Abstract
Purpose:
The objective of this study was to determine how anxiety and/or depressive symptoms differentially affect specific cognitive domains over time in persons with subjective cognitive decline (SCD).
Design:
A longitudinal, observational study was conducted using data from the National Alzheimer’s Coordinating Center-Uniform Data Set. Mean follow-up was 4.1±2.4 years.
Methods:
Using information from a total of 1,401 participants (age 74.0±8.2 years), linear mixed-effects regression models were used to assess longitudinal changes in global cognition, episodic memory, attention, language, and executive function by baseline psychological (anxiety [A] and/or depressive [D]) symptoms in individuals with SCD. Reference was the group having no symptoms (A−/D−).
Findings:
The A+/D− group was not associated with any cognitive changes. The A−/D+ group was associated with a greater decline in episodic memory and executive function. The A+/D+ group had a greater decline in attention. Changes in global cognition and language were not predicted by any psychological symptoms.
Conclusions:
Depressive symptoms predicted lower episodic memory and executive function.
Clinical Relevance:
Nurses need to pay attention to depressive symptoms in older adults with SCD because managing depressive symptoms may help protect against cognitive decline more typical of early Alzheimer’s dementia.
Keywords: Subjective cognitive decline, depressive symptoms, episodic memory, executive function, Alzheimer’s dementia
Globally, around 50 million people are estimated to live with Alzheimer’s and related dementias (World Health Organization, 2020). The Alzheimer’s Association (2019) reported that 5.6 million Americans aged 65 years or older have Alzheimer’s dementia (AD) with 13.8 million expected to contract the disease by 2050. It is the sixth leading cause of death for all adults but the only illness that has no cure among the top 10 death causes in the United States (Alzheimer’s Association, 2019). Given that discovery of a cure for AD remains imperative, there is no doubt that a breakthrough to deter AD is desperately needed. The cognitively normal phase is now considered an opportune time to modify the dementia process prior to objective cognitive deficit.
Subjective cognitive decline (SCD) is a condition in which individuals feel cognitive decline, but the threshold for decline on standard cognitive tests has yet to be reached (Jessen et al., 2014; Molinuevo et al., 2017). The presence of SCD implies a higher risk of future cognitive decline. Incidence of AD was 11.5/1000 person-years in individuals with SCD compared with 10.1/1000 in those without SCD in 11 cohorts from clinic and community across the world (Slot et al., 2019). Therefore, preventive measures to delay cognitive decline are paramount in individuals with SCD. Targeting risk factors such as anxiety and/or depressive symptoms is one of the options to reduce the growing population with AD.
Anxiety and depressive symptoms are common in older adults. The prevalence of anxiety symptoms was up to 15% for anxiety disorders and 52% for general anxiety. The prevalence of depressive symptoms from mild to major depression reached up to 35% in older adults living in the community. Anxiety and depressive symptoms co-exist in up to 50% of older people (Beaudreau & O’Hara, 2009; Hek et al., 2011). Moreover, individuals with SCD were more likely to report anxiety and depressive symptoms than those without SCD (Jenkins, Tree, Thornton, & Tales, 2019).
The gradual deterioration of episodic memory and/or executive function generally marks the early cognitive deficit related to AD (Harrington et al., 2013; Julayanont, Brousseau, Chertkow, Phillips, & Nasreddine, 2014; Morris et al., 2014). Previous studies for participants without AD have suggested that the presence of anxiety and/or depressive symptoms could affect episodic memory, executive function or the development of AD. Anxiety symptoms have increased the risk of AD by 33% to 80% for older adults with mild cognitive impairment (Mah, Binns, & Steffens, 2015; Palmer et al., 2007). Compared with no anxiety symptoms, cognitively normal older adults with anxiety symptoms had lower episodic memory and executive function (de Vito, Calamia, Greening, & Roye, 2019; Yochim, Mueller, & Segal, 2013). Depressive symptoms may increase the risk of AD in older adults by 65% to 76% (Diniz, Butters, Albert, Dew, & Reynolds, 2013; Saczynski et al., 2010). Recurrent depressive symptoms in old age can reduce episodic memory even further (Rapp et al., 2005). In addition to memory loss, more depressive symptoms at baseline also reduced executive function over time in older adults without dementia (Kohler et al., 2010; Park et al., 2018).
While anxiety and depressive symptoms frequently co-occur, studies have rarely examined how anxiety or depressive symptoms and their coexistence differentially affect cognition in older adults with SCD. The purpose of this study was to determine the effects of anxiety and/or depressive symptoms at baseline on changes in cognition over time in older adults with SCD. We hypothesized that individuals with baseline anxiety and/or depressive symptoms would have a greater decline in episodic memory and executive function as compared to those without any anxiety and depressive symptoms.
Methods
Design and participants
The data for the present longitudinal, observational study came from the National Alzheimer’s Coordinating Center’s Uniform Data Set (NACC-UDS). The details of the dataset were described previously (Beekly et al., 2007). Simply put, the Alzheimer’s Disease Centers (ADCs) use standardized forms to provide systematic information to the NACC-UDS. Informed consent was obtained from the institutional review board (IRB) at each ADC. This study was not population-based research, used the de-identified data, and did not require IRB approval. The presence of SCD was determined by an experienced clinician at the initial visit (baseline) after a standardized semi-structured interview to assess whether self-perceived memory impairment was identified (yes/no).
Among persons with SCD, eligibility for the present study required normal cognition and ages 60 years or over at baseline as previously described (Ahn, Mathiason, Salisbury, & Yu, 2020). At baseline, individuals were defined as cognitively normal if they met all three conditions: 1) determination by a qualified clinician, 2) the Mini-Mental State Examination (MMSE) scores ≥ 27 (Folstein, Folstein, McHugh, & Fanjiang, 2001), and 3) the CDR® Dementia Staging Instrument global scores = 0 (Hughes, Berg, Danziger, Coben, & Martin, 1982). Information on individuals collected after March 2015 (n=124) was excluded because different neuropsychological measures were used, resulting in a total of 1,401 participants with SCD who were observed from September 2005 to March 2015 for the current study (Ahn, Mathiason, Lindquist, & Yu, 2021). To be eligible for this study, participants should be English-speaking and community-dwelling. It is not known what proportion of urban and rural participants each ADC recruited.
Measures
The independent variables were the presence of anxiety and/or depressive symptoms at baseline. The dependent variables were global cognition and four cognitive domains (i.e., episodic memory, attention, language, and executive function) measured in the NACC-UDS. Covariates were demographics, medical history, and ADCs.
Anxiety and depressive symptoms
The presence of anxiety and depressive symptoms was assessed at baseline using the Neuropsychiatric Inventory Questionnaire (NPI-Q), a scale for informants to report 12 neuropsychiatric symptoms of participants that have occurred in the past month (Kaufer et al., 2000). The study used the valid sub-domains of anxiety and depressive symptoms (Trzepacz et al., 2013). The presence of anxiety symptoms was determined as “have any signs of nervousness including feeling excessively tense, being unable to relax, shortness of breath, or sighing” and the presence of depressive symptoms was determined as “seem sad or say that they are depressed.” The severity of anxiety and depressive symptoms was rated as mild (noticeable, but not a significant change), moderate (significant, but not a dramatic change), or severe (very marked or prominent, a dramatic change) (Kaufer et al., 2000). In this study, participants were categorized based on the presence (mild, moderate, or severe symptoms) or absence (no symptoms) of the symptoms. Thus, there were four categories: no symptoms (A−/D−), anxiety symptoms only (A+/D−), depressive symptoms only (A−/D+), both anxiety and depressive symptoms (A+/D+).
Cognition
To increase the sensitivity of traditional cognitive measures to subtle cognitive changes, cognition was measured by composite scores of global cognition, episodic memory, attention, language, and executive function (Nandipati, Luo, Schimming, Grossman, & Sano, 2012; Rabin, Smart, & Amariglio, 2017). The components of the composite scores of cognitive domains were the 10 standardized neuropsychological measures assessing episodic memory, attention, language, and executive function, which were available in the NACC-UDS battery. To calculate the composite global score only, the MMSE was used along with those 10 measures. They were described in order below. The process of calculating composite scores is as follows. The neuropsychological measures were normalized by dividing test scores by their possible maximum scores, and then composite scores were created by summing up normalized items (Nandipati et al., 2012). Of note, higher composite scores indicate better cognitive performance.
To assess global cognition, the 10 neuropsychological measures along with the MMSE were used. The MMSE scores range from 0 to 30. The MMSE represented high reliability (0.80 to 0.95) and validity (0.68 to 0.96) (Folstein et al., 2001). The composite global scores ranged from 0 to 11.
To assess episodic memory, the Immediate and the Delayed Recall Tests of the Wechsler Memory Scale-Revised [WMS-R] were used. Each test has scores ranging from 0 to 25. The reliability coefficient for the Immediate Recall and Delayed Recall Test was 0.71 and 0.75, respectively (Elwood, 1991; Wechsler, 1987). The composite episodic memory scores ranged from 0 to 2.
To assess attention, the Digit Span Backward Test and Length of the WMS-R were used. The scores of the Digit Span Backward Test and the Digit Span Backward Length range from 0 to 12 and 0 to 8, respectively. The internal consistency coefficients of the tests were 0.88 (Elwood, 1991; Wechsler, 1987). The composite attention scores ranged from 0 to 2.
To assess language, the Category Fluency Test in animals and vegetables and the Boston Naming Test were used. The scores of the Category Fluency Test and the Boston Naming Test range from 0 to 77 for each category (animals and vegetables) and 0 to 30, respectively. The test-retest reliability coefficient of the Category Fluency Test was 0.68 and the Boston Naming Test showed a high sensitivity (0.80) and specificity (0.70) for the criterion validity (Harrison, Buxton, Husain, & Wise, 2000; Salmon, Jin, Zhang, Grant, & Yu, 1995). The composite language scores ranged from 0 to 3.
To assess executive function, the Trail Making Test A and B and the Digit Symbol Test of the Wechsler Adult Intelligence Scale-Revised [WAIS-R] were used. The scores of the Trail Making Tests indicate time spent to complete the requirements so less time consuming shows better executive function for both tests (Test A ranging from 0 to 150, and Test B ranging from 0 to 300). The test-retest reliability coefficients of the tests were 0.72 (Arnett & Labovitz, 1995). For the consistency in the scoring system, the Trail Making A and B scores were reversed so that higher scores of all measures in this study indicate better cognitive performance. The scores of the Digit Symbol Test range from 0 to 93. The test-retest reliability coefficient was 0.80 (Paolo & Ryan, 1996). The composite executive function scores ranged from 0 to 3.
Covariates
Covariates included baseline information on demographics, medical history, and ADCs. They were previously described in other studies (Ahn et al., 2021; Ahn et al., 2020). Demographic information was gathered from age and education (in total years) as well as sex (men/women), body mass index (BMI) categories (underweight, normal, overweight, obese), race (White or non-White), ethnicity (Hispanic or non-Hispanic), and marital status (Married or non-married). Medical history (yes/no) which could have an impact on cognition included diabetes mellitus, hypercholesterolemia, hypertension, smoking history, stroke, congestive heart failure, atrial fibrillation, traumatic brain injury, thyroid disease, vitamin B12 deficiency, alcohol or other substance abuse, seizures, and Parkinson’s disease. ADCs was a nominal variable that indicates which of the 35 different ADCs the data came from.
Statistical analysis
Participants were divided into four groups on the basis of the presence of anxiety and depressive symptoms at baseline: 1) A−/D−, 2) A+/D−, 3) A−/D+, and 4) A+/D+. Baseline characteristics of demographics and medical history were presented as means (standard deviations) or numbers (percentages) as appropriate based on the four groups. Linear mixed-effects regression models were used to assess longitudinal associations between baseline psychological symptoms (anxiety and/or depressive symptoms) and changes in cognition. The A−/D− group was reference. All coefficients were presented as means (standard error). The main term for psychological symptoms was interpreted as indicating differences in cognition between psychological groups (i.e., A+/D−, A−/D+, or A+/D+ vs. A−/D−) at baseline. Interaction terms with time (psychological groups × time) indicated differences in the rate of changes in cognition between psychological groups (i.e., A+/D−, A−/D+, or A+/D+ vs. A−/D−) as defined at baseline. The models were adjusted for all covariates. A p<0.05 was used to indicate statistical significance. Assuming missing at random based on the missing value pattern, multiple imputation was used for missing values in the neuropsychological battery. Only two percent of data was missing, which is acceptable under the strictest cutoff of five percent to prevent biased statistical analyses (Schlomer, Bauman, & Card, 2010). Data analyses and imputation were performed in SAS version 9.4 (SAS Inc., Cary, NC, USA).
Results
Baseline characteristics
A total of 1,401 participants with SCD were included in the current study. Their mean ages and education levels were 74.0±8.2 and 15.9±2.8 years, respectively. Participants tended to be female [n=946 (67.5%)], married [n=763 (54.5%)], and White [n=1,180 (84.2%)]. The sample included 76 (5.4%) Hispanic participants. The follow-up period was one to nine years (mean 4.1±2.4 years). A total of 147 participants with anxiety symptoms were in mild (111, 75.5%), moderate (31, 21.1%), and severe (5, 3.4%) levels. A total of 221 participants with depressive symptoms were in mild (166, 75.1%), moderate (51, 23.1%), and severe (4, 1.8%) levels. There were 1,112 (79.4%), 68 (4.9%), 142 (10.1%), and 79 (5.6%) participants in the group of A−/D−, A+/D−, A−/D+, and A+/D+, respectively (Table 1).
Table 1.
Baseline Characteristics according to the Psychological Status (N = 1,401)
| A−/D− | A+/D− | A−/D+ | A+/D+ | |
|---|---|---|---|---|
| N | 1112 (79.4) | 68 (4.9) | 142 (10.1) | 79 (5.6) |
| Age, mean (SD) | 74.4 (8.2) | 72.8 (7.4) | 72.7 (8.3) | 72.5 (8.2) |
| Sex (Female) | 740 (66.5) | 47 (69.1) | 97 (68.3) | 62 (78.5) |
| Education, mean (SD) | 15.8 (2.8) | 15.9 (2.7) | 15.7 (3.3) | 16.3 (2.6) |
| Race (White) | 923 (83.0) | 61 (89.7) | 127 (89.4) | 69 (87.3) |
| Ethnicity (Hispanic) | 46 (4.1) | 6 (8.8) | 21 (14.8) | 3 (3.8) |
| Marital status (Married) | 627 (56.4) | 32 (47.1) | 67 (47.2) | 37 (46.8) |
| Parkinson’s disease | 11 (1.0) | 1 (1.5) | 5 (3.5) | 2 (2.5) |
| Seizures | 20 (1.8) | 2 (2.9) | 6 (4.2) | 0 (0) |
| Traumatic brain injury | 116 (10.4) | 6 (8.8) | 12 (8.5) | 10 (12.7) |
| Stroke | 42 (3.8) | 0 (0) | 1 (0.7) | 2 (2.5) |
| Vitamin B12 deficiency | 52 (4.7) | 3 (4.4) | 10 (7.0) | 6 (7.6) |
| Alcohol abuse | 35 (3.1) | 5 (7.4) | 13 (9.2) | 2 (2.5) |
| Substance abuse | 13 (1.2) | 1 (1.5) | 3 (2.1) | 1 (1.3) |
| Atrial fibrillation | 85 (7.6) | 3 (4.4) | 9 (6.3) | 5 (6.3) |
| Congestive heart failure | 24 (2.2) | 3 (4.4) | 4 (2.8) | 2 (2.5) |
| Thyroid disease | 224 (20.1) | 17 (25.0) | 39 (27.5) | 20 (25.3) |
| Diabetes | 125 (11.2) | 3 (4.4) | 13 (9.2) | 11 (13.9) |
| Hypercholesterolemia | 566 (50.9) | 42 (61.8) | 69 (48.6) | 36 (45.6) |
| Hypertension | 576 (51.8) | 34 (50.0) | 69 (48.6) | 35 (44.3) |
| Body mass index | ||||
| Normal weight | 417 (37.5) | 32 (47.1) | 54 (38.0) | 38 (48.1) |
| Underweight | 18 (1.6) | 0 (0) | 3 (2.1) | 2 (2.5) |
| Overweight | 434 (39.0) | 28 (41.2) | 46 (32.4) | 23 (29.1) |
| Obesity | 243 (21.9) | 8 (11.8) | 39 (27.5) | 16 (20.3) |
| Smoking history | ||||
| Lifetime non-smoker | 568 (51.1) | 35 (51.5) | 71 (50.0) | 39 (49.4) |
| Former smoker | 506 (45.5) | 29 (42.6) | 67 (47.2) | 38 (48.1) |
| Current smoker | 38 (3.4) | 4 (5.9) | 4 (2.8) | 2 (2.5) |
Abbreviations: A−/D−, neither anxiety nor depressive symptoms; A+/D−, anxiety symptoms only; A−/D+, depressive symptoms only; A+/D+, both anxiety and depressive symptoms; SD, standard deviation
Values were presented as numbers (percentages) of participants unless otherwise indicated.
Rate of changes in cognition by baseline anxiety and depressive symptoms
Table 2 shows estimated effects of psychological symptoms on cognition after adjusting for all covariates. Using the A−/D− group as reference, the final models for each statistically significant domain are as follows: 1) Episodic memory = 1.497 – 0.010 (A−/D+) + 0.011 (year) – 0.013 ([A−/D+] × [year]), 2) Attention = 1.260 – 0.059 (A+/D+) – 0.007 (year), and 3) Executive function = 3.415 – 0.047 (A−/D+) – 0.020 (year). Any psychological symptoms were not associated with changes in global cognition and language. The A+/D− group did not predict any cognitive changes.
Table 2.
Longitudinal Associations between Baseline Psychological Symptoms and Changes in Cognitive Performance (N = 1,401)
| Coefficient (standard error [SE]) | |||||
|---|---|---|---|---|---|
| Global cognition | Episodic memory | Attention | Language | Executive function | |
| (A+/D−) | 0.059 (0.072) | 0.009 (0.029) | 0.022 (0.028) | 0.008 (0.016) | 0.018 (0.029) |
| (Time) | −0.029 (0.005)** | 0.011 (0.002)** | −0.007 (0.002)** | −0.007 (0.001)** | −0.020 (0.002)** |
| (A+/D−) × (Time) | −0.010 (0.021) | −0.007 (0.009) | −0.004 (0.008) | −0.002 (0.005) | 0.004 (0.009) |
| (A−/D+) | −0.025 (0.052) | −0.010 (0.021) | 0.033 (0.020) | 0.002 (0.012) | −0.047 (0.021) * |
| (Time) | −0.029 (0.005)** | 0.011 (0.002)** | −0.007 (0.002)** | −0.007 (0.001)** | −0.020 (0.002)** |
| (A−/D+) × (Time) | −0.020 (0.015) | −0.013 (0.006) * | −0.011 (0.006) | −0.001 (0.003) | 0.003 (0.006) |
| (A+/D+) | −0.044 (0.067) | −0.021 (0.027) | −0.059 (0.026) * | 0.010 (0.015) | 0.026 (0.027) |
| (Time) | −0.029 (0.005)** | 0.011 (0.002)** | −0.007 (0.002)** | −0.007 (0.001)** | −0.020 (0.002)** |
| (A+/D+) × (Time) | 0.003 (0.019) | −0.015 (0.008) | 0.008 (0.007) | 0.002 (0.004) | 0.007 (0.008) |
p<0.05,
p<0.01
Abbreviations: A−/D−, neither anxiety nor depressive symptoms; A+/D−, anxiety symptoms only; A−/D+, depressive symptoms only; A+/D+, both anxiety and depressive symptoms
Psychological symptoms refer to the presence of anxiety and/or depressive symptoms at baseline.
Reference is participants without anxiety and depressive symptoms (A−/D−).
Coefficient on the psychological status estimates baseline difference in outcome between a group indicator and reference. Coefficient on time estimates average changes in outcome over a year in reference. Coefficient on the psychological status × time estimates average differences in changes over a year in a group indicator compared to reference.
Covariates include baseline demographic information, medical history, and indicators for Alzheimer’s Disease Centers.
For episodic memory, baseline episodic memory scores were similar between the A−/D+ and the A−/D− group (p=0.64). The Episodic memory score increased by an average of 0.011±0.002 points in the A−/D− group every year. A significant interaction indicated that episodic memory scores decreased in the A−/D+ group by an average of 0.013±0.006 points compared to the A−/D− group every year. For attention, the A+/D+ group had an average of 0.059±0.026 lower attention scores than the A−/D− group at baseline. The attention score declined by an average of 0.007±0.002 points in the A−/D− group every year. The rate of changes in attention was similar between the A+/D+ and the A−/D− group over time.
For executive function, the A−/D+ group had an average of 0.047±0.021 lower executive function scores than the A−/D− group at baseline. The executive function score declined by an average of 0.020±0.002 points in the A−/D− group every year. The rate of changes in executive function was similar between the A−/D+ and the A−/D− group over time. Predicted episodic memory, attention, and executive function by significant psychological symptoms were shown in Figure 1. Global cognition and language were not visualized as no psychological symptoms predicted changes in cognitive performance.
Figure 1.

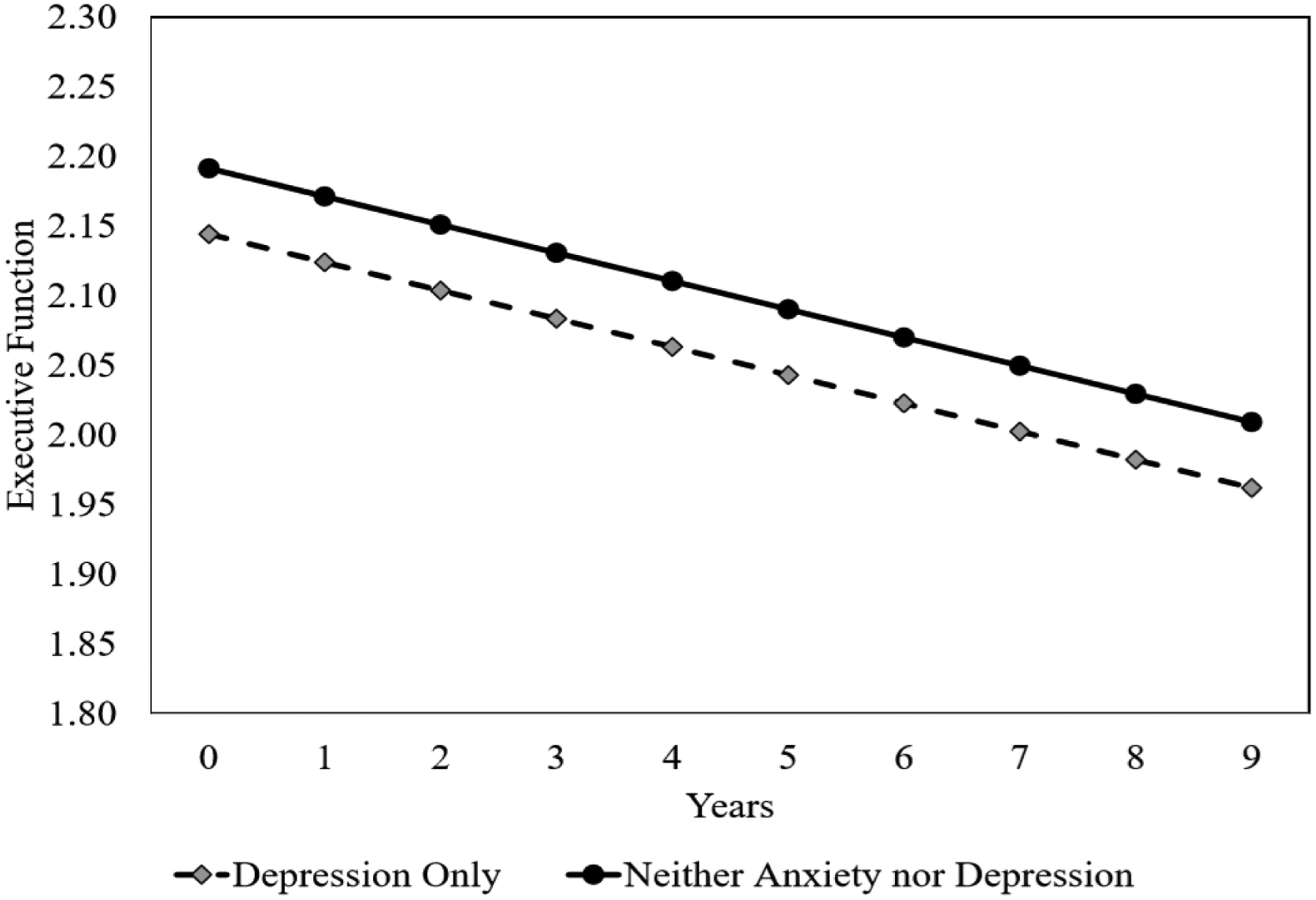
Predicted Episodic Memory, Attention, and Executive Function Scores by Significant Psychological Symptoms
Psychological symptoms refer to the presence of anxiety and/or depressive symptoms at baseline.
Reference is participants without anxiety and depressive symptoms.
Results were from the fully adjusted model by covariates.
Sensitivity analyses
To examine if estimated effects differed, we re-ran the same models by trimming the highest and lowest 1% of the outcome variables. Most of the results were consistent with our original analysis, but three differences were shown (Supplementary Table 1). Unlike the original analysis showing that no factors were associated with global cognition, the A−/D+ group scored 0.077±0.034 points lower in global cognition than the A−/D− group at baseline, and the score differences were maintained over time. In the original analysis, A−/D+ was associated with lower episodic memory and executive function, which was also demonstrated in these further analyses, but A+/D+ also showed statistical significance. The A+/D+ group had 0.061±0.017 lower episodic memory scores than the A−/D− group at baseline. Furthermore, the scores decreased in the A+/D+ group by an average of 0.015±0.007 points every year. But, the A+/D+ group had 0.043±0.017 higher executive function scores than the A−/D− group at baseline and the score differences were maintained over time.
Discussion
To date, studies have rarely delineated the association of anxiety and/or depressive symptoms at baseline with the longitudinal changes in cognition in a large sample of well-characterized older adults with SCD. Our findings showed that the presence of depressive symptoms only is associated with episodic memory and executive function decline. Contrary to the hypothesis, in the original analyses, the presence of both anxiety and depressive symptoms was not associated with episodic memory and executive function decline, but was associated with a decline in attention.
The present study indicated that the presence of anxiety symptoms only was not associated with cognitive decline. In older adults without dementia living in a community, anxiety symptoms were associated with lower episodic memory and executive function (de Vito et al., 2019; Yochim et al., 2013). However, the results were from cross-sectional studies. Research on the relationship between anxiety symptoms and changes in episodic memory over time is limited among older adults. But, a longitudinal study over 17 years showed that the presence of baseline anxiety symptoms was predictive of a decline in learning memory in cognitively normal individuals who were on average 56 years old at baseline (Gallacher et al., 2009). In addition, it was suggested by researchers that effects of the presence of anxiety symptoms on cognition would increase with a longer follow-up period (Petkus, Reynolds, Wetherell, Kremen, & Gatz, 2017). Thus, long-term observation may provide more accurate information on the effects of anxiety symptoms on cognition in older adults with SCD. In the present study, the categorical presence or absence of psychological symptoms served as the exposure of interest. It was indicated that severe anxiety symptoms are more related to cognitive decline than mild to moderate symptoms, but even the mildest severity of depressive symptoms are associated with cognitive decline (Bierman, Comijs, Jonker, & Beekman, 2005). In addition to the follow-up period, the lack of significant results may result from the findings that the majority of anxiety symptoms were mild (111, 75.5%) and moderate (31, 21.1%) levels.
As opposed to the hypothesis, there were no effects of combined anxiety and depressive symptoms on a decline in episodic memory and executive function in the original analyses. The presence of both anxiety and depressive symptoms was only a predictor of attention decline. In a clinic-based sample without AD, older adults with late-onset major depressive disorder (mean ages 84 years) showed deficit in attention (Rapp et al., 2005). However, it was a cross-sectional study and the sample characteristics were different from the current study in which the severity of depressive symptoms was mostly mild (166, 75.1%) and moderate (51, 23.1%). The presence of anxiety symptoms may worsen the attentional network in a sample between the ages of 17 and 32 (Pacheco-Unguetti, Acosta, Callejas, & Lupianez, 2010). But its role in older adults remains unknown. In sensitivity analyses, similar to the original analysis, the presence of anxiety symptoms only was not associated with changes in cognition. But combined anxiety and depressive symptoms were associated with episodic memory and executive function as well as attention. A counterintuitive observation was that combined anxiety and depressive symptoms predicted slightly better executive function than without psychological symptoms as opposed to the findings that combined anxiety and depressive symptoms negatively affected episodic memory and attention. Given these findings, it is still insufficient to explain how combined anxiety and depressive symptoms predicted changes in cognition among older adults with SCD. A follow-up study of this topic seems necessary.
The presence of depressive symptoms only was a predictor of episodic memory and executive function decline in both original and sensitivity analyses, suggesting that depressive symptoms may contribute to the cognitive profile typical of AD in older adults with SCD. Furthermore, a low global cognition by depressive symptoms in sensitivity analyses showed the deleterious effects of depressive symptoms on cognition overall. Our finding supports previous studies among older adults that showed depressive symptoms are associated with the incidence of AD or the decline in episodic memory/executive function (Diniz et al., 2013; Park et al., 2018; Rapp et al., 2005). The potential mechanisms by which depressive symptoms influence cognition may be through AD biomarkers (e.g., aggregated amyloid-beta/tau and shrunk hippocampal volume) which indicate the AD process is underway. Studies reported that depressive symptoms among cognitively healthy older adults can increase amyloid-beta burden or lead to reduced hippocampal volume (Ezzati, Zimmerman, Katz, & Lipton, 2013; Yasuno et al., 2016).
Based on the findings, it is imperative to find ways to manage depressive symptoms in SCD to mitigate the risk of future AD. Physical activity (PA) and cognitive behavioral therapy (CBT) are options to consider. In two meta-analyses, PA was effective to reduce depressive symptoms with moderate to large effect size (Josefsson, Lindwall, & Archer, 2014; Kvam, Kleppe, Nordhus, & Hovland, 2016). In another meta-analysis, CBT was up to 51% more effective to reduce depressive symptoms than any other interventions (van Zoonen et al., 2014). Future research needs to be conducted to see if interventions to reduce depressive symptoms such as PA and CBT can delay the decline in episodic memory and executive function among older adults with SCD.
The effects of independent/combined psychological symptoms on performance in comprehensive cognitive domains were examined in a large, well-characterized sample. However, our study had limitations. First, the study sample may not be representative of the general U.S. population given the race/ethnicity (White 84%/Hispanic 5.4%) and educational level (16 years). Given that depressive symptoms may have a greater impact on cognition in African Americans than in Whites and that higher educational level is one of the protective factors that may prevent AD (Alzheimer’s Association, 2019; Grant, Harries, & Chamberlain, 2018), cognitive changes in this study may not be clinically significant. Thus, to explore the detailed clinical association between psychological symptoms and cognition among older adults with SCD, further studies would be necessary to examine their relationships in diverse race/ethnicity groups and across different educational levels. Second, the study did not involve medication use as covariates. However, covariates included a variety of medical conditions that have been known to influence cognition.
Implications
Our findings have several implications for future research. Given that anxiety symptoms were not associated with changes in cognition during the observation period, further research observing a longer period than the average of four years in the present study may be necessary to see the expected relationships between anxiety symptoms and the decline in episodic memory and executive function. Moreover, in the present study, most of the anxiety and depressive symptoms were mild in severity. The skewed distribution of the severity made it difficult to examine changes in cognition by the severity of psychological symptoms. As indicated above, cognitive changes may vary depending on the severity. It may be the case, especially for anxiety symptoms (Bierman et al., 2005). Therefore, to examine changes in cognition by the severity, further research is required with a sufficient sample in each severity category. In addition, our findings indicate the importance of investigating the effects of changes in psychological symptoms naturally or as the result of medications on changes in cognition over time. Furthermore, adopting AD biomarkers can be considered to understand potential mechanisms. In older adults with SCD, we can figure out whether a decline in episodic memory and executive function by anxiety and/or depressive symptoms would be mediated by AD biomarkers.
Our findings also have clinical implications. Nurses should consider 1) intervention methods that can manage depressive symptoms and 2) how to increase the participation rate of those interventions. Individuals with SCD are by definition aware of and concerned about perceived changes in cognition, which suggests that they would be motivated to participate in any programs to potentially improve their functioning (Rabin et al., 2017). However, persons with depressive symptoms may lack energy and motivation, so nurses should make intervention programs such as PA and CBT which are tailored to participants’ preferences to raise the likelihood of adherence. For example, step-by-step goal achievement would be one of the important aspects (Josefsson et al., 2014; Kvam et al., 2016; van Zoonen et al., 2014). Aerobic and/or strengthening training may be considered as the PA programs. Gradually increasing the intensity of the program may help motivate participants through a sense of accomplishment. Likewise, the CBT program would effectively raise the adherence rate by covering goal-oriented topics gradually on belief, health, spirituality, and/or behaviors.
Conclusions
The presence of depressive symptoms can forecast a decline in episodic memory and executive function in older adults with SCD. Nurses should pay attention to episodic memory and executive function when observing depressive symptoms in older adults with SCD. Furthermore, it is tempting to see whether interventions targeting depressive symptoms may protect against cognitive deterioration in those typical domains related to AD.
Supplementary Material
Acknowledgments
The authors would like to express gratitude for the feedback from Drs. Ruth Lindquist, John Connett, and Dereck Salisbury. The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Footnotes
- Alzheimer’s Association: https://www.alz.org
- National Institute on Aging: https://www.nia.nih.gov
References
- Ahn S, Mathiason M, Lindquist R, & Yu F (2021). Factors predicting episodic memory changes in older adults with subjective cognitive decline: A longitudinal observational study. Geriatric Nursing, 42(1), 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Mathiason M, Salisbury D, & Yu F (2020). Factors predicting the onset of amnestic mild cognitive impairment or Alzheimer’s dementia in persons with subjective cognitive decline. Journal of Gerontological Nursing, 46(8), 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association. (2019). 2019 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, 15(3), 321–387. [Google Scholar]
- Arnett JA, & Labovitz SS (1995). Effect of physical layout in performance of the Trail Making Test. Psychological Assessment, 7(2), 220–221. [Google Scholar]
- Beaudreau SA, & O’Hara R (2009). The association of anxiety and depressive symptoms with cognitive performance in community-dwelling older adults. Psychology and Aging, 24(2), 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, & Kukull WA (2007). The National Alzheimer’s Coordinating Center (NACC) database: The Uniform Data Set. Alzheimer Disease & Associated Disorders, 21(3), 249–258. [DOI] [PubMed] [Google Scholar]
- Bierman EJ, Comijs HC, Jonker C, & Beekman AT (2005). Effects of anxiety versus depression on cognition in later life. American Journal of Geriatric Psychiatry, 13(8), 686–693. [DOI] [PubMed] [Google Scholar]
- de Vito A, Calamia M, Greening S, & Roye S (2019). The association of anxiety, depression, and worry symptoms on cognitive performance in older adults. Aging, Neuropsychology, and Cognition, 26(2), 161–173. [DOI] [PubMed] [Google Scholar]
- Diniz BS, Butters MA, Albert SM, Dew MA, & Reynolds CF 3rd. (2013). Late-life depression and risk of vascular dementia and Alzheimer’s disease: Systematic review and meta-analysis of community-based cohort studies. British Journal of Psychiatry, 202(5), 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood RW (1991). The Wechsler Memory Scale-Revised: Psychometric characteristics and clinical application. Neuropsychology Review, 2(2), 179–201. [DOI] [PubMed] [Google Scholar]
- Ezzati A, Zimmerman ME, Katz MJ, & Lipton RB (2013). Hippocampal correlates of depression in healthy elderly adults. Hippocampus, 23(12), 1137–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, & Fanjiang G (2001). Mini-Mental State Examination user’s guide. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Gallacher J, Bayer A, Fish M, Pickering J, Pedro S, Dunstan F, … Ben-Shlomo Y (2009). Does anxiety affect risk of dementia? Findings from the Caerphilly Prospective Study. Psychosomatic Medicine, 71(6), 659–666. [DOI] [PubMed] [Google Scholar]
- Grant JE, Harries M, & Chamberlain SR (2018). Differences in the cognitive profile of depression between racial groups. Annals of Clinical Psychiatry, 30(1), 32–37. [PMC free article] [PubMed] [Google Scholar]
- Harrington MG, Chiang J, Pogoda JM, Gomez M, Thomas K, Marion SD, … Fonteh AN (2013). Executive function changes before memory in preclinical Alzheimer’s pathology: A prospective, cross-sectional, case control study. PLoS One, 8(11), e79378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JE, Buxton P, Husain M, & Wise R (2000). Short Test of Semantic and Phonological Fluency: Normal performance, validity and test-retest reliability. British Journal of Clinical Psychology, 39(2), 181–191. [DOI] [PubMed] [Google Scholar]
- Hek K, Tiemeier H, Newson RS, Luijendijk HJ, Hofman A, & Mulder CL (2011). Anxiety disorders and comorbid depression in community dwelling older adults. International Journal of Methods in Psychiatric Research, 20(3), 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, & Martin RL (1982). A new clinical scale for the staging of dementia. British Journal of Psychiatry, 140, 566–572. [DOI] [PubMed] [Google Scholar]
- Jenkins A, Tree JJ, Thornton IM, & Tales A (2019). Subjective cognitive impairment in 55–65-year-old adults is associated with negative affective symptoms, neuroticism, and poor quality of life. Journal of Alzheimer’s Disease, 67(4), 1367–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chetelat G, … Wagner M (2014). A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s & Dementia, 10(6), 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson T, Lindwall M, & Archer T (2014). Physical exercise intervention in depressive disorders: Meta-analysis and systematic review. Scandinavian Journal of Medicine & Science in Sports, 24(2), 259–272. [DOI] [PubMed] [Google Scholar]
- Julayanont P, Brousseau M, Chertkow H, Phillips N, & Nasreddine ZS (2014). Montreal Cognitive Assessment Memory Index Score (MoCA-MIS) as a predictor of conversion from mild cognitive impairment to Alzheimer’s disease. Journal of the American Geriatrics Society, 62(4), 679–684. [DOI] [PubMed] [Google Scholar]
- Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, … DeKosky ST (2000). Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. Journal of Neuropsychiatry & Clinical Neurosciences, 12(2), 233–239. [DOI] [PubMed] [Google Scholar]
- Kohler S, van Boxtel MP, van Os J, Thomas AJ, O’Brien JT, Jolles J, … Allardyce J (2010). Depressive symptoms and cognitive decline in community-dwelling older adults. Journal of the American Geriatrics Society, 58(5), 873–879. [DOI] [PubMed] [Google Scholar]
- Kvam S, Kleppe CL, Nordhus IH, & Hovland A (2016). Exercise as a treatment for depression: A meta-analysis. Journal of Affective Disorders, 202, 67–86. [DOI] [PubMed] [Google Scholar]
- Mah L, Binns MA, & Steffens DC (2015). Anxiety symptoms in amnestic mild cognitive impairment are associated with medial temporal atrophy and predict conversion to Alzheimer disease. American Journal of Geriatric Psychiatry, 23(5), 466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinuevo JL, Rabin LA, Amariglio R, Buckley R, Dubois B, Ellis KA, … Jessen F (2017). Implementation of subjective cognitive decline criteria in research studies. Alzheimer’s & Dementia, 13(3), 296–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Blennow K, Froelich L, Nordberg A, Soininen H, Waldemar G, … Dubois B (2014). Harmonized diagnostic criteria for Alzheimer’s disease: Recommendations. Journal of Internal Medicine, 275(3), 204–213. [DOI] [PubMed] [Google Scholar]
- Nandipati S, Luo X, Schimming C, Grossman HT, & Sano M (2012). Cognition in non-demented diabetic older adults. Current Aging Science, 5(2), 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Unguetti AP, Acosta A, Callejas A, & Lupianez J (2010). Attention and anxiety: Different attentional functioning under state and trait anxiety. Psychological Science, 21(2), 298–304. [DOI] [PubMed] [Google Scholar]
- Palmer K, Berger AK, Monastero R, Winblad B, Backman L, & Fratiglioni L (2007). Predictors of progression from mild cognitive impairment to Alzheimer’s disease. Neurology, 68(19), 1596–1602. [DOI] [PubMed] [Google Scholar]
- Paolo AM, & Ryan JJ (1996). Stability of WAIS-R scatter indices in the elderly. Archives of Clinical Neuropsychology, 11(6), 503–511. [PubMed] [Google Scholar]
- Park JH, Lee SB, Lee JJ, Yoon JC, Han JW, Kim TH, … Kim KW (2018). Depression plays a moderating role in the cognitive decline associated with changes of brain white matter hyperintensities. Journal of Clinical Psychiatry, 79(5), e1–10. [DOI] [PubMed] [Google Scholar]
- Petkus AJ, Reynolds CA, Wetherell JL, Kremen WS, & Gatz M (2017). Temporal dynamics of cognitive performance and anxiety across older adulthood. Psychology and Aging, 32(3), 278–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin LA, Smart CM, & Amariglio RE (2017). Subjective cognitive decline in preclinical Alzheimer’s disease. Annual Review of Clinical Psychology, 13, 369–396. [DOI] [PubMed] [Google Scholar]
- Rapp MA, Dahlman K, Sano M, Grossman HT, Haroutunian V, & Gorman JM (2005). Neuropsychological differences between late-onset and recurrent geriatric major depression. American Journal of Psychiatry, 162(4), 691–698. [DOI] [PubMed] [Google Scholar]
- Saczynski JS, Beiser A, Seshadri S, Auerbach S, Wolf PA, & Au R (2010). Depressive symptoms and risk of dementia: The Framingham Heart Study. Neurology, 75(1), 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon DP, Jin H, Zhang M, Grant I, & Yu E (1995). Neuropsychological assessment of Chinese elderly in the Shanghai Dementia Survey. The Clinical Neuropsychologist, 9(2), 159–168. [Google Scholar]
- Schlomer GL, Bauman S, & Card NA (2010). Best practices for missing data management in counseling psychology. Journal of Counseling Psychology, 57(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Slot RER, Sikkes SAM, Berkhof J, Brodaty H, Buckley R, Cavedo E, … van der Flier WM (2019). Subjective cognitive decline and rates of incident Alzheimer’s disease and non-Alzheimer’s disease dementia. Alzheimer’s & Dementia, 15(3), 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzepacz PT, Saykin A, Yu P, Bhamditipati P, Sun J, Dennehy EB, … Cummings JL (2013). Subscale validation of the neuropsychiatric inventory questionnaire: Comparison of Alzheimer’s Disease Neuroimaging Initiative and National Alzheimer’s Coordinating Center cohorts. American Journal of Geriatric Psychiatry, 21(7), 607–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zoonen K, Buntrock C, Ebert DD, Smit F, Reynolds CF 3rd, Beekman AT, & Cuijpers P (2014). Preventing the onset of major depressive disorder: A meta-analytic review of psychological interventions. International Journal of Epidemiology, 43(2), 318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1987). Wechsler Memory Scale-Revised. San Antonio, TX: Psychological Corporation. [Google Scholar]
- World Health Organization. (2020). Dementia. http://www.who.int/news-room/fact-sheets/detail/dementia
- Yasuno F, Kazui H, Morita N, Kajimoto K, Ihara M, Taguchi A, … Nagatsuka K (2016). High amyloid-beta deposition related to depressive symptoms in older individuals with normal cognition: A pilot study. International Journal of Geriatric Psychiatry, 31(8), 920–928. [DOI] [PubMed] [Google Scholar]
- Yochim BP, Mueller AE, & Segal DL (2013). Late life anxiety is associated with decreased memory and executive functioning in community dwelling older adults. Journal of Anxiety Disorders, 27(6), 567–575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


