Abstract
One in 10 persons in the world aged 40 years and older will develop the syndrome of HFpEF (heart failure with preserved ejection fraction), the most common form of chronic cardiovascular disease for which no effective therapies are currently available. Metabolic disturbance and inflammatory burden contribute importantly to HFpEF pathogenesis. The interplay within these two biological processes is complex; indeed, it is now becoming clear that the notion of metabolic inflammation—metainflammation—must be considered central to HFpEF pathophysiology. Inflammation and metabolism interact over the course of syndrome progression, and likely impact HFpEF treatment and prevention. Here, we discuss evidence in support of a causal, mechanistic role of metainflammation in shaping HFpEF, proposing a framework in which metabolic comorbidities profoundly impact cardiac metabolism and inflammatory pathways in the syndrome.
Keywords: HFpEF, Inflammation, Obesity, Immunity, Metabolism
Introduction
The human race is facing the unprecedented challenge of two major epidemics: obesity and heart failure (HF). The World Health Organization reports that in 2016 more than 1.9 billion adults worldwide were overweight and, of these, over 650 million were obese (13% of the world’s adult population).1 Similarly, despite the smaller scale, HF is a burgeoning global public health issue affecting more than 26 million people worldwide. It is pivotal to recognize that these conditions—obesity/metabolic syndrome/diabetes and HF—are epidemiologically and pathophysiological intertwined, culminating in an unprecedented burden on quality of life and global healthcare expenditures. As HF with preserved ejection fraction (HFpEF)—said to be the single greatest unmet need in cardiovascular medicine—is uniquely linked to these burgeoning comorbidities, focus on HFpEF is of paramount importance.
Indeed, the majority of HFpEF individuals are overweight or obese, and increased adiposity is associated with a worsening of functional parameters in HFpEF.2,3 Although obesity also increases the risk of coronary artery disease, which can impair systolic function leading to HF with reduced ejection fraction (HFrEF), obese individuals are at markedly increased risk of HFpEF independent of ischaemic cardiac injury.4,5 Despite their close relationship, mechanisms underlying obesity-induced alterations in HFpEF are poorly understood.
Over the last 10 years, a causal link between adiposity and alterations in cellular and molecular mediators of inflammation has been recognized. This metabolism-induced inflammation has been termed ‘metainflammation’ to describe the chronic low-grade inflammatory response in obesity, diabetes, and other metabolic diseases.6 Metainflammation in the context of metabolic syndrome occurs in several tissues, including the heart. One of the hallmarks of metabolic alterations in cardiovascular diseases is toxic accumulation of lipids (i.e. lipotoxicity). Among several potential mechanisms of lipotoxicity-induced cardiac dysfunction, it is now established that immunometabolic pathways are greatly modulated by lipids and linked to lipotoxicity.
Here, we discuss existing evidence suggesting that HFpEF can be framed as an obesity-associated disease in which metabolic disturbance, inflammation, and impaired cardiac function are intertwined.
Clinical evidence of HFpEF as a cardiometabolic syndrome
HFpEF is a syndrome of epidemic proportions, accounting for at least 50% of HF hospital admissions with rapidly increasing incidence and prevalence, especially among the elderly.7 Defining HFpEF is challenging. Canonical clinical presentations of HF signs and symptoms, together with heterogeneity in the use of diagnostic tools and criteria, as well as the lack of proven-effective treatments, makes HFpEF a complex entity.8
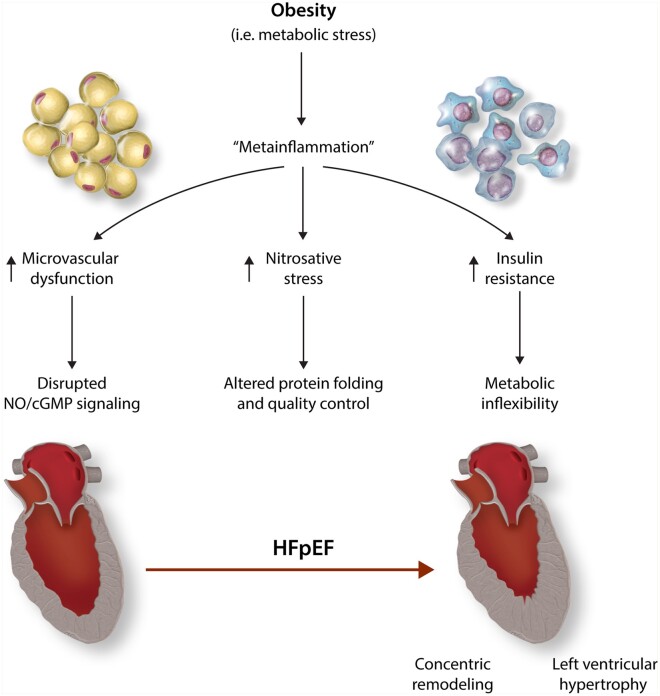
HFpEF cannot be viewed as a single disease.9 Based on epidemiological, clinical, and laboratory findings, multiple phenotypes of HFpEF can be identified.10 Patients with HFpEF comprise elderly women with hypertension and stiff arteries as well as obese/diabetic men with abnormal metabolism and liver and kidney dysfunction.11 Cardiac structural abnormalities are also variably present among the different phenogroups, including left atrial enlargement and various types of left ventricular remodelling and hypertrophy (LVH).12–15 Epidemiological studies have shown concentric, as opposed to eccentric, LVH as a common feature in patients with HFpEF16,17 (Figure 1). Importantly, these differences in clinical and pathophysiological phenotypes drive diverse prognoses and differential responses to therapy.11,18
Figure 1.
Metabolism and Immunity Coupling in HFpEF. Metainflammation is a chronic low-grade systemic state of inflammation associated with obesity. Systemic inflammation has been recognized as a cause of HFpEF through: (i) microvascular dysfunction and downregulation of NO/cGMP signalling; (ii) nitrosative stress and protein quality control impairment, altering the dynamic state of cardiomyocyte molecular constituents. In addition, nutrient overload in obesity has immune and metabolic consequences on insulin sensitivity. Insulin resistance limits cardiac metabolic flexibility and hampers energy provision (iii).
A distinct feature of HFpEF is the presence of multiple comorbidities, in aggregate shaping the complexity of the syndrome. Together with hypertension and ageing,19,20 a main risk factor for HFpEF is obesity.21 Increased body mass index (BMI) has been associated with increased risk of incident HF.21 In particular, it has been reported than 80% of HFpEF patients in the USA are overweight or obese,2 with an average BMI exceeding 35 kg/m2.3 Obese patients with HFpEF present with worse New York Heart Association class, more severe parameters of adverse cardiac remodelling, increased plasma volume, and decreased exercise capacity compared to non-obese HFpEF patients.3,22
It is now becoming clear that adipose tissue is likely involved in HFpEF pathophysiology through multiple mechanisms beyond the simple impact of greater mechanical load with increased body weight.5 In fact, obesity might amplify its deleterious effects both indirectly, promoting other comorbidities, such as insulin resistance and hypertension but also directly, given the fact that adipose tissue is highly metabolically active and capable of releasing regulatory factors—adipokines—involved in promoting a systemic pro-inflammatory state. Thus, the impact of obesity on HFpEF pathophysiology encompasses haemodynamic, neurohumoral, and inflammatory mechanisms (Figure1).
Comorbidity-driven systemic inflammation in HFpEF
Accumulating evidence has emerged on the role of a systemic pro-inflammatory state, predominantly induced by obesity and metabolic stress,10 as a major determinant of HFpEF pathophysiology. For example, it has been proposed that microvascular endothelial inflammation impairs endothelial nitric oxide (NO) production, triggering cardiomyocyte dysfunction.23,24 We and others have demonstrated increased burden of inflammation-dependent oxidative and nitrosative stress in HFpEF.25,26 Additionally, recruitment of inflammatory cells has been recognized in endomyocardial biopsies from HFpEF patients.27 Taken together, these findings position HFpEF as a manifestation of a chronic cardiovascular inflammatory disorder. Intriguingly, the extent to which elements of innate and adaptive immunity participate in the metainflammatory pathophysiology of HFpEF is unknown.
The world’s population is ageing. The global population aged 60 years or over reached nearly 1 billion in 2017, and the number of elderly is expected to double by 2050.28 Based on this, the number of individuals with HF—HFpEF in particular—is expected to rise steadily over the next 20 years.7,29 Whereas HFpEF is epidemiologically linked to ageing, mechanisms whereby senescence contributes to HFpEF pathophysiology are largely unknown. As mentioned, similar to obesity, metabolic syndrome, and diabetes, ageing is characterized by chronic, low-grade, sterile inflammation—a condition that has been termed ‘inflammaging’.30–33 Intriguingly, metainflammatory events may precede and contribute to inflammaging and vice versa, sharing in common a number of signalling pathways and molecular effectors, fuelling both metainflammation and inflammaging as drivers of cardiometabolic disease.34 Metabolic regulation of ageing is complex and involves the repurposing of metabolic pathways towards energy provision for maintenance and reparative processes.35 Nutrient availability impacts longevity meaningfully. Whereas caloric restriction seems to be protective, over-nutrition might accelerate ageing. Interestingly, in both extreme states of nutrient imbalance (malnutrition and over-nutrition) inflammation flourishes.32
In light of this, one fundamental question arises: what is the evidence in support of the notion that comorbidities, and obesity, in particular, act as an upstream source of circulating cytokines inducing a systemic pro-inflammatory state in HFpEF?
As proof-of-concept, one study reported that the comorbidity burden in HFpEF correlates with elevations in circulating levels of C-reactive protein (CRP).36 Reports of elevated circulating inflammatory biomarkers in HFpEF are not limited to CRP but also include soluble interleukin-1 (IL-1) receptor-like 1, growth differentiation factor 15 (GDF15), soluble ST2, and pentraxin-3.37–41 Elevations in these inflammatory markers are of greater magnitude in HFpEF than in HFrEF,37,40,41 or than in other acute and decompensated conditions.42 High levels of tumour necrosis factor alpha (TNFα) are also predictive of incident risk of HFpEF.43 Importantly, increases in inflammatory markers are independently associated with asymptomatic diastolic dysfunction in patients with metabolic syndrome ‘at risk’ for HFpEF. Interestingly, this correlation is stronger in hypertensive patients with metabolic syndrome than in those with hypertension alone.44
Consistent with these data, an elegant network analysis was conducted to infer the most prevalent pathophysiological pathways involved in HFpEF and HFrEF based on circulating biomarker profiles. Analysis of protein–protein interactions has shown that HFpEF biomarkers are specifically related to biological mechanisms of inflammation and extracellular matrix reorganization.45 In addition, indirect evidence for HFpEF as a systemic inflammatory disease is suggested by the presence of extra-cardiac inflammatory manifestations in the syndrome.10,46
Collectively, a growing body of evidence points to a causal role of a comorbidity-driven, systemic pro-inflammatory state in HFpEF. Given the role of inflammation, signals arising from adipose tissue metabolism and from intermediary cardiac metabolism may mutually drive immune responses in obese HFpEF.
Impact of metabolic derangements on immunity
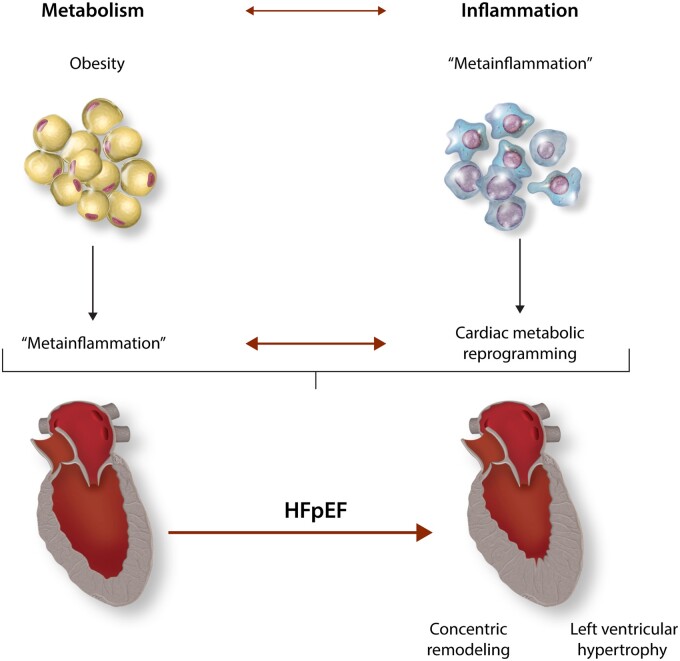
Metabolic alterations are frequently coupled with immune dysregulation. In fact, metabolic processes regulate immune cell responses and vice versa. This bidirectional crosstalk is emerging as a critical component of the pathogenesis of cardiometabolic diseases, such as HFpEF (Figure 2).
Figure 2.
Overview of HFpEF pathophysiology: metabolic inflammation moves centre stage. Metabolic dysregulation is coupled with immune dysregulation. Systemic disruption of metabolic homoeostasis in obesity elicits a chronic low-grade inflammatory response (i.e. metainflammation). In the heart, cytokines and other inflammatory mediators modulate cardiac metabolism. Downstream, these alterations contribute to structural and functional remodelling in HFpEF.
Immunometabolism—the interplay between immunological and metabolic processes—is a growing field of investigation, with extensive implications for cardiovascular disease.47,48 The impact of obesity on HF entails a number of signals and stimuli at the interface of innate and adaptive immunity. Whereas mechanisms of cardiac metabolic reprogramming in response to chronic, systemic, low-grade inflammation in obesity have been explored only partially, immunophenotypic changes occurring in obesity have been investigated in greater depth.
In obesity, adipose tissue expansion leads to secretion of chemokines, thereby initiating macrophage recruitment.49,50 It is known that signals from this microenvironment direct macrophage polarization, and that M1-polarized macrophages display a pronounced pro-inflammatory phenotype. An increased ratio of infiltrating M1 to M2 macrophages is a hallmark of adipose tissue inflammation in obesity.51 In this setting, the increased prevalence of the M1 macrophage subset is responsible for the release of cytokines, producing a systemic pro-inflammatory state. In HFpEF, local inflammation and macrophage infiltration of epicardial adipose tissue (EAT) have been proposed as a potential mechanism contributing to cardiac dysfunction. Despite the fact that experimental evidence of EAT inflammation in HFpEF remains lacking, the notion of a local metainflammatory insult as a contributor to HFpEF pathogenesis merits investigation.52,53
The contribution of inflammatory cells to some elements of HFpEF pathophysiology (e.g. diastolic dysfunction) has been explored in animal models and in human subjects.54 A recent study has reported a causal role of cardiac macrophage expansion and IL-10 production in myocardial fibrosis and diastolic dysfunction in a mouse model of hypertensive HFpEF.55 Despite the lack of metabolic stress in this model, it is clear that immune cells contribute to key pathophysiological features of HFpEF. Similarly, others have provided evidence of both classical and alternative macrophage activation by analysing serum from hypertensive HFpEF patients.56
Metabolic alterations can provoke changes in macrophage functional status. Metabolic reprogramming is a key driver of macrophage polarization in cardiac injury and in the presence of hypertension and obesity.53 In particular, endoplasmic reticulum (ER) stress has been implicated in macrophage activation under conditions of metabolic inflammation.57 Consistent with this, protein quality control is an emerging research focus in HFpEF pathophysiology. Accumulation of misfolded proteins has been uncovered recently in clinical HFpEF by evaluating the presence of wild-type transthyretin amyloidosis in elderly patients.58 Accumulation of unfolded proteins is the leading cause of ER stress, a process highly interconnected with inflammatory pathways.59,60 We and others have shown that in obesity and HFpEF, metabolic inflammation is linked to ER dysfunction through inducible nitric oxide synthase (iNOS)-dependent S-nitrosylation of inositol-requiring enzyme 1 alpha (IRE1α), one of the main arms of unfolded protein response signalling cascade.26,61 Additional studies are required to explore the role of other protein quality control-related pathways in HFpEF, as well as the specific contribution of distinct immune cell populations in metainflammatory mechanisms. Notably, despite the research efforts to date, identification of the predominant cell types (e.g. cardiomyocytes vs. immune cells) responsible for the metabolic alterations observed in HFpEF hearts remains limited. In other words, the extent to which changes in cardiac metabolism and intracellular signalling pathways observed in HFpEF reflect primary changes in inflammatory cell density, composition, and metabolic state as opposed to changes in cardiomyocyte metabolism, is unclear. This knowledge gap reflects the difficulties of dealing with a complex and heterogeneous syndrome, such as HFpEF, for which multiple, complementary research approaches are required. Based on this reality, a call for more integrated clinical and experimental approaches, inclusive of the broad spectrum of HFpEF comorbidities and their metabolic and inflammatory correlates, has been highlighted as a relevant research priority in the field.62
Metabolic intermediates regulating inflammatory pathways
Adaptations of metabolic state greatly impact numerous fundamental cellular regulatory functions. Recognition of signalling properties of metabolic intermediates and their interaction with gene expression pathways is a groundbreaking advance in many areas of biology.63 In immune cells, the dynamics of intermediary metabolism are crucial for a cell’s ability to divide, differentiate, and activate properly.64
Metabolic cycles have evolved to ensure optimal use of cellular resources, taking advantage of a multitude of interconnections with other cellular functions.65 Central to macrophage expansion and polarization in HFpEF, intermediates of the tricarboxylic acid cycle (TCA) can dictate changes in the expression of genes involved in inflammatory pathways.66,67 In addition to its fundamental role in energy provision, the TCA cycle is a specific immunometabolic hub in macrophages.68 Increased macrophage exposure to extracellular glucose, as well as to pro-inflammatory fatty acids (FA), activates nuclear factor-κB (NF-κB) and promotes M1 polarization.53 A distinct metabolic signature of M1-macrophages is up-regulated glycolysis, similar to what is universally known as the ‘Warburg effect’, with suppression of mitochondrial oxidative phosphorylation and, as consequence, the rewiring of metabolic flux through the TCA cycle, leading to accumulation of succinate. Succinate accumulation participates in M1 macrophage polarization, promoting hypoxia inducible factor-1α stabilization and increasing IL-1β production.69
Additional metabolic intermediates of the TCA cycle participate in the regulation of gene expression in immune cells through epigenetic mechanisms. Fumarate and α-ketoglutarate regulate the activity of enzymes required for epigenetic modifications, known as α-ketoglutarate-dependent dioxygenases.70,71 In particular, α-ketoglutarate is a cofactor for Jumonji-C-domain-containing histone demethylases, a histone demethylase, and the ten-eleven translocation family of 5-methylcytosine hydroxylases, involved in DNA demethylation. Increased levels of α-ketoglutarate exert anti-inflammatory effects in macrophages. Conversely, low α-ketoglutarate/succinate ratios promote inflammation.70 Consistently, fumarate, a competitive inhibitor of α-ketoglutarate-dependent dioxygenases, promotes epigenetic modifications leading to increased TNFα and interleukin-6 (IL-6) production.71
Other examples of TCA cycle-derived metabolic intermediates regulating inflammatory gene expression include the synthesis of itaconate from cis-aconitate.72,73 Itaconate levels in macrophages are regulated by iNOS-derived NO.74 This fact supports a model in which NO production by iNOS regulates the production of a metabolite critically involved in regulating metabolic remodelling and cytokine production. Metabolic reprogramming in classically activated macrophages also involves rewiring of metabolic flux towards the aspartate-arginosuccinate shunt, a series of reactions connecting the TCA cycle to the urea cycle and NO production.73
Among the many TCA cycle intermediates active in the regulation of immune cell function, citrate links several biological processes, playing an important role in the metabolic reprogramming of inflammation.75 Inflammatory events upregulate citrate mitochondrial carrier SLC25a1 which exports citrate to the cytosol.76 There, citrate is converted to acetyl-coA by the ATP-citrate lyase. Increased cytosolic acetyl-CoA serves as a cofactor of MEC17 acetyltransferase, leading to tubulin acetylation and increased production of IL-10.77Acetyl-CoA can also be carboxylated to malonyl-CoA and then used for FA biosynthesis, contributing to the production of lipid rafts, prostaglandins, and other inflammatory molecules.78 In addition, accumulation of malonyl-CoA can promote protein malonylation, a lysine-based post-translational modification that has been shown to facilitate TNFα translation.79,80
Whereas all these mechanisms are involved in the metainflammatory reprogramming of macrophages, the impact of metabolic changes in macrophages or other immune cells on cardiac function in HFpEF remains unknown. In aggregate, the totality of evidence supports the role of intermediate metabolites as critical immunomodulating molecules providing potential mechanistic insights into metainflammatory events occurring in HFpEF and other cardiometabolic diseases.
Impact of immunity on cardiac energy metabolism
A distinct feature of cardiac biology and an integral part of myocardial adaptation is metabolic flexibility, i.e. the ability to transition among different energy substrates depending on specific physiological and pathological conditions, substrate availability, and hormonal milieu. The heart can select the most suitable source of energy substrate depending on extant conditions, shifting from one prevailing class of substrate to another, and is therefore often portrayed as an ‘omnivore’ due to this energetic flexibility.81 Under aerobic conditions, FA are the main fuel for the heart, and mitochondrial β-oxidation provides 60–90% of the acetyl-CoA required for cardiac contraction and relaxation.82 Metabolic flexibility occurs in response to changes in oxygen and substrate supply or in response to changes in workload.83 For example, under hypoxic conditions, the heart mainly oxidizes carbohydrates. Reliance of the heart on glucose is an energetically favourable adaptation, as documented in vivo.84 These adaptations are primed by high cardiac ATP demand: with a relatively low ATP content (5 µmol/g wet wt) and a high rate of ATP hydrolysis (∼ 0.5 µmol·g wet wt−1·s−1 at rest), under normal conditions, the complete turnover of the myocardial ATP pool occurs approximately every 10 s85,86 and accelerates in proportion to increases in cardiac workload.87
Tight coupling of cardiac metabolism, energy provision, and contractile function is epitomized by the notion of the failing heart as an ‘engine out of fuel’.88 Nevertheless, the link between energy transfer and cardiac contraction becomes less clear in dysregulated metabolic states, such as diabetes and obesity: under these conditions, even in the absence of overt blood flow alterations, hence despite the uninterrupted supply of energy substrates, the heart is not starved but fails ‘in the midst of plenty’, and its characteristic metabolic flexibility is impaired.89
Mitochondrial metabolism is governed by calcium (Ca2+).90 Specifically, Ca2+ concentrations in the mitochondrial matrix ([Ca2+]m) regulate mitochondrial ATP production.90 Despite the large body of evidence in HFrEF suggesting that [Ca2+]m and, as a consequence, ATP production are decreased, much less is known about Ca2+-dependent regulation of mitochondrial metabolism in HFpEF.91 Recently, cardiac mitochondrial calcium kinetics have been explored in a rat model of metabolically-induced HFpEF.92 Interestingly, in striking contrast to that observed in HFrEF models, [Ca2+]m was increased in HFpEF hearts, coupled with mitochondrial functional alterations and cytosolic Ca2+ mishandling.92 Despite this evidence, the specific role of [Ca2+]m cycling in metabolic remodelling in HFpEF remains elusive.
A large focus of research has been directed towards the metabolic reprogramming of immune cells during the course of cardiac inflammatory processes or towards the role of inflammatory cytokines in cardiac remodelling as occurs in diabetes.93,94 More recently, additional insights into cardiomyocyte metabolic reprogramming in response to metabolic inflammation have emerged. Intriguingly, despite the pivotal role of Ca2+ in regulating many functions of both innate and adaptive immunity,95 Ca2+ alterations in immune cells in the context of metabolic disease remain only partially understood.
Immune regulation of cardiac intermediary metabolism
The heart is highly responsive to external stimuli and changes in workload demand. A chronic inflammatory state, as occurs in HFpEF, impacts cardiac microenvironment and consequently its energetic state. Both immune cells and cardiomyocytes are marked by high metabolic rates, required to meet their energy demands. Therefore, the relative contribution of one or the other cell type to cardiac metabolic adaptation during chronic inflammatory stress and limited access to nutrients is difficult to assess. Crosstalk between different cell types comprises a complex network of signals, involving cytokines and metabolites. Metabolic signals link both cardiac structure and function, and metabolic remodelling is intertwined with—if not causative of—functional and structural remodelling.96 Significant efforts have been made to improve the assessment of myocardial metabolic flux in the elucidation of HFpEF pathophysiology, including employing systems biology approaches.97,98
The limited number of studies currently available focusing on myocardial substrate metabolism in response to inflammation do not specifically refer to HFpEF. In an animal model of high-fat diet and IL-6 infusion, AMP-activated protein kinase (AMPK), an enzyme known to be a crucial energy sensor in cells, is suppressed.99 In this study, blunted AMPK activity was associated with impaired glucose metabolism. Conversely, glucose oxidation increased in a different model of cardiac exposure to metabolic inflammation, featuring TNFα elevation.100 This effect has been mechanistically linked to decreased expression of pyruvate dehydrogenase kinase 4 (PDK4) as a consequence of proliferator-activated-receptor γ coactivator-1 α (PGC-1α) inhibition by binding with p65 subunit of NF-κB.101
Whereas these data raise the prospect of effects of circulating cytokines on cardiac intermediary metabolism, they are not conclusive. As consequence, metainflammatory stress-dependent regulation of cardiac glucose metabolism remains controversial.
NO and iNOS as metabolic regulators
NO is a signalling molecule with a plethora of functions in the cardiovascular system, spanning regulation of endothelial homoeostasis to regulation of cardiac contraction.102,103 Besides its role in vasodilation, NO is implicated in oxygen utilization and mitochondrial respiration, as well as in modulation of cardiac energy substrate metabolism.104,105
It has been proposed that reduced NO bioavailability in HFpEF exists due to diversion of NO to peroxynitrite under conditions of inflammation-induced reactive oxygen species production.23 In light of the connection between NO biology and cardiac metabolism, one could argue that the notion of low NO availability affecting cardiac energy provision in HFpEF is a hypothesis that remains to be tested. Whereas we lack targeted studies, it is worth mentioning that acute or chronic inhibition of NO synthesis in dogs boosts cardiac glucose and lactate oxidation.106–108 Similarly, others have taken advantage of the ex vivo working heart model to show that inhibition of NO synthesis provokes selective enhancement of glucose uptake.109 Specifically, this effect has been related to decreased concentration of cyclic guanosine monophosphate (cGMP), a downstream effector of NO. The functional result of this metabolic shift is unclear. Also, a note of caution is warranted in extending this knowledge to metabolic remodelling in HFpEF, since we still know very little about cardiac metabolic adaptations in this syndrome. And as noted, a substrate shift towards glucose oxidation is a favourable energetic adaptation.84 One might speculate that microvascular dysfunction in HFpEF is a mechanism leading to hypoxia and this might direct substrate preference towards carbohydrate oxidation. However, NO-cGMP signalling is only one of many elements in HFpEF pathophysiology, and other mechanisms may well be involved.
We recently showed that the combination of metabolic stress induced by obesity/diabetes coupled with the mechanical stress induced by hypertension recapitulates in mice most of the clinical features of HFpEF.26 Mechanical stress and metabolic stress exert heterogeneous effects on cardiac energy metabolism. Whereas chronic pressure overload is known to induce a shift in myocardial metabolism towards glucose oxidation,110–112 increased FA availability in obesity enhances cardiac fatty acid oxidation (FAO).113 It is thereby reasonable to speculate that cardiac metabolism in HFpEF is challenged by conflicting metabolic needs. Also, metabolic flexibility might be hampered in the presence of fuel excess, lipotoxicity, and insulin resistance. Deciphering mechanisms of mitochondrial dysfunction and FAO pathways in HFpEF will be critical to the elucidation of specific hallmarks of cardiac metabolic alterations in this syndrome.
Metabolic reprogramming is the result of highly orchestrated interplay of signalling pathways that encompass transcriptional control and biochemical checkpoints. The recent identification of iNOS upregulation in HFpEF highlights the potential of NO-based post-translational protein modification, such as S-nitrosylation, in modulating cardiac metabolic pathways. Mainly known to act as an inflammatory master mediator, iNOS is a metabolic enzyme whose impact on cell metabolism has been explored extensively.114 Given its role in HFpEF, the connection between iNOS activity and insulin resistance, for example, might be of relevance. Indeed, nitrosative stress in skeletal muscle has been associated with protein modifications of key elements of insulin signalling, including insulin receptor substrate-1/2 and Akt, in the context of ageing and obesity.115,116 This heightens the complexity of insulin resistance and metainflammation in HFpEF. Interestingly, nitrosative stress might also induce post-translational modifications of key glycolytic and FAO enzymes, directly influencing metabolic fluxes.117,118
Metainflammation: evolutionary perspective on a potential therapeutic target in HFpEF
Metabolism and inflammation are integrated at the molecular level through highly conserved pathways. Indeed, mechanisms acting at the intersection of metabolism and inflammation underlie aspects of how cells and tissues respond to environmental changes and external stimuli in order to maintain homoeostasis. For example, it is known that pattern recognition receptors are able to operate as metabolic sensors. An example is toll-like receptor 4 activation by free fatty acids that leads to NF-κB signalling activation.119 From an evolutionary perspective, the intersection between metabolism and inflammation can be attributed to complementary means of coping with intermittent nutrient supply and high risk of infectious disease, together promoting survival in hostile conditions.6
The evolutionary underpinnings of the metabolism-inflammation interplay might be informative in our understanding of HFpEF pathophysiology. In cardiac remodelling, a transition from adaptive responses to those that are maladaptive can often be inferred. In the context of HFpEF, one should recognize that a mismatch between our modern environment and human evolutionary history worsens the cost-benefit trade-off of inflammation.120 In other words, environmental factors have changed dramatically in recent centuries, and the evolutionary cost-benefit balance of inflammation in modern human populations is not optimized to the current environment.
Of course, inflammation and metabolism serve different purposes, and it is reasonable that innate immunity may induce an alteration in tissue function when fitness or even survival of the organism is threatened. A cost-benefit trade-off mismatch provides the basis for consideration of clinical intervention as a way to modulate inflammatory responses that are potentially not suited to the environmental conditions. Finally, antagonistic pleiotropy should be taken into consideration.120 This term refers to a phenomenon explaining why biological mechanisms that positively impact fitness at young age occur at a cost in later phases of lifespan. The presence of an inflammatory substrate in many age-related syndromes, such as HF may be an example. On the basis of the role of inflammation in HFpEF, a suboptimal cost-benefit trade-off of immune responses may contribute to increased susceptibility of obese individuals to the syndrome.
Rapidly changing environmental conditions are shaping cardiovascular disease in developed countries.121,122 Cardiac biology, in general, is highly influenced by both environmental stimuli and genetic background.123,124 We have learned to target pathways and molecules involved in cardiac adaptation to stress and to develop therapeutic approaches accordingly. For example, neurohumoral antagonism and haemodynamic unloading are cornerstones of HFrEF therapy.125 Whereas relieving neurohumoral and haemodynamic stresses has been fundamental to combat HFrEF to date, the evolving understanding of HFpEF as a cardio-metabolic disease challenges us to consider a different therapeutic rationale, mirroring inflammatory, and metabolic stress as potential targets of clinical intervention. Metabolic unloading in HF is already recognized to be effective. Examples include the benefit of bariatric surgery and caloric restriction on cardiac function, as well as the recent emergence of sodium/glucose cotransporter-2 (SGLT2) inhibitors in the clinical arena.126,127 However, metabolic stress is only one side of the coin, as metabolic syndrome and fuel over-supply are coupled with inflammatory stress. Restricting fuel supply—i.e. caloric restriction—may provide indirect benefit and diminish the inflammatory burden of metainflammation.128,129
We have argued that innate immunity must evolve in order to be optimized for a given environment and thereby activated appropriately. The dynamic balance of metabolism is fundamental to an organism’s interaction with the environment.130 Metabolic pathways are not only involved in energy provision but also in a two-way dialogue between the cell and the entire organism. Both metabolites and cytokines participate as signals in this dialogue. Metainflammation is a state of disrupted metabolic homoeostasis. Therefore, HFpEF is a paradigmatic example of how metabolic and inflammatory alterations are intertwined in fundamental pathophysiological mechanisms.
Therapeutic modulation of metainflammation
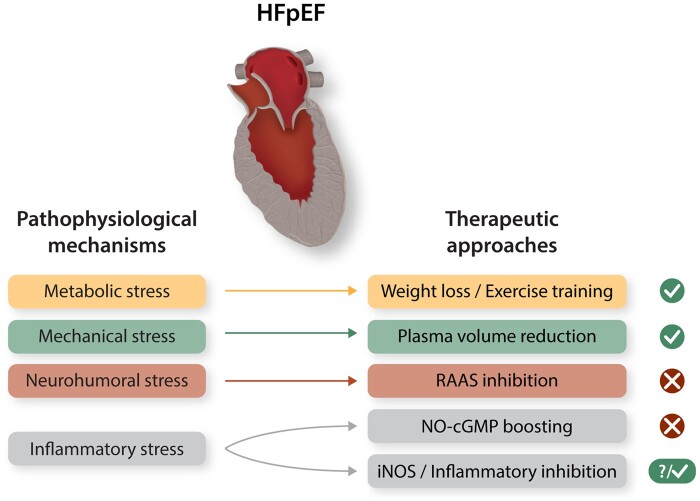
It has been correctly noted that HFpEF represents the greatest unmet medical need in modern cardiology.131 Survival with this condition has not improved over the last decades, and adopting the therapeutic tools available for HFrEF have failed in HFpEF.132 It is now increasingly recognized that HFpEF and HFrEF have distinct pathophysiological mechanisms.133 Despite the fact that these mechanisms may potentially coexist in some cases, a growing body of evidence suggests that tackling HFpEF will require tailored strategies and ad hoc targets134 (Figure 3). We propose that the current understanding of HFpEF involves systemic metabolic inflammation as a key driver, together with fibrosis and altered NO availability as major effects. Implementing this knowledge to develop effective therapeutic strategies is challenging.
Figure 3.
How to fill an empty toolbox? Potential targets and approaches in HFpEF therapy. A promising therapeutic rationale is targeting the stressors prevalent in HFpEF. Possible approaches are shown in association with the prevalent forms of stress they are able to tackle, together with the clinical efficacy they have demonstrated so far. Whereas we still lack evidence of effective HFpEF treatments from large clinical trials, encouraging evidence suggests that targeting metabolic and inflammatory stress, limiting nutrient overload, and antagonizing key inflammatory mediators hold promise and warrant further study. RAAS (Renin–Angiotensin–Aldosterone System).
Anti-fibrotic strategies
One of the major features of HFpEF is reactive fibrosis resulting, at least in part, from transforming growth factor beta (TGF-β) release and collagen deposition. Fibrosis is a highly dynamic process characterized by heterogeneous plasticity across different organs.10,135 In fact, fibrosis in HFpEF can involve many organs beyond the heart. Along these lines, current medications for idiopathic pulmonary fibrosis, such as pirfenidone, have been suggested to exert beneficial effects in HFpEF.136–138 Recently, a clinical trial commenced specifically to test this hypothesis.139 Of note, other cardiovascular drugs with anti-fibrotic properties have been tested in HFpEF, including spironolactone, a mineralocorticoid receptor antagonist with known beneficial effects on extracellular matrix remodelling. However, despite encouraging results in secondary analyses, this study was reported as neutral.140 Importantly, subsequent post hoc analyses of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial revealed possible clinical benefits with spironolactone in patients with HFpEF from the Americas (in contrast with patients enrolled from Russia and Georgia).141,142 Therefore, the significant regional discrepancies observed in the TOPCAT trial have raised legitimate concerns regarding the true therapeutic response to spironolactone in patients with HFpEF.
TGF-β has relevant biological implications in metabolic reprogramming of different tissues and yet this remains incompletely understood in the heart.143 Intermediary metabolism can coordinate extracellular matrix homoeostasis, and metabolic interventions might serve as a strategy to reverse fibrosis.144Proof-of-concept of modulating the immune system for therapeutic purposes emerged recently with the development of chimeric antigen receptor T cells targeting cardiac fibrosis.145,146 Whether this novel strategy might have therapeutic potential in HFpEF has yet to be determined.
Tuning NO availability and anti-inflammatory therapies
Increasing NO availability has been tested in multiple large HFpEF clinical trials; the notion of reduced NO availability as a cause of myocardial stiffness in HFpEF has stimulated investigators around the world to find ways to boost NO-cGMP signalling. Phosphodiesterase 5 (PDE5) inhibitors, NO donors, soluble guanylate cyclase (sGC) stimulators have all been tested in HFpEF as potential therapeutic interventions.147–150 Unfortunately, NO-inducing approaches have failed to improve outcomes with neutral or even negative results.148,151
The recent demonstration of iNOS-dependent metainflammatory events as a major source of nitrosative stress in HFpEF may provide a biological explanation for failure of such strategies. Therefore, turning attention to strategies focusing on reducing mediators of systemic metabolic inflammation, or nitrosative stress26,152 might hold promise in HFpEF therapeutics.114 Counteracting pro-inflammatory mediators in HFpEF also include, for example, IL-1 blockade. Anakinra, an IL-1 receptor antagonist, has been shown to ameliorate, in part, exercise intolerance and oxygen consumption in HFpEF patients.153 These findings were not confirmed in a subsequent phase II study,154 suggesting that targeting specific inflammatory mediators known to be involved in HFpEF pathophysiology, rather than a broad anti-cytokine approach, might represent a more valuable therapeutic strategy.
Metabolic agents with anti-inflammatory properties
Interaction between metabolism and inflammation is also suggested by the anti-inflammatory effects of metabolic agents used to mitigate cardiovascular risk factors, such as cholesterol-lowering and glucose-lowering drugs. These strategies offer the potential to tackle metabolic stress and inflammatory stress simultaneously, providing a therapeutic rationale for targeting these two pathogenetic processes concurrently.
Endomyocardial biopsies from patients with HFpEF treated with statins reveal less myocardial nitrosative stress as well as reduced cardiomyocyte hypertrophy.23 It has also been reported that statin-treated HFpEF patients have diminished probability of developing atrial fibrillation.155 Additional evidence in support of this notion has emerged from registry-based studies showing that statins are associated with improved outcomes in HFpEF, reducing mortality even in the absence of coronary artery disease, the typical targets of these drugs.156,157 Despite the unquestioned need for large clinical trials to test the efficacy of statins in HFpEF, we hypothesize that the pleiotropic anti-inflammatory effects of these cholesterol-lowering drugs will attenuate some of the harmful metainflammatory pathways active in HFpEF.
In additions to statins, several glucose-lowering drugs have been shown to mitigate inflammation, suggesting potential for HFpEF therapeutics.158 For example, recent evidence of the therapeutic benefits of SGLT2-inhibitors in HF even in the absence of diabetes mellitus159 has paved the way to test these drugs in HFpEF. Whereas precise mechanism(s) underlying the beneficial cardiovascular effects of SGLT2 inhibition remain elusive, the possibility that they target metabolic inflammatory pathways has been proposed.160
Of note, metainflammation is a low-grade, systemic state. Previous experience with suppression of pro-inflammatory cytokines in HF led to inconsistent results.161 One can hypothesize that direct anti-inflammatory strategies can increase the risk of unwanted suppression of immune responses. The complexity of immune system responses in shaping cardiovascular adaptions to stress is also evident from the variety of immune cellular subsets present in the human heart.162 Based on this, a targeted, rather than broad and non-specific anti-inflammatory approach, might hold greater promise. Of note, it has been suggested recently that an acute immune response might mediate the beneficial effects of stem cell therapy after myocardial infarction, highlighting the need for fine-tuning manipulation of inflammatory events in cardiovascular disease.163,164 Therefore, we suggest that anti-inflammatory properties of metabolic agents might exert more balanced effects on the immune components of the disease, targeting the primary cause of metainflammation, i.e. metabolic syndrome.
Beneficial effects of exercise training in HFpEF
Exercise intolerance is a hallmark of HFpEF presentation, with obesity and diabetes greatly contributing as underlying mechanisms.165 Physical activity has been shown to have a stronger dose-dependent inverse association with risk in HFpEF as opposed to HFrEF.166 Among the most effective interventions ameliorating exercise intolerance is exercise training. As a consequence, chronic exercise training has emerged as a powerful strategy to mitigate the unfavourable natural history of HFpEF.167,168
Exercise training exerts a number of beneficial effects on cardiovascular function, including improvement of metabolic health, positive modulation of cardiac metabolic profile, and mitigation of mitochondrial dysfunction.169,170 Despite the fact that some of these effects have been related to increased PGC-1α expression induced by endurance exercise, leading to enhanced mitochondrial biogenesis, the intricate mechanisms underlying exercise-induced benefits on cardiovascular function, and in HFpEF in particular, remain elusive. Chronic exercise training is also associated with complex metabolic remodelling in the heart. With respect to lipid metabolism, treadmill training in mice increases both lipid utilization and lipid accumulation,171 suggesting that complex, incompletely characterized, regulation of lipid homoeostasis occurs in the exercised heart. In addition, accumulating evidence suggests that exercise has anti-inflammatory value.172 Cytokines and myokines, secreted by the skeletal muscle, participate in the metabolic/inflammatory reprogramming induced by exercise. For example, irisin is a myokine released in response to physical activity that potentially mediates systemic effects against metabolic inflammation and oxidative stress.173,174 Similarly, other myokines, myostatin, and insulin-like growth factor 1, might represent potential targets in HFpEF as well, given their role in comorbidities frequently associated with HFpEF, such as frailty and sarcopenia in the elderly. Of note, the immunomodulatory properties of skeletal muscle-derived factors integrate the decades-old ‘muscle hypothesis’ of HF.175
Another well-known exercise-induced effect is the upregulation of endothelial nitric oxide synthase (eNOS), resulting in increased bioavailability of NO leading to improved vascular function and reduced oxidative stress.176 Despite this, the disappointing results of NO-increasing strategies in HFpEF suggest strongly that exercise training has much more complex effects on NO signalling than simple increases in NO levels. In the end, the nature and role of cellular and molecular mechanisms linking exercise-dependent metabolic and inflammatory changes in the context of HFpEF warrant further investigation.
Conclusions and perspectives
Metabolic inflammation is emerging as a critical pathophysiological mechanism in HFpEF. Epidemiological evidence pointing to obesity, hypertension, and ageing as risk factors for HFpEF support the notion that a state of subtle systemic inflammation exists in HFpEF, driving key pathophysiological events within the syndrome.
We submit that the field should foster investigations on these topics as HFpEF research priorities.62 The arguably simplistic approach of using successful anti-HFrEF therapies to treat HFpEF has failed. Similarly, many other therapeutic approaches have not provided positive results in clinical trials due to the paucity of preclinical experimental evidence supporting a role for the targeted pathway in HFpEF pathophysiology.
A paradigm shift in HFpEF therapeutics is recommended. Despite the fact that a number of disease modifiers have yet to be identified, elucidation of mechanisms whereby metabolic and inflammatory processes contributes to HFpEF pathogenesis hold promise for therapeutic intervention in this devastating syndrome.
Acknowledgement
Conflict of interest: G.G.S. and J.A.H. are co-inventors on a patent application (PCT/US/2017/037019) that was filed in June 2017 (provisional application filed in June 2016). The patent relates to the diet used for modeling HFpEF.
Funding
This work was supported by grants from the American Heart Association (AHA) and the Theodore and Beulah Beasley Foundation (18POST34060230) to G.G.S. and National Institue of Health (NIH) grants HL-120732, HL-128215, HL-126012, and HL-147933 to J.A.H.
References
- 1.Fact Sheet on Obesity and Overweight. World Health Organization. www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (1 April 2020, date last accessed).
- 2. Kitzman DW, Lam C.. Obese heart failure with preserved ejection fraction phenotype: from pariah to central player. Circulation 2017;136:20–23. [DOI] [PubMed] [Google Scholar]
- 3. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA.. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Packer M. Do most patients with obesity or type 2 diabetes, and atrial fibrillation, also have undiagnosed heart failure? A critical conceptual framework for understanding mechanisms and improving diagnosis and treatment. Eur J Heart Fail 2020;22:214–227. [DOI] [PubMed] [Google Scholar]
- 5. Pandey A, Patel KV, Vaduganathan M, Sarma S, Haykowsky MJ, Berry JD, Lavie CJ.. Physical activity, fitness, and obesity in heart failure with preserved ejection fraction. JACC Heart Fail 2018;6:975–982. [DOI] [PubMed] [Google Scholar]
- 6. Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867. [DOI] [PubMed] [Google Scholar]
- 7. Dunlay SM, Roger VL, Redfield MM.. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017;14:591–602. [DOI] [PubMed] [Google Scholar]
- 8. Roh J, Houstis N, Rosenzweig A.. Why don't we have proven treatments for HFpEF? Circ Res 2017;120:1243–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lüscher TF. Lumpers and splitters: the bumpy road to precision medicine. Eur Heart J 2019;40:3292–3296. [DOI] [PubMed] [Google Scholar]
- 10. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ.. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen JB, Schrauben SJ, Zhao L, Basso MD, Cvijic ME, Li Z, Yarde M, Wang Z, Bhattacharya PT, Chirinos DA, Prenner S, Zamani P, Seiffert DA, Car BD, Gordon DA, Margulies K, Cappola T, Chirinos JA.. Clinical phenogroups in heart failure with preserved ejection fraction: detailed phenotypes, prognosis, and response to spironolactone. JACC Heart Fail 2020;8:172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shah AM, Pfeffer MA.. The many faces of heart failure with preserved ejection fraction. Nat Rev Cardiol 2012;9:555–556. [DOI] [PubMed] [Google Scholar]
- 13. Shah AM. Ventricular remodeling in heart failure with preserved ejection fraction. Curr Heart Fail Rep 2013;10:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rossi A, Gheorghiade M, Triposkiadis F, Solomon SD, Pieske B, Butler J.. Left atrium in heart failure with preserved ejection fraction: structure, function, and significance. Circ Heart Fail 2014;7:1042–1049. [DOI] [PubMed] [Google Scholar]
- 15. Pfeffer MA, Shah AM, Borlaug BA.. Heart failure with preserved ejection fraction in perspective. Circ Res 2019;124:1598–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM.. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation 2007;115:1982–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, Fox J, Chakrabarti S, Sauer AJ, Rich JD, Freed BH, Shah SJ.. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail 2014;7:288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O’Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD.. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail 2014;7:740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sharma K, Kass DA.. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res 2014;115:79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Loffredo FS, Nikolova AP, Pancoast JR, Lee RT.. Heart failure with preserved ejection fraction: molecular pathways of the aging myocardium. Circ Res 2014;115:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS.. Obesity and the risk of heart failure. N Engl J Med 2002;347:305–313. [DOI] [PubMed] [Google Scholar]
- 22. Dalos D, Mascherbauer J, Zotter-Tufaro C, Duca F, Kammerlander AA, Aschauer S, Bonderman D.. Functional status, pulmonary artery pressure, and clinical outcomes in heart failure with preserved ejection fraction. J Am Coll Cardiol 2016;68:189–199. [DOI] [PubMed] [Google Scholar]
- 23. Paulus WJ, Tschope C.. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 24. van Heerebeek L, Hamdani N, Falcao-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ.. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012;126:830–839. [DOI] [PubMed] [Google Scholar]
- 25. Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschope C, Leite-Moreira AF, Musters R, Niessen HW, Linke WA, Paulus WJ, Hamdani N.. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail 2016;4:312–324. [DOI] [PubMed] [Google Scholar]
- 26. Schiattarella GG, Altamirano F, Tong D, French KM, Villalobos E, Kim SY, Luo X, Jiang N, May HI, Wang ZV, Hill TM, Mammen PPA, Huang J, Lee DI, Hahn VS, Sharma K, Kass DA, Lavandero S, Gillette TG, Hill JA.. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019;568:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss H-P, Tschöpe C.. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail 2011;4:44–52. [DOI] [PubMed] [Google Scholar]
- 28.World Population Ageing 2017 - Highlights (ST/ESA/SER.A/397). United Nations, Department of Economic and Social Affairs, Population Division, 2017. 10.18356/10e32e81-en. [DOI]
- 29. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG.. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G.. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2006;908:244–254. [DOI] [PubMed] [Google Scholar]
- 31. Ferrucci L, Fabbri E.. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018;15:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A.. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 2018;14:576–590. [DOI] [PubMed] [Google Scholar]
- 33. Liberale L, Montecucco F, Tardif J-C, Libby P, Camici GG.. Inflamm-ageing: the role of inflammation in age-dependent cardiovascular disease. Eur Heart J 2020:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prattichizzo F, De Nigris V, Spiga R, Mancuso E, La Sala L, Antonicelli R, Testa R, Procopio AD, Olivieri F, Ceriello A.. Inflammageing and metaflammation: the yin and yang of type 2 diabetes. Ageing Res Rev 2018;41:1–17. [DOI] [PubMed] [Google Scholar]
- 35. Finkel T. The metabolic regulation of aging. Nat Med 2015;21:1416–1423. [DOI] [PubMed] [Google Scholar]
- 36. DuBrock HM, AbouEzzeddine OF, Redfield MM.. High-sensitivity C-reactive protein in heart failure with preserved ejection fraction. PLoS One 2018;13:e0201836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Santhanakrishnan R, Chong JP, Ng TP, Ling LH, Sim D, Leong KT, Yeo PS, Ong HY, Jaufeerally F, Wong R, Chai P, Low AF, Richards AM, Lam CS.. Growth differentiation factor 15, ST2, high-sensitivity troponin T, and N-terminal pro brain natriuretic peptide in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail 2012;14:1338–1347. [DOI] [PubMed] [Google Scholar]
- 38. Cheng JM, Akkerhuis KM, Battes LC, van Vark LC, Hillege HL, Paulus WJ, Boersma E, Kardys I.. Biomarkers of heart failure with normal ejection fraction: a systematic review. Eur J Heart Fail 2013;15:1350–1362. [DOI] [PubMed] [Google Scholar]
- 39. D'Elia E, Vaduganathan M, Gori M, Gavazzi A, Butler J, Senni M.. Role of biomarkers in cardiac structure phenotyping in heart failure with preserved ejection fraction: critical appraisal and practical use. Eur J Heart Fail 2015;17:1231–1239. [DOI] [PubMed] [Google Scholar]
- 40. Sanders-van Wijk S, van Empel V, Davarzani N, Maeder MT, Handschin R, Pfisterer ME, Brunner-La Rocca HP, for the TIME-CHF investigators. Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs. reduced left ventricular ejection fraction. Eur J Heart Fail 2015;17:1006–1014. [DOI] [PubMed] [Google Scholar]
- 41. Matsubara J, Sugiyama S, Nozaki T, Sugamura K, Konishi M, Ohba K, Matsuzawa Y, Akiyama E, Yamamoto E, Sakamoto K, Nagayoshi Y, Kaikita K, Sumida H, Kim-Mitsuyama S, Ogawa H.. Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. J Am Coll Cardiol 2011;57:861–869. [DOI] [PubMed] [Google Scholar]
- 42. Abernethy A, Raza S, Sun JL, Anstrom KJ, Tracy R, Steiner J, VanBuren P, LeWinter MM.. Pro‐inflammatory biomarkers in stable versus acutely decompensated heart failure with preserved ejection fraction. JAHA 2018;7:e007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J.. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol 2010;55:2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sciarretta S, Ferrucci A, Ciavarella GM, De Paolis P, Venturelli V, Tocci G, De Biase L, Rubattu S, Volpe M.. Markers of inflammation and fibrosis are related to cardiovascular damage in hypertensive patients with metabolic syndrome. Am J Hypertens 2007;20:784–791. [DOI] [PubMed] [Google Scholar]
- 45. Tromp J, Westenbrink BD, Ouwerkerk W, van Veldhuisen DJ, Samani NJ, Ponikowski P, Metra M, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Lang CC, Ng LL, Zannad F, Zwinderman AH, Hillege HL, van der Meer P, Voors AA.. Identifying pathophysiological mechanisms in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2018;72:1081–1090. [DOI] [PubMed] [Google Scholar]
- 46. Lam CSP, Voors AA, de Boer RA, Solomon SD, van Veldhuisen DJ.. Heart failure with preserved ejection fraction: from mechanisms to therapies. Eur Heart J 2018;39:2780–2792. [DOI] [PubMed] [Google Scholar]
- 47. Hotamisligil GS. Foundations of Immunometabolism and Implications for Metabolic Health and Disease. Immunity 2017;47:406–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oikonomou EK, Antoniades C.. The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol 2019;16:83–99. [DOI] [PubMed] [Google Scholar]
- 49. Osborn O, Olefsky JM.. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med 2012;18:363–374. [DOI] [PubMed] [Google Scholar]
- 50. McNelis JC, Olefsky JM.. Macrophages, immunity, and metabolic disease. Immunity 2014;41:36–48. [DOI] [PubMed] [Google Scholar]
- 51. Saltiel AR, Olefsky JM.. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest 2017;127:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol 2018;71:2360–2372. [DOI] [PubMed] [Google Scholar]
- 53. Mouton AJ, Li X, Hall ME, Hall JE.. Obesity, hypertension, and cardiac dysfunction: novel roles of immunometabolism in macrophage activation and inflammation. Circ Res 2020;126:789–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. DeBerge M, Shah SJ, Wilsbacher L, Thorp EB.. Macrophages in heart failure with reduced versus preserved ejection fraction. Trends Mol Med 2019;25:328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hulsmans M, Sager HB, Roh JD, Valero-Muñoz M, Houstis NE, Iwamoto Y, Sun Y, Wilson RM, Wojtkiewicz G, Tricot B, Osborne MT, Hung J, Vinegoni C, Naxerova K, Sosnovik DE, Zile MR, Bradshaw AD, Liao R, Tawakol A, Weissleder R, Rosenzweig A, Swirski FK, Sam F, Nahrendorf M.. Cardiac macrophages promote diastolic dysfunction. J Exp Med 2018;215:423–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Glezeva N, Voon V, Watson C, Horgan S, McDonald K, Ledwidge M, Baugh J.. Exaggerated inflammation and monocytosis associate with diastolic dysfunction in heart failure with preserved ejection fraction: evidence of M2 macrophage activation in disease pathogenesis. J Card Fail 2015;21:167–177. [DOI] [PubMed] [Google Scholar]
- 57. Shan B, Wang X, Wu Y, Xu C, Xia Z, Dai J, Shao M, Zhao F, He S, Yang L, Zhang M, Nan F, Li J, Liu J, Liu J, Jia W, Qiu Y, Song B, Han JJ, Rui L, Duan SZ, Liu Y.. The metabolic ER stress sensor IRE1alpha suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat Immunol 2017;18:519–529. [DOI] [PubMed] [Google Scholar]
- 58. Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, de Haro-Del Moral FJ, Cobo-Marcos M, Robles C, Bornstein B, Salas C, Lara-Pezzi E, Alonso-Pulpon L, Garcia-Pavia P.. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 2015;36:2585–2594. [DOI] [PubMed] [Google Scholar]
- 59. Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010;140:900–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Amen OM, Sarker SD, Ghildyal R, Arya A.. Endoplasmic reticulum stress activates unfolded protein response signaling and mediates inflammation, obesity, and cardiac dysfunction: therapeutic and molecular approach. Front Pharmacol 2019;10:977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang L, Calay ES, Fan J, Arduini A, Kunz RC, Gygi SP, Yalcin A, Fu S, Hotamisligil GS.. S-Nitrosylation links obesity-associated inflammation to endoplasmic reticulum dysfunction. Science 2015;349:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shah SJ, Borlaug BA, Kitzman DW, McCulloch AD, Blaxall BC, Agarwal R, Chirinos JA, Collins S, Deo RC, Gladwin MT, Granzier H, Hummel SL, Kass DA, Redfield MM, Sam F, Wang TJ, Desvigne-Nickens P, Adhikari BB.. Research priorities for heart failure with preserved ejection fraction. Circulation 2020;141:1001–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McKnight SL. On getting there from here. Science 2010;330:1338–1339. [DOI] [PubMed] [Google Scholar]
- 64. Norata GD, Caligiuri G, Chavakis T, Matarese G, Netea MG, Nicoletti A, O’Neill LAJ, Marelli-Berg FM.. The cellular and molecular basis of translational immunometabolism. Immunity 2015;43:421–434. [DOI] [PubMed] [Google Scholar]
- 65. Baldwin JE, Krebs H.. The evolution of metabolic cycles. Nature 1981;291:381–382. [DOI] [PubMed] [Google Scholar]
- 66. Zasłona Z, O’Neill L.. Cytokine-like roles for metabolites in immunity. Mol Cell 2020;78:814–823. [DOI] [PubMed] [Google Scholar]
- 67. Murphy MP, O’Neill LAJ.. Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell 2018;174:780–784. [DOI] [PubMed] [Google Scholar]
- 68. Ryan DG, O'Neill L.. Krebs cycle reborn in macrophage immunometabolism. Annu Rev Immunol 2020;38:289–313. [DOI] [PubMed] [Google Scholar]
- 69. Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O’Neill LAJ.. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013;496:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu PS, Wang H, Li X, Chao T, Teav T, Christen S, Di Conza G, Cheng WC, Chou CH, Vavakova M, Muret C, Debackere K, Mazzone M, Huang HD, Fendt SM, Ivanisevic J, Ho PC.. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol 2017;18:985–994. [DOI] [PubMed] [Google Scholar]
- 71. Arts RJ, Novakovic B, Ter Horst R, Carvalho A, Bekkering S, Lachmandas E, Rodrigues F, Silvestre R, Cheng SC, Wang SY, Habibi E, Gonçalves LG, Mesquita I, Cunha C, van Laarhoven A, van de Veerdonk FL, Williams DL, van der Meer JW, Logie C, O'Neill LA, Dinarello CA, Riksen NP, van Crevel R, Clish C, Notebaart RA, Joosten LA, Stunnenberg HG, Xavier RJ, Netea MG.. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab 2016;24:807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, Binz T, Wegner A, Tallam A, Rausell A, Buttini M, Linster CL, Medina E, Balling R, Hiller K.. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci USA 2013;110:7820–7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, Ashall J, Everts B, Pearce EJ, Driggers EM, Artyomov MN.. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 2015;42:419–430. [DOI] [PubMed] [Google Scholar]
- 74. Bailey JD, Diotallevi M, Nicol T, McNeill E, Shaw A, Chuaiphichai S, Hale A, Starr A, Nandi M, Stylianou E, McShane H, Davis S, Fischer R, Kessler BM, McCullagh J, Channon KM, Crabtree MJ.. Nitric oxide modulates metabolic remodeling in inflammatory macrophages through TCA cycle regulation and itaconate accumulation. Cell Rep 2019;28:218–230.e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Williams NC, O'Neill L.. A role for the Krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front Immunol 2018;9:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Infantino V, Iacobazzi V, Menga A, Avantaggiati ML, Palmieri F.. A key role of the mitochondrial citrate carrier (SLC25A1) in TNFalpha- and IFNgamma-triggered inflammation. Biochim Biophys Acta 2014;1839:1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang B, Rao YH, Inoue M, Hao R, Lai CH, Chen D, McDonald SL, Choi MC, Wang Q, Shinohara ML, Yao TP.. Microtubule acetylation amplifies p38 kinase signalling and anti-inflammatory IL-10 production. Nat Commun 2014;5:3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Saggerson D. Malonyl-CoA, a key signaling molecule in mammalian cells. Annu Rev Nutr 2008;28:253–272. [DOI] [PubMed] [Google Scholar]
- 79. Galván-Peña S, Carroll RG, Newman C, Hinchy EC, Palsson-McDermott E, Robinson EK, Covarrubias S, Nadin A, James AM, Haneklaus M, Carpenter S, Kelly VP, Murphy MP, Modis LK, O’Neill LA.. Malonylation of GAPDH is an inflammatory signal in macrophages. Nat Commun 2019;10:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Infantino V, Iacobazzi V, Palmieri F, Menga A.. ATP-citrate lyase is essential for macrophage inflammatory response. Biochem Biophys Res Commun 2013;440:105–111. [DOI] [PubMed] [Google Scholar]
- 81. Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Curr Probl Cardiol 1994;19:59–113. [DOI] [PubMed] [Google Scholar]
- 82. Stanley WC, Recchia FA, Lopaschuk GD.. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 2005;85:1093–1129. [DOI] [PubMed] [Google Scholar]
- 83. Goodwin GW, Taylor CS, Taegtmeyer H.. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem 1998;273:29530–29539. [DOI] [PubMed] [Google Scholar]
- 84. Korvald C, Elvenes OP, Myrmel T.. Myocardial substrate metabolism influences left ventricular energetics in vivo. Am J Physiol Heart Circ Physiol 2000;278:H1345–H1351. [DOI] [PubMed] [Google Scholar]
- 85. Neely JR, Morgan HE.. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol 1974;36:413–459. [DOI] [PubMed] [Google Scholar]
- 86. Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC.. Myocardial fatty acid metabolism in health and disease. Physiol Rev 2010;90:207–258. [DOI] [PubMed] [Google Scholar]
- 87. Balaban R, Kantor H, Katz L, Briggs R.. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science 1986;232:1121–1123. [DOI] [PubMed] [Google Scholar]
- 88. Neubauer S. The failing heart—an engine out of fuel. N Engl J Med 2007;356:1140–1151. [DOI] [PubMed] [Google Scholar]
- 89. Taegtmeyer H, Golfman L, Sharma S, Razeghi P, Arsdall M.. Linking gene expression to function: metabolic flexibility in the normal and diseased heart. Ann N Y Acad Sci 2004;1015:202–213. [DOI] [PubMed] [Google Scholar]
- 90. Williams GS, Boyman L, Lederer WJ.. Mitochondrial calcium and the regulation of metabolism in the heart. J Mol Cell Cardiol 2015;78:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kumar AA, Kelly DP, Chirinos JA.. Mitochondrial dysfunction in heart failure with preserved ejection fraction. Circulation 2019;139:1435–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Miranda‐Silva D, Wüst RCI, Conceição G, Gonçalves‐Rodrigues P, Gonçalves N, Gonçalves A, Kuster DWD, Leite‐Moreira AF, Velden J, Sousa Beleza JM, Magalhães J, Stienen GJM, Falcão‐Pires I.. Disturbed cardiac mitochondrial and cytosolic calcium handling in a metabolic risk-related rat model of heart failure with preserved ejection fraction. Acta Physiol 2020;228:e13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Frati G, Schirone L, Chimenti I, Yee D, Biondi-Zoccai G, Volpe M, Sciarretta S.. An overview of the inflammatory signalling mechanisms in the myocardium underlying the development of diabetic cardiomyopathy. Cardiovasc Res 2017;113:378–388. [DOI] [PubMed] [Google Scholar]
- 94. Marelli-Berg FM, Aksentijevic D.. Immunometabolic cross-talk in the inflamed heart. Cell Stress 2019;3:240–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Vig M, Kinet JP.. Calcium signaling in immune cells. Nat Immunol 2009;10:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gibb AA, Hill BG.. Metabolic coordination of physiological and pathological cardiac remodeling. Circ Res 2018;123:107–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Taegtmeyer H, Young ME, Lopaschuk GD, Abel ED, Brunengraber H, Darley-Usmar V, Des Rosiers C, Gerszten R, Glatz JF, Griffin JL, Gropler RJ, Holzhuetter HG, Kizer JR, Lewandowski ED, Malloy CR, Neubauer S, Peterson LR, Portman MA, Recchia FA, Van Eyk JE, Wang TJ.. Assessing cardiac metabolism: a scientific statement from the American Heart Association. Circ Res 2016;118:1659–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lourenço AP, Leite-Moreira AF, Balligand J-L, Bauersachs J, Dawson D, de Boer RA, de Windt LJ, Falcão-Pires I, Fontes-Carvalho R, Franz S, Giacca M, Hilfiker-Kleiner D, Hirsch E, Maack C, Mayr M, Pieske B, Thum T, Tocchetti CG, Brutsaert DL, Heymans S.. An integrative translational approach to study heart failure with preserved ejection fraction: a position paper from the Working Group on Myocardial Function of the European Society of Cardiology. Eur J Heart Fail 2018;20:216–227. [DOI] [PubMed] [Google Scholar]
- 99. Ko HJ, Zhang Z, Jung DY, Jun JY, Ma Z, Jones KE, Chan SY, Kim JK.. Nutrient stress activates inflammation and reduces glucose metabolism by suppressing AMP-activated protein kinase in the heart. Diabetes 2009;58:2536–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Palomer X, Álvarez-Guardia D, Rodríguez-Calvo R, Coll T, Laguna JC, Davidson MM, Chan TO, Feldman AM, Vázquez-Carrera M.. TNF-alpha reduces PGC-1alpha expression through NF-kappaB and p38 MAPK leading to increased glucose oxidation in a human cardiac cell model. Cardiovasc Res 2009;81:703–712. [DOI] [PubMed] [Google Scholar]
- 101. Álvarez-Guardia D, Palomer X, Coll T, Davidson MM, Chan TO, Feldman AM, Laguna JC, Vázquez-Carrera M.. The p65 subunit of NF-kappaB binds to PGC-1alpha, linking inflammation and metabolic disturbances in cardiac cells. Cardiovasc Res 2010;87:449–458. [DOI] [PubMed] [Google Scholar]
- 102. Farah C, Michel LYM, Balligand J-L.. Nitric oxide signalling in cardiovascular health and disease. Nat Rev Cardiol 2018;15:292–316. [DOI] [PubMed] [Google Scholar]
- 103. Massion PB, Feron O, Dessy C, Balligand JL.. Nitric oxide and cardiac function. Circ Res 2003;93:388–398. [DOI] [PubMed] [Google Scholar]
- 104. Recchia FA. Role of nitric oxide in the regulation of substrate metabolism in heart failure. Heart Fail Rev 2002;7:141–148. [DOI] [PubMed] [Google Scholar]
- 105. Suto N, Mikuniya A, Okubo T, Hanada H, Shinozaki N, Okumura K.. Nitric oxide modulates cardiac contractility and oxygen consumption without changing contractile efficiency. Am J Physiol 1998;275:H41–H49. [DOI] [PubMed] [Google Scholar]
- 106. Recchia FA, McConnell PI, Loke KE, Xu X, Ochoa M, Hintze TH.. Nitric oxide controls cardiac substrate utilization in the conscious dog. Cardiovasc Res 1999;44:325–332. [DOI] [PubMed] [Google Scholar]
- 107. d'Agostino C, Labinskyy V, Lionetti V, Chandler MP, Lei B, Matsuo K, Bellomo M, Xu X, Hintze TH, Stanley WC, Recchia FA.. Altered cardiac metabolic phenotype after prolonged inhibition of NO synthesis in chronically instrumented dogs. Am J Physiol Heart Circul Physiol 2006;290:H1721–H1726. [DOI] [PubMed] [Google Scholar]
- 108. Recchia FA, Osorio JC, Chandler MP, Xu X, Panchal AR, Lopaschuk GD, Hintze TH, Stanley WC.. Reduced synthesis of NO causes marked alterations in myocardial substrate metabolism in conscious dogs. Am J Physiol Endocrinol Metab 2002;282:E197–E206. [DOI] [PubMed] [Google Scholar]
- 109. Depre C, Gaussin V, Ponchaut S, Fischer Y, Vanoverschelde JL, Hue L.. Inhibition of myocardial glucose uptake by cGMP. Am J Physiol 1998;274:H1443–H1449. [DOI] [PubMed] [Google Scholar]
- 110. Taegtmeyer H, Overturf ML.. Effects of moderate hypertension on cardiac function and metabolism in the rabbit. Hypertension 1988;11:416–426. [DOI] [PubMed] [Google Scholar]
- 111. Schwartz K, Boheler KR, de la Bastie D, Lompre AM, Mercadier JJ.. Switches in cardiac muscle gene expression as a result of pressure and volume overload. Am J Physiol 1992;262:R364–R369. [DOI] [PubMed] [Google Scholar]
- 112. Li J, Kemp BA, Howell NL, Massey J, Mińczuk K, Huang Q, Chordia MD, Roy RJ, Patrie JT, Davogustto GE, Kramer CM, Epstein FH, Carey RM, Taegtmeyer H, Keller SR, Kundu BK.. Metabolic changes in spontaneously hypertensive rat hearts precede cardiac dysfunction and left ventricular hypertrophy. J Am Heart Assoc 2019;8:e010926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lopaschuk GD, Folmes CDL, Stanley WC.. Cardiac energy metabolism in obesity. Circ Res 2007;101:335–347. [DOI] [PubMed] [Google Scholar]
- 114. Anavi S, Tirosh O.. iNOS as a metabolic enzyme under stress conditions. Free Radic Biol Med 2020;146:16–35. [DOI] [PubMed] [Google Scholar]
- 115. Carvalho-Filho MA, Ueno M, Hirabara SM, Seabra AB, Carvalheira JB, de Oliveira MG, Velloso LA, Curi R, Saad MJ.. S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes 2005;54:959–967. [DOI] [PubMed] [Google Scholar]
- 116. Ropelle ER, Pauli JR, Cintra DE, da Silva AS, De Souza CT, Guadagnini D, Carvalho BM, Caricilli AM, Katashima CK, Carvalho-Filho MA, Hirabara S, Curi R, Velloso LA, Saad MJ, Carvalheira JB.. Targeted disruption of inducible nitric oxide synthase protects against aging, S-nitrosation, and insulin resistance in muscle of male mice. Diabetes 2013;62:466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. White PJ, Charbonneau A, Cooney GJ, Marette A.. Nitrosative modifications of protein and lipid signaling molecules by reactive nitrogen species. Am J Physiol Endocrinol Metabol 2010;299:E868–E878. [DOI] [PubMed] [Google Scholar]
- 118. Brune B, Mohr S.. Protein thiol modification of glyceraldehyde-3-phosphate dehydrogenase and caspase-3 by nitric oxide. Curr Protein Pept Sci 2001;2:61–72. [DOI] [PubMed] [Google Scholar]
- 119. Lumeng CN, Saltiel AR.. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011;121:2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Okin D, Medzhitov R.. Evolution of inflammatory diseases. Curr Biol 2012;22:R733–R740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hill JA. Reflections of the editor-in-chief. Circulation 2017;136:613–614. [DOI] [PubMed] [Google Scholar]
- 122. Nowbar AN, Gitto M, Howard JP, Francis DP, Al-Lamee R.. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes 2019;12:e005375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rappaport SM, Smith MT.. Epidemiology. Environment and disease risks. Science 2010;330:460–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Riggs DW, Yeager RA, Bhatnagar A.. Defining the human envirome: an omics approach for assessing the environmental risk of cardiovascular disease. Circ Res 2018;122:1259–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Uriel N, Sayer G, Annamalai S, Kapur NK, Burkhoff D.. Mechanical unloading in heart failure. J Am Coll Cardiol 2018;72:569–580. [DOI] [PubMed] [Google Scholar]
- 126. Algahim MF, Sen S, Taegtmeyer H.. Bariatric surgery to unload the stressed heart: a metabolic hypothesis. Am J Physiol Heart Circul Physiol 2012;302:H1539–H1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Koutroumpakis E, Jozwik B, Aguilar D, Taegtmeyer H.. Strategies of unloading the failing heart from metabolic stress. Am J Med 2020;133:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gabandé-Rodríguez E, Gómez de las Heras MM, Mittelbrunn M.. Control of inflammation by calorie restriction mimetics: on the crossroad of autophagy and mitochondria. Cells 2019;9:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD.. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 2011;17:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lazar MA, Birnbaum MJ.. Physiology. De-meaning of metabolism. Science 2012;336:1651–1652. [DOI] [PubMed] [Google Scholar]
- 131. Paulus WJ. Unfolding discoveries in heart failure. N Engl J Med 2020;382:679–682. [DOI] [PubMed] [Google Scholar]
- 132. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM.. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 133. Borlaug Barry A, Redfield Margaret M.. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation 2011;123:2006–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Schiattarella GG, Tong D, Hill JA.. Can HFpEF and HFrEF coexist? Circulation 2020;141:709–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Rockey DC, Bell PD, Hill JA.. Fibrosis—a common pathway to organ injury and failure. N Engl J Med 2015;372:1138–1149. [DOI] [PubMed] [Google Scholar]
- 136. Graziani F, Varone F, Crea F, Richeldi L.. Treating heart failure with preserved ejection fraction: learning from pulmonary fibrosis. Eur J Heart Fail 2018;20:1385–1391. [DOI] [PubMed] [Google Scholar]
- 137. Aimo A, Cerbai E, Bartolucci G, Adamo L, Barison A, Lo Surdo G, Biagini S, Passino C, Emdin M.. Pirfenidone is a cardioprotective drug: mechanisms of action and preclinical evidence. Pharmacol Res 2020;155:104694. [DOI] [PubMed] [Google Scholar]
- 138. D’Amario D, Migliaro S, Borovac JA, Restivo A, Vergallo R, Galli M, Leone AM, Montone RA, Niccoli G, Aspromonte N, Crea F.. Microvascular dysfunction in heart failure with preserved ejection fraction. Front Physiol 2019;10:1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lewis GA, Schelbert EB, Naish JH, Bedson E, Dodd S, Eccleson H, Clayton D, Jimenez BD, McDonagh T, Williams SG, Cooper A, Cunnington C, Ahmed FZ, Viswesvaraiah R, Russell S, Neubauer S, Williamson PR, Miller CA.. Pirfenidone in heart failure with preserved ejection fraction-rationale and design of the PIROUETTE trial. Cardiovasc Drugs Ther 2019;33:461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM.. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 141. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O’Meara E, Rouleau J-L, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B.. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131:34–42. [DOI] [PubMed] [Google Scholar]
- 142. de Denus S, O’Meara E, Desai AS, Claggett B, Lewis EF, Leclair G, Jutras M, Lavoie J, Solomon SD, Pitt B, Pfeffer MA, Rouleau JL.. Spironolactone metabolites in TOPCAT—new insights into regional variation. N Engl J Med 2017;376:1690–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zhao X, Kwan JYY, Yip K, Liu PP, Liu FF.. Targeting metabolic dysregulation for fibrosis therapy. Nat Rev Drug Discov 2020;19:57–75. [DOI] [PubMed] [Google Scholar]
- 144. Rabinowitz JD, Mutlu GM.. A metabolic strategy to reverse fibrosis? Nat Metab 2019;1:12–13. [DOI] [PubMed] [Google Scholar]
- 145. Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, Scholler J, Monslow J, Lo A, Han W, Wang T, Bedi K, Morley MP, Linares Saldana RA, Bolar NA, McDaid K, Assenmacher C-A, Smith CL, Wirth D, June CH, Margulies KB, Jain R, Puré E, Albelda SM, Epstein JA.. Targeting cardiac fibrosis with engineered T cells. Nature 2019;573:430–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hill JA. When the CAR targets scar. N Engl J Med 2019;381:2475–2476. [DOI] [PubMed] [Google Scholar]
- 147. Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray J.. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012;380:1387–1395. [DOI] [PubMed] [Google Scholar]
- 148. Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WHW, McNulty SE, Velazquez EJ, Shah MR, Braunwald E.. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015;373:2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, Relax Trial F.. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013;309:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Bonderman D, Pretsch I, Steringer-Mascherbauer R, Jansa P, Rosenkranz S, Tufaro C, Bojic A, Lam CSP, Frey R, Ochan Kilama M, Unger S, Roessig L, Lang IM.. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): a randomized, double-blind, placebo-controlled, single-dose study. Chest 2014;146:1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Komajda M, Lam CS.. Heart failure with preserved ejection fraction: a clinical dilemma. Eur Heart J 2014;35:1022–1032. [DOI] [PubMed] [Google Scholar]
- 152. Jia J, Arif A, Terenzi F, Willard B, Plow EF, Hazen SL, Fox PL.. Target-selective protein S-nitrosylation by sequence motif recognition. Cell 2014;159:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Van Tassell BW, Arena R, Biondi-Zoccai G, Canada JM, Oddi C, Abouzaki NA, Jahangiri A, Falcao RA, Kontos MC, Shah KB, Voelkel NF, Dinarello CA, Abbate A.. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am J Cardiol 2014;113:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Van Tassell Benjamin W, Trankle Cory R, Canada Justin M, Carbone S, Buckley L, Kadariya D, Del Buono Marco G, Billingsley H, Wohlford G, Viscusi M, Oddi-Erdle C, Abouzaki Nayef A, Dixon D, Biondi-Zoccai G, Arena R, Abbate A.. IL-1 blockade in patients with heart failure with preserved ejection fraction. Circ Heart Fail 2018;11:e005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zakeri R, Chamberlain AM, Roger VL, Redfield MM.. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation 2013;128:1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Alehagen U, Benson L, Edner M, Dahlstrom U, Lund LH.. Association between use of statins and mortality in patients with heart failure and ejection fraction of >/=50. Circ Heart Fail 2015;8:862–870. [DOI] [PubMed] [Google Scholar]
- 157. Marume K, Takashio S, Nagai T, Tsujita K, Saito Y, Yoshikawa T, Anzai T.. Effect of statins on mortality in heart failure with preserved ejection fraction without coronary artery disease—report from the JASPER study. Circ J 2019;83:357–367. [DOI] [PubMed] [Google Scholar]
- 158. Pollack RM, Donath MY, LeRoith D, Leibowitz G.. Anti-inflammatory agents in the treatment of diabetes and its vascular complications. Diabetes Care 2016;39:S244–S252. [DOI] [PubMed] [Google Scholar]
- 159. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang C-E, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde A-M.. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 160. Giaccari A. Sodium-glucose co-transporter inhibitors: medications that mimic fasting for cardiovascular prevention. Diabetes Obes Metab 2019;21:2211–2218. [DOI] [PubMed] [Google Scholar]
- 161. Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res 2015;116:1254–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, Epelman S, Kreisel D, Liu Y, Itoh A, Shankar TS, Selzman CH, Drakos SG, Lavine KJ.. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med 2018;24:1234–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AKZ, Schwanekamp JA, York AJ, Huang V, Nahrendorf M, Sadayappan S, Molkentin JD.. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 2020;577:405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Epstein JA, Rosenthal N, Feldman AM.. Teasing the immune system to repair the heart. N Engl J Med 2020;382:1660–1662. [DOI] [PubMed] [Google Scholar]
- 165. Del Buono MG, Arena R, Borlaug BA, Carbone S, Canada JM, Kirkman DL, Garten R, Rodriguez-Miguelez P, Guazzi M, Lavie CJ, Abbate A.. Exercise intolerance in patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:2209–2225. [DOI] [PubMed] [Google Scholar]
- 166. Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, Allen NB, de Lemos JA, Carnethon M, Greenland P, Berry JD.. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol 2017;69:1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Pandey A, Berry JD.. Physical activity in heart failure with preserved ejection fraction: moving toward a newer treatment paradigm. Circulation 2017;136:993–995. [DOI] [PubMed] [Google Scholar]
- 168. Haykowsky M, Brubaker P, Kitzman D.. Role of physical training in heart failure with preserved ejection fraction. Curr Heart Fail Rep 2012;9:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Mann N, Rosenzweig A.. Can exercise teach us how to treat heart disease? Circulation 2012;126:2625–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Roh J, Rhee J, Chaudhari V, Rosenzweig A.. The role of exercise in cardiac aging. Circ Res 2016;118:279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Kolwicz SC Jr. An “exercise” in cardiac metabolism. Front Cardiovasc Med 2018;5:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Lancaster GI, Febbraio MA.. The immunomodulating role of exercise in metabolic disease. Trends Immunol 2014;35:262–269. [DOI] [PubMed] [Google Scholar]
- 173. Mazur-Bialy AI, Bilski J, Pochec E, Brzozowski T.. New insight into the direct anti-inflammatory activity of a myokine irisin against proinflammatory activation of adipocytes. Implication for exercise in obesity. J Physiol Pharmacol 2017;68:243–251. [PubMed] [Google Scholar]
- 174. Silvestrini A, Bruno C, Vergani E, Venuti A, Favuzzi AMR, Guidi F, Nicolotti N, Meucci E, Mordente A, Mancini A.. Circulating irisin levels in heart failure with preserved or reduced ejection fraction: a pilot study. PLoS One 2019;14:e0210320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Coats AJ. The “muscle hypothesis” of chronic heart failure. J Mol Cell Cardiol 1996;28:2255–2262. [DOI] [PubMed] [Google Scholar]
- 176. Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH.. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res 1994;74:349–353. [DOI] [PubMed] [Google Scholar]