Abstract
Background
We assessed the clinical utility of a first-degree breast cancer family history and polygenic risk score (PRS) to inform screening decisions among women aged 30-50 years.
Methods
Two established breast cancer models evaluated digital mammography screening strategies in the 1985 US birth cohort by risk groups defined by family history and PRS based on 313 single nucleotide polymorphisms. Strategies varied in initiation age (30, 35, 40, 45, and 50 years) and interval (annual, hybrid, biennial, triennial). The benefits (breast cancer deaths averted, life-years gained) and harms (false-positive mammograms, overdiagnoses) were compared with those seen with 3 established screening guidelines.
Results
Women with a breast cancer family history who initiated biennial screening at age 40 years (vs 50 years) had a 36% (model range = 29%-40%) increase in life-years gained and 20% (model range = 16%-24%) more breast cancer deaths averted, but 21% (model range = 17%-23%) more overdiagnoses and 63% (model range = 62%-64%) more false positives. Screening tailored to PRS vs biennial screening from 50 to 74 years had smaller positive effects on life-years gained (20%) and breast cancer deaths averted (11%) but also smaller increases in overdiagnoses (10%) and false positives (26%). Combined use of family history and PRS vs biennial screening from 50 to 74 years had the greatest increase in life-years gained (29%) and breast cancer deaths averted (18%).
Conclusions
Our results suggest that breast cancer family history and PRS could guide screening decisions before age 50 years among women at increased risk for breast cancer but expected increases in overdiagnoses and false positives should be expected.
Routine mammography screening starting at age 45 or 50 years has been shown to reduce population breast cancer mortality for women at average risk (1). It remains uncertain whether the current screening guidelines (2-4) are optimal for individual women considering the variability in breast cancer risk at any given age. The American Cancer Society (ACS) and the United States Preventive Services Task Force (USPSTF) recommend that women discuss their individual risk and screening options with their healthcare providers before the age of 45 or 50 years, yet there are limited data to inform such discussions. Risk-based screening has been proposed as a way to inform decisions about the starting age and frequency of screening for women at different levels of risk. Recent discoveries in the field of breast cancer genetics may hold the potential to guide risk-based screening strategies. The risk of developing breast cancer approximately doubles for women with a first-degree family member with breast cancer (5). Approximately 20% of the familial risk is attributable to high or moderate penetrance mutations in genes, including BRCA1, BRCA2, PALB2, ATM, and CHEK2 (6,7). The remaining 80% is due to a combination of more common variations in the DNA sequence (single nucleotide polymorphisms [SNPs]). Currently, about 313 common SNPs have been identified as being associated with breast cancer risk (8). Although these individual variants are associated with small to modest increased risk, their combined effects considered as a polygenic risk score (PRS) could be useful in targeting screening strategies based on risk (9,10).
Two ongoing trials, My Personal Breast Screening and the Women Informed to Screen Depending on Measures of Risk trial, are presently testing age-based vs risk-based screening approaches that include genetic markers and family history information, but results are not expected until 2024-2025 (11). A recent study in the United Kingdom modeled the use of PRS to determine the cost-effectiveness of screening women triennially above a certain risk threshold (12). However, to our knowledge, there are no studies that have compared the impact of screening strategies tailored to risk based on family history of breast cancer and polygenic risk combined. To fill this clinical gap, 2 established Cancer Intervention and Surveillance Modeling Network models that were used to inform the current USPSTF breast cancer screening guidelines (13) were used to estimate the lifetime effects of screening based on family history status and polygenic risk (2). The results are intended to support conversations between women and their healthcare providers about the benefits and harms of starting screening before the age of 50 years. The findings could also provide data to inform policy discussions about risk-tailored screening guidelines.
Methods
This modeling research used deidentified, publicly available data and was considered human participants exempt by the Georgetown University Institutional Review Board, the institutional review board of record.
Model Overview
Breast cancer simulation models developed by the Erasmus University Medical Center (14) and the Georgetown University-Albert Einstein College of Medicine (15) were used to evaluate the lifetime effects of different risk-tailored screening. Briefly, the models projected the lifetime effects of different screening strategies among the 1985 US female birth cohort. This cohort was carefully chosen to make this analysis relevant for women who are now younger than the age of 50 years making choices about screening informed by their breast cancer family history and PRS.
The models portrayed breast cancer molecular subtype–specific incidence and mortality based on estrogen receptor and HER2 status (16). The models included ductal carcinoma in situ (DCIS), where the majority of DCIS progressed to invasive breast cancer, and the rest remained nonlethal as DCIS due to slow progression rates. Screen detection of breast cancer was modeled using age-, first screen–, screening round–, and interval-specific digital mammography sensitivity and specificity by breast density group (low [fatty and scattered density] and high [heterogeneous and extremely dense]) based on Breast Cancer Surveillance Consortium data for women aged 30-74 years (17). We made the simplifying assumption that the distribution of breast density was independent of PRS and family history status.
Screening benefits such as breast cancer deaths averted or greater survival occurred with tumor detection in earlier stages or at smaller tumor sizes compared with that expected without screening. Screening occurred only in the ages specified by the screening strategy; outside of this screening window, cancers can only be diagnosed due to symptoms. At any time, women diagnosed with breast cancer could die of the disease or competing other-cause mortality. Overdiagnosis was defined as screen-detected DCIS and invasive cases that would not have been detected in the absence of screening because of lack of progressive potential or death from competing mortality. In our past research, biennial screening from ages 50 to 74 years resulted in 40% (Model GE) to 60% (Model E) of DCIS cases being overdiagnosed (18), with DCIS accounting for 90% of all overdiagnosed cases.
To evaluate the potential efficacy of different screening strategies, the models assumed that all women received genetic testing and, if diagnosed with cancer, received molecular subtype–specific adjuvant therapy. Treatment effects on survival by tumor subtype were based on systematic reviews of treatment effectiveness trials (19). Model details and input parameters are summarized in the Supplementary Methods and Supplementary Tables 1 and 2 (available online), and model validation has been described elsewhere (13,16,20,21).
Screening Strategies
Risk-based digital mammography screening strategies varied by age at initiation (30, 35, 40, 45, or 50 years) and screening interval (annual, biennial, triennial, and hybrid combinations). Hybrid strategies consisted of screening annually before age 50 years and biennially starting at age 50 years. We compared the results of these strategies with those expected using 3 US screening guidelines, including the USPSTF (biennial screening at ages 50-74 years), the American College of Radiology (annual screening starting at age 40 years), and the ACS (annual screening from ages 45 to 54 years, followed by a choice to continue biennial screening at age 55 years) (2-4). We made the simplifying assumption that all women in the ACS screening strategy switched to biennial screening at age 55 years; otherwise the ACS and ACR strategies would be identical. Although the ACR and ACS guidelines do not specify stopping ages, we used 74 years as the upper age of screening so there was comparability across screening approaches. Note that in biennial or triennial strategies starting at age 45 or 55 years, the last screen occurred at age 72 or 73 years, respectively.
Risk Stratification Based on Family History
We defined 5 family history groups: women who learned in age ranges 30-39 years, 40-49 years, 50-64 years, and 65+ years that they had a first-degree relative with breast cancer; and women with no family history of breast cancer in their lifetimes. The age-specific distribution of family history in the National Health Interview Survey and associated risk levels observed in the Collaborative Breast Cancer Study (22) were used to model breast cancer risk related to family history (Table 1).
Table 1.
Prevalence and risk level according to FH of BC and the 313-SNP PRS
| BC risk and prevalence | BC relative risk level (95% CI) | % of all women |
|---|---|---|
| FH age groupsa | ||
| FH positive between 30 and 39 y | 2.19 (1.72 to 2.77) | 4.7 |
| FH positive between 40 and 49 y | 1.73 (1.74 to1.93) | 4.2 |
| FH positive between 50 and 64 y | 1.39 (1.30 to 1.48) | 5.9 |
| FH positive at age 65 y or older | 1.34 (1.23 to 1.46) | 2.3 |
| No positive FH in life | 0.79 (0.67 to 0.91) | 82.9 |
| Polygenic risk groupsb | ||
| 1 | 0.0 < PRS ≤ 0.5 | 13.2 |
| 2 | 0.5 < PRS ≤ 1.0 | 46.7 |
| 3 | 1.0 < PRS ≤ 1.5 | 25.3 |
| 4 | 1.5 < PRS ≤ 2.0 | 9.3 |
| 5 | 2.0 < PRS ≤ 3.0 | 4.7 |
| 6 | 3.0 < PRS ≤ 5.0 | 0.7 |
| 7 | 5.0 < PRS ≤ 10.0 | 0.0 |
BC risk data by family history status are from the Collaborative Breast Cancer Study (22). A positive first-degree FH was modeled as an increase in risk at the first age-year of each age group. Risk is age specific and relative to the population average in which some women have but most do not have a BC family history. Thus, women in the “no positive FH in life” group have a relative risk below the population average of 1. BC = breast cancer; CI = confidence interval; FH = family history; PRS = polygenic risk score; SNP = single nucleotide polymorphism.
Seven risk groups were established based on the 313-SNP polygenic risk score. For example, women in the 1.5 < PRS ≤ 2.0 group have a 1.5-2.0 increased risk of developing breast cancer compared with the population average due to their polygenic risk.
Risk Stratification Based on Polygenic Risk
Stratification of women by polygenic risk was based on a PRS for all subtypes combined generated from 313 SNPs. The SNP selection was based on analysis of data from 69 studies and validated in 10 studies from the Breast Cancer Association Consortium (8). The PRS is based on a multiplicative risk model for the joint effects of the breast cancer SNPs and is defined as the sum of risk alleles weighted by their effect size as estimated in the combined European ancestry genome wide association studies data (8). The performance of this model has been validated in an external data set and proved to be a reliable predictor of breast cancer risk in women with and without a family history of breast cancer. The area under the receiver operator characteristic curve that measures the discriminatory ability of this PRS is 0.64. The women in the top 1% of the distribution have a predicted risk approximately fourfold greater than the risk in the middle quintile. The lifetime risk of breast cancer in the top 10% of the PRS was 32.6%.
We modeled the distribution of breast cancer risk relative to the average woman without a family history (RR*) as a function of polygenic risk and family history and age:
where FHx is an indicator for first-degree family history of breast cancer (yes = 1, no = 0), is the log relative risk of family history (adjusted for polygenic effects), and σii is the log relative risk associated with a 1-SD change in the PRS in age group i. The values for and were obtained from the original publication by Mavaddat (8) and are included in Supplementary Table 3 (available online). The distribution of breast cancer relative risk as a function of the PRS is displayed in Supplementary Figure 1 (available online). We established 7 PRS groups spanning risk levels from 0 to 10 times the US population average. Using the cut-off risk levels of the 7 defined groups, we calculated the number of women in each risk group (Table 1).
The breast cancer risk levels and prevalences based on the 313-SNP PRS and family history status combined are summarized in Supplementary Table 4 (available online).
Analysis
We examined 47 potential risk groups: 5 family history, 7 polygenic risk, and 35 combinations of both. First, we estimated the harms (overdiagnoses and false positives) and benefits (life-years gained and breast cancer deaths averted) of the 3 comparator screening guidelines (USPSTF, ACS, and ACR). Next, we estimated the harms and benefits of the screening strategies based on age of initiation (30, 35, 40, 45, and 50 years) and interval (annual, biennial, triennial, and hybrid intervals) in each of the 47 risk groups.
To assess the impact of risk-based screening, we analyzed which set of screening strategies maximized the total number of life-years gained under the constraint of maintaining a similar, or better, ratio of life-years gained per mammogram as seen with the screening guidelines, that is, the benchmark. The overall population impact of risk-based screening was quantified by adding the outcomes in the individual risk groups based on their proportions in the population. We used the USPSTF guideline as our base case and included the ACS and ACR guidelines as secondary comparators. Individual model outcomes are included in Supplementary Tables 5-7 (available online).
Sensitivity Analyses
Part of the additional benefits of risk-based screening strategies may accrue simply from an increased number of screens. Therefore, we analyzed what the effects of polygenic risk-based screening would be if the total number of screens were constrained to the number performed in the USPSTF recommendation. All screening strategies were simulated in each PRS group, and we assessed the set of strategies that maximized the overall number of life-years gained across PRS groups under the fixed number of mammograms constraint.
Results
Breast Cancer Screening Guidelines
If women of average breast cancer risk were screened according to the USPSTF guideline (vs no screening), the models project an average of 118 life-years gained (model range = 103-133), 7 (model range = 6.4-6.9) breast cancer deaths averted, 15 (model range = 12.1-16.9) overdiagnoses, and 920 (model range = 918-921) false-positive mammograms per 1000 women screened over their lifetimes vs no screening. If the ACS or ACR guidelines were followed, there were 61% or 178% more mammograms, respectively, than in the USPSTF guideline due to early starting ages and more frequent screening, resulting in substantial increases in benefits but also in harms (Table 2).
Table 2.
Model average benefits, harms, and benefit to harm ratios for digital mammography screening by guideline groups for average risk women per 1000 women screened
| Screening guidelinea | Screening strategy | Screens, No. | LYGb | BC deaths avertedb | Overdiagnoses | False positives | LYG/screens | LYG/ overdiagnoses | BC deaths averted/false positives |
|---|---|---|---|---|---|---|---|---|---|
| USPSTF | Bi 50-74 y | 11 182 | 118 | 6.7 | 14.5 | 920 | 0.0106 | 8.14 | 0.0072 |
| ACR | An 40-74 y | 31 083 | 192 | 9.6 | 21.5 | 2910 | 0.0062 | 8.94 | 0.0033 |
| ACS |
An 45-54, Bi 55-74 |
17 984 | 151 | 7.7 | 16.5 | 1666 | 0.0084 | 9.16 | 0.0046 |
We used age 74 years as the age of the last possible screen for comparability across screening strategies for all analyses. ACR = American College of Radiology; ACS = American Cancer Society; An = annual; BC = breast cancer; Bi = biennial; LYG = life-years gained; USPSTF = United States Preventive Services Task Force.
The LYG and BC deaths averted are relative to the life-years and BC deaths of women at the same level of age-specific BC risk who are never screened.
Breast Cancer Family History
If women with a breast cancer family history started biennial screening at age 40 years (vs 50 years) based on the USPSTF guideline, the life-years gained would increase by 36% (model range = 29%-40%) from 168 to 229 life-years gained, and 20% (model range = 16%-24%) more breast cancer deaths could be averted per 1000 women screened (Table 3). However, overdiagnoses would increase by 21% (model range = 17%-23%) from 16.8 to 20.3 overdiagnoses and false positives by 63% (model range = 62%-64%) from 902 to 1468 per 1000 women screened. Women with a positive family history in their 30s or 40s who followed the ACR or ACS guidelines to begin screening in the 40s would have a better ratio of life-years gained to overdiagnosis than those who followed the USPSTF guideline (Table 3; Figures 1 and 2).
Table 3.
Model average benefits, harms, and benefit to harm ratios for screening based on breast cancer family history per 1000 women screened
| Risk group based on BC FHa | Screening based on | Screening strategy | Screens, No. | LYGb | BC deaths avertedb | Overdiagnoses | False positives | LYG/screens | LYG/overdiagnoses | BC deaths averted/false positives |
|---|---|---|---|---|---|---|---|---|---|---|
| Positive FH age 30-39 y | USPSTF | Bi 50-74 y | 10 814 | 168 | 9.3 | 16.5 | 892 | 0.0156 | 10.18 | 0.0104 |
| ACS | An 45-54 y, Bi 55-74 y | 17 499 | 222 | 11.0 | 19.2 | 1622 | 0.0127 | 11.51 | 0.0068 | |
| ACR | An 40-74 y | 30 173 | 284 | 13.7 | 25.6 | 2830 | 0.0094 | 11.09 | 0.0049 | |
| BC FH | Bi 30-74 y | 20 528 | 254 | 11.9 | 21.7 | 2079 | 0.0124 | 11.73 | 0.0057 | |
| Positive FH age 40-49 y | USPSTF | Bi 50-74 y | 10 904 | 168 | 9.3 | 16.7 | 901 | 0.0154 | 10.03 | 0.0104 |
| ACS | An 45-54 y, Bi 55-74 y | 17 635 | 221 | 11.0 | 19.4 | 1635 | 0.0125 | 11.34 | 0.0067 | |
| ACR | An 40-74 y | 30 406 | 280 | 13.6 | 25.9 | 2851 | 0.0092 | 10.79 | 0.0048 | |
| BC FH | Bi 40-74 y | 15 713 | 229 | 11.3 | 20.3 | 1468 | 0.0145 | 11.28 | 0.0077 |
The primary analysis focuses on screening decisions among women under the age of 50 years. Outcomes among women with a BC FH after age 50 years are included in the Supplementary Material (available online). An = annual; ACR = American College of Radiology; ACS = American Cancer Society; BC = breast cancer; bi = biennial; FH = family history; LYG = life-years gained; USPSTF = United States Preventive Services Task Force.
The LYG and BC deaths averted are relative to the life-years and BC deaths of women at the same level of age-specific BC risk who are never screened.
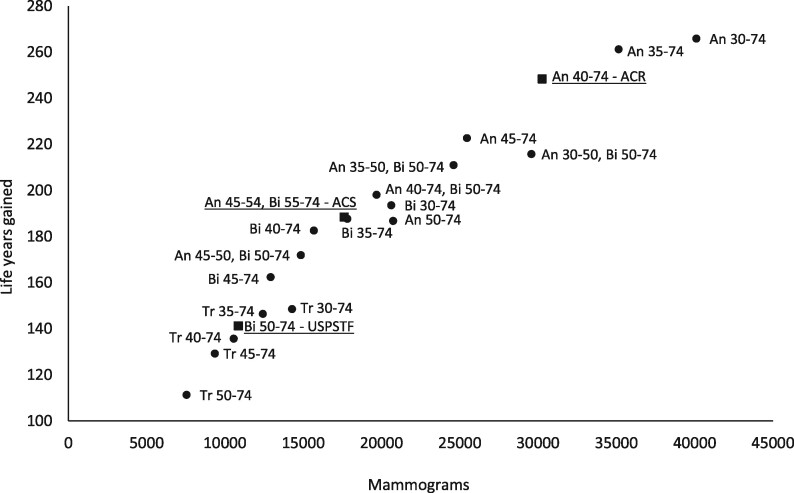
Figure 1.
The number of mammograms and life-years gained associated with different screening strategies among women who learned at age 40 years about a positive first-degree family member with breast cancer. Results from exemplary Model E per 1000 women screened. The estimated harms and benefits associated with these screening strategies are displayed in Figure 2. The underlined strategies are the commonly followed guidelines of the United States Preventive Services Task Force, the American Cancer Society, and the American College of Radiology. An = annual; Bi = biennial.
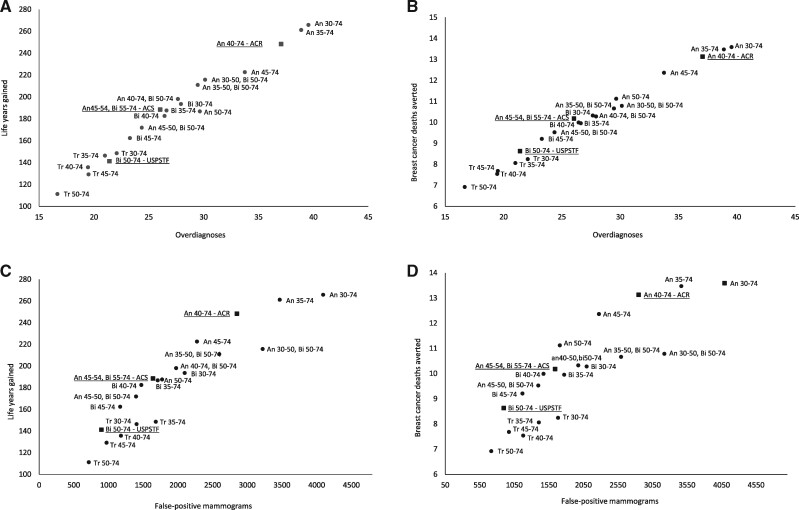
Figure 2.
Harms and benefits associated with different screening strategies among women who learned at age 40 years about a positive first-degree family member with breast cancer. Estimates of Model E per 1000 women screened. The underlined strategies are the commonly followed guidelines of the United States Preventive Services Task Force (USPSTF), the American Cancer Society (ACS), and the American College of Radiology (ACR). The figure shows (A) life-years gained vs overdiagnosed breast cancers; (B) breast cancer deaths averted vs overdiagnosed breast cancers; (C) life-years gained vs false-positive mammograms; and (D) breast cancer deaths averted vs false-positive mammograms.
Polygenic Breast Cancer Risk
In each polygenic risk group, the ACR guideline resulted in the greatest number of life-years gained and breast cancer deaths averted because it is the most intensive screening schedule, but this strategy was also associated with more overdiagnoses and false positives than the ACS and USPSTF guidelines (Table 4). There were several PRS-tailored screening strategies that had comparable or greater benefits than current guidelines, overall increasing life-years gained by an additional 20% (118 [Table 2] to 141 [Table 5]) and breast cancer deaths averted by 11% (6.7 [Table 2] to 7.4 [Table 5]) compared with the USPSTF guideline. The harms increased by 10% (14.5 to 16.0) more overdiagnoses and 26% (920 to 1156) more false positives.
Table 4.
Model average benefits, harms, and benefit to harm ratios for screening based on polygenic risk per 1000 women screened
| Risk group based on PRS | Screening based on | Screening strategy | Screens, No. | LYGa | BC deaths averteda | Over diagnoses | False positives | LYG/screens | LYG/overdiagnoses | BC deaths averted/false positives |
|---|---|---|---|---|---|---|---|---|---|---|
| PRS7 (5.0 < RR < 10.0) | USPSTF | Bi 50-74 y | 8886 | 513 | 27.5 | 28.0 | 726 | 0.0577 | 18.30 | 0.0378 |
| ACS | An 45-54 y, Bi 55-74 y | 15 054 | 685 | 33.1 | 35.2 | 1404 | 0.0455 | 19.45 | 0.0236 | |
| ACR | An 40-74 y | 25 587 | 863 | 40.4 | 48.2 | 2432 | 0.0337 | 17.89 | 0.0166 | |
| Polygenic risk | An 30-74 y | 35 214 | 959 | 42.8 | 53.3 | 3648 | 0.0272 | 18.00 | 0.0117 | |
| PRS6 (3.0 < RR < 5.0) | USPSTF | Bi 50-74 y | 9897 | 352 | 19.3 | 24.8 | 811 | 0.0355 | 14.17 | 0.0238 |
| ACS | An 45-54 y, Bi 55-74 y | 16 369 | 459 | 22.7 | 29.7 | 1522 | 0.0280 | 15.48 | 0.0149 | |
| ACR | An 40-74 y | 28 007 | 578 | 27.8 | 40.4 | 2644 | 0.0206 | 14.31 | 0.0105 | |
| Polygenic risk | An 35-74 y | 32 835 | 616 | 28.9 | 42.6 | 3254 | 0.0188 | 14.48 | 0.0089 | |
| PRS5 (2.0 < RR < 3.0) | USPSTF | Bi 50-74 y | 10 469 | 252 | 14.0 | 21.0 | 859 | 0.0240 | 11.98 | 0.0162 |
| ACS | An 45-54 y, Bi 55-74 y | 17 096 | 325 | 16.3 | 24.6 | 1587 | 0.0190 | 13.21 | 0.0102 | |
| ACR | An 40-74 y | 29 373 | 408 | 20.0 | 33.2 | 2763 | 0.0139 | 12.31 | 0.0072 | |
| Polygenic risk | An 40-50 y, bi 50-74 y | 19 574 | 359 | 17.2 | 26.2 | 1955 | 0.0183 | 13.68 | 0.0088 | |
| PRS4 (1.5 < RR < 2.0) | USPSTF | Bi 50-74 y | 10 845 | 183 | 10.2 | 17.7 | 891 | 0.0169 | 10.34 | 0.0115 |
| ACS | An 45-54 y, Bi 55-74 y | 17 566 | 234 | 11.9 | 20.4 | 1629 | 0.0133 | 11.47 | 0.0073 | |
| ACR | An 40-74 y | 30 268 | 295 | 14.6 | 27.2 | 2839 | 0.0097 | 10.87 | 0.0051 | |
| Polygenic risk | Bi 40-74 y | 15 646 | 242 | 12.1 | 21.2 | 1463 | 0.0154 | 11.39 | 0.0083 | |
| PRS3 (1.0 < RR < 1.5) | USPSTF | Bi 50-74 y | 11 091 | 137 | 7.7 | 15.2 | 912 | 0.0123 | 8.98 | 0.0084 |
| ACS | An 45-54 y, Bi 55-74 y | 17 873 | 174 | 8.8 | 17.3 | 1656 | 0.0097 | 10.06 | 0.0053 | |
| ACR | An 40-74 y | 30 856 | 219 | 10.9 | 22.8 | 2890 | 0.0071 | 9.63 | 0.0038 | |
| Polygenic risk | Bi 40-74 y | 15 923 | 180 | 9.1 | 18.2 | 1487 | 0.0113 | 9.92 | 0.0061 | |
| PRS3 (0.5 < RR < 1.0) | USPSTF | Bi 50-74 y | 11 333 | 90 | 5.1 | 12.4 | 932 | 0.0079 | 7.29 | 0.0055 |
| ACS | An 45-54 y, Bi 55-74 y | 18 171 | 115 | 5.9 | 14.0 | 1683 | 0.0063 | 8.18 | 0.0035 | |
| ACR | An 40-74 y | 31 430 | 144 | 7.2 | 18.0 | 2939 | 0.0046 | 8.02 | 0.0024 | |
| Polygenic risk | Bi 50-74 y | 11 333 | 90 | 5.1 | 12.4 | 932 | 0.0079 | 7.29 | 0.0055 | |
| PRS3 (0.0 < RR < 0.5) | USPSTF | Bi 50-74 y | 11 588 | 40 | 2.3 | 9.1 | 953 | 0.0035 | 4.42 | 0.0024 |
| ACS | An 45-54 y, Bi 55-74 y | 18 484 | 51 | 2.6 | 10.2 | 1710 | 0.0027 | 4.98 | 0.0015 | |
| ACR | An 40-74 y | 32 037 | 64 | 3.2 | 12.4 | 2991 | 0.0020 | 5.16 | 0.0011 | |
| Polygenic risk | Tri 50-74 y | 8020 | 34 | 1.9 | 8.3 | 705 | 0.0042 | 4.10 | 0.0027 |
The life-years gained and breast cancer deaths averted are relative to the life-years and breast cancer deaths of women at the same level of risk who are never screened. ACR = American College of Radiology; ACS = American Cancer Society; An = annual; Bi = biennial; BC = breast cancer; LYG = life-years gained; PRS = polygenic risk score; RR = relative risk; Tri = triennial; USPSTF = United States Preventive Services Task Force.
Table 5.
Model average benefits, harms, and benefit to harm ratios comparison of risk-based screening based on BC FH, PRS, and FH combined with polygenic risk for both the primary analysis and the sensitivity analysis
| Risk-based screening outcomes | Screening based on | Screens, No. | LYG | BC deaths averted | Over diagnoses | False positives | LYG/screens | LYG/overdiagnoses/ | BC deaths averted/false positives |
|---|---|---|---|---|---|---|---|---|---|
| Main analyses | |||||||||
| Risk based | FH (strategies in Table 3) | 11 840 | 125 | 6.9 | 14.9 | 1000 | 0.0105 | 8.35 | 0.0069 |
| Risk based | Polygenic risk (strategies in Table 4) | 12 990 | 141 | 7.4 | 16.0 | 1156 | 0.0109 | 8.85 | 0.0064 |
| Risk based | FH and polygenic riska | 13 089 | 154 | 7.9 | 16.6 | 1169 | 0.0117 | 9.23 | 0.0067 |
| Sensitivity analysis | |||||||||
| Risk based (constrained)b | Polygenic risk (strategies in Table 6) | 10 856 | 135 | 7.1 | 14.0 | 946 | 0.0124 | 9.64 | 0.0075 |
Results per 1000 women screened. The screening strategies and associated harms and benefits are listed in Supplementary Table 6 (available online). BC = breast cancer; FH = family history; LYG = life-years gained; PRS = polygenic risk score.
The constrained risk-based screening approach represents a scenario where the number of screens of the United States Preventive Services Task Force screening guidelines (top row) was not increased but rather was redistributed across the population based on the PRSs (strategies given in Table 6). The number of screens do not exactly match because all women in each risk group were assigned to one of the screening strategies listed in the Methods section.
Benefits increased steeply relative to the USPSTF guideline as polygenic risk increased, so that women with 3 times or higher risk than average could begin screening at age 30 or 35 years, and those with greater than average risk (but <3 times the risk) could initiate screening at age 40 years. In addition, the lowest risk group could be screened triennially from ages 50 to 74 years. Although these benefits were associated with increased harms, the ratio of benefits to harm was generally more favorable or very similar to that seen for average-risk women under USPSTF guidelines (Table 4).
Polygenic Risk and Breast Cancer Family History
Risk stratification using both polygenic risk and family history was estimated to lead to 36 additional life-years gained (29% increases), 1.2 fewer breast cancer deaths (18% increase), 2.1 additional overdiagnoses (15% increase), and 1.16 more false positives in a women’s lifetime (27% increases) than the USPSTF guideline (Tables 2 and 5). Overall, based on benefit to harm ratios, screening based on the combination of family history and polygenic risk generated the maximum life-years gained per screen while having the best ratio of life-years gained to overdiagnosed cases (Table 5).
Sensitivity Analyses
Without increasing the number of mammograms of the USPSTF-recommended screening strategy, polygenic risk-based screening was estimated to slightly increase the population-level benefits and even reduce some of the harms compared with the USPTF guideline (outcomes in Table 5 and screening strategies in Table 6). In this scenario, women in the lowest risk PRS group (0-0.5 the population average) were not screened, women between 0.5 and 2.0 were screened biennially, and above a risk of 2.0 hybrid and annual strategies were found to be optimal. Overall, across risk groups, these strategies resulted in 17 additional life-years gained, 0.4 additional breast cancer deaths averted, 0.5 fewer overdiagnoses, and 26 additional false positives compared with the USPSTF guideline (Table 5).
Table 6.
Screening strategies used in the sensitivity analysis
| Polygenic risk group | Screening strategya |
|---|---|
| Polygenic risk group 1 (0.0 < RR < 0.5) | No screening |
| Polygenic risk group 2 (0.5 < RR < 1.0) | Biennial 50-74 y |
| Polygenic risk group 3 (1.0 < RR < 1.5) | Biennial 45-74 y |
| Polygenic risk group 4 (1.5 < RR < 2.0) | Biennial 45-74 y |
| Polygenic risk group 5 (2.0 < RR < 3.0) | Hybrid 40-74 yb |
| Polygenic risk group 6 (3.0 < RR < 5.0) | Hybrid 40-74 yb |
| Polygenic risk group 7 (5.0 < RR < 10.0) | Annual 30-74 y |
The set of screening strategies in this column followed from a constrained optimization that maximized the overall number of life-years gained by simulating all combinations of screening strategies under the overall constraint of using not more mammograms as seen in the United States Preventive Services Task Force guideline. RR = relative risk.
The hybrid consists of annual screening from ages 40 to 49 years and biennial screening from ages 50 to 74-years.
Discussion
This is the first collaborative modeling study to quantify the effects of tailoring screening based on breast cancer polygenic risk and family history. Compared with the current age-based screening guidelines, our results indicate that risk-based screening based on PRS has greater benefits than that based on breast cancer family history only, but combining PRS and family history maximizes improvement in outcomes. Among women with twice the average population breast cancer risk, initiating annual screening before age 50 years is likely to provide greater benefits than harm than seen under the USPSTF guideline. Women at the lowest end of the risk spectrum could consider screening at triennial intervals.
Our results extend and are consistent with previous work on risk-based screening based on classical risk factors (23,24) and prior research in other countries. Vilaprinyo and colleagues performed an analysis using 4 risk groups based on breast density, family history, and personal history of breast biopsy to guide screening (25). Recently, Pashayan used a life-table model to assess risk-based screening in the United Kingdom based on polygenic risk profile (12). Like our results, both studies concluded that risk-based screening strategies had greater benefit to harm ratios than age-based screening.
Although our findings and the results of others lend support to risk-based breast cancer screening, our approach was unique in evaluating whether the associated increases in benefits of risk-based screening were merely attributable to more screening examinations. When we constrained the number of mammograms, the benefits of risk-based screening moderately persisted and overdiagnoses decreased compared with those seen with the USPSTF guideline. This suggests that allocation of mammography resources across age groups based on risk would be an efficient approach to maximize the benefits of screening programs.
Implementing breast cancer screening based on polygenic risk and family history status would require a one-time saliva sample to determine polygenic risk. The result, together with a questionnaire about family history, could assist women in making choices about more personalized screening options. Ethical aspects of genetic testing such as patient autonomy, accessibility, and increased worry about screening outcomes should be considered before the implementation of polygenic risk-based screening strategies.
Although cost-effectiveness analysis is beyond the scope of this paper, the current commercial laboratory cost of polygenic risk testing is currently $100-$150. This cost is expected to decrease with technology advances and economies of scale. The ultimate feasibility of implementing risk-based screening will depend in part on how much the added costs of testing, counseling, and screening will be offset by savings from less screening in low-risk women and decreases in costs of cancer care from earlier diagnoses among women destined to develop breast cancer.
This study has several important strengths, including consistent results across 2 well-established simulation models, use of US national data, and evaluation of a 313-SNP PRS and family history information to personalize breast cancer screening. There are also several caveats that should be considered in evaluating the results. First, we did not explicitly model the effects of rare, but higher risk variants in genes such as BRCA1, BRCA2, PALB2, CHEK2, or ATM, which are particularly relevant among young women under the age of 50 years. Carriers of high-risk mutations in these genes are typically advised to undergo annual screening with both MRI and mammography (26). Second, although we account for tumor natural history by estrogen receptor and HER2 status, the models assumed that polygenic risk did not directly affect tumor progression (27,28) or mode of detection (29). Third, we did not consider screening after age 74 years or costs and quality-adjusted life-years. These will be important to include in future research. Fourth, we did not consider risk related to second-degree family members with breast cancer due to data limitations. Fifth, to test the efficacy of risk-based screening, we assumed perfect uptake of genetic testing, screening, and receipt of treatment. Actual population impact will be lower. Sixth, the effectiveness of screening in combination with treatment in women under age 40 years has been assessed in case-control studies but not in a randomized controlled trial. Finally, although we considered the effects of breast density on mammography performance, it will be important to conduct future analyses that consider joint distributions of breast density, PRS, and family history as the data evolve.
Overall, this study demonstrated that compared with following general population guideline strategies for women of average risk, risk-tailored screening has the potential to prevent more breast cancer deaths and extend lives for identifiable groups of women at high risk due to their breast cancer family history and polygenic risk.
Funding
This research was supported by the National Institutes of Health under National Cancer Institute (NCI) Grants U01CA12958 and U01CA199218. Collection of Breast Cancer Surveillance Consortium (BCSC) data used in this study was supported by National Cancer Institute-funded grants P01CA154292 and U54CA163303 and contract HHSN261201100031C.
Notes
Role of the funder: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: The authors have no conflicts of interest to disclose.
Disclaimers: The authors are responsible for the research and had full independence in designing the study, interpreting the data, writing, and publishing the report. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Acknowledgments: We thank the Breast Cancer Surveillance Consortium (BCSC) investigators, participating women, mammography facilities, and radiologists for the de-identified data they provided for this study. A list of the BCSC investigators is provided at: http://breastscreening.cancer.gov/. The collection of cancer and vital status data from the BCSC was supported in part by several state public health departments and cancer registries throughout the US. For a full description of these sources, please see: http://breastscreening.cancer.gov/work/acknowledgement.html
Data Availability
The Breast Cancer Surveillance Consortium (BCSC) investigators, provided the de-identified and aggregated data of participating women in mammography facilities in the United States that were used to inform the breast cancer simulation models in this study. The collection of cancer and vital status data from the BCSC was supported in part by several state public health departments and cancer registries throughout the US. For a full description of these sources, please see: http://breastscreening.cancer.gov/work/acknowledgement.html A list of the BCSC investigators is provided at: http://breastscreening.cancer.gov/. The Institutional Review Board at Georgetown University approved the study as exempt based on the use of de-identified data. Other data that was used to inform the models has been described in the Supplementary Table 2.
Supplementary Material
References
- 1.Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380(9855):1778-1786. [DOI] [PubMed] [Google Scholar]
- 2. Siu AL; On behalf of the U.S. Preventive Services Task Force. Screening for breast cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2016;164(4):279-296. [DOI] [PubMed] [Google Scholar]
- 3. Monticciolo DL, Newell MS, Hendrick RE, et al. Breast cancer screening for average-risk women: recommendations from the ACR commission on breast imaging. J Am Coll Radiol. 2017;14(9):1137-1143. [DOI] [PubMed] [Google Scholar]
- 4. Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001;358(9291):1389-1399. [DOI] [PubMed] [Google Scholar]
- 6. Thompson D, Easton D.. The genetic epidemiology of breast cancer genes. J Mammary Gland Biol Neoplasia. 2004;9(3):221-236. [DOI] [PubMed] [Google Scholar]
- 7. Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mavaddat N, Michailidou K, Dennis J, et al. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am J Hum Genet. 2019;104(1):21-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pharoah PD, Antoniou AC, Easton DF, Ponder BA.. Polygenes, risk prediction, and targeted prevention of breast cancer. N Engl J Med. 2008;358(26):2796-2803. [DOI] [PubMed] [Google Scholar]
- 10. Burton H, Chowdhury S, Dent T, Hall A, Pashayan N, Pharoah P.. Public health implications from COGS and potential for risk stratification and screening. Nat Genet. 2013;45(4):349-351. [DOI] [PubMed] [Google Scholar]
- 11. Esserman LJ, Study W, Athena I.. The WISDOM Study: breaking the deadlock in the breast cancer screening debate. NPJ Breast Cancer. 2017;3(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pashayan N, Morris S, Gilbert FJ, Pharoah PDP.. Cost-effectiveness and benefit-to-harm ratio of risk-stratified screening for breast cancer: a life-table model. JAMA Oncol. 2018;4(11):1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different U.S. breast cancer screening strategies. Ann Intern Med. 2016;164(4):215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van den Broek JJ, van Ravesteyn NT, Heijnsdijk EA, de Koning HJ.. Estimating the effects of risk-based screening and adjuvant treatment using the MISCAN-Fadia continuous tumor growth model for breast cancer. Med Decis Making. 2018;38(suppl 1):54S-65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schechter CB, Near AM, Jayasekera J, Chang Y, Mandelblatt JS.. Structure, function, and applications of the Georgetown-Einstein (GE) breast cancer simulation model. Med Decis Making. 2018;38(suppl 1):66S-77S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Plevritis SK, Munoz D, Kurian AW, et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000-2012. JAMA. 2018;319(2):154-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Ravesteyn NT, van den Broek JJ, Li X, et al. Modeling ductal carcinoma in situ (DCIS): an overview of CISNET model approaches. Med Decis Making. 2018;38(suppl 1):126S-139S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peto R, Davies C, Godwin J, et al. Early Breast Cancer Trialists’ Collaborative Group. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van den Broek JJ, van Ravesteyn NT, Mandelblatt JS, et al. Comparing CISNET breast cancer incidence and mortality predictions to observed clinical trial results of mammography screening from ages 40 to 49. Med Decis Making. 2018;38(suppl 1):140S-150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mandelblatt JS, Near AM, Miglioretti DL, et al. Common model inputs used in CISNET collaborative breast cancer modeling. Med Decis Making. 2018;38(suppl 1):9S-23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shiyanbola OO, Arao RF, Miglioretti DL, et al. Emerging trends in family history of breast cancer and associated risk. Cancer Epidemiol Biomarkers Prev. 2017;26(12):1753-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trentham-Dietz A, Kerlikowske K, Stout NK, et al. Tailoring breast cancer screening intervals by breast density and risk for women aged 50 years or older: collaborative modeling of screening outcomes. Ann Intern Med. 2016;165(10):700-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Ravesteyn NT, Miglioretti DL, Stout NK, et al. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years: a comparative modeling study of risk. Ann Intern Med. 2012;156(9):609-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vilaprinyo E, Forne C, Carles M, et al. Cost-effectiveness and harm-benefit analyses of risk-based screening strategies for breast cancer. PLoS One. 2014;9(2):e86858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paluch-Shimon S, Cardoso F, Sessa C, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27:v103-v110. [DOI] [PubMed] [Google Scholar]
- 27. Shimelis H, LaDuca H, Hu C, Hart SNNJ, Thomas A, et al. Triple-negative breast cancer risk genes identified by multigene hereditary cancer panel testing. J Natl Cancer Inst. 2018;110(8):855-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Milne RL, Kuchenbaecker KB, Michailidou K, et al. Identification of ten variants associated with risk of estrogen-receptor-negative breast cancer. Nat Genet. 2017;49(12):1767-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li J, Ugalde-Morales E, Wen WX, et al. Differential burden of rare and common variants on tumor characteristics, survival, and mode of detection in breast cancer. Cancer Res. 2018;78(21):6329-6338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Breast Cancer Surveillance Consortium (BCSC) investigators, provided the de-identified and aggregated data of participating women in mammography facilities in the United States that were used to inform the breast cancer simulation models in this study. The collection of cancer and vital status data from the BCSC was supported in part by several state public health departments and cancer registries throughout the US. For a full description of these sources, please see: http://breastscreening.cancer.gov/work/acknowledgement.html A list of the BCSC investigators is provided at: http://breastscreening.cancer.gov/. The Institutional Review Board at Georgetown University approved the study as exempt based on the use of de-identified data. Other data that was used to inform the models has been described in the Supplementary Table 2.