induction chemotherapy with either gemcitabine alone or gemcitabine plus erlotinib to receive either chemotherapy, or chemoradiation (54 Gy in 30 fractions and concurrent capecitabine)27. On a per-protocol analysis, there was no difference in the OS for the 133 patients treated with chemoradiation (15.2 months) vs. systemic chemotherapy alone (16.5 months), although chemoradiation was associated with improved local control and longer time to re-initiation of treatment.
Initial attempts to utilize higher doses of radiation have been equally discouraging, and historically limited by gastrointestinal toxicity. In a recent analysis of the National Cancer Database by Zhong et al., patients who received SBRT (≥4 Gy per fraction, median BED10 = 72 Gy) were propensity matched with patients receiving CFRT (≤ 2 Gy per fraction), and although a statistically significant survival benefit of 13.9 vs 11.6 months was reported for those receiving SBRT (P<0.001), these survival figures are still no better than systemic chemotherapy alone17. However, more aggressive dose escalation using a protracted hypofractionation approach, initially described by Krishnan et al., showed a survival benefit for those who received a BED of >70 Gy (17.9 vs 15 mo., P=0.03), laying the foundation for our current program at MSKCC.
Using a standardized approach to A-RT planning and delivery, our group at MSKCC has recently reported that dose escalation can translate into durable local control and a promising median survival of 26.8 months from diagnosis and 18.4 months from RT22. These figures are similar to contemporaneous data, such as the phase 3 randomized PREOPANC trial that reported a median OS of 17.3 months in the pre-planned subgroup analysis of patients with BR-PDAC who received chemoradiation followed by resection28. With wide-ranging survival data based on retrospective reports and ongoing research about resectability after induction chemotherapy, it is difficult to determine who is the ideal candidate for resection of locally-advanced tumors29.
At this time, pancreatectomy remains the preferred treatment modality for localized PDAC, despite only 15–20% of patients presenting with resectable disease. Improvements in surgical technique have led to the feasibility of both arterial and venous resection30–32. However, vascular resection or reconstruction in order to fully resect locally advanced tumors is not without additional risk. Beane et al. demonstrated an increase in the need for blood transfusion, venous thromboembolism, septic shock, and a non-significant increase in mortality for pancreaticoduodenectomies requiring venous or arterial resection in a recent propensity matched study of the National Surgical Quality Improvement Project33. Although reports specifically on arterial reconstruction are limited to smaller series, perioperative morbidity has been reported between 38.2–53.0%, with mortality rates between 2.9 and 10.0%30,34,35 . Due to the high morbidity of arterial resection, A-RT may be an attractive option for many patients.
We initially designed our study to compare A-RT to resection in a similar group of patients; however, due to treatment allocation bias, the two cohorts differed substantially in both demographic and disease-specific characteristics, preventing propensity score matching. As such, these two groups are too dissimilar to compare treatment modalities head-to-head, or to generalize the results of patients receiving A-RT to those who would be traditionally offered resection, and vice versa. In this study, 70.2% of patients receiving A-RT would be considered locally advanced by AHPBA/SSO/SSAT criteria for resectability, compared to 19.0% of resected patients, and only 4 (3.8%) required an arterial reconstruction or repair (Supplementary Table 1). The 12.8-month survival difference is presumably multifactorial. Both CA/HA and SMA encasement were associated with adverse survival (1.6- and 1.7-fold, respectively), and more aggressive disease biology may predispose to a higher rate of distant metastases (58% vs. 30% at 18 months). The A-RT cohort was also characterized by more comorbid conditions and poorer functional status, which itself was associated with a 3.5-fold increased risk of mortality. Despite these differences, our results are promising and highlight the potential for A-RT as a modality by which to achieve locoregional tumor control particularly for patients unable to undergo resection in the short-term or indefinitely.
We acknowledge that there are several limitations of this study, including its retrospective nature, small sample size, and differences between cohorts being studied. The differences in fitness, functional status, and vascular involvement are likely due to selection bias and are a limitation of retrospective analysis. Importantly, the higher rate of adverse prognostic factors in patients who received A-RT may underestimate the true efficacy of this modality. Lastly, the assessment of locoregional failure or progression after A-RT is inherently difficult due to peripancreatic fat infiltration which may lead to overestimation of size based on RECIST 1.1 criteria18. Our hypothesis-generating results support further prospective clinical trials to compare surgery to A-RT.
This report is consistent with growing evidence for definitive radiation in the treatment of a variety of solid tumors, including anal, prostate, head and neck, cervical, lung, esophageal and rectal cancer, for which chemoradiation can achieve durable local tumor control in locally advanced disease36. Although less-widely practiced, intraoperative radiotherapy (IORT) to the tumor bed or to intact tumors of 10–20 Gy (BED10 20–60 Gy) after preoperative chemoradiation to 50.4–58.8 Gy (BED10 59.47–69.38 Gy) have been reported. A single-institution series by Keane et al. reported a median OS of 35.1 months for resected LA/BR PDAC and IORT37. Similarly, a separate series from the Massachusetts General Hospital demonstrated a median OS of 46.7 and 23 months for patients with LA/BR PDAC who underwent IORT with and without resection, respectively38; these data are both comparable to our report and reinforce the evidence that dose-escalation strategies may improve survival. At our institution, the phase II, single-arm, open-label MAIBE (Maximal Ablative Irradiation Because of Encasement) trial is enrolling patients with locally advanced tumors to receive the A-RT regimens described in this report with concurrent capecitabine with a primary endpoint of resectability and a secondary endpoint of surgical safety after A-RT (NCT03523312). This approach provides a definitive option for those patients who may not ultimately be candidates for surgery. Thus, the future possibility of combining these modalities may permit optimal outcomes. Of course, systemic regimens must continue to be improved in tandem with locoregional control, as distant failure and generally poor OS plagues the disease course in PDAC.
Conclusion:
Ablative radiation is a promising new treatment option for localized PDAC as part of a multi-modal approach. We report that A-RT provides similar locoregional control compared to pancreatectomy in patients who have received induction chemotherapy. There was a 12.8-month survival difference in favor of resected patients, however patients who completed A-RT had an encouraging median OS of 2-years from diagnosis. These data support future clinical trials to directly compare the outcomes of A-RT to surgical resection in patients with PDAC.
Supplementary Material
Acknowledgements:
We would like to thank Dana Haviland for database management and curation and Erin Patterson for editorial advice.
Grant Support:
All authors are supported by Memorial Sloan Kettering Cancer Center (MSKCC), funded by NIH/NCI Cancer Center Support Grant P30CA008748 and the David M. Rubenstein Center for Pancreatic Cancer Research. JSJ receives support from the Weill Cornell Medical College (WCMC) Clinical and Translational Science Center (CTSC) funded by NIH/NCATS UL1TR002384.
Footnotes
Presentations: This manuscript was presented virtually at the 14th World Congress of the International Hepato-Pancreato-Biliary Association, November 27, 2020.
Disclosures: The authors report no conflicts of interest.
References:
- 1.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2017, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site, April 2020. [Google Scholar]
- 2.Balaban EP, Mangu PB, Yee NS. Locally Advanced Unresectable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline Summary. J Oncol Pract. 2017;13(4):265–269. doi: 10.1200/JOP.2016.017376 [DOI] [PubMed] [Google Scholar]
- 3.Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17(6):801–810. doi: 10.1016/S1470-2045(16)00172-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maggino L, Malleo G, Marchegiani G, et al. Outcomes of Primary Chemotherapy for Borderline Resectable and Locally Advanced Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2019. doi: 10.1001/jamasurg.2019.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gemenetzis G, Groot VP, Blair AB, et al. Survival in Locally Advanced Pancreatic Cancer After Neoadjuvant Therapy and Surgical Resection. Ann Surg. 2019;270(2):340–347. doi: 10.1097/SLA.0000000000002753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and Surgical Implications of Neoadjuvant Treatment With FOLFIRINOX for Locally Advanced and Borderline Resectable Pancreatic Cancer. Ann Surg. 2015;261(1):12–17. doi: 10.1097/SLA.0000000000000867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillen S, Schuster T, Meyer zum Büschenfelde C, Friess H, Kleeff J. Preoperative/Neoadjuvant Therapy in Pancreatic Cancer: A Systematic Review and Meta-analysis of Response and Resection Percentages. Seiler C, ed. PLoS Med. 2010;7(4):e1000267. doi: 10.1371/journal.pmed.1000267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhir M, Malhotra GK, Sohal DPS, et al. Neoadjuvant treatment of pancreatic adenocarcinoma: A systematic review and meta-analysis of 5520 patients. World J Surg Oncol. 2017;15(1):183. doi: 10.1186/s12957-017-1240-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy JE, Wo JY, Ryan DP, et al. Total Neoadjuvant Therapy With FOLFIRINOX in Combination With Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer. JAMA Oncol. 2019;5(7):1020. doi: 10.1001/jamaoncol.2019.0892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philip PA, Lacy J, Portales F, et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study. Lancet Gastroenterol Hepatol. 2020;5(3):285–294. doi: 10.1016/S2468-1253(19)30327-9 [DOI] [PubMed] [Google Scholar]
- 11.Klaassen DJ, MacIntyre JM, Catton GE, Engstrom PF, Moertel CG. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil--an Eastern Cooperative Oncology Group study. J Clin Oncol. 1985;3(3):373–378. doi: 10.1200/JCO.1985.3.3.373 [DOI] [PubMed] [Google Scholar]
- 12.Group GTS. Treatment of Locally Unresectable Carcinoma of the Pancreas: Comparison of Combined-Modality Therapy (Chemotheraphy Plus Radiotherapy) to Chemotheraphy Alone1. JNCI J Natl Cancer Inst. 1988;80(10):751–755. doi: 10.1093/jnci/80.10.751 [DOI] [PubMed] [Google Scholar]
- 13.Oettle H, Post S, Neuhaus P, et al. Adjuvant Chemotherapy With Gemcitabine vs Observation in Patients Undergoing Curative-Intent Resection of Pancreatic Cancer. JAMA. 2007;297(3):267. doi: 10.1001/jama.297.3.267 [DOI] [PubMed] [Google Scholar]
- 14.Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2. Ann Oncol. 2008;19(9):1592–1599. doi: 10.1093/annonc/mdn281 [DOI] [PubMed] [Google Scholar]
- 15.Loehrer PJ, Feng Y, Cardenes H, et al. Gemcitabine Alone Versus Gemcitabine Plus Radiotherapy in Patients With Locally Advanced Pancreatic Cancer: An Eastern Cooperative Oncology Group Trial. J Clin Oncol. 2011;29(31):4105–4112. doi: 10.1200/JCO.2011.34.8904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee S, Hurt CN, Bridgewater J, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): A multicentre, randomised, phase 2 trial. Lancet Oncol. 2013. doi: 10.1016/S1470-2045(13)70021-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong J, Patel K, Switchenko J, et al. Outcomes for patients with locally advanced pancreatic adenocarcinoma treated with stereotactic body radiation therapy versus conventionally fractionated radiation. Cancer. 2017;123(18):3486–3493. doi: 10.1002/cncr.30706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan S, Chadha AS, Suh Y, et al. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int J Radiat Oncol Biol Phys. 2016;94(4):755–765. doi: 10.1016/j.ijrobp.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16(7):1727–1733. doi: 10.1245/s10434-009-0408-6 [DOI] [PubMed] [Google Scholar]
- 20.Reyngold M, Parikh P, Crane CH. Ablative radiation therapy for locally advanced pancreatic cancer: techniques and results. Radiat Oncol. 2019;14(1):95. doi: 10.1186/s13014-019-1309-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crane CH, O’Reilly EM. Ablative Radiotherapy Doses for Locally Advanced. Cancer J. 2017;23(6):350–354. doi: 10.1097/PPO.0000000000000292 [DOI] [PubMed] [Google Scholar]
- 22.Reyngold M, O’Reilly EM, Varghese AM, et al. Association of Ablative Radiation Therapy With Survival Among Patients With Inoperable Pancreatic Cancer. JAMA Oncol. March 2021. doi: 10.1001/jamaoncol.2021.0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 24.Al-Hawary MM, Francis IR, Chari ST, et al. Pancreatic ductal adenocarcinoma radiology reporting template: Consensus statement of the society of abdominal radiology and the american pancreatic association. Radiology. 2014;270(1):248–260. doi: 10.1148/radiol.13131184 [DOI] [PubMed] [Google Scholar]
- 25.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 26.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammel P, Huguet F, van Laethem J-L, et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib. JAMA. 2016;315(17):1844. doi: 10.1001/jama.2016.4324 [DOI] [PubMed] [Google Scholar]
- 28.Versteijne E, Suker M, Groothuis K, et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol. 2020:JCO.19.02274. doi: 10.1200/jco.19.02274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Datta J, Wilson GC, D’Angelica MI, et al. A Call for Caution in Overinterpreting Exceptional Outcomes Following Radical Surgery for Pancreatic Cancer: Let the Data Speak. Ann Surg. October 2020. doi: 10.1097/SLA.0000000000004471 [DOI] [PubMed] [Google Scholar]
- 30.Truty MJ, Colglazier JJ, Mendes BC, et al. En Bloc Celiac Axis Resection for Pancreatic Cancer: Classification of Anatomical Variants Based on Tumor Extent. J Am Coll Surg. 2020;231(1):8–29. doi: 10.1016/j.jamcollsurg.2020.05.005 [DOI] [PubMed] [Google Scholar]
- 31.Ramacciato G, Nigri G, Petrucciani N, et al. Pancreatectomy with Mesenteric and Portal Vein Resection for Borderline Resectable Pancreatic Cancer: Multicenter Study of 406 Patients. Ann Surg Oncol. 2016;23(6):2028–2037. doi: 10.1245/s10434-016-5123-5 [DOI] [PubMed] [Google Scholar]
- 32.Zhang Q, Wu J, Tian Y, et al. Arterial resection and reconstruction in pancreatectomy: surgical technique and outcomes. BMC Surg. 2019;19(1):141. doi: 10.1186/s12893-019-0560-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beane JD, House MG, Pitt SC, et al. Pancreatoduodenectomy with venous or arterial resection: a NSQIP propensity score analysis. HPB (Oxford). 2017;19(3):254–263. doi: 10.1016/j.hpb.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 34.Beane JD, House MG, Pitt SC, et al. Distal pancreatectomy with celiac axis resection: What are the added risks? Hpb. 2015;17(9):777–784. doi: 10.1111/hpb.12453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Chiaro M, Rangelova E, Halimi A, et al. Pancreatectomy with arterial resection is superior to palliation in patients with borderline resectable or locally advanced pancreatic cancer. HPB. 2019;21(2):219–225. doi: 10.1016/j.hpb.2018.07.017 [DOI] [PubMed] [Google Scholar]
- 36.Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018;4(6). doi: 10.1001/jamaoncol.2018.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keane FK, Wo JY, Ferrone CR, et al. Intraoperative Radiotherapy in the Era of Intensive Neoadjuvant Chemotherapy and Chemoradiotherapy for Pancreatic Adenocarcinoma. Am J Clin Oncol. 2018;41(6):607–612. doi: 10.1097/COC.0000000000000336 [DOI] [PubMed] [Google Scholar]
- 38.Harrison JM, Wo JY, Ferrone CR, et al. Intraoperative Radiation Therapy (IORT) for Borderline Resectable and Locally Advanced Pancreatic Ductal Adenocarcinoma (BR/LA PDAC) in the Era of Modern Neoadjuvant Treatment: Short-Term and Long-Term Outcomes. Ann Surg Oncol. 2020;27(5):1400–1406. doi: 10.1245/s10434-019-08084-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


