Abstract
Background
The HIV Prevention Trials Network (HPTN) 083 trial demonstrated that long-acting cabotegravir (CAB-LA) was more effective than tenofovir disoproxil fumarate–emtricitabine (TDF/FTC) in preventing human immunodeficiency virus (HIV) in cisgender men and transgender women who have sex with men. We characterized HIV infections that occurred in the blinded phase of HPTN 083.
Methods
Retrospective testing included HIV testing, viral load testing, quantification of study drugs, and HIV drug resistance testing.
Results
Fifty-eight infections were evaluated, including 51 incident infections (12 in CAB arm and 39 in TDF/FTC arm). In many cases (5 in CAB arm and 37 in TDF/FTC arm), infection was associated with low or unquantifiable study drug concentrations. In 4 cases, infection occurred with on-time CAB-LA injections and expected plasma CAB concentrations. CAB exposure was associated with prolonged viral suppression and delayed antibody expression. In some cases, delayed HIV diagnosis resulted in CAB provision to participants with undetected infection, delayed antiretroviral therapy, and emergence of drug resistance; most of these infections would have been detected earlier with viral load testing.
Conclusions
Early detection of HIV infection and prompt antiretroviral therapy initiation could improve clinical outcomes in persons who become infected despite CAB-LA prophylaxis. Further studies are needed to elucidate the correlates of HIV protection in persons receiving CAB-LA.
Keywords: HIV, preexposure prophylaxis, prevention, cabotegravir, injectable, TDF/FTC, long-acting, men who have sex with men, HPTN 083
Injectable cabotegravir is highly effective for preventing human immunodeficiency virus infection. Infections can occur despite on-time injections and expected drug concentrations. Integrase strand transfer inhibitor resistance is observed in some cases. Viral load testing may help detect incident infections.
Both men and transgender women who have sex with men are at increased risk of human immunodeficiency virus (HIV) infection. Daily oral tenofovir (TFV) disoproxil fumarate–emtricitabine (TDF/FTC) is approved by the US Food and Drug Administration and other agencies for prevention of HIV sexual transmission. Efficacy of daily TDF/FTC preexposure prophylaxis (PrEP) is highly dependent on adherence [1, 2]. Alternative PrEP regimens using long-acting drugs may promote adherence and would increase options for HIV prevention [3].
Long-acting injectable cabotegravir (CAB) (CAB-LA) is an investigational HIV integrase strand transfer inhibitor (INSTI) that is under evaluation for HIV treatment and prevention [1, 4–6]. The HIV Prevention Trials Network (HPTN) 077 trial evaluated the use of CAB-LA in uninfected persons. A regimen that included an oral phase followed by CAB-LA injections achieved pharmacokinetic drug targets in men and women [7]. CAB was quantifiable in plasma 76 weeks after the final injection in 13% of men and 42% of women [8].
HPTN 083 is an ongoing phase 2b/3 trial comparing CAB-LA with TDF/FTC for HIV PrEP in cisgender men who have sex with men and transgender women who have sex with men; 4566 eligible participants who tested negative for HIV infection were enrolled at 43 sites in the United States, Latin America, Asia, and Africa. The trial included a double-blind, double-dummy comparison of the 2 regimens (active CAB plus TDF/FTC placebo vs active TDF/FTC plus CAB placebo; 1:1 randomization). The study protocol included a 5-week oral lead-in phase, 148 weeks of injections, and a 48-week tail phase in which all participants were offered open-label TDF/FTC. The trial was unblinded after a Data Safety Monitoring Board meeting in May 2020. The study demonstrated a 66% reduction in HIV incidence in the CAB arm compared with the TDF/FTC arm (0.41 vs 1.22 per 100 person-years; hazard ratio, 0.34 [95% confidence interval, .18–.62]; P < .001) [9]. In an adherence cohort, pharmacokinetic analysis indicated that 74.2% of participants in the TDF/FTC arm were adherent to the oral drug regimen [9].
Resistance to TFV and FTC has been observed in persons who acquired HIV infection while receiving TDF/FTC PrEP [10–13]. Resistance to CAB and other INSTIs has been observed in HIV treatment trials [14]. TDF/FTC can also suppress viral replication and delay antibody (Ab) production in persons with incident HIV infection who continue to use PrEP because they are not aware of their infection status [12]. These effects could be more pronounced using potent, long-acting PrEP agents. This report includes detailed analysis of HIV infections observed in the blinded phase of HPTN 083, including analysis of drug concentrations obtained with oral CAB and CAB-LA injections, the impact of CAB-LA on detection of HIV infection, and emergence of HIV drug resistance.
METHODS
Samples Used for Analysis
Samples were obtained from participants in HPTN 083 (NCT0272009). Plasma was stored at every study visit (Figure 1). Dried blood spot samples were stored at week 4 and at injection visits starting at week 9. Data are presented for samples collected in the blinded phase of the trial.
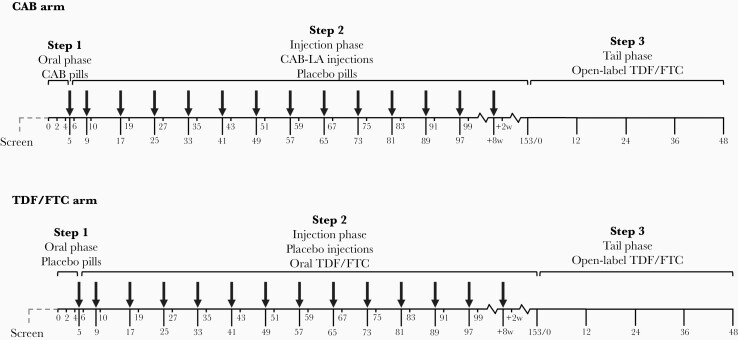
Figure 1.
HIV Prevention Trials Network (HPTN) 083 study design. Figure shows an overview of the HPTN 083 trial design. The study included a 5-week oral lead-in phase (step 1), a 148-week injection phase (step 2), and a 48-week open-label tail phase (step 3). Participants randomized to the tenofovir disoproxil fumarate–emtricitabine (TDF/FTC) arm received daily coformulated tablets with 300 mg of TDF and 200 mg of FTC in steps 1 and 2, with placebo tablets in step 1 and placebo injections in step 2. Participants randomized to the cabotegravir (CAB) arm received oral daily CAB (30-mg tablets) in step 1, followed by long-acting CAB (CAB-LA) injections (600 mg) in step 2, with placebo tablets in both phases. Participants in both arms received open-label daily TDF/FTC in step 3. The first 2 injections were given 4 weeks apart starting at week 5, followed by injections 8 weeks apart starting at week 9 (arrows). A nonreactive rapid test was required before each injection. Study drugs were stopped if the participant had a reactive or positive human immunodeficiency virus test at the study site, if the participant declined drug administration, if this was deemed necessary owing to an adverse event, or for another reason at the discretion of the site principal investigator.
HIV Testing at Study Sites
HIV testing was performed at study sites using locally available tests (Supplementary File 1). All participants had a negative HIV RNA test within 14 days before enrollment. The testing algorithm included a rapid HIV test and a laboratory-based antigen-Ab (Ag/Ab) test. Test results were reviewed by a centralized committee if a reactive or positive result was obtained. HIV DNA testing was performed at the direction of this committee in cases with indeterminate test results. The first site-positive visit was defined as the first visit near the time of HIV diagnosis at when a reactive or positive HIV test was obtained at a study site.
Retrospective Testing
Retrospective testing was performed at the HPTN Laboratory Center and other laboratories in the United States, as described below. Information about the assays and algorithms used for testing is provided in Supplementary Files 1–4.
Identification of HIV Infections
HIV testing was performed in all cases in which a reactive or positive result was obtained at a study site. The testing algorithm included the Architect HIV Ag/Ab Combo Test (Ag/Ab test); the Geenius HIV 1/2 Supplemental Assay (confirmatory Ab test); and the Aptima HIV-1 RNA Qualitative Assay (qualitative RNA test). Additional testing was performed to determine the timing of HIV infection events. HIV test results from study sites and the HPTN Laboratory Center were reviewed by an independent Endpoint Adjudication Committee, which made a final determination of HIV status and identified the first HIV-positive visit. Additional information is provided in Supplementary Files 1 and 2.
Viral Load Testing
Viral load testing was performed using the RealTime HIV-1 Viral Load Assay (limit of quantification, 40 copies/mL). Selected samples were also tested at the University of Pittsburgh using a single-copy HIV RNA assay [15]. Additional information is provided in Supplementary File 2.
Antiretroviral Drug Testing
TFV and CAB measurements in plasma and TFV diphosphate (TFV-DP) measurements in dried blood spots were performed using liquid chromatography-tandem mass spectrometry. The limits of quantification for these assays are as follows: CAB, 0.025 µg/mL; TFV, 0.31 ng/mL; TFV-DP, 31.3 fmol per punch. Additional information is provided in Supplementary Files 2 and 3.
HIV Drug Resistance Testing
HIV resistance testing was performed using the GenoSure PRIme assay, which provides resistance data for nucleoside/nucleotide reverse-transcriptase inhibitors (NRTIs), nonnucleoside reverse-transcriptase inhibitors (NNRTIs), protease inhibitors, and INSTIs. The 2019 drug resistance mutations update from the International Antiviral Society–USA was used to identify resistance-associated mutations [16]. Phenotyping was performed for CAB arm participants using the PhenoSense INSTI assay, which provides data for all licensed INSTIs. CAB was included in the assay. Additional information is provided in Supplementary File 4.
Ethical Considerations
The HPTN 083 protocol was approved by the institutional review boards and/or ethics committees and ministries of health for all participating sites. All participants provided written informed consent.
RESULTS
Identification of HIV Infections
Fifty-eight HIV infections were identified in the blinded phase of HPTN 083 (Table 1). These included 7 infections in participants who were HIV positive at enrollment (4 in the CAB arm and 3 in the TDF/FTC arm; baseline cases) and 51 who acquired HIV infection after enrollment (12 in the CAB arm and 39 in the TDF/FTC arm; incident cases). The 58 cases were divided into groups A–E based on factors related to exposure to study drug (Table 1). Laboratory results and key events for these cases are shown in Figures 2 and 3 and Supplementary Files 5 and 6.
Table 1.
Classification of Human Immunodeficiency Virus Infectionsa
| Infection Type and Study Arm | Infections, No. | Classification Group | Breakthrough Infections, No.b | Case Summaries |
|---|---|---|---|---|
| Baseline | ||||
| CAB arm | 4 (A1–A4) | HIV positive at study enrollment | … | Supplementary File 5 |
| TDF/FTC arm | 3 (E40–42) | HIV positive at study enrollment | … | Supplementary File 6 |
| Incident | ||||
| CAB arm | 5 (B1–B5) | No recent CAB exposurec | 0 | Supplementary File 5 |
| 3 (C1–C3) | Infected during the CAB oral lead-in period | 0 | Supplementary File 5 | |
| 4 (D1–D4) | Infected in the setting of on-time CAB-LA injections | 4d | Figure 2 | |
| TDF/FTC arm | 39 (E1–E39) | Infected after enrollment | 2e | Supplementary File 6 and Figure 3f |
Abbreviations: CAB, cabotegravir; CAB-LA, long-acting injectable cabotegravir; HIV, human immunodeficiency virus; TDF/FTC, tenofovir disoproxil fumarate–emtricitabine.
aTable 1 shows the system used to classify cases in the CAB arm (groups A–D) and the TDF/FTC arm (group E). Groups were assigned based on study arm and timing of HIV infection/exposure to study drug. Each case was assigned a group letter and a case number (eg, case A1).
bIn the TDF/FTC arm, breakthrough infections were defined as having plasma and dried blood spot drug concentrations consistent with good adherence to daily oral dosing at the time of the first HIV-positive visit and at key earlier visits. In the CAB arm, breakthrough infections were defined as having target drug concentration (>8 times the 90% inhibitory concentration) at the first HIV-positive visit.
cGroup B included participants who had no CAB injections or had their last injection ≥6 months before their first HIV-positive visit.
dThese 4 participants had expected drug concentrations at the first HIV-positive visit (D1–D4).
eThese 2 participants had drug concentrations consistent with good adherence to oral TDF/FTC (cases E16 and E34); exposure to HIV resistant to TDF/FTC may have contributed to infection in 1 case (E16).
fCase E16 only.
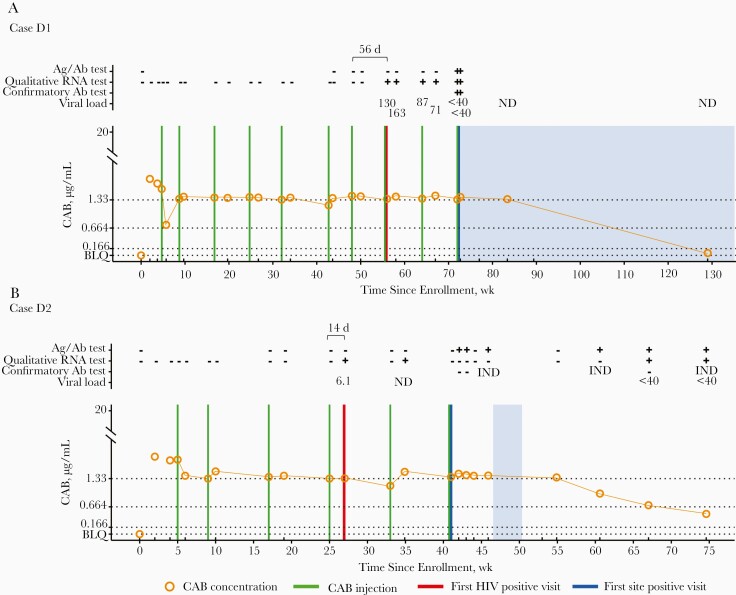
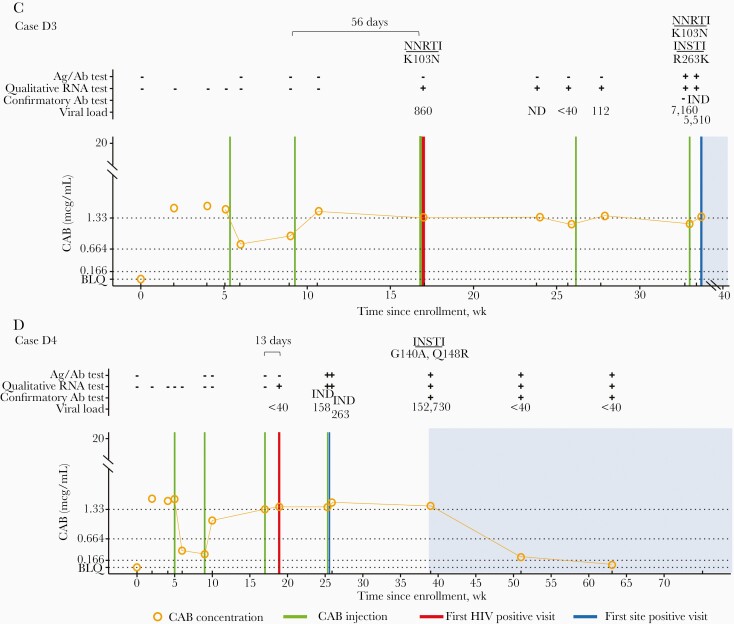
Figure 2.
Case summaries (cabotegravir [CAB] arm; group D). The figure shows a summary of laboratory results and key events for participants in the CAB study arm for cases in which infection occurred despite on-time injections. A, Case D1. B, Case D2. C, Case D3. D, Case D4. Additional information, including results from testing performed at study sites, is provided in Supplementary File 5. Data are shown as a function of time (weeks since enrollment, based on calendar dates). Annotations above graphs show results obtained from testing performed at the HIV Prevention Trials Network (HPTN) Laboratory Center. Plus signs indicate reactive or positive test results; minus signs, nonreactive or negative test results. Viral load values represent the number of human immunodeficiency virus (HIV) RNA copies per milliliter; 1 viral load result for case D2 was obtained using a single-copy RNA assay. Viral load results noted as <40 indicate that HIV RNA was detected at <40 copies/mL. Results from HIV genotyping are shown. All drug resistance mutations are shown for integrase strand transfer inhibitors (INSTIs); major drug resistance mutations are shown for nonnucleoside reverse-transcriptase inhibitors (NNRTIs). Brackets show numbers of days between the last injection and the first HIV-positive visit, graphs show plasma CAB concentrations and key events, and horizontal lines mark the following concentration cutoffs: 1.33 µg/mL (8 times the in vitro protein-adjusted 90% CAB inhibitory concentration [8× PA-IC90], 0.664 µg/mL (4× PA-IC90), and 0.166 µg/mL (1× PA-IC90). Shaded areas indicate that the participant was receiving antiretroviral therapy (cases D1, D3, and D4) or postexposure prophylaxis (case D2). Abbreviations: Ag/Ab: antigen-antibody test; BLQ, below the limit of quantification (<0.025 µg/mL); IND, indeterminate test result; ND, not detected.
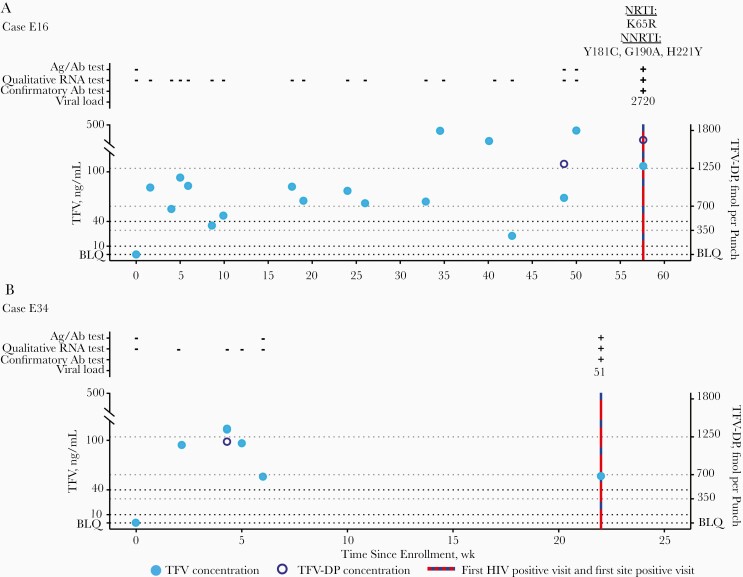
Figure 3.
Case summary (tenofovir [TFV] disoproxil fumarate–emtricitabine [TDF/FTC] arm, cases E16 and E34). Figure shows a summary of laboratory results and key events for 2 cases in the TDF/FTC study arm. A, Case E16. B, Case E34. Data are shown as a function of time (weeks since enrollment, based on calendar dates). Annotations above each graph show results obtained from testing performed at the HIV Prevention Trials Network (HPTN) Laboratory Center. Plus signs indicate reactive or positive test results; minus signs, nonreactive or negative test results. Viral load values indicate numbers of human immunodeficiency virus (HIV) RNA copies per milliliter. Major drug resistance mutations are shown. Graphs show TFV and TFV diphosphate (TFV-DP) concentrations and key events. Black dashed horizontal lines indicate plasma TFV concentrations of 10 and 40 ng/mL, which correspond to 4 and 7 doses per week, respectively; gray dashed lines, TFV-DP concentrations of 350, 700, and 1250 fmol per punch, which correspond to 2, 4, and 7 doses per week, respectively. For case E16 (A), all drug concentrations after the week 9 study visit were consistent with daily oral TDF/FTC dosing, except for the result obtained at week 43; At that visit, the TFV concentration was 22.5 ng/mL, which is consistent with 4 doses per week. For case E34 (B), results from dried blood spot testing were not available at the first HIV-positive visit; this participant had plasma TFV and TFV-DP concentrations consistent with daily TDF/FTC use in the preceding months. Abbreviations: Ag/Ab: antigen-antibody; BLQ, below the limit of quantification (0.31 ng/mL for TFV; 31.3 fmol per punch for TFV-DP); IND, indeterminate test result; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse-transcriptase inhibitor.
Exposure to Study Drugs Before HIV Infection
Plasma CAB concentrations were determined for participants in the CAB arm (Figure 2, Supplementary Files 3 and 5). The in vitro protein-adjusted 90% CAB inhibitory concentration (PA-IC90) is 0.166 µg/mL [17, 18]. In this study, CAB concentrations were stratified into 4 groups: <1× PA-IC90, 1-4× PA-IC90 (0.166–0.664 µg/mL), 4-8× PA-IC90 (0.664–1.33 µg/mL), and ≥8× PA-IC90 (≥1.33 µg/mL) (Supplementary File 3). In 12 of 16 cases in the CAB arm, participants received ≥1 injection. Ten of them had a decline in CAB concentrations in the weeks after the first injection; however, none fell below 1× PA-IC90.
Four baseline infections (group A) and 12 incident infections (groups B–D) were identified in the CAB arm. Five of 12 participants with incident infection had no CAB injections (B2 and B5) or had the last CAB injection >6 months before the first HIV-positive visit (B1, B3, and B4). At the first HIV-positive visit, 2 of these participants had CAB concentrations <1× PA-IC90 (B1 and B3) and 3 did not have quantifiable CAB (B2, B4, and B5). Three of the 12 participants acquired HIV during the oral lead-in phase (group C). Two of these participants had CAB concentrations ≥8× PA-IC90 at the first HIV-positive visit (C1 and C3), and 1 did not have quantifiable CAB at any visit before infection (C2). The remaining 4 participants acquired HIV despite mostly on-time CAB-LA injections (group D; Figure 2). These participants had 2–7 injections before the first HIV-positive visit; the median CAB concentration at that visit (interquartile range) was 1.56 (1.48–1.71) µg/mL. In these 4 cases, CAB concentrations were ≥8× PA-IC90 at 83% of the evaluable visits before the first HIV-positive visit and were ≥4× PA-IC90 at 95% of those visits. These 4 cases were classified as breakthrough infections (Table 1).
Concentrations of plasma TFV and TFV-DP in dried blood spots were determined for participants in the TDF/FTC arm (Figure 3 and Supplementary File 6). The half-lives of TFV and TFV-DP are 17 hours and 17 days, respectively; based on the analytical sensitivity of each assay, plasma and dried blood spots provide detection windows of 1–2 weeks and 2–3 months after TDF/FTC cessation, respectively [19–21]. Two of 39 participants with incident infection had TFV and TFV-DP concentrations consistent with daily TDF/FTC adherence. In 1 case (E16; Figure 3A), TFV and TFV-DP concentrations were 107 ng/mL and 1663 fmol per punch, at the first HIV-positive visit. The other participant had a protective plasma TFV concentration at the first HIV-positive visit (56.7 ng/mL; E34; Figure 3B); a TFV-DP result was not available for that visit. However, this participant had plasma TFV and TFV-DP concentrations consistent with daily TDF/FTC use in the preceding months. These 2 cases were classified as breakthrough infections (Table 1).
Delayed Detection of HIV Infection
In 21 of the 58 cases (36.2%; 11 of 16 [68.8%] in the CAB arm and 10 of 42 [23.8%] in the TDF/FTC arm), testing at study sites failed to detect infection at ≥1 study visits. In 14 of 21 cases (10 or 11 in the CAB arm and 4 of 10 in the TDF/FTC arm), delayed detection of HIV infection resulted in provision/administration of study drug to participants after they were HIV infected (Figures 2 and 3, Supplementary Files 5 and 6).
In the CAB arm, detection of infection was delayed at study sites in all 4 baseline cases and 7 (58.3%) of 12 incident cases (median delay [range], 62 [28–72] days for baseline cases and 98 [35–185] days for incident cases). Viral load testing would have detected infection at the first HIV-positive visit in all 4 baseline cases and 5 of 7 incident cases. The median viral load (range) at the first HIV-positive visit was 24 095 (1360–50 080) copies/mL for the 4 baseline cases and 130 (<40 to 860) copies/mL for the 5 incident cases.
In the TDF/FTC arm, detection of infection was delayed at study sites in all 3 baseline cases and 7 of 39 incident infections (17.9%) (median delay [range], 34 [14–36] days for baseline cases and 31 [7–68] days for incident cases, excluding a case with a visit interval of 372 days). Viral load testing would have detected infection at the first HIV-positive visit in 9 cases. The median (range) viral load at the first HIV-positive visit was 1930 (124–10 000) copies/mL for the 3 baseline cases and 13 230 (<40 to 22 070) copies/mL for the 6 incident cases with detectable viral load.
Table 2 shows results from retrospective testing using an Ag/Ab test, a confirmatory Ab test, qualitative RNA test, and viral load test; more extensive testing was performed in the CAB arm (Supplementary File 1). Persons who received CAB near the first HIV-positive visit (groups A, C, and D) were more likely to have delays in obtaining reactive or positive test results than participants who did not receive CAB near the time of infection (group B). Reversion of the Ag/Ab test from reactive to nonreactive was observed in 4 participants and reversion of the confirmatory Ab test from indeterminate to negative was observed in 1 participant. Assay reversion was not observed in participants who did not receive CAB near the time of infection (group B).
Table 2.
Human Immunodeficiency Virus (HIV) Test Results Associated With Delays in Detection of HIV Infectiona
| CAB Arm | TDF/FTC Arm | ||||
|---|---|---|---|---|---|
| Baseline: Group A (n = 4) | Incident: Group B (n = 5) | Incident: Groups C and D (n = 7 | Baseline (n = 3) | Incident (n = 39) | |
| Delay between 1st reactive qualitative RNA test and 1st reactive Ag/Ab test | |||||
| Participants, no. (%) | 3 (75) | 0 | 7 (100) | 3 (100) | 8 (21) |
| Duration of delay, range, d (among those with delayed Ag/Ab test result) | 14-60b | NA | 35–185 | 14–36 | 7–68c |
| Delay between 1st reactive qualitative RNA test and 1st positive confirmatory Ab test | |||||
| Participants, no. (%) | 4 (100)d | 1 (20) | 7 (100)e | 3 (100) | 18 (46)f |
| Duration of delay, d (among those with delayed confirmatory Ab test result) | 60–248d | 3 | 36–185e | 21–49 | 4-68c,f |
| Participants with assay reversion, no. (%) | |||||
| Ag/Ab test (from reactive to nonreactive) | 2 (50)b | 0 | 2 (29) | 0 | 0 |
| Confirmatory Ab test (from indeterminate to negative) | 0 | 0 | 1 (14)g | 0 | 0 |
| Qualitative RNA test (from reactive to nonreactive) | 2 (50) | 0 | 2 (29) | 0 | 0 |
Abbreviations: Ab, antibody; Ag, antigen; CAB, cabotegravir; NA, not applicable; TDF/FTC, tenofovir disoproxil fumarate–emtricitabine.
aTable 2 shows the delays observed for different human immunodeficiency virus (HIV) test outcomes at the HIV Prevention Trials Network (HPTN) Laboratory Center for the participant groups described in Table 1. The table also shows the number of cases in which reversion of test results was observed for the Ag/Ab test, the confirmatory Ab test, and the qualitative RNA test. Data are shown for visits before initiation of antiretroviral therapy (ART).
bOne case did not have a reactive Ag/Ab test after 41 days of follow-up.
cExcludes 1 case with a 372-day visit window.
dTwo cases did not achieve a positive confirmatory Ab test (longest pre-ART follow-up, 98 days).
eThree cases did not achieve a positive confirmatory Ab test (longest pre-ART follow-up, 333 days).
fEight cases did not achieve a positive confirmatory Ab test (longest pre-ART follow-up, 14 days).
gThis participant did not achieve a positive confirmatory Ab test after 333 days of follow-up.
Prolonged viral suppression was observed in 2 of 4 baseline cases and 6 of 7 incident cases where participants received CAB near the first HIV-positive visit (groups A, C, and D); in 4 of 11 cases, the qualitative RNA assay result reverted from reactive to nonreactive. Prolonged viral suppression and reversion of qualitative RNA assay results were not observed in the TDF/FTC arm or in CAB arm participants who did not receive CAB near the time of infection (group B).
HIV Drug Resistance
HIV genotyping was performed at the first viremic visit (>500 copies/mL) and at subsequent visits before initiation of antiretroviral therapy (ART); phenotypic resistance testing was performed for participants in the CAB arm (Tables 3 and 4). Supplementary File 4 includes additional information about drug resistance testing and descriptions of the 12 cases with resistance to study drugs.
Table 3.
Human Immunodeficiency Virus Drug Resistance in the Cabotegravir Arm
| Drug Resistance Mutationsb | ||||||||
|---|---|---|---|---|---|---|---|---|
| Case | Sample Typea | Visit Type | Subtype | NRTI | NNRTI | PI | INSTI | INSTI Phenotypec, d |
| A1 | 1st Viremic | Enrollment | B | K65R, M184V | L100I, K103N, P225H | L10V, I62V | … | NA (assay failure) |
| A2 | 1st Viremic | Enrollment | C | … | … | M36I, L89M | … | Sensitive to all INSTIs; RC, 101% (95% CI, 64%–160%) |
| Follow-up (60 d later) | wk 6 | … | … | … | M36I, L89M | E138K, Q148K | NA (assay failure) | |
| Follow-up (69 d later) | wk 6 | … | … | … | M36I, L89M | E138K, Q148K | NA (assay failure) | |
| B1 | 1st Viremic | Yearly 1 | B | … | V179T, Y181C, H221Y | I62V | … | NA (assay failure) |
| C1e | 1st Viremic | wk 9 | B | … | … | L10I, M36I | Q148R | NA (assay failure) |
| Follow-up (10 d later) | wk 10 | … | … | … | L10I, M36I | E138E/K, G140G/S, Q148R | NA (assay failure) | |
| Follow-up (14 d later) | wk 10 | … | … | … | L10I, M36I | E138E/K, G140G/S, Q148R | NA (assay failure) | |
| C3 | 1st Viremic | wk 9 | B | … | … | M36I, I62V, A71T | E138A, Q148R | CAB (FC,5.92), EVG (FC above max), RAL (FC, 17), DTG (FC, 1.69), BIC (FC, 1.2); RC, 1.3% (95% CI, .82%–2.1%) |
| Follow-up (1 d later) | Interim visit | … | … | … | M36I, I62V, A71T | E138A, Q148R | CAB (FC, 7.42), EVG (FC above max), RAL (FC, 35), DTG (FC, 2.39), BIC (FC, 1.48); RC, 5.2% (95% CI, 3.3–8.3%) | |
| D3 | 1st Viremic | wk 17 | BF | … | K103N | L10V, M36I | … | Sensitive to all INSTIs; RC, 47% (95% CI, 30%–47%) |
| Follow-up (112 d later) | wk 33 | … | … | K103N | L10V, M36I | R263K | NA (assay failure) | |
| Follow-up (117 d later) | wk 33 | … | … | K103N | L10V, M36I | R263K | CAB (FC, 2.32), EVG (FC, 4.14), RAL (FC, 1.38), DTG (FC, 2.29), BICf (FC, 2.89); RC, 24% (95% CI, 15%–38%) | |
| D4 | 1st Viremic | Follow-up wk 12 | C | … | … | K20R, M36I, L89M | G140A, Q148R | CAB (FC, 13), EVG (FC, 107), RAL (FC, 43), DTG (FC, 2.09), BICf (FC, 2.77); RC, 25% (95% CI, 16%–40%) |
Bold indicates major DRMs. Italic indicates mutations associated with resistance to study drugs.
Bold texts in “INSTI Phenotype” column indicate phenotyping results.
Abbreviations: BIC, bictegravir; CAB, cabotegravir, CI, confidence interval; DTG, dolutegravir; EVG, elvitegravir; FC, fold change; INSTI, integrase strand transfer inhibitor; NA, not available; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse-transcriptase inhibitor; PI, protease inhibitor; RAL, raltegravir; RC, replication capacity.
aThe first viremic visit was the first visit with a viral load >500 copies/mL.
bDrug resistance mutations (DRMs) listed in the 2019 Drug Resistance Mutations update from the International Antiviral Society–USA [16]. The notations E/K and G/S indicate that the DRM was detected as a mixture with the wild-type amino acid.
cRC indicates the ability of recombinant viruses containing the integrase region and the C-terminal region of reverse-transcriptase derived from test samples to replicate in the absence of drug, compared with the RC of a test vector; 95% CIs are shown. An RC of 100% corresponds to the RC of wild-type (INSTI-naive) viruses.
dFC indicates drug susceptibility (ratio of the 50% inhibitory concentration for the test sample compared with the reference).
eCase C1 also had the INSTI polymorphism, L74I.
fPhenotyping results showing partial susceptibility.
Table 4.
Human Immunodeficiency Virus Drug Resistance in Tenofovir Disoproxil Fumarate–Emtricitabine Arm
| Drug Resistance Mutationsb | |||||||
|---|---|---|---|---|---|---|---|
| Case | Sample Typea | Visit Type | Subtype | NRTI | NNRTI | PI | INSTI |
| E3 | 1st Viremic | wk 137 | B | M184I | … | … | … |
| E6 | 1st Viremic | Interim visit after wk 99 | B | … | K103N | M36I, D60E | |
| E7 | 1st Viremic | wk 97 | B | … | A98G, K103N | M36I, I62V | T97A |
| E11 | 1st Viremic | wk 73 | B | … | K103N | … | … |
| E13 | 1st Viremic | wk 65 | B | M184V | K103N | I62V, A71T | … |
| E14 | 1st Viremic | wk 65 | B | … | K103N | … | … |
| E15 | 1st Viremic | wk 4 | B | … | G190S | I62V | … |
| Follow-up | Yearly 1 | … | … | G190S | I62V | … | |
| E16 | 1st Viremic | wk 57 | B | K65R | V179D, Y181C, G190A, Q207E, H221Y, F227L | L10V, D60E, I62V | … |
| E25c | 1st Viremic | wk 43 | BF | M184V | K103N, P225H | M36I | … |
| E32c | 1st Viremic | wk 27 | B | … | K103N | L10V, M36I, I62V | … |
| E39 | 1st Viremic | wk 9 | B | … | K103S | L10I, I62V, A71V | … |
| Follow-up (7 d later) | wk 10 | … | … | K103S | L10I, I62V, A71V | … | |
| E41 | 1st Viremic | Enrollment | B | … | … | … | … |
| Follow-up (34 d later) | wk 4 | … | M184I | … | … | … | |
| E42c | 1st Viremic | Enrollment | B | T369V | M36I | … | |
| Follow-up (14 d later) | wk 2 | … | M184I/V | … | M36I | … | |
Bold indicates major DRMs. Italic indicates mutations associated with resistance to study drugs.
Abbreviations: INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse-transcriptase inhibitor; PI, protease inhibitor.
aThe first viremic visit was the first visit with a viral load >500 copies/mL.
bDrug resistance mutations (DRMs) listed in the 2019 Drug Resistance Mutations update from the International Antiviral Society–USA [16].
cCases E25, E32, and E42 also had the INSTI polymorphism, L74I.
Genotyping results were obtained at the first viremic visit for 13 of 16 cases in the CAB arm (1 failed analysis; 2 had no viremic visits) and 40 of 42 in the TDF/FTC arm (2 had no viremic visit). Nine cases had NNRTI resistance only (2 in the CAB and 7 in the TDF/FTC arm); 4 had NNRTI and NRTI resistance (1 in the CAB and 3 in the TDF/FTC arm); and 1 had NRTI resistance only (in the TDF/FTC arm). All 5 cases with NRTI resistance had K65R and/or M184I/V at the first HIV-positive visit. In 4 cases, virology and pharmacology data suggest that the participants were infected with drug-resistant HIV (cases with little or no TDF/FTC use; A1, E3, E13, and E25). In the fifth case, exposure to virus with K65R may have been responsible for breakthrough infection in a participant with good TDF/FTC adherence (E16). Two participants in the TDF/FTC arm had baseline infection with no resistance at enrollment; both had NRTI resistance at a subsequent visit (1 with M184I and 1 with M184I/V; E41 and E42). These participants likely acquired resistance from study drug exposure (Table 4 and Supplementary File 6).
INSTI resistance mutations were detected in 5 cases in the CAB arm (4 with INSTI resistance only and 1 with INSTI and NNRTI resistance; Table 3). In 2 cases (A2 and D3), resistance was clearly acquired, since there was no resistance at the first HIV-positive visit. In the other 3 cases (C1, C3, and D4), INSTI resistance was detected at the first viremic visit, 35–141 days after the first HIV-positive visit. In these 5 cases, infection was first detected at study sites a median (range) of 47 (35–117) days after the first HIV-positive visit. The participants received 1–3 CAB-LA injections before the site detected the infections. The median CAB concentration (range) at the first visit with INSTI resistance was 1.84 (1.11–3.84) µg/mL.
The participant with baseline infection developed INSTI resistance with E138K + Q148K after receiving 1 CAB-LA injection (A2). The 2 participants infected during the oral lead-in period had INSTI resistance at the first viremic visit after receiving CAB-LA. One participant had L74I + Q148R at the first viremic visit and had L74I + E138E/K + G140G/S + Q148R 10 days later (C1); the other participant had E138A + Q148R at the first viremic visit (C3). In the remaining 2 cases, participants were infected despite on-time CAB-LA injections and had INSTI resistance after receiving 4 CAB-LA injections. One had NNRTI resistance at the first viremic visit and NNRTI resistance and INSTI resistance with R263K 112 days later (D3). The other participant had INSTI resistance with G140A + Q148R at the first viremic visit (D4).
Phenotyping results were obtained for 3 of the 5 participants who had INSTI resistance (1 with E138A + Q148R, 1 with G140A + Q148R, and 1 with R263K; Table 3). The first 2 cases were resistant to CAB, elvitegravir, and raltegravir but susceptible to dolutegravir (DTG); 1 was susceptible to bictegravir (BIC; C3), and 1 was partially susceptible to BIC (D4). The third case was resistant to elvitegravir and partially susceptible to BIC but was susceptible to CAB, DTG, and raltegravir. Viral replication capacity was low in all 3 cases (1.3%, 25%, and 24%).
DISCUSSION
In HPTN 083, HIV incidence was low in both study arms, highlighting the efficacy of both oral TDF/FTC and CAB-LA. In the TDF/FTC arm, 37 of the 39 participants who acquired infection during the study had drug concentrations indicating suboptimal adherence to the daily oral regimen. The oral lead-in phase in the CAB arm also required daily pill taking. Based on drug measurements, adherence to oral CAB was poor in one-third of the participants with incident infection, which may have contributed to infection in 1 case. Owing to barriers with adherence to daily oral PrEP regimens and the good safety profile of CAB-LA, the oral phase before CAB-LA initiation may not be necessary or desirable.
In 6 of the 12 incident cases in the CAB arm, participants had CAB concentrations at the first HIV-positive visit that are expected to be protective against HIV acquisition (≥8× PA-IC90) [17, 22]. Two of these infections occurred during the oral lead-in phase; these infections may have occurred before target drug concentrations were attained. Although pill count data in these cases suggest 96% adherence to oral CAB, these measures are often unreliable [23, 24], and actual adherence is unknown. The other 4 cases were classified as breakthrough infections. In 3 cases, the first HIV-positive visit was 8–13 weeks after a visit with a CAB concentration <8× PA-IC90; this may have increased risk of PrEP failure.
The lag between the decline in CAB concentration and detection of infection may reflect a delay between initial infection in the rectal mucosa and lymph nodes and systemic dissemination of infection. Rectal tissue CAB concentrations are also lower than plasma CAB concentrations (approximately 8%) [25]; however, this alone does not explain variability in HIV risk among study participants. Rectal sexually transmitted infections could increase HIV risk through an increase in mucosal CD4+ cells or mucosal barrier erosion [26]. Rectal sexually transmitted infections were not diagnosed proximal to the first HIV-positive visit in these cases but were common in HPTN 083 and may have been undiagnosed at the time of HIV infection.
In addition to the infections described above, 3 occurred >6 months after the last CAB injection. CAB concentrations were very low or unquantifiable at the first HIV-positive visit in all 3 cases; HIV infection was detected at the site at the first HIV-positive visit, and ART was initiated promptly. INSTI resistance was not detected in any of these 3 cases. Breakthrough infections were observed in 2 participants in the TDF/FTC arm who had drug concentrations consistent with good adherence. In 1 case, exposure to HIV with the K65R mutation may have played a role in PrEP failure. Breakthrough infections with CAB-LA PrEP due to transmitted drug resistance were not observed in this study, which may suggest an important potential benefit of CAB-LA.
Delays in detection of HIV infections at study sites were observed in both study arms, but were more frequent and longer in the CAB arm. In the TDF/FTC arm, most participants in these cases had acute HIV infection at the first HIV-positive visit; those infections were usually detected at the next study visit. In contrast, delayed detection of infection in the CAB arm was usually associated with a long period of viral suppression and delayed Ab expression. HIV infection would have been detected at an earlier visit in almost all of these cases using a viral load assay. This could have limited exposure to study drugs after infection and provided an opportunity for earlier ART initiation. These findings suggest that use of viral load testing to screen for HIV infection in patients on CAB-LA may help prevent acquisition of INSTI resistance. This is being evaluated in the upcoming HPTN 083 open-label extension study.
INSTI resistance was detected in 5 of 14 CAB arm participants (35.7%) with genotyping data. These 5 participants received 1–3 CAB-LA injections after they were infected, before infection was detected at the study sites. Different patterns of CAB mutations were detected in the 5 cases. Susceptibility to DTG and at least partial susceptibility to BIC were retained in the 3 cases with phenotypic resistance test results (1 with E138A + Q148R, 1 with G140A + Q148R, 1 with R263K). This is important, because DTG and BIC are recommended in first-line HIV treatment regimens [27].
The current study provides important information relevant to use of CAB-LA for HIV prevention. The frequency of incident HIV infection during the injection phase among participants with CAB concentrations ≥8× PA-IC90 at the first HIV-positive visit was very low (observed in 4 participants). Long periods of viral suppression and delayed Ab production were observed in participants receiving CAB-LA, which delayed HIV diagnosis, led to drug administration after infection, and delayed ART initiation. Most of these infections would have been detected using a sensitive viral load assay. In 2 cases, INSTI-resistant viruses had low replication capacity and retained susceptibility to DTG/BIC; however, ongoing exposure to CAB-LA in another case, due to delayed ART access, led to accumulation of additional resistance mutations. While CAB-LA is highly protective against HIV infection, infections can occur despite on-time injections with expected CAB concentrations. The open-label extension phase of HPTN 083 will provide additional information about the relationship between pharmacokinetic-pharmacodynamic properties of CAB-LA and HIV protection and the use of viral load testing to improve detection of breakthrough infections.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the HIV Prevention Trials Network (HPTN) 083 study team and participants; the HPTN 083 site principal investigators; the laboratory staff at the study sites and the HPTN Laboratory Center; John Mellors and laboratory staff at the University of Pittsburgh; Michael Seisa, Yolanda Lie, and laboratory staff at Monogram Biosciences; the members of the HPTN 083 Endpoint Adjudication Committee and HIV Alias Committee (William Meyer, Jeanne Marrazzo, Sheila Peel, Carole Wallis, Aida Asmelash, and Eric Daar); expert virology consultants (Grace Aldrovandi and Daniel Kuritzkes); investigators at ViiV Healthcare; and Richard Clark and other investigators at Gilead Sciences.
Author contributions. All authors participated in the study, contributed to manuscript preparation, and reviewed the manuscript.
Designed study: M. A. M., R. J. L., and S. H. E. Coordinated laboratory testing: E. P. M. Coordinated and performed virology testing: V. C. and S. A. Coordinated and performed CAB and tenofovir (TFV) testing: C. D. H. Coordinated and performed TFV diphosphate (TFV-DP) testing: L. R. B. Responsible for TFV-DP testing: P. A. Provided expertise on HIV drug resistance testing: C. P. Responsible for HIV DNA testing: D. Persaud. Provided pharmaceutical support: A. R., M. S. C., and J. F. R. Analyzed data: M. A. M., J. M. F., R. J. L., and S. H. E. Data management and analysis: M. L. and L. W. Provided clinical information for a study participant and scientific input: D. Pryluka. Provided graphic arts support: R. K. Lead Laboratory Pharmacologist: M. A. M. HPTN 083 Protocol Co-Chair: B. G. HPTN Laboratory Center Virologist: J. M. F. HPTN Laboratory Center Deputy Director: E. P. M. HPTN SDMC Data Analyst: M. L. and L. W. HPTN 083 Study Coordinator: M.M. HPTN 083 NIH Medical Officer: A.A. HPTN 083 Site PIs: L. C., A. G., K. M., and N. P. HPTN Leadership and Operations Center PI: M. S. Cohen. HPTN 083 Protocol Pharmacologist: C. W. H. HPTN 083 Statistician: B. H. HPTN 083 Protocol Statistician. D. D. HPTN 083 Protocol Chair: R. J. L. HPTN 083 Virologist: S. H. E. Drafted the manuscript: M. A. M., J. M. F., R. J. L., and S. H. E. Reviewed and edited the proof set: M. A. M., J. M. F., and S. H. E.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, Office of the Director, National Institutes of Health, National Institute on Drug Abuse, and the National Institute of Mental Health (grants UM1AI068619, UM1AI068617, and UM1AI068613).
Potential conflicts of interest. C. P. is an employee, officer, and shareholder of Laboratory Corporation of America. A. R. and M. S. C. are employees of ViiV Healthcare. J. F. R. is an employee and stockholder of Gilead Sciences. R. J. L. has served on scientific advisory boards for Gilead and Merck and has received honoraria from Roche and Janssen. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author roles
References
- 1. Riddell J 4th, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA 2018; 319:1261–8. [DOI] [PubMed] [Google Scholar]
- 2. Grant RM, Lama JR, Anderson PL, et al. ; iPrEx Study Team . Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gulick RM, Flexner C. Long-acting HIV drugs for treatment and prevention. Annu Rev Med 2019; 70:137–50. [DOI] [PubMed] [Google Scholar]
- 4. Stellbrink HJ, Hoffmann C. Cabotegravir: its potential for antiretroviral therapy and preexposure prophylaxis. Curr Opin HIV AIDS 2018; 13:334–40. [DOI] [PubMed] [Google Scholar]
- 5. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017; 390:1499–510. [DOI] [PubMed] [Google Scholar]
- 6. Murray MI, Markowitz M, Frank I, et al. Satisfaction and acceptability of cabotegravir long-acting injectable suspension for prevention of HIV: patient perspectives from the ECLAIR trial. HIV Clin Trials 2018; 19:129–38. [DOI] [PubMed] [Google Scholar]
- 7. Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med 2018; 15:e1002690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Landovitz RJ, Li S, Eron JJ Jr, et al. Tail-phase safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected adults: a secondary analysis of the HPTN 077 trial. Lancet HIV 2020; 7:e472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Landovitz RJ, Donnell D, Clement ME, et al. HPTN 083 interim results: pre-exposure prophylaxis (PrEP) containing long-acting injectable cabotegravir (CAB-LA) is safe and highly effective for cisgender men and transgender women who have sex with men (MSM,TGW). New Engl J Med 2021; In press. [Google Scholar]
- 10. Lehman DA, Baeten JM, McCoy CO, et al. ; Partners PrEP Study Team . Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis 2015; 211:1211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weis JF, Baeten JM, McCoy CO, et al. ; Partners PrEP Study Team . Preexposure prophylaxis-selected drug resistance decays rapidly after drug cessation. AIDS 2016; 30:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sivay MV, Li M, Piwowar-Manning E, et al. ; HPTN 067/ADAPT Study Team . Characterization of HIV seroconverters in a TDF/FTC PrEP Study: HPTN 067/ADAPT. J Acquir Immune Defic Syndr 2017; 75:271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parikh UM, Mellors JW. Should we fear resistance from tenofovir/emtricitabine preexposure prophylaxis? Curr Opin HIV AIDS 2016; 11:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anstett K, Brenner B, Mesplede T, Wainberg MA. HIV drug resistance against strand transfer integrase inhibitors. Retrovirology 2017; 14:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tosiano MA, Jacobs JL, Shutt KA, Cyktor JC, Mellors JW. A simpler and more sensitive single-copy HIV-1 RNA assay for quantification of persistent HIV-1 viremia in Individuals on suppressive antiretroviral therapy. J Clin Microbiol 2019; 57:e01714-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wensing AM, Calvez V, Ceccherini-Silberstein F, et al. 2019 Update of the drug resistance mutations in HIV-1. Top Antivir Med 2019; 27:111–21. [PMC free article] [PubMed] [Google Scholar]
- 17. Andrews CD, Spreen WR, Mohri H, et al. Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science 2014; 343:1151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spreen W, Min S, Ford SL, et al. Pharmacokinetics, safety, and monotherapy antiviral activity of GSK1265744, an HIV integrase strand transfer inhibitor. HIV Clin Trials 2013; 14:192–203. [DOI] [PubMed] [Google Scholar]
- 19. Hendrix CW, Andrade A, Bumpus NN, et al. Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066). AIDS Res Hum Retroviruses 2016; 32:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson PL, Liu AY, Castillo-Mancilla JR, et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 2018; 62:e10710-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gilead. Truvada prescribing information.https://wwwgileadcom/~/media/files/pdfs/medicines/hiv/truvada/truvada_pipdf%202020. Accessed 28 November 2020.
- 22. Andrews CD, Yueh YL, Spreen WR, et al. A long-acting integrase inhibitor protects female macaques from repeated high-dose intravaginal SHIV challenge. Sci Transl Med 2015; 7:270ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amico KR, Marcus JL, McMahan V, et al. Study product adherence measurement in the iPrEx placebo-controlled trial: concordance with drug detection. J Acquir Immune Defic Syndr 2014; 66:530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van der Straten A, Brown ER, Marrazzo JM, et al. ; MTN-003 VOICE Protocol Team for Microbicide Trials Network . Divergent adherence estimates with pharmacokinetic and behavioural measures in the MTN-003 (VOICE) study. J Int AIDS Soc 2016; 19:20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spreen W, Ford SL, Chen S, et al. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr 2014; 67:481–6. [DOI] [PubMed] [Google Scholar]
- 26. Wall KM, Karita E, Nyombayire J, et al. Genital abnormalities, hormonal contraception, and HIV transmission risk in Rwandan serodifferent couples. J Infect Dis 2021; 224:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. US Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV.https://clinicalinfohivgov/en/guidelines/adult-and-adolescent-arv/what-start-initial-combination-regimens-antiretroviral-naive?view=full%202019. Accessed 12 December 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.