Abstract
Context
Inferior petrosal sinus sampling (IPSS) helps differentiate the source of ACTH-dependent hypercortisolism in patients with inconclusive biochemical testing and imaging, and is considered the gold standard for distinguishing Cushing disease (CD) from ectopic ACTH syndrome. We present a comprehensive approach to interpreting IPSS results by examining several real cases.
Evidence Acquisition
We performed a comprehensive review of the IPSS literature using PubMed since IPSS was first described in 1977.
Evidence Synthesis
IPSS cannot be used to confirm the diagnosis of ACTH-dependent Cushing syndrome (CS). It is essential to establish ACTH-dependent hypercortisolism before the procedure. IPSS must be performed by an experienced interventional or neuroradiologist because successful sinus cannulation relies on operator experience. In patients with suspected cyclical CS, it is important to demonstrate the presence of hypercortisolism before IPSS. Concurrent measurement of IPS prolactin levels is useful to confirm adequate IPS venous efflux. This is essential in patients who lack an IPS-to-peripheral (IPS:P) ACTH gradient, suggesting an ectopic source. The prolactin-adjusted IPS:P ACTH ratio can improve differentiation between CD and ectopic ACTH syndrome when there is a lack of proper IPS venous efflux. In patients who have unilateral successful IPS cannulation, a contralateral source cannot be excluded. The value of the intersinus ACTH ratio to predict tumor lateralization may be improved using a prolactin-adjusted ACTH ratio, but this requires further evaluation.
Conclusion
A stepwise approach in performing and interpreting IPSS will provide clinicians with the best information from this important but delicate procedure.
Keywords: IPSS, performance, interpretation, case-based review
Cushing syndrome (CS) can be divided into ACTH-dependent and ACTH-independent hypercortisolism. Inferior petrosal sinus sampling (IPSS) is the “gold standard” to determine the source of hypercortisolism in ACTH-dependent CS. IPSS is indicated following inconclusive imaging but positive, biochemical testing confirming ACTH-dependent hypercortisolemia (1, 2). The sensitivity and specificity of IPSS in diagnosing Cushing disease (CD) can range from 88% to 100% and 67% to 100%, respectively (3). IPSS may not be required in patients who have a sellar mass > 6 mm when dynamic testing is suggestive of CD. A recent study by Yogi-Morren et al. that included 104 patients with CD and 26 patients with ectopic ACTH syndrome (EAS) showed that pituitary tumors > 6 mm have a 96% specificity for CD (vs. EAS) (4, 5). Many clinicians have limited experience performing and interpreting IPSS results. We provide readers a summary of the current literature and offer strategies to help optimize use of this important diagnostic test.
History of IPSS
IPSS was first performed at the Walter Reed Army Medical Center in 1977, with selective and bilateral (but not simultaneous) catheterization via a transjugular approach (6). This evolved to a multistep process that included unilateral sampling (for localization) followed by bilateral and simultaneous catheterization (for localization and lateralization) (7). In 1985, Oldfield and colleagues demonstrated a high rate of remission following hemihypophysectomy to the side showing highest ACTH concentrations on IPSS despite lack of radiological evidence of microadenoma, although the number of patients was small (8). The utility of IPSS has since been further improved by direct stimulation of the corticotroph adenoma using corticotropin-releasing hormone (CRH) or vasopressin (9, 10). In 1991, Oldfield and his National Institutes of Health (NIH) colleagues published on 281 patients with CS, a report that supported the use of CRH to distinguish CD from EAS (11). Prolactin measurement was suggested to measure pituitary venous effluent and confirm successful cannulation in 2004 (12).
IPSS Procedure
Inferior petrosal sinus sampling is most often performed under local anesthesia. The femoral veins are cannulated, and microcatheters are placed in the bilateral petrosal sinuses under fluoroscopic guidance. Venous angiography is used to confirm correct catheter placement, as demonstrated by retrograde flow of contrast into the contralateral cavernous sinus (3). Simultaneous venous blood samples are obtained from the petrosal sinuses and a peripheral vein. The blood samples are drawn at baseline and then at 2, 5, 10, and 15 minutes following CRH (1 μg/kg, maximum 100 μg) or vasopressin (10 μg) administration. Some institutions may use slightly different timepoints for collection.
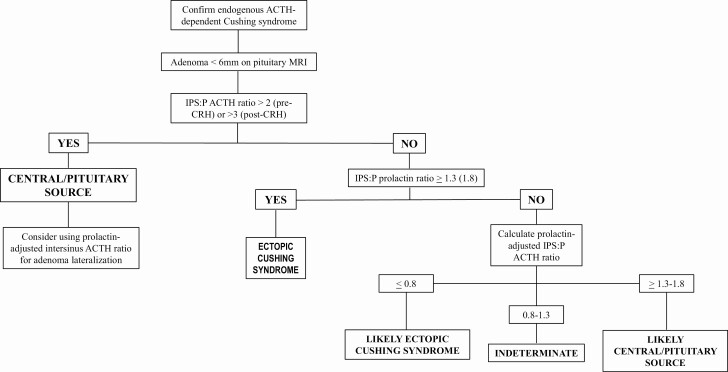
IPS catheter placement is reassessed at various intervals during the procedure to ensure sustained cannulation. Our neuroradiologist checks the position of the IPS catheters at 5 minutes and once sampling is completed. If the catheter locations appear unstable, imaging is obtained prior to each blood draw. The patient is observed for several hours postprocedure. There are rare reports of serious complications, the most notable being stroke and subarachnoid hemorrhage (13-16). These were believed related to aberrant anatomy, leading to transient hypotension and/or vascular injury during the procedure (15). IPSS should be aborted for any significant intraoperative hypotension, hypertension, or change in neurological status. The most common adverse events are groin hematoma (4%) and transient headache (occurs more often if the sinus is small) (17). IPSS is a technically challenging procedure and should be performed by an experienced interventional or neuroradiologist. Although prospective, randomized data are missing, we and others expect better results from centers that have a dedicated radiologist who focuses on successful IPSS. A stepwise approach to IPSS interpretation is given in Fig. 1.
Figure 1.
Our approach to differentiating the etiologies of endogenous, ACTH-dependent Cushing syndrome.
Some centers use DDAVP (desmopressin) for IPSS because of lower cost and past unavailability of CRH. DDAVP stimulates ACTH production via the V2R receptor subtype on corticotroph adenomas and elicits a response similar to CRH (18-20). There is a theoretical risk of false-positive results (positive IPS-to-peripheral [IPS:P] ACTH gradient) using DDAVP in a patient with an ectopic, ACTH-producing tumor if the V2R receptor is present (21). However, the clinical experience seems similar using both CRH and DDAVP. Deipolyi et al. showed comparable diagnostic results using identical IPS:P ACTH gradients after DDAVP and CRH stimulation (10). An earlier study by Tsagarakis et al. demonstrated good IPSS sensitivity and specificity in 54 patients following administration of combination CRH and DDAVP (22).
It is our preference to perform IPSS before starting any antihypercortisolemic therapy, but patients may be referred to our center after already having been prescribed these medications. If we determine that a patient on antihypercortisolemic therapy needs IPSS, our practice is to stop the medication and schedule the procedure once we confirm hypercortisolemia on biochemical testing.
Anatomical Variations
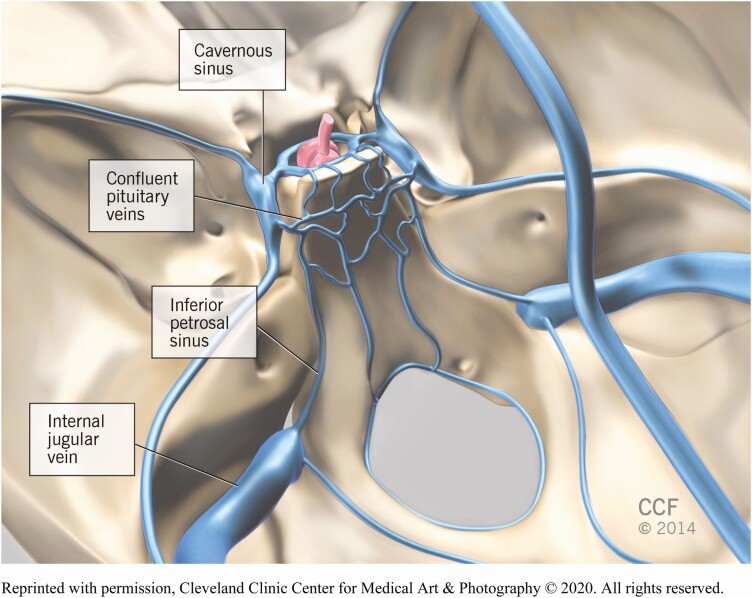
There are anatomical variations in pituitary drainage that can affect the success and interpretation of IPSS. The anterior pituitary drains from the cavernous into the inferior petrosal sinuses. The inferior petrosal sinuses course posterior and caudal and enter the jugular veins at the skull base (23) (Fig. 2). It is not uncommon to have unequal drainage of the cavernous sinuses. Some literature suggests this anatomical variant may be present in 40% of individuals (24, 25). There are other aberrations that make analysis much more difficult. The most notable variants are as follows: (1) inferior petrosal anastomosis to vertebral venous plexus; (2) no connection between the inferior petrosal sinus and the jugular vein; and (3) a hypoplastic inferior petrosal sinus (26). Previous studies have suggested that microcatheters can be used to obtain accurate venous sampling if abnormal anatomy is known or suspected (27). It is also possible to obtain a direct sample from the cavernous sinuses, but this approach has not proved superior to IPSS (28-32). The literature indicates that the prevalence of IPS abnormalities exceeds that of false-negative IPSS results, meaning that adequate catheterization is most often feasible.
Figure 2.
In this schematic, the infundibulum and pituitary gland are marked in red. The pituitary gland sits in the sella. The relevant venous structures are designated in blue.
Case Examples
Case Presentation 1
A 50-year-old woman with progressive weight gain, malaise, and arthralgias presented for evaluation. Her medical history was notable for uncontrolled type 2 diabetes mellitus, steatohepatitis, hypertension, hyperlipidemia, and obstructive sleep apnea. Physical examination showed abdominal obesity, facial plethora, and diffuse acanthosis nigricans. Biochemical testing revealed 24-hour urinary free cortisol (UFC) levels of 94.2 and 152.2 µg (0-50), ACTH levels of 50 and 69 pg/mL (normal range, 10-60 pg/mL), and a cortisol level of 13.8 µg/dL after 1-mg dexamethasone suppression test (DST). Sellar magnetic resonance (MR) imaging demonstrated a 3 × 4-mm focus of early enhancement on the left side of the gland. IPSS was performed using DDAVP; the neuroradiologist reported successful bilateral IPS catheter placement (Table 1).
Table 1.
IPSS results for case presentation 1
| Time (min) | ACTH | ||||
|---|---|---|---|---|---|
| Peripheral (P) | Petrosal sinus | ||||
| Right | Left | ACTH ratio | |||
| R/P | L/P | ||||
| Baseline | 52 | 811 | 469 | 15.6 | 9.0 |
| +3 | 2 | 3015 | 271 | 1507.5 | 135.5 |
| +5 | 59 | 4558 | 741 | 77.3 | 12.6 |
| +10 | 90 | 2359 | 414 | 26.2 | 4.6 |
| +15 | 90 | 1696 | 596 | 18.8 | 6.6 |
Abbreviations: L/P, left IPS/peripheral; R/P, right IPS/peripheral.
Can IPSS differentiate between ACTH-dependent and pseudo-Cushing syndrome?
No, IPSS cannot differentiate between CD and pseudo-Cushing states. A diagnosis of ACTH-dependent CS must be established before a patient is referred for IPSS.
It is critical to establish a diagnosis of endogenous hypercortisolism before pursuing IPSS. This is an invasive procedure and the presence of a positive IPS:P ACTH ratio may precipitate unnecessary surgical exploration. Pseudo-Cushing states have a number of clinical associations including: pregnancy, morbid obesity, severe psychological stress (including major depressive disorder), uncontrolled diabetes mellitus, chronic alcoholism, physical illness or injury, and severe sleep apnea (33). It is thought that higher brain centers stimulate CRH secretion in these conditions, leading to activation of the entire hypothalamic–pituitary–adrenal axis (34). The negative feedback inhibition of cortisol on CRH and ACTH may restrain the resultant hypercortisolemia. It is uncommon to see UFC elevations > 4 times the upper limit of normal in patients with pseudo-Cushing states (1). These same conditions can blunt the circadian rhythm and cause abnormal late-night salivary cortisol concentrations (35-38). Repeat biochemical testing following resolution or treatment of the underlying disease process should be considered. Additional confirmatory testing such as 2-day low-dose DST or a combined dexamethasone suppression/CRH stimulation test may also be used to exclude pseudo-Cushing states. The endocrinology team felt the hypercortisolism in this patient was not secondary to a pseudo-Cushing state and proceeded with an IPSS study.
Researchers at the NIH have demonstrated that there is significant overlap of ACTH concentrations and IPS:P ACTH ratios between CD, normal volunteers, and pseudo-Cushing states (39, 40). Yanovski et al. [37] compared IPSS results from patients with CD (n = 40) to normal subjects (n = 7) and those with pseudo-Cushing states (n = 8) at baseline and 2 minutes post-CRH stimulation (the institutional review board did not allow additional sampling). There was crossover in IPS:P ACTH ratios between the various conditions. This study confirms that IPSS should not be used to distinguish pseudo-Cushing states from CD. Pseudo-Cushing states must be eliminated as potential causes of ACTH-dependent hypercortisolism before referring a patient for IPSS (40).
Is the IPSS result in this patient consistent with Cushing disease?
Yes, the patient has a central to peripheral ACTH ratio > 3 following CRH stimulation. This result confirms CD. She should be referred to an experienced neurosurgeon for surgical exploration of the pituitary gland.
Cushing disease is confirmed when the basal IPS:P ACTH ratio is > 2 and/or the CRH-stimulated ratio is > 3 (8, 41-43). The peripheral ACTH level at 3 minutes post-CRH administration was low (2 pg/mL) and consistent with laboratory error considering her other ACTH values. ACTH must be collected in an EDTA tube and transported swiftly to the laboratory on ice. The sample should then be centrifuged to separate plasma and frozen (if not processed). Delayed or improper handling of the samples during transportation may result in falsely low levels (44). In theory, if a pituitary tumor lacked any CRH receptors, one may not see a rise in ACTH following CRH stimulation. In such an event, the IPS:P ACTH ratio may still be elevated before CRH administration. We have not observed such a clinical scenario in the past (positive IPS:P ACTH ratio before but not after CRH stimulation). This might be because even a low density of CRH receptors on corticotroph cells is enough to generate an increased gradient after CRH stimulation in the highly concentrated IPS environment.”
The patient had successful transsphenoidal resection of a left-sided pituitary mass. Her cortisol nadired following the procedure at 1.7 µg/dL and she required postoperative glucocorticoids. An ACTH-positive corticotroph adenoma was confirmed by surgical pathology.
Is it necessary to confirm hypercortisolism at the time of IPSS?
Yes, IPSS may not be reliable if there is lack of hypercortisolemia at the time of the procedure (39, 45-47).
The absence of sustained hypercortisolism can cause misleading IPSS results. This can happen in a patient with cyclical CD if cortisol is tested during a trough period. The IPSS may reveal an absent IPS:P ACTH gradient because of lack of ACTH production by the corticotroph adenoma and suppression of normal corticotrophs secondary to recent hypercortisolism. This IPSS result may mimic an ectopic source. By contrast, incomplete suppression of normal corticotroph function caused by intermittent ACTH production from a cyclical ectopic tumor might produce a false-positive IPS:P ACTH ratio (48, 49). For these reasons, we measure serum cortisol the morning of scheduled IPSS and proceed only if the value is > 10 μg/dL (50). There are some centers that use midnight salivary cortisol to confirm hypercortisolism the night before IPSS (45, 46). We know of several investigators who require 6 weeks of consistent hypercortisolemia via 24-hour UFC before performing the procedure. This approach requires close monitoring of patients and clinical significance, cost, and practicability require more detailed study.
Table 2 gives an example of IPSS results obtained in a second patient with cyclical Cushing tested during a trough period (49). The patient was confirmed to have cyclic CD by serial measurements of 24-hour UFC, demonstrating 3 elevated and 2 normal/low values (Table 3). IPSS was performed using CRH. There was unsuccessful left-sided IPS cannulation (Table 2). Despite the significant IPS:P gradient on the right side, the authors felt the results were uninterpretable because of a lack of hypercortisolism during the procedure. This is evidenced by the peripheral cortisol and ACTH levels of 6.2 μg/dL and 8 pg/mL at the time of sampling, respectively (49). Swearingen et al. reported 3 cases of false-positive IPSS findings (IPS:P ACTH gradient suggestive of CD) in ectopic tumors. These patients had low peripheral ACTH levels obtained during IPSS, making the IPS:P ratios difficult to interpret (51). After 2 IPSS attempts, during which he was not hypercortisolemic, our patient was referred for pituitary exploration. The transsphenoidal surgery (performed through the nose) yielded an ectopic corticotroph adenoma suspended from the mucosa of the sphenoid sinus (49). The IPSS results are difficult to interpret even retrospectively because we cannot know the venous drainage pattern of this ectopic adenoma. In these rare situations, IPSS may be consistent with either CD or an ectopic ACTH syndrome.
Table 2.
IPSS results obtained during a trough period in our patient with cyclical Cushing
| Time (min) | ACTH (pg/mL) | Cortisol (μg/dL) | ||||
|---|---|---|---|---|---|---|
| Peripheral (P) | Petrosal sinus | Peripheral (P) | ||||
| Right | Left | ACTH ratio | ||||
| R/P | L/P | |||||
| -10 | 8 | 73 | 9 | 9.1 | 1.1 | 6.2 |
| -5 | 8 | 64 | 8 | 8 | 1 | |
| +2 | 7 | 145 | 7 | 21 | 1 | |
| +5 | 7 | 386 | 7 | 55 | 1 | |
| +10 | 7 | 471 | 8 | 67 | 1.1 | |
| +15 | 9 | 2 | 10 | 0.2 | 1.1 | |
Abbreviations: L/P, left IPS/peripheral; R/P, right IPS/peripheral.
Table 3.
Biochemical testing for our patient diagnosed with cyclical ectopic corticotroph adenoma (49)
| Measurements (units) | Time | ||||||
|---|---|---|---|---|---|---|---|
| Presentation | Day 2 | Week 5 | Week 17 | Week 18 | Week 19 | Week 22 | |
| Urinary free cortisol (mcg/d)ª | 119.6 (RAI) | 110.2 (RAI) | 35.9 (RAI) | 343.0 (RAI) | 789.1 (HPLC) | 7.3 (HPLC) | 692.4 (RAI) |
| Urinary creatinine (g/d) | 1.7 | 1.6 | 1.6 | 1.9 | 1.9 | 1.8 | 1.8 |
| Urinary volume (mL) | 1329 | 1318 | 1382 | 1900 | 1250 | 2200 | 1300 |
RAI normal range: 20-100 mcg/d. HPLC normal range: < 45 mcg/d.
Abbreviation: RAI, radioactive iodine.
aUrinary free cortisol measurements were obtained by either RAI or HPLC.
Case Presentation 2
A 51-year-old woman with progressive weight gain, hair loss, emotional lability, and new-onset type 2 diabetes mellitus was referred for possible hypercortisolism. Physical examination revealed abdominal obesity, widespread ecchymoses, excess supraclavicular fat, mild muscle wasting, and proximal muscle weakness. Her biochemical results were as follows: 24-hour UFC, 2000 µg (0-50); morning cortisol 43.8 µg/dL; and ACTH 170 pg/mL following 8 mg DST. Sellar MR imaging (MRI) showed a 5-mm right-sided adenoma. She had an unremarkable chest computed tomography (CT) scan. IPSS was performed using CRH; the neuroradiologist reported successful bilateral IPS catheter placement (Table 4).
Table 4.
IPSS results for case presentation 2
| ACTH (pg/dL) | Prolactin (ng/mL) | Cortisol (μg/dL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peripheral (P) | Petrosal sinus | Peripheral (P) | Petrosal sinus | Peripheral (P) | |||||||
| RT | LT | ACTH ratio | RT | LT | PRL ratio | ||||||
| Time (min) | R/P | L/P | R/P | L/P | |||||||
| -10 | 102 | 125 | 124 | 1.2 | 1.2 | 4.8 | 5.7 | 8.4 | 1.2 | 1.7 | 64 |
| -5 | 100 | 222 | 111 | 2.2 | 1.1 | 5.6 | 8.3 | 6.3 | 1.5 | 1.1 | |
| +2 | 102 | 130 | 129 | 1.3 | 1.2 | 5.0 | 6.2 | 7.1 | 1.2 | 1.4 | |
| +5 | 143 | 220 | 191 | 1.5 | 1.3 | 5.3 | 6.3 | 6.7 | 1.1 | 1.2 | |
| +10 | 176 | 217 | 235 | 1.2 | 1.3 | 5.2 | 5.2 | 6.6 | 1.0 | 1.2 | |
| +15 | 197 | 224 | 232 | 1.1 | 1.2 | 5.0 | 5.5 | 8.5 | 1.1 | 1.1 | |
Abbreviations: LT, left; L/P, left IPS/peripheral; RT, right; R/P, right IPS/peripheral.
Is the lack of an IPS:P ACTH gradient consistent with a diagnosis of ectopic CS in this patient?
Absent a significant IPS:P ACTH ratio, the IPS prolactin level should be used as a surrogate marker of appropriate catheterization/normal IPS venous efflux to avoid a false-negative result.
IPS prolactin can help confirm correct catheter placement during venous sampling. IPS catheterization can be verified using an IPS:P prolactin ratio ≥ 1.3 to 1.8 before and after CRH/DDAVP administration (12, 52). The presence of an IPS:P prolactin ratio < 1.3 and the absence of significant elevation should raise suspicion for either improper cannulation or abnormal IPS venous efflux (53). Prolactin is the most abundant anterior pituitary hormone and spatial separation of normal lactotrophs from corticotropes within the gland make it a good reference hormone (54). Previous studies have advocated use of GH, B-endorphin, and TSH for localization of ACTH-secreting microadenomas, but interpreting these data remain challenging (55). These hormones may also be suppressed in hypercortisolism (most often ectopic ACTH syndrome) (56-58).
There are several possible reasons that radiological confirmation of IPS cannulation and IPS:P prolactin ratios might be discordant. This incongruity is more often attributable to procedural abnormalities rather than sensitivity of the IPS:P prolactin ratio (52). The most common procedural problems include: (1) intermittent displacement of the catheter tip (from the IPS); (2) aspiration of samples from an aberrant collateral vessel (despite proper cannula placement); and (3) the sample does not actually contain venous blood from the pituitary gland (occurs when too small a catheter is used to interrupt laminar flow). As with other technically demanding procedures, the success of IPSS often depends upon the experience of the interventional or neuroradiologist (59).
The absence of an IPS:P ACTH gradient here (Table 4) suggests an ectopic source of hypercortisolism. The only exception is the right IPS:P ACTH ratio at -5 minutes (2.2), a borderline positive value for CD. The neuroradiologist confirmed catheter placement using fluoroscopy. We routinely check IPS prolactin levels at our institution to ensure proper IPS venous efflux before concluding that the ACTH source is ectopic. Some centers limit procedural costs by storing serum during IPSS and checking prolactin concentrations only if ACTH gradients are absent.
What is the role of the prolactin-adjusted IPS:P ACTH ratio when there is lack of IPS catheterization or appropriate IPS venous efflux?
The prolactin-adjusted IPS:P ACTH ratio can improve differentiation between CD and ectopic ACTH syndrome in the absence of proper IPS venous efflux.
Findling and colleagues used prolactin as an index of pituitary venous efflux in 3 cases of surgically proven CD in whom IPSS failed to demonstrate an appropriate IPS:P ACTH gradient (and an ectopic source could not be found). The authors showed that a prolactin-adjusted IPS:P ACTH ratio (dominant post-CRH IPS:P ACTH/ipsilateral pre-CRH IPS:P prolactin) > 0.8 would have identified these 3 patients as having CD (12). The ratio was < 0.6 in 5 EAS patients. A 2013 retrospective study from the NIH examined prolactin-adjusted IPS:P ACTH ratios in CRH-stimulated IPSS samples from 29 patients with ACTH-dependent CS (60). They diagnosed CD using a prolactin-adjusted IPS:P ACTH ratio ≥ 1.3. All ratios ≤ 0.8 corresponded to ectopic ACTH syndrome. Prolactin-adjusted IPS:P ACTH ratios ranging from 0.8 to 1.3 did not discriminate between CD and ectopic ACTH syndrome. The authors concluded that a prolactin-adjusted IPS:P ACTH ratio of: (1) ≤ 0.8 suggests EAS; (2) ≥ 1.3 indicates CD; and (3) 0.8 to 1.3 needs further investigation (60). These findings are similar to those of Grant et al (61).
The use of the prolactin-adjusted ACTH ratio has potential limitations. The pre-CRH prolactin value does not account for erroneous IPS sampling that may occur after CRH injection because of repositioning of the catheter tip. It is also possible that the prolactin-adjusted IPS:P ACTH ratio in ectopic ACTH syndrome may mimic CD if there is unsuccessful IPS cannulation (52). In case 2, using the baseline sample at -10 minutes, the prolactin-adjusted IPS:P ratio is 0.77 and below the threshold of 0.8, which could indicate an ectopic source. If we use the second pre-CRH prolactin value (drawn at the -5 minute timepoint), then the ratio further decreases to 0.63 (very close to the 0.6 cutoff suggested by Findling et al.) (52). Larger studies are needed to confirm the role of the prolactin-adjusted IPS:P ACTH ratio in ACTH-dependent CS and failed IPS cannulation.
Are there additional clues that may suggest lack of proper IPS venous efflux during IPSS?
Yes, the absolute ACTH levels (pre- and post-CRH) in the inferior petrosal sinuses and ACTH response to CRH in the periphery may identify CD when there is lack of a significant central ACTH gradient.
Wind et al. showed that baseline ACTH values < 200 pg/mL and peak post-CRH ACTH values < 400 pg/mL may indicate lack of proper IPS venous efflux (62). The index patient only achieved an ACTH > 200 pg/mL (in the right IPS) at -5 minutes, corresponding to a positive IPS:P ACTH ratio (> 2) at the same timepoint. Additional data suggest that peripheral ACTH response to CRH may help identify false-negative results (the presence of CD but absence of a central IPS:P ACTH gradient) (46, 63). Swearingen et al. studied 9 patients with negative IPSS results and no known ectopic source and evaluated peripheral ACTH response to CRH. Eight of these patients had a significant rise in peripheral ACTH (> 35%) following CRH administration. All 9 patients were later proven to have CD. These findings support using CRH-stimulated peripheral ACTH levels to improve the diagnostic accuracy of IPSS (51). In a recent study from Germany, an increase of ≥ 43% in peripheral ACTH after CRH administration had an 83% sensitivity and 94% specificity for CD (64). Previous studies that compared standard CRH-stimulation testing (measuring cortisol) to IPSS yielded mixed results (65, 66). In our case, the patient had a significant increase in peripheral ACTH following CRH administration, signalling a probable false-negative IPSS result (Table 4).
Our patient elected to pursue transsphenoidal resection of the right-sided pituitary lesion. She was aware that it might have been an incidental finding. Her cortisol and ACTH levels decreased to normal (11.4 μg/dL and 19 pg/mL) during the immediate postoperative period but she did not become hypocortisolemic. Pathology confirmed the presence of a corticotroph adenoma. The patient experienced mild symptoms of (relative) adrenal insufficiency and was discharged on physiologic hydrocortisone. She decided to see her local endocrinologist for further postoperative care and surveillance.
Case Presentation 3
A 30-year-old woman with progressive weight gain, fatigue, muscle weakness, and skin changes suggestive of hypercortisolism presented for endocrine evaluation. Her biochemical testing was as follows: 24-hour UFCs of 824 and 3515 µg (0-50); random plasma ACTHs of 80 and 102 pg/mL, and cortisol of 44.8 µg/dL after 1 mg DST administration. Sellar MR failed to reveal a pituitary adenoma. This patient’s whole-body imaging did not show any source of possible ectopic ACTH syndrome. She underwent IPSS using CRH (Table 5), during which the neuroradiologist reported successful bilateral IPS catheter placement.
Table 5.
IPSS results for case presentation 3
| Time (min) | ACTH (pg/mL) | Prolactin (ng/mL) | Cortisol (μg/dL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peripheral (P) | Petrosal sinus | Peripheral (P) | Petrosal sinus | Peripheral (P) | |||||||
| RT | LT | ACTH ratio | RT | LT | PRL ratio | ||||||
| R/P | L/P | R/P | L/P | ||||||||
| -10 | 73 | 79 | 89 | 1.1 | 1.2 | 14 | 13.4 | 24.1 | 1.0 | 1.8 | 29.1 |
| -5 | 76 | 77 | 85 | 1.0 | 1.1 | 13.1 | 12.5 | 28.6 | 1.0 | 2.2 | |
| +2 | 83 | 108 | 117 | 1.3 | 1.4 | 13.3 | 12.5 | 35.2 | 1.1 | 2.8 | |
| +5 | 210 | 196 | 267 | 0.93 | 1.3 | 13.6 | 11.8 | 38.8 | 1.2 | 3.3 | |
| +10 | 246 | 326 | 315 | 1.3 | 1.3 | 13 | 13.3 | 34.6 | 0.98 | 2.6 | |
| +15 | 288 | 383 | 370 | 1.3 | 1.3 | 13.7 | 12.7 | 34.9 | 1.1 | 2.7 | |
Abbreviations: LT, left; L/P, left IPS/peripheral; RT, right; R/P, right IPS/peripheral.
Is this IPSS result consistent with ectopic ACTH syndrome?
No; the presence of a pituitary adenoma on the contralateral side cannot be excluded when there is only unilateral successful IPS catheterization.
The IPS:P prolactin ratios in this patient indicate lack of appropriate venous efflux or failed cannulation on the right side (all < 1.3). Her IPSS results demonstrate, however, significant peripheral ACTH response to CRH, suggestive of central disease. She had transsphenoidal exploration, which revealed a right-sided adenoma. The tumor stained positive for ACTH on surgical pathology. The patient became hypocortisolemic during the immediate postoperative period confirming CD (2). If there are no intersinus anastomoses, there may not be a positive contralateral IPS:P ACTH gradient in CD (when there is no mixing of blood, the ACTH values represent suppressed normal corticotrophs). Our neuroradiologist has encountered this only once in his 15 years of experience. In the absence of a positive IPS:P ACTH ratio, a unilateral successful IPS cannulation cannot rule out the presence of a corticotroph adenoma on the contralateral side.
Case Presentation 4
A 56-year-old woman with progressive weight gain, easy bruising, and fatigue was evaluated for possible CS. Physical examination revealed abdominal obesity, wide and violaceous striae, excess dorsocervical and supraclavicular fat, facial plethora, and thin extremities. Her medical history was notable for type 2 diabetes mellitus, hypertension, and hyperlipidemia. Her biochemical results showed midnight salivary cortisols of 203 and 341 ng/dL (normal, < 90); 24-hour UFC, 27.8 µg (0-50); morning cortisol, 27.2 µg/dL; and morning ACTH, 126 pg/mL. Sellar MR demonstrated bilateral adenomas (right = 4 mm, left = 2 mm). CT scan of the chest and adrenals was negative. The patient was referred for IPSS. The procedure was performed using CRH and the neuroradiologist reported successful bilateral IPS catheter placement (Table 6).
Table 6.
IPSS results for case presentation 4
| Time | ACTH (pg/mL) | ACTH ratio | PRL (ng/mL) | ACTH/PRL ratio | PRL-adjusted ACTH ratio | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peripheral | RT | LT | LT/RT | Peripheral | RT | LT | RT | LT | RT/LT | |
| -10 | 77 | 1192 | 3295 | 2.8 | 17.5 | 17.5 | 435.0 | 68.1 | 7.6 | 9.0 |
| -5 | 65 | 1585 | 2710 | 1.7 | 16.4 | 43.4 | 457.5 | 36.5 | 5.9 | 6.2 |
| +2 | 81 | 2744 | 12 806 | 4.7 | 15.6 | 21.9 | 282.0 | 125.3 | 45.4 | 2.8 |
| +5 | 131 | 5526 | 14 230 | 2.6 | 16.3 | 35.8 | 391.5 | 154.4 | 36.4 | 4.3 |
| +10 | 153 | 5870 | 9058 | 1.5 | 15.6 | 30.4 | 389.0 | 193.1 | 23.3 | 8.3 |
| +15 | 101 | 4717 | 10 840 | 2.3 | 14.5 | 27.0 | 366.5 | 174.7 | 29.6 | 5.9 |
Abbreviations: LT, left; PRL, prolactin; RT, right.
What is the value of the intersinus ACTH ratio in tumor lateralization?
An intersinus ACTH gradient > 1.4 has limited value in predicting tumor lateralization (8).
There are limited data on the use of IPSS to lateralize pituitary adenomas. In 1985, Oldfield and colleagues proposed an intersinus ACTH gradient of > 1.4 (before CRH administration) for ipsilateral lateralization of pituitary tumors (8). This ratio has proven less reliable as more data have emerged, including a large series of 501 patients published by NIH in 2013 (62). The literature reports correct tumor lateralization using this ratio in 50% to 70% of cases (62, 67-69). There is no evidence to suggest that lateralization improves following CRH administration. In later work from the NIH by Wind et al., the positive predictive value (PPV) for accurate lateralization peaked at 86% using an intersinus ACTH gradient of 60:1. The authors acknowledged, however, a substantive limitation because only 7% of patients who had a unilateral adenoma reached this ratio. This same paper showed that left-sided ACTH lateralization was associated with greater accuracy (reasons unknown). The highest level of accuracy was observed in patients who had consistent lateralization before and after CRH administration (PPV = 72%) (62). These studies reinforce the need for careful neurosurgical exploration of the pituitary gland to identify an adenoma, which may be smaller than 1 mm in the largest diameter (70). This patient’s intersinus ACTH ratio suggested a left-sided lesion. This was inaccurate. Transsphenoidal exploration revealed a right-sided adenoma. The surgical pathology confirmed a corticotroph adenoma.
Does the prolactin-adjusted intersinus ACTH gradient improve lateralization?
The use of a prolactin-adjusted intersinus ACTH gradient > 1.4 improves corticotroph adenoma lateralization during IPSS.
There have been several attempts to improve the predictive value of the intersinus ACTH gradient. In 2012, Mulligan et al. (59) showed improved adenoma lateralization from 54% to 75% using a prolactin-adjusted intersinus ACTH gradient of > 1.4 in their series. The combination of data from pituitary MRI and prolactin-adjusted intersinus ACTH ratio enhanced the lateralization concordance to 82% (55). When successful bilateral IPS catheterization was confirmed using an IPS:P ACTH ratio > 1.3 (n = 14), there were no instances in which the prolactin-adjusted IPS:P ACTH ratio was associated with a contralateral tumor (adenoma was either ipsilateral or centrally located) (59). A later analysis by Qiao et al. again supported improved tumor lateralization using a prolactin-adjusted intersinus ACTH gradient (53). They increased their lateralization from 65% to 77% using an intersinus prolactin-adjusted ACTH > 1.4 following DDAVP administration. They suggested that anatomical variation (such as preexisting communication between the cavernous sinuses) might explain sampling failures (53). They did not comment on the ability of the intersinus prolactin-adjusted ACTH ratio to predict lateralization in patients who had successful bilateral IPS cannulation based on the IPS:P prolactin ratio. There are certain (apparent) situations in which this correction cannot be applied, including rare cases of corticotroph hyperplasia and ectopic pituitary tumors (59). In our patient, the intersinus prolactin-adjusted ACTH ratio correctly indicated a right-sided adenoma.
There are 2 recent studies that are less supportive of using the prolactin-adjusted intersinus ACTH gradient for tumor lateralization but both are small. De Sousa et al. published a retrospective review of IPSS lateralization in 13 patients (71). Their predicted and surgical findings were concordant in only 4 patients regardless of whether the intersinus gradient was corrected for prolactin. The authors concluded that the adjusted ratio could not be used because of consistent co-lateralization of prolactin and ACTH. The study however successfully cannulated both IPSs in only 7 patients (54%). They did not report separately on patients who had adequate sampling and pathology-proven CD (71). A second paper (72) included only 8 patients, 5 of whom had an adenoma > 6 mm (for whom IPSS may not have been indicated). Of the 8 patients, only 1 demonstrated discordant intersinus ACTH and prolactin-adjusted intersinus ACTH ratios. In summary, further evaluation of the prolactin-adjusted ACTH ratio is needed to prove reliable surgical guidance. A careful surgical exploration of the entire pituitary gland in cases where sellar imaging does not reveal a distinct adenoma is needed.
Special Considerations
IPSS in CRH-producing ectopic tumors
CS resulting from ectopic CRH production is very rare. It can be seen in neuroendocrine tumors arising from the pancreas, adrenal glands, or lungs (47, 73-76). There are 2 case reports of IPSS performed on a combined CRH/ACTH-producing pheochromocytoma and a CRH-producing bronchial carcinoid tumor (47, 76). The patient with a pheochromocytoma had a basal IPS:P ACTH gradient < 2 but a peak IPS:P ACTH gradient of 9.1 following CRH administration. The authors theorized that ectopic CRH production prevented suppression of normal corticotrophs that led to a false-positive result (76). The patient with a bronchial carcinoid tumor had a basal IPS:P ACTH gradient > 2, indicative of central disease (the authors did not use CRH or DDAVP). There were no abnormalities on the sellar CT scan and sellar MR was not performed. Surgical exploration of the pituitary gland failed to demonstrate an adenoma. Histopathological examination revealed pituitary hyperplasia. The ectopic CRH production was believed to prevent normal corticotroph suppression leading to a false-positive IPSS result (47).
Inadvertent use of ACTH instead of CRH during IPSS
There have been reports of inadvertent substitution of ACTH for CRH during IPSS. The trade name for ovine CRH (ACTHrel) is similar to ACTH (commercial name Cosyntropin). Carroll et al. analyzed 3 separate cases of IPSS results following accidental cosyntropin administration (77). All patients demonstrated a decrease in IPS ACTH levels after receiving synthetic ACTH. The authors hypothesized that this might be explained by the pharmacokinetics of the immunometric assay used to measure ACTH (78). An IPS:P ACTH ratio that falls following CRH administration should alert clinicians to possible accidental use of cosyntropin. A separate check to confirm medications to be used followed by a time out immediately before CRH administration (similar to that used in the operating room) may eliminate this issue (79).
The role of jugular venous sampling in ACTH-dependent CS
Internal jugular vein sampling (JVS) has been proposed as an easier and safer alternative to IPSS (80-83). Unfortunately, JVS is less sensitive for diagnosing CD than IPSS. Current literature suggests the sensitivity of JVS ranges from 68.7% to 81.3% compared with 93.8% to 98% for IPSS (81, 83). There are several factors that improve JVS sensitivity including: (1) CRH stimulation; (2) positioning catheters against the medial walls of the jugular veins close to the IPS origins; and (3) performing a Valsalva maneuver during the procedure (to facilitate mixing of blood) (82). Erickson et al. enhanced JVS by adjusting the reference ratios for interpreting the results (81). They maximized the sensitivity (as described previously) using a pre-CRH IPS:P ACTH ratio of 1.59 and a post-CRH IPS:P ACTH ratio of 2.47. They observed, however, that during simultaneous JVS and IPSS, the former missed the diagnosis of CD in about 30% of cases (83). Arguably, JVS is less invasive and may be performed by less experienced radiologists. That said, if JVS must be substituted for IPSS, negative results (no central/peripheral gradient) should prompt referral to a tertiary center for IPSS confirmation. In most institutions, the only indication for JVS is unsuccessful IPS cannulation.
The utility of IPSS in pregnancy
The physiologic changes of pregnancy make testing for CS more difficult. The serum cortisol, plasma ACTH, corticosteroid-binding globulin and UFC are increased during the second and third trimesters of normal pregnancy, and response to dexamethasone is blunted (84, 85). There is a paucity of IPSS data during pregnancy because of concerns about fetal radiation exposure (86). Lindsay et al. reported IPSS results from 2 pregnant patents in whom the procedure was safely performed using special precautions including lead shielding and direct catheterization of the jugular veins. IPSS localized the tumor in both patients. The authors cautioned against the potential for false positives (elevated IPS:P ACTH ratio absent CD) during pregnancy because CS resulting from an adrenal lesion can be associated with nonsuppressed ACTH levels (85).
Other approaches to explore the etiology of ACTH-dependent CS
There are alternative strategies to evaluate the source of ACTH-dependent CS that may reduce the need for IPSS. Some studies have used dynamic testing (including the CRH stimulation test, desmopressin stimulation test, and high-dose dexamethasone suppression test) plus advanced imaging to distinguish between CD and EAS with variable results in the literature (87-91). A recent paper by Frete et al. assessed a diagnostic algorithm of CRH and desmopressin stimulation tests and pituitary MRI. The patients with inconclusive results underwent thin-slice whole-body CT scan. The combination of dynamic tests was 73% sensitive for CD. Using a combination of both dynamic tests and MRI scans, followed by CT scan if the diagnosis of CD was disputed, a PPV of 100% for CD was achieved. The authors concluded that IPSS could have been avoided in half of their patients (92).
A recent report in the Journal of Neurosurgery discussed a case of CD in which a 7-tesla (T) MRI was able to localize an otherwise invisible tumor. The authors suggest that 7-T imaging may preempt IPSS in standard and dynamic contrast 1.5-T and 3-T MRI-negative CD (93). This mirrors a previous study that was able to detect 3 pituitary adenomas on 7-T MRI unseen on 1.5-T imaging (94).
A study by Page-Wilson et al. evaluated the utility of neuroendocrine markers proopiomelanocortin and agouti-related protein levels in the diagnostic workup of ACTH-dependent CS. They demonstrated that values of proopiomelanocortin > 36 fmol/mL and agouti-related protein > 280 pg/mL yielded a sensitivity and specificity of 82% and a PPV of 100% for EAS (95).
Walia et al. looked at the use of Gallium-68 (68Ga) tagged CRH during positron emission tomography (PET)-CT to evaluate the etiology of ACTH-dependent CS in 27 patients. The 68Ga CRH PET-CT correctly delineated corticotropinoma in all 24 cases of CD, including 10 tumors of size < 6 mm. The location of the corticotropinoma on 68Ga CRH PET fusion images with MRI scans were concordant with operative findings and confirmed on histopathology. In contrast, there was no pituitary tracer uptake in 2 patients with EAS. There was diffuse pituitary tracer uptake in another patient who was believed to have ectopic CRH production.
Conclusions
IPSS is the most sensitive and specific biochemical test to distinguish pituitary from ectopic ACTH-dependent CS. IPSS cannot be used to confirm the diagnosis of ACTH-dependent CS. There are certain criteria that need be met to ensure the accuracy, precision, and reliability of IPSS, which include the presence of hypercortisolism immediately before as well as at the time of the procedure. IPSS should be performed by an experienced interventional or neuroradiologist to avoid complications and nondiagnostic results. Samples must be collected and processed in a precise manner. Prolactin is an excellent marker to confirm proper IPS venous efflux if the IPS:P ACTH gradient suggests ectopic disease. The value of the intersinus ACTH gradient to predict tumor lateralization may be improved using the prolactin-adjusted ACTH ratio, but further study is needed. We hope that this case-based systematic review will help clinicians use a stepwise approach (Table 7) to interpreting IPSS results.
Table 7.
Important steps in performing and interpreting IPSS results
| 1. Confirmation of endogenous hypercortisolism before the procedure |
| 2. Demonstrate hypercortisolemia before the procedure in patients with cyclical Cushing syndromea |
| 3. Proper processing of blood samples (especially plasma ACTH) |
| 4. Confirmation of adequate IPS venous flux using IPS:P prolactin ratios in patients who lack a significant IPS:P ACTH ratio |
| • The prolactin-adjusted IPS:P ACTH ratio can improve differentiation between Cushing disease and ectopic ACTH syndrome when there is a lack of proper IPS venous efflux based on IPS:P prolactin ratio |
| • An absolute IPS ACTH level < 200 and < 400 pg/mL pre- and post-CRH stimulation and a < 35% increase in ACTH-to-CRH in the periphery may suggest failed IPS cannulation |
| 5. A lack of significant IPS:P ACTH gradient in unilateral successful IPS catheterization does not rule out a corticotroph adenoma to the contralateral gland |
| 6. The value of the intersinus ACTH gradient to predict tumor lateralization may be improved by using a prolactin-adjusted ACTH ratio >1.4 |
Abbreviations: CRH, corticotropin-releasing hormone; IPS, inferior petrosal sinus; IPS:P, inferior petrosal sinus to peripheral ACTH gradient.
a Some experts feel the duration of hypercortisolism before the IPSS may be more important than the presence of hypercortisolemia immediately before the procedure.
Acknowledgments
The authors thank Amanda Mendelsohn, Cleveland Clinic Center, for medical art and photography.
Glossary
Abbreviations
- CD
Cushing disease
- CRH
corticotropin-releasing hormone
- CS
Cushing syndrome
- CT
computed tomography
- DDAVP
desmopressin
- DST
dexamethasone suppression test
- EAS
ectopic ACTH syndrome
- IPS:P
inferior petrosal sinus sampling-to-peripheral gradient
- IPSS
inferior petrosal sinus sampling
- JVS
jugular vein sampling
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- NIH
National Institutes of Health
- PET
positron emission tomography
- PPV
positive predictive value
- T
tesla
- UFC
urinary free cortisol
- 68Ga
gallium 68
Contributor Information
Jordan E Perlman, Johns Hopkins University, Division of Endocrinology, Diabetes and Metabolism, Baltimore, MD, USA.
Philip C Johnston, Regional Center for Endocrinology and Diabetes, Royal Victoria Hospital, Belfast, Northern Ireland, UK.
Ferdinand Hui, Johns Hopkins University, Department of Radiology, Baltimore, MD, USA.
Guy Mulligan, Department of Endocrinology, Diabetes and Metabolism, Cleveland Clinic, Cleveland, OH, USA.
Robert J Weil, Department of Neurosurgery, Rhode Island Hospital, Providence, RI, USA.
Pablo F Recinos, Department of Neurosurgery, Cleveland Clinic, Cleveland, OH, USA.
Divya Yogi-Morren, Department of Endocrinology, Diabetes and Metabolism, Cleveland Clinic, Cleveland, OH, USA.
Roberto Salvatori, Johns Hopkins University, Division of Endocrinology, Diabetes and Metabolism, Baltimore, MD, USA.
Debraj Mukherjee, Johns Hopkins University, Department of Neurosurgery, Baltimore, MD, USA.
Gary Gallia, Johns Hopkins University, Department of Neurosurgery, Baltimore, MD, USA.
Laurence Kennedy, Department of Endocrinology, Diabetes and Metabolism, Cleveland Clinic, Cleveland, OH, USA.
Amir H Hamrahian, Johns Hopkins University, Division of Endocrinology, Diabetes and Metabolism, Baltimore, MD, USA.
Additional Information
Disclosures: The authors have no conflicts to declare.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References. Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nieman LK, Biller BM, Findling JW, et al. ; Endocrine Society . Treatment of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zampetti B, Grossrubatscher E, Dalino Ciaramella P, Boccardi E, Loli P. Bilateral inferior petrosal sinus sampling. Endocr Connect. 2016;5(4):R12-R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yogi-Morren D, Habra MA, Faiman C, et al. Pituitary MRI findings in patients with pituitary and ectopic ACTH-dependent cushing syndrome: does a 6-mm pituitary tumor size cut-off value exclude ectopic ACTH syndrome? Endocr Pract. 2015;21(10):1098-1103. [DOI] [PubMed] [Google Scholar]
- 5. Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593-5602. [DOI] [PubMed] [Google Scholar]
- 6. Corrigan DF, Schaaf M, Whaley RA, Czerwinski CL, Earll JM. Selective venous sampling to differentiate ectopic ACTH secretion from pituitary Cushing’s syndrome. N Engl J Med. 1977;296(15):861-862. [DOI] [PubMed] [Google Scholar]
- 7. Manni A, Latshaw RF, Page R, Santen RJ. Simultaneous bilateral venous sampling for adrenocorticotropin in pituitary-dependent Cushing’s disease: evidence for lateralization of pituitary venous drainage. J Clin Endocrinol Metab. 1983;57(5): 1070-1073. [DOI] [PubMed] [Google Scholar]
- 8. Oldfield EH, Chrousos GP, Schulte HM, et al. Preoperative lateralization of ACTH-secreting pituitary microadenomas by bilateral and simultaneous inferior petrosal venous sinus sampling. N Engl J Med. 1985;312(2):100-103. [DOI] [PubMed] [Google Scholar]
- 9. Landolt AM, Valavanis A, Girard J, Eberle AN. Corticotrophin-releasing factor-test used with bilateral, simultaneous inferior petrosal sinus blood-sampling for the diagnosis of pituitary-dependent Cushing’s disease. Clin Endocrinol. 1986;25(6):687-696. [DOI] [PubMed] [Google Scholar]
- 10. Deipolyi AR, Alexander B, Rho J, Hirsch JA, Oklu R. Bilateral inferior petrosal sinus sampling using desmopressin or corticotropic-releasing hormone: a single-center experience. J Neurointerv Surg. 2015;7(9):690-693. [DOI] [PubMed] [Google Scholar]
- 11. Oldfield EH, Doppman JL, Nieman LK, et al. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing’s syndrome. N Engl J Med. 1991;325(13):897-905. [DOI] [PubMed] [Google Scholar]
- 12. Findling JW, Kehoe ME, Raff H. Identification of patients with Cushing’s disease with negative pituitary adrenocorticotropin gradients during inferior petrosal sinus sampling: prolactin as an index of pituitary venous effluent. J Clin Endocrinol Metab. 2004;89(12):6005-6009. [DOI] [PubMed] [Google Scholar]
- 13. Bonelli FS, Huston J 3rd, Meyer FB, Carpenter PC. Venous subarachnoid hemorrhage after inferior petrosal sinus sampling for adrenocorticotropic hormone. AJNR Am J Neuroradiol. 1999;20(2):306-307. [PMC free article] [PubMed] [Google Scholar]
- 14. Sturrock ND, Jeffcoate WJ. A neurological complication of inferior petrosal sinus sampling during investigation for Cushing’s disease: a case report. J Neurol Neurosurg Psychiatry. 1997;62(5):527-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gandhi CD, Meyer SA, Patel AB, Johnson DM, Post KD. Neurologic complications of inferior petrosal sinus sampling. AJNR Am J Neuroradiol. 2008;29(4):760-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miller DL, Doppman JL, Peterman SB, Nieman LK, Oldfield EH, Chang R. Neurologic complications of petrosal sinus sampling. Radiology. 1992;185(1):143-147. [DOI] [PubMed] [Google Scholar]
- 17. Miller DL, Doppman JL. Petrosal sinus sampling: technique and rationale. Radiology. 1991;178(1):37-47. [DOI] [PubMed] [Google Scholar]
- 18. Castinetti F, Morange I, Dufour H, et al. Desmopressin test during petrosal sinus sampling: a valuable tool to discriminate pituitary or ectopic ACTH-dependent Cushing’s syndrome. Eur J Endocrinol. 2007;157(3):271-277. [DOI] [PubMed] [Google Scholar]
- 19. Machado MC, de Sa SV, Domenice S, et al. The role of desmopressin in bilateral and simultaneous inferior petrosal sinus sampling for differential diagnosis of ACTH-dependent Cushing’s syndrome. Clin Endocrinol (Oxf). 2007;66(1):136-142. [DOI] [PubMed] [Google Scholar]
- 20. Deipolyi AR, Hirsch JA, Oklu R. Bilateral inferior petrosal sinus sampling with desmopressin. J NeuroInterv Surg. 2013;5(5):487-488. [DOI] [PubMed] [Google Scholar]
- 21. Tsagarakis S, Tsigos C, Vasiliou V, et al. The desmopressin and combined CRH-desmopressin tests in the differential diagnosis of ACTH-dependent Cushing’s syndrome: constraints imposed by the expression of V2 vasopressin receptors in tumors with ectopic ACTH secretion. J Clin Endocrinol Metab. 2002;87(4):1646-1653. [DOI] [PubMed] [Google Scholar]
- 22. Tsagarakis S, Vassiliadi D, Kaskarelis IS, Komninos J, Souvatzoglou E, Thalassinos N. The application of the combined corticotropin-releasing hormone plus desmopressin stimulation during petrosal sinus sampling is both sensitive and specific in differentiating patients with Cushing’s disease from patients with the occult ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 2007;92(6):2080-2086. [DOI] [PubMed] [Google Scholar]
- 23. Page RB. Directional pituitary blood flow: a microcinephotographic study. Endocrinology. 1983;112(1):157-165. [DOI] [PubMed] [Google Scholar]
- 24. Mamelak AN, Dowd CF, Tyrrell JB, McDonald JF, Wilson CB. Venous angiography is needed to interpret inferior petrosal sinus and cavernous sinus sampling data for lateralizing adrenocorticotropin-secreting adenomas. J Clin Endocrinol Metab. 1996;81(2):475-481. [DOI] [PubMed] [Google Scholar]
- 25. Lefournier V, Martinie M, Vasdev A, et al. Accuracy of bilateral inferior petrosal or cavernous sinuses sampling in predicting the lateralization of Cushing’s disease pituitary microadenoma: influence of catheter position and anatomy of venous drainage. J Clin Endocrinol Metab. 2003;88(1):196-203. [DOI] [PubMed] [Google Scholar]
- 26. Miller DL, Doppman JL, Chang R. Anatomy of the junction of the inferior petrosal sinus and the internal jugular vein. AJNR Am J Neuroradiol. 1993;14(5):1075-1083. [PMC free article] [PubMed] [Google Scholar]
- 27. Andereggen L, Schroth G, Gralla J, et al. Selective inferior petrosal sinus sampling without venous outflow diversion in the detection of a pituitary adenoma in Cushing’s syndrome. Neuroradiology. 2012;54(5):495-503. [DOI] [PubMed] [Google Scholar]
- 28. Gazioglu N, Ulu MO, Ozlen F, et al. Management of Cushing’s disease using cavernous sinus sampling: effectiveness in tumor lateralization. Clin Neurol Neurosurg. 2008;110(4):333-338. [DOI] [PubMed] [Google Scholar]
- 29. Flitsch J, Lüdecke DK, Knappe UJ, Grzyska U. Cavernous sinus sampling in selected cases of Cushing’s disease. Exp Clin Endocrinol Diabetes. 2002;110(7):329-335. [DOI] [PubMed] [Google Scholar]
- 30. Burkhardt T, Flitsch J, van Leyen P, et al. Cavernous sinus sampling in patients with Cushing’s disease. Neurosurg Focus. 2015;38(2):E6. [DOI] [PubMed] [Google Scholar]
- 31. Doppman JL, Nieman LK, Chang R, et al. Selective venous sampling from the cavernous sinuses is not a more reliable technique than sampling from the inferior petrosal sinuses in Cushing’s syndrome. J Clin Endocrinol Metab. 1995;80(8):2485-2489. [DOI] [PubMed] [Google Scholar]
- 32. Lefournier V, Martinie M, Vasdev A, et al. Accuracy of bilateral inferior petrosal or cavernous sinuses sampling in predicting the lateralization of Cushing’s disease pituitary microadenoma: influence of catheter position and anatomy of venous drainage. J Clin Endocrinol Metab. 2003;88(1):196-203. [DOI] [PubMed] [Google Scholar]
- 33. Findling JW, Raff H. Diagnosis of endocrine disease: differentiation of pathologic/neoplastic hypercortisolism (Cushing’s syndrome) from physiologic/non-neoplastic hypercortisolism (formerly known as pseudo-Cushing’s syndrome). Eur J Endocrinol. 2017;176(5):R205-R216. [DOI] [PubMed] [Google Scholar]
- 34. Gold PW, Loriaux DL, Roy A, et al. Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing’s disease. N Engl J Med. 1986;314(21):1329-1335. [DOI] [PubMed] [Google Scholar]
- 35. Pfohl B, Sherman B, Schlechte J, Stone R. Pituitary-adrenal axis rhythm disturbances in psychiatric depression. Arch Gen Psychiatry. 1985;42(9):897-903. [DOI] [PubMed] [Google Scholar]
- 36. Ross RJ, Miell JP, Holly JM, et al. Levels of GH binding activity, IGFBP-1, insulin, blood glucose and cortisol in intensive care patients. Clin Endocrinol (Oxf). 1991;35(4): 361-367. [DOI] [PubMed] [Google Scholar]
- 37. Liu H, Bravata DM, Cabaccan J, Raff H, Ryzen E. Elevated late-night salivary cortisol levels in elderly male type 2 diabetic veterans. Clin Endocrinol (Oxf). 2005;63(6):642-649. [DOI] [PubMed] [Google Scholar]
- 38. Baid SK, Sinaii N, Wade M, Rubino D, Nieman LK. Radioimmunoassay and tandem mass spectrometry measurement of bedtime salivary cortisol levels: a comparison of assays to establish hypercortisolism. J Clin Endocrinol Metab. 2007;92(8):3102-3107. [DOI] [PubMed] [Google Scholar]
- 39. Yamamoto Y, Davis DH, Nippoldt TB, Young WF, Huston J, Parisi JE. False-positive inferior petrosal sinus sampling in the diagnosis of Cushing’s disease. J Neurosurg. 1995;83(6):1087. doi: 10.3171/jns.1995.83.6.1087. [DOI] [PubMed] [Google Scholar]
- 40. Yanovski JA, CutlerGB, Jr., Doppman JL, et al. The limited ability of inferior petrosal sinus sampling with corticotropin-releasing hormone to distinguish Cushing’s disease from pseudo-Cushing states or normal physiology. J Clin Endocrinol Metab. 1993;77(2):503-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Colao A, Faggiano A, Pivonello R, Pecori Giraldi F, Cavagnini F, Lombardi G; Study Group of the Italian Endocrinology Society on the Pathophysiology of the Hypothalamic-Pituitary-Adrenal Axis . Inferior petrosal sinus sampling in the differential diagnosis of Cushing’s syndrome: results of an Italian multicenter study. Eur J Endocrinol. 2001;144(5): 499-507. [DOI] [PubMed] [Google Scholar]
- 42. McCance DR, McIlrath E, McNeill A, et al. Bilateral inferior petrosal sinus sampling as a routine procedure in ACTH-dependent Cushing’s syndrome. Clin Endocrinol (Oxf). 1989;30(2):157-166. [DOI] [PubMed] [Google Scholar]
- 43. Findling JW, Aron DC, Tyrrell JB, et al. Selective venous sampling for ACTH in Cushing’s syndrome: differentiation between Cushing’s disease and the ectopic ACTH syndrome. Ann Intern Med. 1981;94(5):647-652. [DOI] [PubMed] [Google Scholar]
- 44. Wu ZQ, Xu HG. Preanalytical stability of adrenocorticotropic hormone depends on both time to centrifugation and temperature. J Clin Lab Anal. 2017;31(5). doi: 10.1002/jcla.22081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Atkinson B, Mullan KR. What is the best approach to suspected cyclical Cushing syndrome? Strategies for managing Cushing’s syndrome with variable laboratory data. Clin Endocrinol (Oxf). 2011;75(1):27-30. [DOI] [PubMed] [Google Scholar]
- 46. Albani A, Berr CM, Beuschlein F, et al. A pitfall of bilateral inferior petrosal sinus sampling in cyclic Cushing’s syndrome. BMC Endocr Disord. 2019;19(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 52-1987. A 20-year-old woman with Cushing’s disease and a pulmonary nodule. N Engl J Med. 1987;317(26):1648-1658. doi: 10.1056/NEJM198712243172608. [DOI] [PubMed] [Google Scholar]
- 48. Utz A, Biller BM. The role of bilateral inferior petrosal sinus sampling in the diagnosis of Cushing’s syndrome. Arq Bras Endocrinol Metabol. 2007;51(8):1329-1338. [DOI] [PubMed] [Google Scholar]
- 49. Zerikly RK, Eray E, Faiman C, et al. Cyclic Cushing syndrome due to an ectopic pituitary adenoma. Nat Clin Pract Endocrinol Metab. 2009;5(3):174-179. [DOI] [PubMed] [Google Scholar]
- 50. Johnston PC, Kennedy L, Weil RJ, Hamrahian AH. Ectopic ACTH-secreting pituitary adenomas within the sphenoid sinus. Endocrine. 2014;47(3):717-724. [DOI] [PubMed] [Google Scholar]
- 51. Swearingen B, Katznelson L, Miller K, et al. Diagnostic errors after inferior petrosal sinus sampling. J Clin Endocrinol Metab. 2004;89(8):3752-3763. [DOI] [PubMed] [Google Scholar]
- 52. Mulligan GB, Eray E, Faiman C, et al. Reduction of false-negative results in inferior petrosal sinus sampling with simultaneous prolactin and corticotropin measurement. Endocr Pract. 2011;17(1):33-40. [DOI] [PubMed] [Google Scholar]
- 53. Qiao X, Ye H, Zhang X, et al. The value of prolactin in inferior petrosal sinus sampling with desmopressin stimulation in Cushing’s disease. Endocrine. 2015;48(2):644-652. [DOI] [PubMed] [Google Scholar]
- 54. Heaney AP, Melmed S. Molecular targets in pituitary tumours. Nat Rev Cancer. 2004;4(4):285-295. [DOI] [PubMed] [Google Scholar]
- 55. Crock PA, Pestell RG, Calenti AJ, et al. Multiple pituitary hormone gradients from inferior petrosal sinus sampling in Cushing’s disease. Acta Endocrinol (Copenh). 1988;119(1):75-80. [DOI] [PubMed] [Google Scholar]
- 56. Allolio B, Günther RW, Benker G, Reinwein D, Winkelmann W, Schulte HM. A multihormonal response to corticotropin-releasing hormone in inferior petrosal sinus blood of patients with Cushing’s disease. J Clin Endocrinol Metab. 1990;71(5):1195-1201. [DOI] [PubMed] [Google Scholar]
- 57. McNally PG, Bolia A, Absalom SR, Falconer-Smith J, Howlett TA. Preliminary observations using endocrine markers of pituitary venous dilution during bilateral simultaneous inferior petrosal sinus catheterization in Cushing’s syndrome: is combined CRF and TRH stimulation of value? Clin Endocrinol (Oxf). 1993;39(6):681-686. [DOI] [PubMed] [Google Scholar]
- 58. Zovickian J, Oldfield EH, Doppman JL, CutlerGB, Jr., Loriaux DL. Usefulness of inferior petrosal sinus venous endocrine markers in Cushing’s disease. J Neurosurg. 1988;68(2):205-210. [DOI] [PubMed] [Google Scholar]
- 59. Mulligan GB, Faiman C, Gupta M, et al. Prolactin measurement during inferior petrosal sinus sampling improves the localization of pituitary adenomas in Cushing’s disease. Clin Endocrinol (Oxf). 2012;77(2):268-274. [DOI] [PubMed] [Google Scholar]
- 60. Sharma ST, Nieman LK. Is prolactin measurement of value during inferior petrosal sinus sampling in patients with adrenocorticotropic hormone-dependent Cushing’s syndrome? J Endocrinol Invest. 2013;36(11):1112-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Grant P, Dworakowska D, Carroll P. Maximizing the accuracy of Inferior petrosal sinus sampling: validation of the use of Prolactin as a marker of pituitary venous effluent in the diagnosis of Cushing’s disease. Clin Endocrinol (Oxf). 2012;76(4):555-559. [DOI] [PubMed] [Google Scholar]
- 62. Wind JJ, Lonser RR, Nieman LK, DeVroom HL, Chang R, Oldfield EH. The lateralization accuracy of inferior petrosal sinus sampling in 501 patients with Cushing’s disease. J Clin Endocrinol Metab. 2013;98(6):2285-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nieman LK, Oldfield EH, Wesley R, Chrousos GP, Loriaux DL, Cutler GB Jr. A simplified morning ovine corticotropin-releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 1993;77(5):1308-1312. [DOI] [PubMed] [Google Scholar]
- 64. Ritzel K, Beuschlein F, Berr C, et al. ACTH after 15 min distinguishes between Cushing’s disease and ectopic Cushing’s syndrome: a proposal for a short and simple CRH test. Eur J Endocrinol. 2015;173(2):197-204. [DOI] [PubMed] [Google Scholar]
- 65. Wiggam MI, Heaney AP, McIlrath EM, et al. Bilateral inferior petrosal sinus sampling in the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome: a comparison with other diagnostic tests. J Clin Endocrinol Metab. 2000;85(4):1525-1532. [DOI] [PubMed] [Google Scholar]
- 66. Invitti C, Pecori Giraldi F, Cavagnini F. Inferior petrosal sinus sampling in patients with Cushing’s syndrome and contradictory responses to dynamic testing. Clin Endocrinol (Oxf). 1999;51(2):255-257. [DOI] [PubMed] [Google Scholar]
- 67. Bonelli FS, Huston J III, Carpenter PC, Erickson D, Young WF Jr, Meyer FB. Adrenocorticotropic hormone–dependent Cushing’s syndrome: sensitivity and specificity of inferior petrosal sinus sampling. Am J Neuroradiol. 2000;21(4):690-696. [PMC free article] [PubMed] [Google Scholar]
- 68. Newell-Price J, Trainer P, Besser M, Grossman A. The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr Rev. 1998;19(5):647-672. [DOI] [PubMed] [Google Scholar]
- 69. Lin LY, Teng MM, Huang CI, et al. Assessment of bilateral inferior petrosal sinus sampling (BIPSS) in the diagnosis of Cushing’s disease. J Chin Med Assoc. 2007;70(1):4-10. [DOI] [PubMed] [Google Scholar]
- 70. Johnston PC, Kennedy L, Hamrahian AH, et al. Surgical outcomes in patients with Cushing’s disease: the Cleveland Clinic experience. Pituitary. 2017;20(4):430-440. [DOI] [PubMed] [Google Scholar]
- 71. De Sousa SMC, McCormack AI, McGrath S, Torpy DJ. Prolactin correction for adequacy of petrosal sinus cannulation may diminish diagnostic accuracy in Cushing’s disease. Clin Endocrinol (Oxf). 2017;87(5):515-522. [DOI] [PubMed] [Google Scholar]
- 72. Jarial KDS, Bhansali A, Mukherjee KK, et al. Prolactin-adjusted ACTH ratio in predicting lateralization of ACTH source during simultaneous bilateral inferior petrosal sinus sampling in patients with Cushing’s disease. Indian J Endocrinol Metab. 2019;23(1):56-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bayraktar F, Kebapcilar L, Kocdor MA, et al. Cushing’s syndrome due to ectopic CRH secretion by adrenal pheochromocytoma accompanied by renal infarction. Exp Clin Endocrinol Diabetes. 2006;114(8):444-447. [DOI] [PubMed] [Google Scholar]
- 74. Sauer N, zur Wiesch CS, Flitsch J, et al. Cushing’s syndrome due to a corticotropin-releasing hormone- and adrenocorticotrophic hormone-producing neuroendocrine pancreatic tumor. Endocr Pract. 2014;20(4):e53-e57. [DOI] [PubMed] [Google Scholar]
- 75. Liu J, Heikkilä P, Voutilainen R, Karonen SL, Kahri AI. Pheochromocytoma expressing adrenocorticotropin and corticotropin-releasing hormone; regulation by glucocorticoids and nerve growth factor. Eur J Endocrinol. 1994;131(3):221-228. [DOI] [PubMed] [Google Scholar]
- 76. O’Brien T, Young WF Jr, Davila DG, et al. Cushing’s syndrome associated with ectopic production of corticotrophin-releasing hormone, corticotrophin and vasopressin by a phaeochromocytoma. Clin Endocrinol (Oxf). 1992;37(5):460-467. [DOI] [PubMed] [Google Scholar]
- 77. Carroll TB, Fisco AJH, Auchus RJ, Kennedy L, Findling JW. Paradoxical results after inadvertent use of cosyntropin [adrenocorticotropin hormone (1–24)] rather than Acthrel (ovine corticotropin releasing hormone) during inferior petrosal sinus sampling. Endocr Pract 2014;20(7):646-649. [DOI] [PubMed] [Google Scholar]
- 78. Raff H, Findling JW, Wong J. Short loop adrenocorticotropin (ACTH) feedback after ACTH-(1-24) injection in man is an artifact of the immunoradiometric assay. J Clin Endocrinol Metab. 1989;69(3):678-680. [DOI] [PubMed] [Google Scholar]
- 79. Haynes AB, Weiser TG, Berry WR, et al. ; Safe Surgery Saves Lives Study Group . A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360(5):491-499. [DOI] [PubMed] [Google Scholar]
- 80. Ilias I, Chang R, Pacak K, et al. Jugular venous sampling: an alternative to petrosal sinus sampling for the diagnostic evaluation of adrenocorticotropic hormone-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 2004;89(8):3795-3800. [DOI] [PubMed] [Google Scholar]
- 81. Erickson D, Huston J 3rd, Young WF Jr, et al. Internal jugular vein sampling in adrenocorticotropic hormone-dependent Cushing’s syndrome: a comparison with inferior petrosal sinus sampling. Clin Endocrinol (Oxf). 2004;60(4):413-419. [DOI] [PubMed] [Google Scholar]
- 82. Doppman JL, Oldfield EH, Nieman LK. Bilateral sampling of the internal jugular vein to distinguish between mechanisms of adrenocorticotropic hormone-dependent Cushing syndrome. Ann Intern Med. 1998;128(1):33-36. [DOI] [PubMed] [Google Scholar]
- 83. Radvany MG, Quinones-Hinojosa A, Gallia GL, Wand GS, Salvatori R. Venous sampling for cushing disease: comparison of internal jugular vein and inferior petrosal sinus sampling. Endocr Pract. 2016;22(9):1057-1061. [DOI] [PubMed] [Google Scholar]
- 84. Carr BR, Parker CR Jr, Madden JD, MacDonald PC, Porter JC. Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. Am J Obstet Gynecol. 1981;139(4):416-422. [DOI] [PubMed] [Google Scholar]
- 85. Lindsay JR, Jonklaas J, Oldfield EH, Nieman LK. Cushing’s syndrome during pregnancy: personal experience and review of the literature. J Clin Endocrinol Metab. 2005;90(5):3077-3083. [DOI] [PubMed] [Google Scholar]
- 86. Pinette MG, Pan YQ, Oppenheim D, Pinette SG, Blackstone J. Bilateral inferior petrosal sinus corticotropin sampling with corticotropin-releasing hormone stimulation in a pregnant patient with Cushing’s syndrome. Am J Obstet Gynecol. 1994;171(2):563-564. [DOI] [PubMed] [Google Scholar]
- 87. Reimondo G, Paccotti P, Minetto M, et al. The corticotrophin-releasing hormone test is the most reliable noninvasive method to differentiate pituitary from ectopic ACTH secretion in Cushing’s syndrome. Clin Endocrinol (Oxf). 2003;58(6):718-724. [DOI] [PubMed] [Google Scholar]
- 88. Barbot M, Trementino L, Zilio M, et al. Second-line tests in the differential diagnosis of ACTH-dependent Cushing’s syndrome. Pituitary. 2016;19(5):488-495. [DOI] [PubMed] [Google Scholar]
- 89. Moro M, Putignano P, Losa M, Invitti C, Maraschini C, Cavagnini F. The desmopressin test in the differential diagnosis between Cushing’s disease and pseudo-Cushing states. J Clin Endocrinol Metab. 2000;85(10):3569-3574. [DOI] [PubMed] [Google Scholar]
- 90. Newell-Price J, Perry L, Medbak S, et al. A combined test using desmopressin and corticotropin-releasing hormone in the differential diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab. 1997;82(1):176-181. [DOI] [PubMed] [Google Scholar]
- 91. Aron DC, Raff H, Findling JW. Effectiveness versus efficacy: the limited value in clinical practice of high dose dexamethasone suppression testing in the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 1997;82(6):1780-1785. [DOI] [PubMed] [Google Scholar]
- 92. Frete C, Corcuff JB, Kuhn E, et al. Non-invasive diagnostic strategy in ACTH-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 2020;105(10). doi: 10.1210/clinem/dgaa409. [DOI] [PubMed] [Google Scholar]
- 93. Law M, Wang R, Liu C-SJ, et al. Value of pituitary gland MRI at 7 T in Cushing’s disease and relationship to inferior petrosal sinus sampling: case report. J Neurosurg. 2018;130(2):347. doi: 10.3171/2017.9.Jns171969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. de Rotte AA, Groenewegen A, Rutgers DR, et al. High resolution pituitary gland MRI at 7.0 tesla: a clinical evaluation in Cushing’s disease. Eur Radiol. 2016;26(1):271-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Page-Wilson G, Freda PU, Jacobs TP, et al. Clinical utility of plasma POMC and AgRP measurements in the differential diagnosis of ACTH-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 2014;99(10):E1838-E1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References. Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.