Abstract
Objectives
To describe the clinical profile of Asian Indian patients with Takayasu’s arteritis (TAK) and to compare clinical features and outcome of childhood-onset Takayasu’s arteritis (cTAK) with adult-onset TAK (aTAK).
Methods
Data related to clinical features and response to treatment of patients with cTAK (age of onset <16 years) and aTAK from a large observational cohort in our tertiary care teaching hospital were noted and compared.
Results
Altogether, 602 patients (cTAK = 119; aTAK = 483) were studied. Patients with cTAK had a blunted female: male ratio; but fever, elevated acute phase reactants, involvement of abdominal aorta or its branches, hypertension, abdominal pain, elevated serum creatinine and cardiomyopathy were more common in cTAK as compared with aTAK. Patients with aTAK were more likely to have aortic-arch disease and claudication than cTAK. During follow-up, complete remission was more common in cTAK (87% vs 66%; P < 0.01), but subsequent relapses were equally common (30% vs 27%; P = 0.63). Independent associations of disease duration at presentation with disease extent [Disease Extent Index in TAK (DEI.Tak)] and damage [TAK Damage Score (TADS)] were observed (P ≤ 0.01). Moreover, 54% of patients with symptom duration of >5 years at presentation still continued to have elevated CRP suggesting continued and active inflammation warranting escalation or inititation of immunosuppression.
Conclusion
Patients with cTAK are more likely to have arterial disease below the diaphragm, systemic inflammation and achieve remission. Disease of the aortic arch is more common in patients with aTAK. Longer duration of symptoms prior to initiation of immunosuppression, thereby leading to extensive disease and damage, reflects ongoing disease activity as the rule rather than exception in untreated TAK.
Keywords: Takayasu’s arteritis, childhood vasculitis, childhood Takayasu’s arteritis, large-vessel vasculitis, vasculitis, autoimmune, India
Rheumatology key messages
Infra-diaphragmatic disease is more common in cTAK while aortic-arch disease is more common in aTAK.
cTAK patients more commonly present with systemic inflammation and have more favourable outcomes than aTAK.
Longer duration of symptoms of TAK is associated with extensive disease and damage.
Introduction
Takayasu’s arteritis (TAK) is a large-vessel vasculitis associated with inflammation and damage to the aorta, its main branches, and at times, the pulmonary arteries. Untreated inflammation may lead to narrowing or ectasias of these vessels [1, 2]. The estimated incidence of TAK ranges from 1 to 2.6 cases/million/year as per Asian, Turkish and American studies; European countries report lower estimates in the range of 0.3–1 cases/million/year. Clinical series from different parts of the world have studied varying clinical aspects of this disease. Barring a few single-centre studies from NIH (USA), India, Turkey and China, most series are retrospective [3–16]. Childhood-onset TAK (cTAK) has also been studied in several case series from centres across the world, but there is paucity of data from large, single-centre series comparing clinical characteristics of cTAK with adult-onset disease (aTAK). Over the past 18 years, we have maintained one of the largest single-centre cohorts of TAK in our tertiary care teaching hospital setting. The objectives of the present study included: (i) to describe the clinical profile of Asian Indian patients with TAK at the time of presentation; and (ii) to compare clinical profiles, patterns of arterial involvement and response to treatment between cTAK and aTAK.
Methods
Data extraction
Among the 608 patients treated as TAK in our department between January 1998 and June 2017, 602 patients satisfied either the 1990 ACR classification criteria, Ishikawa’s criteria, Sharma’s modification of Ishikawa criteria or the EULAR/PRINTO/PReS criteria for cTAK and were included in this study [17–20]. Clinical data including age, sex, disease duration, angiographic type, extent of clinical disease using Disease Extent Index in TAK (DEI.Tak) score, CRP, and ESR, were recorded for 123 of the patients with TAK as a part of an earlier study on the derivation of the DEI.Tak score [21]. Additional data related to baseline demographics, symptoms and signs were extracted from electronic medical records for 70 of these 123 patients. The remaining 479 patients were included from our extended cohort of patients with TAK. In the extended cohort, data of 233 patients was recorded retrospectively from electronic medical records of our institution, while 246 patients were recruited prospectively. Clinical data extracted include age, sex, disease duration, angiographic type, extent of clinical disease using DEI.Tak score, CRP, ESR, details of treatment, damage and disease outcome. The symptoms and signs known to adversely affect the prognosis or those that are potentially organ threatening were listed as complications [10, 22, 23, 24]. A summary flow chart is provided in Fig. 1.
Fig. 1.
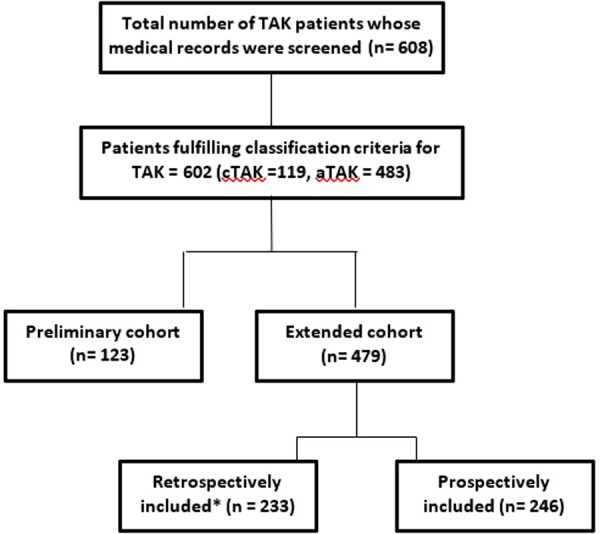
Flow chart of patients included in this study
Available baseline details for both groups of patients (preliminary and extended cohorts) were analysed together in the combined cohort. Details of the longitudinal disease course in the extended cohort have been previously published; however, comparison of clinical outcomes between our cases of cTAK and aTAK has not been reported [25]. This study complies with the norms laid down by the Declaration of Helsinki and has been approved by the Institutional Review Board and Ethics Committee of Christian Medical College, Vellore. As the data was collected in a retrospective manner from the anonymized medical records, informed consent from patients was not obtained.
Study definitions
In our centre, it is standard practice to use 16 years of age as the cut-off between adult and paediatric-onset rheumatic diseases. Hence, patients with onset of symptoms related to TAK at ≤16 years of age were classified as cTAK; patients with onset of disease >16 years of age were referred to as aTAK.
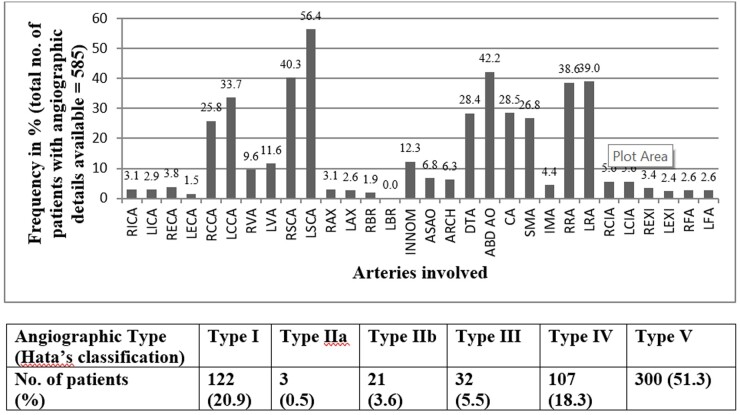
The patients with TAK were subclassified into five types according to Hata’s angiographic classification criteria. In addition, coronary involvement (C+) and pulmonary involvement (P+) were noted.
Disease extent at the baseline visits was assessed by DEI.Tak score [21].
Disease activity was assessed by the composite clinical score Indian Takayasu Arteritis Activity Score (ITAS-A) (CRP) [26].
Active disease was defined as follows:
-
Clinical activity by presence of any one of the following:
1a.ITAS ≥ 2 (not attributable by in-stent restenosis), or
1b.ITAS-A (CRP) ≥ 3, but at least one point should be contributed by clinical criteria as in ITAS pro forma.
Imaging activity by presence of de novo lesion on follow-up angiography or stenosis of the same vessel extending beyond stent margins.
Laboratory activity by persistently raised CRP or ESR on two consecutive visits without any alternative explanation, such as infection.
Disease damage at presentation was assessed using the Takayasu arteritis damage score (TADS) [27].
Outcome parameters were defined as follows:
Complete response (CR): attainment of ITAS-A (CRP) = 0.
Partial response (PR): ITAS 2010 = 1 or ITAS-A (CRP) = 2.
Persistently stable disease: maintenance of ITAS-A (CRP) of 0 throughout the entire follow-up period.
Relapse: Increase in ITAS-A (CRP) to ≥2 or a need for dose escalation of the immunosuppressive agent after prior attainment of complete response.
Persistently active disease: Inability to attain ITAS 2010 = 1 or persistently raised CRP.
Statistical analysis: Demographic variables are depicted as mean (s.d.) or median [interquartile range (IQR)], wherever applicable. Glucocorticoid dose at baseline was categorized into three groups as 0.75–1 mg/kg/day, 0.5–0.74 mg/kg/day, and <0.5 mg/kg/day of prednisolone equivalent. Intergroup comparisons were performed using non-parametric test (Mann–Whitney U test) for continuous variables and chi-squared test for categorical variables. Pearson’s coefficient was used to determine correlation between two continuous variables. Logistic regression was performed for relevant variables with P < 0.1 in univariate analysis, to adjust for effect of potential confounders on significant associations. Analyses related to disease extent, damage and outcome were also adjusted for possible confounders namely age at onset, symptom duration, use of immunosuppression and glucocorticoid dose. The relation between ordinal variables was assessed using Kendall’s tau test and ordinal regression. SPSS Version 18 was used for all analyses.
Results
Baseline demographics, clinical presentation and comorbidities
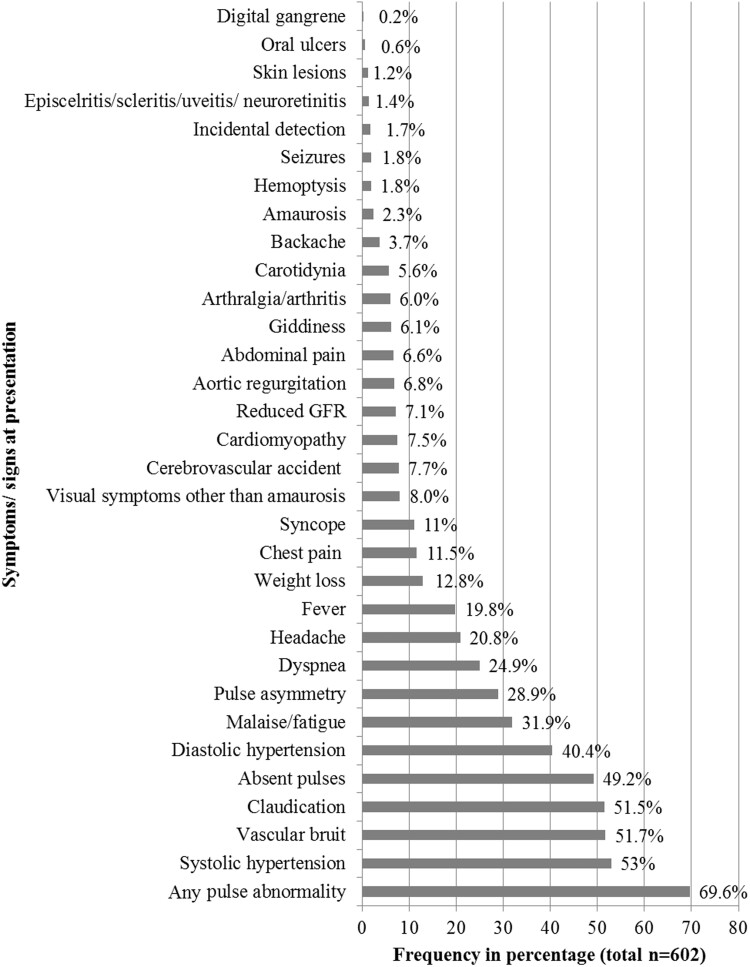
A total of 602 patients were studied. The majority of patients (n = 466, 77.4%) were female with mean age at the initial visit of 28.6 (11.4) years and mean age at onset of symptoms of 25.5 (11.1) years. Median disease duration prior to the initial evaluation, recorded in 587 patients, was 12 (IQR: 6–48) months. All the patients were of Asian Indian ethnicity. Baseline clinical presentations are depicted in Fig. 2. Arterial pulse abnormality (pulse loss and asymmetry) was the most common presentation, followed by systolic hypertension, vascular bruit and limb claudication.
Fig. 2.
Baseline clinical characteristics of all patients with Takayasu’s arteritis
One hundred and thirty-nine patients (23.1%) had a current or prior history of complications at the time of presentation, with cerebrovascular accidents and cardiomyopathy being the most common complications. Details of complications and coexisting medical conditions have been listed in Supplementary Table S1, available at Rheumatology online.
One hundred and nineteen (19.8%) patients met the definition of cTAK with lowest age at disease onset of 1 year, and 483 patients (80.2%) had aTAK. Median age at onset was 14 (IQR: 11–15) years and 26 (IQR: 21–33) years for patients with cTAK and aTAK, respectively. Full clinical details were available for 108 patients with cTAK and 447 patients with aTAK.
Comparison of clinical characteristics of cTAK with aTAK revealed female predominance to be less striking in cTAK compared with aTAK (70.6% vs 79.1%; P = 0.05) (Table 1). There was no difference in duration of symptoms prior to presentation between the two groups. Fever, headache, abdominal pain, systolic hypertension, cardiomyopathy and raised serum creatinine were more frequent in cTAK, while claudication at presentation was more common in aTAK. The two most common presenting features in cTAK were systolic hypertension (66.4%) and pulse abnormality (61.3%), while arterial pulse abnormality (70.8%) was the most common presentation in aTAK. Aortic regurgitation (7.7%) and stroke (7.5%) were the most frequent complications in aTAK.
Table 1.
Comparison of presenting clinical and angiographic features between childhood and adult-onset Takayasu’s arteritis
| Parameter | cTAK (n = 119) | aTAK (n = 483) | P-value |
P-value
[gender-adjusted OR (95% CI)]** |
|---|---|---|---|---|
| Baseline demography | ||||
| Female : Male | 84:35 (2.4:1) | 382:101 (3.8:1) | 0.05 | – |
| Median age at onset (years) | 14 (11–15) | 26 (21–33) | <0.001 | – |
| Median age at presentation (years) | 15 (12–18) | 30 (25–38) | <0.001 | – |
| Median symptom duration (months) | 12 (6–36) | 16 (6–48) | 0.29 | – |
| Clinical symptoms and signs at presentation (recorded for 555 patients) | ||||
| n = 108 | n = 447 | |||
| Fever at presentation (%) | 35 (29.4) | 84 (17.4) | <0.01 |
<0.01 [0.5 (0.31, 0.79)] |
| Malaise/ fatigue | 40 (33.6) | 152 (31.5) | 0.57 | – |
| Weight loss | 12 (10.1) | 65 (13.4) | 0.36 | – |
| Claudication | 46 (38.7) | 264 (54.6) | <0.01 |
<0.01 [1.8 (1.19, 2.7)] |
| Visual disturbance | 14 (11.8) | 34 (7.0) | 0.09 | – |
| Headache | 37 (31.1) | 88 (18.2) | <0.01 |
<0.01 [0.47 (0.3, 0.75)] |
| Syncope | 9 (7.6) | 57 (11.8) | 0.20 | – |
| Cerebrovascular accident | 10 (8.4) | 36 (7.5) | 0.69 | – |
| Abdominal pain | 13 (10.9) | 27 (5.6) | 0.03 |
0.06 [0.51 (0.25, 1.03)] |
| Dyspnoea | 28 (23.5) | 121 (25.1) | 0.81 |
0.87 [1.04 (0.65, 1.7)] |
| Chest pain | 11 (9.2) | 57 (11.8) | 0.47 | – |
| Systolic hypertension | 79 (66.4) | 234 (48.4) | <0.01 |
<0.01 [0.50 (0.33, 0.77)] |
| Diastolic hypertension | 52 (43.7) | 188 (38.9) | 0.19 |
0.43 [0.85 (0.56, 1.3)] |
| Raised creatinine | 19 (15.9) | 23 (4.7) | <0.01 |
<0.01 [0.29 (1.5, 0.5)] |
| Carotidynia | 5 (4.2) | 29 (6) | 0.66 |
0.53 [1.38 (0.52, 3.7)] |
| Vascular bruit | 55 (46.2) | 251 (51.9) | 0.22 | – |
| Pulse loss/ asymmetry | 73 (61.3) | 342 (70.8) | 0.06 |
0.06 [1.5 (0.99, 2.3)] |
| Aortic regurgitation | 3 (2.5) | 37 (7.7) | 0.06 |
0.04 [3.5 (1.1, 11.7)] |
| Cardiomyopathy | 18 (15.1) | 26 (5.4) | <0.01 |
<0.01 [0.32 (0.17, 0.61)] |
| Angiographic distribution of disease (recorded for 585 patients) | ||||
| n = 116 | n = 469 | |||
| Complete occlusion of vessel | 55 (47.4) | 273 (58.2) | 0.03 |
0.05 [0.66(0.44, 0.998)] |
| Aneurysmal disease | 10 (8.6) | 48 (1.0) | 0.61 | |
| Type 1 disease | 6 (5.1) | 106 (22.6) | <0.01 |
<0.01 [4.98 (2.25, 11.01)] |
| Type 4 disease | 29 (25.0) | 67 (14.3) | <0.01 |
<0.01 [0.50 (0.31, 0.81)] |
| Type 5 disease | 61 (56.5) | 223 (47.5) | 0.64 | – |
| Type 2 disease | 5 (4.3%) | 24 (5.1%) | 1.00 | – |
| Type 3 disease | 6 (5.2) | 23 (4.9) | 0.88 | – |
| RSCA | 41 (35.3) | 195 (41.5) | 0.08 |
0.15 [1.38 (0.89, 2.13)] |
| LSCA | 57 (49.1) | 273 (58.2) | 0.05 |
0.098 [1.4 (0.94, 2.15)] |
| RCCA | 19 (16.3) | 132 (28.1) | 0.01 |
<0.01 [1.9 (1.1, 3.2)] |
| LCCA | 25 (21.6) | 172 (36.7) | <0.01 |
<0.01 [1.9 (1.1, 3.2)] |
| RVA | 11 (9.5) | 44 (9.4) | 0.98 | – |
| LVA | 9 (7.8) | 58 (12.4) | 0.16 | – |
| Innominate artery | 9 (7.8) | 61 (13.0) | 0.10 | – |
| Ascending aorta | 8 (6.9) | 36 (7.7) | 0.77 | – |
| Arch of aorta | 5 (4.3) | 32 (6.8) | 0.32 | – |
| DTA | 32 (27.6) | 130 (27.7) | 0.98 | – |
| Abdominal aorta | 59 (50.9) | 185 (39.4) | <0.01 |
0.05 [0.66 (0.44, 0.99)] |
| SMA stenosis | 38 (32.8) | 117 (24.9) | 0.10 | – |
| IMA | 4 (3.4) | 19 (4.1) | 1.0 | – |
| Right renal artery | 63 (54.3) | 154 (32.8) | <0.01 |
<0.01 [0.40 (0.27, 0.63)] |
| Left renal artery | 55 (47.4) | 168 (35.8) | <0.01 |
0.01 [0.59 (0.39, 0.88)] |
| Coeliac artery | 38 (32.8) | 129 (27.5) | 0.30 | – |
| RCIA | 7 (6.0) | 26 (5.5) | 0.82 | – |
| LCIA | 7 (6.0) | 26 (5.) | 0.82 | – |
| C+ disease * | 6 (8.3) | 53 (20.6) | 0.02 |
0.01 [2.70 (1.20, 5.80)] |
| P+ disease * | 5 (11.9) | 37 (18.4) | 0.27 | – |
Coronary and pulmonary angiograms were performed for 329 (72 of cTAK and 257 of aTAK) and 290 patients (42 of cTAK and 201 of aTAK), respectively, and the percentages are calculated out of these numbers.
All parameters were adjusted for sex.
RSCA, right subclavian; LSCA, left subclavian; RCCA, right common carotid; LCCA, left common carotid; RVA, right vertebral artery; LVA, left vertebral artery; DTA, descending thoracic aorta; SMA, superior mesenteric; IMA, inferior mesenteric; RCIA, right common iliac; LCIA, left common iliac; C+, coronary involvement, P+, Pulmonary artery involvement.
Angiographic extent of disease and vascular complications
Complete angiographic data were available for 585 patients, which included 116 and 469 patients with cTAK and aTAK, respectively (Fig. 3). Hata type 5 disease was the most common type (n = 298, 50.9%). A total of 487 patients underwent coronary angiography, and coronary involvement (C+ disease) was present in 78 (16%) of them; similarly, pulmonary involvement (P+ disease) was present in 47 of 453 (10.4%) patients who underwent pulmonary angiography. Ectasias or arterial aneurysms, with or without stenotic lesions, were observed in 58 (9.6%) patients, while the rest had only the stenotic variety of disease. The left subclavian artery was the most frequently involved artery (56.4% of patients), followed by the abdominal aorta (42.2%), and the right subclavian artery (40.3%) (Fig. 3).
Fig. 3.
Distribution of arterial lesions assessed by angiography in 585 patients with Takayasu's arteritis
ABD AO, abdominal aorta; ARCH, aortic arch; ASAO, ascending aorta; CA, coeliac artery; DTA, descending thoracic aorta; IMA, inferior mesenteric artery; INNOM, innominate artery; LAX, left axial artery; LBR, left brachial artery; LCCA, left common carotid artery; LCIA, left common iliac artery; LECA, left internal carotid artery; LEXI, left external iliac artery; LFA, left femoral artery; LICA, left internal carotid artery; LRA, left renal artery; LSCA, left subclavian artery; LVA, left vertebral artery; RAX, right axillary artery; RBR, right brachial artery; RCCA, right common carotid artery; RCIA, right common iliac artery; RECA, right external carotid artery; REXI, right external iliac artery; RFA, right femoral artery; RICA, right internal carotid artery; RRA, right renal artery; RSCA, right subclavian artery; RVA, right vertebral artery; SMA, superior mesenteric artery.
Differences in the distribution of angiographically identified lesions in cTAK and aTAK are listed in Table 1. Angiographic type 5 was the most common type in both the cTAK and aTAK patients. However, type 1 disease was relatively more common in aTAK (5.1 vs 22.6%; P < 0.01), while type 4 disease occurred more frequently in cTAK (24.0 vs 14.3%; P < 0.01). Left subclavian, bilateral common carotids and coronary artery involvement were more common in aTAK, while abdominal aorta and bilateral renal arterial involvement were more frequently noted in cTAK. Complete occlusive disease was more common in aTAK compared with cTAK. These results remained the same, even after adjustment for sex in logistic regression (Table 1).
Disease extent, activity, damage assessment indices, and laboratory markers at presentation
Median DEI.Tak score for 599 patients was 9 (IQR: 6–12, range: 0–27). Median baseline TADS score in 479 patients was 6 (IQR: 4–9, range: 0–22). There was a positive correlation between duration of symptoms and DEI.Tak score (r = 0.10; P = 0.01) and TADS (r = 0.15; P = 0.001). Linear regression analysis, adjusting for age at onset, showed an independent association of duration of symptoms with DEI.Tak score [regression coefficient = 0.106 (95% CI: 0.002, 0.018); P = 0.01] as well as with TADS [regression coefficient = 0.152 (95% CI: 0.004, 0.017); P = 0.01].
A subset analysis of 97 patients for whom information about prior immunosuppression was available at the baseline visit was performed. Fifty-eight patients were treatment-naïve, while 39 were on lower dose and/or shorter duration of immunosuppressive agents. A significant association was found between DEI.Tak and duration of symptoms [regression coefficient = 0.02 (95% CI: 0.01, 0.04); P = 0.01], independent of age at onset, prior glucocorticoid dose, or use of non-glucocorticoid immunosuppressive agents. The association between TADS and disease duration did not reach statistical significance [regression coefficient = 0.01 (95% CI: −0.004, 0.024); P = 0.14].
Elevated CRP (≥6 mg/l) and ESR (≥20 mm/1st h) at baseline was observed in 311 (54.2%) and 415 (71.9%) patients, respectively. Among patients presenting within 5 years of onset of symptoms, the proportion of patients with elevated CRP levels kept increasing with longer disease duration at presentation; i.e. high CRP was present in 49.5%, 57.1%, 57.7%, and 62.3% of patients with symptom duration at presentation of <1 year, 1–2 years, 2–3 years, and 3–5 years, respectively [regression coefficient = 0.39 (95% CI: 0.024, 0.72); P = 0.037]. However, this trend was blunted in patients presenting >5 years after the onset of symptoms (54%), a time when all patients in the cohort were also taking non-glucocorticoid immunosuppressive medications.
Raised CRP values were more frequently observed in cTAK compared with aTAK. Median ITAS 2010 score at presentation was also higher in cTAK, while there was no difference in the DEI.Tak and TADS between the two subsets (Table 2).
Table 2.
Comparison of disease activity, extent and damage scores between cTAK and aTAK
| Parameter | Childhood-onset TAK (n = 119) | Adult-onset TAK (n = 483) | P-value |
|---|---|---|---|
| Median ITAS 2010 (IQR) | 7 (2–14) | 5 (0–11) | <0.01 |
| Median DEI.Tak (IQR) | 9 (5–13) | 9 (6–12) | 0.81 |
| Median TADS (IQR) | 6 (3–10) | 6 (4–9) | 0.82 |
| No. of patients with raised ESR (>20 mm/1st hr) (%) | 41/107 (61.0%) | 106/423 (74.7%) | <0.01 |
| No. of patients with raised CRP (≥6 mg/l) (%) | 61/106 (56.8%) | 169/421 (41%) | <0.01 |
ITAS, Indian Takayasu Activity Score; DEI.Tak, Disease Extent Index-Takayasu arteritis; TADS, Takayasu Arteritis Damage Score; IQR, interquartile range.
Medical and surgical therapies
Data on medical management was captured for 104 patients with cTAK and for 376 patients with aTAK (Table 3). Immunosuppressive therapy was initiated for most patients. The majority of patients received glucocorticoids (79.8% of cTAK and 75.9% of aTAK) and glucocorticoid-sparing immunosuppressants (80.7% of cTAK and 96% of aTAK). However, the initial dose (mg/kg/d) of glucocorticoids was higher in patients with cTAK compared with aTAK. Mycophenolate was the most commonly used glucocorticoid-sparing agent in both subsets. Biological DMARDS were used in 5% of patients in both subsets.
Table 3.
Comparison of treatment details and outcomes between patients with cTAK and aTAK
| Parameter | Childhood-onset TAK | Adult-onset TAK | P-value |
|---|---|---|---|
|
Treatment initiated |
|||
|
(details available) n |
104 | 376 | |
| On glucocorticoid, n (%) | 83 (79.8%) | 285 (75.9%) | NS |
| Prednisolone equivalent dose | |||
| 0.75–1 mg/kg/d | 50 (42.2%) | 128 (26.2%) | <0.01 |
| 0.5 mg/kg/d | 32 (26.9%) | 196 (40.6%) | <0.01 |
| <0.5 mg/kg/d | 13 (10.9%) | 42 (8.7%) | <0.01 |
| Traditional DMARDs, n (%) | 96 (80.7%) | 361 (96%) | NS |
| Mycophenolate | 58 (48.7%) | 241 (49.9%) | NS |
| Azathioprine | 17 (14.3%) | 68 (14.1%) | NS |
| Methotrexate | 15 (12.6%) | 31 (6.4%) | 0.03 |
| Biologics, n (%) | 6 (5%) | 20 (5%) | NS |
| Tocilizumab | 6 (5%) | 19 (5%) | |
| TNF inhibitors | 0 | 1 (0.2%) | |
| Endovascular revascularization procedure, n (%) | 63 (52.9%) | 241 (49.9%) | NS |
| Open surgical procedure, n (%) | 3 (2.9%) | 25 (6.7%) | NS |
| Outcome | |||
| Follow-up duration (months) [median (IQR)] | 32 (10–61) | 27 (10–59) | NS |
| Complete remission, n (%) | 67 (87%) | 190 (66.2%) | <0.01 |
| Partial remission, n (%) | 7 (9%) | 55 (19.2%) | |
| Relapse in patients after a complete remission, n (%) | 20 (29.9%) | 50 (26.6%) | NS |
| Persistently stable disease, n (%) | 48 (62.3%) | 135 (47.5%) | 0.03 |
TAK, Takayasu’s arteritis; DMARDs, disease-modifying anti-rheumatic drugs; IQR, interquartile range; NS, not significant.
Vascular interventions during or prior to first visit to our institution were performed in 66 patients with cTAK and 253 patients with aTAK. The majority of patients in both subsets [63 (52.9%) with cTAK and 241 (49.9%) with aTAK] underwent endovascular interventions, including balloon angioplasty with or without stent insertion, and aneurysmal repair. Eight patients underwent endovascular repair of aneurysms, aortic valve, or dissections. Open surgery, with or without endovascular procedures, were performed in 3 patients with cTAK and 25 patients with aTAK. These procedures included arterial bypass and graft, renal auto-transplant (n = 9), nephrectomy due to refractory secondary hypertension (n = 2), aortic valve repair (n = 2), and Bentall’s surgery (n = 1).
Comparison of clinical outcomes between cTAK and aTAK
Follow-up data was available for 77 patients with cTAK and 287 patients with aTAK. Median follow-up duration was 32 (10–61) months for cTAK and 27 (10–59) months for aTAK. Overall, 74 (96%) patients with cTAK and 241 (85.4%) with aTAK had partial, complete or delayed response to treatment (P = 0.006). Complete remission was attained more frequently in cTAK (n = 67, 87%) than aTAK (n = 190, 66.2%) (P = 0.004), which was significant even after adjustment for baseline dose of glucocorticoids [OR: 3.8 (1.8–7.9); P = 0.001]. Among patients with initial complete remission, frequency of relapse during further follow-up was similar in cTAK (n = 20, 29.9%) compared with aTAK (n = 50, 26.6%) (P = 0.63). Persistently stable disease course was more common in cTAK (n = 48, 62.3%) than in aTAK patients (n = 135, 47.5%) (P = 0.029), with an odds ratio of 1.97 (95% CI: 1.2, 3.4; P = 0.014), after adjusting for the duration of symptoms prior to the first study visit, baseline glucocorticoid dose, and length of follow-up.
Discussion
In this report, we describe the baseline clinical presentation of TAK from a large cohort of patients, representative of South Asia, predominantly the Indian subcontinent. A major objective of this study was to compare the clinical phenotype and outcome of patients with cTAK vs aTAK. With 119 patients with cTAK, this is one of the largest studies to date in cTAK and thereby, it enabled us to carry out a meaningful comparison with the data of patients with adult-onset disease.
Findings from the present study confirm several prior observations in cTAK (Supplementary Table S3, available at Rheumatology online). There was a lower female predominance in patients with cTAK compared with aTAK. This observation parallels recent smaller series comparing the two groups [28, 29]. Headache, cardiomyopathy, renal failure, and systolic hypertension at presentation were significantly more common in cTAK, while destructive arterial disease and resultant vascular symptoms like claudication were more frequent in aTAK. These findings are similar to a pooled set of data from 241 paediatric-onset TAK and 844 adult cases by Brunner et al. [30]. Type 4 disease, abdominal aorta and renal artery involvement, were more common in patients with cTAK, consistent with prior reports [28, 29, 31–34]. Patients with cTAK had higher CRP and disease activity scores at presentation, consistent with already published reports describing more severe and aggressive disease in childhood-onset TAK [28, 29].
Beyond confirming findings from prior studies regarding differences between childhood- vs adult-onset TAK, the present study elaborates on understanding of these differences, particularly with respect to long-term outcomes. Prior smaller studies described more refractory disease and higher relapse rates in cTAK [28, 29]. In contrast, in this study, more patients with cTAK compared with aTAK (87% vs 66%), achieved complete response. Among patients who achieved complete remission, relapse rates, however, were high and similar in both subsets. Using validated disease activity indices, patients with cTAK had more active disease at presentation compared with patients with aTAK. Additionally, patients with cTAK had more systemic features of inflammation such as fever and elevated acute phase reactants, which may relate to the seemingly better clinical response to immunosuppressive treatments observed in this subset. Alternatively, could the better response to treatment in patients with cTAK be due to higher initial doses of glucocorticoids frequently used in this population? This is unlikely, as improved response to treatment in patients with cTAK compared with aTAK was observed, even after adjusting for differences in glucocorticoid regimens. Divergent causal factors may underlie the differences in the distribution of arterial lesions and resultant clinical presentation, along with differences in clinical outcomes observed in cTAK compared with aTAK. Further studies to characterize potential genetic and immunologic differences in childhood- vs adult-onset TAK are warranted.
Compared with cohorts of TAK from other parts of the world, there were some notable differences in our cohort. Female predominance was relatively less prominent (79%) compared with American, Japanese, Turkish and Italian cohorts (∼90% female), but it was similar to that in Chinese populations [6, 8, 9, 16, 23, 35, 36]. The majority (98.5%) of our patients had disease onset prior to age 40. This was similar to all earlier series with the exception of the Japanese series and a subset of Caucasian patients in a multi-ethnic French series reporting ∼40% of Caucasian patients having disease onset after 40 years of age [13, 36]. Past or concurrent tuberculosis was less frequent in our series (5.6%) compared with the series from China (7.2%) and Mexico (48%) [5, 15]. Only 3 of our patients had evidence of inflammatory bowel disease, which was much lower than that reported in the French study and in a North American cohort [23, 37]. Altogether, 9.6% of our patients had presence of true aneurysms or significant ectasia (excluding post-stenotic dilation), a much lower value than that reported in the French study (24%) (Supplementary Table S2, available at Rheumatology online).
Beyond differences in clinical features of disease, there are contrasting data regarding the association between disease duration and disease extent at the time of presentation in TAK. Reports from Japan observed no effect of diagnostic delay with disease extent and inferred that the extent of disease is determined during the initial phase of disease itself and is dependent on sex and age at onset rather than the duration [36]. However, in our series, duration of symptoms before initiation of immunosuppressant drugs (12 months, IQR: 6–48) was positively associated with disease extent and damage as reflected by DEI.Tak and TADS. Similar associations of treatment delay and disease extent or damage were reported in cohorts from the United States and Italy [8, 14]. Raised CRP levels occurred in a substantial proportion of our patients presenting to us even after >5 years of disease. This along with our previous study, implies that, if not treated medically, systemic inflammation frequently continues unabated in TAK [25].
Important strengths of this study include the size of the cohort. It is the largest single-centre series of TAK, second only to the Japanese nationwide multi-centric survey of >1000 patients with TAK [36]. This real-world study used objective disease assessment instruments like the DEI.Tak, ITAS and TADS to assess disease extent, activity and damage in a large cohort.
Limitations of our study include restricted data entry in our preliminary cohort and retrospective arm of the cohort. Our findings should be validated using larger studies across various populations to address geo-ethnic variations in TAK, including cTAK.
Our preliminary cohort had missing data, but baseline data was missing only in <10% of our patients belonging to both subsets. It was predominantly the outcome data that was missing, for 33.8% and 40.6% of patients with cTAK and aTAK, respectively.
In conclusion, this largest single-centre study cohort of patients with TAK demonstrated higher rates of cardiac and renal complications, higher disease activity including general systemic features, and a better response to immunosuppression, in cTAK compared with aTAK. Whether this paradoxical finding is due to a more aggressive immunosuppression in our cTAK cohort as reflected in the baseline data or due to possible underlying, unique genetic factors, needs to be explored in future studies. Differences between clinical features of disease in relation to age of disease onset further highlight the need to study whether the patients with cTAK have different pathophysiologic mechanisms than those with aTAK. Nevertheless, it appears that ongoing disease activity leading to damage is common in TAK in general; this finding underscores the potential role of treatment with immunosuppressive drugs early in the course of TAK irrespective of age of onset to limit disease extent and damage.
Funding: No specific funding was received from any funding body in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: P.A.M. reports receiving funds for the following activities: Consulting: AbbVie, AstraZeneca, Biogen, Boeringher-Ingelheim, Bristol-Myers Squibb, Celgene, ChemoCentryx, CSL Behring, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, InflaRx, Insmed, Jannsen, Kiniksa, Sparrow; and research support from AstraZeneca, Boeringher-Ingelheim, Bristol-Myers Squibb, Celgene, ChemoCentryx, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, InflaRx, Kypha, TerumoBCT. The other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Numano F, Kakatu T.. Takayasu arteritis—five doctors in the history of Takayasu arteritis. Int J Cardiol 1996;54:S1–10. [DOI] [PubMed] [Google Scholar]
- 2. Numano F. The story of Takayasu arteritis. Rheumatology 2002;41:103–6. [DOI] [PubMed] [Google Scholar]
- 3. Kerr GS, Hallahan CW, Giordano J. et al. Takayasu arteritis. Ann Intern Med 1994;120:919–29. [DOI] [PubMed] [Google Scholar]
- 4. Valsakumar AK, Valappil UC, Jorapur V. et al. Role of immunosuppressive therapy on clinical, immunological, and angiographic outcome in active Takayasu’s arteritis. J Rheumatol 2003;30:1793–8. [PubMed] [Google Scholar]
- 5. Yang L, Zhang H, Jiang X. et al. Clinical manifestations and longterm outcome for patients with Takayasu arteritis in China. J Rheumatol 2014;41:2439–46. [DOI] [PubMed] [Google Scholar]
- 6. Maksimowicz-McKinnon K, Clark TM, Hoffman GS.. Limitations of therapy and a guarded prognosis in an American cohort of Takayasu arteritis patients. Arthritis Rheum 2007;56:1000–9. [DOI] [PubMed] [Google Scholar]
- 7. Jain S, Kumari S, Ganguly NK, Sharma BK.. Current status of Takayasu arteritis in India. Int J Cardiol 1996;54:S111–6. [DOI] [PubMed] [Google Scholar]
- 8. Schmidt J, Kermani TA, Bacani AK. et al. Diagnostic features, treatment, and outcomes of Takayasu arteritis in a US Cohort of 126 patients. Mayo Clin Proc 2013;88:822–30. [DOI] [PubMed] [Google Scholar]
- 9. Ohigashi H, Haraguchi G, Konishi M. et al. Improved prognosis of Takayasu arteritis over the past decade. Circ J 2012;76:1004–11. [DOI] [PubMed] [Google Scholar]
- 10. Ishikawa K, Maetani S.. Long-term outcome for 120 Japanese patients with Takayasu’s disease. Clinical and statistical analyses of related prognostic factors. Circulation 1994;90:1855–60. [DOI] [PubMed] [Google Scholar]
- 11. Hong S, Bae S-H, Ahn SM. et al. Outcome of Takayasu arteritis with inactive disease at diagnosis: the extent of vascular involvement as a predictor of activation. J Rheumatol 2014;42:489–94. [DOI] [PubMed] [Google Scholar]
- 12. Moriwaki R, Numano F.. Takayasu arteritis: follow-up studies for 20 years. Heart Vessels Suppl 1992;7:138–45. [DOI] [PubMed] [Google Scholar]
- 13. Arnaud L, Haroche J, Limal N. et al. Takayasu arteritis in France: a single-center retrospective study of 82 cases comparing white, North African, and black patients. Medicine (Baltimore) 2010;89:1–17. [DOI] [PubMed] [Google Scholar]
- 14. Vanoli M, Daina E, Salvarani C, Itaka Study Group et al. Takayasu’s arteritis: a study of 104 Italian patients. Arthritis Rheum 2005;53:100–7. [DOI] [PubMed] [Google Scholar]
- 15. Lupi-Herrera E, Sánchez-Torres G, Marcushamer J. et al. Takayasu’s arteritis. Clinical study of 107 cases. Am Heart J 1977;93:94–103. [DOI] [PubMed] [Google Scholar]
- 16. Fan L, Zhang H, Cai J. et al. Clinical course and prognostic factors of childhood Takayasu’s arteritis: over 15-year comprehensive analysis of 101 patients. Arthritis Res Ther 2019;21:31. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6341556/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ishikawa K. Diagnostic approach and proposed criteria for the clinical diagnosis of Takayasu’s arteriopathy. J Am Coll Cardiol 1988;12:964–72. [DOI] [PubMed] [Google Scholar]
- 18. Sharma BK, Jain S, Suri S, Numano F.. Diagnostic criteria for Takayasu arteritis. Int J Cardiol 1996;54:S141–7. [DOI] [PubMed] [Google Scholar]
- 19. Arend WP, Michel BA, Bloch DA. et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 1990;33:1129–34. [DOI] [PubMed] [Google Scholar]
- 20.Ozen S, Ruperto N, DillonMJet al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis 2005;65:936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sivakumar MR, Misra RN, Bacon PA.. Op14. The Indian perspective of Takayasu arteritis and development of a Disease Extent Index (DEI.Tak) to assess Takayasu arteritis. Rheumatology 2005;44:iii6–7. [Google Scholar]
- 22. Subramanyan R, Joy J, Balakrishnan KG.. Natural history of aortoarteritis (Takayasu’s disease). Circulation 1989;80:429–37. [DOI] [PubMed] [Google Scholar]
- 23. Comarmond C, Biard L, Lambert M. et al. Long-term outcomes and prognostic factors of complications in Takayasu arteritis: a multicenter study of 318 patients. Circulation 2017;136:1114–22. [DOI] [PubMed] [Google Scholar]
- 24. Goel R, Danda D, Joseph G. et al. Long-term outcome of 251 patients with Takayasu arteritis on combination immunosuppressant therapy: single centre experience from a large tertiary care teaching hospital in Southern India. Semin Arthritis Rheum 2018;47:718–26. [DOI] [PubMed] [Google Scholar]
- 25. Misra R, Danda D, Rajappa SM. et al. Development and initial validation of the Indian Takayasu Clinical Activity Score (ITAS2010). Rheumatology 2013;52:1795–801. [DOI] [PubMed] [Google Scholar]
- 26. Goel R, Kumar TS, Danda D et al. Childhood-onset Takayasu arteritis—experience from a tertiary care center in South India. J Rheumatology 2014;41:1183–9. [DOI] [PubMed] [Google Scholar]
- 27.Danda D, Goel R, Ravindran R, Joseph G. Damage assessment in Takyasu arteritis using Takayasu Arteritis Damage Score (TADS). 2014 ACR/ARHP Annual Meeting Archives. ACR Abstract No.: 810. http://acrabstracts.org/abstracts/damage-assessment-in-takyasu-arteritis-using-takayasu-arteritis-damage-score-tads/.
- 28. Jales-Neto LH, Levy-Neto M, Bonfa E, de Carvalho JF, Pereira RMR.. Juvenile-onset Takayasu arteritis: peculiar vascular involvement and more refractory disease. Scand J Rheumatol 2010;39:506–10. [DOI] [PubMed] [Google Scholar]
- 29. Aeschlimann FA, Barra L, Alsolaimani R. et al. Presentation and disease course of childhood- versus adult-onset Takayasu arteritis. Arthritis Rheumatol 2018;71:315–23. [DOI] [PubMed] [Google Scholar]
- 30. Brunner J, Feldman BM, Tyrrell PN. et al. Takayasu arteritis in children and adolescents. Rheumatology 2010;49:1806–14. [DOI] [PubMed] [Google Scholar]
- 31. Clemente G, Hilario MOE, Lederman H. et al. Takayasu arteritis in a Brazilian multicenter study: children with a longer diagnosis delay than adolescents. Clin Exp Rheumatol 2014;32(3 Suppl 82):S128–33. [PubMed] [Google Scholar]
- 32. Szugye HS, Zeft AS, Spalding SJ.. Takayasu Arteritis in the pediatric population: a contemporary United States-based single center cohort. Pediatr Rheumatol Online J 2014;12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Misra DP, Aggarwal A, Lawrence A, Agarwal V, Misra R.. Pediatric-onset Takayasu’s arteritis: clinical features and short-term outcome. Rheumatol Int 2015;35:1701–6. [DOI] [PubMed] [Google Scholar]
- 34. Cong X-L, Dai S-M, Feng X. et al. Takayasu’s arteritis: clinical features and outcomes of 125 patients in China. Clin Rheumatol 2010. ;29:973–81. [DOI] [PubMed] [Google Scholar]
- 35. Bicakcigil M, Aksu K, Kamali S. et al. Takayasu’s arteritis in Turkey—clinical and angiographic features of 248 patients. Clin Exp Rheumatol 2009;27(1 Suppl 52):S59–64. [PubMed] [Google Scholar]
- 36. Watanabe Y, Miyata T, Tanemoto K.. Current clinical features of new patients with Takayasu arteritis observed from a cross-country research in Japan: age and sex specificity. Circulation 2015;132:1701–9. [DOI] [PubMed] [Google Scholar]
- 37. Sy A, Khalidi N, Dehghan N. et al. Vasculitis in patients with inflammatory bowel diseases: a study of 32 patients and systematic review of the literature. Semin Arthritis Rheum 2016;45:475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.