In this multicentre study, Arnaldi et al. show that combining dopaminergic brain imaging with clinical features allows the identification of idiopathic REM sleep behaviour disorder patients at high risk of short-time phenoconversion to an overt synucleinopathy. Cut-off values to be used in single subjects are provided.
Keywords: REM sleep behaviour disorder, Parkinson’s disease, dementia with Lewy bodies, SPECT
Abstract
This is an international multicentre study aimed at evaluating the combined value of dopaminergic neuroimaging and clinical features in predicting future phenoconversion of idiopathic REM sleep behaviour (iRBD) subjects to overt synucleinopathy. Nine centres sent 123I-FP-CIT-SPECT data of 344 iRBD patients and 256 controls for centralized analysis. 123I-FP-CIT-SPECT images were semiquantified using DaTQUANTTM, obtaining putamen and caudate specific to non-displaceable binding ratios (SBRs). The following clinical variables were also analysed: (i) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale, motor section score; (ii) Mini-Mental State Examination score; (iii) constipation; and (iv) hyposmia. Kaplan-Meier survival analysis was performed to estimate conversion risk. Hazard ratios for each variable were calculated with Cox regression. A generalized logistic regression model was applied to identify the best combination of risk factors. Bayesian classifier was used to identify the baseline features predicting phenoconversion to parkinsonism or dementia. After quality check of the data, 263 iRBD patients (67.6 ± 7.3 years, 229 males) and 243 control subjects (67.2 ± 10.1 years, 110 males) were analysed. Fifty-two (20%) patients developed a synucleinopathy after average follow-up of 2 years. The best combination of risk factors was putamen dopaminergic dysfunction of the most affected hemisphere on imaging, defined as the lower value between either putamina (P < 0.000001), constipation, (P < 0.000001) and age over 70 years (P = 0.0002). Combined features obtained from the generalized logistic regression achieved a hazard ratio of 5.71 (95% confidence interval 2.85–11.43). Bayesian classifier suggested that patients with higher Mini-Mental State Examination score and lower caudate SBR asymmetry were more likely to develop parkinsonism, while patients with the opposite pattern were more likely to develop dementia. This study shows that iRBD patients older than 70 with constipation and reduced nigro-putaminal dopaminergic function are at high risk of short-term phenoconversion to an overt synucleinopathy, providing an effective stratification approach for future neuroprotective trials. Moreover, we provide cut-off values for the significant predictors of phenoconversion to be used in single subjects.
Introduction
α-Synucleinopathies are neurodegenerative disorders, characterized by the abnormal accumulation of α-synuclein aggregates in neuronal or glial cells. The main phenotypes of α-synucleinopathies are Parkinson’s disease, dementia with Lewy bodies and multiple system atrophy, and they all have a long prodromal stage in which symptoms or signs of neurodegeneration are detectable, but full clinical disease has not developed yet (McKeith et al., 2020).
Several promising neuroprotective treatments are now being developed, and prodromal synucleinopathies are likely the best target to test neuroprotective therapy, because the neurodegenerative process is still in an early stage and thus the likelihood to rescue both brain structure and function is higher. However, identifying patients in a prodromal neurodegenerative stage may be challenging. Several prodromal synucleinopathy markers have been proposed, including autonomic dysfunction, cognitive impairment, olfaction loss and sleep disorders (Berg et al., 2015). Among these, the presence of idiopathic REM sleep behaviour disorder (iRBD) is by far the strongest risk factor (Berg et al., 2015). Indeed, patients with iRBD are at high risk of developing a synucleinopathy over time and >70% of iRBD patients develop an overt neurodegenerative syndrome after 12 years of follow-up (Postuma et al., 2019). Thus, iRBD cohorts are likely the most ready neuroprotective trial populations.
The feasibility of neuroprotective studies in prodromal synucleinopathies is still debated. One relevant problem is the length of a hypothetical trial. Even accepting iRBD patients as the target population, the long interval between the diagnosis and the development of the overt neurodegenerative disease makes the viability of this choice questionable. Indeed, a clinical trial would unlikely last more than 5 years, and certainly not more than 10. To overcome this problem, risk factors of short-time phenoconversion have been investigated in iRBD patients. For example, the prodromal Parkinson’s disease criteria have good sensitivity and specificity in predicting the phenoconversion to synucleinopathy (Postuma et al., 2019). Moreover, the presence of nigro-striatal dopaminergic dysfunction, as investigated by dopamine transporter (DAT) single photon emission tomography (SPECT), was able to identify iRBD patients at high risk of short-time phenoconversion to overt synucleinopathy (Iranzo et al., 2017; Li et al., 2017).
The European Medicines Agency (EMA) supports the use of DAT-SPECT neuroimaging as an enrichment biomarker to be used in early Parkinson’s disease neuroprotection trials (Conrado et al., 2018). Indeed, DAT imaging allows the identification of early Parkinson’s disease patients with more rapid clinical decline and the exclusion of subjects without evidence of DAT deficit, thus allowing reduction of trial size and preventing exposure of subjects who unlikely experience important clinical worsening to experimental treatments (Conrado et al., 2018). However, available literature data on DAT-SPECT in iRBD are inhomogeneous (Bauckneht et al., 2018) and definite results on its ability to efficiently predict future phenoconversion are not achieved yet. Moreover, to date only one single-centre study explored the combined predictive value of presynaptic dopaminergic imaging with clinical risk factors for short-term phenoconversion to overt synucleinopathy in iRBD (Arnaldi et al., 2020).
123I-Ioflupane SPECT (123I-FP-CIT-SPECT) is currently the most studied and available DAT-SPECT imaging modality. Clinical imaging pathological data confirm the utility of DAT-SPECT, including DaTQUANTTM, in differentiating Lewy body disease from non-Lewy body disease disorders (Maltais et al., 2020). The aim of this international multicentre study was to investigate the combined value of 123I-FP-CIT-SPECT and the main clinical features in predicting future phenoconversion of iRBD patients to an overt synucleinopathy.
Material and methods
Subjects
This is a retrospective international multicentre study. Inclusion criteria were the diagnosis of iRBD according to current criteria and the availability of 123I-FP-CIT-SPECT on file. The presence of RBD was confirmed by polysomnography in all patients. Exclusion criteria were history of stroke, head trauma, brain injury or any other major neurological or psychiatric disease, including parkinsonism and dementia. All patients underwent routine clinical follow-up during which systematic assessment for parkinsonism and dementia was performed, including semi-structured interview with patients and caregivers. Centres were also asked to send on file 123I-FP-CIT-SPECT and demographic data of healthy controls (i.e. subjects who were judged to be free of neurological disorders at the end of diagnostic work-up that included 123I-FP-CIT-SPECT).
All participants signed an informed consent form in compliance with the Declaration of Helsinki of 1975. Ethics approval was obtained from the local institutional boards.
123I-FP-CIT-SPECT
Nigro-striatal dopaminergic functioning was evaluated by means of 123I-FP-CIT-SPECT, as a marker of striatal DAT density. Images were acquired after intravenous administration of 123I-FP-CIT (DaTSCAN, GE Healthcare) according to the European Association of Nuclear Medicine (EANM) guidelines (Darcourt et al., 2010) in all centres. SPECT equipment and other technical details are summarized in Supplementary Table 1. DAT-SPECT images were exported in Digital Imaging and Communications in Medicine (DICOM) format and sent to the coordinating centre (Genoa) for analysis, except for the Rochester centre, which sent the quantified data using the same semiquantification software instead of the DICOM files. Quality of images was checked by a nuclear medicine physician with specific expertise in dopaminergic imaging (S.M.).
DaTQUANTTM software (GE Healthcare) was used for semiquantification of DAT-SPECT images. Bilateral specific to non-displaceable binding ratios (SBRs) at putamen and caudate levels were computed, using the occipital lobes uptake as the background reference region. Bilateral putamen/caudate ratios as well as putamen and caudate asymmetries were also computed and used in statistical analyses. Asymmetry values were calculated with the formula: SBR_As = 2 × abs (SBR_Left − SBR_Right)/(SBR_Left + SBR_Right). Both putamen and caudate SBR in either the most affected (MAH) or least affected hemisphere (LAH) were also computed (i.e. the lower/higher value between left and right hemisphere, respectively).
Baseline clinical variables
Available baseline clinical variables include: (i) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale, motor section (MDS-UPDRS-III) (Goetz et al., 2007) for standardized motor examination (1987 version of the UPDRS-III were converted into MDS-UPDRS-III) (Hentz et al., 2015) and only MDS-UPDRS-III scores were used for statistical analysis; (ii) Mini-Mental State Examination (MMSE) (Folstein et al., 1975) or Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005) as a marker of global cognition; MoCA scores were converted into MMSE scores (van Steenoven et al., 2014) and only MMSE was used for statistical analysis; (iii) SCOPA-AUT (Visser et al., 2004), constipation questionnaire (Szewczyk-Krolikowski et al., 2014), or clinical interview to assess constipation; and (iv) University of Pennsylvania Smell Identification Test (Doty et al., 1984), Sniffin' Sticks 16 items odour identification test (Hummel et al., 1997), Odor Stick Identification Test for Japanese (Kobayashi et al., 2006) and Smell Diskettes Olfaction test (Briner and Simmen, 1999) to assess olfaction. For statistical analysis, constipation and hyposmia were dichotomized as abnormal or normal according to the cut-off point of each test.
Follow-up and disease phenoconversion
All centres prospectively followed patients with in-person evaluation to diagnose phenoconversion to defined parkinsonism (defined as bradykinesia plus at least one of rigidity or rest tremor) (Postuma et al., 2015) or dementia (defined as functional impairment in activities of daily living and with evidence of cognitive impairment on standardized testing) (American Psychiatric Association, 2013). For patients with parkinsonism as the primary disease manifestation, the primary diagnosis (Parkinson’s disease/multiple system atrophy) was made according to the treating neurologist. This differential diagnosis incorporated all available follow-up information (i.e. any patient who was initially diagnosed with Parkinson’s disease at phenoconversion but who was subsequently found to have multiple system atrophy would be included as multiple system atrophy). For dementia converters, all met the 2017 criteria for probable dementia with Lewy bodies (McKeith et al., 2017). All iRBD patients who developed Parkinson’s disease, dementia with Lewy bodies or multiple system atrophy at follow-up were considered converters for statistical analysis, while the remaining patients were considered non-converters.
Statistical analysis
DAT-SPECT data, MMSE and MDS-UPDRS-III scores were corrected by age, sex and centre, obtaining adjusted data to be used in further analysis. Subsequently, adjusted DAT-SPECT data were compared between iRBD patients and control subjects by receiver operation characteristic (ROC) analysis.
A first survival Kaplan-Meier analysis was performed to estimate conversion risk on the eight adjusted DAT-SPECT features data (i.e. most affected and least affected hemisphere putamen and caudate SBR, bilateral putamen/caudate ratios, putamen and caudate asymmetries) and on the clinical features (i.e. adjusted MMSE and adjusted MDS-UPDRS-III scores, olfaction, constipation, and age). Continuous variables were categorized as above or below a cut-point identified by the Youden method. Censoring time was set at the time of last assessment for non-converters and the time of conversion for converter patients. Hazard ratios (HR) for each variable were calculated with Cox regression.
To define the best combination of risk factors predicting conversion, a generalized backward stepwise logistic regression was applied, using the conversion outcome (dichotomous variable) as the dependent variable, and the best DAT-SPECT feature (i.e. the one with the highest HR), MMSE score (continuous variable), MDS-UPDRS-III score (continuous variable), olfaction (dichotomous variable), constipation (dichotomous variable) and age (continuous variable) as independent variables. Logistic regression results were used to compute a combined features score for each patient, using the best combination of risk factors for conversion. Then, Kaplan-Meier analysis and Cox regression were applied using the combined features scores. In all analyses, we used the clinical and DAT-SPECT variables adjusted by age, sex and centre, thus no further correction was applied. All analyses were cross-validated by a bootstrap (500×) approach to test the reliability of the identified cut-off values.
Subsequently, sample size requirements for a future neuroprotective trial were estimated. Sample size calculation followed the Freedman formula to compute the number of events (Freedman, 1982) and a parametric estimation of the overall probability of an uncensored observation by the end of the study (Klein et al., 2014). A categorical definitive end point (defined disease phenoconversion), with two groups (placebo versus a single-dose of active treatment), two-sided α = 0.05, and 80% power was assumed. An agent that reduces phenoconversion with HR = 0.5 was assumed. Therefore, the number of events needed is 65. A time-to-event analysis was performed, with phenoconversion as the end-point and the risk factors obtained by the logistic regression analysis as screening criteria. The Weibull fit of the Kaplan-Meier curve was used as estimate of the survival function.
Finally, we performed an explorative analysis to investigate whether baseline clinical and imaging features were able to predict phenoconversion to Parkinson’s disease or to dementia with Lewy bodies. To reduce the number of the variables and to minimize multicollinearity a principal component analysis (PCA) was applied to DAT-SPECT variables. Then, a random forest classifier was trained to identify the best predictors of conversion (variable importance). The two most significant predictors were fed to a Bayesian classifier to visualize the likely decision boundary on the two predictors plane. Statistical analyses were performed using MATLAB R2018B (The MathWorks, Inc., Natick, MA, USA).
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
A total of 344 DAT-SPECT of iRBD patients and 256 DAT-SPECT of healthy controls from nine centres were collected (Supplementary Table 2). One hundred and eighty iRBD patients (52.3%) were included in a previous study (Postuma et al., 2019), but in the present study the patients had a longer (2-year) follow-up.
Some of the DAT-SPECT scans were not readable by the DaTQUANTTM software or were excluded from the analysis for either insufficient quality of images or other technical reasons. Moreover, the availability of the requested clinical features was checked in each patient and only those with all clinical features were included in the study. In total, 263 iRBD patients and 243 control subjects were included in the analysis. Clinical and demographic data of the two groups are summarized in Table 1. Thirteen control subjects were excluded because the scans were not readable by the software for technical reasons. Among the 81 iRBD patients who were excluded, 62 were ruled out because scans were not managed by the semiquantification software, four for insufficient quality and 15 for missing clinical variables. No significant differences were found in demographic and clinical data (including conversion rate) between these excluded patients and those entering the statistical model. Raw and adjusted DAT-SPECT data are shown in Fig. 1.
Table 1.
Clinical and demographic data of iRBD patients and controls
| iRBD | Controls | |
|---|---|---|
| n | 263 | 243 |
| Age, years | 67.6 ± 7.3 | 67.2 ± 10.1 |
| Sex, males | 229 (87.1%) | 110 (45.3%) |
| MMSE score | 27.2 ± 2.3 | – |
| MDS-UPDRS-III score | 5.1 ± 4.6 | – |
| Constipation | 111 (42.2%) | – |
| Hyposmia | 159 (60.5%) | – |
| Follow-up, months | 26.2 ± 20.8 | – |
| Phenoconversion | 52 (19.8%) | – |
| Parkinson’s disease | 33 (63.5%) | |
| Dementia with Lewy bodies | 18 (34.6%) | |
| Multiple system atrophy | 1 (1.9%) |
.
Figure 1.
Box plot of raw (A) and adjusted (B) DAT-SPECT data. Imaging data were adjusted by age, sex and centre. The x-axis shows DAT-SPECT specific to non-displaceable binding ratios (SBRs). The y-axis shows the centres involved in the study. LAH = least affected hemisphere; MAH = most affected hemisphere.
ROC analysis between iRBD and control subjects
Area under the curve (AUC) values for each DAT-SPECT variable varied from 0.51 to 0.62 and are shown in Supplementary Table 3. Overall, 77 of 263 iRBD patients (29.3%) had an abnormal scan [defined as at least 1.5 standard deviations (SD) below the average value of controls, adjusted for age, in at least one putamen].
Survival analysis
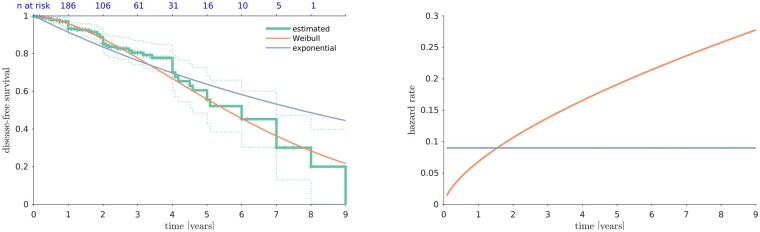
Fifty-two patients (19.8%) developed an overt synucleinopathy over an average follow-up time of ∼2 years (Table 1 and Fig. 2). Mean conversion time was 24.3 ± 26.4 (median 20) months after DAT-SPECT. The risk of conversion over time did not follow an exponential function (i.e. constant hazard rate) but was best expressed by a Weibull distribution (increasing hazard rate). Therefore, it is not appropriate to calculate a fixed annual conversion rate. In the present sample, the risk of conversion was of 14.7% after 2 years, 30% after 4 years, and 54.8% after 6 years.
Figure 2.
Disease free survival and hazard rate over time in iRBD patients. The left plot shows disease-free survival (i.e. free of parkinsonism and dementia) over time (years) in iRBD patients. The right plot shows the hazard rate over time (years) in iRBD patients computed on the exponential and Weibull survivor functions.
Kaplan-Meyer curves of DAT-SPECT and clinical features are shown in Supplementary Fig. 1. On Cox proportional hazards analysis, all baseline variables—except hyposmia—significantly predicted outcome (Table 2). To make the results usable in the context of a single centre, the adjusted cut-off values were complemented with their equivalent numbers on raw values, with the addition of minimum and maximum bounds. These bounds are computed as min/max on the whole raw data variability due to the covariates (centre, age and sex), constrained to the adjusted cut-off values. This means that, for instance, the range of values on raw data for the MMSE adjusted cut-off = 26 is between 24 and 28. Therefore, abnormal values are those <24.
Table 2.
Baseline predictors of follow-up phenoconversion to a synucleinopathy in iRBD patients
| Variable | Raw cut-off | Adjusted cut-off (bounds) | HR (95% CI) |
|---|---|---|---|
| aPutamen MAH | 1.98 | 2.14 (1.31–2.85) | 4.35 (2.35–8.05) |
| aCaudate MAH | 2.49 | 2.67 (1.69–3.53) | 3.17 (1.73–5.80) |
| aPutamen LAH | 2.17 | 2.34 (1.52–3.05) | 4.00 (2.13–7.53) |
| aCaudate LAH | 2.33 | 2.48 (1.50–3.31) | 3.03 (1.74–5.29) |
| aP/C ratio Left | 0.75 | 0.75 (0.68–0.78) | 2.51 (1.44–4.39) |
| aP/C ratio Right | 0.75 | 0.75 (0.68–0.78) | 2.53 (1.45–4.40) |
| aPutamenAs | 9.49 | 8.85 (7.25–13.18) | 2.83 (1.62–4.95) |
| aCaudateAs | 10.66 | 10.42 (8.57–12.50) | 3.30 (1.89–5.75) |
| aMMSE | 26.37 | 26.37 (24.02–28.22) | 2.09 (1.17–3.75) |
| aUPDRSIII | 7.36 | 7.36 (1.94–12.85) | 1.88 (1.05–3.38) |
| Constipation | n/a | n/a | 2.24 (1.23–4.08) |
| Hyposmia | n/a | n/a | 2.12 (0.95–4.72) |
| Age | 70 | n/a | 2.91 (1.64–5.18) |
| Combined features | −0.49 | n/a | 5.71 (2.85–11.43) |
As = asymmetry; CI = confidence interval; LAH = least affected hemisphere; MAH = most affected hemisphere; n/a = not applicable.
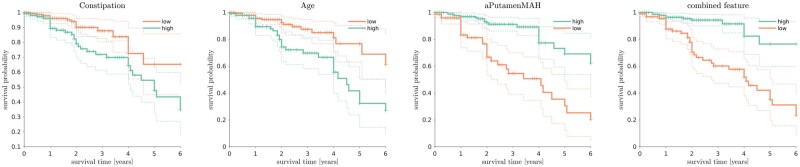
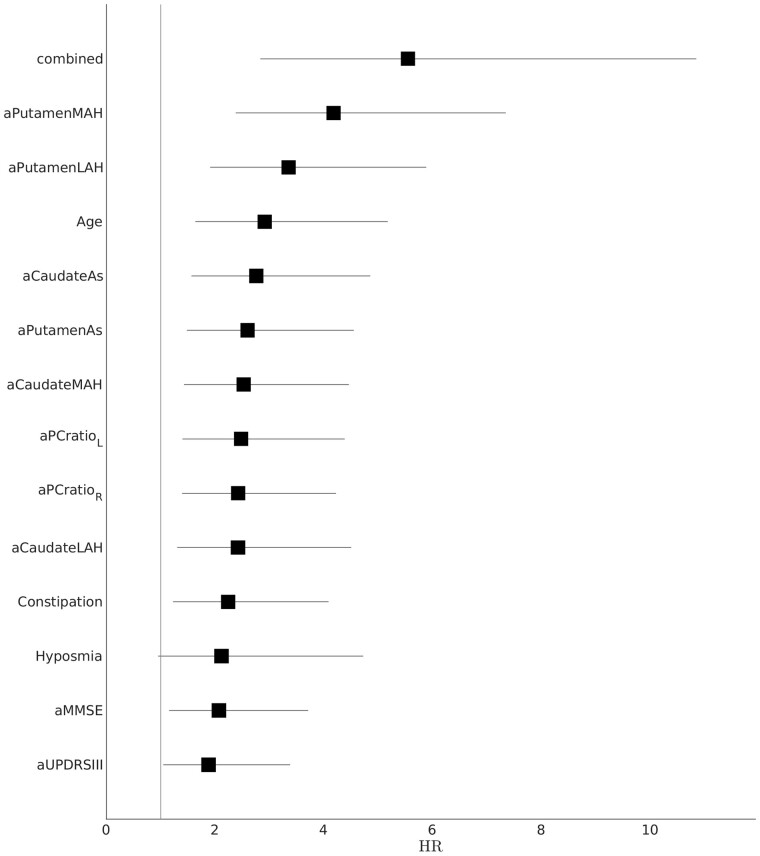
Putamen SBR in the most affected hemisphere reached the highest HR numerically (HR = 4.35) among DAT-SPECT variables and was therefore included in subsequent analysis. The generalized logistic regression was highly significant in predicting conversion (P < 0.000001), and the best combination of risk factors included the most affected hemisphere putamen SBR (cut-off 2.14, bounds 1.31–2.85; P < 0.000001), constipation, (P < 0.000001) and age (cut-off 70; P = 0.00015). The combined features obtained from the generalized logistic regression achieved an HR of 5.71 (Fig. 3) and were numerically higher than the other risk factor HRs (Fig. 4), although not significantly higher than most other risk factor HRs. However, the combined features’ HR was significantly higher than the clinical data HR (P < 0.05).
Figure 3.
Kaplan-Meier disease-free survival plots for iRBD patients according to the best predictors of phenoconversion and the combined feature obtained by the generalized logistic regression.
Figure 4.
Forest plot showing the risk factors’ HR. The lines show 95% confidence intervals. LAH = least affected hemisphere; MAH = most affected hemisphere.
Sample size calculation
Sample size requirements for a future neuroprotective trial are summarized in Table 3. The number of events needed to achieve statistical significance and the number of patients per arm, stratified according to trial duration, are reported. In this analysis, the combined features did not have the lowest number because the sample size analysis only considered the arm of patients with abnormal values, while the HR accounts for both patients below or above the cut-off, and the distance between the two lines in Kaplan-Meier analysis.
Table 3.
Sample size requirements for a future neuroprotective trial
| Number of patients per arm |
|||
|---|---|---|---|
| 1-year duration | 2-year duration | 3-year duration | |
| All iRBD | 1055 | 352 | 190 |
| Constipation | 678 | 245 | 140 |
| Age | 653 | 222 | 123 |
| Putamen most affected hemisphere | 408 | 155 | 93 |
| Combined features | 517 | 185 | 106 |
Comparison between Parkinson’s disease and dementia with Lewy bodies converters
The PCA on DAT-SPECT data identified four components (Supplementary Fig. 2). According to the random forest classifier, the best predictors were the MMSE score and PCA 3 (Supplementary Fig. 3), which was mainly expressed by the caudate SBR asymmetry. Therefore, it may be suggested that patients with higher MMSE and lower caudate asymmetry are more likely to develop Parkinson’s disease, while patients with lower MMSE and higher caudate asymmetry are more likely to develop dementia with Lewy bodies (Supplementary Fig. 4). However, the caudate asymmetry was not by itself a significant predictor of dementia with Lewy bodies versus Parkinson’s disease phenoconversion [AUC 0.63 (0.45–0.77)]. On the contrary, the MMSE was an independent predictor of dementia, as expected [AUC 0.71 (0.56–0.83)]. More advanced analysis was not appropriate due to the limited number of converted patients. Supplementary Fig. 5 shows individual DAT-SPECT data, according to the final diagnosis (i.e. Parkinson’s disease, dementia with Lewy bodies or still idiopathic).
Discussion
This large international, multicentre study aimed to investigate the combined value of DAT-SPECT and clinical features in predicting future phenoconversion to synucleinopathy in iRBD patients. Fifty-two patients (19.8%) developed an overt synucleinopathy after an average follow-up time of 2 years, and the risk of phenoconversion was 14.7% after 2 years, 30% after 4 years, and 54.8% after 6 years. This was higher than previous data showing a risk of 17.9% after 3 years, 31.3% after 5 years, and 51.4% after 8 years (Postuma et al., 2019). However, as the present dataset can be considered to mostly not overlap with the previous international RBD study (Postuma et al., 2019), this result may be explained by the heterogeneity of the iRBD patients. Another possible explanation could be that in the present study the phenoconversion time was calculated starting from the DAT-SPECT date, whereas in previous studies it was calculated from RBD diagnosis. We found that risk of conversion was not a fixed annual rate but rather an increasing hazard rate. While complex and still partially known pathophysiological factors may play a role, increasing age and role of co-morbidities may at least partially explain such an increasing hazard rate, since it is known that ageing is a significant risk factor for phenoconversion, as confirmed in this present study.
For multivariate analysis, the best combination of risk factors for phenoconversion included putaminal dopaminergic dysfunction of the most affected hemisphere seen on imaging, constipation, and age over 70 years.
Several studies have shown nigro-striatal dopaminergic dysfunction in iRBD patients (Bauckneht et al., 2018). Moreover, nigro-putaminal dopaminergic deafferentation has been proposed as a suitable prognostic marker of subsequent phenoconversion to full-blown neurodegenerative disease (Iranzo et al., 2017; Li et al., 2017). However, available data on presynaptic radionuclide neuroimaging in patients with iRBD are largely heterogeneous, especially for DAT-SPECT analysis and semiquantification methodology (Bauckneht et al., 2018). It is interesting to note that the power of DAT-SPECT in predicting phenoconversion of iRBD patients was only moderate (HR = 1.98) in the largest study conducted so far (Postuma et al., 2019). However, there, the entire examination was simply dichotomized to abnormal versus normal. In contrast, in the present study, with standardized semiquantification, all eight DAT-SPECT features achieved a good prediction power, with putaminal SBR in the more affected hemisphere being the single highest risk factor (HR = 4.35).
Subsequently, we performed a multivariate analysis in order to determine the best combination of clinal and imaging risk factors, and only age and constipation were retained in the model along with the most affected hemisphere putaminal SBR. The presence of age as a significant risk factor was not surprising. Indeed, age is one the most important risk factors for any neurodegenerative disease, and is one of the major modifiers in the computation of the prodromal Parkinson’s disease risk (Berg et al., 2015). Constipation was the only measure of dysautonomia in our study. Early signs of dysautonomia have been repeatedly found as significant predictors of phenoconversion in RBD patients (Li et al., 2017; Postuma et al., 2019), therefore, in our sample, constipation likely expressed the part of the variance related to dysautonomia. It is, however, possible that performing more comprehensive and advanced measures of dysautonomia may further increase the weight of this aspect in predicting future phenocoversion in iRBD patients.
We define cut-offs that need to be applied and verified in independent cohorts of iRBD patients. The presence of constipation was defined by using well-known, validated and easily reproducible questionnaires, and a cut-off of 70 years of age resulted from the survival analysis. As for the DAT-SPECT feature, considering the retrospective nature of the study, which is its main limitation, only the semiquantification procedure has been harmonized, while the acquisition and reconstruction SPECT protocols, as well as the SPECT equipment were inhomogeneous between centres. Thus, for each feature, we calculated the boundaries expressing the variability due to the co-variates (centre, age and sex), constrained to the adjusted cut-off values. This means that, as long as the EANM guidelines are followed in performing 123I-FP-CIT-SPECT scans, and the data are semiquantified with the DaTQUANTTM tool, a restrictive threshold of 1.31 SBR (the lowest bound, Table 2) in the MAH putamen should identify those iRBD patients at high risk of short-time phenoconversion to an overt synucleinopathy. This threshold should be confirmed and validated in future, independent studies.
This approach would allow a sensitive reduction in sample size requirement for future neuroprotection trials. Indeed, according to our results, selecting iRBD patients over 70 years of age, with constipation and reduced nigro-putaminal dopaminergic function for a 2-year clinical trial would notably decrease the sample size requirement to ∼185 patients per arm. Other factors need to be taken into account to calculate the exact sample size, according to the specifics of a clinical trial.
Finally, we performed an exploratory analysis to investigate whether, based on baseline DAT-SPECT and clinical features, iRBD patients eventually developing Parkinson’s disease could be differentiated from those eventually developing dementia with Lewy bodies. In our sample, iRBD patients with higher MMSE and lower caudate SBR asymmetry were more likely to develop Parkinson’s disease, while patients with lower MMSE and higher caudate SBR asymmetry were more likely to develop dementia with Lewy bodies. These findings are in agreement with previous data showing that iRBD patients with cognitive impairment are more likely to develop dementia (Génier Marchand et al., 2018). Moreover, patients with dementia with Lewy bodies have clear nigro-striatal dopaminergic impairment, especially at the caudate level (Walker et al., 2004), and a lower caudate/putamen ratio compared with Parkinson’s disease patients (Joling et al., 2018), On the other hand, Parkinson’s disease patients have greater putamen asymmetry compared with patients with dementia with Lewy bodies (Walker et al., 2004). Indeed, it has been suggested that the nigro-caudate dopaminergic function is more related to cognition, while the nigro-putaminal function would be more related to motor symptoms (Nobili et al., 2010). Therefore, it may be argued that, in our sample, caudate asymmetry may reflect an early involvement of the nigro-caudate dopaminergic pathway that would anticipate the emergence of dementia with Lewy bodies instead of Parkinson’s disease. This result should be taken with caution because of the limited number of converted patients. Indeed, the same DAT-SPECT signatures (i.e. Parkinson’s disease patients with greater putamen asymmetry and dementia with Lewy bodies patients with lower nigro-caudate dopaminergic function) were not present in our dataset (data not shown).
In the present study, 1 of 52 converters (1.9%) developed multiple system atrophy. While a median multiple system atrophy conversion rate of 3.8% in iRBD patients has been reported in a recent meta-analysis (Galbiati et al., 2019), the slightly lower conversion rate in the present study may be explained by the still relatively limited number of converters. The Parkinson’s disease to dementia with Lewy bodies ratio in conversion rates may also vary between studies. In a recent meta-analysis, 44% among all converters developed Parkinson’s disease and 25% developed dementia with Lewy bodies (Galbiati et al., 2019), thus close to a 2:1 ratio and similar to our ratio since among the 52 converters in our study, 63.5% developed Parkinson’s disease and 34.6% developed dementia with Lewy bodies.
About half of the patients enrolled in the present study were also included in a previous study (Postuma et al., 2019). However, the patients in the present study had a longer follow-up. Moreover, the novelty of the present study is that DAT-SPECT data were analysed by a standardized semiquantitative analysis, while in the previous study a dichotomous (abnormal versus normal) approach was used. Furthermore, in the present study, for the first time imaging and clinical risk factors were combined for prediction using a multivariate approach.
The main limitation of the study is that has been conducted retrospectively, with already existing DAT-SPECT data. Therefore, acquisition and the reconstruction protocols were not harmonized prior to the study. The main strength of the present study, besides the remarkable sample size, is that the semiquantification of DAT-SPECT scans has been conducted by means of a standardized postprocessing process, with a widely available registered tool.
In conclusion, we found that iRBD patients older than 70, with constipation and reduced nigro-putaminal dopaminergic function in the more affected hemisphere (SBR < 1.31) are at high risk of phenoconversion to an overt synucleinopathy within 3 years of follow-up. These data could be used to stratify patients to be enrolled in a future neuroprotective clinical trial.
Supplementary Material
Acknowledgements
This work was developed within the framework of the DINOGMI Department of Excellence of MIUR 2018-2022 (legge 232 del 2016).
Funding
The Oxford Discovery cohort is funded by the Monument Trust Discovery Award from Parkinson’s UK and supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre based at Oxford University Hospitals NHS Trust and University of Oxford, the NIHR Clinical Research Network and the Dementias and Neurodegenerative Diseases Research Network (DeNDRoN). The funding for DAT-SPECT at one centre (Mayo Clinic Rochester) was provided by GE Healthcare; the none of the staff at GE Healthcare were involved in the analyses or interpretation of the data, or in the writing or review of this manuscript. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
D.A. received fees from Fidia for lectures and board participation. K.S. has received consultancy and lecture fees from UCB, Sanofi, Angelini and Stada and participated in clinical trials: Flamel-Avadel, Jazz and Luitpold Pharmaceutical. B.B. has served as an investigator for clinical trials sponsored by Biogen, Alector, EIP Pharma and GE Healthcare. He serves on the Scientific Advisory Board of the Tau Consortium. He receives research support from the NIH, the Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program, the Little Family Foundation, and the Turner Family Foundation. V.C.D.C. has received speaking honoraria from Orkyn, LVL medical, HomePerf and Aguettant. She receives research support from Orkyn and Aguettant. N.T. received a research grant from Novartis Pharma K.K. S.M. received speaking honoraria from G.E. healthcare. P.D. received funding from Czech Ministry of Health, grant No. 15-25602A and No. NV19-04-00120, Czech Science Foundation, grant No. 16-07879S, European Union’s Horizon 2020 research and innovation programme, grant No. 633190, advisory board payment from Alexion pharmaceuticals, and honoraria for clinical trials from BenevolentAI Bio Limited and Retrophin. V.L. is a consultant for Bayer Schering Pharma, Philips Molecular Imaging, Piramal Imaging, AVID Radiopharmaceuticals and GE Healthcare and receives research support from GE Healthcare, Siemens Molecular Imaging, and AVID Radiopharmaceuticals. F.N. received fees from BIAL for consultation, from G.E. healthcare for teaching talks, from Roche for board participation. All other authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
Glossary
- DAT =
dopamine transporter
- iRBD =
idiopathic REM sleep behaviour disorder; MDS-UPDRS-III = Movement Disorder Society-Unified Parkinson's Disease Rating Scale, motor section
- MMSE =
Mini-Mental State Examination
- SBR =
specific to non-displaceable binding ratio
- SPECT =
single photon emission tomography
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edn. Washington, DC: American Psychiatric Publishing, Inc.; 2013.
- Arnaldi D, Chincarini A, De Carli F, Famà F, Girtler N, Brugnolo A, et al. The fate of patients with REM sleep behavior disorder and Mild Cognitive Impairment. Sleep Med 2020; Feb 29: S1389-9457(20)30084-8. doi: 10.1016/j.sleep.2020.02.011 [DOI] [PubMed] [Google Scholar]
- Bauckneht M, Chincarini A, De Carli F, Terzaghi M, Morbelli S, Nobili F, et al. Presynaptic dopaminergic neuroimaging in REM sleep behavior disorder: a systematic review and meta-analysis. Sleep Med Rev 2018; 41: 266–74. [DOI] [PubMed] [Google Scholar]
- Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, et al. MDS research criteria for prodromal Parkinson's disease. Mov Disord 2015; 30: 1600–9. [DOI] [PubMed] [Google Scholar]
- Briner HR, Simmen D.. Smell diskettes as screening test of olfaction. Rhinology 1999; 37: 145–8. [PubMed] [Google Scholar]
- Conrado DJ, Nicholas T, Tsai K, Macha S, Sinha V, Stone J, et al. Dopamine transporter neuroimaging as an enrichment biomarker in Early Parkinson's disease clinical trials: a disease progression modeling analysis. Clin Transl Sci 2018; 11: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcourt J, Booij J, Tatsch K, Varrone A, Vander Borght T, Kapucu OL, et al. EANM procedure guidelines for brain neurotransmission SPECT using (123)I-labelled dopamine transporter ligands, version 2. Eur J Nucl Med Mol Imaging 2010; 37: 443–50. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Dann M.. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 1984; 32: 489–502. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR.. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res 1975; 12: 189–98. [DOI] [PubMed] [Google Scholar]
- Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. Stat Med 1982; 1: 121–9. [DOI] [PubMed] [Google Scholar]
- Galbiati A, Verga L, Giora E, Zucconi M, Ferini-Strambi L.. The risk of neurodegeneration in REM sleep behavior disorder: a systematic review and meta-analysis of longitudinal studies. Sleep Med Rev 2019; 43: 37–46. [DOI] [PubMed] [Google Scholar]
- Génier Marchand D, Postuma RB, Escudier F, De Roy J, Pelletier A, Montplaisir J, et al. How does dementia with Lewy bodies start? prodromal cognitive changes in REM sleep behavior disorder. Ann Neurol 2018; 83: 1016–26. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord 2007; 22: 41–7. [DOI] [PubMed] [Google Scholar]
- Hentz JG, Mehta SH, Shill HA, Driver-Dunckley E, Beach TG, Adler CH.. Simplified conversion method for unified Parkinson's disease rating scale motor examinations. Mov Disord 2015; 30: 1967–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G.. ‘Sniffin’ sticks': olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 1997; 22: 39–52. [DOI] [PubMed] [Google Scholar]
- Iranzo A, Santamaria J, Valldeoriola F, Serradell M, Salamero M, Gaig C, et al. Dopamine transporter imaging deficit predicts early transition to synucleinopathy in idiopathic REM sleep behavior disorder. Ann Neurol 2017; 82: 419–28. [DOI] [PubMed] [Google Scholar]
- Joling M, Vriend C, van der Zande JJ, Lemstra AW, van den Heuvel OA, Booij J, et al. Lower (123)I-FP-CIT binding to the striatal dopamine transporter, but not to the extrastriatal serotonin transporter, in Parkinson's disease compared with dementia with Lewy bodies. Neuroimage Clin 2018; 19: 130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JP, van Houwelingen HC, Ibrahim JG, Scheike TH.. Handbook of survival analysis. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2014. [Google Scholar]
- Kobayashi M, Saito S, Kobayakawa T, Deguchi Y, Costanzo RM.. Cross-cultural comparison of data using the odor stick identification test for Japanese (OSIT-J). Chem Senses 2006; 31: 335–42. [DOI] [PubMed] [Google Scholar]
- Li Y, Kang W, Yang Q, Zhang L, Zhang L, Dong F, et al. Predictive markers for early conversion of iRBD to neurodegenerative synucleinopathy diseases. Neurology 2017; 88: 1493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltais DD, Jordan LG 3rd, Min HK, Miyagawa T, Pryzbleski S, Lesnick TG, et al. Confirmation of 123I-FP-CIT SPECT Quantification Methods in Dementia with Lewy Bodies and Other Neurodegenerative Disorders. J Nucl Med 2020; 61: 1628–1635. [DOI] [PMC free article] [PubMed]
- McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017; 89: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Ferman TJ, Thomas AJ, Blanc F, Boeve BF, Fujishiro H, et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology 2020; 94: 743–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–9. [DOI] [PubMed] [Google Scholar]
- Nobili F, Campus C, Arnaldi D, De Carli F, Cabassi G, Brugnolo A, et al. Cognitive-nigrostriatal relationships in de novo, drug-naive Parkinson's disease patients: a [I-123]FP-CIT SPECT study. Mov Disord 2010; 25: 35–43. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015; 30: 1591–601. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Iranzo A, Hu M, Hogl B, Boeve BF, Manni R, et al. Risk and predictors of dementia and Parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain 2019; 142: 744–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk-Krolikowski K, Tomlinson P, Nithi K, Wade-Martins R, Talbot K, Ben-Shlomo Y, et al. The influence of age and gender on motor and non-motor features of early Parkinson's disease: initial findings from the Oxford Parkinson Disease Center (OPDC) discovery cohort. Parkinsonism Relat Disord 2014; 20: 99–105. [DOI] [PubMed] [Google Scholar]
- van Steenoven I, Aarsland D, Hurtig H, Chen-Plotkin A, Duda JE, Rick J, et al. Conversion between mini-mental state examination, montreal cognitive assessment, and dementia rating scale-2 scores in Parkinson's disease. Mov Disord 2014; 29: 1809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Marinus J, Stiggelbout AM, Van Hilten JJ.. Assessment of autonomic dysfunction in Parkinson's disease: the SCOPA-AUT. Mov Disord 2004; 19: 1306–12. [DOI] [PubMed] [Google Scholar]
- Walker Z, Costa DC, Walker RW, Lee L, Livingston G, Jaros E, et al. Striatal dopamine transporter in dementia with Lewy bodies and Parkinson disease: a comparison. Neurology 2004; 62: 1568–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.