Abstract
Mycoplasma (M.) hyopneumoniae is the main pathogen of porcine enzootic pneumonia (PEP). Its controlling is challenging, and requires alternative strategies. This study aimed to develop an oral vaccine against M. hyopneumoniae using a nanostructured mesoporous silica (SBA-15) as an adjuvant, and compare its effect with an intramuscular (IM) commercial vaccine (CV). Fifty 24 day-old M. hyopneumoniae-free piglets composed five equal groups for different immunization protocols, consisting of a CV and/or oral immunization (OI). Control piglets did not receive any form of immunization. All piglets were challenged with M. hyopneumoniae strain 232 on D49 by tracheal route. IgA antibody response in the respiratory tract, bacterial shedding and serum IgG were evaluated. The piglets were euthanized on 28 (D77) and 56 (D105) days post-infection. Lung lesions were macroscopically evaluated; lung fragments and bronchoalveolar fluid (BALF) were collected for estimation of bacterial loads by qPCR and/or histopathology examination. All immunization protocols induced reduction on Mycoplasma-like macroscopic lung lesions. IgA Ab responses anti-M. hyopneumoniae, the expression of IL-4 cytokine and a lower expression of IL-8 were induced by CV and OI vaccines, while IgG was induced only by CV. Oral immunization using silica as a carrier-adjuvant can be viable in controlling M. hyopneumoniae infection.
Subject terms: Infectious diseases, Bacterial infection, Adjuvants
Introduction
Mycoplasma hyopneumoniae (M. hyopneumoniae) is the main causative pathogen of porcine enzootic pneumonia (PEP), a chronic respiratory disease in pigs, and one of the main pathogens involved in the porcine respiratory disease complex (PRDC)1. The infections caused by this bacterium are highly prevalent worldwide and result in financial losses for the pig industry, mainly due to the costs of treatment and vaccination, decreased performance, and increased mortality from secondary infections2.
The microorganism's adhesion to the respiratory epithelium, the stimulation of a prolonged inflammatory reaction, the suppression and modulation of innate and adaptive immune responses favoring the pathogen are recognized as important steps in the colonization and infection by this microorganism. As a result, infected animals become more susceptible to infections by other respiratory pathogens1. As in other animals, most porcine pathogens cross mucous surfaces when ingested or inhaled, due to contamination of food, environment and fecal matter. Systemic vaccination generally promotes little stimulation of mucosal associated lymphoid tissue (MALT) and, therefore, the host immune system can only fight against the pathogen after its entering into the body3,4.
In the mucosal lymphoid tissues, mature T cells and B cells are stimulated by antigen and induce IgA antibody response. These cells migrate from the submucosal lymphoid tissue by the bloodstream to the lamina propria, where B cells differentiate into plasma cells secreting dimeric IgA antibodies. Many of these cells return to the original mucosal surface, but others can be found at different mucosal surfaces, so that oral immunization can lead to a migration of IgA precursor B cells to the bronchi, which subsequently secreted IgA antibodies in the bronchial mucosa5.
Previous studies with other pathogens have demonstrated the feasibility of using oral immunization as a strategy for inducing protective immunity in the swine reproductive tract, reinforcing the interconnection between different mucosal sites6. The secretory IgA (SIgA) specific antibodies have been considered as a crucial factor in protecting pigs against infection by M. hyopneumoniae7, while local humoral immunity seems to play an important role in this infection. SIgA is the main effector of respiratory tract mucosa immunity, which can form a protective barrier to eliminate respiratory invading pathogens and prevent infection and active colonization8. Since mucosal immunity has the potential to control pathogens at their portal of entry, it would be advantageous to develop vaccines that trigger a mucosal and systemic immune response rather than simply stimulating the systemic immune system9.
Promising M. hyopneumoniae bacterin formulations have been identified based on their capacity to induce strong innate immune responses10. Limitations in the use of adjuvants for vaccine formulation can be found in the literature, such as toxicity, the ability to lead to an immune response against the agent and not against the adjuvant, induction of adverse reactions, among other factors11. Since the silica has the characteristic of incorporating and releasing molecules12, the mesoporous silica particles have gained attention due to its potential role as adjuvants. Its toxicity to respiratory cells has been described13,14 and depends on the physicochemical properties of particles, size and concentration15. On the other hand, its use may enhance antigen-specific cellular immune responses in dendritic and Langerhans cells15. In addition, it is able to stimulate the immune system in a similar or superior way than other adjuvants, such as the Incomplete Freund's Adjuvant16. Moreover, an improvement in the recruitment of defense cells was observed, which led to an increase in phagocytosis and processing by the gut antigen-presenting cells17–20.
Based on the importance of developing oral vaccine adjuvants that are efficient in presenting antigens to the cells of mucosal lymphoid tissues (MALT), the objectives of this study were (i) to develop an oral vaccine specific to M. hyopneumoniae by encapsulating a blend of proteins of this bacterium into the silica (SBA-15); (ii) to stimulate the immune responses of the respiratory mucosa; (iii) to evaluate the efficacy of the protection induced by this vaccine against experimental infection with a virulent strain of M. hyopneumoniae, compared to a commercial inactivated vaccine.
Results
Mycoplasma-like macroscopic and microscopic lung lesion score at slaughter
At slaughter, gross lesions were mainly located in the apical and cardiac lung lobes, cranio-ventral portions of diaphragmatic lobe, and in portions of the intermediate lobe. All immunized groups showed lower lung lesion scores when compared to the control.
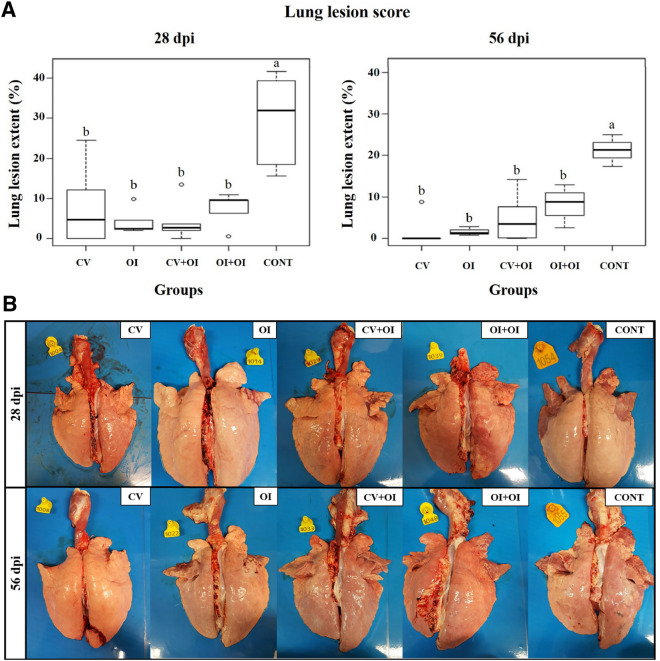
The medians of macroscopic lung lesion scores at the first slaughter (28dpi), followed by the lowest and higher scores observed in each group, were: CV-4.8% (0–24.5%), OI-2.5% (2–9.8%), CV + OI-2.7% (0–13.5%), OI + OI-9.6% (0.5–10.9%) and CONT-32.0% (15.6–41.7%). At the second slaughter (56 dpi), the values obtained were: CV-0.0% (0–8.8%), OI-1.3% (0.8–2.9%), CV + OI-3.5% (0–14.3%), OI + OI-8.8% (2.6–12.9%) and CONT-21.3% (17.4–24.9%). All immunized groups showed significant differences (Dunn test, p-value = 0.0229) in the total Mycoplasma-like lung lesion area when compared with the percentage obtained in the control. Significant differences were observed between the immunized groups and the control, but not between the immunized groups at both post-infection time-points (Fig. 1A). A difference in consolidation lung lesion score of 85% in CV, 92% in OI, 91% in CV + OI, 70% in OI + OI was observed in the first slaughter, while in the second slaughter the difference was of 100% in CV, 94% in OI, 83% in CV + OI, and 59% in OI + OI. Representative photos of consolidation lung lesion from each group are shown below (Fig. 1B).
Figure 1.
(A) Comparison of Mycoplasma-like macroscopic lung lesions extent observed at slaughter of piglets, 28- and 56-days post-infection with M. hyopneumoniae. Means followed by the same letter do not differ statistically from each other (Tukey test). (B) Representative photos of Mycoplasma-like macroscopic lung lesions extent observed at slaughter of piglets, 28- and 56-days post-infection with M. hyopneumoniae.
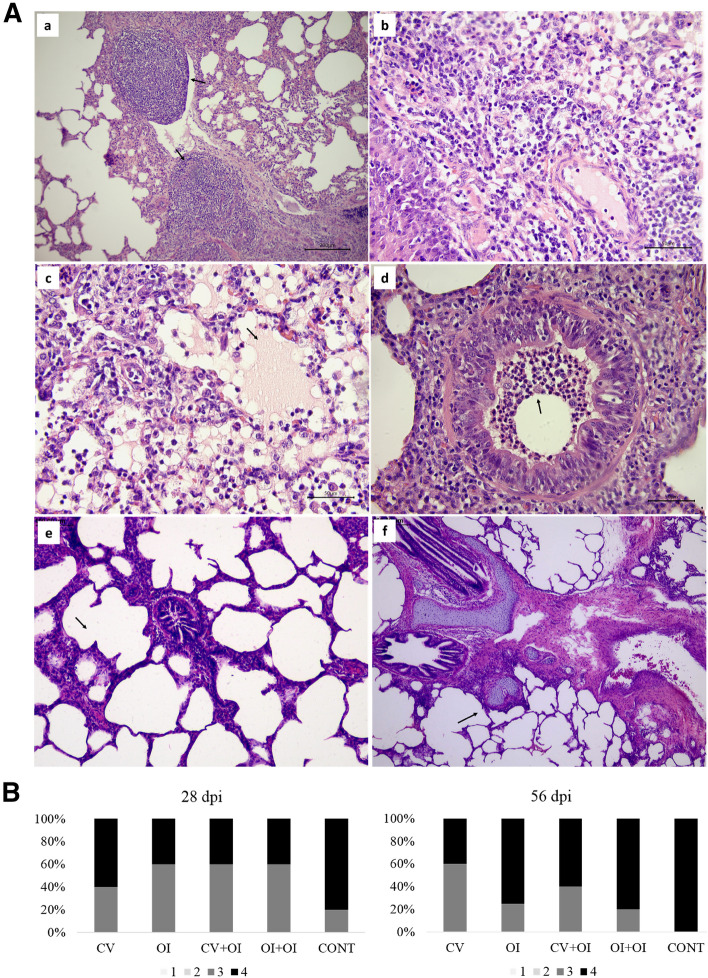
Microscopically, all groups showed histological lesions score varying between 3 and 4, characterized as PEP-specific (Fig. 2A,B). No significant differences were found, neither between groups, nor between the two post-infection intervals. No significant correlations were observed between macro and microscopic analysis (Kruskall-Wallis, p-value = 0.341).
Figure 2.
(A) Photomicrography of histological lung lesion characterized by (a) hyperplasia of lymphoid follicles (arrow) (100 ×); (b) inflammatory infiltrate predominantly compound by macrophages (400 ×); (c) amorphous and acidophilic material in addition to inflammatory infiltrate in the light of the alveoli (arrow) (100 ×); (d) inflammatory infiltrate in bronchioles (arrow) (400 ×); (e) light of the alveoli without noteworthy changes (arrow) (100 ×); (f) normal lymphoid follicle (arrow) (40 ×). (B) Percentage of animals showing different microscopic lung lesion score (0–4) according to each vaccination protocol. No significant differences were observed between groups.
Detection of IgA and IgG anti-M. hyopneumoniae antibodies in nasal swabs, serum and BALF samples
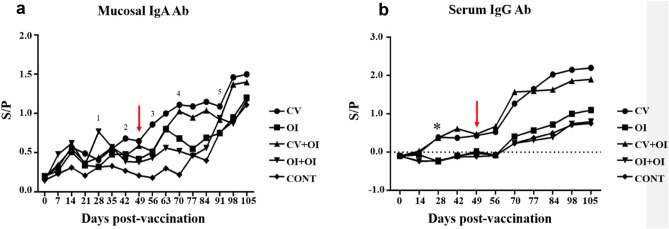
All immunized groups showed IgA Ab response in nasal swabs at 14 days post immunization, while the control group only became positive for IgA Abs 28 days post infection (Fig. 3a). The mean values with the respective standard deviation of each group and day of sampling, as well as statistical differences, are shown in Supplementary Table S3. On D28, OI + OI was statistically different from the other groups (for p-values, see Supplementary Table S5 online). The IgA responses of CV and OI at D42 and D49 were significantly different from the others. At D56, CV was statistically different from the other groups, while OI and CV + OI were statistically similar to each other and different from CONT. At D70, only CV and CV + OI IgA Ab responses were significantly higher than other groups, and OI was different from CONT. From D91 onwards, no differences were found between experimental groups. All piglets from immunized groups were IgA positive before challenge. When comparing the moments of slaughter (28 dpi and 56 dpi), significant differences (Two Sample t-test) were found for CV group (p = 0.045), CV + OI group (p = 0.028) and for CONT (p = 0.0001). No significant correlation was observed for any experimental group between the IgA Ab levels and the macroscopic lung lesion scores either at 28 or 56 dpi intervals.
Figure 3.
(a) Mucosal IgA antibody response related to different immunization protocols (D0) against M. hyopneumoniae along the experimental period. Dots represent the mean values of each group in each day of sampling. Positive S/P values > 0.4. (b) Serum IgG antibody response obtained in different immunization protocols (D0) against M. hyopneumoniae along experimental period. Piglets challenged with M. hyopneumoniae on D49 (red arrows). Dots represent the mean values of each group in each day of sampling. Positive S/P values > 0.3. Kruskall-Wallis test was used.
The oral vaccine did not induce a serum IgG Ab response, whereas the commercial vaccine induced the highest levels of this antibody isotype (Fig. 3b). This result has to be further investigated by other experiments with larger concentration of antigens in SBA-15, providing no aggregation of proteins. It is important to point out that the concentration of antigen administered by the oral vaccine is much smaller than that the one used in the injected CV. The mean values with the respective standard deviation of each group and day of sampling, as well as statistical differences, are shown in Supplementary Table S4. All animals from the CV and CV + OI groups produced IgG anti-M. hyopneumoniae antibodies, and the Ab response started between D14 and D28, remaining with high levels until slaughter. From the moment that seroconversion was detected, the levels of IgG Ab were statistically similar for these two groups, but they differed from the others until the end of the experimental period (for p-values, see Supplementary Table S6 online). The animals from groups OI, OI + OI, and CONT seroconverted for IgG antibodies only four weeks after challenge with the pathogen (D70), and produced levels of IgG Ab statistically similar until the end of the experimental period. Comparing the differences between the moments of slaughter, significant differences (Two sample t-test) were found between the levels of IgG antibodies for CV (p = 0.008) and OI (p = 0.0003). No significant correlation was found for any experimental group between the IgG Ab levels and the macroscopic lung lesion scores, neither at 28 or 56 dpi intervals.
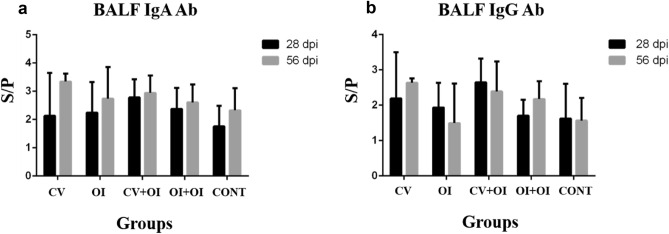
Regarding BALF, all groups showed positive responses to both IgA and IgG anti-M. hyopneumoniae Ab on 28 dpi and 56 dpi (Fig. 4). No statistical differences were found between groups for any of the evaluated immunoglobulins. All correlation analysis tested between variables are shown in Supplementary Table S7.
Figure 4.
ELISA S/P (mean ± sd) results for BALF on 28- and 56 dpi regarding (a) IgA antibody response against M. hyopneumoniae; (b) IgG antibody response against M. hyopneumoniae. Positive S/P values > 0.4.
Detection and quantification of p102 gene of M. hyopneumoniae by qPCR
The samples of nasal swabs tested by qPCR indicated that at 7th dpi there were piglets already shedding the pathogen in all experimental groups in different proportions, and by the end of the evaluated period, most of the piglets from all groups were shedding intermittently M. hyopneumoniae (Table 1). No significant differences were found between groups along the experiment.
Table 1.
Number of piglets shedding M. hyopneumoniae in nasal swabs collected from the 7th to the 56th day post-infection with the pathogen, detected by qPCR.
| 7 dpi | 14 dpi | 21 dpi | 28 dpi | 35 dpi | 42 dpi | 49 dpi | 56 dpi | |
|---|---|---|---|---|---|---|---|---|
| CV | 2/10 | 7/10 | 7/10 | 4/5 | 5/5 | 2/5 | 4/5 | 5/5 |
| OI | 4/10 | 9/10 | 4/10 | 2/5 | 3/5 | 3/5 | 4/5 | 5/5 |
| CV + OI | 3/10 | 5/10 | 5/10 | 0/5 | 4/5 | 3/5 | 4/5 | 5/5 |
| OI + OI | 4/10 | 4/10 | 7/10 | 3/5 | 4/5 | 5/5 | 4/5 | 5/5 |
| CONT | 5/10 | 6/10 | 5/10 | 2/5 | 5/5 | 5/5 | 5/5 | 5/5 |
Mycoplasma hyopneumoniae was detected and quantified in lungs and BALF of all piglets from all groups, and the respective estimate loads and standard deviation are shown in Table 2. No statistical differences (Two Sample t-test) were found for all groups in both sampled time-points (28 and 56 dpi). Considering the correlation between macroscopic lung lesion and M. hyopneumoniae estimate quantification in lungs, a lower quantification was found to be strongly correlated with a reduced macroscopic lung lesion in CV + OI at 28 dpi (Spearman’s correlation coefficient R = 1; p = 0.01667), with a trend on group CV (p = 0.0538, R = 0.87). A lower quantification was also correlated with a reduced M. hyopneumoniae quantification in the BALF (28dpi) of CV (R = 0.99, p = 0.0051), OI (R = 0.97, p = 0.0299), CV + OI (R = 0.96, p = 0.03), and on 56 dpi in OI + OI (R = 0.99, p = 0.0004).
Table 2.
Results of p102 gene estimate quantification by qPCR in lungs and BALF of piglets from all experimental groups at the 28th and 56th days post-infection.
| p102 gene estimate quantification by qPCR | ||||
|---|---|---|---|---|
| Groups | Lungs (± sd) 28 dpi | Lungs (± sd) 56 dpi | BALF (± sd) 28 dpi | BALF (± sd) 56 dpi |
| CV | 3.1 × 104 (± 3.3 × 104) | 2.3 × 104 (± 4.8 × 104) | 4.3 × 105 (± 3.0 × 105) | 3.2 × 105 (± 2.7 × 105) |
| OI | 2.5 × 104 (± 2.8 × 104) | 4.4 × 104 (± 3.6 × 104) | 2.1 × 106 (± 1.9 × 105) | 3.4 × 105 (± 3.5 × 105) |
| CV + OI | 1.3 × 104 (± 1.7 × 104) | 3.0 × 104 (± 3.6 × 104) | 1.5 × 106 (± 1.5 × 106) | 3.0 × 105 (± 3.4 × 105) |
| OI + OI | 1.3 × 105 (± 1.6 × 105) | 4.7 × 104 (± 5.8 × 104) | 2.3 × 106 (± 1.5 × 106) | 9.5 × 105 (± 1.3 × 106) |
| CONT | 1.0 × 105 (± 7.4 × 104) | 1.7 × 105 (± 2.0 × 105) | 1.0 × 106 (± 7.0 × 105) | 2.2 × 106 (± 2.4 × 106) |
No statistical differences were observed between groups or time-points.
Cytokine coding gene’s expression and correlation with antibody levels and bacterial loads
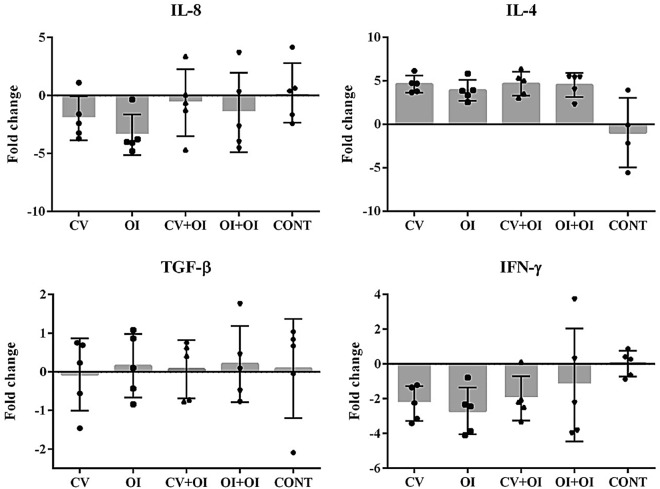
Interleukin 8 was downregulated in immunized groups at 28 dpi compared to the control group, with a slight non-significant difference in the CV + OI group (Kruskall-Wallis test). Similarly, IFN-γ was less expressed in the immunized groups when compared to the control group, especially in CV, OI and CV + OI, with no significant differences between them. A significant and negative correlation was found between IFN-γ and IgA Ab response at 28 dpi (Pearson’s correlation coefficient R = − 0.43; p-value = 0.028). On the other hand, IL-4 expression was up regulated in all immunized groups, while it was less expressed in the control group. TGF-β expression was reported in all groups, with non-statistical differences between them (Fig. 5). TGF-β expression was positively correlated with IgA Ab response in the upper respiratory tract (nasal swabs) of piglets (Pearson’s correlation coefficient R = 0.95; p-value = 0.0401). All correlation analyses between variables are shown in Supplementary Table S7.
Figure 5.
Bar graphs representing the fold change of cytokine gene expression (Fold Change mean ± sd) in lung lesion samples of five pigs per group, 28 days after experimental infection with M. hyopneumoniae strain 232, previously submitted to different immunization protocols. Target gene expression was normalized based on rpl-4 gene expression. No statistical differences were found between groups (Kruskall-Wallis test).
Discussion
Oral vaccines are desirable in pig industry due to the relative ease of administration for large populations. In the present study, an oral vaccine for weaned piglets was developed by using the SBA-15 nanostructured mesoporous silica as a vehicle for a blend of M. hyopneumoniae proteins. Four immunization protocols were tested, followed by an experimental inoculation of M. hyopneumoniae strain 232, to evaluate the action of the oral and the intramuscular vaccines, alone or combined, and the results were compared with a non-immunized group. According to the manufacturer (MSD Animal Health), the commercial vaccine contains the proprietary dual adjuvant EMUNADE, capable of producing a rapid and prolonged immune response due to the aluminum hydroxide and an oil emulsion, respectively. As we have no information regarding the bacterial load present in M + PAC® and different routes of administration were performed, comparing its efficacy to the experimental oral vaccine is challenging. However, this IM vaccine was found to be an interesting option as a basis for comparison since it has been used worldwide and provided satisfactory results. In addition, most works with oral or aerosol vaccination against M. hyopneumoniae in piglets did not perform experimental challenge with this pathogen, which can limit the comparison of our results, such as bacterial shedding and quantification in the lugs, with other vaccines.
All immunization protocols showed reduced Mycoplasma-like lung lesion, and all groups presented a significant difference in lung lesion score when compared with the control group, being the highest differences observed in the medians of OI and CV + OI in the first slaughter (reduction of 92% and 91% of consolidated area, respectively), and in CV and OI (reduction of 100% and 94%, respectively) at the second slaughter. The oral and the commercial vaccines offered effective protection individually, but no combination effect with the two immunization ways were observed. These are promising findings, especially when compared to previous works in which no significant differences in lung lesions were found between vaccinated and non-vaccinated groups on the field with natural infection21, and with homologous and heterologous vaccines followed by experimental infection with M. hyopneumoniae22. On the other hand, microscopic lung lesions were statistically similar in all groups, vaccinated or not, with histological findings characteristics of M. hyopneumoniae infection, as also observed by Almeida23, after experimental infection using the same M. hyopneumoniae 232 virulent strain. An oral vaccine with recombinant E. rhusiopathiae strain expressing the M. hyopneumoniae P97 protein reduced the severity of pneumonic lung lesions caused by M. hyopneumoniae infection24. Similarly, a number of M. hyopneumoniae commercial inactivated vaccines administered by IM route provided such type of protection25–27.
Mycoplasma hyopneumoniae induces innate and adaptive immune responses, which is able to prevent significant systemic spread of the organisms. However, the immune system is unable to rapidly clear pulmonary airways infection, resulting in a prolonged localized inflammatory and cellular immune response, responsible for the majority of gross and microscopic lesions1. The vaccines and vaccination programs tested in the current study were able to induce IgG (commercial vaccine only) and IgA (commercial and experimental oral vaccines) antibodies against M. hyopneumoniae. Only the groups that received IM vaccination were able to produce serum IgG Ab detectable by ELISA test. It is well known that systemic anti-M. hyopneumoniae antibodies are considered to play a minor role in protection against PEP28,29. On the contrary, it is believed that specific locally secreted IgA may play a protective role by preventing the adhesion of the pathogen to the ciliate epithelium30. Independently on the presence of serum IgG Ab, all vaccinated animals showed a significant reduction of consolidation lung lesions, which allows to infer that mucosal IgA Ab probably participates in the protection and prevention against M. hyopneumoniae invasion and adherence, corroborating Martelli30. In this study, both oral and IM vaccines were capable of inducing IgA Ab production, which was found in the respiratory tract, as all immunized piglets were positive for this Ab at 14 days post-vaccination. Feng31 also observed the presence of IgA Ab in nasal cavities 14 days after administration of an aerosol vaccine against M. hyopneumoniae, although no challenge was performed by these authors.
Our results differ from previous ones that only detected significant antibody immune response after challenge infection24, and from studies using commercial vaccine which were not able to induce IgA Ab responses31,32. We have found that vaccinated pigs presented significantly higher antibody levels than the non-vaccinated ones, which may indicate a memory humoral immune response, either for IgA or IgG Abs. It is noteworthy that antibodies induced by IM vaccination may occur between 3 to 4 weeks after vaccination33, while seroconversion in natural M. hyopneumoniae infected pigs usually occurs around 8–24 weeks of age34–36. In the current study, seroconversion in the challenged piglets occurred 3 weeks post-infection. This early immune response, when compared to naturally infected ones, may be due to the differences in experimental and natural bacterial loads. The infecting dose under field conditions is expected to be lower, especially in farms with good management and biosecurity practices37. Determining the ideal time point to experimentally infect the piglets after vaccination is challenging, once vaccination and infections occur dynamically in field conditions. Furthermore, the primary or boosted immune response must be considered in the dynamics of M. hyopneumoniae infection. In the present study, the infectious challenge occurred two weeks after the booster vaccination, and the piglets from CV + OI and OI + OI could be still experiencing the effects of the immunization at that time. For this reason, further studies evaluating different moments of immunization in the field are necessary to a better understanding of this situation.
When considering the Ab immune response in BALF at slaughter (28 dpi and 56 dpi), all groups showed high S/P values for both IgA and IgG Ab, despite the statistically non-significance. As these samples were taken only at slaughter, it was not possible to determine the contribution of each vaccination protocol in inducing the antibody immune response in BALF, once all piglets were challenged with the pathogen and an immune response was expected at 28 dpi. However, it is possible to observe that a substantial Ab detection occurred in piglets previously submitted to both IM and oral vaccination when compared to the control. It may be due not only to the capacity of vaccines to induce memory cells, but also to a previous local presence of these antibodies, as observed in the respiratory tract by nasal swabs. It was expected that vaccinated animals showed higher mucosal IgA Ab responses compared to the non-vaccinated ones after challenge28,38.
The number of organisms colonizing a pig possibly depends on cumulated infectious doses, capacity of the M. hyopneumoniae strain to multiply in the lungs, and time1. In the present study, a high dose of inoculum containing M. hyopneumoniae organisms was provided. M. hyopneumoniae replication in the lungs was lower in vaccinated pigs in both slaughter points compared to control, and quantification in lungs and BALF was lower at 56 dpi in vaccinated groups than in control, despite non-significant differences in bacterial load estimate quantification. The lower lung lesion score at 56 dpi was expected for the immunized groups, as previously observed39. However, it also indicates that vaccination alone does not significantly reduce the bacterial load in the lower respiratory tract of pigs, and would not eliminate infection with this pathogen from pig herds24,27, which may justify nasal shedding not differing between groups along the time37. In addition, several factors such as the challenge dose, the time post-infection, the strain virulence and individual immune responses of pigs may influence the number of M. hyopneumoniae organisms and its nasal shedding23,33.
The evaluation of cytokines production 30 days after challenge with M. hyopneumoniae could better illustrate the resistance status in the vaccinated pigs after infection40. Thus, in the current study, the expression of some cytokine genes at 28 dpi of vaccinated animals point out to a possible role of T cell response after pathogen exposure, so that the oral administration of M. hyopneumoniae antigens incorporated to the silica adjuvant may induce an activation of Treg lymphocytes, which can reduce part of the inflammatory response caused by this pathogen. In this context, it is known that mesenteric Treg lymphocytes of animals submitted to tolerance by the oral route secrete TGF-β with variable amounts of IL-441. Additionally, TGF-β expression was positively correlated with IgA Ab response in the upper respiratory tract, raising the hypothesis that the presence of TGF-β further enhances both secreted IgA and the number of IgA producing cells, as reported42.
T helper (Th) cells are essential to initiate the B-cell activation and generation of antibody responses, which will result in antibody production for T-dependent antigens5. The higher gene expression level of IL-4 in lungs of immunized piglets could also be associated with a positive regulation of a Th2-mediated immune response30, positively influencing the IgA Ab local secretion. Th lymphocytes activation is an important pathway for generating protective immune response against M. hyopneumoniae43.
Interferon-γ secretion is important for the control of several infectious diseases44, and it is usually evident between 4 to 8 weeks post vaccination against M. hyopneumoniae30,38. Although the piglets were tested only at 28 days post-infection, its lower expression in immunized groups may be involved with an immunosuppressed environment caused by the inhibition of Th1 differentiation, since IL-4 suppresses the production of IFN-γ by Th1 cells45. In addition, IFN-γ was negatively correlated with IgA Ab response in the upper respiratory tract. These findings may be justified either by the suppressive effect of IL-4 or the effect of TGF-β on IFN-γ secretion and the direct correlation of IL-4 and IgA Ab response. IL-8 was found to be less expressed in all immunized groups of this study, and its suppression at this time point may be similar to IFN-γ. This chemokine is mainly produced by tissue macrophages in response to infection, and it is an important neutrophil recruiter46. M. hyopneumoniae organisms stimulate the production of proinflammatory and immunoregulatory cytokines by the alveolar macrophages and lymphocytes, inducing lung inflammation and lymphoid hyperplasia. As colonization by M. hyopneumoniae progresses, there is an increase in cytokine secretion due to the increase in the number of inflammatory cells recruited1. In non-vaccinated challenged piglets23, IL-8 gene expression was positively correlated with M. hyopneumoniae bacterial loads, suggesting an intense inflammatory response being required at lung lesion sites due to the presence of the pathogen. In our study, IL-8 and IFN-γ were found downregulated in vaccinated piglets, which may be due to modulation effect caused by the vaccines, preventing an intense inflammatory and immune responses in lungs, consequently leading to a lower consolidation lung lesion.
Under the conditions of this study, the immune response induced by IM and oral vaccination involved both humoral and likely T-cell immune responses. Thus, oral vaccination with a blend of M. hyopneumoniae antigens incorporated into the silica induced local humoral immunity in the gut, measured in this study as IgA anti-M. hyopneumoniae antibodies in respiratory secretions, which were comparable to those obtained by the intramuscular administration of the commercial inactivated vaccine with oil adjuvant. All vaccination protocols reduced the severity of macroscopic lung lesions in the challenged pigs. It suggests that mucosal antibodies and the inflammatory responses were involved in the mechanism of immune-protection, or by raising IL-4 and reducing the IL-8 expression, or even likely by down-regulating the expression of other pro-inflammatory cytokines in the vaccinated animals, which were not evaluated in the current study.
As the protection induced by M. hyopneumoniae vaccines evaluated here and conferred by others conventional vaccines do not prevent colonization and shedding of this microorganism, the immune-protection induced by these vaccines is often incomplete47. Considering that, better results may be achieved whether vaccination is combined with good management and biosecurity procedures in pig farms. Moreover, under field conditions, improved results should be expected for the vaccines tested in this study, once a lower infection challenge dose probably results in lower number of microorganisms in the respiratory tract21. Thus, future studies applying the oral vaccination against M. hyopneumoniae in pigs reared in field conditions could elucidate this point and bring more consistent results for the use of inactivated oral vaccines in the control of this important respiratory pathogen for pigs, combined with the advantage of being a needle-free strategy of M. hyopneumoniae control.
The oral vaccine developed in this research with the SBA-15 nanostructured silica proved to effectively reduce macroscopic lung lesions in challenged pigs and induce mucosal humoral immune response. Moreover, its efficacy was similar to the one conferred by the commercial vaccine parentally administered. The immunogenicity characterization determined in the current study provided useful data for the further development of this oral vaccine, which requires studies under field conditions to elucidate its potential for the effective control and prevention of Mycoplasma hyopneumoniae injuries in the pig production.
Methods
Oral vaccine preparation
Cultivation and preparation of M. hyopneumoniae for protein obtainment
A pure pathogenic strain of M. hyopneumoniae (232) was purchased from Iowa State University48, certified free of any other pathogens, and a small fraction was removed for cultivation in Friis medium. Initially, this fraction was inoculated into two sterile graduated vials (Corning®, USA) containing 5 ml of Friis medium, kept in a shaking oven at 37 °C, which color was observed daily until indicating bacterial growth (CCU). After approximately five days, 2 ml of the culture were used to inoculate each of two flasks containing 200 ml of Friis medium, which were maintained in the same incubation conditions and presented color changing about a week later. The determination of the concentration of M. hyopneumoniae was carried out by successive dilutions of the samples in Friis medium, varying from 10–1 to 10–8, which reached a concentration of 107, and the negative control remained unchanged. Sterility tests were performed for all flasks on blood agar and McConkey media, left in an oven at 37 °C for three days, proving the absence of contamination with other pathogens by the absence of colonies growth.
The contents were centrifuged in appropriate tubes, previously autoclaved, in an ultracentrifuge (Sorvall) at 13,700×g for 45 min. The bacterial cells were deposited at the bottom of the tubes, forming pellets, which were resuspended in 15 mL of Phosphate Buffered Saline 1X (PBS, Sigma-Aldrich, USA), pH 7.4, for washing. The tubes were centrifuged three times at 21,000×g for 10 min until a clean pellet was obtained, which was resuspended in 10 mL of 1X PBS and stored in sterile falcon tubes. The resuspended content underwent a sonication process, and before sonication, an aliquot of whole cell preparation was taken for Dynamic Light Scattering (DLS) evaluation, which will be further discussed. For sonication, the flask was kept on ice and sonicated for three consecutive times in a sonicator (Soni-tech Ultrasonic Cleaning) in 20 Hz frequency for 1 min, with one-minute breaks between processes. To determine the concentration of proteins in the cell lysate, the Bradford method was used (Thermofisher Scientific, USA) followed by spectrophotometer reading (NanoDrop One, Thermofisher Scientific, USA), which provided a concentration of 1048 µg/mL.
Characterization and development of the oral vaccine
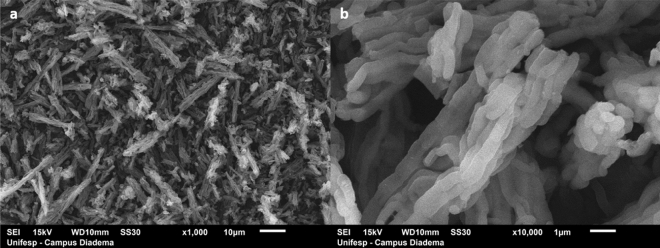
A partnership was established with the Department of Applied Physics, of the Physics Institute of the University of São Paulo (USP-SP), Department of Chemistry, Federal University of São Paulo (UNIFESP/Diadema) and the Butantan Institute (São Paulo), which have been conducting researches with an innovative immunogenic complex for human oral vaccines. They are based on the use of ordered mesoporous silica (OMS, SBA-15 type) as protective vehicle of antigens. The SBA-15 sample was synthesized according to Cavalcante49. It is composed by a bi-dimensional matrix of hexagonally ordered mesopores (diameter around 10 nm), high specific surface (around 1240 m2 g−1) and pore volume 1.8 cm3 g−1 (see Supplementary Fig. S1 online), with amorphous silica walls and rod-like morphology, formed by aggregates of rods connected as rope-like domains (Fig. 6), capable of encapsulating different molecules into their macropores and mesopores. The immunogenic complex has to be able to cross the digestive tract in order to be absorbed by the intestinal mucosa.
Figure 6.
Scanning electron microscopy images of SBA-15 (Santa Barbara Amorphous silica) before antigen adsorption in macro pores. (a) 1000x magnification; (b) 10,000x magnification.
To protect the oral vaccine from the harsh stomach medium, the commercial polymer Eudragit® (Evonik Industries) was used for coating. This polymer is insoluble in acidic pH and dissolves in contact with the intestinal basic medium, providing slow release of the desired proteins12.
The preparation and characterization of the vaccine particles were carried out at the Crystallography Laboratory of the Department of Applied Physics at USP. The process of including the antigen in the pores of the SBA-15 was carried out considering the concentration of the obtained proteins. The 1:35 antigen-to-silica ratio was established (w/w), based on previous work with Hepatitis B encapsulation in SBA-1518,20. In the present study, the silica SBA-15, from a closed recipient (to avoid contamination and humidity) was weighed on a precision scale (± 1 μg), and immediately macerated with a glass stick. After that, the total amount of the silica was mixed with the flask containing the liquid antigen (concentration determined previously). The wet mixture was spread in a glass recipient and partially covered by a glass plate, and placed in an oven at 35 (± 2) °C for total drying. This drying procedure took 48 h. Then, the product was covered with Eudragit L30 D-55 (Evonik®), by mixing the polymer with the silica + antigen, using a glass spatula to homogenize the final product. The amount of Eudragit was equal (in weight) to the amount of silica added to the liquid antigen until a paste was formed, which was taken again to an oven at 35 (± 2) °C for drying, during 48 h. The dry contents were stored in a refrigerator to protect the antigen until use. Since the polymer has the ability to dissolve at a pH higher than 5.5, the compound was supplied to piglets in acidified water (pH 4.5).
Dynamic light scattering (DLS) evaluation of particle diameter and nitrogen adsorption/desorption isotherms of SBA-15:Antigen sample
Dynamic Light Scattering (DLS) evaluation was performed at the Physics Institute, USP-SP, using a DLS (DynaPro NanoStar, Wyatt) equipment at 90° scattering angle with a 100 mW He–Ne laser and wavelength of 658 nm. An aliquot of the whole-cells (concentration of 106 CCU/mL) dispersed in 25 mL of PBS (pH 7.4) was used to fill the sample holder. The same process was done to investigate by DLS the sonicated sample (cell lysate), having a concentration of 1048 µg/ml. The size distribution was obtained by the equipment software, calculated by the CONTIN method50. The antigen encapsulation, depending on its size, can occur in the silica mesopores, with an average diameter of ~ 10 nm, or in the morphological macrospores, with dimensions greater than 50 nm. The average diameter of the molecules present in the whole-cell preparation sample was 460 nm, while the protein preparation was 218 nm, which allowed inferring that cell lysis occurred, and the antigen would be adsorbed on the silica macro porosity, regarding the sizes of the antigen in solution compared with the mean diameter of the mesopores.
By nitrogen adsorption/desorption isotherms of SBA-15:Antigen sample (Supplementary Figs. S2 and S3 online) were evidenced the preservation of mesostructured SBA-15, which exhibited a type IV isotherm and type H1 hysteresis loops, according to the IUPAC classification51 that are characteristic of hexagonal cylindrical channel mesoporous such as SBA-15, with a pore size about 10 nm, as reported in the literature52. The reduction of specific surface area (336 m2 g−1) and pore volume (0.91 cm3 g−1) of SBA-15:Antigen, comparing to pure SBA-15, has evidenced antigen into the macropores of SBA-15.
Oral vaccine administration
After a pilot test, a concentration of 200 µg of protein antigen of M. hyopneumoniae per animal was established. The doses of the compound were weighed on a precision scale, considering the proportion between antigen, silica and polymer. The vaccine was provided by gavage using an esophageal tube with the aid of a laryngoscope, administered in individual doses diluted in 5 ml of filtered water plus acidifier (Selko pH, Trouw Nutrition). Each dose was diluted at the moment of administration.
Experimental design and sample collection
Experimental design
Fifty piglets were randomly separated into five groups (n = 10) to receive different vaccine protocols, consisting of a commercial vaccine (CV) or oral immunization (OI). Random numbers were generated using the standard = RAND() function in Microsoft Excel. Group 1 (CV) piglets received a single dose commercial vaccine at 24 days of age (D0). Group 2 (OI) piglets received a single dose of oral vaccine at D0. Group 3 (CV + OI) received a dose of the commercial vaccine at D0 and a booster with the oral vaccine at D28. Group 4 (OI + OI) received a dose of the oral vaccine on D0 and a booster with the same vaccine on D28; piglets of Group 5 (CONT) were the control group, which did not receive any form of immunization. The commercial vaccine chosen for this work was M+PAC (Merck Animal Health, USA), which promotes an effective protection against M. hyopneumoniae as it contains aluminum hydroxide, responsible for a rapid immune response, and an oil emulsion, which promotes a prolonged immune response by its slow release in the organism. At about 70 days of age (D49), all piglets were challenged by the tracheal route with 5 mL of Friis medium containing 106 CCU/mL of M. hyopneumoniae virulent strain 232, homologous to the vaccine.
Weekly, nasal swabs were collected since D0 for IgA measurement (ELISA), and, after the challenge, were used to evaluate M. hyopneumoniae shedding as well. Fortnightly, all piglets underwent blood collections to obtain serum for IgG measurement (ELISA). Half of the animals in each group were euthanized 28 days post-infection (D77), and the other half was euthanized 56 days post-infection (D105). At slaughter, lungs of all animals were macroscopically evaluated following the European Pharmacopeia methodology, biological samples such as lung fragments were collected for qPCR and histopathology, and bronchoalveolar fluid (BALF) for qPCR. A schematic design is shown in the Supplementary Fig. S4 online.
Animal selection
Fifty 21-day-old piglets of commercial lineage (Landrace × Large White) and average weight between 6 and 7 kg were purchased from a certified M. hyopneumoniae-free commercial farm. The piglets were housed in the experimental barn of the Swine Medicine Laboratory of School of Agricultural and Veterinarian Sciences (FCAV), remaining in the nursery pens until 65 days of age (3 pigs/m2), and then transferred to fattening pens (1 pig/m2), where they remained until 130 days of age. Biosecurity standards were met to avoid cross-infections and minimize external interferences, such as shower-in/shower-out, specific and clean clothes for pig husbandry and no contact with any other pig during the experimental period. The animals received feed according to the production phase, free of antibiotics, and water ad libitum. Upon arrival, blood samples were taken from all piglets to obtain serum for measurement of antibodies, in addition to laryngeal swabs to confirm the absence of the pathogen. Piglets went through an adaptation period of three days before the beginning of the experiment.
All procedures described here were conducted in accordance with the Federal Council of Veterinary Medicine (Brazil), submitted for approval by the Ethics Committee on the Use of Animals (CEUA) of the School of Agricultural and Veterinarian Sciences, São Paulo State University—Campus Jaboticabal, being approved and registered under the protocol number 005174/18. The study was carried out in compliance with the ARRIVE guidelines53.
Blood serum and nasal swabs collection
The titers and duration of antibodies in serum and nasal swabs were assessed along the experimental period. The blood was collected every two weeks to obtain blood serum by puncture the jugular vein, using sterile disposable needles and syringes, deposited in tubes with clot activator and centrifuged at 1500×g for 10 min. The serum was aliquoted in duplicate and stored at − 20 °C until the time of analysis. Samples of nasal swabs were weekly collected to obtain quantitative data on the immune response induced by different protocols of immunization (ELISA), and on the dynamics of excretion of M. hyopneumoniae (qPCR). The animals were restrained, and the swab samples were collected from a light rubbing of the swab on the nasal mucosa and deposited in 2 mL graduated plastic microtubes (Kasvi, Brazil) containing 500 μL of 1 × PBS, and stored at − 80 °C until analysis.
Slaughtering of piglets, lung lesion scoring, BALF and lung fragments collection
Four and eight weeks after the challenge, half of the piglets of each group was euthanized with an intramuscular administration of a combination of ketamine and xylazine (6 mg/kg and 4 mg/kg, respectively), followed by intravenous administration of saturated potassium chloride solution (approved by Federal Council of Veterinary Medicine, Brazil). After evisceration, the respiratory set (trachea + lung) of each animal was separated to collect bronchoalveolar fluid (BALF), with the introduction of 20 mL of PBS 1 × in the cranial portion of the trachea. After pouring all the liquid, the lung was lightly massaged and the liquid was aspirated by the pipette, recovering an approximate volume of 10 mL. The aspirate was aliquoted in duplicate in 2 mL graduated microtubes, free of DNAses and RNAses (Kasvi, Brazil), while the remaining volume was deposited in sterile Falcon tubes (Kasvi, Brazil) and stored at – 20 °C until analysis by qPCR.
The lung was evaluated for the extent of lung lesions followed by photo documentation by a unique blinded investigator, and the extent of the lesions was quantified using the European Pharmacopeia method, in which the percentage of each lobe affected area is multiplied by the lobe relative weight and summed to provide the total weight percentage of affected lung54. The lung tissue fragments for the qPCR were collected with individual scalpel blades and sterile tweezers. The tweezers were kept in boiling water in the interval between collections, while each fragment was collected with a disposable scalpel blade. These fragments were collected in duplicate in the shortest possible time after the animal's death, and all samples were bathed in liquid nitrogen and later stored in a freezer at − 80 °C.
Histopathological evaluation
The histopathological analysis aimed at evaluating lung lesions caused by Mycoplasma hyopneumoniae in different groups, in order to classify qualitatively histological lesions as PEP-specific or non-specific. For that, during the necropsy, lung fragments with lesions caused by PEP resources were collected, mainly in the transition area between healthy and affected tissue. Samples of tissues apparently healthy were also collected for control. The fragments were collected with a thickness of approximately 5 mm and initially stored submerged in a 10% buffered formalin solution (pH 7.0) in an approximate ratio of 10: 1 formalin: tissue. After 24 h in formalin solution, the fragments were routinely processed for Hematoxylin/Eosin staining.
The slides were blind read under a light microscope and microscopic lesions on the tissues were classified into five different degrees55, in which: 0 = absence of lesion; 1 = lesions of interstitial pneumonia and/or purulent bronchopneumonia; 2 = light to moderate infiltrates of macrophages, lymphocytes and neutrophils into airways and alveoli; 3 = perivascular and peribronchiolar lymphoplasmacytic hyperplasia, type II pneumocyte hyperplasia, alveolar spaces with edema fluid, neutrophils, macrophages and plasma cells; 4 = lesions with characteristics of grade 3, together with peribronchial and perivascular lymphoid nodules. Grades 1 and 2 injuries were nonspecific, while injuries 3 and 4 were considered PEP-specific.
Detection and quantification of IgA and IgG antibodies by enzyme-linked immunosorbent assay
The detection and quantification of antibodies (IgA and IgG) in blood serum, nasal secretions and tracheobronchial lavage were carried out by enzyme-linked immunosorbent assay (ELISA). To detect serum IgG Ab, the commercial kit M.hyo Ab test (Idexx, USA) was used. For the detection of IgA Ab in nasal swab, and IgA and IgG Abs in BALF samples, standardization was performed using the sensitized plates and components of the kit mentioned above, with modifications. Initially, the plate was blocked with 1.5% ovalbumin in PBS, followed by incubation at 37 °C for 30 min. Then, a first procedure was performed to determine the ideal dilutions of the samples (positive and negative controls for anti-M. hyopneumoniae antibodies) and the anti-IgA or anti-IgG peroxidase conjugates. For standardization, samples of nasal swabs from animals known to be positive and negative for M. hyopneumoniae were used, and part of the negative and positive samples were homogenized in the form of a pool to compose the negative (CN) and positive (CP) controls, respectively.
To detect IgA Ab in nasal swabs, 100 μL of the sample's liquid fraction was used, as the swabs were deposited in 500 μL of PBS, which were quickly homogenized in a vortex and placed, without further dilution, in each microplate well. The same procedure was performed with the controls, which were tested in duplicate, followed by plate incubation for 60 min at room temperature. The conjugate from the kit was replaced by an immunoenzymatic conjugate of goat anti-Pig IgA Antibody HRP Conjugated (Bethyl Laboratories Inc., USA), at a dilution of 1:500 using the diluent provided by the kit, followed by incubation for 60 min at room temperature. The washing processes and all the following steps were performed according to the protocol of the kit M.hyo Ab test (Idexx, USA).
For BALF IgA Ab detection, the samples were diluted 1:10 using the diluent from the kit, and the same conjugate was used in the proportion of 1:800. For IgG detection in the BALF, the samples were diluted in the proportion of 1:2 and the conjugate (Pig IgG-Fc Fragment Antibody HRP, Bethyl Laboratories Inc., USA) in the proportion of 1:5000. In all microplates, the conjugate was tested in separate wells to determine its non-specific adsorption in the absence of samples. The plate was read in an absorbance microplate reader (iMark, Bio-Rad Laboratories Inc., USA), at a wavelength of 650 nm.
The mean optical densities (OD) for each of the test samples (ODs) were related with the OD found for the negative and positive controls (NC in order to calculate the S/P values (sample/positive ratio) according to the formula: S/P = . The threshold between positive and negative samples was calculated from the value of S/P + 2 × standard deviation. Serum samples were considered positive if S/P > 0.3; nasal swabs S/P > 0.4. BALF S/P > 0.4.
Detection and quantification of p102 gene fragment of M. hyopneumoniae by qPCR
DNA extraction from nasal swabs, lung and BALF samples
The DNA extraction was carried out by Tris–HCl protocol56. For nasal swabs and BALF samples, a centrifugation (Centrifuge 5804 R, Eppendorf, Germany) at 13,000×g at 4 °C for 20 min was performed previously to the DNA extraction protocol. For lung samples, 0.05 g of lung tissue were used. After DNA extraction, the samples were stored at − 20 °C until qPCR analysis. The measurement of the DNA concentration of the samples was made through spectrophotometry, with the aid of the Thermo Scientific NanoDrop 2000 Spectrophotometer (Thermo FisherScientific®, USA), having as exclusion factor the samples that did not reach the purity of 1.8 to 2.0 in the 260/280 ratio to perform the qPCR technique. To rule out the presence of inhibitors in the extracted DNA samples and the occurrence of false negatives in the qPCR for M. hyopneumoniae, all samples were submitted to a conventional PCR targeting the endogenous gene Glyceraldehyde-3-phosphate dehydrogenase (gapdh), and the conventional PCR technique57. The amplified products of gapdh gene with 437 bp were detected after horizontal electrophoresis on a 1% agarose gel stained with Ethidium Bromide (0.5 μL/mL) in TEB running buffer pH 8.0 at a current of 90 V/50 mA for 90 min.
qPCR assay
Absolute real-time quantitative PCR analysis (qPCR) was used to detect p102 gene fragment in nasal swab samples, and to detect and quantify it in lung fragments and bronchoalveolar fluids. For M. hyopneumoniae, the primers used in the reaction were based on the bacterium p102 adhesion protein gene sequence. All samples were tested in duplicate and the qPCR reaction was optimized from a previous published protocol58, adapted by Almeida23. The nucleotide sequences used were forward primer 5′-AAGGGTCAAAGTCAAAGTC-3′, reverse primer 5′-AAATTAAAAGCTGTTCAAATGC-3′ and hydrolysis probe 5′-FAM-AACCAGTTTCCACTTCATCGCC-§BHQ2-3′.
The results were only accepted for those with a standard deviation lesser than or equal to 0.5 cycle, and quantification data were used only if the efficiency obtained was between 90 and 105%57, otherwise, the samples were retested in triplicates. As a negative control in the qPCR reactions, sterile ultrapure water was used (Nuclease-Free Water, Promega®, Madison, Wisconsin, USA) q.s.p. Serial dilutions were made to determine the standard curve generated with different concentrations of synthetic DNA (GBlock®, IDT, USA) containing the target sequence (107 copies/μL to 101 copies/μL), that were also used as positive controls. The synthetic DNA was diluted according to the manufacturer's guidelines and maintained in a stock concentration of 107 molecules∕µL.
Quantification was performed using serial tenfold dilutions (starting at 107 until 101 copies∕ μL) of synthetic DNA (GBlock®, IDT, Iowa City, IA, USA) containing the 150 bp fragment amplified by the primer pair used in the qPCR. Quantification data based on the standard curve generated was only validated if the reaction efficiency was between 90 and 105%59. qPCR parameters are shown in Supplementary Table S1.
Cytokine coding gene expression in lung samples
RNA extraction and cDNA synthesis
Total RNA was extracted from 0.02 g of lung tissue samples collected on the first slaughter (28 dpi) using RNeasy Blood and Tissue Plus kit (Qiagen, USA), and 500 ng of extracted RNA were used per reaction to convert the extracted RNA into cDNA by Superscript IV First Strand Synthesis kit (Thermo Fisher, USA), both according to the manufacturer’s guidelines. RNA purity and integrity were assessed by Bioanalyzer® (Thermo Scientific, USA), and immediately stored at − 80 °C until use. Samples were only used for gene expression analysis if they had RNA Integrity Number (RIN) > 7.0. Oligo d(T)20 targeted the poly-A tail of mRNA for the synthesis of this molecule into cDNA, instead of other types of RNA. Reactions were all performed in a MyCycler thermocycler (Bio Rad, USA), and the cDNA was stored at − 20 °C until use for qPCR.
Detection and quantification of cytokine coding gene’s expression
The reference gene used to normalize the expression of target genes was standardized23, and the best result was obtained with Ribosomal protein L4 (rpl-4), which was also used in this study. Quantification of cytokine coding gene’s expression was performed using relative quantification of cDNA produced. Transcript levels of inflammatory (IL-8) and specific immunity regulation (IFN-γ IL-4 and TGF-β) cytokines evaluated to compare the immune response promoted by each type of immunization with the control group. Specific primers targeting the genes of IL-8 (CXCL-8) and IFN-γ were based on previous work23, while genes of IL-4 and TGF- β were designed based on the reference sequences deposited in GenBank and using software Primer360. To avoid genomic DNA interference, all primers were designed comprising the exon-exon span. The specific primers are shown in Table 3.
Table 3.
Details of the primer sequences of cytokines used for quantitative SYBR Green real-time PCR amplification. The rpl-4 gene was used as reference.
| Target gene | Primer sequence | Access number | qPCR efficiency (%) | Slope | R2 | Amplicon size (bp) | Amplicon melting temperature (°C) |
|---|---|---|---|---|---|---|---|
| rpl-4 | (F)CAAGAGTAACTACAACCTTC | NC_010443.5 | 96.79 | 3.401 | 0.994 | 122 | 75 |
| (R)GAACTCTACGATGAATCTTC | |||||||
| IL-8 | (F)AGGAAAAGTGGGTGCAGAAG | NM_213867.1 | 97.67 | 3.379 | 0.991 | 190 | 78 |
| (R)CAACCCTATGTCTGACCAGC | |||||||
| IFN-γ | (F)ATTGGAAAGAGGAGAGTGAC | NM_213948.1 | 96.54 | 3.408 | 0.997 | 168 | 76.5 |
| (R)CATTCAGTTTCCCAGAGCTA | |||||||
| IL-4 | (F)AGAGCTCTATTCATGGGTCT | NM_214123.1 | 99.88 | 3.325 | 0.997 | 209 | 82 |
| (R)CTTCTCCGTCGTGTCTCT | |||||||
| TGF-β | (F)GGATACCAACTACTGCTTCA | NM_214015.2 | 100.85 | 3.302 | 0.996 | 228 | 83 |
| (R)TTGTACAGAGCCAGGACTT |
The qPCR reactions were performed as previously described23, using the Quantitect® Sybr Green master mix (Qiagen, USA) and 1 μL of cDNA template, totalizing 10 μL of final volume per reaction, in a real time thermocycler CFX 96 (Bio Rad, USA). The dissociation curve was used to assess the specificity of the amplicons at the end of 40 cycles, with a maximum variation of ± 0.5 °C. Serial tenfold dilutions (107 copies∕μL until 101 copies∕μL) of positive controls (Gblock®, IDT, USA) of synthetic DNA containing the amplified fragment of each primer pair was used to assess the efficiency, which was only accepted between 90 and 105%59. In order to normalize the target gene expression, the 2−ΔΔCq calculation61 was performed, and, to attend this methodology, all qPCR reaction’s efficiency had to be close to 100% with a maximum difference of 5% between them. All parameters of cytokine gene expression qPCR are shown Supplementary Table S2.
Data analysis
The variables of each group for each moment were assessed for normality and homoscedasticity by the Shapiro–Wilk and Bartlet tests, respectively. The difference between the means was calculated using the Tukey test (p < 0.05). Variables that did not meet the assumptions were subjected to the Kruskal–Wallis non-parametric test (p < 0.05) and in cases which significance was observed, the Dunn test (Post hoc) was applied. Differences between the slaughter times for the quantitative variables were subjected to parametric analysis by the unpaired T-test, and the variables that did not meet the assumptions were subjected to the Wilcoxon–Mann–Whitney non-parametric test. The difference between the means (post hoc) was calculated by the last square mean adjusted by the Tukey method. For proportion analysis, the differences between groups by date were calculated using the Wilson score interval method. The correlation analysis of parametric data were subjected to Pearson's correlation test (p < 0.05), and non-parametric data, to Spearman's test. Correlation analysis to assess whether two variables can be considered dependent was performed using Kendall's nonparametric test. For this, the software R was used, with the following packages “agricolae”62; "LmerTest"63, "arm"64; "Emmeans"65; "Car"66; "Nortest"67; "MASS"68 with the software R69. Normalization of cytokine gene expression and the graphs were performed on software GraphPad Prism 6 (La Jolla, CA-USA).
Supplementary Information
Acknowledgements
The authors are thankful for the research grant and PhD scholarship provided by São Paulo State Research Foundation (FAPESP—#2019/19710-4, #2018/12742-5, #2017/17844-8), and to Farmabase Animal Health for the financial support.
Author contributions
M.L.M.D. and L.G.O. designed the overall project. M.L.M.D., H.M.S.A., K.S., M.A.C.M., F.A.M.P., B.B.Z. and M.M.F. performed the animal experimentation. M.L.M.D., H.M.S.A., K.S. and H.J.M. performed the acquisition and analysis of data. G.Y.S. performed all statistical analysis. T.S.M., H.J.M., O.A.S., M.C.A.F. designed, produced and characterized the oral vaccines. All authors reviewed the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-01883-2.
References
- 1.Pieters MG, Maes D. Mycoplasmosis. In: Zimmermann JJ, editor. The Diseases of Swine. Wiley-Blackwell; 2019. [Google Scholar]
- 2.Holst S, Yeske P, Pieters M. Elimination of Mycoplasma hyopneumoniae from breed-to-wean farms: A review of current protocols with emphasis on herd closure and medication. J. Swine. Health. Prod. 2015;23:6. [Google Scholar]
- 3.Murtaugh MP. Advances in swine immunology help move vaccine technology forward. Vet. Immunol. Immunopathol. 2014;159:3–4. doi: 10.1016/j.vetimm.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasternak JA, Ng SH, Wilson HL. A single, low dose oral antigen exposure in newborn piglets primes mucosal immunity if administered with CpG oligodeoxynucleotides and polyphosphazene adjuvants. Vet. Immunol. Immunopathol. 2014;161:3–4. doi: 10.1016/j.vetimm.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Chase C, Lunney JK. Immune system. In: Zimmermann JJ, editor. The Diseases of Swine. Wiley-Blackwell; 2019. pp. 264–291. [Google Scholar]
- 6.Hyland K, Foss DL, Johnson CR, Murtaugh MP. Oral immunization induces local and distant mucosal immunity in swine. Vet. Immunol. Pathol. 2004;102:3. doi: 10.1016/j.vetimm.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Lin JH, Weng CN, Liao CW, Yeh KS, Pan MJ. Protective effects of oral microencapsulated Mycoplasma hyopneumoniae vaccine prepared by co-spray drying method. J. Vet. Med. Sci. 2003;2:7. doi: 10.1292/jvms.65.69. [DOI] [PubMed] [Google Scholar]
- 8.Hua LZ, et al. Comparative analysis of mucosal immunity to Mycoplasma hyopneumoniae in Jiangquhai porcine lean strain and DLY piglets. Genet. Mol. Res. 2014;13:3. doi: 10.4238/2014.July.7.13. [DOI] [PubMed] [Google Scholar]
- 9.Wilson HL, Obradovic MR. Evidence for a common mucosal immune system in the pig. Mol. Immunol. 2015;66:1. doi: 10.1016/j.molimm.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthijs AM, et al. Systems immunology characterization of novel vaccine formulations for Mycoplasma hyopneumoniae bacterins. Front. Immunol. 2019;10:1087. doi: 10.3389/fimmu.2019.01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrovsky N, Aguilar JC. Vaccine adjuvants: Current state and future trends. Immunol. Cell Biol. 2004;82:5. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 12.Mariano-Neto F, et al. Physical properties of ordered mesoporous SBA-15 silica as immunological adjuvant. J. Phys. D. Appl. Phys. 2014;47:42. doi: 10.1088/0022-3727/47/42/425402. [DOI] [Google Scholar]
- 13.Ko JW, et al. Silica dioxide nanoparticles aggravate airway inflammation in an asthmatic mouse model via NLRP3 inflammasome activation. Regul. Toxicol. Pharmacol. 2020;112:104618. doi: 10.1016/j.yrtph.2020.104618. [DOI] [PubMed] [Google Scholar]
- 14.Ravinayagam V, Jermy BR. Nanomaterials and their negative effects on human health. In: Khan F, editor. Applications of Nanomaterials in Human Health. Springer; 2020. pp. 249–273. [Google Scholar]
- 15.Chen L, et al. The toxicity of silica nanoparticles to the immune system. Nanomedicine. 2018;13:1939–1962. doi: 10.2217/nnm-2018-0076. [DOI] [PubMed] [Google Scholar]
- 16.Mercuri LP, et al. Ordered mesoporous silica SBA-15: A new effective adjuvant to induce antibody response. Small. 2006;2:2. doi: 10.1002/smll.200500274. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho LV, et al. Immunological parameters related to the adjuvant effect of the ordered mesoporous silica SBA-15. Vaccine. 2010;28:50. doi: 10.1016/j.vaccine.2010.09.087. [DOI] [PubMed] [Google Scholar]
- 18.Scaramuzzi K, et al. Nanostructured SBA-15 silica: An effective protective vehicle to oral hepatitis B vaccine immunization. Nanomed. Nanotechnol. 2016;12:8. doi: 10.1016/j.nano.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Lopes JLS, et al. Antigenic and physicochemical characterization of Hepatitis B surface protein under extreme temperature and pH conditions. Vaccine. 2019;37:43. doi: 10.1016/j.vaccine.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen MK, et al. 3D visualisation of hepatitis B vaccine in the oral delivery vehicle SBA-15. Sci. Rep. 2019;9:1. doi: 10.1038/s41598-019-42645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villarreal I, et al. The effect of vaccination on the transmission of Mycoplasma hyopneumoniae in pigs under field conditions. Vet. J. 2011;188:1. doi: 10.1016/j.tvjl.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 22.Villarreal I, et al. Effect of challenge of pigs previously immunised with inactivated vaccines containing homologous and heterologous Mycoplasma hyopneumoniae strains. Vet. Res. 2012;8:1. doi: 10.1186/1746-6148-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida HM, et al. Cytokine expression and Mycoplasma hyopneumoniae burden in the development of lung lesions in experimentally inoculated pigs. Vet. Microbiol. 2020;1:108647. doi: 10.1016/j.vetmic.2020.108647. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa Y, et al. Oral vaccination against mycoplasmal pneumonia of swine using a live Erysipelothrix rhusiopathiae vaccine strain as a vector. Vaccine. 2009;27:33. doi: 10.1016/j.vaccine.2009.04.081. [DOI] [PubMed] [Google Scholar]
- 25.Dawson A, Thevasagayam SJ, Sherington J, Harvey RE, Peters AR. Studies of the field efficacy and safety of a single-dose Mycoplasma hyopneumoniae vaccine for pigs. Vet. Rec. 2002;151:18. doi: 10.1136/vr.151.18.535. [DOI] [PubMed] [Google Scholar]
- 26.Moreau IA, Miller GY, Bahnson PB. Effects of Mycoplasma hyopneumoniae vaccine on pigs naturally infected with M. hyopneumoniae and porcine reproductive and respiratory syndrome virus. Vaccine. 2004;22:17–18. doi: 10.1016/j.vaccine.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 27.Meyns T, et al. Comparison of transmission of Mycoplasma hyopneumoniae in vaccinated and non-vaccinated populations. Vaccine. 2006;24:49–50. doi: 10.1016/j.vaccine.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Djordjevic S, et al. Serum and mucosal antibody responses and protection in pigs vaccinated against Mycoplasma hyopneumoniae with vaccines containing a denatured membrane antigen pool and adjuvant. Austr. Veter. J. 1997 doi: 10.1111/j.1751-0813.1997.tb14383. [DOI] [PubMed] [Google Scholar]
- 29.Thacker EL, Thacker BJ, Boettcher TB, Jayappa H. Comparison of antibody production, lymphocyte stimulation, and protection induced by four commercial Mycoplasma hyopneumoniae bacterins. J. Swine Health Prod. 1998;6:107–112. [Google Scholar]
- 30.Martelli P, et al. Systemic and local immune response in pigs intradermally and intramuscularly injected with inactivated Mycoplasma hyopneumoniae vaccines. Vet. Microbiol. 2014;168:2–4. doi: 10.1016/j.vetmic.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 31.Feng ZX, et al. Development and validation of an attenuated Mycoplasma hyopneumoniae aerosol vaccine. Vet. Microbiol. 2013;167:3–4. doi: 10.1016/j.vetmic.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Bai Y, et al. Application of a sIgA-ELISA method for differentiation of Mycoplasma hyopneumoniae infected from vaccinated pigs. Vet. Microbiol. 2018 doi: 10.1016/j.vetmic.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 33.Sibila M, et al. Chronological study of Mycoplasma hyopneumoniae infection, seroconversion and associated lung lesions in vaccinated and non-vaccinated pigs. Vet. Microbiol. 2007;122:1–2. doi: 10.1016/j.vetmic.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Andreasen M, Nielsen JP, Bækbo P, Willeberg P, Bøtner A. A longitudinal study of serological patterns of respiratory infections in nine infected Danish swine herds. Prev. Vet. Med. 2000;45:3. doi: 10.1016/S0167-5877(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 35.Djordjevic SP, Eamens GJ, Romalis LF, Saunders MM. An improved enzyme linked immunosorbent assay (ELISA) for the detection of porcine serum antibodies against Mycoplasma hyopneumoniae. Vet. Microbiol. 1994;39:3–4. doi: 10.1016/0378-1135(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 36.Leon EA, Madec F, Taylor NM, Kobisch M. Seroepidemiology of Mycoplasma hyopneumoniae in pigs from farrow-to-finish farms. Vet. Microbiol. 2001;78:4. doi: 10.1016/S0378-1135(00)00303-5. [DOI] [PubMed] [Google Scholar]
- 37.Villarreal I, et al. Infection with a low virulent Mycoplasma hyopneumoniae isolate does not protect piglets against subsequent infection with a highly virulent M. hyopneumoniae isolate. Vaccine. 2009 doi: 10.1016/j.vaccine.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Thacker EL, Thacker BJ, Kuhn M, Hawkins PA, Waters WR. Evaluation of local and systemic immune responses induced by intramuscular injection of a Mycoplasma hyopneumoniae bacterin to pigs. Am. J. Vet. Res. 2000;61:1384–1389. doi: 10.2460/ajvr.2000.61.1384. [DOI] [PubMed] [Google Scholar]
- 39.Vranckx K, Maes D, Sacristán RDP, Pasmans F, Haesebrouck F. A longitudinal study of the diversity and dynamics of Mycoplasma hyopneumoniae infections in pig herds. Vet. Microbiol. 2012 doi: 10.1016/j.vetmic.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Kick AR, Tompkins MB, Hammer JM, Routh PA, Almond GW. Evaluation of peripheral lymphocytes after weaning and vaccination for Mycoplasma hyopneumoniae. Res. Vet. Sci. 2011;91:3. doi: 10.1016/j.rvsc.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Wawrzyniak M, O’Mahony L, Akdis M. Role of regulatory cells in oral tolerance. Allergy Asthma Immunol. Res. 2017;9:2. doi: 10.4168/aair.2017.9.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore KW, O’Garra A, Malefyt RD, Vieira P, Mosmann TR. Interleukin-10. Annu. Rev. Immunol. 1993;11:1. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Morante B, et al. Potential use of local and systemic humoral immune response parameters to forecast Mycoplasma hyopneumoniae associated lung lesions. PLoS ONE. 2017;12:4. doi: 10.1371/journal.pone.0175034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen Y, Hu W, Wei Y, Feng Z, Yang Q. The immune mechanism of Mycoplasma hyopneumoniae 168 vaccine strain through dendritic cells. Vet. Res. 2017;13:1. doi: 10.1186/s12917-017-1194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004;75:2. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 46.Palomino DCT, Marti LC. Chemokines and immunity. Einstein. 2015;13:3. doi: 10.1590/S1679-45082015RB3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haesebrouck F, et al. Efficacy of vaccines against bacterial diseases in swine: What can we expect? Vet. Microbiol. 2004 doi: 10.1016/j.vetmic.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Minion FC, et al. The genome sequence of Mycoplasma hyopneumoniae strain 232, the agent of swine mycoplasmosis. J. bacterial. 2004;186:21. doi: 10.1128/JB.186.21.7123-7133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cavalcante CT, Molina C, Martins TS. Synthesis and characterization of ordered mesoporous silica containing di-ureasil hybrid/phosphotungstic acid and Eu 3+ J. Mater. Sci. Mater. Electron. 2019;30:18. doi: 10.1007/s10854-019-01626-0. [DOI] [Google Scholar]
- 50.Provencher SW. CONTIN: A general-purpose constrained regularization program for inverting noisy linear algebraic and integral-equations. Comput. Phys. Commun. 1982;27:3. [Google Scholar]
- 51.Thommes M, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report) Pure Appl. Chem. 2015;87:1051–1069. doi: 10.1515/pac-2014-1117. [DOI] [Google Scholar]
- 52.Jardim AAMLF, Bacani R, Gonçalves NS, Fantini MCA, Martins TS. SBA-15:TiO2 nanocomposites: II direct and post-synthesis using acetylacetone. Microporous Mesoporous Mater. 2017;239:235–243. doi: 10.1016/j.micromeso.2016.10.009. [DOI] [Google Scholar]
- 53.Du Sert NP, et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020;18:e3000411. doi: 10.1371/journal.pbio.3000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ph. Eur. (European Pharmacopoeia) Monograph 04/2013:2448. Porcine enzootic pneumonia vaccine (inactivated) Ph. Eur. (8th Edit.) (2013) Strasbourg, France. Garcia-Morante, B. et al. Assessment of Mycoplasma hyopneumoniae-induced pneumonia using different lung lesion scoring systems: a comparative review. J. Comp. Pathol.154, 2–3 (2016). 10.1016/j.jcpa.2015.11.003. [DOI] [PubMed]
- 55.Calsamiglia M, Collins JE, Pijoan C. Correlation between the presence of enzootic pneumonia lesions and detection of Mycoplasma hyopneumoniae in bronchial swabs by PCR. Vet. Microbiol. 2000;6:3. doi: 10.1016/S0378-1135(00)00245-5. [DOI] [PubMed] [Google Scholar]
- 56.Kuramae-Izioka EE. A Rapid, easy and high yield protocol for total genomic DNA isolation of Colletotrichum gloeosporioides and Fusarium oxysporum. Unimar. 1997;13:3. [Google Scholar]
- 57.Birkenheuer AJ, Levy MG, Breitschwerdt EB. Development and evaluation of a seminested PCR for detection and differentiation of Babesia gibsoni (Asian Genotype) and B. canis DNA in canine blood samples. J. Clin. Microbiol. 2003;41:9. doi: 10.1128/JCM.41.9.4172-4177.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fourour S, et al. A new multiplex real-time TaqMan® PCR for quantification of Mycoplasma hyopneumoniae, M. hyorhinis and M. flocculare: exploratory epidemiological investigations to research mycoplasmal association in enzootic pneumonia-like lesions in slaughtered pigs. J. Appl. Microbiol. 2018;125:2. doi: 10.1111/jam.13770. [DOI] [PubMed] [Google Scholar]
- 59.Bustin S, et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 60.Untergasser A, et al. Primer3-new capabilities and interfaces. Nucleic Acids Res. 2014 doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2-ΔΔCT method. Methods. 2001 doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 62.De Mendiburu, F., de Mendiburu, M. F. Package ‘agricolae’. R Package, Version, 1.2–1. https://CRAN.R-project.org/package=agricolae (2019).
- 63.Bates D, Mächle M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 64.Gelman, A. & Hill, J. Data analysis using regression and multilevel/hierarchical models. Cambridge University Press. R package, Version, 9. https://CRAN.R-project.org/package=arm (2016).
- 65.Lenth R, Lenth MR. Package ‘lsmeans’. Am. Stat. 2018;34:216–221. [Google Scholar]
- 66.Fox, J. et al. Package ‘car’. Vienna: R Foundation. https://cran.r-project.org/web/packages/car/index.html (2012).
- 67.Gross, J., Ligges, U., Ligges, M.U. Package ‘nortest, Version, 1.0–4. Five omnibus tests for testing the composite hypothesis of normality. https://CRAN.R-project.org/package=nortest (2015).
- 68.Venables, W. N., Ripley, B. D. Modern Applied Statistics with S. (Springer, 2002) https://www.stats.ox.ac.uk/pub/MASS4/, https://CRAN.R-project.org/package=MASS.
- 69.R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2018). https://www.R-project.org/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.