Abstract
Aims
Non-invasive assessment and risk stratification of coronary artery disease in patients with large body habitus is challenging. We aim to examine whether body mass index (BMI) modifies the prognostic value and diagnostic utility of stress cardiac magnetic resonance imaging (CMR) in a multicentre registry.
Methods and results
The SPINS Registry enrolled consecutive intermediate-risk patients who presented with a clinical indication for stress CMR in the USA between 2008 and 2013. Baseline demographic data including BMI, CMR indices, and ratings of study quality were collected. Primary outcome was defined by a composite of cardiovascular death and non-fatal myocardial infarction. Of the 2345 patients with available BMI included in the SPINS cohort, 1177 (50%) met criteria for obesity (BMI ≥ 30) with 531 (23%) at or above Class 2 obesity (BMI ≥ 35). In all BMI categories, >95% of studies were of diagnostic quality for cine, perfusion, and late gadolinium enhancement (LGE) sequences. At a median follow-up of 5.4 years, those without ischaemia and LGE experienced a low annual rate of hard events (<1%), across all BMI strata. In patients with obesity, both ischaemia [hazard ratio (HR): 2.14; 95% confidence interval (CI): 1.30–3.50; P = 0.003] and LGE (HR: 3.09; 95% CI: 1.83–5.22; P < 0.001) maintained strong adjusted association with the primary outcome in a multivariable Cox regression model. Downstream referral rates to coronary angiography, revascularization, and cost of care spent on ischaemia testing did not significantly differ within the BMI categories.
Conclusion
In this large multicentre registry, elevated BMI did not negatively impact the diagnostic quality and the effectiveness of risk stratification of patients referred for stress CMR.
Keywords: stress cardiac MRI, obesity, prognosis
Introduction
Obesity [body mass index (BMI) ≥30 kg/m2] is a growing public health concern, with a prevalence approaching 40% in adult Americans and 15% in adult Europeans.1,2 Diagnostic evaluation and risk stratification of obese patients with chest pain syndromes remain challenging. Basic exercise treadmill testing may be limited by patient’s inability to reach target heart rate or workload. Stress echocardiography and single-photon emission tomography (SPECT) perfusion can be limited by suboptimal image quality, due to poor acoustic windows and soft tissue attenuation artefacts, respectively. Diagnostic quality of computed tomography angiography (CCTA) may also be hampered in obese patients due to increased noise from fewer photons reaching the detectors.3,4 In addition, obese patients undergoing SPECT or CCTA are subject to higher doses of ionizing radiation.3,5
Single-centre studies have demonstrated that stress cardiac magnetic resonance imaging (CMR) is feasible and effective in prognosticating obese patients.6,7 In this study, we sought to better understand the impact of BMI on stress CMR performance in the multicentre, observational Stress CMR Perfusion Imaging in the United States (SPINS) study.
Methods
Patient population
The rationale and design of the SPINS Registry have been previous described in detail.8,9 In brief, SPINS was a retrospective, multicentre study of patients with chest pain syndromes, ECG changes, or other presentations suspicious for coronary artery disease (CAD) referred for a clinical stress CMR in the USA. Between 1 January 2008 and 31 December 2013, we enrolled consecutive patients with completed CMR studies at 13 sites in the USA if they had suspicion of underlying CAD and at least two of the following risk factors: age > 50 years for male or > 60 years for female, history of diabetes, hypertension, or hypercholesterolaemia; family history of premature coronary disease; BMI ≥30 kg/m2; history of peripheral vascular disease; history of percutaneous coronary intervention (PCI) or myocardial infarction (MI). Exclusion criteria were as previously published.9 Site were included if they demonstrated at least 10 years of experience performing vasodilator stress CMR, had the ability to contribute between 100 and 500 consecutive subjects, and could dedicate either a cardiac research nurse or fellow to perform patient follow-up. Pulse sequence protocols included cine, stress perfusion, and late gadolinium enhancement (LGE) imaging of infarction. The study was approved by local institutional review boards with a waiver of written informed consent.
Imaging analysis
Scanner model, field strength, vasodilator stress perfusion protocols, and CMR sequences were determined by local practices. CMR results were based on sites’ interpretation at the time of study performance. All studies were reviewed by a COCATS Level II or III trained reader and each site had at least one COCATS Level III supervising reader. CMR variables analysed included: left ventricular dimensions, volumes and function, segmental stress perfusion, and LGE. A perfusion defect was considered present in a region of hypoenhancement densest in the endocardium with a transmural gradient across the wall thickness, which persisted beyond peak myocardial enhancement and conformed to a coronary distribution. Inducible ischaemia was defined as the presence of a stress perfusion defect in at least one segment with absence of matching LGE.10 MI was defined as the presence of LGE in a pattern consistent with infarction in any segment. Mild, moderate, and severe defects were defined as the involvement of 1–2, 3–5, and ≥6 myocardial segments, respectively. All analyses were recorded according to the 16-segment (for perfusion) or 17-segment (for LGE) American Heart Association (AHA) nomenclature. Study image quality was rated on a 1–5 scale for cine, perfusion, and LGE sequences using the following criteria: 5 = excellent quality, no artefacts; 4 = good quality, mild artefacts; 3 = fair quality, moderate artefacts; 2 = poor quality, severe artefacts; and 1 = non-diagnostic. We a priori defined a diagnostic quality study as a score ≥3 out of 5.
Data collection and study endpoints
At the beginning of the study, investigators were trained using group webinars and study documents on specific definitions of all key variables required. Clinical variables and follow-up data were collected by local investigators using medical visits documented in electronic medical records or contact via telephone and standardized checklist questionnaire. The mortality status of study participants was further verified via the Social Security Death Index at the end of the study period. An encrypted web-based database (www.CMRCOOP.org) was used for sites to enter PHI-free variables. Baseline collected variables included: demographics, indication for study, cardiovascular risk factors, and prior cardiac history. Class 1 obesity (BMI 30.0—34.9), Class 2 obesity (BMI 35—39.9), and Class 3 obesity (BMI ≥ 40) were as defined by the World Health Organization criteria.11
Follow-up for clinical events was mandated for at least 4 years post-index stress CMR and was verified by each site’s principal investigator. The adjudication of events was performed using standardized clinical definitions per current guidelines of clinical endpoints,12 blinded to the imaging findings, by the consensus of site investigators. Primary outcome was defined as cardiovascular death or non-fatal MI. Secondary outcome was defined as a composite of cardiovascular death, non-fatal MI, hospitalization for unstable angina, hospitalization for congestive heart failure (CHF), and unplanned late coronary artery bypass grafting (CABG) performed >6 months after the index stress CMR. Cardiovascular deaths were defined as deaths preceded by an acute MI, malignant ventricular arrhythmia, or decompensated heart failure. The diagnosis of acute MI required chest pain or anginal equivalent and abnormal temporal changes in troponins consistent with myocardial injury. Hospitalization for unstable angina was defined as an unscheduled hospitalization due to worsening chest pain or anginal equivalent, with evidence of ischaemia by imaging or significant coronary stenosis by angiography. Heart failure hospitalization was defined as an unscheduled hospitalization of >24 h, due to worsening or new symptoms, and intensification of heart failure treatment.
In addition, enrolling centres collected data on all invasive and non-invasive testing for myocardial ischaemia which occurred following the index stress CMR, namely, SPECT, CCTA, stress echocardiography, exercise treadmill test, repeat stress CMR, and coronary angiography during the study follow-up period. Revascularization procedures, such as PCI or CABG were also recorded. Corresponding costs of downstream tests were extrapolated using the published average national payment rates from the Medicare Hospital Outpatient Prospective Payment System, as previously described.9 Data will be made available upon written request.
Statistical analysis
Continuous data were expressed as means ± SD or median with inter-quartile range (IQR) for normal and skewed distributions. Categorical variables were expressed as counts with percentages. Comparison between groups was performed with ANOVA and Kruskal–Wallis test for continuous data. χ2 test was used to compare categorical data and the Cochran–Armitage test was used to establish trend. Event-free survival, stratified by category of BMI and CMR findings, was estimated by the Kaplan–Meier method and compared using a log-rank test. In patients with obesity (BMI ≥30 kg/m2), we used univariable Cox regression models to estimate unadjusted hazard ratio (HR) of clinical and CMR covariates for primary and secondary outcomes. The prognostic value of inducible ischaemia or presence of LGE was evaluated using a multivariable Cox model constructed by inclusion of all significant covariates on univariable screen. We then used a forward selection algorithm with P < 0.05 for model entry and retention and forcing BMI into the model a priori. We tested for significant interaction between BMI and CMR-detected ischaemia and LGE. Proportional hazards assumption was evaluated using visual inspection of the log–log survival curves and the Schoenfeld residuals test. All analyses were performed using SAS version 9.4 (SAS Institute, North Carolina, USA) and a P < 0.05 was used to establish statistical significance.
Results
Baseline patient demographics and CMR characteristics
Overall, 2349 patients from 13 centres across the USA met inclusion and exclusion criteria for the SPINS study. Four subjects had incomplete information on BMI due to missing weight or height. The remaining 2345 subjects formed the cohort for this analysis. Baseline demographic and clinical characteristics are summarized in Table 1, stratified by BMI. The mean age in the overall cohort was 63 ± 11 years with 47% female. Obesity was present in 1177 (50%) patients, with 531 (23%) patients having Class 2 or 3 obesity (BMI ≥ 35). Patients with increased BMI were younger and more likely to be female. Across higher BMI categories, there was increasing proportion of patients with risk factors of hypertension and diabetes (both P for trend <0.001). The proportion of patients with prior history of MI was lower across higher BMI categories (P for trend =0.001), whereas the proportion of patients with prior history of PCI or CHF did not differ.
Table 1.
Demographics and baseline characteristics
| Overall (N = 2345) | 30>BMI (N = 1168) | 35>BMI ≥ 30 (N = 646) | BMI ≥ 35 (N = 531) | P-value | |
|---|---|---|---|---|---|
| Follow-up (years), median (IQR) | 5.4 (4.6–6.8) | 5.5 (4.5–6.8) | 5.5 (4.7–6.9) | 5.2 (4.5–6.6) | 0.08 |
| Age (years), mean ± SD | 63 ± 11 | 64 ± 11 | 62 ± 11 | 59 ± 11 | <0.001 |
| Female, n (%) | 1102 (47) | 509 (44) | 288 (45) | 305 (57) | <0.001 |
| Height (m), mean ± SD | 1.70 ± 0.11 | 1.71 ± 0.10 | 1.70 ± 0.11 | 1.68 ± 0.11 | <0.001 |
| Weight (kg), mean ± SD | 90 ± 22 | 76 ± 13 | 94 ± 12 | 116 ± 21 | <0.001 |
| BMI (kg/m2), mean ± SD | 31 ± 7 | 26 ± 2.9 | 32 ± 1.4 | 41 ± 5.9 | <0.001 |
| Cardiac risk factors, n (%) | |||||
| Hypertension | 1841 (79) | 881 (75) | 512 (79) | 448 (84) | <0.001 |
| Hypercholesterolaemia | 1644 (70) | 843 (72) | 446 (69) | 355 (67) | 0.07 |
| Diabetes mellitus | 664 (28) | 253 (22) | 193 (30) | 218 (41) | <0.001 |
| Smoking | 757 (33) | 374 (32) | 193 (30) | 190 (36) | 0.09 |
| Family history of CAD | 761 (34) | 387 (35) | 196 (32) | 178 (35) | 0.39 |
| History of PCI, n (%) | 538 (23) | 275 (24) | 150 (23) | 113 (21) | 0.61 |
| History of MI, n (%) | 357 (15) | 201 (17) | 98 (15) | 58 (11) | 0.005 |
| History of HF, n (%) | 245 (10) | 119 (10) | 64 (10) | 62 (12) | 0.54 |
| Stress CMR | |||||
| Required sedation, n (%) | 50 (2) | 22 (2) | 15 (2) | 13 (2) | 0.70 |
| Magnet field strength | |||||
| 1.5 T, n (%) | 1532 (65) | 755 (65) | 384 (59) | 393 (74) | <0.001 |
| 3.0 T, n (%) | 813 (35) | 413 (35) | 262 (41) | 138 (26) | — |
| Bore size | |||||
| 60 cm, n (%) | 1630 (70) | 846 (72) | 479 (74) | 305 (57) | <0.001 |
| 70 cm, n (%) | 715 (30) | 322 (28) | 167 (26) | 226 (43) | — |
| Quality of cine sequence | |||||
| Score, median (IQR) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 0.09 |
| Score 3–5, n (%) | 2335 (100) | 1165 (100) | 643 (100) | 527 (99) | 0.34 |
| Score 1–2, n (%) | 10 (0) | 3 (0) | 3 (0) | 4 (1) | — |
| Quality of perfusion sequence | |||||
| Score, median (IQR) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 0.07 |
| Score 3–5, n (%) | 2251 (100) | 1111 (100) | 622 (100) | 518 (99) | 0.36 |
| Score 1–2, n (%) | 10 (0) | 3 (0) | 3 (0) | 4 (2) | — |
| Quality of LGE sequence | |||||
| Score, median (IQR) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 0.01 |
| Score 3–5, n (%) | 2252 (99) | 1112 (100) | 621 (99) | 519 (99) | 0.33 |
| Score 1–2, n (%) | 11 (1) | 3 (0) | 4 (1) | 4 (1) | — |
| LVEF (%), median (IQR) | 63 (54–70) | 62 (54–69) | 64 (55–71) | 64 (56–71) | 0.009 |
| Inducible ischaemia, n (%) | 405 (17) | 244 (21) | 90 (14) | 71 (13) | <0.001 |
| Ischaemic segments | |||||
| Mild (1–2), n (%) | 175 (8) | 102 (9) | 38 (6) | 35 (7) | 0.001 |
| Moderate (3–5), n (%) | 128 (5) | 82 (7) | 26 (4) | 20 (4) | — |
| Severe (≥ 6), n (%) | 102 (4) | 60 (5) | 26 (4) | 16 (3) | — |
| Prior infarct by LGE, n (%) | 572 (24) | 312 (27) | 154 (24) | 106 (20) | 0.01 |
| Ischaemia or LGE, n (%) | 766 (33) | 422 (36) | 199 (31) | 145 (27) | 0.001 |
BMI, body mass index; CMR, cardiovascular magnetic resonance; CAD, coronary artery disease; HF, heart failure; IQR, inter-quartile range; LVEF, left ventricular ejection fraction; LGE, late gadolinium enhancement; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Use of sedation prior to stress CMR was 2% and did not differ significantly across the BMI categories (P = 0.40). The overall study cohort had preserved left ventricular ejection fraction (LVEF) with median of 63% (IQR 54–70%). Overall, 17% of the cohort had evidence of ischaemia and 24% had evidence of LGE. Across increasing BMI categories, there was a lower proportion of patients with either ischaemia (P for trend <0.001) or LGE (P for trend =0.002).
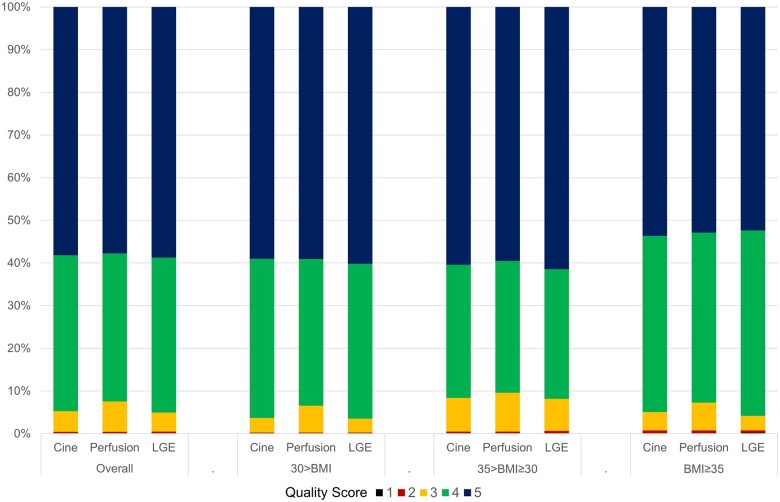
Image quality scores for cine, perfusion, and LGE sequences were available in 100%, 96%, and 97% of patients, respectively. Diagnostic image quality for perfusion sequence, as defined by a quality score ≥3 out of 5 was consistently present in >99% of all scans scored, across all BMI strata (Table 1 and Figure 1). Similarly, diagnostic image quality for cine and LGE sequences were achieved in over 99% of all scans scored, with no significant difference across the BMI strata. Patients with higher BMI were more likely to require a large bore magnet (70 cm). Patients requiring a large bore magnet had overall lower score for image quality, compared with those who did not, for cine [4 (IQR 4–5) vs. 5 (IQR 4–5)], perfusion [4 (IQR 4–5) vs. 5 (IQR 4–5)], and LGE [4 (IQR 4–5) vs. 5 (IQR 4–5)] sequences (all P < 0.001).
Figure 1.
Study quality. Quality rating of cine, perfusion, and LGE sequences, according to BMI category. BMI, body mass index; LGE, late gadolinium enhancement.
Clinical outcomes and prognostic value of stress CMR
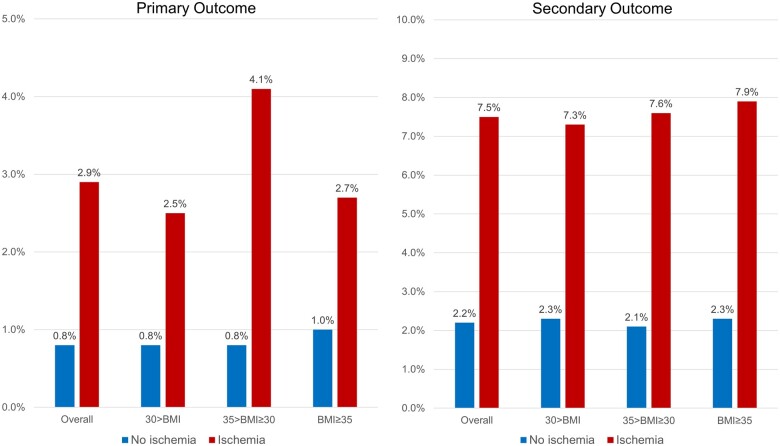
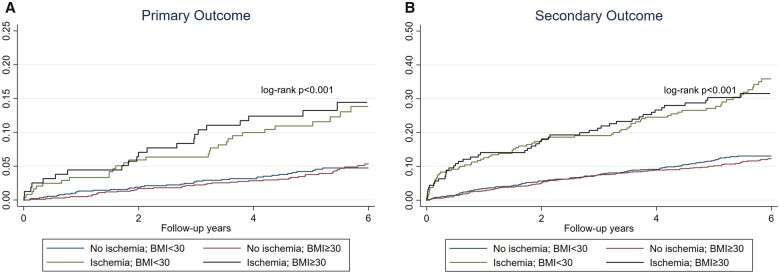
Median follow-up duration in the overall cohort was 5.4 years (IQR 4.6–6.8 years), with 97.7% of patients achieving a follow-up of at least 4 years. In the entire cohort, 152 patients achieved the primary outcome of cardiovascular death or non-fatal MI, whereas 373 patients achieved the secondary outcome. Patients with no evidence of inducible ischaemia had a low annualized rate of primary outcome (≤1%) across all BMI strata (Figure 2). Patients with neither ischaemia nor LGE had an annualized rate of 0.6–0.7% for primary outcome. Kaplan–Meir cumulative incidence curves for primary and secondary outcome, stratified by presence of ischaemia and presence of obesity are presented in Figure 3A and B. In both obese and non-obese patients, CMR detected ischaemia was associated with a lower event-free survival, for primary and secondary outcomes (log-rank P < 0.001). In the absence of ischaemia, the event-free survival was similar in obese and non-obese patients. Kaplan–Meir cumulative incidence curves for primary outcome, according to the presence of obesity and extent of ischaemia is shown in Supplementary data online, Figure S1A and B.
Figure 2.
Cardiovascular outcomes event rates. Annualized rates of primary (left) and secondary (right) outcomes, stratified by presence vs. absence of ischaemia, according to BMI category. Primary outcome = cardiovascular death or non-fatal MI. Secondary outcome = cardiovascular death, non-fatal MI, hospitalization for unstable angina, hospitalization for congestive heart failure, and unplanned late CABG. BMI, body mass index; CABG, coronary artery bypass grafting; MI, myocardial infarction.
Figure 3.
Cumulative incidence rate. Time-to-event curves for primary (A) and secondary (B) outcomes, stratified by presence vs. absence of ischaemia and obesity.
In obese patients, univariable analysis for association with primary and secondary outcomes is summarized in Tables 2 and 3. Age, sex, history of diabetes, history of smoking, prior MI, prior PCI, prior CHF, LVEF, presence of ischaemia, and presence of LGE were all significantly associated with primary outcome. In a multivariable Cox regression model, inducible ischaemia [HR: 2.14; 95% confidence interval (CI): 1.30–3.50; P = 0.003] and presence of LGE (HR: 3.09; 95% CI: 1.83–5.22; P < 0.001) remained independently associated with primary outcome, after adjusting for age, BMI, history of smoking, history of prior MI, and history of prior CHF (Table 2). After adjusting for the same covariates, each segment of ischaemia (HR: 1.09; 95% CI: 1.03–1.16; P = 0.005) and LGE (HR: 1.08; 95% CI: 1.03–5.22; P = 0.001) maintained significant association with the primary outcome. In the multivariable model, inducible ischaemia (HR 2.27; 95% CI: 1.61–3.19; P < 0.001), and LGE (HR: 2.28; 95% CI: 1.61–3.23; P < 0.001) were also independently associated with secondary outcome. There was no significant interaction between BMI and CMR-detected ischaemia (P = 0.21) or LGE (P = 0.60). Visual inspection of the log–log survival curves and calculation of the Schoenfeld residuals did not demonstrate violation of the proportionality assumption.
Table 2.
Univariable and multivariable cox association of clinical and stress cardiac magnetic resonance indices with primary outcome in patients with obesity
| Characteristics | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Demographics | ||||||
| Age (per year) | 1.02 | 1.00–1.04 | 0.03 | 1.03 | 1.01–1.05 | 0.009 |
| Female | 0.53 | 0.33–0.83 | 0.006 | — | ||
| BMI (per 1 kg/m2) | 1.01 | 0.97–1.05 | 0.62 | 1.02 | 0.98–1.06 | 0.27 |
| Cardiac risk factors | ||||||
| Hypertension | 2.04 | 0.98–4.23 | 0.06 | — | ||
| Hypercholesterolaemia | 1.26 | 0.77–2.06 | 0.36 | — | ||
| Diabetes mellitus | 1.69 | 1.09–2.63 | 0.02 | — | ||
| Smoking | 2.40 | 1.54–3.75 | <0.001 | 1.97 | 1.25–3.10 | 0.003 |
| Family history of CAD | 0.69 | 0.41–1.17 | 0.17 | — | ||
| History of PCI | 2.69 | 1.72–4.20 | <0.001 | — | ||
| History of MI | 4.27 | 2.70–6.75 | <0.001 | 1.98 | 1.20–3.27 | 0.008 |
| History of HF | 4.18 | 2.57–6.81 | <0.001 | 2.36 | 1.42–3.92 | 0.001 |
| Stress CMR | ||||||
| LVEF (per +5% Δ) | 0.82 | 0.77–0.88 | <0.001 | — | ||
| Presence of inducible ischaemia | 3.86 | 2.43–6.12 | <0.001 | 2.14 | 1.30–3.50 | 0.003 |
| Extent of ischaemia (per segment) | 1.10 | 1.04–1.16 | 0.001 | — | ||
| Presence of LGE | 5.81 | 3.70–9.11 | <0.001 | 3.09 | 1.83–5.22 | <0.001 |
| Extent of LGE (per segment) | 1.12 | 1.08–1.17 | <0.001 | — | ||
BMI, body mass index; CMR, cardiovascular magnetic resonance; CI, confidence interval; CAD, coronary artery disease; HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; LGE, late gadolinium enhancement; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Table 3.
Univariable and multivariable Cox association of clinical and stress cardiac magnetic resonance indices with secondary outcome in patients with obesity
| Characteristics | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Demographics | ||||||
| Age (per year) | 1.01 | 1.00–1.02 | 0.11 | 1.01 | 1.00–1.03 | 0.13 |
| Female | 0.82 | 0.61–1.11 | 0.19 | — | ||
| BMI (per 1 kg/m2) | 1.02 | 0.99–1.04 | 0.14 | 1.03 | 1.00–1.05 | 0.04 |
| Cardiac risk factors | ||||||
| Hypertension | 2.17 | 1.31–3.57 | 0.002 | — | ||
| Hypercholesterolemia | 1.44 | 1.03–2.03 | 0.04 | — | ||
| Diabetes mellitus | 1.49 | 1.11–2.02 | 0.009 | — | ||
| Smoking | 1.69 | 1.25–2.29 | 0.001 | 1.41 | 1.04–1.91 | 0.03 |
| Family history of CAD | 0.95 | 0.68–1.32 | 0.75 | — | ||
| History of PCI | 2.96 | 2.19–4.00 | <0.001 | — | ||
| History of MI | 3.24 | 2.34–4.48 | <0.001 | 1.89 | 1.32–2.73 | 0.001 |
| History of HF | 3.10 | 2.19–4.41 | <0.001 | 2.16 | 1.50–3.11 | <0.001 |
| Stress CMR | ||||||
| LVEF (per +5% Δ) | 0.84 | 0.80–0.88 | <0.001 | — | ||
| Presence of inducible ischaemia | 3.52 | 2.55–4.84 | <0.001 | 2.27 | 1.61–3.19 | <0.001 |
| Extent of ischaemia (per segment) | 1.11 | 1.07–1.15 | <0.001 | — | ||
| Presence of LGE | 3.89 | 2.89–5.24 | <0.001 | 2.28 | 1.61–3.23 | <0.001 |
| Extent of LGE (per segment) | 1.09 | 1.06–1.12 | <0.001 | — | ||
BMI, body mass index; CMR, cardiovascular magnetic resonance; CI, confidence interval; CAD, coronary artery disease; HR, hazard ratio; HF, heart failure; LVEF, left ventricular ejection fraction; LGE, late gadolinium enhancement; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Downstream testing, revascularization, and cost
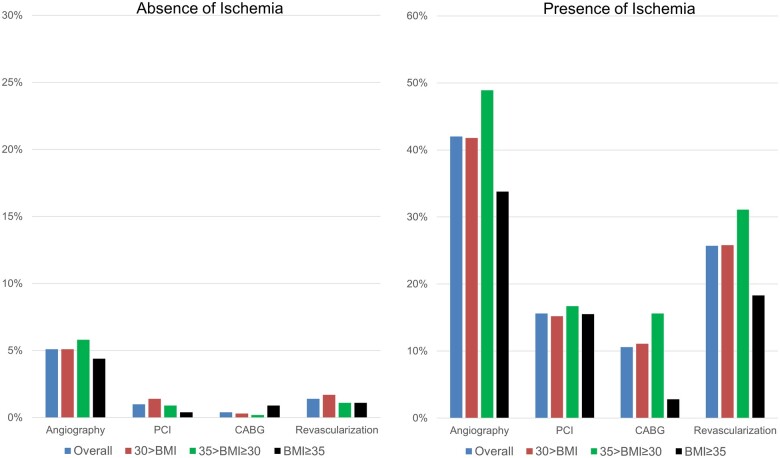
Referral rates to invasive coronary angiography and subsequent performance of revascularization procedures at 90 days following index CMR, stratified by CMR ischaemia, and BMI categories are shown in Figure 4. Rates of angiography according to extent of ischaemia are shown in Supplementary data online, Figure S2. Overall, the rate of referral to coronary angiography and subsequent revascularization was 5.1% and 1.4%, respectively, for those without ischaemia and 42.0% and 25.7%, respectively, for those with ischaemia. In patients with ischaemia, the rates of referral to angiography and revascularization were not affected by the category of obesity (P = 0.16 and P = 0.18, respectively), although the rates of CABG at 90 days was lower in patients with BMI ≥35. Potential reasons for this finding include lower burden of ischaemia amongst obese patients in our cohort and clinicians’ preference to using PCI over CABG in obese patients. Similar referral patterns in obese patients have been previously reported in the literature.13
Figure 4.
Coronary angiography and revascularization at 90 days. Referral to invasive coronary angiography and revascularization at 90-day post-stress cardiac magnetic resonance imaging according to BMI category, by the presence (right panel) and absence (left panel) of ischaemia. BMI, body mass index.
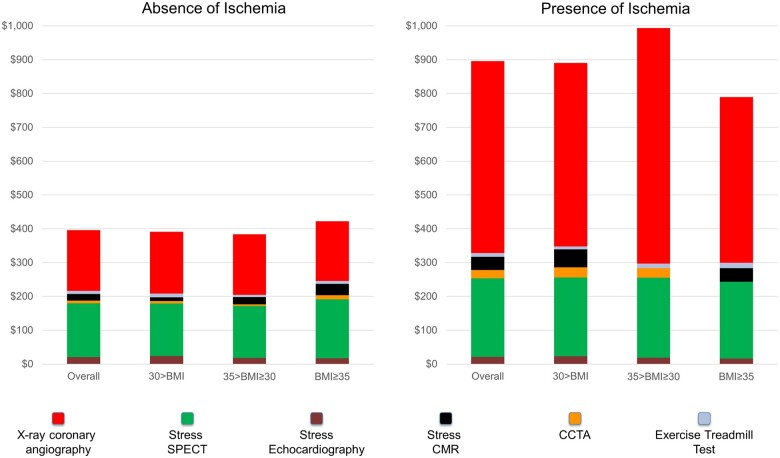
At 4 year of follow-up, average cumulative cost per patient spent on downstream ischaemic testing was $896 in those with ischaemia vs. $396 in those without (P < 0.001). Figure 5 illustrates the average cost according to CMR findings and BMI categories. Overall, coronary angiography and SPECT accounted for most of spending on downstream ischaemia testing. In patients with ischaemia, coronary angiography was the main driver of increased costs. The overall amount of spending as well as the proportion of spending accounted by the different modalities did not significantly differ with increasing BMI.
Figure 5.
Costs of downstream ischaemia testing at 4 years. Cumulative costs of downstream cardiac tests incurred during follow-up with breakdown by modality and across BMI categories, by presence (right panel) and absence (left panel) of ischaemia. Costs are in US dollars spent per patient. BMI, body mass index.
Discussion
In this multicentre cohort with long-term follow-up, we observed several key findings. First, an obese body habitus did not appear to diminish the prognostic value of stress CMR. Across BMI categories, stress CMR findings of ischaemia and LGE maintained strong association with cardiovascular events. In obese patients whose CMR had no ischaemia, annualized hard event rate was low at ≤1%. Secondly, obese body habitus did not lead to deteriorated image quality in the current CMR environment. We observed that only 2% of patients (across the BMI strata) required sedation and diagnostic image quality was achieved in >95% of cases for all three key CMR sequences. Finally, downstream referral to invasive angiography and revascularization, as well as long-term cost for ischaemia testing, was not influenced by BMI categories.
Non-invasive evaluation for coronary disease remains an important challenge, especially in the growing obese population. The two most widely used imaging modality in the USA, namely, SPECT perfusion and stress echocardiography14 have limitations in this population. Echocardiography remains operator dependent and image quality may be limited by poor acoustic windows. One study performed in bariatric patients reported a 45% prevalence for technically difficult studies, although the use of contrast agents significantly alleviated these concerns.15 In obese patients referred for clinical evaluation, Shah et al.16 reported low annualized event rate of 0.95% in patients with negative stress echocardiography. Follow-up in that study, however, was relatively short with mean of 18 months; hence intermediate- and long-term prognoses remain unclear.
Similarly, SPECT perfusion remains challenging in the obese individual. Because radiotracer dosing is determined by patient weight, obese individuals are subject to substantially higher exposure to ionizing radiation. To limit radiation exposure and improve image quality, a 2-day imaging protocol is generally favoured,17 which can be more logistically challenging for patients. Obese patients are also vulnerable to soft tissue attenuation, which can lower test specificity, although the systematic use of attenuation correction can improve image interpretation.18 More recently, the development of gamma cameras using cadmium–zinc telluride detectors have demonstrated improved image quality despite lower radiotracer use. Such technology has been studied in obese patients and demonstrated good negative prognostic value.19 Cardiac rubidium 82 (Rb-82) positron emission tomography (PET) has also been studied in obese patients, with improved specificity compared with SPECT.20 In a study of 7061 patients undergoing clinical PET studies, Chow et al.21 reported very low (<0.5%) rate of annual cardiac death in both overweight and obese patients with negative studies.
Stress CMR has been shown in many studies to be an excellent prognosticating tool in patients suspected of having CAD,22–25 providing multiparametric highly accurate information on ventricular function, inducible ischaemia, and prior infarction. For obese patients, stress CMR represents a reasonable alternative option, as image quality is not subject to poor acoustic window or attenuation artefacts, and there is no need for ionizing radiation. Few studies have, however, directly evaluated the impact of BMI on the predictive performance of stress CMR. In a study of 285 obese patients referred for vasodilator stress CMR, Shah et al.6 determined that diagnostic quality imaging was achieved in >89% of patients. In those who underwent stress CMR protocol, sedation was used in only 7%. Importantly, at a median follow-up period of 2.1 years, stress CMR was predictive of hard cardiovascular events. Patients with neither ischaemia nor LGE had an annualized event rate of 0.3%. Another study, reported by Kelle et al.,26 examined the prognostic impact of BMI on dobutamine stress CMR. In 501 obese patients with suspected or known CAD, dobutamine stress CMR had significant prognostic value at mean follow-up of 3 years. Obese patients with negative studies had a cumulative hard event rate of 0.6%. Unlike vasodilator perfusion studies, dobutamine stress CMR does not routinely use contrast agents, instead focusing on inducible wall motion abnormalities. As such, information on the presence of prior infarcts via LGE is not readily available. The results presented within SPINS significantly expand upon prior literature on the prognostic value of stress CMR in obese patients. In our cohort of 1177 obese patients, stress CMR demonstrated excellent negative long-term predictive value. In this multicentre study, annual hard event rates were <1% in patients without ischaemia and LGE, and this held true irrespective of BMI category.
Previous reports have examined the impact of BMI on stress echocardiography and SPECT image quality. There has been, to our knowledge, no such specific analysis for stress CMR. Although image quality in CMR is not subject to soft tissue attenuation or poor acoustic windows, there are theoretical means by which elevated BMI could affect image quality. Large body habitus often requires increased field of view to prevent wrap artefacts, which, in turn, increases voxels size and decreases spatial resolution. In SPINS, we examined image quality for cine, perfusion, and LGE sequences and found no stepwise effect of BMI on the proportion of diagnostic studies. Within every BMI category, >95% of studies were categorized as diagnostic.
The increased prevalence of obesity has been associated with a dramatic increase in overall cost of medical spending.27 SPINS is the first study to examine the impact of BMI on subsequent referral to invasive investigations and revascularization in patients who undergo stress CMR. Although the presence and extent of myocardial ischaemia9 were significantly associated with early (< 90 days) referral to angiography and coronary revascularization, this association was not modified by the presence and category of obesity. In addition, cost of downstream investigations for myocardial ischaemia, up to the 4 years of mandated follow-up period, was not affected by the presence and category of obesity. This finding is important because obese patients without myocardial ischaemia are not subject to increased downstream testing, compared with a non-obese reference group, and likely reflects the high proportion of good-quality studies throughout the BMI categories.
Limitations
A few limitations of our study deserve mention. First, SPINS was a retrospective study that predominantly included higher volume CMR centres and hence it is uncertain whether the results could generalize to less experienced centres. Secondly, our study only included patients with completed CMR studies, and hence we were unable to determine the proportion of patients who were referred but aborted a CMR study across all the sites, as no DICOM images were generated. However, at the Brigham and Women’s Hospital in Boston, this represents <2% of our referred patients. Thirdly, due to study design, we cannot ascertain the presence or extent of a bias due to local referral patterns in the obese population. Some morbidly obese patients may not fit into current scanner specifications. Nevertheless, in our study, 50% met the definition of obesity of BMI ≥ 30, which is similar to USA data on prevalence of obesity in the general population.1 Finally, assessment of study quality were qualitative and did not include any specific measures of signal to noise ratio or delta signal intensity during first pass perfusion.28 Despite these limitations, our study demonstrated that a stress CMR can achieve adequate diagnostic quality and is an effective imaging tool in risk stratifying obese patients.
Conclusions
Risk stratification by vasodilator stress CMR was effective in obese patients referred for chest pain syndromes. Irrespective of BMI category, a study without ischaemia or LGE was associated with low yearly incidence (<1%) of hard cardiovascular events. Qualitative scoring of cine, perfusion, and LGE sequences demonstrates that diagnostic quality studies are achieved in the vast majority of cases, irrespective of body habitus.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Funding
The Stress CMR Perfusion Imaging in the United States (SPINS) Study of the Society for Cardiovascular Magnetic Resonance (SCMR) Registry was funded by the SCMR, using a research grant jointly sponsored by Siemens Healthineers (Erlangen, Germany) and Bayer AG (Leverkusen, Germany). These sponsors to SCMR provided financial support for the study but did not play a role in study design, data collection, analysis, interpretation, or manuscript drafting. Clinical trial registration information: http://www.clinicaltrials.gov. Unique identifier: NCT03192891.
Conflict of interest: P.A. has received research funding from the Swiss National Science Foundation (grant P2LAP3_184037), the Novartis Foundation for Medical-Biological Research, the Bangerter-Rhyner Foundation, and the SICPA Foundation. A.E.A. has research agreements with Siemens, Bayer, and Circle Cardiovascular Imaging. W.P.B. is the principal investigator of one of the Bayer-sponsored GadaCAD2 (Gadavist-Enhanced Cardiac Magnetic Resonance Imaging to Detect Coronary Artery Disease) sites. A.R.P. has received a research grant from and served on the Speakers Bureau of Astellas. J.S.-M. has research agreements with Siemens and serves on the Advisory Board of Bayer. M.S. has received non-monetary research support from Siemens Healthineers. S.V.R. and O.P.S. receive institutional research support from Siemens. R.Y.K. receives research support from Myokardia Inc. and Alnylam Pharmaceuticals. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Supplementary Material
References
- 1. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL.. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA 2018;319:1723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marques A, Peralta M, Naia A, Loureiro N, de Matos MG.. Prevalence of adult overweight and obesity in 20 European countries, 2014. Eur J Public Health 2018;28:295–300. [DOI] [PubMed] [Google Scholar]
- 3. Mangold S, Wichmann JL, Schoepf UJ, Litwin SE, Canstein C, Varga-Szemes A. et al. Coronary CT angiography in obese patients using 3(rd) generation dual-source CT: effect of body mass index on image quality. Eur Radiol 2016;26:2937–46. [DOI] [PubMed] [Google Scholar]
- 4. Leschka S, Stinn B, Schmid F, Schultes B, Thurnheer M, Baumueller S. et al. Dual source CT coronary angiography in severely obese patients: trading off temporal resolution and image noise. Invest Radiol 2009;44:720–7. [DOI] [PubMed] [Google Scholar]
- 5. Ghanem MA, Kazim NA, Elgazzar AH.. Impact of obesity on nuclear medicine imaging. J Nucl Med Technol 2011;39:40–50. [DOI] [PubMed] [Google Scholar]
- 6. Shah RV, Heydari B, Coelho-Filho O, Abbasi SA, Feng JH, Neilan TG. et al. Vasodilator stress perfusion CMR imaging is feasible and prognostic in obese patients. JACC Cardiovasc Imaging 2014;7:462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wallace EL, Morgan TM, Walsh TF, Dall'Armellina E, Ntim W, Hamilton CA. et al. Dobutamine cardiac magnetic resonance results predict cardiac prognosis in women with known or suspected ischemic heart disease. JACC Cardiovasc Imaging 2009;2:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kwong RY, Petersen SE, Schulz-Menger J, Arai AE, Bingham SE, Chen Y, The Global Cardiovascular Magnetic Resonance Registry (GCMR) Investigators et al. The global cardiovascular magnetic resonance registry (GCMR) of the society for cardiovascular magnetic resonance (SCMR): its goals, rationale, data infrastructure, and current developments. J Cardiovasc Magn Reson 2017;19:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kwong RY, Ge Y, Steel K, Bingham S, Abdullah S, Fujikura K. et al. Cardiac magnetic resonance stress perfusion imaging for evaluation of patients with chest pain. J Am Coll Cardiol 2019;74:1741–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG. et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J Cardiovasc Magn Reson 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL.. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obes 1998;22:39–47. [DOI] [PubMed] [Google Scholar]
- 12. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A. et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 2015;66:403–69. [DOI] [PubMed] [Google Scholar]
- 13. De Santo LS, Moscariello C, Zebele C.. Implications of obesity in cardiac surgery: pattern of referral, physiopathology, complications, prognosis. J Thorac Dis 2018;10:4532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kini V, McCarthy FH, Dayoub E, Bradley SM, Masoudi FA, Ho PM. et al. Cardiac stress test trends among US patients younger than 65 years, 2005-2012. JAMA Cardiol 2016;1:1038–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Supariwala A, Makani H, Kahan J, Pierce M, Bajwa F, Dukkipati SS. et al. Feasibility and prognostic value of stress echocardiography in obese, morbidly obese, and super obese patients referred for bariatric surgery. Echocardiography 2014;31:n/a–85. [DOI] [PubMed] [Google Scholar]
- 16. Shah BN, Zacharias K, Pabla JS, Karogiannis N, Calicchio F, Balaji G. et al. The clinical impact of contemporary stress echocardiography in morbid obesity for the assessment of coronary artery disease. Heart 2016;102:370–5. [DOI] [PubMed] [Google Scholar]
- 17. Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ.. ASNC imaging guidelines for SPECT nuclear cardiology procedures: stress, protocols, and tracers. J Nucl Cardiol 2016;23:606–39. [DOI] [PubMed] [Google Scholar]
- 18. Duvall WL, Croft LB, Corriel JS, Einstein AJ, Fisher JE, Haynes PS. et al. SPECT myocardial perfusion imaging in morbidly obese patients: image quality, hemodynamic response to pharmacologic stress, and diagnostic and prognostic value. J Nucl Cardiol 2006;13:202–9. [DOI] [PubMed] [Google Scholar]
- 19. De Lorenzo A, Peclat T, Amaral AC, Lima RS.. Prognostic evaluation in obese patients using a dedicated multipinhole cadmium-zinc telluride SPECT camera. Int J Cardiovasc Imaging 2016;32:355–61. [DOI] [PubMed] [Google Scholar]
- 20. Freedman N, Schechter D, Klein M, Marciano R, Rozenman Y, Chisin R.. SPECT attenuation artifacts in normal and overweight persons: insights from a retrospective comparison of Rb-82 positron emission tomography and TI-201 SPECT myocardial perfusion imaging. Clin Nucl Med 2000;25:1019–23. [DOI] [PubMed] [Google Scholar]
- 21. Chow BJ, Dorbala S, Di Carli MF, Merhige ME, Williams BA, Veledar E. et al. Prognostic value of PET myocardial perfusion imaging in obese patients. JACC Cardiovasc Imaging 2014;7:278–87. [DOI] [PubMed] [Google Scholar]
- 22. Bodi V, Sanchis J, Lopez-Lereu MP, Nunez J, Mainar L, Monmeneu JV. et al. Prognostic value of dipyridamole stress cardiovascular magnetic resonance imaging in patients with known or suspected coronary artery disease. J Am Coll Cardiol 2007;50:1174–9. [DOI] [PubMed] [Google Scholar]
- 23. Bingham SE, Hachamovitch R.. Incremental prognostic significance of combined cardiac magnetic resonance imaging, adenosine stress perfusion, delayed enhancement, and left ventricular function over preimaging information for the prediction of adverse events. Circulation 2011;123:1509–18. [DOI] [PubMed] [Google Scholar]
- 24. Shah R, Heydari B, Coelho-Filho O, Murthy VL, Abbasi S, Feng JH. et al. Stress cardiac magnetic resonance imaging provides effective cardiac risk reclassification in patients with known or suspected stable coronary artery disease. Circulation 2013;128:605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heitner JF, Kim RJ, Kim HW, Klem I, Shah DJ, Debs D. et al. Prognostic value of vasodilator stress cardiac magnetic resonance imaging: a multicenter study with 48000 patient-years of follow-up. JAMA Cardiol 2019;4:256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kelle S, Giusca S, Buss SJ, Fleck E, Katus HA, Korosoglou G.. BMI does not influence the prediction of cardiac events using stress CMR. Int J Cardiol 2015;179:31–3. [DOI] [PubMed] [Google Scholar]
- 27. Finkelstein EA, Trogdon JG, Cohen JW, Dietz W.. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28:w822–31. [DOI] [PubMed] [Google Scholar]
- 28. Klinke V, Muzzarelli S, Lauriers N, Locca D, Vincenti G, Monney P. et al. Quality assessment of cardiovascular magnetic resonance in the setting of the European CMR registry: description and validation of standardized criteria. J Cardiovasc Magn Reson 2013;15:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.