Abstract
Care coordination challenges for patients with cancer continue to grow as expanding treatment options, multimodality treatment regimens, and an aging population with comorbid conditions intensify demands for multidisciplinary cancer care. Effective teamwork is a critical yet understudied cornerstone of coordinated cancer care delivery. For example, comprehensive lung cancer care involves a clinical “team of teams”—or clinical multiteam system (MTS)—coordinating decisions and care across specialties, providers, and settings. The teamwork processes within and between these teams lay the foundation for coordinated care. Although the need to work as a team and coordinate across disciplinary, organizational, and geographic boundaries increases, evidence identifying and improving the teamwork processes underlying care coordination and delivery among the multiple teams involved remains sparse. This commentary synthesizes MTS structure characteristics and teamwork processes into a conceptual framework called the cancer MTS framework to advance future cancer care delivery research addressing evidence gaps in care coordination. Included constructs were identified from published frameworks, discussions at the 2016 National Cancer Institute-American Society of Clinical Oncology Teams in Cancer Care Workshop, and expert input. A case example in lung cancer provided practical grounding for framework refinement. The cancer MTS framework identifies team structure variables and teamwork processes affecting cancer care delivery, related outcomes, and contextual variables hypothesized to influence coordination within and between the multiple clinical teams involved. We discuss how the framework might be used to identify care delivery research gaps, develop hypothesis-driven research examining clinical team functioning, and support conceptual coherence across studies examining teamwork and care coordination and their impact on cancer outcomes.
The 2013 Institute of Medicine (now National Academy of Medicine) report on the cancer care delivery system identified suboptimal care coordination as a common, costly, and growing public health problem (1). Fragmented care contributes to excessive costs, patient and clinician workload, suboptimal clinical outcomes, and treatment-related medical errors (2,3). Improving interprofessional team-based care was noted as one strategy to reduce fragmentation and improve care coordination in a 2019 workshop of the National Cancer Policy Forum (4). Development of generalizable, team-based interventions as recommended is challenging, however, because the effectiveness of team-based care is affected by factors at multiple levels, including the patient, provider, and organizational levels. Further, intervention development is also challenged to consider the different providers, specialties, and practice settings that may be involved in care delivery, given that multiple care teams must work together interdependently as a “team of teams”—or clinical multiteam system (MTS)—to coordinate care. Conceptual frameworks and construct taxonomies are important tools for research aimed at developing such multilevel interventions and distilling the teamwork behaviors critical for optimal care coordination within and between teams. Frameworks and taxonomies identify key variables and develop a shared language that informs testable hypotheses, supports future cross-study comparisons, enhances replicability in implementation science, and informs practice.
However, health care delivery researchers currently lack a conceptual framework from which to study the mechanisms of effective, interdisciplinary, team-based cancer care and guide intervention development. To address this gap, we describe a conceptual framework identifying inputs such as MTS structure (ie, the “anatomy” of the care team), teaming processes, and emergent states (ie, the “physiology” or mediating processes that turn inputs into outputs) as well as outcomes relevant for understanding and intervening to improve teaming in cancer care delivery. The synthesized framework draws together teamwork frameworks from health care delivery and the organizational sciences, which have a long history of studying coordination and teamwork in a variety of high-risk environments. We adopt the example of lung cancer throughout this article to illustrate elements of the framework and related research questions.
Lung Cancer as an Exemplar
Lung cancer is a substantial public health challenge with an estimated annual worldwide incidence of 2.1 million new cases and projected 1.8 million deaths in 2018 (5). Despite a downward trend in the United States, lung cancer remains the leading cause of cancer mortality worldwide and in the United States (6,7). The challenges to delivering interdisciplinary team-based lung cancer care are growing rapidly with expanding diagnostic, staging, and treatment options; increasing use of multimodality treatment; and high rates of age- and tobacco-related comorbidity.
We use a case study to illustrate these challenges and the concepts discussed throughout our framework (8). A summary of the case study is provided here for reference (described in detail and illustrated in a timeline in the Supplementary Materials, available online).
Case Study
In March 2016, Mr S, a 61-year-old Black man from Mississippi, was found to have 3 lung nodules on a computed tomography (CT) scan done in his local hospital’s emergency department (ED) for unrelated reasons. He was referred to a pulmonologist for follow-up. After multiple referrals (involving 7 different specialists) and repeated radiologic testing, Mr S was diagnosed with lung cancer in October 2016 (6 months after his ED visit and the initial detection of a high-risk lesion).
Seen in a multidisciplinary lung cancer clinic (MDC) in mid-November 2016, he was an active 1-pack-per-day smoker since age 16 years despite above-knee amputation for peripheral vascular disease, 2 episodes of cerebrovascular accident, and 2 episodes of acute myocardial infarction. In the MDC, Mr S’s risk of a perioperative cardiac event was deemed prohibitive, and curative-intent stereotactic body radiation therapy (SBRT) was recommended. This was communicated to Mr S and his referring pulmonologist.
In December 2016, on a routine postvisit telephone call, the MDC navigator learned that Mr S had been referred to hospice care “because nothing else could be done for his lung cancer.” Reminded that his lung cancer was potentially curable with SBRT, Mr S agreed to see a radiation oncologist 3 hours from where he lived. Housed in the American Cancer Society’s Hope Lodge, he received SBRT in 5 fractions in February 2017. It took approximately 1 year for Mr S to move from initial lesion detection to definitive treatment.
As evidenced in Mr S’s case, multiple nonbiologic factors at the patient, provider, and organizational levels exacerbate the care fragmentation that lung cancer patients face (9). These patients often have age- and tobacco-related comorbidities, advanced disease at diagnosis (10), and challenges related to social determinants of health (11-13). At the provider level, there are several diagnostic, staging, and treatment approaches (14), which are often controlled by different types of clinicians with different practice cultures and skill sets, any of which may or may not be relevant to an individual patient’s care (15). Such differences across specialties and providers challenge patients, their families, and providers to coordinate their efforts across team members to develop and execute an effective treatment plan. Health-care systems are often not organized to cope effectively with the complexity of decision making required for optimal lung cancer care. Fragmentation of information, knowledge, and care delivery processes adds an organizational layer to the complexity of the care-delivery challenge (15).
The complexity, poor outcomes, and disparities of this case suggest an opportunity to use teamwork concepts to comprehensively reexamine the structures, processes, and outcomes of lung cancer care, promoting the development of deep insights for quality improvement. Using Mr S’s case as an example, we present a multilevel, theoretical framework grounded in organizational and cancer care delivery research and highlight the pitfalls many cancer patients face in care delivery.
MTS: A Path for Conceptualizing and Improving Cancer Care Delivery
The complexity of the care delivery system makes cancer care coordination research challenging. Overcoming this challenge requires developing conceptual coherence and common understanding about the care team structure variables and teamwork constructs underlying coordination of care. Though anecdotally described as a single cancer care team, the different teams and specialists involved in health care often do not work, or view themselves, as a single integrated team (16,17). Rather, they work as an interdependent “team of teams,” or MTS, coordinating comprehensive care across specialties, allied health providers, and care settings (18-20). MTSs are networks of interdependent teams simultaneously working toward at least 1 overarching shared goal—such as providing timely high-quality care for a particular person with cancer—as well as local team goals, such as ensuring that appointments within their clinic run on time (18,21). The MTS concept has been applied across a range of phenomena, including multiagency disaster response (22), space exploration (23), and health care delivery (24-26).
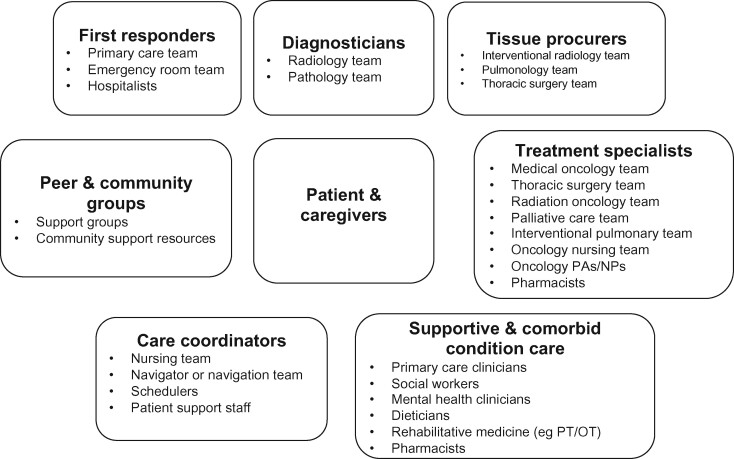
In lung cancer care, representatives of no fewer than 6 types of teams are typically involved during diagnostic workup, staging, treatment planning, and delivery (see Figure 1). These teams must work together to optimally coordinate care across inpatient, outpatient, community, and tertiary care settings. For example, 1 study found that 19% of lung cancer patients received diagnostic and/or treatment-related care at 2 or more health systems in the year following diagnosis (27). To better understand the role that dispersed care and MTS structures play in cancer care delivery, we describe existing theoretical models of team functioning and present a synthesized conceptual framework describing inputs (eg, care MTS structure), teamwork processes and emergent states, and multilevel outputs of cancer care delivery.
Figure 1.
Example of a clinical multiteam system for lung cancer diagnosis and active treatment. Adapted from Osarogiagbon, 2018 (15). NP = nurse practitioner; OT = occupational therapist; PA = physician assistant; PT = physical therapist.
Conceptual Framework Development
Framework development began at the National Cancer Institute-American Society of Clinical Oncology Teams in Cancer Care Delivery Workshop, an in-person meeting of over 95 researchers, clinicians, and patient advocates held in February 2016 (28) (meeting materials may be accessed here: https://www.asco.org/practice-guidelines/cancer-care-initiatives/team-based-care-oncology). Workshop presentations aimed to identify key team structure variables, teamwork processes, and other group-level variables contributing to care quality and patient outcomes in a diverse mix of case studies spanning the cancer care continuum. Presentations were then developed into a set of 19 articles and 4 invited commentaries published in the November 2016 issue of the Journal of Oncology Practice (29) (special series may be accessed here: https://ascopubs.org/toc/jop/12/11).
At the workshop, participants identified the need to further organize the lists of variables identified into an overarching framework or taxonomy to drive future research. Therefore, a small working group of organizational science, clinical, and health-care researchers who attended the workshop volunteered to create a cancer care coordination framework. The group identified additional constructs from published frameworks in the teams, care coordination, and care quality literatures. We identified existing frameworks from several prior reviews (30-35), suggestions from working group members, other frameworks cited in the Journal of Oncology Practice special series on health-care teams, and references cited in these reviews and relevant frameworks. We aimed to highlight the seminal models and frameworks most applicable to cancer care delivery, not to conduct an exhaustive systematic review. Therefore, additional relevant frameworks or models may exist beyond those cited here [see Driskell (36) and Mathieu (37) for detailed reviews]. Variables from other frameworks that could be identified by the working group were cross-walked with the draft framework. We used Mr S’s lung cancer case study to provide practical grounding for further framework development. The working group iteratively revised the framework during a series of virtual meetings with input from 3 consultants with expertise in effective collaborations in complex multiorganizational systems.
Analysis of Existing Team and MTS Performance Frameworks
There is a long history of research into teams and teamwork in the social and organizational sciences. Consequently, multiple theoretical frameworks focus on the development, contributors, processes, and states of teams and teamwork outside the health care context. We highlight several frameworks, including those specific to teamwork in health care delivery, to bridge these 2 areas and provide a foundation for our current understanding of teaming within the complex domain of cancer care (Table 1). Early theoretical teamwork models conceptualized team performance in terms of the inputs-processes-and-outputs (38), which were later revised to reflect a broader conceptualization of the inputs-mediators, moderators-outputs-and subsequent inputs of team performance (39). Although these frameworks provided the impetus for many conceptual models of team functioning, they also had limitations, such as failing to account for the dynamic and evolving nature of teams and team composition over time, as was done by later models such as the Marks (40) temporal taxonomy of team processes and the Salas “Big Five” teamwork framework (41).
Table 1.
Other teamwork models or conceptual frameworksa
| Framework or model | Framework contributions to literature |
|---|---|
| General teamwork and MTS frameworks | |
| Hackman (38) IPO framework | Characterized team performance in terms of a series of inputs that lead to processes that, in turn, lead to outcomes |
| Ilgen et al. (39) IMOI framework | Advanced the IPO framework to incorporate inputs, mediators, outputs, and, in turn, new inputs. Emphasizes that a broader range of variables mediate the input-outcome relationship (eg, learning, conflict management, or processes for managing differences of opinion) and that team outcomes, in turn, serve as inputs of causal feedback for future team interactions and performance |
| Marks et al. (40) Temporal taxonomy of team processes | Advanced nuanced definition of team processes to better differentiate team process variables from team emergent state variables and presented a taxonomy of 10 critical team processes clustered into higher order temporal categories (transition processes, action processes, and interpersonal processes) |
| Salas et al. (41) Big Five | Argued for 5 core components of teamwork: team leadership, mutual performance monitoring, backup behavior, adaptability, and team orientation |
| Zaccaro et al. (42) MTS framework | Differentiates intrateam processes (ie, processes within a given team) from interteam processes (ie, processes unfolding across and connecting different teams). Also identified compositional, linkage, and development attributes that influence these processes and, in turn, system outcomes. |
| Shuffler et al. (43) MTS effectiveness framework | Input-mediator-output framework for MTSs synthesizing key factors contributing to MTS effectiveness based on review of extant literature |
| Health care delivery–focused teamwork and MTS frameworks | |
| Reader et al. (44) Team performance framework for the ICU | Based on prior IPO teamwork frameworks, this framework identifies input characteristics (of the team, task, and team leader), teamwork processes (communication, leadership, coordination, decision making), and outcomes (patient outcomes, team outcomes) relevant to ICU care teams. |
| Flin et al. (45) Anesthetists’ nontechnical skills framework | Advanced 4 categories of teamwork processes (task management, team working, situation awareness, and decision making) and related behavioral markers that could be rated by trained observers during cases |
| Lemieux-Charles et al. (46) Health-care team effectiveness framework | Framework suggests task type, task features, and team composition directly affect team processes and team psychosocial traits with team effectiveness being the ultimate output |
| Weaver et al. (18) Care coordination framework | Differentiates the strategies used to coordinate care within and between care teams from the teamwork-oriented behaviors underlying coordinated activity and related outcomes |
IMOI = input-mediator-outcome-input; IPO = input-process-outcome; MTS = multiteam system.
MTS frameworks move team science a step further to theorize how larger, more complex teams of teams function. For instance, Zaccaro’s (42) MTS framework differentiates intrateam processes (ie, processes within a given team) from interteam processes (ie, processes unfolding across and connecting different teams). The framework also identified a series of compositional, linkage, and development attributes that influence these processes and, in turn, system outcomes. Similarly, Shuffler (43) developed an input-mediator-output framework for MTSs synthesizing key factors contributing to MTS effectiveness. For example, the framework suggests that interventions to improve MTS effectiveness must focus on improving both intra- and interteam processes.
Building from these broader team and MTS frameworks, several have been adapted and developed specifically for the health care delivery context. For example, Reader (44) developed a team performance framework for intensive care postulating that team, task, and leader characteristics influence the processes of communication, leadership, coordination, and decision making, which result in patient and team outcomes. In related work, Flin (45) and colleagues developed and tested a framework outlining 4 categories of teamwork and nontechnical behaviors among anesthesiology and surgical teams. Lemieux-Charles (46) developed a more generalized health care teamwork framework, positing that task type, task features, and team composition directly affect team processes and team psychosocial traits with team effectiveness being the ultimate output.
Finally, Weaver (18) moved health-care theory beyond the team level, drawing from prior MTS and other coordination frameworks from the organizational sciences, to develop a conceptual framework of coordination for chronic disease care when multiple teams are involved. The Weaver et al. (18) framework differentiates the tools used to coordinate care within and between care teams from the teamwork-oriented behaviors underlying coordinated activity and related outcomes. However, it fails to consider the full range of teamwork processes and factors that may affect care coordination and the additional role that context and organizational structure features may play. Our proposed cancer MTS (cMTS) framework addresses this gap and leverages contributions from each of the described frameworks to provide a conceptual foundation for studying teamwork in cancer care delivery.
cMTS: A Conceptual Framework for Research on Cancer Multiteam Systems
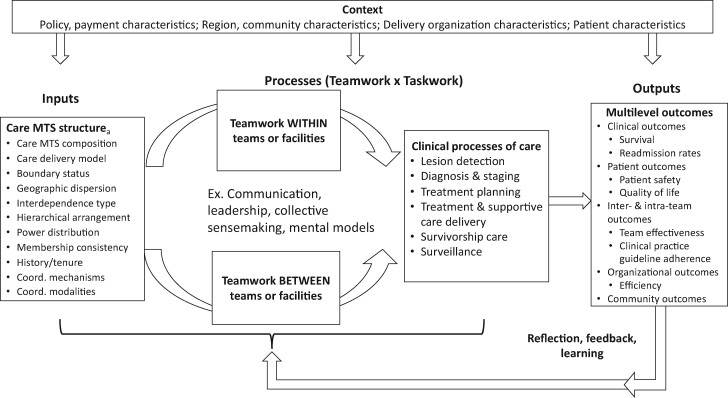
At its core, the cMTS follows an input-mediator-output-input structure by incorporating feedback loops (see Figure 2). This cyclical perspective highlights the episodic nature of team performance, where the outputs of 1 performance episode become the inputs for subsequent performance episodes over time (47). Further, the inputs of this framework apply MTS theory (42) to describe different factors that define the composition of cancer care MTSs, which directly affect subsequent teamwork and taskwork processes both within and between teams. In turn, outputs at the patient, provider, and organizational levels are described. Finally, our framework illustrates the integrative role that contextual features, such as characteristics of the health-care organization (eg, resources, payment characteristics), community, and patient, may have for each of these relationships by serving as a moderator (Figure 2). The following sections describe each of these cMTS components in depth.
Figure 2.
Cancer multiteam system (MTS) conceptual model. Variables listed are examples only, not exhaustive lists. aMTS and team structure characteristics may differ for each patient or may be similar for groups of patients that follow similar care pathways. Coord. = coordination.
Inputs: Multiteam System Structure
MTS structure variables characterize the composition and organization of the interdependent groups working as part of a clinical MTS and serve as the inputs of our framework. Specifically, they characterize the team context in which care is playing out, the players involved, and the prior (or not) relationships among the players. For example, in the case study, Mr S’s cancer MTS included the ED team, pulmonologist, radiologist teams, and MDC providers, who likely had varying degrees of prior experience working together. This familiarity deficit among the members/teams composing the MTS may have been the reason that Mr S faced delays in care and experienced miscommunications regarding treatment, illustrating how MTS structure variables (ie, MTS composition, MTS history/tenure) influence subsequent teamwork behaviors and dictate which behaviors or processes are needed. Zaccaro (42) outlined a variety of structure variables likely to influence MTS functioning and outcomes generally. The current framework integrates several of the structural elements identified in their model relevant for MTSs involved in delivering cancer care. For example, cMTS composition variables may include the number and type of teams working interdependently to care for a particular patient or population of patients. Even relatively simple lung cancer care involves multiple teams for lesion identification, histologic diagnosis, staging, selection between different treatment options, treatment execution, and survivorship care (12). Patients with multiple conditions, such as Mr S, increase the number of teams and team representatives that compose the cMTS and pose greater challenges for care, requiring additional teamwork processes and coordinating teams, which complicates the composition of the MTS.
The care delivery model is another MTS structure characteristic that broadly refers to the way in which care services are organized and delivered. For example, care may be delivered through a serial referral model in which providers are informally connected through serial interdependencies and “hand off” patients to one another in less structured ways. Alternatively, care can also occur in structured, team-based models, as evidenced across MDCs. For example, Mr S’s care was initially in the serial referral model, with limited follow-up or continuity. When he entered the MDC, his care coordination greatly improved. In this model, patients see providers from multiple specialties on the same day and at the same location, with greater provider interactivity, collaboration, and awareness of interdependence. Beyond shared infrastructure, providers in the MDC model are more likely to share incentives, quality initiatives, and team goals. Additional aspects of key MTS component teams’ structures are defined in Table 2. Overall, the team structure variables in this framework are posited to influence the quality of interdependent teamwork and processes that unfold among the different teams, their clinical taskwork, and outcomes. Combined, these MTS structural factors compose the inputs of cancer care delivery, and the processes of cancer care are defined by the component teams’ teamwork and taskwork activities and emergent states.
Table 2.
Key conceptual model variables, definitions, and examplesa
| Variables | Definition | Examples (or types) |
|---|---|---|
| Inputs: care MTS structure | ||
| Care MTS composition | Number and type of component teams working interdependently toward overarching goal(s) (42) |
Case study: 10 different specialists and their respective teams were involved in patient care from ED admission to eventual treatment, approximately 1 year later. Goals of each team and comprehensive plan for follow-up unclear across MTS |
| Type of multidisciplinary care approach | The overarching care delivery model used to coordinate care across multiple clinical care providers (48) | Conference, clinic, navigator, referral, combination, other |
| Boundary status | Proportion of component teams working within the same organization, facility, or health system (42) |
Case study: teams comprising the MDC (eg, radiation oncology, surgical oncology, medical oncology) all worked in the same organization, making them less differentiated than peripheral teams, such as the ED and pulmonologist, where coordinating across teams, providers, and organizations was more challenging. Before reaching the MDC, care providers remained siloed and failed to work as a collective to develop a comprehensive care plan for the patient as he received a nondiagnostic CT and subsequent staging tests. |
| Geographic dispersion | Degree to which component teams are colocated vs dispersed (42) | Case study: MTS teams were dispersed across facilities and departments. However, the MDC was collocated, allowing providers to meet to establish a treatment plan and the patient to see multiple providers in 1 day. |
| Type of interdependence | Way that inputs, process, and outcomes among members of different component teams are interrelated (49) |
Sequential: certain component teams enact and complete their tasks before handing their work off to the next team or teams in the chain. The case study followed a more sequential form of interdependence, where the patient was handed off from 1 team to the next. Reciprocal: component teams complete different parts of the task in a give-and-take and cyclical fashion. This type of interdependence implies some degree of flexibility in the ordering of tasks and, because of the cyclical nature of the workflow, adjustments between component teams occur iteratively. Intensive: task demands that require the concerted action of multiple component teams mutually adjusting in real time |
| Hierarchical arrangement | Ordering of teams according to levels of responsibility (42) |
Clinical care teams often fail to establish clear hierarchies. Hierarchical arrangements are continually redefined for each case or patient and as cases evolve over time. For cancer patients undergoing active treatment, it is likely that the cancer care team will be the leader of the MTS. |
| Power distribution | The relative influence of teams within the MTS (42) |
The patient and their caregiver play a key role in cancer MTS power distribution because they have final say in all decision making and possess financial power over treatment plans. From a clinical perspective, distribution of power within cancer care MTS is unclear, but cancer MTSs tend to disperse power across the system based on the specialty of care required for patient. |
| History or tenure | Previous experience working together or anticipated duration of the MTS (42) | MDC has established protocol and system for long-term MTS functioning, but peripheral teams (eg, ED, PCP) continually change across patients. |
| Coordination mechanisms | The tools, strategies used to time, sequence, and align interdependent care tasks (50) | Designated coordinator, shared clinical pathways, etc |
| Coordination modalities | The modes used to share information and resources across component teams (33) | In-person, phone, virtual, EMR/fax, etc |
| Processes: teamwork within and between teams | ||
| Information exchange or closed loop communication | Pattern of communication characterized by information sharing; the message being received, interpreted, and acknowledged by intended receiver; and follow-up from sender to ensure message received and appropriately interpreted (31) |
Case study: lack of closed loop communication evident across each care team transition. For example, after having a diagnostic CT-guided biopsy performed that was nondiagnostic, the patient faced a 2-month delay in care and then had staging procedures (PET and CT scans) preformed, although no formal diagnosis had been made. Following the nondiagnostic CT, there is a clear need for a follow-up plan to be established. |
| Coordination or adaptability | Altering course of action across MTS component teams in response to changing conditions (51) |
Case study: interdisciplinary coordination within the MDC led to a patient-centered determination of best care for this particular individual, at this particular time, with a consideration of resources available. Thoracic surgeon and cardiologist in the MDC determined that Mr S had occluded both right and circumflex coronary arteries and his risk of a perioperative cardiac event was high; therefore, general anesthesia and surgery were not safe, so they decided that his best option was curative-intent SBRT, for what was determined to be cT2aN0M0 (stage IB) adenocarcinoma. |
| Boundary spanning | Actions of component teams to establish linkages and interactions with one another and other parties (52) |
Case study: tumor boards and the MDC serve as key boundary-spanning activities between cancer care teams. Need similar boundary-spanning activities to occur across all phases of care |
| Situation monitoring | Tracking fellow team member work and situational factors while carrying out own work (31) |
Case study: care teams failed to monitor patient after referring him to the pulmonologist and receiving the diagnostic CT-guided biopsy that was nondiagnostic. Patient waited 2 months for the next step. Later, MDC followed up with patient in routine, postvisit telephone call and learned patient had been referred to hospice by PCP and mislead to believe there were no other treatment options. |
| Collective sensemaking | Negotiation and creation of shared meaning, or understanding, about a given piece of information or situation among a group (53) |
Case study: PCP and MDC failed to establish collective understanding, which led PCP to refer patient to hospice, stating nothing else could be done, while cancer care team knew cancer was treatable. MDC nurse informed patient that cancer was treatable and established a clear understanding between the MDC teams and patient and caregiver team. |
| Group decision making | Negotiation and creation of group approach to identified problem or challenge (31) |
Tumor board or multidisciplinary clinic meeting to discuss case and develop a unified treatment plan together Case study: the MDC |
| Mutual support or back-up behavior | Anticipating other team members’ needs by having accurate knowledge about their responsibilities. This includes the ability to shift workload among members if appropriate during periods of high workload or pressure (41). |
Occurs when team members step up and fulfill other’s responsibilities as needed Case study: the nurse navigators in the MDC worked together to get the patient an appointment with the radiation oncologist associated with the MDC 3 hours away from where Mr S lived. |
| Shared leadership | Transference of leadership functions among team members to take advantage of member strengths (eg, knowledge, skills, attitudes, perspectives, contacts, time) as dictated by environmental demands, task, etc (54) | Case study: oncologist and PCP share leadership where the PCP oversees and leads the management of chronic care while the oncology teams (MDC) lead cancer treatment. |
| Conflict resolution and management of conflicting, missing information | Establishing conditions to prevent, control, guide, and work through disagreements, differences of opinion, or interpersonal conflict (31) | Case study: MDC and primary care delivery team did not have clear processes for managing comorbidities and failed to establish a shared mental model of the patient’s care plan, resulting in the PCP providing the patient with incorrect information regarding his cancer treatment. This conflict was resolved by a routine postvisit telephone call from the MDC and the patient was able to receive appropriate care. |
| Psychological safety | Shared belief that the team is safe for interpersonal risk taking (55) | Team members feel like they can speak up within their team and to other component team members (eg, to intervene or suggest alternative treatment modalities) without fear of being reprimanded. |
| Trust | Willingness to be vulnerable to the actions of another based on expectations that the party will perform actions irrespective of the ability to control (56) | Team members believe that other members and care teams will do their jobs appropriately and that cancer has been properly staged and diagnosed before treatment. |
| Cohesion | Commitment to the task and to each other (57) | Within component teams, team members form interpersonal relationships that strengthen their commitment to one another and the team to facilitate care delivery. |
| Cooperation | Attitude or predisposition held by the involved parties to be concerned about the overall collaborative goal rather than their own individual goal (58) | Case study: in the ED, the team was more focused on their proximal goals within the ED than the MTS goal of managing Mr S’s long-term care, leading to care delays and lack of follow through. Focusing on systems goals is critical in MTSs to drive MTS-focused actions. |
| Mental models | Sharedness and accuracy of mental models among key care team members (59) |
Case study: Mr S’s care team lacked a clear understanding of the care plan and next steps across the system (eg, between teams), resulting in multiple delays in care. Collective sensemaking can facilitate the development of shared mental models. |
| Transactive memory | Cooperative division of labor for learning, remembering, and communicating relevant team knowledge (60) |
Team members understand who is responsible for each task across the system and communicate relevant team knowledge during tumor boards and MDC. Established follow-up plans and clear division of responsibilities within and between care teams Transactive memory across teams can facilitate backup behaviors. |
| Outputs: multilevel outcomes of cancer MTSs | ||
| Clinical outcomes | Clinical outcomes encompass cancer-related health outcomes and can be assessed across organizational levels (eg, individual patient vs hospital morbidity) (61) | Common clinical outcomes included patient survival, disease control, patient morbidity and mortality, and readmission rates. |
| Patient outcomes | Affective outcomes of the patient; often assessed at the patient level but can be aggregated across levels (61) | Patient outcomes included patient safety, quality of life, financial burden, and patient experience. |
| Inter- and intrateamwork outcomes | Measures assessing team performance both within teams and across teams. This can be assessed at the team- and meso-level (43). |
Examples included team viability, team effectiveness, CPG adherence, and patient-centered care. Case study: if evaluating the CPG adherence of Mr S’s care across all teams, they would not be adherent. However, on being referred to the MDC, care became more adherent because of patient follow-up. |
| Organizational outcomes | Measures at the organizational level of analysis, which are often the result of numerous factors beyond the providers control (eg, patient preferences) (62) | Common organizational measures include efficiency [eg, avoiding waste of equipment, supplies, ideas, time, and energy (63)], return on investments, turnover costs. |
| Community outcomes | Measures evaluating impacts that interventions or studies have on the community (64) | Long-term outcomes within the community include community health, such as morbidity and mortality within the community and prevalence. |
CPG = clinical practice guideline; CT = computed tomography; ED = emergency department; MDC = multidisciplinary clinic for lung cancer; MTS = multiteam system; PCP = primary care provider; PET = positron emission tomography; SBRT = stereotactic body radiation therapy. Case study refers to Mr S’s case and was used for all constructs illustrated in the case study when possible.
Processes: Teamwork and Taskwork
Team processes consist of teamwork and taskwork. Whereas taskwork defines what functions are completed throughout the continuum of care (eg, the clinical activities such as accurate interpretation of radiologic studies, safe performance of a biopsy procedure), teamwork depicts how these tasks are effectively carried out (eg, ensuring secure, timely communication of findings to the correct providers) (40). Although the clinical processes of care that encompass taskwork (eg, Mr S’s positron emission tomography and CT scans, MDC visit) are critical for care delivery, teamwork processes set the conditions for taskwork, as illustrated by the delays in treatment Mr S faced because of miscommunications between his providers. Therefore, our framework provides a comprehensive review of teamwork processes most critical for cancer care. The resulting set of processes illustrates how teamwork unfolds within and between clinical teams and defines how inputs (eg, clinical MTS structure) are converted to outputs (eg, clinical team performance and patient outcomes).
Teamwork processes and emergent states refer to the cognitive, behavioral, and affective mechanisms that encompass effective “teaming” across interdependent team members and teams. Team processes and states are inherently complex because of their multilevel nature of emergence (eg, individuals embedded in team, which is embedded in MTS). That is, team processes and states emerge by combining lower level attributes, such as individual team members’ predisposition to trust to form higher level variables (eg, team processes and states), such as team trust (65,66). MTSs add an additional layer of complexity to teamwork given that individuals are embedded within teams, which are embedded within a system, creating an intricate network of goal hierarchies and task interdependencies within and between teams (67). Given the multilevel nature of these phenomena, some constructs may be most critical within a given team, whereas others will be pivotal for optimal between-team or system-level functioning (68,69).
For example, exchange of information and closed loop communication are key team processes both within and between teams. Indeed, when performing the initial diagnostic CT scan for Mr S, members within the radiology team had to communicate effectively to ensure they performed all necessary tests and procedures (ie, intrateam information exchange). However, when the CT-guided biopsy was nondiagnostic, there was a failure of communication between the radiology team, pathology team, pulmonologist, and patient (ie, interteam) regarding follow-up care. Rather than preforming additional diagnostic tests to confirm Mr S’s suspected lung cancer, he was forced to wait 2 months for a staging positron emission tomography-CT, although diagnosis had not been made, leading to additional delays in care. Mr S’s experience highlights the need for a strategic approach to care delivery that establishes contingency plans for diagnosis, staging, and treatment.
Boundary spanning is a key between-team process that can mitigate such delays in care. This process encompasses actions of MTS members that connect different component teams across the system to facilitate subsequent processes, such as information sharing across teams to foster a shared understanding among cMTS members. Tumor boards and MDCs can serve as boundary-spanning structures that link MTS component teams. This is evidenced in Mr S’s case, where his delays in care and treatment improved after joining the lung MDC. However, such boundary-spanning structures and their activities need to encompass all phases of care and teams involved from lesion detection, through histologic diagnosis, staging, treatment planning and execution, to survivorship care and must engage all the relevant participants (Figure 1).
Although there may be differences in how team processes and states impact within-team and between-team functioning, the cMTS conceptual framework aims to outline elements critical both within and across teams (see Table 2). The cMTS is not intended to be all-inclusive of the within- and between-team effects for each construct but rather focuses on the integral role these teamwork processes play in the clinical process of care.
Outputs: Multilevel Outcomes of Cancer MTSs
The inputs and processes of cancer care MTSs compose multilevel phenomena and, subsequently, have multilevel results. The functioning of cancer care MTSs influences outcomes at the patient, provider, team, institution, and community levels. Although much existing research regarding team-based care touts numerous patient benefits from successful coordination, this is truly a multilevel issue that requires analysis across levels to understand the differential outcomes at the patient, team, and MTS levels. For example, although no outcomes were formally assessed in Mr S’s case, he likely experienced reduced satisfaction at the patient level, failed to adhere to clinical practice guidelines at the team level, and may have had little impact at the organizational level. However, if cases like his were recurrent across the MTS, the prevalence of poor care coordination would likely affect outcomes at the organizational level too.
Overall, enhanced teamwork processes can improve patient satisfaction, quality of life, and survival and enhance shared decision making; improve team outcomes such as effectiveness and learning and reduce provider burnout; and, at the organizational level, enhance institutional quality measures, service expansion and, potentially in a value-based reimbursement world, commercial viability. Currently, much of the research on health-care teams fail to consider these multilevel outcomes. Although prior studies have assessed outcomes like adherence to clinical practice guidelines (70) and clinician perceptions of communication, the majority evaluate outcomes only at 1 level (eg, patient outcomes) (71). Given the cyclical nature of cMTS performance, where outcomes of 1 performance episode determine the inputs for future events, understanding both the multilevel inputs and outputs of cMTSs is imperative for developing generalizable interventions.
Feedback Loops and Learning
The inputs, processes, emergent states, and outcomes discussed thus far present a snapshot of how teams may interact and perform at 1 point in time. However, in addition to patient care being a multilevel phenomenon, it also follows a cyclical pattern, where the results and outcomes of 1 performance episode become inputs for subsequent cMTS functioning over time (40). Team processes and states do not occur in a vacuum; the actions and outcomes of cMTS performance at 1 point in time will influence the inputs, processes, and states that define how the team will work together in the future. For example, following the conclusion of a performance episode (eg, after the MDC learned Mr S was referred to hospice despite his cancer being treatable), the cMTSs should receive feedback that spans across all component teams to improve their teamwork processes and enhance future outcomes (21). This feedback can be on both an individual and team level and administered in both formal and informal capacities. Teams may also alter their future actions by engaging in team self-correction, where team members modify their attitudes, behaviors, and cognitions without outside intervention (72). For example, care teams may engage in debriefing after particularly challenging cases or after facing coordination problems as a form of self-correction. In Mr S’s case study, such self-corrective activities would be beneficial to mitigate future delays in care.
Moderators: Context
Finally, contextual factors depicted in the box along the top of Figure 2 serve as conditions that may influence the interrelationships among inputs, processes or states, and outcomes. Specifically, characteristics of patients, care delivery organizations, the external community or region, and policies or payment models may all affect the direction or strength of the relationships among the team inputs, processes, and outcomes previously discussed. For example, although the delays in care Mr S experienced probably had a negative impact on his quality of life and overall satisfaction, this was likely further exasperated when he had to travel 3 hours to receive treatment. Similar interactions can be seen at the organizational level, where providers working across different organizations, especially in the volume-based, fee-for-service reimbursement model, face conflicting organizational factors and hard boundaries that impede teamwork. In contrast, providers jointly participating in an Accountable Care Organization, integrated health system, or other value-based payment model may face relatively fewer barriers to sharing information or accessing records, which in turn may strengthen their ability to work as an effective team and deliver high quality care. Understanding how different contextual variables influence clinical team structure, functioning, and their joint effects on patient outcomes is critical for understanding which clinical team structures work best for particular patient populations and the conditions under which certain structures or team processes produce optimal outcomes.
Discussion
Taken together, the cMTS framework defines the anatomy (ie, inputs such as MTS structure), physiology (ie, the teaming processes and states), and outcomes relevant for understanding and intervening to improve teaming in cancer care delivery. Although prior frameworks describe organizational factors and processes that may influence care delivery, our framework applies multilevel theory and evaluates cancer care delivery through an MTS lens. Applying this framework, future research can disentangle not only what intra- and interteam processes are most critical for cancer care delivery but also when certain teaming aspects are imperative for MTS functioning. Given that cancer care delivery spans multiple phases of care and care teams, the inter- and intrateam processes required by the cMTS to facilitate care coordination will vary at different points in time. For example, team processes such as collective sense-making and group decision making are critical for teams involved in MDCs or tumor boards, but these processes are less critical once the patient is referred to the primary care provider for survivorship care. At that point, boundary-spanning processes and closed-loop communication would be critical to ensure that care plans are received and appropriately interpreted. This example illustrates the dynamic nature of care coordination and highlights the need for better understanding how inputs of the MTS structure, team processes and emergent states, and contextual factors of the organization, providers, and patient interact to affect care coordination.
We hope the cMTS framework proves a valuable tool for identifying key variables that affect care coordination, which can inform testable hypotheses. By understanding how different care teams form an MTS, the organizational and structural features that influence their functioning, and the teaming processes that emerge within and between teams to influence the clinical processes of care, we can advance the science and practice of cancer care delivery.
Funding
The authors received no specific funding for this work.
Notes
Role of funder: Not applicable.
Disclosures: Dr Osarogiagbon discloses: Grant funding from the National Cancer institute (NCI UG1CA189873), stock ownership (Eli Lilly, Gilead Sciences and Pfizer); consulting or advisory roles with the American Cancer Society, the Association of Community Cancer Centers, AstraZeneca and Triptych Healthcare Partners; patents for a Lung Cancer Specimen Kit; founder, Oncobox Device Inc. The other authors report no conflicts of interest.
Prior presentations: Verhoeven, D. C., Chollette, V., Lazzara, E. H., Shuffler, M. L., Osarogiagbon, R. U., Weaver, S. J. (2020). The Anatomy and Physiology of Teaming in Cancer Care Delivery: A Conceptual Framework. Organizational Theory in Health Care Conference. Virtual.
Acknowledgments: This commentary was prepared as part of the official duties of some of the authors as employees of the US Federal Government.
Disclaimer: The views expressed are those of the authors and do not necessarily represent the official position of the National Cancer Institute, the National Institute of Health, or Department of Health and Human Services.
Author contributions: All authors contributed to the development of the conceptual model and contributed to drafting of the manuscript. RO developed the case study and provided clinical lung cancer expertise. All authors reviewed the final framework and contributed to the final manuscript.
Data Availability
No new data were generated or analyzed in support of this research.
Supplementary Material
References
- 1.Institute of Medicine (IOM) Committee on improving the quality of cancer. In: Levit LA, Balogh EP, Nass SJ, Ganz PA, eds. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington (DC: ): The National Academies Press; 2013;19-90.doi: 10.17226/18359. [DOI] [PubMed] [Google Scholar]
- 2.The Joint Commission. Sentinel Event Data: Root Causes by Event Type. Oakbrook Terrace, IL: Elsevier; 2015. [Google Scholar]
- 3. Walsh KE, Dodd KS, Seetharaman K, et al. Medication errors among adults and children with cancer in the outpatient setting. J Clin Oncol. 2009;27(6):891-896. [DOI] [PubMed] [Google Scholar]
- 4.National Academies of Sciences Engineering and Medicine. Developing and Sustaining an Effective and Resilient Oncology Careforce: Proceedings of a Workshop. Washington, DC: The National Academies Press;2019. doi: 10.17226/25533. [DOI] [PubMed]
- 5.World Health Organization. Cancer Fact Sheet. 2018. https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed February 21, 2020.
- 6.National Cancer Institute. Surveillance, epidemiology, and end results program cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed March 3, 2020.
- 7. Ward EM, Sherman RL, Henley SJ, et al. Annual report to the nation on the status of cancer, featuring cancer in men and women age 20-49 years. JNCI J Natl Cancer Inst. 2019;111(12):1279-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weaver SJ. From teams of experts to mindful expert teams and multiteam systems. J Oncol Pract. 2016;12(11): doi: 10.1200/JOP.2016.018184. [DOI] [PubMed] [Google Scholar]
- 9. Taplin SH, Anhang Price R, Edwards HM, et al. Introduction: understanding and influencing multilevel factors across the cancer care continuum. J Natl Cancer Inst Monogr. 2012;2012(44):2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knight SB, Crosbie PA, Balata H, Chudziak J, Hussell T, Dive C.. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7(9):170070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Torre LA, Siegel RL, Jemal A.. Lung cancer statistics. In: Ahmad A, Gadgeel S, eds. Lung Cancer and Personalized Medicine. Advances in Experimental Medicine and Biology. Vol 893. Cham, Switzerland: Springer; 2016:1-19. [DOI] [PubMed] [Google Scholar]
- 12. Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD.. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg. 2008;135(2):247-254. [DOI] [PubMed] [Google Scholar]
- 13. Little AG, Gay EG, Gaspar LE, Stewart AK.. National survey of non-small cell lung cancer in the United States: epidemiology, pathology and patterns of care. Lung Cancer. 2007;57(3):253-260. [DOI] [PubMed] [Google Scholar]
- 14. Sineshaw HM, Wu XC, Flanders WD, Osarogiagbon RU, Jemal A.. Variations in receipt of curative-intent surgery for early-stage non-small cell lung cancer (NSCLC) by state. J Thorac Oncol. 2016;11(6): doi: 10.1016/j.jtho.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 15. Osarogiagbon RU. Achieving better quality of lung cancer care. In: Tanoue L, Detterbeck F, eds. Lung Cancer: A Practical Approach to Evidence-Based Clinical Evaluation and Management. St. Louis, MO: Elsevier; 2018:167-182. [Google Scholar]
- 16. Frasier LL, Pavuluri Quamme SR, Becker AM, et al. Health care providers’ perceptions of team identity and teamwork in the operating room. J Am Coll Surg. 2015;221(4):S126. [Google Scholar]
- 17. Doekhie KD, Buljac-Samardzic M, Strating MMH, Paauwe J.. Who is on the primary care team? Professionals’ perceptions of the conceptualization of teams and the underlying factors: a mixed-methods study. BMC Fam Pract. 2017;18(1):111. doi: 10.1186/s12875-017-0685-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weaver SJ, Che XX, Petersen LA, Hysong SJ.. Unpacking care coordination through a multiteam system lens. Med Care. 2018;56(3):247-259. [DOI] [PubMed] [Google Scholar]
- 19. Weaver SJ, Jacobsen PB.. Cancer care coordination: opportunities for healthcare delivery research. Transl Behav Med. 2018;8(3):503-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taplin SH, Weaver S, Chollette V, et al. Teams and teamwork during a cancer diagnosis: interdependency within and between teams. J Oncol Pract. 2015;11(3): 231-238. doi: 10.1200/JOP.2014.003376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mathieu JE, Marks MA, Zaccaro SJ.. Multiteam systems. In: Anderson N., Ones D.S., Sinangil H.K., Viswesvarin C., eds. Handbook of Industrial, Work and Organizational Psychology. 2nd ed. Thousand Oaks, CA; SAGE Publishing; 2001:289-313. http://maorhan.com/wp-content/uploads/2014/11/Handbook_of_Industrial_Work_and_Organizational_Psychology_Vol_2_2005.pdf#page=318. Accessed September 4, 2018. [Google Scholar]
- 22. DeChurch LA, Burke CS, Shuffler ML, Lyons R, Doty D, Salas E.. A historiometric analysis of leadership in mission critical multiteam environments. Leadersh Q. 2011;22(1):152-169. [Google Scholar]
- 23. Pendergraft JG, Carter DR, Tseng S, Landon LB, Slack KJ, Shuffler ML.. Learning from the past to advance the future: the adaptation and resilience of NASA’s Spaceflight Multiteam Systems across four eras of spaceflight. Front Psychol. 2019;10(JULY):1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DiazGranados D, Dow AW, Perry SJ, Palesis JA.. Understanding patient care as a multiteam system. Res Manag Groups Teams. 2014;16:95-113. [Google Scholar]
- 25. Noyes K, Monson JRT, Rizvi I, Savastano A, Green JSA, Sevdalis N.. Regional multiteam systems in cancer care delivery. J Oncol Pract. 2016;12(11):1059-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gerber DE, Reimer T, Williams EL, et al. Resolving rivalries and realigning goals: challenges of clinical and research multiteam systems. J Oncol Pract. 2016;12(11):1020-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clarke CA, Glaser SL, Leung R, Davidson-Allen K, Gomez SL, Keegan THM.. Prevalence and characteristics of cancer patients receiving care from single vs. multiple institutions. Cancer Epidemiol. 2017;46:27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ASCO. Team-based care in oncology. https://www.asco.org/practice-policy/cancer-care-initiatives/team-based-care-oncology. Accessed October 1, 2020.
- 29.Special Series: NCI-ASCO Teams. J Oncol Pract. 2016;12(11). https://ascopubs.org/toc/jop/12/11. Accessed October 1, 2020.
- 30. Schmutz JB, Meier LL, Manser T.. How effective is teamwork really? The relationship between teamwork and performance in healthcare teams: a systematic review and meta-analysis. BMJ Open. 2019;9(9):e028280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salas E, Rosen MA, Burke CS, Goodwin GF, The wisdom of collectives in organizations: an update of the teamwork competencies. In: Salas E, Goodwin GF, Burke CS, eds. Team Effectiveness in Complex Organizations: Cross Disciplinary Perspectives and Approaches. New York, NY: Routledge; 2008:39-79. [Google Scholar]
- 32. Shuffler ML, Rico R, Salas E. Pushing the boundaries of multiteam systems in research and practice: an introduction. In: Shuffler ML, Rico R, Salas E, eds. Pushing the Boundaries: Multiteam Systems in Research and Practice (Research on Managing groups and teams, Vol. 16). Bingley, United Kingdom: Emerald Group Publishing Limited; 2014:3-16. doi: 10.1108/S1534-085620140000016001
- 33. Shuffler ML, Jiménez-Rodríguez M, Kramer WS.. The science of multiteam systems. Small Gr Res. 2015;46(6):659-699. [Google Scholar]
- 34. Taplin SH, Weaver S, Salas E, et al. Reviewing cancer care team effectiveness. J Oncol Pract. 2015;11(3):239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buljac-Samardzic M, Doekhie KD, Van Wijngaarden JDH.. Interventions to improve team effectiveness within health care: a systematic review of the past decade. Hum Resour Health. 2020;18(1):2. doi: 10.1186/s12960-019-0411-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Driskell JE, Salas E, Driskell T.. Foundations of teamwork and collaboration. Am Psychol. 2018;73(4):334-348. [DOI] [PubMed] [Google Scholar]
- 37. Mathieu JE, Wolfson MA, Park S.. The evolution of work team research since Hawthorne. Am Psychol. 2018;73(4):308-321. [DOI] [PubMed] [Google Scholar]
- 38. Hackman JR, The design of work teams. In: Lorsch JW, ed. Handbook of Organizational Behavior. Englewood Cliffs, NJ: Prentice-Hall; 1987:315-342. [Google Scholar]
- 39. Ilgen DR, Hollenbeck JR, Johnson M, Jundt D.. Teams in organizations: from input-process-output models to IMOI models. Annu Rev Psychol. 2005;56(1):517-560. [DOI] [PubMed] [Google Scholar]
- 40. Marks MA, Mathieu JE, Zaccaro SJ.. A temporally based framework and taxonomy of team processes. AMR. 2001;26(3):356-376. [Google Scholar]
- 41. Salas E, Sims DE, Burke CS.. Is there a “Big Five” in teamwork? Small Gr Res. 2005;36(5):555-599. [Google Scholar]
- 42. Zaccaro SJ, Marks MA, DeChurch LA. . Multiteam Systems: An Introduction. In: Zaccaro SJ, Marks MA, DeChurch L, eds. Multiteam Systems: An Organization Form for Dynamic and Complex Environments. Newyork, NY: Taylor and Francis Group, LLC; 2012:3-32. doi: 10.4324/9780203814772. [Google Scholar]
- 43. Shuffler ML, Jimenez-Rodriguez M, Kramer WS.. The science of multiteam systems: a review and future research agenda. Small Gr Res. 2015;46(6). doi: 10.1177/1046496415603455. [Google Scholar]
- 44. Reader TW, Flin R, Mearns K, Cuthbertson BH.. Developing a team performance framework for the intensive care unit. Crit Care Med. 2009;37(5):1787-1793. [DOI] [PubMed] [Google Scholar]
- 45. Flin R, Patey R, Glavin R, Maran N.. Anaesthetists’ non-technical skills. Br J Anaesthesia. 2010;105(1):38-44. [DOI] [PubMed] [Google Scholar]
- 46. Lemieux-Charles L, McGuire WL.. What do we know about health care team effectiveness? A review of the literature. Med Care Res Rev. 2006;63(3):263-300. [DOI] [PubMed] [Google Scholar]
- 47. Marks MA, Mathieu JE, Zaccaro SJ.. A temporally based framework and taxonomy of team processes. Acad Manag Rev. 2001;26(3). doi: 10.5465/AMR.2001.4845785. [Google Scholar]
- 48. Prabhu Das I, Baker M, Altice C, Castro KM, Brandys B, Mitchell SA.. Outcomes of multidisciplinary treatment planning in US cancer care settings. Cancer. 2018;124(18):3656-3667. [DOI] [PubMed] [Google Scholar]
- 49. Rico R, Hinsz VB, Davison RB, Salas E.. Structural influences upon coordination and performance in multiteam systems. Hum Resour Manag Rev. 2018;28(4):332-346. [Google Scholar]
- 50. Schroder C, Medves J, Paterson M, et al. Development and pilot testing of the collaborative practice assessment tool. J Interprof Care. 2011;25(3):189-195. [DOI] [PubMed] [Google Scholar]
- 51. Burke CS, Stagl KC, Salas E, Pierce L, Kendall D.. Understanding team adaptation: a conceptual analysis and model. J Appl Psychol. 2006;91(6):1189-1207. [DOI] [PubMed] [Google Scholar]
- 52. Marrone JA. Team boundary spanning: a multilevel review of past research and proposals for the future. J Manage. 2010;36(4):911-940. [Google Scholar]
- 53. Weick KE, Sutcliffe KM, Obstfeld D.. Organizing and the process of sensemaking. Organ Sci. 2005;16(4):409-421. [Google Scholar]
- 54. Burke CS, Fiore SM, Salas E.. The role of shared cognition in enabling shared leadership and team adaptability. In: Pearce CLConger JA, eds. Shared Leadership: Reframing the Hows and Whys of Leadership. Thousand Oaks, CA: S AGE Publications Inc.; 2003;103-122; doi: 10.4135/9781452229539.n5. [Google Scholar]
- 55. Edmondson A. Psychological safety and learning behavior in work teams. Adm Sci Q. 1999;44(2):350-383. [Google Scholar]
- 56. Mayer RC, Davis JH, Schoorman FD.. An integrative model of organizational trust. AMR. 1995;20(3):709-734. [Google Scholar]
- 57. Mathieu JE, Kukenberger MR, D'Innocenzo L, Reilly G.. Modeling reciprocal team cohesion-performance relationships, as impacted by shared leadership and members’ competence. J Appl Psychol. 2015;100(3):713-734. [DOI] [PubMed] [Google Scholar]
- 58. Bedwell WL, Ramsay PS, Salas E.. Helping fluid teams work: a research agenda for effective team adaptation in healthcare. Transl Behav Med. 2012;2(4). doi: 10.1007/s13142-012-0177-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mohammed S, Klimoski R, Rentsch JR.. The measurement of team mental models: we have no shared schema. Organ Res Methods. 2000;3(2):123-165. [Google Scholar]
- 60. Ren Y, Argote L.. Transactive memory systems 1985-2010: an integrative framework of key dimensions, antecedents, and consequences. Annals. 2011;5(1):189-229. [Google Scholar]
- 61. Ong WL, Schouwenburg MG, van Bommel ACM, et al. A standard set of value-based patient-centered outcomes for breast cancer: The International Consortium for Health Outcomes Measurement (ICHOM) initiative. JAMA Oncol. 2017;3(5):677. [DOI] [PubMed] [Google Scholar]
- 62.Agency for Health Research and Quality. Types of health care quality measures. https://www.ahrq.gov/talkingquality/measures/types.html. Published July 2015. Accessed September 13, 2020.
- 63.Agency for Health Research and Quality. Six domains of health care quality. https://www.ahrq.gov/talkingquality/measures/six-domains.html. Published November 2018. Accessed September 13, 2020.
- 64. Dymek C, Johnson M Jr, McGinnis P, Buckley D, Fagnan L, Mardon R, Hassell SC.. Clinical-Community Relationships Measures Atlas. (Prepared under Contract No. HHSA 290-2010-00021. Westat prime contractor) AHRQ Publication No. 13-0041-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2013. https://www.ahrq.gov/sites/default/files/publications/files/ccrmatlas.pdf. Accessed September 13, 2020.
- 65. Kozlowski S, Klein K.. A multilevel approach to theory and research in organizations: contextual, temporal, and emergent processes. In: Klein K, Kozlowski S, eds. Multilevel Theory, Research, and Methods in Organizations: Foundations, Extensions, and New Directions. San Francisco, CA: Jossey-Bass; 2000:3-90. [Google Scholar]
- 66. Bell ST, Brown SG, Colaneri A, Outland N.. Team composition and the ABCs of teamwork. Am Psychol. 2018;73(4). doi: 10.1037/amp0000305. [DOI] [PubMed] [Google Scholar]
- 67. Luciano MM, DeChurch LA, Mathieu JE.. Multiteam systems: a structural framework and meso-theory of system functioning. J Manage. 2018;44(3):1065-1096. [Google Scholar]
- 68. Shuffler ML, Kramer WS, Carter DR, Thayer AL, Rosen MA.. Leveraging a team-centric approach to diagnosing multiteam system functioning: the role of intrateam state profiles. Hum Resour Manag Rev. 2018;28(4):361-377. [Google Scholar]
- 69. Mathieu JE, Luciano MM, Dechurch LA.. Multiteam systems: the next chapter. In: Ones DS, Anderson N, Viswesvaran C, Sinangil HK, eds. Handbook of Industrial, Work, and Organizational Psychology. Thousand Oaks, CA: SAGE Publications Ltd; 2014. doi: 10.4135/9781473914957.n16. [Google Scholar]
- 70. Tomcavage J, Littlewood D, Salek D, Sciandra J.. Advancing the role of nursing in the medical home model. Nurs Adm Q. 2012;36(3). doi: 10.1097/NAQ.0b013e3182588b6a [DOI] [PubMed] [Google Scholar]
- 71. Gorin SS, Haggstrom D, Han PKJ, Fairfield KM, Krebs P, Clauser SB.. Cancer care coordination: a systematic review and meta-analysis of over 30 years of empirical studies. Ann Behav Med. 2017;51(4):532-546. [DOI] [PubMed] [Google Scholar]
- 72. Blickensderfer E, Cannon-Bowers JA, Salas E.. Theoretical bases for team self-correction: fostering shared mental models. In: Advances in Interdisciplinary Studies of Work Teams. Greenwich, CT: JAI; 1997;249-279. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analyzed in support of this research.