Abstract
Objectives
Recent guidelines advise limiting opioid prescriptions for acute pain to a three-day supply; however, scant literature quantifies opioid use patterns after an emergency department (ED) visit. We sought to describe opioid consumption patterns after an ED visit for acute pain.
Design
Descriptive study with data derived from a larger interventional study promoting safe opioid use after ED discharge.
Setting
Urban academic emergency department (>88,000 annual visits).
Subjects
Patients were eligible if age >17 years, not chronically using opioids, and newly prescribed hydrocodone-acetaminophen and were included in the analysis if they returned the completed 10-day medication diary.
Methods
Patient demographics and opioid consumption are reported. Opioid use is described in daily number of pills and daily morphine milligram equivalents (MME) both for the sample overall and by diagnosis.
Results
Two hundred sixty patients returned completed medication diaries (45 [17%] back pain, 52 [20%] renal colic, 54 [21%] fracture/dislocation, 40 [15%] musculoskeletal injury [nonfracture], and 69 [27%] “other”). The mean age (SD) was 45 (15) years, and 59% of the sample was female. A median of 12 pills were prescribed. Patients with renal colic used the least opioids (total pills: median [interquartile range {IQR}] = 3 [1–7]; total MME: median [IQR] = 20 [10–50]); patients with back pain used the most (total pills: median [IQR] = 12 [7–16]; total MME: median [IQR] = 65 [47.5–100]); 92.5% of patients had leftover pills.
Conclusions
In this sample, pill consumption varied by illness category; however, overall, patients were consuming low quantities of pills, and the majority had unused pills 10 days after their ED visit.
Keywords: Opioid, Pain Management, Emergency Department
Introduction
The ongoing opioid epidemic in the United States has resulted in efforts at the national, state, and health system levels to curb unnecessary prescription opioids. Nearly half of all states adopted prescribing limits for acute pain [1], and recently published guidelines from the Centers for Disease Control and Prevention (CDC) make a “category A” recommendation that, when prescribing opioids for acute pain, “three days or less will often be sufficient; more than seven days will rarely be needed” [2]. The three-day supply recommendation was based on previously published guidelines and expert opinion (“based on clinical experience”) with a goal of controlling pain while simultaneously minimizing the hazards of unnecessary opioid exposure or overprescribing (e.g., increased likelihood of physical dependence, opioid diversion).
The CDC guidelines recommend prescribing “no greater quantity than needed for the expected duration of pain severe enough to require opioids” [2]. However, the quantity is determined by expected pain duration rather than both duration and frequency of dosing. For example, if an opioid analgesic is prescribed with a dosing frequency of “every four hours as needed for pain,” some patients may dose once daily, taking only three pills in the first three days, whereas others will dose every four hours, taking 18 pills in the same time frame, a sixfold difference. Prescribing based on duration, but not frequency of use, may unintentionally contribute to overprescribing and excess opioids. These excess opioids are rarely disposed of properly [3,4], increasing the risk of exposure to children, other household members, and friends [3,5–7].
Acute care clinicians may be overtreating pain (with risk of diversion and misuse, as described above) or may be undertreating pain based on these three-day guidelines. The problem of treating acute pain extends beyond the emergency department (ED) and involves immediate care centers as well as physicians and advance care providers in multiple other specialties (e.g., trauma surgery, urology, orthopedics, internal medicine). Given these concerns and the limited strength of data on which the days’ supply–based guidelines were developed, there is a clear need to formulate quantity-based opioid prescribing guidelines. Yet, there is a paucity of available literature on which to formulate quantity-based recommendations after an encounter for acutely painful diagnoses. We therefore sought to characterize actual pill consumption trajectories by major diagnosis group in order to better inform prescribing guidelines and practices.
Methods
Study Setting and Participants
These data are part of a larger study at an urban academic ED (>88,000 annual visits) investigating a multifaceted strategy to improve patients’ understanding and safe use of prescription opioid pain relievers [8]. The larger study from which these data were derived was a randomized controlled trial and is registered at clinicaltrials.gov (NCT02431793). The data reported herein are not the results of the trial, but rather a subanalysis. As part of that study, patients completed a 10-day medication diary documenting their medication consumption (including both analgesics and other home medications). The medication diary is the data source for this analysis.
Patients were enrolled in the larger study if they were English speaking, age >17 years, received a prescription for hydrocodone-acetaminophen, and did not chronically use opioids. Chronic use of opioids was based on self-report of daily or near daily use of opioids for the past 90 days [9]. Patients were included in this analysis if they returned the completed medication diary. The institutional review board approved all study procedures, and participants provided written, informed consent.
Study Protocol and Variables
As part of the larger study, patients were randomized to receive either standard care or additional information about opioids. Patients in the intervention arms received information on multiple topics related to the safe use of opioids (e.g., avoiding concomitant sedating medications, avoiding double-dipping with acetaminophen, safe disposal); however, none of the educational information contained specific advice about medication dosing or frequency of use. Patients in the intervention arm also had changes made to the wording of their label (Take-Wait-Stop) to make the label more patient-centered and encourage safe use [8,10]. The goal of these changes was not to decrease opioid use, but rather to encourage safe use in compliance with the prescription instructions (e.g., not exceeding recommended daily dosage).
The medication diary instructions asked patients to document all medications taken (including prescription and over-the-counter medications, as well as their chronic daily home medications). In addition to recording their medication(s), patients were asked to report a pain score at the time of the analgesic dose using the 0–10 numeric pain rating scale. Patients who did not take any analgesics in a given day did not record pain scores. The patients were instructed to start the diary immediately following discharge, and therefore the first “day” of completed entries represents the day of discharge (and may have been a partial day depending upon time of discharge).
At the time of enrollment, patients were provided with a paid envelope to return the medication diary. Medication diary details were transcribed by trained research assistants from the paper format into the electronic Research Electronic Data Capture (REDCap) database [11].
Demographic variables included age, race, gender, education, literacy level, income, insurance status, self-reported health status, and prior exposure to opioids (self-report). Literacy level was measured using the Newest Vital Sign (NVS) [12]. Visit characteristics included first and last recorded pain scores (0–10), ED visit length of stay, exposure to opioids during the ED visit, and discharge diagnosis. Opioid prescription characteristics included morphine milligram equivalents (MME) and number of pills prescribed.
Outcome Measurement
The primary outcome was the number of hydrocodone-acetaminophen pills (and corresponding MME) consumed each day after discharge by diagnosis. We focused solely on hydrocodone-acetaminophen, given that this is the predominating opioid type prescribed at our institution. Secondary measures used to illustrate dosing patterns included the proportion of patients in each diagnosis group using opioids on each postdischarge day and the mean days on which patients in each diagnosis group took opioid pills. To most accurately describe the overall analgesic use in this population, the use of nonsteroidal anti-inflammatory medications (NSAIDs), acetaminophen, and muscle relaxants (both skeletal muscle relaxants and benzodiazepines) is also reported.
Additionally, we sought to characterize the number of pills per diagnosis that would satisfy the requirements of 80% and 95% of the study population. Hill et al., in the surgical literature, recommended prescribing a pill quantity by diagnosis that would “satisfy the opioid requirements” of 80% of the population to balance opioid risk against analgesic needs and the potential inconvenience of returning for a refill [13]. In a single-center study of Canadian ED patients, Daoust et al. replicated Hill et al.’s 80% analysis for different categories of ED diagnoses and also calculated quantities that would satisfy the opioid requirements of 95% of all ED patients for the first three days [14]. However, this analysis used a combination of phone reports of pill use and medication diaries and rounded up (e.g., 1–5 pills consumed = 5) before calculations. The 95% number was calculated for all diagnoses combined, but not for individual diagnosis categories. Building upon this prior work, we calculated the pill quantity that would satisfy both 80% and 95% of patients’ opioid requirements for the first three days after discharge (defined as the day of discharge [partial day] and first three full days).
Analysis
Descriptive statistics were calculated for sociodemographic characteristics and descriptors of the ED visit. Chi-square, one-way analysis of variance (ANOVA), and Wilcoxon rank-sum tests were used, as appropriate, to test for differences between participants who returned medication diaries and those who did not. Pill quantities are presented as median (interquartile range [IQR]) and mean (SD), as appropriate, based on the data distribution. Although the intervention described above was not designed to directly influence medication use frequency, we additionally evaluated for any influence of the intervention on the study outcomes using bivariate analysis and the chi-square test as appropriate. All analyses were performed using SAS 9.4.
Results
Sample
A total of 652 patients were enrolled. Two hundred sixty patients returned medication diaries and were eligible for analysis; 57.1% were female, with a mean age of 46 years (Table 1). Participants returning medication diaries differed significantly from the overall sample enrolled. Participants who returned diaries were older, with higher educational attainment, literacy, and household earnings, and were less likely to be uninsured or from a racial/ethnic minority. Nearly three-quarters of the sample who returned diaries had adequate health literacy, and over a third of the sample had been previously prescribed hydrocodone (although, per inclusion criteria, they were not taking it on a daily or near daily basis before enrollment). The sample was fairly evenly split between diagnosis categories: back pain (N = 45, 17.3%,), renal colic (N = 52, 20.0%), fracture/dislocation (N = 54, 20.8%), musculoskeletal injury (nonfracture; N = 40, 15.4%), and other diagnoses (N = 69, 26.5%).
Table 1.
Participant demographic and ED visit characteristics
| Characteristic | Study Sample (Returned Medication Diary) | Excluded (Did Not Return Medication Diary) | P Value |
|---|---|---|---|
| N = 260 | N = 392 | ||
| Demographic characteristics | |||
| Age, mean (SD), y | 45.0 (14.5) | 40.4 (13.2) | <0.05 |
| Female gender, % | 59.2 | 55.6 | 0.36 |
| Race, % | <0.05 | ||
| White | 57.7 | 40.6 | |
| African American | 25.4 | 35.2 | |
| Other | 16.9 | 23.9 | |
| Education, % | <0.05 | ||
| High school grad or less | 13.1 | 21.2 | |
| Some college | 28.1 | 34.3 | |
| College graduate | 33.5 | 29.9 | |
| Graduate degree | 25.4 | 14.6 | |
| Income level, %+ | <0.05 | ||
| ≤$40,000 | 22.3 | 36.1 | |
| >$40,000–$100,000 | 33.9 | 35.2 | |
| >$100,000 | 43.8 | 28.7 | |
| Health literacy, % | <0.05 | ||
| Low+marginal | 28.5 | 37.0 | |
| Adequate | 71.5 | 63.0 | |
| Primary insurance, %++ | <0.05 | ||
| Medicaid | 12.6 | 21.8 | |
| Medicare | 7.5 | 5.7 | |
| Private/managed care | 72.8 | 58.6 | |
| Self- or no insurance | 3.2 | 8.8 | |
| Other | 3.9 | 5.2 | |
| Self-reported health status, % | <0.05 | ||
| Excellent | 16.2 | 14.1 | |
| Very good | 38.1 | 34.5 | |
| Good | 34.2 | 30.1 | |
| Fair | 9.2 | 19.3 | |
| Poor | 2.3 | 2.1 | |
| Previously prescribed hydrocodone, %+ | 40.3 | 38.01 | 0.57 |
| ED visit characteristics | |||
| Triage acuity, % | 0.78 | ||
| 1 & 2 | 8.2 | 8.7 | |
| 3 | 57.0 | 54.2 | |
| 4 & 5 | 34.8 | 37.1 | |
| Triage pain score, mean (SD) | 7.6 (2.3) | 7.7 (2.3) | 0.44 |
| Total length of stay, median (IQR), h | 4.2 (3.1–5.4) | 3.7 (2.7–5.1) | <0.05 |
| Exposure to opioids in the ED, % | 36.2 | 33.7 | 0.51 |
ED = emergency department; IQR = interquartile range.
Pain Scores
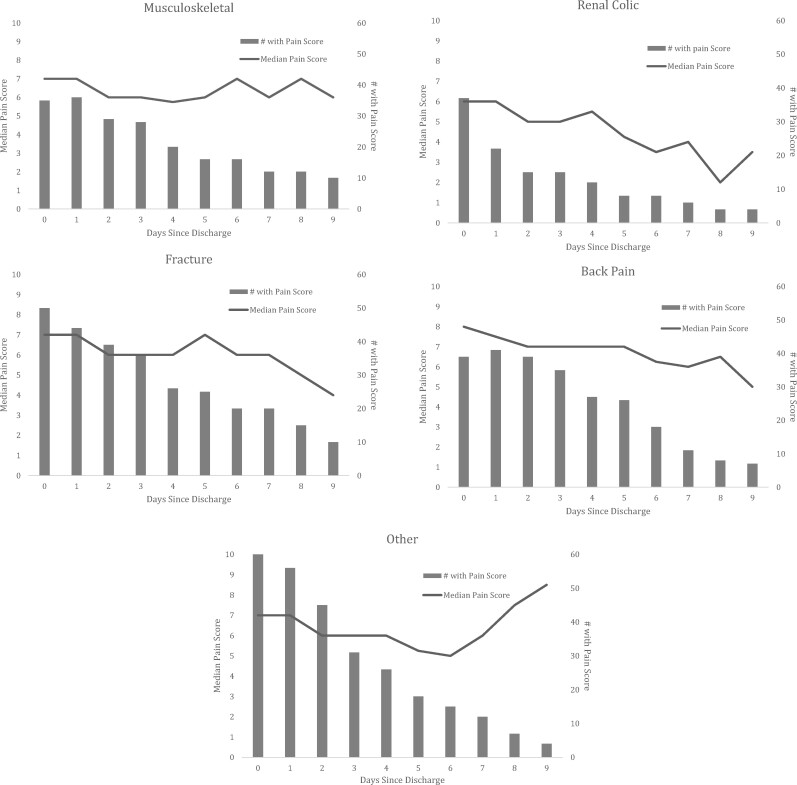
Pain scores for the sample were only recorded on those patients continuing to dose their opioid analgesics. Among that group, pain scores showed a slow but steady decline throughout the 10-day medication dairy, with the exception of the “other” diagnosis group, wherein pain scores of those continuing to dose their medication first decreased before returning to and exceeding baseline levels (day of discharge: median = 7; postdischarge day 6: median = 5; postdischarge day 9: median = 8.5) (Figure 1). Patients with back pain had the highest median pain scores (IQR) on the day of discharge (8 [6.5–9]), and renal colic patients the lowest (6 [3–7]) (Supplementary Data).
Figure 1.
Pain scores by diagnosis over time for patients continuing to use analgesics. Only those patients taking an analgesic on a given day reported a pain score. The number of patients included each day is noted with the bar graph, and the pain score change overtime is represented with the line graph.
Prescription Characteristics
The median quantity of opioid pills prescribed (IQR) was 12 (12–20). The median MME prescribed (IQR) was 90 (60–120). Both the number of pills and MME prescribed varied by diagnosis (Table 2), with renal colic diagnoses receiving the greatest MME (120) and musculoskeletal injuries receiving the least MME (60).
Table 2.
Opioid use, by diagnosis
| Total | Back Pain | Renal Colic | Fracture/Dislocation | Musculoskeletal Injury | Other | |
|---|---|---|---|---|---|---|
| (N = 260) | (N = 45) | (N = 52) | (N = 54) | (N = 40) | (N = 69) | |
| Tabs dispensed, median (IQR) | 12 (12–20) | 12 (10–20) | 14.5 (12–24) | 12 (12–24) | 12 (10–18) | 12 (12–18) |
| MME dispensed, median (IQR) | 90 (60–120) | 80 (60–120) | 120 (67.5–135) | 95 (60–120) | 60 (60–120) | 70 (60–120) |
| Total MME left, median (IQR) | 50 (20–90) | 35 (5–60) | 75 (50–120) | 45 (20–65) | 35 (15–60) | 40 (20–100) |
| Proportion of patients taking opioid by medication diary day, % | ||||||
| Day of discharge | 86.2 | 88.9 | 71.2 | 92.6 | 90.0 | 88.4 |
| Post-DC day 1 | 77.3 | 91.1 | 44.2 | 81.5 | 90.0 | 82.6 |
| Post DC day 2 | 65.0 | 86.7 | 30.8 | 72.2 | 75.0 | 65.2 |
| Post-DC day 3 | 57.7 | 80.0 | 30.8 | 68.5 | 72.5 | 46.4 |
| Post-DC day 4 | 43.5 | 62.2 | 25.0 | 48.1 | 50.0 | 37.7 |
| Post-DC day 5 | 36.5 | 57.8 | 17.3 | 48.1 | 40.0 | 26.1 |
| Post-DC day 6 | 30.8 | 42.2 | 17.3 | 38.9 | 40.0 | 21.7 |
| Post-DC day 7 | 24.2 | 26.7 | 13.5 | 37.0 | 30.0 | 17.4 |
| Post-DC day 8 | 18.5 | 17.8 | 7.7 | 29.6 | 30.0 | 11.6 |
| Post-DC day 9 | 13.8 | 15.6 | 7.7 | 18.5 | 25.0 | 7.2 |
| Tabs taken per day among those consuming any opioid, mean (SD)* | ||||||
| Day of discharge | 1.7 (1.0) | 2.1 (1.2) | 1.4 (0.7) | 1.7 (1.0) | 1.8 (0.8) | 1.6 (0.9) |
| Post-DC day 1 | 2.3 (1.2) | 2.6 (1.4) | 2.0 (1.2) | 2.4 (0.9) | 1.9 (1.2) | 2.2 (1.2) |
| Post DC day 2 | 2.3 (1.4) | 2.6 (1.4) | 2.2 (1.4) | 2.4 (2.0) | 2.0 (1.2) | 2.0 (1.1) |
| Post-DC day 3 | 2.0 (1.3) | 2.2 (1.1) | 1.8 (1.5) | 2.2 (1.8) | 1.6 (0.9) | 2.2 (1.3) |
| Post-DC day 4 | 2.0 (1.3) | 2.1 (1.2) | 1.6 (0.7) | 2.4 (1.5) | 1.6 (0.9) | 1.8 (1.0) |
| Post-DC day 5 | 1.9 (1.1) | 2.0 (1.2) | 1.5 (0.9) | 1.8 (1.0) | 1.7 (0.9) | 2.0 (0.9) |
| Post-DC day 6 | 1.8 (1.2) | 2.0 (1.2) | 1.8 (1.2) | 1.9 (1.7) | 15 (0.8) | 1.6 (0.8) |
| Post-DC day 7 | 1.7 (1.3) | 2.1 (2.0) | 1.3 (0.5) | 1.9 (1.3) | 1.5 (0.8) | 1.5 (0.7) |
| Post-DC day 8 | 1.6 (1.2) | 2.0 (1.6) | 1.0 (0.0) | 1.8 (1.4) | 1.5 (0.8) | 1.3 (0.5) |
| Post-DC day 9 | 1.6 (1.1) | 1.8 (1.2) | 0.8 (0.4) | 1.9 (1.4) | 1.6 (1.0) | 1.3 (0.5) |
| Days on which an opioid was consumed, median (IQR)* | 4 (2–7) | 6 (4–8) | 2 (1–5) | 6 (4–8) | 5.5 (4–7) | 3 (2–6) |
| Total tabs used, median (IQR) | 8 (3–13) | 12 (7–16) | 3 (1–7) | 10.5 (4–14) | 8.5 (4–11) | 6 (3–11) |
| Total MME used, median (IQR) | 45 (20–80) | 65 (47.5–100) | 20 (10–50) | 60 (25–110) | 47.5 (20–80) | 37.5 (16.3–77.5) |
| Patients reporting leftover pills (N = 226), % | 92.5 | 85.3 | 98.0 | 93.0 | 97.1 | 88.9 |
IQR = interquartile range; MME = morphine milligram equivalents; post-DC = postdischarge.
Among those who reported taking opioids.
Total Opioid Consumption
For all diagnoses combined, the median number of pills consumed in the 10 days postdischarge (IQR) was eight (3–13), corresponding to a total MME median (IQR) of 45 (20–80). Patients with renal colic reported taking pills for a median (IQR) of two (1–5) days, whereas those in the “other” category took pills for a median (IQR) of three (2–6) days. The remaining three diagnosis groups took pills for a median of five or more days. Patients with renal colic used the fewest opioids (total pills: median [IQR] = 3 [1–7]; total MME: median [IQR] = 20 [10–50]); patients with back pain used the most (total pills: median [IQR] = 12 [7–16]; total MME: median [IQR] = 65 [47.5–100]). There was no influence of the overall study intervention on total opioid consumption, defined as total pills, total MME, and total days used.
Frequency of Daily Opioid Consumption by Diagnosis
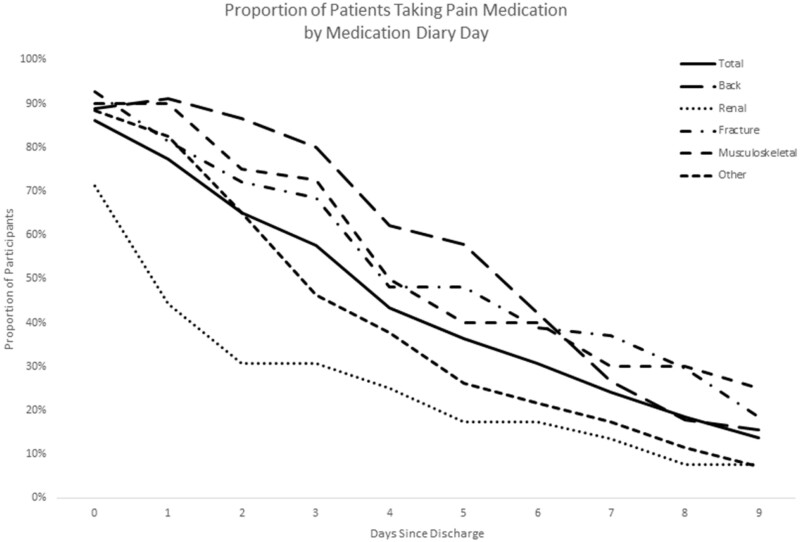
On the day of ED discharge (day 0), 86% of patients consumed an opioid at home (88.9% back pain, 71.2% renal colic, 92.6% fracture/dislocation, 90.0% musculoskeletal injury, 88.4% other). Slightly more than one-third of the patients (36.2%) had also received an opioid (oral or intravenous) in the ED on day 0. On day 1 after ED discharge, the proportion of patients consuming opioids decreased for those with renal colic (44.2%), fracture/dislocation (81.5%), and other diagnoses (82.6%), remained stable for those with musculoskeletal pain (90.0%), and increased for those with back pain (91.1%). Figure 2 displays the proportion of patients reporting opioid consumption by postdischarge day.
Figure 2.
Proportion of patients continuing to take pain medication.
Regardless of the diagnosis category or the postdischarge day, the number of pills consumed on a given day was low, with patients typically using one to two pills daily among those continuing to take medication (Table 2).
Use of Other Analgesics
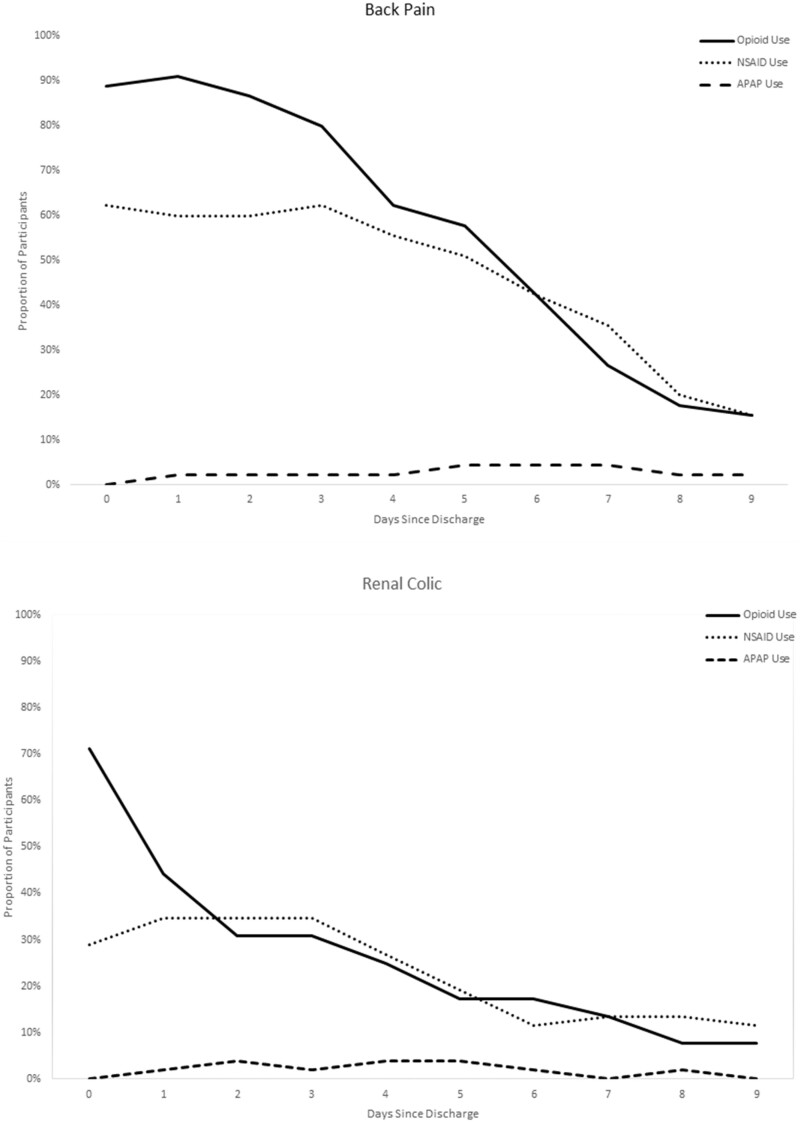
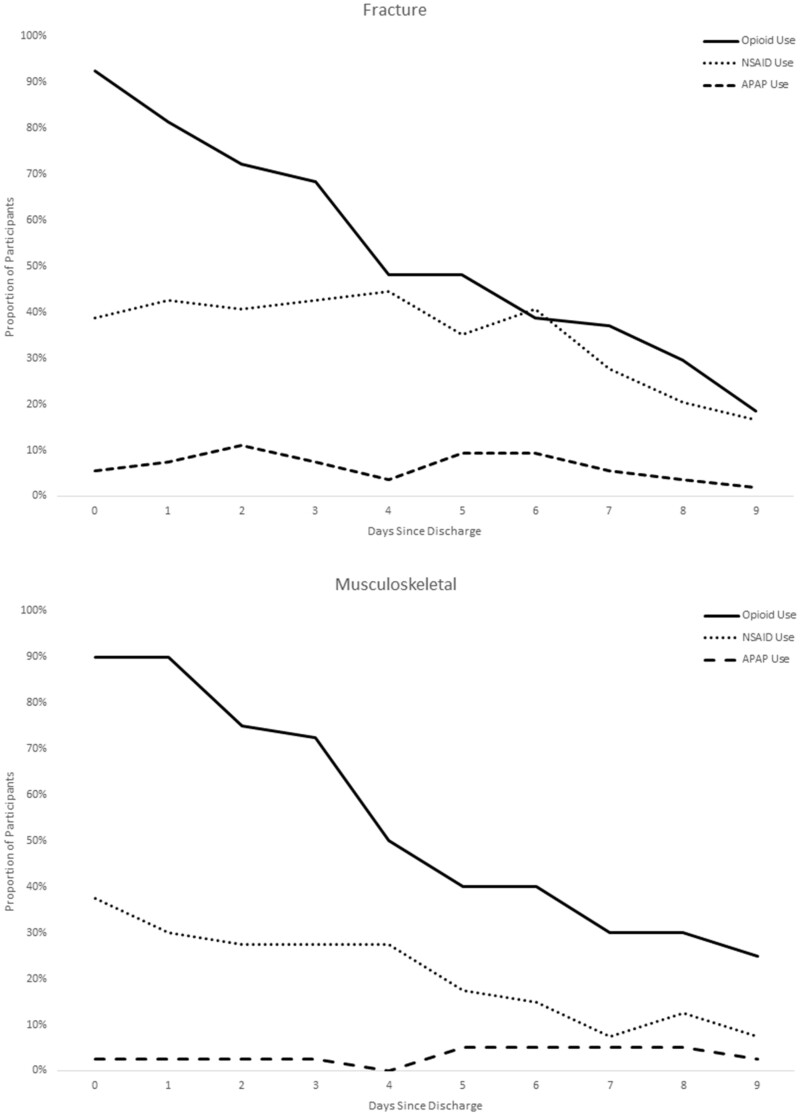
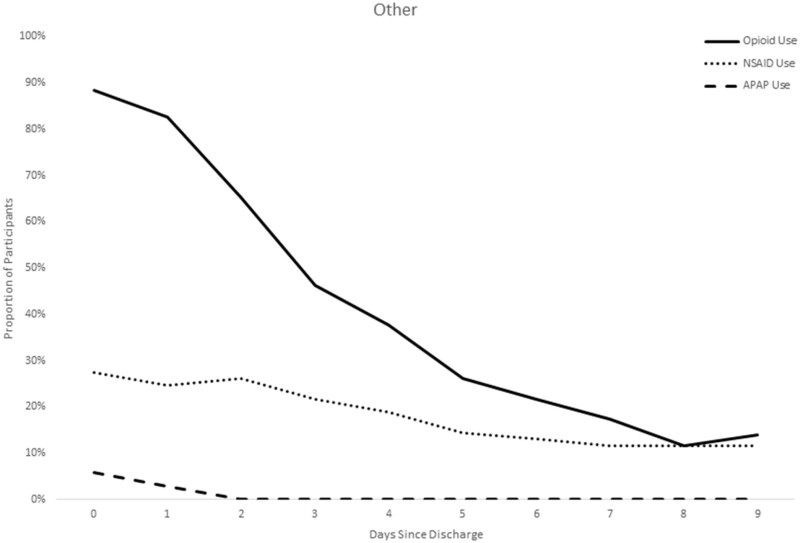
NSAIDs were used fairly consistently across time in the sample, with patients reporting NSAID use for a median of 5.3 days, but was most frequently used among the patients with a back pain diagnosis. Not surprisingly, benzodiazepine use was also most common among back pain patients (51.1%). In contrast, acetaminophen as a standalone medication (rather than in a combination formulation with hydrocodone) was used very infrequently as an analgesic, with only 8.5% of the sample using acetaminophen at any time in the 10 days postdischarge. Figure 3 displays the timing of use of the analgesics, demonstrating that not only did the overall use of NSAIDs differ by diagnosis, but the NSAID patterns followed a similar trajectory to that of the opioids, with a decreasing proportion of patients taking them on each subsequent day (rather than “replacing” their opioid with nonopioid alternatives) (Table 3).
Figure 3.
Patterns of analgesic use by diagnosis over time.
Table 3.
Use of other medications, by diagnosis
| Total | Back Pain | Renal Colic | Fracture/Dislocation | Musculoskeletal Injury | Other | |
|---|---|---|---|---|---|---|
| (N = 260) | (N = 45) | (N = 52) | (N = 54) | (N = 40) | (N = 69) | |
| NSAID use at any time, % | 52.7 | 77.8 | 42.3 | 57.4 | 50.0 | 42.0 |
| Days on which an NSAID was consumed, mean (SD) | 5.3 (3.0) | 6.0 (3.0) | 5.4 (3.0) | 6.1 (3.1) | 4.2 (2.9) | 4.3 (2.7) |
| Muscle relaxant use at any time, % | 12.7 | 51.1 | 0.0 | 7.4 | 7.5 | 4.35 |
| Days on which a muscle relaxant was consumed, mean (SD) | 5.5 (2.7) | 5.0 (2.5) | 0.0 (0.0) | 4.5 (2.5) | 9.7 (0.6) | 6.3 (2.3) |
| Acetaminophen use at any time, % | 8.5 | 4.4 | 7.7 | 13.0 | 10.0 | 7.3 |
| Days on which acetaminophen was consumed, mean (SD) | 3.5 (3.0) | 6.0 (4.2) | 2.5 (1.9) | 5.0 (2.9) | 3.3 (3.9) | 1.2 (0.5) |
NSAID = nonsteroidal anti-inflammatory drug.
Unused Opioids
Ninety-two point five percent of patients had leftover pills. Among the 260 patients, a total of 3,975 pills of hydrocodone-acetaminophen were prescribed, representing 26,155 MME. At the end of their assessment, 2,077.5 pills (52.2%) were unused.
Calculated Quantities by Diagnosis
The opioid quantities required to fully supply the opioid needs for 80% and 95% of patients for the first three days after discharge (day of discharge + postdischarge days 1, 2, and 3) are as follows: back pain 12 pills/19 pills, renal colic five pills/nine pills, fracture/dislocation nine pills/15 pills, musculoskeletal injury nine pills/12 pills, “other” diagnoses eight pills/13 pills. If all diagnoses were grouped together, 13 pills would supply the needs of 95% of patients for the first three days after discharge.
Limitations
This analysis was part of a larger interventional study. Although the intervention was not designed to decrease opioid use (but rather improve safe use) and there was no measured effect of the intervention on patients’ overall pill use, the study context may have influenced dosing in an unmeasured way. The study took place at a single urban site, limiting generalizability. Further, medication diaries had a low rate of return, are subject to patient recall bias, and were more likely to be returned among patients with higher literacy, educational attainment, and income. It is possible that this population of patients has different pill use patterns in both frequency and duration than those who did not return medication diaries.
Patients’ prescribed pill quantity was not controlled and was not equal between diagnoses or individuals; therefore, there may be a ceiling effect wherein patients appear to “only” use 12 pills because they were supplied 12, artificially lowering the calculated 80% and 95% numbers. This limitation is mitigated by the fact that even among the diagnosis groups with the highest use (back pain) and the highest proportion of patients with continued use at nine days post-ED (musculoskeletal injury), there were high rates of unused opioids at the end of the 10-day diary. Finally, there was no check for patients obtaining prescriptions from other, non-ED sources.
Discussion
In this prospective observational study of opioid pill consumption after ED discharge, patient-reported opioid use varied by painful diagnosis. Patients with renal colic were prescribed the highest quantity of opioid pills and ultimately used the lowest number of pills, perhaps due to the acute, intermittent nature of pain from renal colic. All diagnoses displayed a similar daily use pattern over time, with patients consuming low pill numbers on a given day and a progressive decline in the proportion of patients reporting opioid use with each passing day.
These data add to the existing literature in several ways. First, these findings highlight the unique opioid consumption trajectory of renal colic and indicate a potential mismatch between prescriber expectations of painful symptom trajectory and actual patient-reported symptom trajectory. Additionally, they demonstrate that ED patients rarely take as-needed medications at the maximum daily dosing limits, and even a low pill quantity may allow for continued use up to a week or beyond. Finally, the number of unused opioid pills 10 days after ED discharge remains high; 93% of study participants reported leftover opioids. This finding is notable because all prescriptions in this study had pill quantities that complied with the days’ supply–based guidelines, yet still resulted in >2,000 unused opioid pills from only 260 patients. A prior cross-sectional study of national EDs revealed similar quantities of opioid pills prescribed (mean = 16.6), also fitting well within the days’ supply–based guidelines [15].
We are aware of at least one other study evaluating opioid consumption trajectories after ED discharge. Daoust et al. reported that patients were prescribed a median of 150 MME and consumed a median of 35 MME over two weeks of follow-up, resulting in 68% of prescribed pills remaining unused. When stratified by painful diagnosis, opioid consumption was significantly lower among diagnoses of renal colic and abdominal pain than extremity fracture and musculoskeletal pain [14]. In comparison, we found that patients were prescribed a median of 90 MME and consumed a median of 45 MME over 10 days of follow-up, resulting in 52% of opioid pills remaining unused. Similar to Daoust et al., we found that opioid consumption was lowest in patients with renal colic.
Based on these data, Daoust et al. calculated that 15 pills of morphine 5 mg (i.e., a total of 75 MME) would adequately supply 95% of the population for undifferentiated acute pain diagnoses for three days. By comparison, we found that 13 pills of hydrocodone/acetaminophen 5 mg/325 mg (same MME as morphine) would be needed to supply 95% of the population for undifferentiated acute pain diagnoses over three days. Overall, these results are very similar despite different populations and data collection techniques. Notably, these data demonstrate that adopting a “one size fits all” quantity would have a different impact on a renal colic patient than a back pain patient.
In contrast, if one were to adopt the 95% calculated quantities by diagnosis (19 for back pain, nine for renal colic, 15 for fracture/dislocation, 12 for musculoskeletal injury, and 13 for “other” diagnoses), there would perhaps be fewer excess pills for some diagnoses, but many patients would still have excess pills (e.g., median back pain used 12 pills). Another possible effect of these different quantities relates to the “message” that a prescription (and quantity) sends to the patient. Howard et al. recently examined opioid prescribing and use following surgical procedures across 33 health systems in Michigan; they found that the quantity of opioids prescribed was associated with the amount consumed by patients. They surmised that the quantity prescribed may act as a “psychologic heuristic” or “mental anchor by which patients estimate their analgesic needs” [16].
Although our results closely approximate those of Daoust et al. [17], there are several notable distinctions. First, the Daoust study was conducted in Canada, which has a health care system fundamentally different from that of the United States and has not experienced the same severity of opioid overprescribing and overdose historically. It is therefore necessary to evaluate opioid use patterns among US ED visits. Second, the most common opioid prescribed in the Canadian cohort was morphine, whereas the most common opioid prescribed in our cohort—and nationally in the United States [18]—was hydrocodone-acetaminophen. The use of a combination opioid-acetaminophen formulation may influence use patterns. Notably, in reference to other analgesics, the Daoust et al. study reported high rates of NSAID (45.1%) and acetaminophen use (67.9%) but did not have daily consumption pattern data. Our data show that routine use of these over-the-counter analgesics in our population is different than in the Canadian sample, with much lower rates of acetaminophen use. However, patients were already receiving the benefits of acetaminophen via the combination formulation and were counseled to avoid double-dipping with acetaminophen. Interestingly, despite the higher rates of over-the-counter analgesics reported by Daoust et al., their patients had similar overall patterns of opioid consumption.
Our intent in publishing these data is not that our findings be interpreted as firm parameters for quantity-based limits of prescribed opioids, particularly given the limitations of a single-center observational study. Rather, these findings build upon and validate a growing body of literature identifying distinct pain and pill consumption trajectories for common acutely painful conditions. Further, these data help to underscore that opioid prescriptions for acute pain are not a “one size fits all” situation [1]. These data also confirm the findings from Daoust et al. that renal colic, in particular, follows a pain intensity and opioid consumption trajectory distinct from musculoskeletal diagnoses and fracture/dislocation. Ideally, additional studies would confirm our data in more diverse populations, eventually informing a more evidence-driven approach to acute prescribing limits.
Importantly, any change in guidelines will require thoughtful consideration of the impact on individual physicians and patients, as well as the more collective perspective of overall public health. This approach must include a relative prioritization of the competing goals of minimizing excess opioid exposure while ensuring adequate pain relief, as well as a consensus definition of the optimal target for population-level quantity-based guidelines (i.e., meeting the needs of 80% vs 95% of the population’s acute pain needs vs some other target). Diagnosis-specific opioid dosing evidence is one aspect to consider; however, not just dose but also anticipated duration of opioid use should be considered, and this work enhances that understanding. Further, any acute prescribing guidelines should preserve individual prescriber flexibility in responding to unique patient and clinical circumstances. There is a wide response range to opioids, pain is subjectively experienced, and other considerations, including patient comorbidities and the timeliness and ability to connect to follow-up care, all contribute to prescribing decisions (analgesic choice, strength, and quantity) [19].
In summary, the ED patients returning medication diaries in our sample consumed only half (45 MME) of the prescribed quantity of opioids (90 MME). Total opioids prescribed and consumed varied by diagnosis, with renal colic representing the largest quantity of opioids prescribed but the lowest quantity of opioids consumed. These data should be considered in the creation of any future opioid prescribing guidelines.
Authors’ Contributions
DMM, DMC, HSK, MSW, PML, and LMC conceived the study, designed the trial, and obtained research funding. DMM supervised the conduct of the trial and data collection. KP and CA undertook recruitment of patients and managed the data. SIH, LMC, and LO provided statistical advice on study design and analyzed the data. DMM and HSK drafted the manuscript, and all authors contributed substantially to its revision. DMM takes responsibility for the paper as a whole.
Supplementary Material
Acknowledgments
We would like to acknowledge the research assistants, project coordinators, and analysts who assisted with data collection and preliminary analysis, as well as the remaining co-investigators for the larger study (Kenzie A. Cameron, Abbie E. Lyden, Surrey M. Walton, Enid Montague, and Kwang-Youn A. Kim).
Supplementary Data
Supplementary data are available at Pain Medicine online.
Funding sources: This project was supported by grant number R18HS023459 (PI: McCarthy) from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. REDCap is supported at FSM by the Northwestern University Clinical and Translational Science (NUCATS) Institute. Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest: None.
Prior presentation: Presented at American College of Emergency Physicians Scientific Assembly 2018, San Diego.
Trial registration: The larger study from which these data were derived was a randomized controlled trial (RCT) and is registered at clinicaltrials.gov (NCT02431793). The data reported herein are not the results of the RCT, but rather a subanalysis.
References
- 1. Lowenstein M, Grande D, Delgado MK.. Opioid prescribing limits for acute pain-striking the right balance. N Engl J Med 2018;379(6):504–6. [DOI] [PubMed] [Google Scholar]
- 2. Dowell D, Haegerich TM, Chou R.. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016;65(1):1–49. [DOI] [PubMed] [Google Scholar]
- 3. Kennedy-Hendricks A, Gielen A, McDonald E, McGinty EE, Shields W, Barry CL.. Medication sharing, storage, and disposal practices for opioid medications among US adults. JAMA Int Med 2016;176(7):1027–9. [DOI] [PubMed] [Google Scholar]
- 4. Lewis ET, Cucciare MA, Trafton JA.. What do patients do with unused opioid medications? Clin J Pain 2014;30(8):654–62. [DOI] [PubMed] [Google Scholar]
- 5. Finkelstein Y, Macdonald EM, Gonzalez A, Sivilotti MLA, Mamdani MM, Juurlink DN.. Overdose risk in young children of women prescribed opioids. Pediatrics 2017;139(3):e20162887. [DOI] [PubMed] [Google Scholar]
- 6. Khan NF, Bateman BT, Landon JE, Gagne JJ.. Association of opioid overdose with opioid prescriptions to family members. JAMA Int Med 2019;179(9):1186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM.. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 National Survey on Drug Use and Health. Ann Intern Med 2017;167(5):293–301. [DOI] [PubMed] [Google Scholar]
- 8. McCarthy DM, Courtney DM, Lank PM, et al. Electronic medication complete communication strategy for opioid prescriptions in the emergency department: Rationale and design for a three-arm provider randomized trial. Contemp Clin Trials 2017;59:22–9. [DOI] [PubMed] [Google Scholar]
- 9. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009;10(2):113–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McCarthy DM, Davis TC, King JP, et al. Take-wait-stop: A patient-centered strategy for writing PRN medication instructions. J Health Commun 2013;18(Suppl 1):40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: The newest vital sign. Ann Fam Med 2005;3(6):514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hill MV, McMahon ML, Stucke RS, Barth RJ Jr.. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg 2017;265(4):709–14. [DOI] [PubMed] [Google Scholar]
- 14. Daoust R, Paquet J, Cournoyer A, et al. Quantity of opioids consumed following an emergency department visit for acute pain: A Canadian prospective cohort study. BMJ Open 2018;8(9):e022649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoppe JA, Nelson LS, Perrone J, et al. Opioid Prescribing in a Cross Section of US Emergency Departments. Ann Emerg Med 2015;66(3):253–9.e251. [DOI] [PMC free article] [PubMed]
- 16. Howard R, Fry B, Gunaseelan V, et al. Association of opioid prescribing with opioid consumption after surgery in Michigan. JAMA Surg 2018;154:e184234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Special Advisory Committee on the Epidemic of Opioid Overdoses. National Report: Apparent Opioid-Related Deaths in Canada (January 2016 to December 2018). Web Based Report. Ottawa: Public Health Agency of Canada; 2019. Available at: https://health-infobase.canada.ca/datalab/national-surveillance-opioid-mortality.html (accessed July 2019).
- 18. Rui P, Kang K, Ashman JJ. National Hospital Ambulatory Medical Care Survey: 2016 emergency department summary tables. 2016. Available at: https://http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2016_ed_web_tables.pdf (accessed July 2019).
- 19. Yealy DM, Green SM.. Opioids and the emergency physician: Ducking between pendulum swings. Ann Emerg Med 2016;68(2):209–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.