Abstract
Context
Male hypogonadism is associated with low bone mineral density (BMD) and increased fragility fracture risk. Patients with type 2 diabetes (T2D) have relatively higher BMD, but greater fracture risk.
Objective
Evaluate the skeletal response to testosterone therapy in hypogonadal men with T2D compared with hypogonadal men without T2D.
Methods
Single arm, open-label clinical trial (NCT01378299) involving 105 men (40-74 years old), with average morning testosterone <300 ng/dL. Subjects were injected intramuscularly with testosterone cypionate (200 mg) every 2 weeks for 18 months. Testosterone and estradiol were assessed by liquid chromatography/mass spectrometry; serum C-terminal telopeptide of type I collagen (CTX), osteocalcin and sclerostin by enzyme-linked immunosorbent assay; glycated hemoglobin (HbA1c) by high-performance liquid chromatography, areal BMD (aBMD) and body composition by dual-energy x-ray absorptiometry; tibial volumetric BMD (vBMD) and bone geometry by peripheral quantitative computed tomography.
Results
Among our population of hypogonadal men, 49 had T2D and 56 were non-T2D. After 18 months of testosterone therapy, there were no differences in circulating testosterone and estradiol between the groups. Hypogonadal men with T2D had increased osteocalcin, reflecting increased osteoblast activity, compared with non-T2D men (P < .01). T2D men increased lumbar spine aBMD (P < .05), total area at 38% tibia (P < .01) and periosteal and endosteal circumferences at the same site (P < .01 for both). T2D men had reduced tibial vBMD (P < .01), but preserved bone mineral content (P = .01). Changes in HbA1c or body composition were similar between the 2 groups.
Conclusion
Testosterone therapy results in greater improvements in the skeletal health of hypogonadal men with T2D than their nondiabetic counterparts.
Keywords: Type 2 diabetes, hypogonadism, bone geometry, bone mineral density, testosterone
Type 2 diabetes (T2D) and male hypogonadism are both considered risk factors for fragility fractures (1-3). While patients with T2D have an increased risk despite normal or relatively high bone mineral density (BMD) (1, 3, 4), low testosterone (T) levels in hypogonadal men result in low BMD (2, 5). Although BMD increases with increasing body mass index (BMI) even in hypogonadal men (6), and T2D is often associated with obesity, the increased risk of fragility fractures in T2D patients was demonstrated to be independent of BMI (1, 7, 8). For a given BMD, in fact, T2D patients have reduced skeletal strength and increased cortical porosity compared with age-matched individuals without diabetes (9). On the other hand, the reduced T levels in hypogonadal men results in low circulating estrogen, the main sex hormone responsible for skeletal health, lack of which leads to low BMD, fragility fractures, and disability (2, 10).
A substantial percentage of men with T2D suffer from hypogonadism (11, 12). The interplay between glucose and androgen metabolism has been suggested by reports showing that low T is associated with glucose intolerance (13) and that hyperglycemia is commonly found in men with hypogonadism (12). It is thus not surprising to note that T2D is considered as risk factor for hypogonadism and that high T levels are protective against T2D development (14). Considering the common association between the 2 conditions, there is a suggestion to screen for low T levels in men with T2D (15). Despite the growing awareness of the association between T2D and hypogonadism, there is no established treatment for subjects suffering from both. Whether the approved treatment for osteoporosis or hypogonadism would be helpful for patients with both, T2D and low T remains unclear, as there are no randomized controlled studies investigating the effect of these agents in these patients (16).
The majority of drugs used in the treatment of osteoporosis have an antiresorptive action, while the use of anabolic agents is reserved only to patients with severe osteoporosis. Our group reported that hypogonadal men with T2D have suppressed bone turnover similar to subjects who only have T2D (17). Accordingly, the use of antiresorptive drugs in hypogonadal patients with T2D may result bone turnover oversuppression (18). Similarly, T use for the treatment of male hypogonadism may result in the oversuppression of bone turnover in those who also have T2D, since the effect of T is mediated by its conversion to estradiol (E2), an inhibitor of bone resorption. Although T has been shown to improve bone mineral density and bone quality in men with hypogonadism, with the exception of a small published study reporting an increase in osteocalcin via T therapy (11), little information is available on the skeletal effect of T supplementation in hypogonadal men with T2D (19, 20). The objective of this study was to evaluate the effect of T replacement in men with both T2D and hypogonadism compared with hypogonadal men without T2D. In this study, we investigated the skeletal effects of T therapy on bone turnover, BMD, and bone structure and geometry in men with both T2D and hypogonadism. We hypothesized that T therapy improves the skeletal health of hypogonadal men with T2D because of its anabolic effect on the osteoblasts.
Materials and Methods
Study Design and Study Population
This study is a secondary analysis of longitudinal data obtained from hypogonadal male veterans who volunteered to participate in an open label clinical trial investigating the effect of genetics on T therapy (21), carried out from October 2016 to November 2017 (ClinicalTrials.gov identifier: NCT01378299). Information regarding study design, inclusion and exclusion criteria of the subjects, as well as details of T therapy have been published elsewhere (21). In brief, hypogonadism was defined as an average total T of <300 ng/dL from 2 samples taken in the morning. The study was conducted at the New Mexico VA Health Care System (NMVAHCS) and at the Michael E. DeBakey VA Medical Center (MEDVAMC) in accordance with the guidelines of the Declaration of Helsinki for the ethical treatment of human subjects. The protocol was approved by the Institutional Review Boards of the University of New Mexico and of Baylor College of Medicine. Participants were recruited from patients attending the Endocrine, Urology, and Primary Care Clinics of the NMVAHCS and MEDVAMC. Recruitment was accomplished either through flyers or letters to physicians about patients who may qualify for the study. Written informed consent was obtained from each subject. The inclusion criteria were males between 40 and 75 years of age with no medical problems that may prevent them from finishing the study. Exclusion criteria included treatment with bone-acting drugs (eg, bisphosphonates, teriparatide, denosumab, glucocorticoids, sex steroid compounds, selective estrogen receptor modulators, androgen deprivation therapy, and anticonvulsants) and finasteride. Additional exclusion criteria included osteoporosis and history of fragility fractures or diseases known to affect bone metabolism, such as hyperparathyroidism, chronic liver disease, uncontrolled or untreated hyperthyroidism, and significant renal impairment (creatinine of >1.5 mg/dL). Those with a history of prostate cancer, breast cancer, and untreated sleep apnea also met the criteria for exclusion. The presence of T2D was ascertained from the medical history and intake of medications for diabetes. When these data were not available, gycosylated hemoglobin (HbA1c) ≥6.5% at study entry was used as criteria to assess T2D condition. None of our subjects had type 1 diabetes mellitus.
Testosterone Therapy
Therapy consisted of intramuscular injection of 200 mg of T cypionate administered every 2 weeks. The dose was subsequentially adjusted to reach the T serum target level of 17.3 to 27.7 nmol/L (500-800 ng/dL). However, after the third year of the study, upon the direction of the FDA, this target was changed to 17.3 to 20.8 nmol/L (300-600 ng/dL). This change affected the last 6 months of data for 16 subjects at NMVAHCS and of all 15 subjects at MEDVAMC. We did not detect significant difference in T levels among those affected and those not affect by the change as published previously (21). For all subjects, safety monitoring and dose adjustments were performed based on T and prostate-specific antigen (PSA) levels, hematocrit, and lipid profile; dose adjustment modalities have been published elsewhere (21). The participants were given T therapy for 18 months.
Biochemical Measurements
Fasting blood samples were collected at baseline; serum samples were extracted and stored at –80°C until analysis except for the baseline screening of T levels. Baseline serum T represents an average from 2 determinations taken 30 minutes apart between 8:00 and 11:00 am, and measured using automated immunoassay, detection range 10 to 3200 ng/dL (Vitros®, Ortho Clinical Diagnostics, Rochester, NY). The coefficient of variation (CV) for T assay was ≤20% for T <50 ng/dL and ≤10% for T 200 to 1000 ng/dL. At the end of the study, T and E2 for each timepoint were measured by liquid chromatography/mass spectrometry (Mayo Clinic Laboratories, Mayo Clinic, Rochester, MN, USA). Testosterone intra-assay CVs were 7.4, 6.1, 9.0, 2.3, and 0.9% at 0.65, 4.3, 48, 118, and 832 ng/dL, respectively. Interassay CVs were 8.9, 6.9, 4.0, 3.6, and 3.5% at 0.69, 4.3, 45, 117, and 841 ng/dL, respectively. The detection range was 0.5 to 2000 ng/dL. E2 assay sensitivity was 0.23 pg/mL to 405 pg/mL, intra-assay CV was 1.4% to 11.8% and interassay CV was 4.8% to 10.8% (17). Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) were assessed by a third-generation chemiluminescent assay, while alkaline phosphatase (ALP) by Vitros Systems at the Michael E. DeBakey and Albuquerque VA Medical Centers. Fasting glucose was measured using a Unicel DxC 800 auto-analyzer (Beckman Coulter, Fullerton, CA, USA) and HbA1c by high-performance liquid chromatography (Tosoh G8, South San Francisco, CA, USA). The following were measured using enzyme-linked immunosorbent assay kits: C-terminal telopeptide of type I collagen (CTX), marker of bone resorption (Crosslaps; Immunodiagnostic System Inc., Gaithersburg, MD, USA), osteocalcin, marker of bone formation, (Metra OC; Quidel Corporation, San Diego, CA, USA), and sclerostin (TECO medical Sclerostin HS Enzyme Immunoassay Kit, Quidel Corp, San Diego, CA, USA). The CVs for the above assays in our laboratory are <10% and <3.5% for HbA1c.
Body Mass Index and Body Composition
Body weight was measured using a standard weighing scale and height was obtained using a stadiometer. BMI was calculated as body weight in kilograms (kg) divided by the square of the height in meters (m2) and expressed as kg/m2. Body composition assessment was performed by dual-energy x-ray absorptiometry (DXA) (Hologic-Discovery; Hologic Inc, Bedford, MA, USA; Enhanced Whole Body 11.2 software version; Hologic) as described elsewhere (10). Images were analyzed, following the manufacturer’s instructions. The CV for lean and fat mass assessment in our laboratory is 1.5%. BMI and body composition were assessed at baseline, 6, 12 and 18 months.
Areal Bone Mineral Density by Dual Energy X-Ray Absorptiometry
Areal BMD was measured by DXA of the lumbar spine and proximal femur using Hologic Discovery (Hologic Inc, Bedford, MA, USA). Regions of interest in the femur include the total hip and femoral neck. The CVs at our center are ~1.1% for the lumbar spine and 1.2% for the proximal femur (21).
Volumetric Bone Mineral Density, Bone Morphology, and Structure by Peripheral Quantitative Computed Tomography
Peripheral quantitative computed tomography was performed on the left tibia (except for those who had surgery or metal plates on the left tibia where the right tibia was used) using Stratec XCT-2000 (Stratec GmbH, Pforzheim, Germany) as previously described (17). The voxel size was 500 μm, scan speed 30 mm/s, and slice thickness 2.4 mm. The x-ray current was between 0.14 to 0.22 mA and the voltage was 58 kV. Scanning was performed by first obtaining a scout view on the lower leg to identify anatomical landmarks. From the scout view, the cortical endplates of the tibia were identified visually and used as anatomical landmarks. A reference line was fixed to bisect the distal tibial endplate in a transverse plane set at right angles to the long axis of the tibia. Scans were then obtained at regions of interest equal to a percentage of the limb length measured from the distal to proximal direction. A slice was taken at 4% and 38% for assessment of bone volume and bone quality of the tibia.
For bone assessments, trabecular and total vBMD, bone mineral content (BMC) and bone area were measured distally (4%) while cortical and total vBMD, BMC, bone area and cortical thickness more proximally (38%). The short-term CV for these measurements in our center is 0.96%. Different thresholds were applied to separate trabecular, cortical, and soft tissues from each other. At 4% tibia, we applied the following thresholds: 180 mg/cm3 and 45% of the area for the evaluation of total and trabecular vBMD, BMC, and total area; and thresholds 280 mg/cm3 and 400 mg/cm3 for the analysis of the trabecular area. The threshold for separating trabecular and cortical bone at 38% was 710 mg/cm3. All images were reviewed for motion grade by the Principal Investigator and the researcher acquiring the scan at the end of each study.
Statistical Analysis
The experimental design to show the temporal effect of T therapy in T2D consisted of comparison between the 2 groups (T2D and non-T2D) at each of 4 time points (baseline, 6, 12, and 18 months). Data are presented in tables as baseline means and mean percentage change from baseline ± SD. In graphs, percentage change data are plotted as mean percent ± standard error (SE). The analyses were paired analyses; significance within groups are determined by paired t-tests and between the 2 groups are determined by 2-tailed, 2-sample t-tests of the percent differences. P ≤ .05 was considered statistically significant.
Results
A total of 105 subjects participated in the study, 49 had T2D and 56 non-T2D. The clinical features of the study population, including medications for T2D treatment, have been published elsewhere (17). Briefly, there was a significant difference in mean age between the groups; men with T2D were significantly older (63.0 ± 5.9 vs 56.8 ± 9.5 years old, P < .001) and tended to have a higher BMI (33.2 ± 5.3 vs 31.3 ± 5.2 kg/m2, P = .07) than non-T2D men. As expected, HbA1C was higher in men with T2D than in those without T2D (7.9 ± 2.0 vs 5.7 ± 0.4%, P < .001). At 18 months, absolute changes in fasting glucose and HbA1c did not significantly differ between groups (T2D: 0.71 ± 92.89 vs non-T2D: –8.42 ± 17.28 ng/dL for fasting glucose; T2D: –0.05 ± 1.50 vs non-T2D: 0.05 ± 0.26% for HbA1C, with P = .58 and .70, respectively). There were no differences in T levels between the 2 groups at baseline (T2D: 264.33 ± 80.59 ng/dL vs non-T2D: 275.64 ± 87.12 ng/dL, P = .51) and at the end of the study (T2D: 504.26 ± 268.38 ng/dL vs non-T2D: 590.61 ± 283.49 ng/dL, P = .16). There were no between group differences in E2 levels at baseline (T2D: 17.25 ± 5.64 pg/mL vs non-T2D: 16.56 ± 6.74 pg/mL, P = .59) and at the end of the study (T2D: 21.29 ± 21.25 pg/mL vs non-T2D: 29.06 ± 20.15 pg/mL, P = .09). There were no between group differences in FSH (T2D: 7.4 ± 5.7 vs non-T2D: 6.8 ± 9.6 mIU/mL, P = .65) and LH (T2D: 4.8 ± 3.8 vs non-T2D: 4.0 ± 6.1 mIU/mL, P = .41) levels at baseline. Twelve of the 56 subjects without and 17 of 49 with T2D did not finish the 18-month study, but the dropout rate between the groups was not statistically significant (P = .19).
Bone Turnover
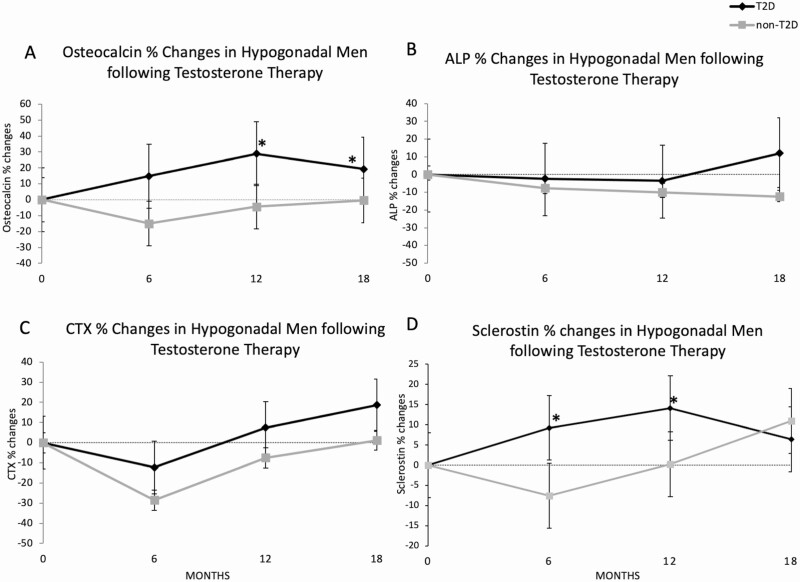
Analysis of bone turnover markers shows significantly lower osteocalcin and CTX at baseline in men with T2D but comparable sclerostin and ALP levels between the groups (Table 1). With T therapy, patients with T2D experienced an increase in osteocalcin levels at 12 and 18 months compared with non-T2D, who experienced a suppression at the 6- and 12-month time points (Fig. 1A). The between group difference in osteocalcin levels was significant after 12 and 18 months of T therapy (P < .001) (Table 1 and Fig. 1A). No significant changes were observed for ALP; similar reduction in both groups at 6 and 12 months followed by a slight increase in the T2D patients at 18 months were found (Fig. 1B). We did not detect significant differences in the changes in CTX between the groups after T therapy; both groups showed the same degree of CTX suppression within the first 6 months of therapy which seemed to wear off with continued treatment in men with T2D; although no between group differences were noted at all timepoints, mean CTX of the T2D group was above baseline at 18 months compared with the persistent suppression observed in non-T2D men (Fig. 1C). Sclerostin levels increased in men with T2D at 6 and 12 months compared with non-T2D men who experienced a reduction (P < .05 for both). However, there was no significant difference in sclerostin levels changes between the groups at 18 months (Fig. 1D).
Table 1.
Baseline levels and percent changes in bone turnover markers with testosterone therapy according to the presence of T2D
| T2D | non-T2D | P value | |
|---|---|---|---|
| CTX | |||
| Baseline (ng/mL) | 0.25 ± 0.14 | 0.40 ± 0.20 | <.0001 |
| 6 months (%) | –12.3 ± 70.7 | –28.5 ± 27.6 | .92 |
| 12 months (%) | 7.4 ± 55.9 | –7.5 ± 41.6 | .49 |
| 18 months (%) | 18.6 ± 69.2 | 1.2 ± 62.8 | .20 |
| Osteocalcin | |||
| Baseline (ng/mL) | 5.15 ± 3.04 | 7.44 ± 5.10 | .01 |
| 6 months (%) | 14.8 ± 64.5 | –15.0 ± 46.3 | .10 |
| 12 months (%) | 28.9 ± 107.1 | –4.3 ± 81.8 | <.05 |
| 18 months (%) | 19.3 ± 78.1 | –0.4 ± 87.8 | <.001 |
| Alkaline phosphatase | ` | ||
| Baseline (IU/L) | 82,2 ± 22.0 | 80.4 ± 21.0 | .67 |
| 6 months (%) | –2.32 ± 46.0 | –7.7 ± 14.7 | .45 |
| 12 months (%) | –3.5 ± 29.5 | –10.0 ± 16.5 | .22 |
| 18 months (%) | 12.0 ± 102.4 | –12.4 ± 16.2 | .18 |
| Sclerostin | |||
| Baseline (ng/mL) | 0.84 ± 0.22 | 0.81 ± 0.28 | .49 |
| 6 months (%) | 9.2 ± 35.3 | –7.6 ± 29.6 | <.05 |
| 12 months (%) | 14.1 ± 41.9 | 0.2 ± 44.3 | <.05 |
| 18 months (%) | 6,4 ± 44.8 | 10.9 ± 47.0 | .58 |
Values are reported as means ± SD. Significant P values are reported in bold.
Abbreviation: T2D, type 2 diabetes mellitus.
Figure 1.
Changes in bone turnover markers and sclerostin after 18 months of testosterone therapy. Osteocalcin (A), alkaline phosphatase (ALP) (B), C-terminal telopeptide of type I collagen (CTX) (C), and sclerostin (D) percentage changes following 6, 12, and 18 months of testosterone therapy in hypogonadal patients with (T2D) and without (non-T2D) type 2 diabetes.
Areal Bone Mineral Density
Baseline total hip aBMD was higher in the T2D group than in the non-T2D one (P = .02). A detailed information of the baseline characteristics of our population can be found in our previously published article by Colleluori et al. (17) (also Table 1 (23)). Comparison of changes in aBMD adjusted for age between T2D and non-T2D hypogonadal men after T therapy showed no significant differences at all sites except for the lumbar spine aBMD at 18 months. At this timepoint, T2D men exhibited a significant increase in lumbar spine BMD (P < .05) compared with non-T2D men (Table 2).
Table 2.
Percent changes in areal bone mineral density with testosterone therapy in hypogonadal men with and without T2D
| T2D | non-T2D | P value | |
|---|---|---|---|
| Spine (%) | |||
| 6 months | 2.6 ± 3.3 | 1.4 ± 2.7 | .65 |
| 12 months | 3.3 ± 2.9 | 2.6 ± 2.6 | .47 |
| 18 months | 4.5 ± 3.4 | 3.2 ± 3.4 | <.05 |
| Total hip (%) | |||
| 6 months | 0.2 ± 2.7 | 0.5 ± 3.4 | .95 |
| 12 months | 0.2 ± 2.8 | 0.7 ± 4.0 | .94 |
| 18 months | 1.3 ± 2.8 | 0.5 ± 3.2 | .13 |
| Femoral neck (%) | |||
| 6 months | 0.2 ± 4.8 | –0.3 ± 4.3 | .39 |
| 12 months | 0.4 ± 4.8 | 0.0 ± 3.6 | .90 |
| 18 months | 1.5 ± 5.3 | –0.8 ± 4.2 | .18 |
| Whole body (%) | |||
| 6 months | 0.8 ± 1.9 | 0.8 ± 1.9 | .82 |
| 12 months | 0.9 ± 1.7 | 1.1 ± 2.6 | .34 |
| 18 months | 2.1 ± 2.2 | 1.5 ± 2.3 | .48 |
Values are reported as mean ± SD; all P values are adjusted for age. Significant P values are reported in bold.
Abbreviation: T2D, type 2 diabetes mellitus.
Volumetric Bone Mineral Density
Baseline bone size at the 38% tibia was smaller in the T2D group compared to the non-T2D as previously reported (17) (see Table 1 (23) (Colleluori, 2021 #404)). Analysis of vBMD, bone structure, and morphology by peripheral quantitative computed tomography showed that there were no significant differences in changes in any parameter in the trabecular compartment of bone (4% tibia) (Table 3). However, significant differences in the changes in the cortical bone compartment (38% tibia) were observed at 18 months. The T2D group experienced an increase in the total bone area, and endosteal and periosteal circumferences (P < .001 for both) and relatively maintained the bone total content (P < .01) compared with the decrease in these parameters experienced by the non-T2D men. However, there was a greater reduction in total BMD at the same site in patients with T2D than euglycemic patients (P < 0.01).
Table 3.
Percent changes in bone volumetric density, content, and geometry with testosterone therapy in hypogonadal men with and without T2D
| Changes at 6 months (%) | Changes at 18 months (%) | |||||
|---|---|---|---|---|---|---|
| 4% tibia | T2D | non-T2D | P value | T2D | non-T2D | P value |
| Trabecular density | –1.1 ± 6.4 | –0.8 ± 7.0 | .70 | –0.9 ± 6.4 | –0.7 ± 8.8 | .49 |
| Trabecular content | –0.8 ± 8.1 | –0.9 ± 9.0 | .59 | –0.2 ± 9.0 | 1.4 ± 14.4 | .71 |
| Trabecular area | 0.2 ± 3.0 | –0.1 ± 3.7 | .43 | 0.7 ± 4.4 | 1.8 ± 6.5 | .30 |
| Total density | –1.2 ± 5.4 | –1.1 ± 5.8 | .39 | –0.3 ± 5.7 | –1.5 ± 7.0 | .66 |
| Total content | –1.0 ± 6.3 | –1.3 ± 6.5 | .87 | 0.3 ± 6.7 | 0.2 ± 8.9 | .80 |
| Total area | 0.2 ± 3.0 | –0.1 ± 3.7 | .44 | 0.7 ± 4.4 | 1.8 ± 6.5 | .29 |
| 38% tibia | ||||||
| Cortical density | –1.2 ± 4.4 | –1.1 ± 4.9 | .36 | –0.8 ± 4.1 | –1.6 ± 5.4 | .09 |
| Cortical content | –2.0 ± 6.2 | –1.5 ± 5.9 | .99 | –1.3 ± 5.0 | –2.5 ± 6.3 | .61 |
| Cortical area | –0.9 ± 3.8 | –0.5 ± 2.1 | .36 | –0.5 ± 1.8 | –1.0 ± 2.1 | .24 |
| Cortical thickness | 0.3 ± 3.8 | 0.6 ± 4.2 | .85 | –1.9 ± 4.2 | –0.1 ± 3.9 | .12 |
| Total density | –0.3 ± 5.4 | –0.5 ± 6.1 | .93 | –2.1 ± 4.9 | –1.2 ± 5.8 | <.01 |
| Total content | –2.0 ± 5.6 | –0.9 ± 8.7 | .73 | –0.6 ± 4.9 | –2.5 ± 6.5 | .01 |
| Total area | –1.6 ± 4.4 | –1.3 ± 5.5 | .43 | 1.7 ± 4.9 | –1.2 ± 5.7 | <.001 |
| Endosteal circumference | –1.7 ± 4.9 | –1.3 ± 7.5 | .75 | 2.9 ± 6.9 | –0.9 ± 7.8 | <.001 |
| Periosteal circumference | –0.8 ± 2.2 | –0.7 ± 2.8 | .43 | 0.8 ± 2.4 | –0.6 ± 2.8 | <.001 |
Values are reported as mean ± SD; all P values are adjusted for age. Significant P values are reported in bold.
Abbreviation: T2D, type 2 diabetes mellitus.
Body Composition
The T2D group had mild weight loss compared with the non-T2D group who gained weight after 18 months of therapy (–0.73 ± 4.05 kg vs 1.69 ± 4.13 kg, respectively, P = .02). However, we did not detect significant differences in changes in body composition as both groups equally reduced total (T2D: –2.107 ± 2.393 and non-T2D: –1.66 ± 5.63 kg) and truncal fat (T2D: –1.0 ± 1.77 and non-T2D: –0.34 ± 2.12 kg) and improved total (T2D: 1.64 ± 2.57 and non-T2D: 2.66 ± 2.44 kg) and appendicular lean mass (T2D: 2.39 ± 7.45 and non-T2D: 1.58 ± 2.11 kg) at 18 months.
Discussion
Our study demonstrates that 18 months of T therapy in hypogonadal men with T2D results in a marked increase in circulating osteocalcin, possibly reflecting increased osteoblastic activity, compared with hypogonadal non-T2D men. Consistently, the same group had increased lumbar spine aBMD and improved tibia cortical bone geometry compared with hypogonadal non-T2D men. We did not detect differences in changes in glucose, HbA1c and body composition between groups. Our results suggest that T therapy can improve bone turnover and skeletal health of hypogonadal men with T2D.
Testosterone levels progressively decline starting at age 40 (15), a process that is not only associated with bone loss and increased risk for fragility fractures (2), but also with the development of T2D (12, 14). Glucose and androgen homeostasis are interconnected, ie, hyperglycemia is associated with reduced production of T, while low T levels are associated with glucose intolerance (5, 12, 14). It is thus not surprising that at least one third of men with T2D have low T levels (11, 12, 24). Fragility fractures are serious complications associated with both hypogonadism and T2D (1, 2). While the association between hypogonadism and fragility fractures is attributed to the bone loss that follows reduction in T levels (2), the mechanisms underlying the association between T2D and fragility fractures are more complex (1). Animal and human studies have consistently showed that T2D is associated with a state of low bone turnover, hence with a defect in bone formation (25-27). The inactive bone remodeling and ensuing accumulation of old bone of poor quality in conjunction with the deposition of advanced glycation end products in collagen may foster bone brittleness and contribute to poor bone quality in T2D (1, 22, 28). T treatment reduces bone turnover, improves BMD (29) and bone quality of hypogonadal men (30). However, according to our previously published data, hypogonadal men with T2D have suppressed bone turnover compared with hypogonadal and eugonadal men without T2D (17). Considering their low bone turnover, whether hypogonadal men with T2D derive skeletal benefits from T therapy remains undetermined.
Our current data suggest that in hypogonadal men with T2D, T therapy leads to a substantial and significant increase in bone formation as reflected by increases in osteocalcin levels after 12 and 18 months of treatment compared with the non-T2D group who experienced reduction in osteocalcin. Furthermore, the trend in bone formation was paralleled by a similar trend of bone resorption at 12 and 18 months, although the size of the changes did not differ significantly between the 2 groups. These data suggest increased bone turnover in T2D patients with low T following T therapy, with coupling of formation and resorption, but the increase in the former far exceeded the increase in the latter at the end of the study. By contrast, both markers of bone formation and resorption were suppressed in hypogonadal subjects without T2D. T-induced inhibition of bone turnover among the non-T2D group was not surprising considering that T bone action is mainly mediated by its conversion into E2, which inhibits bone resorption, a process coupled with bone formation. Taken together these findings suggest a predominant anabolic effect of T on the skeleton of men with T2D, while the antiresorptive effect seems to prevail among those without T2D. In agreement with our findings was the result of the study by Ghanim et al., showing an increase in bone turnover in 22 men with subnormal free T and T2D following 23 weeks of T therapy compared with those treated with placebo (11).
Our study also showed that changes in circulating osteocalcin levels were accompanied by a similar alteration in sclerostin concentrations, which increased at 6 and 12 months in the T2D group compared with the non-T2D group. The significance of these findings in our study remains unclear. Sclerostin is produced by osteocytes which arise from osteoblast and are mechanosensors contributing to bone strength. It is thus possible, that the stimulatory effect of T on osteoblast proliferation and inhibition of osteocyte apoptosis from increased E2 (31) resulted in increased osteoblasts and osteocyte numbers ultimately leading to increased osteocalcin and sclerostin levels, respectively. Furthermore, in a study by Mödder et al. conducted on 59 men whose endogenous T and estrogen production was eliminated by gonadotropin-releasing hormone agonist administration, replacement with E2 in the absence of T led to a reduction in sclerostin levels, and replacement of T in the absence of E2 led to an increase (32). On the other hand, no changes in sclerostin levels were observed when both T and E2 were administered. Men without T2D in our study experienced similar changes in sclerostin levels as the ones observed by Mödder and colleagues following E2 replacement, without T administration. This evidence suggests that in these men, the effect of E2 predominates, a finding consistent with the observed changes in CTX and osteocalcin levels.
Our data showed a greater increase in spine BMD in men with T2D from T therapy than men without T2D. Furthermore, coherent with the stimulatory effect of T on periosteal apposition (33), men with low T and T2D had an increase in total bone area of the tibia compared with the decrease in men without T2D. Consistently, men with low T and T2D increased endosteal and periosteal circumference compared with the decreases in these parameters in men without T2D. The load-bearing capacity of bone depends not only on the amount of bone (BMD) but also on the spatial distribution (ie, size, shape) and intrinsic properties of the materials that make up the bone (34). Androgens contribute to bone strength by their stimulatory effect on periosteal apposition resulting in bone expansion, which explains the wider or bigger bones in men than in women (33). A bigger bone area is associated with a higher capacity to adjust to mechanical loading thereby increasing bone strength and resistance to compression forces that elicit fractures (35). Patients with T2D are reported to have smaller bone size, which may contribute to the increased risk for fractures in these patients (36). Moreover, our previous data demonstrated a smaller bone size in hypogonadal men with T2D than in hypogonadal men without T2D (17). An increase in bone size in these men would mean an improvement in bone geometry, bone quality, and resistance to fractures. Likewise, in the study by Ghanim et al., the authors demonstrated that patients on T had an improvement in bone strength as assessed by hip structure analysis (11). However, this latter study was performed on a small sample size with only 20 patients in the T arm and 14 in the placebo arm finishing the study in a very short period of time. The mild reduction in cortical bone area and content in the non-T2D group could be explained by the trend of bone turnover markers, for which reduction in bone suppression and formation was coupled at all timepoints. However, all markers reached their baseline values by the end of the study, suggesting reversal and the possibility of future improvement in bone area and content at later timepoints if the treatment was continued. On the other hand, the relative reduction in the tibial bone density observed in the T2D group could be the consequence of the increase in bone area which was accompanied by lack of changes in bone mineral content at the same site.
The mechanism for the skeletal effects of androgens in males remains a controversial issue as T, the major androgen, is also the primary source of E2 (by aromatase activity), and E2 has long been recognized as the dominant regulator of bone metabolism in men (37, 38). Added to this complexity is the fact that both androgen and estrogen receptors are detected in several bone cells including osteoblasts, osteoclasts, and mesenchymal stromal cells (latter cells differentiate into osteoblasts) (39, 40). Data from in vitro studies showed that androgen treatment results in osteoblast differentiation, proliferation, matrix production, and mineralization (41). However, to what extent this anabolic effect of androgens on bone influences the skeleton separately from E2 is uncertain. Furthermore, the reason why anabolic effect of T seems to selectively affect the skeleton of men with T2D is unclear. It is possible that T therapy may have differential effects on bone depending on the underlying pathophysiology, ie, mainly antiresorptive in those with high bone turnover and anabolic in those with low bone turnover. Alternatively, although recently disputed (42, 43), some studies suggest that osteocalcin (undercarboxylated) improves insulin sensitivity (44-46) and promotes T secretion by Leydig cells and insulin secretion by the pancreas (44). The improved insulin sensitivity in addition to increased T levels can result in enhanced anabolic effect selectively on the bone (47, 48) of patients with T2D, who at baseline could have insulin resistance in multiple tissues and organs perhaps including bone. However, we did not detect significant differences in changes in A1c or fasting glucose upon T treatment between our study groups, indicating that studies specifically evaluating local T/insulin action in osteoblast are needed (16).
Our study has limitations. We have a dropout rate of 28% and the remaining sample size may have limited our ability to detect highly significant differences in the changes in parameters examined. In addition, the study was conducted for only 18 months and longer duration of follow-up may uncover significant differences in outcomes.
In summary, our results suggest an activation of bone turnover with T therapy in hypogonadal men with T2D who had low bone turnover at baseline, mainly through an anabolic effect on osteoblastic activity. This resulted in a greater increase in spine BMD and a trend for increase in bone size compared with men without T2D. However, our findings should be verified by larger studies with a longer duration of follow-up and with bone strength or fractures as the primary outcome.
Acknowledgments
This study was supported by the resources at the New Mexico VA Health Care System in Albuquerque, NM, USA; the Biomedical Research of New Mexico Albuquerque, NM, USA; the Michael E. DeBakey VA Medical Center, Houston, TX, USA, and the Center for Translational Research in Inflammatory Diseases at the Michael E. DeBakey VA Medical Center, Houston, TX, USA.
Financial Support: This study was supported by the VA Merit Review (5101CX00042403), 1 R01 HD093047-01 and Alkek Fundation; 5R01-HD093047-03.
Clinical Trial Information : Clinical trial registration number NCT01378299 (registered June 22, 2011).
Glossary
Abbreviations
- aBMD
areal bone mineral density
- ALP
alkaline phosphatase
- BMC
bone mineral content
- BMD
bone mineral density
- BMI
body mass index
- CTX
C-terminal telopeptide of type I collagen
- CV
coefficient of variation
- DXA
dual-energy x-ray absorptiometry
- T
testosterone
- T2D
type 2 diabetes
- vBMD
volumetric bone mineral density
Additional Information
Disclosures: Authors have no conflict of interest to disclose. The contents of this manuscript do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Ethics Approval : All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1. Shanbhogue VV, Mitchell DM, Rosen CJ, Bouxsein ML. Type 2 diabetes and the skeleton: new insights into sweet bones. Lancet Diabetes Endocrinol. 2016;4(2):159-173. [DOI] [PubMed] [Google Scholar]
- 2. Finkelstein JS, Klibanski A, Neer RM, Greenspan SL, Rosenthal DI, Crowley WF Jr. Osteoporosis in men with idiopathic hypogonadotropic hypogonadism. Ann Intern Med. 1987;106(3):354-361. [DOI] [PubMed] [Google Scholar]
- 3. Napoli N, Strollo R, Paladini A, Briganti SI, Pozzilli P, Epstein S. The alliance of mesenchymal stem cells, bone, and diabetes. Int J Endocrinol. 2014;2014:690783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL; IOF Bone and Diabetes Working Group . Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13(4):208-219. [DOI] [PubMed] [Google Scholar]
- 5. Russo V, Chen R, Armamento-Villareal R. Hypogonadism, type-2 diabetes mellitus, and bone health: a narrative review. Front Endocrinol (Lausanne). 2020;11:607240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aguirre LE, Colleluori G, Dorin R, et al. Hypogonadal men with higher body mass index have higher bone density and better bone quality but reduced muscle density. Calcif Tissue Int. 2017;101(6):602-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burghardt AJ, Issever AS, Schwartz AV, et al. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95(11):5045-5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vigevano F, Gregori G, Colleluori G, et al. In men with obesity, T2DM is associated with poor trabecular microarchitecture and bone strength, and low bone turnover. J Clin Endocrinol Metab. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kurra S, Fink DA, Siris ES. Osteoporosis-associated fracture and diabetes. Endocrinol Metab Clin North Am. 2014;43(1):233-243. [DOI] [PubMed] [Google Scholar]
- 10. Aguirre LE, Colleluori G, Fowler KE, et al. High aromatase activity in hypogonadal men is associated with higher spine bone mineral density, increased truncal fat and reduced lean mass. Eur J Endocrinol. 2015;173(2):167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghanim H, Dhindsa S, Green K, et al. Increase in osteocalcin following testosterone therapy in men with type 2 diabetes and subnormal free testosterone. J Endocr Soc. 2019;3(8):1617-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Corona G, Monami M, Rastrelli G, et al. Type 2 diabetes mellitus and testosterone: a meta-analysis study. Int J Androl. 2011;34(6 Pt 1):528-540. [DOI] [PubMed] [Google Scholar]
- 13. Navarro G, Xu W, Jacobson DA, et al. Extranuclear actions of the androgen receptor enhance glucose-stimulated insulin secretion in the male. Cell Metab. 2016;23(5):837-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295(11):1288-1299. [DOI] [PubMed] [Google Scholar]
- 15. Bhasin S, Cunningham GR, Hayes FJ, et al. ; Task Force, Endocrine Society . Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559. [DOI] [PubMed] [Google Scholar]
- 16. Russo VC, Chen G, Mediwala R, et al. Testosterone therapy and bone quality in men with diabetes and hypogonadism: Study design and protocol. Contemp Clin Trials Commun. 2021;21:100723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Colleluori G, Aguirre L, Dorin R, et al. Hypogonadal men with type 2 diabetes mellitus have smaller bone size and lower bone turnover. Bone. 2017;99:14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Armamento-Villareal R, Napoli N, Panwar V, Novack D. Suppressed bone turnover during alendronate therapy for high-turnover osteoporosis. N Engl J Med. 2006;355(19):2048-2050. [DOI] [PubMed] [Google Scholar]
- 19. Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81(12):4358-4365. [DOI] [PubMed] [Google Scholar]
- 20. Wang C, Swerdloff RS, Iranmanesh A, et al. Effects of transdermal testosterone gel on bone turnover markers and bone mineral density in hypogonadal men. Clin Endocrinol (Oxf). 2001;54(6):739-750. [DOI] [PubMed] [Google Scholar]
- 21. Aguirre LE, Colleluori G, Robbins D, et al. Bone and body composition response to testosterone therapy vary according to polymorphisms in the CYP19A1 gene. Endocrine. 2019;65(3):692-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Colleluori G, Chen R, Turin CG, et al. Aromatase inhibitors plus weight loss improves the hormonal profile of obese hypogonadal men without causing major side effects. Front Endocrinol (Lausanne). 2020;11:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Colleluori G, Aguirre L, NapoliN, et al. Testosterone therapy effects on bone mass and turnover in hypogonadal men with type 2 diabetes. Supplementary Tables. Datadryads. Deposited February 17, 2021. https://datadryad.org/stash/share/OwHTt9q9rWEfLyKQ0TeQHN2bgej4I5m692EPhLM9ADo [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab. 2004;89(11):5462-5468. [DOI] [PubMed] [Google Scholar]
- 25. Verhaeghe J, van Herck E, Visser WJ, et al. Bone and mineral metabolism in BB rats with long-term diabetes. Decreased bone turnover and osteoporosis. Diabetes. 1990;39(4):477-482. [DOI] [PubMed] [Google Scholar]
- 26. Starup-Linde J, Eriksen SA, Lykkeboe S, Handberg A, Vestergaard P. Biochemical markers of bone turnover in diabetes patients–a meta-analysis, and a methodological study on the effects of glucose on bone markers. Osteoporos Int. 2014;25(6):1697-1708. [DOI] [PubMed] [Google Scholar]
- 27. Manavalan JS, Cremers S, Dempster DW, et al. Circulating osteogenic precursor cells in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97(9):3240-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Piccoli A, Cannata F, Strollo R, et al. Sclerostin regulation, microarchitecture, and advanced glycation end-products in the bone of elderly women with type 2 diabetes. J Bone Miner Res. 2020;35(12):2415- 2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84(6):1966-1972. [DOI] [PubMed] [Google Scholar]
- 30. Snyder PJ, Kopperdahl DL, Stephens-Shields AJ, et al. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: a controlled clinical trial. JAMA Intern Med. 2017;177(4):471-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kousteni S, Bellido T, Plotkin LI, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104(5):719-730. [PubMed] [Google Scholar]
- 32. Mödder UI, Clowes JA, Hoey K, et al. Regulation of circulating sclerostin levels by sex steroids in women and in men. J Bone Miner Res. 2011;26(1):27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vandewalle S, Taes Y, Fiers T, et al. Associations of sex steroids with bone maturation, bone mineral density, bone geometry, and body composition: a cross-sectional study in healthy male adolescents. J Clin Endocrinol Metab. 2014;99(7):E1272-E1282. [DOI] [PubMed] [Google Scholar]
- 34. Bouxsein ML, Seeman E. Quantifying the material and structural determinants of bone strength. Best Pract Res Clin Rheumatol. 2009;23(6):741-753. [DOI] [PubMed] [Google Scholar]
- 35. Haapasalo H, Kontulainen S, Sievänen H, Kannus P, Järvinen M, Vuori I. Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: a peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone. 2000;27(3):351-357. [DOI] [PubMed] [Google Scholar]
- 36. Petit MA, Paudel ML, Taylor BC, et al. ; Osteoporotic Fractures in Men (MrOs) Study Group . Bone mass and strength in older men with type 2 diabetes: the Osteoporotic Fractures in Men Study. J Bone Miner Res. 2010;25(2):285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Falahati-Nini A, Riggs BL, Atkinson EJ, O’Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106(12):1553-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khosla S, Melton LJ 3rd, Atkinson EJ, O’Fallon WM. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab. 2001;86(8):3555-3561. [DOI] [PubMed] [Google Scholar]
- 39. Colvard DS, Eriksen EF, Keeting PE, et al. Identification of androgen receptors in normal human osteoblast-like cells. Proc Natl Acad Sci U S A. 1989;86(3):854-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Manolagas SC, O’Brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol. 2013;9(12):699-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wiren KM. Androgens and bone growth: it’s location, location, location. Curr Opin Pharmacol. 2005;5(6):626-632. [DOI] [PubMed] [Google Scholar]
- 42. Moriishi T, Ozasa R, Ishimoto T, et al. Osteocalcin is necessary for the alignment of apatite crystallites, but not glucose metabolism, testosterone synthesis, or muscle mass. PLoS Genet. 2020;16(5):e1008586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Diegel CR, Hann S, Ayturk UM, et al. An osteocalcin-deficient mouse strain without endocrine abnormalities. PLoS Genet. 2020;16(5):e1008361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oury F, Ferron M, Huizhen W, et al. Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J Clin Invest. 2013;123(6):2421-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Colleluori G, Napoli N, Phadnis U, Armamento-Villareal R, Villareal DT. Effect of weight loss, exercise, or both on undercarboxylated osteocalcin and insulin secretion in frail, obese older adults. Oxid Med Cell Longev. 2017;2017:4807046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oury F, Sumara G, Sumara O, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144(5):796-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dhindsa S, Ghanim H, Batra M, et al. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care. 2016;39(1):82-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ghanim H, Dhindsa S, Batra M, et al. Testosterone increases the expression and phosphorylation of AMP kinase alpha in men with hypogonadism and type 2 diabetes. J Clin Endocrinol Metab. 2020;105(4):1169-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Ethics Approval : All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki declaration and its later amendments or comparable ethical standards.