Abstract
Background
The protozoan Trypanosoma cruzi is auxotrophic for purines and causes Chagas’ disease (CD), a neglected illness affecting >6 million people. Combining the 3-deoxyribofuranose part of cordycepin with the modified purine ring of a nucleoside ‘hit’ led to the discovery of 4-amino-5-(4-chlorophenyl)-N7-(3′-deoxy-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (Cpd1), revealing promising anti-T. cruzi activity.
Objectives
To further evaluate Cpd1 in vitro and in vivo to fully assess its therapeutic potential against CD, covering cell culture sterilization through washout assays, drug combination with benznidazole and long-term administration in T. cruzi-infected mice.
Results
Although less susceptible to Cpd1 than amastigotes, trypomastigotes present an impaired capacity to successfully establish intracellular infection of cardiac cultures. Combination of benznidazole with Cpd1 indicated no interaction (additive effect) (FIC index = 0.72) while administration to mice at one-tenth of the optimal dose (2.5 mg/kg and 10 mg/kg for Cpd1 and benznidazole, respectively) suppressed parasitaemia but failed to avoid mortality. Long-term treatment (60 days) gave a rapid drop of the parasitaemia (>98% decline) and 100% mice survival but only 16% cure. In vitro washout experiments demonstrated that although parasite release into the supernatant of infected cardiac cultures was reduced by >94%, parasite recrudescence did occur after treatment.
Conclusions
Parasite recrudescence did occur after treatment corroborating the hypothesis of therapeutic failure due to subpopulations of dormant forms and/or genetic factors in persister parasites involved in natural drug resistance.
Introduction
American trypanosomiasis, known as Chagas’ disease (CD), was discovered in 1909 by Carlos Chagas and is largely ignored in terms of R&D investment.1 CD is caused by the protozoan Trypanosoma cruzi and has two clinical disease stages. The asymptomatic or oligosymptomatic acute phase lasts up to 2 months and is characterized by patent parasitaemia. Due to an efficient host immune response, the parasitism is controlled but not eliminated and the disease moves to the chronic stage in which most infected people will present an asymptomatic profile. After years or even decades for reasons not fully known yet, 30%–40% develop progressive cardiac and/or digestive complications with fatal outcome.2–4 The nature of the host–parasite interaction as well as the host’s and parasite’s genetic backgrounds may play a role in these symptomatic manifestations.5 Also, comorbidity with the new coronavirus SARS-CoV-2 is a threat for chagasic patients.6
Substantial improvement in vector control was achieved in several endemic countries, and the number of new cases dropped considerably. However, it still poses a public concern due to remaining/new triatome foci,7 besides congenital and oral transmissions.8
Only two old nitro-derivative drugs are available: benznidazole and nifurtimox.9 Both have low efficacy in the chronic stage, limiting a therapeutic benefit for millions of infected individuals.10,11 Also, they require long-term administration and elicit several side effects leading to low patient adherence or discontinuation of treatment. Finally, the nitro-drugs are contraindicated in pregnant women and in people suffering from liver or kidney failure or neurological disorders.9,12,13 Novel, safer and more efficacious drugs are therefore mandatory.14
Like other trypanosomatid protozoa, T. cruzi depends on uptake of host purines as it is not able to synthetize purine rings de novo, rendering interference with the purine salvage attractive.15,16 In fact, purine nucleoside analogues have a wide spectrum of activity, including antiviral,17 antitumoral18 and antibacterial.19 Some nucleoside derivatives exhibit nano- and submicromolar in vitro activity against African trypanosomes,20Leishmania spp.21 and T. cruzi.22,23
After revisiting the anti-trypanosomatid activity of the natural nucleoside antibiotic tubercidin and a series of C-7 modified analogues, phenyl-substituted compounds showed encouraging efficacy against intracellular forms of T. cruzi, being more potent than benznidazole, which motivated us to combine 7-substituted 7-deazapurine moieties with the carbohydrate group of cordycepin.22,23 After rounds of optimization, compound 1 (Cpd1) or 4-amino-5-(4-chlorophenyl)-N7-(3′-deoxy-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (Figure 1) revealed outstanding efficacy in an acute mouse model of CD, however, without resulting in parasitological cure.23
Figure 1.
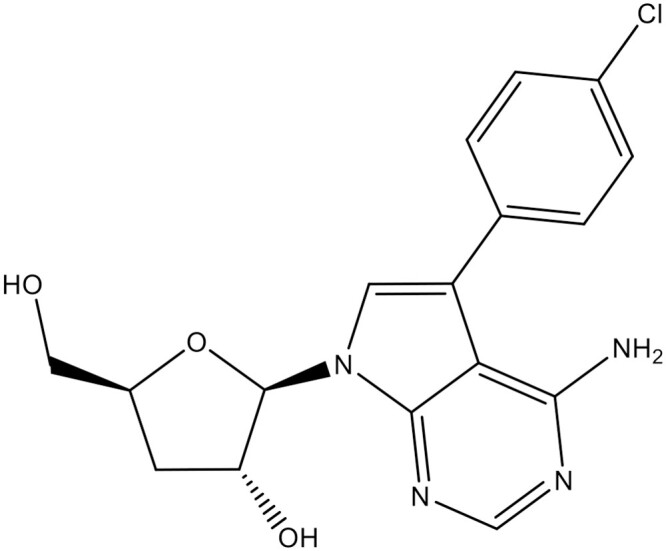
Chemical structure of Cpd1: 4-amino-5-(4-chlorophenyl)-N7-(3′-deoxy-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine.
In the present study, additional in vitro and in vivo protocols were performed to assess and improve the efficacy of Cpd1, including drug combination approaches with benznidazole, washout assays, pretreatment of trypomastigotes prior to incubation with host cells, and dose-titration and long-term (60 days) drug administration experiments in mice aiming to control parasite relapses.
Materials and methods
Compounds
Benznidazole (LAFEPE, Brazil) was used as reference drug. Cpd1 (Figure 1) was synthesized as described by Hulpia et al.23 The identity and purity of Cpd1 was confirmed via 1H NMR, 13C, HRMS and analytical LC/MS respectively. The analytical data match those previously reported.23 Purity of Cpd1 was >95%. Stock solutions of benznidazole and Cpd1 were prepared in 100% DMSO, with the final in-test concentration never exceeding 0.6% for in vitro experiments to avoid non-specific toxicity.24
Mammalian cell culture
Primary cultures of mouse embryonic cardiac cells (CC) were obtained as described.25,26 L929 cell lines were cultivated (4.000 cells/well) in 96-well microplates at 37 °C in RPMI 1640 medium (Sigma–Aldrich).27
Parasites
Bloodstream trypomastigotes (BT) of Y strain (DTU II) were obtained as reported.24 The trypomastigote forms of Tulahuen strain (DTU VI) expressing the Escherichia coli β-galactosidase gene were collected from the supernatant of L929 cell cultures previously infected (host: parasite cell ratio 10:1).27 For both strains, purified parasites were added to RPMI 1640 medium supplemented with 5% FBS to perform assays at 37 °C in 5% CO2.
In vitro assays
The infections were performed using parasite: host cell ratio 10:1, at least two assays in biological duplicate. Half inhibitory concentration (EC50) and statistical analysis (95% CI of SD) were obtained through non-linear regression analysis by GraphPad Prism Version 9.1.0.
Pretreated BT forms
Bloodstream trypomastigotes were incubated for 24 h with Cpd1 and benznidazole at their corresponding EC50 (23 ± 4 and 11.5 ± 1.1 μM, respectively).23 Parasites were rinsed to remove compound, and the number of live and motile parasites determined by light microscopic quantification to adjust the parasite: host cell ratio. After 24 h of interaction, the infected CC were rinsed to get rid of non-internalized parasites, fixed with Bouin or submitted to additional incubation up to 72 h.28 After fixation and staining with Giemsa, light microscopic analysis was performed to determine the number of infected host cells, parasites per infected cell and infection index (percentage of infected cells multiplied by the average number of intracellular parasites per infected host cell).
Washout assays
After 24 h of plating, CC were infected for 24 h at 37 °C with BT (Y strain). The cultures were rinsed to remove free parasites and treated for 168 h at 37 °C with the compounds at the non-toxic concentration of 5 μM in culture medium that was replaced every 48 h. After 168 h of drug exposure, the cultures were rinsed using PBS and drug-free culture medium was added for another 168 h of incubation. Parasites released into the medium were quantified using light microscopy.22
Drug combination assays
The association of benznidazole + Cpd1 was assessed by colorimetric readout using L929 cells that were infected for 2 h with trypomastigote forms (Tulahuen-β-gal strain), followed by rinsing the cultures to remove non-internalized parasites and further incubated for 24 h before drug administration using a fixed-ratio method.29,30 Predetermined EC50 values were used to determine the top concentrations of each drug ensuring that EC50 fell at the midpoint of a seven-point 2-fold dilution series. The fixed ratios of 5:0, 4:1, 3:2, 2:3, 1:4 and 0:5 were used.30,31 At least two independent experiments in triplicate were performed. The FIC index (FICI) at the EC50 of each drug was calculated as EC50 when in combination/EC50 of drug alone. The sum of FICI of each compound (ΣFICI) was obtained.31 The mean ΣFICI (xΣFICI) was calculated as the average of ΣFICI. Isobolograms were built by plotting the FICI values. The ΣFICI was used to classify the nature of interaction as recommended by Odds:30 synergy for xΣFICI ≤0.5, no interaction for xΣFICI 0.5–4 and antagonism for xΣFICI >4.
In vivo activity
Male Swiss Webster mice (18–20 g, 4–5 weeks of age) obtained from the animal facilities of the Institute of Science and Biomodels Technology (ICTB) Fiocruz were housed at a maximum of six per cage, kept in a specific-pathogen-free room at 20 °C to 24 °C under a 12 h light and 12 h darkness cycle, and provided sterilized water and chow ad libitum. The animals were acclimated for 7 days before starting the experiments. Infection was performed by intraperitoneal (IP) injection of 104 bloodstream trypomastigotes (BT) (Y strain). The animals were divided into the following groups (n ≥ 5): untreated (infected but treated only with citrate buffer vehicle) and treated (infected and treated with the compounds). T. cruzi (Y strain)-infected mice were treated by oral gavage for 60 consecutive days (except for weekends) starting at 5 days post-infection (dpi), which corresponds to the parasitaemia onset, using 0.25–25 mg/kg (q12h) of Cpd1 or 100 mg/kg/day benznidazole. Co-administration for 11 days at suboptimal doses of Cpd1 (2.5 mg/kg/day) + benznidazole (10 mg/kg/day) was also performed. Benznidazole at 100 mg/kg/day as the optimal dose was run in parallel. Cpd1 was diluted in 10% (v/v) EtOH, 0.1 M aqueous citrate buffer (pH 3.02, 1.8 mg/mL and then dosed according to body weight of the animals and drug concentration). The drug formulations were freshly prepared before each administration. Only mice with positive parasitaemia were used in the infected groups. Parasitaemia was individually checked by direct microscopic counting of parasites in 5 μL blood, and mice were examined daily for mortality until 261 dpi when blood was collected from surviving animals for qPCR analysis. Animal survival is expressed as the percentage survival rate at the endpoint. For blood qPCR analysis, 500 μL of blood was diluted in a 1:2 volume of guanidine solution and heated for 60 s in boiling water, followed by DNA purification using a High Pure PCR Template Preparation Kit (Roche Applied Science) and quantitative Realtime multiplex PCR assays. TaqMan probes were used for quantification of both T. cruzi satellite nuclear DNA and internal amplification control (IAC) (Figure S1, available as Supplementary data at JAC-AMR Online).32 Standard curves were constructed for absolute quantification through the serial dilution of total DNA, ranging from 105 parasite equivalents to 1 parasite equivalents per mL of blood, obtained with a negative blood sample in guanidine-EDTA. Parasite load was then expressed as equivalent of parasite DNA per mL of blood.33 All animal studies were carried out in strict accordance with the guidelines established by the FIOCRUZ Committee of Ethics for the Use of Animals (CEUA L038-2017).
Statistical analysis
Statistical analysis was performed using ANOVA with the level of significance set at P ≤ 0.05.
Results
Pretreatment of BT
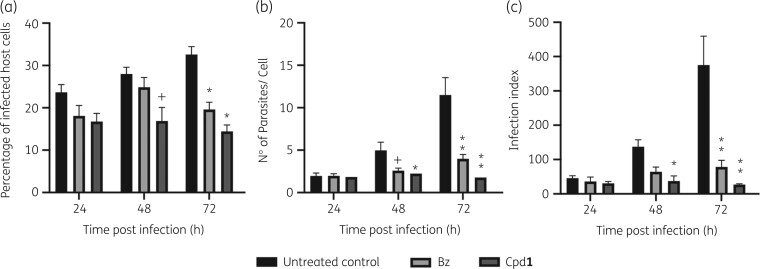
BT were incubated with EC50 of Cpd1 or benznidazole. Drug-treated and untreated parasites were rinsed, and live and motile trypomastigotes quantified before infection of CC. After 24 h, infected cultures were rinsed to remove non-internalized trypomastigotes and followed-up for up to 72 h (Figure 2). Although not presenting high potency, Cpd1 induced morphological alterations (round-shape like phenotype) suggestive of impaired fitness. When treated parasites were used to infect CC, although no major effect could be found after 24 h post-infection (hpi), a high and statistically significant (P < 0.025) drop in all parameters (percentage of infected host cells, parasites per infected cell and infection index rates) was observed for Cpd1 pretreated trypomastigotes after 48 and 72 h of CC infection. With benznidazole pretreated parasites, the mean parasites per infected cell values gave a statistically relevant decrease (P = 0.0135) only at 48 hpi, while after 72 h all parameters were reduced (P ≤ 0.0093) (Figure 2a–d). Cpd1 and benznidazole interfere with parasite fitness, impairing the parasite’s ability to successfully establish and sustain infection in vitro, affecting the proliferative capacity as clearly shown at 72 hpi with earlier and greater reduction rates triggered by Cpd1 leading to >90% decrease in the infection indexes (Figure 2c).
Figure 2.
Pretreatment assays of T. cruzi (Y strain) with Cpd1 or benznidazole (Bz) before parasite–host cell interaction. BT were treated or not (untreated control) for 24 h with the compounds (corresponding EC50), rinsed and used to infect cardiac cell cultures (moi 10:1) in drug-free medium. The mean and standard deviation values were plotted according to percentage of infected host cells (a), number of parasites/cell (b) and infection index (c), at each timepoint. Statistical analysis was performed by two-way multiple comparison ANOVA Tukey’s test (P < 0.05, 95% CI). +P < 0.05, *P < 0.01 and **P < 0.001.
Drug combination
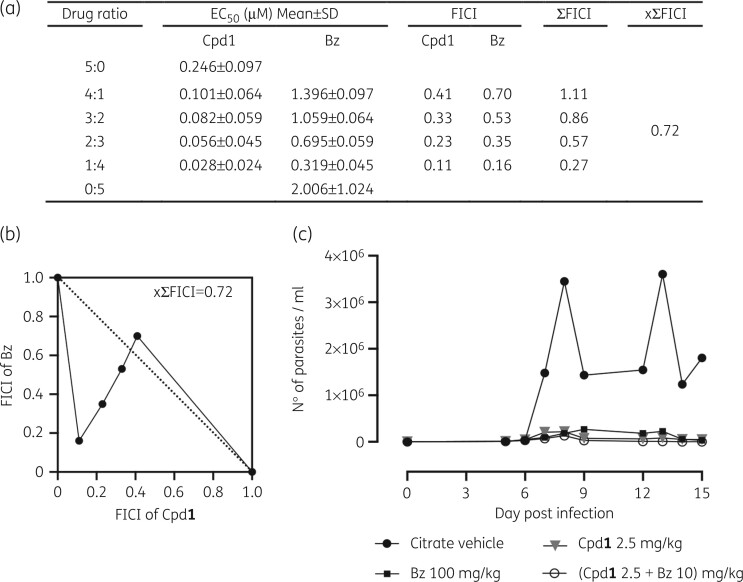
In vitro effect of co-treatment of Cpd123 and benznidazole was assessed by colorimetric readout, using L929 cells infected with β-gal Tulahuen strain with a fixed-ratio method.29–31The result of xΣFICI equal to 0.72 obtained from Cpd1 and benznidazole combination confers the status of a merely additive effect (Figure 3). The most promising results were obtained with the drug ratio of 1:4 (Cpd1: benznidazole) leading to the lowest IC50 and ΣFICI = 0.27 revealing a synergistic profile (Figure 3a). The isobolograms (Figure 3b) show the drug interaction profile with almost all ratios below the graphic threshold, except for 4:1 Cpd1: benznidazole ratio (ΣFICI = 1.11).
Figure 3.
In vitro (a and b) and in vivo (c) analysis of Cpd1 and benznidazole (Bz) combination. Isobolograms (b) of FICI values (a) were calculated based on the in vitro activity (EC50) against intracellular forms of T. cruzi after 168 h of drug exposure. (c) In vivo evaluation of Cpd1 in T. cruzi mouse model of acute infection displaying parasitaemia levels of animal groups. Male Swiss mice were infected (IP) with 104 BT (Y strain) and drug administration (11 days) started at 5 dpi. Benznidazole alone was given at 100 mg/kg (q24h, orally) as a reference drug given at the optimal dose. Co-administration of benznidazole (10 mg/kg, q24h, orally) and Cpd1 (2.5 mg/kg, q12h) is depicted (c). Control group received only vehicle (citrate buffer, q12h).
A proof-of-concept in vivo co-administration study was conducted with Cpd1 and benznidazole in male Swiss mice infected with Y strain (five per group).27 The chosen drug ratio was near to that which gave the best results in vitro (synergy profile reached at 1:4 of Cpd1: benznidazole ratio). The treatment started on the onset of parasitaemia (5 dpi) and was given orally for 11 days q12h for Cpd1 and q24h for benznidazole. Mice also received each drug alone (Cpd1 at 2.5 mg/kg and benznidazole 10 mg/kg), benznidazole at its optimal dose (100 mg/kg) or just vehicle.
Cpd1, benznidazole and their combination gave similar declines in parasite load, reaching ≥94% at peak on 8 dpi (Figure 3c). Cpd1 (2.5 mg/kg) given alone resulted in 60% of animal survival. However, mice treated with benznidazole + Cpd1 showed increased death rates (80% mortality) and enhanced toxicity, exhibiting urination impairment, prostatic and hunched postures with weight loss. All surviving mice in the different groups showed parasite relapse after interrupting drug administration. Based on the still promising results of Cpd1 in monotherapy at 2.5 mg/kg producing >97% reduction in blood parasitaemia, follow-up experiments were conducted adopting dose-dependent analysis with reduced Cpd1 doses and introducing long-term drug administration (60 days) aiming to check whether sterile cure could be achieved.
In vivo dose-titration and long-term drug administration
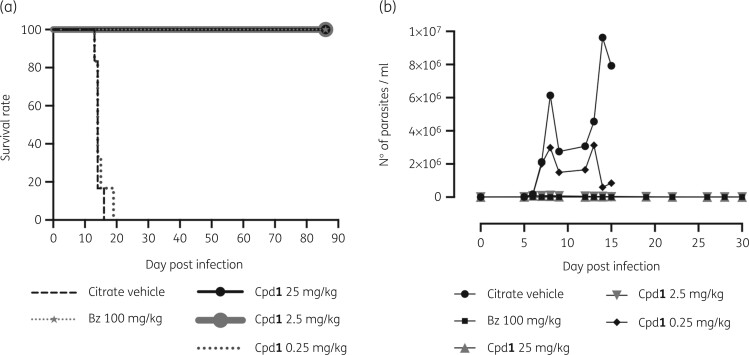
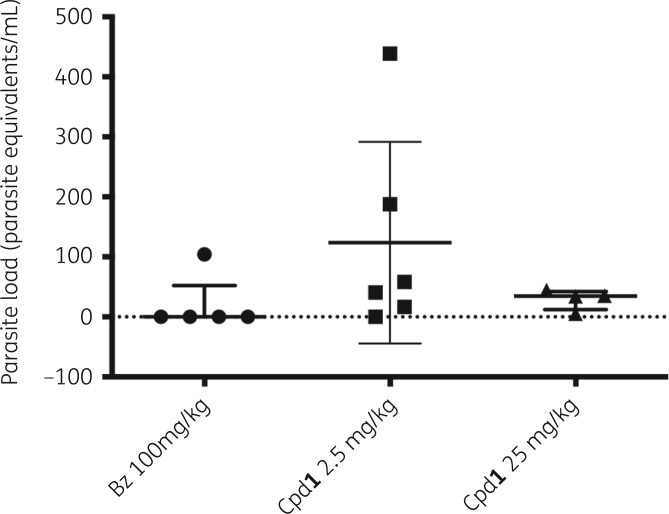
To check for parasite sterilization, an extended and dose-response protocol of drug administration (Cpd1 at 0.25–25 mg/kg for 60 days, excluding weekends) was used in the same mouse model of acute T. cruzi infection. Cpd1 showed a dose-dependent decrease in both parasitaemia levels and mortality rates (Figure 4a and b). At 25 and 2.5 mg/kg, a sharp drop (>98%) in blood parasite load was reached similarly to benznidazole at 100 mg/kg (Figure 4a). Cpd1 at 0.25 mg/kg resulted in >50% of parasitaemia decline although was not able to prevent animal mortality (Figure 4a and b). Blood analysis by qPCR of the surviving animals33 showed that the standard curves ranged from 105 to 1 parasite equivalents per mL of blood. Parasite load referred as equivalent of parasite DNA per mL of blood sample (parasite equivalents/mL) showed 76% efficiency for the satellite nuclear DNA target with a linearity coefficient of 0.996 (Figure S1b), confirming the sensitivity and accuracy of parasite detection and quantification. When infected mice were treated with benznidazole, three out of five animals presented negative blood qPCR (60% of cure) while another showed very low amplification (0.0247 parasite equivalents/mL) (Figure 5). Doses of 25 and 2.5 mg/kg of Cpd1 also decreased the blood parasite load but to a lesser extent than benznidazole, with the one animal from the 25 mg/kg group displaying negative qPCR (17% cure) (Figure 5). Statistical analysis indicated no significant differences between the groups (ANOVA P > 0.31).
Figure 4.
In vivo evaluation of Cpd1 in a mouse model of T. cruzi acute infection. (a) Survival rates (b) and parasitaemia levels of animal groups. Male Swiss mice were infected (IP) with 104 BT (Y strain). Cpd1 administration (0.25–25 mg/kg, q12h, orally) was initiated at 5 dpi and given for 60 days. Benznidazole (Bz) was included as a reference drug (100 mg/kg, once a day, orally). Control group received only vehicle (citrate buffer, q12h).
Figure 5.
Blood qPCR analysis of surviving Swiss male mice infected with T. cruzi (Y strain) and orally treated with Cpd1 (25 and 2.5 mg/kg, q12h) and benznidazole (Bz) (100 mg/kg, q24h). Parasite load is referred as number of parasite equivalents per mL. Each symbol on the scatter dot plots represents an individual value. Bars represent the median values and whiskers represent the IQR. Statistical analysis was performed by one-way multiple comparison ANOVA Tukey’s test between the experimental groups (P < 0.05, 95% CI), all displaying no significant (ns) difference: 0.31 < P < 0.43.
Washout assays
The number of released parasites into the supernatant of the infected CC cultures was quantified by light microscopy at different timepoints (Figure 6a).
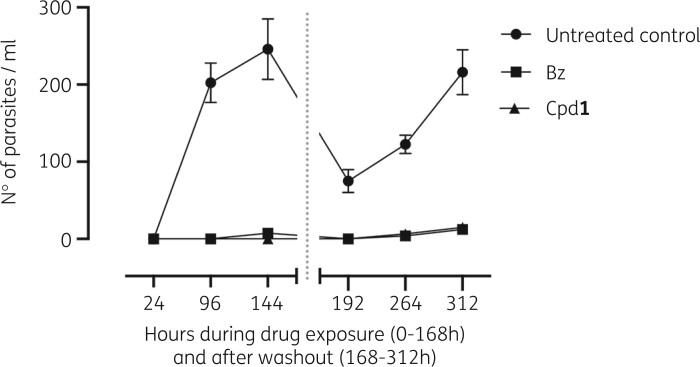
Figure 6.
Washout experiments of T. cruzi-infected cardiac cell cultures. Number of culture-released trypomastigotes as a function of incubation time. Infected cultures (Y strain) were incubated for 168 h with Cpd1 or benznidazole (Bz) and then another 168 h with drug-free medium before assay readout. Data represent mean ± SD of two independent experiments. The grey dotted line indicates the timepoint at which compound exposure is halted by changing to drug-free medium. Statistically significant values between control and treated groups were calculated by Welsh multiple non-parametric t-test (P < 0.05, 95% CI) and P values ranging 0.000009 ≤ P < 0.002.
Both benznidazole and Cpd1-treated cultures sustained a marked and significant (P ≤ 0.001) reduction in the number of released parasites compared with the untreated samples, which showed a continuous discharge of trypomastigotes into the culture medium peaking at 144 h with >200 parasites/mL (Figure 6a and b). Before drug withdrawal, Cpd1 fully suppressed parasitism (light microscopy observation) while benznidazole resulted in a minor parasite release (≈8 parasites/mL) at 144 hpi (Figure 6a). After medium washout at 168 h, benznidazole and Cpd1 were able to sustain the absence of parasites into the culture supernatant for 24 h, while untreated cultures exhibited >75 parasites/mL. However, trypomastigotes were identified from the fourth day of drug removal (at 264 h) in benznidazole as well as Cpd1-treated cultures. Benznidazole and Cpd1 incubation resulted in comparable parasitism (6 and 10 parasites/mL, respectively) that was at least 19-fold lower than the untreated group (180 parasites/mL). At the last timepoint (312 h or sixth day post-drug withdrawal), the number of released parasites reached 15 (Cpd1) and 13 (benznidazole) parasites/mL, being about 15-fold lower than the untreated control (>210 parasites/mL). Washout assays demonstrated a similar capacity of Cpd1 and benznidazole to temporarily arrest parasite growth, ensuing a reduced number of released parasites into the supernatant of infected CC cultures. Cpd1 could not provide definite sterile cure (Figure 6) even upon using long-term drug administration (Figures 4 and 5).
Discussion
After more than five decades since their introduction into clinical use, the two old nitro-heterocyclic drugs benznidazole and nifurtimox still represent the only available therapeutic arsenal to treat CD patients who mostly belong to a vulnerable socioeconomic group.34,35 This scenario has become even worse under the COVID-19 syndemic since the 30%–40% of chronic chagasic patients are more vulnerable to have a health outcome harmed or worsened by SARS-CoV-2 infection.36 Despite these serious challenges, some recent improvement has been achieved based on the development of a paediatric formulation of benznidazole37 and the potential adoption of a shorter benznidazole treatment regimen.38 Fexinidazole, a 5-nitroimidazole derivative recently approved for sleeping sickness therapy39 is under repurposing analysis in chronic chagasic patients.40 However, no novel chemical entity has yet been discovered or implemented for this silent disease although some pre-clinical studies have reported promising drug candidates for CD, including nucleoside derivatives that act on the purine salvage pathway.41 Our group reassessed the natural antibiotic tubercidin and a series of 7-substituted analogues aiming to identify novel hits against Trypanosoma brucei, the agent of human African trypanosomiasis.42 Besides being very active against T. brucei, some phenyl-substituted analogues also presented high in vitro potency against T. cruzi, which motivated us to explore related 7-substituted 7-deazapurine moieties with the carbohydrate group of cordycepin (i.e. 3′-deoxy-d-ribofuranose). Some of these derivatives, such as Cpd1, showed an acceptable safety and efficacy profile in mouse models of T. cruzi infection.23 Cpd1 also presented desirable drug-like properties, such as good oral bioavailability and in vitro metabolic stability, which are essential characteristics to meet the target product profile (TPP) and allow potential clinical translation. The current criteria for ‘hit’ and ‘lead’ candidate selection for CD comprises definition of compound profile activity (preferably trypanocidal),43 efficacy against different T. cruzi discrete type units (DTUs) and parasite forms relevant for human infection,44,45 host–parasite interaction exploitation,14 capacity to sustain sterile cure in vitro43 and parasitaemia suppression and survival rates with sterile cure in vivo.46 Most of these criteria were investigated for Cpd1, which proved highly potent against intracellular forms from different parasite strains and DTUs, displayed very high selectivity indices and was metabolically stable in the presence of mouse, human, rat and dog S9 microsomal fractions.23 Unfortunately, no sterile cure could be achieved despite its high capacity to fully suppress parasitaemia and provide 100% survival in mouse models of T. cruzi infection.23 As observed with other purine nucleoside analogues,22,47,48 Cpd1 was inactive against BT despite its outstanding intracellular activity in infected CC cultures (EC50 = 0.029 ± 0.006 μM) and L929 cell lines (EC50 = 0.25 ± 0.17 μM).23 At that time, the lack of in vivo sterilization by Cpd1 was attributed at least in part to its inefficacy towards BT and/or to the short treatment period (only 5 days) adopted in the initial in vivo study.
Our present study demonstrated that although Cpd1 is not potent to induce a rapid lysis of the trypomastigotes, it induced morphological alterations (round-shape like phenotype) suggestive of impaired fitness. To check if these treated and surviving parasites were able to invade host cells and differentiate into intracellular amastigotes, a pretreatment assay on BT before infection was performed, revealing impairment of the parasite’s ability to develop successfully in CC. The pre-incubation of BT with Cpd1 followed by rinsing and quantification of the live and motile forms to normalize the parasite: host cell ratio resulted in reduced infection indices (>90%) as compared with the infected untreated parasites.
When evaluated in a mouse model of acute T. cruzi infection under extended drug administration (60 days), Cpd1 could control parasitaemia but was unable to induce sterile cure as confirmed by qPCR analysis. In vitro washout assays corroborated these findings as Cpd1 was unable to accomplish sterilization even though the number of released trypomastigotes was >90% lower than in untreated controls. As proposed in immunocompromised CD patients, it is probable that trypomastigotes can flourish from ‘dormant forms’ located in different organs, including the gastrointestinal tract.49,50 Our present assays argue in favour of dormant forms since even though it was not able to lyse trypomastigotes, Cpd1 impairs their ability to establish a successful in vitro infection. Despite outstanding efficacy against intracellular forms (Figure 7), Cpd1 is not able to induce in vitro sterilization possibly due to its inefficacy against low metabolic latent (e.g. dormant) parasites.
Figure 7.
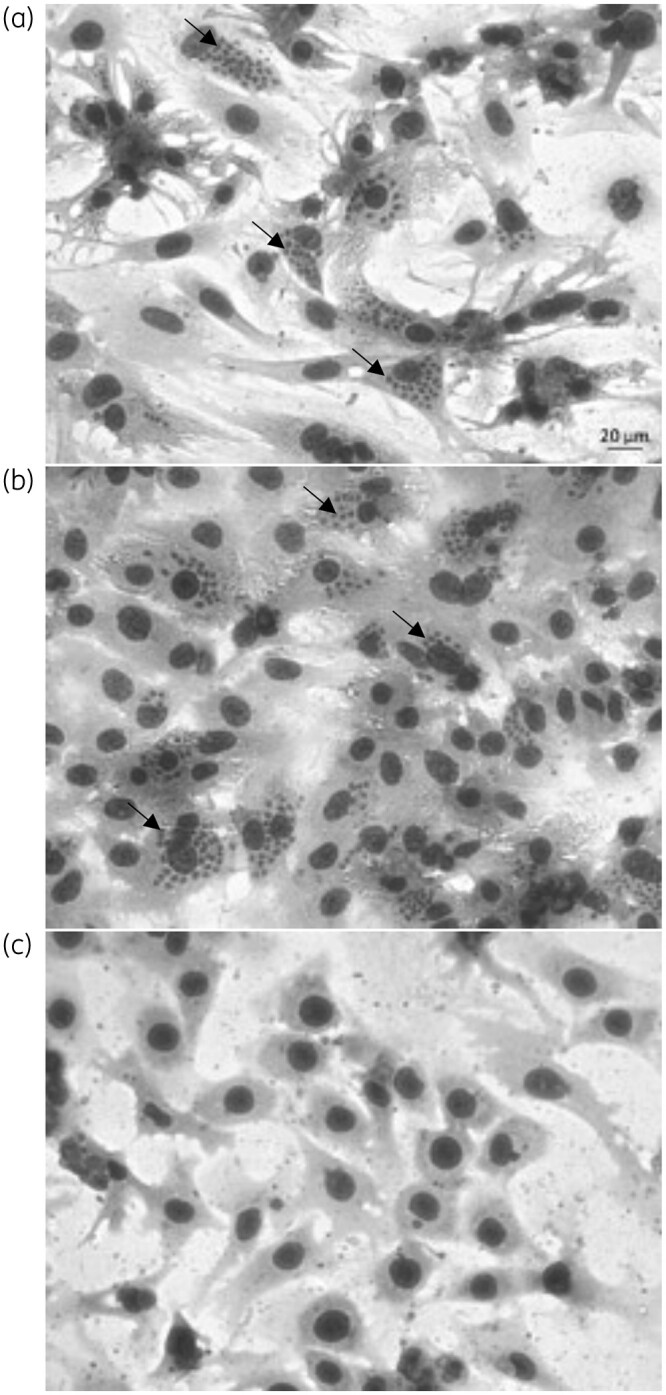
Light microscopy of cardiac cell cultures infected with T. cruzi (Y strain). (a) Untreated and treated with 1 μM (b) benznidazole (Bz) and (c) Cpd1. Arrows: intracellular amastigotes.
Drug combinations have largely been encouraged to find more efficient, shorter and safer therapies for different diseases, thereby also mitigating drug resistance.51 For more than one decade, combinations of nifurtimox/eflornithine (NECT)52 and sodium stibogluconate/paromomycin (SSG&PM)53 have been successfully used for human African trypanosomiasis and cutaneous leishmaniasis, respectively, but no combination therapy is yet available for CD.54 Our data indicated an additive interaction profile when benznidazole + Cpd1 was used in vitro. In vivo co-administration using suboptimal doses of both agents did not improve the outcomes of each compound used separately. Conversely, an unexpected toxic profile (urination impairment) was noted that could be related to the exacerbation of renal injuries induced by T. cruzi infection.55
Our drug research complies with current criteria for drug discovery programmes of neglected tropical diseases (NTDs) caused by kinetoplastids, seeking robust proof-of-concept in preclinical trials to infer the ability of novel compounds to eliminate residual parasite nests and avoid relapses. The excellent efficacies of Cpd1 on intracellular amastigote forms and on trypomastigote fitness were not enough to achieve in vitro nor in vivo sterilization, possibly due to the presence of dormant forms of T. cruzi. It remains attractive to evaluate nucleoside prodrugs to check for improved permeability and/or improved in vivo efficacy as novel drug candidates for NTDs like CD.
Supplementary Material
Acknowledgements
We thank Marcos Meuser Batista for the excellent technical assistance. We thank the Fortalecimento dos Programas de Gestão Estratégica de Pesquisa da Fiocruz Rede de Plataformas Fiocruz (VPPLR-001-Fio 14) and the Programa de Excelência Acadêmica (PROEX) from CAPES.
Funding
The present study was supported by grants from Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional Desenvolvimento científico e Tecnológico (CNPq), Fundação Oswaldo Cruz, PAEF/CNPq/Fiocruz, CAPES. M.N.C.S. and O.C.M. are research fellows of CNPq and CNE and JCNE researchers.
Transparency declarations
None to declare.
Supplementary data
Figure S1 is available as Supplementary data at JAC-AMR Online.
References
- 1. Coura JR, Dias JCP.. Epidemiology, control and surveillance of Chagas disease:- 100 years after its discovery. Mem Inst Oswaldo Cruz 2009; 104: 31–40. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Key Facts. Chagas Disease (Also Known as American Trypanosomiasis). https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis)
- 3. Rassi A Jr, Rassi A, Marcondes de Rezende J.. American trypanosomiasis (Chagas disease). Infect Dis Clin North Am 2012; 26: 275–91. [DOI] [PubMed] [Google Scholar]
- 4. Dias JCP, Ramos AN Jr, Gontijo ED. et al. 2nd Brazilian Consensus on Chagas disease, 2015. Rev Soc Bras Med Trop 2016; 49 Suppl 1: 3–60. [DOI] [PubMed] [Google Scholar]
- 5. Pérez-Mazliah D, Ward AI, Lewis MD.. Host-parasite dynamics in Chagas disease from systemic to hyper-local scales. Parasite Immunol 2021; 43: e12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zaidel EJ, Forsyth CJ, Novick G. et al. COVID-19: implications for people with Chagas disease. Glob Heart 2020; 15: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dias JVL, Queiroz DRM, Martins HR. et al. Spatial distribution of triatomines in domiciles of an urban area of the Brazilian Southeast region. Mem Inst Oswaldo Cruz 2016; 111: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drugs for Neglected Diseases Initiative. Annual Report 2018. Making Medical History to Meet the Needs of Neglected Patients. https://www.dndial.org/wp-content/uploads/2020/03/DNDi_2018_AnnualReport.pdf?x96328.
- 9. Castro JA, De Mecca MM, Bartel LC.. Toxic side effects of drugs used to treat Chagas’ disease (American trypanosomiasis). Hum Exp Toxicol 2006; 25: 471–9. [DOI] [PubMed] [Google Scholar]
- 10. Olivera MJ, Cucunubá ZM, Álvarez CA. et al. Safety profile of nifurtimox and treatment interruption for chronic chagas disease in Colombian adults. Am J Trop Med Hyg 2015; 93: 1224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drugs for Neglected Diseases Initiative. Disease Factsheet. Chagas Disease: Progress Towards Shorter, Better Treatments to Stop a Silent Killer. https://dndi.org/wp-content/uploads/2020/10/DNDi-Factsheet-Chagas-2019.pdf.
- 12. Berenstein AJ, Falk N, Moscatelli G. et al. Adverse events associated with nifurtimox treatment for Chagas disease in children and adults. Antimicrob Agents Chemother 2021; 65: e01135-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Urbina JA, Docampo R.. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol 2003; 19: 495–501. [DOI] [PubMed] [Google Scholar]
- 14. Rao SPS, Barrett MP, Dranoff G. et al. Drug discovery for kinetoplastid diseases: future directions. ACS Infect Dis 2019; 5: 152–7. [DOI] [PubMed] [Google Scholar]
- 15. Berg M, Van der Veken P, Goeminne A. et al. Inhibitors of the purine salvage pathway: a valuable approach for antiprotozoal chemotherapy? Curr Med Chem 2010; 17: 2456–81. [DOI] [PubMed] [Google Scholar]
- 16. Ceron CR, Caldas RD, Felix CR. et al. Purine metabolism in trypanosomatids. J Protozool 1979; 26: 479–83. [DOI] [PubMed] [Google Scholar]
- 17. Jordheim LP, Durantel D, Zoulim F. et al. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat Rev Drug Discov 2013; 12: 447–64. [DOI] [PubMed] [Google Scholar]
- 18. Galmarini CM, Mackey JR, Dumontet C.. Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol 2002; 3: 415–24. [DOI] [PubMed] [Google Scholar]
- 19. Seki JI. Nucleoside analogues. Nippon Nogeikagaku Kaishi 1992; 66: 1349–53. [Google Scholar]
- 20. Hulpia F, Campagnaro GD, Alzahrani KJ. et al. Structure-activity relationship exploration of 3′-deoxy-7-deazapurine nucleoside analogues as anti- Trypanosoma brucei agents. ACS Infect Dis 2020; 6: 2045–56. [DOI] [PubMed] [Google Scholar]
- 21. Azzouz S, Lawton P.. In vitro effects of purine and pyrimidine analogues on Leishmania donovani and Leishmania infantum promastigotes and intracellular amastigotes. Acta Parasitol 2017; 62: 582–8. [DOI] [PubMed] [Google Scholar]
- 22. Lin C, Hulpia F, Da Silva CF. et al. Discovery of pyrrolo[2,3- b]pyridine (1,7-dideazapurine) nucleoside analogues as anti- Trypanosoma cruzi agents. J Med Chem 2019; 62: 8847–65. [DOI] [PubMed] [Google Scholar]
- 23. Hulpia F, Van Hecke K, França Da Silva C. et al. Discovery of novel 7-aryl 7-deazapurine 3′-deoxy-ribofuranosyl nucleosides with potent activity against Trypanosoma cruzi. J Med Chem 2018; 61: 9287–300. [DOI] [PubMed] [Google Scholar]
- 24. Batista DDGJ, Batista MM, De Oliveira GM. et al. Arylimidamide DB766, a potential chemotherapeutic candidate for Chagas’ disease treatment. Antimicrob Agents Chemother 2010; 54: 2940–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meirelles MN, de Araujo-Jorge TC, Miranda CF. et al. Interaction of Trypanosoma cruzi with heart muscle cells: ultrastructural and cytochemical analysis of endocytic vacuole formation and effect upon myogenesis in vitro. Eur J Cell Biol 1986; 41: 198–206. [PubMed] [Google Scholar]
- 26. Timm BL, Da Silva PB, Batista MM. et al. In vitro investigation of the efficacy of novel diamidines against Trypanosoma cruzi. Parasitology 2014; 141: 1272–6. [DOI] [PubMed] [Google Scholar]
- 27. Romanha AJ, de Castro SL, Soeiro M de NC. et al. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem Inst Oswaldo Cruz 2010; 105: 233–8. [DOI] [PubMed] [Google Scholar]
- 28. Coutinho L, Ferreira MA, Cosson A. et al. Inhibition of Trypanosoma cruzi proline racemase affects host-parasite interactions and the outcome of in vitro infection. Mem Inst Oswaldo Cruz 2009; 104: 1055–62. [DOI] [PubMed] [Google Scholar]
- 29. Santos CC, Lionel JR, Peres RB. et al. In vitro, in silico, and in vivo analyses of novel aromatic amidines against Trypanosoma cruzi. Antimicrob Agents Chemother 2018; 62: e02205-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 2003; 52: 1. [DOI] [PubMed] [Google Scholar]
- 31. Trinconi CT, Reimão JQ, Coelho AC. et al. Efficacy of tamoxifen and miltefosine combined therapy for cutaneous leishmaniasis in the murine model of infection with Leishmania amazonensis. J Antimicrob Chemother 2016; 71: 1314–22. [DOI] [PubMed] [Google Scholar]
- 32. Duffy T, Cura CI, Ramirez JC. et al. Analytical performance of a multiplex real-time PCR assay using TaqMan probes for quantification of Trypanosoma cruzi satellite DNA in blood samples. PLoS Negl Trop Dis 2013; 7: e2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guedes-Da-Silva FH, Batista DDGJ, Meuser MB. et al. In vitro and in vivo trypanosomicidal action of novel arylimidamides against Trypanosoma cruzi. Antimicrob Agents Chemother 2016; 60: 2425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ben Beard C. Forgotten people, forgotten diseases: the neglected tropical diseases and their impact on global health and development. Emerg Infect Dis 2009; 15: 510–1. [Google Scholar]
- 35. Hotez PJ, Damania A, Bottazzi ME.. Central Latin America: two decades of challenges in neglected tropical disease control. PLoS Negl Trop Dis 2020; 14: e0007962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nishiga M, Wang DW, Han Y. et al. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol 2020; 17: 543–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Drugs for Neglected Dieseases Initiative. LAFEPE Benznidazol 12,5 mg. Pediatric Dosage Form of Benznidazole. https://dndi.org/wp-content/uploads/2009/03/Product_launch_PaedBenz_dossier_ENG.pdf.
- 38. Molina-Morant D, Fernández ML, Bosch-Nicolau P. et al. Efficacy and safety assessment of different dosage of benznidazol for the treatment of Chagas disease in chronic phase in adults (MULTIBENZ study): study protocol for a multicenter randomized Phase II non-inferiority clinical trial. Trials 2020; 21: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lindner AK, Lejon V, Chappuis F. et al. New WHO guidelines for treatment of gambiense human African trypanosomiasis including fexinidazole: substantial changes for clinical practice. Lancet Infect Dis 2020; 20: e38–46. [DOI] [PubMed] [Google Scholar]
- 40. Deeks ED. Fexinidazole: first global approval. Drugs 2019; 79: 215–20. [DOI] [PubMed] [Google Scholar]
- 41. Mazzeti AL, Capelari-oliveira P, Bahia MT. et al. Review on experimental treatment strategies against Trypanosoma cruzi. J Exp Pharmacol 2021; 13: 409–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hulpia F, Mabille D, Campagnaro GD. et al. Combining tubercidin and cordycepin scaffolds results in highly active candidates to treat late-stage sleeping sickness. Nat Commun 2019; 10: 5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cal M, Ioset JR, Fügi MA. et al. Assessing anti-T. cruzi candidates in vitro for sterile cidality. Int J Parasitol Drugs Drug Resist 2016; 6: 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Katsuno K, Burrows JN, Duncan K. et al. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat Rev Drug Discov 2015; 14: 751–8. [DOI] [PubMed] [Google Scholar]
- 45. Franco CH, Alcântara LM, Chatelain E. et al. Drug discovery for Chagas disease: impact of different host cell lines on assay performance and hit compound selection. Trop Med Infect Dis 2019; 4: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guedes-da-Silva FH, Batista DDGJ, Da Silva CF. et al. Successful aspects of the coadministration of sterol 14α-demethylase inhibitor VFV and benznidazole in experimental mouse models of Chagas disease caused by the drug-resistant strain of Trypanosoma cruzi. ACS Infect Dis 2019; 5: 365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lin C, Fiuza LFA, Santos CC. et al. 6-Methyl-7-aryl-7-deazapurine nucleosides as anti-Trypanosoma cruzi agents: structure-activity relationship and in vivo efficacy. ChemMedChem 2021; 16: 2231–53. [DOI] [PubMed] [Google Scholar]
- 48. Bouton J, Ferreira de Almeida Fiuza L, Cardoso Santos C. et al. Revisiting pyrazolo[3,4-d]pyrimidine nucleosides as anti-Trypanosoma cruzi and antileishmanial agents. J Med Chem 2021; 64: 4206–38. [DOI] [PubMed] [Google Scholar]
- 49. Pinesi HT, Strabelli TMV, Aiello VD.. Case 4/2019 - 26-year-old man with congenital Chagas disease and heart transplantation. Arq Bras Cardiol 2019; 113: 286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Perez CJ, Lymbery AJ, Thompson RCA.. Reactivation of Chagas disease: implications for global health. Trends Parasitol 2015; 31: 595–603. [DOI] [PubMed] [Google Scholar]
- 51. Sun W, Sanderson PE, Zheng W.. Drug combination therapy increases successful drug repositioning. Drug Discov Today 2016; 21: 1189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yun O, Priotto G, Tong J. et al. NECT is next: implementing the new drug combination therapy for Trypanosoma brucei gambiense sleeping sickness. PLoS Negl Trop Dis 2010; 4: e720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Musa A, Khalil E, Hailu A. et al. Sodium stibogluconate (SSG) & paromomycin combination compared to SSG for visceral leishmaniasis in East Africa: a randomised controlled trial. PLoS Negl Trop Dis 2012; 6: e1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Urbina JA. Recent clinical trials for the etiological treatment of chronic Chagas disease: advances, challenges and perspectives. J Eukaryot Microbiol 2015; 62: 149–56. [DOI] [PubMed] [Google Scholar]
- 55. De Oliveira GM, Da Silva TM, Batista WS. et al. Acute Trypanosoma cruzi experimental infection induced renal ischemic/reperfusion lesion in mice. Parasitol Res 2009; 106: 111–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.