Abstract
Zinc is a critical component in a number of conserved processes that regulate female germ cell growth, fertility, and pregnancy. During follicle development, a sufficient intracellular concentration of zinc in the oocyte maintains meiotic arrest at prophase I until the germ cell is ready to undergo maturation. An adequate supply of zinc is necessary for the oocyte to form a fertilization-competent egg as dietary zinc deficiency or chelation of zinc disrupts maturation and reduces the oocyte quality. Following sperm fusion to the egg to initiate the acrosomal reaction, a quick release of zinc, known as the zinc spark, induces egg activation in addition to facilitating zona pellucida hardening and reducing sperm motility to prevent polyspermy. Symmetric division, proliferation, and differentiation of the preimplantation embryo rely on zinc availability, both during the oocyte development and post-fertilization. Further, the fetal contribution to the placenta, fetal limb growth, and neural tube development are hindered in females challenged with zinc deficiency during pregnancy. In this review, we discuss the role of zinc in germ cell development, fertilization, and pregnancy with a focus on recent studies in mammalian females. We further detail the fundamental zinc-mediated reproductive processes that have only been explored in non-mammalian species and speculate on the role of zinc in similar mechanisms of female mammals. The evidence collected over the last decade highlights the necessity of zinc for normal fertility and healthy pregnancy outcomes, which suggests zinc supplementation should be considered for reproductive age women at risk of zinc deficiency.
Keywords: zinc, mammal, female
An overview of the recent discoveries on the role of zinc in the reproductive processes of mammalian females including oogenesis, folliculogenesis, ovulation, maturation, fertilization, and pre- and post-implantation development.
Introduction
Zinc is an essential nutrient involved in a multitude of physiological processes necessary for growth and survival. As a transition metal, zinc is indispensable for the catalytic activity of hundreds of enzymes, including two that are key for DNA synthesis: thymidine kinase and DNA polymerase [1, 2]. An estimated 3000 proteins bind zinc to maintain structural integrity and function, such as the major ribosomal RNA-transcribing enzyme, RNA polymerase I [3, 4]. Epigenetic reprogramming through histone and DNA methylation, metal-response element-regulated gene expression, and chromatin compaction are all regulated by the concentration of free intracellular zinc [5–9]. Additionally, transient changes in the intracellular zinc content and zinc efflux into the local extracellular space are used as intra- and intercellular signaling mechanisms in various cells and tissues [10–13]. For instance, zinc is co-stored and co-released with insulin in beta cells to mediate autocrine and paracrine signaling, and altered zinc homeostasis in the pancreas is associated with diabetes [14].
As zinc plays an integral role in cell physiology and biochemistry, zinc levels are tightly regulated at the cellular level. In general, cellular zinc is present in one of two pools: (1) zinc that is tightly bound to proteins, such as zinc finger proteins, to maintain the protein structure and stability and where zinc is relatively unavailable for the other cellular functions (for review, see [15]) or (2) labile zinc that is sequestered in the cytoplasm by proteins, such as metallothionein, or in various intracellular compartments, including mitochondria and secretory vesicles. Labile zinc is readily accessible for cellular signaling and other physiological processes. Twenty-four mammalian zinc transporters have been identified, which are expressed across cytoplasmic and organelle membranes and move zinc in and out of various compartments. Of these transporters, the zinc transporter (ZnT), or solute carrier 30 (Slc30) family, consists of 10 proteins that control efflux from the cytoplasm to either the extracellular space or into organelles. A second family, the Zrt- and Irt-like protein (Zip), or solute carrier 39A (Slc39a) family, consists of 14 transporters that control influx into the cytoplasm from organelle stores or the extracellular milieu. These zinc transporters are reviewed extensively in [16–18].
Mammals lack an inherent tissue that can act as a zinc reserve to store or supply zinc in response to zinc availability in the diet, therefore, an adequate and regular intake of dietary zinc is necessary to balance the loss through excretion and to maintain normal zinc homeostasis [19]. The recommended daily intake of zinc ranges from 4.7 to 18.6 mg, depending on sex, age, and other physiological factors [20]. Individuals from low-income regions, particularly children, women of reproductive age, and the elderly, have an elevated risk of zinc deficiency (ZD) [20, 21]. Worldwide, an estimated one in seven to one in five people are at risk of ZD [22, 23]. Even in high-income regions, ZD is estimated to affect 7.5% of the population and may affect up to 30% of the population in poorer regions [22, 23].
Given the many roles for cellular zinc, it is not surprising that ZD contributes to a wide range of pathological processes that include impaired growth and development, increased oxidative stress, elevated inflammatory signaling, and apoptosis induction [24–27]. Zinc deficiency exacerbates negative health outcomes in individuals with chronic diseases, such as diabetes, chronic liver disease, Alzheimer’s, and cardiovascular diseases [28–31]. Mutations in certain zinc transporters have been linked to intellectual disabilities, developmental delays and short stature [32], and skeletal dysplasia, among other disorders [33]. Specifically, mutations in the Znt2 transporter in breastfeeding mothers are associated with neonatal ZD due to low milk zinc concentration [34]. Alternatively, zinc excess, while rare, is also detrimental with symptoms that include nausea and headaches during acute zinc toxicity to reduced immune function and neuropathy in chronic zinc excess [21].
The essential need for zinc is particularly notable within the mammalian reproductive system where ZD causes abnormal or failed development of germ cells in both sexes, which can result in infertility. In fact, approximately 9% of couples [35] face issues with infertility where dietary zinc status and/or dysregulation of the metal may play a significant role. Zinc deficiency during pregnancy increases the risk of adverse outcomes that include miscarriage, fetal growth retardation, impaired neural development, and placental dysfunction, supporting the recommendations for pregnant and breastfeeding women to increase their zinc intake [19, 36–39].
Over the past decade, work from many labs have uncovered many zinc-dependent processes that regulate mammalian female reproduction (Table 1). These discoveries were made using various techniques for the visualization and quantification of zinc ions in cells (Table 2). However, many questions remain unanswered. Revealing the roles of zinc in female reproduction, in addition to the reproductive defects and impaired fertility caused by ZD, is therefore critical for the identification, treatment, and prevention of infertility and ZD-associated risks to prenatal and postnatal health. The aim of this review is to highlight recent advancements in our understanding of zinc-mediated reproductive processes in mammalian female reproduction and the pathologies and phenotypes caused by ZD in reproductive health.
Table 1.
Studies showing the importance of zinc according to species and stage of development.
| Species | Oocyte development, maturation, and ovulation | Fertilization and partheno-genesis | Preconception and periconception effects | Reference(s) | ||||
|---|---|---|---|---|---|---|---|---|
| In vitro | In vivo | In vitro | In vivo | Early embryo | Fetal and placenta development | |||
| DO | COC | |||||||
| Mouse | X | [127] | ||||||
| X | X | X | [56, 87] | |||||
| X | X | [10, 104, 117, 121, 124, 125, 132] | ||||||
| X | X | [154] | ||||||
| X | [118] | |||||||
| X | X | X | X | [98] | ||||
| X | X | X | X | [6] | ||||
| X | [11, 233] | |||||||
| X | X | [141] | ||||||
| X | [163, 164, 202] | |||||||
| X | X | [88] | ||||||
| X | [210, 216, 226] | |||||||
| Rat | X | [197] | ||||||
| Primate | X | X | [10] | |||||
| Human | X | [11] | ||||||
| Bovine | X | [151, 152] | ||||||
| X | X | [123] | ||||||
| X | X | X | [150] | |||||
| Porcine | X | [103, 234] | ||||||
| X | X | [235] | ||||||
| X | X | X | [143, 155] | |||||
Table 2.
Examples of commonly used tools and methods to study zinc.
| Type | Method | General description |
|---|---|---|
| Manipulation of zinc availability | Membrane-impermeable chelators | Compounds with high zinc affinity that are unable to cross the lipid bilayer of cells to manipulate zinc availability in media. One example is zinc-diethylenetriamine pentaacetate (Zn-DTPA) that has a high affinity for zinc but also forms complexes with other heavy metals such as actinides |
| Membrane-permeable chelators | Compounds with zinc affinity that cross the plasma membrane to enter cells to sequester intracellular zinc. The most commonly used in reproductive biology is TPEN N,N,N′,N′-tetrakis(2-pyridylmethyl)ethane-1,2-diamine, which has a high affinity for zinc but also binds other transition metal ions such as copper and iron | |
| Zinc ionophore | Increases intracellular zinc uptake. A potent zinc ionophore is zinc pyrithione, a derivative of pyrithione that is also known to mediate influx of other ions such as copper, is used in vitro | |
| Dietary ZD | Used in animal models to assess in vivo effects of ZD. Zinc-deficient diets typically consist of <3 mg zinc/kg in the diet compared to control diets of >= 29 mg zinc/kg | |
| Fluorescence probing (reviewed in [236, 237] | Small molecule indicators | Zinc-selective fluorophores that range in sensitivity, typically have cell permeable and impermeable versions, and are easy to use in cell culture. However, these probes typically have similar excitation and emission spectra, and assumptions that these indicators only bind labile zinc appear to be invalid. Examples used commonly in reproductive biology include FluoZin-3 and Zinquin |
| Peptide-based zinc indicators | Exploits naturally derived zinc-binding peptides with fluorescent sensors. These probes have a greater range of excitation and emission wavelengths and can target specific subcellular compartments; however, they are more difficult to use and have a smaller dynamic range compared to small molecule indicators. Includes single protein-based zinc fluorophores, Forster resonance energy transfer (FRET)- and bioluminescence resonance energy transfer (BRET)-based probes. Examples include ZnGreen, Zap, and BLZinCh | |
| Total elemental imaging and mapping (reviewed in [236]) | X-ray fluorescence microscopy | High-resolution technique that can image total (free and bound) zinc localization and quantify zinc abundance by the atom. Includes synchrotron X-ray fluorescence, X-ray absorption spectroscopy, and energy-dispersive spectroscopy |
| Mass-spectrometry-based imaging | Employs ionization methods to detect particles by their mass-to-charge ratio. Examples include laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) and secondary ion mass spectrometry (SIMS) |
Zinc control of ovarian function
Primordial follicles form during fetal development or shortly after birth in mammals when oogonia, arrested at the diplotene stage of prophase I, become surrounded by somatic cells [40–45]. Interestingly, the somatic cells destined to enclose oogonia and become granulosa cells (GCs) can arise from either the coelomic or the ovarian surface epithelium [44, 45]. Primordial follicles remain in a quiescent and dormant state until they are “activated” to begin follicle growth. Follicle activation involves a complex interaction between germ cells and somatic factors to ensure steady maintenance of the growing follicle pool [46, 47]. Little information is available on the importance of zinc in these early stages, however, recent work in nematodes suggests a necessary role for zinc in regulating early meiosis [48] (see discussion below).
Following activation, gonadotropin-independent growth of the primary oocyte and proliferation of the somatic GCs contribute to the follicle development to the preantral stage [49, 50]. Shortly before the preantral to antral transition, growth of preantral follicles becomes dependent on circulating gonadotropins, particularly follicle stimulating hormone (FSH). Gonadotropin support is essential to stimulate further growth of antral follicles to the preovulatory stage. The GCs also develop along two distinct lineages during antrum formation; the cumulus GCs (CGCs), which immediately surround the oocyte, and the mural GCs (MGCs) that line the follicular wall [51–54]. Paracrine signaling among theca cells, GCs, and the oocyte regulates many processes in the follicle, including proliferation, steroidogenesis, metabolism, meiosis, and ovulation [53–75]. As with the early germ cell development, there is little information on the role of zinc in the follicle assembly, activation, or preantral follicular growth. However, there have been several recent studies showing effects of in vivo or in vitro zinc depletion on the development of antral follicles and function of the cumulus–oocyte complex (COC). These are reviewed in the following sections.
Zinc in early germ cell meiosis
There is no information to date on the role of zinc in early follicular or germ cell development in mammals. However, two recent studies suggest an important role for zinc ions during both early and late germ cell developments in nematodes. Most notably, Caenorhabditis elegans grown under zinc-limiting conditions using the chelator N,N,N′,N′-tetrakis(2-pyridylmethyl)ethane-1,2-diamine (TPEN) show a severe reduction in fertility, which is mostly due to the impaired oocyte development rather than the deficiencies in sperm production [48, 76]. Caenorhabditis elegans are hermaphrodites and progress through four larval stages (L1–L4) before reaching adulthood [77, 78]. The gonad is composed of two U-shaped gonads, each composed of a distal arm, gonadal bend, proximal arm, and ending in a common uterus [79]. In the distal gonad, a stem cell population supplies germ cells for spermatogenesis during the L3 larval stage but switches to oogenesis beginning around L4 [80, 81]. During oogenesis, there is incomplete cell division in the distal gonad, resulting in the formation of a syncytium called the rachis. Germ cells enter meiosis and progress to the pachytene stage of prophase I while joined with other germ cells in the rachis. As cells enter the proximal gonad, they progress through the final stages of meiosis and become enclosed by a membrane to produce an orderly arrangement of fully grown oocytes which will ovulate in succession.
Oocyte development in worms normally progresses through diplotene and eventually diakinesis in the proximal gonadal arm [79]. However, in TPEN-treated worms, there is a notable extension of pachytene germ cells well into the proximal gonadal arm [48]. These data may indicate that zinc is essential for early meiotic progression; however, details on the cellular mechanisms affected by zinc depletion remain to be determined. The early germ cell arrest could be the result of disruption in the mitogen-activated protein kinase (MAPK) signaling pathway, which promotes meiotic progression in worms and is known to be regulated by zinc in other contexts [82, 83]. Ablation of the MAPK homolog mitogen-activated protein kinase 1 (MPK-1) results in pachytene arrest in C. elegans [84, 85]. The MPK-1 interacts with the zinc-containing protein and mutants for both gla-3 and show a similar gonadal phenotype to the TPEN-treated worms [86]. It will be interesting to examine the details of zinc involvement in early meiosis in mammals. In late meiosis, around ovulation, there are many similarities between worms and mammals, suggesting a robust conserved pathway. For example, as in mice (see below), there are defects in the polar body extrusion and spindle formation, ultimately resulting in aneuploidy, in the TPEN-treated worms [76].
Zinc in antral follicular development
Antral follicles are actively growing, highly steroidogenic structures that are regulated by autocrine, endocrine, and paracrine signals during growth and ovulation. Recently, a series of studies using dietary zinc depletion during antral follicular development and ovulation revealed an important role for zinc during follicle rupture and cumulus expansion [6, 87, 88]. In these studies, newly weaned mice ~18 days of age were fed a control (>30 mg zinc/kg) or zinc-deficient (<1 mg/kg) diet for up to 10 days. The timing of the dietary treatments was important because, at weaning, there is a cohort of large preantral follicles that are poised to make the preantral to antral transition and form a synchronous follicular wave. These follicles will ovulate if stimulated with exogenous hormones. Using this paradigm, the authors show that neither a 3- nor a 5-day treatment with a ZD diet causes any decrease in the ovulation rate in response to exogenous hormonal stimulation [6, 87]. However, a 10-day treatment with a ZD diet resulted in an almost complete ovulation block. Interestingly, the block in follicle rupture was not due to the failure of follicular development. In fact, antral follicles grew quite large in the ZD group, but did not rupture, even after hormonal stimulation with an ovulatory dose of human chorionic gonadotropin [87]. Impaired follicle rupture could be due to the decreased activity of zinc-dependent enzymes, such as matrix metalloproteinases, which are important for the degradation of the follicular wall during ovulation [89–92]. However, effects on other cellular proteins or pathways cannot be ruled out.
Another zinc-dependent pathway has recently been discovered in the ovarian cells of the Atlantic croaker and zebrafish [93, 94]. In these species, testosterone-mediated zinc signaling regulates cell survival (croaker) and oocyte maturation (zebrafish). In GCs, mammary cancer, and prostate cells of these model species, androgens signal through the activation of a previously identified zinc transport protein, Zip9 [93, 95, 96]. These studies provide compelling evidence that Zip9 functions as both a zinc transporter and membrane androgen receptor coupled to both zinc transport and G-protein activation. While, to date, no studies have identified a role for Zip9 in mammalian ovaries, androgen-mediated signaling through Zip9 could represent another zinc-mediated pathway that is important for the ovarian function in mammals, particularly since Zip9 in zebrafish is required to generate a zinc spark, a phenomenon that is conserved across many species (Table 3) [97].
Table 3.
Studies that highlight the evolutionarily conserved zinc efflux upon egg activation.
Zinc in the COC
In antral follicles, the COC is particularly sensitive to zinc deprivation. The effects of zinc depletion on oocyte function are discussed below. Here, we consider how zinc ions may contribute to the function of the cumulus cells. In unstimulated COCs, chelation of transition metals, including zinc, with TPEN causes an acute increase in steroidogenic transcripts, such as Cyp11a1 and Star mRNA, and an increase in progesterone accumulation in the culture medium [87, 98]. This is likely due to zinc depletion because feeding a zinc-deficient diet for 10 days recapitulates the increase in steroidogenic transcripts in COCs [87]. Interestingly, luteal tissue cultured ex vivo also responds to TPEN chelation with an increase in the abundance of steroidogenic transcripts and progesterone accumulation in culture media [98]. Collectively, these observations suggest that zinc may play an inhibitory role in the onset and maintenance of progesterone production. However, this idea remains to be tested rigorously. The increase in progesterone under zinc-depleted conditions could also be due to the removal of inhibitory pathways. Indeed, signaling through the zinc-binding SMAD (Mothers against decapentaplegic homolog) transcriptional pathway is known to inhibit progesterone production [99–101], and TPEN treatment potently suppresses SMAD2/3 phosphorylation in COCs [87]. The suppression of SMAD2/3 signaling also leads to other defects including a complete failure of cumulus expansion as seen in the TPEN-treated COCs or COCs from animals fed with a zinc-deficient diet [87]. Further, CGCs isolated from mouse COCs cultured in TPEN show a dramatic increase in the expression of Cyp11a1 and Lhcgr typically associated with the MGCs [87]. While phosphorylation of SMAD2, which suppresses mural transcripts and enables cumulus expansion following epidermal growth factor (EGF) stimulation, is also markedly reduced in CGCs by zinc chelation, EGF-induced mitogen-activated protein kinase 3/1 (MAPK3/1) phosphorylation does not appear to be disrupted [51, 87].
Together, these findings indicate that zinc has a role in maintaining the cumulus phenotype and may be involved in preventing the premature upregulation of progesterone production in the COC [98]. Thus, a model is emerging wherein zinc ions are required for the SMAD activation to prevent the premature upregulation of progesterone and to enable cumulus expansion. The SMAD2/3 activation is reduced by 6–8 h following the onset of cumulus expansion [51], which may be a similar timeframe for a shift in free intracellular zinc from the CGCs to the oocyte [56]. It is tempting to speculate that the release of zinc from the CGCs may play a functional role as a switch in the CGC physiology at the time of ovulation. This idea requires further experimental study and pathways other than SMAD activation may be involved. Nevertheless, it is interesting that CGCs, which have extensive gap junctional communication with the oocyte [102], have much higher free intracellular zinc stores than the oocyte before maturation [56].
It seems that the CGCs themselves produce a factor (zinc inhibitory factor (ZIF)) to regulate the level of free intracellular zinc in the oocyte before ovulation. Removal of CGCs leads to an abrupt increase in the oocyte free intracellular zinc which is reversed after co-culture with CGCs. Thus, ZIF is a secreted product that reduces free intracellular zinc in the oocyte [56]. Treatment with EGF abolishes the ZIF activity, allowing free intracellular zinc in the oocyte to increase [56, 103, 104]. However, metaphase II (MII) oocytes remain sensitive to ZIF since the CGC co-culture reduces free intracellular zinc in these cells. Whether the zinc that accumulates in the in vivo maturing oocyte originates from the extracellular milieu, through a paracrine mechanism that involves flux of labile zinc from the CGCs to the oocyte, or a combination thereof and to what degree, is unknown [56].
Zinc regulation of oocyte development and maturation
Prophase I is maintained in the germinal vesicle (GV) oocytes through an orchestrated mechanism where MGCs synthesize natriuretic peptide precursor C (NPPC) that signals through its receptor, NPPC receptor 2 (NPR2), in CGCs to produce cyclic guanosine monophosphate (cGMP) production [67, 105]. The cGMP diffuses to the oocyte via the connexin 37 (Cx37)-predominant gap junctions to inhibit phosphodiesterase 3A (PDE3A), thus blocking cyclic adenosine monophosphate (cAMP) breakdown and inhibiting the activation of maturation induced downstream by maturation promoting factor (MPF) [64, 67, 105]. Ovulatory signals abolish this inhibitory pathway, leading to a decrease in cAMP, MPF activation, and subsequent GV breakdown (GVBD) and progression to MII (Figure 1).
Figure 1.
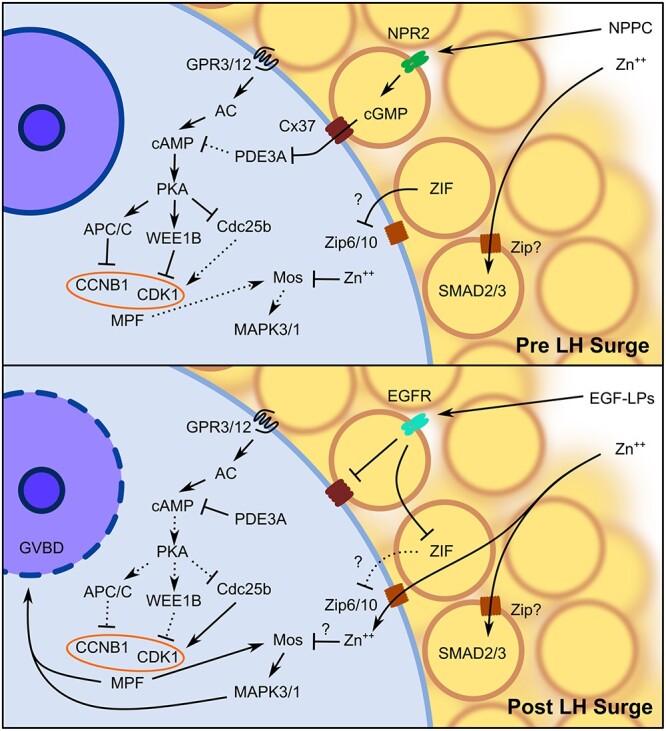
Diagram of the signaling pathways that regulate GV arrest and GVBD. The NPPC secreted from peripheral MGCs binds to NPR2 in CGCs to upregulate cGMP synthesis. The cGMP passively diffuses into the GV oocyte via Cx37 predominant gap junctions to inhibit PDE3A, thus maintaining an elevated concentration of cAMP in the oocyte through constitutive activation of AC by GPR3/12. The high concentration of cAMP activates PKA to directly inhibit MPF through APC/C proteasomal degradation of CCNB1 and WEE1B phosphorylation of CDK1 in addition to inhibiting Cdc25b activity. The CGCs also secrete ZIF that prevents uptake of free intracellular zinc by the oocyte, possibly through inhibition of Zip6/10. In the CGCs, zinc is required for SMAD2/3 activation to maintain the cumulus phenotype, while in GV oocytes, zinc inhibits premature activation of the Mos–MAPK3/1 pathway. Following the LH surge, EGF-LPs secreted by MGCs bind to the EGFR in CGCs to initiate the closure of CGC–oocyte gap junctions, thus preventing further cGMP diffusion into the oocyte. Activation of the EGFR in CGCs also inhibits ZIF activity, allowing free intracellular zinc accumulation in the oocyte during maturation. The reduction in intra-oocyte cGMP concentration increases PDE3A activity to degrade cAMP. As PKA activity is subsequently reduced, MPF is activated by increased CCNB1 availability and CDK1 dephosphorylation by Cdc25b, leading to both activation of Mos–MAPK3/1 and GVBD. Abbreviations: AC, adenylyl cyclase; EGFR, epidermal growth factor receptor; Zn++, zinc. Arrows indicate activation and bars represent inhibition. Solid lines indicate active pathways, while dashed lines represent inactive pathways.
Sufficient free intracellular zinc levels are required to maintain prophase I arrest in GV-stage oocytes, but once maturation is initiated, a rise in intracellular zinc is necessary for the oocyte to progress through meiosis I, extrude an asymmetric polar body, establish a second meiotic spindle, and arrest at MII. Consequently, insufficient zinc availability severely disrupts oocyte maturation, which results in a diverse set of phenotypic abnormalities depending on the severity, duration, and maturation stage in which the ZD occurs. These disordered morphologies include the formation of a large polar body, symmetric cell division, reduced cumulus expansion, and meiotic failure before MII. All of these defects result in a failure of maturation or subsequent fertilization and embryonic development. This section will outline the physiological functions of intracellular zinc during oocyte maturation and how misregulation of the transition metal may lead to developmental failure.
Follicular signaling pathways in ovulation
Depending on the species, one or multiple preovulatory follicles are induced to ovulate by a surge of circulating luteinizing hormone (LH) released by the anterior pituitary. The LH receptors (LHRs) in MGCs respond to the LH surge by increasing cytosolic cAMP to activate protein kinase A (PKA), resulting in the release of the EGF-like peptides (EGF-LPs) amphiregulin (AREG), epiregulin (EREG), and betacellulin (BTC), which have autocrine and paracrine functions [65, 66, 106]. These EGF-LPs, particularly AREG and EREG, bind to the EGF receptors present in CGCs, which generally lack LHRs in mice, to reduce the cGMP production and terminate Cx37-predominant gap junctions between the CGCs and the GV oocyte [51, 66, 106, 107]. Both effects result in a reduction of intra-oocyte cGMP, allowing an increase of PDE3A, and resulting in the degradation of cAMP which was high in GV oocyte through constitutively activity of G-protein coupled receptor 3 (GPR3) and G-protein coupled receptor 12 (GPR12) [64, 105, 108–111]. The cAMP degradation inactivates PKA and thus facilitates the activation of MPF, a protein complex consisting of an enzymatic cyclin-dependent kinase (CDK1) and PKA-regulated cyclin B1 (CCNB1) [112]. The MPF activation occurs through both the reduction of WEE1B activity that phosphorylates and deactivates CDK1 at Tyr15 and the inhibition of the anaphase-promoting complex/cyclosome (APC/C) proteasome to reduce CCNB1 degradation. The active MPF protein complex induces GVBD and meiotic resumption [64, 67, 105, 108, 109, 113]. Additionally, EGF receptor activation and gap junction closure initiate CGC expansion and mucification [64, 67, 105].
Following the LH surge, the mammalian oocyte will undergo a period of meiotic maturation for approximately 12–16 h until arresting at MII, where it will await possible activation at fertilization. During this short window, the oocyte will undergo a wholesale reorganization of the cytoplasm [114] as well as remodeling of the transcriptome and proteome [115, 116] to prepare for early embryonic development. Proper maturation to the MII stage is critical in producing a high-quality oocyte, which largely determines the fertilization success and the development of healthy embryos and progeny.
Intracellular zinc maintains GV arrest
Interestingly, in vitro zinc sequestration by TPEN alone induces GVBD in GV oocytes arrested with a PDE inhibitor, suggesting that sufficient zinc is necessary for maintaining prophase I arrest [87, 117]. The GV-stage oocytes maintained in media containing TPEN undergo GVBD and subsequently arrest prematurely at telophase I. However, oocytes that undergo only a transient TPEN exposure to induce GVBD and then are cultured without the chelator and sufficient zinc can successfully mature to the MII stage, while maintaining normal developmental competency to blastocysts [87, 117]. The role of zinc in meiotic prophase I arrest appears to be independent of the physiological mechanisms that regulate maturation as TPEN-induced GVBD occurs even in the presence of various PDE inhibitors and without a reduction in the intra-oocyte cAMP concentrations [87]. Instead, GVBD induced by zinc chelation occurs through the activation of the Mos–MAPK pathway [117, 118].
In mouse oocytes, Mos is only expressed after the induction of maturation and acts to maintain MII arrest by maintaining MPF prior to fertilization, however, injection of Mos can induce GVBD [119, 120]. The TPEN-induced maturation in prophase I-arrested oocytes appears to be through premature activation of Mos–MAPK, which precedes GVBD and MPF activation, while cAMP, which maintains prophase I arrest, increases [87, 117, 118]. Surprisingly, while TPEN-induced GVBD is regulated by the expression of Mos, the failure to transition from meiosis I to meiosis II during ZD is unrelated to the Mos–MAPK pathway even though similar phenotypes are observed in Mos insufficiency [118]. Similar abnormalities have been observed both in vivo using a ZD diet and in vitro using zinc chelators, where early GVBD occurs without an ovulatory signal and results in the premature meiotic arrest that impairs both ovulation and oocyte transport to the oviduct [87].
Accumulation and localization of zinc during maturation
Some of the first studies that identified the necessary role of zinc in mammalian reproduction observed the importance of the transition metal for oocyte maturation. During this short developmental window, the total intracellular zinc content increases by 30–50%, an approximate influx of 20 billion atoms [104, 121]. However, the amount of zinc accumulated during maturation is likely greater as these studies employed denuded oocytes for which the removal of CGCs prompts an immediate influx of zinc in the GV-arrested oocytes to approximately 2-fold after 15 min and to 4-fold after 1 h [56]. The accumulated zinc is predominantly stored in vesicles that are located symmetrically along the oocyte cortex at the GV stage and polarize toward the vegetal pole at the MII stage [10, 121–124]. Intriguingly, these zinc-positive vesicles relocalize to and from the cortical region in a meiotic-stage dependent manner, forming a hemispherical pattern by the MII stage with zinc-positive vesicles localized away from the spindle [121, 125].
Zinc in meiotic progression and spindle formation
The transporters Zrt- and Irt-like protein 6 (Zip6) and Zrt- and Irt-like protein 6 (Zip10) both belong to the Slc39a family that regulates zinc influx into the cytoplasm, and both localize to the oocyte cortex [122]. These two transporters are necessary for the oocyte maturation in mice as the functional blocking of the proteins with anti-Zip6 and anti-Zip10 antibodies or morpholino-induced knockdown of translational capacity for either transporter disrupt this process. The mechanism of disrupted oocyte maturation likely involves interference of Zip6–Zip10 heteromer formation observed in zebrafish [126], which produces phenotypes that resemble zinc-chelated oocytes [122]. Restricting intracellular zinc with the chelator TPEN during mouse in vitro oocyte maturation results in the meiotic arrest at telophase I, formation of an abnormal spindle, and in some cases, formation of an abnormally large polar body or even symmetric cellular division [104, 118]. While telophase I-arrested mouse oocytes are fertilization-competent as pronuclear formation still occurs, embryonic progression to the blastocyst is impaired [104].
A sufficient intracellular zinc supply is critical immediately prior to polar body extrusion and meiotic I exit. Zinc supplementation of maturing oocytes cultured in the zinc chelator TPEN rescues MII morphology when provided prior to telophase I arrest but not after [104]. Additionally, TPEN-mediated zinc chelation during the MI to MII transition, at approximately 7.5 h after GVBD, prevents MII spindle formation [127]. The failure of zinc-insufficient oocytes to transition from meiosis I to meiosis II is due to impaired MPF activity [118]. The CCNB1, a component of MPF, through its translation and degradation, is one control point for MPF activity during the MI to MII transition [118, 127, 128]. The MPF activity is increased in the oocyte to induce GVBD, is reduced during meiosis I and extrusion of the first polar body, and then is again upregulated to establish the MII spindle [129]. However, zinc-insufficient oocytes fail to accumulate CCNB1 and upregulate the MPF activity following meiosis I. As a result, they fail to enter MII and remain in telophase I arrest [118, 127]. Early mitotic inhibitor 2 (EMI2), a zinc-binding APC/C proteasome inhibitor, is an integral component of the cytostatic factor (CSF) to initiate MII entry and maintains MII arrest which is disrupted in zinc-insufficient maturing oocytes [127, 130–132]. Reduced zinc binding to EMI2 leads to an overactive APC/C proteasome which increases CCNB1 degradation and reduces MPF activity, resulting in early meiotic arrest [127].
Zinc in actin nucleation and meiotic spindle migration
Abnormal meiotic progression and large polar body extrusion resulting from zinc chelation appears to be caused by the disruption of actin cytoskeletal nucleation and thus spindle formation and localization to the cortex. Culture in TPEN results in reduced F-actin in a dose-dependent manner, and disruption of Zip6 and Zip10 by morpholinos significantly reduces actin as measured by phalloidin intensity [124]. A highly specialized actin cytoskeleton facilitates spindle formation and positioning as oocytes from many species lack centrosomes typically used to facilitate mitotic division. The lack of centrosomes may be beneficial during polar body extrusion by allowing the meiotic spindle to localize more closely to the cortex and by reducing the cytoplasmic loss during division [133–135]. This actin cytoskeleton is nucleated through interactions between Formin-2 and Spire1/2 which localize to Rab11a-positive vesicles, which serve as the site for nucleation and drive asymmetric division [135–137]. Importantly, Spire1/2 contains a cysteine-rich zinc finger domain that is crucial for its localization, and zinc content regulates Formin-2/Spire1/2 localization and actin nucleation [124, 138]. Further, Spire1/2 co-localizes with punctate zinc-positive vesicles within the oocyte [124].
Spire1/2 knockdown or mutation of the zinc-binding domain results in reduced cytoplasmic actin mesh formation, premature meiotic arrest, and abnormal polar body extrusion similar to zinc chelation by TPEN [104, 124, 137]. Both zinc chelation and specific mutation of the Spire1/2 zinc finger disrupts its localization, thus dispersing the nucleator throughout the cytoplasm and reducing contact with Formin-2 [124]. While zinc chelation results in a loss in MPF activity after the first meiotic division, impaired actin mesh formation by zinc chelation is not recovered by supplementing MPF activity with a non-degradable form of CCNB1 [118, 124]. However, this could be due to zinc acting through cell division cycle 25 (Cdc25) to regulate MPF through CDK1, thus supplementation of the other component of MPF may not be sufficient to rescue the MPF activity [139]. Importantly, Spire1/2 contains a zinc finger motif and co-localizes with zinc to cortical granules (CGs) along the oocyte membrane, where zinc chelation and a Spire mutant lacking the zinc-binding domain both impair Spire localization [121, 124, 136, 137].
Together, these studies have identified zinc as a critical mediator throughout the oocyte maturation process to maintain prophase I arrest, regulate the meiosis I to meiosis II transition, and initiate MII arrest.
Zinc in fertilization and egg activation
Mature eggs arrest at MII of meiosis and await fertilization and egg activation (Figure 2). The MII arrest is maintained by a CSF which we now know is dependent on the accumulation of enough free intracellular zinc during maturation. As described earlier, accumulated zinc in maturing oocytes binds to and activates EMI2 which disrupts the association of cell division cycle 20 (Cdc20) with the APC/C proteasome complex and thereby inhibits the degradation of CCNB1 to maintain MPF-mediated meiotic arrest [127, 130–132]. Upon fusion of the sperm and egg, phosphoinositide phospholipase C y1 (PLCy1) is delivered from the sperm to the oocyte to induce the conversion of inositol trisphosphate (IP3) and diacylglycerol from phosphatidylinositol 4,5-bisphosphate at the oocyte membrane, thus stimulating calcium release from the intracellular stores and activating protein kinase C, respectively [123, 140]. The resulting transient calcium oscillations induce zinc sparks, which are short bursts of zinc released into the extracellular milieu that result in a reduction of the total zinc status of the fertilized egg [10, 11, 121, 123, 141].
Figure 2.
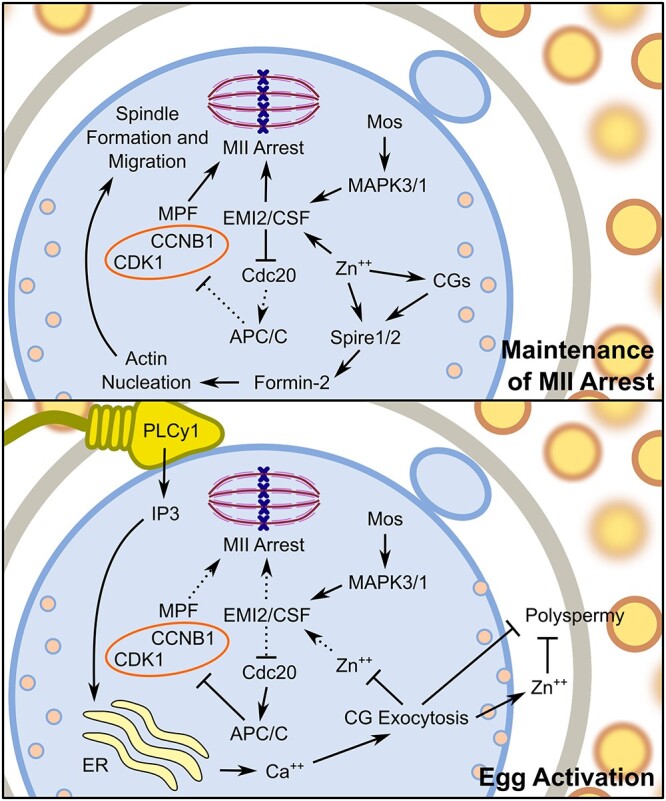
Model of signaling pathways that modulate MII arrest and egg activation. A high intracellular concentration of zinc establishes and maintains MII arrest through activation of EMI2 to prevent degradation of CCNB1 by the APC/C proteasome and maintains high MPF activity to maintain MII arrest. Additionally, zinc accumulation facilitates Spire1/2 localization with Formin-2 to nucleate actin cytoskeleton and to facilitate meiotic spindle formation and migration to the cortex. Following sperm binding and the acrosomal reaction, PLCy1 increases IP3 availability that initiates calcium release from the ER. This calcium influx promotes exocytosis of CGs, releasing zinc and other factors important for establishing the block to polyspermy. The subsequent reduced intracellular concentration of zinc reduces EMI2 activity. Subsequent activation of the APC/C proteasome by Cdc20 leads to the degradation of CCNB1 to reduce MPF activity, thus allowing completion of meiosis. Abbreviations: Ca++, calcium; ER, endoplasmic reticulum; Zn++, zinc. Arrows indicate activation and bars represent inhibition. Solid lines indicate active pathways, while dashed lines represent inactive pathways.
One to five zinc spark events are triggered within 90 min of fertilization or parthenogenesis in mammals, and approximately half of the 20 billion zinc atoms accrued during oocyte maturation are released prior to the cleavage to form the two-cell embryo [10, 11, 121, 123, 141]. While multiple zinc sparks may be initiated in a single cell, each preceded by an intracellular calcium oscillation, only one is required for the successful resumption of meiosis II and transition from an egg into an embryo [10, 11, 121, 123, 141]. Instead, it is the amplitude of the zinc released during these events that is critical as a sufficient drop in intracellular zinc is necessary for meiotic resumption [10]. The amount of zinc released positively correlates with the chance that the resulting embryo will develop into a morula and blastocyst [141]. Additionally, the zinc spark event is stage-dependent as GV oocytes respond to the parthenogenetic activation with a much smaller amplitude relative to MII oocytes [11].
The quick release of zinc leads to a reduction in the total intracellular zinc available to bind to and activate EMI2, thus relieving the inhibition on the APC/C proteasome to increase CCNB1 degradation and alleviate MPF-mediated meiotic arrest [10, 121, 127, 130, 142]. The calcium oscillations stimulated by IP3 work synergistically to resume meiosis II by signaling for the degradation of EMI2, further reducing APC/C proteasomal inhibition [127, 130]. Calcium release is not required for in vitro meiotic resumption in MII oocytes as zinc chelation by TPEN induces MII exit through the APC/C proteasome pathway to result in parthenogenesis without calcium mobilization [130, 143]. However, zinc chelation-induced parthenogenesis causes significant reductions in developmental potential, with successfully activated oocytes rarely progressing beyond the two-cell embryonic stage [130]. In separate studies with pig oocytes, brief TPEN exposure for 30–60 min promoted, while longer exposures inhibited the blastocyst development [143, 144]. Conversely, zinc excess also is detrimental to developmental potential as displayed in experiments with the ionophore zinc pyrithione that increased cortical actin and blocked pronuclear formation in eggs which had successfully completed zinc spark events prior to treatment [10].
Extra-oocyte role of zinc sparks
In addition to inducing MII exit upon successful fertilization, the zinc spark events also act to prevent polyspermy. Approximately, 500–600 million atoms or 5% of the total zinc released following egg activation is incorporated into the surrounding ZP without affecting the exocytosis of CGs or ZP protein cleavage [125]. The accumulation of zinc along the ZP increases fibril connectivity along the glycoprotein matrix, which reduces the number of sperms that are capable to bind and reach the recently fertilized egg [125, 145, 146]. Additionally, zinc released into the extracellular milieu reduces the local sperms’ forward motility to further reduce the number of sperms reaching the egg [146]. This was a particularly interesting finding as hardening of the ZP to prevent additional sperm binding does not occur until around 30 min after fertilization, while the zinc spark events are initiated only a few minutes after fertilization, indicating that early zinc efflux is used to slow sperm to allow more time for cleavage and inhibition of sperm-binding proteins along the outer zona matrix and to completely block polyspermy [146].
Epigenetic defects in fertilized zinc-deficient oocytes
The research discussed above outlines some of the mechanisms of zinc-mediated processes during maturation and fertilization using in vitro methods; however, there is ample evidence that zinc depletion in vivo impairs the female reproductive function. An acute bout of ZD prior to ovulation reduces the in vitro fertilization potential even when the ovulation rates are unaffected [6]. Although in vivo fertilization rates are not as dramatically reduced in ZD mice compared to in vitro studies, the negative effects of ZD are present in the fertilized eggs from these animals, which displayed a disorganized chromatin structure and the resulting embryos had reduced developmental competency compared with controls [6]. As discussed below, ZD impairs histone (H3K4me3) and DNA methylation. Indeed, deletion of mixed-lineage leukemia 2 (Mll2), also known as lysine methyltransferase 2D (Kmt2d), the methyltransferase responsible for H3K4me3, results in maturation failure and oocyte death [147]
Supplementation with the methyl donor S-adenosylmethionine (SAM) during in vitro maturation restores H3K4me3 and improves the proportion of ZD oocytes that are successfully fertilized and progress to the two-cell embryo stage, indicating that disrupted histone methylation is a hallmark of disrupted fertilization and early embryonic development caused by ZD [6]. While sperm may enter the oocyte and decondense to initiate some of the early stages of fertilization in zinc-insufficient oocytes, they fail to form blastocysts as a result of either a failure to reach MII or pronuclear fusion [6, 68, 141]. In vivo, preconception ZD in mice reduces fertilization success by 20% after 3 days, but fertilization rate is reduced by over 90% after 5 days of ZD, nearly as effective as in vitro zinc chelation [88]. Since biological zinc reserves are low, animals are likely able to maintain a sufficient supply of zinc for oocyte maturation for only a few days under ZD conditions. Despite the similarities of ZD in vivo and chelation in vitro on oocyte maturation and fertilization, the exact mechanisms altered by ZD in vivo remain to be fully identified but do include alterations in the epigenetic programing of the oocyte and perhaps zygote as discussed below. Overall, it is likely that zinc concentrations fall below the threshold necessary for successful oocyte maturation and pregnancy.
Zinc supplementation in IVM and IVF
Prior to uncovering the role of zinc in regulating mammalian oocyte maturation and fertilization, experiments with COCs supplemented with zinc during IVM showed a positive correlation between zinc concentration and the rate of blastocyst formation following IVF [148]. Increased intra-oocyte, but not intra-CGC, glutathione (GSH) concentrations and reduced DNA damage in CGCs are associated with dose-dependent increases in zinc in cows [148, 149]. Additionally, superoxide dismutase (SOD) activity increases with zinc supplementation during IVM with bovine COCs, and although cumulus expansion is unaffected, the proportion of CGCs undergoing apoptosis is reduced [150]. Further, zinc supplementation increases blastocyst rates for COCs, denuded oocytes, and denuded oocytes cultured with CGCs, indicating that the mechanism that employs extracellular zinc to reduce reactive oxygen species (ROS) is not entirely dependent on the somatic cells [150]. Supplemental zinc during culture of COC from small antral follicles, containing transcriptionally active bovine oocytes, enhances both transcription and competence to undergo maturation, suggesting that zinc during oocyte growth is also important for full developmental potential [151]. Both zinc supplementation and hormones such as estradiol, LH, and FSH modulate the zinc quota and expression of zinc transporters in both CGCs and oocytes during bovine IVM [152]. This dynamic regulation is similar to the changes observed in mouse oocytes [56, 104] and highlights the tight regulation of zinc ions during maturation.
Zinc supplementation during IVM and IVF in mice displays similar trends with a dose-dependent increase in MII formation, successful fertilization, and blastocyst development [153]. Conversely, zinc chelation increases ROS that is associated with reduced MII oocyte quality and impaired embryonic development [154]. While zinc supplementation during IVM of porcine oocytes does not enhance the percentage of MII oocytes, blastocyst formation rate following IVF is increased in association with reduced ROS and increased GSH [155]. Similarly, increasing concentrations of zinc supplementation of yak oocytes enhances the percentage of embryos reaching the blastocyst stage and is also associated with reduced ROS and increased intracellular GSH concentrations and SOD activity in matured oocytes [156]. By contrast, zinc supplementation during IVM in horses does not improve oocyte maturation, cleavage, or blastocyst formation [157]. Together, these studies indicate a dose-dependent relationship between zinc supplementation during IVM and reduced ROS, which is conserved across many mammalian species. However, excess zinc supplementation during IVM may curtail the positive benefits of zinc as the percentage of embryos that reach the blastocyst stage appear to slightly reduce after ~1–1.5 mg/mL zinc [148, 155, 156]. As these experiments did not use zinc concentrations above 2 mg/mL during IVM, additional studies are necessary to assess the concentrations of zinc that become toxic.
While zinc supplementation during IVM improves IVF outcomes, how extracellular zinc enhances the buffering of ROS in oocytes and COCs is unknown. Zinc has both protective and toxic properties dependent on the concentration and cell type. In primary neuronal astrocytes derived from rats, high zinc concentrations in the media inhibited GSH redox cycling to promote ROS and cell death [158]. Conversely, human adult retinal pigment epithelial cells or rat vascular endothelial cells treated with similar zinc concentrations were protected against oxidative stress by elevating GSH through Nrf2-mediated transcription of glutamate-cysteine ligase, the rate-limiting enzyme for GSH synthesis [159, 160]. For oocytes, the latter mechanism is likely taking place as depletion or disruption of Nrf2 reduces CCNB1/CDK1 expression and disrupts spindle organization, chromosomal alignment, and polar body extrusion similar to zinc depletion [161, 162].
Zinc in preimplantation development
Upon completion of meiosis II and successful fertilization, the mammalian egg transitions into a preimplantation embryo that shifts from meiotic to mitotic division. Initially, cell division occurs without the intervening growth of daughter cells, resulting in smaller and smaller embryonic cells that fit within the ZP. Maternally derived transcripts stored in the egg prior to oocyte maturation provide the necessary gene products for early embryogenesis until embryonic genome activation (two- to eight-cell stage). As the embryo develops and increases in cell count, compaction and cell polarization define the outside and inside of the embryo, while localized signaling determines the cell differentiation and lineage.
Total bound and labile zinc is an order of magnitude higher (~4- to 10-fold) in the preimplantation embryo compared to iron and copper, with the total amount of zinc atoms at similar levels to the GV oocyte [163]. This high concentration of zinc persists throughout early embryo development from the one-cell to at least the eight-cell stage. Zinc is primarily localized to discrete punctate structures that are enriched around the cortex [104, 163]. It is therefore no surprise that zinc status is just as important for embryogenesis as it is for oocyte maturation and fertilization.
In vitro culture of embryos with TPEN at the one-, two-, and four-cell stages results in the dose-dependent developmental arrest and a reduction in blastomere quality that was not reversible following culture in TPEN-free media [163]. The phenotypes observed were associated with more tightly packed DNA, particularly around the nucleolus, and a reduction in global transcriptional activity [163]. It is possible that ZD or zinc chelation, particularly during the one-cell stage, may inhibit the activation of the embryonic genome that occurs around the two-cell stage in mice. Then, as maternally derived transcripts are continuously degraded, the overall translational capacity would be reduced and lead to developmental arrest. Alternatively, maternally derived transcripts may still be available, but an insufficient zinc pool instead reduces the translational capacity through the inhibition of ribosomal RNA synthesis by RNA polymerase I [3]. Culture of blastocysts in a low-zinc environment results in a somewhat delayed formation of inner cell mass (ICM) and visceral endoderm, which may be due to the increased oxidative stress and apoptosis [164].
In vivo, preimplantation mouse embryos are unable to recover from maternal ZD even if zinc is later supplemented, with a critical period of zinc requirement appearing to be between fertilization and the four-cell stage [165, 166]. Additionally, early blastocyst development and competence is adversely affected following the preconception ZD in mice [6]. Even an acute bout (3–5 days) of preconception ZD delayed blastocyst progression through reduced expression of imprinting genes, such as the growth-promoting genes, Igf2 and H19 [6]. Further, preconception ZD compromises the later stages of embryo development, placentation, and survival throughout pregnancy likely through altered epigenetic programming of the oocyte and/or early embryo (see discussion below) [6, 88].
Epigenetic programming of oocytes and early embryos
Germ cells and early embryos are particularly susceptible to epigenetic disruption. Oocytes acquire high levels of chromatin methylation as ovulation approaches [167–169], but after fertilization, the paternal genome is actively demethylated, while the maternal genome is passively demethylated [170–173]. As preimplantation embryo development proceeds, there is extensive re-methylation of both the ICM and trophectoderm (TE) lineages [170, 173, 174]. However, TE lineages have a dramatically different epigenetic landscape from the epiblast cells with extensive methylation at CpG islands and promoter regions, particularly the promoters of pluripotency genes such as Oct4 and Nanog [175–177]. In both lineages, DNA methylation is necessary to establish the imprinted (monoallelic) gene expression [178] and for silencing of damaging repetitive elements [179, 180]. The DNA methylation is maintained at imprinted control regions (ICRs) [181–183] and on imprinted alleles through the de novo methylation in the zygote/early embryo [184].
Failure to maintain imprinted DNA methylation at imprint control regions [170, 185] causes epigenetic defects that are transmitted to the offspring [6, 186, 187]. Importantly, imprinted genes are crucial for placenta development, and their dysregulation is associated with impaired function and adverse pregnancy outcomes [188, 189]. Previous work using acute dietary ZD shows that preconception ZD causes chromatin hypomethylation in oocytes [6]. Interestingly, supplementation of zinc-depleted oocytes with SAM during in vitro maturation restores DNA methylation and partially rescues fertilization defects [6], suggesting that methyl donors could help correct the epigenetic defects caused by lack of zinc. A similar phenomenon occurs in mice supplemented with a methyl donor-rich diet that increases DNA methylation and alters gene expression at specific loci [190].
Epigenetic effects of ZD on postimplantation embryo and placenta development
Zinc deficiency prior to fertilization can have lasting effects on the development of the embryo and supporting tissues, even when the zinc intake becomes adequate during pregnancy (Figure 3). Acute ZD during the preovulatory period results in reduced DNA and histone methylation with an increased abundance of repetitive elements in the mouse GV oocytes [6]. These epigenetic defects are associated with growth delays and defects in embryonic and placental development later in pregnancy. For example, following acute ZD, embryos are 31% smaller in size with a high 46% mortality rate on embryonic day 10.5 (E10.5) [88]. These defects were specific to ZD of the embryo itself rather than the uterus as embryo transfer of ZD embryos to females fed with a normal, zinc-sufficient diet had reduced the implantation rates and smaller embryos following implantation [88]. Strikingly, as few as 5 days on a ZD diet prior to ovulation reduces the embryonic implantation rate by up to 75%, with successfully implanting embryos reduced in size by 38% and trophoblast outgrowth area reduced by 40% [88]. Preconception ZD also has long-lasting effects on the developing embryo as the fetal contribution to the placenta is significantly reduced and defects in limb buds and neural tube development were noticed even when zinc was sufficient throughout the pregnancy [88]. The impairments in neural tube development caused by preconception ZD could be related to the increase in cell death and reduction in ectoderm formation observed in zinc-deficient embryos [164] and to a reduction in working memory seen during gestational ZD [191].
Figure 3.
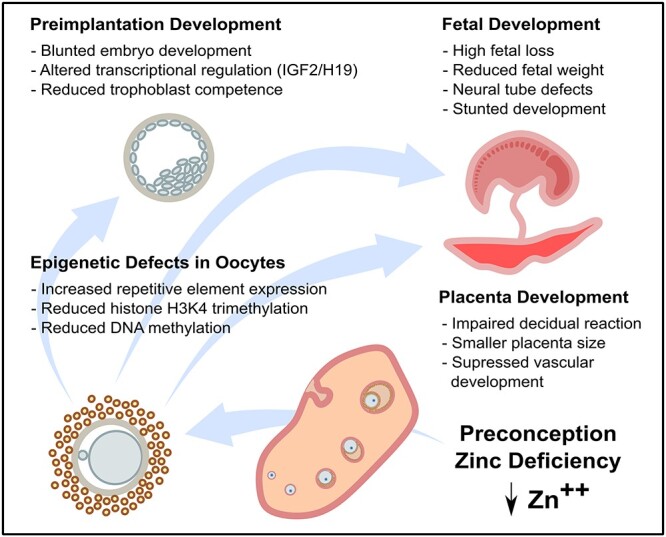
Epigenetic defects caused by preconception ZD. A 3–5 day period of dietary ZD is sufficient to cause a reduction in DNA and histone methylation and increased abundance of transcripts from the repetitive elements in the oocyte. These defects are associated with impaired preimplantation development, including reduced abundance of Igf2/H19 transcripts and impaired trophoblast differentiation. However, even later in pregnancy after normal dietary zinc status is restored, defects in placenta formation, fetal survival, and fetal development persist, suggesting a long-lasting impairment of developmental potential.
Effects of ZD during gestation on fetal development
Zinc is an essential micronutrient known to be of paramount importance for successful fetal development during pregnancy. Severe skeletal and cardiac abnormalities occur when there is not enough zinc in the diet [36, 88, 192–197]. Zinc deficieny during gestation also results in defects in neural tube development as fetal neural progenitor cell proliferation is impaired by decreased phosphorylation of ERK1/2 [198]. However, proliferation of a neuroblast-like cell line challenged with ZD can be recovered with the inhibition of protein phosphatase 2 [198]. While similar abnormalities are observed in embryos that underwent preovulatory ZD or ZD during gestation, whether the same signaling mechanisms are involved is not certain. What is certain however is that ZD disrupts cellular proliferation and increases apoptosis, particularly in cell types that have high proliferation rates, such as along the neural tube. This observation is sensible as zinc serves as a cofactor in many metalloenzymes involved in DNA transcription and protein synthesis [3, 199–201].
Interestingly, the plasma membrane transporter, Znt1, which belongs to the Slc30 family of transporters involved in zinc efflux from the cytoplasm, is essential for embryonic development as mouse homozygous Znt1 knockout (Znt1−/−) in embryos result in death by embryonic day 9 (E9) [202]. While normal embryonic growth occurs during early embryogenesis, morphological changes are observed in the Znt1−/− animals following implantation around embryonic day 7 (E7). These changes are attributed to the failure of zinc passage through the embryo-derived visceral yolk sac and extraembryonic tissues to supply zinc to the embryo [202]. Additionally, zinc supplementation to the mothers of the Znt1−/− mice does not rescue the E7–E9 morphological changes and death, suggesting Znt1 is the critical mediator of zinc flux to the postimplantation embryo [202]. Furthermore, a higher proportion of Znt1+/− heterozygotes display delayed development, and higher rates of offspring with abnormalities occur when the mothers are challenged with ZD during pregnancy [202].
During postimplantation development, the embryo responds to ZD by upregulation of Zip4 in the embryonic visceral yolk sac [203]. Homozygous knockout of Zip4 is embryonically lethal in mice around mid-gestation at E9, while heterozygous embryos were more susceptible to ZD [204]. While the expression of other zinc transporters may also be regulated in response to ZD, Zip1 and Zip5 appear to have similar mRNA content regardless of the maternal zinc content [203].
Zinc deficiency in later but pre-placental embryonic stages has teratogenic effects, such as necrosis and gross malformation, particularly along the neural tube [205–207]. These teratogenic effects are also observed following exposure to chemicals that affect zinc homeostasis [208, 209]. After placenta formation, zinc transporter expression within the tissue responds to dietary zinc uptake; however, these changes do not appear to save the embryo from severe insults of ZD [210].
Zinc and placenta development
The importance of zinc for placenta development and function is relatively unexplored but is potentially significant. There is evidence that both preconception and pregnancy zinc status are important for proper placenta function. Insufficient zinc in the diet could be a major contributing factor in poor placenta development and subsequent adverse pregnancy outcomes. For example, there is a strong association between ZD and risk of pre-eclampsia [39, 211–214], and ZD in pregnant rats during the last third of gestation produces fetuses with smaller brains and livers, while the placentas were less affected [215]. Recent findings demonstrate that marginal ZD causes a compensatory change to the labyrinth zone of the placenta and in maternal cardiovascular adaptations during pregnancy in mice [216]. Recent work in mice shows that preconception ZD for 3–5 days impairs the trophoblast proliferation, implantation, and placenta development [6, 88]. The reduction in placental mass appears to be specific to a reduction in the area occupied by the fetal labyrinth zone, which is also associated with a reduction of the expression of many key placental genes [88].
Maternal to fetal zinc transport is essential for fetal growth and appears to be regulated by the umbilical blood flow [217], the presence of zinc-binding ligands that include albumin [218], metallothionein [219], an unidentified low molecular weight placental zinc-binding protein [220], and a zinc/potassium exchanger [221]. Zinc content is greater in the maternal blood and mid-disk region compared to the fetus [222–224] and so requires active processes of delivering zinc to the fetus. There are 24 known zinc transport proteins, and of these, mRNA for Znt1, Znt2, Znt4, and Znt5 but not Znt3 have been detected in human placentas, with ZnT1 and ZnT5 protein expressed along the apical membrane of the placental syncytiotrophoblast [210, 225]. The Znt1-7 transcripts have also been quantified in mouse placentas, however, Znt3 is lowly expressed and is only detectable at higher PCR cycles [226]. Importantly, the expression of both Znt1 and Znt5 respond to the dietary zinc availability in mouse placentas and may therefore significantly contribute to the maintenance of fetal zinc content [210, 225]. The Zip1 is also differentially expressed in the mouse placenta in response to dietary zinc [210]. While Zip4 is associated with inherited ZD disorders and is detected in the mouse embryonic visceral yolk sack [203], it is not expressed in the human placenta [225]. Given the many roles of the placenta (endocrine, metabolic, and developmental) and the widespread involvement of zinc in these processes, it is likely that we have only begun to understand the importance of zinc in placental development and function.
Perspectives, conclusions, and translational relevance
Although zinc was first recognized as a critical nutrient in mammalian female reproduction in the 1940s [24], recent discoveries over the last decade have greatly expanded our knowledge of the role of zinc in a multitude of reproductive processes, including oocyte growth and maturation, fertilization, epigenetic programing, and subsequent embryonic, fetal and placental development (Figure 4) [6, 10, 76, 87, 88, 104, 125, 127]. The wide-ranging effects of zinc are mediated by the many zinc-binding proteins present in mammalian cells. By one estimate, up to 10% of human proteins are known or predicted to bind zinc ions [227]. It will be a challenge, in the years to come, to sort out the many pathways regulated by zinc ions in the ovary. Nevertheless, it is already clear that the active and dynamic movements of zinc ions, particularly in and out of the oocyte and zygote, are important signals regulating gamete function and embryo development. One of the current most clinically relevant zinc discoveries is the use of measuring zinc efflux during IVF to evaluate the developmental potential of individual oocytes. Assessing activated eggs by the magnitude of their zinc spark events could be a useful tool in selecting embryos with a high likelihood of implantation success. Additionally, supplementation of zinc in the IVM and IVF media for human oocytes has the potential to improve the outcomes of both techniques.
Figure 4.
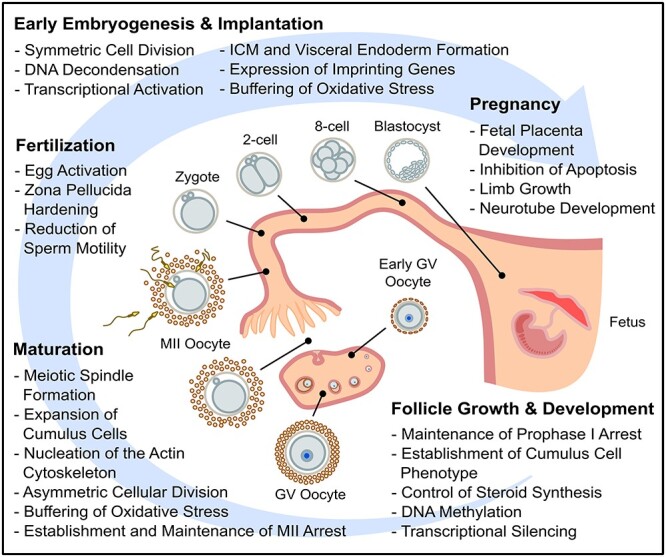
Summary of the events and processes in mammalian female reproduction that zinc is known to play a role.
Given the important functions of zinc in female reproduction, it is not surprising that a lack of zinc in the diet (3–5 days preconception) or during in vitro maturation severely impairs fertility in mice though combined disruption of meiosis, fertilization, preimplantation, and postimplantation development. Likewise, in humans, dietary ZD severely impairs reproduction. The similarities and differences in zinc signaling in rodents versus humans or other mammals remains to be fully revealed, but the extensive commonalities shown in Table 1 strongly suggest that zinc ions are important for multiple conserved reproductive processes. Whether these are an important consideration in human reproduction remains to be determined. Zinc deficiency is common in many parts of the world [22], especially in pregnant women and in disadvantaged populations in the USA and abroad [228–230]. An estimated 82% of pregnant women worldwide do not consume the recommended dietary allowance for zinc [231]. Thus, the mechanisms of zinc action revealed in animals and the prevalence of ZD in humans underscore a pressing need to understand the significance of zinc and the consequences of ZD to human reproductive health.
Conflict of interest
The authors have declared that no conflict of interest exists.
Grant Support: This work was supported, in part, by NIH Grant T32GM108563, Research Training in Physiological Adaptations to Stress.
Contributor Information
Tyler Bruce Garner, Huck Institutes of the Life Sciences, Integrative and Biomedical Physiology Program, The Pennsylvania State University, University Park, PA, USA.
James Malcolm Hester, Huck Institutes of the Life Sciences, Integrative and Biomedical Physiology Program, The Pennsylvania State University, University Park, PA, USA.
Allison Carothers, Huck Institutes of the Life Sciences, Integrative and Biomedical Physiology Program, The Pennsylvania State University, University Park, PA, USA.
Francisco J Diaz, Huck Institutes of the Life Sciences, Integrative and Biomedical Physiology Program, The Pennsylvania State University, University Park, PA, USA; Department of Animal Science, The Pennsylvania State University, University Park, PA, USA.
References
- 1. Swenerton H, Shrader R, Hurley LS. Zinc-deficient embryos: Reduced thymidine incorporation. Science 1969; 166:1014–1015. [DOI] [PubMed] [Google Scholar]
- 2. Duncan JR, Hurley LS. Thymidine kinase and DNA polymerase activity in normal and zinc deficient developing rat embryos. Proc Soc Exp Biol Med 1978; 159:39–43. [DOI] [PubMed] [Google Scholar]
- 3. Chanfreau GF. Zinc’ing down RNA polymerase I. Transcription 2013; 4:217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maret W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv Nutr 2013; 4:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wallwork JC, Duerre JA. Effect of zinc deficiency on methionine metabolism, methylation reactions and protein synthesis in isolated perfused rat liver. J Nutr 1985; 115:252–262. [DOI] [PubMed] [Google Scholar]
- 6. Tian X, Diaz FJ. Acute dietary zinc deficiency before conception compromises oocyte epigenetic programming and disrupts embryonic development. Dev Biol 2013; 376:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koutmos M, Pejchal R, Bomer TM, Matthews RG, Smith JL, Ludwig ML. Metal active site elasticity linked to activation of homocysteine in methionine synthases. Proceedings of the National Academy of Sciences 2008; 105:3286–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loenen WA. S-adenosylmethionine: Jack of all trades and master of everything? Biochem Soc Trans 2006; 34:330–333. [DOI] [PubMed] [Google Scholar]
- 9. Iii BAP, Garrow TA. Random mutagenesis of the zinc-binding motif of betaine-homocysteine Methyltransferase reveals that Gly 214 is essential. Arch Biochem Biophys 2002; 399:73–80. [DOI] [PubMed] [Google Scholar]
- 10. Kim AM, Bernhardt ML, Kong BY, Ahn RW, Vogt S, Woodruff TK, O’Halloran TV. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem Biol 2011; 6:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duncan FE, Que EL, Zhang N, Feinberg EC, O’Halloran TV, Woodruff TK. The zinc spark is an inorganic signature of human egg activation. Sci Rep 2016; 6:24737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liang X, Dempski RE, Burdette SC. Zn(2+) at a cellular crossroads. Curr Opin Chem Biol 2016; 31:120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Besser L, Chorin E, Sekler I, Silverman WF, Atkin S, Russell JT, Hershfinkel M. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J Neurosci 2009; 29:2890–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li YV. Zinc and insulin in pancreatic beta-cells. Endocrine 2014; 45:178–189. [DOI] [PubMed] [Google Scholar]
- 15. Padjasek M, Kocyla A, Kluska K, Kerber O, Tran JB, Krezel A. Structural zinc binding sites shaped for greater works: Structure-function relations in classical zinc finger, hook and clasp domains. J Inorg Biochem 2020; 204:110955. [DOI] [PubMed] [Google Scholar]
- 16. Jeong J, Eide DJ. The SLC39 family of zinc transporters. Mol Aspects Med 2013; 34:612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang L, Tepaamorndech S. The SLC30 family of zinc transporters--a review of current understanding of their biological and pathophysiological roles. Mol Aspects Med 2013; 34:548–560. [DOI] [PubMed] [Google Scholar]
- 18. Schweigel-Röntgen M. The families of zinc (SLC30 and SLC39) and copper (SLC31) transporters. Curr Top Membr 2014; 73:321–355. [DOI] [PubMed] [Google Scholar]
- 19. Hotz C, Brown KH. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 2004; 25:94–204. [PubMed] [Google Scholar]
- 20. Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol 2006; 20:3–18. [DOI] [PubMed] [Google Scholar]
- 21. King JC, Brown KH, Gibson RS, Krebs NF, Lowe NM, Siekmann JH, Raiten DJ. Biomarkers of nutrition for development (BOND)-zinc review. J Nutr 2016; 146:858S–885S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wuehler SE, Peerson JM, Brown KH. Use of national food balance data to estimate the adequacy of zinc in national food supplies: Methodology and regional estimates. Public Health Nutr 2005; 8:812–819. [DOI] [PubMed] [Google Scholar]
- 23. Wessells KR, Brown KH. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One 2012; 7:e50568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Day HG, McCollum EV. Effects of acute dietary zinc deficiency in the rat. Proc Soc Exp Biol Med 1940; 45:282–284. [Google Scholar]
- 25. Sunderman FW Jr. The influence of zinc on apoptosis. Ann Clin Lab Sci 1995; 25:134–142. [PubMed] [Google Scholar]
- 26. Liuzzi JP, Guo L, Yoo C, Stewart TS. Zinc and autophagy. Biometals 2014; 27:1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suzuki T, Katsumata S-I, Matsuzaki H, Suzuki K. Dietary zinc deficiency induces oxidative stress and promotes tumor necrosis factor-a- and interleukin-1ß-induced RANKL expression in rat bone. J Clin Biochem Nutr 2016; 58:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kinlaw WB, Levine AS, Morley JE, Silvis SE, McClain CJ. Abnormal zinc metabolism in type II diabetes mellitus. Am J Med 1983; 75:273–277. [DOI] [PubMed] [Google Scholar]
- 29. Mohammad MK, Zhou Z, Cave M, Barve A, McClain CJ. Zinc and liver disease. Nutr Clin Pract 2012; 27:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brewer GJ. Copper excess, zinc deficiency, and cognition loss in Alzheimer’s disease. Biofactors 2012; 38:107–113. [DOI] [PubMed] [Google Scholar]
- 31. Ranasinghe P, Wathurapatha WS, Ishara MH, Jayawardana R, Galappatthy P, Katulanda P, Constantine GR. Effects of zinc supplementation on serum lipids: A systematic review and meta-analysis. Nutr Metab 2015; 12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boycott KM, Beaulieu CL, Kernohan KD, Gebril OH, Mhanni A, Chudley AE, Redl D, Qin W, Hampson S, Küry S, Tetreault M, Puffenberger EGet al. Autosomal-recessive intellectual disability with cerebellar atrophy syndrome caused by mutation of the manganese and zinc transporter gene SLC39A8. Am J Hum Genet 2015; 97:886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Giunta C, Elçioglu NH, Albrecht B, Eich G, Chambaz C, Janecke AR, Yeowell H, Weis M, Eyre DR, Kraenzlin M, Steinmann B. Spondylocheiro dysplastic form of the Ehlers-Danlos syndrome—An autosomal-recessive entity caused by mutations in the zinc transporter gene SLC39A13. Am J Hum Genet 2008; 82:1290–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chowanadisai W, Lönnerdal B, Kelleher SL. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J Biol Chem 2006; 281:39699–39707. [DOI] [PubMed] [Google Scholar]
- 35. Connolly MP, Ledger W, Postma MJ. Economics of assisted reproduction: Access to fertility treatments and valuing live births in economic terms. Hum Fertil 2010; 13:13–18. [DOI] [PubMed] [Google Scholar]
- 36. Jameson S. Zinc status in pregnancy: The effect of zinc therapy on perinatal mortality, prematurity, and placental ablation. Ann N Y Acad Sci 1993; 678:178–192. [DOI] [PubMed] [Google Scholar]
- 37. Ota E, Mori R, Middleton P, Tobe-Gai R, Mahomed K, Miyazaki C, Bhutta ZA. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev 2015; 2:CD000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abe SK, Balogun OO, Ota E, Takahashi K, Mori R. Supplementation with multiple micronutrients for breastfeeding women for improving outcomes for the mother and baby. Cochrane Database Syst Rev 2016; 2:CD010647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilson RL, Grieger JA, Bianco-Miotto T, Roberts CT. Association between maternal zinc status, dietary zinc intake and pregnancy complications: A systematic review. Nutrients 2016; 8:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Juengel JL, Sawyer HR, Smith PR, Quirke LD, Heath DA, Lun S, Wakefield SJ, McNatty KP. Origins of follicular cells and ontogeny of steroidogenesis in ovine fetal ovaries. Mol Cell Endocrinol 2002; 191:1–10. [DOI] [PubMed] [Google Scholar]
- 41. Choi Y, Rajkovic A. Genetics of early mammalian folliculogenesis. Cell Mol Life Sci 2006; 63:579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology 2007; 148:3580–3590. [DOI] [PubMed] [Google Scholar]
- 43. Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 2001; 234:339–351. [DOI] [PubMed] [Google Scholar]
- 44. Mork L, Maatouk DM, McMahon JA, Guo JJ, Zhang P, McMahon AP, Capel B. Temporal differences in granulosa cell specification in the ovary reflect distinct follicle fates in mice. Biol Reprod 2012; 86:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zheng W, Zhang H, Gorre N, Risal S, Shen Y, Liu K. Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Hum Mol Genet 2014; 23:920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang H, Liu K. Cellular and molecular regulation of the activation of mammalian primordial follicles: Somatic cells initiate follicle activation in adulthood. Hum Reprod Update 2015; 21:779–786. [DOI] [PubMed] [Google Scholar]
- 47. Kim JY. Control of ovarian primordial follicle activation. Clin Exp Reprod Med 2012; 39:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hester J, Hanna-Rose W, Diaz F. Zinc deficiency reduces fertility in C. elegans hermaphrodites and disrupts oogenesis and meiotic progression. Comp Biochem Physiol C Toxicol Pharmacol 2017; 191:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gougeon A. Dynamics of follicular growth in the human: A model from preliminary results. Hum Reprod 1986; 1:81–87. [DOI] [PubMed] [Google Scholar]
- 50. Gougeon A. Regulation of ovarian follicular development in primates: Facts and hypotheses. Endocr Rev 1996; 17:121–155. [DOI] [PubMed] [Google Scholar]
- 51. Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci 2007; 120:1330–1340. [DOI] [PubMed] [Google Scholar]
- 52. Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Dev Biol 2007; 305:300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001; 122:829–838. [DOI] [PubMed] [Google Scholar]
- 54. Russell DL, Robker RL. Molecular mechanisms of ovulation: Co-ordination through the cumulus complex. Hum Reprod Update 2007; 13:289–312. [DOI] [PubMed] [Google Scholar]
- 55. Tiwari M, Prasad S, Tripathi A, Pandey AN, Singh AK, Shrivastav TG, Chaube SK. Involvement of reactive oxygen species in meiotic cell cycle regulation and apoptosis in mammalian oocytes. Reactive Oxygen Species 2016; 1:110–116. [Google Scholar]
- 56. Lisle RS, Anthony K, Randall MA, Diaz FJ. Oocyte-cumulus cell interactions regulate free intracellular zinc in mouse oocytes. Reproduction 2013; 145:381–390. [DOI] [PubMed] [Google Scholar]
- 57. Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum Reprod Update 2008; 14:159–177. [DOI] [PubMed] [Google Scholar]
- 58. Su Y-Q, Sugiura K, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ. Mouse oocytes enable LH-induced maturation of the cumulus-oocyte complex via promoting EGF receptor-dependent signaling. Mol Endocrinol 2010; 24:1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Diaz FJ, Anderson LE, Wu YL, Rabot A, Tsai SJ, Wiltbank MC. Regulation of progesterone and prostaglandin F2a production in the CL. Mol Cell Endocrinol 2002; 191:65–80. [DOI] [PubMed] [Google Scholar]
- 60. Su Y-Q, Wigglesworth K, Pendola FL, O’Brien MJ, Eppig JJ. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology 2002; 143:2221–2232. [DOI] [PubMed] [Google Scholar]
- 61. Su Y-Q, Denegre JM, Wigglesworth K, Pendola FL, O’Brien MJ, Eppig JJ. Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte–cumulus cell complex. Dev Biol 2003; 263:126–138. [DOI] [PubMed] [Google Scholar]
- 62. Jamnongjit M, Gill A, Hammes SR. Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc Natl Acad Sci U S A 2005; 102:16257–16262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: One of two paths to meiotic resumption. Development 2008; 135:3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 2009; 136:1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Park J-Y, Su Y-Q, Ariga M, Law E, Jin S-LC, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 2004; 303:682–684. [DOI] [PubMed] [Google Scholar]
- 66. Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 2007; 27:1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vaccari S, Weeks JL 2nd, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod 2009; 81:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Su Y-Q, Sugiura K, Wigglesworth K, O’Brien MJ, Affourtit JP, Pangas SA, Matzuk MM, Eppig JJ. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 2008; 135:111–121. [DOI] [PubMed] [Google Scholar]
- 69. Sugiura K, Su Y-Q, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, O’Brien MJ, Matzuk MM, Shimasaki S, Eppig JJ. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development 2007; 134:2593–2603. [DOI] [PubMed] [Google Scholar]
- 70. Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ. FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor (s) secreted by the oocyte. Dev Biol 1990; 138:16–25. [DOI] [PubMed] [Google Scholar]
- 71. Fortune JE, Armstrong DT. Androgen production by theca and granulosa isolated from proestrous rat follicles. Endocrinology 1977; 100:1341–1347. [DOI] [PubMed] [Google Scholar]
- 72. Young JM, McNeilly AS. Theca: The forgotten cell of the ovarian follicle. Reproduction 2010; 140:489–504. [DOI] [PubMed] [Google Scholar]
- 73. Orisaka M, Tajima K, Tsang BK, Kotsuji F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J Ovarian Res 2009; 2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tajima K, Orisaka M, Mori T, Kotsuji F. Ovarian theca cells in follicular function. Reproductive BioMedicine Online 2007; 15:591–609. [DOI] [PubMed] [Google Scholar]
- 75. Richards JS, Ren YA, Candelaria N, Adams JE, Rajkovic A. Ovarian follicular theca cell recruitment, differentiation, and impact on fertility: 2017 update. Endocr Rev 2018; 39:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mendoza AD, Woodruff TK, Wignall SM, O’Halloran TV. Zinc availability during germline development impacts embryo viability in Caenorhabditis elegans. Comp Biochem Physiol C Toxicol Pharmacol 2017; 191:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Madl JE, Herman RK. Polyploids and sex determination in Caenorhabditis elegans. Genetics 1979; 93:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Corsi AK, Wightman B, Chalfie MA. Transparent window into biology: A primer on Caenorhabditis elegans. WormBook 2015; 200:387–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hubbard EJA, Greenstein D. Introduction to the germ line. WormBook 2005; 20:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. L’Hernault SW. Spermatogenesis. WormBook 2006; 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Austin J, Kimble J. Glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell 1987; 51:589–599. [DOI] [PubMed] [Google Scholar]
- 82. Bruinsma JJ, Jirakulaporn T, Muslin AJ, Kornfeld K. Zinc ions and cation diffusion facilitator proteins regulate Ras-mediated signaling. Dev Cell 2002; 2:567–578. [DOI] [PubMed] [Google Scholar]
- 83. Yoder JH, Chong H, Guan K-L, Han M. Modulation of KSR activity in Caenorhabditis elegans by Zn ions, PAR-1 kinase and PP2A phosphatase. EMBO J 2004; 23:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lee M-H, Ohmachi M, Arur S, Nayak S, Francis R, Church D, Lambie E, Schedl T. Multiple functions and dynamic activation of MPK-1 extracellular signal-regulated kinase signaling in Caenorhabditis elegans germline development. Genetics 2007; 177:2039–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Church DL, Guan KL, Lambie EJ. Three genes of the MAP kinase cascade, mek-2, mpk-1/Sur-1 and let-60 ras, are required for meiotic cell cycle progression in Caenorhabditis elegans. Development 1995; 121:2525–2535. [DOI] [PubMed] [Google Scholar]
- 86. Kritikou EA, Milstein S, Vidalain P-O, Lettre G, Bogan E, Doukoumetzidis K, Gray P, Chappell TG, Vidal M, Hengartner MO. C. elegans GLA-3 is a novel component of the MAP kinase MPK-1 signaling pathway required for germ cell survival. Genes Dev 2006; 20:2279–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tian X, Diaz FJ. Zinc depletion causes multiple defects in ovarian function during the periovulatory period in mice. Endocrinology 2012; 153:873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tian X, Anthony K, Neuberger T, Diaz FJ. Preconception zinc deficiency disrupts postimplantation fetal and placental development in mice. Biol Reprod 2014; 90:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Peluffo MC, Murphy MJ, Talcott Baughman S, Stouffer RL, Hennebold JD. Systematic analysis of protease gene expression in the rhesus macaque ovulatory follicle: Metalloproteinase involvement in follicle rupture. Endocrinology 2011; 152:3963–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tsafriri A. Ovulation as a tissue remodelling process: Proteolysis and cumulus expansion. Adv Exp Med Biol 1995; 377:121–140. [DOI] [PubMed] [Google Scholar]
- 91. Goldman S, Shalev EMMPS. TIMPS in ovarian physiology and pathophysiology. Front Biosci 2004; 9:2474–2483. [DOI] [PubMed] [Google Scholar]
- 92. Tallant C, Marrero A, Gomis-Rüth FX. Matrix metalloproteinases: Fold and function of their catalytic domains. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2010; 1803:20–28. [DOI] [PubMed] [Google Scholar]
- 93. Berg AH, Rice CD, Rahman MS, Dong J, Thomas P. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: I. discovery in female Atlantic croaker and evidence ZIP9 mediates testosterone-induced apoptosis of ovarian follicle cells. Endocrinology 2014; 155:4237–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li J, Huang D, Sun X, Li X, Cheng CHK. Zinc mediates the action of androgen in acting as a downstream effector of luteinizing hormone on oocyte maturation in zebrafish. Biol Reprod 2018; 100:468–478. [DOI] [PubMed] [Google Scholar]
- 95. Thomas P, Pang Y, Dong J, Berg AH. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis. Endocrinology 2014; 155:4250–4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Converse A, Zhang C, Thomas P. Membrane androgen receptor ZIP9 induces croaker ovarian cell apoptosis via stimulatory G protein alpha subunit and MAP kinase Signaling. Endocrinology 2017; 158:3015–3029. [DOI] [PubMed] [Google Scholar]
- 97. Converse A, Thomas P. The zinc transporter ZIP9 (Slc39a9) regulates zinc dynamics essential to egg activation in zebrafish. Sci Rep 2020; 10:15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tian X, Anthony K, Diaz FJ. Transition metal Chelator induces progesterone production in mouse cumulus-oocyte complexes and corpora Lutea. Biol Trace Elem Res 2017; 176:374–383. [DOI] [PubMed] [Google Scholar]
- 99. Chang H-M, Cheng J-C, Christian Klausen A, PCK L. BMP15 suppresses progesterone production by down-regulating StAR via ALK3 in human granulosa cells. Mol Endocrinol 2013; 27:2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fang L, Chang H-M, Cheng J-C, Leung PCK, Sun Y-P. TGF-ß1 downregulates StAR expression and decreases progesterone production through Smad3 and ERK1/2 Signaling pathways in human granulosa cells. J Clin Endocrinol Metab 2014; 99:E2234–E2243. [DOI] [PubMed] [Google Scholar]
- 101. Miró F, Smyth CD, Hillier SG. Development-related effects of recombinant activin on steroid synthesis in rat granulosa cells. Endocrinology 1991; 129:3388–3394. [DOI] [PubMed] [Google Scholar]
- 102. Kidder GM, Mhawi AA. Gap junctions and ovarian folliculogenesis. Reproduction 2002; 123:613–620. [DOI] [PubMed] [Google Scholar]
- 103. Zhao M-H, Kwon J-W, Liang S, Kim S-H, Li Y-H, Oh J-S, Kim N-H, Cui X-S. Zinc regulates meiotic resumption in porcine oocytes via a protein kinase C-related pathway. PLoS One 2014; 9:e102097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kim AM, Vogt S, O’Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol 2010; 6:674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhang M, Su Y-Q, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 2010; 330:366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Panigone S, Hsieh M, Fu M, Persani L, Conti M. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol 2008; 22:924–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Eppig JJ, Pendola FL, Wigglesworth K. Mouse oocytes suppress cAMP-induced expression of LH receptor mRNA by granulosa cells in vitro. Molecular reproduction and development: Incorporating. Gamete Research 1998; 49:327–332. [DOI] [PubMed] [Google Scholar]
- 108. Mehlmann LM, Jones TLZ, Jaffe LA. Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science 2002; 297:1343–1345. [DOI] [PubMed] [Google Scholar]
- 109. Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science 2004; 306:1947–1950. [DOI] [PubMed] [Google Scholar]
- 110. Hinckley M, Vaccari S, Horner K, Chen R, Conti M. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol 2005; 287:249–261. [DOI] [PubMed] [Google Scholar]
- 111. DiLuigi A, Weitzman VN, Pace MC, Siano LJ, Maier D, Mehlmann LM. Meiotic arrest in human oocytes is maintained by a Gs signaling pathway. Biol Reprod 2008; 78:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Labbé JC, Capony JP, Caput D, Cavadore JC, Derancourt J, Kaghad M, Lelias JM, Picard A, Dorée M. MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO J 1989; 8:3053–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Han SJ, Chen R, Paronetto MP, Conti M. Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol 2005; 15:1670–1676. [DOI] [PubMed] [Google Scholar]
- 114. Albertini DF. Cytoplasmic reorganization during the resumption of meiosis in cultured preovulatory rat oocytes. Dev Biol 1987; 120:121–131. [DOI] [PubMed] [Google Scholar]
- 115. Su Y-Q, Sugiura K, Woo Y, Wigglesworth K, Kamdar S, Affourtit J, Eppig JJ. Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev Biol 2007; 302:104–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang S, Kou Z, Jing Z, Zhang Y, Guo X, Dong M, Wilmut I, Gao S. Proteome of mouse oocytes at different developmental stages. Proc Natl Acad Sci U S A 2010; 107:17639–17644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kong BY, Bernhardt ML, Kim AM, O’Halloran TV, Woodruff TK. Zinc maintains prophase I arrest in mouse oocytes through regulation of the MOS-MAPK pathway. Biol Reprod 2012; 87:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bernhardt ML, Kim AM, O’Halloran TV. Zinc requirement during meiosis I–meiosis II transition in mouse oocytes is independent of the MOS-MAPK pathway. Biology of 2011; 84:526–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Paules RS, Buccione R, Moschel RC, Vande Woude GF, Eppig JJ. Mouse Mos protooncogene product is present and functions during oogenesis. Proc Natl Acad Sci U S A 1989; 86:5395–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Choi T, Fukasawa K, Zhou R, Tessarollo L, Borror K, Resau J, Vande Woude GF. The Mos/mitogen-activated protein kinase (MAPK) pathway regulates the size and degradation of the first polar body in maturing mouse oocytes. Proc Natl Acad Sci U S A 1996; 93:7032–7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Que EL, Bleher R, Duncan FE, Kong BY, Gleber SC, Vogt S, Chen S, Garwin SA, Bayer AR, Dravid VP, Woodruff TK, O’Halloran TV. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat Chem 2015; 7:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kong BY, Duncan FE, Que EL, Kim AM, O’Halloran TV, Woodruff TK. Maternally-derived zinc transporters ZIP6 and ZIP10 drive the mammalian oocyte-to-egg transition. Mol Hum Reprod 2014; 20:1077–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Que EL, Duncan FE, Lee HC, Hornick JE, Vogt S, Fissore RA, O’Halloran TV, Woodruff TK. Bovine eggs release zinc in response to parthenogenetic and sperm-induced egg activation. Theriogenology 2019; 127:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Jo Y-J, Lee I-W, Jung S-M, Kwon J, Kim N-H, Namgoong S. Spire localization via zinc finger–containing domain is crucial for the asymmetric division of mouse oocyte. The FASEB Journal 2019; 33:4432–4447. [DOI] [PubMed] [Google Scholar]
- 125. Que EL, Duncan FE, Bayer AR, Philips SJ, Roth EW, Bleher R, Gleber SC, Vogt S, Woodruff TK, O’Halloran TV. Zinc sparks induce physiochemical changes in the egg zona pellucida that prevent polyspermy. Integr Biol 2017; 9:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Taylor KM, Muraina IA, Brethour D, Schmitt-Ulms G, Nimmanon T, Ziliotto S, Kille P, Hogstrand C. Zinc transporter ZIP10 forms a heteromer with ZIP6 which regulates embryonic development and cell migration. Biochem J 2016; 473:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bernhardt ML, Kong BY, Kim AM, O’Halloran TV. Woodruff TK. A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes. Biol Reprod 2012; 86:114–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gautier J, Minshull J, Lohka M, Glotzer M, Hunt T, Maller JL. Cyclin is a component of maturation-promoting factor from Xenopus. Cell 1990; 60:487–494. [DOI] [PubMed] [Google Scholar]
- 129. Fulka J Jr, Jung T, Moor RM. The fall of biological maturation promoting factor (MPF) and histone H1 kinase activity during anaphase and telophase in mouse oocytes. Mol Reprod Dev 1992; 32:378–382. [DOI] [PubMed] [Google Scholar]
- 130. Suzuki T, Yoshida N, Suzuki E, Okuda E, Perry ACF. Full-term mouse development by abolishing Zn2+−dependent metaphase II arrest without Ca2+ release. Development 2010; 137:2659–2669. [DOI] [PubMed] [Google Scholar]
- 131. Shoji S, Muto Y, Ikeda M, He F, Tsuda K, Ohsawa N, Akasaka R, Terada T, Wakiyama M, Shirouzu M, Yokoyama S. The zinc-binding region (ZBR) fragment of Emi2 can inhibit APC/C by targeting its association with the coactivator Cdc20 and UBE2C-mediated ubiquitylation. FEBS Open Bio 2014; 4:689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ohe M, Kawamura Y, Ueno H, Inoue D, Kanemori Y, Senoo C, Isoda M, Nakajo N, Sagata N. Emi2 inhibition of the anaphase-promoting complex/cyclosome absolutely requires Emi2 binding via the C-terminal RL tail. Mol Biol Cell 2010; 21:905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Severson AF, Dassow G, Bowerman B. Oocyte meiotic spindle assembly and function. Curr Top Dev Biol 2016; 116:65–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mogessie B, Scheffler K, Schuh M. Assembly and positioning of the oocyte meiotic spindle. Annu Rev Cell Dev Biol 2018; 34:381–403. [DOI] [PubMed] [Google Scholar]
- 135. Uraji J, Scheffler K, Schuh M. Functions of actin in mouse oocytes at a glance. J Cell Sci 2018; 131:1–6. [DOI] [PubMed] [Google Scholar]
- 136. Schuh M. An actin-dependent mechanism for long-range vesicle transport. Nat Cell Biol 2011; 13:1431–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Pfender S, Kuznetsov V, Pleiser S, Kerkhoff E, Schuh M. Spire-type actin nucleators cooperate with Formin-2 to drive asymmetric oocyte division. Curr Biol 2011; 21:955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Tittel J, Welz T, Czogalla A, Dietrich S. Membrane targeting of the Spir· formin actin nucleator complex requires a sequential handshake of polar interactions. Journal of Biological 2015; 290:6428–6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Sun L, Chai Y, Hannigan R, Bhogaraju VK, Machaca K. Zinc regulates the ability of Cdc25C to activate MPF/cdk1. J Cell Physiol 2007; 213:98–104. [DOI] [PubMed] [Google Scholar]
- 140. Yu Y, Halet G, Lai FA, Swann K. Regulation of diacylglycerol production and protein kinase C stimulation during sperm-and PLC?-mediated mouse egg activation. Biol Cell 2008; 100:633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhang N, Duncan FE, Que EL, O’Halloran TV, Woodruff TK. The fertilization-induced zinc spark is a novel biomarker of mouse embryo quality and early development. Sci Rep 2016; 6:22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Suzuki T, Suzuki E, Yoshida N, Kubo A, Li H, Okuda E, Amanai M, Perry ACF. Mouse Emi2 as a distinctive regulatory hub in second meiotic metaphase. Development 2010; 137:3281–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lee K, Davis A, Zhang L, Ryu J, Spate LD, Park K-W, Samuel MS, Walters EM, Murphy CN, Machaty Z, Prather RS. Pig oocyte activation using a Zn2+ chelator, TPEN. Theriogenology 2015; 84:1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Macedo MP, Glanzner WG, Rissi VB, Gutierrez K, Currin L, Baldassarre H, Bordignon V. A fast and reliable protocol for activation of porcine oocytes. Theriogenology 2019; 123:22–29. [DOI] [PubMed] [Google Scholar]
- 145. Aonuma S, Okabe M, Kawaguchi M, Kishi Y. Zinc effects on mouse spermatozoa and in-vitro fertilization. J Reprod Fertil 1981; 63:463–466. [DOI] [PubMed] [Google Scholar]
- 146. Tokuhiro K, Dean J. Glycan-Independent Gamete Recognition Triggers Egg Zinc Sparks and ZP2 Cleavage to Prevent Polyspermy. Dev Cell 2018; 46:627–640.e5. [DOI] [PMC free article] [PubMed]
- 147. Andreu-Vieyra CV, Chen R, Agno JE, Glaser S, Anastassiadis K, Stewart AF, Matzuk MM. MLL2 is required in oocytes for bulk histone 3 lysine 4 trimethylation and transcriptional silencing. PLoS Biol 2010; 8:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Picco SJ, Anchordoquy JM, Matos DG, Anchordoquy JP, Seoane A, Mattioli GA, Errecalde AL, Furnus CC. Effect of increasing zinc sulphate concentration during in vitro maturation of bovine oocytes. Theriogenology 2010; 74:1141–1148. [DOI] [PubMed] [Google Scholar]
- 149. Picco SJ, Seoane AI, Anchordoquy JM, Anchordoquy JP, Rosa DE, Fazzio LE, Mattioli GA, Furnus CC. Effect of zinc on DNA integrity of cumulus cells during oocyte in vitro maturation. J Basic Appl Genet 2009; 19:21–25. [Google Scholar]
- 150. Anchordoquy JM, Anchordoquy JP, Sirini MA, Picco SJ, Peral-García P, Furnus CC. The importance of having zinc during in vitro maturation of cattle cumulus--oocyte complex: Role of cumulus cells. Reprod Domest Anim 2014; 49:865–874. [DOI] [PubMed] [Google Scholar]
- 151. Lodde V, Garcia Barros R, Dall’Acqua PC, Dieci C, Robert C, Bastien A, Sirard M-A, Franciosi F, Luciano AM. Zinc supports transcription and improves meiotic competence of growing bovine oocytes. Reproduction 2020; 159:679–691. [DOI] [PubMed] [Google Scholar]
- 152. Pascua AM, Nikoloff N, Carranza AC, Anchordoquy JP, Quintana S, Barbisán G, Díaz S, Anchordoquy JM, Furnus CC. Reproductive hormones influence zinc homeostasis in the bovine cumulus-oocyte complex: Impact on intracellular zinc concentration and transporters gene expression. Theriogenology 2020; 146:48–57. [DOI] [PubMed] [Google Scholar]
- 153. Geravandi S, Azadbakht M, Pourmoradi M, Nowrouzi F. Zinc supplementation of vitrification medium improves in vitro maturation and fertilization of oocytes derived from vitrified-warmed mouse ovaries. Cryobiology 2017; 74:31–35. [DOI] [PubMed] [Google Scholar]
- 154. Yahfoufi ZA, Bai D, Khan SN, Chatzicharalampous C, Kohan-Ghadr H-R, Morris RT, Abu-Soud HM. Glyphosate induces metaphase II oocyte deterioration and embryo damage by zinc depletion and overproduction of reactive oxygen species. Toxicology 2020; 439:152466. [DOI] [PubMed] [Google Scholar]
- 155. Jeon Y, Yoon JD, Cai L, Hwang S-U, Kim E, Zheng Z, Lee E, Kim DY, Hyun S-H. Supplementation of zinc on oocyte in vitro maturation improves preimplatation embryonic development in pigs. Theriogenology 2014; 82:866–874. [DOI] [PubMed] [Google Scholar]
- 156. Xiong X, Lan D, Li J, Lin Y, Zi X. Effects of zinc supplementation during in vitro maturation on meiotic maturation of oocytes and developmental capacity in yak. Biol Trace Elem Res 2018; 185:89–97. [DOI] [PubMed] [Google Scholar]
- 157. Choi Y-H, Gibbons JR, Canesin HS, Hinrichs K. Effect of medium variations (zinc supplementation during oocyte maturation, perifertilization pH, and embryo culture protein source) on equine embryo development after intracytoplasmic sperm injection. Theriogenology 2016; 86:1782–1788. [DOI] [PubMed] [Google Scholar]
- 158. Bishop GM, Dringen R, Robinson SR. Zinc stimulates the production of toxic reactive oxygen species (ROS) and inhibits glutathione reductase in astrocytes. Free Radic Biol Med 2007; 42:1222–1230. [DOI] [PubMed] [Google Scholar]
- 159. Ha K-N, Chen Y, Cai J, Sternberg P. Increased glutathione synthesis through an ARE-Nrf2–dependent pathway by zinc in the RPE: Implication for protection against oxidative stress. Invest Ophthalmol Vis Sci 2006; 47:2709–2715. [DOI] [PubMed] [Google Scholar]
- 160. Cortese MM, Suschek CV, Wetzel W, Kröncke K-D, Kolb-Bachofen V. Zinc protects endothelial cells from hydrogen peroxide via Nrf2-dependent stimulation of glutathione biosynthesis. Free Radic Biol Med 2008; 44:2002–2012. [DOI] [PubMed] [Google Scholar]
- 161. Ma R, Li H, Zhang Y, Lin Y, Qiu X, Xie M, Yao B. The toxic effects and possible mechanisms of Brusatol on mouse oocytes. PLoS One 2017; 12:e0177844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ma R, Liang W, Sun Q, Qiu X, Lin Y, Ge X, Jueraitetibaike K, Xie M, Zhou J, Huang X, Wang Q, Chen L. Sirt1/Nrf2 pathway is involved in oocyte aging by regulating Cyclin B1. Aging 2018; 10:2991–3004. [DOI] [PMC free article] [PubMed]
- 163. Kong BY, Duncan FE, Que EL, Xu Y, Vogt S, O’Halloran TV, Woodruff TK. The inorganic anatomy of the mammalian preimplantation embryo and the requirement of zinc during the first mitotic divisions: Zinc requirement in embryo development. Dev Dyn 2015; 244:935–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Hanna LA, Clegg MS, Momma TY, Daston GP, Rogers JM, Keen CL. Zinc influences the in vitro development of peri-implantation mouse embryos. Birth Defects Res A Clin Mol Teratol 2003; 67:414–420. [DOI] [PubMed] [Google Scholar]
- 165. Hurley LS, Shrader RE. Abnormal development of preimplantation rat eggs after three days of maternal dietary zinc deficiency. Nature 1975; 254:427–429. [DOI] [PubMed] [Google Scholar]
- 166. Peters JM, Wiley LM, Zidenberg-Cherr S, Keen CL. Influence of short-term maternal zinc deficiency on the in vitro development of preimplantation mouse embryos. Proc Soc Exp Biol Med 1991; 198:561–568. [DOI] [PubMed] [Google Scholar]
- 167. Kageyama S-I, Liu H, Kaneko N, Ooga M, Nagata M, Aoki F. Alterations in epigenetic modifications during oocyte growth in mice. Reproduction 2007; 133:85–94. [DOI] [PubMed] [Google Scholar]
- 168. Kim J-M, Liu H, Tazaki M, Nagata M, Aoki F. Changes in histone acetylation during mouse oocyte meiosis. J Cell Biol 2003; 162:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Tomizawa S-I, Nowacka-Woszuk J, Kelsey G. DNA methylation establishment during oocyte growth: Mechanisms and significance. Int J Dev Biol 2012; 56:867–875. [DOI] [PubMed] [Google Scholar]
- 170. Messerschmidt DM, Knowles BB. Solter D. DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev 2014; 28:812–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Kourmouli N, Jeppesen P, Mahadevhaiah S, Burgoyne P, Wu R, Gilbert DM, Bongiorni S, Prantera G, Fanti L, Pimpinelli S, Shi W, Fundele Ret al. Heterochromatin and tri-methylated lysine 20 of histone H4 in animals. J Cell Sci 2004; 117:2491–2501. [DOI] [PubMed] [Google Scholar]
- 172. Morgan H, Santos F, Green K, Dean W, Reik W. Epigenetic reprogramming in mammals. Molecular Genetics 2005; 14:R47–R58. [DOI] [PubMed] [Google Scholar]
- 173. Santos F, Hendrich B, Reik W, Dean W. Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev Biol 2002; 241:172–182. [DOI] [PubMed] [Google Scholar]
- 174. Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science 2001; 293:1089–1093. [DOI] [PubMed] [Google Scholar]
- 175. Senner CE, Krueger F, Oxley D, Andrews S, Hemberger M. DNA methylation profiles define stem cell identity and reveal a tight embryonic--extraembryonic lineage boundary. Stem Cells 2012; 30:2732–2745. [DOI] [PubMed] [Google Scholar]
- 176. Farthing CR, Ficz G, Ng RK, Chan C-F, Andrews S, Dean W, Hemberger M, Reik W. Global mapping of DNA methylation in mouse promoters reveals epigenetic reprogramming of pluripotency genes. PLoS Genet 2008; 4:e1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Hattori N, Nishino K, Ko Y-G, Hattori N, Ohgane J, Tanaka S, Shiota K. Epigenetic control of mouse Oct-4 gene expression in embryonic stem cells and trophoblast stem cells. J Biol Chem 2004; 279:17063–17069. [DOI] [PubMed] [Google Scholar]
- 178. Bartolomei MS, Tilghman SM. Genomic imprinting in mammals. Annu Rev Genet 1997; 31:493–525. [DOI] [PubMed] [Google Scholar]
- 179. Lees-Murdock DJ, De Felici M, Walsh CP. Methylation dynamics of repetitive DNA elements in the mouse germ cell lineage. Genomics 2003; 82:230–237. [DOI] [PubMed] [Google Scholar]
- 180. Walsh CP, Chaillet JR, Bestor TH. Transcription of IAP endogenous retroviruses is constrained by cytosine methylation. Nat Genet 1998; 20:116–117. [DOI] [PubMed] [Google Scholar]
- 181. Nakamura T, Arai Y, Umehara H, Masuhara M, Kimura T, Taniguchi H, Sekimoto T, Ikawa M, Yoneda Y, Okabe M, Tanaka S, Shiota Ket al. PGC7/Stella protects against DNA demethylation in early embryogenesis. Nat Cell Biol 2007; 9:64–71. [DOI] [PubMed] [Google Scholar]
- 182. Messerschmidt DM, Vries W, Ito M, Solter D, Ferguson-Smith A, Knowles BB. Trim28 is required for epigenetic stability during mouse oocyte to embryo transition. Science 2012; 335:1499–1502. [DOI] [PubMed] [Google Scholar]
- 183. Hirasawa R, Chiba H, Kaneda M, Tajima S, Li E, Jaenisch R, Sasaki H. Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development. Genes Dev 2008; 22:1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Matsuzaki H, Okamura E, Takahashi T, Ushiki A, Nakamura T, Nakano T, Hata K, Fukamizu A, Tanimoto K. De novo DNA methylation through the 5′-segment of the H19 ICR maintains its imprint during early embryogenesis. Development 2015; 142:3833–3844. [DOI] [PubMed] [Google Scholar]
- 185. Stewart KR, Veselovska L, Kim J, Huang J, Saadeh H, Tomizawa S-I, Smallwood SA, Chen T, Kelsey G. Dynamic changes in histone modifications precede de novo DNA methylation in oocytes. Genes Dev 2015; 29:2449–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Jenkins TG, Aston KI, Pflueger C, Cairns BR, Carrell DT. Age-associated sperm DNA methylation alterations: Possible implications in offspring disease susceptibility. PLoS Genet 2014; 10:e1004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Lambrot R, Xu C, Saint-Phar S, Chountalos G, Cohen T, Paquet M, Suderman M, Hallett M, Kimmins S. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat Commun 2013; 4:2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Kappil MA, Green BB, Armstrong DA, Sharp AJ, Lambertini L, Marsit CJ, Chen J. Placental expression profile of imprinted genes impacts birth weight. Epigenetics 2015; 10:842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Novakovic B, Saffery R. The ever growing complexity of placental epigenetics--role in adverse pregnancy outcomes and fetal programming. Placenta 2012; 33:959–970. [DOI] [PubMed] [Google Scholar]
- 190. Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A 2007; 104:13056–13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Halas ES, Hunt CD, Eberhardt MJ. Learning and memory disabilities in young adult rats from mildly zinc deficient dams. Physiol Behav 1986; 37:451–458. [DOI] [PubMed] [Google Scholar]
- 192. Blamberg DL, Blackwood UB, Supplee WC, Combs GF. Effect of zinc deficiency in hens on hatchability and embryonic development. Proc Soc Exp Biol Med 1960; 104:217–220. [DOI] [PubMed] [Google Scholar]
- 193. Shah D, Sachdev HP. Effect of gestational zinc deficiency on pregnancy outcomes: Summary of observation studies and zinc supplementation trials. Br J Nutr 2001; 85:S101–S108. [DOI] [PubMed] [Google Scholar]
- 194. Keen CL, Clegg MS, Hanna LA, Lanoue L, Rogers JM, Daston GP, Oteiza P, Uriu-Adams JY. The plausibility of micronutrient deficiencies being a significant contributing factor to the occurrence of pregnancy complications. J Nutr 2003; 133:1597S–1605S. [DOI] [PubMed] [Google Scholar]
- 195. Uriu-Adams JY, Keen CL. Zinc and reproduction: Effects of zinc deficiency on prenatal and early postnatal development. Birth Defects Res B Dev Reprod Toxicol 2010; 89:313–325. [DOI] [PubMed] [Google Scholar]
- 196. Apgar J. Zinc and reproduction. Annu Rev Nutr 1985; 5:43–68. [DOI] [PubMed] [Google Scholar]
- 197. Liu C, He X, Hong X, Kang F, Chen S, Wang Q, Chen X, Hu D, Sun Q. Suppression of placental metallothionein 1 and zinc transporter 1 mRNA expressions contributes to fetal heart malformations caused by maternal zinc deficiency. Cardiovasc Toxicol 2014; 14:329–338. [DOI] [PubMed] [Google Scholar]
- 198. Nuttall JR, Supasai S, Kha J, Vaeth BM, Mackenzie GG, Adamo AM, Oteiza PI. Gestational marginal zinc deficiency impaired fetal neural progenitor cell proliferation by disrupting the ERK1/2 signaling pathway. J Nutr Biochem 2015; 26:1116–1123. [DOI] [PubMed] [Google Scholar]
- 199. Vallee BL, Auld DS. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry 1990; 29:5647–5659. [DOI] [PubMed] [Google Scholar]
- 200. Coleman JE. Zinc enzymes. Curr Opin Chem Biol 1998; 2:222–234. [DOI] [PubMed] [Google Scholar]
- 201. Ahn YH, Kim YH, Hong SH, Koh JY. Depletion of intracellular zinc induces protein synthesis-dependent neuronal apoptosis in mouse cortical culture. Exp Neurol 1998; 154:47–56. [DOI] [PubMed] [Google Scholar]
- 202. Andrews GK, Wang H, Dey SK, Palmiter RD. Mouse zinc transporter 1 gene provides an essential function during early embryonic development. Genesis 2004; 40:74–81. [DOI] [PubMed] [Google Scholar]
- 203. Dufner-Beattie J, Kuo Y-M, Gitschier J, Andrews GK. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5. J Biol Chem 2004; 279:49082–49090. [DOI] [PubMed] [Google Scholar]
- 204. Dufner-Beattie J, Weaver BP, Geiser J, Bilgen M, Larson M, Xu W, Andrews GK. The mouse acrodermatitis enteropathica gene Slc39a4 (Zip4) is essential for early development and heterozygosity causes hypersensitivity to zinc deficiency. Hum Mol Genet 2007; 16:1391–1399. [DOI] [PubMed] [Google Scholar]
- 205. Hurley LS, Gowan J, Swenerton H. Teratogenic effects of short-term and transitory zinc deficiency in rats. Teratology 1971; 4:199–204. [Google Scholar]
- 206. Hurley LS, Swenerton H. Congenital malformations resulting from zinc deficiency in rats. Proc Soc Exp Biol Med 1966; 123:692–696. [DOI] [PubMed] [Google Scholar]
- 207. Record IR, Tulsi RS, Dreosti IE, Fraser FJ. Cellular necrosis in zinc-deficient rat embryos. Teratology 1985; 32:397–405. [DOI] [PubMed] [Google Scholar]
- 208. Peters JM, Taubeneck MW, Keen CL, Gonzalez FJ. Di (2-Ethylhexyl) phthalate induces a functional zinc deficiency during pregnancy and teratogenesis that is independent of peroxisome proliferator-activated receptor. Teratology 1997; 56:311–316. [DOI] [PubMed] [Google Scholar]
- 209. Lee J, Park J, Jang B, Knudsen TB. Altered expression of genes related to zinc homeostasis in early mouse embryos exposed to di-2-ethylhexyl phthalate. Toxicol Lett 2004; 152:1–10. [DOI] [PubMed] [Google Scholar]
- 210. Helston RM, Phillips SR, McKay JA, Jackson KA, Mathers JC, Ford D. Zinc transporters in the mouse placenta show a coordinated regulatory response to changes in dietary zinc intake. Placenta 2007; 28:437–444. [DOI] [PubMed] [Google Scholar]
- 211. Zhu Q, Zhang L, Chen X, Zhou J, Liu J, Chen J. Association between zinc level and the risk of preeclampsia: A meta-analysis. Arch Gynecol Obstet 2016; 293:377–382. [DOI] [PubMed] [Google Scholar]
- 212. Ferdousi S, Akhtar S, Begum S. Copper and zinc status in patients with preeclampsia in Bangladesh. Mymensingh Med J 2015; 24:780–786. [PubMed] [Google Scholar]
- 213. Bilodeau-Goeseels S. Bovine oocyte meiotic inhibition before in vitro maturation and its value to in vitro embryo production: Does it improve developmental competence? Reprod Domest Anim 2012; 47:687–693. [DOI] [PubMed] [Google Scholar]
- 214. Wu Y-L, Wiltbank MC. Transcriptional regulation of the cyclooxygenase-2 gene changes from protein kinase (PK) A- to PKC-dependence after luteinization of granulosa cells. Biol Reprod 2002; 66:1505–1514. [DOI] [PubMed] [Google Scholar]
- 215. McKenzie JM, Fosmire GJ, Sandstead HH. Zinc deficiency during the latter third of pregnancy: Effects on Fetal rat brain, liver, and Placenia. J Nutr 1975; 105:1466–1475. [DOI] [PubMed] [Google Scholar]
- 216. Wilson RL, Leemaqz SY, Goh Z, McAninch D, Jankovic-Karasoulos T, Leghi GE, Phillips JA, Colafella KM, Tran C, O’Leary S, Buckberry S, Pederson Set al. Zinc is a critical regulator of placental morphogenesis and maternal hemodynamics during pregnancy in mice. Sci Rep 2017; 7:15137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Simmer K, Dwight JS, Brown IM, Thompson RP, Young M. Placental handling of zinc in the Guinea pig. Biol Neonate 1985; 48:114–121. [DOI] [PubMed] [Google Scholar]
- 218. Paterson PG, Mas A, Sarkar B, Zlotkin SH. The influence of zinc-binding ligands in fetal circulation on zinc clearance across the in situ perfused Guinea pig placenta. J Nutr 1991; 121:338–344. [DOI] [PubMed] [Google Scholar]
- 219. Goyer RA, Haust MD, Cherian MG. Cellular localization of metallothionein in human term placenta. Placenta 1992; 13:349–355. [DOI] [PubMed] [Google Scholar]
- 220. Honey S, Dhall GI, Nath R. Purification and characterization of a low molecular weight zinc binding protein from human placenta. Mol Cell Biochem 1994; 136:77–83. [DOI] [PubMed] [Google Scholar]
- 221. Aslam N, McArdle HJ. Mechanism of zinc uptake by microvilli isolated from human term placenta. J Cell Physiol 1992; 151:533–538. [DOI] [PubMed] [Google Scholar]
- 222. Manci EA, Blackburn WR. Regional variations in the levels of zinc, iron, copper, and calcium in the term human placenta. Placenta 1987; 8:497–502. [DOI] [PubMed] [Google Scholar]
- 223. Herman Z, Greeley S, King JC. Placenta and maternal effects of marginal zinc deficiency during gestation in rats. Nutr Res 1985; 5:211–219. [Google Scholar]
- 224. Tsuchiya H, Mitani K, Kodama K, Nakata T. Placental transfer of heavy metals in normal pregnant Japanese women. Arch Environ Health 1984; 39:11–17. [DOI] [PubMed] [Google Scholar]
- 225. Ford D. Intestinal and placental zinc transport pathways. Proc Nutr Soc 2004; 63:21–29. [DOI] [PubMed] [Google Scholar]
- 226. Asano N, Kondoh M, Ebihara C, Fujii M, Nakanishi T, Soares MJ, Nakashima E, Tanaka K, Sato M, Watanabe Y. Expression profiles of zinc transporters in rodent placental models. Toxicol Lett 2004; 154:45–53. [DOI] [PubMed] [Google Scholar]
- 227. Andreini C, Banci L, Bertini I, Rosato A. Counting the zinc-proteins encoded in the human genome. J Proteome Res 2006; 5:196–201. [DOI] [PubMed] [Google Scholar]
- 228. Torheim LE, Ferguson EL, Penrose K, Arimond M. Women in resource-poor settings are at risk of inadequate intakes of multiple micronutrients. J Nutr 2010; 140:2051S–2058S. [DOI] [PubMed] [Google Scholar]
- 229. Schneider JM, Fujii ML, Lamp CL, Lönnerdal B, Zidenberg-Cherr S. The prevalence of low serum zinc and copper levels and dietary habits associated with serum zinc and copper in 12-to 36-month-old children from low-income families at risk for iron deficiency. J Am Diet Assoc 2007; 107:1924–1929. [DOI] [PubMed] [Google Scholar]
- 230. Rahman S, Ahmed T, Rahman AS, Alam N, Ahmed AMS, Ireen S, Chowdhury IA, Chowdhury FP, Rahman SMM. Status of zinc nutrition in Bangladesh: The underlying associations. J Nutr Sci 2016; 5:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Caulfield LE, Zavaleta N, Shankar AH, Merialdi M. Potential contribution of maternal zinc supplementation during pregnancy to maternal and child survival. Am J Clin Nutr 1998; 68:499S–508S. [DOI] [PubMed] [Google Scholar]
- 232. McGinnis LA, Lee HJ, Robinson DN. MAPK3/1 (ERK1/2) and myosin light chain kinase in mammalian eggs affect myosin-II function and regulate the metaphase II state in a calcium-and zinc-dependent manner. Biology of 2015; 92:146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Bleher R, Gleber SC, Vogt S, Woodruff TK. Zinc sparks induce physiochemical changes in the egg zona pellucida that prevent polyspermy. Integrative 2017; 9:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Zhao M-H, Kim N-H, Cui X-S. Zinc depletion activates porcine metaphase II oocytes independently of the protein kinase C pathway. In Vitro Cell Dev Biol Anim 2014; 50:945–951. [DOI] [PubMed] [Google Scholar]
- 235. Jeon Y, Yoon JD, Cai L, Hwang S-U, Kim E, Zheng Z, Jeung E, Lee E, Hyun S-H. Zinc deficiency during in vitro maturation of porcine oocytes causes meiotic block and developmental failure. Mol Med Rep 2015; 12:5973–5982. [DOI] [PubMed] [Google Scholar]
- 236. Carpenter MC, Lo MN, Palmer AE. Techniques for measuring cellular zinc. Arch Biochem Biophys 2016; 611:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Pratt EPS, Damon LJ, Anson KJ, Palmer AE. Tools and techniques for illuminating the cell biology of zinc. Biochim Biophys Acta Mol Cell Res 1868; 2021:118865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Lee HC, Edmonds ME, Duncan FE, O’Halloran TV, Woodruff TK. Zinc exocytosis is sensitive to myosin light chain kinase inhibition in mouse and human eggs. Mol Hum Reprod 2020; 26:228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Hu Q, Duncan F, Nowakowski AB, Antipova OA, Woodruff TK, O’Halloran TV, Wolfner MF. Zinc dynamics during drosophila oocyte maturation and egg activation. IScience 2020; 23:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Wozniak KL, Bainbridge RE, Summerville DW, Tembo M, Phelps WA, Sauer ML, Wisner BW, Czekalski ME, Pasumarthy S, Hanson ML, Linderman MB, Luu CHet al. Zinc protection of fertilized eggs is an ancient feature of sexual reproduction in animals. PLoS Biol 2020; 18:e3000811. [DOI] [PMC free article] [PubMed] [Google Scholar]


