Abstract
Objective
Despite empirical support for interdisciplinary pain rehabilitation programs improving functioning and quality of life, access to this treatment approach has decreased dramatically over the last 20 years within the United States but has grown significantly in the Department of Veterans Affairs (VA). Between 2009 and 2019, VA pain rehabilitation programs accredited by the Commission on Accreditation of Rehabilitation Facilities increased 10-fold in the VA, expanding from two to 20. The aim of this collaborative observational evaluation was to examine patient outcomes across a subset of six programs at five sites.
Methods
Outcomes were assessed using agreed-upon measures of patient-reported pain intensity, pain interference across various domains, pain catastrophizing, and sleep.
Results
A total of 931 patients enrolled in the selected VA interdisciplinary pain programs, with 84.1% of participants completing the full course of treatment. Overall, all programs showed significant improvements from pretreatment to posttreatment in nearly all patient-reported outcomes. The effect sizes ranged from medium to large. Notably, the results demonstrate that positive outcomes were typical despite differences in structure and resources across programs.
Conclusions
The adverse impacts of opioid use have highlighted the importance of chronic pain treatment approaches that emphasize team-based care focused on functional improvements. This study represents the first and largest analysis of outcomes across chronic pain rehabilitation programs and demonstrates the need for increased access to similar comprehensive approaches to pain management across the health care system. Further, it suggests that a variety of structures may be effective, encouraging flexibility in adopting this interdisciplinary approach.
Keywords: Interdisciplinary, Chronic Pain, Veterans, Pain Program, Multidisciplinary
Introduction
Although the complexity of pain is acknowledged and the biopsychosocial model is touted as the ideal heuristic [1–3], the US health care system typically offers fragmented care for pain. Patients are treated in silos, shuffled among primary care and specialists with limited coordination and integration of perspectives. A team-based approach to pain treatment in which a variety of therapeutic disciplines and modalities are used is recognized as the recommended model to adequately address the complex needs of individuals with persistent pain, particularly the 20 million with high-impact chronic pain affecting various functional domains [4, 5]; however, for most people, this option is absent or largely inaccessible. In the 1940s, pioneering physician John Bonica identified multidisciplinary treatment as being essential for pain care. John Loeser and William Fordyce furthered the cause by introducing interdisciplinary treatment programs in which patients were not simply treated concurrently across disciplines but by an integrated team with a shared philosophy and cohesive treatment plan. According to Loeser, “the great success of the program was due to the interaction between the various disciplines of the team members rather than to any specific intervention that was applied” [6].
Pain rehabilitation programs offer an exemplar of how evidence-based approaches for a variety of chronic pain conditions can be integrated into a cohesive treatment modality that provides multiple interventions informed by the biopsychosocial approach [7, 8]. The evidence for pain rehabilitation programs as an effective option for reducing pain and related disability has been well established in the literature [9–15]. They provide a “one-stop shop” to receive multimodal intervention, which has been shown repeatedly to be more therapeutic than the use of single modalities to address pain [8]. The structure and constitution of programs vary, but the common core components include cognitive behavioral treatments, physical therapy, occupational therapy, and medical management [16]. Philosophically, the focus is on improving functioning and quality of life while restoring levels of independence and self-efficacy [17].
These programs proliferated in the 1970s and 1980s, but third-party payers lost enthusiasm by the turn of the century despite significant empirical support [17]. The number of programs in the United States accredited by the Committee on Accreditation of Rehabilitation Facilities (CARF) declined from 210 in 1998 to 87 in 2019, according to CARF International (CARF International, written communication, August 2019). CARF is an independent nonprofit health and human services accreditor that provides a set of rigorous standards by which health care facilities may voluntarily elect to pursue accreditation in various areas (e.g., behavioral health, aging services, medical rehabilitation). Surveys are performed on a regular basis, and accreditation signifies that a high level of qualitative standards are embraced by accredited programs. Although it is common both inside and outside the Department of Veterans Affairs (VA) for pain programs to opt out of the CARF process for a variety of reasons (e.g., finances, staff shortages), the number of CARF-accredited pain rehabilitation programs provides a concrete, albeit imperfect, reference point for high-functioning pain treatment when there is no other similar systems-level data for such information. The decline in CARF-accredited and other pain rehabilitation programs is likely multifactorial, but undoubtedly payment for services is a key issue. Although the initial investment is greater than that for most unimodal services, the long-term benefits are proven; unfortunately, insurance carriers are hesitant to support participation in an interdisciplinary chronic pain program because the risk of switching carriers in the short term is valid [7, 18] and investment may never pay off for them. In addition, Chapman [18] notes that “concurrent with the decline in intensive programs is the rise of procedural interventions and medication, which receive a great deal of support from medical technology and pharmaceutical companies.”
The availability of interdisciplinary pain rehabilitation programs in the United States has grown in the VA, largely due to the 2009 Veterans Health Administration (VHA) Directive [19] that formally established a Stepped Care Model for Pain Management (SCM-PM) in the VA, which was critical for several reasons. The directive [19] established a population-based stepped approach to pain management that focused on pain prevention and management at all levels [20]. It outlined a new standard of multimodal pain care at each level, founded on a biopsychosocial patient-centered base, where individuals could move seamlessly between steps based on key factors such as comorbidities and treatment refractoriness [20]. In this context, the tertiary level—or “step 3”—was intended to help address the needs of the most complex patients. The directive mandated that each of the then 21 designated regions, known within the VA as Veteran Integrated Service Networks (VISNs), have at least one CARF-accredited interdisciplinary pain rehabilitation program by September 2014. When the directive was published in 2009, only two CARF-accredited pain programs existed within the VA, both in southeastern VISN 8 (Tampa, Florida, inpatient and San Juan, Puerto Rico, outpatient); therefore, the expansion was an ambitious request, particularly when not backed by funding. Between 2009 and 2019, the number of VA CARF-accredited pain programs increased 10-fold from two to 20 [7]. Despite the lack of direct fiscal support, organizational features of the VA facilitated development in other ways.
In 2013, the VA CARF Pain Programs Leadership Committee was initiated by and formed as an extension of the VA National Pain Management Strategy Coordinating Committee, the system’s highest-level pain advisory group chartered by the VA Central Office and responsible for supporting changes across the health care system. This increased efforts to achieve the goals set forth in the directive [19] and facilitate development of interdisciplinary pain rehabilitation programs. A subgroup of multidisciplinary health care professionals responsible for their local programs formed a voluntary Outcomes Workgroup as part of the CARF Pain Programs Leadership Committee. As the number of CARF programs grew, so too did clinicians’ innate desire to compare happenings across facilities and collaborate to improve patient care. The initial goal of the group was to establish a set of core outcome measures to fulfill this desire and serve as guidance to other pain rehabilitation programs. This started by first surveying all programs to determine the measurement tools being used across the country. With that information, those who participated in the workgroup agreed to use the same three core measures for standardized program evaluation and later agreed to participate in this observational evaluation. Because the evidence to support the clinical and cost effectiveness of interdisciplinary pain programs is robust and the studies are numerous [9–12, 14], we sought to examine the effectiveness of these programs and to compare similarities and differences across programs. We hypothesized that participation in these pain rehabilitation programs would contribute to significant improvements across outcome domains, despite the heterogeneous nature of the programs’ resources and structures. This study represents the first and largest analysis of patient outcomes across interdisciplinary pain rehabilitation programs to date.
Methods
Program Descriptions
Each program included in this evaluation developed pain programs based on a variety of factors. The James A. Haley Veterans’ Hospital in Tampa was identified as a training site, as it has a long-standing CARF-accredited inpatient program as well as an outpatient program. Because infrastructures and resources varied across sites, each facility created its own approach with support from hospital and VISN leadership. Core components are the foundation, whereas the inclusion of other pain treatment modalities is influenced uniquely at each site by availability and pain program leadership. The following programs are those that voluntarily chose to adopt a core set of mutually agreeable patient outcome measures. They represent a diverse group of the VA’s CARF pain rehabilitation offerings. Most programming is provided in a group format, but some services, such as initial evaluations or psychotherapy, may be completed individually. Details regarding each program format are provided.
Albuquerque
The New Mexico VA Health Care System’s Interdisciplinary Pain Rehabilitation Pain Program received initial accreditation from CARF in 2014. The program runs for 2 consecutive days for 5 weeks. Groups consist of cognitive behavioral therapy, neurophysiology education, physical therapy exercise, occupational therapy, yoga or tai chi, neuroscience education, auricular acupuncture, nutrition classes, family support classes and education, and medication safety classes. Patients meet with the entire treatment team on multiple occasions throughout the course of treatment to discuss progress; to address barriers, goal-setting, and values-based activities; and to answer any questions they may have while participating in the program. In addition, the program provides nursing aftercare follow-up calls at 1 week and 1 month following treatment to identify any initial barriers to success maintenance.
Cleveland
The Cleveland VA Pain Management Center’s Intensive Outpatient Program became CARF accredited in 2013. The program is held 1 day per week and requires that patients attend for 6 hours. The programming day includes group exercise, cognitive behavioral therapy, mental health occupational therapy, and aqua therapy. Vocational rehabilitation services, as well as individual and group appointments with a dietician, are available to interested program participants. This rolling admissions–based program allows patients to attend up to 12 weeks of treatment with a flexible discharge date that can be shortened in accordance with the patient’s progress toward treatment goals.
Puget Sound
The Outpatient Functional Restoration Pain Program is located at the VA Puget Sound Health Care System’s American Lake Division and has been CARF accredited since 2015. The program is held 2 days per week for 4 hours per day for 8 weeks. Groups consist of cognitive behavior–based therapy and psychoeducation, mind-body medicine, neuroplasticity education, physical therapy exercise and neuroscience education, and pharmacy education. Patients are seen individually to discuss progress, address challenges, set goals, and receive answers to any questions they may have while participating in the program.
San Francisco
The San Francisco VA Health Care System’s Intensive Pain Rehabilitation Program received its first CARF accreditation in 2014 and has an outpatient structure of 3 half-days per week for 12 weeks. Patients are admitted to the program following a team evaluation by an anesthesiologist, psychologist, and physical therapist. The program uses a rolling admissions format, with patients being admitted to and discharged from the program each month. In addition to individual and team meetings with providers on an as-needed basis, patients attend groups focused on cognitive behavioral therapy, acceptance and commitment therapy, physical therapy, pharmacy education, nutritional counseling, pain education that includes neuroscience, mindfulness instruction facilitated by an occupational therapist, and spiritual support. The program focuses on patients developing pain self-management skills and working toward individualized, functional goals based on patients’ values.
Tampa
The Chronic Pain Rehabilitation Program (CPRP) at the James A. Haley Veterans’ Hospital in Tampa, Florida, has CARF accreditation for both its inpatient and outpatient programs. The inpatient pain program was founded in 1988, and its initial CARF accreditation occurred in 1996; the outpatient program followed and has been CARF accredited since 2011. The inpatient CPRP accepts referrals from across the country for both military veterans and active-duty service members. The program has 12 physical medicine and rehabilitation beds with 24-hour nursing coverage; nurses are responsible for dispensing medications and providing other standard monitoring. Patients are admitted on a Sunday and spend the next 19 days (i.e., 3 weeks with two weekends) engaging in a comprehensive program that includes tapering off of opioid medications. The outpatient CPRP is held 2 days per week for 8 weeks. Following a medical and psychiatric evaluation that ensures appropriateness for the CPRP, patients and staff collaboratively determine which path of treatment (i.e., inpatient or outpatient) will be selected. For those from outside the Tampa catchment area and the state of Florida (i.e., over 50% of all inpatient participants), only the inpatient program is available, and the initial evaluation and follow-up care are provided via video-based telehealth and/or phone. For both programs, participants engage in a cognitive behavioral–based comprehensive program to help those with pain improve their pain self-efficacy and quality of life by learning ways to minimize and manage pain. Routine program components include medical consultation with medication adjustments, physical and occupational therapy, aquatic therapy in a heated pool, pain neuroscience education, vocational rehabilitation, recreational therapy, and psychological or behavioral therapy, as well as tai chi, yoga, the use of virtual reality, and various other classes taught by multidisciplinary staff (e.g., dietician, chaplain). Table 1 represents a summary of pain program strcuture and components by sites.
Table 1.
Summary of pain programs
| ABQ | CLE | PS | SF | TPA-IN | TPA-OUT | ||
|---|---|---|---|---|---|---|---|
| Program time commitment | Length of program | 5 wk | 12 wk* | 8 wk | 12 wk | 3 wk | 8 wk |
| Days per week | 2 d/wk | 1 d/wk | 2 d/wk | 3 d/wk | 5 d/wk | 2 d/wk | |
| Hours per day | 7 h/d | 6 h/d | 4 h/d | 3.5 h/d | 8.5 h/d | 5 h/d | |
| Total treatment hours | 70 h | 72 h | 64 h | 126 h | 127.5 h | 80 h | |
| Time points for outcomes collection | Pretreatment | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Midtreatment | ✓ | ||||||
| Posttreatment | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| One month posttreatment | ✓ | ✓ | ✓ | ||||
| Three months posttreatment | ✓ | ✓ | ✓ | ||||
| Six months posttreatment | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Twelve months posttreatment | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Sample program components | Behavioral therapy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Medication management | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Physical therapy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Neuroscience education | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Occupational therapy | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Yoga and/or tai chi | ✓ | ✓ | ✓ | ||||
ABQ = Albuquerque; CLE = Cleveland; PS = Puget Sound; SF = San Francisco; TPA = Tampa, IN = inpatient; OUT = outpatient.
Description of each program’s respective time commitments and time points for outcomes collection.
Flexible discharge time frame.
Program Participation
Referrals to all of the interdisciplinary pain programs typically come from within the VA system, with the only exception being those programs that accept active-duty military service members. The most common sources for consultations are primary care, pain clinics, rehabilitative care services, mental health, and women’s health. There are also referrals from specialties such as surgery, orthopedics, and neurosurgery. Patients must have pain of at least 3 months’ duration that is associated with functional impairment. They must be medically and psychiatrically cleared for the program and may be excluded if they have an acute medical issue requiring attention (e.g., unmanaged cardiac or pulmonary issues), untreated or unstable mental health or substance use disorder (e.g., florid psychosis), or are actively engaged in a pain-related workers’ compensation case. Those who are engaged in treatment for a mental health or substance use disorder generally are able to participate in an interdisciplinary pain rehabilitation program while receiving those other treatments concurrently, although these determinations are made on an individual basis. Cognitive concerns that would prohibit individuals from benefiting from the treatment may also prevent participation.
These tertiary-level interdisciplinary programs aim to be inclusive and to provide access to patients with chronic pain who are interested in treatment and who may benefit from a comprehensive whole-person approach. As tertiary pain care centers, these programs generally serve those with lengthy pain histories, higher levels of pain-related impairment, and complicated medical and psychiatric multimorbidities. Outside multiple pain conditions, common medical concerns include type 2 diabetes, chronic obstructive pulmonary disease, hypertension, hyperlipidemia, and obstructive sleep apnea. Psychosocial stressors often abound (e.g., lack of employment, relationship difficulties, financial hardship), and mental health issues such as depression and anxiety are frequent. In addition, regardless of military history, trauma-related conditions are often associated with chronic pain [21], and those with posttraumatic stress disorder (PTSD) are encountered routinely within pain rehabilitation programs. Demographics vary across programs. Overall sample demographcis are represented in Table 2, while demographics per each site are shown in Table 3.
Table 2.
Total sample description
| Characteristic | Percentage |
|---|---|
| Sex | |
| Female | 22% |
| Male | 78% |
| Race | |
| White | 58% |
| African American | 21% |
| Hispanic | 14% |
| Other | 8% |
| Pain site | |
| Back | 56% |
| Extremity | 15% |
| Headache | 4% |
| Neck | 8% |
| Other | 17% |
Table 3.
Veteran sample characteristics per site
| Sex, F (%) |
Race, F (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | N | African American | White | Hispanic | Other | Age, M (SD) | ||
| Site | |||||||||
| ABQ | Completers | 103 (88%) | 14 (12%) | 117 | 5 (4%) | 54 (46%) | 48 (41%) | 10 (2%) | 51.35 (10.97) |
| Noncompleters | 14 (78%) | 4 (22%) | 18 | 1 (6%) | 16 (88%) | 1 (6%) | 0 (0%) | 49.00 (11.64) | |
| CLE | Completers | 53 (79%) | 14 (21%) | 67 | 22 (33%) | 40 (60%) | 3 (4%) | 2 (3%) | 56.31 (11.55) |
| Noncompleters | 24 (77%) | 7 (23%) | 31 | 11 (36%) | 19 (61%) | — | 1 (3%) | 52.78 (13.79) | |
| PS | Completers | 101 (70%) | 44 (30%) | 145 | 20 (14%) | 107 (74%) | 12 (8%) | 6 (4%) | 51.11 (11.65) |
| Noncompleters | 23 (74%) | 8 (26%) | 31 | 5 (16%) | 22 (71%) | 3 (10%) | 1 (3%) | 48.43 (11.31) | |
| SF | Completers | 36 (80%) | 9 (20%) | 45 | 7 (16%) | 26 (57%) | 4 (9%) | 8 (17%) | 54.07 (14.39) |
| Noncompleters | 26 (93%) | 2 (7%) | 28 | 5 (18%) | 11 (39%) | 1 (4%) | 11 (39%) | 53.11 (12.64) | |
| TPA-IN | Completers | 285 (78%) | 79 (22%) | 364 | 94 (26%) | 205 (56%) | 40 (11%) | 25 (7%) | 52.71 (11.06) |
| Noncompleters | 21 (84%) | 4 (16%) | 25 | 5 (20%) | 14 (56%) | 4 (16%) | 2 (8%) | 52.08 (9.98) | |
| TPA-OUT | Completers | 31 (69%) | 14 (31%) | 45 | 15 (33%) | 12 (27%) | 17 (38%) | 1 (2%) | 55.51 (12.47) |
| Noncompleters | 10 (67%) | 5 (33%) | 15 | 2 (13%) | 10 (67%) | 2 (13%) | 1 (7%) | 51.47 (12.32) | |
| Total | Completers | 609 (84%) | 174 (85%) | 783 (84%) | 163 (21%) | 444 (57%) | 124 (16%) | 54 (7%) | 53.51 (11.49) |
| Noncompleters | 118 (16%) | 30 (15%) | 148 (16%) | 29 (20%) | 92 (62%) | 11 (7%) | 16 (10%) | 51.15 (12.07) | |
| Total enrolled | 727 | 204 | 931 | 192 (21%) | 536 (58%) | 135 (14%) | 70 (8%) | 52.33 (11.58) | |
ABQ = Albuquerque; CLE = Cleveland; PS = Puget Sound; SF = San Francisco; TPA = Tampa, IN = inpatient; OUT = outpatient.
Outcome Measures
When the CARF Pain Program Leadership Committee Outcomes Workgroup commenced, information was gathered via the general CARF Pain Programs Leadership Committee mail group regarding what measures were currently being used across all sites. The data gathered were reviewed and informed the selection of the three most commonly implemented “core measures,” whereas other tools could be used at the discretion of the individual sites based on their needs and interests for evaluation. Those participating in the Outcomes Workgroup agreed to adopt the three core measures, which required changes for several sites. The core pain measures were chosen by consensus because they represented measures already used most commonly across the system to assess various pain-related functional domains, pain-related cognitions or mindset, and sleep. It was agreed that these components constitute an overall picture of pain interference in quality of life and reflect the focus of pain rehabilitation that targets physical, emotional, and social functional restoration. Details regarding these measures are provided are in the following sections.
Pain Outcomes Questionnaire-For Veterans
The Pain Outcomes Questionnaire-For Veterans (POQ-VA) [22] is a multidomain pain assessment instrument developed and validated specifically for veterans. The POQ-VA assesses treatment outcomes across the major pain-related domains of functioning identified by the Rehabilitation Accreditation Commission (2002) [22] as being essential for comprehensive outcomes measurement. The POQ-VA scales include average pain intensity via the 0–10 pain numeric rating scale (pain NRS), pain interference in activities of daily living (ADL) and mobility (MOB), negative affect (NA), vitality (VIT), and pain-related fear (Fear). The POQ-VA scales have been shown to have high internal reliability and good stability, strong generalizability, and good discriminant and concurrent validity, and they have demonstrated sensitivity to treatment-related change [22–24].
Pain Catastrophizing Scale
The Pain Catastrophizing Scale (PCS) [25] is a 13-item self-report measure designed to assess an individual’s negative cognitions and feelings accompanying the experience or anticipation of pain. It is composed of three subscales representing the dimensions of rumination (e.g., “I can’t seem to get it out of my mind”), magnification (e.g., “I wonder if something serious may happen”), and helplessness (e.g., “There’s nothing I can do to reduce the intensity of the pain”). The alpha for the total PCS score is 0.92 and is 0.85, 0.75, and 0.86 for rumination, magnification, and helplessness, respectively [25]. Each item is rated using a 5-point Likert scale (where 0 represents “not at all” and 4 represents “all the time”). The total score represents a single construct of general catastrophizing and ranges from 0 to 52, with higher scores indicating a greater degree of pain-related catastrophic thinking. The PCS is a widely used measure among a variety of chronic pain populations and has shown good reliability and validity [26].
Insomnia Symptom Inventory
The Insomnia Symptom Inventory (ISI) [27] is a seven-item self-report measure of the nature, impact, and severity of insomnia. Respondents report “current” (i.e., last 2 weeks) sleep difficulties. The 5-point Likert scale ranging from 0 (“no problem”) to 4 (“very severe problem”) is used to rate each item, yielding a total score ranging from 0 to 28 (i.e., no clinically significant insomnia to severe insomnia). Previous studies have reported adequate psychometric properties. The ISI has been shown to have adequate psychometric properties (i.e., internal consistency, concurrent validity, factor structure) and can be used as a reliable and valid instrument to evaluate insomnia [28].
Data Analyses
All statistical analyses were performed using Microsoft Excel software, as it was available at each site. Analyses were conducted separately at each site using methods harmonized across all sites. Parametric tests were used for all analyses. Demographic information and descriptive statistics for all outcome measures have been provided. To assess differences in pretreatment and posttreatment scores for outcome variables, paired t tests were performed. To assess differences between completers and noncompleters, independent-samples t tests were performed. For all analyses, P<0.05 was considered significant. Data from noncompleters were excluded from the outcome analyses. Missing data from completers were not imputed. Missing data per site is shown in Table 6. Finally, to explore the size of improvement in outcome scores across all sites, the standardized mean differences (SMDs) were estimated and calculated for each site with their 95% confidence intervals (CIs). Pooled SMDs were calculated using fixed-effects analyses for each outcome measure. Statistical heterogeneity among sites was assessed using the Higgins I2 test Higgins & Thompson 2002. Exploratory subgroup analyses were conducted on the association of program duration with treatment outcomes. The result was considered to be statistically significant when the P value was less than 0.05.
Table 6.
Missing values for completers
| NRS | POQ | PCS | ISI | Total N | |
|---|---|---|---|---|---|
| ABQ | 0% | 0% | 0% | 0% | 117 |
| CLE | 14% | 2% | 0% | 3% | 67 |
| PS | 20% | 19% | 22% | 1% | 145 |
| SF | 0% | 0% | 0% | 4% | 45 |
| TPA-IN | 0% | 0% | 0% | 0% | 364 |
| TPA-OUT | 0% | 0% | 2% | 0% | 45 |
NRS = numeric rating scale; POQ = Pain Outcomes Questionnaire; PCS = Pain Catastrophizing Scale; ISI = Insomnia Sleep Index; ABQ = Albuquerque; CLE = Cleveland; PS = Puget Sound; SF = San Francisco; TPA = Tampa, IN = inpatient; OUT = outpatient.
Results
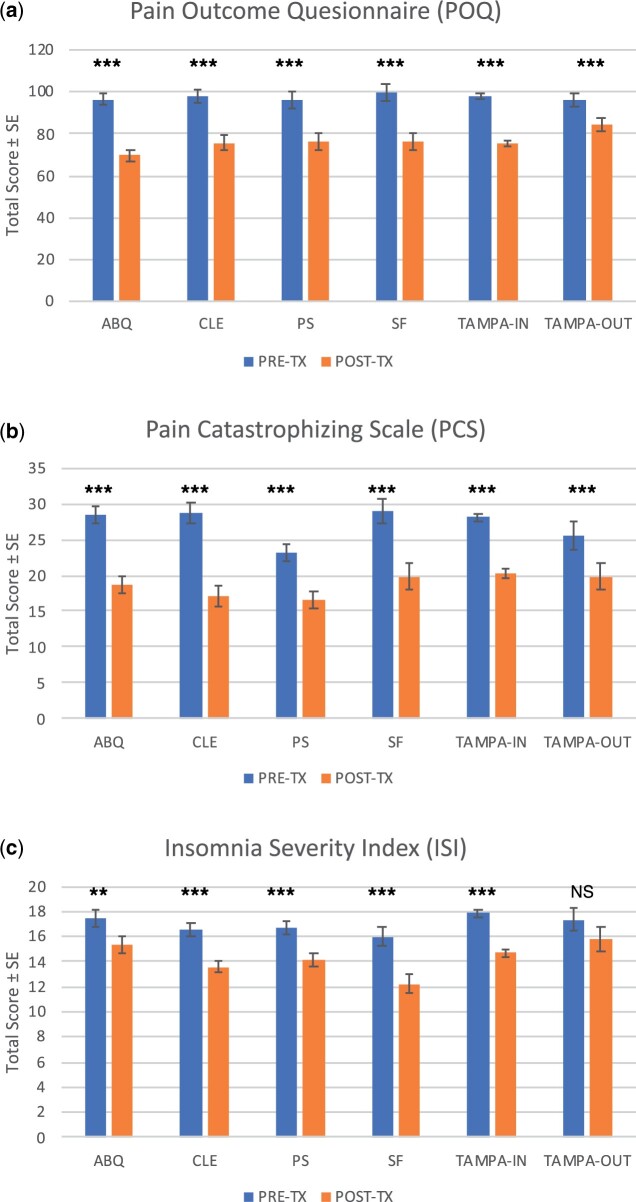
A total of 931 veterans enrolled in the selected VA tertiary-level interdisciplinary pain programs, with 84.1% of participants completing the full course of treatment. Table 2 summarizes the main demographics of the overall sample, indicating that the majority of participants were White (57%) men (78%) with chronic back pain (55%). Table 3 provides veteran sample characteristics for each site, whereas Table 4 provides each site’s pretreatment and posttreatment outcomes with corresponding paired t test statistics (corrected for multiple comparisons). Across all programs, we observed decreases in average scores for every variable, consistent with improved outcomes for each sample. Average total scores for outcome variables by site are presented in Figure 1. Finally, the results of independent-samples t tests comparing baseline outcome variables between completers and noncompleters are shown in Table 5.
Table 4.
Pretreatment and posttreatment patient outcomes
| POQ |
PCS | ISI | Pain NRS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Program | MOB | ADL | VIT | NA | Fear | Total | Total | Total | Average | |
| ABQ | Pretreatment | 23.86 (9.94) | 9.94 (10.11) | 19.15 (5.43) | 27.10 (11.88) | 9.75 (4.95) | 96.55 (31.45) | 28.33 (13.02) | 17.43 (7.70) | 6.74 (1.53) |
| Posttreatment | 17.04 (10.02) | 6.35 (8.35) | 13.60 (5.42) | 21.53 (11.50) | 5.50 (3.88) | 69.56 (31.81) | 18.65 (12.04) | 15.31 (7.64) | 5.53 (1.82) | |
| t (df)* | 7.36 (116)* | 4.17 (116)* | 11.01 (116)* | 5.13 (116)* | 10.31 (116)* | 9.19 (116)* | 8.32 (116)* | 2.98 (116)* | 7.72 (116)* | |
| CLE | Pretreatment | 25.73 (8.25) | 10.70 (9.75) | 19.93 (4.73) | 24.18 (11.03) | 10.79 (3.38) | 98.07 (28.03) | 28.61 (13.01) | 16.58 (7.32) | 6.75 (1.71) |
| Posttreatment | 21.22 (9.98) | 7.86 (9.40) | 15.05 (4.78) | 18.30 (11.17) | 8.24 (4.22) | 75.63 (30.60) | 17.16 (10.33) | 13.60 (6.51) | 5.59 (2.25) | |
| t (df)* | 4.45 (62)* | 2.80 (62)* | 7.63 (62)* | 4.76 (62)* | 4.71 (62)* | 6.66 (62)* | 7.82 (63)* | 5.86 (61)* | 4.25 (55)* | |
| PS | Pretreatment | 24.18 (9.76) | 10.22 (9.19) | 18.60 (5.28) | 26.12 (11.16) | 9.49 (4.73) | 96.29 (29.85) | 23.13 (12.51) | 16.73 (5.96) | 6.42 (1.69) |
| Posttreatment | 19.14 (9.85) | 8.64 (8.58) | 14.75 (4.89) | 26.44 (10.98) | 6.93 (4.08) | 76.45 (31.28) | 16.52 (10.36) | 14.10 (6.88) | 5.31 (2.02) | |
| t (df)* | 4.00 (116)* | 1.31 (116) | 6.20 (116)* | 3.63 (116) | 4.11 (116)* | 4.88 (116)* | 5.92 (112)* | 4.83 (143)* | 5.15 (115)* | |
| SF | Pretreatment | 23.44 (10.60) | 9.47 (10.23) | 20.80 (4.98) | 26.80 (12.51) | 12.78 (4.61) | 99.51 (31.61) | 28.93 (14.27) | 15.98 (6.63) | 6.22 (2.09) |
| Posttreatment | 18.11 (10.03) | 7.49 (8.16) | 15.42 (5.32) | 21.07 (11.71) | 9.13 (4.64) | 76.11 (31.15) | 19.80 (12.81) | 12.20 (7.01) | 5.00 (2.22) | |
| t (df)* | 3.68 (44)* | 1.64 (44) | 6.71 (44)* | 3.82 (44)* | 4.31 (44)* | 5.99 (44)* | 5.08 (44)* | 5.06 (42)* | 3.93 (44)* | |
| TPA-IN | Pretreatment | 25.37 (9.5) | 14.16 (10.6) | 19.55 (5.07) | 27.92 (11.32) | 11.29 (4.57) | 98.29 (27.9) | 28.07 (12.49) | 17.89 (5.72) | 6.87 (1.53) |
| Posttreatment | 18.49 (10.12) | 9.77 (9.49) | 15.79 (4.89) | 22.97 (12.08) | 8.01 (4.29) | 75.07 (31.47) | 20.2 (13.42) | 14.68 (6.75) | 5.91 (1.79) | |
| t (df)* | 15.55 (363)* | 9.63 (363)* | 12.75 (362)* | 10.07 (362)* | 13.43 (362)* | 17.26 (362)* | 12.96 (363)* | 10.18 (363)* | 10.37 (363)* | |
| TPA-OUT | Pretreatment | 24.24 (9.6) | 15.98 (11.65) | 18.22 (4.13) | 24.71 (12.89) | 12.64 (3.98) | 95.80 (32.71) | 25.57 (13.1) | 17.36 (7.12) | 6.89 (1.34) |
| Posttreatment | 21.73 (10.57) | 12.73 (10.93) | 15.89 (5.55) | 24.07 (12.66) | 9.71 (4.73) | 84.13 (36.37) | 19.87 (13.34) | 15.8 (7.16) | 6.38 (1.83) | |
| t (df)* | 2.45 (44)* | 2.62 (44)* | 3.01 (44)* | 0.51 (44) | 3.94 (44)* | 3.71 (44)* | 2.98 (43)* | 1.63 (44) | 1.94 (44) | |
POQ = Pain Outcomes Questionnaire; PCS = Pain Catastrophizing Scale; ISI = Insomnia Sleep Index; NRS = numeric rating scale; MOB = mobility; ADL = activities of daily living; VIT = vitality; NA = negative affect; ABQ = Albuquerque; CLE = Cleveland; PS = Puget Sound; SF = San Francisco; TPA = Tampa, IN = inpatient; OUT = outpatient; t=t score; df = degrees of freedom.
Paired t test analyses were used to identify statistically significant changes from pretreatment to posttreatment.
Significant at 0.05 level.
Figure 1.
Change in Patient Reported Outcomes by Site Total score averages on Pain Outcome Questionnaire (POQ) (A), Pain Catastrophizing Scale (PCS) (B) and Insomnia Severity Index (ISI) (C) collected before and after completing behavioral intervention at each participating site. Note: *< 0.05, **< 0.01, ***< 0.001.
Table 5.
Baseline outcome measures for completers and noncompleters
| POQ |
PCS | ISI | Pain NRS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Program | MOB | ADL | VIT | NA | Fear | Total | Total | Total | Average | |
| ABQ | Completers | 23.86 (9.94) | 9.94 (10.11) | 19.15 (5.43) | 27.10 (11.88) | 9.75 (4.95) | 96.55 (31.45) | 28.33 (13.02) | 17.43 (7.70) | 6.74 (1.53) |
| Noncompleters | 22.67 (9.66) | 10.61 (10.69) | 18.11 (4.55) | 32.17 (7.96) | 10.33 (4.68) | 100.06 (23.5) | 35.06 (11.26) | 19.72 (7.09) | 6.17 (1.38) | |
| t (df)* | 0.48 (133) | −0.26 (133) | 0.77 (133) | −1.75 (133) | −0.47 (133) | −0.45 (133) | −3.62 (133)* | −1.19 (133) | 1.49 (133) | |
| CLE | Completers | 25.73 (8.25) | 10.70 (9.75) | 19.93 (4.73) | 24.18 (11.03) | 10.79 (3.38) | 98.07 (28.03) | 28.61 (13.01) | 16.58 (7.32) | 6.75 (1.71) |
| Noncompleters | 26.21 (9.81) | 10.48 (10.52) | 19.55 (6.08) | 19.55 (6.08) | 10.03 (4.64) | 102.12 (28.54) | 26.48 (13.79) | 16.79 (6.95) | 7.21 (1.43) | |
| t (df)* | 0.85 (96) | 0.74 (96) | 0.91 (96) | 0.91 (96) | 1.36 (96) | 1.16 (96) | 1.22 (96) | 0.76 (96) | 1.71 (96) | |
| PS | Completers | 24.25 (9.27) | 10.51 (9.27) | 18.60 (5.35) | 26.12 (11.16) | 9.55 (4.67) | 96.13 (29.94) | 23.13 (12.54) | 16.73 (5.96) | 6.48 (1.71) |
| Noncompleters | 27.17 (7.29) | 11.52 (10.79) | 18.88 (8.54) | 25.94 (9.36) | 8.00 (4.94) | 98.82 (25.23) | 23.84 (11.12) | 17.68 (5.55) | 6.35 (1.85) | |
| t (df)* | −1.47 (146) | 0.36 (146) | 0.17 (146) | −0.07 (146) | −1.36 (146) | 5.14 (146) | 0.29 (142) | −0.63 (173) | 5.14 (145) | |
| SF | Completers | 23.44 (10.60) | 9.47 (10.23) | 20.80 (4.98) | 26.80 (12.51) | 12.78 (4.61) | 99.51 (31.61) | 28.93 (14.27) | 15.98 (6.63) | 6.22 (2.09) |
| Noncompleters | 24.25 (9.47) | 10.82 (8.97) | 17.50 (5.42) | 28.86 (10.68) | 11.46 (4.32) | 99.89 (28.03) | 26.58 (13.41) | 17.04 (6.88) | 7.00 (1.72) | |
| t (df)* | 0.33 (71) | 0.58 (71) | −2.66 (71)* | 0.72 (71) | −1.21 (71) | 0.05 (71) | −0.69 (69) | 0.64 (68) | 1.65 (71) | |
| TPA-IN | Completers | 25.37 (9.5) | 14.16 (10.6) | 19.55 (5.07) | 27.92 (11.32) | 11.29 (4.57) | 98.29 (27.9) | 28.07 (12.49) | 17.89 (5.72) | 6.87 (1.53) |
| Noncompleters | 24.83 (8.89) | 13.04 (11.61) | 20.33 (6.06) | 29.79 (10.10) | 12.38 (5.01) | 100.38 (32.67) | 25.87 (13.89) | 18.13 (5.04) | 7.17 (1.49) | |
| t (df)* | 0.27 (386) | 0.49 (386) | −0.72 (386) | −0.79 (386) | −1.12 (386) | −0.39 (386) | 0.82 (385) | −0.19 (386) | −0.91 (386) | |
| TPA-OUT | Completers | 24.24 (9.6) | 15.98 (11.65) | 18.22 (4.13) | 24.71 (12.89) | 12.64 (3.98) | 95.80 (32.71) | 25.57 (13.1) | 17.36 (7.12) | 6.89 (1.34) |
| Noncompleters | 26.07 (7.92) | 15.86 (10.09) | 20.93 (4.49) | 25.50 (14.34) | 12.71 (4.84) | 101.07 (31.53) | 22.07 (14.06) | 15.71 (6.63) | 6.79 (1.72) | |
| t (df)* | −0.65 (57) | 0.04 (57) | −2.09 (57)* | −0.19 (57) | −0.05 (57) | −0.53 (57) | 0.86 (56) | 0.77 (57) | 0.24 (57) | |
POQ = Pain Outcomes Questionnaire; PCS = Pain Catastrophizing Scale; ISI = Insomnia Sleep Index; NRS = numeric rating scale; MOB = mobility; ADL = activities of daily living; VIT = vitality; NA = negative affect; ABQ = Albuquerque; CLE = Cleveland; PS = Puget Sound; SF = San Francisco; TPA = Tampa, IN = inpatient; OUT = outpatient; t=t score; df = degrees of freedom.
Independent-samples t test analyses were used to identify statistically significant differences between completers and noncompleters.
Significant at 0.05 level.
Albuquerque
A total of 135 patients enrolled in the 5-week intensive pain rehabilitation program, with 86.6% (117) completing the full course of treatment. Those who completed the program were primarily men (80%) who were White (46%) or Hispanic (41%) with an average age of 51 years (SD = 10.97; Table 3). Average scores for all the outcome variables significantly decreased from pretreatment to posttreatment (Table 4) as expected. The results of independent-samples t tests shown in Table 5 indicate that after correcting for multiple comparisons, completers and noncompleters did not differ significantly on any of the baseline outcome variables.
Cleveland
A total of 98 participants enrolled in the 12-week intensive pain rehabilitation program, with 68% (67) completing the full course of treatment. As shown in Table 3, the participants who completed the program were primarily White (60%) men (79%) with an average age of 56 years (SD = 11.55). Average scores for all outcome variables significantly decreased from pretreatment to posttreatment as expected (Table 4). The results of independent-samples t tests shown in Table 5 indicate that completers and noncompleters did not differ significantly on any of the baseline outcome variables.
Puget Sound
A total of 176 veterans enrolled in the 8-week intensive pain rehabilitation program, with 82% (145) completing the full course of treatment. Participants who completed the program were primarily White (74%) men (70%) with an average age of 51 years (SD=11.65; Table 3). Average scores for all outcome variables decreased from pretreatment to posttreatment as expected, with statistically significant improvements shown in all but activities of daily living (Table 4). As shown in Table 5, there were no statistically significant differences between completers and noncompleters for any of the outcome variables.
San Francisco
As shown in Table 3, a total of 73 patients enrolled in the 12-week intensive pain rehabilitation program, with 61.6% (45) completing the full course of treatment. Those who completed the program were primarily White (57.6%) men (80.0%) with an average age of 54 years (SD = 14.4). Average scores for all outcome variables decreased from pretreatment to posttreatment as expected, with statistically significant improvements shown in all but activities of daily living (Table 4). The results of independent-samples t tests shown in Table 5 indicate that completers and noncompleters did not differ significantly on any of the baseline outcome variables, except for mean vitality scores (t[71] = –2.66, P=0.01).
Tampa Inpatient
As shown in Table 3, a total of 389 patients enrolled in the 3-week inpatient intensive pain rehabilitation program, with 93.6% (364) completing the full course of treatment. Those who completed the program were primarily White (56.3%) men (78.3%) with an average age of 52.7 years (SD = 11.06). Average scores for all outcome variables decreased significantly from pretreatment to posttreatment (Table 4) as expected. The results from independent-samples t tests shown in Table 5 indicate that completers and noncompleters did not differ significantly on any of the baseline outcome variables.
Tampa Outpatient
As shown in Table 3, a total of 60 patients enrolled in the 8-week outpatient intensive pain rehabilitation program, with 75% (45) completing the full treatment. Those who completed the program were primarily Hispanic (37.8%) men (68.9%) with an average age of 55.5 years (SD = 12.47). Average scores for all outcome variables decreased from pretreatment to posttreatment (Table 4) as expected, but decreases in pain intensity (t[45]=1.94, P=0.059), negative affect (t[45]=0.51, P=0.61), and sleep (t[45]=1.63, P=0.11) were not statistically significant. The results from independent-samples t tests shown in Table 5 indicate that after correcting for multiple comparisons, completers and noncompleters did not differ significantly on any of the baseline outcome variables.
Exploratory Analyses
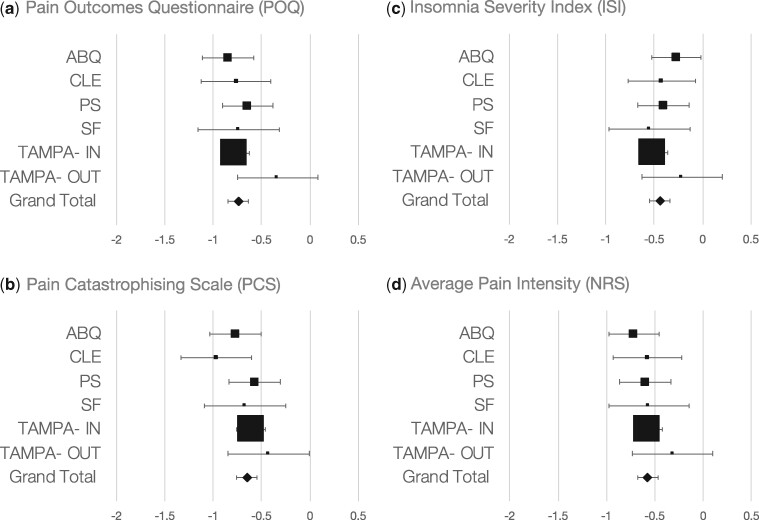
Figure 2 demonstrates the SMDs with their 95% CIs for improvement in patient outcomes for each site examined. Significant improvement was observed for all outcome measures (pain NRS: Hedges’ g [SMD] = −0.58; 95% CI, −0.69 to −0.48; P<0.001; I2=0.0%; POQ-VA: Hedges’ g [SMD] = −0.74; 95% CI, −0.8 to −0.63; P<0.001; I2=2.6%; PCS: Hedges’ g [SMD] = −0.65; 95% CI, −0.75 to −0.54; P<0.001; I2=8.5%; ISI: Hedges’ g [SMD] = −0.44; 95% CI, −0.54 to −0.33; P<0.001; I2=0.0%). There was no significant association between program duration and reported outcomes (P>0.05).
Figure 2.
Treatment effect mean differences in pain outcomes. Standardized Mean Difference (SMD) with 95% confidence intervals for Pain Outcome Questionnaire (POQ) (A), Pain Catastrophizing Scale (PCS) (B) Insomnia Severity Index (ISI) (C) and Average Pain Intensity (NRS) (D). Significant improvement was observed for all outcomes measures [Pain NRS: Hedges’g (SMD)= −0.58, 95% CI −0.69 to −0.48], P < 0.001, I2 = 0.0%), POQ: Hedges’g (SMD)= −0.74, 95% CI −0.8 to −0.63, P < 0.001, I2 = 2.6%, PCS: Hedges'g (SMD)= −0.65, 95% CI −0.75 to −0.54, P < 0.001, I2 = 8.5%, ISI: Hedges’g (SMD)= −0.44, 95% CI −0.54 to −0.33, P < 0.001, I2 = 0.0%]. There was no association between program duration and reported outcomes (P’s > 0.05).
Discussion
The objective of this study was to examine the effectiveness of and the similarities and differences across VA interdisciplinary pain rehabilitation programs that used the same core measures for program evaluation. A total of six programs at five sites were included, which represents the largest analysis of patient-reported outcomes across interdisciplinary chronic pain rehabilitation programs to date. Overall, we found that nearly all patient-reported outcomes showed statistically significant improvements across the programs examined, despite differences in structure and intensity. We found average reductions of 22% (range, 12–28%) in pain-related domains of functioning (e.g., mobility) [22], a 31% (range, 22–40%) reduction in pain catastrophizing, and a 16% (range, 9–24%) reduction in sleep difficulties. We also found that the length, contact, and intensity of treatment did not seem to have an overall effect on the investigated outcomes. For the outcomes included, the program dosage was not generally associated with better effect sizes, and an optimal dosage could not be determined.
Among all six programs examined, the largest effect was on pain catastrophizing. The profound impact of pain catastrophizing on both experimental and clinical pain cannot be overstated. It has been shown that individuals reporting high levels of pain catastrophizing also report greater pain in response to controlled, laboratory-induced, noxious stimuli [30–34]. In the clinical setting, in both cross-sectional and prospective studies, individuals reporting high levels of pain catastrophizing show increased vulnerability to developing chronic pain conditions [35], increased chronic pain severity and pain-related disability [36–39], and increased affective distress [40] and are less likely to respond to treatments [41]. Conversely, improvements in levels of pain catastrophizing predict decreased levels of high disability and disability days following treatment [42], with some suggesting that pain catastrophizing plays a larger role in predicting disability levels than depression [43]. Furthermore, levels of pain catastrophizing play an important role in trauma by mediating the effects of posttraumatic stress on pain chronification [44]. Recent work also found that levels of pain catastrophizing significantly predicted suicidal ideation and behaviors in patients with chronic pain and opioid use [45]. Thus, focusing on lowering levels of pain catastrophizing through value-based actions, cognitive restructuring, and other psychological techniques that are the focus of the interdisciplinary pain rehabilitation programs evaluated herein may be critical in increasing functional improvements in the veteran population.
Only one of the six programs included in this study (Tampa VA inpatient) included opioid tapering as a specific and required component of treatment. The other programs offered tapering through various mechanisms, and although not always part of participation, veterans frequently decreased opioid usage. Examples for ways that deprescribing occurred included continuing an established opioid taper plan initiated by a referring provider, working collaboratively with patients’ primary care teams to reduce medications during treatment or following completion of the program, or pain program prescribers assuming pain medication management and developing an individualized plan with patients during programming.
Despite the clear benefits of interdisciplinary pain rehabilitation programs to those individuals with the most treatment-refractory and high-impact pain, within the United States these programs have grown in the VA while struggling in the private sector. The VA’s 20 CARF-accredited pain programs represent almost one-fourth of what is currently available in the entire country despite the fact that veterans represent approximately 7% of the US population. Furthermore, interdisciplinary programs outside the VA are often targeted to workers’ compensation cases, as the treatment is financially supported by insurers in select states (e.g., Texas, California). It is an undeniable benefit that the VHA has the ability to adopt new models of care delivery that address many of the structural, socioeconomic, and clinical limitations present in a community or private health care system [20] and is not reliant on reimbursement by third-party payers, the primary obstacle in the private sector. However, implementing the stepped care model, a paradigm that could be emulated across settings, has also helped. Enhanced pain treatment at the primary and secondary levels provides adequate services for many, whereas those most in need of intensive pain rehabilitation can be cared for in a setting with increased scaffolding and support. In addition, the successful expansion of these programs within the largest health care system in the United States suggests that their realization may be more flexible than previously understood.
As evidenced in the current evaluation, the VA’s CARF-accredited pain programs have a variety of forms that were tailored to fit the unique needs of each facility. Our results demonstrate that despite these differences, positive outcomes are typical and treatment is beneficial to patients across domains of function. Although these programs do require collaboration and resources, the variety of effective formats suggests that there is room for flexibility in programming. The development of programs can and should be approached creatively, as a spectrum of options may produce similar effects. Although a setting that offers around-the-clock care is helpful in many ways and perhaps indicated for those most in need, programs can take numerous shapes while still providing quality care to individuals with pain. As Dr. Bonica [46] said, perhaps it is the “magic” of the team approach that contributes most to the powerful rehabilitative effects [47]—like-minded clinicians working together, alongside the patient, with both compassion and effective strategies for taking back control over pain. Instead of fragmented care without shared goals and communication, it models the treatment needed to address complex conditions. In fact, pain as a “team sport” has been espoused more loudly in recent years with a call for integrated, multimodal, interdisciplinary treatment recommended by the National Pain Strategy [47] and more strongly mandated by the Comprehensive Addiction and Recovery Act [48], with all VA facilities required to create multidisciplinary pain management teams.
The important takeaway is that interdisciplinary pain programs are effective, worthy of investment, and should be attended to and promoted as a mainstay for optimal pain treatment. Although we have seen changes on the policy side related to increased restrictions on opioids, we have not seen commensurate increases in the availability of comprehensive pain management. This is both unjust and unethical to those who suffer from chronic pain and is related in part to the lack of reimbursement for nonpharmacological options such as interdisciplinary pain rehabilitation programs, which have more evidence than many biomedical treatments that are covered by insurers. Population-based health strategy should include increasing access to interdisciplinary pain programs by revising reimbursement policies to enable those with chronic pain to benefit. The treatment model deserves the attention and support of legislators, insurance companies, and the health care industry. Sadly, the lack of these programs is a reflection of the misguided practice of offering unidimensional “solutions” that are often disappointing and neglect the need for whole-person pain care. Rather than focusing on the reduction of opioid medications as a primary outcome, the programs included in this study used treatments to increase pain management strategies and empower individuals with pain.
This study has several notable limitations. First, it involved a retrospective analysis of program evaluation data across sites, so the treatment was not randomized and we did not have a control group for comparison. It included only outcomes at program completion, and no follow-up information was available for this analysis. Future analysis will explore the long-term outcomes (i.e., 6 and 12 months), which will strengthen our conclusions and add to the literature. In addition, as there were numerous sites involved, the clinical information was collected somewhat differently at each location, which could have affected patient reporting. Furthermore, only five sites and six programs volunteered to participate in the CARF Outcomes Workgroup. These programs agreed to adopt the core measures established based on use in the field; however, their inclusion may result in a degree of selection bias and may not adequately represent all VA CARF pain programs. Despite that, based on the information tracked through the national leadership committee, the variability in the programs represented is typical of approaches across the system; hence, we believe that results would be similar with more sites included. Furthermore, as this sample was largely military veterans, it was predominantly male, and therefore women are underrepresented compared with the US population. However, the percentage of women served across the programs was 22%, which is higher than that of women in the general VA at 7.5% [49]. Finally, we realize the limitations to generalizability in the private sector given current barriers (e.g., lack of reimbursement) that do not support similar interdisciplinary care. Rather than ignore the evidence of these outcomes, however, we ask insurance companies and other health care systems to consider the beneficial clinical and financial impacts of this treatment approach for individuals who are often the highest utilizers of health care services.
Our outcomes support the effectiveness of interdisciplinary pain rehabilitation programs and reinforce the need to reconsider a model that exemplifies an integrated, coordinated, team-based approach to pain care.
Conclusion
The adverse impacts of increased prescription opioid use have shifted the focus to nonpharmacological approaches for chronic pain management, emphasizing the importance of biopsychosocially informed care that is patient centered, multidisciplinary, and focused on improvements in quality of life and functioning [50]. This is not a new approach to those working in an interdisciplinary pain rehabilitation program—it is the approach and has informed programming for decades. The question is how to leverage the current climate to precipitate the development, implementation, and reimbursement for this important treatment option. It is our hope that the current study will highlight the utility and benefits that may be gleaned from this long-standing and proven treatment milieu.
Acknowledgments
The authors would like to thank Ms. Nidhi S. Anamkath for helping with data organization. They would also like to extend recognition and gratitude to the leaders and members of the VA National Pain Management Strategy Coordinating Committee, who were instrumental in advocating for the expansion of interdisciplinary pain rehabilitation programs through the 2009 VA Pain Directive.
Current affiliations: Dr. Murphy is now with the VA Central Office and Dr. Van Keuren is with the Cleveland Clinic.
Funding sources: This work was supported in part by the US Department of Veterans Affairs I01-CX-000816 and I01-CX-001652 and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award No. U19AR076737 and UH2AR076724 (Irina A. Strigo).
Conflicts of interest: The authors have no conflicts of interest to report.
Disclosure: The information in this study does not represent the views of the Department of Veterans Affairs or the US government.
References
- 1. Gatchel RJ. The conceptual foundations of pain management: Historical overview. In: Gatchel RJ, ed. Clinical Essentials of Pain Management. Washington, DC: American Psychological Association; 2005:3–18. [Google Scholar]
- 2. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC.. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull 2007;133(4):581–624. [DOI] [PubMed] [Google Scholar]
- 3. Dueña M, Ojeda B, Salazar A, Mico JA, Failde I.. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res 2016;9:457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67(36):1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services. Report on pain management best practices: Updates, gaps, inconsistencies, and recommendations. 2019. Available at: https://www.hhs.gov/ash/advisory-committees/pain/reports/index.html (accessed August 2019).
- 6. Loeser JD. Multidisciplinary pain management. In: Merskey H, Loeser JD, Dubner R, eds. The Paths of Pain, 1975-2005. Seattle, WA: IASP Press; 2005:503–11. [Google Scholar]
- 7. Murphy JL, Schatman M.. Interdisciplinary chronic pain management: Overview and lessons from the public sector. In: Ballantyne JC, Fishman SM, Rathmell JP, eds. Bonica’s Management of Pain. 5th ed. Philadelphia, PA: Wolters Kluwer; 2018:1709–16 . [Google Scholar]
- 8. Nielson WR, Weir R.. Biopsychosocial approaches to the treatment of chronic pain. Clin J Pain 2001;17(suppl 4):S114–27. [DOI] [PubMed] [Google Scholar]
- 9. Flor H, Fydrich T, Turk DC.. Efficacy of multidisciplinary pain treatment centers: A meta-analytic review. Pain 1992;49(2):221–30. [DOI] [PubMed] [Google Scholar]
- 10. Turk DC, Okifuji A.. Treatment of chronic pain patients: Clinical outcomes, cost-effectiveness, and cost-benefits of multidisciplinary pain centers. Crit Rev Phys Rehabil Med 1998;10(2):181–208. [Google Scholar]
- 11. Turk DC. Clinical effectiveness and cost-effectiveness of treatments for patients with chronic pain. Clin J Pain 2002;18(6):355–65. [DOI] [PubMed] [Google Scholar]
- 12. Guzmán J, Esmail R, Karjalainen K, Malmivaara A, Irvin E, Bombardier C.. Multidisciplinary rehabilitation for chronic low back pain: Systematic review. BMJ 2001;322(7301):1511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guzmán J, Esmail R, Karjalainen K, Malmivaara A, Irvin E, Bombardier C.. Multidisciplinary bio-psycho-social rehabilitation for chronic low back pain: Systematic review. BMJ 2001;350:h444 (doi:10.1136/bmj.h444). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bujak BK, Regan E, Beattie PF, Harrington S.. The effectiveness of interdisciplinary intensive outpatient programs in a population with diverse chronic pain conditions: A systematic review and meta-analysis. Pain Manag 2019;9(4):417–29. [DOI] [PubMed] [Google Scholar]
- 15. Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: Cochrane systematic review and meta-analysis. BMJ 2015;350:h444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gatchel RJ, McGeary DD, McGeary CA, Lippe B.. Interdisciplinary chronic pain management: Past, present, and future. Am Psychol 2014;69(2):119–30. [DOI] [PubMed] [Google Scholar]
- 17. Schatman ME. The demise of multidisciplinary pain management clinics? Pract Pain Manag 2006;6(1):30–41. [Google Scholar]
- 18. Chapman SL. Chronic pain rehabilitation: Lost in a sea of drugs and procedures? Am Pain Soc Bull 2000;10(suppl 3):8–9. [Google Scholar]
- 19.Department of Veterans Affairs Veterans Health Administration. VHA Directive 2009-053: Pain Management. 2009. Available at: https://www.va.gov/painmanagement/docs/vha09paindirective.pdf (accessed August 2019).
- 20. Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin 2016;34(2):357–78. [DOI] [PubMed] [Google Scholar]
- 21. Moeller-Bertram T, Keltner J, Strigo IA.. Pain and post traumatic stress disorder - Review of clinical and experimental evidence. Neuropharmacology 2012;62(2):586–97. [DOI] [PubMed] [Google Scholar]
- 22. Clark ME, Gironda RJ, Young RW.. Development and validation of the Pain Outcomes Questionnaire-VA. J Rehabil Res Dev 2003;40(5):381–95. [DOI] [PubMed] [Google Scholar]
- 23. Gironda RJ, Azzarello L, Clark ME.. Test-retest reliability of the National Pain Data Bank V. 2.0. Am J Pain Mgmt 2002;12:24–30. [Google Scholar]
- 24. Gironda RJ, Clark ME.. Cluster analysis of the pain outcomes questionnaire. Pain Med 2008;9(7):813–23. [DOI] [PubMed] [Google Scholar]
- 25. Sullivan MJL, Bishop SR, Pivik J.. The Pain Catastrophizing Scale: Development and validation. Psychol Assess 1995;7(4):524–32. [Google Scholar]
- 26. Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O’Neill E.. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med 1997;20(6):589–605. [DOI] [PubMed] [Google Scholar]
- 27. Morin CM, Belleville G, Bélanger L, Ivers H.. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34(5):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bastien CH, Vallières A, Morin CM.. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 29. Higgins JPT, , Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 30. Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA.. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain 2006;22(8):730–7. [DOI] [PubMed] [Google Scholar]
- 31. Edwards RR, Campbell CM, Fillingim RB.. Catastrophizing and experimental pain sensitivity: Only in vivo reports of catastrophic cognitions correlate with pain responses. J Pain 2005;6(5):338–9. [DOI] [PubMed] [Google Scholar]
- 32. Dixon KE, Thorn BE, Ward LC.. An evaluation of sex differences in psychological and physiological responses to experimentally-induced pain: A path analytic description. Pain 2004;112(1–2):188–96. [DOI] [PubMed] [Google Scholar]
- 33. France CR, France JL, al’Absi M, Ring C, McIntyre D.. Catastrophizing is related to pain ratings, but not nociceptive flexion reflex threshold. Pain 2002;99(3):459–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rhudy JL, Maynard LJ, Russell JL.. Does in vivo catastrophizing engage descending modulation of spinal nociception? J Pain 2007;8(4):325–33. [DOI] [PubMed] [Google Scholar]
- 35. Bérubé M, Choinière M, Laflamme YG, Gélinas C.. Acute to chronic pain transition in extremity trauma: A narrative review for future preventive interventions (part 2). Int J Orthop Trauma Nurs 2017;24:59–67. [DOI] [PubMed] [Google Scholar]
- 36. Wolff B, Burns JW, Quartana PJ, Lofland K, Bruehl S, Chung OY.. Pain catastrophizing, physiological indexes, and chronic pain severity: Tests of mediation and moderation models. J Behav Med 2008;31(2):105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edwards RR, Dworkin RH, Turk DC, et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain 2016;157(9):1851–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA.. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol 2011;7(4):216–24. [DOI] [PubMed] [Google Scholar]
- 39. Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD.. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain 2016;17(suppl 9):T70–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Edwards RR, Bingham CO III, Bathon J, Haythornthwaite JA.. Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis Rheum 2006;55(2):325–32. [DOI] [PubMed] [Google Scholar]
- 41. Meints SM, Edwards RR.. Evaluating psychosocial contributions to chronic pain outcomes. Prog Neuropsychopharmacol Biol Psychiatry 2018;87(pt B):168–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scott EL, Kroenke K, Wu J, Yu Z.. Beneficial effects of improvement in depression, pain catastrophizing, and anxiety on pain outcomes: A 12-month longitudinal analysis. J Pain 2016;17(2):215–22. [DOI] [PubMed] [Google Scholar]
- 43. Arnow BA, Blasey CM, Constantino MJ, et al. Catastrophizing, depression and pain-related disability. Gen Hosp Psychiatry 2011;33(2):150–6. [DOI] [PubMed] [Google Scholar]
- 44. Andersen TE, Karstoft K-I, Brink O, Elklit A.. Pain-catastrophizing and fear-avoidance beliefs as mediators between post-traumatic stress symptoms and pain following whiplash injury: A prospective cohort study. Eur J Pain 2016;20(8):1241–52. [DOI] [PubMed] [Google Scholar]
- 45. Brown LA, Lynch KG, Cheatle M.. Pain catastrophizing as a predictor of suicidal ideation in chronic pain patients with an opiate prescription. Psychiatry Res 2020;286:112893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Butler S. A personal experience learning from two pain pioneers, J.J. Bonica and W. Fordyce: Lessons surviving four decades of pain practice. Scand J Pain 2010;1(1):34–7. [DOI] [PubMed] [Google Scholar]
- 47.US Office of the Assistant Secretary for Health. National pain strategy: A comprehensive population health strategy for pain. 2016. Available at: https://cha.com/wp-content/uploads/2018/01/National-Pain-Strategy-A-Comprehensive-Populationn-Health-Level-Strategy-for-Pain.pdf (accessed September 2019).
- 48.Comprehensive Addiction and Recovery Act, Pub L No. 114-198, 130 Stat 695 (2016).
- 49.US Department of Veterans Affairs. Sourcebook: Women veterans in the Veterans Health Administration. Volume 4: Longitudinal trends in sociodemographics, utilization, health profile, and geographic distribution. 2018. Available at: https://www.womenshealth.va.gov/WOMENSHEALTH/docs/WHS_Sourcebook_Vol-IV_508c.pdf (accessed October 2019).
- 50.US Department of Health and Human Services. Facing addiction in America: The surgeon general’s report on alcohol, drugs, and health. 2016. Available at: https://addiction.surgeongeneral.gov/sites/default/files/surgeon-generals-report.pdf (accessed December 2019). [PubMed]