Abstract
Aims
We investigated whether patients with atrial fibrillation (AF) demonstrate detectable changes in biomarkers including high-sensitivity troponin T (hsTnT), N-terminal B-type natriuretic peptide (NT-proBNP), and growth differentiation factor-15 (GDF-15) over 12 months and whether such changes from baseline to 12 months are associated with the subsequent risk of stroke or systemic embolic events (S/SEE) and bleeding.
Methods and results
ENGAGE AF-TIMI 48 was a randomized trial of the oral factor Xa inhibitor edoxaban in patients with AF and a CHADS2 score of ≥2. We performed a nested prospective biomarker study in 6308 patients, analysing hsTnT, NT-proBNP, and GDF-15 at baseline and 12 months. hsTnT was dynamic in 46.9% (≥2 ng/L change), NT-proBNP in 51.9% (≥200 pg/mL change), GDF-15 in 45.6% (≥300 pg/mL change) during 12 months. In a Cox regression model, upward changes in log2-transformed hsTnT and NT-proBNP were associated with increased risk of S/SEE [adjusted hazard ratio (adj-HR) 1.74; 95% confidence interval (CI) 1.36–2.23 and adj-HR 1.27; 95% CI 1.07–1.50, respectively] and log2-transformed GDF-15 with bleeding (adj-HR 1.40; 95% CI 1.02–1.92). Reassessment of ABC-stroke (age, prior stroke/transient ischaemic attack, hsTnT, and NT-proBNP) and ABC-bleeding (age, prior bleeding, haemoglobin, hsTnT, and GDF-15) risk scores at 12 months accurately reclassified a significant proportion of patients compared with their baseline risk [net reclassification improvement (NRI) 0.50; 95% CI 0.36–0.65; NRI 0.42; 95% CI 0.33–0.51, respectively].
Conclusion
Serial assessment of hsTnT, NT-proBNP, and GDF-15 revealed that a substantial proportion of patients with AF had dynamic values. Greater increases in these biomarkers measured over 1 year are associated with important clinical outcomes in anticoagulated patients with AF.
Keywords: Atrial fibrillation, Biomarkers, Stroke, Haemorrhage, Risk assessment
Graphical Abstract
Serial assessment of biomarkers and the risk of stroke or systemic embolism and bleeding in patients with atrial fibrillation. hsTnT, high-sensitivity troponin T; NT-proBNP, N-terminal pro-brain natriuretic peptide; GDF-15, growth differentiation factor-15; AF, atrial fibrillation; ABC, age, biomarker, and clinical history.
See page 1707 for the editorial comment on this article (doi: 10.1093/eurheartj/ehab146)
Introduction
Circulating cardiovascular biomarkers may reflect underlying myocardial injury, hemodynamic stress, and inflammation that contribute to cardiac electrical and structural remodelling in patients with atrial fibrillation (AF).1 In the ENGAGE AF-TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-TIMI 48) trial, we previously identified that a multimarker risk score incorporating cardiac troponin, N-terminal B-type natriuretic peptide (NT-proBNP), and D-dimer enhanced prognostic accuracy for stroke or systemic embolic events (S/SEE) and death compared with the CHA2DS2-VASc score.2 Moreover, when combined with clinical parameters in the ABC-stroke [age, prior stroke/transient ischaemic attack, high-sensitivity cardiac troponin T (hsTnT), and NT-proBNP] and ABC-bleeding [age, prior bleeding, haemoglobin, hsTnT, and growth differentiation factor-15 (GDF-15)] risk scores, biomarkers improved prediction of stroke and bleeding risks, respectively, when studied in several trial-based cohorts.3 , 4 We previously demonstrated that, assessed at baseline in the ENGAGE AF-TIMI 48 trial, the ABC-stroke and ABC-bleeding scores were well calibrated and outperformed the CHA2DS2-VASc and HAS-BLED scores to predict stroke and bleeding, respectively, in this trial population.5 However, few studies have examined the changes of these biomarkers in patients with AF over time, and the duration of follow-up in such analyses has been limited.6 , 7 Thus, little is known about the change in these three biomarkers in patients with AF over the longer term and whether the changes in biomarkers are associated with subsequent clinical adverse outcomes.
Therefore, in a cohort from the ENGAGE AF-TIMI 48 trial, we investigated whether patients with AF demonstrate detectable changes in these biomarkers over 12 months and whether such changes from baseline to 12 months are associated with subsequent risk of S/SEE and major bleeding.
Methods
Study design and population
The ENGAGE AF-TIMI 48 trial was a multinational randomized double-blind trial of the once daily, oral factor Xa inhibitor edoxaban vs. warfarin for the prevention of S/SEE in 21 105 patients with AF and CHADS2 score ≥2.8 Patients were randomly allocated to warfarin (adjusted to an international normalized ratio of 2.0–3.0), higher-dose edoxaban (60 mg/day with reduction to 30 mg/day in selected patients), or lower-dose edoxaban (30 mg/day with reduction to 15 mg/day in selected patients). The median follow-up was 2.8 years. Participation in a prospective nested biomarker substudy was offered to all enrolled patients at sites that elected to participate in the biomarker substudy until ≈9000 patients were recruited. The trial, including the biomarker substudy, was approved by the governing institutional review board/ethics committee at each site and written informed consent for participation was obtained from each participant.
Biomarkers
In this prespecified biomarker substudy, blood samples were collected on the day of randomization, and at 12 months after randomization. Samples were collected in EDTA anticoagulant tubes and isolated plasma was stored at −20°C or colder and then shipped frozen to the TIMI Clinical Trials Laboratory (Boston, MA), where they were stored at −80°C or colder until thawed and analysed by personnel blinded to treatment allocation and clinical outcomes. hsTnT, NT-proBNP, and GDF-15 concentrations were measured with immunoassays on the Cobas e601 (Roche Diagnostics; see the Biomarker Assay Parameters section in the online-only Data Supplement). Haemoglobin was measured separately in a commercial core laboratory during the conduct of the trial.
Clinical outcomes
The outcomes of interest for this analysis were the time to the first adjudicated stroke (ischaemic and hemorrhagic), or SEE, and adjudicated major bleeding during treatment, as defined by the International Society on Thrombosis and Haemostasis.9 Major bleeding included the following fatal bleeding: bleeding in a critical area or organ such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, pericardial, or intramuscular with compartment syndrome; or bleeding causing a fall in haemoglobin level of ≥2 g/dL (adjusted for transfusion) or leading to transfusion of ≥2 U whole blood or red cells. Based on our previous work with these biomarkers, the outcome of S/SEE was of primary interest for hsTnT and NT-proBNP, and the outcome of bleeding was of primary interest for GDF-15 and hsTnT. An independent clinical endpoint committee blinded to randomized treatment assignment and biomarker levels adjudicated all events during conduct of trial.
Statistical analysis
On the basis of the skewed distribution of biomarker values and their absolute changes between baseline and 12 months, continuous data were log2-transformed. Since there are no established thresholds for change in biomarker concentration in this setting, we evaluated both continuous relationships as well as several thresholds according to the distribution of change in biomarker concentration.
Event rates were estimated and displayed as annualized event rates. Univariable analyses of the relationship between each biomarker and the relevant outcome(s) are presented. For analyses using the 12-month biomarker concentration or change in biomarker concentration, landmark analyses of S/SEE and bleeding outcomes starting at 12 months were performed. Adjusted estimates of the association between individual biomarkers and their changes over time with S/SEE were calculated using a Cox proportional hazards model with the biomarker as an independent variable, along with estimated glomerular filtration rate (eGFR) and each of the elements of the CHA2DS2-VASc score [age, sex, history of heart failure, history of hypertension, vascular disease (prior myocardial infarction, peripheral arterial disease or aortic plaque), diabetes mellitus, and history of stroke or transient ischaemic attack]. Similarly, adjusted estimates of the association between individual biomarkers and major bleeding were calculated from a Cox proportional hazards model with the biomarker as an independent variable, along with eGFR and elements of the HAS-BLED score (age, history of hypertension, history of abnormal renal or liver function, history of stroke or transient ischaemic attack, history of major bleeding, medication use predisposing to bleeding, and alcohol use). International normalized ratio lability (a component of the HAS-BLED score) was not included because there were no available international normalized ratio data before randomization and 40% of patients enrolled in the trial were naive to vitamin K antagonists.
In addition to the analysis of the biomarkers individually, we evaluated the biomarkers in the context of established biomarker-based clinical risk scores. The ABC-stroke and ABC-bleeding risk score factors were analysed using a Cox model with coefficients from ENGAGE AF-TIMI 48. Annualized S/SEE event rates were stratified according to categorical subgroups (<1%, 1–2%, >2%) defined using 1-year S/SEE risk probabilities predicted by the ABC-stroke scores at baseline and 12 months with biomarkers at baseline and 12 months, respectively. Similarly, annualized major bleeding event rates were stratified according to categorical subgroups (<2%, 2–4%, >4%) defined using 1-year major bleeding risk probabilities predicted by the ABC-bleeding score at baseline and 12 months with biomarkers and haemoglobin at baseline and 12 months, respectively.
The prognostic discriminatory performance of the biomarkers, CHA2DS2-VASc, and HAS-BLED scores for clinical outcomes after 12 months were assessed using Harrell’s c-index.2 , 5 The ability of change in biomarkers during 12 months, CHA2DS2-VASc, and HAS-BLED scores reassessed at 12 months to enhance discrimination and correctly reclassify patients were additionally evaluated with the integrated discrimination improvement and the net reclassification improvement (NRI).2 , 5 All analyses were performed with SAS software version 9.4 (SAS Institute Inc, Cary, NC, USA). Unless otherwise stated, all tests are 2-sided, and a P-value of <0.05 was considered statistically significant. No adjustment for multiplicity was performed.
Results
Analysis population
For this analysis, collected samples were available for 6806 patients at 12 months after trial enrolment and for 6308 patients both at baseline (trial enrolment) and 12 months. The characteristics of this analysis cohort were similar to the overall population enrolled in the ENGAGE AF-TIMI 48 trial (Supplementary material online, Table S1).
Changes in biomarkers
The concentration distributions of each biomarker at baseline and at 12 months and their changes are shown in Supplementary material online, Table S2. On average, the changes in biomarker concentration between baseline and 12 months were qualitatively very small [median (25th percentile to 75th percentile)]: 0.5 ng/L (−1.2 to +2.4 ng/L) for hsTnT, −1.9 pg/mL (−217 to +216 pg/mL) for NT-proBNP, and 89 pg/mL (−145 to +385 pg/mL) for GDF-15. However, examination of the distribution of absolute changes (Figure 1) reveals that hsTnT was dynamic in 46.9% (≥2 ng/L change), NT-proBNP in 51.9% (≥200 pg/mL change), and GDF-15 in 45.6% (≥300 pg/mL change). Quantitatively larger changes (≥6 ng/L for hsTnT, ≥600 pg/mL for NT-proBNP, and ≥600 pg/mL for GDF-15) were evident in 15.2%, 22.0%, and 25.0% of the population, respectively. The distribution of relative (%) changes is reported in Supplementary material online, Figure S1. Framed categorically, 7.7% shifted from low hsTnT (<14 ng/L) to high hsTnT (≥14 ng/L), 9.4% from low NT-proBNP (<900 pg/mL) to high (≥900 pg/mL), and 10.6% from low GDF-15 (<1800 pg/mL) to high (≥1800 pg/mL) over 12 months.
Figure 1.
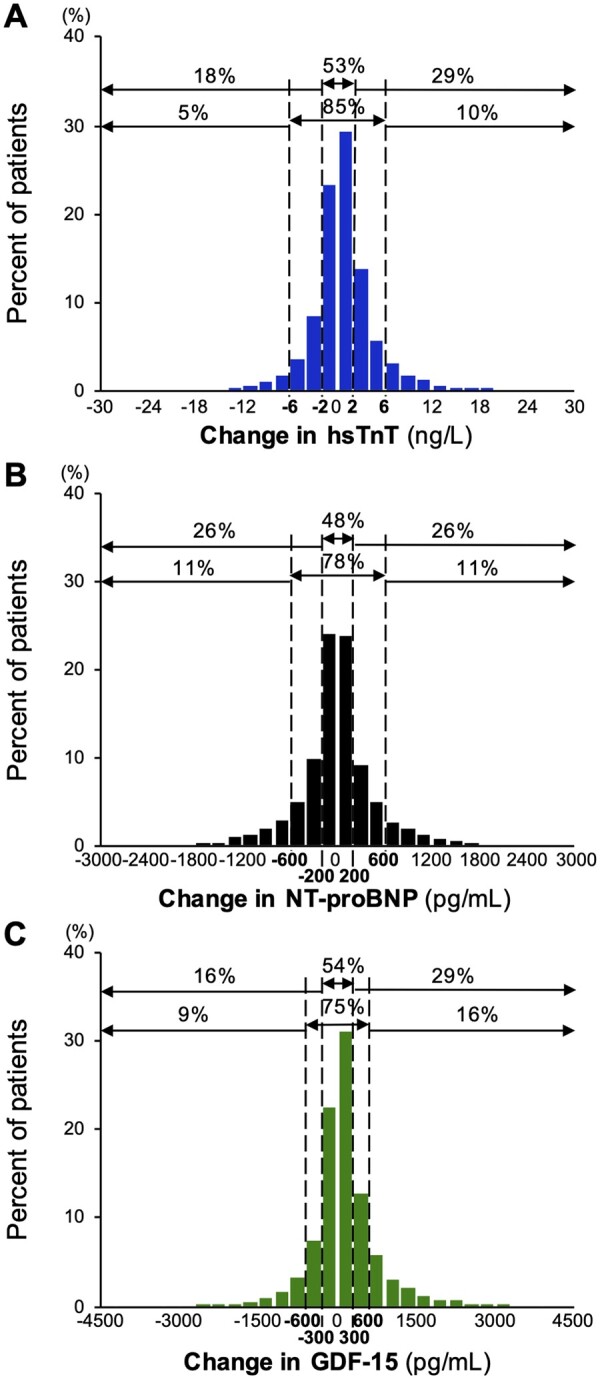
Distribution of patients by the absolute change in biomarker concentrations between baseline and 12 months. One percentage of observations is truncated from each side for illustrative purposes only in this figure. GDF-15, growth differentiation factor-15; hsTnT, high-sensitivity troponin T; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
There was no effect of randomized anticoagulant therapy on the change in biomarker concentration (P > 0.05, for each).
Individual biomarkers at 12 months and subsequent clinical outcomes
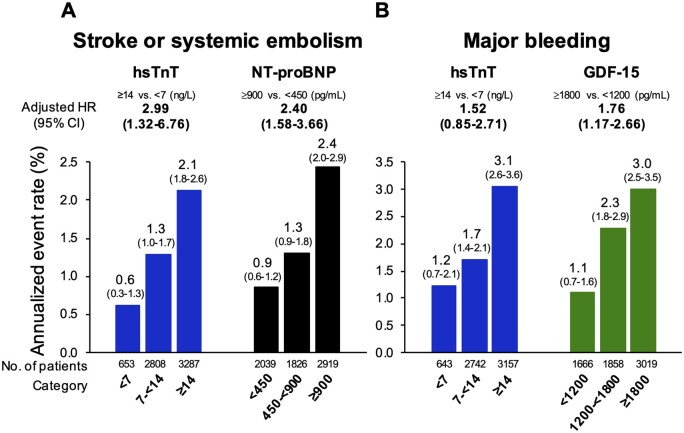
First, examining the biomarker value at a fixed timepoint of 12 months revealed a graded relationship between biomarker concentrations and outcomes (Figure 2). After adjustment for each element of the CHA2DS2-VASc score and eGFR, hsTnT and NT-proBNP at 12 months were each independently associated with a more than two-fold higher rate of subsequent S/SEE in a comparison of the highest and lowest biomarker categories for each biomarker (P < 0.001 for each; Figure 2A). After adjustment for the elements of the HAS-BLED score and eGFR, GDF-15 was independently associated with a 1.76-fold higher rate of subsequent major bleeding (P < 0.001), whereas the association with hsTnT was no longer significant after adjustment (Figure 2B).
Figure 2.
Biomarker values at 12 months and annualized subsequent event rates after 12 months. CI, confidence interval; ; GDF-15, growth differentiation factor-15; HR, hazard ratio; hsTnT, high-sensitivity troponin T; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Changes in biomarkers and subsequent clinical outcomes
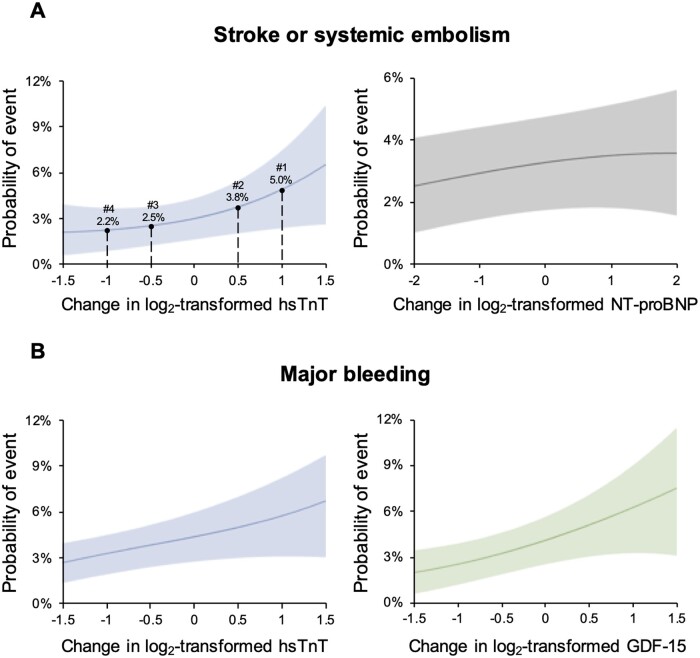
When the changes in biomarker concentrations between baseline and 12 months were analysed as a continuous variable, after adjustment for their baseline value, eGFR, and each element of the CHA2DS2-VASc score, changes upward or downward in log2-transformed hsTnT and NT-proBNP were both independently associated with the risk of subsequent S/SEE (Figure 3A and Supplementary material online, Figure S2A). For example, patients with a two-fold increase in hsTnT or NT-proBNP from baseline to 12 months had 74% and 27% higher risks for S/SEE, respectively [adjusted hazard ratio (adj-HR) 1.74; 95% confidence interval (CI) 1.36–2.23; P < 0.001; adj-HR 1.27; 95% CI 1.07–1.50; P = 0.007]. Similarly, after adjustment for baseline value, eGFR, and elements of the HAS-BLED score, the change in log2-transformed GDF-15 was associated with the risk of subsequent major bleeding, whereas the change in log2-transformed hsTnT was not (Figure 3B and Supplementary material online, Figure S2B). For example, patients with a two-fold increase in GDF-15 during 12 months had a 40% higher risk for major bleeding (adj-HR 1.40; 95% CI 1.02–1.92; P = 0.037). After adding the interaction between treatment and changes in log2-transformed biomarker values in the Cox model for the risk of subsequent S/SEE or bleeding, the interactions were all not significant.
Figure 3.
Changes in biomarkers from baseline to 12 months as a continuous variable and subsequent risks of clinical events. Example patients in panel A indicate specific changes in high-sensitivity troponin T; #1 with 8 ng/L at baseline and 16 ng/L at 12 months, #2 with 31 ng/L at baseline and 45 ng/L at 12 months, #3 with 33 ng/L at baseline and 23 ng/L at 12 months, and #4 with 17 ng/L at baseline and 8 ng/L at 12 months. ; GDF-15, growth differentiation factor-15; hsTnT, high-sensitivity troponin T; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
When analysed in a categorical manner, defining patients as moving between low and high values, compared with patients with stably low biomarker values, patients with values of hsTnT that were either persistently elevated or that increased from baseline to 12 months and those with values of NT-proBNP that were persistently elevated had a significantly higher incidence of subsequent S/SEE (Supplementary material online, Figure S3). Similarly, compared with patients with stably low values of biomarkers, patients with persistently elevated hsTnT or GDF-15 at baseline and 12 months had a higher incidence of subsequent major bleeding (Supplementary material online, Figure S3). Analyses categorized by absolute change (compared with the group with no change as reference) also identified patients with larger increases in hsTnT, NT-proBNP, and GDF-15 from baseline to 12 months as being at significantly higher risk of subsequent S/SEE (P-trend < 0.001 for hsTnT; P-trend = 0.048 for NT-proBNP), and major bleeding (P-trend = 0.001 for GDF-15; Supplementary material online, Figure S4). Event rates for S/SEE and bleeding stratified by baseline concentration of the biomarker and categorized by absolute change are shown in Supplementary material online, Figures S5 and S6.
Adding the change in biomarker concentration from baseline to 12 months to the baseline value of biomarker appropriately reclassified the risk of S/SEE using NT-proBNP (NRI 0.24; 95% CI 0.10–0.38), but not significantly for hsTnT. Bleeding risk was also appropriately reclassified by adding the change in hsTnT or GDF-15 to the baseline value of those biomarkers (NRI 0.29; 95% CI 0.17–0.42; NRI 0.22; 95% CI 0.10–0.34, respectively) (Supplementary material online, Table S3).
Serial assessment by the ABC-stroke and ABC-bleeding scores
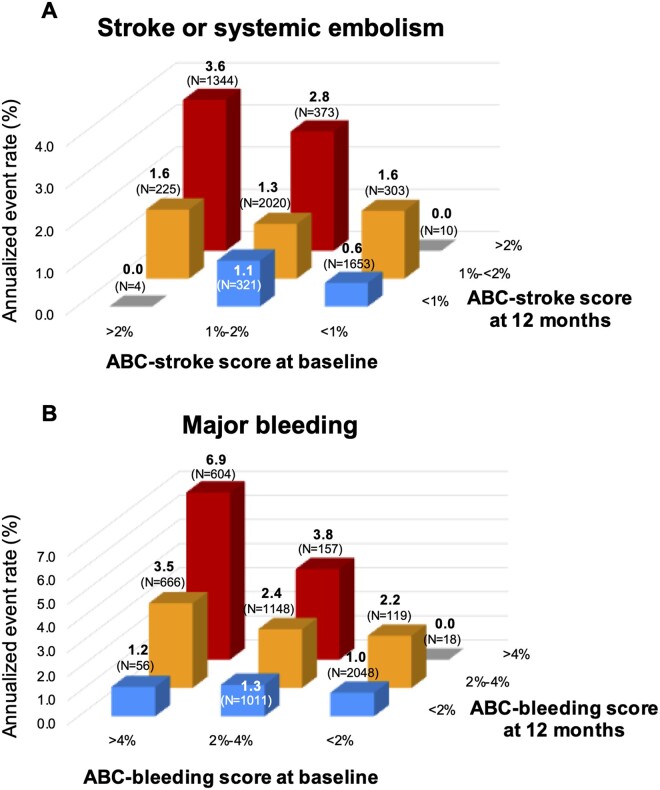
The ABC-stroke and ABC-bleeding risk scores at 12 months were well calibrated with the observed incidence rates of subsequent S/SEE and major bleeding within each category of risk matching the 1-year risks predicted by the ABC-stroke and ABC-bleeding scores (Supplementary material online, Figure S7). Moreover, reassessment of the ABC-stroke and ABC-bleeding risk scores at 12 months accurately reclassified a significant proportion of patients compared with their baseline risk (NRI 0.50; 95% CI 0.36–0.65; NRI 0.42; 95% CI 0.33–0.51, respectively; Figure 4 and Supplementary material online, Table S3). For example, patients with moderate risk (1–2% per each year) for S/SEE at baseline who have a higher ABC-stroke score at 12 months would be appropriately reclassified into a higher-risk category after 12 months. Moreover, patients with moderate risk (2–4% per each year) for bleeding at baseline who had a higher ABC-bleeding score at 12 months were appropriately reclassified towards higher risk after 12 months.
Figure 4.
Annualized subsequent event rates stratified by age, biomarker, and clinical history score at baseline and 12 months for stroke or systemic embolism (A) and major bleeding (B). ABC, age, biomarker, and clinical history.
Discussion
In this nested prospective biomarker study from the ENGAGE AF-TIMI 48 trial, we found that a substantial proportion of patients with AF had dynamic values in hsTnT, NT-proBNP, and GDF-15 between baseline and 12 months. Furthermore, we demonstrated that patients with greater increases in hsTnT or NT-proBNP over 1 year had a significantly higher risk of subsequent S/SEE and those with a larger increase in GDF-15 had a significantly higher risk of subsequent major bleeding. Repeated assessment of the ABC risk scores at 12 months appropriately reclassified a substantial proportion of patients compared with their baseline risk (Graphical Abstract). Our findings suggest that serial biomarker-based risk assessment may allow clinicians to accurately monitor changes in risk in patients with AF over time, which is in line with recommendation for serial assessment with clinical risk scores in the 2020 European Society of Cardiology guidelines for the management of AF.10 With further investigation, these findings might translate into a strategy that would inform decision-making regarding the timing of initiation of anticoagulant therapy in otherwise clinically low-risk patients or more intensive monitoring on the basis of changes in bleeding risk over time to mitigate bleeding.
Substantial epidemiological evidence now supports a potential role for biomarkers for clinical risk assessment in patients with AF.1 The RE-LY (Randomized Evaluation of Long-Term Anticoagulant Therapy) investigators first demonstrated that cardiac troponin measured at baseline was independently associated with risk of stroke or major bleeding events, and NT-proBNP with that of stroke.7 Subsequent studies validated this finding and additionally showed that GDF-15 is strongly associated with the risk of bleeding in anticoagulated patients with AF.11 The ABC-stroke and ABC-bleeding scores, incorporating hsTnT, NT-proBNP, or GDF-15, were developed and validated for risk stratification for stroke and bleeding, respectively, in patients with AF and have offered superior prognostic performance to the CHA2DS2-VASc and HAS-BLED scores in several populations.3 , 5
AF is perpetuated by progressive remodelling marked by myocardial ischaemia, volume and pressure overload, changes in microvascular blood flow, and atrial dysfunction.12 This natural progression engenders a need for iterative assessment of clinical status over time. For example, repeated assessment of CHA2DS2-VASc has showed that most patients with AF develop ≥1 new clinical risk factor in the CHA2DS2-VASc score before presentation with a future ischaemic stroke, and that the change of CHA2DS2-VASc score is a predictor for ischaemic stroke.13 Serial assessment of clinical risk scores for identifying high-risk AF patients for adverse clinical outcomes is a new recommendation in the 2020 European Society of Cardiology guideline for the management of AF.10 However, few studies have examined the changes of troponins and NT-proBNP for S/SEE over time in patients with AF, and the duration of follow-up in these analyses has been limited.6 , 7 In 2514 stable patients with AF, serial measurement of cardiac troponin I (cTnI) with qualitative testing and NT-proBNP revealed that patients with persistently detectable cTnI at baseline and 3 months had substantially higher rates of S/SEE during a median follow-up of 2 years compared with those with an undetectable value at either time point.7 In contrast, in a study of 4796 patients with AF, repeated measurement of hsTnI, hsTnT, and NT-proBNP at baseline and 2 months did not provide any incremental prognostic value for stroke during a median follow-up of 1.8 years.6 Moreover, serial measurement using troponins and GDF-15 for major bleeding had not yet been explored.
In the present study, we quantitatively assessed hsTnT and NT-proBNP, as well as GDF-15, over a longer period of time (1 year) and in a larger population of patients with a median follow-up of 2.8 years. hsTnT and NT-proBNP at 12 months were independently associated with the risk of subsequent S/SEE and GDF-15 with the risk of subsequent major bleeding. Moreover, we found that larger increases in biomarkers over 12 months were associated with higher risks of subsequent S/SEE (hsTnT), and major bleeding (GDF-15). Furthermore, in a framework that may support clinical implementation, we found that reassessment of the ABC risk scores at 12 months accurately reclassified a significant proportion of patients compared with their baseline risk pointing towards a possible new direction for monitoring patients with AF and a dynamic risk of future events. This insight establishes a conceptual basis from which future strategies for tailoring preventive therapy in patients with AF might emerge and would need to be prospectiveliy studied. For example, recognition of increasing bleeding risk may allow clinicians to appropriately reconsider the therapeutic agent or dosing, such as switching from warfarin to a direct oral anticoagulant with a more favourable bleeding profile, or using lower-dose non-vitamin K antagonist oral anticoagulants (NOACs), where approved; although these possible strategies would need to be confirmed in clinical trials.8 , 14 Very-low-dose edoxaban 15 mg vs. placebo has recently shown to reduce S/SEE but not to increase major bleeding among AF patients ≥80 years of age that are high-risk population for bleeding.15 In similar fashion, lower-dose NOACs might be considered using biomarker-based risk stratification to detect high-risk patients for bleeding. Moreover, this finding forms a basis for future investigation of strategies in which serial assessment of biomarkers might inform decision-making regarding when to initiate oral anticoagulant therapy in patients with AF and a CHA2DS2-VASc score of 0–1. An ongoing trial (ABC-AF, ClinicalTrials.gov NCT03753490) that is prospectively testing use of the ABC risk scores to guide decision-making in patients with new or established AF will provide additional insight regarding the usefulness of such a framework. Our findings suggest that serial biomarker-based risk assessment has the potential to contribute towards precision medicine in patients with AF with the goal of optimizing net clinical outcome from stroke and bleeding.
Study limitations
Several limitations of this analysis should be acknowledged. First, ENGAGE AF-TIMI 48 was a clinical trial that enrolled higher-risk patients with a larger burden of comorbid diseases compared with the clinical trial patients in which the ABC scores were derived.3 , 4 , 8 While the performance of the ABC risk scores has been robust and outperformed the CHA2DS2-VASc and HAS-BLED scores in several trial populations, the prognostic performance of a modified ABC-bleeding score (excluding GDF-15) was not significantly better than the HAS-BLED risk score in a smaller non-clinical trial cohort from the Murcia Atrial Fibrillation Project (n = 1120),16 raising the possibility that the performance of the ABC scores may be diminished in populations with a higher prevalence of non-AF comorbidities. Although our data demonstrate consistency across multiple trial populations and timeframes, additional well-powered studies in non-clinical trial cohorts would be helpful. Second, because all patients in the trial were anticoagulated and had at least two risk factors for stroke, application of our findings in patients with AF who are not anticoagulated and those at low risk for stroke would require prospective investigation. Third, by necessary exclusion of the first 1 year of events, our power was diminished in the landmark analyses performed starting at 12 months. Fourth, because blood samples were available for the biomarker substudy only at baseline and 12 months, our analyses could not assess prognostic performance at intermediate time periods (e.g. 3–6 months).Finally, it is important to note that these biomarkers or the ABC scores using them are not specific to cardioembolic events or bleeding in AF. As such, the ABC score risk score may identify patients with overall poor prognosis. For example, GDF-15 is predictive of mortality in patients with a variety of other inflammatory and cardiovascular conditions, including heart failure.17–19 Moreover, in the Murcia Atrial Fibrillation Project, the ABC scores had similar associations with other outcomes, including myocardial infarction, acute heart failure, and all-cause death.20 The application of biomarkers to supplement clinical risk assessment in AF ought also weigh issues of cost and practicality, as well as recognize that biomarkers may be subject to laboratory variability, inter-assay differences, and diurnal variation and may change in individual patients depending on changes in other risk factors and treatments over time.21
Conclusions
In an analysis of patients with AF treated with anticoagulation from the ENGAGE AF-TIMI 48 trial, serial assessment of hsTnT, NT-proBNP, and GDF-15 revealed a substantial proportion of patients with AF had dynamic values. Greater increases in these three biomarkers measured over 1 year are associated with important clinical outcomes in anticoagulated patients with AF.
Supplementary material
Supplementary material is available at European Heart Journal online.
Data availability
We encourage parties interested in collaboration and data sharing to contact the corresponding author directly for further discussions.
Funding
The ENGAGE AF-TIMI 48 was supported by Daiichi Sankyo Pharma Development. The present analysis was supported by a grant from Roche Diagnostics.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT00781391.
Conflict of interest: K.O. reports a grant from JSPS Overseas Research Fellowships. R.P.G. reports research grants from Amgen, Anthos Therapeutics, Daiichi Sankyo, and Merck; honoraria from Amgen, Daiichi Sankyo, and Merck; and consulting fees from Amarin, Amgen, Boehringer-Ingelheim, Bristol-Myers-Squibb, CVS Caremark, Daiichi Sankyo, GlaxoSmithKline, Lexicon, Merck, Portola, Pfizer, SAJA, and Servier. D.D.B. reports research support from Harvard Catalyst KL2/CMeRIT (NIH/NCATS UL 1TR002541) and institutional research grant support to TIMI Study Group at Brigham & Women’s Hospital from AstraZeneca. C.T.R. reports research grants from Daiichi Sankyo, Anthos Therapeutics, and Boehringer-Ingelheim and consulting fees from Daiichi Sankyo, Boehringer-Ingelheim, Bayer, Portola, and Janssen. P.J. reports research support from Abbott Laboratories, Amgen, Inc., AstraZeneca, LP, Daiichi Sankyo, Inc., Eisai, Inc., GlaxoSmithKline, Merck & Co., Inc., Regeneron Pharmaceuticals, Inc., Roche Diagnostics Corporation, Siemens Healthineers, Takeda Global Research and Development Center, and Waters Technologies Corporation and consulting fees from Roche Diagnostics Corporation. S.A.M. report research grant support through Brigham and Women’s Hospital from Abbott Laboratories, Amgen, AstraZeneca, Critical Diagnostics, Daiichi Sankyo, Eisai, Genzyme, Gilead, GlaxoSmithKline, Intarcia, Janssen Research and Development, The Medicines Company, MedImmune, Merck, Novartis, Poxel, Pfizer, Roche Diagnostics, and Takeda. H.J.L. and M.A.G. report employment by Daiichi Sankyo. E.M.A. reports research grants from Eli Lilly. E.B. reports research grants from Daichi Sankyo. D.A.M. reports research grants from Abbott Labs, Amgen, Anthos Therapeutics, AstraZeneca, Daichi Sankyo, Eisai, GSK, Merck, Novartis, Pfizer, Regeneron, Roche, Siemens, and Takeda, as well as consulting fees from AstraZeneca, Bayer, InCarda, Merck, Novartis, and Roche.
Supplementary Material
Contributor Information
Kazuma Oyama, TIMI Study Group, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, 60 Fenwood Road, Suite 7022, Boston, MA 02115, USA; Department of Cardiovascular Medicine, Tohoku University Graduate School of Medicine, 1-1 Seiryo-machi, Aoba-ku, Sendai 980-8574, Japan.
Robert P Giugliano, TIMI Study Group, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, 60 Fenwood Road, Suite 7022, Boston, MA 02115, USA.
David D Berg, TIMI Study Group, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, 60 Fenwood Road, Suite 7022, Boston, MA 02115, USA.
Christian T Ruff, TIMI Study Group, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, 60 Fenwood Road, Suite 7022, Boston, MA 02115, USA.
Petr Jarolim, Division of Pathology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Minao Tang, TIMI Study Group, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, 60 Fenwood Road, Suite 7022, Boston, MA 02115, USA.
Sabina A Murphy, TIMI Study Group, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, 60 Fenwood Road, Suite 7022, Boston, MA 02115, USA.
Hans J Lanz, Daiichi Sankyo, Zielstattstraße 48, München 81379, Germany.
Michael A Grosso, Daiichi Sankyo, 211 Mt Airy Rd, Basking Ridge, NJ 07920, USA.
Elliott M Antman, TIMI Study Group, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, 60 Fenwood Road, Suite 7022, Boston, MA 02115, USA.
Eugene Braunwald, TIMI Study Group, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, 60 Fenwood Road, Suite 7022, Boston, MA 02115, USA.
David A Morrow, TIMI Study Group, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, 60 Fenwood Road, Suite 7022, Boston, MA 02115, USA.
References
- 1. Hijazi Z, Oldgren J, Siegbahn A, Granger CB, Wallentin L. Biomarkers in atrial fibrillation: a clinical review. Eur Heart J 2013;34:1475–1480. [DOI] [PubMed] [Google Scholar]
- 2. Ruff CT, Giugliano RP, Braunwald E, Murphy SA, Brown K, Jarolim P, Mercuri M, Antman EM, Morrow DA. Cardiovascular biomarker score and clinical outcomes in patients with atrial fibrillation: a subanalysis of the ENGAGE AF-TIMI 48 randomized clinical trial. JAMA Cardiol 2016;1:999–1006. [DOI] [PubMed] [Google Scholar]
- 3. Hijazi Z, Lindbäck J, Alexander JH, Hanna M, Held C, Hylek EM, Lopes RD, Oldgren J, Siegbahn A, Stewart RAH, White HD, Granger CB, Wallentin L. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker-based risk score for predicting stroke in atrial fibrillation. Eur Heart J 2016;37:1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hijazi Z, Oldgren J, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JW, Ezekowitz MD, Held C, Hylek EM, Lopes RD, Siegbahn A, Yusuf S, Granger CB, Wallentin L. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet 2016;387:2302–2311. [DOI] [PubMed] [Google Scholar]
- 5. Berg DD, Ruff CT, Jarolim P, Giugliano RP, Nordio F, Lanz HJ, Mercuri MF, Antman EM, Braunwald E, Morrow DA. Performance of the ABC scores for assessing the risk of stroke or systemic embolism and bleeding in patients with atrial fibrillation in ENGAGE AF-TIMI 48. Circulation 2019;139:760–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hijazi Z, Lindahl B, Oldgren J, Andersson U, Lindbäck J, Granger CB, Alexander JH, Gersh BJ, Hanna M, Harjola V-P, Hylek EM, Lopes RD, Siegbahn A, Wallentin L. Repeated measurements of cardiac biomarkers in atrial fibrillation and validation of the ABC stroke score over time. J Am Heart Assoc 2017;6:e004851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Ezekowitz MD, Hohnloser SH, Reilly PA, Siegbahn A, Yusuf S, Wallentin L. Importance of persistent elevation of cardiac biomarkers in atrial fibrillation: a RE-LY substudy. Heart 2014;100:1193–1200. [DOI] [PubMed] [Google Scholar]
- 8. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 9. Schulman S, Kearon C; the Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 10. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan G-A, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau J-P, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, Kirchhof P, Kühne M, Aboyans V, Ahlsson A, Balsam P, Bauersachs J, Benussi S, Brandes A, Braunschweig F, Camm AJ, Capodanno D, Casadei B, Conen D, Crijns HJGM, Delgado V, Dobrev D, Drexel H, Eckardt L, Fitzsimons D, Folliguet T, Gale CP, Gorenek B, Haeusler KG, Heidbuchel H, Iung B, Katus HA, Kotecha D, Landmesser U, Leclercq C, Lewis BS, Mascherbauer J, Merino JL, Merkely B, Mont L, Mueller C, Nagy KV, Oldgren J, Pavlović N, Pedretti RFE, Petersen SE, Piccini JP, Popescu BA, Pürerfellner H, Richter DJ, Roffi M, Rubboli A, Scherr D, Schnabel RB, Simpson IA, Shlyakhto E, Sinner MF, Steffel J, Sousa-Uva M, Suwalski P, Svetlosak M, Touyz RM, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan G-A, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau J-P, Lettino M, Lip GYH, Pinto FJ, Neil Thomas G, Valgimigli M, Van Gelder IC, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 11. Wallentin L, Hijazi Z, Andersson U, Alexander JH, De Caterina R, Hanna M, Horowitz JD, Hylek EM, Lopes RD, Åsberg S, Granger CB, Siegbahn A. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation 2014;130:1847–1858. [DOI] [PubMed] [Google Scholar]
- 12. Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 2002;54:230–246. [DOI] [PubMed] [Google Scholar]
- 13. Chao TF, Lip GYH, Liu CJ, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Liao JN, Chung FP, Chen TJ, Chen SA. Relationship of aging and incident comorbidities to stroke risk in patients with atrial fibrillation. J Am Coll Cardiol 2018;71:122–132. [DOI] [PubMed] [Google Scholar]
- 14. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener H-C, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 15. Okumura K, Akao M, Yoshida T, Kawata M, Okazaki O, Akashi S, Eshima K, Tanizawa K, Fukuzawa M, Hayashi T, Akishita M, Lip GYH, Yamashita T; ELDERCARE-AF Committees and Investigators. Low-dose edoxaban in very elderly patients with atrial fibrillation. N Engl J Med 2020;383:1735–1745. [DOI] [PubMed] [Google Scholar]
- 16. Esteve-Pastor MA, Rivera-Caravaca JM, Roldan V, Vicente V, Valdés M, Marín F, Lip GYH. Long-term bleeding risk prediction in ‘real world’ patients with atrial fibrillation: comparison of the HAS-BLED and ABC-Bleeding risk scores. The Murcia Atrial Fibrillation Project. Thromb Haemost 2017;117:1848–1858. [DOI] [PubMed] [Google Scholar]
- 17. Anand IS, Kempf T, Rector TS, Tapken H, Allhoff T, Jantzen F, Kuskowski M, Cohn JN, Drexler H, Wollert KC. Serial measurement of growth-differentiation factor-15 in heart failure: relation to disease severity and prognosis in the Valsartan Heart Failure Trial. Circulation 2010;122:1387–1395. [DOI] [PubMed] [Google Scholar]
- 18. Ban N, Siegfried CJ, Lin JB, Shui YB, Sein J, Pita-Thomas W, Sene A, Santeford A, Gordon M, Lamb R, Dong Z, Kelly SC, Cavalli V, Yoshino J, Apte RS. GDF15 is elevated in mice following retinal ganglion cell death and in glaucoma patients. JCI Insight 2017;2:e91455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Myhre PL, Prebensen C, Strand H, Røysland R, Jonassen CM, Rangberg A, Sørensen V, Søvik S, Røsjø H, Svensson M, Berdal JE, Omland T. Growth differentiation factor-15 provides prognostic information superior to established cardiovascular and inflammatory biomarkers in unselected patients hospitalized with COVID-19. Circulation 2020;142:2128–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Camelo-Castillo A, Rivera-Caravaca JM, Marín F, Vicente V, Lip GYH, Roldán V. Predicting adverse events beyond stroke and bleeding with the ABC-stroke and ABC-bleeding scores in patients with atrial fibrillation: the Murcia AF Project. Thromb Haemost 2020;120:1200–1207. [DOI] [PubMed] [Google Scholar]
- 21. Lip GYH, Banerjee A, Boriani G, Chiang CE, Fargo R, Freedman B, Lane DA, Ruff CT, Turakhia M, Werring D, Patel S, Moores L. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest 2018;154:1121–1201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We encourage parties interested in collaboration and data sharing to contact the corresponding author directly for further discussions.