Abstract
Intra-articular (IA) glucocorticoids (GC) are commonly used for clinical management of both osteoarthritis and rheumatoid arthritis, but their efficacy is limited by the relatively short duration of action and associated side effects. To provide sustained efficacy and to improve the safety of GCs, we previously developed a N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer-based dexamethasone (Dex) prodrug. Serendipitously, we discovered that, by increasing the Dex content of the prodrug to unusually high levels, the aqueous solution of the polymeric prodrug becomes thermoresponsive, transitioning from a free-flowing liquid at 4°C to a hydrogel at 30°C or greater. Upon IA injection, the prodrug solution forms a hydrogel (ProGel-Dex) that is retained in the joint for more than 1 month, where it undergoes gradual dissolution, releasing the water-soluble polymeric prodrug. The released prodrug is swiftly internalized and intracellularly processed by phagocytic synoviocytes to release free Dex, resulting in sustained amelioration of joint inflammation and pain in rodent models of inflammatory arthritis and osteoarthritis. The low molecular weight (6.8 kDa) of the ProGel-Dex ensures rapid renal clearance once it escapes the joint, limiting systemic GC exposure and risk of potential off-target side effects. The present study illustrates the translational potential of ProGel-Dex as a potent opioid-sparing, locally delivered adjuvant analgesic for sustained clinical management of arthritis pain and inflammation. Importantly, the observed thermoresponsive properties of the prodrug establishes ProGel as a platform technology for the local delivery of a broad spectrum of therapeutic agents to treat a diverse array of pathological conditions.
Keywords: polymeric prodrug, thermoresponsive, hydrogel, glucocorticoid, arthritis, pain
Graphical Abstract
One Sentence Summary:
A thermoresponsive polymeric dexamethasone prodrug provides sustained resolution of arthritis pain and inflammation without apparent glucocorticoid-associated toxicity.
Introduction
Arthritis is an umbrella term for joint pathologies encompassing more than 100 different types.[1] Osteoarthritis (OA) is the most common form of arthritis, affecting more than 30 million individuals in the United States.[2] OA is characterized by progressive loss of articular cartilage and alterations in peri-articular bone that are associated with chronic pain, inflammation, and loss of mobility. Currently, no treatment has been shown to alter the progression of joint structural damage or promote repair in patients with OA. Effective joint pain control is the primary objective of management of the disease.[3] Osteoarthritis Research Society International (OARSI) guidelines recommend intra-articular (IA) administration of glucocorticoids (GC) as an appropriate treatment for all OA patient subgroups.[4] IA-GC injections are also commonly used as an adjunctive therapy to manage joint pain and inflammation in rheumatoid arthritis (RA), which is a prototypical form of inflammatory arthritis, affecting 0.6% of the population in the United States.[5] For both OA and RA, IA-GC injections provide effective short-term pain relief and improve function. The utility of IA-GC for long-term pain management has been hampered by the short half-life of GC in the joint and resultant short duration of the anti-inflammatory and analgesic effects. Therefore, there remains an unmet clinical need for an effective and safe IA-GC therapy that can provide sustained pain relief and suppression of joint inflammation for clinical management of OA and RA.
To address the limitations of the conventional GC therapies, we have developed a N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer-based water-soluble dexamethasone (Dex) prodrug (P-Dex) for the systemic treatment of inflammatory and autoimmune diseases, including RA.[6–13] A hydrazone bond was used as the linker chemistry to conjugate Dex to the HPMA copolymer, enabling acidity-driven subcellular prodrug activation in the arthritic joints. The Dex content of the P-Dex employed in these previous studies was generally maintained at ~10 w/w% to ensure the water-solubility of the prodrug for systemic administration. Upon systemic administration, the prodrug passively targets to the inflammatory pathologies via what we have termed, “Extravasation through Leaky Vasculature and subsequent Inflammatory cell-mediated Sequestration” (ELVIS) mechanism,[14, 15] resulting in sustained anti-inflammatory and disease-modifying activities.[13, 14] The passive targeting of P-Dex necessitates the maintenance of a long serum half-life, which entails the need for a higher molecular weight (Mw) of the prodrug.[7, 11] Inadvertently, the long serum half-life also significantly increases systemic exposure to the prodrug and the associated risk of potential off-target toxicities.
Serendipitously, we recently discovered that when the Dex content in P-Dex was increased to an unusually high level (e.g., 24 w/w%), its aqueous solution demonstrated a thermoresponsive phase transition behavior with the prodrug concentration maintained at 20 w/v%. This formulation, which we have designated “ProGel-Dex”, is a free-flowing liquid at 4°C but forms a hydrogel when the temperature is elevated to ~ 30°C (Figure 1a). The Mw of ProGel-Dex was found to have limited impact on the gelation temperature (Tgel). We hypothesized that the unexpected thermoresponsive properties of the polymeric prodrug would provide a unique solution to the seemingly irreconcilable demands of higher Mw P-Dex to allow ELVIS-mediated targeting of inflammation and of lower Mw P-Dex to evade the unwanted capture by the mononuclear phagocytic system (MPS, e.g., liver and spleen), as well as to ensure efficient renal clearance. As we have shown previously, the P-Dex with highest Mw would achieve the highest serum half-life in an arthritis rat model. While it resulted in the highest P-Dex levels in the arthritis joints, it also led to very high deposition to the liver and spleen. As a plausible solution to the problem, backbone degradable HPMA copolymers have been successfully developed.[16–18] As another potential solution, we envisioned that ProGel-Dex would form a hydrogel when administered in vivo, resulting in physical entrapment of the polymeric prodrug at the injection site. Upon interaction with the body fluids, we further hypothesized that the gel would gradually dissolve, providing sustained prodrug release and activation at the site of pathology. Due to its low Mw, the released ProGel-Dex molecules will be quickly cleared through the kidney after draining away from the injection site, thus circumventing the unwanted systemic redistribution to the MPS. Although the thermoresponsive behavior precludes ProGel-Dex from systemic administration, it does provide a unique drug delivery technology for treatment of local pathologies. In this manuscript, we investigated the thermoresponsive behavior and rheology of ProGel-Dex. Its outstanding safety and unique capacity to provide sustained amelioration of inflammation and pain in arthritic joints were validated in two rodent models of inflammatory arthritis and a rodent model of osteoarthritis pain.
Figure 1.

The characterization of the ProGel-Dex. (a) The thermoresponsive phase transition of ProGel-Dex (24 w/w% Dex content in ProGel-Dex, 20 w/v% ProGel-Dex concentration). Left panel: aqueous ProGel-Dex solution was free-flowing at 4°C. Right panel: aqueous ProGel-Dex solution formed a hydrogel at 30°C. (b) The rheological properties of ProGel-Dex (24 w/w% Dex content in ProGel-Dex, 20 w/v% ProGel-Dex concentration). The first temperature (21.9 °C), at which the G’ value (storage modulus) equaled the G” value (loss modulus), was identified as the gelation temperature (Tgel) of ProGel-Dex. The second temperature (33.5 °C), at which the G’ value equaled the G” value, was identified as the syneresis temperature (Tsyn) of ProGel-Dex. (c) ProGel-Dex (24 w/w% Dex content in ProGel-Dex, 20 w/v% ProGel-Dex concentration) viscosity vs. shear rate profiles at different temperatures. (d) Sol-gel-syn phase transition diagram of ProGel-Dex constructed using the test tube-tilting method. The gelation and syneresis temperatures of ProGel-Dex with three different Dex contents (20, 24, and 28 w/w%) were recorded with the concentrations of the prodrug solution ranging from 15 w/v% to 40 w/v% with 2.5 w/v% increments.
Results and Discussion
Characterization of the Thermoresponsive ProGel-Dex
Given the significance of the newly discovered thermoresponsive behaviors, we first performed a series of rheologic studies to quantitatively characterize the ProGel-Dex formulation. Using a rheometer (DHR-2, TA Instrument, USA), we determined that the gelation temperature Tgel of the original ProGel-Dex formulation (Dex content = 24 w/w%; ProGel-Dex concentration = 20 w/v%) was 21.9°C, at which the storage and loss modulus (G’ and G”, respectively) were equal in value under an angular frequency of 10 rad/s and 0.5% strain (Figure 1b). The syneresis temperature (Tsyn, at which point liquid is expelled from the gel) was 33.5°C, where G’ and G” values were equal for the second time. This result confirms the originally observed thermoresponsive phase transition of the ProGel-Dex formulation (Figure 1a). Analysis of its viscosity in a flow shear rate sweep study at four different temperatures (4, 10, 20, and 30°C) showed the typical shear-thinning behavior (non-Newtonian) and the viscosity decreased as a function of shear rate. At lower temperatures (4 and 10°C), the viscosity was low, suggesting a free-flowing liquid, suitable for the purpose of IA injection. When the temperature was elevated to 20°C (close to Tgel), the viscosity of the system increased substantially. At 30°C, which is the estimated intra-articular knee joint temperature,[19] the viscosity further increased by several orders of magnitude in a low range of shear rate, confirming the formation of a hydrogel (Figure 1c). The yield stress of ProGel-Dex was 51.4 Pa in a strain sweep experiment with the angular frequency of 10 rad/s at 30°C, indicating the formation of a firm hydrogel that required high stress to deform. Such property insures the physical stability of the formulation against the effects of repeated mechanical joint loading.
To assess the impact of the Dex content on the thermoresponsive properties of ProGel-Dex, we synthesized the polymeric prodrug with Dex contents at approximately 20, 24, and 28 w/w%. Using the test tube-tilting method,[20] we measured the solution-hydrogel-syneresis (sol-gel-syn) transition temperatures of these prodrugs at the different concentrations. The Tgel and Tsyn vs. ProGel-Dex concentrations were plotted to generate phase transition diagrams of the prodrug (Figure 1d), which illustrates a large range of feasible formulation parameters for ProGel-Dex’ in vivo utility. Clearly, both the Dex content in ProGel-Dex and the ProGel-Dex concentration can be used to regulate the gelation behavior of the formulation. It is interesting to note that ProGel-Dex with a Dex content at 28 w/w% showed the highest Tgel, which seems to contradict our speculation that the thermoresponsive behavior of ProGel-Dex may involve the hydrophobicity-driven aggregation of Dex molecules (to be further discussed in later sections). With the highest Dex content, the observation of the lowest Tgel for this ProGel-Dex would have been more reasonable. We cannot explain this observation at present. Additional studies will be needed to better understand this finding. As another structural parameter, the Mw of ProGel-Dex may also have an impact on its thermoresponsive properties. To assess this possibility, we used the test tube-tilting method to measure the Tgel values of ProGel-Dex with similar Dex content (~24 w/w%) but different Mw (6.8, 9.8, 14.0 and 35.0 kDa). Only a small increase in corresponding Tgel values (22, 25, 24, 28.5°C) was observed with the increase of Mw, suggesting a limited impact.
The influence of linker chemistry on the activation of P-Dex and Dex release has been examined extensively in a previous study.[10] Given that the aggregation of the hydrophobic Dex may have resulted in the thermoresponsive behavior of ProGel-Dex, we posit that the ProGel-Dex phase transition into a hydrogel can also affect the kinetics of Dex release through modification of its molecular environment. As the in vitro Dex release data shows (Figure 2, Figure S1), the increase of Dex content in ProGel-Dex, not the change of ProGel-Dex concentration, would slow down the Dex activation and release. Comparing to the non-thermoresponsive P-Dex,[10] the in vitro Dex activation and release from the ProGel-Dex formulations are slower. This can be partially attributed to the increased hydrophobic aggregation associated with the higher Dex content in ProGel-Dex. Limited exposure of ProGel-Dex to the releasing media (on top of the hydrogel only, see Materials and Methods section) is another contributing factor. Different from its in vitro performance, we anticipate the in vivo Dex activation and release from ProGel-Dex will be significantly increased. This is because ProGel-Dex will be fully exposed to the synovial fluid once administered into the joint. Mechanical impact of repeated joint articulation will further fragment the hydrogel into smaller pieces, which increases the surface area of ProGel-Dex, resulting in accelerated prodrug dissolution and activation.
Figure 2.
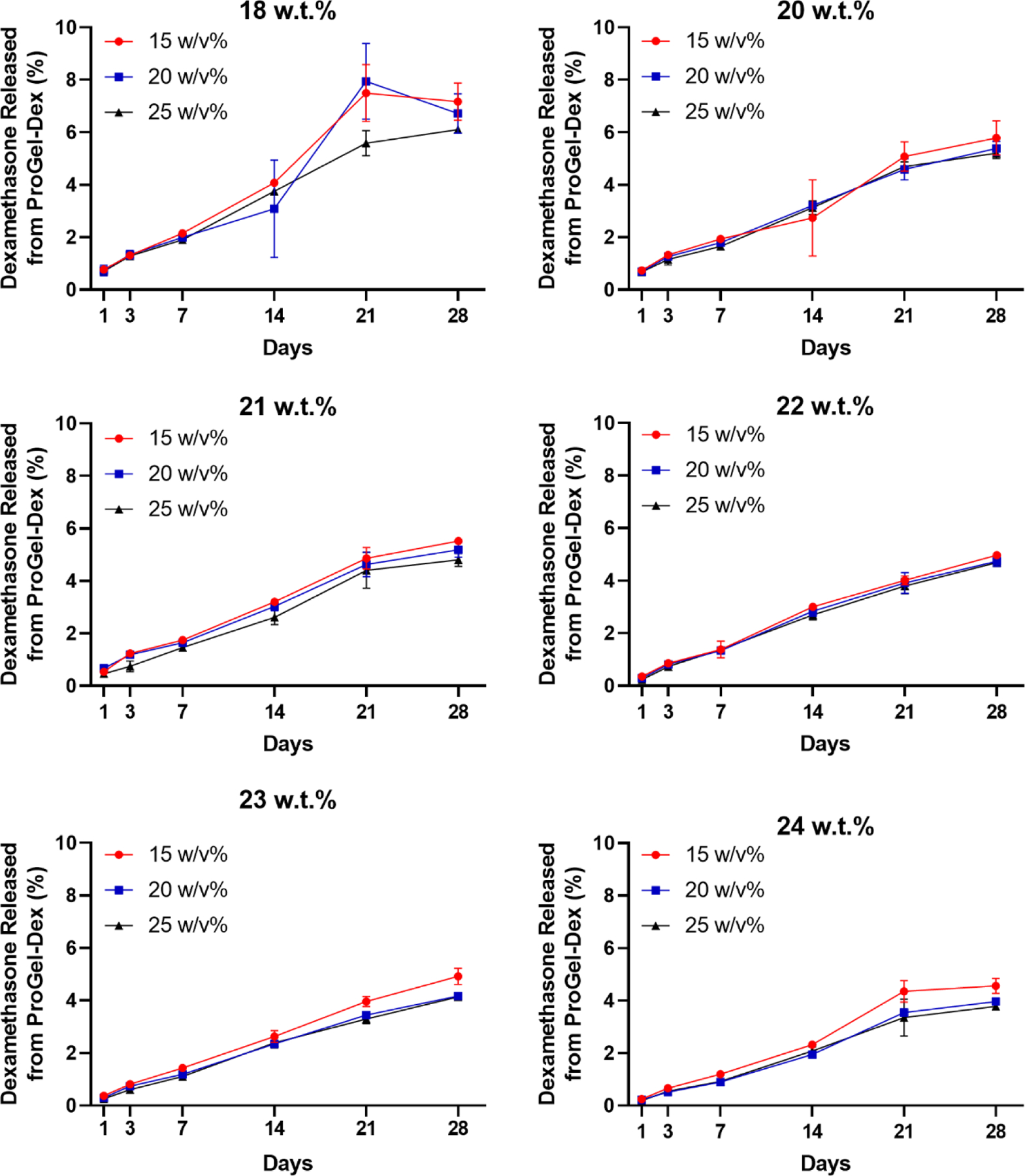
In vitro release kinetics of the Dex released from ProGel-Dex. ProGel-Dex formulations (Dex content ranging from 18 to 24 w/w%) were prepared in a releasing buffer (pH = 5.0). The red dot, blue square, and black triangle represented each ProGel-Dex at 15, 20, 25 w/v% concentration, respectively. The percentage of Dex released represents that of the total Dex loaded in ProGel-Dex.
Having demonstrated the unique characteristics of the ProGel-Dex, we selected the formulation with Dex content at 24 w/w% and ProGel-Dex concentration at 20 w/v% for all the in vivo studies. The non-aromatic hydrazone at C-3 position of the Dex was identified as the favorable linker chemistry[10] and the Mw of ProGel-Dex was maintained at 6.8 kDa. The selection of these formulation parameters was made with the consideration of the ease of IA administration (low resistance through a 30-gauge needle), the limited IA injection volume in rodents (≤ 10 μL), the Dex loading, activation and release kinetics necessary to maintain long-term resolution of pathology (e.g., months of OA joint pain relief after a single administration). Given the well-established kidney glomerular filtration threshold (< 45 kDa) for HPMA copolymers[21] and the limited dependence of the Tgel of ProGel-Dex on Mw, we hypothesized that the ProGel-Dex with a Mw of 6.8 kDa would undergo swift renal clearance once it dissolves from the gel surface and drains into the circulation, avoiding off-target systemic exposure and associated toxicities. We anticipated that this particular ProGel-Dex formulation would demonstrate sustained amelioration of arthritis pain and inflammation in vivo, with very limited systemic exposure and associated toxicities.
There is a large body of literature documenting thermoresponsive polymers and their biomedical applications.[22–27] The most widely used polymers include poly(N-isopropylacrylamide) (PNIPAAm), poly(ethylene oxide) (PEO)/poly(propylene oxide) (PPO) block copolymers (commercially available as Pluronics®), and polylactides (PLA)/polyethylene glycol (PEG) block copolymers. These polymers are widely used in the biomedical field because their lower critical solution temperatures (LCST) are close to the body temperature. These polymers typically contain a mixture of hydrophilic and hydrophobic molecular elements or blocks. At lower temperatures, the hydrogen bonding between hydrophilic elements of the polymer chain and water molecules are strong, presenting the system as a clear solution. At elevated temperatures, the interactions among the hydrophobic structural elements are intensified. Hydrogen bonding, on the other hand, is disrupted as the temperature increases. Together, these changes result in the hydrophobic collapse of the polymers marked by cloudiness of the solution, their conformational change from a coil to globule state,[28–30] followed by self-aggregation and hydrogel formation.[31]
Similar to the aforementioned thermoresponsive polymeric systems, the newly developed ProGel-Dex is an amphiphilic polymer consisting of the hydrophilic HPMA copolymer carrier and the hydrophobic Dex payload. The serendipitous discovery, in which the accidental doubling of Dex content in P-Dex resulted in the formation of the thermoreponsive ProGel-Dex, suggests that the phase transition process may be similar to that of PNIPAAm and is driven by the increase of hydrophobic structural elements. As the initial attempt to understand the mechanism responsible for the thermoresponsive behavior of ProGel-Dex, we performed molecular dynamic simulations of ProGel-Dex. As shown in Figure 3, ProGel-Dex polymer chains simulated at 30°C adopt a more interconnected conformation in which the hydrophobic Dex molecules from different polymer chains coalesce, resulting in the formation of a more compact conformation. At 4°C, however, the aggregation of the hydrophobic Dex appears to be reduced, resulting in limited intermolecular entanglement of the simulated ProGel-Dex polymer chains and a more extended conformation. Using dynamic light scattering (DLS), we assessed the average hydrodynamic diameters (Dh) of ProGel-Dex (1 mg/mL in ddH2O, Dex content = 24 w/w%, Mw = 6.8 kDa) at different temperatures. The Dh of the prodrug self-assembly increased gradually from ~ 400 nm to 1,200 nm with the elevation of the temperature from 4°C to 24°C. It then decreased to ~ 200 nm (Supporting Information, Table S1) as the temperature increased to 30°C. While more extensive studies would be necessary to fully understand the mechanism of the thermoresponsive behavior of ProGel-Dex, these initial assessments suggest a gelation process like PNIPAAm and other well-studied thermoresponsive polymeric systems.[22–27]
Figure 3.
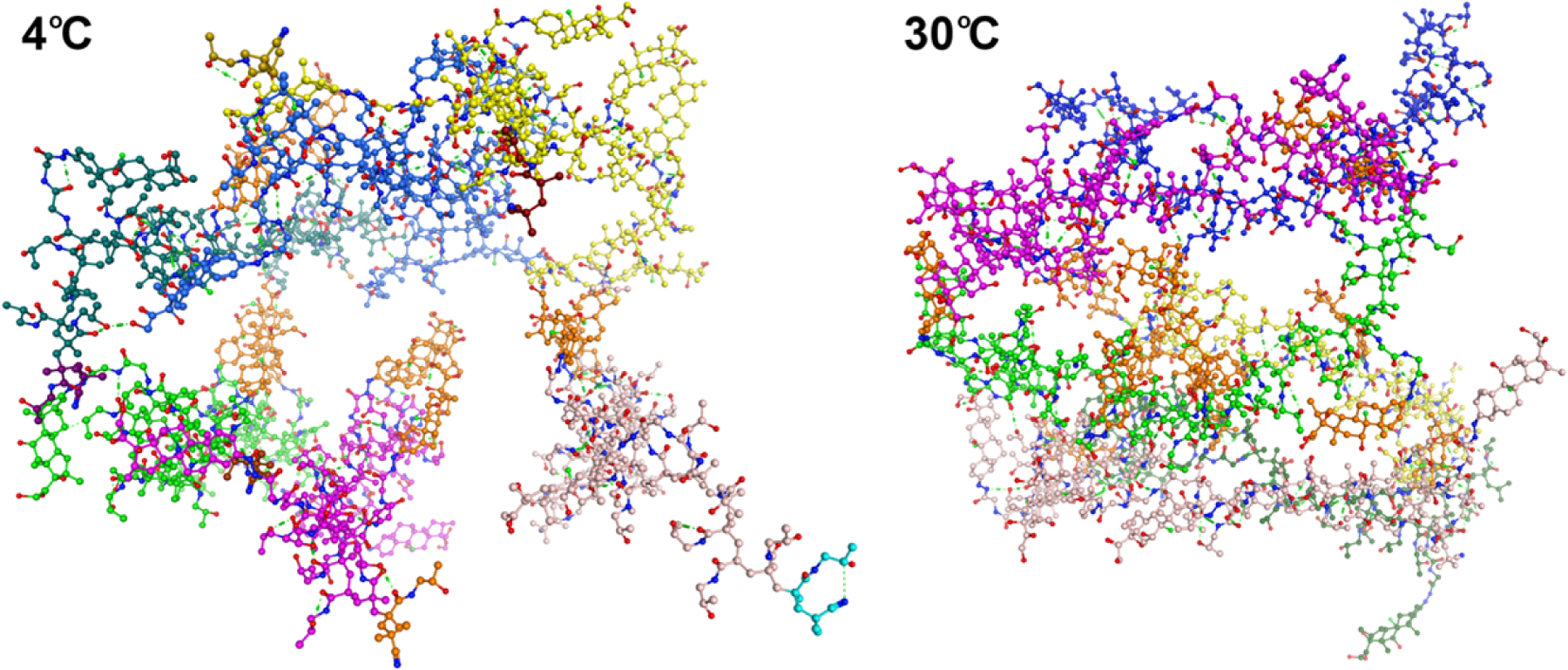
Molecular dynamic simulations of ProGel-Dex in water at 4°C and 30°C. Six ProGel-Dex polymer molecules are colored as yellow, blue, dark green, bright green, magenta, and pale colors. The Dex molecules are colored in orange. The size of the six-molecule system was 59 Å × 62 Å × 71 Å at 4°C and 52 Å × 61 Å × 40 Å at 30°C, respectively. This suggests that the ProGel-Dex polymer chains simulated at higher temperature (30°C) adopts a more interconnected conformation with aggregation of the hydrophobic Dex molecules (orange color) from different polymer chains, leading to the formation of a more compact conformation. At lower temperature (4°C), however, the aggregation of the hydrophobic Dex (orange color) appeared to be reduced, resulting in less intermolecular entanglement of the simulated ProGel-Dex polymer chains and a more extended conformation.
For local drug delivery purposes, it is important to recognize that the ProGel is distinctively different from PNIPAAm, Pluronics®, and PLA/PEG/PLA in that it is a polymeric prodrug in nature with the drug payload chemically conjugated to the polymeric carrier. For the other delivery systems, the drug molecules are incorporated via physical entrapment. Such a difference in the design of the delivery system would result in different mechanisms of release, cell internalization, elimination, and redistribution after local administration.
Visualization of the distribution of ProGel-Dex
We envision the main in vivo advantage of ProGel-Dex is its capacity to be directly delivered to and retained at sites of pathology. After injection, ProGel-Dex rapidly undergoes gelation at the site of pathology where its presence is locally sustained. The hydrogel then undergoes slow dissolution, releasing the ProGel-Dex molecules, which are preferentially internalized by the pathogenic phagocytic cells (e.g., synoviocytes), which provide the prodrug a unique cell selectivity for uptake and processing. Prodrug that is not internalize rapidly drains through lymph nodes and returns to the circulation. The low Mw of the water-soluble polymeric prodrug renders a swift renal clearance with very limited systemic redistribution. To validate this model, the thermoresponsive polymeric prodrug was labeled with IRDye 800CW or Alexa Fluor 647 and administered IA into arthritic joints. Three different animal models of arthritis were used in the present study: the rat polyarticular adjuvant-induced arthritis (AA) model; the rat monoarticular adjuvant-induced arthritis (MAA) model; and the monoiodoacetate-induced (MIA) mouse model of OA-like pain. The use of the AA polyarthritis model in this study allows us to determine whether the locally administered ProGel-Dex redistributes and ameliorates symptoms in the contralateral arthritic joint. With MAA rats, this single joint arthritis model allows the use of an incapacitance tester to assess the pain behavior after ProGel-Dex local administration. Different from the highly inflammatory AA and MAA models, the MIA model is a standard model for osteoarthritis (OA) pain. Validation of ProGel-Dex’ distribution and efficacy in amelioration of OA-like pain in MIA mice further confirms its versatility as a potent adjunct analgesic in managing pain associated with diverse pathologies.
Dissection of the decalcified MAA knee joint at 7 days post IA injection (Figure 4a) and dissection of the AA ankle joint at 28 days (Figure 4b) illustrate the presence of the labeled ProGel-Dex (light blue/green color, attributed to IRDye 800CW) in the joint cavity. To assess the redistribution pattern of the prodrug, MAA rats were euthanized at different time points post IA injection. The organs and tissues were then imaged with near-infrared (NIR) optical imaging and compared. As shown in Figure 4c and 4d, the extra-articular distribution of the IRDye 800CW-labeled ProGel-Dex was limited to low levels in the kidneys, consistent with renal clearance of the prodrug. Sequential live imaging of the MAA rats supported the continuous presence of the IRDye 800CW-labeled ProGel-Dex in the arthritic joints up to the time of necropsy at 21 days (Figure 4e). Similar studies were also performed in the AA and MIA models, revealing sustained ProGel-Dex presence in the arthritic joints (> 1 month; Supporting Information, Figure S2–S4), similar to that seen in the MAA rats. Semiquantitative analysis of the NIR signal from each organ or tissue (Figure 4f) on day 1 post-administration demonstrated 4-fold higher fluorescent signal intensity in the arthritic joints than in the kidney, 20 times higher signal intensity than in the liver, 40 times higher signal intensity than in the contralateral knee joint, and 102- to 103-fold higher signal intensity than in the spleen, lung and heart, consistent with a joint-specific distribution pattern. This pattern of tissue distribution was sustained until the experimental end point on day 21. These data provide evidence confirming the proposed mechanism by which the ProGel-Dex formulation provides sustained joint retention in animal models of inflammatory arthritis and OA with very limited redistribution to extra-articular organs and tissues.
Figure 4.
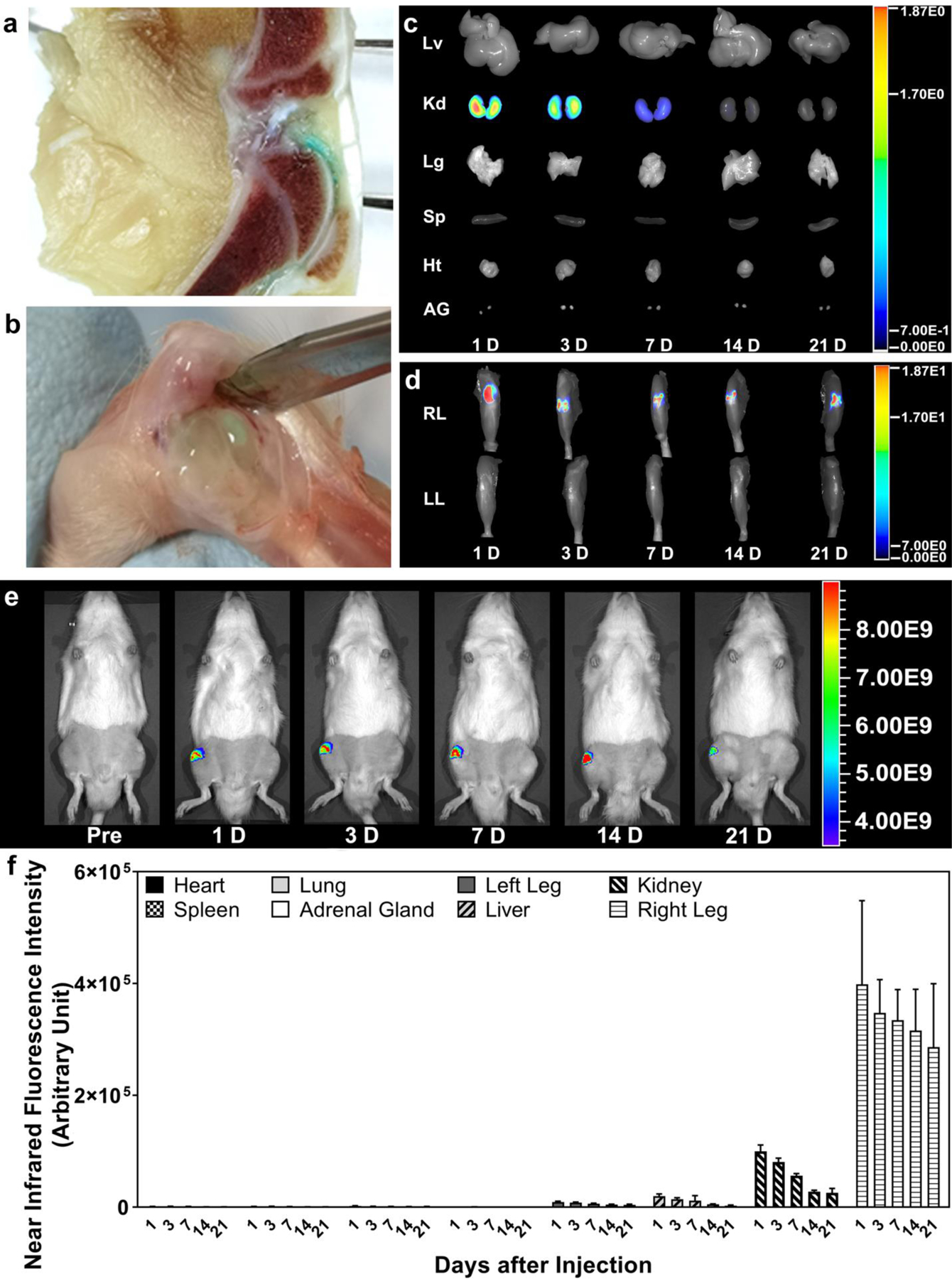
The sustained presence of the ProGel-Dex in arthritic joints. (a) Visualization of ProGel-Dex in the knee joints of MAA rats at 7 days post IA administration of the IRDye 800CW-labeled polymeric prodrug. The fixed and fully decalcified joint was cut sagittally. The blue/green-colored area indicates the presence of ProGel-Dex in the synovial cavity. (b) The presence of IRDye 800CW-labeled ProGel-Dex in the dissected ankle joint of AA rats at 28 days post IA injection. The blue/green color is attributed to the IRDye 800CW dye. The near-infrared optical images of different organs (c) and hind limbs (d) isolated at different time points post IA injection of ProGel-Dex into the knee joints of MAA rats (e), which were analyzed semi-quantitatively (f). The distribution of the ProGel-Dex in the non-target organ and tissues was much lower than that in the injected joint at all the time points examined; low level signal was detected in the kidney only at the early time points.
Compared to Dex, one of the main advantages of the design of ProGel-Dex is its capacity to selectively localize the release of free Dex in highly phagocytic cells, which are the major sources of proinflammatory mediators and small molecules targeting pain pathways. To test this hypothesis, MAA rat hind limbs from the NIR imaging study were collected, followed by fixation, decalcification, and paraffin embedding. Tissue sections (20 μm) were then prepared for immunofluorescence analysis using mouse anti-rat CD68, which identifies macrophage-like synoviocytes[32] and anti-rat P4HB, which identifies activated fibroblast-like synoviocytes.[33] Sections were further incubated with Alexa Fluor dye-labeled goat anti-mouse secondary antibodies and images analyzed using a confocal microscope. As shown in Figure 5, colocalization of the red and green colors confirmed the internalization of the Alexa 647-labeled ProGel-Dex by P4HB (fibroblasts)- and CD68 (monocytes/macrophages)-positive cells of the synovial lining. Interestingly, both cell phenotypes have been implicated in the joint inflammation and tissue destruction associated with rheumatoid arthritis.[34, 35] Further study is necessary to understand what mediated the synoviocytes’ phagocytosis of ProGel-Dex.
Figure 5.
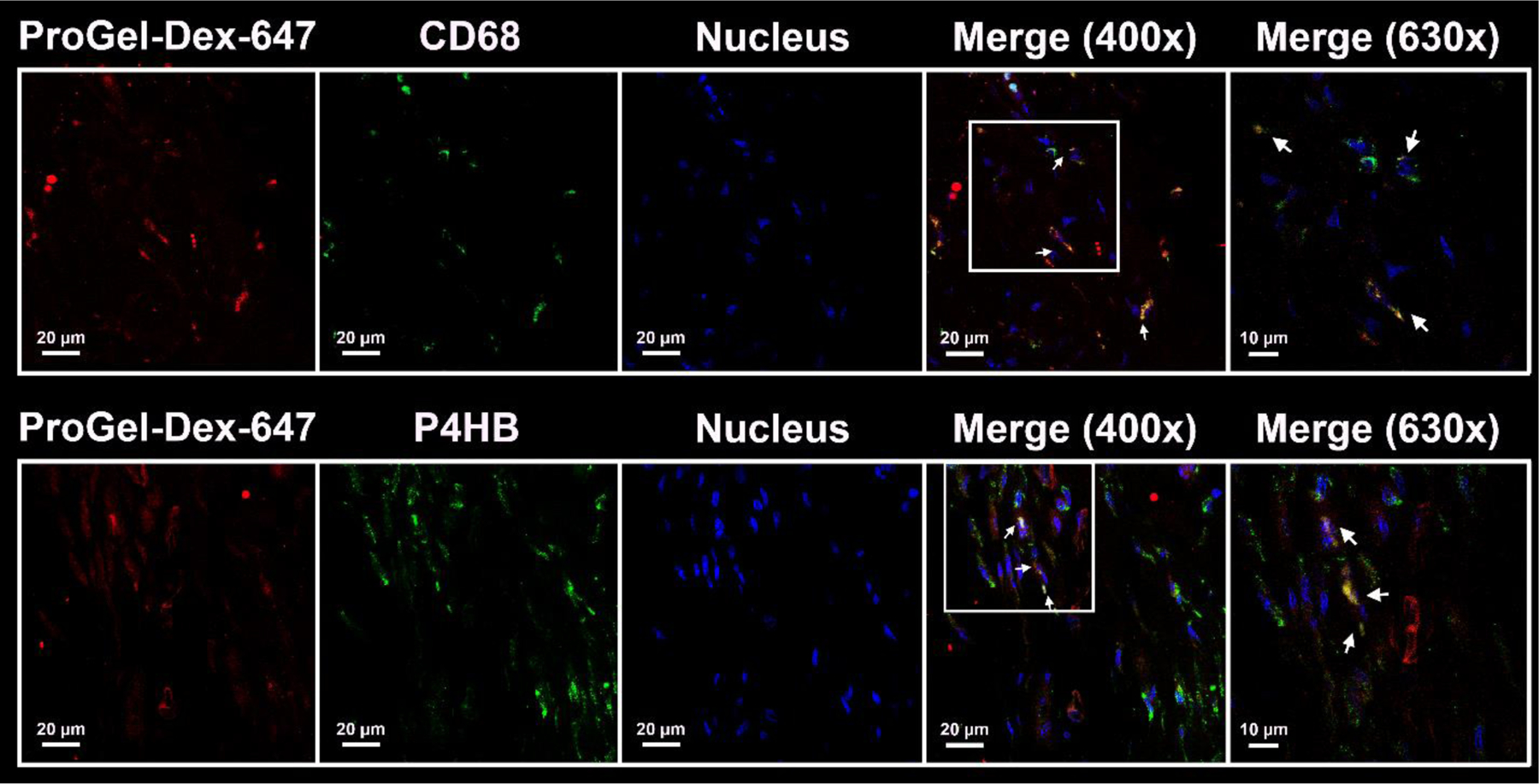
Representative confocal images of anti-CD68 and anti-P4HB antibody stained sections of decalcified MAA rat knee joints. Each panel is composed of five subpanels: Anti-CD68 or anti-P4HB signal (green), Alexa 647-labeled ProGel-Dex (red), DAPI signal (blue), and two merged images at 400× and 630× magnification. The colocalization of the red and green colors confirmed the internalization of the Alexa 647-labeled ProGel-Dex by P4HB-positive cells (fibroblasts) and CD68-positive cells (monocytes/macrophages) of the synovial lining. Arrows point to the colocalization of cell markers and Alexa 647-labeled ProGel-Dex.
Evaluation of the therapeutic efficacy and safety of ProGel-Dex
The unique biodistribution pattern of ProGel-Dex holds promise for providing sustained delivery, therapeutic efficacy, and excellent safety in the management of arthritis. Three rodent models of arthritis were used in the in vivo investigation to assess its efficacy and safety profile. For the AA rat model, subcutaneous injection of heat-killed Mycobacterium tuberculosis at the base of the tail produces a rapid immune response characterized by the development of polyarthritis with progressive swelling of distal joints, accompanied by articular cartilage loss and peri-articular bone erosion.[6, 27] As shown in Figure 6a, IA injection of ProGel-Dex into the inflamed right ankle joints resulted in significant and progressive reduction of joint swelling for 1 month, as assessed by caliper-measured joint diameter. Although a trend of improvement was also detected for the contralateral left ankle joints, it was not statistically significant, suggesting ProGel-Dex’ therapeutic effect is mostly localized at the site of the administration.
Figure 6.

Therapeutic effect of the ProGel-Dex in inflammatory arthritis models. (a) The change of ankle joint diameter in the AA rats. ProGel-Dex-IPSI represents the ankle joint treated with ProGel-Dex locally and ProGel-Dex-CL represents the contralateral ankle with no local injection. (b) The change of knee joint diameter in the MAA rats. (c) Knee joint pain in MAA rats was evaluated using static weight distribution method. (d) Mechanical hyperalgesia was assessed by measuring withdrawal threshold ratio after pressure application to MAA rats’ knee joints. T1 = pre-induction; T2 = pre-injection.
Placement in the incapacitance test apparatus for assessment of pain behaviors[36] in AA rats was technically challenging due to the polyarticular nature of the arthritis. Therefore, for pain behavior analysis, the efficacy of the ProGel-Dex was evaluated in the MAA rat model, which is associated with inflammation in a single knee joint. Similar to the observations in the AA rats, ProGel-Dex treatment produced progressive and significant amelioration of joint swelling (Figure 6b) over the 21 days of assessment. Dose-equivalent free Dex produced an acute improvement in joint swelling, but this effect was transient. Saline had no effect on joint swelling. Static weight distribution in the hind limbs of the MAA rats was assessed to evaluate the effect of ProGel-Dex on weight-bearing behavior using an incapacitance tester, as described previously.[36] Briefly, the MAA rats were placed on the incapacitance tester with their hind paws centered on the two force transducers, and the average body weight distribution in grams was measured. The weight bearing score (WBS) was expressed as a ratio of the weight placed through the ipsilateral limb versus the sum of the weights placed through both the contralateral and ipsilateral limbs, with a ratio of 50% resulting from equal weight distribution across both hind limbs. As shown in Figure 6c, ProGel-Dex normalized the WBS within 1-week post-IA administration. In contrast, treatment with dose equivalent Dex did not affect the WBS. To further evaluate the therapeutic efficacy of ProGel-Dex, we performed mechanical knee hyperalgesia assessment using a Pressure Application Measurement (PAM) device[37] to determine the effect of treatment on arthritis pain behavior. Free Dex treatment was associated with an initial desensitization to mechanical pressure applied to the arthritic joints, but this effect was transient. In contrast, ProGel-Dex treatment produced gradual improvement in pain pressure desensitization that was sustained during the entire course of the study (Figure 6d).
Metaphyseal bone (secondary spongiosa) quality was analyzed at 21 days post treatment in the MAA rats. Micro-CT analysis of sagittal sections of the distal femur illustrated preservation of bone structure in the ProGel-Dex-treated animals with thinning of metaphyseal bone in dose-equivalent Dex-treated animals and saline controls (Figure 7a). Quantitative assessment of bone mineral density (BMD), bone volume fraction (BV/TV), trabecular number (Tb.N), and trabecular thickness (Tb.Th) demonstrated preservation of bone structure in the ProGel-Dex treated animals with loss of metaphyseal bone structural elements in the dose-equivalent, Dex-treated animals and saline controls (Figure 7b). Further analysis demonstrated mineralization of the growth plate in the ProGel-Dex-treated animals with thinning and hypomineralization of the resting, proliferative, and hypertrophic zones, suggesting growth plate closure. This observation agrees with the previous reports of the sensitivity of the growth plate to GC exposure.[38] These joints were subsequently decalcified, sectioned, and stained with H&E and Safranin O for histological analysis by a pathologist blinded to the group arrangement. As shown in Figure 7d, treatment with ProGel-Dex led to reduction of synovial inflammatory cell infiltration, cartilage loss, and bone erosion. Semi-quantitative assessment (Figure 7c) confirmed these findings. The reduced inflammation in the ProGel-Dex group correlated with the observed preservation of metaphyseal bone, as demonstrated in the micro-CT analyses (Figure 7a, b).
Figure 7.
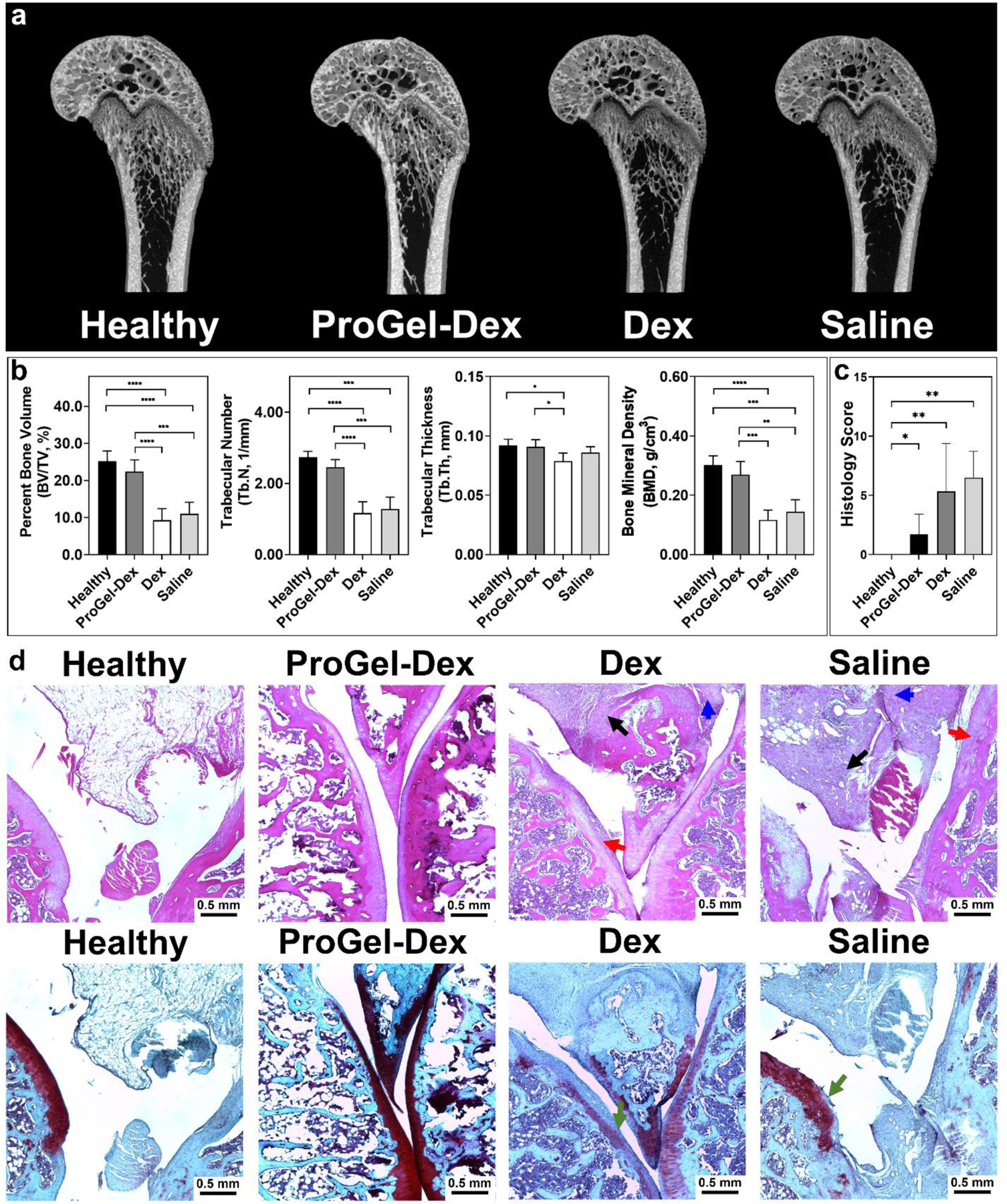
Bone quality and histology evaluation of the ProGel-Dex treated MAA rats. (a) Representative reconstructed knee joint images of MAA rats, including the growth plate and associated resting, proliferative, and hypertrophic zones. (b) Micro-CT quantitative analysis of femoral trabecular bone quality of the MAA rats. (c) Sum of histology scores of H&E stained sections from MAA rats evaluated and scored by a professional pathologist, who was blinded to the group arrangement. (d) Histology of knee joints photographed at 400× magnification. Upper panel, H&E stained; lower panel, Safranin O stained. Cellular infiltration in periarticular soft tissue, bone, and cartilage destruction are clearly evident in the Dex and Saline groups. Black arrow: synovial cell lining hyperplasia; red arrow: pannus formation; blue arrow: mononuclear cell infiltration; green arrow: cellular infiltration in cartilage. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.
Both AA and MAA rat models are generally recognized as models of inflammatory arthritis. The MIA model of arthritis recapitulates many of the pathological features of human OA, including the progressive development of pain and pain behavior. This model was used, therefore, to assess the effects of IA ProGel-Dex injection (at 1 day after induction of MIA) on preservation of articular cartilage and amelioration of OA-like pain behavior. At 28 days post treatment, animals were euthanized, and their arthritic joints isolated, decalcified, and processed for histological analysis. As shown in Figure 8a, the sections stained with H&E and Safranin O demonstrated that ProGel-Dex treatment provided significant protection from cartilage loss compared to dose-equivalent Dex and Saline controls. Semi-quantitative scoring of the sections by a professional pathologist, who was blinded to the group arrangement, confirmed the findings (Figure 8b). To determine the efficacy of the ProGel-Dex treatment on OA-like pain behavior, static weight distribution between the two hind limbs was analyzed daily after model induction. As shown in Figure 8c, ProGel-Dex treatment effectively mitigated joint pain within 2 days. The observed pain relief was sustained for 28 days until the end of the study. In contrast, dose equivalent Dex or Saline control did not modify joint pain-related behavior.
Figure 8.

The effect of the ProGel-Dex treatment in the MIA OA-like pain mouse model. (a) Histology of knee joint sections photographed at 400× magnification. Upper panel, H&E-stained sections; Lower panel, Safranin-O-stained sections. Black arrow: synovial cell lining hyperplasia; red arrow: pannus formation; blue arrow: mononuclear cell infiltration; green arrow: cellular infiltration in cartilage. (b) The sum of histology scores of H&E-stained sections from MIA mice evaluated and scored by a professional pathologist. (c) Static weight distribution of MIA mice. T1 = pre-induction; T2 = pre-injection. *, P ≤ 0.05; **, P ≤ 0.01.
For safety assessment of ProGel-Dex, full panels of hematology, liver and kidney function tests, and organ weight were evaluated in both MAA rats and MIA mice treated with ProGel-Dex and all were within the normal ranges[39–42] (Supporting Information, Figure S5–S10). We detected moderate adrenal gland weight reduction in MAA rats treated with ProGel-Dex (Supporting Information, Figure S7), but not in MIA mice (Supporting Information, Figure S10). All MIA mice (ProGel-Dex, Saline, and free Dex) showed slightly higher liver and kidney weights than the healthy control (Supporting Information, Figure S10), which may be attributed to the model establishment. In addition, micro-CT analyses were consistent with increased mineralization and sealing of the growth plate of the MAA rats, which would potentially limits the clinical application of ProGel-Dex in an adolescent patient cohort. Based on the findings in these rodent models, the toxicology is consistent with a favorable safety profile for the ProGel-Dex system.
IA-GC interventions are recommended by the American Academy of Orthopaedic Surgeons (AAOS), Osteoarthritis Research Society International (OARSI), and American College of Rheumatology (ACR) for the management of OA joint pain and inflammation and as an adjunctive therapy in selected patients with RA. The effectiveness of the previously available GC formulations for IA administration has been limited by their relatively short duration of clinical efficacy. In 2017, the United State Food and Drug Administration (US FDA) approved Zilretta™, a triamcinolone acetonide extended-release poly(lactide-co-glycolide) (PLGA) microparticular formulation from Flexion Therapeutics for the treatment of OA knee pain, and in 2019, TLC599, a liposomal formulation of dexamethasone sodium phosphate developed by Taiwan Liposome Company for the treatment of knee OA, entered a Phase III trial. These IA-GC formulations have demonstrated sustained clinical efficacy persisting for up to 12 weeks. Although the safety profiles, in general, have been favorable, injection site reactions have been reported with these formulations.[43] Different from Zilretta™ and TLC599, which are physical formulations of low molecular weight GC, ProGel-Dex is the only polymeric prodrug-based formulation under development. It provides sustained presence of Dex at sites of deposition and pathology (i.e., arthritic joint) via both physical (thermoresponsive hydrogel formation) and biochemical (prodrug activation) mechanisms.
The discovery of the thermoresponsive ProGel-Dex formulation presents multiple unique advantages. First, unlike conventional water-soluble polymeric prodrug formulations that have been developed for systemic delivery, the thermoresponsive ProGel-Dex formulation is designed to be administered locally at sites of pathology. After injection it rapidly transitions into a stable hydrogel, which through gradual dissolution slowly releases the prodrug. Second, ProGel-Dex molecules enter cells via endocytosis rather than passive diffusion. After IA injection and in-situ formation of a hydrogel, the ProGel-Dex molecules released from the gel surface are preferentially internalized by activated synoviocytes with high phagocytic activity (Figure 5); this differs from the non-specific diffusive cell entry of free Dex. Thus, the ProGel-Dex formulation offers a unique cellular pharmacology that targets the highly phagocytic cells present at sites of joint inflammation (i.e., the disease amplifier[44]), which by targeting the cell populations that play a key role in the pathogenesis of joint inflammation, not only potentiates the therapeutic efficacy of Dex, but also reduces the risk of potential GC toxicity that might adversely affect other joint cells, such as articular chondrocytes. Third, the thermoresponsive properties of ProGel-Dex is insensitive to its Mw. Importantly, IA administration of ProGel-Dex provided potent and sustained relief of local inflammation and pain for more than a month, even at a Mw of 6.8 kDa. For conventional polymeric prodrugs, however, a much higher Mw (> 20 kDa) is necessary to attain a sufficient serum half-life that will allow passive targeting through vascular extravasation of the prodrug to the site of oncologic or inflammatory pathology via the so called “EPR” or “ELVIS mechanisms”.[14, 45, 46] As a consequence, sequestration of the polymeric prodrugs by the mononuclear phagocyte (MPS) system, which includes cells in the liver and spleen, is frequently observed. In contrast, the low Mw ProGel-Dex molecules released from the gel, if not internalized by the highly phagocytic synoviocytes, enter the circulation via lymphatic drainage and are rapidly removed by renal clearance. This restricts the systemic redistribution of ProGel-Dex to non-targeted organs (Figure 4), further mitigating the risk of potential off-target, GC-associated toxicities.
After IA-GC administration, the sustained resolution of joint inflammation and pain in the AA, MAA and MIA models (Figure 6–8) provides strong evidence of the design advantages of the ProGel-Dex formulation. In the AA rats, the marked difference in joint inflammation in the treated and untreated legs demonstrates that the therapeutic effect of ProGel-Dex is localized to the injected joint. Furthermore, the comprehensive safety assessment demonstrates that all the hematological parameters and liver and kidney panels of the animals treated with ProGel-Dex were within normal range with only moderate adrenal gland weight decrease observed in the MAA rats, but not in the MIA mice (Supporting Information, Figure S5–S10). This confirms the excellent safety profile of the ProGel-Dex. It is important to note that the HPMA copolymer carrier system has been extensively used in polymeric prodrug design, with several having been evaluated clinically.[47] No polymer-related toxicity has been reported, even at maximum tolerated doses,[48] and the HPMA homopolymer is regarded as a neutral, water-soluble, biocompatible synthetic polymer carrier with low immunogenicity and no significant effect on the complement system.[49–52] These prior reports regarding the safety of the polymeric carrier system further boosts the clinical translation potential of ProGel-Dex.
From the clinical perspective, the administration of the ProGel-Dex formulation fits well into the current patterns of practice. Given the urgent demand for opioid-sparing novel analgesics, the data presented in this study strongly support the translational potential of ProGel-Dex for more effective and safer clinical management of arthritis pain. In addition to arthritis, the ProGel-Dex formulation also holds promise as a local therapy for a broad spectrum of joint and musculoskeletal conditions, including chronic discogenic and facet arthropathy lower back pain, tendonitis, and bursitis, for which local GC injections are commonly prescribed in clinical practice.
From the polymeric prodrug design prospective, we speculate that the potential drug payload for the ProGel system may not be limited to Dex or other GC molecules. Given our initial findings that suggests that the thermoresponsive behavior of ProGel-Dex may be driven by the hydrophobic interactions between the conjugated Dex molecules, it is reasonable to believe that other neutral hydrophobic drugs may also be incorporated into the ProGel if they have useful chemical handles for the polymer conjugation. For drug molecules with ionizable components, the situation will be complex. The initial thermoresponsive behavior of the polymeric prodrug and its subsequent release and activation will depend heavily upon the pKa of the drug molecules and environmental pH of the prodrug deposition site, which is often regulated by the pathophysiology of the diseases.
Conclusion
In summary, we report the discovery of a thermoresponsive polymeric prodrug design (ProGel-Dex) based on a N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer-dexamethasone (Dex) conjugate. The unique thermoresponsive phase transition property of ProGel-Dex allows it to be deposited and retained in the synovial cavity for a protracted period of > 1 month. The collective results from three rodent arthritis models demonstrated its capacity to provide sustained therapeutic efficacy in ameliorating joint pain and inflammation, while exhibiting a highly favorable safety profile. Our results strongly supports the utility and clinical translational potential of ProGel-Dex as a novel therapy for management of arthritis pain and inflammation. Additional in-depth studies are needed to fully understand the mechanism(s) underlying the thermoresponsive behavior of ProGel-Dex.
Materials and Methods
Synthesis of ProGel-Dex
ProGel-Dex and fluorescent dye labeled ProGel-Dex were synthesized according to the same procedure of P-Dex as reported previously,[6–13] with significantly higher Dex-containing monomer feed-in ratio (See Supplementary Materials, Scheme S1). Briefly, HPMA (400 mg, 2.79 mmol), MA-Gly-Gly-NHN=Dex (Dex-containing monomer, 256.7 mg, 0.44 mmol)(10), were dissolved in methanol (5 mL). The initiator 2,2”-azobisisobutyronitrile (AIBN, 38.8 mg, 0.24 mmol) and RAFT agent S,S’-bis(α,α’-dimethyl-α”-acetic acid)-trithiocarbonate (CTA, 37.1 mg, 0.13 mmol) were then added. The solution was purged with argon and polymerized at 55°C for 2 days. The resulting polymer was first purified on a LH-20 column to remove the unreacted low molecular weight compounds, and then dialyzed. The polymer solution was lyophilized to obtain the final ProGel-Dex.
Characterization of polymeric prodrugs
Size exclusion chromatography (SEC, Superdex 75 10/300 GL, mobile phase 30% acetonitrile in 1×PBS) with an ÄKTA™ pure fast protein liquid chromatography (FPLC) system equipped with Multi Angle Light Scattering (MALS, Wyatt) and Optilab T-rEX refractive index detector (Wyatt) were used to determine the molecular weights (Mw, Mn) and dispersity (Ð) of the copolymers. To quantify Dex content in ProGel-Dex, the copolymers (2 mg/mL) were hydrolyzed in 0.2 N HCl in 50% methanol overnight. The resulting solution was neutralized using 0.2 N NaOH in 50% methanol and analyzed on an Agilent 1260 Infinity II HPLC system with a reverse phase analytical C18 column (InfinityLab Poroshell 120 EC-C18, 4.6×250 mm, 2.7 μm, mobile phase, acetonitrile/water 4:6; detection, UV 240 nm; flow rate, 1 mL/min; injection volume, 10 μL) based on a Dex standard curve. The analyses were performed in triplicate. The mean value and standard deviation were obtained using Microsoft Excel.
Construction of the sol-gel-syn phase transition diagram of the thermoresponsive ProGel-Dex
The phase transition temperature of the thermoresponsive ProGel-Dex with different structural parameters (Dex content and ProGel-Dex concentration) were assessed using a tube-tilting method.[20] Eppendorf tubes (2 mL) containing ProGel-Dex (0.4 mL) were placed in a dry block heater with precise temperature control (Multi-Blok Digital Dry Incubator 2002, Thermo Scientific, USA). A thermometer with an accuracy of 0.1°C was immersed in the 2 mL Eppendorf tubes and the phase transition temperature of each ProGel-Dex sample was recorded when significant alteration of the rheologic behavior of the ProGel-Dex (e.g., free flow to gelation) was observed.
Rheological analysis
The rheological behavior of ProGel-Dex was quantitatively assessed using a rheometer (DHR-2 with a 20 mm parallel plate geometry, TA Instrument, USA). The sample was uniformly loaded between the Peltier plate of the rheometer using a syringe and the sample thickness was set at 500 μm. The linear viscoelastic range (LVR) was pre-determined at 30°C by using the oscillation amplitude function, and the following condition: strain of 0.5%, and angular frequency of 10 rad/s. The rheological parameters used in all tests were within LVR. The gelation temperature was determined by measuring the storage modulus (G’) and loss modulus (G”) of samples in the oscillation temperature ramp (4–50°C) at a fixed frequency of 10 rad/s and 0.5% strain with a heating rate of 0.5°C /min. The gelation temperature was defined as the temperature at which G’ and G” become equal. The viscosity of the gel phase was also investigated at constant temperature by flow shear rate sweep (4, 10, 20 and 30°C, shear rate from 0.0001 to 1000 s−1). The shear yield stress was defined as the stress at which the value of G’ equals the value of G”[53] during the oscillation strain sweep (0.01%-100%) performed at 30°C under 10 rad/s.
In vitro release
A series of ProGel-Dex formulations with Dex content ranging from 18 to 24 w/w% were prepared, characterized, and included in this study. The polymers were dissolved into saline at 15, 20, 25 w/v% concentration respectively at 4 °C, and then deposited into pre-cooled Eppendorf tubes (1.5 mL) followed by gentle spinning. The polymer solutions at the bottom of the tubes were allowed to form a hydrogel by placing the tubes in a preheated dry block heater at 30 °C with precise temperature control (Multi-Blok Digital Dry Incubator 2002, Thermo Scientific, USA). The weights of the hydrogels were measured using an analytical balance. The acidic pH releasing buffer (acetated buffer, pH=5.0) was chosen to simulate the acidosis associated with arthritic joint inflammation and the acidity within the lysosomal compartment where the polymeric prodrug is sequestered. The acetated buffer (1 mL, pre-heated to 30 °C) with sodium azide (0.02 w/v%) as preservative were added to the top of the tubes to serve as a releasing buffer. The addition of Pluronic F127 (1 w/v %) to the releasing buffer ensured the sink condition. During the release study, the hydrogels remained at the bottom of the tube without detachment. Triplicates were prepared under each concentration for each polymer, and agitated in a shaking incubator (60 r/min) at 30 °C. The supernatants (200 μL) were withdrawn at predesigned time points for free Dex extraction in 1200 μL methyl tert-butyl ether (MTBE). MTBE solutions (1000 μL) were collected and evaporated using a vacuum evaporator. The residues were reconstituted into 100 μL H2O/MeOH solution (H2O/MeOH=1:1) resulting in samples for HPLC analyses under the same HPLC conditions used in the Dex content analysis. The MTBE extraction recovery efficiency were analyzed with the Dex concentration ranging 1–100 μg/mL.
Molecular Dynamic Simulation
The polymer was built in MOE software (Molecular Operating Environment, MOE2019.0101 version) by linking twenty-five HPMA monomers with five MA-Gly-Gly-Dex monomers evenly distributed along the chain using a five HPMA monomers interval, according to the characterization of ProGel-Dex used in the in vivo experiments (Mw = 6.8 kDa, Dex content = 24 w/w%). The first MA-Gly-Gly-Dex monomer is situated at a chain terminus of the polymer. To simulate the ProGel-Dex thermoresponsive properties, six ProGel-Dex molecules were placed in parallel in a droplet water model (38,250 water molecules) with 15 Å radius from the edge of the polymer. The solvated system was minimized using AMBER force field (AMBER14:EHT) in MOE with AMBER partial charges being used. The model was simulated with 2 fs time-step, with 100 ps of equilibration, followed by 5000 ps of production run, and carried out at 277°K (4°C) and 303°K (30°C), respectively. At the end of the simulation, the molecular geometry of ProGel-Dex molecules was investigated. The ball-and-sticks models were produced in MOE for presentation.
Animal experiments
Polyarticular adjuvant-induced arthritis (AA) rat model
Male Lewis rats (175–200g) were obtained from Charles River Laboratories (Wilmington, MA) for the adjuvant-induced arthritis models. After one week of acclimation, the animals were induced as previously described.[6, 27, 36] Briefly, rats received one adjuvant mixture containing heat-killed Mycobacterium tuberculosis (H37RA, 1mg) and N,N-dioctadecyl-N′,N′-bis(2-hydroxyethyl)-1,3-propanediamine (LA, 5 mg) in paraffin oil (100 μL) via subcutaneous injection at the base of the rat tail. The inflamed ankle joints were observed from day 11 post-immunization. The established AA rats reached an inflammatory plateau on day 14 after which they were randomly divided into 3 groups: 1. Saline control group (208.3 μL/kg); 2. ProGel-Dex treated group (20 w/v% in saline, dose equivalent of Dex = 10 mg/kg, 208.3 μL/kg); and 3. Free Dex treated group (dexamethasone sodium phosphate 63.4 mg/mL in saline, Dex equivalent dose = 10 mg/kg, 208.3 μL/kg). All treatments were administered via single IA injection into the right ankle on day 14 post-induction. An additional group of healthy rats was used as a negative control. Joint inflammation and body weight change were monitored daily from day 11.
Monoarticular adjuvant induced arthritis (MAA) rat model
Male Lewis rats (175–200 g) were purchased from Charles River Laboratories (Wilmington, MA). Monoarticular adjuvant induced arthritis was established as described previously.[36] Briefly, the rats received two adjuvant immunizations subcutaneously at different sites on their back with a week interval. The adjuvant mixture contained methylated bovine serum albumin (mBSA, 2mg/mL, in water, 0.25mL) and heat-killed Mycobacterium tuberculosis (H37RA, 2 mg/mL, in paraffin oil, 0.25 mL). Two weeks after the second immunization, rats received a booster intraarticular injection of mBSA (10 g/L, in water, 50 μL) in the right knee joint. The treatment was initiated on the following day of the booster injection when the onset of the arthritis in the right knee joints was confirmed by the presence of joint swelling. The rats with established arthritis were randomly assigned into the following treatment groups on day 0: Saline control group (208.3 μL/kg), ProGel-Dex treated group (20 w/v% in saline, dose equivalent of Dex = 10 mg/kg, 208.3 μL/kg), and Free Dex treated group (dexamethasone sodium phosphate 63.4 mg/mL in saline, Dex equivalent dose: 10 mg/kg, 208.3 μL/kg). Age-matched healthy control rats were included. The width of the rat knee joint was measured using a digital Vernier caliper. The incapacitance test and pressure application measurement (PAM) were performed to assess the pain-related behaviors. Blood samples were collected weekly through the tail artery for complete blood count (CBC), and liver and kidney function analyses. Serum samples were separated from the whole blood samples and stored at −20°C for further evaluation. The rats were euthanized on day 21. The heart, kidney, liver, spleen, lung, and adrenal gland were collected and weighted. The hind legs were also collected and fixed in the buffered formalin for further micro-CT and histological analyses.
Monoiodoacetate (MIA)-induced osteoarthritis model
C57BL/6 mice (9 weeks old) from the Jackson Laboratory were used for the MIA model. The MIA model was established as reported previously.[54] Briefly, under anesthesia, mice received monoiodoacetate (MIA, 1 mg/10 μL per mice) via intraarticular injection into the right knee joint. For better dispersion of the MIA, knee joints were manually extended and flexed for 30 s. The MIA model was established on the following day post-MIA injection. The mice were then randomly assigned into the following treatment groups: 1. Saline treated group (416.6 μL/kg); 2. ProGel-Dex treated group (20 w/v% in saline, dose equivalent of Dex = 20 mg/kg, 416.6 μL/kg); and 3. Free Dex treated group (dexamethasone sodium phosphate 63.4 mg/mL in saline, Dex equivalent dose: 20 mg/kg, 416.6 μL/kg). Additional mice were used as healthy controls. The knee joint widths were measured using a digital Vernier caliper. The incapacitance test was performed to assess the pain-related behaviors. After euthanasia, blood samples were collected via cardiac puncture for CBC, and liver and kidney function analyses.
Near infrared optical imaging of rats after IA administration of IRDye 800CW-labeled ProGel-Dex
The IRDye 800CW-labeled ProGel-Dex (ProGel-Dex-IRDye) and ProGel-Dex were mixed and administered to MAA rats (n = 5) (IRDye dose = 4.5 × 10−7 mol IRDye/kg, Dex equivalent dose = 10 mg/kg). The animals were then imaged with a Xenogen IVIS Spectrum in vivo system under anesthesia at predesigned time points with the following conditions: excitation: 778 nm (filter: 745 nm); emission: 794 nm (filter: 800 nm); exposure times: 2 s; field of view: 24.5 cm; binning factor: 8; f number: 2. The captured images were analyzed using the Living Image 4.5 software (PerkinElmer Inc.). At the predesigned time points of euthanasia, the animals were perfused with saline. Vital organs and the hind limbs were collected and imaged using a Pearl® In Vivo Imaging System (LI-COR Biosciences, Lincoln, NE, USA) with the following conditions: dual light channels (800 nm and white); resolution, 85 μm.
Pain behavior assessment
- Static weight distribution
To test the effect of IA ProGel-Dex treatment on amelioration of arthritic joint pain, the static weight distribution between the hind limbs of rats and mice was measured using an incapacitance meter (Columbus Instruments, Columbus, OH), which consists of two force transducers capable of measuring the body weight that the animal places on each hind limb. Animals were placed on the apparatus with their hind paws centered on the two force transducers, and the average body weight distribution in grams was recorded. The weight-bearing score was expressed as a ratio of the weight placed through the ipsilateral limb versus the sum of the weights placed through both the contralateral and ipsilateral limbs, with a ratio of 50% resulting from equal weight distribution across both hind limbs. Weight distribution was measured before model establishment, before drug administration, and daily after the initiation of the treatment.
- Mechanical knee hyperalgesia
Pressure application measurement (PAM) was utilized to test mechanical hyperalgesia of MAA rats. The PAM apparatus consisted of a force transducer mounted on a unit fitted to the operator’s thumb. The thumb unit was connected to a recording base unit containing the control panel and digital readout display. The apparatus had a force transducer with a range of 0–1,500 g and the diameter of the circular contact was 5 mm. The animals were gently, but securely held by the operator with the thumb unit pressed against the medial side of the ipsilateral knee, while the forefinger gently held the lateral side of the knee. A gradually increasing squeeze force was applied across the joint at a rate of approximately 300 grams per second with a maximum test duration of 5 s. After calibration of the instrumentation, the force in grams applied was displayed on the digital screen and recorded. The test endpoint was determined when the animal withdrew its limb or showed behavioral signs of discomfort or distress, such as freezing of whisker movement or wriggling. The peak force applied immediately prior to limb withdrawal was recorded, and this value was designated as the limb withdrawal threshold (LWT), which was used to assess mechanical knee hyperalgesia levels in each group.
Micro-CT analysis
To evaluate the therapeutic effects and potential toxicity of ProGel-Dex, hind limbs of experimental rats and mice were isolated and fixed at completion of the study for micro-CT (Skyscan 1172, Bruker, Belgium) analysis. For mouse bones, the scanning parameters were set to 55 kV, 181 μA, 8.88 μm, 0.5 mm aluminum filter, 0.4 rotation step, 4 frames averaging and 180° scans. For rat bones, the scanning parameters were set to 70 kV, 141 μA, 13.47 μm, 0.5 mm aluminum filter, 0.4 rotation step, 6 frames averaging and 180° scans. Two standard hydroxyapatite rods were scanned at each condition, serving as standards for bone mineral density (BMD) calculation. The datasets were reconstructed using NRecon software (Skyscan) and analyzed using CTAn software. All datasets were realigned and 3D-registered before analysis. Distal femur metaphyseal bone was analyzed to evaluate the effects of the treatments. Bone morphometric parameters, including bone mineral density (BMD), trabecular number (Tb.N), bone volume fraction (BV/TV), etc., were analyzed.
Histological assessment.
Upon completion of decalcification of the joints, the tissues were paraffin-embedded and sectioned (8 μm) for staining with H&E and Safranin O. The stained sections were histologically graded by a pathologist, who was blinded to treatment group arrangement. The histopathologic features were graded as previously reported[6, 27]: synovial cell lining hyperplasia (0 to 2); pannus formation (0 to 3); mononuclear cell infiltration (0 to 3); polymorphonuclear leukocyte infiltration in periarticular soft tissue (0 to 3); cellular infiltration and bone erosion at the distal femur and proximal tibia (0 to 3); and cellular infiltration of cartilage (0 to 2). Scores for all histopathologic features were summed for each animal.
Immunohistochemical (IHC) analysis
Alexa Fluor 647-labeled ProGel-Dex (ProGel-Dex-647) was mixed and administered IA to MAA rats (n = 5). Rat hind limbs were collected and decalcified using 14% EDTA solution (pH = 7.4) after perfusion. The knee joints were paraffin embedded, and sectioned (20 μm). The slides were stained for immunohistochemical (IHC) analysis using the following antibodies, mouse anti-rat CD68 (Bio-Rad, MCA341R, dilution 1:100) and rabbit anti-rat P4HB (Abcam, ab85564, dilution 1:50), respectively, overnight at 4°C after antigen retrieval using sodium citrate buffer and blocked using 10% normal goat serum. Slides incubated with mouse anti-rat CD68 were further incubated with Alexa Fluor 488-labeled goat anti-mouse secondary antibody (Thermo Fisher scientific, A11001, dilution 1:1000) and slides incubated with rabbit anti-rat P4HB were incubated with Alexa Fluor 546-labeled goat anti-rabbit secondary antibody (Thermo Fisher scientific, A11008, dilution 1:1000) for another 1 h at 21°C in the dark. The stained slides were imaged using a ZEISS LSM 800 confocal microscope after being mounted in ProLong Gold antifade with DAPI (Thermo Fisher scientific, P36931, Waltham, MA) for the staining of nuclei.
Statistical analysis
One-way or two-way analysis of variance (ANOVA), followed by Tukey’s post hoc test to account for multiple comparisons, was used in data analysis using GraphPad Prism 8. P-values ≤ 0.05 were considered as statistically significant.
Supplementary Material
Highlights.
HPMA copolymer-based dexamethasone prodrug demonstrates thermoresponsive properties
The thermoresponsive prodrug (ProGel-Dex) can be intraarticularly administered
ProGel-Dex forms hydrogel and remains in the joint for more than one month
ProGel-Dex provides sustained pain relief in three models of arthritis
ProGel-Dex does not have any measurable glucocorticoid side effects
Acknowledgement
This study was supported in part by National Institutes of Health (R01AI119090, R44DA051278). NRC received a scholarship (CSC ID: 201707060010) from China Scholarship Council. The content is solely the responsibility of the coauthors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT author statement
Gang Zhao: Conceptualization, Methodology, Validation, Formal Analysis, Investigation, Writing - Original Draft. Rongguo Ren: Investigation, Validation. Xin Wei: Methodology, Validation, Formal Analysis, Investigation, Writing - Original Draft, Visualization. Zhenshan Jia: Methodology, Investigation. Ningrong Chen: Investigation. Yuanyuan Sun: Investigation. Zhifeng Zhao: Investigation. Subodh M. Lele: Investigation. Haizhen A. Zhong: Investigation. Mary B. Goldring: Writing - Review & Editing. Steven R. Goldring: Conceptualization, Writing - Review & Editing. Dong Wang: Conceptualization, Methodology, Writing - Review & Editing, Supervision, Project administration, Funding acquisition.
Competing Interest Statement
DW, SRG, GZ, RGR, ZSJ, and XW are co-inventors of a PCT patent application covering ProGel technology. DW, SRG and GZ claim equity positions in Ensign Pharmaceutical, Inc, a start-up company that has licensed ProGel technology for further preclinical and translational development. The rest of the coauthors declare no competing financial interest.
Data availability statement
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
References
- [1].Wedmore IS, Butler FK Jr., Battlefield Analgesia in Tactical Combat Casualty Care, Wilderness & environmental medicine, 28 (2017) S109–s116. [DOI] [PubMed] [Google Scholar]
- [2].Cisternas MG, Murphy L, Sacks JJ, Solomon DH, Pasta DJ, Helmick CG, Alternative methods for defining osteoarthritis and the impact on estimating prevalence in a US population-based survey, Arthritis care & research, 68 (2016) 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sokolove J, Lepus CM, Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations, Therapeutic advances in musculoskeletal disease, 5 (2013) 77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott JH, Bhandari M, Blanco FJ, Espinosa R, Haugen IK, Lin J, Mandl LA, Moilanen E, Nakamura N, Snyder-Mackler L, Trojian T, Underwood M, McAlindon TE, OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis, Osteoarthritis and Cartilage, 27 (2019) 1578–1589. [DOI] [PubMed] [Google Scholar]
- [5].Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD, Merkel PA, Pillemer SR, Reveille JD, Stone JH, Workgroup NAD, Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I, Arthritis & Rheumatism, 58 (2008) 15–25. [DOI] [PubMed] [Google Scholar]
- [6].Liu X-M, Quan L-D, Tian J, Alnouti Y, Fu K, Thiele GM, Wang D, Synthesis and evaluation of a well-defined HPMA copolymer-dexamethasone conjugate for effective treatment of rheumatoid arthritis, Pharmaceutical research, 25 (2008) 2910–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Quan L.-d., Yuan F, Liu X.-m., Huang J.-g., Alnouti Y, Wang D, Pharmacokinetic and Biodistribution Studies of N-(2-Hydroxypropyl)methacrylamide Copolymer-Dexamethasone Conjugates in Adjuvant-Induced Arthritis Rat Model, Molecular Pharmaceutics, 7 (2010) 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ren K, Purdue PE, Burton L, Quan L.-d., Fehringer EV, Thiele GM, Goldring SR, Wang D, Early detection and treatment of wear particle-induced inflammation and bone loss in a mouse calvarial osteolysis model using HPMA copolymer conjugates, Molecular pharmaceutics, 8 (2011) 1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ren K, Yuan H, Zhang Y, Wei X, Wang D, Macromolecular glucocorticoid prodrug improves the treatment of dextran sulfate sodium-induced mice ulcerative colitis, Clinical Immunology, 160 (2015) 71–81. [DOI] [PubMed] [Google Scholar]
- [10].Jia Z, Zhao G, Wei X, Kong D, Sun Y, Zhou Y, Lele SM, Fehringer EV, Garvin KL, Goldring SR, Wang D, Structural optimization of HPMA copolymer-based dexamethasone prodrug for improved treatment of inflammatory arthritis, Journal of Controlled Release, 324 (2020) 560–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wei X, Li F, Zhao G, Chhonker YS, Averill C, Galdamez J, Purdue PE, Wang X, Fehringer EV, Garvin KL, Goldring SR, Alnouti Y, Wang D, Pharmacokinetic and Biodistribution Studies of HPMA Copolymer Conjugates in an Aseptic Implant Loosening Mouse Model, Molecular pharmaceutics, 14 (2017) 1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yuan F, Tabor DE, Nelson RK, Yuan H, Zhang Y, Nuxoll J, Bynoté KK, Lele SM, Wang D, Gould KA, A Dexamethasone Prodrug Reduces the Renal Macrophage Response and Provides Enhanced Resolution of Established Murine Lupus Nephritis, PLOS ONE, 8 (2013) e81483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yuan F, Nelson RK, Tabor DE, Zhang Y, Akhter MP, Gould KA, Wang D, Dexamethasone prodrug treatment prevents nephritis in lupus-prone (NZB × NZW)F1 mice without causing systemic side effects, Arthritis & Rheumatism, 64 (2012) 4029–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yuan F, Quan L.-d., Cui L, Goldring SR, Wang D, Development of macromolecular prodrug for rheumatoid arthritis, Advanced drug delivery reviews, 64 (2012) 1205–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhao G, Wei X, Wang D, Macromolecular Therapeutics: Development and Delivery Engineering, Springer, 2017. [Google Scholar]
- [16].Zhang R, Luo K, Yang J, Sima M, Sun Y, Janát-Amsbury MM, Kopeček J, Synthesis and evaluation of a backbone biodegradable multiblock HPMA copolymer nanocarrier for the systemic delivery of paclitaxel, Journal of controlled release : official journal of the Controlled Release Society, 166 (2013) 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pan H, Sima M, Yang J, Kopeček J, Synthesis of long-circulating, backbone degradable HPMA copolymer-doxorubicin conjugates and evaluation of molecular-weight-dependent antitumor efficacy, Macromolecular bioscience, 13 (2013) 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Duangjai A, Luo K, Zhou Y, Yang J, Kopeček J, Combination cytotoxicity of backbone degradable HPMA copolymer gemcitabine and platinum conjugates toward human ovarian carcinoma cells, European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e. V, 87 (2014) 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim YH, Baek SS, Choi KS, Lee SG, Park SB, The Effect of Cold Air Application on Intra-Articular and Skin Temperatures in the Knee, Yonsei Med J, 43 (2002) 621–626. [DOI] [PubMed] [Google Scholar]
- [20].Kashyap N, Viswanad B, Sharma G, Bhardwaj V, Ramarao P, Ravi Kumar MNV, Design and evaluation of biodegradable, biosensitive in situ gelling system for pulsatile delivery of insulin, Biomaterials, 28 (2007) 2051–2060. [DOI] [PubMed] [Google Scholar]
- [21].Kopeček J, Soluble polymers in medicine, Williams DF (Ed.), Systemic Aspects of Biocompatibility, vol. II, CRC Press, Boca Raton, Florida, (1981) 159–180. [Google Scholar]
- [22].Qiu Y, Park K, Environment-sensitive hydrogels for drug delivery, Adv Drug Deliv Rev, 53 (2001) 321–339. [DOI] [PubMed] [Google Scholar]
- [23].Xu X, Liu Y, Fu W, Yao M, Ding Z, Xuan J, Li D, Wang S, Xia Y, Cao M, Poly(N-isopropylacrylamide)-Based Thermoresponsive Composite Hydrogels for Biomedical Applications, Polymers, 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bordat A, Boissenot T, Nicolas J, Tsapis N, Thermoresponsive polymer nanocarriers for biomedical applications, Advanced Drug Delivery Reviews, 138 (2019) 167–192. [DOI] [PubMed] [Google Scholar]
- [25].Doi M, Gel Dynamics, Journal of the Physical Society of Japan, 78 (2009) 052001. [Google Scholar]
- [26].Kulthe SS, Choudhari YM, Inamdar NN, Mourya V, Polymeric micelles: authoritative aspects for drug delivery, Designed Monomers and Polymers, 15 (2012) 465–521. [Google Scholar]
- [27].Wei X, Wu J, Zhao G, Galdamez J, Lele SM, Wang X, Liu Y, Soni DM, Purdue PE, Mikuls TR, Development of a Janus kinase inhibitor prodrug for the treatment of rheumatoid arthritis, Molecular pharmaceutics, 15 (2018) 3456–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Halperin A, Kröger M, Winnik FM, Poly (N-isopropylacrylamide) phase diagrams: fifty years of research, Angewandte Chemie International Edition, 54 (2015) 15342–15367. [DOI] [PubMed] [Google Scholar]
- [29].Wang X, Wu C, Light-Scattering Study of Coil-to-Globule Transition of a Poly(N-isopropylacrylamide) Chain in Deuterated Water, Macromolecules, 32 (1999) 4299–4301. [Google Scholar]
- [30].Bischofberger I, Trappe V, New aspects in the phase behaviour of poly-N-isopropyl acrylamide: systematic temperature dependent shrinking of PNiPAM assemblies well beyond the LCST, Sci Rep, 5 (2015) 15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hoffman AS, Stimuli-responsive polymers: biomedical applications and challenges for clinical translation, Adv Drug Deliv Rev, 65 (2013) 10–16. [DOI] [PubMed] [Google Scholar]
- [32].Holness C, Simmons D, Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins, Blood, 81 (1993) 1607–1613. [PubMed] [Google Scholar]
- [33].Zhang Z, Yu B, Gu Y, Zhou S, Qian T, Wang Y, Ding G, Ding F, Gu X, Fibroblast-derived tenascin-C promotes Schwann cell migration through β1-integrin dependent pathway during peripheral nerve regeneration, Glia, 64 (2016) 374–385. [DOI] [PubMed] [Google Scholar]
- [34].Firestein GS, Budd RC, Harris EDJ, McInnes IB, Ruddy S, Sergent JS, Kelley’s Textbook of Rheumatology 8th Edition, Elsevier Health Sciences, 2008. [Google Scholar]
- [35].Firestein GS, Budd RC, Harris EDJ, McInnes IB, Ruddy S, Sergent JS, Kelley’s Textbook of Rheumatology 8th Edition, Elsevier Health Sciences, 2008. [Google Scholar]
- [36].Weber L, Wang X, Ren R, Wei X, Zhao G, Yang J, Yuan H, Pang H, Wang H, Wang D, The Development of a Macromolecular Analgesic for Arthritic Pain, Molecular Pharmaceutics, 16 (2019) 1234–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Amorim D, David-Pereira A, Pertovaara A, Almeida A, Pinto-Ribeiro F, Amitriptyline reverses hyperalgesia and improves associated mood-like disorders in a model of experimental monoarthritis, Behavioural Brain Research, 265 (2014) 12–21. [DOI] [PubMed] [Google Scholar]
- [38].Lui JC, Baron J, Effects of glucocorticoids on the growth plate, Karger Publishers, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Stahl FR, Jung R, Jazbutyte V, Ostermann E, Tödter S, Brixel R, Kemmer A, Halle S, Rose-John S, Messerle M, Arck PC, Brune W, Renné T, Laboratory diagnostics of murine blood for detection of mouse cytomegalovirus (MCMV)-induced hepatitis, Scientific Reports, 8 (2018) 14823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Korstanje R SK, Churchill G, Yang H. Variation over time for C57BL/6J controls, multi-system survey of mouse physiology. MPD:CGDpheno2. Mouse Phenome Database web resource (RRID:SCR_003212), The Jackson Laboratory, Bar Harbor, Maine USA. https://phenome.jax.org in, 2012. [Google Scholar]
- [41].Otto GP, Rathkolb B, Oestereicher MA, Lengger CJ, Moerth C, Micklich K, Fuchs H, Gailus-Durner V, Wolf E, de Angelis MH, Clinical chemistry reference intervals for C57BL/6J, C57BL/6N, and C3HeB/FeJ mice (Mus musculus), Journal of the American Association for Laboratory Animal Science, 55 (2016) 375–386. [PMC free article] [PubMed] [Google Scholar]
- [42].Biochemistry and Hematology for Lewis Rat Colonies in North American for January 2008 – December 2011, in, https://www.criver.com/products-services/find-model/lewis-rat.
- [43].Spitzer AI, Richmond JC, Kraus VB, Gomoll A, Jones DG, Huffman KM, Peterfy C, Cinar A, Lufkin J, Kelley SD, Safety and Efficacy of Repeat Administration of Triamcinolone Acetonide Extended-release in Osteoarthritis of the Knee: A Phase 3b, Open-label Study, Rheumatology and Therapy, 6 (2019) 109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kinne RW, Stuhlmüller B, Burmester G-R, Cells of the synovium in rheumatoid arthritis. Macrophages, Arthritis Research & Therapy, 9 (2007) 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Matsumura Y, Maeda H, A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs, Cancer research, 46 (1986) 6387–6392. [PubMed] [Google Scholar]
- [46].Kopecek J, Yang J, Polymer nanomedicines, Adv Drug Deliv Rev, 156 (2020) 40–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yang J, Kopeček J, The light at the end of the tunnel—second generation HPMA conjugates for cancer treatment, Current Opinion in Colloid & Interface Science, 31 (2017) 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sirova M, Mrkvan T, Etrych T, Chytil P, Rossmann P, Ibrahimova M, Kovar L, Ulbrich K, Rihova B, Preclinical evaluation of linear HPMA-doxorubicin conjugates with pH-sensitive drug release: efficacy, safety, and immunomodulating activity in murine model, Pharmaceutical research, 27 (2010) 200–208. [DOI] [PubMed] [Google Scholar]
- [49].Říhová B, Kopečeh J, Ulbrich K, Pospíšil M, Mančal P, Effect of the chemical structure of N-(2-hydroxypropyl) methacrylajnide copolymers on their ability to induce antibody formation in inbred strains of mice, Biomaterials, 5 (1984) 143–148. [DOI] [PubMed] [Google Scholar]
- [50].Říhová B, Kopeček J, Ulbrich K, Chytrý V, Immunogenicity of N-(2-hydroxypropyl) methacrylamide copolymers, Die Makromolekulare Chemie: Macromolecular Chemistry and Physics, 9 (1985) 13–24. [Google Scholar]
- [51].Říhová B, Ulbrich K, Kopeček J, Mančal P, Immunogenicity of N-(2-hydroxypropyl)-methacrylamide copolymers—potential hapten or drug carriers, Folia microbiologica, 28 (1983) 217–227. [DOI] [PubMed] [Google Scholar]
- [52].Šimečková J, Ríhová B, Plocová D, Kopeček J, The activity of complement in the presence of N-(2-hydroxypropyl) methacrylamide copolymers, Journal of Bioactive and Compatible Polymers, 1 (1986) 20–31. [Google Scholar]
- [53].Abbadessa A, Landín M, Oude Blenke E, Hennink WE, Vermonden T, Two-component thermosensitive hydrogels: Phase separation affecting rheological behavior, European Polymer Journal, 92 (2017) 13–26. [Google Scholar]
- [54].Au - Pitcher T, Au - Sousa-Valente J, Au - Malcangio M, The Monoiodoacetate Model of Osteoarthritis Pain in the Mouse, JoVE, (2016) e53746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.


