Abstract
SARS-CoV-2 infection results in a spectrum of outcomes from no symptoms to widely varying degrees of illness to death. A better understanding of the immune response to SARS-CoV-2 infection and subsequent, often excessive, inflammation may inform treatment decisions and reveal opportunities for therapy. We studied immune cell subpopulations and their associations with clinical parameters in a cohort of 26 patients with COVID-19. Following informed consent, we collected blood samples from hospitalized patients with COVID-19 within 72 h of admission. Flow cytometry was used to analyze white blood cell subpopulations. Plasma levels of cytokines and chemokines were measured using ELISA. Neutrophils undergoing neutrophil extracellular traps (NET) formation were evaluated in blood smears. We examined the immunophenotype of patients with COVID-19 in comparison to that of SARS-CoV-2 negative controls. A novel subset of pro-inflammatory neutrophils expressing a high level of dual endothelin-1 and VEGF signal peptide-activated receptor (DEspR) at the cell surface was found to be associated with elevated circulating CCL23, increased NETosis, and critical-severity COVID-19 illness. The potential to target this subpopulation of neutrophils to reduce secondary tissue damage caused by SARS-CoV-2 infection warrants further investigation.
Subject terms: Immunology, Diseases
Introduction
Overactivation of the immune system is thought to play a role in the pathophysiology of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection1–3; the mainstays of therapy for severe disease include reduction in viral load, corticosteroids, and IL-1 and IL-6 inhibitory treatments, which act to suppress the immune response4,5. Proposed mechanisms of immune dysfunction in severe COVID-19 include T cell deficiencies and dysregulation of lymphocyte response6, excessive inflammation7, and aberrant complement activation8,9.
Increased numbers of circulating neutrophils are typically seen during COVID-1910. High neutrophils in combination with a low number of circulating lymphocytes predict a poor outcome11. The increased number of immature CD10 negative neutrophils has been described and also correlates with the severity of COVID-1912. Infiltration of neutrophils in the lungs has also been documented in patients infected with SARS-Cov-2, specifically in those developing acute respiratory distress syndrome13. However, not only the number but also the functional status of neutrophils is different in COVID-19 subjects14. Factors contributing to the shift in their functional properties include mobilization of immature cells from the bone marrow and activation of neutrophils in the circulation, resulting in increased heterogeneity in the pool of circulating neutrophils.
An increased number of immature CD10 negative neutrophils has been described and correlates with the severity of COVID-1912. Blood accumulation of abnormal neutrophils occurs15,16 and likely indicates the activation of immature neutrophils with pro-inflammatory factors, released during a systemic inflammatory response17. While mature and properly activated neutrophils are protective, aberrant activation of immature neutrophils may contribute to excessive inflammatory and secondary tissue damage. The development of targeted therapeutic approaches to prevent unnecessary immune cell-mediated tissue damage requires further investigation of neutrophil heterogeneity18.
The Dual Endothelin-1 and VEGF signal peptide-activated Receptor (DEspR) is a single transmembrane receptor coupled to a Ca2+-mobilizing transduction pathway19. DEspR is essential for embryonic angiogenesis and neuroepithelial development. DEspR deficiency is associated with embryonic lethality20. Endothelin-1 is an endogenous ligand that binds DEspR within the range of low nanomolar concentrations20. Both endothelin-1 and Ca2+ intracellular signaling play an important role in activating innate immunity21,22. However, the role of DEspR in the regulation of myeloid cells, and specifically in neutrophils, has not been described.
The goals of this study were to characterize the expression of DEspR in subpopulations of immune cells in patients with COVID-19 and healthy donors and to determine the associations between the number of immune cells expressing a high level of DEspR (DEspRhigh), inflammatory factors, and the severity and clinical features of COVID-19.
Results
Study subjects
A convenience sample of 26 patients with COVID-19 and 12 control samples were analyzed. Among our COVID-19 study group, 6 patients (23%) were characterized by mild illness, 9 subjects (35%) had a severe illness, and 11 subjects (42%) were classified with a critical illness.
There were no differences in age or sex between study subjects and controls. A comparison of the demographics and clinical characteristics of COVID-19 and control subjects is provided in Table 1.
Table 1.
Characteristics of control subjects and subjects with COVID-19.
| Characteristic | Control subjects (n = 12) | Subjects with COVID-19 (n = 26) |
|---|---|---|
| Age, mean ± SD | 67 ± 9.6 | 63.7 ± 15.4 |
| Male, n (%) | 8 (66.7) | 16 (61.5) |
| Race, (%) | White (100) | White (100) |
| BMI, mean ± SD | 28.2 ± 5 | 32 ± 7 |
| COVID severity*, # (%) | ||
| Mild, severe, critical | 6, 9, 11 (23, 35, 42) | |
| Estimated FiO2 (%), median (IQR) | 36, (29, 75) | |
| Days since symptom onset, mean ± SD | 8.0 ± 4.8 | |
| Antibiotics, # (%) | 17 (65.4) | |
| Corticosteroids, # (%) | 21 (80.8) | |
| Hemodialysis, # (%) | 3 (11.5) | |
| Thrombosis, # (%) | 6 (23.1) | |
| Hospital LOS, median (IQR) | 8 (4, 14) | |
| Discharge survival, # (%) | 21 (80.8) | |
IQR interquartile range, FiO2 fraction of inspired oxygen, LOS length of stay.
*Mild: no need for supplemental oxygen; Severe: supplemental oxygen required; Critical: critically ill with respiratory failure.
The number of DEspRhigh cells is increased in patients with COVID-19 and correlates to disease severity
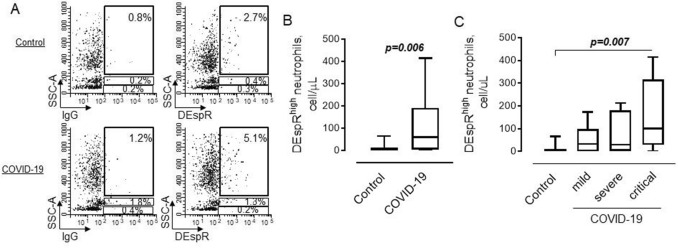
To determine the cell surface expression of DEspR on circulating immune cells, we used flow cytometric analysis. Gates for cells expressing a high level of DEspR within major subpopulations of SSChigh neutrophils, SSCintermediate monocytes, and SSClow lymphocytes were set up using isotype IgG’s as shown in Fig. 1A. The majority of cells with a high level of DEspR at their surface are characterized by high intracellular granularity (SSChigh), which allowed us to identify these cells as neutrophils. These cells were found in both healthy subjects and COVID-19 patients. However, the number of DEspRhigh neutrophils was increased in COVID-19 patients compared to controls (Fig. 1B). The analysis also revealed the accumulation of DEspRhigh cells in the blood of critically ill COVID-19 patients (Fig. 1C).
Figure 1.
Flow cytometric strategy to determine DEspRhigh cells in the peripheral circulation. Freshly obtained blood cells were analyzed after erythrocytes lysis. (A) Flow cytometric plots showing gating strategy for cells expressing a high level of cell surface DEspR in control (upper) and COVID-19 (lower) subjects. (B) Graphical representation of data from flow cytometric analysis on the number of DEspRhigh/SSChigh neutrophils in control (n = 12) and COVID-19 (n = 26) patients; Mann–Whitney test. (C) The number of DEspRhigh/SSChigh neutrophils in control subjects and patients with mild (n = 6), severe (n = 9), or critical (n = 11) COVID-19. Kruskal–Wallis test, Dunn’s multiple comparisons test; P value is shown.
DEspRhigh neutrophils express CD10 and elevated levels of CD14
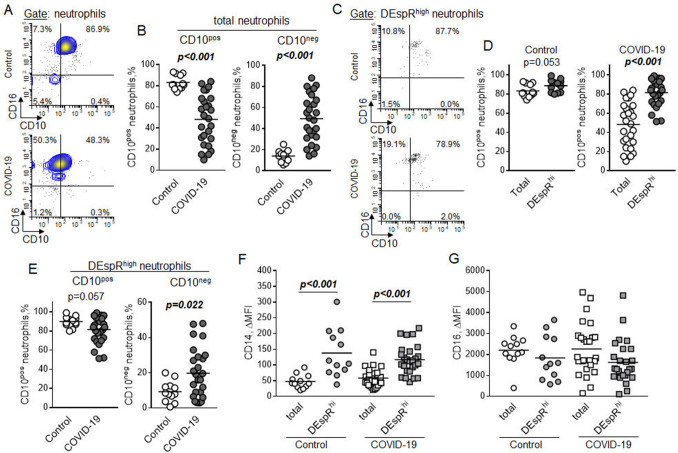
To better characterize the antigenic phenotype of cells with high expression of DEspR, we performed the analysis of markers associated with neutrophils maturation and activation, CD10 and CD16, in the subpopulation of total neutrophils and subset of DEspRhigh neutrophils. High level expression of CD16 was found on the surface of more than ninety percent of cells in the total subpopulation of SSChigh neutrophils in both control subjects and patients with COVID-19 (Fig. 2A). The majority of neutrophils from the control subjects were also characterized by the expression of CD10, whereas neutrophils from patients with COVID-19 did not express CD10, a marker associated with neutrophils maturation23, on their surfaces (Fig. 2B).
Figure 2.
Expression of CD10, CD14, and CD16 on DEspRhigh neutrophils in control subjects and COVID-19 patients. The expression of cell surface markers was determined in the subpopulation of peripheral blood SSChigh neutrophils (total neutrophils) or a subset of neutrophils with high expression of DEspR (DEspRhigh neutrophils) in control (n = 12) and COVID-19 (n = 26) subjects. (A) Representative flow cytometric plots showing expression of CD10 on total neutrophils in control (upper) and COVID-19 (lower) subjects. (B) Number of CD10 positive (left) and CD10 negative neutrophils. Mann–Whitney test. (C) Flow cytometric plots demonstrating expression of CD10 on DEspRhigh neutrophils in control (upper) and COVID-19 (lower) subjects. (D) Percentage of CD10 positive cells in a subpopulation of total neutrophils (Total) or a subset of DEspRhigh neutrophils in control (left) and COVID-19 (right) subjects. Mann–Whitney test. (E) Percentage of CD10 positive (left) and CD10 negative (right) neutrophils in control subjects and COVID-19 patients. Mann–Whitney test. (F,G) Expression of (F) CD14 and (G) CD16 on total and DEspRhigh neutrophils in control and COVID-19 subjects. The expression is represented by the mean fluorescence intensity that corresponds to the level of cell surface CD14 and CD16. ΔMFI was calculated by subtracting the mean fluorescence intensity of isotype controls from the mean fluorescent intensity of specific antibodies. Two-way ANOVA with Tukey multiple comparisons test; P values are indicated.
We also determined the percentage of CD16 and CD10 positive cells in the subset of DEspRhigh neutrophils. Similar to the subpopulation of total neutrophils, the majority of DEspRhigh neutrophils expressed CD16 (Fig. 2C). Although not statistically significant, this subset of DEspRhigh neutrophils demonstrated a trend toward a higher percentage of CD10 expressing cells compared to total neutrophils in control subjects (Fig. 2D). The high percentage of neutrophils that express CD10 within the subset of DEspRhigh neutrophils indicated their more mature state compared to total neutrophils in COVID-19 patients. There was a difference in the maturation state of DEspRhigh neutrophils between control and COVID-19 subjects. A trend toward a lower percentage of CD10 positive neutrophils and a significantly higher percentage of CD10 negative neutrophils were found in the subset of DEspRhigh neutrophils in patients with COVID-19 compared to control subjects (Fig. 2E).
CD14 is associated with pro-inflammatory activation of neutrophils24,25. DEspRhigh neutrophils were characterized by significantly higher expression of CD14 compared to total neutrophils in both groups of study subjects (Fig. 2F). No differences were found in the level of CD14 on the surface of total and DEspRhigh neutrophils between control and COVID-19. There was no difference in the cell surface expression of CD16 between total and DEspRhigh neutrophils or neutrophils in the groups of control subjects and patients with COVID-19 (Fig. 2G).
Lack of association between the number of DEspRhigh neutrophils and lymphocyte count in COVID-19
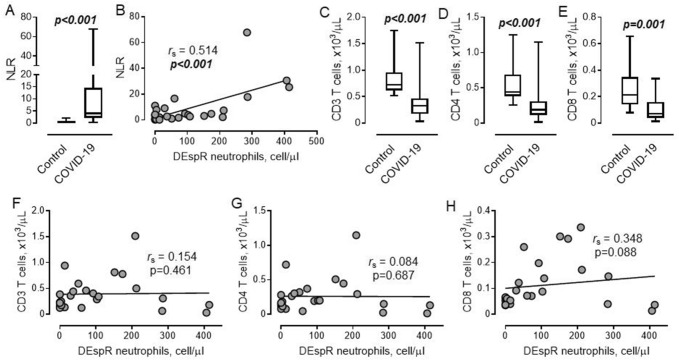
Our data demonstrate that the neutrophil-to-lymphocyte ratio (NLR) is significantly increased in patients with COVID-19 compared to control (Fig. 3A). We also found a positive correlation between the number of DEspRhigh neutrophils and NLR in COVID-19 (Fig. 3B). Because neutrophils possess immunosuppressive properties26, we separately examined the potential relationship between both DEspRhigh neutrophils and lymphocytes in the circulation.
Figure 3.
High neutrophil to lymphocyte ratio is associated with an increased number of DEspRhigh neutrophils. (A) Neutrophil–lymphocyte ratio (NLR) in control (n = 12) and COVID-19 (n = 26) subjects. Mann–Whitney test. (B) Relationship between NLR and number of DEspRhigh neutrophils. Spearman correlation coefficient is indicated. (C–E) Number of (C) CD3 T lymphocytes, (D) CD3/CD4 and (E) CD3/CD8 T cells in control and COVID-19 subjects. Mann–Whitney test. (F–H) Association between the number of DEspRhigh neutrophils and (F) total T lymphocytes, (G) CD3/CD4, and (H) CD3/CD8 T cells. Spearman correlation coefficients and P values are indicated.
The number of CD3 lymphocytes and major subsets of CD4 and CD8 T cells were significantly decreased in COVID-19 compared to control subjects, indicating enhanced systemic immunosuppression (Fig. 3C–E). However, no associations were found between the number of DEspRhigh neutrophils and the total number of T cells and CD4 lymphocytes (Fig. 3F,G). Although not statistically significant, a trend toward a weak positive association was found between DEspRhigh neutrophils and CD8 T cells in patients with COVID-19 (Fig. 3H).
Circulating CCL23 is increased in COVID-19
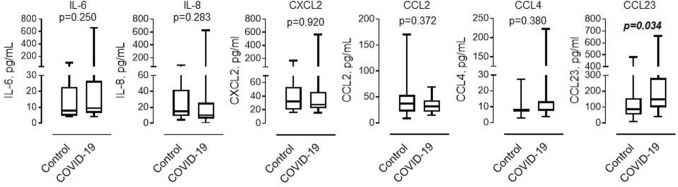
In parallel with the flow cytometric analysis of DEspRhigh neutrophils, we determined the level of cytokines and chemokines that contribute to the activation and recruitment of neutrophils. As shown in Fig. 4, the level of CCL23 was significantly increased in patients with COVID-19 compared to control subjects. No differences were found between the two groups in the levels of IL-6, IL-8, CXCL2, CCL2, or CCL4.
Figure 4.
Level of circulating pro-inflammatory factors in control and COVID-19 subjects. Levels of circulating cytokines and chemokines were determined in platelet-free plasma in groups of control subjects (n = 12) and COVID-19 patients (n = 25). One patient with extracorporeal membrane oxygenation support was excluded from the analysis due to the potential effect of cytokine adsorption during ECMO therapy. Mann–Whitney test.
DEspRhigh neutrophils are associated with increased CCL23
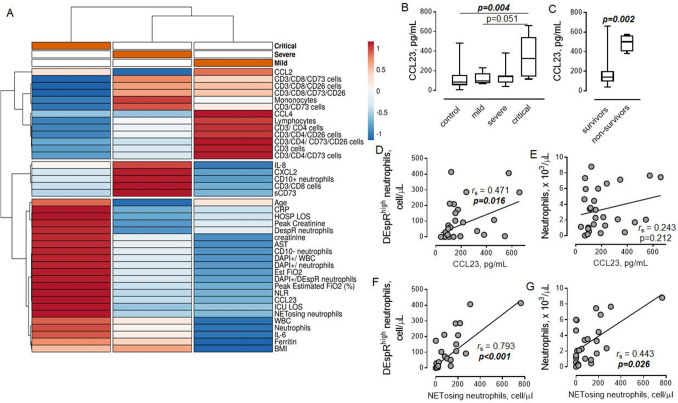
To determine the potential relationship between DEspRhigh neutrophils and other measured parameters in relation to disease severity, a hierarchical clustering analysis in a group of patients with COVID-19 was performed. As shown in Fig. 5A, the majority of lymphocyte subpopulations, CCL2, and CCL4, were clustered with mild illness. As expected, DespRhigh neutrophils were clustered within the group of critically ill patients with COVID-19. In addition, we found that CCL23 is also associated with a critical illness (Fig. 5B), and that CCL23 is significantly increased in non-survivors (Fig. 5C).
Figure 5.
Association between DEspRhigh neutrophils and CCL23. (A) Heat map associated with hierarchical clustering analysis of tested parameters in groups of mild (n = 6), severe (n = 9), and critical (n = 10) COVID-19 patients obtained using ClustVis 2.0. Columns with similar annotations are collapsed by taking the median inside each group. Rows are centered; unit variance scaling is applied to rows. Both rows and columns are clustered using correlation distance and average linkage. Color keys show the relative strength of the signal in each cluster group. Annotations on top of the heatmap show clustering of the samples. AST aspartate aminotransferase, BMI body mass index, CRP C-reactive protein, DAPI+ 4′,6-diamidino-2-phenylindole viability dye positive dead cells, Est FiO2 estimated fraction of inspired oxygen, HOSP LOS hospital length of stay, ICU LOS intensive care unit length of stay, sCD73 soluble CD73. (B) Association between the level of circulating CCL23 and COVID-19 severity. Spearman correlation coefficient and P value are indicated. (C) The level of CCL23 in COVID-19 survivors (n = 21) and non-survivors (n = 4). Mann–Whitney test. (D,E) Associations between the level of CCL23 and number of (D) DEspRhigh neutrophils and (E) total neutrophils in COVID-19 patients. (F,G) Relationships between the number of NETosing neutrophils and number of (F) DEspRhigh neutrophils and (G) total neutrophils in COVID-19 patients. (D–G) Spearman correlation coefficients and P values are indicated.
A positive correlation was found between the number of DEspRhigh neutrophils, but not the total number of neutrophils, and the level of CCL23 (Fig. 5D,E), indicating there may be a direct relationship between these two factors in the development of critical COVID-19. In addition, our analysis revealed positive correlations between the number of NETosing neutrophils and the number of both DEspRhigh and the total subpopulation of neutrophils (Fig. 5F,G).
Discussion
This study demonstrates the presence of a newly defined subset of neutrophils characterized by high cell surface expression of DEspR in human blood. The number of these DEspRhigh neutrophils is increased in patients with COVID-19 and correlates with disease severity. Elevated expression of CD10 and CD14, markers of maturation and activation, may indicate the high pro-inflammatory potential of these neutrophils. An association was seen between the number of DEspRhigh neutrophils, NETosing neutrophils, the blood plasma level of CCL23, and critical illness in COVID-19.
Accumulating evidence suggests that neutrophils are major players in the development of hyperinflammation associated with poor outcomes in patients with SARS-CoV-2 infection27. Neutrophils may contribute to disease progression via several mechanisms, including immunosuppression28, production of pro-inflammatory factors29, and activation of coagulation and thrombosis30. Recently it has been recognized that circulating neutrophils are a heterogeneous population of cells31. A subset of mature CD10-expressing neutrophils is characterized by the ability to suppress T lymphocytes23, which may contribute to systemic immunosuppression and prevent effective viral clearance32. It has also been shown that decreased lymphocyte count is associated with severe and critical conditions in COVID-1933,34. Clustering analysis of data generated in our study demonstrated an association between immunosuppressive factors such as soluble CD7335, CD10 positive neutrophils23 and IL-836, and severe COVID-19. We also found that DEspRhigh neutrophils are characterized by the high expression of CD10. Our analysis revealed a positive correlation between DEspRhigh neutrophils and NLR and a significantly decreased number of lymphocytes in patients with COVID-19. In addition, we found differential clustering of DEspRhigh neutrophils to the group of critically ill patients compared to lymphocyte clustering in the group of patients with mild COVID-19 disease, pointing toward potential immunosuppressive properties of neutrophils expressing a high level of DEspR. No correlations, however, were found between DEspRhigh neutrophils and different subsets of circulating CD3 lymphocytes in the current study. Thus, our data indicate that DEspRhigh neutrophils are unlikely to be involved in the regulation of systemic lymphocyte trafficking or suppression. Further studies are warranted to determine the direct effect of DEspRhigh neutrophils on lymphocyte activation locally in inflamed tissue.
While the majority of DEspRhigh neutrophils express CD10 in both study groups, the percent of CD10 negative neutrophils within the DEspRhigh subset is higher in patients with COVID-19 compared to control, reflecting the increased mobilization of immature neutrophils from the bone marrow in COVID-19. This also indicates that the upregulation of DEspR expression on neutrophils is not dependent on their maturation and is initiated in the peripheral circulation. This should result in their accumulation in the blood, however, the overall percent of DEspRhigh neutrophils is low. One possible explanation is related to the egress of DEspRhigh neutrophils out of the bloodstream due to their migration into tissues. It has been shown previously that endothelin-1, a DEspR ligand, enhances the adhesion of neutrophils to endothelial cells37–39 and promotes the recruitment of neutrophils into inflamed tissue40–42.
Another important observation from this study is related to the high expression of CD14 on DEspRhigh neutrophils. CD14 is a coreceptor for toll-like receptors43,44. Upon pro-inflammatory activation, the cell surface expression of CD14 is increased45 and promotes synthesis and secretion of pro-inflammatory factors, such as tumor necrosis factor-alpha46. CD14 is involved in the activation of both monocytes and neutrophils in vasculitis47,48, one of the common cardiovascular complications in COVID-1949. While high expression of CD14 is associated with activation of neutrophils, our data demonstrate that DEspRhigh neutrophils also express a high level of CD16 comparable to the level of expression found on most neutrophils in the blood. A high level of CD16 expression is usually found on resting, non-activated neutrophils50. Apoptotic neutrophils, subsets of immature or activated neutrophils, are characterized by a significant reduction in the cell surface expression of CD1650. The high levels of CD14 expression on DEspRhigh neutrophils may indicate their enhanced pro-inflammatory potential compared to the overall neutrophil population.
Chemokines are critical factors in the recruitment of immune cells to the area of inflammation51. Our study demonstrated elevated levels of circulating CCL23 in patients with COVID-19. CCL23 is a potent chemoattractant for the peripheral blood mononuclear cells, including monocytes, dendritic cells, and resting lymphocytes52,53. Both monocytes and neutrophils produce CCL23 in response to a variety of toll-like receptor ligands54. However, in contrast to the majority of cytokines and chemokines secreted at a significantly higher level from monocytes, neutrophils produce high levels of CCL23. Furthermore, toll-like receptor ligands induce early neutrophils response compared to delayed upregulation and secretion of CCL23 in monocytes54. Neutrophils may represent a major source of CCL23 considering the significant increase in the number of neutrophils during the systemic inflammatory response. The expression of CCL23 by brain tissue-infiltrating neutrophils after a stroke has been reported55. Our analysis revealed a positive correlation between the number of DEspRhigh neutrophils, but not total neutrophils, and the level of circulating CCL23 in patients with COVID-19. Both DEspRhigh neutrophils and CCL23 were significantly increased in critically ill patients with COVID-19. Given a high expression of CD14 that promotes toll-like receptor signaling, it is plausible that DEspRhigh neutrophils produce CCL23 to further promote inflammation. This is supported by our data showing that a high level of CCL23 is associated with poor outcomes in patients with COVID-19. However, further studies are required to determine the potential role of DEspRhigh neutrophils in CCL23 secretion.
Neutrophil extracellular traps (NET) propagate inflammation and microvascular thrombosis in patients with COVID-1956. Our data reveal a positive correlation between the number of DEspRhigh neutrophils and NETosing neutrophils in the peripheral circulation of patients with COVID-19, indicating potential involvement of DEspR in the regulation of NET. NETosing neutrophils may induce the secretion of endothelin-1 from endothelial cells57, suggesting the presence of an amplification loop that may further promote both NETosis and inflammatory activation of endothelial cells via mechanisms involving the generation of DEspRhigh neutrophils.
This study included a broad population of patients with COVID-19, and the sample size of subgroups was small, so important variability of individuals within the categories of mild, moderate, and severe disease may either be lost or exaggerated. Most patients were receiving corticosteroids, which may modulate the cellular immune response and could affect the behavior or activation status of lymphocytes and neutrophils; the effect on DEspR expression in vivo is unknown. Critically ill patients also received sedatives, analgesics, and sometimes neuromuscular blocking agents, all of which may affect the inflammatory response58,59. Because the study was conducted over 5 months of intensive COVID-19 research and evolving clinical standards, care of early and later patients may have varied somewhat, potentially affecting the immunoinflammatory response. Nonetheless, this study is among the first to characterize a newly identified subset of DEspRhigh neutrophils in COVID-19, and ultimately its inclusion of healthy controls and patients with the full spectrum of severity of COVID-19 is a strength. Furthermore, the population studied reflects a “real-world” cohort with corresponding complexity and variability, making the strength of our findings even more relevant and clinically important.
In summary, the systemic inflammatory response in patients with COVID-19 is associated with an accumulation of neutrophils expressing a high level of DEspR at the cell surface. Antigenic immunophenotype identifies DEspRhigh neutrophils as mature neutrophils with high pro-inflammatory properties. A high number of circulating DEspRhigh neutrophils is associated with an increased level of CCL23 and NETosing neutrophils in critically ill COVID-19 patients, identifying DEspRhigh neutrophils as an intermediate biomarker to track in clinical trials of therapies for COVID-19 and a new potential therapeutic target for the prevention of immune cell-driven hyperinflammation.
Methods
Patients
The study and our informed consent process were approved by the Maine Medical Center Institutional Review Board. Informed consent was obtained from the patient or their legally authorized representative (LAR) using a secure electronic consent form (to prevent disease transmission), either in-person (with the patient) or by telephone (with the LAR). All methods were performed in accordance with the relevant guidelines and regulations. A convenience sample included hospitalized patients that provided informed consent between July and December 2020 within 72 h of being hospitalized with PCR-confirmed SARS-CoV-2 infection (real-time RT-PCR test, NorDx Laboratories, Portland, ME). We excluded patients under 18 years of age, those representing a vulnerable population, and those with hemoglobin < 8 g/dL.
The severity of COVID-19 disease was defined using the following criteria from the US Center for Disease Control (CDC)60, (1) mild illness individuals who have any signs and symptoms of COVID-19 (e.g., fever, cough, sore throat, malaise, headache, muscle pain) without shortness of breath, dyspnea, or abnormal chest imaging, (2) severe illness individuals who have respiratory frequency > 30 breaths per minute, SpO2 < 94% on room air at sea level (or, for patients with chronic hypoxemia, a decrease from baseline of > 3%), a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) < 300 mmHg, or lung infiltrates > 50%, and (3) critical illness individuals who have respiratory failure, septic shock, and/or multiple organ dysfunction.
Blood samples were additionally obtained from asymptomatic and SARS-CoV-2 negative subjects to serve as a control population. Pertinent demographic and clinical data were collected from the electronic medical record for all study participants.
Standards of care
Patients received an evolving standard of care during their hospitalization. This included the antiviral drug remdesivir61 and the synthetic adrenocortical glucocorticoid dexamethasone62; many also received systemic anticoagulation63, and antibiotics for community-acquired pneumonia64 until it was determined they did not have a concurrent bacterial infection. Our cohort included a few patients who were asymptomatic but determined to have a SARS-CoV-2 infection on administrative testing, although most had a severe or critical disease. Mechanically ventilated patients received lung-protective ventilation according to international clinical practice guidelines65, proning for refractory hypoxemia66, and standard evidence-based critical care therapies (e.g., neuromuscular blocking agents, sedation, and analgesia)67. Patients may also have received renal replacement therapy (i.e., intermittent hemodialysis or continuous veno-venous hemofiltration) or underwent venovenous extracorporeal membrane oxygenation, as indicated.
Blood sample collection
Subjects underwent phlebotomy on the day of enrollment. Venous blood (8.5 mL) was collected from COVID-19 and control subjects using BD Vacutainer ACD tubes. Blood aliquots (50 μL) for flow cytometric analysis underwent erythrocyte lysis with ammonium chloride lysing solution (150 mmol/L NH4Cl, 10 mmol/L NaHCO3, and 1 mmol/L EDTA, pH 7.4). Blood smears were prepared and fixed in cold 100% methanol; slides were stored at − 20 °C until shipment on dry ice for third-party NETosis analysis.
Platelet-free plasma (PFP) was prepared at room temperature using 2-step centrifugation, each at 2000×g for 20 min. After processing, plasma was stored at − 80 °C until further analysis. No more than one freeze/thaw cycle was allowed for PFP samples to prevent protein degradation.
Flow cytometric analysis
After red blood cell lysis, white blood cells (WBC, 106 cells/mL) were treated with whole molecule mouse and human IgGs to prevent nonspecific binding, followed by incubation with relevant antibodies for 25 min at 4 °C. Cells were washed once with ten volumes of cold PBS/0.5% BSA/2 mM EDTA before data acquisition.
Subpopulations of WBC were analyzed using the following antibodies: CD3 (UCHT1), CD4 (OKT4), CD8a (HIT8a), CD10 (HI10a), CD14 (HCD14), CD16 (3G8), CD26 (BA5b), CD38 (HIT2), CD45 (HI30), CD73 (AD2), HLA-DR (LN2430) (all purchased from BioLegend). Human anti-human/mouse DEspR antibody (NCTX-01) was provided by NControl Therapeutics (Medfield, MA). Human IgG4-S228P isotype control was obtained from Syd Labs (Southborough, MA). Both anti-DEspR and isotype IgGs were conjugated with Alexa Fluor 647 using Molecular Probes® Alexa Fluor® Antibody Labeling Kits (ThermoFisher Scientific). Subpopulations of cells were defined as follows: side scatter (SSC)high/CD16high/CD14low/−/HLA-DR negative neutrophils, SSCintermediate/CD14high/HLA-DR positive monocytes, SSClow/CD14negative lymphocytes. Subsets of CD4 and CD8 T cells coexpressing CD73 and CD26 were determined within the subpopulation of CD3 positive cells.
The total number of WBC was determined using TruCount™ Tubes (BD Biosciences). Viable and non-viable cells were distinguished using 4′,6-diamidino-2-phenylindole (DAPI) to detect dead nucleated cells. Data acquisition was performed on a MacsQuant Analyzer 10 (Miltenyi Biotec, Inc), and the data were analyzed using WinList 5.0 and FlowJo 10.7 software.
Analysis of circulating IL-6, IL-8, MCP-1, CCL4, CCL23, CXCL2, and CD73
Plasma levels of IL-6, IL-8, MCP-1, CCL4, CCL23, CXCL2, and soluble CD73 were measured using ELISA kits (Bio-techne/R & D Systems).
Fixed cell imaging of blood smears (NETosing quantification)
Immunofluorescence imaging was performed as a contract research service at Nikon Imaging Laboratory (Cambridge, MA) as follows. Slides were imaged with a Nikon Ti2-E Widefield microscope equipped with a Plan Apo λ 20× objective and Spectra LED light source and controlled by NIS-Elements. An automated JOBS routine in NIS-Elements was used to image 100 evenly spaced positions along with an entire slide. At each position, automatic focus adjustment with the Perfect Focus System (PFS) was followed by sequential imaging with the 395 (Blue), 470 (Green), and 555 (Red) nm LED light to detect DAPI (nuclei), Alexa Fluor 488 (CD11b) and Alexa Fluor 568 (DEspR, hu6g8), respectively. A General Analysis 3 algorithm was used to process image stacks to segment the nuclei, measure circularity, and quantify signal intensity. Generated CSV files were imported to Excel for scoring. Cells were separated into non-NETosing (Circularity > 0.8) and NETosing (Circularity < 0.8) groups. Scoring of CD11b and DEspR expression was based on the mean signal intensities of the respective fluorophore stainings relative to background fluorescence. Boolean operations were used to count the number of cells in each subgroup as graded by the three markers (Yes/No NETosis, +/− CD11b, +/− DEspR).
Statistical analysis
Data in this study are expressed as mean value and standard error for normal distribution or as median and interquartile range if the distribution is skewed. Comparisons between two groups were performed using Mann–Whitney tests. Comparisons between more than two groups were performed using Kruskal–Wallis test with Dunn’s multiple-comparisons test, or two-way ANOVA with Tukey multiple comparisons test. Correlation analysis was performed using a Spearman (skewed distribution) correlation. ClustVis v2.0 was used to compute hierarchical clustering and heat map on measured clinical and laboratory parameters68. P < 0.05 was considered significant.
Acknowledgements
We are grateful to David Groft and the NorDx lab, Dr. Anne Breggia, Sue LaPierre, and the MMC BioBank, a Core Facility, for subject recruitment; Divya Guthikonda and Meghan P. Searight for providing logistical support during sample transportation. We would like to thank our research subjects and their families for their contribution to this project and to offer condolences to the families of those that did not survive.
Author contributions
J.T.d.K., S.R., J.R., I.E. and D.B.S. designed the study. J.R., A.E., C.L., T.M., R.R.R. and D.B.S. organized the recruitment, consent, and blood collection from subjects. J.T.d.K. and S.R. performed flow cytometry analysis and ELISA. V.L.M.H. and N.R.O. developed a methodology and performed the analysis of NET. J.T.d.K., S.R. and D.B.S. wrote the manuscript, which was critically reviewed by D.J.G., I.E., T.M., D.B.S. and R.R.R.
Funding
This work was supported by the Maine Medical Center Cardiovascular Research Institute 2015 Pilot Project Program, NIH/NIGMS under Grants P30GM106391, COBRE in Stem and Progenitor Cell Biology, and Regenerative Medicine and U54GM115516, Northern New England Clinical and Translational Research Network (Translational Technologies Core and Clinical Research Design, Epidemiology & Biostatistics Core), P20GM139745, COBRE in Acute Care Research and Rural Disparities, and Boston Biomedical Innovation Center Drive Grant, NHLBI U54HL119145. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by an unrestricted Grant from NControl Therapeutics, Inc.
Competing interests
Victoria L. M. Herrera and Nelson Ruiz-Opazo are co-inventors on Boston University patents on DEspR; co-scientific founders of NControl Therapeutics, Inc., with the option to license DEspR intellectual property from Boston University. All other co-authors have no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sergey Ryzhov, Email: sryzhov@mmc.org.
David B. Seder, Email: sederd@mmc.org
References
- 1.Yazdanpanah F, Hamblin MR, Rezaei N. The immune system and COVID-19: Friend or foe? Life Sci. 2020;256:117900–117900. doi: 10.1016/j.lfs.2020.117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C. Immune response in COVID-19: Addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Target Ther. 2020;5:84. doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gustine JN, Jones D. Immunopathology of hyperinflammation in COVID-19. Am. J. Pathol. 2021;191:4–17. doi: 10.1016/j.ajpath.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavalli G, Larcher A, Tomelleri A, Campochiaro C, Della-Torre E, De Luca G, Farina N, Boffini N, Ruggeri A, Poli A, Scarpellini P, Rovere-Querini P, Tresoldi M, Salonia A, Montorsi F, Landoni G, Castagna A, Ciceri F, Zangrillo A, Dagna L. Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with COVID-19 and hyperinflammation: A cohort study. Lancet Rheumatol. 2021;3:e253–e261. doi: 10.1016/S2665-9913(21)00012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Q, Meng Y, Wang K, Zhang X, Chen W, Sheng J, Qiu Y, Diao H, Li L. Inflammation and antiviral immune response associated with severe progression of COVID-19. Front. Immunol. 2021;12:631226–631226. doi: 10.3389/fimmu.2021.631226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aboudounya MM, Heads RJ. COVID-19 and toll-like receptor 4 (TLR4): SARS-CoV-2 may bind and activate TLR4 to increase ACE2 expression, facilitating entry and causing hyperinflammation. Mediat. Inflamm. 2021;2021:8874339. doi: 10.1155/2021/8874339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim AHJ, Wu X, Atkinson JP. The beneficial and pathogenic roles of complement in COVID-19. Cleve Clin. J. Med. 2020 doi: 10.3949/ccjm.87a.ccc065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chouaki Benmansour N, Carvelli J, Vivier E. Complement cascade in severe forms of COVID-19: Recent advances in therapy. Eur. J. Immunol. 2021;51(7):1652–1659. doi: 10.1002/eji.202048959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavalcante-Silva LHA, Carvalho DCM, de Almeida Lima É, et al. Neutrophils and COVID-19: The road so far. Int. Immunopharmacol. 2021;2021(90):107233. doi: 10.1016/j.intimp.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, Zhang M, Tan J, Xu Y, Song R, Song M, Wang L, Zhang W, Han B, Yang L, Wang X, Zhou G, Zhang T, Li B, Wang Y, Chen Z, Wang X. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J. Transl. Med. 2020;18:206. doi: 10.1186/s12967-020-02374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carissimo G, Xu W, Kwok I, Abdad MY, Chan Y-H, Fong S-W, Puan KJ, Lee CY-P, Yeo NK-W, Amrun SN, Chee RS-L, How W, Chan S, Fan BE, Andiappan AK, Lee B, Rötzschke O, Young BE, Leo Y-S, Lye DC, Renia L, Ng LG, Larbi A, Ng LFP. Whole blood immunophenotyping uncovers immature neutrophil-to-VD2 T-cell ratio as an early marker for severe COVID-19. Nat. Commun. 2020;11:5243. doi: 10.1038/s41467-020-19080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buja LM, Wolf DA, Zhao B, Akkanti B, McDonald M, Lelenwa L, Reilly N, Ottaviani G, Elghetany MT, Trujillo DO, Aisenberg GM, Madjid M, Kar B. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc. Pathol. 2020;48:107233–107233. doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulte-Schrepping J, Reusch N, Paclik D, Baßler K, Schlickeiser S, Zhang B, Krämer B, Krammer T, Brumhard S, Bonaguro L, De Domenico E, Wendisch D, Grasshoff M, Kapellos TS, Beckstette M, Pecht T, Saglam A, Dietrich O, Mei HE, Schulz AR, Conrad C, Kunkel D, Vafadarnejad E, Xu CJ, Horne A, Herbert M, Drews A, Thibeault C, Pfeiffer M, Hippenstiel S, Hocke A, Müller-Redetzky H, Heim KM, Machleidt F, Uhrig A, Bosquillon de Jarcy L, Jürgens L, Stegemann M, Glösenkamp CR, Volk HD, Goffinet C, Landthaler M, Wyler E, Georg P, Schneider M, Dang-Heine C, Neuwinger N, Kappert K, Tauber R, Corman V, Raabe J, Kaiser KM, Vinh MT, Rieke G, Meisel C, Ulas T, Becker M, Geffers R, Witzenrath M, Drosten C, Suttorp N, von Kalle C, Kurth F, Händler K, Schultze JL, Aschenbrenner AC, Li Y, Nattermann J, Sawitzki B, Saliba AE, Sander LE. Severe COVID-19 Is marked by a dysregulated myeloid cell compartment. Cell. 2020;182:1419–1440. doi: 10.1016/j.cell.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nazarullah A, Liang C, Villarreal A, Higgins RA, Mais DD. Peripheral blood examination findings in SARS-CoV-2 infection. Am. J. Clin. Pathol. 2020;154:319–329. doi: 10.1093/ajcp/aqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zini G, Bellesi S, Ramundo F, d'Onofrio G. Morphological anomalies of circulating blood cells in COVID-19. Am. J. Hematol. 2020;95:870–872. doi: 10.1002/ajh.25824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosales C. Neutrophil: A cell with many roles in inflammation or several cell types? Front. Physiol. 2018;9:113–113. doi: 10.3389/fphys.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grieshaber-Bouyer R, Nigrovic PA. Neutrophil heterogeneity as therapeutic opportunity in immune-mediated disease. Front. Immunol. 2019;10:346. doi: 10.3389/fimmu.2019.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz-Opazo N, Hirayama K, Akimoto K, Herrera VL. Molecular characterization of a dual endothelin-1/Angiotensin II receptor. Mol. Med. (Cambridge, Mass) 1998;4:96–108. [PMC free article] [PubMed] [Google Scholar]
- 20.Herrera VL, Ponce LR, Bagamasbad PD, VanPelt BD, Didishvili T, Ruiz-Opazo N. Embryonic lethality in dear gene-deficient mice: New player in angiogenesis. Physiol. Genomics. 2005;23:257–268. doi: 10.1152/physiolgenomics.00144.2005. [DOI] [PubMed] [Google Scholar]
- 21.Halim A, Kanayama N, Maradny EEI, Maehara K, Terao T. Activated neutrophil by endothelin-1 caused tissue damage in human umbilical cord. Thromb. Res. 1995;77:321–327. doi: 10.1016/0049-3848(95)93835-n. [DOI] [PubMed] [Google Scholar]
- 22.Immler R, Simon SI, Sperandio M. Calcium signalling and related ion channels in neutrophil recruitment and function. Eur. J. Clin. Investig. 2018;48:e12964. doi: 10.1111/eci.12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marini O, Costa S, Bevilacqua D, Calzetti F, Tamassia N, Spina C, De Sabata D, Tinazzi E, Lunardi C, Scupoli MT, Cavallini C, Zoratti E, Tinazzi I, Marchetta A, Vassanelli A, Cantini M, Gandini G, Ruzzenente A, Guglielmi A, Missale F, Vermi W, Tecchio C, Cassatella MA, Scapini P. Mature CD10(+) and immature CD10(-) neutrophils present in G-CSF-treated donors display opposite effects on T cells. Blood. 2017;129:1343–1356. doi: 10.1182/blood-2016-04-713206. [DOI] [PubMed] [Google Scholar]
- 24.Rodeberg DA, Morris RE, Babcock GF. Azurophilic granules of human neutrophils contain CD14. Infect. Immun. 1997;65:4747–4753. doi: 10.1128/iai.65.11.4747-4753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner C, Deppisch R, Denefleh B, Hug F, Andrassy K, Hänsch GM. Expression patterns of the lipopolysaccharide receptor CD14, and the FCgamma receptors CD16 and CD64 on polymorphonuclear neutrophils: Data from patients with severe bacterial infections and lipopolysaccharide-exposed cells. Shock. 2003;19:5–12. doi: 10.1097/00024382-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 26.El-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J. Immunol. 1987;139:2406–2413. [PubMed] [Google Scholar]
- 27.Didangelos A. COVID-19 hyperinflammation: What about neutrophils? mSphere. 2020;5:e00367. doi: 10.1128/mSphere.00367-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, Ulfman LH, Leenen LP, Pickkers P, Koenderman L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J. Clin. Investig. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hellebrekers P, Vrisekoop N, Koenderman L. Neutrophil phenotypes in health and disease. Eur. J. Clin. Investig. 2018;48:e12943. doi: 10.1111/eci.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C, Weber A, Barnes BJ, Egeblad M, Woods RJ, Kanthi Y, Knight JS. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng LG, Ostuni R, Hidalgo A. Heterogeneity of neutrophils. Nat. Rev. Immunol. 2019;19:255–265. doi: 10.1038/s41577-019-0141-8. [DOI] [PubMed] [Google Scholar]
- 32.Fung M, Babik JM. COVID-19 in immunocompromised hosts: What we know so far. Clin. Infect. Dis. 2021;72:340–350. doi: 10.1093/cid/ciaa863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): Systematic review and meta-analysis. J. Intens. Care. 2020;8:36. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Deng Y, Weng Z, Yang L. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis. Int. J. Infect. Dis. 2020;96:131–135. doi: 10.1016/j.ijid.2020.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmadi P, Hartjen P, Kohsar M, Kummer S, Schmiedel S, Bockmann J-H, Fathi A, Huber S, Haag F, Schulze zur Wiesch J. Defining the CD39/CD73 axis in SARS-CoV-2 infection: The CD73- phenotype identifies polyfunctional cytotoxic lymphocytes. Cells. 2020;9:1750. doi: 10.3390/cells9081750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.David JM, Dominguez C, Hamilton DH, Palena C. The IL-8/IL-8R axis: A double agent in tumor immune resistance. Vaccines (Basel) 2016;4:22. doi: 10.3390/vaccines4030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zouki C, Baron C, Fournier A, Filep JG. Endothelin-1 enhances neutrophil adhesion to human coronary artery endothelial cells: Role of ET(A) receptors and platelet-activating factor. Br. J. Pharmacol. 1999;127:969–979. doi: 10.1038/sj.bjp.0702593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.López Farré A, Riesco A, Espinosa G, Digiuni E, Cernadas MR, Alvarez V, Montón M, Rivas F, Gallego MJ, Egido J, et al. Effect of endothelin-1 on neutrophil adhesion to endothelial cells and perfused heart. Circulation. 1993;88:1166–1171. doi: 10.1161/01.cir.88.3.1166. [DOI] [PubMed] [Google Scholar]
- 39.Boros M, Massberg S, Baranyi L, Okada H, Messmer K. Endothelin 1 induces leukocyte adhesion in submucosal venules of the rat small intestine. Gastroenterology. 1998;114:103–114. doi: 10.1016/s0016-5085(98)70638-9. [DOI] [PubMed] [Google Scholar]
- 40.Fagan KA, McMurtry IF, Rodman DM. Role of endothelin-1 in lung disease. Respir. Res. 2001;2:90. doi: 10.1186/rr44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarpelon AC, Pinto LG, Cunha TM, Vieira SM, Carregaro V, Souza GR, Silva JS, Ferreira SH, Cunha FQ, Verri WA., Jr Endothelin-1 induces neutrophil recruitment in adaptive inflammation via TNFα and CXCL1/CXCR2 in mice. Can. J. Physiol. Pharmacol. 2012;90:187–199. doi: 10.1139/y11-116. [DOI] [PubMed] [Google Scholar]
- 42.Bhavsar T, Liu XJ, Patel H, Stephani R, Cantor JO. Preferential recruitment of neutrophils by endothelin-1 in acute lung inflammation induced by lipopolysaccharide or cigarette smoke. Int. J. Chron. Obstruct. Pulmon. Dis. 2008;3:477–481. doi: 10.2147/copd.s2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baumann CL, Aspalter IM, Sharif O, Pichlmair A, Blüml S, Grebien F, Bruckner M, Pasierbek P, Aumayr K, Planyavsky M, Bennett KL, Colinge J, Knapp S, Superti-Furga G. CD14 is a coreceptor of Toll-like receptors 7 and 9. J. Exp. Med. 2010;207:2689–2701. doi: 10.1084/jem.20101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raby A-C, Holst B, Le Bouder E, Diaz C, Ferran E, Conraux L, Guillemot J-C, Coles B, Kift-Morgan A, Colmont CS, Szakmany T, Ferrara P, Hall JE, Topley N, Labéta MO. Targeting the TLR co-receptor CD14 with TLR2-derived peptides modulates immune responses to pathogens. Sci. Transl. Med. 2013;5:185. doi: 10.1126/scitranslmed.3005544. [DOI] [PubMed] [Google Scholar]
- 45.Kuuliala K, Orpana A, Leirisalo-Repo M, Repo H. Neutrophils of healthy subjects with a history of reactive arthritis show enhanced responsiveness, as defined by CD11b expression in adherent and non-adherent whole blood cultures. Rheumatology. 2007;46:934–937. doi: 10.1093/rheumatology/kem039. [DOI] [PubMed] [Google Scholar]
- 46.Haziot A, Tsuberi BZ, Goyert SM. Neutrophil CD14: biochemical properties and role in the secretion of tumor necrosis factor-alpha in response to lipopolysaccharide. J. Immunol. 1993;150:5556–5565. [PubMed] [Google Scholar]
- 47.Hattar K, van Bürck S, Bickenbach A, Grandel U, Maus U, Lohmeyer J, Csernok E, Hartung T, Seeger W, Grimminger F, Sibelius U. Anti-proteinase 3 antibodies (c-ANCA) prime CD14-dependent leukocyte activation. J. Leukoc. Biol. 2005;78:992–1000. doi: 10.1189/jlb.0902442. [DOI] [PubMed] [Google Scholar]
- 48.Takeshita S, Nakatani K, Kawase H, Seki S, Yamamoto M, Sekine I, Yoshioka S. The role of bacterial lipopolysaccharide-bound neutrophils in the pathogenesis of Kawasaki disease. J. Infect. Dis. 1999;179:508–512. doi: 10.1086/314600. [DOI] [PubMed] [Google Scholar]
- 49.Becker RC. COVID-19-associated vasculitis and vasculopathy. J. Thromb. Thrombol. 2020;50:499–511. doi: 10.1007/s11239-020-02230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riera N, Canalejo K, Aixalá M, Rosso M, Gaddi E, Bracco MM, Galassi N. Detection of CD16low neutrophil subpopulations. Cytometry B Clin. Cytom. 2003;51:45–46. doi: 10.1002/cyto.b.10004. [DOI] [PubMed] [Google Scholar]
- 51.Struyf S, Gouwy M, Dillen C, Proost P, Opdenakker G, Van Damme J. Chemokines synergize in the recruitment of circulating neutrophils into inflamed tissue. Eur. J. Immunol. 2005;35:1583–1591. doi: 10.1002/eji.200425753. [DOI] [PubMed] [Google Scholar]
- 52.Nardelli B, Tiffany HL, Bong GW, Yourey PA, Morahan DK, Li Y, Murphy PM, Alderson RF. Characterization of the signal transduction pathway activated in human monocytes and dendritic cells by MPIF-1, a specific ligand for CC chemokine receptor 1. J. Immunol. 1999;162:435–444. [PubMed] [Google Scholar]
- 53.Nardelli B, Morahan DK, Bong GW, Semenuk MA, Kreider BL, Garotta G. Dendritic cells and MPIF-1: Chemotactic activity and inhibition of endogenous chemokine production by IFN-γ and CD40 ligation. J. Leukoc. Biol. 1999;65:822–828. doi: 10.1002/jlb.65.6.822. [DOI] [PubMed] [Google Scholar]
- 54.Arruda-Silva F, Bianchetto-Aguilera F, Gasperini S, Polletti S, Cosentino E, Tamassia N, Cassatella MA. Human neutrophils produce CCL23 in response to various TLR-agonists and TNFα. Front. Cell. Infect. Microbiol. 2017;7:176. doi: 10.3389/fcimb.2017.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simats A, García-Berrocoso T, Penalba A, Giralt D, Llovera G, Jiang Y, Ramiro L, Bustamante A, Martinez-Saez E, Canals F, Wang X, Liesz A, Rosell A, Montaner J. CCL23: A new CC chemokine involved in human brain damage. J. Intern. Med. 2018;283:461–475. doi: 10.1111/joim.12738. [DOI] [PubMed] [Google Scholar]
- 56.Veras FP, Pontelli MC, Silva CM, Toller-Kawahisa JE, de Lima M, Nascimento DC, Schneider AH, Caetité D, Tavares LA, Paiva IM, Rosales R, Colón D, Martins R, Castro IA, Almeida GM, Lopes MIF, Benatti MN, Bonjorno LP, Giannini MC, Luppino-Assad R, Almeida SL, Vilar F, Santana R, Bollela VR, Auxiliadora-Martins M, Borges M, Miranda CH, Pazin-Filho A, da Silva LLP, Cunha LD, Zamboni DS, Dal-Pizzol F, Leiria LO, Siyuan L, Batah S, Fabro A, Mauad T, Dolhnikoff M, Duarte-Neto A, Saldiva P, Cunha TM, Alves-Filho JC, Arruda E, Louzada-Junior P, Oliveira RD, Cunha FQ. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020;217:e20201129. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aldabbous L, Abdul-Salam V, McKinnon T, Duluc L, Pepke-Zaba J, Southwood M, Ainscough AJ, Hadinnapola C, Wilkins MR, Toshner M, Wojciak-Stothard B. Neutrophil extracellular traps promote angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2016;36:2078–2087. doi: 10.1161/ATVBAHA.116.307634. [DOI] [PubMed] [Google Scholar]
- 58.Forel JM, Roch A, Marin V, Michelet P, Demory D, Blache JL, Perrin G, Gainnier M, Bongrand P, Papazian L. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit. Care Med. 2006;34:2749–2757. doi: 10.1097/01.CCM.0000239435.87433.0D. [DOI] [PubMed] [Google Scholar]
- 59.Smith MA, Hibino M, Falcione BA, Eichinger KM, Patel R, Empey KM. Immunosuppressive aspects of analgesics and sedatives used in mechanically ventilated patients: An underappreciated risk factor for the development of ventilator-associated pneumonia in critically ill patients. Ann. Pharmacother. 2014;48:77–85. doi: 10.1177/1060028013510698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/ (Accessed 28 October 2021). [PubMed]
- 61.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh M-d, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC. Remdesivir for the treatment of covid-19—Final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santoro F, Núñez-Gil IJ, Viana-Llamas MC, Maroun Eid C, Romero R, Fernández Rozas I, Parisi A, Becerra-Muñoz VM, García Aguado M, Huang J, Maltese L, Cerrato E, Alfonso-Rodriguez E, Castro Mejía AF, Marin F, Raposeiras Roubin S, Pepe M, Moreno Munguia VH, Feltes G, Navas JV, Cortese B, Buzón L, Liebetrau C, Ramos-Martinez MR, Fernandez-Ortiz A, Estrada V, Brunetti ND. Anticoagulation therapy in patients with coronavirus disease 2019: Results from a multicenter international prospective registry (health outcome predictive evaluation for corona virus disease 2019 [HOPE-COVID19]) Crit. Care Med. 2021;49:e624. doi: 10.1097/CCM.0000000000005010. [DOI] [PubMed] [Google Scholar]
- 64.Grasselli G, Scaravilli V, Mangioni D, Scudeller L, Alagna L, Bartoletti M, Bellani G, Biagioni E, Bonfanti P, Bottino N, Coloretti I, Cutuli SL, De Pascale G, Ferlicca D, Fior G, Forastieri A, Franzetti M, Greco M, Guzzardella A, Linguadoca S, Meschiari M, Messina A, Monti G, Morelli P, Muscatello A, Redaelli S, Stefanini F, Tonetti T, Antonelli M, Cecconi M, Foti G, Fumagalli R, Girardis M, Ranieri M, Viale P, Raviglione M, Pesenti A, Gori A, Bandera A. Hospital-acquired infections in critically-ill COVID-19 patients. Chest. 2021;160:454. doi: 10.1016/j.chest.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, Adhikari NKJ, Amato MBP, Branson R, Brower RG, Ferguson ND, Gajic O, Gattinoni L, Hess D, Mancebo J, Meade MO, McAuley DF, Pesenti A, Ranieri VM, Rubenfeld GD, Rubin E, Seckel M, Slutsky AS, Talmor D, Thompson BT, Wunsch H, Uleryk E, Brozek J, Brochard LJ. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2017;195:1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 66.Shelhamer MC, Wesson PD, Solari IL, Jensen DL, Steele WA, Dimitrov VG, Kelly JD, Aziz S, Gutierrez VP, Vittinghoff E, Chung KK, Menon VP, Ambris HA, Baxi SM. Prone positioning in moderate to severe acute respiratory distress syndrome due to COVID-19: A cohort study and analysis of physiology. J. Intens. Care Med. 2021;36:241–252. doi: 10.1177/0885066620980399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche J-D, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent J-L, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Crit. Care Med. 2017;43:304. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 68.Metsalu T, Vilo J. ClustVis: A web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015;43:W566–W570. doi: 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]