Abstract
Background
There is an urgent need to identify factors specifically associated with aggressive prostate cancer (PCa) risk. We investigated whether rare pathogenic, likely pathogenic, or deleterious (P/LP/D) germline variants in DNA repair genes are associated with aggressive PCa risk in a case-case study of aggressive vs nonaggressive disease.
Methods
Participants were 5545 European-ancestry men, including 2775 nonaggressive and 2770 aggressive PCa cases, which included 467 metastatic cases (16.9%). Samples were assembled from 12 international studies and germline sequenced together. Rare (minor allele frequency < 0.01) P/LP/D variants were analyzed for 155 DNA repair genes. We compared single variant, gene-based, and DNA repair pathway-based burdens by disease aggressiveness. All statistical tests are 2-sided.
Results
BRCA2 and PALB2 had the most statistically significant gene-based associations, with 2.5% of aggressive and 0.8% of nonaggressive cases carrying P/LP/D BRCA2 alleles (odds ratio [OR] = 3.19, 95% confidence interval [CI] = 1.94 to 5.25, P = 8.58 × 10-7) and 0.65% of aggressive and 0.11% of nonaggressive cases carrying P/LP/D PALB2 alleles (OR = 6.31, 95% CI = 1.83 to 21.68, P = 4.79 × 10-4). ATM had a nominal association, with 1.6% of aggressive and 0.8% of nonaggressive cases carrying P/LP/D ATM alleles (OR = 1.88, 95% CI = 1.10 to 3.22, P = .02). In aggregate, P/LP/D alleles within 24 literature-curated candidate PCa DNA repair genes were more common in aggressive than nonaggressive cases (carrier frequencies = 14.2% vs 10.6%, respectively; P = 5.56 × 10-5). However, this difference was non-statistically significant (P = .18) on excluding BRCA2, PALB2, and ATM. Among these 24 genes, P/LP/D carriers had a 1.06-year younger diagnosis age (95% CI = -1.65 to 0.48, P = 3.71 × 10-4).
Conclusions
Risk conveyed by DNA repair genes is largely driven by rare P/LP/D alleles within BRCA2, PALB2, and ATM. These findings support the importance of these genes in both screening and disease management considerations.
Prostate cancer (PCa) is the second leading cause of cancer death in the United States and fifth worldwide among men (1). The 5-year cancer-specific survival rate of men diagnosed with localized or regional PCa is nearly 100%, with those diagnosed with higher Gleason grade disease requiring more aggressive treatment. However, only approximately 30% of men diagnosed with metastatic PCa survive beyond 5 years (2). To reduce both the number of deaths from PCa and overtreatment of lower-risk patients, it is critical to identify men at high risk of aggressive disease.
Multiple lines of evidence support a genetic contribution to aggressive PCa risk, including concordance of PCa survival duration between fathers and sons (3), familial aggregation of incident and fatal PCa (4,5), and several genomic regions implicated by linkage studies of aggressive PCa (6-10). However, the specific variants and genes implicated by linkage studies have yet to be identified, and few common variants have been associated with risk of aggressive as opposed to nonaggressive PCa (11,12). An important component of the genetic architecture of aggressive PCa may include multiple rare variants, which represent a sizable spectrum of human genetic variation yet to be comprehensively examined for aggressive disease.
Germline sequencing studies have reported that rare pathogenic and deleterious variants within DNA repair genes may predispose individuals to earlier PCa onset (13,14), aggressive PCa (15-20), and response to PCa treatment (21,22). Among these studies, BRCA2 is the most consistently reported gene, with evidence also reported for ATM, CHEK2, MSH2, and NBN, which are typically associated with increased aggressive PCa risk (13-22). Because of the extreme rarity of pathogenic variants, larger sample sizes are needed to identify genes with statistically significant and consistent associations. Guidelines now recommend germline genetic testing for a panel of DNA repair genes at the time of initial PCa diagnosis for men with a family history or high-risk, regional, or metastatic PCa to inform disease management (23); identifying the specific genes that impact aggressive disease risk would likely improve the clinical utility of such testing, which in the future could be offered prior to the diagnosis of PCa to inform screening decisions. However, previous studies have focused on a small number of candidate DNA repair genes, and whole-exome sequencing studies have been conducted in small samples (15,24). A large-scale investigation of DNA repair genes in aggressive PCa has yet to be conducted.
Here, we examined the involvement of rare pathogenic, likely pathogenic, and deleterious (P/LP/D) germline variants within a comprehensive panel of 155 DNA repair genes in PCa using a case-case investigation of 5545 men of European ancestry comparing aggressive PCa (death from PCa, metastatic disease, stage T4, or stage T3 and Gleason ≥8 tumors) with nonaggressive PCa cases (stages T1/T2 and Gleason ≤6 tumors). In addition to single variant associations, we tested gene- and pathway-based associations to examine the aggregate effect of rare P/LP/D variants on aggressive PCa and age at disease diagnosis.
Methods
Participants and Genetic Sequencing
After excluding 18 men whose DNA samples failed quality control, 5545 men of European ancestry selected from 12 large epidemiological studies across Australia, Finland, the United Kingdom, the United States, and Sweden were included in analyses. Participants were selected without knowledge or suspicion of genetic alleles carried (see Supplementary Methods, available online, for study recruitment details and sample quality control). Of these, 2775 had nonaggressive PCa and 2770 had aggressive PCa. Aggressive cases were men who either died from PCa or had metastatic disease, stage T4, or both stage T3 and a Gleason score of 8 or higher at diagnosis. Nonaggressive cases were men diagnosed with localized disease (stage T1/T2) and a Gleason score of 6 or less tumors (71.3% of nonaggressive cases additionally had follow-up indicating that they were alive and without recurrence for ≥10 years). Variants within DNA repair genes were extracted from whole-exome sequencing data generated at the Center for Inherited Diseases Research with 56X mean targeted exon coverage (details in Supplementary Methods, available online). All participants provided informed consent, and study protocols were approved by respective institutional review boards.
DNA Repair Gene and Pathway Selection
DNA repair pathways were based on previous curations (25-28) and included homologous recombination and/or Fanconi anemia (HR/FA), ATM signaling (ATM), base excision repair (BER), nucleotide excision repair (NER), nonhomologous end joining (NHEJ), mismatch repair (MMR), RECQ helicase family (RECQ), translesion synthesis (TLS), cross-link repair (XLR), and other miscellaneous DNA repair genes with functions including endonuclease and/or exonuclease activity and modification of chromatin structure (Other). From these curations and another DNA repair gene investigation (16), we identified 194 genes of which 188 were sequenced and 155 contained variants meeting the inclusion criteria of our study (Supplementary Table 1, available online). We also curated a candidate subset of 24 DNA repair genes based on previous literature supporting an association between germline variants in these genes and PCa risk or disease aggressiveness (13-16,18,21,29) (Supplementary Table 1, available online).
Pathogenic, Likely Pathogenic, and Deleterious Variant Definition
P/LP/D variants analyzed were rare (minor allele frequency <0.01) and had either 1) a variant effect predictor impact score of “high” (30), representing variants with deleterious (protein truncating or splice altering) functional consequences, or 2) a pathogenic or likely pathogenic Clinvar classification (31) to identify known pathogenic variants, including nonsynonymous substitutions. We excluded variant c.9976A>T (rs11571833) in BRCA2, because it is a known low-to-moderate PCa risk variant (32).
Statistical Analyses
Single variant, gene-based, and pathway-based analyses were performed for aggressive vs nonaggressive PCa, metastatic vs nonaggressive PCa, and age at PCa diagnosis. As a secondary analysis, we assessed lethal (ie, death from PCa) vs nonaggressive PCa. Single variants were analyzed using Firth logistic regression models (33) and the likelihood-ratio test. Gene-based and pathway-based analyses were performed by comparing P/LP/D carriers with noncarriers. Carrier status was compared between aggressive statuses using logistic regression models and tested for associations with age at diagnosis using linear regression models, with P values calculated using the likelihood-ratio test. Gene-based analyses excluded genes with 5 or fewer carriers of qualifying variants.
Analyses included covariates for study, country, age at PCa diagnosis, and 3 principal components of ancestry to account for potential population stratification. Analyses of individual variants, genes, and pathways were corrected for multiple testing for each outcome using the Benjamini-Hochberg (34) adjustment. An adjusted P value of less than .05 was considered statistically significant, whereas an unadjusted P value of less than .05 was considered nominally statistically significant (P values described within the “Results” section are unadjusted). All tests of statistical significance are 2-sided. Top findings for each outcome were further investigated in analyses stratified by age at PCa diagnosis (younger than 60 years and 60 years or older), PCa family history (available for 79.2% [n = 4390] of participants), and country. Top findings were also further investigated comparing nonaggressive cases with subgroups of nonmetastatic aggressive cases, including those diagnosed with 1) T1/T2 and a Gleason score less than 8, 2) T1/T2 and a Gleason score of 8 or higher, 3) T3/T4 and a Gleason score less than 8, and 4) T3/T4 and a Gleason score of 8 or higher. Analyses investigating age at diagnosis excluded 543 nonaggressive Australian participants because the selection criterion applied to these samples included age at diagnosis (Supplementary Methods, available online).
Results
Participants
Of aggressive PCa cases, 74.1% (n = 2052) died from PCa, 16.9% (n = 467) had metastatic disease, 67.2% (n = 1862) had a Gleason score of 8 or higher, and 69.7% (n = 1931) had stage T3 or T4 (Table 1). Of cases who died from PCa, only 11.5% (n = 319) had stage T1/T2 disease and a tumors with a Gleason score less than 8 at diagnosis. Aggressive cases were younger at diagnosis than nonaggressive cases (66.1 years [SD = 8.8] vs 67.5 [SD = 7.0], respectively).
Table 1.
Clinical characteristics of study participants by study (n = 5545)
| Characteristic | Overall | Australia |
Finland | United States |
United Kingdom | Sweden |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APCS | MCCS | PCFS | ATBC | CPS-II | MEC | PLCO | ICR | CAPS | PROCAP | STHM1 | STHM2 | ||
| Total No. | 5545 | 219 | 413 | 442 | 916 | 360 | 203 | 394 | 1061 | 690 | 248 | 205 | 394 |
| Aggressive, No. | 2770 | 219 | 114 | 198 | 466 | 169 | 106 | 190 | 530 | 452 | 31 | 67 | 228 |
| Age at dx, mean (SD) | 66.1 (8.8) | 64.9 (5.8) | 70.6 (7.4) | 57.9 (8.4) | 70.4 (6.1) | 73.3 (6.9) | 70.7 (9.0) | 70.1 (6.4) | 57.1 (5.2) | 67.3 (7.6) | 63.2 (5.2) | 69.6 (7.6) | 70.0 (8.7) |
| Family history, No. (%)a | |||||||||||||
| Yes | 331 (11.9) | 0 (0) | 15 (13.2) | 32 (16.2) | 27 (5.8) | 22 (13.0) | 6 (5.7) | 18 (9.5) | 111 (20.9) | 79 (17.5) | 10 (32.3) | 11 (16.4) | 0 (0) |
| No | 1776 (64.1) | 2 (0.9) | 67 (58.8) | 137 (69.2) | 380 (81.5) | 147 (87.0) | 90 (84.9) | 168 (88.4) | 351 (66.2) | 373 (82.5) | 6 (19.4) | 55 (82.1) | 0 (0) |
| Death from PCa, No. (%)a | |||||||||||||
| Yes | 2052 (74.1) | 21 (9.6) | 89 (78.1) | 156 (78.8) | 386 (82.8) | 127 (75.1) | 61 (57.5) | 117 (61.6) | 530 (100.0) | 362 (80.1) | 31 (100.0) | 66 (98.5) | 106 (46.5) |
| No | 311 (11.2) | 0 (0) | 0 (0) | 0 (0) | 80 (17.2) | 0 (0) | 18 (17.0) | 0 (0) | 0 (0) | 90 (19.9) | 0 (0) | 1 (1.5) | 122 (53.5) |
| Metastatic disease, No. (%)a | |||||||||||||
| Yes | 467 (16.9) | 4 (1.8) | 17 (14.9) | 10 (5.1) | 186 (39.9) | 0 (0) | 29 (27.4) | 47 (24.7) | 174 (32.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No | 663 (23.9) | 3 (1.4) | 0 (0) | 1 (0.5) | 234 (50.2) | 0 (0) | 74 (69.8) | 131 (68.9) | 220 (41.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Stage, No. (%)a | |||||||||||||
| 1 | 410 (14.8) | 14 (6.4) | 59 (51.8) | 109 (55.1) | 63 (13.5) | 77 (45.6) | 31 (29.2) | 25 (13.2) | 16 (3.0) | 0 (0) | 4 (12.9) | 12 (17.9) | 0 (0) |
| 2 | 367 (13.2) | 0 (0) | 2 (1.8) | 10 (5.1) | 109 (23.4) | 0 (0) | 0 (0) | 36 (18.9) | 66 (12.5) | 71 (15.7) | 27 (87.1) | 15 (22.4) | 31 (13.6) |
| 3 | 1277 (46.1) | 194 (88.6) | 34 (29.8) | 67 (33.8) | 91 (19.5) | 92 (54.4) | 43 (40.6) | 77 (40.5) | 164 (30.9) | 315 (69.7) | 0 (0) | 33 (49.3) | 167 (73.2) |
| 4 | 654 (23.6) | 11 (5.0) | 19 (16.7) | 12 (6.1) | 203 (43.6) | 0 (0) | 29 (27.4) | 52 (27.4) | 230 (43.4) | 64 (14.2) | 0 (0) | 4 (6.0) | 30 (13.2) |
| Gleason score, No (%)a | |||||||||||||
| ≤6 | 197 (7.1) | 0 (0) | 4 (3.5) | 1 (0.5) | 109 (23.4) | 22 (13.0) | 4 (3.8) | 28 (14.7) | 9 (1.7) | 18 (4.0) | 0 (0) | 0 (0) | 2 (0.9) |
| 7 | 490 (17.7) | 2 (0.9) | 28 (24.6) | 80 (40.4) | 88 (18.9) | 36 (21.3) | 27 (25.5) | 38 (20.0) | 16 (3.0) | 133 (29.4) | 9 (29.0) | 0 (0) | 33 (14.5) |
| 8-10 | 1862 (67.2) | 217 (99.1) | 70 (61.4) | 115 (58.1) | 134 (28.8) | 82 (48.5) | 67 (63.2) | 114 (60.0) | 484 (91.3) | 299 (66.2) | 22 (71.0) | 67 (100.0) | 191 (83.8) |
| Nonaggressive, No. | 2775 | 0 | 299 | 244 | 450 | 191 | 97 | 204 | 531 | 238 | 217 | 138 | 166 |
| Age at dx, mean (SD) | 67.5 (7.0) | — | 71.5 (6.7) | 65.0 (6.5) | 71.9 (4.7) | 68.3 (5.0) | 67.8 (6.8) | 68.4 (5.5) | 62.6 (6.0) | 66.9 (7.4) | 63.7 (5.1) | 73.4 (3.8) | 67.0 (7.9) |
| Family history, No. (%)a | |||||||||||||
| Yes | 467 (16.8) | — | 47 (15.7) | 56 (23.0) | 17 (3.8) | 53 (27.7) | 14 (14.4) | 20 (9.8) | 123 (23.2) | 50 (21.0) | 58 (26.7) | 29 (21.0) | 0 (0) |
| No | 1816 (65.4) | — | 199 (66.6) | 137 (56.1) | 385 (85.6) | 138 (72.3) | 80 (82.5) | 181 (88.7) | 350 (65.9) | 188 (79.0) | 49 (22.6) | 109 (79.0) | 0 (0) |
| Stage, No. (%) | |||||||||||||
| 1 | 2383 (85.9) | — | 262 (87.6) | 198 (81.1) | 267 (59.3) | 191 (100.0) | 97 (100.0) | 78 (38.2) | 531 (100.0) | 238 (100.0) | 217 (100.0) | 138 (100.0) | 166 (100.0) |
| 2 | 392 (14.1) | — | 37 (12.4) | 46 (18.9) | 183 (40.7) | 0 (0) | 0 (0) | 26 (61.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Gleason score ≤6, No. (%)a | 2773 (99.9) | — | 299 (100.0) | 244 (100.0) | 450 (100.0) | 191 (100.0) | 97 (100.0) | 204 (100.0) | 531 (100.0) | 238 (100.0) | 217 (100.0) | 136 (98.6) | 166 (100.0) |
Numbers do not sum to the total sample size due to missing data. Dx = diagnosis; PCa = prostate cancer; APCS = Aggressive Prostate Cancer Case-Control Study; MCCS = Melbourne Collaborative Cohort Study; PCFS = Australian Prostate Cancer Family Study; ATBC = Alpha-Tocopherol, Beta-Carotene Prevention Study; CPS-II = American Cancer Society Cancer Prevention Study II; MEC = Multiethnic Cohort; PLCO = The Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Cohort; ICR = The Institute of Cancer Research; CAPS = The Cancer of the Prostate in Sweden Study; PROCAP = Progression of Cancer in the Prostate; STHM1 = The Stockholm-1 Study; STHM2 = The Stockholm-2 Study; Em dash (—) = missing.
Aggressive vs Nonaggressive PCa
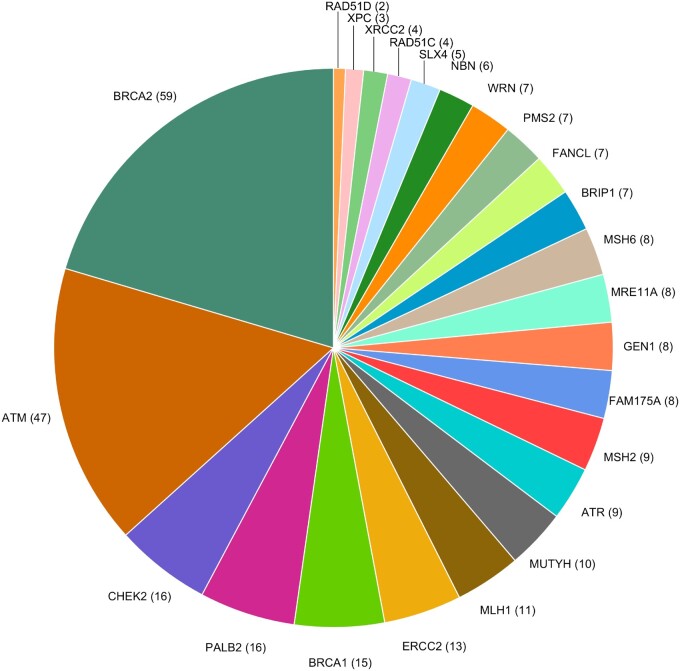
Among the 155 DNA repair genes, 858 P/LP/D variants were identified in the sample of 5545 men (Supplementary Figure 1 and Supplementary Table 2, available online), which included 289 P/LP/D variants in the 24 candidate genes (Figure 1; Supplementary Figure 2, available online). Owing to their rare frequencies, associations between single P/LP/D variants and aggressive PCa were non-statistically significant (Supplementary Figure 3 and Supplementary Table 3, available online).
Figure 1.
Distribution of 289 rare pathogenic/likely pathogenic/deleterious variants among 24 candidate prostate cancer DNA repair genes. Genes (no. of variants) are shown.
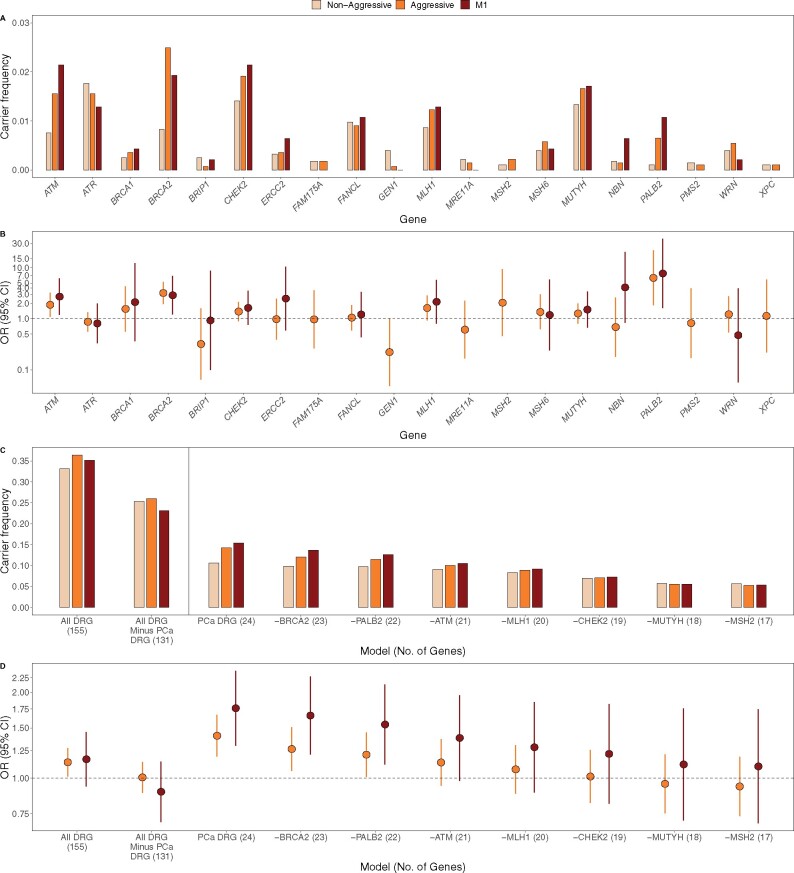
BRCA2 and PALB2 had the strongest gene-based associations with aggressive PCa (Table 2; Supplementary Table 4 and Supplementary Figure 4, available online). We observed that 2.5% of aggressive and 0.8% of nonaggressive cases carried P/LP/D BRCA2 alleles (odds ratio [OR] = 3.19, 95% confidence interval [CI] = 1.94 to 5.25, P = 8.58 × 10-7) and 0.65% of aggressive and 0.11% of nonaggressive cases carried P/LP/D PALB2 alleles (OR = 6.31, 95% CI = 1.83 to 21.68, P = 4.79 × 10-4). ATM was nominally associated with aggressive PCa, with 1.6% of aggressive and 0.8% of nonaggressive cases carrying P/LP/D ATM alleles (OR = 1.88, 95% CI = 1.10 to 3.22, P = .02). Effects of these 3 genes were similar or only slightly larger when comparing metastatic cases with nonaggressive cases (Table 2). Although 6 genes were nominally associated with metastatic disease, none were statistically significant after adjusting for multiple testing (Supplementary Table 4 and Supplementary Figure 4, available online). Associations with lethal PCa were similar in magnitude to aggressive disease, with slightly stronger effects (Supplementary Table 4 and Supplementary Figure 4, available online). Carrier frequencies and effects of the candidate PCa genes by disease aggressiveness are shown in Figure 2, A and B.
Table 2.
Association results for top 15 DNA repair genes and 24 candidate PCa genes
| Gene | Rank/81a | Chr | No. of var | Carrier frequencies |
Aggressive vs nonaggressive |
Metastatic vs nonaggressive |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-agg | Agg | M1 | OR (95% CI) | P b | P adj | OR (95% CI) | P b | P adj | ||||
| Top 15 genes | ||||||||||||
| BRCA2 c | 1 | 13 | 59 | 0.00829 | 0.02491 | 0.01927 | 3.19 (1.94 to 5.25) | 8.58 x 10-7 d | 6.95 x 10-5 | 2.88 (1.22 to 6.83) | .02 | .25 |
| PALB2 c | 2 | 16 | 16 | 0.00108 | 0.00650 | 0.01071 | 6.31 (1.83 to 21.68) | 4.79 x 10-4 d | .02 | 7.71 (1.62 to 36.72) | .009 | .19 |
| TP53BP1 | 3 | 15 | 2 | 0.00360 | 0.00072 | 0 | 0.18 (0.04 to 0.86) | .01 | .36 | — | .07 | .51 |
| ATM c | 4 | 11 | 47 | 0.00757 | 0.01552 | 0.02141 | 1.88 (1.10 to 3.22) | .02 | .38 | 2.71 (1.19 to 6.2) | .02 | .25 |
| GEN1 c | 5 | 2 | 8 | 0.00396 | 0.00072 | 0 | 0.22 (0.05 to 1.02) | .03 | .42 | — | .23 | .64 |
| ALKBH3 | 6 | 11 | 5 | 0.00865 | 0.00469 | 0 | 0.50 (0.25 to 1.01) | .049 | .53 | — | .01 | .21 |
| APLF | 7 | 2 | 5 | 0.00216 | 0.00036 | 0 | 0.17 (0.02 to 1.44) | .05 | .53 | — | .06 | .51 |
| RECQL | 8 | 12 | 10 | 0.00757 | 0.00433 | 0 | 0.52 (0.25 to 1.07) | .07 | .53 | — | .005 | .19 |
| FANCG | 9 | 9 | 3 | 0.00180 | 0.00036 | 0 | 0.17 (0.02 to 1.55) | .07 | .53 | NA | NA | NA |
| MLH1 c | 10 | 3 | 11 | 0.00865 | 0.01227 | 0.01285 | 1.62 (0.94 to 2.82) | .08 | .53 | 2.15 (0.8 to 5.75) | .15 | .63 |
| FANCM | 11 | 14 | 11 | 0.00829 | 0.01300 | 0.01071 | 1.61 (0.93 to 2.79) | .08 | .53 | 1.1 (0.39 to 3.13) | .85 | .94 |
| DCLRE1C | 12 | 10 | 4 | 0.00180 | 0.00036 | 0 | 0.20 (0.02 to 1.72) | .09 | .53 | NA | NA | NA |
| MDC1 | 13 | 6 | 2 | 0.00180 | 0.00036 | 0 | 0.20 (0.02 to 1.78) | .10 | .53 | — | .31 | .72 |
| EXO1 | 14 | 1 | 6 | 0.00577 | 0.00975 | 0.00857 | 1.70 (0.90 to 3.23) | .10 | .53 | 1.43 (0.46 to 4.47) | .56 | .87 |
| FANCD2 | 15 | 3 | 4 | 0.00216 | 0.00072 | 0 | 0.28 (0.06 to 1.42) | .10 | .53 | — | .12 | .61 |
| Remaining candidate PCa DNA repair genes | ||||||||||||
| BRIP1 c | 16 | 17 | 7 | 0.00252 | 0.00072 | 0.00214 | 0.32 (0.06 to 1.59) | .13 | .67 | 0.93 (0.1 to 8.66) | .95 | .97 |
| CHEK2 c | 17 | 22 | 16 | 0.01405 | 0.01913 | 0.02141 | 1.38 (0.89 to 2.14) | .14 | .68 | 1.63 (0.76 to 3.51) | .23 | .64 |
| MUTYH c | 28 | 1 | 10 | 0.01333 | 0.01661 | 0.01713 | 1.27 (0.81 to 1.99) | .30 | .81 | 1.51 (0.67 to 3.4) | .34 | .73 |
| MSH2 c | 31 | 2 | 9 | 0.00108 | 0.00217 | 0.00214 | 2.06 (0.46 to 9.28) | .33 | .81 | NA | NA | NA |
| BRCA1 c | 37 | 17 | 15 | 0.00252 | 0.00361 | 0.00428 | 1.55 (0.56 to 4.29) | .39 | .85 | 2.11 (0.37 to 12.21) | .42 | .80 |
| MSH6 c | 39 | 2 | 8 | 0.00396 | 0.00578 | 0.00428 | 1.36 (0.62 to 2.98) | .44 | .89 | 1.19 (0.24 to 5.86) | .84 | .94 |
| MRE11A c | 41 | 11 | 8 | 0.00216 | 0.00144 | 0 | 0.61 (0.17 to 2.25) | .45 | .89 | — | .19 | .64 |
| ATR c | 48 | 3 | 9 | 0.01766 | 0.01552 | 0.01285 | 0.87 (0.57 to 1.34) | .53 | .90 | 0.81 (0.33 to 1.99) | .64 | .90 |
| NBN c | 50 | 8 | 6 | 0.00180 | 0.00144 | 0.00642 | 0.69 (0.18 to 2.60) | .58 | .93 | 4.11 (0.83 to 20.3) | .10 | .59 |
| WRN c | 52 | 8 | 7 | 0.00396 | 0.00542 | 0.00214 | 1.22 (0.55 to 2.74) | .62 | .96 | 0.48 (0.06 to 3.95) | .45 | .80 |
| PMS2 c | 62 | 7 | 7 | 0.00144 | 0.00108 | 0.00214 | 0.82 (0.17 to 3.96) | .81 | .98 | NA | NA | NA |
| FANCL c | 67 | 2 | 7 | 0.00973 | 0.00903 | 0.01071 | 1.05 (0.60 to 1.85) | .87 | .98 | 1.21 (0.44 to 3.35) | .72 | .92 |
| XPC c | 70 | 3 | 3 | 0.00108 | 0.00108 | 0.00214 | 1.14 (0.22 to 5.85) | .88 | .98 | NA | NA | NA |
| FAM175A c | 79 | 4 | 8 | 0.00180 | 0.00181 | 0 | 0.97 (0.26 to 3.60) | .97 | .98 | NA | NA | NA |
| ERCC2 c | 80 | 19 | 13 | 0.00324 | 0.00361 | 0.00642 | 0.98 (0.39 to 2.46) | .97 | .98 | 2.48 (0.59 to 10.46) | .24 | .64 |
| RAD51C c | — | 17 | 4 | 0.00036 | 0.00144 | 0 | NA | NA | NA | NA | NA | NA |
| RAD51D c | — | 17 | 2 | 0.00036 | 0.00072 | 0.00214 | NA | NA | NA | NA | NA | NA |
| SLX4 c | — | 16 | 5 | 0.00108 | 0.00072 | 0 | NA | NA | NA | NA | NA | NA |
| XRCC2 c | — | 7 | 4 | 0.00036 | 0.00108 | 0 | NA | NA | NA | NA | NA | NA |
Ranking is based on the P values for aggressive vs nonaggressive PCa. Em dash (—) = effect could not be calculated because no alleles were present in metastatic cases; Agg = aggressive cases; CI = confidence interval; M1 = metastatic cases; NA = test not performed because the minor allele count was 5 or less between nonaggressive and metastatic cases; Non-agg = nonaggressive cases; OR = odds ratio; P adj = Benjamini-Hochberg adjusted P values, calculated using an alpha 0.05; PCa = prostate cancer; Chr = chromosome; Var = number of pathogenic, likely pathogenic, and deleterious variants identified.
P values are calculated using the likelihood-ratio test. All tests of statistical significance are 2-sided.
Subset of 24 literature-curated candidate PCa genes.
Statistically significant.
Figure 2.
Carrier frequencies and effects of candidate prostate cancer (PCa) DNA repair genes (DRG). Carrier frequencies (A) and effects (B) of candidate PCa genes by disease aggressiveness (RAD51C, RAD51D, SLX4, and XRCC2 were not evaluated in gene-based tests, as our sample had ≤5 carriers). Aggregate carrier frequencies (C) and aggregate effects (D) of DNA repair genes, sequentially removing the strongest genes. Left panels aggregate all DNA repair genes, including and excluding the 24 candidate PCa DRG genes. Right panels aggregate the 24 candidate PCa DRG genes, sequentially removing the 7 genes with the strongest risk-increasing effects. The remaining PCa DRG genes had no aggregate effect on aggressive disease (excluding top 3 genes: BRCA2, PALB2, and ATM, P = .18; excluding top 7 genes: BRCA2, PALB2, ATM, MLH1, CHEK2, MUTYH, and MSH2, P = .59). CI = confidence interval; OR = odds ratio.
In aggregate, P/LP/D alleles within the 155 DNA repair genes were more common in aggressive than nonaggressive PCa cases (carrier frequency = 36.4% vs 33.1%, respectively; P = .03) but did not statistically significantly differ between metastatic and nonaggressive cases (P = .17; Figure 2, C and D; Supplementary Table 5, available online). Larger differences were observed in the 24 candidate PCa genes, with nonaggressive cases having a statistically significantly lower carrier frequency (10.6%) than aggressive cases (14.2%; P = 5.56 × 10-5) and metastatic cases (15.4%; P = 3.61 × 10-4). Upon removing the 24 candidate genes from the 155 DNA repair genes, the remaining 131 genes were not associated with aggressive PCa risk (Figure 2, C and D). Further, the observed association with the 24 candidate genes was determined only by a small number of genes; upon sequentially removing genes with the strongest risk-increasing effects, the remaining genes had no aggregate effect on aggressive disease (excluding BRCA2, PALB2, and ATM, P = .18; excluding BRCA2, PALB2, ATM, MLH1, CHEK2, MUTYH, and MSH2, P = .59). Removing these genes similarly led to decreased aggregate effects on metastatic disease, with a residual non-statistically significant effect observed after excluding the 7 genes (OR = 1.10, 95% CI = 0.69 to 1.74, P = .69). P/LP/D alleles in BRCA2, PALB2, and ATM were found in 1.7% of nonaggressive vs 4.7% of aggressive (P = 5.46 × 10-10) and 5.1% of metastatic cases (P = 6.54 × 10-5; Supplementary Table 5, available online).
The HR/FA pathway was the only pathway with a statistically significant association, with carriers of P/LP/D HR/FA alleles having 1.27-fold increased risk of PCa death (95% CI = 1.05 to 1.53, P = .004); however, this association was statistically non-significant after excluding BRCA2 (P = .47; Supplementary Table 6 and Supplementary Figure 5, available online). The NER and MMR pathways were associated with a 1.48-fold and 1.29-fold increased risk of aggressive PCa, respectively, although neither was statistically significant (95% CI = 1.00 to 2.18, P = .045, and 95% CI = 0.95 to 1.76, P = .10, respectively).
Age at PCa Diagnosis
P/LP/D alleles within BRCA2, NBN, ATM, and CCNH had nominal (P < .05) associations with younger age at diagnosis; however, none were statistically significant after correcting for multiple testing (Supplementary Figure 6, A and B and Supplementary Table 7, available online). Carrying P/LP/D alleles within the 155 DNA repair genes was associated with a 0.59-year younger age at PCa diagnosis (95% CI = -1.00 to -0.19, P = .004; Supplementary Table 5, available online). Upon removing the 24 candidate PCa genes, the remaining 131 genes were associated with a 0.41-year younger age at diagnosis, although this did not reach statistical significance (95% CI = -0.84 to 0.03, P = .07). A larger effect was observed for the 24 candidate genes, with carriers having a 1.06-year younger age at diagnosis (95% CI = -1.65 to -0.48, P = 3.71 × 10-4), which reduced to a 0.55-year younger age at diagnosis after removing BRCA2, PALB2, and ATM (95% CI = -1.21 to 0.11, P = .10; Supplementary Figure 6, C, available online). P/LP/D alleles in the BER pathway were nominally associated with a younger age at diagnosis by 0.74 years (95% CI = -1.43 to -0.06, P = .03), although this was not statistically significant after correcting for multiple testing (Supplementary Table 6, available online). Associations with age at diagnosis did not statistically significantly differ in analyses stratified by disease aggressiveness (Supplementary Tables 5-7, available online).
Stratified Analyses
BRCA2, PALB2, ATM, and the aggregate 155 DNA repair genes and 24 candidate genes were further assessed in stratified analyses. We observed larger effects of P/LP/D BRCA2 alleles on aggressive PCa, PCa death, and metastatic disease among men diagnosed younger than 60 years vs those 60 years or older; however, these results did not statistically significantly differ between age strata (Supplementary Table 8, available online). The effects of PALB2, ATM, the aggregate 155 DNA repair genes, and the aggregate 24 candidate genes did not statistically significantly differ by age at diagnosis. No statistically significant differences were observed in analyses stratified by PCa family history (Supplementary Table 9, available online).
Risk associated with BRCA2 statistically significantly differed by country (P = .04; Supplementary Table 10, available online), with the strongest associations with aggressive disease observed in men from the United Kingdom (OR = 10.11, 95% CI = 2.23 to 45.76, P = 1.22 × 10-4), followed by Australia (OR = 5.60, 95% CI = 1.65 to 19.05, P = .002), the United States (OR = 2.84, 95% CI = 0.85 to 9.41, P= .07), and Sweden (OR = 1.91, 95% CI = 0.76 to 4.81, P = .16), with no evidence of association in Finnish men (OR = 0.69, 95% CI = 0.15 to 3.14, P = .62). Differences were also observed for the aggregate 24 candidate genes (P = .01), with the strongest associations with aggressive disease observed in men from the United Kingdom (OR = 2.24, 95% CI = 1.50 to 3.35, P = 4.92 × 10-5), followed by Finland (OR = 1.88, 95% CI = 1.17 to 3.02, P = .008), with non-statistically significant effects observed in men from Sweden, the United States, and Australia (ORs < 1.30). These differences remained statistically significant after excluding BRCA2, PALB2, and ATM (P = .03; Supplementary Table 10, available online), indicating the potential importance of the remaining 21 genes for certain populations.
Among nonmetastatic aggressive cases, the highest BRCA2 carrier frequency was observed in those with T1/T2 and a Gleason score of 8 or higher (4.7%, P = 3.65 × 10-7), followed by those with T3/T4 and a Gleason score of 8 or higher (2.5%, P = 3.55 × 10-5), T3/T4 and a Gleason scores less than 8 (1.9%, P = .33), and T1/T2 and a Gleason score less than 8 (1.6%, P = .20) relative to nonaggressive cases (0.8%) (Supplementary Table 11, available online). The aggregate 24 candidate PCa genes also had the highest carrier frequency in nonmetastatic aggressive cases with T1/T2 and a Gleason score of 8 or higher tumors (15.8%, P = .01), followed by cases with T3/T4 and a Gleason score less than 8 (14.3%, P = .11), T3/T4 and a Gleason score of 8 or higher (14.0%, P = .03), and T1/T2 and a Gleason score less than 8 (11.6%, P = .44) (Supplementary Table 11, available online).
Discussion
In this international case-case investigation of 5545 men with PCa, we investigated whether rare P/LP/D variants in 155 DNA repair genes differentiate risk of aggressive vs nonaggressive disease. BRCA2 and PALB2 were associated with the greatest risk, with P/LP/D BRCA2 carriers having 3.2-fold increased risk of aggressive PCa and P/LP/D PALB2 carriers having 6.3-fold increased risk of aggressive PCa. ATM had nominal evidence of association, with P/LP/D ATM carriers having 1.9-fold increased risk of aggressive PCa. Our candidate set of 24 DNA repair genes had higher aggregate carrier frequencies in aggressive (14.2%) and metastatic (15.4%) than nonaggressive (10.6%) PCa cases; however, these differences were largely driven by BRCA2, PALB2, and ATM.
Although PALB2 has been suspected to be a PCa susceptibility gene, because of the rarity of pathogenic variants in this gene, little statistical evidence has supported an association between PALB2 and PCa (35). PALB2 is an important biological link between BRCA1 and BRCA2 needed for homologous recombination repair after double-strand breaks (36), and rare pathogenic PALB2 variants have been reported to increase risk of breast, ovarian, and pancreatic cancer (37-39). One investigation reported marginal evidence of pathogenic PALB2 variants being associated with a 3.5-fold increased risk (95% CI = 0.7 to 10.3, P = .05) of metastatic PCa when compared with cancer-free controls in the Exome Aggregation Consortium (16). A recent study found that PALB2 was an important risk factor for overall and aggressive PCa in African American and Ugandan men, in addition to BRCA2 and ATM (20), which is of particular importance given that men of African descent have increased risk of aggressive PCa (40). Other studies have also reported ATM to be associated with increased risk of aggressive PCa (16,17), providing external support for the nominal ATM associations we observed.
The associations we identified between BRCA2 and increased risk of aggressive PCa are consistent with previous studies (16,17,19,41). We identified heterogeneous BRCA2 effects between populations, with larger effects seen in men from the United Kingdom and null effects in Finnish men, consistent with previous null findings in this population (42). Although we report fairly similar carrier frequencies among metastatic cases for 20 DNA repair genes investigated by Pritchard et al. (16) (Supplementary Table 12, available online), BRCA2 is a notable exception, being substantially more common among metastatic cases in this previous report (5.35%) than the current study (1.93%), and less common in TCGA primary PCa cases (0.20%), used as their comparison group, than our nonaggressive cases (0.83%). Another recent study (17) reported a similar BRCA2 carrier frequency among high-grade PCa cases (2.55%) as our aggressive cases (2.49%); however, they reported a lower frequency among low-grade cases (0.20%) than our nonaggressive cases (0.83%). “Winner’s curse” may contribute to the larger BRCA2 effect observed in these previous studies given their smaller sample sizes (43). Differences in carrier frequencies and/or effect sizes between studies may also be attributed to different compositions of aggressive and nonaggressive comparison groups.
We observed suggestive evidence of associations between the MMR pathway, which is associated with Lynch syndrome (44) and Lynch syndrome genes MLH1 and MSH2 contributing to risk of aggressive PCa. Although additional studies are needed to validate these findings, MMR variant carriers have been reported to have increased PCa risk, higher Gleason scores, and younger PCa diagnoses (45), and loss of MSH2 protein has been observed among high-grade primary PCa tumors (46).
The aggregate 24 candidate PCa genes were associated with younger age at PCa diagnosis, with some residual effect remaining after excluding the strongest risk-increasing genes: BRCA2, PALB2, and ATM. Although gene-based associations with age at diagnosis were not statistically significant after correcting for multiple testing, our nominal association between BRCA2 and younger age at diagnosis is consistent with previous studies (47,48). We also observed suggestive evidence for greater risk of aggressive PCa in BRCA2 carriers with a younger vs older age at diagnosis, which builds on previous reports of overall PCa risk being greater in younger than older BRCA2 carriers (49). Younger disease onset is typically attributed to stronger genetic predisposition, which may be partially attributable to P/LP/D BRCA2 variants for PCa.
Although our investigation represents the largest DNA repair gene sequencing study of PCa to date, the study was still underpowered to detect statistically significant associations in single variant and gene burden testing. For example, to detect an odds ratio of 2.0 with 90% power and a 0.25% carrier frequency in nonaggressive cases, more than 25 000 total cases would be needed. Until such samples are available, it will be difficult to nominate specific genes for personalized risk prediction of PCa and/or aggressive disease based on statistical evidence. This is supported by our observation that a multigene burden test of candidate DNA repair genes was no longer predictive of aggressive disease after removing the top 3 genes—BRCA2, PALB2, and ATM (OR = 1.14, 95% CI = 0.94 to 1.37, P = .18)—with further risk reduction observed when removing the top 7 genes (OR = 0.94, 95% CI = 0.74 to 1.19, P = .59, for the remaining 17 genes). A larger sample will also be necessary to identify genetic factors that distinguish subgroups of aggressive disease. Further, among our top findings, we observed association differences by country; although this can likely be partly attributed to genetic differences, it is possible that differences in the composition of aggressive and nonaggressive cases by country (Table 1) also contributed to these differences.
Our results suggest that PCa risk conveyed by DNA repair genes is largely driven by rare P/LP/D alleles within BRCA2, PALB2, and ATM, with suggestive evidence that MLH1, CHEK2, MUTYH, and MSH2 are also associated with increased risk of aggressive and metastatic disease. It was recently recommended that BRCA2 carrier status be factored into determining the initial age of PCa screening and intervals of subsequent screenings and BRCA2 and ATM be factored into high-risk and advanced PCa disease management (50). Our findings support the importance of these genes as well as PALB2 in both screening and disease management considerations. The decision to undergo genetic testing in men without PCa is typically based on family history; however, it was recently shown that men with PCa who do not have a family history carry P/LP alleles (51). Universal genetic testing to tailor PSA screening will require additional research and support of the clinical availability of such genetic testing. Although the modest risk conveyed by P/LP/D alleles within 24 candidate DNA repair genes provides important information regarding disease etiology, particularly given the sparsity of known risk factors for aggressive PCa beyond obesity (52), genes with larger effects, such as BRCA2, PALB2, and ATM, should be prioritized in future genetic risk prediction testing for PCa. In addition to the need to better understand the relative risks of each of these genes in aggressive and nonaggressive disease compared with cancer-free controls, research is needed to understand the role of rare coding variation in genes that function outside of DNA repair in overall and aggressive PCa.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health grant (R01 CA196931) and an award from the Achievement Rewards for College Scientists Foundation Los Angeles Founder Chapter. A full list of research support can be found in the Supplementary Materials.
Notes
Role of the funder: Funders provided financial support for the investigation and were not involved in study design, data analysis, or interpretation of the results.
Author contributions: Study conception/design: CAH, DVC, ZK-J; Data analysis: BFD, TD, XS, LYX; Data acquisition: XS, PW, LP, LYX, SC, SIB, SMG, VS, DA, SJW, VG, GGG, TN-D, RLM, MP, JAS, LM, WJC, RJM, MCS, RAE, FW, ZK-J, DVC, CAH; Data generation: KNH, KFD, Interpretation of results: BFD, TD, ES, RJM, ZK-J, DVC, CAH; Drafted the manuscript: BFD, CAH; Major revisions: BFD, CAH, ES, RJM, ZK-J, DVC; All authors read and approved of the final manuscript.
Disclosures: The authors have no conflicts of interest to report.
Acknowledgments: Sequencing services were provided by the Center for Inherited Disease Research (CIDR). CIDR is fully funded through a federal contract from the National Institutes of Health to the Johns Hopkins University, contract number HHSN268201200008I.
Prior presentations: Parts of this study have been presented during the poster session at the American Society of Human Genetics Annual Meeting, Houston, TX, October 15-19, 2019, and the International Genetic Epidemiology Society Annual Meeting, Virtual, July 2-3, 2020.
Data availability
Whole-exome sequencing data along with the clinical status of each participant in this investigation is available through the database of genotypes and phenotypes (dbGaP, accession number: phs001524.v1.p1).
Supplementary Material
Contributor Information
Burcu F Darst, Center for Genetic Epidemiology, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Tokhir Dadaev, The Institute of Cancer Research, London, UK.
Ed Saunders, The Institute of Cancer Research, London, UK.
Xin Sheng, Center for Genetic Epidemiology, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Peggy Wan, Center for Genetic Epidemiology, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Loreall Pooler, Center for Genetic Epidemiology, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Lucy Y Xia, Center for Genetic Epidemiology, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Stephen Chanock, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Sonja I Berndt, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Susan M Gapstur, American Cancer Society, Atlanta, GA, USA.
Victoria Stevens, American Cancer Society, Atlanta, GA, USA.
Demetrius Albanes, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Stephanie J Weinstein, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Vincent Gnanapragasam, Department of Surgery and Oncology, Academic Urology Group, University of Cambridge, Cambridge, UK.
Graham G Giles, Cancer Epidemiology Division, Cancer Council Victoria, Melbourne, Victoria, Australia; Precision Medicine, School of Clinical Sciences at Monash Health, Monash University, Melbourne, Victoria, Australia; Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Victoria, Australia.
Tu Nguyen-Dumont, Precision Medicine, School of Clinical Sciences at Monash Health, Monash University, Melbourne, Victoria, Australia; Department of Clinical Pathology, The University of Melbourne, Victoria, Australia.
Roger L Milne, Cancer Epidemiology Division, Cancer Council Victoria, Melbourne, Victoria, Australia; Precision Medicine, School of Clinical Sciences at Monash Health, Monash University, Melbourne, Victoria, Australia; Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Victoria, Australia.
Mark Pomerantz, Dana Farber Cancer Institute, Boston, MA, USA.
Julie A Schmidt, University of Oxford, Oxford, UK.
Lorelei Mucci, Harvard University, Cambridge, MA, USA.
William J Catalona, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Kurt N Hetrick, Department of Genetic Medicine, Center for Inherited Disease Research, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Kimberly F Doheny, Department of Genetic Medicine, Center for Inherited Disease Research, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Robert J MacInnis, Cancer Epidemiology Division, Cancer Council Victoria, Melbourne, Victoria, Australia; Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Victoria, Australia.
Melissa C Southey, Cancer Epidemiology Division, Cancer Council Victoria, Melbourne, Victoria, Australia; Precision Medicine, School of Clinical Sciences at Monash Health, Monash University, Melbourne, Victoria, Australia; Department of Clinical Pathology, The University of Melbourne, Victoria, Australia.
Rosalind A Eeles, The Institute of Cancer Research, London, UK; The Royal Marsden NHS Foundation Trust, London, UK.
Fredrik Wiklund, Karolinska Institute, Solna, Sweden.
Zsofia Kote-Jarai, The Institute of Cancer Research, London, UK.
David V Conti, Center for Genetic Epidemiology, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Christopher A Haiman, Center for Genetic Epidemiology, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin . 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3. Hemminki K, Ji J, Forsti A, et al. Concordance of survival in family members with prostate cancer. J Clin Oncol. 2008;26(10):1705–1709. [DOI] [PubMed] [Google Scholar]
- 4. Brandt A, Sundquist J, Hemminki K. Risk for incident and fatal prostate cancer in men with a family history of any incident and fatal cancer. Ann Oncol. 2012;23(1):251–256. [DOI] [PubMed] [Google Scholar]
- 5. Jansson KF, Akre O, Garmo H, et al. Concordance of tumor differentiation among brothers with prostate cancer. Eur Urol. 2012;62(4):656–661. [DOI] [PubMed] [Google Scholar]
- 6. Witte JS, Goddard KA, Conti DV, et al. Genomewide scan for prostate cancer-aggressiveness loci. Am J Hum Genet. 2000;67(1):92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schaid DJ, Stanford JL, McDonnell SK, et al. Genome-wide linkage scan of prostate cancer Gleason score and confirmation of chromosome 19q. Hum Genet. 2007;121(6):729–735. [DOI] [PubMed] [Google Scholar]
- 8. Slager SL, Schaid DJ, Cunningham JM, et al. Confirmation of linkage of prostate cancer aggressiveness with chromosome 19q. Am J Hum Genet. 2003;72(3):759–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Witte JS, Suarez BK, Thiel B, et al. Genome-wide scan of brothers: replication and fine mapping of prostate cancer susceptibility and aggressiveness loci. Prostate. 2003;57(4):298–308. [DOI] [PubMed] [Google Scholar]
- 10. Stanford JL, McDonnell SK, Friedrichsen DM, et al. Prostate cancer and genetic susceptibility: a genome scan incorporating disease aggressiveness. Prostate. 2006;66(3):317–325. [DOI] [PubMed] [Google Scholar]
- 11. Schumacher FR, Al Olama AA, Berndt SI, et al. ; the Profile Study. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet. 2018;50(7):928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amin Al Olama A, Kote-Jarai Z, Schumacher FR, et al. ; the UK Genetic Prostate Cancer Study Collaborators/British Association of Urological Surgeons’ Section of Oncology. A meta-analysis of genome-wide association studies to identify prostate cancer susceptibility loci associated with aggressive and non-aggressive disease. Hum Mol Genet. 2013;22(2):408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leongamornlert DA, Saunders EJ, Wakerell S, et al. Germline DNA repair gene mutations in young-onset prostate cancer cases in the UK: evidence for a more extensive genetic panel. Eur Urol. 2019;76(3):329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Na R, Zheng SL, Han M, et al. Germline mutations in ATM and BRCA1/2 distinguish risk for lethal and indolent prostate cancer and are associated with early age at death. Eur Urol. 2017;71(5):740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mijuskovic M, Saunders EJ, Leongamornlert DA, et al. Rare germline variants in DNA repair genes and the angiogenesis pathway predispose prostate cancer patients to develop metastatic disease. Br J Cancer. 2018;119(1):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu Y, Yu H, Li S, et al. Rare germline pathogenic mutations of DNA repair genes are most strongly associated with grade group 5 prostate cancer. Eur Urol Oncol. 2020;3(2):224–230. [DOI] [PubMed] [Google Scholar]
- 18. Leongamornlert D, Saunders E, Dadaev T, et al. ; the UKGPCS Collaborators. Frequent germline deleterious mutations in DNA repair genes in familial prostate cancer cases are associated with advanced disease. Br J Cancer. 2014;110(6):1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31(14):1748–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matejcic M, Patel Y, Lilyquist J, et al. Pathogenic variants in cancer predisposition genes and prostate cancer risk in men of African ancestry. J Clin Oncol Precision Oncol. 2020;4(4):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marshall CH, Sokolova AO, McNatty AL, et al. Differential response to olaparib treatment among men with metastatic castration-resistant prostate cancer harboring BRCA1 or BRCA2 versus ATM mutations. Eur Urol. 2019;76(4):452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(5):479–505. [DOI] [PubMed] [Google Scholar]
- 24. Koboldt DC, Kanchi KL, Gui B, et al. Rare variation in TET2 is associated with clinically relevant prostate carcinoma in African Americans. Cancer Epidemiol Biomarkers Prev. 2016;25(11):1456–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wood RD, Mitchell M, Sgouros J, et al. Human DNA repair genes. Science. 2001;291(5507):1284–1289. [DOI] [PubMed] [Google Scholar]
- 26. Saunders EJ, Dadaev T, Leongamornlert DA, et al. ; the UK Genetic Prostate Cancer Study Collaborators. Gene and pathway level analyses of germline DNA-repair gene variants and prostate cancer susceptibility using the iCOGS-genotyping array. Br J Cancer. 2016;114(8):945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kang J, D’Andrea AD, Kozono D. DNA repair pathway-focused score for prediction of outcomes in ovarian cancer treated with platinum-based chemotherapy. J Natl Cancer Inst. 2012;104(9):670–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ming M, He YY. PTEN in DNA damage repair. Cancer Lett. 2012;319(2):125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hart SN, Ellingson MS, Schahl K, et al. Determining the frequency of pathogenic germline variants from exome sequencing in patients with castrate-resistant prostate cancer. BMJ Open. 2016;6(4):e010332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McLaren W, Gil L, Hunt SE, et al. The Ensembl Variant Effect Predictor. Genome Biol. 2016;17(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Landrum MJ, Lee JM, Riley GR, et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucl Acids Res. 2014;42(D1):D980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meeks HD, Song H, Michailidou K, et al. BRCA2 polymorphic stop Codon K3326X and the risk of breast, prostate, and ovarian cancers. J Natl Cancer Inst. 2016;108(2):djv315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Firth D. Bias reduction of maximum-likelihood-estimates. Biometrika. 1993;80(1):27–38. [Google Scholar]
- 34. Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 35. Southey MC, Winship I, Nguyen-Dumont T. PALB2: research reaching to clinical outcomes for women with breast cancer. Hered Cancer Clin Pract. 2016;14(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang F, Ma J, Wu J, et al. PALB2 links BRCA1 and BRCA2 in the DNA-damage response. Curr Biol. 2009;19(6):524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3(9):1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hu C, LaDuca H, Shimelis H, et al. Multigene hereditary cancer panels reveal high-risk pancreatic cancer susceptibility genes. J Clin Oncol Precis Oncol. 2018;2(2):1–28., [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang X, Leslie G, Doroszuk A, et al. Cancer risks associated with germline PALB2 pathogenic variants: an international study of 524 families. J Clin Oncol. 2020;38(7):674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Group USCSW. U.S. Cancer Statistics Visualizations Tool, based on November 2017 submission data (1999-2015). 2017. http://www.cdc.gov/cancer/dataviz. Accessed October 1, 2019.
- 41. Edwards SM, Evans DG, Hope Q, et al. ; the UK Genetic Prostate Cancer Study Collaborators and BAUS Section of Oncology. Prostate cancer in BRCA2 germline mutation carriers is associated with poorer prognosis. Br J Cancer. 2010;103(6):918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ikonen T, Matikainen MP, Syrjakoski K, et al. BRCA1 and BRCA2 mutations have no major role in predisposition to prostate cancer in Finland. J Med Genet. 2003;40(8):e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kraft P. Curses–winner’s and otherwise–in genetic epidemiology. Epidemiology. 2008;19(5):649–651; discussion 657–658. [DOI] [PubMed] [Google Scholar]
- 44. Lynch HT, Smyrk T, Lynch J. An update of HNPCC (Lynch syndrome). Cancer Genet Cytogenet. 1997;93(1):84–99. [DOI] [PubMed] [Google Scholar]
- 45. Grindedal EM, Moller P, Eeles R, et al. Germ-line mutations in mismatch repair genes associated with prostate cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(9):2460–2467. [DOI] [PubMed] [Google Scholar]
- 46. Guedes LB, Antonarakis ES, Schweizer MT, et al. MSH2 loss in primary prostate cancer. Clin Cancer Res. 2017;23(22):6863–6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Page EC, Bancroft EK, Brook MN, et al. Interim results from the IMPACT study: evidence for prostate-specific antigen screening in BRCA2 mutation carriers. Eur Urol. 2019;76(6):831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kote-Jarai Z, Leongamornlert D, Saunders E, et al. ; the UKGPCS Collaborators. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: Implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105(8):1230–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91(15):1310–1316. [DOI] [PubMed] [Google Scholar]
- 50. Giri VN, Knudsen KE, Kelly WK, et al. Role of genetic testing for inherited prostate cancer risk: Philadelphia Prostate Cancer Consensus Conference 2017. J Clin Oncol. 2018;36(4):414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nicolosi P, Ledet E, Yang S, et al. Prevalence of germline variants in prostate cancer and implications for current genetic testing guidelines. JAMA Oncol. 2019;5(4):523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Su LJ, Arab L, Steck SE, et al. Obesity and prostate cancer aggressiveness among African and Caucasian Americans in a population-based study. Cancer Epidemiol Biomarkers Prev. 2011;20(5):844–853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Whole-exome sequencing data along with the clinical status of each participant in this investigation is available through the database of genotypes and phenotypes (dbGaP, accession number: phs001524.v1.p1).