Research reports on contraceptive development have reached the “big leagues” with this report from Chang et al., appearing in a recent issue of Nature Communications [1]. What I mean is that such research is for the most part published in respectable but less widely viewed journals that are specialized in reproductive biology. This report is the culmination of a team effort from the laboratory of and directed by Wei Yan who is well known not only for the importance of his research but also presently as Co-Editor-in-Chief of Biology of Reproduction. Development of a non-hormonal male contraceptive is the overarching aim of this research. The latest advances from experimental studies to develop methods of birth control for use by men are documented in the recent Special Issue on Contraception Contraceptive Development published by Biology of Reproduction in July 2020 [2]. Male contraception has received widespread attention in the form of user acceptance and importantly government and private sector funding [3]. The rationale for a male contraceptive drug is that men have a responsibility to share in family planning. The requirements for a male contraceptive drug include that it is reversible, there are no adverse side effects, that it approaches 100% efficacy, and that administration is user-friendly such as in the form of a daily pill. The authors note that “among all of the currently available contraceptives, ‘oral pills remain the most popular method. However, oral pills are available only for women, and despite five decades of efforts there remain no non-hormonal male contraceptive pills.” Also, these requirements place contraceptive drug development research under even more stringent controls than required for therapeutic use. According to this report the more that is known about mechanism of action of a drug, the more efficient will be the search that meets these criteria. With this goal in mind Chang et al. [1] have taken a mechanistic approach to establish how the antifertility properties of a common Chinese herb Tripterygium wilfordii Hook F (Figure 1A) can be the basis for a contraceptive drug. The antifertility effect of refined extracts of the roots of this herb was observed years ago in male mice and rats [4]. Of greater interest were the observations that when used in Chinese medicine, men taking daily oral doses of the plant root extract to treat rheumatoid arthritis and psoriasis became infertile. Lue et al. [5] reported that one of the ingredients of the extract, triptolide, at a dose level that induces complete infertility in rats, acts primarily on epididymal sperm [6]. However, key findings reported by Chang et al. [1] were that triptonide, another ingredient displays reversible male contraceptive effects on spermatids in both mice and monkeys. The rationale leading to this finding is what makes the report of interest to an audience beyond reproductive biologists. The research moves the field forward by utilizing state-of-the-art technology to develop a male contraceptive “pill” based on those properties of triptonide that cause deformation of sperm. Yan’s team formulated a strategy to impair spermiogenesis resulting in structurally deformed nonfunctional sperm at a late enough stage in the process that spermatogenesis continues, and the effect is reversible. That is, they defined the end stage of the process and then planned to disrupt it rather approaching drug discovery by the commonly used trial and error methods. Observations from several decades of studies on genes encoding proteins expressed in elongating and elongated spermatids [7] compared the triptonide “head bent-back” effect with a phenotype that resembled that of Spem1 KO sperm [8]. Detailed analyses of testes and sperm from treated mice revealed by scanning electron microscopy that bending of sperm heads occurred inside seminiferous tubules. To determine whether the effect would also occur in primates, triptonide was administered to male “cynomolgus” monkeys. Treated monkeys showed deformed sperm, were infertile and importantly suffered no systemic side effects. Furthermore, male infertility induced by triptonide was reversible in both mice and monkeys (Figure 1B and C). These observations supported the premise that triptonide could be the basis for a potent and safe male contraceptive agent.
Figure 1 .
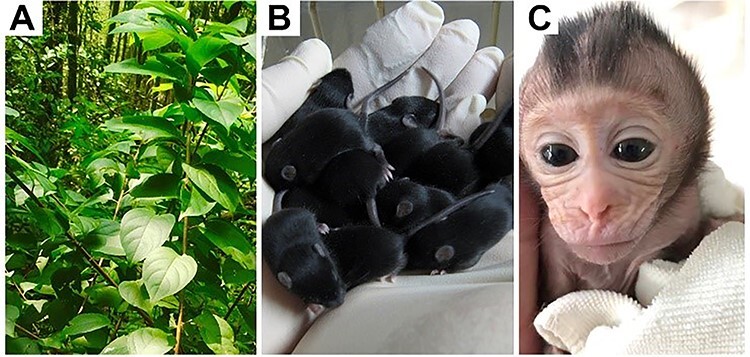
Triptonide is one of the numerous compounds purified from the Chinese herb Tripterygium wilfordii Hook F (Thunder God vine) (A). Single daily oral doses of triptonide induce deformed sperm with minimal or no forward motility with close to 100% penetrance and consequently male infertility in 3–4 and 5–6 weeks in mice and cynomolgus monkeys, receptively. Once the treatment is stopped, the male fertility comes back in ~4–6 weeks, and the fertility recovered male mice and monkeys can produce healthy pups (B) and babies (C), respectively. (Photo credit: Wei Yan).
These observations then led to a series of experiments designed to identify the molecular target that mediates the effect of triptonide. A series of testis extract were probed by binding assays followed by mass spectrometry that identified junction plakoglobin/gamma-catenin as the probable target. Also, and as noted above targeted disruption of SPEM1 is phenocopied in triptonide treated testes. SPEM-null mice are completely infertile because of deformed sperm characterized by a bent head wrapped around by the neck and the middle piece of the tail. Protein structural analyses predicted that the N-terminus of SPEM1 acts as a domain for protein–protein interactions. The N-terminal portion of SPEM1 (~28 a.a.) was synthesized and used to probe testicular lysates. Five proteins were identified to bind only to these N-terminus peptides, including junction plakoglobin/gamma-catenin, heat shock-related 70 kDa protein 2 (HSP70-2), L-lactate dehydrogenase C chain (LDHC), Y-box-binding protein 2, and retinal dehydrogenase 1. LDHC, Y-box-binding protein 2, and retinal dehydrogenase 1 also bind triptonide! These findings led the authors to speculate that interference with the interaction between SPEM1 and junction plakoglobin/gamma-catenin results in the “bent back” head phenomenon in spermatids. As an aside, the observation that there is an interaction between LDHC and triptonide is, of course, of interest to the author of this review, since LDHC has been proposed as a male contraceptive target [9].
The report by Chang et al. [1] contains a wealth of rigorously obtained experimental data which were obtained by state-of-the-art methodology in experiments designed to move forward our precise knowledge of the molecular events of sperm development. The key intellectual contribution from this research was the “Top Down” approach to drug discovery. Define your target in terms of the desired effect which here is to impair sperm function and then establish experimentally that a compound involved can be the basis for contraceptive drug development. This design and the success of its conceptual foundation explain the broad interest in the work. Of at least equal importance is the increased prospect of developing a male contraceptive and demonstrating proof-of-concept efficacy and reversibility of triptonide as a potential non-hormonal male contraceptive. These first animal studies represent a major milestone that will allow the research team to move towards safety studies in humans.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. Chang Z, Qin W, Zheng H, Schegg K, Han L, Liu X, Wang Y, Wang Z, McSwiggin H, Peng H, Yuan S, Wu Jet al. Triptonide is a reversible non-hormonal male contraceptive agent in mice and non-human primates. Nat Commun 1253, 2021; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matzuk MM, Georg GI, Yan W. A special issue on contraceptive development: past, present, and future. Biol Reprod 2020; 103:145–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnston DS, Goldberg E. Preclinical contraceptive development for men and women. Biol Reprod 2020; 103:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matlin SA, Belenguer A, Stacey VE, Qlan SZ, Xu Y, Zhang JW, Sanders JK, Amor SR, Pearce CM. Male antifertility compounds from Tripterygium wilfordii Hook F. Contraception 1993; 47:387–400. [DOI] [PubMed] [Google Scholar]
- 5. Lue Y, Sinha Hikim AP, Wang C, Leung A, Baravarian S, Reutrakul V, Sangsawan R, Chaichana S, Swerdloff RS. Triptolide: a potential male contraceptive. J Androl 1998; 19:479–486. [PubMed] [Google Scholar]
- 6. Hikim AP, Lue YH, Wang C, Reutrakul V, Sangsuwan R, Swerdloff RS. Posttesticular antifertility action of triptolide in the male rat: evidence for severe impairment of cauda epididymal sperm ultrastructure. J Androl 2000; 21:431–437. [PubMed] [Google Scholar]
- 7. Yan W. Male infertility caused by spermiogenic defects: lessons from gene knockouts. Mol Cell Endocrinol 2009; 306:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng H, Stratton CJ, Morozumi K, Jin J, Yanagimachi R, Yan W. Lack of Spem1 causes aberrant cytoplasm removal, sperm deformation, and male infertility. Proc Natl Acad Sci U S A 2007; 104:6852–6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldberg E. The sperm specific form of lactate dehydrogenase (LDHC4) is required for fertility and is an attractive target for male contraception a review. Biol Reprod 2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.


