Abstract
Objectives
To analyse the impact of cefiderocol use on outcome in patients admitted to the ICU for severe COVID-19 and further diagnosed with carbapenem-resistant Acinetobacter baumannii (CR-Ab) infection.
Methods
Retrospective multicentre observational study was performed at four Italian hospitals, from January 2020 to April 2021. Adult patients admitted to ICU for severe COVID-19 and further diagnosed with CR-Ab infections were enrolled. Patients treated with cefiderocol, as compassionate use, for at least 72 h were compared with those receiving alternative regimens. Primary endpoint was all-cause 28 day mortality. The impact of cefiderocol on mortality was evaluated by multivariable Cox regression model.
Results
In total, 107 patients were enrolled (76% male, median age 65 years). The median time from ICU admission to CR-Ab infection diagnosis was 14 (IQR 8–20) days, and the main types of CR-Ab infections were bloodstream infection (58%) and lower respiratory tract infection (41%). Cefiderocol was administered to 42 patients within a median of 2 (IQR 1–4) days after CR-Ab infection diagnosis and as monotherapy in all cases. The remaining patients received colistin, mostly (82%) administered as combination therapy. All-cause 28 day mortality rate was 57%, without differences between groups (cefiderocol 55% versus colistin 58% P = 0.70). In multivariable analysis, the independent risk factor for mortality was SOFA score (HR 1.24, 95% CI 1.15–1.38, P < 0.001). Cefiderocol was associated with a non-significant lower mortality risk (HR 0.64, 95% CI 0.38–1.08, P = 0.10).
Conclusions
Our study confirms the potential role of cefiderocol in the treatment of CR-Ab infection, but larger clinical studies are needed.
Introduction
Antibiotic resistance has been recognized as a public health concern due to its impact on patient morbidity and mortality.1 Carbapenem-resistant Acinetobacter baumannii (CR-Ab) is the top-ranked pathogen in the WHO priority list.2 Indeed, CR-Ab infections are associated with very high mortality rates, partially due to the hosts, which are generally represented by critically ill and/or immunocompromised patients, and due to the limited therapeutic options, which are mostly unable to safely achieve the optimal pharmacokinetic/pharmacodynamic (PK/PD) targets for severe infections.3 In addition, the burden of CR-Ab has been increasing during the COVID-19 pandemic; the weakening in antimicrobial resistance surveillance activities, the need for reorganizing ICU spaces and activities during pandemic waves and the overuse of antibiotics for COVID-19 pneumonia are some of the factors contributing to this increase.4,5
Cefiderocol is a new drug developed to overcome challenges presented by common carbapenem-resistance mechanisms; it is active against a variety of drug-resistant pathogens including CR-Ab.6 Clinical evidence on its efficacy in treating severe CR-Ab infections is still limited, consisting of one randomized controlled trial (RCT) and a few case series.7–10 The RCT reported higher rates of mortality at 14 and 28 days in patients with severe CR-Ab infections treated with cefiderocol versus ‘best available therapy’,7 while the case series reported high rates of clinical cure and survival associated with cefiderocol use.8–10 To overcome these controversial results, additional clinical data from either RCT or observational studies on the use of cefiderocol in severe CR-Ab infections are needed.
The aim of our study was to describe the antibiotic treatment of patients diagnosed with CR-Ab infection during COVID-19 pandemic; the impact of cefiderocol use on 28 day mortality was further investigated.
Methods
Study design
We performed a retrospective multicentre cohort study of patients with CR-Ab infection admitted to the ICU from January 2020 to April 2021.
Carbapenem resistance was defined according to EUCAST criteria.11 Infection was defined according to CDC criteria.12 The databases of Microbiology Laboratories were used as a data source. Clinical charts and hospital records of each patient with CR-Ab isolation during the study period were reviewed to assess the presence of CR-Ab infection and for data collection. CR-Ab infection was assessed by a senior investigator (R.P.) blinded to therapeutic management and patient outcome.
The study was approved by our Ethics Committee (Comitato Etico Indipendente di Area Vasta Emilia Centro, no. 283/2020/Oss/AOUBo).
Population
All consecutive adult (≥18 years) patients admitted to the ICU and diagnosed with CR-Ab infection during the study period were included. Exclusion criteria were: (i) rectal or respiratory CR-Ab colonization without symptoms and/or signs of infection; and (ii) clinical data not available. Patients were considered only once, at the time of first CR-Ab infection diagnosis.
Setting
Participating hospitals consist of three facilities (IRCCS Policlinico di Sant’Orsola, Bellaria Hospital and Maggiore Hospital) from the metropolitan area of Bologna, Emilia Romagna Region, with on average 75 ICU beds for adult patients. The other centre is San Salvatore Hospital from Pesaro, Marche region, with 40 ICU beds. Due to the COVID-19 pandemic, during the study period the number of ICU beds increased and they were mostly dedicated to the management of patients with critical COVID-19. An infection control programme was active in all the sites and included weekly surveillance for CR-Ab on respiratory and rectal specimens during ICU stay.
Variables and definitions
For the assessment of endpoints, Time 0 was defined as the day of sample collection yielding CR-Ab infection. The primary endpoint was all-cause mortality within 28 days after CR-Ab infection diagnosis (Time 0). Secondary endpoints were assessed at 14 days after CR-Ab infection diagnosis and included clinical cure, defined by resolution of fever and hypotension, and microbiological cure, defined by the clearance of follow-up blood cultures and/or respiratory samples that were performed in all patients as per local policy.
The main exposure variable was treatment with cefiderocol for at least 72 h, assessed from infection onset to ICU discharge or death. Cefiderocol was obtained through the compassionate Shionogi Europe Early Access Program in patients fulfilling the following criteria: age ≥18 years, hospitalization, clinically documented infection, identification or suspicion of carbapenem-resistant Gram-negative pathogen and a failed/unavailable existing treatment option due to other concurrent clinical conditions. Exclusion criteria included: history of hypersensitivity/allergic reaction to cephalosporins, penicillins or carbapenems, central nervous system infection, pregnancy or breastfeeding and uncontrolled seizure disorders. Stocks unemployed on patients for whom they were originally requested were initiated in patients for whom a new request was performed, according to the patient inclusion and exclusion criteria of the programme, under the responsibility of the clinician, and after obtaining Ethics Committee approval case by case. The drug was administered intravenously at a standard dose of 2 g every 8 h, with dosage adjustments for renal impairment as recommended by the manufacturers.13
The other exposure variables were assessed at ICU admission and included age, sex and underlying conditions recorded according to the Charlson comorbidity index.14 Immunosuppression included neutropenia (neutrophil count <500 cells/mm3); solid organ transplantation; haematopoietic stem cell transplantation; corticosteroid therapy at a dosage higher than or equivalent to prednisone 16 mg/day for 15 days; or uncontrolled HIV infection (CD4 cell count <200 cells/mm3).
Infection types were established according to CDC criteria.12 In the absence of a recognized source, bloodstream infection (BSI) was considered as the primary source. BSI was defined as complicated when the infection source was not fully removable.15 Two investigators (R.P., Z.P.) independently assessed the diagnosis of lower respiratory tract infection (LRTI) following CDC criteria.12 Disagreements were resolved through discussion or by consulting a third senior investigator (M.G.). Clinical severity at infection onset was assessed according to SOFA score and new septic shock criteria.16
Empirical therapy was defined as antibiotics administered before the susceptibility report was available. It was considered appropriate when at least one in vitro active drug (according to the susceptibility pattern of the isolate) was administered within 24 h from infection onset. Delayed or no active antibiotic administration within this period was considered as inappropriate empirical therapy.
Regarding the SARS-CoV-2 infection, defined as a positive RT-PCR test on nasopharyngeal swabs, clinical severity at ICU admission, antiviral and immunomodulatory treatment and the need for mechanical ventilation were collected. Clinical severity of SARS-CoV-2 infection was defined according to WHO criteria.17
Microbiology
During the study period, CR-Ab isolates were tested for antimicrobial susceptibility using the MicroScan Walkaway-96 automated system (Beckman Coulter, Brea, CA, USA). Antimicrobial susceptibility testing for cefiderocol was performed with a broth microdilution panel using iron-depleted CAMHB (ID-CAMHB).18 In brief, ID-CAMHB was prepared by the removal of divalent cations using a cation-binding Chelex® 100 resin (Bio-Rad Laboratories, Hercules, CA, USA). Cation-depleted medium was filtered with a 0.2 μm pore-size filter and autoclaved. Then, medium was supplemented with CaCl2, MgCl2 and ZnSo4 at final concentrations of 25 mg/L, 12.5 mg/L and 1.0 mg/L, respectively. The cefiderocol powder (Shionogi & Co. Ltd) was dissolved in sterile normal saline and the 96-well plate microdilution panel was inoculated with a final concentration of 5 × 105 cfu/mL and incubated for 20 h at 35 ± 1°C.
The MICs of cefiderocol were determined following EUCAST guidelines by evaluating the relative growth reduction (button of <1 mm) in comparison to the ID-CAMHB growth control well.19 For cefiderocol MIC ≥2 mg/L, antimicrobial resistance was confirmed by disc diffusion antimicrobial susceptibility testing, according to EUCAST standard methodology for non-fastidious organisms on regular Mueller–Hinton agar.
Blood cultures were incubated using the Bactec FX Automated Blood Culture System (Becton Dickinson, Franklin Lakes, NJ, USA). All positive blood cultures were processed with the MALDI Biotyper MALDI-TOF MS system (Bruker Daltonics, Bremen, Germany) for rapid and reliable species identification of microorganisms.
Statistical analysis
For descriptive analysis, categorical variables were presented as absolute numbers and their relative frequencies and continuous variables were presented as mean ± standard deviation (SD) if normally distributed, or as median and IQR if non-normally distributed. Patients treated with and without cefiderocol were compared using the χ2 test, the exact test was used when appropriate; continuous variables were compared using the non-parametric Mann–Whitney test. To assess the impact of cefiderocol on 28 day mortality, univariable and multivariable analyses of risk factors for 28 day mortality were performed considering cefiderocol treatment as the variable of interest. All the variables with P < 0.1 at univariable analysis were introduced in the multivariable Cox regression model using the stepwise backward approach; patients were considered from the day of infection diagnosis to death or 28 days, whichever occurred first. The analysis was further adjusted for steroid use, as this variable was different among patients treated with and without cefiderocol, and for bloodstream infection for clinical relevance. Statistical significance was defined as a P value <0.05. All the analyses were carried out using SPSS 21.00 software.
Results
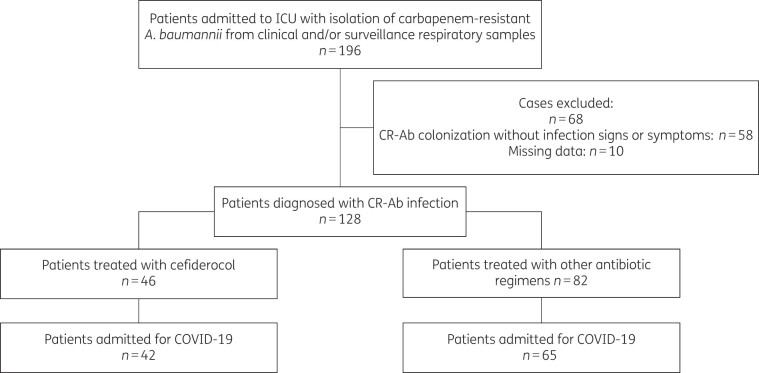
Over the study period, CR-Ab isolation was reported in 196 patients. Of them, 68 were excluded: 58 patients had CR-Ab colonization and for 10 patients clinical data were not available. Thus, 128 patients were analysed; 46 received cefiderocol and 82 received other regimens. Hospital distribution of patients is showed in Table S1 (available as Supplementary data at JAC-AMR Online), and the characteristics of the overall population are shown in Table S2. Since for most patients the underlying condition for being admitted to ICU was severe COVID-19, we decided to restrict the analysis to this subgroup in order to study a homogeneous setting (see study flow chart depicted in Figure 1).
Figure 1.
Study population flow chart.
Thus, we analysed 107 patients admitted to ICU for severe COVID-19 and further diagnosed with CR-Ab infection. Of them, 76% of patients were male, the median age was 65 (IQR 59–72) years, the median Charlson comorbidity index was 3 (IQR 2–4) and 31.8% of them had an impaired renal function. Almost all patients required mechanical ventilation (98%) for COVID-19 pneumonia. The median time from ICU admission to the diagnosis of CR-Ab infection was 14 (IQR 8–20) days. Almost all patients (105/107, 98%) were found to be CR-Ab carriers before infection diagnosis. The main types of CR-Ab infection were bloodstream infection and lower respiratory tract infection in 58% and 41% of cases, respectively. At CR-Ab infection onset, median SOFA score was 8 (IQR 6–11), and 43 (41%) patients fulfilled septic shock criteria. All-cause 14 and 28 day mortality rates were 46% and 57%, respectively (data shown in Table 1).
Table 1.
Characteristics of patients admitted to the ICU for severe COVID-19 who developed CR-Ab superinfection, and comparison between cefiderocol and other treatment groups
| Total, N = 107 | Cefiderocol, N = 42 | Other treatment, N = 65 | P a | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years, median (IQR) | 65 (59–72) | 64 (55–73) | 65 (60–71) | 0.972 |
| Male sex, n (%) | 82 (76) | 32 (76) | 50 (77) | 0.930 |
| Comorbidities | ||||
| Charlson comorbidity index, median (IQR) | 3 (2–4) | 2 (1–3) | 3 (2–4) | 0.316 |
| Immunosuppression, n (%) | 9 (8.4) | 3 (7) | 6 (9) | 0.704 |
| Impaired renal function at baseline (serum Cr >1.2 mg/dL), n (%) | 34 (31.8) | 13 (31) | 21 (32.3) | 0.883 |
| COVID-19 at time of A. baumannii infection, n (%) | ||||
| Critical COVID-19 | 103 (96) | 42 (100) | 61 (94) | 0.443 |
| Mechanical ventilation due to COVID-19 | 105 (98) | 42 (100) | 63 (97) | 0.251 |
| COVID-19 therapy | ||||
| Tocilizumab | 58 (54) | 24 (57) | 34 (52) | 0.624 |
| Steroid therapy | 89 (83) | 42 (100) | 47 (72) | <0.001 |
| A. baumannii infection | ||||
| Source of infection, n (%) | ||||
| LRTI | 44 (41) | 14 (33) | 30 (46) | 0.18 |
| BSI | 62 (58) | 27 (64) | 35 (54) | 0.28 |
| Source of A. baumannii BSI | ||||
| CVC related | 8 (13) | 2 (7.4) | 6 (17) | |
| Lower respiratory tract | 31 (50) | 17 (63) | 14 (40) | |
| Clinical severity at BSI onset | ||||
| SOFA score, median (IQR) | 8 (6–11) | 9 (5–11) | 8 (5–12) | 0.666 |
| Septic shock, n (%) | 43 (41) | 18 (46) | 25 (38) | 0.441 |
| Polymicrobial infection, n (%) | 34 (32) | 12 (27) | 22 (34) | 0.567 |
| Complicated infection, n (%) | 8 (7.5) | 5 (11.9) | 3 (4.6) | 0.153 |
| Therapeutic management of A. baumannii infection | ||||
| Appropriate empirical therapy | 41 (38) | 20 (47) | 21 (32) | 0.108 |
| Time from infection diagnosis to active antibiotic initiation, days, median (IQR) | 1 (1–3) | 1 (1–2) | 1 (1–3) | 0.628 |
| Outcomes | ||||
| Time to microbiological cure, days, median (IQR) | 7 (5–12) | 6 (4–14) | 7 (6–12) | 0.951 |
| Outcome at Day 14 | ||||
| Clinical cure (14 days) | 41 (38) | 17 (40) | 24 (36) | 0.453 |
| Microbiological cure (14 days) | 26 (24) | 12 (28) | 14 (21) | 0.246 |
| SOFA score at Day 14, median (IQR) | 0 (0–4) | 1 (0–5) | 0 (0–2) | 0.063 |
| Assessment of renal function according to RIFLE classification | ||||
| Risk (increased serum Cr ×1.5 or GFR decrease >25%) | 3 (2.8) | 1 (2.4) | 2 (3.1) | 0.831 |
| Injury (increased serum Cr ×2 or GFR decrease >50%) | 5 (4.7) | 2 (4.8) | 3 (4.6) | 0.972 |
| Failure (increased serum Cr ×3 or GFR decrease >75% or Cr ≥4 mg per 100 mL) | 1 (0.9) | 0 (0) | 1 (1.5) | 0.251 |
| End stage renal disease | 1 (0.9) | 0 | 1 | 0.421 |
| Composite acute kidney injury | 10 (9.3) | 4 (9.5) | 6 (9.2) | 0.944 |
| Death (14 days) | 50 (46) | 17 (40) | 33 (51) | 0.147 |
| Outcome at Day 28 | ||||
| Death | 61 (57) | 23 (55) | 38 (58) | 0.706 |
Cr, creatinine; CVC, central venous catheter; GFR, glomerular filtration rate.
Significant differences are highlighted in bold.
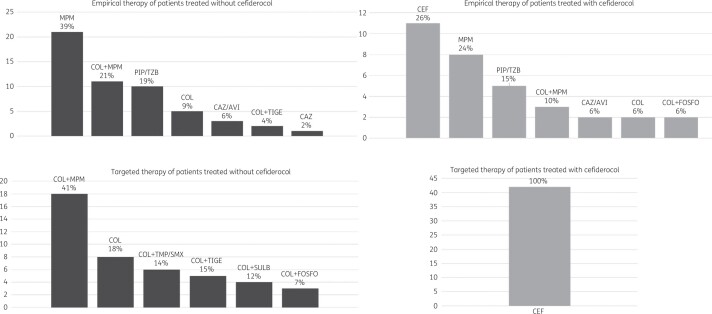
Forty-two patients (39%) were treated with cefiderocol; it was administered as empirical and definitive therapy in 11/42 (26.2%) patients and only as definitive therapy in the remaining 31/42 (73.8%) patients, and it was always employed as monotherapy (see Figure 2).
Figure 2.
Description of empirical and definitive regimens with and without cefiderocol. COL, colistin; MPM, meropenem; PIP/TZB, piperacillin/tazobactam; CAZ, ceftazidime; AVI, avibactam; TIGE, tigecycline; FOSFO, fosfomycin; TMP/SMX, trimethoprim/sulfamethoxazole; SULB, sulbactam; CEF, cefiderocol.
The median time to cefiderocol administration from CR-Ab infection diagnosis was 2 (IQR 1–4) days. Cefiderocol was tested in all isolates obtained from patients receiving this drug to confirm susceptibility: the median MIC was 1 (IQR 0.5–1) mg/L. The remaining 65 patients were treated with colistin-based regimens (data shown in Figure 2).
The only significant difference between cefiderocol and non-cefiderocol treated patients was steroid treatment for COVID-19 (100% versus 72%, P < 0.001). There were no differences in 14 day and 28 day mortality rates (data shown in Table 1). Furthermore, we analysed the subset of patients with CR-Ab BSI and found no significant differences in mortality rates in this subgroup (data shown in Table S4).
Univariable analysis of risk factors for 28 day mortality is shown in Table S3. On multivariable analysis, the independent risk factor for mortality was SOFA score (HR 1.24, 95% CI 1.15–1.38, P < 0.001); cefiderocol treatment remained in the final model as a protective factor but with a non-statistically significant value (HR 0.64, 95% CI 0.38–1.08, P = 0.10) (data shown in Table 2). The subgroup analysis including only patients with CR-Ab BSI confirmed these findings (Tables S5 and S6).
Table 2.
Multivariable analysis of independent risk factors for 28 day mortality adjusted for male sex, SOFA score, septic shock, steroid treatment for COVID-19, bloodstream infection and cefiderocol as the variable of interest
| HR (95% CI) | P | |
|---|---|---|
| SOFA score | 1.24 (1.15–1.38) | <0.001 |
| Cefiderocol | 0.64 (0.38–1.08) | 0.10 |
Discussion
We analysed a large cohort (n = 107) of patients with CR-Ab superinfection during ICU admission for severe COVID-19, of them more than one-third (39%) were treated with cefiderocol. Underlying conditions and clinical severity at CR-Ab infection onset between cefiderocol- and non-cefiderocol-treated patients were similar. On multivariable analysis for 28 day mortality, there was a non-statistically significant lower mortality in patients treated with cefiderocol.
This is one of the largest real-life experiences of cefiderocol use for severe CR-Ab infections in current clinical practice; in addition, comparison with non-cefiderocol-treated patients was performed. Although there was no randomization, the two groups were similar with regard to underlying conditions, severity of COVID-19 and severity of CR-Ab superinfection. The only difference consisted of steroid use for COVID-19, which was more frequent among the cefiderocol group. For this reason, we adjusted the multivariable analysis of risk factors for 28 day mortality for steroid use.
In our study, the main types of CR-Ab infection were BSI and LRTI, where cefiderocol has been associated with higher mortality rates in the CREDIBLE-CR trial.7 One may argue that the diagnosis of LRTI in COVID-19 patients is extremely difficult and subjective. Indeed, clinical and radiological criteria may overlap with COVID-19, and laboratory criteria may be altered by immunomodulatory treatments administered for the hyperinflammatory syndrome.20,21 To overcome these issues two senior ID consultants, blinded to antibiotic treatments and patient outcome, reviewed all the records of patients with a CR-Ab isolation to establish the final diagnosis of infection, with a third advisor in case of disagreements. In addition, we adjusted the multivariable analysis for the presence of BSI considering this as an unequivocal index of true and severe infection. Furthermore, we performed a subgroup analysis of patients with BSI without finding differences in outcome.
A compassionate programme allowed access to cefiderocol during the study period. In such contexts, administrative and ethical issues may cause a delay in drug onset that is crucial in time-dependent severe infections22 such as septic shock, which was present in 41% of our patients. For this reason, this variable was also included in the multivariable analysis. Therefore, it is worth mentioning that the access to cefiderocol was very well organized, indeed the median time to cefiderocol initiation was 2 (IQR 1–4) days, often prompted by the knowledge of carriage status, susceptibility of colonizing pathogen and the local epidemiology during pandemic waves.4
Cefiderocol is a very appealing resource in the management of carbapenem-resistant infections, in particular of CR-Ab, for its mechanism of action, in vitro activity, PK/PD characteristics and safety profile.23 However, as stated in the introduction, current clinical data are extremely controversial.7,8,10,22 We found that cefiderocol remained as a protective factor against mortality in our multivariable analysis but at a non-statistically significant value. It remains to be established whether the lack of significance was true or related to the limitations of our study. In the first case, another element to explore should be the relationship between cefiderocol exposure and treatment failure as recently shown for ceftazidime/avibactam in a large Italian real-life experience.24
Our study has several limitations. First, the small sample size may have hampered a powerful analysis. However, this is one of the largest real-life experiences with cefiderocol use in patients with CR-Ab infections compared with alternative regimens. Second, the high mortality rate associated with critical COVID-19 could make difficult to evaluate the effect of cefiderocol treatment on medium- and long-term outcome (i.e. 6 month or 1 year mortality). On the other hand, the selection of patients admitted to the ICU for the same reason could have mitigated the influence of the underlying cause of ICU admission on early outcomes (i.e. 14 day and 28 day mortality).
To conclude, our experience suggests that cefiderocol could be an effective treatment option for CR-Ab infection in critically ill patients admitted for COVID-19. Large studies are needed to confirm the role of cefiderocol in the therapeutic armamentarium against severe carbapenem-resistant infections.
Funding
This study was carried out as part of our routine work and supported by internal funding.
Transparency declarations
None to declare.
Supplementary data
Tables S1 to S6 are available as Supplementary data at JAC-AMR Online.
Supplementary Material
References
- 1. Cassini A, Hogberg LD, Plachouras D. et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 2019; 19: 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tacconelli E, Carrara E, Savoldi A. et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18: 318–27. [DOI] [PubMed] [Google Scholar]
- 3. Piperaki ET, Tzouvelekis LS, Miriagou V. et al. Carbapenem-resistant Acinetobacter baumannii: in pursuit of an effective treatment. Clin Microbiol Infect 2019; 25: 951–7. [DOI] [PubMed] [Google Scholar]
- 4. Pascale R, Bussini L, Gaibani P. et al. Carbapenem-resistant bacteria in an intensive care unit during the coronavirus disease 2019 (COVID-19) pandemic: a multicenter before-and-after cross-sectional study. Infect Control Hosp Epidemiol 2021; doi:10.1017/ice.2021.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perez S, Innes GK, Walters MS. et al. Increase in hospital-acquired carbapenem-resistant Acinetobacter baumannii infection and colonization in an acute care hospital during a surge in COVID-19 admissions - New Jersey, February-July 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 1827–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heil EL, Tamma PD.. Cefiderocol: the Trojan horse has arrived but will Troy fall? Lancet Infect Dis 2021; 21: 153–5. [DOI] [PubMed] [Google Scholar]
- 7. Bassetti M, Echols R, Matsunaga Y. et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis 2021; 21: 226–40. [DOI] [PubMed] [Google Scholar]
- 8. Falcone M, Tiseo G, Nicastro M. et al. Cefiderocol as rescue therapy for Acinetobacter baumannii and other carbapenem-resistant Gram-negative infections in intensive care unit patients. Clin Infect Dis 2021; 72: 2021–4. [DOI] [PubMed] [Google Scholar]
- 9. Zingg S, Nicoletti GJ, Kuster S. et al. Cefiderocol for extensively drug-resistant Gram-negative bacterial infections: real-world experience from a case series and review of the literature. Open Forum Infect Dis 2020; 7: ofaa185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trecarichi EM, Quirino A, Scaglione V. et al. Successful treatment with cefiderocol for compassionate use in a critically ill patient with XDR Acinetobacter baumannii and KPC-producing Klebsiella pneumoniae: a case report. J Antimicrob Chemother 2019; 74: 3399–401. [DOI] [PubMed] [Google Scholar]
- 11. EUCAST. Clinical Breakpoints - Breakpoints and Guidance. 2021. https://eucast.org/clinical_breakpoints/.
- 12. Horan TC, Andrus M, Dudeck MA.. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36: 309–32. [DOI] [PubMed] [Google Scholar]
- 13. Summary of Product Characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/fetcroja-epar-product-information_en.pdf.
- 14. Charlson ME, Pompei P, Ales KL. et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–83. [DOI] [PubMed] [Google Scholar]
- 15. Friedman ND, Kaye KS, Stout JE. et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002; 137: 791–7. [DOI] [PubMed] [Google Scholar]
- 16. Singer M, Deutschman CS, Seymour CW. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. WHO. Clinical Management of COVID-19. 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1.
- 18. Hackel MA, Tsuji M, Yamano Y. et al. In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of Gram-negative bacilli collected worldwide in 2014 to 2016. Antimicrob Agents Chemother 2018; 62: e01968-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. EUCAST. Guidance Document on Broth Microdilution Testing of Cefiderocol, December 2020. 2021. https://eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Guidance_documents/Cefiderocol_MIC_testing_EUCAST_guidance_document_201217.pdf.
- 20. Sterne JAC, Murthy S, Diaz JV. et al. ; WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020; 324: 1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pelaia C, Calabrese C, Garofalo E. et al. Therapeutic role of tocilizumab in SARS-CoV-2-induced cytokine storm: rationale and current evidence. Int J Mol Sci 2021; 22: 3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oliva A, Ceccarelli G, De Angelis M. et al. Cefiderocol for compassionate use in the treatment of complicated infections caused by extensively and pan-resistant Acinetobacter baumannii. J Glob Antimicrob Resist 2020; 23: 292–6. [DOI] [PubMed] [Google Scholar]
- 23. Jorda A, Zeitlinger M.. Pharmacological and clinical profile of cefiderocol, a siderophore cephalosporin against gram-negative pathogens. Expert Rev Clin Pharmacol 2021; 14: 777–91. [DOI] [PubMed] [Google Scholar]
- 24. Tumbarello M, Raffaelli F, Giannella M. et al. Ceftazidime-avibactam use for KPC-Kp infections: a retrospective observational multicenter study. Clin Infect Dis 2021; doi:10.1093/cid/ciab176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.