Abstract
The parent–adolescent relationship is important for adolescents’ emotion regulation (ER), yet little is known regarding the neural patterns of dyadic ER that occur during parent–adolescent interactions. A novel measure that can be used to examine such patterns is cross-brain connectivity (CBC)—concurrent and time-lagged connectivity between two individuals’ brain regions. This study sought to provide evidence of CBC and explore associations between CBC, parenting, and adolescent internalizing symptoms. Thirty-five adolescents (mean age = 15 years, 69% female, 72% Non-Hispanic White, 17% Black, 11% Hispanic or Latino) and one biological parent (94% female) completed an fMRI hyperscanning conflict discussion task. Results revealed CBC between emotion-related brain regions. Exploratory analyses indicated CBC is associated with parenting and adolescent depressive symptoms.
Parent–child social interactions are highly influential in the development of emotion regulation (ER), the ability to recognize and modulate one’s emotions, throughout childhood and adolescence (Eisenberg et al., 2005). For instance, emotional support, guidance, and warmth observed during parent–adolescent interactions has consistently predicted more effective adolescent ER skills as well as lower levels of internalizing and externalizing symptoms (Criss, Morris, Ponce-Garcia, Cui, & Silk, 2016; Yap, Schwartz, Byrne, Simmons, & Allen, 2010). Increasingly, research has begun to uncover how parenting behaviors influence adolescent emotion-related brain networks that support the development of ER and risk for psychopathology (e.g., Silk et al., 2017). Few studies, however, have explored neural mechanisms underlying social cognitive processes during dynamic, real-time parent–adolescent interactions. This is particularly important to examine during adolescence when many forms of psychopathology emerge (Guyer, Silk, & Nelson, 2016). By examining these neural mechanisms in a dyadic context, using novel methods such as functional magnetic resonance imaging (fMRI) hyperscanning (i.e., the simultaneous scanning of two interacting individuals), we can gain valuable insight into dynamic emotion-related processes.
Research indicates that parenting behaviors and practices influence the development of adolescent emotion-related brain networks. For example, a study by Lee and colleagues (2015) found that adolescents showed greater activation in subcortical limbic regions associated with emotional reactivity when listening to maternal critical statements. During this same task, adolescents also showed reduced activation in brain regions associated with cognitive control (e.g., dorsolateral prefrontal cortex [dlPFC]) and social cognition (e.g., posterior cingulate cortex [PCC]), suggesting adolescents may have difficulty recruiting regulatory regions when faced with maternal criticism. A second study examining maternal statements and adolescent brain function found that adolescent girls with higher levels of depression and anxiety symptoms showed greater activaton in the right amygdala when listening to maternal criticism but reduced activity in the left amygdala when listening to maternal criticism and praise compared to girls with lower levels of internalizing symptoms (Aupperle et al., 2016). A longitudinal study of adolescents with a history of anxiety found higher levels of adolescent-perceived maternal warmth predicted lower activation in regions involved in the affective salience network when listening to maternal criticism versus neutral statements (Butterfield et al., 2020). The affective salience network (e.g., anterior insula [AI], amygdala) is involved in the orientation of attention to relevant emotional stimuli (Phillips, Drevets, Rauch, & Lane, 2003), suggesting adolescent-perceived supportive parenting practices may lessen the negative effects of parental criticism through downregulation in these regions, thus shifting attention away from the negative stimulus. Taken together, these findings suggest that evaluative feedback from parents is associated with the development of adolescent neurocircuitry, specifically in regions involved in ER, processing, and reactivity.
Researchers have also used simultaneous fMRI scanning to examine social and emotional processes in parent–adolescent dyads. Kerr and colleagues (2020) measured activity in the brains of parent–adolescent dyads while completing a dyadic error-processing task designed to elicit disappointment during simultaneous fMRI scanning. This study revealed that positive parenting practices were associated with greater activity in the parent ventromedial prefrontal cortex (vmPFC), a region implicated in suppression of negative emotion as well as empathetic responding, when witnessing their adolescent’s error. Another study using the same task revealed that parents who showed decreased activity in the medial prefrontal cortex (mPFC) and PCC when witnessing their adolescent’s error had adolescents with greater depressive and anxiety symptoms (Cosgrove et al., 2020). In addition, adolescents who showed increased activity in the AI when witnessing their parent’s error had parents with greater anxiety symptoms. These studies demonstrate the utility of simultaneous fMRI scanning in the examination of parent–adolescent cross-brain associations and suggest that emotion-related neurobiology may be better understood within a dyadic context. However, fMRI hyperscanning, which refers to the simultaneous scanning of two interacting individuals, differs slightly from simultaneous scanning in that researchers are able to use this technology to examine brain activity in two individuals during a reciprocal interaction (e.g., conversation [Misaki et al., 2021]). Thus, hyperscanning allows us to examine dyadic brain function simultaneously during reciprocal and unpredictable interactions, making this technology indispensable when considering the neurobiological mechanisms associated with parent–adolescent social interactions. Furthermore, fMRI hyperscanning allows for the examination of whole-brain activity, including the limbic system, that cannot be captured in other hyperscanning modalities such as electroencephalogram (EEG) and dual functional near-infrared spectroscopy (fNIRS).
Hyperscanning using EEG (e.g., Astolfi et al., 2011), fNIRS (e.g., Reindl, Gerloff, Scharke, & Konrad, 2018), and MEG (e.g., Hirata et al., 2014) has been used to study interbrain synchrony. Interbrain synchrony, also termed brain-to-brain synchrony or neural synchrony, refers to concurrent functional connectivity between the same brain regions in two interacting individuals. The concept of interbrain synchrony stems from a vast literature spanning decades of research on emotional, behavioral, and biological synchrony between socially interacting individuals (e.g., Feldman, 2007). The research focused on the parent–child dyad suggests that these forms of synchrony may be related to caregiver attachment, parent–child relationship quality, social competence, empathetic responding, and importantly, self-regulation (Barber, Bolitho, & Bertrand, 2001; Feldman, 2007). Indeed, empirical findings have consistently shown that parent–child synchrony is related to the development of self-regulation strategies from infancy to adolescence (Barber et al., 2001), which in turn is related to lower levels of internalizing symptoms in adolescence (Criss et al., 2016; Yap et al., 2010).
A recent study examining parent–child interbrain synchrony using fNIRS hyperscanning found increased synchrony in the dlPFC and frontopolar cortex during parent–child cooperation but not during competition between parents and children (Reindl et al., 2018). When children completed the tasks with an adult stranger, evidence of interbrain synchrony was not found, suggesting that synchrony may relate to social connectedness between parents and children. A similar study by Miller and colleagues (2019) also found increased interbrain synchrony in the dlPFC and frontopolar cortex of mother–child dyads while completing a cooperative task versus one completed independently. Lastly, an fNIRS hyperscanning study found higher levels of parenting stress was associated with reduced interbrain synchrony in the left mPFC among mothers and their children when watching animated videos together (Azhari et al., 2019).
Building on these findings, we suggest that cross-brain connectivity (CBC) may provide insight into the patterns of social and emotion-related processes that occur during real-time interactions between parents and adolescents. CBC extends interbrain synchrony by including both concurrent and time-lagged functional connectivity between the same and different brain regions in two interacting individuals (Bilek et al., 2015). Considering developmental differences as well as the distinct roles of parents and children during interactions, it is likely that parents and adolescents may recruit different regions to process social and emotional stimuli while engaging in conversation. Moreover, the inclusion of time-lagged functional connectivity within our definition is crucial in order to accommodate the natural delay that occurs between the speaker and the listener during real-life conversations. To date and to the best of our knowledge, no studies have examined CBC in the parent–child dyad. However, Bilek and colleagues (2015) examined CBC in stranger dyads completing a joint attention task while undergoing fMRI hyperscanning. The cooperative task required the “sender” to convey the location of a given target by shifting their gaze to the correct location, and the “receiver” used this nonverbal information to accurately guess the position of the target. They found evidence of CBC between the sender’s right temporoparietal junction and the receiver’s mPFC and orbitofrontal cortex. They also noted dyads who reported larger social networks showed greater CBC during the task. Thus, like interbrain synchrony, CBC appears to play a role in supporting social interactions.
The Current Study
CBC may reveal the nuances of social cognition, including emotion-related processes, during real-time parent–adolescent social interactions. Moreover, fMRI hyperscanning, which offers a high spatial resolution of both cortical and deep-brain structures, provides a method of assessing CBC between interacting parents and adolescents. Thus, we sought to explore parent–adolescent CBC between emotion-related brain regions during an fMRI hyperscanning task as well as associations between CBC and parent emotion socialization behaviors, parent–adolescent interaction quality, and adolescent internalizing symptoms. Specifically, we sought to address the following questions: (a) is there evidence of parent–adolescent CBC during a conflict discussion task?; (b) is CBC between emotion-processing regions in the adolescent and parent brain related to adolescent self-reported depressive and anxiety symptoms?; (c) is CBC between emotion-processing regions of the adolescent and parent brain related to adolescent–perceived parent emotion socialization behavior?; and (d) is CBC between emotion-processing regions of the parent and adolescent brain related to the valence of a parent–adolescent social interaction during a conflict discussion task? Each of our research questions were exploratory in nature. Directional hypotheses were unable to be derived given that this is the first study to examine CBC in parents and adolescents as well as the first study to examine CBC during a reciprocal verbal interaction using fMRI hyperscanning. Regions of interest in this study were determined a priori and included the the bilateral AI, dlPFC, ventrolateral prefrontal cortex (vlPFC), and amygdala, as these regions have been identified in meta-analyses of ER and processing (Frank et al., 2014; Kohn et al., 2014; Xu et al., 2014; Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011).
Methods
Participants
Parent–adolescent dyads were recruited from public middle and high schools in a southern mid-west city in the United States using flyers sent via a digital platform used by school districts in the area. Adolescent participants were 14–16 years of age. We selected a minimum age of 14 in order to limit the effects of puberty-related brain changes. These data are taken from a larger study that sought to predict adolescent-onset psychopathology at longitudinal follow ups, necessitating an upper age limit of 16. Parents were biological and co-residing with the focal adolescent at least four or more days per week. The study was approved by the university’s institutional review board. Parents provided written informed consent for themselves as well as their adolescent. Adolescents provided written informed assent. All participants were financially compensated for their participation.
All participants included in the study were right-handed (assessed using the Edinburgh Handedness Inventory; Oldfield, 1971) and fluent in English. Exclusion criteria for the parent and adolescent included a current medical condition known to influence fMRI, use of psychotropic medication within 3 weeks (36 hours for stimulant use) of scanning, or current pregnancy. In addition, parents were excluded for current psychiatric diagnosis, and adolescents were excluded for any current or past psychiatric diagnosis. Both parents and adolescents were excluded if under the influence of alcohol or a psychoactive drug on the day of scanning, as determined by breathalyzer and saliva testing.
Following the initial screening, 39 parent–adolescent dyads completed the hyperscanning session. One dyad was excluded for clinical abnormalities (i.e., structural abnormality significantly affecting data quality or brain function), and three dyads were excluded due to issues with the audio system during the scan. This resulted in a final sample of 35 adolescents (M age = 15.2 years; 69% female; 72% Non-Hispanic White, 17% Black, 11% Hispanic or Latino) and 35 parents (M age = 42.6 years; 94% female; 74% Non-Hispanic White, 17% Black, 9% Hispanic or Latino). The demographic characteristics of the original sample (n = 39) closely matched those of the (city name removed for blinding) metro area population from which they were drawn. Unfortunately, some of those excluded for the issues described above were Native American or Asian American, which affected these groups’ representation in our final data set. For additional demographic information, please see Table 1.
Table 1.
Sample demographics
| Parents (n = 35) | Adolescents (n = 35) | |
|---|---|---|
| Female | 33 | 24 |
| Age (years; M [SD]) | 43 (6) | 15 (1) |
| Parent education | ||
| High school graduate/GED | 3 | — |
| Some college/trade school | 8 | — |
| College graduate | 16 | — |
| Graduate degree | 8 | — |
| Race | ||
| Black | 6 | 6 |
| White | 29 | 29 |
| Ethnicity | ||
| Hispanic or latino | 3 | 4 |
| Not hispanic or latino | 32 | 31 |
Note. The two dyads including fathers were father–daughter dyads.
Procedure
The study protocol consisted of an initial phone screen, a 2-hour screening and survey visit, and a 4-hour scanning visit. Individuals interested in the study were directed to call the study research assistant. Upon initial contact, the parent completed a brief phone screen to determine eligibility for the screening visit. The phone screen included questions regarding MRI safety (e.g., surgical history) as well as a brief mental and physical health screener for both the adolescent (parent reported) and parent. If the dyad met initial eligibility requirements, the parent and adolescent were scheduled to attend the 2-hour screening and survey visit.
During the 2-hour visit, dyads completed a battery of surveys assessing parent–child relationship quality, mental and physical health, and other demographic information. Parents and adolescents completed surveys separately. In addition, parents and adolescents completed the Mini International Neuropsychiatric Interview (parent—MINI 7.0 [Sheehan et al., 1997]; adolescent—MINI KID 7.0 [Sheehan et al., 2010]) to determine current and past psychiatric diagnoses. In our final sample of 35 parents, five had a prior psychiatric diagnosis. Of these, four met the criteria for a history of major depressive disorder (no current symptoms) as determined by the MINI 7.0. Additionally, one parent self-reported a diagnosis of attention-deficit/hyperactivity disorder. Parents completed the MRI screening questionnaire developed by the Institute for Magnetic Resonance Safety, Education, and Research (IMR-SER) for both themselves and their adolescent to ensure the dyad met MRI safety screening criteria. Additionally, the adolescent completed a brief version of this measure to assess criteria affecting MRI safety that may be unknown to the parent (e.g., piercings). Height, weight, and blood pressure were obtained for all participants.
If eligible following the screening and survey visit, dyads were scheduled to attend the 4-hour scanning visit typically within 3–4 weeks of their initial visit. During the scanning visit, parents and adolescents completed surveys assessing parent–child conflict. In addition, parents were asked to complete the MRI safety screening assessment for both themselves and their adolescent a second time. Parents and adolescents completed a breathalyzer and saliva test to screen for alcohol and drug use, and female participants were required to take a pregnancy test prior to scanning.
Functional magnetic resonance imaging hyperscanning technology was used to collect brain imaging data. fMRI hyperscanning is an innovative methodology that utilizes simultaneous fMRI to measure neural responses of two interacting individuals in separate scanners. Two identical GE MR750 3.0T scanners with GE 8 channel head coils were used to obtain all imaging data (see Supplement for imaging parameters and preprocessing). During the scan, dyads completed the conflict discussion task (see Measures).
Measures
Adolescent Symptoms of Depression
Adolescents completed the 33-item Mood and Feelings Questionnaire (MFQ), which assesses symptoms of depression in the past 2 weeks (Angold & Costello, 1987). MFQ example items include, “I didn’t enjoy anything at all,” and “I felt miserable or unhappy,” and were completed on a 3-point Likert scale (0 = “not true,” 1 = “sometimes,” or 2 = “true”). Scores were summed to compute the adolescent depression variable. Cronbach’s α was .80.
Adolescent Symptoms of Anxiety
Adolescents completed the 41-item Screen for Child Anxiety Related Disorders (SCARED; Birmaher et al., 1997) which assesses symptoms of anxiety over the past 3 months. SCARED example items included, “I worry about being as good as other kids,” and “I get really frightened for no reason at all.” Questions are completed on a 3-point Likert scale (0 = “Not True or Hardly Ever True,” 1 = “Somewhat True or Sometimes True,” or 2 = “Very True or Often True”). Scores were summed to compute the adolescent anxiety variable. Cronbach’s α was .91.
Parenting Emotion Socialization
Adolescents completed the Emotions as a Child Scale (EAC; Magai & O’Neal, 1997) which assesses parent responses to their child’s negative emotions. Example items included, “When you have been upset (angry, sad), how often did your (focal parent) not notice?” and “When you have been upset (angry, sad), how often did your (focal parent) ask you about it?” The “focal parent” (mother or father) was automatically generated and included in the item. Questions are completed on a 3-point Likert scale (1 = “Not at All,” 3 = “Sometimes,” or 5 = “Very Much”). The EAC has five subscales assessing parent response: Reward, Punish, Magnify, Override, and Neglect; however, based on a factor analysis conducted by Cui and colleagues (2020), a two-factor structure for the EAC is an acceptable alternative for use with adolescents. The two factors, Supportive and Usupportive socialization strategies, are comprised of the Reward and Override subscales and the Punish, Magnify, and Neglect subscales, respectively. Cronbach’s α was .70 for the Unsupportive factor and .80 for the Supportive factor. The EAC includes identical subscales assessing four different negative emotions: sadness, anger, fear, and shame. However, in this study, only sadness and anger were evaluated and were combined in one subscale. We expected sadness and anger to be particularly salient given the nature of the conflict discussion task, and both sadness and anger dysregulation have been shown to predict internalizing issues in adolescents (e.g., Cui et al., 2020).
fMRI Conflict Discussion Task
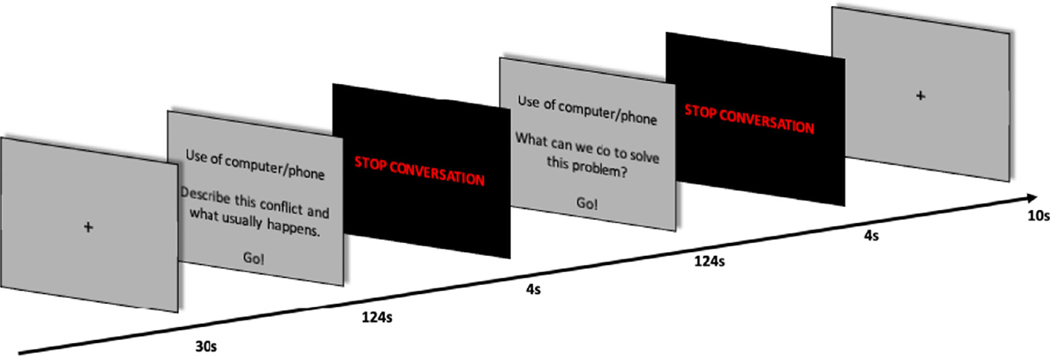
Before scanning, parents and adolescents completed the Conflict Frequency Questionnaire (Melby et al., 1998) to determine the dyad’s most frequent conflict. The 33-item questionnaire consists of 32 possible conflict topics as well as an “other” category to list any topics not covered in the questionnaire. Example topics include, “Activities with friends,” “Attitude or respect,” “Chores at home,” “Homework,” and “Use of computer or phone.” Parents and adolescents were asked to rate how often in the past year they had each disagreement with their parent or adolescent on a 5-point Likert scale from “Never” to “Very Often.” The three topics rated highest were selected for use in the conflict discussion task, with topics rated highly by both dyad members being prioritized. These conflicts were programmed into the task (see Figure 1), with one topic being presented during each scanning run.
Figure 1.
Conflict discussion task completed by parent and adolescent during fMRI hyperscanning.
While undergoing fMRI hyperscanning, dyads discussed each of the three conflicts for 4 min per conflict, for a total of 12 min, using headsets equipped with headphones and a microphone (OptoActive II NC Microphones and ANC Headphones, Opto acoustics Ltd.). During the first 2 min, the dyad was prompted to describe the conflict presented on the screen. During the next 2 min, the dyad was prompted to come up with a solution for the conflict. A timer was present on the screen counting down in 5-second increments and then by 1-second increment for the final 10 seconds of the task.
Audio from the conflict discussion task was recorded, transcribed, and coded for positive and negative statements based on the coding manual used by Eisenberg and colleagues (2008). Transcriptions were coded by personnel trained in qualitative and verbal data coding. In the case of disagreements between the two coders, consensus was attempted through discussion, but if consensus could not be reached, the statement was not coded. Reliability coding (i.e., coding by both the primary coder and reliability coder) was completed for 26% of the transcriptions with 96% agreement (Cohen’s κ = .92, p < .0005) found between the coders. Positive statement categories included validation, agree, humor, elicit opinion, and offer solution; and negative statement categories included disagree, put down, derisive humor, coerce, interrupt, and stonewall. Statements that did not fall into one of these categories were considered noncoded statements. To assess the quality of the parent–adolescent interaction during the conflict discussion task, the ratio of parent negative statements to positive statements was quantified as “parent negativity.” Similarly, “adolescent negativity” was quantified by the ratio of adolescent negative statements to positive statements during the conflict discussion task.
Statistical Analyses
Neuroimaging Analyses
To infer neural responses of socially interacting brains, we employed a CBC approach. CBC is an extension of conventional within-brain functional connectivity analysis to between-brain connectivity by utilizing lagged cross-correlation analysis (Bilek et al., 2015). In this study, CBC was measured by calculating cross-correlations between the BOLD signal time course of one member of the dyad’s emotion-related brain regions, referred to as seed regions, and the other member of the dyad’s BOLD signal time course for all voxels in the brain during the describe and solution blocks of the conflict discussion.
The signal time series during the describe and solution blocks of the discussion was extracted from the residual signal of the noise regression. The initial four fMRI volumes in each block were removed to account for hemodynamic response delay. These signals reflect brain activity across time during each block of the task. Cross-correlations between the parent and adolescent signals were then calculated for each block. Lagged cross-correlation analysis was used to account for variability in brain response, as CBC is mediated by a behavioral interaction, which may result in a longer delay due to the timing of the behavioral interaction and transmission of an emotion-related communication. To accommodate this, cross-correlation analysis with lags of + or − 6 seconds were performed consistent with prior studies examining neural coupling during conversation (Stephens, Silbert, & Hasson, 2010), and the maximum correlation in the positive and negative lags were extracted, respectively, as well as the zero-lag correlation. The cross-correlation values applied unbiased normalization to make the possible range −1 to 1 and then were transformed to z-values with Fisher’s transformation.
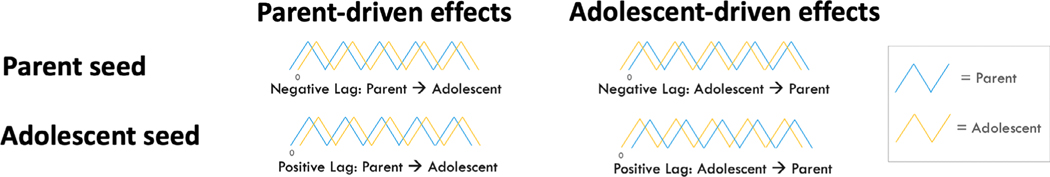
For cross-correlations between the adolescent seed region and the parent whole brain, a negative lag indicates adolescent brain activity precedes parent brain activity; whereas, a positive lag indicates parent brain activity precedes adolescent brain activity. For the cross-correlations between the parent seed region and the adolescent whole brain, a negative lag indicates parent brain activity precedes adolescent brain activity; whereas, a positive lag indicates adolescent brain activity precedes parent brain activity. For ease of interpretation, we refer to all instances in which parent brain activity precedes adolescent brain activity as parent-driven effects. Instances in which adolescent brain activity precedes parent brain activity are referred to as adolescent-driven effects. Please see Figure 2 for a visual representation.
Figure 2.
The negative lag correlation indicates the seed brain activation in one individual precedes the brain activation of the other individual and vice versa for the positive lag. Instances in which parent brain activity precedes adolescent brain activity are referred to as parent-driven effects. Instances in which adolescent brain activity precedes parent brain activity are referred to as adolescent-driven effects.
Seed regions were selected a priori and included the bilateral AI, dlPFC, vlPFC, and amygdala. For all ROIs except the amygdala, sphere ROIs centered at coordinates reported in previous research were used in order to capture functional specificity. A sphere size of 6mm was selected following our empirical observation showing that spatial smoothness of the residual image of GLM analysis was about 6 mm full width at half maximum on average in the whole brain. Spheres centered around MNI coordinates identified in meta-analyses were used to create the following seed regions: bilateral AI: (+ or −36, 19, 1; Xu et al., 2014); bilateral dlPFC: (+ or −45, 34, 30), identified using the search term “dlPFC” on Neurosynth (Yarkoni et al., 2011); bilateral vlPFC: (+ or −46, 22, 8), the midpoint between two previously identified vlPFC seed regions (Frank et al., 2014; Kohn et al., 2015). The amygdala seed regions were defined anatomically using masks based on the MNI brain created by FreeSurfer. (For small subcortical regions such as the amygdala, the anatomical mask is more refined than a 6mm radius sphere mask.)
Group-level analysis was conducted using linear mixed-effect model analysis for the cross-correlation values as the dependent variable with fixed effects of the lag (positive or negative or 0), adolescent gender, interaction between lag and adolescent gender, and adolescent age, and a random effect of dyad on intercept. Linear mixed-effect model analysis was completed for both the describe block and the solution block separately. The lme4 package (Bates, Maechler, Bolker, & Walker, 2015) in R language and statistical computing software (R core Team, 2019) was used for the voxel-wise analysis. We performed ad hoc testing for the significance of the cross-correlation value in each lag using t-tests with multiple testing correction by critical values from multivariate t-distribution determined with lsmeans package (Lenth, 2016). A voxel-wise threshold of p < .001 was applied to the results followed by cluster-size family-wise error correction at p < .05. Cluster-size threshold was evaluated with 3dClust-Sim in AFNI using an improved spatial autocorrelation function (Cox, Chen, Glen, Reynolds, & Taylor, 2017), resulting in a cluster threshold of 103 voxels.
Exploratory Behavioral Analyses
For region pairs exhibiting statistically significant CBC, the resulting cross-correlation (normalized and Fisher’s r-to-z transformed) were averaged across the three runs, creating a value reflecting the degree of parent–adolescent CBC between the two regions for each dyad during the conflict discussion task. To determine the relationship between CBC and measures of interest, these values were used in a region-based Bayesian multilevel (BML) approach using the AFNI program RBA (Chen et al., 2019). The Bayesian approach attempts to estimate parameters of an underlying distribution using a prior probability distribution and evidence from the observed distribution. The region-based BML approach resolves the issue of multiplicity at the ROI-level, often encountered when using null hypothesis significance testing, by assuming a Gaussian distribution for the ROIs in a single multilevel model. This results in partial pooling among the ROIs, in contrast to univariate approaches which assume ROIs are unrelated, allowing for greater predictive accuracy and model efficiency (Chen et al., 2019). The region-based BML uses the brms R package (Bürkner, 2017), which employs the R interface to Stan, or RStan, and the C++ math library. Stan is a probabilistic programming language that uses No-U-Turn Sampling (NUTS) for Bayesian inferences, an extension of the Hamiltonian Monte Carlo (HMC) Samplers under Markov chain Monte Carlo (MCMC) algorithms (Stan Development Team, 2017).
In this study, two Bayesian analyses were performed. The first model included all parent-driven CBC region pairs and explanatory variables, including measures of adolescent symptoms of depression and anxiety, parent emotion socialization behaviors (supportive and unsupportive), and measures of parent–adolescent interaction quality during the conflict discussion task (parent negativity and adolescent negativity). Bivariate correlations between behavioral measures can be found in Table 2. The second model included all adolescent-driven CBC region pairs and the aforementioned explanatory variables. For both models, four Markov chains were implemented for each parameter. An initial predetermined 5,000 iterations were run with 2,500 thrown away as burn-in iterations, and 10,000 post burn-in iterations were specified. To assess chain convergence, the split R statistic was used. The diagnostic indicated convergence was obtained for all chains. Parameters assessing the number of effective sampling draws following burn-in showed the effective sample sizes in relation to the total sampling draws were large enough to ensure accuracy when obtaining the quantile intervals for each posterior distribution.
Table 2.
Bivariate Correlations Between Behavioral Measures
| Variable | Mean | SD | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|---|
| 1. Adolescent MFQ | 5.83 | 4.82 | |||||
| 2. Adolescent SCARED | 15.77 | 10.83 | 0.67*** | ||||
| 3. Parent negativity | 0.28 | 0.21 | 0.20 | 0.00 | |||
| 4. Adolescent negativity | 0.26 | 0.30 | 0.42* | 0.19 | 0.56*** | ||
| 5. EAC unsupportive | 15.74 | 5.29 | 0.29 | 0.45*** | 0.11 | 0.22 | |
| 6. EAC supportive | 22.29 | 5.33 | 0.26 | 0.31 | −0.07 | −0.10 | 0.02 |
Note. EAC = Emotions as a Child Scale; MFQ = Mood and Feelings Questionnaire; SCARED = Screen for Child Anxiety-Related Disorders.
p < .05.
p < .001.
We utilized the default prior settings (Gaussian distribution) in the RBA program for the ROIs, subjects, and response variables. In addition, we implemented uninformative priors for the effects at the population level and weakly informative priors for the scaling parameters. The selection of priors was based on recommendations from Chen and colleagues (2019). The graphical posterior predictive check tool (PPC), which graphically compares observed data to the model prediction, was used to assess model fit. Visual inspection of the resulting model indicated good model quality and fitness.
Results
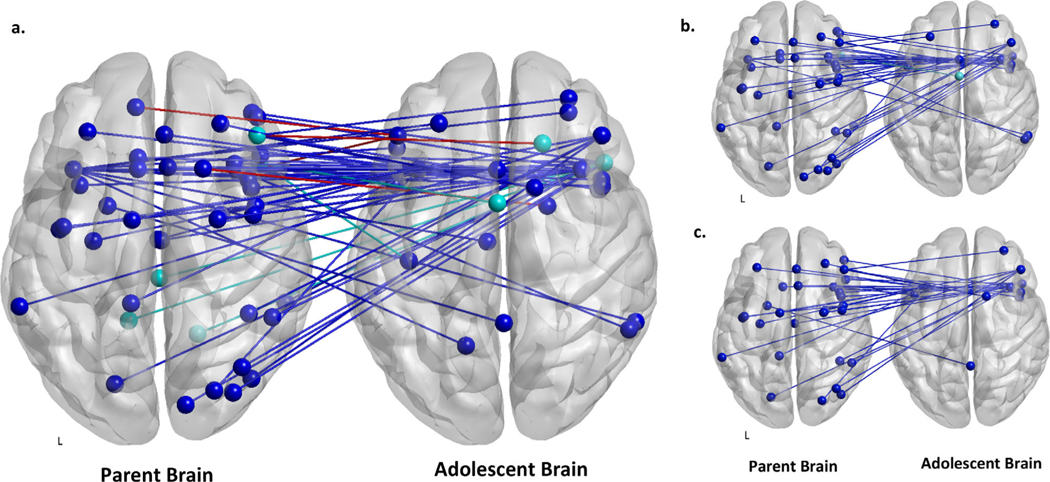
The cross-brain correlation analysis of fMRI hyperscanning data revealed lagged CBC between multiple emotion-related brain regions in the parent and adolescent brains, while no significant zero-lag correlation between the brains was found. Figure 3a shows the significant CBC region pairs for both parent-driven and adolescent-driven effects. The brain networks were visualized with the BrainNet viewer (Xia, Wang, & He, 2013; http://www.nitrc.org/projects/bnv/). Lists of all statistically significant CBC region pairs can be found in Supplement, Tables S1 and S2. CBC region pairs were found during the describe block of the conflict discussion task unless otherwise noted. Notably, we found that most significant CBCs were primarily driven by the parent (parent brain activity precedes adolescent) and only a few were adolescent-driven (adolescent brain activity precedes parent). We summarize the results in the following two sections for the region pairs by parent-driven effect and adolescent-driven effects. Associations between regions reflect positive connectivity unless otherwise noted.
Figure 3.
Results of the cross-brain connectivity (CBC) analysis and Bayesian analyses. Blue lines represent parent-driven effects. Teal lines represent adolescent-driven effects. (a) Results of the CBC analysis. Nodes represent locations of region pairs showing statistically significant CBC between parents and adolescents. Red lines represent negative connectivity. (b) Results of the Bayesian analysis (see Figures S1 and S8) examining associations between significant CBC region pairs and adolescent-perceived parent emotion socialization behaviors (EAC). All parent-driven CBC between these regions was associated with fewer supportive parent emotion socialization behaviors. The only instance of adolescent-driven CBC between these regions was associated with greater unsupportive parent emotion socialization behaviors (teal line). (c) Results of the Bayesian analysis (see Figure S5) examining associations between significant CBC region pairs and adolescent-reported depressive symptoms (MFQ). All parent-driven CBC between these regions was associated with fewer adolescent depressive symptoms. No associations between adolescent-driven CBC and adolescent depressive symptoms were found.
CBC: Parent-Driven Effects
Parent Seed Region and Adolescent Whole Brain
The cross-correlation analyses revealed 54 significant parent-driven region pairs. Here we highlight significant region pairs between emotion-related brain regions (all parent-driven effects can be found in the Supplement, Table S1). Parent activation in cortical seed regions, predominantly the AI, often preceded activity in the adolescent AI (see Figure 3a). For example, activity in the parent right dlPFC seed was associated with activity in the adolescent right AI. Activity in the parent left AI seed region was associated with activity in the adolescent left AI, and activity in the parent right AI seed region was associated with activity in the adolescent bilateral AI. In addition, parent activation in the left AI seed region preceded activity in multiple adolescent cortical regions, including the left inferior frontal gyrus (IFG), left middle frontal gyrus, right superior frontal gyrus (SFG), and the dorsal left anterior cingulate gyrus; whereas, activation in the parent right AI seed region predicted activity in only the adolescent bilateral AI.
Parent Whole Brain and Adolescent Seed Region
In regard to whole-brain activation in the parent, results indicated parent cortical regions were predominately related to activity in the adolescent bilateral AI seed regions (see Figure 3a). For example, activation in the parent bilateral middle frontal gyrus, right medial frontal gyrus, and left dorsal anterior cingulate gyrus was associated with activation in the adolescent left AI seed. Activation in the parent bilateral middle frontal gyrus, right IFG, and right medial frontal gyrus was associated with activation in the adolescent right AI seed region. Activity in the parent bilateral AI was associated with activity in both the left and right adolescent AI seed regions. In addition, some parent cortical regions were associated with activity in adolescent cortical seed regions aside from the AI (see Figure 3a). For example, activity in the parent right IFG and the left SFG was associated with activity in the adolescent right dlPFC seed region, and activity in the parent right IFG and right precuneus was associated with activity in the adolescent right vlPFC seed region. Few associations demonstrated negative connectivity; however, one to note is the negative association between activity in the parent right SFG and the adolescent right amygdala seed region. This is the only finding showing an association between a parent cortical region and adolescent subcortical region involved in emotion reactivity (see Figure 3a). Parent subcortical regions involved in emotion reactivity, including the bilateral lentiform nucleus and right thalamus, preceded activity in the left and right adolescent AI seed as well.
CBC: Adolescent-Driven Effects
Adolescent Seed Region and Parent Whole Brain
Results of the cross-correlation analyses revealed comparatively fewer adolescent-driven effects (see Table S2), which may suggest neural processes underlying parent–adolescent social interactions are often parent led. Only one adolescent seed region predicted activation in the parent—activity in the adolescent right vlPFC seed region was associated with activity in the parent bilateral culmen during the solution block of the conflict discussion task.
Adolescent Whole Brain and Parent Seed Region
Regarding whole-brain activation in the adolescent, results indicated activity in adolescent cortical regions predicted activity in the parent cortical seed regions. Activity in adolescent right SFG was negatively associated with activity in the parent right dlPFC seed region. Activity in the adolescent left SFG and left precentral gyrus was associated with activity in the parent right vlPFC seed region. Both of these CBC region pairs were found during the solution block.
CBC: Exploratory Behavioral Results
Exploratory analyses were conducted using the region-based BML approach (Chen et al., 2019), which provides the probability of an effect being either positive or negative based on the specified model and the sample. All posterior density plots with P+ values can be found in Supplement, Figures S1–S12. P+ is the probability that the effect is positive conditional on the current model and data set. It can be used to assess evidence for region effects, with p+ values close to 1 providing stronger evidence that the effect is positive and smaller values providing stronger evidence that the effect is negative (1-P+). In the following sections, we highlight associations between CBC and parent emotion socialization behaviors and adolescent depressive symptoms. We did not find evidence for associations between CBC and adolescent anxiety or parent–adolescent overall interaction quality (i.e., parent negativity and adolescent negativity) during the conflict discussion task.
CBC and Parent Emotion Socialization
CBC between parent cortical regions and adolescent bilateral AI seed regions was associated with fewer adolescent-reported supportive parent emotion socialization behaviors (see Figure 3b and Figure S1). For example, CBC between the parent left cingulate gyrus and the adolescent bilateral AI was associated with fewer supportive parent emotion socialization behaviors (left: p+ = .028; right: p+ = .023). CBC between the parent left middle frontal gyrus and the adolescent right AI was associated with fewer supportive behaviors (p+ = .014), and CBC between the parent right IFG and the adolescent right AI was associated with fewer supportive behaviors (p+= .034). CBC between the parent right inferior parietal lobule and the adolescent right dlPFC was associated with fewer supportive behaviors (p+= .039). CBC between the parent right angular gyrus and the adolescent right dlPFC seed and CBC between the parent right precuneus and the adolescent right vlPFC were associated with fewer supportive behaviors (p+ = .017; p+ = .012, respectively).
CBC between parent subcortical regions and adolescent bilateral AI seed regions was also associated with fewer adolescent-reported supportive parent emotion socialization behaviors. For example, CBC between the parent right thalamus and the adolescent right AI was associated with fewer supportive behaviors (p+ = .037). CBC between the parent left lentiform nucleus and the adolescent left AI was associated with fewer supportive behaviors (p+ = .033), and CBC between the parent right lentiform nucleus and the adolescent right AI was also associated with fewer supportive behaviors (p+ = .024). Lastly, one instance of adolescent-driven CBC between the adolescent left SFG and the parent right vlPFC seed was associated with greater adolescent reported unsupportive behaviors (p+ = .934).
CBC and Adolescent Depression
In regard to adolescent depressive symptoms, parent activation in several cortical regions was associated with activation in the adolescent bilateral AI seed region. CBC between these regions was associated with fewer depressive symptoms (see Figure 3c and Figure S5). For example, CBC between the parent right middle frontal gyrus and the adolescent left AI seed region was associated with fewer adolescent depressive symptoms (p+ = .089). CBC between the parent right IFG and the adolescent right AI seed region and CBC between the parent right dlPFC seed region and the adolescent right AI were also associated with fewer adolescent depressive symptoms (p+ = .077; p+ = .058, respectively).
Parent activation in subcortical regions was also related to activation in the adolescent bilateral AI seed region and adolescent-reported depression. For instance, CBC between the parent right AI seed region and the adolescent right AI was associated with fewer adolescent depressive symptoms (p+ = .065). CBC between the parent left lentiform nucleus and the adolescent left AI seed region was associated with fewer adolescent depressive symptoms (p+ = .082).
Discussion
In this study, we provide evidence of parent–adolescent CBC between several emotion-related brain regions during a naturalistic fMRI hyperscanning task. This is the first study to our knowledge to show patterns of CBC between parents and adolescents. Interestingly, effects were primarily driven by parents, and several brain regions consistently emerged, including the AI. Furthermore, we explored associations between these CBC region pairs, parenting behavior, and adolescent depressive symptoms. We found that parent–adolescent CBC is related to both parent emotional socialization behaviors and adolescent depressive symptoms. Specifically, we found that CBC between multiple emotion-related brain regions was associated with fewer adolescent-perceived supportive parent emotion socialization behaviors. Additionally, we found CBC between emotion-related brain regions was associated with fewer adolescent-reported depressive symptoms. Our findings provide novel insight into the role of CBC in social cognitive processes underlying parent–adolescent dynamic, social interactions.
In regard to our first research question, we found evidence of CBC between emotion-related regions of parents’ and adolescents’ brains during the conflict discussion task. Sixty total significant CBC region pairs were found. Notably, of those, 54 were parent-driven effects while only six were adolescent-driven effects. Given a large number of parent-driven effects, we further explored the temporal unfolding of the discussion (i.e., parent-initiated versus adolescent-initiated) using descriptive statistics. Descriptive statistics indicated parents initiated 67% of the conversations, yet 90% of the significant effects were found to be parent-driven. Moreover, when parents did initiate the conversation, they were equally likely to initiate the conversation in either the describe or solution block (48% and 52%, respectively). If the effects did indeed simply reflect who initiated the conversation, we might expect an equal number of parent-driven effects in both the describe and solution blocks. However, we see only two of the 54 parent-driven effects were found in the solution block. These findings suggest that parent initiation of the conversation cannot fully account for a large number of parent-driven effects. For the six adolescent-driven effects, three occurred during the solution block and three during the describe block. However, adolescents were slightly more likely to initiate the conversation during the describe block (55% of adolescent-initiated conversations vs. 45% in the solution block). Although research suggests parent–child emotion dynamics become more egalitarian in structure throughout adolescence (Branje, 2018), parents still remain influential socializing agents through the process of emotion co-regulation (Morris, Criss, Silk, & Houltberg, 2017). It is plausible that parents’ emotion-related neurocircuitry may relate to adolescents’ through co-regulation strategies during real life, dynamic interactions (Kerr et al., 2018).
Due to the large number of region pairs identified in the CBC analysis, identified regions are organized by both structure and function for discussion. Results showed CBC across several cortical regions, including the IFG, middle and medial frontal gyri, and precuneus, among others, all involved in the regulation of emotion (Frank et al., 2014). The AI is often considered a hub for coordinating complex cognitive processes related to emotion (Uddin, Nomi, Hebert-Seropian, Ghaziri, & Boucher, 2017). Because of its unique role in emotion processing, we differentiate it from other cortical regions involved in regulation. In addition, we found CBC across subcortical regions, such as the amygdala, thalamus, and lentiform nucleus. These regions have consistently been implicated in emotion reactivity (Frank et al., 2014). When examining significant parent-driven CBC region pairs, several patterns emerge between these emotion-related regions. First, in over one-third of these region pairs, activity in the parent cortical regions preceded activity in the adolescent bilateral AI during the conflict discussion task. These cortical regions are involved in numerous social cognitive functions including perspective-taking, memory encoding and retrieval, decision making, and cognitive control processes that support ER (Ochsner & Gross, 2005). The AI is one of several regions involved in the processing of cognitive and affective stimuli during social interactions and undergoes considerable development during adolescence, both in structure and connectivity (Guyer et al., 2016). Specifically, research shows connectivity between the AI and the brain’s cognitive control network, encompassing regions important for self-regulation, is strengthened throughout adolescence (Smith, Steinberg, & Chein, 2014). Thus, in this context, activation in the adolescent AI following activity in parent cortical regions may represent the integration of the cognitive and emotional stimuli received from the parent to inform the adolescent’s own regulatory response.
A second pattern to emerge from discovered parent-driven CBC region pairs was parent activation in cortical regions preceding activity in the adolescent bilateral dlPFC and right vlPFC seed regions. Research with adolescents suggests both the vlPFC and dlPFC are recruited during ER strategies, specifically cognitive reappraisal, to modulate activity in the amygdala and that negative connectivity between these prefrontal regions and the amygdala increases with age (Silvers et al., 2017). Adolescents’ recruitment of regulatory regions may be influenced by the parents’ behavior during the interaction.
Lastly, notable parent-driven effects involving subcortical regions implicated in emotion reactivity were identified. Specifically, activity in the parent right thalamus and bilateral lentiform nucleus preceded activity in the adolescent AI. Additionally, parent activity in the right SFG preceded lower activity in the adolescent right amygdala. In studies examining functional connectivity, the SFG has been shown to be involved in emotional downregulation; specifically, the SFG reduced activity in the amygdala through recruitment of the middle frontal gyrus when faced with negative emotional stimuli (Frank et al., 2014). Based on our findings, a similar process may be occurring interindividually in parent–adolescent dyads.
Overall, 43 of the 54 parent-driven CBC region pairs included either the parent or adolescent AI. This highlights the importance of the AI in social cognitive processes. Moreover, bearing in mind its role in the integration of social and emotional stimuli, it is not surprising we would find considerable evidence of CBC between other brain regions involved in social cognition and the AI. The AI is recruited when processing emotions related to the self and others, including during empathy and compassion-related tasks (Lamm & Singer, 2010). Thus, given the importance of the AI in processing social and emotional stimuli, it will be crucial to further explore these discovered patterns in future research.
The comparatively fewer number of adolescent-driven CBC region pairs resulted in only one identifiable pattern. For three of the six adolescent-driven findings, adolescent cortical regions, including the right and left SFG and left precentral gyrus, preceded activity in the parent right dlPFC and left vlPFC. Interestingly, this specific pattern of effects occurred only during the solution block of the task in which interactions were more reciprocal. This is in contrast to the parent-driven effects where nearly all occurred during the describe block. Similar to studies of parent–child interbrain synchrony during cooperative tasks (Miller et al., 2019), CBC between these regions may reflect problem solving. Adolescents may be more engaged when asked to consider solutions for a problem rather than describe the problem itself; however, additional research is needed to further understand these findings.
In addition, the exploratory Bayesian analyses revealed several potentially interesting associations between CBC, parenting behaviors, and adolescent depressive symptoms. However, given the novelty of these findings and dearth of research on CBC more generally, we interpret these results with caution. In regard to our second research question, we found that CBC was associated with fewer adolescent-reported depressive symptoms but not symptoms of anxiety. The overwhelming pattern to emerge from this analysis was CBC between parent cortical regions and adolescent AI was associated with fewer adolescent depressive symptoms. Moreover, in regard to our third research question, unexpectedly we found parent-driven CBC was associated with fewer supportive parent emotion socialization behaviors, while one instance of adolescent-driven CBC was associated with greater unsupportive parent emotion socialization behaviors. Similar to past findings of parent–child synchrony, CBC may be indicative of a typical, healthy parent–adolescent relationship. Our finding that lower CBC values are related to both adolescent depressive symptoms and supportive parenting practices at first glance seems contradictory. Moreover, many of the CBC regions pairs significantly associated with both of these behavioral measures overlap. However, past studies have also found that associations between parenting, neurobiology, and emotion-related behavior differ in the context of adolescent internalizing symptoms (Butterfield et al., 2020). It should also be noted that findings are specific to an emotion-inducing task, the conflict discussion, and this study sample is psychiatrically healthy. Future studies may consider testing whether low CBC reflects a disruption in the parent–adolescent relationship in the context of adolescent depressive symptoms and emotional contexts such as a conflict discussion. It is possible that supportive behaviors by the parent could be made in response to their adolescent’s internalizing symptoms, but are not reflected by CBC during the task.
In regard to our fourth research question, we did not find any significant evidence relating CBC and the quality of the interaction during the conflict discussion task. This finding was surprising given past research suggesting CBC and interbrain synchrony are particularly dependent on context, whether it be the type of task (e.g., cooperative versus competitive; Reindl et al., 2018) or the relationship between the dyad (e.g., stranger dyads versus romantic partners; Bilek et al., 2015). However, previous studies did not examine CBC during a naturalistic conversation paradigm as in this study. It is entirely possible that CBC underlies social interactions, specifically conversation, within the same contexts and relationships regardless of the quality of the interaction. In dyads with a history of interactions, such as parents and children, patterns of CBC may be embedded throughout conversations, as each member of the dyad anticipates and responds in predictable ways. Simply put, the parent–adolescent relationship itself may be more important than the observed interaction in determining levels of CBC. Another plausible explanation may be that our analyses, which examined the overall level of CBC between brain regions across each block, did not capture fluctuating levels of CBC that may have occurred in direct response to negative versus positive statements. Additional research is warranted to determine how context influences levels of CBC in parent–child interactions.
This study had some limitations. The sample size was relatively small when considering the various exploratory behavioral analyses conducted. However, in comparison to univariate approaches, Bayesian analyses are particularly robust in the face of smaller samples, assuming the correct specification of the prior distributions. Moreover, Bayesian approaches resolve the issue of multiple comparisons through the use of “partial pooling,” or sharing the information among the identified brain regions in one model, unlike univariate approaches which assume the brain regions are unrelated to each other (Chen et al., 2019). Although we did covary for sex, due to our small sample size, we were unable to fully explore sex differences. Future research should consider sex differences among adolescents, parents, and dyad types (i.e., mother–daughter, mother–son, father–son, father–daughter), as these may influence levels of CBC during social interactions. Additionally, a more diverse sample including more variation in socioeconomic status and race and ethnicity would increase generalizeability. Future research should also consider the influence of genetic factors influencing CBC by examining both adoptive and biological parent–child dyads. The cross-sectional study design limits our understanding of the directionality of the findings. Further elucidation of the direction of the associations described is needed to identify additional implications of parent–adolescent CBC in adolescent brain development as well as adjustment outcomes. Moreover, a longitudinal design encompassing a larger age range (e.g., 12–18 years) that allows for examination of age-related differences may provide valuable insight into the ways patterns of social cognition change during the transition from childhood to adolescence as well as from adolescence to young adulthood. An additional limitation is that the use of seed-based analyses necessitated the selection of certain emotion-related brain regions to the exclusion of others that are likely involved in parent–adolescent CBC. For example, the vmPFC has previously been associated with two-person, dyadic emotional interactions (Healey & Grossman, 2018; Hiser & Koenigs, 2018), but we were unable to include this region due to significant signal dropout in our sample, partially due to orthodontic retainers in the adolescent sample. Lastly, our sample consisted of psychiatrically healthy parents and adolescents. Future research would benefit from examining CBC in both healthy control dyads and dyads in which adolescents are at greater risk for psychopathology, thus identifying possible differences in dyadic neurocircuitry that may contribute to the development of mental health issues.
In summary, this study utilized the capabilities of fMRI hyperscanning to examine parent–adolescent CBC during an ecologically valid task. We explored both cortical and deep-brain function of socially interacting parent–adolescent dyads in real time. Such a study can only be done using fMRI hyperscanning. To date, this is the first study to our knowledge to examine parent–adolescent CBC using fMRI hyperscanning, and, as such, highlights the potential for this technology in the study of parent–child interactions. These findings expand our understanding of the neural basis of dynamic, parent–adolescent social interactions and provide a foundation for future research to continue to uncover the role of cross-brain influences involved in dyadic ER and emotion-related contexts.
Supplementary Material
Acknowledgments
The research reported in this manuscript was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number (P20GM109097). Development of the fMRI hyperscanning system was supported by Laureate Institute of Brain Research, The William K. Warren Foundation, and in part by the P20GM121312 award from the National Institute of General Medical Sciences, National Institutes of Health. The content in this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Footnotes
Ethics Statement
We confirm that all procedures performed in this study involving human participants were in accordance with the ethical standards of the Oklahoma State University Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The informed consent/assent was obtained from all individual participants included in the study.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher’s website:Supplementary Material
Contributor Information
Erin L. Ratliff, Oklahoma State University.
Kara L. Kerr, Oklahoma State University
Kelly T. Cosgrove, Laureate Institute for Brain Research and University of Tulsa
Danielle C. DeVille, Laureate Institute for Brain Research and University of Tulsa
Deanna M. Barch, Washington University
W. Kyle Simmons, Oklahoma State University.
Amanda Sheffield Morris, Oklahoma State University and Laureate Institute for Brain Research.
Masaya Misaki, Laureate Institute for Brain Research.
Andrew J. Moore, Laureate Institute for Brain Research
Jennifer S. Silk, University of Pittsburgh
Susan F. Tapert, University of California
Jerzy Bodurka, Laureate Institute for Brain Research and University of Oklahoma.
References
- Angold A, & Costello EJ (1987). Mood and feelings questionnaire (MFQ). Durham: Developmental Epidemiology Program, Duke University. [Google Scholar]
- Astolfi L, Toppi J, De Vico Fallani F, Vecchiato G, Cincotti F, Wilke C, … Babiloni F. (2011). Imaging the social brain by simultaneous hyperscanning during subject interaction. IEEE Intelligent Systems, 26, 38. 10.1109/MIS.2011.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, Morris AS, Silk JS, Criss MM, Judah MR, Eagleton SG, … Alvarez RP (2016). Neural responses to maternal praise and criticism: Relationship to depression and anxiety symptoms in high-risk adolescent girls. NeuroImage: Clinical, 11, 548–554. 10.1016/j.nicl.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, & Gee JC (2008). Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis, 12, 26–41. 10.1016/j.media.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhari A, Leck WQ, Gabrieli G, Bizzego A, Rigo P, Setoh P, … Esposito G. (2019). Parenting stress undermines mother-child brain-to-brain synchrony: A hyperscanning study. Scientific Reports, 9, 1–9. 10.1038/s41598-019-47810-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber JG, Bolitho F, & Bertrand L. (2001). Parent-child synchrony and adolescent adjustment. Child and Adolescent Social Work Journal, 18, 51–64. 10.1023/A:1026673203176 [DOI] [Google Scholar]
- https://doi.org/Bates D, Maechler M, Bolker B, & Walker S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- https://doi.org/Bilek E, Ruf M, Sch€afer A, Akdeniz C, Calhoun VD, Schmahl C, … Meyer-Lindenberg A. (2015). Information flow between interacting human brains: Identification, validation, and relationship to social expertise. Proceedings of the National Academy of Sciences, 112, 5207–5212. 10.1073/pnas.1421831112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, & Neer SM (1997). The screen for child anxiety related emotional disorders (SCARED): Scale construction and psychometric characteristics. Journal of the American Academy of Child & Adolescent Psychiatry, 36, 545–553. [DOI] [PubMed] [Google Scholar]
- https://doi.org/Branje S. (2018). Development of parent–adolescent relationships: Conflict interactions as a mechanism of change. Child Development Perspectives, 12, 171–176. 10.1111/cdep.12278 [DOI] [Google Scholar]
- https://doi.org/Bürkner P. (2017). Bayesian regression models using stan. R Package Version, 1. [Google Scholar]
- https://doi.org/Butterfield R, Silk J, Lee KH, Siegle G, Dahl R, Forbes E, … Ladouceur C. (2020). Parents still matter! Parental warmth predicts adolescent brain function and anxiety and depressive symptoms two years later. Development and Psychopathology, 32, 1–14. 10.1017/S0954579419001718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Chen G, Xiao Y, Taylor PA, Rajendra JK, Riggins T, Geng F, … Cox RW (2019). Handling multiplicity in neuroimaging through Bayesian lenses with multilevel modeling. Neuroinformatics, 17, 515–545. 10.1007/s12021-018-9409-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Cosgrove KT, Kerr KL, Aupperle RL, Ratliff EL, DeVille DC, Silk JS, … Morris AS (2020). Corrigendum to “Always on my mind: Cross-brain associations of mental health symptoms during simultaneous parent-child scanning”. Developmental Cognitive Neuroscience, 41, 100751. 10.1016/j.dcn.2019.100751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Cox RW, Chen G, Glen DR, Reynolds RC, & Taylor PA (2017). FMRI clustering in AFNI: False-positive rates redux. Brain Connectivity, 7, 152–171. 10.1089/brain.2016.0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Criss MM, Morris AS, Ponce-Garcia E, Cui L, & Silk JS (2016). Pathways to adaptive emotion regulation among adolescents from low-income families. Family Relations, 65, 517–529. 10.1111/fare.12202 [DOI] [Google Scholar]
- https://doi.org/Cui L, Criss MM, Ratliff E, Wu Z, Houltberg BJ, Silk JS, & Morris AS (2020). Longitudinal links between maternal and peer emotion socialization and adolescent girls’ socioemotional adjustment. Developmental Psychology, 56, 595. 10.1037/dev0000861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Eisenberg N, Hofer C, Spinrad TL, Gershoff ET, Valiente C, Losoya SH, … Maxon E. (2008). Understanding mother-adolescent conflict discussions: Concurrent and across-time prediction from youths’ dispositions and parenting. Monographs of the Society for Research in Child Development, 73, vii–viii, 1–160. 10.1111/j.1540-5834.2008.00470.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Eisenberg N, Zhou Q, Spinrad TL, Valiente C, Fabes RA, & Liew J. (2005). Relations among positive parenting, children’s effortful control, and externalizing problems: A three-wave longitudinal study. Child Development, 76, 1055–1071. 10.1111/j.1467-8624.2005.00897.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Feldman R. (2007). Parent–infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry, 48, 329–354. 10.1111/j.1469-7610.2006.01701.x [DOI] [PubMed] [Google Scholar]
- https://doi.org/Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, … Sabatinelli D. (2014). Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neuroscience and Biobehavioral Reviews, 45, 202–211. 10.1016/j.neubiorev.2014.06.010 [DOI] [PubMed] [Google Scholar]
- https://doi.org/Guyer AE, Silk JS, & Nelson EE (2016). The neurobiology of the emotional adolescent: From the inside out. Neuroscience and Biobehavioral Reviews, 70, 74–85. 10.1016/j.neubiorev.2016.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Healey ML, & Grossman M. (2018). Cognitive and affective perspective-taking: Evidence for shared and dissociable anatomical substrates. Frontiers in Neurology, 9, 491. 10.3389/fneur.2018.00491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Hirata M, Ikeda T, Kikuchi M, Kimura T, Hiraishi H, Yoshimura Y, & Asada M. (2014). Hyperscanning MEG for understanding mother–child cerebral interactions. Frontiers in Human Neuroscience, 8, 118. 10.3389/fnhum.2014.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Hiser J, & Koenigs M. (2018). The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biological Psychiatry, 83, 638–647. 10.1016/j.biopsych.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Jo HJ, Saad ZS, Simmons WK, Milbury LA, & Cox RW (2010). Mapping sources of correlation in resting state FMRI, with artifact detection and removal. NeuroImage, 52, 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Kerr KL, Cosgrove KT, Ratliff EL, Burrows K, Misaki M, Moore AJ, … Morris AS (2020). TEAMwork: Testing emotional attunement and mutuality during parent-adolescent fMRI. Frontiers in Human Neuroscience, 14, 24. 10.3389/fnhum.2020.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Kerr KL, Moore AJ, Misaki M, Ratliff EL, Cosgrove KT, Deville DC, … Morris AS (2018). A free-speech paradigm for studying dyadic interactions with fMRI hyperscanning. Paper presented at the Society for Neuroscience Conference, San Diego, CA. [Google Scholar]
- https://doi.org/Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, & Habel U. (2014). Neural network of cognitive emotion regulation—an ALE meta-analysis and MACM analysis. NeuroImage, 87, 345–355. 10.1016/j.neuroimage.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, & Habel U. (2015). Corrigendum to “Neural network of cognitive emotion regulation — An ALE meta-analysis and MACM analysis”. NeuroImage, 111, 631. 10.1016/j.neuroimage.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Lamm C, & Singer T. (2010). The role of anterior insular cortex in social emotions. Brain Structure and Function, 214, 579–591. 10.1007/s00429-010-0251-3 [DOI] [PubMed] [Google Scholar]
- https://doi.org/Lee KH, Siegle GJ, Dahl RE, Hooley JM, & Silk JS (2015). Neural responses to maternal criticism in healthy youth. Social Cognitive and Affective Neuroscience, 10, 902–912. 10.1093/scan/nsu133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Lenth RV (2016). Least-squares means: The R package lsmeans. Journal of Statistical Software, 69, 1–33. [Google Scholar]
- https://doi.org/Magai C, & O’Neal CR (1997). Emotions as a child (child version). Brooklyn: Long Island University. Unpublished manuscript. [Google Scholar]
- https://doi.org/Melby J, Conger R, Book R, Rueter M, Lucy L, Repinski D, & Scaramella L. (1998). The Iowa family interaction rating scales. Ames, IA: Institute for Social and Behavioral Research, Iowa State University. [Google Scholar]
- https://doi.org/Miller JG, Vrticka P, Cui X, Shrestha S, Hosseini SH, Baker JM, & Reiss AL (2019). Inter-brain synchrony in mother-child dyads during cooperation: An fNIRS hyperscanning study. Neuropsychologia, 124, 117–124. 10.1016/j.neuropsychologia.2018.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Misaki M, Kerr KL, Ratliff EL, Cosgrove KT, Simmons WK, Morris AS, & Bodurka J. (2021). Beyond synchrony: The capacity of fMRI hyperscanning for the study of human social interaction. Social Cognitive and Affective Neuroscience, 16, 84–92. 10.1093/scan/nsaa143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Morris AS, Criss MM, Silk JS, & Houltberg BJ (2017). The impact of parenting on emotion regulation during childhood and adolescence. Child Development Perspectives, 11, 233–238. 10.1111/cdep.12238 [DOI] [Google Scholar]
- https://doi.org/Ochsner KN, & Gross JJ (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9, 242–249. 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- https://doi.org/Oldfield RC (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- https://doi.org/Phillips ML, Drevets WC, Rauch SL, & Lane R. (2003). Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry, 54, 504–514. 10.1016/S0006-3223(03)00168-9 [DOI] [PubMed] [Google Scholar]
- https://doi.org/R Core Team (2019). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://doi.org/https://www.R-project.org/ [Google Scholar]
- https://doi.org/Reindl V, Gerloff C, Scharke W, & Konrad K. (2018). Brain-to-brain synchrony in parent-child dyads and the relationship with emotion regulation revealed by fNIRS-based hyperscanning. NeuroImage, 178, 493–502. 10.1016/j.neuroimage.2018.05.060 [DOI] [PubMed] [Google Scholar]
- https://doi.org/Sheehan DV, Lecrubier Y, Sheehan KH, Janavs J, Weiller E, Keskiner A, … Dunbar GC (1997). The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. European Psychiatry, 12, 232–241. 10.1016/S0924-9338(97)83297-X [DOI] [Google Scholar]
- https://doi.org/Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, … Wilkinson B. (2010). Reliability and validity of the mini international neuropsychiatric interview for children and adolescents (MINIKID). The Journal of Clinical Psychiatry, 10.4088/JCP.09m05305whi [DOI] [PubMed] [Google Scholar]
- https://doi.org/Silk JS, Lee KH, Elliott RD, Hooley JM, Dahl RE, Barber A, & Siegle GJ (2017). ‘Mom—I don’t want to hear it’: Brain response to maternal praise and criticism in adolescents with major depressive disorder. Social Cognitive and Affective Neuroscience, 12, 729–738. 10.1093/scan/nsx014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin RE, … Ochsner KN (2017). vlPFC–vmPFC–amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cerebral Cortex, 27, 3502–3514. 10.1093/cercor/bhw073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Smith AR, Steinberg L, & Chein J. (2014). The role of the anterior insula in adolescent decision making. Developmental Neuroscience, 36, 196–209. 10.1159/000358918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Stan Development Team. (2017). Stan modeling language users guide and reference manual, Version 2.17.0. Retrieved from https://doi.org/http://mc-stan.org
- https://doi.org/Stephens GJ, Silbert LJ, & Hasson U. (2010). Speaker–listener neural coupling underlies successful communication. Proceedings of the National Academy of Sciences, 107, 14425–14430. 10.1073/pnas.1008662107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, & Boucher O. (2017). Structure and function of the human insula. Journal of Clinical Neurophysiology, 34, 300–306. 10.1097/WNP.0000000000000377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Xia M, Wang J, & He Y. (2013). BrainNet viewer: A network visualization tool for human brain connectomics. PLoS One, 8, e68910. 10.1371/journal.pone.0068910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Xu Y, Tong Y, Liu S, Chow HM, AbdulSabur NY, Mattay GS, & Braun AR (2014). Denoising the speaking brain: Toward a robust technique for correcting artifact contaminated fMRI data under severe motion. NeuroImage, 103, 33–47. 10.1016/j.neuroimage.2014.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://doi.org/Yap MB, Schwartz OS, Byrne ML, Simmons JG, & Allen NB (2010). Maternal positive and negative interaction behaviors and early adolescents’ depressive symptoms: Adolescent emotion regulation as a mediator. Journal of Research on Adolescence, 20, 1014–1043. 10.1111/j.1532-7795.2010.00665.x [DOI] [Google Scholar]
- https://doi.org/Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, & Wager TD (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods, 8, 665–670. 10.1038/nmeth.1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.