Abstract
Ticks are regarded as one of the most ancient, unique, and highly evolved ectoparasites. They can parasitize diverse vertebrates and transmit a number of widespread infections. Once acquired from infected hosts, many tick-borne pathogens, like Borrelia burgdorferi, are confined within the tick gut lumen and are surrounded by discrete gut barriers. Such barriers include the peritrophic membrane (PM) and the dityrosine network (DTN), which are in close contact with resident microbiota and invading pathogens, influencing their survival within the vector. Herein, we review our current state of knowledge about tick-microbe interactions involving the PM and DTN structures. As a model, we will focus on Ixodes ticks, their microbiome, and the pathogen of Lyme disease. We will address the most salient findings on the structural and physiological roles of these Ixodes gut barriers on microbial interactions, with a comparison to analogous functions in other model vectors, such as mosquitoes. We will distill how this information could be leveraged towards a better understanding of the basic mechanisms of gut biology and tick-microbial interactions, which could contribute to potential therapeutic strategies in response to ticks and tick-borne infections.
Keywords: arthropod vectors, gut barrier, Ixodes ticks, Borrelia burgdorferi
Graphical Abstract
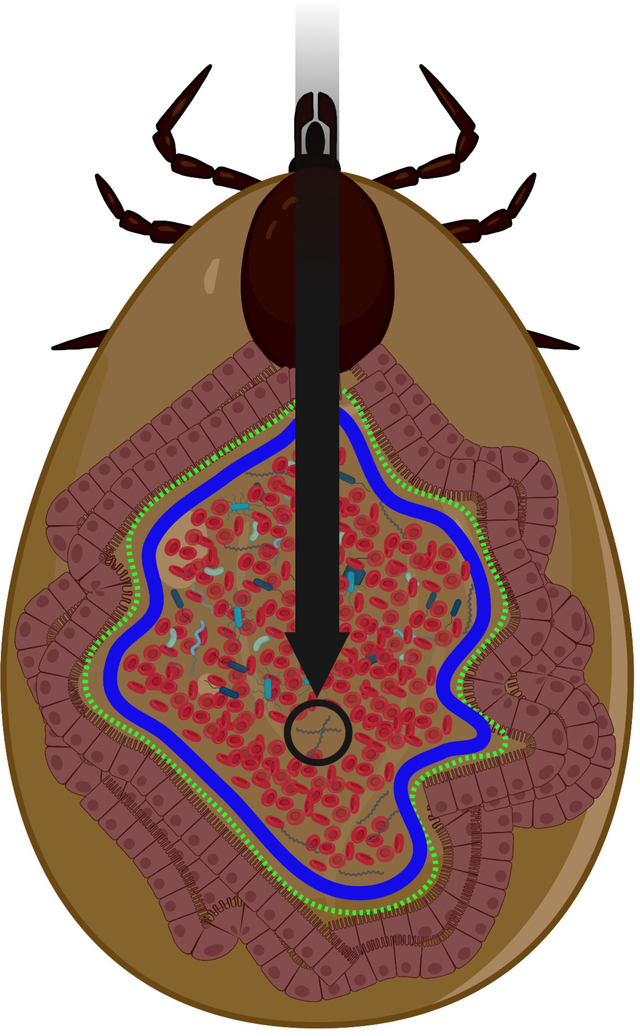
Most tick-borne pathogens, such as the Lyme disease spirochetes (black circle), are acquired within the tick gut along with the host-derived blood meal. Acellular gut barriers like peritrophic membrane (solid blue line) and dityrosine network (green dotted line) in tick gut potentially cover the gut epithelial cells and physically separate them from the lumen, ultimately influencing the persistence of many tick-borne pathogens. This review highlights our current state of knowledge about tick-microbe interactions involving these gut barriers. This image was created using BioRender (https://biorender.com/).
Introduction
Ticks represent a major group of arthropod vectors that are present worldwide and transmit a number of serious infections (Parola & Raoult, 2001). They exhibit a high degree of heterogeneity in terms of their habitat, biology, and genome, which possibly dictates their remarkable vectorial competence, as evidenced by their ability to harbor a diverse range of pathogenic agents, including viruses, bacteria, protozoa, and nematodes (Jia et al., 2020). The hematophagous and ectoparasitic behavior of ticks facilitates the acquisition and transmission of certain pathogens between reservoir hosts and ticks, as well as frequent transmission from ticks to incidental hosts. Many human and animal diseases are caused by tick-borne pathogens. For example, tick-borne encephalitis (TBE) (Beaute, Spiteri, Warns-Petit, & Zeller, 2018) is elicited by a virus of the family Flaviviridae, the TBE virus (Mansfield et al., 2009), which occurs in many European countries, northern China, Mongolia, and the Russian Federation, while Lyme disease, or Lyme borreliosis, is caused by the bacteria Borrelia burgdorferi sensu lato, which is prevalent in North America and Eurasia (Radolf, Caimano, Stevenson, & Hu, 2012). The latest estimate from the United States Center for Disease Control and Prevention (CDC) suggests that, in recent years, there were likely over 400,000 new cases of Lyme disease annually in the U.S. alone (Kugeler, Schwartz, Delorey, Mead, & Hinckley, 2021). This infection is predominantly transmitted by Ixodes scapularis ticks, in addition to closely related species, such as I. pacificus, I. ricinus, and I. persulcatus, that are found across specific geographical regions of North America, Eurasia, and Asia. While many distinct tick species transmit various human diseases, Ixodes ticks are considered as one of the most prolific vectors; besides Lyme disease and TBE, they also transmit numerous other human infections, such as anaplasmosis, babesiosis, Powassan virus, and B. miyamotoi disease. In fact, 13 newly recognized tick-borne pathogens have been identified in the Western Hemisphere during the last two decades, including ones transmitted by Ixodes ticks (Paddock, Lane, Staples, & Labruna, 2016). Despite the high incidence of tick-borne diseases and an increasing awareness of ticks as prolific vectors of prevalent human diseases, there remains an unmet need for preventive vaccines, as well as a deeper understanding of the fundamental molecular biology of ticks as disease vectors.
Hard ticks like Ixodes spp. can quest for days, and once latched on a host, they usually remain attached to the dermis for several days while ingesting copious amounts of blood, as much as 100-fold greater than their own body volume. A large blood meal is fundamental to tick survival throughout intermolt periods, which are characterized by many months of feeding deprivation; it is also a requirement for the support of the molting process, the subsequent growth of new organs (such as an additional pair of legs or reproductive organs in fed nymphs), and egg-laying in fed females (Sauer, 1986; Sonenshine, 1993). Additionally, blood meal ingestion provides an opportunity for pathogens to be acquired by or transmitted via sub-adult or adult ticks. Ticks also maintain a diverse microbiome (Greay et al., 2018) in various organs, often including pathogenic microorganisms (Bonnet & Pollet, 2021), which are transmitted to the next host during a subsequent blood meal, following a defined route. Of note, the duration of microbial transmission to the host in relation to tick attachment varies greatly by the type of pathogen (Eisen, 2018). For example, upon acquisition from an infected host, the Lyme disease agent B. burgdorferi exclusively resides within the gut of an Ixodes tick, often through the lengthy period of intermolt development. During the tick’s subsequent blood meal, spirochetes exit the gut and move to the hemocoel and ultimately the salivary gland, where they can be transferred to the host dermis along with tick saliva (Piesman, Mather, Sinsky, & Spielman, 1987; Piesman, Oliver, & Sinsky, 1990). In contrast, most other tick-borne pathogens, like agents of anaplasmosis or TBE, do not colonize the tick gut for long periods of time; rather, they cross the gut barriers and colonize the salivary gland for subsequent transmission to hosts (de la Fuente et al., 2017). Such “saliva-assisted” transmission of pathogens has been reported for many blood-feeding arthropods, including many examples in ticks (Nuttall, 2019).
The ability of ticks to ingest and store the components of their blood meal in gut epithelia, while simultaneously maintaining their resident microorganisms, indicates an intimate interaction between gut components and invading pathogens. In addition to the microbicidal cellular or humoral immune responses of the gut epithelia, specific acellular or tissue barriers also exist within the tick gut that impact the survival or dissemination of vector-transmitted pathogens. One acellular component called the peritrophic membrane or peritrophic matrix (PM), which lines the gut of arthropods (Hegedus, Toprak, & Erlandson, 2019), is formed during blood feeding in ticks (Kariu, Smith, Yang, & Pal, 2013; Yang et al., 2021; Zhu, Gern, & Aeschlimann, 1991). The PM appears to provide digestive and immunological or defensive roles for the vector. Composed of chitin and proteins or glycoproteins, it serves as a molecular sieve to separate the lumen from the ectoperitrophic space, thereby selectively controlling the transfer of molecules from the partially digested blood meal components between these two areas. The PM has been shown to exhibit a protective function for the epithelial gut cells by acting as a mechanical barrier against abrasive foodstuffs, invasive microbes, and secreted toxins from pathogens (Hegedus et al., 2019). Furthermore, its structural integrity has been correlated with the ability of pathogens to colonize epithelial cells, as the disruption of the PM in I. scapularis ticks has been shown to affect the persistence of B. burgdorferi spirochetes in the gut (Yang et al., 2021). Overall, a complex relationship exists in ticks between pathogens and the PM, as the presence or disruption of the PM can either positively or negatively affect the survival of specific tick-borne pathogens.
Relatively recent studies have identified another acellular structure that can immunologically separate the lumen from the gut epithelium. An event of tyrosine cross-linking within the extracellular matrix occurs in the guts of feeding mosquitoes, forming a proteinaceous molecular barrier termed the dityrosine network (DTN) (Kumar, Molina-Cruz, Gupta, Rodrigues, & Barillas-Mury, 2010), which is primarily catalyzed by a transmembrane gut enzyme complex called dual oxidase (Duox) (Ameziane-El-Hassani et al., 2005; Donko, Peterfi, Sum, Leto, & Geiszt, 2005). Duox, along with another enzyme with potential heme peroxidase activity work cooperatively in forming the DTN (Kumar et al., 2010). The formation of the DTN, potentially over the gut epithelia, appears to reduce the permeability of epithelial cells to immune elicitors, thereby protecting the beneficial gut microbiota, which in turn supports invading pathogens, such as Plasmodium parasites in the Anopheles mosquito gut (Kumar et al., 2010) or B. burgdorferi in the I. scapularis tick gut (Yang, Smith, Williams, & Pal, 2014). Duox in arthropods is likely a multi-functional enzyme; in addition to the DTN, it also regulates gut homeostasis and the microbiome in the Drosophila gut via the production of reactive oxygen species (ROS) (Ha, Oh, Bae, & Lee, 2005). In the following paragraphs, we will review tick gut barriers, specifically the PM and DTN, and their impact on invading pathogens in the feeding gut upon acquisition from infected hosts. In particular, we will focus on I. scapularis ticks and their responses to Lyme disease pathogens as a model. We will attempt to summarize studies that are pertinent to the basic concepts of tick immune and physiological responses, emphasizing the less-appreciated roles of the intricate tick gut tissue barriers that influence pathogen survival or dissemination within the vector.
The peritrophic matrix in the gut
One of the relatively well-characterized insect gut barriers is called the peritrophic membrane or peritrophic matrix (PM). This acellular structure lines the gut epithelia of arthropods and separates the endoperitrophic area of the lumen from the ectoperitrophic zone (Hegedus et al., 2019; Lehane, 1997). The porous matrix along the surface of the gut allows for the selective transport of compounds. Compositionally, the PM contains chitin, which is an N-acetylglucosamine polymer, as well as proteins and additional carbohydrates or glycoproteins. Chitin is synthesized by the chitin synthase (CS) enzyme. In insect species, CS is often encoded by two genes, CHS-A and CHS-B; it has been observed that the expression of CHS-B is restricted to gut epithelial cells that produce the PM (Arakane et al., 2004), and that the experimental inhibition of chitin synthesis by a chitin inhibitor named Dimilin impaired PM formation in the Locusta migratoria locust (Clarke, Temple, & Vincent, 1977). The I. scapularis genome encodes for multiple CS enzymes (Gulia-Nuss et al., 2016). Proteins that participate in PM structures can vary in number and function across various arthropod species. Studies have focused on a specific group of proteins, called peritrophins, due to their ability to affect chitin interactions through the formation of disulfide bonds between their cysteines and the cysteines present in the chitin-binding domain (Tellam, Wijffels, & Willadsen, 1999; Toprak et al., 2016). Heavily glycosylated peritrophins appear to share functional similarities with mammalian mucins, including the protection of epithelial cells against pathogens and proteolytic events, the lubrication of foodstuffs during passage through the gut, and the selective transport of molecules across the matrix (Strous & Dekker, 1992; Tellam et al., 1999).
Two major types of PM have been identified in arthropods: Type I, which originates from the gut, and Type II, which is formed by specialized tissues in the cardia (anterior gut). The PM often creates a highly ordered orthogonal or hexagonal lattice, as indicated by electron microscopic analysis (Hegedus et al., 2019). The PM can be a permanent or temporary structure, likely depending on the specific feeding pattern and biology of each organism. It has been shown that blood ingestion serves as the signal for PM production, but it is not clear as to how this process is controlled (Hegedus et al., 2019; Yang et al., 2021; Zhu et al., 1991).
The PM appears to have multiple functions in arthropod physiology. It accounts for the functional compartmentalization of the gut lumen, which likely supports the digestive processes. Macromolecules are first processed in the endoperitrophic space; then, the digested products are translocated through the PM to the ectoperitrophic area for final digestion (Bolognesi, Terra, & Ferreira, 2008; Caldeira, Dias, Terra, & Ribeiro, 2007; Jordao & Terra, 1991). In addition, the PM seems to act as a mechanical barrier that protects gut epithelial cells from abrasive food particles. The midgut epithelium of a PM-lacking Bombyx mori mutant was highly abraded, and membranous bodies were released from its surface to the bolus (Sudha & Muthu, 1988).
Furthermore, several studies have shown the PM’s defensive role against invasive pathogens and their released toxins. In insects, a negative correlation was recorded between the abundance of pathogens in the gut and the presence of the PM. In D. melanogaster, higher susceptibility to Pseudomonas entomophila infection, in addition to higher lethality (probably owing to the increased deleterious action of its pore-forming toxin, monalysin), were linked to a reduction in PM thickness, due to a mutation in the drosocrystallin (dcy) gene; as Dcy is a chitin-binding protein expressed in the gut, these studies provide genetic evidence that the PM protects the fly against intestinal bacterial infections (Kuraishi, Binggeli, Opota, Buchon, & Lemaitre, 2011). A knockdown of the gene that encodes for transglutaminase (TG), an enzyme that helps to stabilize PM by crosslinking with Dcy, was lethal in Drosophila and induced the apoptosis of gut epithelial cells after oral infection with P. entomophila (Shibata et al., 2015). In the A. coluzzii mosquito gut, the synthesis and integrity of the PM were supported by microbiota, while the PM influenced the persistence and containment of Enterobacteriaceae bacteria within the gut, preventing a systemic infection, and participated in the restoration of gut homeostasis after a blood meal (Rodgers, Gendrin, Wyer, & Christophides, 2017). Likewise, Cry toxins, Cry1Aa and Cry1Ac, produced by the Gram-positive bacteria Bacillus thuringiensis were found to be entrapped by the PM in B. mori; interestingly, the binding of Cry1Ac to the PM was inhibited by N-acetylgalactosamine (GalNAc), and the pretreatment of Cry1Ac with GalNAc completely restored Cry1Ac passage, indicating that Cry1Ac binds a PM protein via GalNAc on a sugar side chain (Hayakawa, Shitomi, Miyamoto, & Hori, 2004). Additionally, in insecticide-resistant Aedes aegypti mosquitoes, an extensive layer of PM was found to be burdened with DDT (Abedi & Brown, 1961). In A. aegypti, the PM was shown to participate in the protection of the midgut cells from the potentially toxic effects of heme that is generated after hemoglobin catabolism, by specifically binding heme, and therefore playing an important role in heme detoxification (Pascoa et al., 2002).
In another study, it was shown that PM formation is necessary for the survival of Leishmania major in the midgut of the Phlebotomus papatasi vector, as the activity of exogenous chitinase led to complete blockage of PM formation and subsequent loss of midgut parasites infections. (Pimenta, Modi, Pereira, Shahabuddin, & Sacks, 1997); an early parasite mortality (within four hours) was also observed in the absence of exogenous chitinase, which was associated with the activity of digestive enzymes present in the gut after the blood meal and was reversed with the addition of a trypsin inhibitor or Allosamadin, a specific inhibitor of chitinase. Together, these studies suggest that PM favors parasites persistence by regulating their exposure to proteolytic enzyme present in the fed midgut. The disruption of PM formation, following the knockdown of the A. aegypti chitin synthase (AeCs) gene via RNA interference (RNAi), impaired P. gallinaceum infectivity, as measured by oocyst intensity (Kato et al., 2008). In the midgut of the A. gambiae mosquito, reduced infection with P. falciparum parasites was observed upon disrupting the function of fibrinogen-related protein 1 (FREP1). FREP1, which is secreted from the midgut epithelial cells, was shown to localize in and interact with the PM. As FREP1 also binds Plasmodium gametocytes and ookinetes, this protein also has proposed roles in PM penetration and epithelial invasion by parasites (Zhang et al., 2015).
Additional microbes seem to have evolved to manipulate PM structure or function in arthropods, affecting pathogen persistence in the gut. Increased infection was observed in baculovirus-treated Trichoplusia ni larvae, due to a factor present in baculovirus occlusion bodies that likely consists of a metalloprotease called enhancin (Derksen & Granados, 1988; Wang & Granados, 1997). Entomopoxvirus (EPV) spindles enhance infection by disrupting the PM in host insects, possibly using a protein called fusolin that has a conserved region with a potential chitin-binding domain (Mitsuhashi et al., 2007). Through a remarkable mechanism adopted by trypanosome surface coat proteins, called variant surface glycoproteins (VSGs), African trypanosomes can interfere with the PM function by modulating the transcriptional profile of cardia cells, which are responsible for the PM synthesis, to successfully establish infection in the tsetse gut (Aksoy et al., 2016). Babesia microti have been found to penetrate the PM in I. dammini ticks by using the arrowhead, a highly specialized organelle that forms in some Babesia parasites during their development and invasion through the tick PM (Rudzinska, Spielman, Lewengrub, Piesman, & Karakashian, 1982). A chitinase gene, named PbCHT1, which encodes for putative proenzyme with chitin-binding domains, was identified in the rodent malaria parasite P. berghei and shown to facilitate midgut invasion in A. stephensi mosquitoes (Dessens et al., 2001). Based on its homology to other chitinases and experimental evidence, PbCHT1 seems to be the only chitinase in P. berghei, and it was suggested that the gene’s role is different than directly targeting the PM, since its disruption was correlated with a significant reduction in parasite infectivity not only in mosquitoes, but also in ookinete-feeds where the midgut invasion occurs before the PM formation (Dessens et al., 2001). Similarly, the chitinase PfCHT1 gene in P. falciparum was shown to be essential for the parasites to invade the A. freeborni midgut (Tsai, Hayward, Langer, Fidock, & Vinetz, 2001). Taken together, these studies highlight PM’s important role in vector gut physiology and its profound influence on pathogen persistence in arthropods.
The PM is formed in the midgut of I. scapularis (Yang et al., 2021) or related ticks (Zhu et al., 1991) during blood meal engorgement on a vertebrate host (Figure 1). More recently, the nano-LC-MS/MS proteomic analysis of isolated PMs from fed I. scapularis ticks resulted in the identification of a few constituent PM proteins (Kariu et al., 2013). Amongst these, one of the most abundant was a 60-kDa protein which shares homology with arthropod chitin deacetylase (CDA), termed as I. scapularis CDA-like protein (IsCDA). Although the RNAi-mediated silencing of IsCDA in ticks had no effects on PM structure or spirochete levels in the gut, the treatment of ticks with IsCDA antibodies significantly affected B. burgdorferi persistence, without influencing the levels of total gut bacteria (Kariu et al., 2013). Additional studies indicated the significant role of another protein, termed peritrophic membrane chitin binding protein (PM_CBP), which also resembles CDA from B. mori (Yang et al., 2021). The silencing of PM_CBP in ticks via RNAi led to a significant decrease in the thickness and function of the PM in 48-hr fed ticks, although there was no marked impact on spirochete persistence. Notably, when infected ticks were treated with PM_CBP antibodies, both the PM structure and Borrelia burden were altered in ticks. Consequently, the transmission of B. burgdorferi from antibody-treated ticks to naïve mice was also affected. Similarly, after feeding on infected mice that were actively immunized with PM_CBP, the acquisition of Borrelia by I. scapularis ticks was affected (Yang et al., 2021). Additional research also highlighted PM regulation via the STAT signaling pathway and its importance to pathogen persistence in ticks. In experiments where the expression of Ixodes transcription factor STAT was diminished, a reduction in the expression of a PM protein called peritrophin-1 was also observed, in addition to a significant decrease in PM thickness. The levels of tick-borne pathogens, including B. burgdorferi, were also affected in nymphs after the targeted silencing of peritrophin-1 via RNAi. Fewer epithelium-bound spirochetes were observed in nymphs after the knockdown of STAT or peritrophin-1, suggesting that the STAT signaling pathway and the PM may impact spirochete colonization in I. scapularis ticks (Narasimhan et al., 2014). In addition, the colonization of I. scapularis by other tick-borne pathogens, like Anaplasma phagocytophilum, was correlated with decreased expression levels of certain peritrophin genes. Reduced PM thickness was also observed in nymphs that fed on A. phagocytophilum-infected mice, as compared to nymphs that fed on control mice. The RNAi-mediated silencing of those peritrophin genes significantly enhanced the colonization of A. phagocytophilum in the tick gut and salivary glands, indicating that A. phagocytophilum infection, as acquired from the host, induces changes in the gut barrier (Abraham et al., 2017).
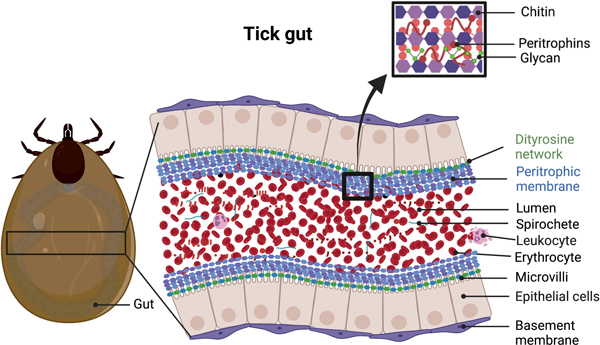
Figure 1:
A schematic diagram of a part of the tick gut. The diagram shows the occurrence of the peritrophic matrix (PM) and the possible location of the dityrosine network (DTN) in the gut of a fed I. scapularis tick. The Lyme disease pathogens exist in the gut lumen and are surrounded by the PM of an infected I. scapularis tick. This figure was created using BioRender (https://biorender.com/).
A newly recognized immune barrier in the gut: the dityrosine network
In Drosophila, it has been shown that dual oxidase (Duox) regulates the microbiome and homeostasis of the gut through ROS production (Ha et al., 2009). Duox proteins are named as dual oxidases because they have an NADPH oxidase domain (gp91phox) and an extracellular N-terminal domain, with high homology to mammalian peroxidases. The Duox peroxidase-like domain probably does not bind heme like other peroxidases, as it lacks the essential amino acids that are needed for heme binding. Between these NADPH oxidase and N-terminal domains, there is an EF-hand calcium-binding cytosolic region, indicating that calcium ions control their activity (De Deken, Wang, Dumont, & Miot, 2002; Donko et al., 2005; Sumimoto, 2008). It has been shown that Duox enzymes generate hydrogen peroxide (H2O2) in a calcium-dependent manner, but not superoxide, which is produced by the other family members (Ameziane-El-Hassani et al., 2005; Dupuy, Deme, Kaniewski, Pommier, & Virion, 1988). Duox homologs were identified in lower organisms, including Caenorhabditis elegans, sea urchins, D. melanogaster, A. gambiae mosquitoes, and I. scapularis ticks (Edens et al., 2001; Heinecke & Shapiro, 1992; Kumar et al., 2010; Yang et al., 2014). In C. elegans and sea urchins, Duox enzymes appear to play an additional crucial role in extracellular matrix formation, providing hydrogen peroxide for the crosslinking of dityrosine and trityrosine proteins (Edens et al., 2001). Duox knockdown in C. elegans using RNAi resulted in cuticle morphological defects and the elimination of dityrosine and trityrosine linkage formation (Edens et al., 2001). In response to blood feeding, gut epithelial cells in A. gambiae secrete a heme immunomodulatory peroxidase (IMPer), which works alongside a dual oxidase to catalyze the formation of a proteinaceous barrier termed as the dityrosine network (DTN). This network seems to protect gut epithelial cells from invasive microorganisms and create a suitable lumen environment for the proliferation of bacteria by effectively controlling gut permeability to immune elicitors. It also supports the development of malarial parasites within the midgut lumen without detection by epithelial immunity. Pathogen-specific immune responses were observed after the disruption of the DTN (Kumar et al., 2010).
The I. scapularis genome encodes a single Duox protein and about 16 proteins with possible peroxidase activity. It has been shown that the Duox enzyme is expressed in ticks after blood meal engorgement, and that an initial upregulation is potentially correlated with gut microbiome replication (Yang et al., 2014). Interestingly, Duox expression was observed to be 3-fold higher in ticks that fed on B. burgdorferi-infected mice, as compared to ticks that fed on naïve mice. In addition to Duox expression, immunofluorescence microscopy analysis revealed the formation of a DTN in the tick gut (Yang et al., 2014); a schematic diagram showing the possible location of DTN is presented (Figure 1). RNAi-mediated Duox knockdown has been shown to interfere with DTN formation and negatively regulate B. burgdorferi persistence in the tick gut (Yang et al., 2014). Additionally, two peroxidases, annotated as ISCW017368 and ISCW002528, are dramatically upregulated upon spirochete invasion, with ISCW017368 also affecting DTN formation and spirochete levels in ticks. A reduced abundance of B. burgdorferi could be partially attributed to nitric oxide (NO) synthase induction and NO production, as observed in ticks when the DTN was impaired and the expression of ISCW017368 was knocked down (Yang et al., 2014). Further studies on the function of the Duox enzyme (and other peroxidases) are warranted to better understand the mechanisms that govern DTN formation and the regulation of pathogen persistence.
Gut barriers and their molecular constituents as targets for vector- or pathogen-specific vaccines:
As studies suggest that gut barriers, like the PM and DTN, influence vector physiology and pathogen persistence, these structures or their molecular constituents serve as potential targets for vaccines against vector-borne infections. Experimental studies have demonstrated that the disruption of genes important to PM formation can affect pathogen persistence in the gut or impose lethal effects, as seen with D. melanogaster (Buchon, Broderick, & Lemaitre, 2013). The targeting of the chitinase enzyme, which is released by specific parasites to invade the PM, impaired the persistence of invading microbes in the guts of mosquitoes (Dessens et al., 2001; Tsai et al., 2001). In I. scapularis ticks, antibodies raised against proteins that resemble CDA were found to affect spirochete burdens in the vector (Kariu et al., 2013). Additionally, B. burgdorferi levels were reduced after the silencing of peritrophin-1, although the disruption of certain peritrophin genes significantly enhanced the colonization of A. phagocytophilum in the I. scapularis tick gut and salivary glands (Abraham et al., 2017; Narasimhan et al., 2014). Taken together, these studies indicate that specific molecules that are critical to the structure and formation of the PM, or are utilized by pathogens to penetrate the PM or invade the gut epithelia, could serve as possible targets for vaccines against vector-borne infections. Nevertheless, future studies are warranted to elucidate PM formation and its functions in disease vectors, including ticks. In addition, as the DTN has also been shown to influence the burden of malarial parasites or Lyme disease agents within their respective vectors, further research is required to better understand the structure and roles of DTN components, particularly as targets for vaccines against vector-borne pathogens.
Concluding Remarks and Future Prospects:
Evolving hundreds of millions of years ago, ticks have adapted sophisticated hematophagy processes and evolved an ability to survive in diverse environments across the globe. Given the remarkable diversity among arthropods, tick biology is likely unique, with significant divergence from model arthropod species. In fact, recent studies on the biology and vectorial competence of ticks highlight their roles as atypical ectoparasites and acknowledge their distinction as prolific vectors of many serious human diseases. Although the environmental acquisition of microbes by ticks is possible, these excellent blood feeders primarily acquire pathogens in their guts during the ingestion of a blood meal from a vertebrate host. Enhancing the roadmap of our existing knowledge of the gut barrier functions that are relevant to tick-pathogen associations, with an emphasis on the cellular and molecular mechanisms that govern gut biology and homeostasis, will plant new seeds of innovative research. While much is known about the tick salivary gland, the identities of tick gut proteins, especially those that are integral to the structure and function of the gut barriers, remain enigmatic. Although it is unclear as to precisely how gut barrier disruption impacts pathogen persistence, physical separation and decreased interaction with the epithelial cells, resulting in altered microbicidal responses, remain a likely general explanation (Kumar et al., 2010; Yang et al., 2014). Elucidating the components of the PM and DTN, and how these structures drive functional interactions with the microbiome and invading pathogens to determine vector-host interactions, will enrich our knowledge of the intricate biology of tick-pathogen interactions in the gut, which may contribute to the identification of novel strategies to control vector-borne infections, including tick-transmitted diseases.
Acknowledgement
The work in our laboratories is supported by grants from the National Institute of Allergy and Infectious Diseases, Award Numbers R01AI080615, R01AI116620, and P01AI138949 to U.P. The authors are thankful to Kathryn Nassar for assistance with the preparation of this manuscript.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Abedi ZH, & Brown AWA (1961). Peritrophic membrane as vehicle for DDT and DDE excretion in Aedes aegypti larvae. Ann. Entomol. Soc. Am, 54, 539–542. [Google Scholar]
- Abraham NM, Liu L, Jutras BL, Yadav AK, Narasimhan S, Gopalakrishnan V, . . . Fikrig E (2017). Pathogen-mediated manipulation of arthropod microbiota to promote infection. Proc Natl Acad Sci U S A, 114(5), E781–E790. doi: 10.1073/pnas.1613422114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy E, Vigneron A, Bing X, Zhao X, O’Neill M, Wu YN, . . . Aksoy S (2016). Mammalian African trypanosome VSG coat enhances tsetse’s vector competence. Proc Natl Acad Sci U S A, 113(25), 6961–6966. doi: 10.1073/pnas.1600304113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameziane-El-Hassani R, Morand S, Boucher JL, Frapart YM, Apostolou D, Agnandji D, . . . Dupuy C (2005). Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem, 280(34), 30046–30054. doi:M500516200 [pii] 10.1074/jbc.M500516200 [DOI] [PubMed] [Google Scholar]
- Arakane Y, Hogenkamp DG, Zhu YC, Kramer KJ, Specht CA, Beeman RW, . . . Muthukrishnan S (2004). Characterization of two chitin synthase genes of the red flour beetle, Tribolium castaneum, and alternate exon usage in one of the genes during development. Insect Biochem Mol Biol, 34(3), 291–304. doi: 10.1016/j.ibmb.2003.11.004 [DOI] [PubMed] [Google Scholar]
- Beaute J, Spiteri G, Warns-Petit E, & Zeller H (2018). Tick-borne encephalitis in Europe, 2012 to 2016. Euro Surveill, 23(45). doi: 10.2807/1560-7917.ES.2018.23.45.1800201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognesi R, Terra WR, & Ferreira C (2008). Peritrophic membrane role in enhancing digestive efficiency. Theoretical and experimental models. J Insect Physiol, 54(10–11), 1413–1422. doi: 10.1016/j.jinsphys.2008.08.002 [DOI] [PubMed] [Google Scholar]
- Bonnet SI, & Pollet T (2021). Update on the intricate tango between tick microbiomes and tick-borne pathogens. Parasite Immunol, 43(5), e12813. doi: 10.1111/pim.12813 [DOI] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, & Lemaitre B (2013). Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat Rev Microbiol, 11(9), 615–626. doi: 10.1038/nrmicro3074 [DOI] [PubMed] [Google Scholar]
- Caldeira W, Dias AB, Terra WR, & Ribeiro AF (2007). Digestive enzyme compartmentalization and recycling and sites of absorption and secretion along the midgut of Dermestes maculatus (Coleoptera) larvae. Arch Insect Biochem Physiol, 64(1), 1–18. doi: 10.1002/arch.20153 [DOI] [PubMed] [Google Scholar]
- Clarke L, Temple GH, & Vincent JF (1977). The effects of a chitin inhibitor-dimilin- on the production of peritrophic membrane in the locust, Locusta migratoria. J Insect Physiol, 23(2), 241–246. doi: 10.1016/0022-1910(77)90037-3 [DOI] [PubMed] [Google Scholar]
- De Deken X, Wang D, Dumont JE, & Miot F (2002). Characterization of ThOX proteins as components of the thyroid H(2)O(2)-generating system. Exp Cell Res, 273(2), 187–196. doi: 10.1006/excr.2001.5444 [DOI] [PubMed] [Google Scholar]
- de la Fuente J, Antunes S, Bonnet S, Cabezas-Cruz A, Domingos AG, Estrada-Pena A, . . . Rego ROM (2017). Tick-Pathogen Interactions and Vector Competence: Identification of Molecular Drivers for Tick-Borne Diseases. Front Cell Infect Microbiol, 7, 114. doi: 10.3389/fcimb.2017.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen AC, & Granados RR (1988). Alteration of a lepidopteran peritrophic membrane by baculoviruses and enhancement of viral infectivity. Virology, 167(1), 242–250. doi: 10.1016/0042-6822(88)90074-8 [DOI] [PubMed] [Google Scholar]
- Dessens JT, Mendoza J, Claudianos C, Vinetz JM, Khater E, Hassard S, . . . Sinden RE (2001). Knockout of the rodent malaria parasite chitinase pbCHT1 reduces infectivity to mosquitoes. Infect Immun, 69(6), 4041–4047. doi: 10.1128/IAI.69.6.4041-4047.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donko A, Peterfi Z, Sum A, Leto T, & Geiszt M (2005). Dual oxidases. Philos Trans R Soc Lond B Biol Sci, 360(1464), 2301–2308. doi: 10.1098/rstb.2005.1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy C, Deme D, Kaniewski J, Pommier J, & Virion A (1988). Ca2+ regulation of thyroid NADPH-dependent H2O2 generation. FEBS Lett, 233(1), 74–78. doi: 10.1016/0014-5793(88)81358-9 [DOI] [PubMed] [Google Scholar]
- Edens WA, Sharling L, Cheng G, Shapira R, Kinkade JM, Lee T, . . . Lambeth JD (2001). Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J Cell Biol, 154(4), 879–891. doi: 10.1083/jcb.200103132154/4/879 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L (2018). Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks. Ticks Tick Borne Dis, 9(3), 535–542. doi: 10.1016/j.ttbdis.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greay TL, Gofton AW, Paparini A, Ryan UM, Oskam CL, & Irwin PJ (2018). Recent insights into the tick microbiome gained through next-generation sequencing. Parasit Vectors, 11(1), 12. doi: 10.1186/s13071-017-2550-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulia-Nuss M, Nuss AB, Meyer JM, Sonenshine DE, Roe RM, Waterhouse RM, . . . Hill CA (2016). Genomic insights into the Ixodes scapularis tick vector of Lyme disease. Nat Commun, 7, 10507. doi: 10.1038/ncomms10507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha EM, Lee KA, Seo YY, Kim SH, Lim JH, Oh BH, . . . Lee WJ (2009). Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in Drosophila gut. Nat Immunol, 10(9), 949–957. doi:ni.1765 [pii] 10.1038/ni.1765 [DOI] [PubMed] [Google Scholar]
- Ha EM, Oh CT, Bae YS, & Lee WJ (2005). A direct role for dual oxidase in Drosophila gut immunity. Science, 310(5749), 847–850. doi:310/5749/847 [pii] 10.1126/science.1117311 [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Shitomi Y, Miyamoto K, & Hori H (2004). GalNAc pretreatment inhibits trapping of Bacillus thuringiensis Cry1Ac on the peritrophic membrane of Bombyx mori. FEBS Lett, 576(3), 331–335. doi: 10.1016/j.febslet.2004.09.029 [DOI] [PubMed] [Google Scholar]
- Hegedus DD, Toprak U, & Erlandson M (2019). Peritrophic matrix formation. J Insect Physiol, 117, 103898. doi: 10.1016/j.jinsphys.2019.103898 [DOI] [PubMed] [Google Scholar]
- Heinecke JW, & Shapiro BM (1992). The respiratory burst oxidase of fertilization. A physiological target for regulation by protein kinase C. J Biol Chem, 267(12), 7959–7962. [PubMed] [Google Scholar]
- Jia N, Wang J, Shi W, Du L, Sun Y, Zhan W, . . . Cao WC (2020). Large-Scale Comparative Analyses of Tick Genomes Elucidate Their Genetic Diversity and Vector Capacities. Cell, 182(5), 1328–1340 e1313. doi: 10.1016/j.cell.2020.07.023 [DOI] [PubMed] [Google Scholar]
- Jordao BP, & Terra WR (1991). Regional distribution and substrate specificity of digestive enzymes involved in terminal digestion in Musca domestica hind-midguts. Arch Insect Biochem Physiol, 17(2–3), 157–168. doi: 10.1002/arch.940170209 [DOI] [PubMed] [Google Scholar]
- Kariu T, Smith A, Yang X, & Pal U (2013). A Chitin Deacetylase-Like Protein Is a Predominant Constituent of Tick Peritrophic Membrane That Influences the Persistence of Lyme Disease Pathogens within the Vector. PLoS ONE, 8(10), e78376. doi: 10.1371/journal.pone.0078376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N, Mueller CR, Fuchs JF, McElroy K, Wessely V, Higgs S, & Christensen BM (2008). Evaluation of the function of a type I peritrophic matrix as a physical barrier for midgut epithelium invasion by mosquito-borne pathogens in Aedes aegypti. Vector Borne Zoonotic Dis, 8(5), 701–712. doi: 10.1089/vbz.2007.0270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugeler KJ, Schwartz AM, Delorey MJ, Mead PS, & Hinckley AF (2021). Estimating the Frequency of Lyme Disease Diagnoses, United States, 2010–2018. Emerg Infect Dis, 27(2), 616–619. doi: 10.3201/eid2702.202731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Molina-Cruz A, Gupta L, Rodrigues J, & Barillas-Mury C (2010). A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science, 327(5973), 1644–1648. doi: 10.1126/science.1184008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraishi T, Binggeli O, Opota O, Buchon N, & Lemaitre B (2011). Genetic evidence for a protective role of the peritrophic matrix against intestinal bacterial infection in Drosophila melanogaster. Proc Natl Acad Sci U S A, 108(38), 15966–15971. doi: 10.1073/pnas.1105994108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehane MJ (1997). Peritrophic matrix structure and function. Annu Rev Entomol, 42, 525–550. doi: 10.1146/annurev.ento.42.1.525 [DOI] [PubMed] [Google Scholar]
- Mansfield KL, Johnson N, Phipps LP, Stephenson JR, Fooks AR, & Solomon T (2009). Tick-borne encephalitis virus - a review of an emerging zoonosis. J Gen Virol, 90(Pt 8), 1781–1794. doi: 10.1099/vir.0.011437-0 [DOI] [PubMed] [Google Scholar]
- Mitsuhashi W, Kawakita H, Murakami R, Takemoto Y, Saiki T, Miyamoto K, & Wada S (2007). Spindles of an entomopoxvirus facilitate its infection of the host insect by disrupting the peritrophic membrane. J Virol, 81(8), 4235–4243. doi: 10.1128/JVI.02300-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Rajeevan N, Liu L, Zhao YO, Heisig J, Pan J, . . . Fikrig E (2014). Gut Microbiota of the Tick Vector Ixodes scapularis Modulate Colonization of the Lyme Disease Spirochete. Cell Host Microbe, 15(1), 58–71. doi: 10.1016/j.chom.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall PA (2019). Tick saliva and its role in pathogen transmission. Wien Klin Wochenschr. doi: 10.1007/s00508-019-1500-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Lane RS, Staples JE, & Labruna MB (2016). CHANGING PARADIGMS FOR TICK-BORNE DISEASES IN THE AMERICAS. National Academies of Sciences, Engineering, and Medicine. Washington (DC): National Academies Press (US), Forum on Microbial Threats; Board on Global Health. [Google Scholar]
- Parola P, & Raoult D (2001). Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis, 32(6), 897–928. doi: 10.1086/319347 [DOI] [PubMed] [Google Scholar]
- Pascoa V, Oliveira PL, Dansa-Petretski M, Silva JR, Alvarenga PH, Jacobs-Lorena M, & Lemos FJ (2002). Aedes aegypti peritrophic matrix and its interaction with heme during blood digestion. Insect Biochem Mol Biol, 32(5), 517–523. doi: 10.1016/s0965-1748(01)00130-8 [DOI] [PubMed] [Google Scholar]
- Piesman J, Mather T, Sinsky R, & Spielman A (1987). Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol, 25, 557–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J, Oliver J, & Sinsky R (1990). Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini). Am J Trop Med Hyg, 42, 352–357. [DOI] [PubMed] [Google Scholar]
- Pimenta PF, Modi GB, Pereira ST, Shahabuddin M, & Sacks DL (1997). A novel role for the peritrophic matrix in protecting Leishmania from the hydrolytic activities of the sand fly midgut. Parasitology, 115 (Pt 4), 359–369. [DOI] [PubMed] [Google Scholar]
- Radolf JD, Caimano MJ, Stevenson B, & Hu LT (2012). Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol, 10(2), 87–99. doi:nrmicro2714 [pii] 10.1038/nrmicro2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers FH, Gendrin M, Wyer CAS, & Christophides GK (2017). Microbiota-induced peritrophic matrix regulates midgut homeostasis and prevents systemic infection of malaria vector mosquitoes. PLoS Pathog, 13(5), e1006391. doi: 10.1371/journal.ppat.1006391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzinska MA, Spielman A, Lewengrub S, Piesman J, & Karakashian S (1982). Penetration of the peritrophic membrane of the tick by Babesia microti. Cell Tissue Res, 221(3), 471–481. [DOI] [PubMed] [Google Scholar]
- Sauer J. R. a. H., J. A (1986). Morphology, Physiology and Biology of Ticks (Vol. Vol-1). New York: Ellis Horwood Limited and John Wiley and Sons. [Google Scholar]
- Shibata T, Maki K, Hadano J, Fujikawa T, Kitazaki K, Koshiba T, & Kawabata S (2015). Crosslinking of a Peritrophic Matrix Protein Protects Gut Epithelia from Bacterial Exotoxins. PLoS Pathog, 11(10), e1005244. doi: 10.1371/journal.ppat.1005244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE (1993). Biology of Ticks (Vol. Vol-1). New York: Oxford University Press. [Google Scholar]
- Strous GJ, & Dekker J (1992). Mucin-type glycoproteins. Crit Rev Biochem Mol Biol, 27(1–2), 57–92. doi: 10.3109/10409239209082559 [DOI] [PubMed] [Google Scholar]
- Sudha PM, & Muthu SP (1988). Damage to the midgut epithelium caused by food in the absence of peritrophic membrane. Curr. Sci. , 57, 624–625. [Google Scholar]
- Sumimoto H (2008). Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J, 275(13), 3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x [DOI] [PubMed] [Google Scholar]
- Tellam RL, Wijffels G, & Willadsen P (1999). Peritrophic matrix proteins. Insect Biochem Mol Biol, 29(2), 87–101. [DOI] [PubMed] [Google Scholar]
- Toprak U, Erlandson M, Baldwin D, Karcz S, Wan L, Coutu C, . . . Hegedus DD (2016). Identification of the Mamestra configurata (Lepidoptera: Noctuidae) peritrophic matrix proteins and enzymes involved in peritrophic matrix chitin metabolism. Insect Sci, 23(5), 656–674. doi: 10.1111/1744-7917.12225 [DOI] [PubMed] [Google Scholar]
- Tsai YL, Hayward RE, Langer RC, Fidock DA, & Vinetz JM (2001). Disruption of Plasmodium falciparum chitinase markedly impairs parasite invasion of mosquito midgut. Infect Immun, 69(6), 4048–4054. doi: 10.1128/IAI.69.6.4048-4054.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, & Granados RR (1997). An intestinal mucin is the target substrate for a baculovirus enhancin. Proc Natl Acad Sci U S A, 94(13), 6977–6982. doi: 10.1073/pnas.94.13.6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Koči J, Smith AA, Zhuang X, Sharma K, Dutta S, . . . Pal U (2021). A novel tick protein supports integrity of gut peritrophic matrix impacting existence of gut microbiome and Lyme disease pathogens. Cell Microbiol, 23(2), e13275. doi: 10.1111/cmi.13275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Smith AA, Williams MS, & Pal U (2014). A Dityrosine Network Mediated by Dual Oxidase and Peroxidase Influences the Persistence of Lyme Disease Pathogens within the Vector. J Biol Chem, 289(18), 12813–12822. doi: 10.1074/jbc.M113.538272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Niu G, Franca CM, Dong Y, Wang X, Butler NS, . . . Li J (2015). Anopheles Midgut FREP1 Mediates Plasmodium Invasion. J Biol Chem, 290(27), 16490–16501. doi: 10.1074/jbc.M114.623165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Gern L, & Aeschlimann A (1991). The peritrophic membrane of Ixodes ricinus. Parasitol Res, 77(7), 635–641. [DOI] [PubMed] [Google Scholar]