Abstract
Acral lentiginous melanoma (ALM) is a rare histological subtype of cutaneous malignant melanoma that typically presents on the palms and soles. To characterize the demographic and treatment characteristics of ALM, we used the National Cancer Database (NCDB) to describe a large multi-institutional cohort of ALM patients, consisting of 4,796 ALM patients from 2004–2015.
ALM was more likely to be diagnosed at a later stage overall compared to non-ALM cutaneous melanomas, and more likely to be thicker, ulcerated, lymph node positive, and have lymphovascular invasion and positive margins. When stratified by stage, ALM had worse survival compared to non-ALM patients, most notably in stage III patients with 5-year survival of 47.5% versus 56.7%, respectively (p<0.001).
In ALM patients, older age, male sex, higher comorbidity burden, increased tumor thickness and ulceration, positive lymph nodes, and positive metastasis were independently associated with lower 5-year survival. Multimodality therapy, defined as surgery in addition to systemic therapy and/or radiation therapy, was associated with higher survival in stage III patients but not in other stages. These results call for further investigation into possible treatment intensification in the ALM population in the future.
SIGNIFICANCE:
Acral lentiginous melanoma is (ALM) is a rare histological subtype of cutaneous malignant melanoma that historically has had a worse prognosis compared to non-ALM cutaneous melanoma. This updated National Cancer Database (NCDB) study describes demographic and treatment characteristics in ALM patients. Importantly, we report the survival of ALM patients stratified by stage and identified that stage III patients have significantly poorer outcomes compared to non-ALM patients. We believe that the findings of this study will add to the limited body of literature on this rare diagnosis and can help further possible treatment intensification in the ALM population in the future.
Keywords: melanoma, skin neoplasms, hand dermatoses, foot dermatoses, databases, clinical research, epidemiology
INTRODUCTION
Acral lentiginous melanoma (ALM) is a rare histological subtype of melanoma, accounting for 1–3% of cutaneous malignant melanomas (CMM) (Bradford et al., 2009; Huang et al., 2020). It was first described by Reed in 1976 as a “distinct clinicopathologic entity… pigmented lesions on the extremities, particularly on plantar regions, like the palms of the hands and soles of the feet” (Reed, 1976). The anatomic descriptor “acral” means extremity while “lentiginous” describes the histologic side-by-side arrangement of melanocytes.
Histologically, distinguishing features of ALM include acanthosis, lentiginous proliferation of melanocytes in epidermis, spindle cell makeup of the dermal component, and poor circumscription. ALMs also have features common in other melanoma subtypes, including asymmetry, irregular borders, color variation, nodular components, and presence of ulceration (D. Elder et al., 2018; D. E. Elder et al., 2020; Reed, 1976). No standard histologic criteria have been established for ALM, and many experts use clinical features in addition to the above histologic features (Kuchelmeister et al., 2000a).
In contrast to other CMM, ALM has a similar incidence across racial groups, suggesting that ALM is less related to sun-exposure. Indeed, molecular studies suggest a lack of UV-related mutational signatures seen in non-ALM CMM (Hayward et al., 2017; Liang et al., 2017). Consequently, the proportion to ALM to other CMM is higher in darker skinned populations compared to lighter skinned populations. Compared to CMM, ALM usually presents later, and more aggressively (Bradford et al., 2009; Cormier et al., 2006; Huang et al., 2020; Wang et al., 2016). Additionally, ALM tends to affect older patients (mean 63 years versus 59 for CMM overall) (Huang et al., 2020). Although trauma, genetics, carcinogen exposure, and viral infection are hypothesized predisposing factors for ALM, there is insufficient data to establish a causal relationship for these factors.
Clinical outcomes of ALM versus non-ALM CMM also differ. Population-based studies show lower survival in ALM compared to non-ALM CMM. A recent study SEER study reported 5-year disease-specific survival rates of 81% in ALM compared to 93% in non-ALM CMM (Huang et al., 2020). Some hypothesize that this lower survival is due to ALM presenting at later stages (CASCINELLI et al., 1994a; Jimbow et al., 1984; Ridgeway et al., 1995; Teramoto et al., 2018; Wada et al., 2017; Weyers et al., 1999). However, others suggest that ALM has a poorer prognosis even after controlling for tumor thickness and stage (Bello et al., 2013; Bradford et al., 2009; Huang et al., 2020). Other factors that have been reported to impact prognosis are older age, race, and socioeconomic status (York et al., 2016; Zemelman et al., 2014).
Due to ALM’s rarity, outcomes studies are limited. To date, a few population-based studies using the Surveillance, Epidemiology, and End Results (SEER) database and the National Cancer Database (NCDB) have described the survival patterns (Behbahani et al., 2020; Bradford et al., 2009; Huang et al., 2020; Huayllani et al., 2020). The SEER registry acquires population data from 20 U.S. geographic regions, while the NCDB records data from over 1,500 hospitals across the U.S. accredited by the American College of Surgeons’ Commission on Cancer (COC). In this study, we use the NCDB to describe a large multi-institutional cohort of ALM patients versus non-ALM patients. We also present prognostic and treatment factors associated with ALM survival. Finally, we report on the clinical management and survival of ALM when stratified by stage.
MATERIALS AND METHODS
This study was reviewed and approved by our institutional IRB. We used data from the NCDB, a nationwide database of cancer registry information created by the American College of Surgeons’ Commission on Cancer (COC) and the American Cancer Society in 1987. It represents more than 70% of newly diagnosed cancer cases nationwide and more than 34 million historical records.
We identified patients aged 18–90 years with a diagnosis of cutaneous melanoma from 2004–2015. In our initial analysis, we included patients with malignant tumor behavior, an AJCC 6th or 7th edition cancer stage group I-IV and follow up or vital status.
Patient characteristics included age, sex, race, Charlson-Deyo comorbidity index, year of diagnosis, and histology. Charlson-Deyo comorbidity index is a validated method for measuring patient comorbidity based ICD (International Classification of Diseases) diagnosis codes, using integers to denote the number of comorbidities(Deyo et al., 1992). Tumor characteristics included primary site, laterality, tumor thickness, ulceration, grade, positive lymph nodes, lymphovascular invasion, metastasis, surgical margin, and stage group. Treatment characteristics included receipt of surgery, radiation, chemotherapy, or immunotherapy. The NCDB database does not differentiate among types of immunotherapy and includes new immune checkpoint inhibitors and older cytokine-based immune modulators. Surgical technique was divided into three groups: 1) wide local excision (WLE) or amputation (AMP), 2) local excision, including excisional biopsy, gross excision or Mohs with <1cm margins, or surgery NOS, and 3) No surgery. Radiation, chemotherapy, and immunotherapy were analyzed as binary variables. We defined multimodality therapy as surgery in combination with either chemotherapy, immunotherapy, or radiation therapy.
In a descriptive analysis, we separated the cohort into ALM and non-ALM histologies. Differences in patient characteristics, lesion characteristics, and treatment between these two groups were tested using the Wilcoxon test for continuous variables, and a χ2 test for categorical variables. For overall survival (OS) calculations, we used the time from diagnosis to the time of death, censoring for loss to follow-up. We used a Kaplan-Meier analysis to compare OS between ALM and non-ALM groups overall and for each stage and tested for statistical significance using Log rank tests. We reported the median and interquartile ranges (IQR) for continuous variables that were not normally distributed, and the frequency counts and percentages were reported for the categorical variables.
We then narrowed our survival analysis to ALM-only histologies, excluding non-ALM histologies (n=346,352) and patients without follow up or survival data (n=522), patients with missing data regarding surgery type (n=56), surgical margin status (n=159), ulceration data (n=1,146) or lymph node positivity data (n=405).
In the ALM-only cohort, we used univariable and multivariable analysis to examine associations between patient, tumor, and treatment characteristics and survival. For univariable analysis, we created Kaplan-Meier curves to compare survival between groups and Log Rank tests to assess if unadjusted differences in survival were statistically significant. Cox regression was used in multivariable analysis to assess the association of patient characteristics with mortality. We tested the proportional hazard assumption using Schoenfeld residuals. Lymph node positivity appeared to violate this assumption, so we modeled it as a time-varying covariate by interacting the variable with yearly increments, which we accomplished using the PHREG procedure. Variables in univariable analysis included age, gender, race, Charlson-Deyo comorbidity index, T stage, ulceration, N stage, M stage, surgical margin, surgery type, chemotherapy, immunotherapy, and radiation. Laterality, grade and primary site were excluded from the multivariable analysis as they were nonsignificant on univariable analysis. Lymphovascular invasion was excluded due to >50% missing data. Patients were followed for up to five years. Significance tests were two-tailed, with α=0.05. All analyses were performed using SAS software v. 9.4 (SAS Institute Inc.).
RESULTS
ALM versus non-ALM CMM cohorts
We identified 351,148 patients with CMM from 2004–2015 in the NCDB. There were 4,796 patients with ALM (1.4%) and 346,352 patients with non-ALM. The most common non-ALM histologies were superficial spreading melanoma (30.8%), nodular melanoma (9.8%), and lentigo maligna melanoma (5.1%). Melanoma NOS accounted for 49.1% of the sample.
ALM and non-ALM patients were demographically different (Table 1). ALM patients were older than non-ALM patients (66 years and 62 years, respectively, p<0.01). ALM had a slight female predominance while non-ALM had a slight male predominance. White, non-Hispanic patients constituted a majority of ALM and non-ALM diagnoses (86.5% in ALM versus 98.8% in non-ALM). However, the incidence of ALM was proportionally higher in patients of other races: 20.9% of Black patients, 7.8% of White Hispanic patients, and 11.1% of Asian/Pacific Islander patients, compared to 1.1% of White non-Hispanic patients. The Charlson-Deyo comorbidity index was lower in non-ALM patients, and slightly more likely diagnosed after 2010 compared to non-ALM patients (p<0.01).
Table 1.
Patient, tumor, and treatment characteristics of patients with ALM versus non-ALM histology
| ALM (N=4796) | Non-ALM (N=346352) | p-value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Patient Characteristics | |||
| Age | <0.01 | ||
| < 60 years | 1672 (34.9) | 155772 (45.0) | |
| ≥ 60 years | 3124 (65.1) | 190580 (55.0) | |
| Sex | <0.01 | ||
| Male | 2261 (47.1) | 200642 (57.9) | |
| Female | 2535 (52.9) | 145710 (42.1) | |
| Race | <0.01 | ||
| White | 4099 (86.5) | 338163 (98.8) | |
| Black | 433 (9.1) | 1642 (0.5) | |
| Other | 205 (4.3) | 2359 (0.7) | |
| Charlson-Deyo Comorbidity Index | <0.01 | ||
| 0 | 3906 (81.4) | 300141 (86.7) | |
| 1 | 706 (14.7) | 37487 (10.8) | |
| 2–3 | 184 (3.8) | 8724 (2.5) | |
| Year of Diagnosis | 0.04 | ||
| 2004–2010 | 2366 (49.3) | 176103 (50.9) | |
| 2011–2015 | 2430 (50.7) | 170249 (49.2) | |
| Tumor Characteristics | |||
| Primary Site | <0.01 | ||
| Head and Neck | 0 (0) | 73913 (21.3) | |
| Trunk | 0 (0) | 107434 (31.0) | |
| Upper extremity | 820 (17.1) | 84664 (24.4) | |
| Lower extremity | 3701 (77.2) | 60900 (17.6) | |
| Other | 275 (5.7) | 19441 (5.6) | |
| Laterality | <0.01 | ||
| Not paired | 63 (1.3) | 89735 (14.4) | |
| Primary right | 2322 (48.4) | 130069 (37.6) | |
| Primary left | 2354 (49.1) | 139148 (40.2) | |
| Other | 57 (1.2) | 27400 (7.9) | |
| Stage Group | <0.01 | ||
| I | 2140 (44.6) | 221003 (63.8) | |
| II | 1366 (28.5) | 62577 (18.1) | |
| III | 1177 (24.5) | 40695 (11.8) | |
| IV | 113 (2.4) | 22077 (6.4) | |
| Tumor thickness (T stage) | <0.01 | ||
| T1: <=1.0mm | 840 (18.0) | 95536 (28.7) | |
| T2: 1.01 – 2.0mm | 983 (21.0) | 59406 (17.8) | |
| T3: 2.01 – 4mm | 987 (21.1) | 37235 (11.2) | |
| T4: >4mm | 906 (19.4) | 31445 (9.5) | |
| Unknown | 962 (20.6) | 109299 (32.8) | |
| Ulceration | <0.01 | ||
| No | 1863 (53.5) | 135335 (67.0) | |
| Yes | 1621 (46.5) | 66627 (33.0) | |
| Grade | 0.92 | ||
| Well differentiated | 10 (17.9) | 719 (16.7) | |
| Moderately differentiated | 13 (23.2) | 980 (22.7) | |
| Poorly differentiated | 26 (46.4) | 1924 (44.6) | |
| Undifferentiated | 7 (12.5) | 689 (16.0) | |
| Lymphovascular Invasion† | <0.01 | ||
| Present | 261 (10.8) | 7961 (5.3) | |
| Not present | 2146 (89.2) | 142350 (94.7) | |
| Positive lymph nodes | <0.01 | ||
| 0 | 2405 (68.3) | 174466 (82.0) | |
| 1+ | 1117 (31.7) | 38212 (18.0) | |
| Metastasis | <0.01 | ||
| M0 | 4683 (97.6) | 324275 (93.6) | |
| M1 | 113 (2.4) | 22077 (6.4) | |
| Surgical margin status | <0.01 | ||
| Positive | 257 (5.6) | 11072 (3.3) | |
| Negative | 4325 (93.3) | 309224 (90.7) | |
| No primary site surgery | 52 (1.1) | 20523 (6.0) | |
| Treatment Characteristics | |||
| Surgery | <0.01 | ||
| Amputation | 741 (15.5) | 1652 (0.5) | |
| Wide local excision | 2605 (54.3) | 205514 (59.4) | |
| Local excision/biopsy | 1382 (28.8) | 117251 (33.9) | |
| Surgery, unknown | 17 (0.4) | 1208 (0.4) | |
| No Surgery | 52 (1.1) | 20523 (5.9) | |
| Chemotherapy | <0.01 | ||
| Yes | 105 (2.3) | 10689 (3.2) | |
| No | 4568 (97.8) | 326682 (96.8) | |
| Immunotherapy | <0.01 | ||
| Yes | 362 (7.7) | 16978 (5.0) | |
| No | 4370 (92.4) | 326233 (95.1) | |
| Radiation | <0.01 | ||
| Yes | 101 (2.1) | 14704 (4.3) | |
| No | 4660(97.9) | 329699 (95.7) | |
| No treatment | 37 (0.8) | 8407 (2.4) | <0.01 |
Note: Missing data: race (n=4247), tumor thickness (n=13549), ulceration (n=145702), grade (n=346780), positive lymph nodes (n=134948), lymph vascular invasion (n=198430), surgical margin status (n=5695), surgery (n=204), chemotherapy (n=9104), immunotherapy (n=3205), and radiation (n=1984). Percent may not add up to 100 due to rounding.
Lymph vascular invasion data is only available for edition 7 and from 2010 onwards.
Overall, ALM was more likely diagnosed at a later stage. In ALM patients, 44.6% presented as stage I, 28.5% as stage II, 24.5% as stage III, and 2.4% as stage IV. In non-ALM patients, 63.8% presented as stage I, 18.1% as stage II, 11.8% as stage III, and 6.4% as stage IV (p<0.01). ALM was also more likely thicker, ulcerated, lymph node positive, and have lymphovascular invasion and positive margins compared to non-ALM (p<0.01). ALM patients were more likely to receive major amputation and immunotherapy and less likely to receive chemotherapy and radiation therapy (p<0.01).
Unadjusted 5-year survival was lower in ALM: 67.3% in ALM versus 75.8% in non-ALM (p<0.001) (Figure 1). Survival was lower in ALM even after stratifying by stage, with differences most pronounced in stage III. Comparing ALM to non-ALM, 5-year survival was 88.6% versus 84.6% in stage I (p<0.001), 64.0% versus 62.1% in stage II (p=0.72), 56.7% versus 47.5% in stage III (p<0.001), and 16.4% versus 16.2% in stage IV (p=0.02).
Figure 1:
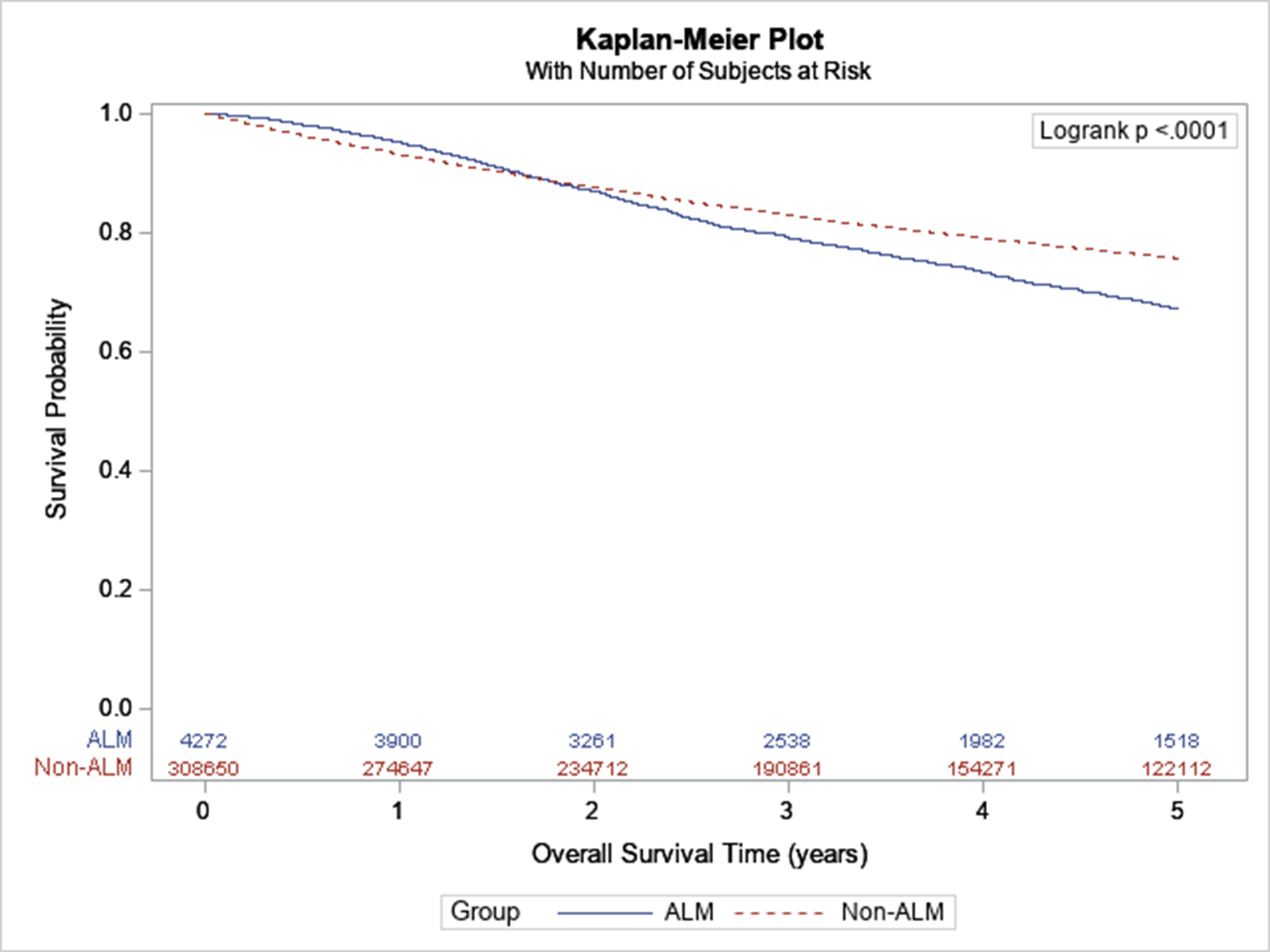
Survival of ALM versus non-ALM
ALM cohort
We identified 2,245 patients for our ALM-only analysis. On univariable analysis, older age, male sex, higher Charlson-Deyo comorbidity index, and earlier year of diagnosis were significantly associated with worse survival (Appendix Figures 1–3). Lymphovascular invasion, greater tumor thickness, ulceration, positive lymph nodes, metastases, and positive surgical margins were also associated with worse survival. Amputation was associated with worse survival compared to local excision and wide local excision. Receipt of chemotherapy, immunotherapy, and radiation were also predictors of worse survival. Unadjusted 5-year survival by stage is shown in Figure 2.
Figure 2:
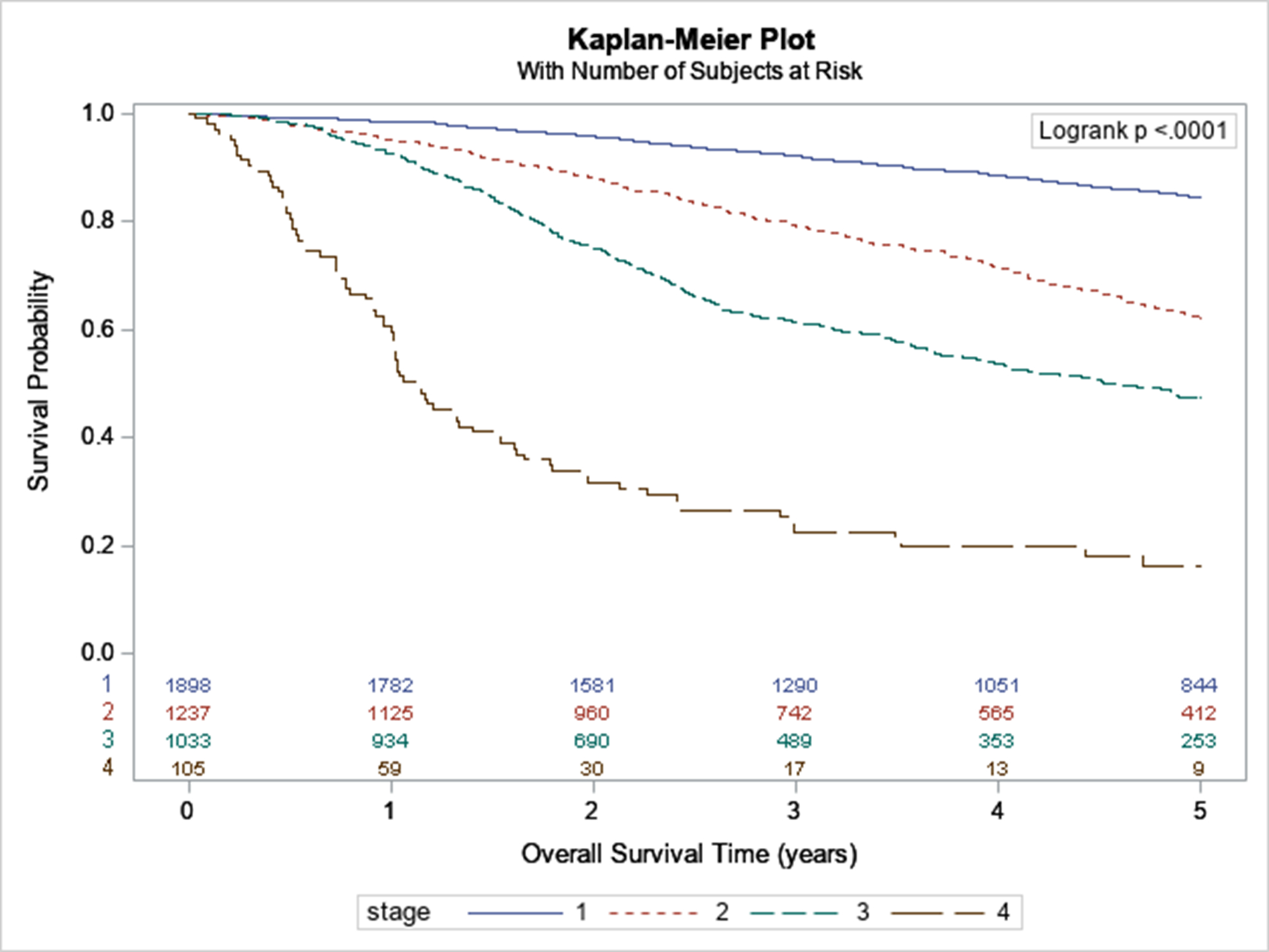
Survival of ALM by Stage
In multivariable analysis, we found that only older age, male sex, Charlson-Deyo comorbidity index >1, earlier year of diagnosis, tumor thickness >2mm, ulceration, positive lymph nodes, and metastatic disease were significant predictors of worse survival (Table 2). Receipt of immunotherapy was associated with better survival on multivariable analysis, while radiation therapy was nonsignificant. The lymph node positivity variable did not fulfill the proportional hazards assumption and was therefore modeled as a time dependent covariate. While lymph node positivity was associated with increased mortality, the hazard ratio decreased over time (from 1 to 5 years).
Table 2.
Cox Regression on Mortality in ALM patients
| Covariate | Hazard Ratio (95% CI) | p-value |
|---|---|---|
| Age | ||
| < 60 years | Reference | |
| ≥ 60 years | 1.92 (1.59, 2.32) | <0.01 |
| Sex | ||
| Male | 1.40 (1.20, 1.64) | <0.01 |
| Female | Reference | |
| Race | ||
| White | Reference | |
| Black | 1.11 (0.87, 1.42) | 0.41 |
| Other | 0.89 (0.60, 1.33) | 0.57 |
| Charlson-Deyo Comorbidity Index | ||
| 0 | Reference | |
| 1 | 1.01 (0.83, 1.24) | 0.93 |
| 2–3 | 1.76 (1.30, 2.39) | <0.01 |
| Year of Diagnosis | ||
| 2004–2010 | 1.18 (1.00, 1.39) | 0.05 |
| 2011–2015 | Reference | |
| Tumor thickness (T stage) | ||
| T1: <=1.0mm | Reference | |
| T2: 1.01 – 2.0mm | 1.37 (0.91, 2.06) | 0.13 |
| T3: 2.01 – 4mm | 2.00 (1.35, 2.98) | <0.01 |
| T4: >4mm | 3.29 (2.22, 4.88) | <0.01 |
| Ulceration | ||
| Yes | 1.45 (1.23, 1.72) | <0.01 |
| No | Reference | |
| Positive lymph nodes × Years | <0.01 | |
| ≥1 vs none, at ≤1 year | 3.40 (2.55, 4.55) | <0.01 |
| ≥1 vs none, at 1.01 to 2 years | 2.75 (2.25, 3.37) | <0.01 |
| ≥1 vs none, at 2.01 to 3 years | 2.23 (1.88, 2.65) | <0.01 |
| ≥1 vs none, at 3.01 to 4 years | 1.80 (1.44, 2.26) | <0.01 |
| ≥1 vs none, at 4.01 to 5 years | 1.46 (1.05, 2.02) | 0.02 |
| Metastasis | ||
| M0 | Reference | |
| M1 | 2.73 (1.79, 4.16) | <0.01 |
| Surgery | ||
| Amputation | 1.17 (0.93, 1.48) | 0.19 |
| Wide local excision | 0.91 (0.75, 1.10) | 0.33 |
| Local excision/biopsy | Reference | |
| Surgical margin status at any COC facility | ||
| Positive | 1.07 (0.79, 1.45) | 0.67 |
| Negative | Reference | |
| Chemotherapy | ||
| Yes | 0.84 (0.53, 1.31) | 0.43 |
| No | Reference | |
| Immunotherapy | ||
| Yes | 0.79 (0.63, 1.00) | 0.05 |
| No | Reference | |
| Radiation | ||
| Yes | 0.97 (0.65, 1.44) | 0.87 |
| No | Reference |
Treatment characteristics by stage are shown in Table 3. In stages I and II, most patients were treated with amputation/wide-local excision (AMP/WLE) or local excision alone (70.5% and 29.1% for stage I, 75.3% and 18.9% for stage II, respectively). Only 0.4% of stage I and 5.7% stage of II patients received surgery with multimodality therapy. For stage III patients, 51.3% received AMP/WLE alone, 15.9% received local excision alone, and 32.8% received surgery and multimodality therapy. For stage IV patients, 35.0% received AMP/WLE alone, 10.0% received local excision alone, and 45.0% received surgery and multimodality therapy.
Table 3:
Treatment by Stage in ALM Patients
| Treatment | Stage I N (%) |
Stage II N (%) |
Stage III N (%) |
Stage IV N (%) |
|---|---|---|---|---|
| WLE/AMP only | 537 (70.5) | 628 (75.3) | 401 (51.3) | 14 (35.0) |
| Local Excision only | 222 (29.1) | 158 (18.9) | 124 (15.9) | 4 (10.0) |
| WLE/AMP + Multimodality Therapy | 2 (0.3) | 41 (4.9) | 207 (26.5) | 16 (40.0) |
| Local Excision + Multimodality Therapy | 1 (0.1) | 7 (0.8) | 49 (6.3) | 6 (15.0) |
WLE = wide local excision, AMP = amputation
When stratified by stage, survival for each of the four treatment categories was not statistically different in stages I, II and IV (p=0.32 for stage I, p=0.82 for stage II, p=0.56 for stage IV). Multimodality therapy was associated with higher survival compared to surgery alone (Figure 3).
Figure 3:
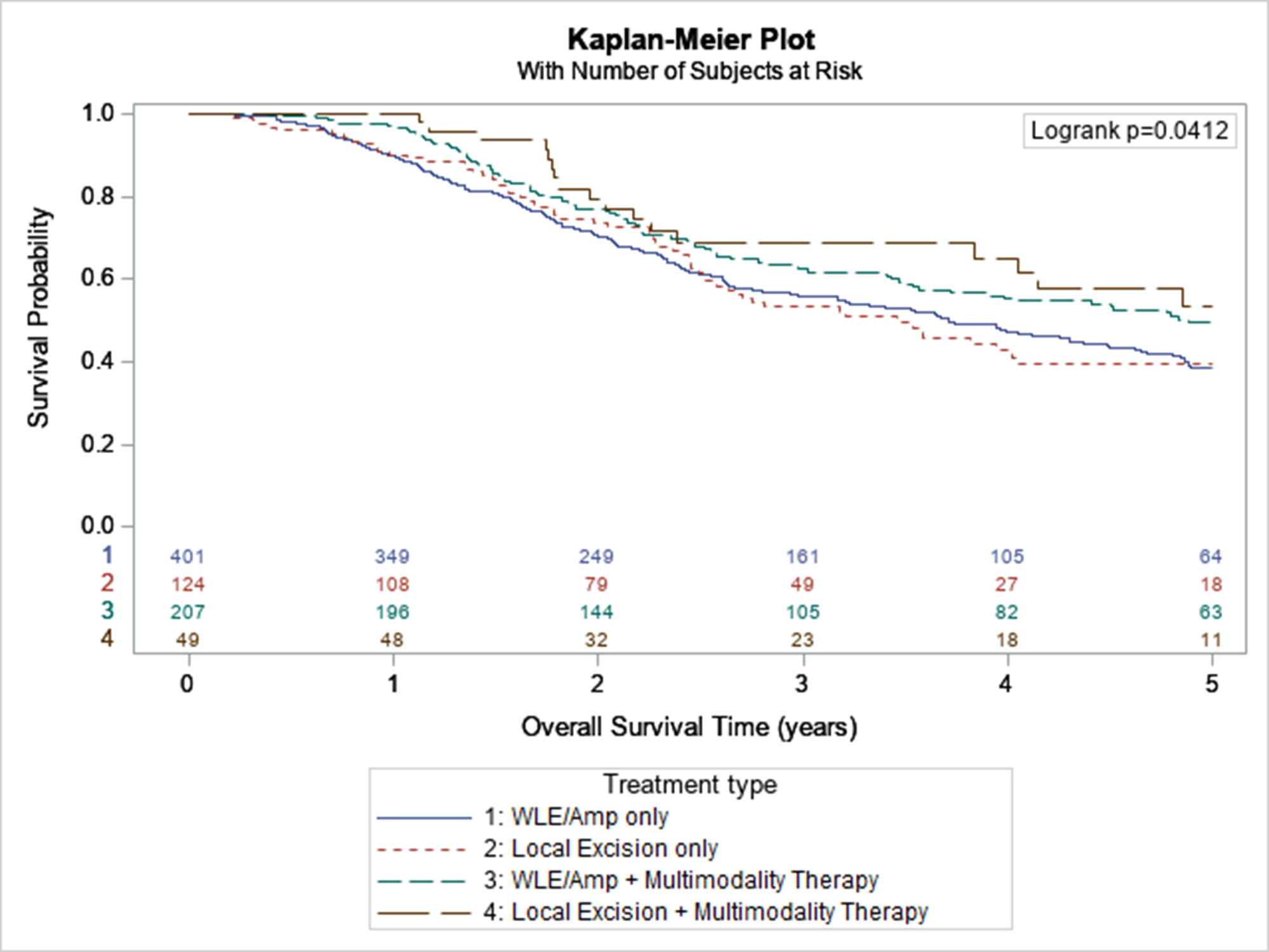
Survival of Stage 3 ALM by Treatment Modality
DISCUSSION
ALM is a rare histologic entity representing 1–3% of melanomas. This study using data from the NCDB describes a large cohort of 2,245 ALM patients.
ALM versus non-ALM cohorts
ALM is clinically distinct from other melanomas, has a similar incidence in darker and lighter skinned individuals, is less correlated with sun exposure, and presents with later stage disease (Basurto-Lozada et al., 2020; Bradford et al., 2009; Cormier et al., 2006; Hayward et al., 2017; Huang et al., 2020; Liang et al., 2017; Wang et al., 2016). It is the least frequent of the four major histologic subtypes of cutaneous melanoma, representing only 1.4% of our CMM cohort.
Compared with other melanoma subtypes, we found that ALM affects a somewhat different population. Our findings are consistent with prior studies that report a larger proportion of ALM in non-white racial groups (34–36% Black, 18–23% Asian/Pacific Islander versus 9% Hispanic White and 1% non-Hispanic White) (Bradford et al., 2009; Huang et al., 2020). We also corroborate other studies demonstrating older age, female predominance, and higher comorbidity burden among ALM patients compared to non-ALM patients (Basurto-Lozada et al., 2020; Bradford et al., 2009; Cormier et al., 2006; Huang et al., 2020).
There is a predilection for lower extremity location within the ALM population (77% versus 17% of lower versus upper extremity in our study). Some studies hypothesize that the pathogenesis of ALM is related to trauma, with feet bearing the brunt of daily trauma from walking (Jung et al., 2013). However, other studies contradict this hypothesis making the causal relationship unclear (Coleman et al., 1980; Kaplan & Youngleson, 1972; Shah JP, 1971).
ALM patients were more likely to have a later year of diagnosis in our study. There is a trend of increased ALM diagnoses in the last 10–15 years in the literature (Bradford et al., 2009). This may reflect the growing population and growing awareness in the medical community of this rare histologic subtype (Basurto-Lozada et al., 2020; Criscito & Stein, 2017; Soon et al., 2003). Our study also showed that ALM was more likely to be diagnosed at a later stage and more likely to be thicker, ulcerated, lymph node positive, and have lymphovascular invasion and positive margins. However non-ALM melanoma was more likely to have metastatic disease at presentation (2.4% versus 6.4%, ALM versus non-ALM respectively).
Although studies indicate a worse prognosis in ALM compared to other melanomas (CASCINELLI et al., 1994a; Chang et al., 1998; Harmelin et al., 1998; Kuchelmeister et al., 2000b; O’Leary JA, Berend KR, Johnson JL, Levin LS, 2000; Slingluff et al., 1990; Tan et al., 2007), it is controversial whether this is due to presentation at a later stage or worse tumor biology. It is well established that ALM presents at a later stage. A large SEER analysis reported that 38% of ALM presented at stage 1 compared to 68% of non-ALM. In contrast, 33% of ALM presented at stage III compared to 12% of non-ALM (Bradford et al., 2009). Our study had similar findings, with Stage I and III disease in 45% and 25% for ALM and 64% and 12% in non-ALM patients, respectively. Huang et. al reported an updated analysis of the 2006–2015 SEER data with similar numbers with stage I and stage III presentation in 45% and 23% of the ALM cohort compared to 76% and 8% of the non-ALM cohort, respectively. Even when corrected for tumor stage, these large population-based studies (Balch, 1998; Bello et al., 2013) report persistently worse survival for ALM patients, indicating a potentially more aggressive tumor biology. Our study also found a worse prognosis in ALM overall and for stages I, III, and IV, compared to non-ALM. The absolute difference in overall survival between ALM and non-ALM in stages I, II, and IV was small (≤ 4%) perhaps suggesting that standard therapies are similarly effective for early disease and ineffective for advanced disease. ALM patients with stage III disease may represent an important population in which standard therapy is not defined and thus heterogenous management may amplify the disease’s inherent biological differences from non-ALM.
Survival differences after accounting for disease stage could be due to biologic differences between ALM and non-ALM. For example, ALM has a significantly lower BRAF mutation rate than non-ALM (Beadling et al., 2008; Curtin et al., 2005; Greaves et al., 2013; Maldonado et al., 2003; Viros et al., 2008), which may make targeted therapies less effective. They also have other genetic differences, including focal amplifications and deletions such as increased KIT mutations/amplifications, NRAS mutations, and PI3 Kinase activation (Ascierto et al., 2013; Curtin et al., 2006; Lee et al., 2011; Omholt et al., 2011; Woodman & Davies, 2010; Yeh et al., 2019). Additionally, studies suggest that ALM is less susceptible to immune checkpoint inhibitors (ICIs) due to a low number of tumor infiltrating lymphocytes and lower tumor mutational burden with lack of the UV mutational signature seen in non-ALM (Castaneda et al., 2017; Hayward et al., 2017; Kaunitz et al., 2017; Liang et al., 2017). Much of the melanoma survival gains in the last decade have resulted from the development of ICIs and the use of BRAF mutation-targeting therapies (Hamid et al., 2017; Robert et al., 2011, 2015), which may be less effective in ALM.
ALM patients were more likely to receive major amputation and immunotherapy but less likely to receive chemotherapy and radiation therapy (p<0.01). The increased amputation rate may be due to ALM’s exclusive involvement of distal extremities, more frequently necessitating amputation to obtain clear margins. The cause of differences in receipt of systemic therapy and radiation therapy is not obvious but could be due to differences in disease presentation and lack of consensus regarding optimal treatment paradigms. As with other CMM, increased use of neoadjuvant systemic treatment may mitigate the need for amputation, and further research into the use of neoadjuvant treatments is needed for this subset of patients.
ALM cohort
In adjusted analysis, we confirmed that older age, male sex, and increased comorbidity score were associated with worse survival as previously reported(Balch, 1998; CASCINELLI et al., 1994b; Chang et al., 1998; Chen et al., 1999; Massi et al., 1999; Slingluff et al., 1990). Later year of diagnosis was also independently associated with improved survival, suggesting that ALM diagnosis, treatment, or both have improved over the years(Kato et al., 1999). Additionally, race was not a significant predictor of survival, similar to previous studies.(CASCINELLI et al., 1994a; Cormier et al., 2006; Krementz et al., 1976; Ridgeway et al., 1995).
All the determinants of stage (tumor thickness, ulceration, lymph node positivity, and metastasis) were independently associated with survival. In our study, survival differences were noted for tumor thickness greater than 2mm. We also found a time-based survival detriment for lymph node positivity, with the largest change in HR for mortality present at <1 year, decreasing yearly to 5 years.
Our data suggests that surgery type is not independently associated with survival, but receipt of immunotherapy is. After stratifying for stage, we found that multimodality therapy, defined as surgery in addition to another treatment modality, significantly increased survival in stage III but not in other stages. This may be due to the efficacy of standard local therapies in early stage and inefficacy of treatments for metastatic disease. Current treatment recommendations do not distinguish between ALM and other melanomas. The generally accepted treatment paradigm is for wide local excision or amputation for localized disease (stage I-II), a combination of surgery, immunotherapy, and/or radiation for locally advanced disease (stage III), and systemic therapy and/or palliative radiation therapy for metastatic disease (stage IV) (NCCN, n.d.). Stage III ALM patients may represent a population in which the optimal therapy has yet to be determined and as such the heterogenous therapies administered result in varying effects on patient outcome. Further study on the treatment of stage III patients may better elucidate the optimal therapy for locally advanced ALM. Although disease recurrence is not well captured in population-based databases, some data suggest higher rates of locoregional recurrence in patients with localized ALM compared to non-ALM CMM (Gumaste et al., 2014). Improved understanding of the clinicopathological behavior of ALM may help guide possible treatment intensification in this unique patient population.
There are important limitations to our study. The retrospective and multi-institutional nature of the NCDB makes the study vulnerable to potential coding and clerical errors in addition to a lack of granularity in disease characteristics and outcomes. As with other observational studies using administrative databases, our results could be biased by residual confounding from unobserved patient and disease characteristics. Our study only describes associations and cannot provide causal evidence. Furthermore, the NCDB database draws from COC-approved hospitals, which excludes many outpatient treatment centers. Since earlier stages of melanoma can be diagnosed and treated in outpatient settings, it is less well captured in the NCDB and other population databases. This may limit the accuracy of the database in reflecting the true incidence and outcome in the general population. Also, a large proportion of non-ALM CMM were missing exact histologic subtype. A similar proportion of missing data was found in prior SEER studies with no impact on statistical analyses (Bradford et al., 2009; Huang et al., 2020).
Strengths of our study include using an updated national dataset that captures detailed patient, disease, and treatment characteristics over a modern period in a wide variety of treatment facilities. Compared to previous large registry analyses, where only 60% of cases were staged (Bradford et al., 2009; Huang et al., 2020), our publication contains staging information for over 95% of patients. Data is currently very scarce for this rare disease and having such valuable clinical information provides an opportunity for clinicians around the country to better address questions such as treatment modalities, disease outcomes, and any disparities or barriers to treatment for certain groups.
Supplementary Material
Appendix Figure 3: Survival of ALM by Treatment Characteristics
A) Surgery type, B) Chemotherapy, C) Immunotherapy, D) Radiation
Abbreviations: chemo = chemotherapy, immuno = immunotherapy
Appendix Figure 1: Survival of ALM by Patient Characteristics
A) Age, B) Sex, C) Race, D) Charlson-Deyo Comorbidity Index, E) Year of diagnosis
Abbreviations: under60 = age under 60, cc = Charlson-Deyo Comorbidity Index, year_dx_cat = year of diagnosis
Appendix Figure 2: Survival of ALM by Tumor Characteristics
A) Primary site, B) Laterality, C) Stage, D) Tumor thickness, E) Ulceration, F) Grade, G) Number of positive lymph nodes, H) Lymphovascular invasion, I) Metastasis, J) Surgical Margin Status
Abbreviations: lat = laterality, t_thick = tumor thickness in cm, pos_nodes = number of positive lymph nodes, lymph_vasc_invasion = lymphovascular invasion, surg_margin_status = surgical margin status
ACKNOWLEDGEMENTS
We’d like to acknowledge Caron Park, MS, from the Division of Biostatistics, Department of Preventive Medicine, Keck School of Medicine at the University of Southern California, for assisting with statistical support for this manuscript.
This work was supported partly by grants UL1TR001855 and UL1TR000130 from the National Center for Advancing Translational Science (NCATS), the National Cancer Institute grant number P30-CA014089 (USC Norris Comprehensive Cancer Center) and grant K08 DK118213 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Footnotes
CONFLICTS OF INTEREST
The authors state no conflict of interest.
DATA AVAILABILITY STATEMENT
All data used in this publication are publicly available through the National Cancer Database.
REFERENCES
- Ascierto PA, Schadendorf D, Berking C, Agarwala SS, van Herpen CML, Queirolo P, Blank CU, Hauschild A, Beck JT, St-Pierre A, Niazi F, Wandel S, Peters M, Zubel A, & Dummer R (2013). MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: A non-randomised, open-label phase 2 study. The Lancet Oncology, 14(3), 249–256. 10.1016/S1470-2045(13)70024-X [DOI] [PubMed] [Google Scholar]
- Balch CM (1998). Surgical management of melanoma: Results of prospective randomized trials. Annals of Surgical Oncology, 5(4), 301–309. 10.1007/BF02303492 [DOI] [PubMed] [Google Scholar]
- Basurto-Lozada P, Molina-Aguilar C, Castaneda-Garcia C, Vázquez-Cruz ME, Garcia-Salinas OI, Álvarez-Cano A, Martínez-Said H, Roldán-Marín R, Adams DJ, Possik PA, & Robles-Espinoza CD (2020). Acral lentiginous melanoma: Basic facts, biological characteristics and research perspectives of an understudied disease. In Pigment Cell and Melanoma Research. Blackwell Publishing Ltd. 10.1111/pcmr.12885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadling C, Jacobson-Dunlop E, Hodi FS, Le C, Warrick A, Patterson J, Town A, Harlow A, Cruz F, Azar S, Rubin BP, Muller S, West R, Heinrich MC, & Corless CL (2008). KIT gene mutations and copy number in melanoma subtypes. Clinical Cancer Research, 14(21), 6821–6828. 10.1158/1078-0432.CCR-08-0575 [DOI] [PubMed] [Google Scholar]
- Behbahani S, Malerba S, & Samie FH (2020). Acral lentiginous melanoma: clinicopathological characteristics and survival outcomes in the US National Cancer Database 2004–2016. In British Journal of Dermatology (Vol. 183, Issue 5, pp. 952–954). Blackwell Publishing Ltd. 10.1111/bjd.19211 [DOI] [PubMed] [Google Scholar]
- Bello DM, Chou JF, Panageas KS, Brady MS, Coit DG, Carvajal RD, & Ariyan CE (2013). Prognosis of acral melanoma: A series of 281 patients. Annals of Surgical Oncology, 20(11), 3618–3625. 10.1245/s10434-013-3089-0 [DOI] [PubMed] [Google Scholar]
- Bradford PT, Goldstein AM, McMaster ML, & Tucker MA (2009). Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986–2005. Archives of Dermatology, 145(4), 427–434. 10.1001/archdermatol.2008.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASCINELLI N, ZURRIDA S, GALIMBERTI V, BARTOLI C, BUFALINO R, DEL PRATO I, MASCHERONI L, TESTORI A, & CLEMENTE C (1994a). Acral Lentiginous Melanoma: A Histological Type without Prognostic Significance. The Journal of Dermatologic Surgery and Oncology, 20(12), 817–822. 10.1111/j.1524-4725.1994.tb03711.x [DOI] [PubMed] [Google Scholar]
- CASCINELLI N, ZURRIDA S, GALIMBERTI V, BARTOLI C, BUFALINO R, DEL PRATO I, MASCHERONI L, TESTORI A, & CLEMENTE C (1994b). Acral Lentiginous Melanoma: A Histological Type without Prognostic Significance. The Journal of Dermatologic Surgery and Oncology, 20(12), 817–822. 10.1111/j.1524-4725.1994.tb03711.x [DOI] [PubMed] [Google Scholar]
- Castaneda CA, Torres-Cabala C, Castillo M, Villegas V, Casavilca S, Cano L, Sanchez J, Dunstan J, Calderon G, De La Cruz M, Cotrina JM, Gomez HL, Galvez R, & Abugattas J (2017). Tumor infiltrating lymphocytes in acral lentiginous melanoma: a study of a large cohort of cases from Latin America. Clinical and Translational Oncology, 19(12), 1478–1488. 10.1007/s12094-017-1685-3 [DOI] [PubMed] [Google Scholar]
- Chang AE, Karnell LH, & Menck HR (1998). The national cancer data base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. Cancer, 83(8), 1664–1678. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Wu CY, Chen JT, Shen JL, Chen CC, & Wang HC (1999). Clinicopathologic analysis of malignant melanoma in Taiwan. Journal of the American Academy of Dermatology, 41(6), 945–949. 10.1016/S0190-9622(99)70251-3 [DOI] [PubMed] [Google Scholar]
- Coleman WP, Loria PR, Reed RJ, & Krementz ET (1980). Acral Lentiginous Melanoma. Archives of Dermatology, 116(7), 773–776. 10.1001/archderm.1980.01640310043015 [DOI] [PubMed] [Google Scholar]
- Cormier JN, Xing Y, Ding M, Lee JE, Mansfield PF, Gershenwald JE, Ross MI, & Du XL (2006). Ethnic differences among patients with cutaneous melanoma. Archives of Internal Medicine, 166(17), 1907–1914. 10.1001/archinte.166.17.1907 [DOI] [PubMed] [Google Scholar]
- Criscito MC, & Stein JA (2017). Melanoma Management part of Improving the diagnosis and treatment of acral melanocytic lesions. 4(2), 113–123. 10.2217/mmt-2016-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JA, Busam K, Pinkel D, & Bastian BC (2006). Somatic activation of KIT in distinct subtypes of melanoma. Journal of Clinical Oncology, 24(26), 4340–4346. 10.1200/JCO.2006.06.2984 [DOI] [PubMed] [Google Scholar]
- Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Bröcker EB, LeBoit PE, Pinkel D, & Bastian BC (2005). Distinct sets of genetic alterations in melanoma. New England Journal of Medicine, 353(20), 2135–2147. 10.1056/NEJMoa050092 [DOI] [PubMed] [Google Scholar]
- Deyo RA, Cherkin DC, & Ciol MA (1992). Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of Clinical Epidemiology, 45(6), 613–619. 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- Elder D, Barnhill R, & Bastian B (2018). WHO Classification of tumours Pathology & Genetics of Skin Tumours (Elder DE WR, Massi D, Scolyer RA (ed.); 4th ed). [Google Scholar]
- Elder DE, Bastian BC, Cree IA, Massi D, & Scolyer RA (2020). The 2018 World Health Organization classification of cutaneous, mucosal, and uveal melanoma detailed analysis of 9 distinct subtypes defined by their evolutionary pathway. In Archives of Pathology and Laboratory Medicine (Vol. 144, Issue 4, pp. 500–522). College of American Pathologists. 10.5858/arpa.2019-0561-RA [DOI] [PubMed] [Google Scholar]
- Greaves WO, Verma S, Patel KP, Davies MA, Barkoh BA, Galbincea JM, Yao H, Lazar AJ, Aldape KD, Medeiros LJ, & Luthra R (2013). Frequency and spectrum of BRAF mutations in a retrospective, single-institution study of 1112 cases of melanoma. Journal of Molecular Diagnostics, 15(2), 220–226. 10.1016/j.jmoldx.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumaste PV, Fleming NH, Silva I, Shapiro RL, Berman RS, Zhong J, Osman I, & Stein JA (2014). Analysis of recurrence patterns in acral versus nonacral melanoma: Should histologic subtype influence treatment guidelines? JNCCN Journal of the National Comprehensive Cancer Network, 12(12), 1706–1712. 10.6004/jnccn.2014.0172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid O, Puzanov I, Dummer R, Schachter J, Daud A, Schadendorf D, Blank C, Cranmer LD, Robert C, Pavlick AC, Gonzalez R, Hodi FS, Ascierto PA, Salama AKS, Margolin KA, Gangadhar TC, Wei Z, Ebbinghaus S, Ibrahim N, & Ribas A (2017). Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. European Journal of Cancer, 86, 37–45. 10.1016/j.ejca.2017.07.022 [DOI] [PubMed] [Google Scholar]
- Harmelin ES, Holcombe RN, Goggin JP, Carbonell J, & Wellens T (1998). Acral lentiginous melanoma. Journal of Foot and Ankle Surgery, 37(6), 540–545. 10.1016/S1067-2516(98)80033–1 [DOI] [PubMed] [Google Scholar]
- Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, Patch AM, Kakavand H, Alexandrov LB, Burke H, Jakrot V, Kazakoff S, Holmes O, Leonard C, Sabarinathan R, Mularoni L, Wood S, Xu Q, Waddell N, … Mann GJ (2017). Whole-genome landscapes of major melanoma subtypes. Nature, 545(7653), 175–180. 10.1038/nature22071 [DOI] [PubMed] [Google Scholar]
- Huang K, Fan J, & Misra S (2020). Acral Lentiginous Melanoma: Incidence and Survival in the United States, 2006–2015, an Analysis of SEER Registry. Journal of Surgical Research, 251, 329–339. 10.1016/j.jss.2020.02.010 [DOI] [PubMed] [Google Scholar]
- Huayllani MT, RESTREPO DJ, BOCZAR D, AVILA FR, BAGARIA SP, SPAULDING AC, RINKER BD, & FORTE AJ (2020). National Comprehensive Analysis of Characteristics of Acral Lentiginous Melanoma. Anticancer Research, 40(6), 3411–3415. 10.21873/anticanres.14325 [DOI] [PubMed] [Google Scholar]
- Jimbow K, Takahashi H, Miura S, Ikeda S, & Kukita A (1984). Biological behavior and natural course of acral malignant melanoma. Clinical and histologic features and prognosis of palmoplantar, subungual, and other acral malignant melanomas. The American Journal of Dermatopathology, 6 Suppl, 43–53. http://www.ncbi.nlm.nih.gov/pubmed/6528942 [PubMed] [Google Scholar]
- Jung HJ, Kweon SS, Lee JB, Lee SC, & Yun SJ (2013). A clinicopathologic analysis of 177 acral melanomas in Koreans: Relevance of spreading pattern and physical stress. JAMA Dermatology, 149(11), 1281–1288. 10.1001/jamadermatol.2013.5853 [DOI] [PubMed] [Google Scholar]
- Kaplan I, & Youngleson J (1972). Malignant melanomas in the South African Bantu. British Journal of Plastic Surgery, 25(C), 65–68. 10.1016/S0007-1226(72)80018-3 [DOI] [PubMed] [Google Scholar]
- Kato T, Suetake T, Tabata N, Takahashi K, & Tagami H (1999). Epidemiology and prognosis of plantar melanoma in 62 Japanese patients over a 28-year period. International Journal of Dermatology, 38(7), 515–519. 10.1046/j.1365-4362.1999.00736.x [DOI] [PubMed] [Google Scholar]
- Kaunitz GJ, Cottrell TR, Lilo M, Muthappan V, Esandrio J, Berry S, Xu H, Ogurtsova A, Anders RA, Fischer AH, Kraft S, Gerstenblith MR, Thompson CL, Honda K, Cuda JD, Eberhart CG, Handa JT, Lipson EJ, & Taube JM (2017). Melanoma subtypes demonstrate distinct PD-L1 expression profiles. Laboratory Investigation, 97(9), 1063–1071. 10.1038/labinvest.2017.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krementz ET, Sutherland CM, Carter RD, & Ryan RF (1976). Malignant melanoma in the American Black. Annals of Surgery, 183(5), 533–542. 10.1097/00000658-197605000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchelmeister C, Schaumburg-Lever G, & Garbe C (2000a). Acral cutaneous melanoma in caucasians: Clinical features, histopathology and prognosis in 112 patients. British Journal of Dermatology, 143(2), 275–280. 10.1046/j.1365-2133.2000.03651.x [DOI] [PubMed] [Google Scholar]
- Kuchelmeister C, Schaumburg-Lever G, & Garbe C (2000b). Acral cutaneous melanoma in caucasians: Clinical features, histopathology and prognosis in 112 patients. British Journal of Dermatology, 143(2), 275–280. 10.1046/j.1365-2133.2000.03651.x [DOI] [PubMed] [Google Scholar]
- Lee JH, Choi JW, & Kim YS (2011). Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: A meta-analysis. British Journal of Dermatology, 164(4), 776–784. 10.1111/j.1365-2133.2010.10185.x [DOI] [PubMed] [Google Scholar]
- Liang WS, Hendricks W, Kiefer J, Schmidt J, Sekar S, Carpten J, Craig DW, Adkins J, Cuyugan L, Manojlovic Z, Halperin RF, Helland A, Nasser S, Legendre C, Hurley LH, Sivaprakasam K, Johnson DB, Crandall H, Busam KJ, … Trent J (2017). Integrated genomic analyses reveal frequent TERT aberrations in acral melanoma. Genome Research, 27(4), 524–532. 10.1101/gr.213348.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado JL, Fridlyand J, Patel H, Jain AN, Busam K, Kageshita T, Ono T, Albertson DG, Pinkel D, & Bastian BC (2003). Determinants of BRAF mutations in primary melanomas. Journal of the National Cancer Institute, 95(24), 1878–1880. 10.1093/jnci/djg123 [DOI] [PubMed] [Google Scholar]
- Massi D, Franchi A, Borgognoni L, Reali UM, & Santucci M (1999). Thin cutaneous malignant melanomas (≤1.5 mm): Identification of risk factors indicative of progression. Cancer, 85(5), 1067–1076. [DOI] [PubMed] [Google Scholar]
- NCCN. (n.d.). National Comprehensive Cancer Network. Cutaneous Melanoma (Version 3.2020). https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma_blocks.pdf. Accessed August 14, 2020.
- O’Leary JA, Berend KR, Johnson JL, Levin LS, S. H. (2000). Subungual melanoma. A review of 93 cases with identification of prognostic variables. Clin Orthop Relat Res, 378, 206–212. [PubMed] [Google Scholar]
- Omholt K, Grafström E, Kanter-Lewensohn L, Hansson J, & Ragnarsson-Olding BK (2011). KIT pathway alterations in mucosal melanomas of the vulva and other sites. Clinical Cancer Research, 17(12), 3933–3942. 10.1158/1078-0432.CCR-10-2917 [DOI] [PubMed] [Google Scholar]
- Reed R (1976). New Concepts in Surgical Pathology of the Skin. John Wiley & Sons. [Google Scholar]
- Ridgeway CA, Hieken TJ, Ronan SG, Kim DK, & Das Gupta TK (1995). No Title. 130(1), 88–92. 10.1001/archsurg.1995.01430010090019 [DOI] [PubMed] [Google Scholar]
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbé C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, … Ascierto PA (2015). Nivolumab in Previously Untreated Melanoma without BRAF Mutation. New England Journal of Medicine, 372(4), 320–330. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain J-F, Testori A, Grob J-J, Davidson N, Richards J, Maio M, Hauschild A, Miller WH, Gascon P, Lotem M, Harmankaya K, Ibrahim R, … Wolchok JD (2011). Ipilimumab plus Dacarbazine for Previously Untreated Metastatic Melanoma. New England Journal of Medicine, 364(26), 2517–2526. 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- Shah JP GH (1971). Malignant melanoma in the North American Negro. Surg Gynecol Obstet, 133, 437–439. [PubMed] [Google Scholar]
- Slingluff CL, Vollmer R, & Seigler HF (1990). Acral with melanoma: A review of 185 patients identification of prognostic variables. Journal of Surgical Oncology, 45(2), 91–98. 10.1002/jso.2930450207 [DOI] [PubMed] [Google Scholar]
- Soon SL, Solomon AR, Papadopoulos D, Murray DR, McAlpine B, & Washington CV (2003). Acral lentiginous melanoma mimicking benign disease: The Emory experience. Journal of the American Academy of Dermatology, 48(2 SUPPL.), 183–188. 10.1067/mjd.2003.63 [DOI] [PubMed] [Google Scholar]
- Tan KB, Moncrieff M, Thompson JF, McCarthy SW, Shaw HM, Quinn MJ, Li LXL, Crotty KA, Stretch JR, & Scolyer RA (2007). Subungual melanoma: A study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. American Journal of Surgical Pathology, 31(12), 1902–1912. 10.1097/PAS.0b013e318073c600 [DOI] [PubMed] [Google Scholar]
- Teramoto Y, Keim U, Gesierich A, Schuler G, Fiedler E, Tüting T, Ulrich C, Wollina U, Hassel JC, Gutzmer R, Goerdt S, Zouboulis C, Leiter U, Eigentler TK, & Garbe C (2018). Acral lentiginous melanoma: a skin cancer with unfavourable prognostic features. A study of the German central malignant melanoma registry (CMMR) in 2050 patients. British Journal of Dermatology, 178(2), 443–451. 10.1111/bjd.15803 [DOI] [PubMed] [Google Scholar]
- Viros A, Fridlyand J, Bauer J, Lasithiotakis K, Garbe C, Pinkel D, & Bastian BC (2008). Improving melanoma classification by integrating genetic and morphologic features. PLoS Medicine, 5(6), 0941–0952. 10.1371/journal.pmed.0050120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M, Ito T, Tsuji G, Nakahara T, Hagihara A, Furue M, & Uchi H (2017). Acral lentiginous melanoma versus other melanoma: A single-center analysis in Japan. Journal of Dermatology, 44(8), 932–938. 10.1111/1346-8138.13834 [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao Y, & Ma S (2016). Racial differences in six major subtypes of melanoma: Descriptive epidemiology. BMC Cancer, 16(1). 10.1186/s12885-016-2747-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyers W, Euler M, Diaz-Cascajo C, Schill WB, & Bonczkowitz M (1999). Classification of cutaneous malignant melanoma: A reassessment of histopathologic criteria for the distinction of different types. Cancer, 86(2), 288–299. [DOI] [PubMed] [Google Scholar]
- Woodman SE, & Davies MA (2010). Targeting KIT in melanoma: A paradigm of molecular medicine and targeted therapeutics. In Biochemical Pharmacology (Vol. 80, Issue 5, pp. 568–574). Biochem Pharmacol. 10.1016/j.bcp.2010.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh I, Jorgenson E, Shen L, Xu M, North JP, Shain AH, Reuss D, Wu H, Robinson WA, Olshen A, Von Deimling A, Kwok PY, Bastian BC, & Asgari MM (2019). Targeted genomic profiling of acral melanoma. Journal of the National Cancer Institute, 111(10), 1068–1077. 10.1093/jnci/djz005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- York K, Dlova NC, Wright CY, Khumalo NP, Kellett PE, Kassanjee R, & Mosam A (2016). Primary cutaneous malignancies in the Northern Cape Province of South Africa: A retrospective histopathological review. South African Medical Journal, 107(1), 83. 10.7196/samj.2016.v107.i1.10924 [DOI] [PubMed] [Google Scholar]
- Zemelman VB, Valenzuela CY, Sazunic I, & Araya I (2014). Malignant melanoma in Chile: Different site distribution between private and state patients. Biological Research, 47(1). 10.1186/0717-6287-47-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Figure 3: Survival of ALM by Treatment Characteristics
A) Surgery type, B) Chemotherapy, C) Immunotherapy, D) Radiation
Abbreviations: chemo = chemotherapy, immuno = immunotherapy
Appendix Figure 1: Survival of ALM by Patient Characteristics
A) Age, B) Sex, C) Race, D) Charlson-Deyo Comorbidity Index, E) Year of diagnosis
Abbreviations: under60 = age under 60, cc = Charlson-Deyo Comorbidity Index, year_dx_cat = year of diagnosis
Appendix Figure 2: Survival of ALM by Tumor Characteristics
A) Primary site, B) Laterality, C) Stage, D) Tumor thickness, E) Ulceration, F) Grade, G) Number of positive lymph nodes, H) Lymphovascular invasion, I) Metastasis, J) Surgical Margin Status
Abbreviations: lat = laterality, t_thick = tumor thickness in cm, pos_nodes = number of positive lymph nodes, lymph_vasc_invasion = lymphovascular invasion, surg_margin_status = surgical margin status
Data Availability Statement
All data used in this publication are publicly available through the National Cancer Database.


