Abstract
The serine/threonine kinase Akt (also known as protein kinase B) (Akt/PKB) is activated upon T-cell antigen receptor (TCR) engagement or upon expression of an active form of phosphatidylinositide (PI) 3-kinase in T lymphocytes. Here we report that the small GTPase Rac1 is implicated in this pathway, connecting the receptor with the lipid kinase. We show that in Jurkat cells, activated forms of Rac1 or Cdc42, but not Rho, stimulate an increase in Akt/PKB activity. TCR-induced Akt/PKB activation is inhibited either by PI 3-kinase inhibitors (LY294002 and wortmannin) or by overexpression of a dominant negative mutant of Rac1 but not Cdc42. Accordingly, triggering of the TCR rapidly stimulates a transient increase in GTP-Rac content in these cells. Similar to TCR stimulation, L61Rac-induced Akt/PKB kinase activity is also LY294002 and wortmannin sensitive. However, induction of Akt/PKB activity by constitutive active PI 3-kinase is unaffected when dominant negative Rac1 is coexpressed, placing Rac1 upstream of PI 3-kinase in the signaling pathway. When analyzing the signaling hierarchy in the pathway leading to cytoskeleton rearrangements, we found that Rac1 acts downstream of PI 3-kinase, a finding that is in accordance with numerous studies in fibroblasts. Our results reveal a previously unrecognized role of the GTPase Rac1, acting upstream of PI 3-kinase in linking the TCR to Akt/PKB. This is the first report of a membrane receptor employing Rac1 as a downstream transducer for Akt/PKB activation.
Engagement of the T-cell antigen receptor (TCR) by antigen in a major histocompatibility complex context or by antibodies that cross-link this receptor triggers a complex series of signaling events that lead to reorganization of the cytoskeleton as well as transcriptional activation of multiple genes and culminate in T-lymphocyte activation and proliferation (9). One of the earliest events triggered by TCR engagement is the activation of protein tyrosine kinases (PTKs). Activation of the Src tyrosine kinase Lck is necessary to phosphorylate the cytoplasmic tails of the CD3 complex on tyrosine residues within the immunoreceptor tyrosine-based activation motifs (ITAMs). Phosphorylation of the ITAMs provides docking sites for the Src homology domains (SH2) of the Syk family PTKs which, once recruited, become activated and cause subsequent tyrosine phosphorylation of multiple substrates. One such substrate is the integral membrane protein LAT (linker for activation of T cells), whose phosphorylation allows recruitment of a whole range of signaling molecules, including Grb2, PLC-γ, GADs, SLP-76, Cbl, Vav, and the regulatory subunit p85 of phosphatidylinositide (PI) 3-kinase, through either direct or indirect interactions (46). With respect to PI 3-kinase, the TCR is endowed with at least two other putative modes of activation: via a direct mechanism, by binding of the p85 regulatory subunit of PI 3-kinase to the tyrosine phosphorylated ITAM (11, 25), or in an indirect way, through activation of Ras (12), which in turn could interact with and activate the p110 catalytic subunit of PI 3-kinase (31, 32). PI 3-kinase catalyzes the phosphorylation of phosphoinositides at the D3 hydroxyl of the myoinositol ring, generating polyphosphoinositides PtdIns(3)P, PtdIns(3,4)P2, and PtdIns(3,4,5)P3, which act as second messengers to recruit and activate downstream effectors.
One well-characterized PI 3-kinase effector is Rac1 (27), a GTPase which controls cytoskeletal organization and cell morphology (24). In various cell types, activation of Rac1 in response to growth factors elicits actin polymerization at the plasma membrane to produce lamellipodia and membrane ruffles (30). In T cells, membrane ruffling is induced in response to the T-cell growth factor interleukin 2 (IL-2) via a pathway also involving PI 3-kinase and Rac1 (3). Another major target of PI 3-kinase signaling is the serine/threonine kinase Akt (also known as protein kinase B) (Akt/PKB). This kinase regulates critical functions, such as insulin signaling, cell survival, and cell cycle progression (reviewed in reference 10). Akt/PKB is activated by a number of receptors that activate PI 3-kinase in various cell types and by various ligands, such as growth factors including insulin, epidermal growth factor (EGF), platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), or cytokines, such as IL-2, IL-3, IL-4, granulocyte-macrophage colony-stimulating factor, or the B-cell antigen receptor (17). In these systems, it has been shown that activation of PI 3-kinase is necessary for the induction of activation of Akt/PKB. In mature T cells, Akt/PKB has also been shown to protect against cell death and to control cell cycle progression, two events essential for proper clonal expansion (1, 7). In these cells, stimulation of Akt/PKB by the TCR is also strictly dependent on the activity of PI 3-kinase since it is blocked by the PI 3-kinase inhibitors wortmannin and LY294002 (15). Moreover, ectopic expression of constitutively active forms of PI 3-kinase stimulates Akt/PKB (15, 26).
Considerable progress has been made toward understanding how PI 3-kinase activates Akt/PKB (5). The generation of the polyphosphoinositides by PI 3-kinase serves to localize Akt/PKB at the plasma membrane, through binding of its PH domain. This event is thought to expose Akt/PKB to phosphoinositide-dependent kinases (PDKs). Akt/PKB is then phosphorylated on threonine 308 in the activation loop of the kinase domain by PDK1 and on serine 473 at the carboxy terminus by an as-yet-unidentified kinase often referred to as PDK2. In COS-1 cells, it was shown that phosphorylation of either threonine 308 or serine 473 leads to partial activation of the enzyme in vitro and that phosphorylation of both residues results in a synergistic activation of Akt/PKB catalytic function (2). Activated Akt/PKB next detaches from the membrane and phosphorylates substrates in the cytosol and nucleus.
In order to delineate the roles of small GTPases in effector pathways downstream of the TCR (16, 44), we screened for a possible involvement of members of the Ras and Rho family of GTPases in the activation of Akt/PKB. Our previous studies have revealed that in Jurkat T cells, TCR-induced Akt/PKB activation is indeed mediated by PI 3-kinase but occurs independently of Ras (15). We now report that Rac1 is a key player in the activation of Akt/PKB. Akt/PKB activity is induced by a constitutively active mutant of Rac1 in a PI 3-kinase-dependent manner, positioning this GTPase between the receptor and the lipid kinase. Furthermore, we provide evidence that, within the same cell, PI 3-kinase and Rac1 are placed in opposite positions in the pathways resulting in Akt/PKB activation versus cytoskeletal rearrangement.
MATERIALS AND METHODS
Cells and reagents.
JHM1, a subline of the human T-acute lymphocytic lymphoma cell line Jurkat (15), was maintained in RPMI medium supplemented with 5% heat-inactivated fetal calf serum and 2 mg of geneticin (Gibco BRL) per ml at 37°C under 5% CO2. Cells were transferred to geneticin-free medium 48 h prior to the experiments. Reagents were from Sigma. Antibodies UCHT1 (reactive with human CD3), 12CA5 (antihemagglutinin [anti-HA] tag), and 9E10 (anti-myc tag) were affinity purified from hybridoma supernatants at the Imperial Cancer Research Fund (ICRF) by standard protocols. Antibodies against Akt/PKB were from U.B.I. or New England Biolabs, and anti-Rac1 and anti-Cdc42 antibodies were obtained from Santa Cruz Biotechnology. Peroxidase-labeled antibodies used in the Western blotting protocol were from Amersham International (Amersham, Buckinghamshire, United Kingdom), and LY294002 and wortmannin were from Calbiochem. Histone 2B (H2B) was from Boehringer Mannheim.
Plasmids and reporter constructs.
Plasmids directing expression of wild-type or mutated N-terminal-HA-tagged Akt/PKB were used in kinase assays. pSG5 HA-wtAkt/PKB, pcDNA3 EE-T308A Akt/PKB, pSG5 HA-S473A-Akt/PKB, and pSG5 HA-ΔPH-Akt/PKB have been described previously (8, 34, 38). Constitutively active, myc-tagged L61Rac and its mutants and L61Cdc42 were subcloned from PRK5 into pEFBOS vector (22). The chimera construct of PI 3-kinase, pEFrCD2p110, and the pSG5p110K227E mutant have also been described previously (27, 33). All plasmids were purified by equilibrium centrifugation in CsCl-ethidium bromide gradients by using standard procedures.
Cells and transfections.
Cells were transfected via electroporation (Gene pulser; Bio-Rad, Hemel Hempstead, United Kingdom) as previously described (16). Briefly, cells were pulsed (at 1.5 × 107 cells/0.5 ml) in complete medium at 960 μF and 310 V. Cells transfected with similar plasmid mixtures were pooled and realiquoted before treatment with stimulating antibodies. The amounts of DNA transfected in each experiment were kept constant by adding empty vector.
Rac1 activation assay.
The assay was performed essentially as described previously (21). The GST-PAK70-106 (GST-CRIB) was a generous gift from E. Manser (23). For each point, 4.5 million cells were treated with phosphate-buffered saline (PBS), in the absence or presence of 10 μg of UCHT1 antibody per ml or 0.5 mM carbachol for the time periods indicated. Cells were lysed by incubation for 15 min at 4°C in a buffer containing 25 mM HEPES (pH 7.3), 0.15 M NaCl, 5 mM MgCl2, 0.5 mM EGTA (pH 8), 20 mM beta-glycerophosphate (pH 7.5), 0.5% Triton X-100, 4% glycerol, 10 mM NaF, 2 mM sodium orthovanadate, 5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 5 μg of leupeptin per ml, and 5 μg of pepstatin per ml. The lysate was centrifuged at 14,000 × g for 10 min at 4°C. The supernatant was next incubated with 40 μg of bacterially expressed glutathione S-transferase (GST)-CRIB prebound to 20 μl of gluthathione agarose and was tumbled for 15 min at 4°C. The beads were collected in a spin column and washed with 500 μl of lysis buffer. Forty microliters of 1× reducing sample buffer was added, followed by an incubation at 100°C for 5 min. Eluted proteins were resolved by sodium dodecyl sulfate–15% polyacrylamide gel electrophoresis (SDS–15% PAGE) and Western blotting with an anti-Rac1 antiserum (Transduction Labs).
Kinase assays and Western blot analysis.
For Akt/PKB kinase assays, cells were transfected with an HA-tagged version of wtAkt/PKB together with various expression plasmids. At 18 to 24 h after transfection, cells were harvested and equivalent numbers of living cells were subjected to immunoprecipitation using 12CA5 antibodies as previously described (15). The kinase reaction was performed using H2B as a substrate in the presence of [γ-32P]ATP. After incubation at room temperature for 30 min, the reaction was stopped with Laemmli sample buffer and kinase reaction products were analyzed by SDS–15% PAGE. The lower half of the gel was dried down, and 32P incorporation into H2B was quantitated using a PhosphorImager (Molecular Dynamics). The upper part of the gel containing immunoprecipitated tagged protein kinase was transferred onto polyvinyldifluoridine membrane membranes (Immobilon; Millipore). Membranes were blocked in PBS–0.05% Tween 20 (PBST) plus 5% milk (5% bovine serum albumin for phosphospecific antibodies) for 2 h. Appropriate dilutions of the relevant antibodies were incubated with the membrane overnight at 4°C. After extensive washes, membranes were incubated with the second antibody in PBST for 1 h at room temperature. Membrane-bound antibodies were visualized by autoradiography using ECL Western blotting detection reagents (Amersham) and Kodak XS1 films. The amount of Akt/PKB detected by Western blotting was determined by scanning the autoradiography followed by processing of the data with the Adobe Photoshop program. H2B phosphorylation was normalized for the amount of HA-Akt/PKB in each sample and thus expressed as fold increase of the control activity. To assess protein expression from the transfected plasmid, proteins were acetone precipitated from supernatants of immunoprecipitates for 1 h at −20°C, pelleted, and resuspended in reducing Laemmli sample buffer. After separation by SDS-PAGE, proteins were transferred to polyvinylidene difluoridine membranes (Immobilon; Millipore) followed by Western blotting as described above.
Analysis of morphological changes.
Cells were cotransfected with 5 μg of pEF.BOS.GFP and either 45 μg of the empty vector or N17Rac, V12Rac, L61Rac, L61RacF37A, L61RacY40C, or rCD2p110 construct. After 48 h, living cells were purified on Ficoll-Hypaque gradients and analyzed for morphological changes as previously described (3). Experiments were performed with cells kept in suspension at 37°C in a saline solution (140 mM NaCl, 5 mM KCl, 10 mM HEPES [pH 7.4], 1 mM MgCl2) containing 1 million cells/ml. Fetal calf serum was added at 0.3% to reduce adhesion of cells to the glass coverslips. When the effect of wortmannin (100 nM) or LY294002 (10 μM) was being tested, the compound was added 20 min prior to morphological analysis.
RESULTS
Activated Rac1 is a strong activator of Akt/PKB in T cells.
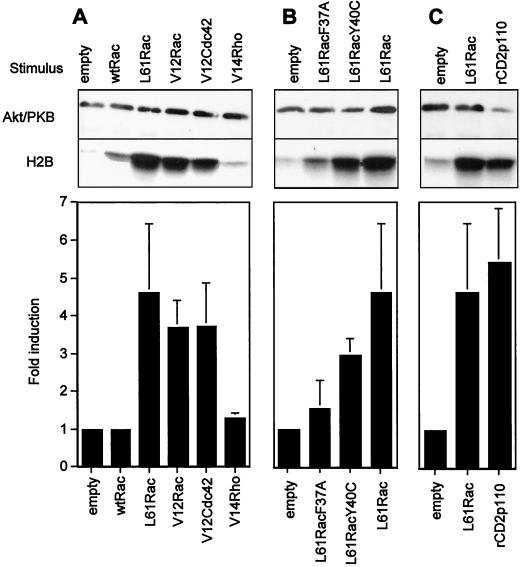
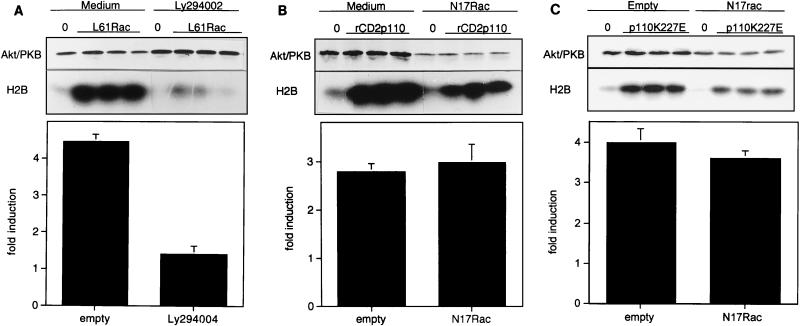
Because engagement of the TCR stimulates Akt/PKB activity in a PI 3-kinase-dependent (LY294002-sensitive) yet Ras-independent manner (15), we investigated if other monomeric GTPases, in particular members of the Rho family, could stimulate this protein kinase. For this purpose, constitutively active forms of Rho, Rac, or Cdc42 GTPases were transiently expressed in cells from the Jurkat J.HM1 T-cell line and Akt/PKB activity was measured in an in vitro kinase assay using H2B as a substrate. Constitutively activated Rac1 or Cdc42 (mutation at position 12 or 61) raised basal levels of Akt/PKB activity 4.5- and 4-fold, respectively, whereas constitutively active RhoA showed no induction (Fig. 1A). This effect was specific for the GTP-bound form, since wild-type Rac1, which in its resting state binds GDP in vivo, was unable to activate Akt/PKB. To further characterize the Rac1 effector pathway that regulates Akt/PKB activity, we tested the effector-loop mutants L61RacF37A and L61RacY40C. These mutants still display constitutive activity but have selectively lost interaction with some downstream effectors (22, 40). The L61RacY40C mutant retains the ability to efficiently induce Akt/PKB activity, whereas the L61RacF37A mutant was less effective (Fig. 1B). Expression of the Rac mutants was verified by Western blotting (data not shown). To attest that these Rac1 mutants discriminate between Rac1 effector pathways in T cells, we measured their ability to induce Jun-N-terminal kinase 2 activity in Jurkat T cells and obtained data consistent with those found in other cell types (data not shown and references 22 and 40). To compare Akt/PKB induction by activated forms of Rac1 (L61Rac) with PI 3-kinase-mediated stimulation, we expressed a chimeric construct of PI 3-kinase, rCD2p110, in which p110 has been fused to the extracellular domain of the CD2 cell surface marker (rat CD2) (27). The chimera warrants recruitment of the catalytic subunit to the plasma membrane and somehow mimics the role of p85. Similar levels of Akt/PKB activity were obtained when using either L61Rac or rCD2p110 (Fig. 1C). These results establish that GTP-bound Rac1 and Cdc42 are strong activators of Akt/PKB in T cells. Rac1 employs a downstream effector that retains the ability to bind the L61RacY40C mutant.
FIG. 1.
Activated Rac1 stimulates Akt/PKB in T cells. (A) Jurkat cells (15 million per condition) were transfected with plasmids encoding HA-Akt/PKB (10 μg), together with the empty vector (20 μg), the plasmid encoding wild-type Rac1 (wtRac1, 20 μg), or constitutively active forms of either Rac1 or one of the other small GTPases (each 20 μg). Eighteen hours later, cells were harvested and equal numbers of living cells were lysed and subjected to an immunokinase assay as described in Materials and Methods. Results from one representative experiment are shown in the top panel, whereas the histogram presents the mean ± standard deviation of at least three experiments. (B) Conditions were the same as those for panel A except that Rac1 and Rac1-effector-loop mutants were compared for their induction of Akt/PKB kinase activity (mean ± standard deviation of three experiments). (C) Conditions were the same as those for panel A except that 20 μg of the empty vector or the plasmid encoding constitutively activated forms of either Rac1 (L61Rac) or PI 3-kinase chimera (rCD2p110) was cotransfected with HA-Akt/PKB (mean ± standard deviation of seven experiments).
Akt/PKB activation by L61Rac1 is not mediated through an autocrine loop.
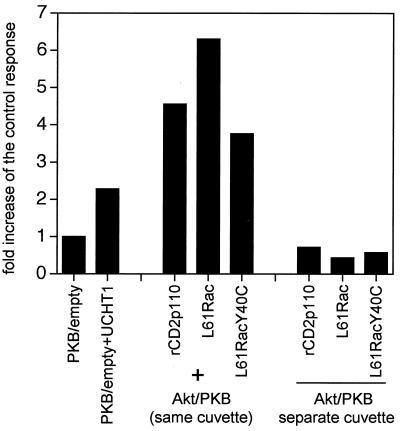
Because transient-transfection experiments were carried out over a relatively long time period (24 h), we could not rule out the possibility that Akt/PKB activation might be a consequence of the induction of autocrine growth factors, subsequently activating PI 3-kinase in L61-Rac1-transfected cells. To investigate if the stimulatory potential of activated Rac1 involved a factor that was released in the medium, we employed a cell mixing protocol as follows. Either activated Rac1 (L61Rac) was cotransfected with epitope-tagged Akt/PKB (added in the same cuvette) or Rac1 and tagged Akt/PKB were transfected into separate populations of cells (two separate cuvettes), which were mixed after electroporation and cultured together until the cells were harvested and subjected to the kinase assay. In parallel, the experiment was also performed with the constitutively activated PI 3-kinase chimera (rCD2p110) as a positive control. Elevated Akt/PKB activity was observed only when both constructs, L61Rac and tagged-Akt/PKB, were expressed in the same cell, and not when they were expressed in neighboring cells kept in the same culture (Fig. 2). Similar results were obtained with the activated V12 or L61Cdc42 constructs (data not shown). It is therefore unlikely that Rac1 activates Akt/PKB through an autocrine mechanism.
FIG. 2.
Rac1-induced PKB activation is not mediated by an autocrine factor. Jurkat cells were transfected with plasmids encoding HA-Akt/PKB (10 μg) together with either an empty vector or with the plasmid encoding L61Rac or rCD2p110 (first set); in parallel, HA-Akt/PKB, L61Rac, and rCD2p110 were transfected into separate cuvettes which were subsequently mixed in combination (second set) as indicated. Akt/PKB kinase activity was assessed 24 h posttransfection. TCR stimulation was for 10 min. Kinase activity was determined as described for Fig. 1.
UCHT1-mediated TCR triggering stimulates Rac1 activation.
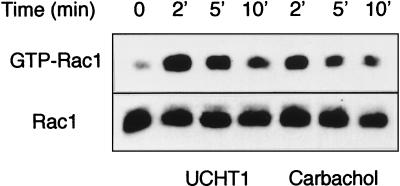
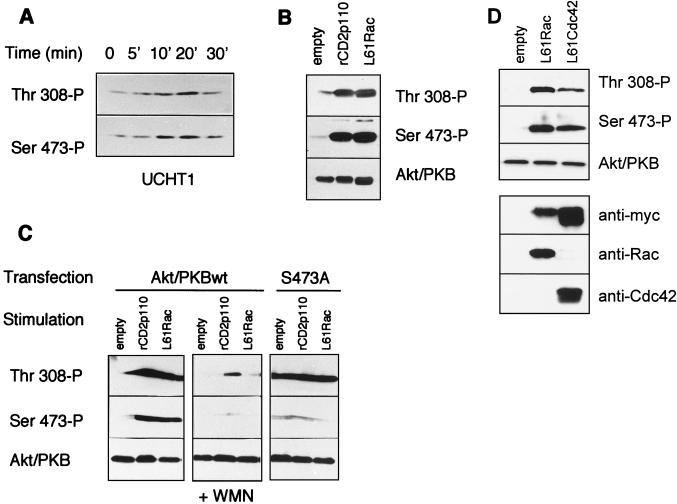
To assess the physiological implication of Rac1 in T-cell signaling following TCR stimulation with the UCHT1 antibody (reactive with the ɛ-chain of the TCR), we investigated the activation of endogenous Rac1. Relative GTP-Rac levels were estimated using an in vitro binding assay that measures a GTP-dependent absorption of Rac to the GTPase-binding amino terminus of the p21-activated kinase (PAK1) (23). We treated Jurkat cells with UCHT1 for 2, 5, or 10 min and performed the GTP-Rac1 pull-down experiment (21). A transient increase was observed in the amount of precipitated Rac1, with a maximal fourfold increase at 2 min of incubation (Fig. 3). Because the clone of Jurkat cells used in these experiments stably expresses the muscarinic acetylcholine receptor, we tested carbachol-mediated Rac1 stimulation as a positive control and found a response with similar magnitude. These results show that TCR triggering elicits a rapid and transient increase in the GTP-Rac content in Jurkat cells.
FIG. 3.
UCHT1 stimulates an increase in GTP-Rac. Jurkat cells (4.5 million per condition) were incubated in PBS in the absence or presence of UCHT1 (10 μg/ml) or carbachol (0.5 mM). At the times indicated, cells were lysed, and after partial purification, Rac1 binding to the CRIB domain was assessed as described in Materials and Methods. Western blot detection of Rac1 with anti-tag antibodies is shown. The top gel represents GTP-loaded Rac eluted from the beads; the bottom gel quantitates Rac1 from the total lysate to verify equivalent levels of myc-tagged Rac1 expression.
TCR-induced Akt/PKB activation is sensitive to PI 3-kinase inhibitors as well as N17Rac.
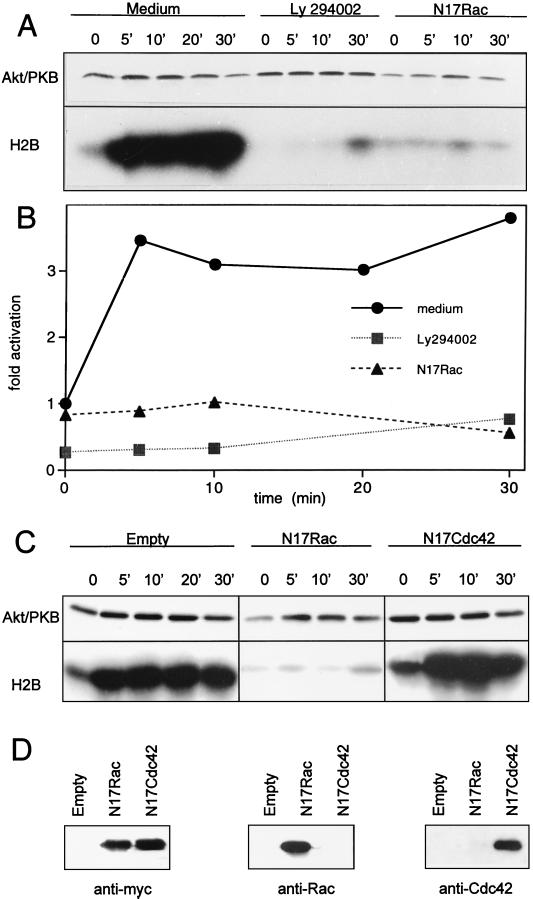
We next investigated whether Rac1 or Cdc42 could be components of the signaling pathway that couples the TCR to Akt/PKB. For this purpose, we ectopically expressed the inhibitory mutant for Rac1, N17Rac, or N17Cdc42 together with a tagged Akt/PKB construct. As a control, the consequences of pharmacological inhibition of PI 3-kinase on TCR-induced Akt/PKB activation were assessed. After 24 h of culture of transfected cells, the activity of Akt/PKB was measured in an in vitro kinase assay at various time points after TCR engagement. Triggering of the TCR induced a rapid increase in Akt/PKB activity that was sustained for at least 30 min (Fig. 4A and B). The presence of N17Rac, or the treatment with the LY249002 inhibitor, suppressed protein kinase activity at all time points tested. In contrast, N17Cdc42 showed no effect (Fig. 4C and D). Taken together, these results indicate that a functional Rac1 molecule is required for TCR-mediated Akt/PKB activation.
FIG. 4.
TCR-induced Akt/PKB activation is inhibited by LY294002 or N17Rac. (A) Jurkat cells were transfected with tagged Akt/PKB (10 μg) together with either an empty vector or the dominant negative N17Rac construct (20 μg). On the next day, cells were harvested and split into three sets. The first set, transfected with control vector, was incubated in control medium. The second set, transfected with control vector, was incubated in medium containing the PI 3-kinase inhibitor LY294002 (10 μM, for 30 min at 37°C). The third set, transfected with N17Rac, was grown in control medium. Aliquots containing equal cell numbers were incubated for the time indicated with 10 μg of UCHT1 antibody per ml, and kinase activity (shown in panel B) was determined as described for Fig. 1. The figure represents one experiment out of three. (C) Jurkat cells were transfected with tagged Akt/PKB (10 μg) together with either an empty vector, the dominant negative N17Rac construct (20 μg), or the dominant negative N17Cdc42 construct (20 μg). On the next day, each set of cells was harvested, aliquots containing equal cell numbers were made, and kinase activity was determined as described for Fig. 1. (D) Expression of the inhibitory mutants was assessed by Western blotting using either anti-myc tag, or anti-Rac1 or anti-Cdc42 antibodies. The figure represents one experiment out of three.
L61Rac activates Akt/PKB upstream of PI 3-kinase.
Although the major pathway for Akt/PKB activation involves a functional PI 3-kinase, other mechanisms have been described. In particular, an increase in the intracellular concentration of calcium (45) or cyclic AMP (14) stimulates Akt/PKB activity in a wortmannin- or LY294002-insensitive manner. It was therefore important to determine whether Rac1-mediated activation of Akt/PKB was sensitive to PI 3-kinase inhibitors. Results from these experiments are presented in Fig. 5A and show that the PI 3-kinase inhibitor LY294002 almost completely inhibited L61Rac induction of Akt/PKB activity. These results strongly suggest that PI 3-kinase is located downstream of Rac1 in the signaling pathway leading to activation of Akt/PKB. The fact that both the LY249002 compound and the N17Rac mutant block TCR-mediated Akt/PKB induction prompted us to investigate the possibility that Rac1 and PI 3-kinase are positioned in a linear pathway, downstream of the TCR. We therefore performed the reverse experiment, for which we used constitutively active PI 3-kinase mutants and studied the effects of dominant negative Rac. Two mutants of PI 3-kinase were used, the rCD2p110 mutant activated through membrane localization (27) and the p110K227E mutant activated by point mutation (33). N17Rac did not significantly affect the induction mediated by either mutant of PI 3-kinase (Fig. 5B and C). H2B phosphorylation was slightly reduced in N17Rac-transfected cells, but Western blot experiments showed that Akt/PKB expression was also diminished, meaning that the specific kinase activity was unchanged in these cells. We conclude that Rac1 acts upstream of PI 3-kinase in the TCR-mediated Akt/PKB activation pathway.
FIG. 5.
Rac1-mediated activation of Akt/PKB requires functional PI 3-kinase, but PI 3-kinase-mediated activation of Akt/PKB does not require functional Rac1. (A) Cells were transfected with Akt/PKB together with either the empty vector or the construct encoding constitutively active Rac1 (L61Rac), in the presence or in the absence of LY294002 inhibitor, in triplicate. Eighteen hours later, samples were harvested and assayed for kinase activity. Kinase activity was calculated as fold induction of basal activity (bottom) and presented as the mean ± standard deviation of triplicate samples of one representative experiment out of four. (B) Cells were transfected with Akt/PKB together with either the empty vector or the construct encoding active PI 3-kinase (rCD2p110), in the presence or in the absence of N17Rac, in triplicate. Eighteen hours later, samples were harvested and assayed for kinase activity, which is presented as the mean fold induction ± standard deviation of triplicate samples (bottom). (C) Conditions were the same as those in panel B except that rCD2p110 was replaced by p110K227E to simulate Akt/PKB activity.
Phosphorylation of threonine 308 and integrity of the PH domain are critical for Rac1- and PI 3-kinase-dependent Akt/PKB activation.
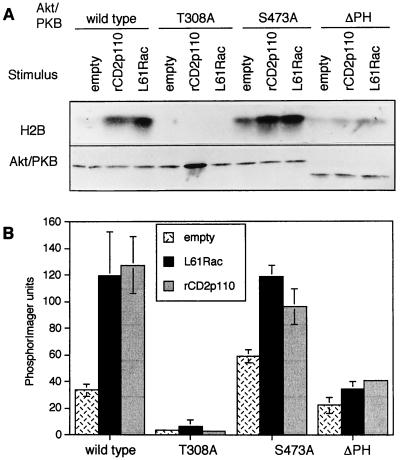
The mechanism by which Akt/PKB is activated has been investigated extensively but is not yet fully elucidated (5). Translocation to the plasma membrane has not always been found necessary for activation (14). In addition, phosphorylation of threonine 308 appears to be more important for Akt/PKB activation than phosphorylation of serine 473, since replacing threonine 308 by a nonphosphorylable amino acid (alanine) always completely ablates Akt/PKB activity, whereas mutation of serine 473 has a more variable effect (2, 5, 39). We next studied the requirement for the PH domain and phosphorylation at sites 308 and 473 in TCR-, L61Rac-, or rCD2p110-mediated activation of Akt/PKB. We employed a variety of Akt/PKB constructs, either mutated at these phosphorylation sites or with the PH domain deleted, to prevent phosphorylation or translocation, respectively. Western blot experiments showed that all mutants were expressed at similar levels in Jurkat cells (Fig. 6). When the PH domain of Akt/PKB was lacking, the kinase could no longer be activated by L61Rac, rCD2p110 (Fig. 6), or TCR stimulation (data not shown). When threonine 308 was replaced by an alanine residue, Akt/PKB induction in response to either rCD2p110 or L61Rac was also ablated. In contrast, a mutation at site 473 had little or no effect on Akt/PKB activation by rCD2p110 or L61Rac. Unexpectedly, this mutant had elevated basal activity in comparison to wild-type Akt/PKB. Combined with the results presented in Fig. 5, these data indicate that TCR-, rCD2p110-, and L61Rac-mediated activation of Akt/PKB require both an intact PH domain and phosphorylation at residue 308.
FIG. 6.
Activation of Akt/PKB by Rac1 or PI 3-kinase requires phosphorylation at residue 308 and a PH domain. (A) Cells were transfected with wtAkt/PKB or with mutants T308A, S473A, or Akt/PKB lacking the PH domain, together with either the empty vector, the construct encoding constitutively active Rac1 (L61Rac), or the construct encoding constitutively active PI 3-kinase (rCD2p110). Eighteen hours later, samples were harvested and assayed for kinase activity. (B) The histogram presents mean values ± standard deviation obtained from two independent experiments.
TCR, L61Rac, and rCD2p110 regulate Akt/PKB phosphorylation in a similar manner.
We next addressed the question whether activation of the TCR, expression of rCD2p110, or L61Rac would induce phosphorylation of Akt/PKB at positions 308 and 473. For this purpose, we used phosphospecific antibodies directed against Akt/PKB regulatory phosphorylation sites in the Western blot experiments. We first investigated TCR-mediated phosphorylation of endogenous Akt/PKB in a time course experiment. In the absence of stimulation, basal phosphorylation was observed on both residues, which was substantially increased at both sites, following TCR stimulation by UCHT1 (Fig. 7A). Phosphorylation of Akt/PKB at both residues was transient, with a peak at 10 to 20 min and a decline to basal levels at 30 min. When rCD2p110 or L61Rac was expressed in these cells, a similar phosphorylation pattern was seen (albeit in a nontransient fashion), with an increase in both threonine 308 and serine 473 phosphorylations (Fig. 7B). We also found that treatment of the cells with the PI 3-kinase inhibitor wortmannin ablates rCD2p110- or L61Rac-induced phosphorylation at these sites (Fig. 7C). By using the Akt/PKB S473A mutant, we further showed that mutation of this site does not impair phosphorylation at threonine 308, as previously described for COS-1 cells (2). Lastly, we verified the effect of L61Cdc42 and found a phosphorylation pattern similar to that induced by L61Rac1 (Fig. 7D). Taken together, these results indicate that TCR, L61Rac, or rCD2p110 induce Akt/PKB phosphorylation at both sites, indicative of a similar way of activation.
FIG. 7.
TCR stimulation, L61Rac, or rCD2p110 induce phosphorylation of Akt/PKB at threonine 308 and serine 473. (A) Jurkat cells were washed three times in serum-free medium and incubated at 37°C for 10 min before stimulation. UCHT1 (10 μg/ml) was then added for the indicated times, and samples were prepared for Western blot analysis. Phosphorylation on threonine 308 and serine 473 was assessed with phosphospecific antibodies in parallel, using two identical sets of samples for the detection. (B) Cells were cotransfected with wtAkt/PKB (10 μg) together with 30 μg of the empty vector, L61Rac, or rCD2p110. On the next day, cells were collected and washed and cell lysates were made for Western blot analysis. Phosphorylation on threonine 308 and serine 473 was assessed with phosphospecific antibodies in parallel, as described for panel A, and a third set of sample was used to assess the total Akt/PKB content in these transfected cells. (C) Cells were transfected with 15 μg of plasmids encoding rCD2p110 or L61Rac together with either 10 μg of wtAkt/PKB or 10 μg of the Akt/PKB S473A mutant, or cells were transfected with wtAkt/PKB and treated with wortmannin (+ WMN) (100 nM) or untreated as indicated. Akt/PKB phosphorylation status on threonine 308 and serine 473 was assessed as described above. (D) Cells were transfected with 30 μg of plasmids encoding L61Rac or L61Cdc42, together with 10 μg of wtAkt/PKB. Akt/PKB phosphorylation was assessed as described above, and expression of the stimulatory mutant was verified by Western blotting using anti-myc, anti-Rac, or anti-Cdc42 antibodies.
Rac1 is positioned downstream of PI 3-kinase in the pathway controlling spike formation in T cells.
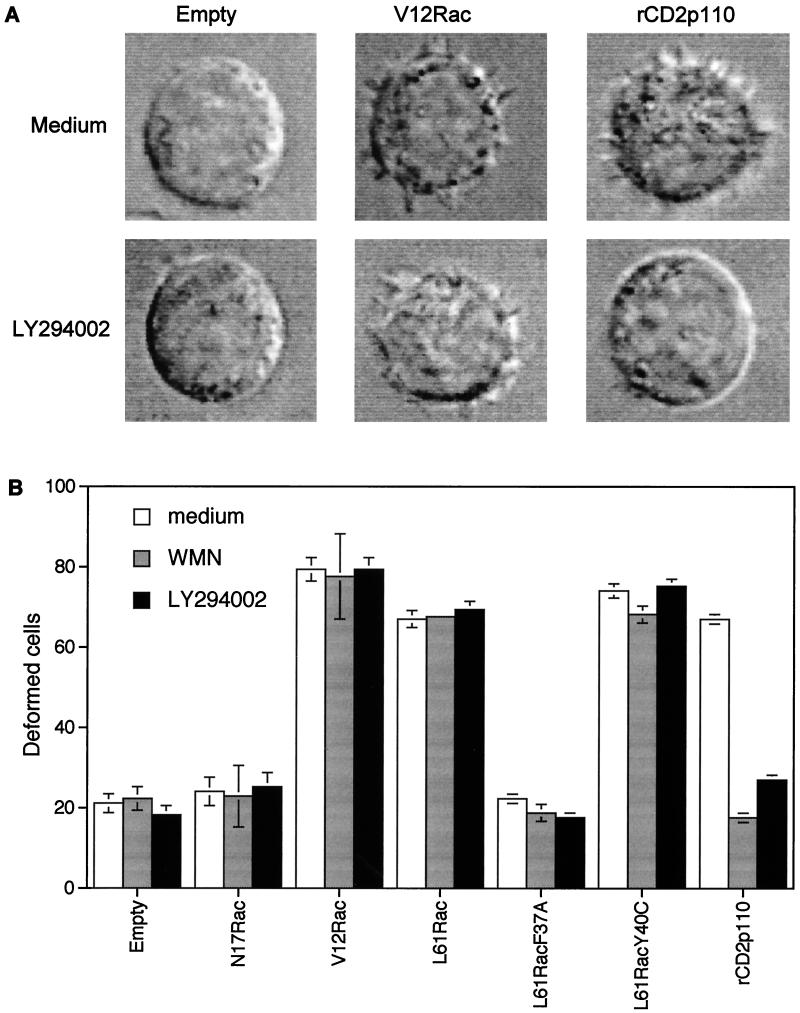
One of the major biological effects mediated by Rac1 is to control actin polymerization at the leading edge of the plasma membrane, leading to the extension of lamellipodia and the subsequent formation of membrane ruffles in adherent cells. In this process, Rac1 is a clear downstream effector of PI 3-kinase. Our results prompted us to investigate whether a similar positioning applies for cytoskeletal changes in T lymphocytes. We therefore examined the ability of various dominant negative and constitutively active mutants of PI 3-kinase and Rac1 to alter T-cell morphology in cells kept in suspension. In these experiments, green fluorescent protein (GFP) was cotransfected with the various constructs to allow selection of transfected cells for microscopic analysis as already described (3). Cells transfected with constitutively active Rac1 mutants (either V12Rac or L61Rac) or PI 3-kinase (rCD2p110) exhibited profound morphological changes, defined as membrane spikes (Fig. 8A). The changes in cell morphology triggered by both constructs were qualitatively the same. Constitutively active Rac1-mediated changes were, however, not sensitive to LY294002 or wortmannin, whereas rCD2p110-induced changes were completely inhibited. We also tested Rac1-effector loop mutants and found that L61RacY40C was as effective as L61Rac, whereas L61RacF37A was inactive (Fig. 8B). These results are identical to those found in fibroblasts (22) and confirm that with respect to the formation of membrane spikes in T cells, a similar cascade is employed as described for adherent cells, a cascade in which Rac1 is positioned downstream of PI 3-kinase.
FIG. 8.
Alteration in cell shape mediated by ectopic expression of activated Rac1 is insensitive to the PI 3-kinase inhibitor wortmannin. (A) Jurkat cells were cotransfected with the plasmid encoding GFP together with either the empty vector or plasmids encoding V12Rac or rCD2p110. Forty-eight hours later, living cells were isolated and analyzed for morphological changes under nonadherent conditions. Each set of cells was incubated with (bottom) or without (top) LY294002 for 20 min prior to analysis. (B) Jurkat cells were cotransfected with GFP as in panel A and with either the empty vector, plasmids encoding mutated forms of Rac1, or the plasmid encoding active PI 3-kinase. Forty-eight hours later, living cells were isolated and analyzed for morphological changes in the presence of wortmannin (100 nM) or LY294002 (10 μM). Data are presented as percentages of deformed cells among cells expressing GFP (mean ± standard error of the mean of three experiments).
DISCUSSION
We demonstrate that Akt/PKB is activated by the TCR in a PI 3-kinase- and Rac1-dependent manner. Constitutively active Rac1 alone is sufficient to fully activate Akt/PKB in T cells, and moreover, L61Rac-mediated stimulation of Akt/PKB activity is indistinguishable from that mediated by the active form of PI 3-kinase, rCD2p110, or engagement of the TCR. Phosphorylation at threonine 308 and integrity of the PH domains were found essential for activation of Akt/PKB by the three stimuli mentioned above, whereas phosphorylation of serine 473 appeared not essential. These results position Rac1 as an upstream regulator of PI 3-kinase in the activation of Akt/PKB in Jurkat lymphoid cells. Although L61Cdc42 could also induce activation of Akt/PKB, the dominant negative mutant N17Cdc42 did not prevent TCR-mediated Akt/PKB activation. This finding suggests that although L61Cdc42 can activate Akt/PKB, such a pathway is not operative downstream of the TCR for Akt/PKB stimulation.
Our findings apparently contrast with the current idea that Rac1 and Akt/PKB are activated in parallel, downstream of a PI 3-kinase-controlled signaling pathway, as demonstrated in endothelial cells (39), in COS cells (20), or in neuronal cells (38). It is reasonable to assume that differences in PI 3-kinase effector pathways in distinct cell lineages may, at least in part, account for these discrepancies. In particular, differences between regulatory pathways controlling Akt/PKB can be expected in nonadherent and adherent cells, taking into account the important role of focal adhesion complexes in the regulation of the PI 3-kinase–Akt/PKB pathway. In nonadherent cells, a different type of regulation is certain to be put in place to warrant Akt/PKB-controlled cell survival in the absence of these complexes. Moreover, evidence has been provided that PI 3-kinase can be either upstream or downstream of the Rho family of GTPases, depending on which event is analyzed (29, 36). For instance, Rac1 is an effector of PI 3-kinase for membrane ruffling (27), but Rac1 has been positioned upstream of PI 3-kinase in the regulation of integrin-mediated cell motility and invasiveness (19). The involvement of a lipid kinase different from PI 3-kinase, downstream of Rac1, is also described for T-cell spreading on fibronectin (13). The existence of different pools of GTPases that are spatially restricted, already suggested by other studies, provides a plausible explanation for these results (27). The coexistence of functionally distinct pools of a GTPase within the same cell could regulate disparate effector targets in response to a similar upstream signal. Alternatively, distinct PI 3-kinase isoforms may be involved in these pathways, responding to distinct upstream signaling (differential interactions). This possibility has been clearly illustrated in studies with macrophages, where it was shown that distinct isotypes modulate discrete cellular responses: colony-stimulating factor 1 (CSF1)-induced DNA synthesis is under the control of p110α, whereas actin organization and migration depend on a functional p110β or p110δ (37). Since the lipid kinase activities of most of the PI 3-kinase enzymes, including p110α, p110β, and p110δ, exhibit comparable sensitivities to inhibition by wortmannin or the LY294002 compound, these inhibitors would not discriminate between different p110 isoforms in our study.
Although PI 3-kinase can be recruited to the phosphorylated ITAMs upon TCR stimulation (11, 25) and in theory could be activated by Ras through direct interaction with the p110 catalytic subunit, these pathways appear not to be operational with respect to Akt/PKB activation. These direct links do not explain the inhibitory effect of dominant negative Rac1 on TCR responses, nor do they explain how Rac1 can activate Akt/PKB. Our data therefore favor an indirect pathway, in which an intermediate component is required to activate Rac1 prior to activation of PI 3-kinase. Recent studies have given proof of such a mechanism, where the TCR activates the guanine nucleotide exchange factor Vav, which in turn brings Rac1 in a GTP-bound state, thus allowing for downstream signaling (21). It is through such a mode of action that we envision Rac to be involved in the activation of PI 3-kinase. The following question remains: why could these direct pathways not bypass the requirement for Rac1 in activation of Akt/PKB? A possible explanation comes from studies of Akt/PKB regulation in adipocytes for which it has been shown that the site of activation of PI 3-kinase is essential in determining activation of downstream effectors (41). In these cells, PDGF activates PI 3-kinase through direct binding to its receptor, but PDGF is not very effective in activating Akt/PKB and is totally ineffective in stimulating glycogen synthesis (which involves PI 3-kinase and Akt/PKB). Insulin, on the contrary, which activates PI 3-kinase through insulin receptor substrate 1 (IRS-1), is very effective in stimulating Akt/PKB and in inducing glycogen synthesis. Apparently, the localization of the PI 3-kinase is different between the two stimuli, with PDGF PI 3-kinase activity residing mainly in the membrane, whereas with insulin, the lipid kinase activity is found in a high-speed pellet associated with a cytoskeleton-like protein complex. In T lymphocytes, LAT could represent the equivalent of IRS-1, and with respect to TCR stimulation, ITAM-mediated activation of PI 3-kinase might have different cellular effects from the LAT-mediated activation of PI 3-kinase. Because there are several different isoforms of both the regulatory and catalytic subunits of PI 3-kinase, different isoforms of PI 3-kinase may couple uniquely to different effectors in distinct microdomains.
We have shown that activation of Akt/PKB in Jurkat cells requires membrane localization and subsequent phosphorylation at residue Thr-308 in the catalytic domain (activation loop). The following question remains: how is Rac1 involved in Akt/PKB regulation? Rac1 could interfere at the level of membrane recruitment, through regulation of PI 3-kinase, or at the level of phosphorylation at threonine 308, through regulation of PDK1. Evidence for interference at the level of generation of PI-3,4,5-triphosphate is provided by several reports that describe a direct interaction of Rac1 or Cdc42 with the p85 regulatory subunit of PI 3-kinase in a GTP-dependent manner (6, 43). In particular, it was shown that the bcr domain (breakpoint cluster) of p85 is instrumental in this interaction (35), which results in activation of PI 3-kinase (47). The results of our study would favor the model in which Rac1, in its GTP-bound state, binds to p85, an interaction that is required for the activation of the p110 catalytic subunit. It has been demonstrated that p85β preferentially binds to members of the Rho family of GTPases (C. M. Yballe, D. A. Fruman, and L. C. Cantley, Abstr. Kestone Meet. Specificity Signal Transduction, abstr. p62, 1999). Interestingly, this isoform is selectively regulated in response to activation of the TCR by UCHT1 (28). LAT, whose expression is restricted to T, NK, and mast cells, could serve as a unique scaffold for this complex, bringing together p85β, Rac1, and Vav (46). Interestingly, LAT and Rac1 have been found to be permanently present in glycosphingolipid-enriched microdomains in hematopoietic cells (4). Our observation that N17Rac has no effect on activation of PKB by constitutively active variants of PI 3-kinase makes it unlikely that Rac1 acts at the level of regulation of PDK1. However, it remains possible that constitutively active PI 3-kinase recruits or activates a PDK1 that differs from the one activated by the TCR. In this case, one can envision that Rac1 is necessary for activation or proper localization of PDK1 in response to engagement of the TCR but that this role is not required when constitutive active PI 3-kinase comes into play (42). Further analysis is required to elucidate how Rac1, PI 3-kinase, and PDK1 are linked with the TCR in the signal transduction pathway that leads to activation of Akt/PKB.
In conclusion, our data reveal that Rac1 and Akt/PKB are not always on separated pathways downstream of PI 3-kinase as previously thought and that these GTPases can be either upstream or downstream of PI 3-kinase, depending on the cellular event considered. Rac1 is a selective regulator of the serine/threonine kinase Akt/PKB and operates upstream of PI 3-kinase in the signal transduction pathway in Jurkat T lymphocytes.
ACKNOWLEDGMENTS
This work was supported by a Wellcome Foundation Grant DMIH 3468. E.M.G. is a member of the INSERM organization. C.A. was supported from a fellowship from the Fondation pour la Recherche Medicale.
We are grateful to Sarah Beach for initial technical assistance, Ludowijk Dekker for help with radioactive experiments, Alan Hall and Julian Downward for various plasmids, Alain Trautmann and Peter Parker for helpful discussions, Bart Vanhaesebroeck and Jacques Nunes for advice, and Doreen Cantrell and Robert Lechler for constant support.
REFERENCES
- 1.Ahmed N N, Grimes H L, Bellacosa A, Chan T O, Tsichlis P N. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 3.Arrieumerlou C, Donnadieu E, Brennan P, Keryer G, Bismuth G, Cantrell D A, Trautmann A. Involvement of phosphoinositides 3-kinase and rac in membrane ruffling induced by IL2 in T cells. Eur J Immunol. 1998;28:1877–1885. doi: 10.1002/(SICI)1521-4141(199806)28:06<1877::AID-IMMU1877>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 4.Arudchandran R, Brown M J, Peirce M J, Song J S, Zhang J, Siraganian R P, Blank U, Rivera J. The src homology 2 domain of vav is required for its compartmentation to the plasma membrane and activation of c-Jun NH(2)-terminal kinase 1. J Exp Med. 2000;191:47–60. doi: 10.1084/jem.191.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellacosa A, Chan T O, Ahmed N N, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 6.Bokoch G M, Vlahos C J, Wang Y, Knaus U G, Traylor-Kaplan A E. Rac GTPase interacts specifically with phosphatidylinositol 3-kinase. Biochem J. 1996;315:775–779. doi: 10.1042/bj3150775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan P, Babbage J W, Burgering B M T, Groner B, Reif K, Cantrell D A. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7:679–689. doi: 10.1016/s1074-7613(00)80388-x. [DOI] [PubMed] [Google Scholar]
- 8.Burgering B M, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 9.Cantrell D A. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 10.Coffer P J, Jin J, Woodgett J R. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Aos I M, Metzger H, Exley M, Dahl C E, Misra S, Zheng D, Varticovski L, Terhorst C, Sancho J. Tyrosine phosphorylation of the CD3-epsilon subunit of the T cell antigen receptor mediates enhanced association with phosphatidylinositol 3-kinase in Jurkat T cells. J Biol Chem. 1997;272:25310–25318. doi: 10.1074/jbc.272.40.25310. [DOI] [PubMed] [Google Scholar]
- 12.Downward J, Graves J D, Warne P H, Rayter S, Cantrell D A. Stimulation of p21ras upon T-cell activation. Nature. 1990;346:719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- 13.D'souza-schorey C, Boettner B, Van Aelst L. Rac regulates integrin-mediated spreading and increased adhesion of T lymphocytes. Mol Cell Biol. 1998;18:3936–3946. doi: 10.1128/mcb.18.7.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filippa N, Sable C L, Filloux C, Hemmings B, Van Obberghen E. Mechanism of protein kinase B activation by cyclic AMP-dependent protein kinase. Mol Cell Biol. 1999;19:4989–5000. doi: 10.1128/mcb.19.7.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Génot E, Reif K, Beach S, Kramer I M, Cantrell D A. p21ras initiates Rac-1 but not phosphatidylinositol 3-kinase/PKB mediated signaling pathways in T lymphocytes. Oncogene. 1998;17:1731–1738. doi: 10.1038/sj.onc.1202101. [DOI] [PubMed] [Google Scholar]
- 16.Génot E, Cleverley S, Henning S, Cantrell D A. Multiple p21ras effector pathways regulate Nuclear Factor of Activated T cell, NFAT. EMBO J. 1996;15:3923–3933. [PMC free article] [PubMed] [Google Scholar]
- 17.Gold M R, Scheid M P, Santos L, Dang-Lawson M, Roth R A, Matsuuchi L, Duronio V, Krebs D L. The B cell antigen receptor activates the Akt (protein kinase B)/glycogen synthase kinase-3 signaling pathway via phosphatidylinositol 3-kinase. J Immunol. 1999;163:1894–1905. [PubMed] [Google Scholar]
- 18.Goldsmith M A, Desai D M, Schultz T, Weiss A. Function of a heterologous muscarinic receptor in T cell antigen receptor signal transduction mutants. J Biol Chem. 1989;264:17190–17197. [PubMed] [Google Scholar]
- 19.Keely P J, Westwick J K, Whitehead I P, Der C J, Parise L. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 20.Klippel A, Kavanaugh W M, Pot D, Williams L T. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhne M, Ku G, Weiss A. A guanine nucleotide exchange factor-independent function of Vav1 in transcriptional activation. J Biol Chem. 1999;275:2185–2190. doi: 10.1074/jbc.275.3.2185. [DOI] [PubMed] [Google Scholar]
- 22.Lamarche N, Tapon N, Stowers L, Burbelo P D, Aspenstrom P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independent of p65PAK and the JNK/SAPK MAPkinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- 23.Manser E, Loo T H, Koh C G, Zhao Z S, Chen X Q, Tan L, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- 24.Nobes C, Hall A. Rho, Rac and Cdc42 GTPases regulate the assembly of multimolecular complexes associated with actin stress fibers lamellipodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 25.Osman N, Turner H, Lucas S, Reif K, Cantrell D A. The protein interactions of the immunoglobulin receptor family tyrosine-based activation motifs present in the T cell receptor zeta subunits and the CD3 gamma, delta and epsilon chains. Eur J Immunol. 1996;26:1063–1068. doi: 10.1002/eji.1830260516. [DOI] [PubMed] [Google Scholar]
- 26.Reif K, Lucas S, Cantrell D A. A negative role for phosphatidylinositol 3-kinase in T cell antigen receptor function. Curr Biol. 1997;7:285–293. doi: 10.1016/s0960-9822(06)00151-5. [DOI] [PubMed] [Google Scholar]
- 27.Reif K, Nobes K, Thomas G, Hall A, Cantrell D A. Phosphatidylinositol 3-kinase activates a selective subset of Rac/Rho-dependent effector pathways. Curr Biol. 1996;6:1445–1455. doi: 10.1016/s0960-9822(96)00749-x. [DOI] [PubMed] [Google Scholar]
- 28.Reif K, Gout I, Waterfield M D, Cantrell D A. Divergent regulation of phosphatidylinositol 3-kinase p85 alpha and p85 beta isoforms upon T cell activation. J Biol Chem. 1993;268:10780–10788. [PubMed] [Google Scholar]
- 29.Ren X D, Schwartz M A. Regulation of inositol lipid kinases by Rho and Rac. Curr Opin Genet Dev. 1998;8:63–67. doi: 10.1016/s0959-437x(98)80063-4. [DOI] [PubMed] [Google Scholar]
- 30.Ridley A J, Paterson H F, Johneson C L, Dieckman D, Hall A. The small GTP-binding protein Rac regulates growth factor induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Viciana P, Warne P, Khwaja A, Marte M M, Pappin D, Das P, Waterfield P M, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Viciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Viciana P, Warne P H, Vanhaesebroeck B, Waterfield M D, Downward J. Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. EMBO J. 1996;15:2442–2451. [PMC free article] [PubMed] [Google Scholar]
- 34.Stokoe D L, Stephens R, Copeland T, Gaffney P R, Reese C B, Painter G F, Holmes A B, McCormick F, Hawkins P T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 35.Tolias K F, Cantley L C, Carpenter C L. Rho family GTPases bind to phosphoinositide kinases. J Biol Chem. 1995;270:17656–17659. doi: 10.1074/jbc.270.30.17656. [DOI] [PubMed] [Google Scholar]
- 36.Van Aelst L, D'souza-schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 37.Vanhaesebroeck B, Jones G, Alen W E, Zicha D, Hooshmand-Rad R, Sawyer T, Wells C, Waterfield M, Ridley A. Distinct PI3K mediate mitogenic signalling and cell migration in macrophages. Nat Cell Biol. 1999;1:69–72. doi: 10.1038/9045. [DOI] [PubMed] [Google Scholar]
- 38.Van Weering D H, De Rooij J, Marte B M, Downward J, Bos J L, Burgering B M. Protein kinase B activation and lamellipodium formation are independent phosphoinositide 3-kinase-mediated events differentially regulated by endogenous Ras. Mol Cell Biol. 1998;18:1802–1811. doi: 10.1128/mcb.18.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welch H, Eguinoa A, Stephens L R, Hawkins P T. Protein kinase B and Rac are activated in parallel within a phosphatidylinositide 3OH-kinase-controlled signaling pathway. J Biol Chem. 1998;273:11248–11256. doi: 10.1074/jbc.273.18.11248. [DOI] [PubMed] [Google Scholar]
- 40.Westwick J K, Lambert Q T, Clark G J, Symons M, Van Aelst L, Pestell R G, Der C J. Rac regulation of transformation, gene expression, and actin organization by multiple, PAK-independent pathways. Mol Cell Biol. 1997;17:1324–1335. doi: 10.1128/mcb.17.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitehead J P, Clark S F, Urso B, James D E. Signalling through the insulin receptor. Curr Opin Cell Biol. 2000;12:222–228. doi: 10.1016/s0955-0674(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 42.Williams M, Simon J, Arthur C, Balendran A, Van der Kaay J, Poli A, Coher P, Alessi D. The role of 3-phosphoinositide-dependent protein kinase1 in activating AGC kinases defined in embryonic stem cells. Curr Biol. 2000;10:439–448. doi: 10.1016/s0960-9822(00)00441-3. [DOI] [PubMed] [Google Scholar]
- 43.Wymann M P, Pirola L. Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1998;1436:127–150. doi: 10.1016/s0005-2760(98)00139-8. [DOI] [PubMed] [Google Scholar]
- 44.Yablonski D, Kane L P, Qian D, Weiss A. A Nck-Pak1 signalling module is required for T cell receptor mediated activation of NFAT, but not of JNK. EMBO J. 1998;17:5647–5657. doi: 10.1093/emboj/17.19.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yano S, Tokumitsu H, Soderling T R. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Sloan-Lancaster J, Kitchen J, Trible R P, Samelson L E. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 47.Zheng Y, Bagrodia S, Cerione R A. Activation of phosphoinositide 3-kinase activity by Cdc42HS binding to p85. J Biol Chem. 1994;269:18727–18730. [PubMed] [Google Scholar]