Abstract
Zika virus (ZIKV) deoxyribonucleic acid vaccine VRC5283 encoding viral structural genes has been shown to be immunogenic in humans. Recognizing that antigenically related flaviviruses cocirculate in regions with ZIKV activity, we explored the degree of antibody cross-reactivity elicited by this vaccine candidate using genetically diverse flaviviruses. The antibody response of vaccinated individuals with no evidence of prior flavivirus infection or vaccine experience had a limited capacity to bind heterologous viruses. In contrast, vaccine-elicited antibodies from individuals with prior flavivirus experience had a greater capacity to bind, but not neutralize, distantly related flaviviruses. These findings suggest that prior flavivirus exposure shapes the humoral immune response to vaccination.
Keywords: cross-reactive antibodies, DNA vaccine, neutralization, Zika virus
Antibodies elicited by ZIKV DNA vaccination of individuals with no evidence of prior flavivirus experience had a limited capacity to bind or neutralize heterologous flaviviruses. More extensive cross-reactivity was observed in vaccinated individuals with prior flavivirus exposure.
Flaviviruses are positive-stranded ribonucleic acid viruses responsible for human disease worldwide. The expanding range of arthropod vectors, potential for extensive transmission in naive populations, and cocirculation of antigenically related viral species make predicting and managing flavivirus outbreaks challenging. Zika virus (ZIKV) was introduced into the Americas in 2015 and caused over 700 000 suspected or laboratory-confirmed human disease cases by December of 2016 [1]. This outbreak revealed clinical presentations not previously associated with flavivirus infections, including Guillain-Barré Syndrome in adults and a spectrum of congenital neurodevelopmental diseases in infants, such as microcephaly. Declaration of the ZIKV outbreak as a public health emergency of international concern stimulated the rapid development of multiple ZIKV vaccine candidates.
Flaviviruses are enveloped virions produced from 3 viral structural proteins (capsid [C], premembrane [prM], and envelope [E]). The E protein mediates virus entry into cells and is the principal target of virus type-specific (TS) and cross-reactive (CR) antibodies [2]. The CR antibodies are elicited frequently by infection, have a limited capacity to confer protection, and complicate serological testing [3]. Moreover, CR antibodies produced after infection by one of the 4 circulating dengue virus (DENV) serotypes may exacerbate disease after secondary heterologous DENV infection by enhancing the infection of Fc-receptor (FcR)-expressing cells. Antibody-dependent enhancement (ADE) of infection or disease severity has limited the use of the tetravalent DENV vaccine Dengvaxia [4]. In addition, several classes of antibodies bind features shared among DENV and ZIKV [5]. Epidemiological studies of ZIKV incidence in DENV-experienced populations suggest that prior DENV infection is protective [6]. Recent studies in mice and nonhuman primate models demonstrated that prior ZIKV infection impacts the magnitude of subsequent DENV infections [7, 8]. In humans, an association between prior ZIKV infection and an increased risk of symptomatic DENV serotype 2 virus (DENV2) infections and severe disease was recently reported [9]. Because vaccine-elicited antibodies may be a safety concern for DENV infection, and prior flavivirus experience has the potential to shape a vaccine-elicited immune response [10], a detailed understanding of CR antibody responses is warranted.
In a phase 1 clinical trial, ZIKV deoxyribonucleic acid (DNA) vaccine VRC5283, expressing prM-E structural genes, was found to be safe and immunogenic in healthy adults [11]. This vaccine was evaluated in a placebo-controlled randomized phase 2/2b clinical trial in multiple sites in the Americas, where populations are expected to have experience with antigenically related flaviviruses (results are pending). In this study, we evaluated the extent of serum antibody cross-reactivity elicited by VRC5283. We created a panel of reporter virus particles (RVPs) using the structural proteins of diverse flaviviruses and used them in a screen for CR antibodies in naive, convalescent, and phase 1 vaccine-immune human sera. Our study demonstrated that the VRC5283 vaccine-elicited antibody response of flavivirus-naive individuals was largely TS. However, VRC5283 vaccination of individuals with prior flavivirus exposure resulted in a detectable increase in CR, but not cross-neutralizing, antibodies.
METHODS
Human Sera
Convalescent sera from 4 volunteers were collected with informed consent within 3–6 weeks of symptomatic ZIKV disease onset as described previously (Supplementary Experimental Procedures and Supplementary Table 1) [12]. Human sera collected during a phase 1, randomized, open-label clinical trial of ZIKV DNA vaccine VRC5283 were obtained from 14 volunteers for inclusion in these studies (Supplementary Experimental Procedures) [11]. Flavivirus-immune status was not an exclusion criterion during enrollment of this clinical trial, and 6 volunteers had evidence of prior flavivirus exposure at the time of vaccination (Supplementary Table 1).
Structural Gene Plasmids
Plasmids encoding the structural gene (C-prM-E) sequences from 16 flavivirus strains were obtained or newly generated as described in Supplementary Table 2, Supplementary Figure 1, and Supplementary Experimental Procedures. This panel included C-prM-E expression constructs for ZIKV strains H/PF/2013 (ZIKV H/PF) and MR766 (ZIKV MR766), Japanese encephalitis (JEV), DENV1, DENV2, DENV4, West Nile (WNV), Langat (LGTV), Modoc (MODV), Murray Valley encephalitis (MVEV), Ntaya (NTAV), Powassan (POWV), Spondweni (SPOV), St. Louis encephalitis (SLEV), Usutu (USUV), and yellow fever (YFV) viruses.
Neutralization and Enhancement Assays
The RVP neutralization assays were performed as previously described and detailed in the Supplementary Experimental Procedures [12]. Serial dilutions of monoclonal antibodies (mAbs) or heat-inactivated serum were incubated with RVPs and used to infect Raji-DCSIGNR cells. Green fluorescent protein expression was detected by flow cytometry. Nonlinear regression was used to estimate the mAb concentration or dilution of sera required to inhibit infection by 50% (EC50). The ADE assays were performed identically, with the exception that RVP-antibody complexes were used to infect FcR-expressing K562 cells.
RESULTS
We generated RVPs using the structural proteins of 16 flaviviruses representing endemic, emerging, and potential human pathogens (Supplementary Table 2). The RVP production efficiency was evaluated by the titer achieved after infection of Raji-DCSIGNR cells (Figure 1A), and the specific infectivity was calculated for each preparation (Supplementary Figure 2 and Supplementary Experimental Procedures). Together, these data indicated that our methods yielded RVPs of sufficient titers for use in quantitative antibody assays.
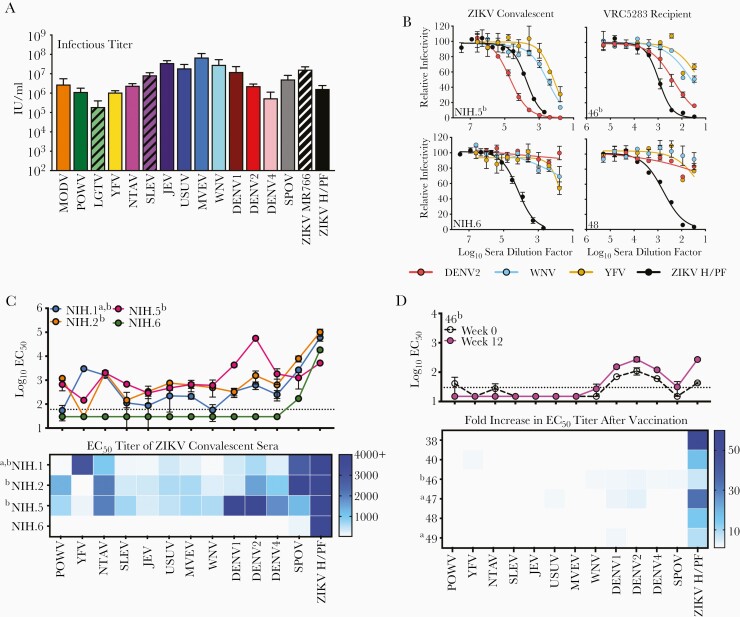
Figure 1.
Neutralization sensitivity of virus- and vaccine-induced antibody responses against a panel of reporter virus particles (RVPs). Flavivirus RVPs were produced and infectious titers were quantified as detailed in the Supplementary Experimental Procedures. (A) Infectious titers for the entire panel of RVPs were estimated using the most linear portions of the virus dose-infectivity curves and expressed as infectious units per milliliter (IU/mL). Shown is the average infectious titer calculated from 4 independent preparations of RVPs, each performed in duplicate; error bars represent the standard deviation. (B) Representative dose-response neutralization curves of Zika virus (ZIKV) convalescent sera from confirmed infection or VRC5283 ZIKV deoxyribonucleic acid vaccine recipient sera (12 weeks postfirst vaccine dose) against indicated RVPs are shown. Serum was serially diluted, incubated with indicated RVPs, and added to Raji-DCSIGNR cells. Green fluorescent protein-expressing cells were measured 36–48 hours postinfection using flow cytometry. Data were fit using nonlinear regression analysis in which the bottom of the curve was constrained to zero and used to estimate the serum dilution required to inhibit infection by 50% (EC50). Error bars represent the range of duplicate technical replicates. (C) The average EC50 neutralization titer for 4 ZIKV convalescent samples against the panel of RVPs is displayed. Each line connects the EC50 of a ZIKV convalescent individual against the designated 13 strains. Error bars depict the range of 2 independent experiments. These EC50 values are displayed in a comprehensive heat map below. A preinfection sample was not available for these individuals. (D) Average EC50 neutralization titers for prevaccination and 12 weeks postvaccination serum samples from 6 VRC5283 vaccine recipients against the designated panel of RVPs were determined (see complete data set in Supplementary Figure 5). In the top panel, an example of the primary data from a single subject is shown, connecting dashed and solid lines represent the average EC50 titers obtained with pre- and postvaccination serum from the designated recipient, respectively. Errors bars represent the range of 2 independent experiments. In the bottom panel, the fold increase in EC50 between pre- and postvaccination samples was calculated for each recipient and displayed as a heat map. The fold-increase is defined as the quotient of the postvaccination titer by the prevaccination titer. The horizontal dotted line represents the limit of detection (LOD) of the assay, defined here by the smallest dilution of sera tested. Regression analysis was not used to forecast EC50 titers if sera did not inhibit more than 50% infection at the highest dilution factor; therefore, any measurement below the LOD was assigned a value of half the LOD (a value of 30 for ZIKV convalescent sera, 15 for ZIKV vaccine clinical sera). aYellow fever virus (YFV) vaccine immunization history; bpositive for dengue virus (DENV) IgG at time of collection or vaccination.
To explore the sensitivity of this diverse RVP panel to neutralization by CR antibodies, we studied mAbs that bind the highly conserved E domain II fusion loop (EDII-FL) (Supplementary Figure 3). E60 and E53 are murine mAbs that bind overlapping EDII-FL epitopes and are capable of neutralizing multiple flaviviruses [13, 14]. Neutralization studies revealed variable sensitivity to these EDII-FL mAbs among the panel of RVPs (Supplementary Figure 4). All but 3 RVP types were sensitive to at least 1 mAb, although a majority were incompletely neutralized at the highest concentration of antibody tested.
To investigate the breadth of CR antibody activity in ZIKV convalescent sera collected during the 2015 ZIKV epidemic (Supplementary Table 1), we performed neutralization studies with 13 heterologous RVP types (Figure 1B and C). The MODV and LGTV RVPs were excluded from this analysis because we lacked a mAb or immune sera suitable for use as a positive control. Sera from all 4 volunteers robustly neutralized ZIKV (log10 EC50 range, 3.72 to 5.00) and moderately inhibited infection of closely related SPOV (log10 EC50 range, 2.23 to 3.90). Although sera from volunteer NIH.6 revealed a relatively TS pattern of neutralization, the others exhibited varied but considerable CR neutralizing activity against heterologous viruses (Figure 1B and C). These individuals had extensive documented travel in flavivirus-endemic regions and had screened positive for DENV-reactive antibodies by enzyme-linked immunosorbent assay (ELISA) at the time of collection. Volunteer NIH.1 was also previously vaccinated against YFV (Supplementary Table 1), and sera from this individual robustly neutralized YFV. Studies with this panel of distantly related flaviviruses reveal complex patterns of CR neutralizing activities among convalescent ZIKV individuals with varied infection and vaccination histories.
We next explored the degree of cross-reactivity elicited by VRC5283 using sera collected during a phase 1 clinical study [11]. We first screened sera collected from 6 individuals at the time of first vaccination and 12-weeks thereafter for the capacity to neutralize ZIKV and heterologous RVPs in our panel (Figure 1B and D and Supplementary Figure 5). Vaccine-elicited increases in neutralization activity between the pre- and postvaccination samples are depicted in Figure 1D. Postvaccination sera neutralized both Asian (H/PF) and African (MR766) lineage strains of ZIKV to a similar degree as anticipated [12] (average log10 EC50 titer ± standard deviation, 2.51 ± 1.93 and 2.61 ± 2.05, respectively), but it did not consistently inhibit infection of the closely related SPOV above the limit of detection of our assay (Supplementary Figure 6). Thus, although neutralization of SPOV by ZIKV convalescent sera was observed here and in prior studies (Figure 1C and D) [5], VRC5283 vaccination is unlikely to confer heterologous protection to this related virus. Overall, minimal vaccine-elicited CR neutralization activity was observed (Figure 1D and Supplementary Figure 5). Although significant CR neutralizing antibody titers were observed at the time of enrollment in vaccine recipient 46, who was previously identified as DENV seropositive, and recipients 47 and 49, who were previously immunized against YFV, these CR titers were only boosted modestly by ZIKV vaccination (average 1.65-fold increase in EC50 titer). Altogether, comparisons of neutralization activity in pre- and postvaccination samples revealed that antibodies elicited by this vaccine candidate were largely TS.
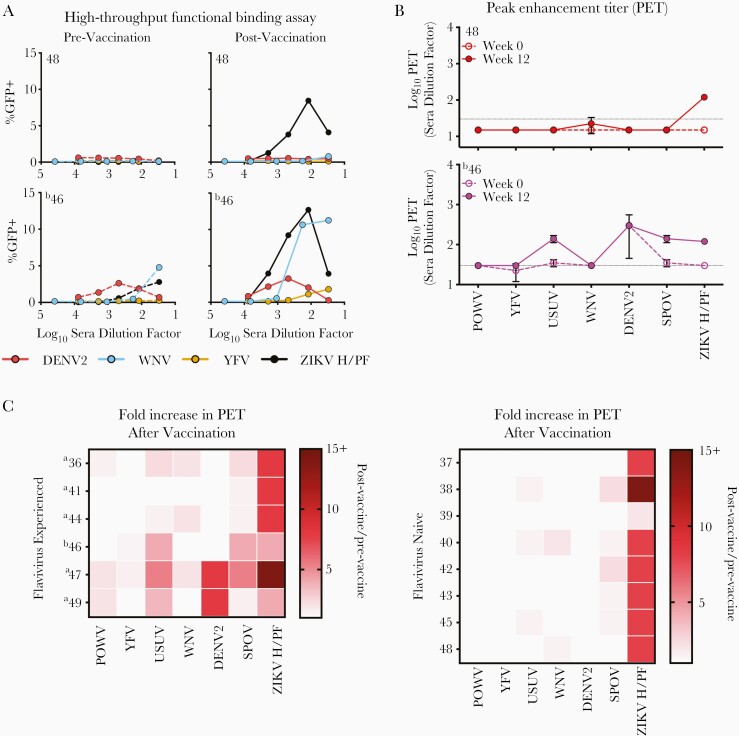
Antibodies may decorate virions without reducing viral infectivity [15]. We took advantage of the capacity of relatively small numbers of antibody molecules to enhance infection of FcR-expressing cells to screen quantitatively for CR-binding antibodies irrespective of their neutralization capacity [16]. These ADE assays are rapid, exploit the quantitative strengths of a flow cytometry format, and allow measures of virus-antibody interactions at steady-state and under conditions of antibody excess (Supplementary Figure 7) [16]. Furthermore, this technique allows the measurement of antibody binding to intact functional virus particles displaying quaternary epitopes without risk of distortion of the virion that occurs when anchored to solid substrates involved in many ELISA formats. High-throughput ADE assays were performed using FcR+ K562 cells and sera from 14 VRC5283 vaccine recipients in the presence of RVPs (Figure 2A). Comparisons between the serum titer that maximally enhanced infection (peak enhancement titer [PET]) among pre- and postvaccination samples were used to identify vaccine-elicited CR antibody responses. Samples were scored positive for ADE when infection increased ≥3-fold above background infection levels (Figure 2B and Supplementary Figures 7 and 8). Serum from all individuals tested detected vaccine-elicited antibody binding against ZIKV (average 9.6-fold increase in PET postvaccination) in agreement with our neutralization studies (Figure 2C). The most consistent vaccine-induced increase in heterologous antibody binding was observed for SPOV (average 2.0-fold increase in PET, range 1.0- to 5.3-fold), consistent with its antigenic similarity to ZIKV (Supplementary Table 3). Individuals with no evident flavivirus pre-exposure had limited vaccine-elicited binding activity against heterologous flaviviruses (average 1.1-fold increase in PET, range 1.0- to 3.5-fold). In contrast, sera from vaccine recipients with documented previous flavivirus experience revealed CR antibody binding against heterologous viruses of varying magnitude, most frequently against USUV, DENV2, and SPOV (up to a 5.3-, 8.0-, and 5.3-fold increase in PET, respectively). Neutralization assays were performed to confirm that the observed CR antibody binding was not cross-neutralizing except where prior exposure was documented (Supplementary Table 4).
Figure 2.
Cross-reactive antibody binding of vaccine-induced antibody responses in humans. Antibody-dependent enhancement (ADE) assays were performed as a sensitive measure of antibody binding to infectious virions in solution. Serum was serially diluted, incubated with indicated reporter virus particles (RVPs), and used to infect semipermissive FcγR-expressing K562 cells. Enhancement was scored by green fluorescent protein (GFP) expression and measured after 36–48 hours using flow cytometry. (A) Representative data using pre- and postvaccination sera from 2 VRC5283 vaccine recipients are shown. (B) The serum dilution corresponding to the highest level of signal (peak enhancement titer [PET]) was determined. The PET was considered positive if ≥3-fold above the background level of infection for each RVP in the absence of antibody. Prevaccination and 12 weeks postfirst vaccine dose serum samples were studied. Dashed and solid lines represent the average PET obtained with pre- and postvaccination serum, respectively. The horizontal dotted line represents the limit of detection (LOD) of the assay. Any measurement below the LOD was assigned a value of half the LOD. Error bars represent the range of 2 independent experiments. (C) The change in PET occurring after vaccination of flavivirus experienced (left panel) or flavivirus naive (right panel) recipients was calculated and displayed as the fold-difference, or quotient of the postvaccination PET by the prevaccination PET, on a heat map. For samples with no detectable binding activity, the half value of the number assigned to the LOD was used in these calculations (a value of 15). Smaller PET fold-differences are indicated in lighter red; larger differences are indicated in dark red. aYellow fever virus (YFV) vaccine immunization history; bpositive for dengue virus (DENV) IgG at time of vaccination.
Discussion
Flavivirus prM and E proteins display conserved structural features recognized by CR antibodies with the potential to contribute to protection [5]. To study flavivirus-reactive antibodies, we developed a panel of RVPs using the structural genes of a diverse group of mosquito- and tick-borne flaviviruses. We demonstrate that vaccination with VRC5283 elicits a highly TS neutralizing antibody response with limited potential to inhibit the infection of heterologous flaviviruses, even closely related SPOV. Neutralizing titers to other flavivirus species were boosted modestly, if at all, by vaccination. Because neutralizing assays may have limited sensitivity, a second approach was used to detect antibodies that do not bind with a stoichiometry sufficient for neutralization. Our studies demonstrate that CR binding antibodies elicited by VRC5283 are present in circulation at very low titers, if at all, in individuals with no evidence of prior flavivirus antigen-experience. In contrast to our neutralization studies, vaccine administration markedly increased titers of CR binding antibodies in some flavivirus antigen-experienced individuals. The presence of heterologous flavivirus antibodies before vaccination had little impact on the titer of vaccine-elicited neutralizing ZIKV antibodies, although this will be explored further in phase 2 clinical studies.
Recent studies suggest that antibodies elicited by ZIKV have the potential to modulate DENV pathogenesis in animal models via an ADE mechanism [3]. A role for VRC5283-elicited CR antibodies in vaccine-mediated protection or the outcome of subsequent heterologous flavivirus infection remains to be explored in animal models. In humans, prior ZIKV infection may influence the severity of secondary infections by some DENV strains [10]. An influence of CR antibodies after vaccination could ultimately be revealed by clinical studies in flavivirus-endemic regions of the Americas [9]. Although an ADE assay was used here as a sensitive measure of antibody binding, this is not an established predictor of in vivo outcomes. The potential for low levels of CR antibodies to change the magnitude and functional quality of the antibody response to vaccination or modulate disease after secondary DENV infections warrants careful study [9, 10]. The quantitative approaches to measure flavivirus-reactive antibody activity detailed here will have significant utility for interpreting ZIKV vaccine responses in clinical trials.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: Keystone Symposia: Molecular Approaches to Vaccines and Immune Monitoring, February 10–14, 2019, Keystone, CO, USA.
Acknowledgments. We thank the clinical trial participants for their contribution and commitment to vaccine research and acknowledge the contributions of the VRC 320 clinical trial study team. We also thank Dr. Leah Katzelnick (Laboratory of Infectious Diseases, National Institutes of Allergy and Infectious Diseases, National Institutes of Health) for helpful suggestions and discussions on the manuscript.
Financial support. Funding was provided by the Division of Intramural Research, National Institute of Allergy and Infectious Disease and by the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. K. A. D., J. E. L., J. R. M., B. S. G., and T. C. P. are inventors on patent applications describing the VRC5283 DNA vaccine (U.S. patent application number 16/334 099 and PCT/2018/018809). The other authors declare that they have no competing interests. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hills SL, Fischer M, Petersen LR. Epidemiology of Zika virus infection. J Infect Dis 2017; 216:868–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rey FA, Stiasny K, Vaney MC, Dellarole M, Heinz FX. The bright and the dark side of human antibody responses to flaviviruses: lessons for vaccine design. EMBO Rep 2018; 19:206–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maciejewski S, Pierson TC. Cross-reactive flavivirus antibody: friend and foe? Cell Host Microbe 2018; 24:622–4. [DOI] [PubMed] [Google Scholar]
- 4. Halstead SB. Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine 2017; 35:6355–8. [DOI] [PubMed] [Google Scholar]
- 5. Salazar V, Jagger BW, Mongkolsapaya J, et al. Dengue and zika virus cross-reactive human monoclonal antibodies protect against spondweni virus infection and pathogenesis in mice. Cell Rep 2019; 26:1585–97.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Whitehead SS, Pierson TC. Effects of dengue immunity on Zika virus infection. Nature 2019; 567:467–8. [DOI] [PubMed] [Google Scholar]
- 7. Kawiecki AB, Christofferson RC. Zika virus-induced antibody response enhances dengue virus serotype 2 replication in vitro. J Infect Dis 2016; 214:1357–60. [DOI] [PubMed] [Google Scholar]
- 8. George J, Valiant WG, Mattapallil MJ, et al. Prior exposure to zika virus significantly enhances peak dengue-2 viremia in rhesus macaques. Sci Rep 2017; 7:10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Katzelnick LC, Narvaez C, Arguello S, et al. Zika virus infection enhances future risk of severe dengue disease. Science 2020; 369:1123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malafa S, Medits I, Aberle JH, et al. Impact of flavivirus vaccine-induced immunity on primary Zika virus antibody response in humans. PLoS Negl Trop Dis 2020; 14:e0008034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaudinski MR, Houser KV, Morabito KM, et al. ; VRC 319; VRC 320 study teams . Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. Lancet 2018; 391:552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dowd KA, DeMaso CR, Pelc RS, et al. Broadly neutralizing activity of zika virus-immune sera identifies a single viral serotype. Cell Rep 2016; 16:1485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oliphant T, Nybakken GE, Engle M, et al. Antibody recognition and neutralization determinants on domains I and II of West Nile virus envelope protein. J Virol 2006; 80:12149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goo L, DeMaso CR, Pelc RS, et al. The Zika virus envelope protein glycan loop regulates virion antigenicity. Virology 2018; 515:191–202. [DOI] [PubMed] [Google Scholar]
- 15. Peiris JS, Porterfield JS. Antibody-mediated enhancement of flavivirus replication in macrophage-like cell lines. Nature 1979; 282:509–11. [DOI] [PubMed] [Google Scholar]
- 16. Pierson TC, Xu Q, Nelson S, et al. The stoichiometry of antibody-mediated neutralization and enhancement of West Nile virus infection. Cell Host Microbe 2007; 1:135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.