Abstract
Background
Robotic ventral hernia repair (VHR) has seen rapid adoption, but with limited data assessing clinical outcome or cost. This systematic review compared robotic VHR with laparoscopic and open approaches.
Methods
This systematic review was undertaken in accordance with PRISMA guidelines. PubMed, MEDLINE, Embase, and Cochrane databases were searched for articles with terms relating to ‘robot-assisted’, ‘cost effectiveness’, and ‘ventral hernia’ or ‘incisional hernia’ from 1 January 2010 to 10 November 2020. Intraoperative and postoperative outcomes, pain, recurrence, and cost data were extracted for narrative analysis.
Results
Of 25 studies that met the inclusion criteria, three were RCTs and 22 observational studies. Robotic VHR was associated with a longer duration of operation than open and laparoscopic repairs, but with fewer transfusions, shorter hospital stay, and lower complication rates than open repair. Robotic VHR was more expensive than laparoscopic repair, but not significantly different from open surgery in terms of cost. There were no significant differences in rates of intraoperative complication, conversion to open surgery, surgical-site infection, readmission, mortality, pain, or recurrence between the three approaches.
Conclusion
Robotic VHR was associated with a longer duration of operation, fewer transfusions, a shorter hospital stay, and fewer complications compared with open surgery. Robotic VHR had higher costs and a longer operating time than laparoscopic repair. Randomized or matched data with standardized reporting, long-term outcomes, and cost-effectiveness analyses are still required to weigh the clinical benefits against the cost of robotic VHR.
Ventral hernias affect around one-quarter of adults, and incisional hernias develop in 10–15 per cent of open abdominal incisions. Robotic repair has seen widespread adoption globally, yet there are limited data assessing its clinical or cost outcomes. In this systematic review, the evidence shows that robotic ventral hernia repair is associated with a longer duration of operation, fewer transfusions, shorter hospital stay, and lower total complication rates compared with open repair. Robotic ventral hernia repair has greater costs and a longer operating time than laparoscopic repair. Further randomized and matched data with standardized reporting, long-term outcomes, and cost-effectiveness analyses are required.
Introduction
Adult ventral hernias are common1, and include epigastric, umbilical, Spigelian, and incisional hernias. Incisional hernias develop after 10–15 per cent of laparotomies2, and the risk of recurrence increases with each subsequent repair3. Over 60 per cent of ventral hernias are repaired using an open approach, although there has been a nearly 45-fold increase in repairs using robotics technology over the past decade4.
Morbidity rates associated with open repair are high owing to patient factors and hernia complexity, with short-term complication rates of up to 40 per cent5. Laparoscopic repair has been recommended for large epigastric or umbilical hernias by the European and Americas Hernia Societies6, largely on the basis of decreased wound morbidity. Robotic surgery augments the laparoscopic approach with its magnified three-dimensional visualization of the operative field, stable platform, and superior range of motion7 that may be particularly beneficial for complex hernias.
The cost of robotic surgery for ventral hernia repair (VHR) is also unknown. Acquisition and implementation require substantial investment along with expenses for annual maintenance contracts, instrument purchases, staff and training, and infrastructure upgrades8. It is imperative to weigh the costs of robotic surgery relative to clinical efficacy.
A systematic review9 in 2019 reported on limited short-term outcomes following VHR but without cost outcomes. New studies, including two RCTs, have since been published. The present systematic review analysed intraoperative and postoperative clinical outcomes and costs of robotic VHR compared with laparoscopic and open approaches.
Methods
This review formed part of a larger report commissioned by the Department of Veterans Affairs on clinical and cost outcomes of robotic procedures for cholecystectomy, inguinal hernia repair, and VHR10. PRISMA standards11 were adhered to, and the a priori protocol registered in PROSPERO (CRD42020156945).
Literature search
English-language articles in PubMed, MEDLINE, Embase, and Cochrane (all databases) from 1 January 2010 to 10 November 2020 were searched. Search terms relating to ‘robotic surgical procedures’ or ‘robot-assisted’, ‘cost effectiveness’, and ‘ventral hernia’ or ‘incisional hernia’ were used. Studies published before 2010 were not included, as robotic procedures were not widely performed then, and many surgeons and their support staff may have been in the early adoption phase for both the technique and implementation of the robotic platform.
Study selection and data collection
All stages of review were completed by two independent team members, and disagreements were resolved through discussion. RCTs and observational studies comparing robotic VHR with either laparoscopic or open approaches were included. Studies with fewer than 10 patients per arm, and those that evaluated only emergency repairs, used a hybrid approach (open VHR with robotic transversus abdominis release (TAR)), or assessed parastomal hernias were excluded. Studies that used the same national databases with duplicate patients were excluded if there was greater than 1-year overlap, with an exception made for one study12 that reported a unique outcome. When selecting studies for inclusion using the same databases, peer-reviewed studies with superior methodology (propensity matching) or longer time span were preferentially included. The same inclusion and exclusion criteria were applied to the economic analysis.
Data were collected on study design, sample size, patient and hernia characteristics, intraoperative outcomes, short-term postoperative outcomes, long-term outcomes, and length of follow-up. Patient characteristics included: age, race/ethnicity, sex, BMI, ASA fitness grade, and co-morbidities. Hernia characteristics included: hernia area or length, whether the hernia was primary or recurrent, whether the hernia was midline, and repair technique. Intraoperative outcomes included: operating room (OR) time, estimated blood loss, transfusions, intraoperative complications, conversions to open surgery, use of mesh, rate of fascial closure, method of mesh fixation, and presence of concurrent procedures. Short-term outcomes were defined as those occurring 30 days or less after surgery, including length of hospital stay, surgical-site infection (SSI), readmission rate, reoperation rate, emergency department visits, all complications, and mortality. Studies13,14 reporting ‘postoperative infection’ provided an estimated SSI outcome. Long-term outcomes were defined as those occurring after 30 days, and included readmission rate, mesh infection, chronic pain, recurrence, and quality of life. Economic analyses included the source and type of cost data and estimated mean or median costs for each approach. Costs originally calculated in US dollars were converted to euros at an exchange rate of US $1.2 to €1.
Risk of bias and certainty of evidence
The risk of bias in RCTs was assessed with the Cochrane risk-of-bias tool15. Each observational study was assessed for risk of bias using the Cochrane Risk Of Bias In Non-Randomized Studies of Interventions (ROBINS-I) tool16.
Criteria of the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) working group were used to assess the overall certainty of the evidence17.
Statistical analysis
The data synthesis is narrative; meta-analysis was not conducted owing to sources of heterogeneity in clinical and cost outcomes of the RCTs and observational studies. RCTs and studies that performed propensity matching were considered more valuable in terms of summarizing the data than non-matched studies that did not account for clinical differences between patient cohorts.
The mean (or median) and a measure of variation (s.d., i.q.r., range, or c.i.) were extracted for continuous outcomes. For binary outcomes, a count and percentage were retrieved. P < 0.050 was considered significant. When data were presented only for subgroups, pooled values were back-calculated.
Graphical representations of risk and mean differences with 95 per cent confidence intervals were plotted when available or estimated using counts and sample sizes using R 4.0.218. Annotations were made where significance differed between the study-reported P value and calculated risk or mean differences and 95 per cent confidence intervals. For rare outcome events, risk differences (RDs) were used preferentially during analysis.
Results
Literature search
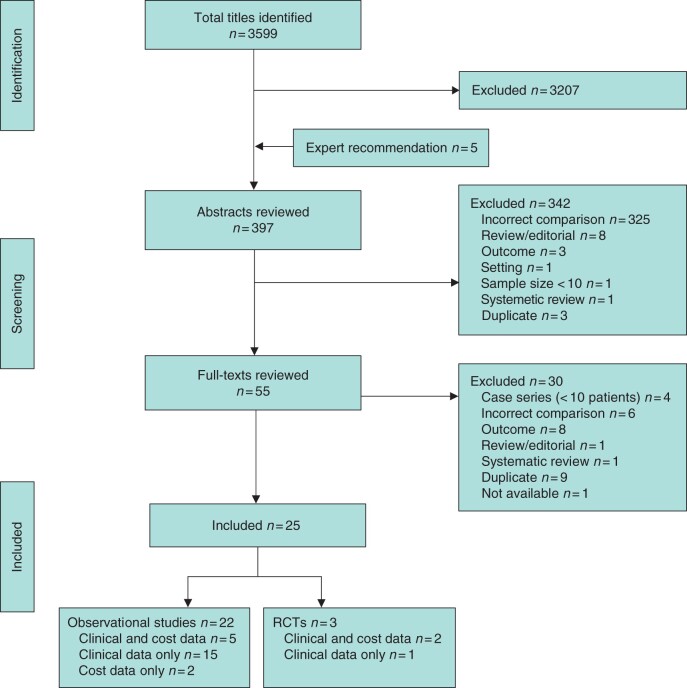
A total of 3604 citations were identified, 3599 potentially relevant citations from databases and five publications recommended by experts. From these, 397 abstracts were included for screening, and 55 articles for full-text review. Of these, 30 full-text publications were excluded for the following reasons: duplicate data (9), no outcomes of interest (8), incorrect comparison (6), case series (4), review or editorial (2), and full text not available (1). In total, 25 studies met the inclusion criteria: three RCTs19–21 and 2212–14,22–40 observational studies, of which two37,39 were included for cost outcomes only (Fig. 1).
Fig. 1.
PRISMA flow diagram showing selection of articles for review
Study characteristics
One RCT19, of 38 patients comparing robotic with laparoscopic VHR, was published as a conference abstract from a single institution in Brazil. Details of the operative techniques were not provided, and data supporting intraoperative outcomes and the majority of postoperative outcomes were not reported. This study was judged to have a high risk of bias and was omitted from the final synthesis (Table 1).
Table 1.
Risk-of-bias assessment for RCTs using Cochrane risk-of-bias tool
| Reference, year | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other sources of bias |
|---|---|---|---|---|---|---|---|
| Abdalla et al.19 2017* | ○ | ● | ● | ● QoL | ○ | ● | |
| Olavarria et al.20 2020 | ○ | ○ | ● Surgeon not blinded; patient and rest of research team blinded | ○ | ○ | ○ | ● Study supported by investigator-initiated grant from Intuitive |
| Petro et al.21 2021 | ○ | ○ | ● Single-blinded | ● Not stated whether outcome assessor was blinded; patient-recorded outcomes concealed | ○ | ○ | ● Study funded by grant from Intuitive; 6 authors (including 1st author) received grants from Intuitive |
Conference abstract. ○, Low risk of bias; ● high risk of bias; QoL, quality of life.
The remaining two RCTs compared robotic with laparoscopic intraperitoneal onlay mesh (IPOM) repair (Table 2). One20 was multi-institutional and included 123 patients, and the other21 included 75 patients at a single institution.
Table 2.
Study, hernia, and patient characteristics in studies comparing robotic versus laparoscopic surgery
| Study characteristics |
Hernia characteristics |
Patient data |
||||||
|---|---|---|---|---|---|---|---|---|
| Reference, year, country | Sample size |
Hernia area (cm2)* |
Age (years)* |
BMI (kg/m2)* |
||||
| Robotic | Laparoscopic | Robotic | Laparoscopic | Robotic | Laparoscopic | Robotic | Laparoscopic | |
| RCTs | ||||||||
| Olavarria et al.20 2020, USA | 65 | 58 | 3.0 (2.0–5.0)††§ | 3.0 (1.0–4.5)††§ | 50.1(13.3) | 48.0(12.9)** | 32.4(4.6) | 31.8(5.4)** |
| Petro et al.21 2021, USA | 39 | 36 | 5 (3–8)††§ | 5 (2–8)††§ | 56 (50–70) | 55 (49–60)** | 35 (31–39)†† | 31 (27– 36)††,†††† |
| Studies with propensity matching | ||||||||
| Altieri et al.23¶ 2018, USA | 679 | 2089 | – | – | > 55: 67.6% | > 55: 47.4%†††† | – | – |
| LaPinska et al.30 2020, USA | 615 | 615 | 4(2)§ | 4(3)§ | 55(14) | 56(14)** | 33(7) | 33(8)** |
| Song et al.36# 2017, USA | 94 | 94 | – | – | – | – | – | – |
| Walker et al.38¶ 2018, USA | 142 | 73 | 4.3(3.2)§ | 4.1(2.1)§ | 53.2(13.2) | 49.5(13.3)** | 31.6(5.1) | 35.7(7.9)†††† |
| Studies without propensity matching | ||||||||
| Alimi et al.22# 2020, USA | 46 | 100 | 17.5 | 119.5 | – | – | 28.8 | 31.6** |
| Armijo et al.13 2018, USA | 465 | 6829 | – | – | 59(13.1) | 57(13.2)** | – | – |
| Chen et al.26 2017, USA | 39 | 33 | 3.07 (1–9)‡§ | 2.07 (0.5–5)‡§ | 47.2 (24–69)‡ | 46.6 (27–68)‡** | 33 (23–53) | 32 (25–45)‡** |
| Coakley et al.14 2017, USA | 351 | 32 243 | – | – | 59.4(14.6) | 57.4(14.9)†††† | – | – |
| Gonzalez et al.28 2015, USA | 67 | 67 | – | – | 56.6(14.5) | 55.0(13.2)** | 34.7(9.0) | 33.5(9.5)** |
| Khorgami et al.29 2019, USA | 99 | 3600 | – | – | – | – | – | – |
| Lu et al.31 2019, USA | 86 | 120 | 7.1(2.6)§ | 5.5(1.8)§ | 50.8(12.8) | 53.2(14.6)** | 34.4(7.4) | 31.3(6.1)†††† |
| Mudyanadzo et al.33 2020, USA | 16 | 19 | – | – | – | – | – | – |
| Tan et al.37# 2018, USA | 46 | 47 | – | – | 55.1 | 61.6†††† | – | –** |
| Warren et al.39 2017, USA | 53 | 103 | 82.5(69.8) | 88.0(94.0) | 52.9(12.3) | 60.2(13.4)†††† | 34.7(7.4) | 35.7(9.5)** |
| Zayan et al.40 2019, USA | 16 | 33 | – | – | 49.0 (42.2– 55.2)†† | 51.5 (46.5– 56.2)††** | 48.97 (42.15– 55.23)†† | 33.71 (30.84– 42.88)††,†††† |
Values are mean(s.d.) unless indicated otherwise; values are ††mean (i.q.r.) and ‡mean (range). §Hernia length in centimetres. ¶Unmatched data presented, as matched demographic data not reported. #Conference abstract. **P not significant. ††††P < 0.050. A version of this table featuring additional data is available as Table S1 online.
Twenty-two studies were observational, of which four22,34,36,37 were conference abstracts, and two37,39 provided cost outcomes only. Eleven studies12–14,23–25,27,29,30,36,39 used data from prospectively maintained databases, nine12,13,24,25,27,32,34–36 compared robotic VHR with open repair (Table 3), and 1513,14,22,23,26,28–31,33,36–40 compared robotic surgery with laparoscopy. All observational studies were performed in the USA, except for two studies27,35 from Austria and Australia. Ten studies12–14,23,25,29,30,32,35,38 were multi-institutional, 1122,24,26–28,31,33,34,37,39,40 were from a single institution, and one study36 did not specify the number of centres. Propensity matching was performed in six studies23,25,30,32,36,38. The sample size varied from 26 to 46 799 patients.
Table 3.
Study, hernia, and patient characteristics in studies comparing robotic versus open surgery
| Study characteristics |
Hernia characteristics |
Patient data |
||||||
|---|---|---|---|---|---|---|---|---|
| Reference, year, country | Sample size |
Hernia area (cm2)* |
Age (years)* |
BMI (kg/m2)* |
||||
| Robotic | Open | Robotic | Open | Robotic | Open | Robotic | Open | |
| Studies with propensity matching | ||||||||
| Carbonell et al.25 2018, USA | 111 | 222 | 87.96(67.57) | 80.13(74.02) | 55.59(12.36) | 55.08(13.76)# | 33.88(7.30) | 33.23(7.39)# |
| Martin-del-Campo et al.32 2018, USA | 38 | 76 | 13.5(4.5)§ | 13.5(4.5)§ | 58.9(12.7) | 58.8(11.8)# | 33.1(8.8) | 33.51(5.7)# |
| Song et al.36 2017, USA | 96 | 96 | – | – | – | – | – | – |
| Studies without propensity matching | ||||||||
| Armijo et al.13 2018, USA | 465 | 39 505 | – | – | 59(13.1) | 57(13.3)# | – | – |
| Bittner et al.24 2018, USA | 26 | 76 | 235(107) | 260(209) | 52.4(12.9) | 54.6(14)# | 33.4(9) | 32.1(7)# |
| Dauser et al.27 2020, Austria | 16 | 10 | – | – | 71 | 62# | 28.4 (22.0–40.5)† | 25.7 (23.6–29.8)†# |
| Guzman-Pruneda et al.12 2020, USA | 42 | 194 | 61 (40–120)‡ | 193 (106–300)‡ | 59 (54–65)‡ | 62 (53–68)‡# | 32 (28–39)‡ | 31 (28–35)‡# |
| Nguyen et al.34, 2019, USA | 27 | 16 | 216 | 242 | 55.4(12.4) | 58.6(10.4)# | 32.2(6.4) | 33.3(5.5)# |
| Reeves et al.35 2020, Australia | 13 | 13 | – | – | 69.9(13.3) | 64.8(14.7)# | – | – |
*Values are mean(s.d.) unless indicated otherwise; values are †median (range) and ‡median (i.q.r.). §Hernia length in centimetres. ¶Conference abstract. P not significant. A version of this table featuring additional data is available as Table S2 online.
Hernia characteristics, such as size, whether the hernia was recurrent or midline, repair technique, fascial closure, and mesh fixation technique, were reported inconsistently. Where reported, about three-quarters of ventral hernias were primary. The most common repair technique in studies comparing robotic and open approaches was retrorectus VHR with or without TAR, whereas IPOM repair was more common in studies comparing robotic and laparoscopic approaches. With regard to mesh fixation, tacks were used more frequently in laparoscopic VHRs, whereas sutures and adhesives were used primarily in robotic and open repairs.
The two RCTs included in the final analysis were deemed to have a moderate risk of bias (Table 1). Both suffered from lack of blinding of the surgeon given the nature of the trial design, and both were funded by the manufacturer of the robotic platform (Intuitive Surgical, Sunnyvale, CA, USA).
The majority of observational studies had a high risk of confounding bias, as baseline characteristics differed between approaches. Selection bias, bias in the measurement classification of interventions, bias owing to deviation from intended interventions, and bias in selection of the reported result were generally low. Studies reporting only short-term outcomes were presumed to have minimal loss to follow-up and therefore to be at low risk of bias because of missing data. Studies with long-term outcomes were classified as being at higher risk of bias owing to missing data if follow-up rates were low (less than 70 per cent) or not reported. For bias in measurement of outcomes, self-reported outcomes relating to pain and quality of life had a moderate risk of bias because of the subjectivity of these measurements. Objective assessments, such as length of hospital stay, complications, OR time, recurrence, and pain as assessed by narcotic use, had a low risk of bias. Only six studies were propensity-matched, but there was inconsistency concerning the matched variables (such as patient characteristics or hernia size) (Table 4). The non-matched studies had a high risk of bias, whereas the matched studies were deemed to have a moderate risk of bias. Seven24,25,30–32,38,39 studies disclosed author involvement with Intuitive Surgical to varying extents.
Table 4.
Risk of bias in observational studies determined using ROBINS-I tool
| Reference | Confounding | Selection bias | Bias in measurement classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of reported result | Other source of bias |
|---|---|---|---|---|---|---|---|---|
| Alimi et al.22 2020 | Serious: very large differences in hernia size, limited characteristics reported; not propensity-matched | Serious: institutional data, not stated whether consecutive series | Low | Low | Moderate: unknown follow-up | Low: complications | Moderate: limited outcomes reported | |
| Altieri et al.23 2018 | Moderate: differences in ethnicity, sex, BMI; propensity-matched but characteristics not reported | Low: database | Low | Low | Low | Low: complications | Moderate: matched outcomes poorly reported and inconsistent with tables | |
| Armijo et al.13 2018 | Moderate: similar characteristics except sex and co-morbidities; not propensity-matched | Low: database | Low | Low | Low | Low: narcotic use, complications, cost | Low | |
| Bittner et al.24 2018 | Serious: differences in co-morbidities, smoking status, sex, hernia size; not-propensity- matched | Low | Low | Low | Low | Low: complications | Moderate: no data on recurrences at 90 days | 1st author is consultant for Intuitive |
| Carbonell et al.25 2018 | Low: similar characteristics, including proportion of TARs performed; propensity-matched | Low: database | Low | Low | Low | Low: complications | Low | 6 authors (including 1st author) received honoraria from Intuitive; 2 authors received educational funds from Intuitive |
| Chen et al.26 2017 | Moderate: similar characteristics except for sex; not propensity-matched | Low | Low | Low | Low | Low: complications, recurrence | Low | |
| Coakley et al.14 2017 | Low: similar baseline characteristics; not propensity-matched | Low: database | Low | Low | Low | Low: complications, cost | Low | |
| Dauser et al.27 2020 | Moderate: similar baseline characteristics except sex; not propensity-matched | Serious: institutional data, not stated whether consecutive series | Low | Low | Low | Low: complications | Low | |
| Gonzalez et al.28 2015 | Low: similar baseline characteristics; not propensity-matched | Low | Low | Low | Moderate: unknown follow-up | Low: complications, recurrence | Low | |
| Guzman-Pruneda et al.12 2020 | Serious: large difference in sex, smoking status, hernia size; not propensity-matched | Low: database | Low | Low | Low | Low: complications, recurrence Moderate: QoL | Low | Operative techniques (e.g., drain placement) were significantly different between comparison groups |
| Khorgami et al.29 2019 | Serious: unable to assess characteristics, as data were pooled for multiple procedures; not propensity-matched | Low: database | Low | Low | Low | Low: LOS, cost | Serious: no other outcomes besides LOS | |
| LaPinska et al.30 2020 | Low: similar baseline characteristics with propensity matching | Low: database | Low | Low | Moderate: 83–85% short-term follow-up rates | Low: complications | Low | 1st author receives personal fees from Intuitive; Intuitive funded independent editorial support and data analysis |
| Lu et al.31 2019 | Moderate: similar baseline characteristics except for sex and co-morbidities; not propensity-matched | Low | Low | Low | Serious: large difference in 1-year follow-up rates between groups | Low: complications, recurrence | Low | Senior author has received honoraria for speaking engagements and consulting for Intuitive |
| Martin-del-Campo et al.32 2018 | Low: similar baseline characteristics except for ASA; propensity-matched for hernia size | Low | Low | Low | Low | Low: complications | Low | 2 authors are consultants for Intuitive |
| Mudyanadzo et al.33 2020 | Serious: baseline characteristics not reported; not propensity-matched | Serious: institutional data, not stated whether consecutive series | Low | Low | Low | Low: pain, narcotic use | Low | |
| Nguyen et al.34 2019 | Moderate: similar characteristics except hernia size; not propensity-matched | Serious: institutional data, not stated whether consecutive series | Low | Low | Low | Low: complications | Low | |
| Reeves et al.35 2020 | Moderate: similar characteristics except certain co-morbidities (i.e., diabetes); not propensity-matched | Serious: institutional data, not stated whether consecutive series | Low | Low | Low | Low: complications | Low | Large difference in postoperative drain placement between comparisons |
| Song et al.36 2017 | Moderate: characteristics not explicitly reported; propensity-matched | Low: database | Low | Low | Low | Low: complications, narcotic use, cost | Low | |
| Tan et al.37 2018 | Serious: significantly different age, other characteristics not explicitly reported; not propensity-matched | Serious: institutional data, not stated whether consecutive series | Low | Low | Low | Low: cost | Low | |
| Walker et al.38 2018 | Moderate: similar baseline characteristics except for sex; propensity-matched except for sex, and matched characteristics not reported | Serious: institutional data, not stated whether consecutive series | Low | Low | Moderate: unknown follow-up | Low: complications, recurrence | Moderate: matched outcomes only reported selectively | 2 authors (including senior author) receive honoraria to proctor for Intuitive |
| Warren et al.39 2017 | Serious: similar characteristics except for sex, recurrent hernia, and whether TAR performed concurrently; not propensity-matched | Low: database | Low | Low | Low | Low: narcotic use, complication | Low | 1st and senior authors are speakers for Intuitive |
| Zayan et al.40 2019 | Serious: difference in sex, BMI, smoking status, baseline QoL; not propensity-matched | Serious: institutional data, not stated whether consecutive series | Low | Low | Moderate: unknown follow-up | Low: recurrence Moderate: QoL | Moderate: no outcomes relating to other complications |
ROBINS-I, Risk Of Bias In Non-Randomized Studies of Interventions; TAR, transversus abdominis release; Qol, quality of life; LOS, length of hospital stay.
Intraoperative outcomes
Robotic VHR took longer than both open and laparoscopic surgery in most studies (Tables 5 and 6). Seven12,24,25,32,34–36 of eight studies, including all three with propensity matching, demonstrated increased operating time with robotic compared with open surgery by 66–88 min, whereas there was no statistically significant difference in the remaining non-matched study27. For the robotic versus laparoscopic comparison, all nine studies20,21,26,28,30,31,36,38,40 reporting duration of surgery, including two RCTs and two propensity-matched studies, reported a longer operating time with robotic VHR by a median of 54 min.
Table 5.
Intraoperative outcomes in studies comparing robotic with open surgery
| Reference | Intraoperative outcomes |
|||||
|---|---|---|---|---|---|---|
| Duration of operation (min)* |
Intraoperative complications (%) |
|||||
| Robotic | Open | P | Robotic | Open | P | |
| Studies with propensity matching | ||||||
| Carbonell et al.25 | > 2 h: 45.1% | > 2 h: 12.6% | < 0.001 | 1.8 | 1.4 | 1.000 |
| Martin-del-Campo et al.32 | 299(95) | 211(63) | < 0.001 | 0 | 0 | 1.000 |
| Song et al.36‡ | 231 (101) | 163 (101) | < 0.001 | 1.0 | 1.0 | 1.000 |
| Studies without propensity matching | ||||||
| Bittner et al.24 | 365(78) | 287(121) | < 0.010 | 0 | 5.3 | 0.57¶ |
| Dauser et al.27 | 253.5 (158–380)†§ | 211.5 (112–303)†§ | 0.085 | – | – | – |
| Guzman-Pruneda et al.12 | >4 h: 33% | >4 h: 18% | 0.010 | 0 | 0 | 1.000 |
| Nguyen et al.34‡ | 272.1 | 206.5 | < 0.001 | – | – | – |
| Reeves et al.35 | 260.0(78.9) | 185.7(64.5) | 0.017 | – | – | – |
Values are mean(s.d.) unless indicated otherwise;
values are median (range).
Conference abstract.
Skin-to-skin time.
Outcome significant when risk difference calculated.
Table 6.
Intraoperative outcomes of robotic versus laparoscopic surgery
| Reference | Intraoperative outcomes |
|||||
|---|---|---|---|---|---|---|
|
Duration of operation (min)
*
|
Intraoperative complications (%) |
|||||
| Robotic | Laparoscopic | P | Robotic | Laparoscopic | P | |
| RCTs | ||||||
| Olavarria et al.20 | 141(56)§ | 77(37)§ | < 0.001 | – | – | – |
| Petro et al.21 | 146 (123–192)† | 94 (69–116)† | < 0.001 | 5.1 | 5.6 | > 0.99 |
| Studies with propensity matching | ||||||
| LaPinska et al.30 | >2 h: 42.9% | >2 h: 21.5% | < 0.001 | 0.98 | 1.3 | 0.591 |
| Song et al.36¶ | 231(101) | 169(108) | < 0.001 | 1.1 | 4.3 | 1.000 |
| Studies without propensity matching | ||||||
| Chen et al.26 | 156.6 (77–261)‡ | 65.9 (25–128)‡ | < 0.001 | – | – | – |
| Gonzalez et al.28 | 107.6(33.9)§ | 87.9(53.1)§ | 0.012 | – | – | – |
| Lu et al.31 | 174.7(44.9) | 120.4(35.0) | < 0.001 | – | – | – |
| Walker et al.38# | 116.9(47.9)§ | 98.7(56.6)§ | 0.03 | – | – | – |
| Zayan et al.40 | 139 (108–186)† | 86 (67–104)† | 0.009 | – | – | – |
Values are mean(s.d.) unless indicated otherwise; values are †median (i.q.r.) and
median (range).
Skin-to-skin time.
Conference abstract.
Unmatched data presented, as study propensity-matched for limited outcomes.
The rate of intraoperative complications was 0–5 per cent among the five studies that compared this outcome between robotic and open surgery, of which four12,25,32,36, including three with propensity matching, did not show a statistically significant difference (Table 5). One non-matched study24 found a decreased intraoperative complication rate with robotic surgery when the RD was calculated. Intraoperative complication rates for robotic and laparoscopic VHR ranged between 1 and 6 per cent among the three studies21,30,36 reporting this outcome and were no different between the approaches (Table 6).
Three propensity-matched studies assessed transfusion outcomes between the three approaches. Transfusion events were rare, with a rate ranging between 0 and 7 per cent among all studies. Both studies32,36 assessing transfusion in robotic and open VHR found a reduced transfusion rate with robotic surgery (0 versus 6.6 per cent; RD −0.066, 95 per cent c.i. −0.121 to −0.010; 0 versus 5.2 per cent, P = 0.02). Comparing robotic with laparoscopic VHR, one study36 demonstrated fewer transfusions during robotic surgery (0 versus 5.3 per cent; P = 0.02), whereas the other30 found no significant difference between the two approaches (0.2 versus 0 per cent; P = 1.000).
Four studies reported the rate of conversion to open from minimally invasive approaches, which ranged from 0 to 4 per cent. Two propensity-matched studies30,36 reported fewer conversions to open operation with robotic surgery compared with laparoscopy (0.5 versus 2.3 per cent, P = 0.007; 2.1 versus 13.9 per cent, P = 0.003); however, the RCT20 and one non-matched study28 did not find a difference in conversion rates between the approaches (1.5 versus 1.7 per cent, P = 0.84; 1.5 versus 4.5 per cent, P = 0.310).
Short-term postoperative outcomes
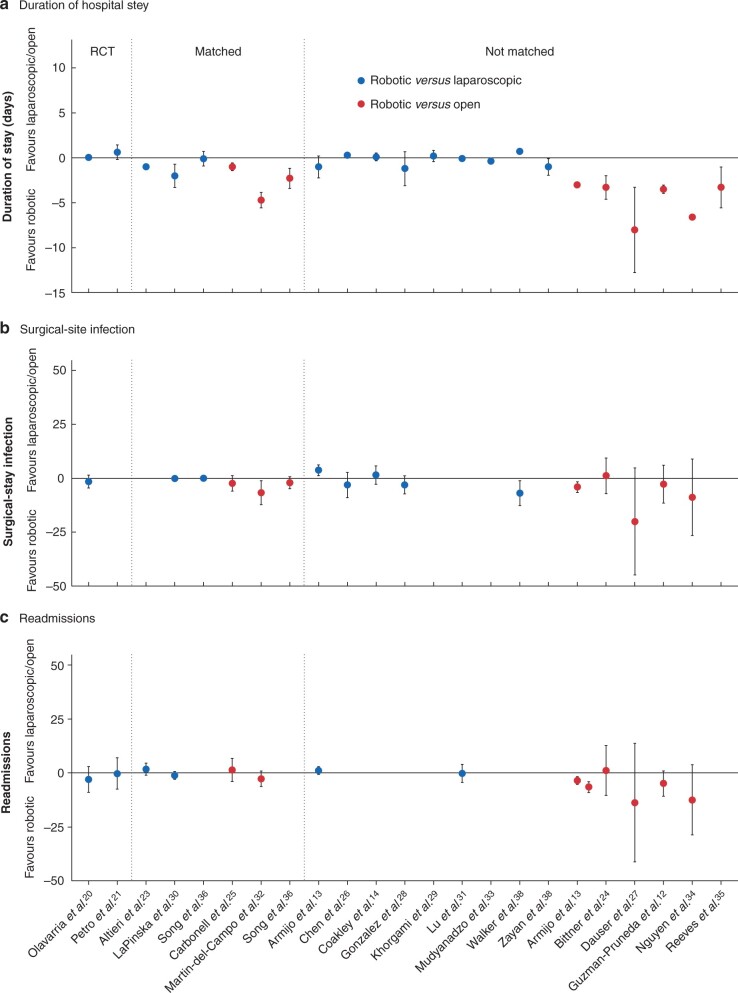
Comparing robotic with open VHR, all nine studies12,13,24,25,27,32,34–36, including three that were propensity-matched, demonstrated a shorter hospital stay in the robotic cohort, with one study27 reporting an absolute reduction of 8 days (Fig. 2 and Table 7). There were smaller differences between robotic and laparoscopic VHR; four23,30,33,40 of 14 studies, including two with propensity matching, reported a decreased duration of hospital stay after robotic surgery, with a mean absolute difference of 1 day (Table 8). Nine13,14,20,21,26,28,29,31,36 of the remaining 10 studies, which included both RCTs and one propensity-matched study, did not find any difference in length of stay between robotic and laparoscopic VHR.
Fig. 2.
Forest plot of short-term postoperative outcomes
a Duration of hospital stay, b surgical-site infection, and c readmission rates. The point estimate for the risk and mean differences are plotted with 95 per cent confidence intervals for robotic ventral hernia repair versus laparoscopic or open approaches.
Table 7.
Short-term postoperative outcomes of robotic versus open surgery
| Reference | Short-term postoperative outcomes |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Duration of hospital stay (days)* |
Surgical-site infection (%)§ |
Total complications (%)§ |
|||||||
| Robotic | Open | P | Robotic | Open | P | Robotic | Open | P | |
| Studies with propensity matching | |||||||||
| Carbonell et al.25 | 2 (1–3)† | 3 (2–5)† | < 0.001 | 2 | 4 | 0.5 | 29.7 | 43.2 | –# |
| Martin-del-Campo et al.32 | 1.3(1.3) | 6(3.4) | < 0.001 | 0 | 6.6 | 0.106# | 0 | 17.1 | 0.007 |
| Song et al.36¶ | 3.0(2.4) | 5.3(5.2) | 0.003 | 0 | 2.1 | 0.50 | 17.7 | 39.6 | 0.001 |
| Studies without propensity matching | |||||||||
| Armijo et al.13 | 2 (1–4)† | 5 (3–8)† | < 0.050 | 1.7 (0.8, 3.4) | 2.8 (2.7, 3.0) | n.s.# | 7.3 (5.1, 10.0) | 11.4 (11.1, 11.75) | < 0.050 |
| Bittner et al.24 | 3.8(1.5) | 7.1(5.4) | < 0.010 | 3.8 | 2.6 | 1.00 | 19.2 | 30.2 | 0.32 |
| Dauser et al.27 | 4.5 (2–10)‡ | 12.5 (6–25)‡ | < 0.001 | 0 | 20.0 | –** | 12.5 | 50.0 | –# |
| Guzman-Pruneda et al.12 | 1.5 (1–2.8)† | 5 (4–6)† | < 0.010 | 0 | 1.5 | 1 | 9.5 | 15.5 | –** |
| Nguyen et al.34¶ | 3.0 | 9.6 | < 0.001 | 3.7 | 12.5 | –** | – | – | – |
| Reeves et al.35 | 3.6(2.1) | 6.9(3.6) | 0.007 | – | – | – | 15.4 | 23.1 | 0.619 |
Values are mean(s.d.) unless indicated otherwise; values are †median (i.q.r.) and
median (range);
values in parentheses are 95 per cent confidence intervals.
Conference abstract. n.s., Not significant.
Outcome significant when risk difference calculated;
utcome not significant when risk difference calculated.
Table 8.
Short-term postoperative outcomes of robotic versus laparoscopic surgery
| Reference | Short-term postoperative outcomes |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Duration of hospital stay (days)* |
Surgical-site infection (%)§ |
Total complications (%)§ |
|||||||
| Robotic | Laparoscopic | P | Robotic | Laparoscopic | P | Robotic | Laparoscopic | P | |
| RCTs | |||||||||
| Olavarria et al.20 | 0 | 0 | 0.82 | 0 | 1.7 | 1.00 | 21.5 | 19.0 | 0.80 |
| Petro et al.21 | 25 h (10–30)† | 10 h (8–31)† | 0.17 | – | – | – | 5.1 | 8.3 | > 0.99 |
| Studies with propensity matching | |||||||||
| Altieri et al.23 | Median difference (robotic versus laparoscopic): −1 day | < 0.001 | – | – | – | 14.60 | 20.35 | 0.013 | |
| LaPinska et al.30 | 2(7) | 4(13) | < 0.001 | 0.76 | 0.97 | 0.716 | 10.5 | 11.5 | 0.613 |
| Song et al.36¶ | 3.0(2.4) | 3.2(3.0) | 0.67 | 0.0 | 0.0 | 1.00 | 17.0 | 24.5 | 0.21 |
| Studies without propensity matching | |||||||||
| Armijo et al.13 | 2 (1–4)† | 3 (2–4)† | n.s. | 1.7 (0.8, 3.4) | 0.7 (0.5, 0.9) | < 0.05 | 7.3 (5.1, 10.1) | 3.5 (3.1, 4.0) | < 0.050 |
| Chen et al.26 | 0.49 (0–3)‡# | 0.21 (0–1)‡# | 0.09 | 0 | 3.03 | 0.458 | 7.7 | 9.1 | 1 |
| Coakley et al.14 | 3.5(3.6) | 3.4(2.6) | 0.211 | 0.85 | 0.47 | 0.234 | 20.24 | 18.73 | –†† |
| Gonzalez et al.28 | 2.5(4.1) | 3.7(6.6) | 0.461 | 2 | 0 | –†† | 3.0 | 10.4 | 0.084 |
| Khorgami et al.29 | 2.9(3.1) | 2.7(1.9) | –†† | – | – | – | – | – | – |
| Lu et al.31 | 0.1(0.5) | 0.2(0.9) | 0.294 | – | – | – | 2.3 | 9.2 | 0.046 |
| Mudyanadzo et al.33 | 1.3(0.1) | 1.7(0.2) | n.s.‡‡ | – | – | – | – | – | – |
| Walker et al.38** | 1.4(0.4) | 0.7(0.3) | 0.09‡‡ | 0 | 6.8 | < 0.01 | – | – | – |
| Zayan et al.40 | 22.1 (9.4–33.7) h† | 46.3 (26.3–65.6) h† | 0.044 | – | – | – | – | – | – |
Values are mean(s.d.), unless indicated otherwise; values are †median (i.q.r.) and
median (range);
values in parentheses are 95 per cent confidence intervals.
Conference abstract.
Reported only for those who required admission in the robotic (14) and laparoscopic (7) groups. n.s., Not significant.
Unmatched data presented, as study propensity-matched for limited outcomes.
Outcome significant when risk difference calculated;
utcome not significant when risk difference calculated.
With regard to SSI rates, two13,32 of eight studies, which included one propensity-matched study, found a decreased incidence associated with robotic repair compared with the open approach when RDs were calculated (Table 7). The remaining six12,24,25,27,34,36 showed no statistically significant difference. In the robotic and laparoscopic comparison, one non-matched study38 demonstrated a lower SSI rate in the robotic cohort, whereas another13 found a higher SSI rate in the robotic group (Table 8). The remaining six studies14,20,26,28,30,36, which included one RCT and two propensity-matched studies, did not report a difference in SSI rates between robotic and laparoscopic hernia repair.
Of the seven studies that assessed readmission rate after robotic and open VHR, one non-matched study13 found decreased rates following robotic repair, and the remaining six12,24,25,27,32,34, including two matched studies, found no difference. None of the six studies13,20,21,23,30,31 evaluating readmission rates showed a difference between robotic and laparoscopic VHR.
Total complication rates were reported in eight studies comparing robotic with open VHR (Table 7). Five studies13,25,27,32,36, including three with matching, noted a lower postoperative morbidity rate after robotic surgery, whereas the remaining three12,24,35 non-matched studies found no difference. Of note, one non-matched study27 pooled both intraoperative and postoperative complications. Of the 10 studies evaluating complication rates in robotic and laparoscopic VHR, two23,31 demonstrated decreased morbidity rates following robotic surgery, included one matched study (Table 8). One non-matched study13 reported an increased morbidity rate after robotic VHR. The remaining seven studies14,20,21,26,28,30,36, including two RCTs and two matched studies, found that total complication rates did not stays differ between the robotic and laparoscopic approaches.
Short-term mortality was rare among all approaches, with rates of between 0 and 1 per cent. Mortality rates did not differ in any of the four studies13,24,25,32 comparing robotic and open hernia repair, or the four13,14,28,30 comparing robotic and laparoscopic VHR.
Pain
Three studies evaluated short-term pain outcomes in robotic and open VHR. One matched study36 found decreased narcotic use measured as milligram morphine equivalents and a trend toward decreased patient-controlled analgesia use following robotic repair. Neither of the two remaining studies13,25, one matched and one not matched, demonstrated a difference in pain between the robotic and open approaches assessed in terms of readmission owing to pain and narcotic requirements.
Five studies evaluated short-term pain outcomes in robotic and laparoscopic VHR. One of the RCTs21 demonstrated a greater improvement in pain from baseline following laparoscopic surgery, as assessed by the Patient-Reported Outcomes Measurement Information System Pain Intensity short form 3a; however, there was no difference in any of the other reported pain metrics in this trial. One non-matched study33 reported a reduced narcotic requirement rate at 6–8 weeks after robotic VHR. The remaining three studies13,20,36, including one RCT and one matched study, did not show differences in change in pain rating from baseline, patient-controlled analgesia use, rate of opiate prescription, or perioperative analgesia between approaches.
Hernia recurrence
One non-matched study12 evaluated 1-year recurrence rates in robotic and open VHR, and reported no difference.
Of the seven studies assessing recurrence rates after robotic and laparoscopic repairs, study-specific follow-up varied from 1 month to nearly 2 years. One matched study38 with a mean follow-up of approximately 6 weeks found a decrease in recurrence rate in the robotic cohort. However, the remaining six20,22,26,28,31,40, including one RCT, found no differences in hernia recurrence rates.
Cost
Nine studies13,14,20,21,27,29,36,37,39 reported cost data for robotic VHR compared with laparoscopic and open approaches (Table 9). One study37 reported cost data only and no clinical outcomes; one database study39 was included for cost outcomes only, and excluded from analyses of clinical outcomes owing to overlapping clinical data. Three studies13,27,36 compared robotic with open VHR, and eight13,14,20,21,29,36,37,39 compared robotic with laparoscopic surgery. Of the three studies comparing robotic with open VHR, one13 found the robotic approach to be more expensive, whereas the two others27,36 reported no difference in costs between the two approaches. Five studies13,14,20,21,29, including two RCTs, found the robotic approach to be more expensive than laparoscopic surgery, one39 reported that the robotic approach showed a non-significant trend towards higher costs than laparoscopy, and two36,37 found that robotic surgery and laparoscopy were no different with respect to costs.
Table 9.
Cost outcomes
| Reference | Source of cost data |
Cost outcomes (€)* |
||||
|---|---|---|---|---|---|---|
| Type of cost data | Robotic | Laparoscopic | Open | P | ||
| RCTs | ||||||
| Olavarria et al.20 | Costs including all patient visits, admissions, and procedural costs from operation through first 90 postoperative days came from hospital administration accounting system. Cost did not include surgeons’ fees or initial acquisition cost of robotic or laparoscopic platforms | Mean costs | 19 038 (5854) | 15 546 (6763) | – | 0.004 |
| Petro et al.21 | Values for cost reported as ratios. Total cost includes OR cost (as calculated by cost per minute of OR time required for the procedure) and disposable/reusable cost, which was calculated to include disposable materials as well as reusable materials including robotic instruments | Disposable/reusable median cost ratio | 0.97 (0.85–1.51)† | 1.00 (0.87–1.19)† | – | 0.60 |
| OR time cost ratio | 1.25 (0.98–1.49)† | 0.85 (0.67–1.00)† | – | < 0.001 | ||
| Total cost ratio | 1.13 (0.90–1.52)† | 0.97 (0.85–1.16)† | – | 0.03 | ||
| Studies with propensity matching | ||||||
| Song et al.36¶ | Total cost included direct cost and overhead cost, adjusted for inflation to 2015 US dollars | Total cost | 12 422 | – | 12 989 | n.s. |
| 12 562 | 12 908 | – | n.s. | |||
| Studies without propensity matching | ||||||
| Armijo et al.13 | Ratio of cost-to-charge method applied for estimating cost of patient care | Total direct cost | 12 000 (8400, 16 800)‡ | 8400 (6000, 10 800)‡ | 10 800 (7200, 19 200)‡ | < 0.050# |
| Coakley et al.14 | Total hospital charges | Adjusted mean charges (controlling for CCI, geography, public versus private, etc.) | 73 446(1717) | 50 293(310) | – | < 0.001 |
| Dauser et al.27 |
|
Total procedure-related costs | 5394.41 | – | 1987.19 | – |
| Cost of inpatient stay | 2714.53 | – | 6662.93 | – | ||
| Total cost | 8108.93 | – | 8650.12 | – | ||
| Khorgami et al.29 | Hospital total charges converted to cost estimates using hospital specific cost-to-charge ratios provided by HCUP. Admissions with total charges below 0.1th percentile or above 99.9th percentile were considered outliers and excluded from analysis | Average cost estimate | 16 093(6648) | 12 887(5774) | – | < 0.050 |
| Tan et al.37¶ |
|
Median OR costs | 3714 (3 532–3988)† | 4069 (3204–5074)† | – | 0.056 |
| Median total variable costs | 5234 (4571–6433)† | 5461 (4234–7399)† | – | 0.609 | ||
| Warren et al.39 | No details provided | Mean direct hospital cost | 23 438 | 16 732 | – | 0.07 |
Values are mean(s.d.) unless indicated otherwise;
values are median (i.q.r.);
values in parentheses are 95 per cent confidence intervals. Original charges in US dollars were converted to euros at an exchange rate of US $1.2 to €1.
Conference abstract. OR, operating room; n.s., not significant; CCI, Charlson Co-morbidity Index; HCUP, Healthcare Cost and Utilization Project; #Two-way comparisons of robotic versus open and robot versus laparoscopic.
Discussion
Overall, the evidence for the comparison between robotic and open VHR had low certainty (Table 10). Robotic VHR has a longer operating time and shorter hospital stay than open repair, supported by evidence of moderate certainty. There is low certainty that robotic VHR is associated with fewer transfusions and very low certainty that it is associated with a decreased total complication rate compared with open repair. There is low or very low certainty of evidence for no difference in intraoperative complications, SSI, readmissions, hernia recurrence, and cost.
Table 10.
GRADE summary of findings and certainty of evidence
| Study limitations | Consistency | Directness | Precision | Certainty of evidence | |
|---|---|---|---|---|---|
| Intraoperative outcomes | |||||
| Duration of operation |
|
||||
| Robotic > open | Consistent | Direct | Precise | Moderate | |
| Robotic > laparoscopic | Consistent | Direct | Precise | High | |
| Intraoperative complications |
|
||||
| Robotic = open | Consistent | Direct | Imprecise | Low | |
| Robotic = laparoscopic | Consistent | Direct | Imprecise | Moderate | |
| Transfusion | Matched observational studies: moderate | ||||
| Robotic < open | Consistent | Direct | Imprecise | Low | |
| Robotic = laparoscopic | Inconsistent | Direct | Imprecise | Very low | |
| Conversion to open surgery |
|
||||
| Robotic = laparoscopic | Inconsistent | Direct | Imprecise | Low | |
| Postoperative short-term outcomes | |||||
| Length of hospital stay |
|
||||
| Robotic < open | Consistent | Direct | Precise | Moderate | |
| Robotic = laparoscopic | Inconsistent | Direct | Precise | Moderate | |
| Surgical-site infection |
|
||||
| Robotic = open | Consistent | Direct | Imprecise | Low | |
| Robotic = laparoscopic | Consistent | Direct | Imprecise | Moderate | |
| Readmissions |
|
||||
| Robotic = open | Consistent | Direct | Imprecise | Low | |
| Robotic = laparoscopic | Consistent | Direct | Imprecise | Moderate | |
| Mortality |
|
||||
| Robotic = open/laparoscopic | Consistent | Direct | Imprecise | Low | |
| Total complications |
|
||||
| Robotic < open | Inconsistent | Indirect | Imprecise | Very low | |
| Robotic = laparoscopic | Inconsistent | Indirect | Imprecise | Low | |
| Postoperative functional outcomes | |||||
| Pain |
|
||||
| Robotic = open | Inconsistent | Indirect | Imprecise | Very low | |
| Robotic = laparoscopic | Inconsistent | Indirect | Imprecise | Low | |
| Hernia recurrence |
|
||||
| Robotic = open | – | Direct | Imprecise | Very low | |
| Robotic = laparoscopic | Consistent | Direct | Imprecise | Moderate | |
| Cost | |||||
| Cost |
|
||||
| Robotic = open | Inconsistent | Indirect | Imprecise | Very low | |
| Robotic > laparoscopic | Inconsistent | Indirect | Imprecise | Low | |
GRADE, Grading of Recommendations, Assessment, Development, and Evaluation.
There is high certainty of evidence that robotic VHR takes longer than laparoscopic repair, with no evidence of any difference in length of hospital stay or intraoperative complications, SSI, readmissions, or hernia recurrence between these approaches. There is low certainty that robotic VHR has greater costs than laparoscopic repair. There is low or very low certainty of evidence for no difference in rates of conversion to open surgery, transfusion, mortality, and total complications, or pain. Based on current data, there is no high-quality evidence that either approach is superior to the other with regard to clinical outcomes, excluding duration of operation.
The longer operating time for robotic VHR compared with open surgery or laparoscopic VHR is consistent with similar findings for inguinal hernia repair and cholecystectomy41,42. This is likely related to a variety of factors, including robot docking, surgeon and staff efficiency, learning curve, and patient selection. Most studies in the present review acknowledged the learning curve as a potential contributing factor; however, only one27 evaluated operative times longitudinally, which decreased after the first six procedures.
Two matched studies reported lower transfusion rates associated with robotic VHR compared with open surgery. This may be due to magnified visualization, smaller cut surfaces, and intra-abdominal pressure tamponade, although the certainty of evidence is limited as transfusions were rare. This topic should be explored further, as transfusion is a critical clinical outcome and determinant of resource use43.
Robotic VHR is also associated with shorter hospital stay compared with open surgery, probably reflecting earlier mobilization and faster functional recovery. Earlier functional recovery may contribute to the decreased complications of robotic VHR compared with open surgery.
The European and Americas Hernia Societies guidelines6 do not recommend a preferred surgical approach for minimizing pain outcomes. Pain assessments varied widely in the present review. Small differences in pain outcomes may not have been detected because of lack of standardized reporting. A meta-epidemiological study44 noted that effect estimates for subjective outcomes were exaggerated when studies had unclear allocation concealment or lacked blinding, such as in the included RCTs, yet trials with objective outcomes had little evidence of bias. Future work should report objective measures consistently. Chronic pain should be evaluated with follow-up of at least 6 months and include assessments for pain requiring intervention.
This review has limitations. Only two RCTs met the inclusion criteria, and a third limited RCT abstract was excluded. The remaining data were observational, although six of the studies were propensity-matched, the result of non-matched studies were mostly congruent with matched and randomized data. Conclusions about the robotic approach compared with open VHR were based only on observational data. Although the earliest RCTs20,21 looking at robotic VHR were included, these patients had small hernias (3–5 cm) that were repaired mostly on an outpatient basis (median hospital stay 0–1 days), reflecting overall lower complexity. Therefore, the findings may not be generalizable to larger (over 10 cm) and more complex hernias, for which a minimally invasive approach may be less appropriate. Most studies reported only short-term outcomes, and the RCTs were underpowered to detect differences in pain or recurrence. Technical factors, such as hernia size, primary versus recurrent hernia, operative urgency, technique, mesh fixation, and fascial closure, were reported inconsistently. Hernia recurrence was reported infrequently, with only one study evaluating the robotic versus open approaches. Follow-up for hernia recurrence ranged from 47 days to nearly 2 years. Current guidelines for inguinal hernia repair recommend 3–5-year follow-up for recurrences44; however, that time frame is often not feasible (for example because of patient attrition or high cost) and follow-up should be standardized to at least 1 year. Seven of 22 studies disclosed financial relationships with Intuitive Surgical, introducing potential for author bias (Tables 1 and 3).
The methodology of the cost studies was limited, specifically how values were derived. Cost estimates for the robotic approach varied considerably (from €5234 to 73 446). The majority of studies did not define the type of cost or charge, time frame, or items included in cost estimates, follow cost-reporting guidelines45, or report staff costs—the largest component of operative costs8. Several studies relied on administrative databases and used cost-to-charge ratios to estimate hospital costs13,29,46, which are prone to bias47. Furthermore, the overall cost of care was not available, and no formal cost-effectiveness analyses were performed.
Randomized or matched data are still needed to account for patient and technical factors and, particularly, to evaluate large, complex hernias. Standardization of outcome measurements and long-term follow-up, and more detailed economic analyses are all necessary.
Taken the high morbidity rates after open VHR, particularly for large complex hernias, the robotic platform may be an effective minimally invasive approach capable of delivering genuine clinical benefit. Randomized studies of better quality are needed.
Funding
L.Y. is supported in part by the H. H. Lee Research Program. Funding was provided by the Veterans Affairs Quality Enhancement Research Initiative. The funders participated in setting the scope of the review, the interventions to be compared, the outcomes of interest, and review of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Supplementary Material
Acknowledgements
The authors thank B. Emr, T. Frankel, T. Heimann, and F. Luchette for their role on the Veterans Affairs Technical Expert Panel, and their expertise in guiding topic refinement, input on key questions, and feedback; and acknowledge S. Yagyu for performing the literature search and A. J. Chen for assistance in compiling the supplementary tables.
Disclosure. The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
References
- 1. Bedewi MA, El-Sharkawy MS, Al Boukai AA, Al-Nakshabandi N.. Prevalence of adult paraumbilical hernia. Assessment by high-resolution sonography: a hospital-based study. Hernia 2012;16:59–62. [DOI] [PubMed] [Google Scholar]
- 2. Kingsnorth A, LeBlanc K.. Hernias: inguinal and incisional. Lancet 2003;362:1561–1571. [DOI] [PubMed] [Google Scholar]
- 3. Flum DR, Horvath K, Koepsell T.. Have outcomes of incisional hernia repair improved with time? A population-based analysis. Ann Surg 2003;237:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sheetz KH, Claflin J, Dimick JB.. Trends in the adoption of robotic surgery for common surgical procedures. JAMA Netw Open 2020;3:e1918911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poruk KE, Farrow N, Azar F, Burce KK, Hicks CW, Azoury SC. et al. Effect of hernia size on operative repair and post-operative outcomes after open ventral hernia repair. Hernia 2016;20:805–810. [DOI] [PubMed] [Google Scholar]
- 6. Henriksen NA, Montgomery A, Kaufmann R, Berrevoet F, East B, Fischer J. et al. ; European and Americas Hernia Societies (EHS and AHS). Guidelines for treatment of umbilical and epigastric hernias from the European Hernia Society and Americas Hernia Society. Br J Surg 2020;107:171–190. [DOI] [PubMed] [Google Scholar]
- 7. Peters BS, Armijo PR, Krause C, Choudhury SA, Oleynikov D.. Review of emerging surgical robotic technology. Surg Endosc 2018;32:1636–1655. [DOI] [PubMed] [Google Scholar]
- 8. Childers CP, Maggard-Gibbons M.. Estimation of the acquisition and operating costs for robotic surgery. JAMA 2018;320:835–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Henriksen NA, Jensen KK, Muysoms F.. Robot-assisted abdominal wall surgery: a systematic review of the literature and meta-analysis. Hernia 2019;23:17–27. [DOI] [PubMed] [Google Scholar]
- 10. Maggard-Gibbons M, Girgis M, Ye L, Shenoy R, Mederos M, Childers CP. et al. Robot-Assisted Procedures in General Surgery: Cholecystectomy, Inguinal and Ventral Hernia Repairs. Los Angeles: Evidence Synthesis Program, Health Services Research and Development Service. Office of Research and Development, Department of Veterans Affairs, 2020, contract no. 05-226. Washington, DC. [PubMed]
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guzman-Pruneda FA, Huang LC, Collins C, Renshaw S, Narula V, Poulose BK.. Abdominal core quality of life after ventral hernia repair: a comparison of open versus robotic-assisted retromuscular techniques. Surg Endosc 2021;35:241–248. [DOI] [PubMed] [Google Scholar]
- 13. Armijo P, Pratap A, Wang Y, Shostrom V, Oleynikov D.. Robotic ventral hernia repair is not superior to laparoscopic: a national database review. Surg Endosc 2018;32:1834–1839. [DOI] [PubMed] [Google Scholar]
- 14. Coakley KM, Sims SM, Prasad T, Lincourt AE, Augenstein VA, Sing RF. et al. A nationwide evaluation of robotic ventral hernia surgery. Am J Surg 2017;214:1158–1163. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD. et al. ; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schünemann HBJ, Guyatt G, Oxman A (eds.). GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations.https://gdt.gradepro.org/app/handbook/handbook.html (accessed 30 July 2020).
- 18. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2020.
- 19. Abdalla R, Santo M, Gontijo C, Frade Said D, Cecconello I, Costa T.. Randomized clinical trial: comparison between robotic assisted and laparoscopic incisional hernia repair. Hernia 2017;21:S115. [Google Scholar]
- 20. Olavarria OA, Bernardi K, Shah SK, Wilson TD, Wei S, Pedroza C. et al. Robotic versus laparoscopic ventral hernia repair: multicenter, blinded randomized controlled trial. BMJ 2020;370:m2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Petro CC, Zolin S, Krpata D, Alkhatib H, Tu C, Rosen MJ. et al. Patient-reported outcomes of robotic vs laparoscopic ventral hernia repair with intraperitoneal mesh: the PROVE-IT randomized clinical trial. JAMA Surg 2021;156:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alimi YR, Nasher N, Lofthus A, Bhanot P.. Robotic vs laparoscopic hernia repair: early outcomes. J Am Coll Surg 2020;231:e137. [Google Scholar]
- 23. Altieri MS, Yang J, Xu J, Talamini M, Pryor A, Telem DA.. Outcomes after robotic ventral hernia repair: a study of 21 565 patients in the state of New York. Am Surg 2018;84:902–908. [PubMed] [Google Scholar]
- 24. Bittner JG, Alrefai S, Vy M, Mabe M, Del Prado PAR, Clingempeel NL.. Comparative analysis of open and robotic transversus abdominis release for ventral hernia repair. Surg Endosc 2018;32:727–734. [DOI] [PubMed] [Google Scholar]
- 25. Carbonell AM, Warren JA, Prabhu AS, Ballecer CD, Janczyk RJ, Herrera J. et al. Reducing length of stay using a robotic-assisted approach for retromuscular ventral hernia repair: a comparative analysis from the Americas Hernia Society Quality Collaborative. Ann Surg 2018;267:210–217. [DOI] [PubMed] [Google Scholar]
- 26. Chen YJ, Huynh D, Nguyen S, Chin E, Divino C, Zhang L.. Outcomes of robot-assisted versus laparoscopic repair of small-sized ventral hernias. Surg Endosc 2017;31:1275–1279. [DOI] [PubMed] [Google Scholar]
- 27. Dauser B, Hartig N, Vedadinejad M, Kirchner E, Trummer F, Herbst F.. Robotic-assisted repair of complex ventral hernia: can it pay off? J Robot Surg 2021;15:45–52. [DOI] [PubMed] [Google Scholar]
- 28. Gonzalez AM, Romero RJ, Seetharamaiah R, Gallas M, Lamoureux J, Rabaza JR.. Laparoscopic ventral hernia repair with primary closure versus no primary closure of the defect: potential benefits of the robotic technology. Int J Med Robot 2015;11:120–125. [DOI] [PubMed] [Google Scholar]
- 29. Khorgami Z, Li WT, Jackson TN, Howard CA, Sclabas GM.. The cost of robotics: an analysis of the added costs of robotic-assisted versus laparoscopic surgery using the National Inpatient Sample. Surg Endosc 2019;33:2217–2221. [DOI] [PubMed] [Google Scholar]
- 30. LaPinska M, Kleppe K, Webb L, Stewart TG, Olson M.. Robotic-assisted and laparoscopic hernia repair: real-world evidence from the Americas Hernia Society Quality Collaborative (AHSQC). Surg Endosc 2021;35:1331–1341. [DOI] [PubMed] [Google Scholar]
- 31. Lu R, Addo A, Ewart Z, Broda A, Parlacoski S, Zahiri HR. et al. Comparative review of outcomes: laparoscopic and robotic enhanced-view totally extraperitoneal (eTEP) access retrorectus repairs. Surg Endosc 2020;34:3597–3605. [DOI] [PubMed] [Google Scholar]
- 32. Martin-Del-Campo LA, Weltz AS, Belyansky I, Novitsky YW.. Comparative analysis of perioperative outcomes of robotic versus open transversus abdominis release. Surg Endosc 2018;32:840–845. [DOI] [PubMed] [Google Scholar]
- 33. Mudyanadzo TA, Hunter JD, Rider PF, Richards WO.. An evaluation of robotic ventral hernia repair. Am Surg 2020;86:e45–e46. [PubMed] [Google Scholar]
- 34. Nguyen B, David B, Gosch K, Sorensen GB.. Comparisons of abdominal wall reconstruction for ventral hernia repairs, open versus robotic. Surg Endosc 2019;33:S354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reeves J, Mehta S, Prabha RD, Salama Y, Mittal A.. Robotic versus open transversus abdominis release and incisional hernia repair: a case–control study. Laparosc Endosc Robot Surg 2020;3:59–62. [Google Scholar]
- 36. Song C, Liu E, Shi L, Marcus D.. Comparative effectiveness for ventral hernia repairs among an obese patient population. Surg Endosc 2017;31:S136. [Google Scholar]
- 37. Tan WH, McAllister J, Feaman S, Blatnik JA.. Cost comparison of laparoscopic versus robotic ventral hernia repairs. Surg Endosc 2018;32:S18. [Google Scholar]
- 38. Walker PA, May AC, Mo J, Cherla DV, Santillan MR, Kim S. et al. Multicenter review of robotic versus laparoscopic ventral hernia repair: is there a role for robotics? Surg Endosc 2018;32:1901–1905. [DOI] [PubMed] [Google Scholar]
- 39. Warren JA, Cobb WS, Ewing JA, Carbonell AM.. Standard laparoscopic versus robotic retromuscular ventral hernia repair. Surg Endosc 2017;31:324–332. [DOI] [PubMed] [Google Scholar]
- 40. Zayan NE, Meara MP, Schwartz JS, Narula VK.. A direct comparison of robotic and laparoscopic hernia repair: patient-reported outcomes and cost analysis. Hernia 2019;23:1115–1121. [DOI] [PubMed] [Google Scholar]
- 41. Shenoy R, Mederos MA, Ye L, Mak SS, Begashaw MM, Booth MS. et al. Intraoperative and postoperative outcomes of robot-assisted cholecystectomy: a systematic review. Syst Rev 2021;10:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ye L, Tang AB, Shenoy R, Mederos MA, Mak SS, Booth MS. et al. ; GLA ESP Team. Clinical and cost outcomes of robot-assisted inguinal hernia repair: a systematic review. J Am Coll Surg 2021;232:746.e2–763.e2. [DOI] [PubMed] [Google Scholar]
- 43. Ruiz J, Dugan A, Davenport DL, Gedaly R.. Blood transfusion is a critical determinant of resource utilization and total hospital cost in liver transplantation. Clin Transplant 2018;32:e13164. [DOI] [PubMed] [Google Scholar]
- 44. HerniaSurge Group: M P Simons, M Smietanski, H J Bonjer, et al. International guidelines for groin hernia management. Hernia 2018;22:1–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ. 2013;346:f1049. [DOI] [PubMed] [Google Scholar]
- 46. Childers CP, , DworskyJQ, , RussellMM, , Maggard-Gibbons M.. Comparison of Cost Center-Specific vs Hospital-wide Cost-to-Charge Ratios for Operating Room Services at Various Hospital Types. JAMA Surg 2019;154:557–558. 10.1001/jamasurg.2019.0146 30892567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sanders GD, , NeumannPJ, , BasuA, , BrockDW, , FeenyD, , Krahn Met al. . Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016;316:1093–1103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.