Abstract
Major Depressive Disorder (MDD) is a common and debilitating mood disorder that is more prevalent in women than men. In humans, PET imaging of microglia activation is currently being explored as a potential biomarker of MDD and suicidal ideation. Stress is a trigger for many mood disorders, including MDD. Microglial changes in morphology and activation state in response to stress has been reported in various brain regions, but most studies only examined male subjects. Here we report changes in microglia morphology in the nucleus accumbens (NAc) and subregions of the hippocampus (HPC) in both male and female mice following variable stress of 6 or 28 days in duration. Our data demonstrate that after 6 days of stress, microglia in the female NAc and dentate gyrus have a reduction in homeostatic associated morphology and an increase in primed microglia. After 28 days some of these sex specific stress effects were still present in microglia within the NAc but not the dentate gyrus. There were no effects of stress in either sex at either timepoint in CA1. In female mice, anti-inflammatory activation of microglia using rosiglitazone promoted sociability behavior after 6 days of stress. Furthermore, both drug and stress have impact on microglia morphology and activation state in the NAc. These data suggest that microglia morphology and activation state are altered by 6 days of variable stress in a region-specific manner and may contribute to, or potentially compensate for, the onset of stress susceptibility rather than impacting long term exposure to stress.
Highlights
-
•
There are region and sex specific effects of stress on microglia.
-
•
Female microglia respond more strongly to shorter periods of stress.
-
•
Rosiglitazone increases social interaction in stressed female mice.
-
•
Rosiglitazone does not block stress induced microglia activation in females.
1. Introduction
Microglia are resident innate immune cells of the central nervous system and comprise ∼10–15% of the total glia in the brain (Norden and Godbout, 2013; Rabinowitz and Gordon, 1991; Rosen et al., 2017). In the adult brain, these cells are highly mobile and scan the brain for pathogens and provide neuronal support in their homeostatic or surveillant state (Nimmerjahn et al., 2005; Ransohoff and Perry, 2009). Disturbances in brain homeostasis activate microglia, producing a shift in morphological state and function, and changes in secreted cytokines (Banati et al., 1993; Du et al., 2017; Nelson and Lenz, 2017; Nelson et al., 2017). It was initially thought that activated microglia existed as a dichotomy, expressing classical pro-inflammatory (M1 polarized) state or an alternative anti-inflammatory (M2 polarized) state (Colton, 2009). Classically activated microglia secrete pro-inflammatory cytokines, such as Il-1β, IL-6 and TNF-α, produce reactive oxygen species, and engage in phagocytosis of anything that is recognized as dangerous, impaired, or non-self (Banati et al., 1993; Cherry et al., 2014; Du et al., 2017; Hanisch and Kettenmann, 2007; Nelson and Lenz, 2017; Nelson et al., 2017; Orihuela et al., 2016).They can also engulf synapses and engage in the process of trogocytosis (synapse nibbling) in which they selectively remove proteins rather than phagocytose the entire synapse (Weinhard et al., 2018a). Alternatively activated microglia are involved in inhibiting inflammation, restoring homeostasis, and produce anti-inflammatory cytokines, such as IL-4, IL-10 and IL-13 but also engage in phagocytosis (Akhmetzyanova et al., 2019; Cherry et al., 2014). Microglia are dynamic and plastic cells that have the ability to transform back and forth between states and functions (Streit et al., 1999). As such, more recent research demonstrates that microglia can express markers of both forms, exist on a spectrum, and engage in multiple forms of alternative activation with different functions (Cherry et al., 2014; Mecha et al., 2016).
Microglia activation is a hallmark of many psychiatric illnesses including depression, schizophrenia, autism spectrum disorder, Parkinson's disease and Alzheimer's disease (Gandal et al., 2018a, 2018b; Hansen et al., 2018; Setiawan et al., 2015; Tang and Le, 2016; Tsilioni et al., 2019). Postmortem analysis from brain tissue of people that committed suicide identifies microglia activation in the prefrontal cortex (PFC) and limbic regions, including HPC, NAc, amygdala and thalamus across different disorders (Steiner et al., 2006, Steiner et al., 2008; Suzuki et al., 2019; Yirmiya et al., 2015). Recent developments in positron emission tomography imaging have used translocator protein (TSPO) as a marker of microglia activation in living people (Setiawan et al., 2015). These studies report increased TSPO signals in people who were experiencing suicidal ideation (Holmes et al., 2018). Unfortunately, TSPO is not a specific marker for microglia activation and is also expressed in endothelial cells and some astrocytes (Notter et al., 2018a, 2018b). Therefore, these studies demonstrate at most a basal state of immune activation in the brain. A recent study comparing transcriptional patterns of expression across disorders in humans found greater regulation of microglia-associated genes in schizophrenia and autism spectrum disorder compared to depression (Gandal et al., 2018a). All these disorders have reported sex differences in symptoms and rate of occurrence (Cover et al., 2014; Hodes and Epperson, 2019), yet to date none of the studies in humans have examined sex as an independent variable. Postmortem studies of transcriptional signatures of depression from men and women with a diagnosis of MDD demonstrated dramatic sex differences across multiple brain regions (Labonte et al., 2017; Seney et al., 2018). Many of the reported pathways involved immune regulation (Rainville et al., 2021). In mice, the variable stress paradigm has been validated as a tool to explore many of these transcriptional sex differences associated with MDD (Hodes et al., 2015; Labonte et al., 2017; LaPlant et al., 2009; Pfau et al., 2016). Specifically, there is significant overlap in sex specifically regulated immune associated genes between women and female mice in the NAc, an area of the brain that integrates hedonic experience and emotional processing (Cathomas et al., 2019; Christoffel et al., 2015; Menard et al., 2017; Wang et al., 2018). Importantly, these data highlight that even when the behaviors of males and females are affected similarly by stress or depression, different mechanisms may be responsible and there is a need to identify these differences to develop better treatments for both sexes.
Preclinical studies have identified important sex differences in microglia that are both age- and region-dependent. Microglia contribute to male and female specific brain development during the pre- and early postnatal periods, even contributing to the masculinization of brain regions and subsequent adult sexual behavior (Lenz and McCarthy, 2015; Lenz et al., 2013; Nugent et al., 2015; Schwarz et al., 2012). In adult animals (13 weeks) males were reported to have a greater density of microglia in cortex, HPC and amygdala, larger soma size and with greater ability of those microglia to present antigens than females (Guneykaya et al., 2018). Female microglia have faster maturation during development, and a higher expression of genes associated with inflammation, apoptosis and response to stimulation with the endotoxin lipopolysaccharide (Hanamsagar et al., 2017). HPC female microglia were also found to mature faster and have an earlier peak in phagocytic capacity compared to males, although basal sex differences were absent by adolescence (Weinhard et al., 2018b).
Stress is a trigger for the onset of a depressive episode in humans and as such has been used to examine the underlying biology in animals that contributes to onset of mood disorders (Menard et al., 2016; Otte et al., 2016; Yang et al., 2015). Preclinical studies strongly suggest that microglia from males and females have different responses to stress, but this is region specific (Liu et al., 2019; Yin et al., 2019). Higher proportions of primed to surveillant microglia at baseline were identified in medial PFC of female rats compared to males; furthermore, both acute and chronic stress decreased the proportion of primed microglia in females but did not alter microglia in males (Bollinger et al., 2016). Opposite sex differences in microglia activation were also observed following unpredictable chronic stress in the NAc and HPC in the rats that were prenatally exposed to dexamethasone, a glucocorticoid agonist (Gaspar et al., 2021).
Here we examine whether subchronic variable stress (6 days) or chronic variable stress (28 days) impacts microglia activation in the NAc and subregions of the HPC. We examined these time points because after 6 days of stress female mice show greater behavioral stress susceptibility, whereas males are generally resilient (Hodes et al., 2015; Johnson et al., 2021; LaPlant et al., 2009; Williams et al., 2020). After 21–28 days of stress both males and females engage in stress susceptible behavioral responses (Bittar et al., 2021; Johnson et al., 2021; Labonte et al., 2017; Muir et al., 2020). These two brain structures were chosen for investigation as there are known sex differences of the effects of stress on neuronal plasticity and glutamatergic signaling (Brancato et al., 2017; Dalla et al., 2009; Shors et al., 2001), both of which have been implicated as altered in human MDD (Barch et al., 2019; Pizzagalli et al., 2009; Roddy et al., 2019). Additionally, recent preclinical studies have demonstrated that manipulation of a glutamatergic pathway between these regions impacts stress related behavior in a sex specific manner (Muir et al., 2020; Williams et al., 2020).
2. Methods
2.1. Animals
C577BL/6J male and female mice (Jackson Laboratory) were used between 8 and 12 weeks of age. Mice were group housed (5 per cage) in paper bedding and maintained on a 12-h light/dark cycle with ad libitum access to food and water. Procedures were performed in accordance with the Institutional Animal Care and Use Committee guidelines at Virginia Tech.
2.2. Variable stress
Variable stress was performed as described previously (Hodes et al., 2015; Johnson et al., 2021; Labonte et al., 2017; LaPlant et al., 2009) for the duration of either 6 (sub-chronic variable stress or SCVS) or 28 days (long term chronic stress or LTVS). The variable stress paradigm (Fig. 1A) consisted of three different stressors: foot shock for 1 h (100 foot shocks for 2 s at 0.45 mA, male and female mice were put in separate chambers), tail suspension for 1 h and restraint tube stress for 1 h (mice were placed in 50 ml ventilated conical tubes in their home cages). Experimental mice were exposed to one of three stressors each day for 1 h in the following order: foot shock, tail suspension, restraint tube (Fig. 1A) These stressors were then repeated in the same order for either the next 3 days (for SCVS) or for the next 25 days (for LTVS).
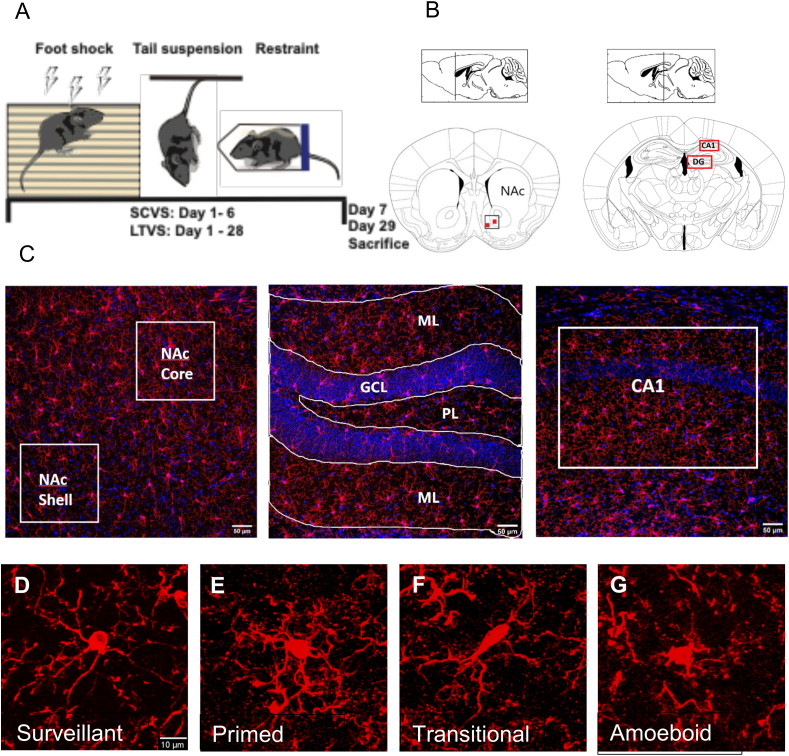
Fig. 1.
Microglial cell morphology and classification. (A) Schematic of the timeline of study. (B) NAc and HPC coordinates. (C) Representative images from the NAc, dentate gyrus and CA1 regions of HPC. (D) Surveillant microglia (small body and long thin processes). (E) Primed microglia (larger body, thicker processes, secondary branching). (F) Transitional microglia (highly reduced branching, thickened processes, often elongated body). (G) Amoeboid microglia (enlarged body, with little to no branching).
2.3. Rosiglitazone treatment
Sixty female C57BL/6J 8 week old mice were grouped into 4 groups: a non-stressed control group treated with vehicle, a stress group treated with vehicle, a non-stressed control group treated with rosiglitazone (8.5 mg/kg) and stress group treated with rosiglitazone (8.5 mg/kg). Rosiglitazone and vehicle treatment was administered daily in the drinking water. Treatment started one week prior to the variable stress and during 6 days of stress and behavioral testing (social interaction). Variable stress consisted of the same stressors described above. Twenty-four hours after the last stressor, 40 of the mice underwent social interaction testing, and the other 20 were sacrificed, perfused and had their brains harvested.
2.3.1. Social interaction test
The social interaction test was performed under the red lights. Forty mice (n= 10 per condition) were placed in a novel (42 x 42 × 42 cm) arena with a small cage placed at one end of the arena. Mice movements were monitored and recorded (Ethovision 3.0; Noldus Information Technology) for 2.5 min in the absence of the novel target mouse (female C57BL/6 mouse) (target absent phase), followed by 2.5 min when the novel mouse was present in the cage (target present phase). Social interaction ratio was then calculated as the ratio of the time spent in the interaction zone when the target mouse was present, divided by the time spend in the interaction zone with the target absent. The ratio above 1 indicates resilience whereas below 1 indicates susceptibility. Corner time was recorded as the amount of time the mice spent in the corner at the furthest point away from the novel conspecific. Locomotor activity was combined distance traveled during the target absent trials.
2.4. Perfusion and tissue processing
Twenty-four hours after the last stressor, mice were deeply anesthetized using tribromoethanol (Avertin) and transcardially perfused with cold phosphate-buffered saline (PBS, pH 7.4) followed by fixation with cold 4% paraformaldehyde (PFA) in PBS. Brains were removed and post-fixed for 48 h in the same fixative used for perfusion, then fixed brains were cryoprotected by immersion in 10% sucrose in PBS for 24 h, followed by immersion in 20% sucrose in PBS for the next 24 h, and finally followed by immersion in 30% sucrose in PBS until sank. Brains were flash-frozen and serial coronal sections of 40 μm (30 μm from the rosiglitazone study) were collected using a freezing microtome (Leica SM, 2010 R) and stored at −20 °C in cryoprotectant solution (ethylene glycol 300 ml, glycerol 250 ml, 0.2M phosphate buffer 250 ml and dH2O 200 ml) until immunofluorescence processing.
2.5. Immunofluorescence staining
Coronal sections (three per animal) containing NAc (from bregma 1.70 to 0.98 mm, according to Paxinos and Franklin, 2001) and HPC (from bregma −1.34 to −1.82) (Fig. 1B) were mounted on the Superfrost Plus slides; afterwards they were washed in PBS for 30 min (3 × 10 min), sections were incubated in blocking solution (3% normal donkey serum (NDS), 0.3% Triton-X in PBS) for 2 h at room temperature and gently shaken. Then sections were incubated in primary antibody solution (rabbit anti-Iba1(1:600; 019–19741, Wako Chemicals USA Corporation), 3% NSD, 0.3% Tween-20 in PBS) at 4˚C overnight under gentle shaking. After washing in PBS (3 × 10 min) at room temperature, sections were incubated in secondary antibody solution (donkey anti-rabbit Cy5 1:200; Jackson ImmunoResearch Laboratories, Inc) for 2 h at room temperature under gentle shaking. Afterwards, sections were washed in PBS (3 × 10 min) and cover-slipped in VECTASHIELD with DAPI (H-1200-10, Vector Laboratories). NAc coronal sections from the rosiglitazone study, were mounted on the Superfrost Plus slides and washed in PBS for 30 min (3 × 10 min). They were incubated in blocking solution (5% normal goat serum (NGS), 0.1% Triton-X in PBS) for 2 h at room temperature and then incubated in primary antibody solution (rabbit anti-Iba1(1:1000; 019–19741, Wako Chemicals USA Corporation), anti-mouse CD68 (1:500; 137,002, Biolegend) 5% NGS, 0.1% Tween-20 in PBS) overnight at 4˚C overnight under gentle shaking. After washing in PBS (3 × 10 min) at room temperature, sections were incubated in secondary antibody solution (goat anti-rabbit DyLight 594 (1:500; 35,561, Invitrogen); goat anti-rat DyLight 488 (1:500; SA510018, Invitrogen) for 2 h at room temperature under gentle shaking. Afterwards, sections were washed in PBS (3 × 10 min) and cover-slipped in VECTASHIELD with DAPI (H-1200-10, Vector Laboratories).
2.6. Imaging and analysis
Z-stack images of 20 μm thickness (0.8 μm interval) were acquired with a Nikon 1A confocal microscope at 20× magnification (40× magnification from the rosiglitazone study). Microglial morphology analysis was performed on 3 coronal sections of the NAc and averaged within mice. Mice were then averaged by group (n=5 per condition) to provide a group mean. The same method was used in the dentate gyrus (DG) and CA1 sub-regions of HPC (n = 4–5 animals per condition) using ImageJ 1.52i analysis software (National Institute of Health). For Iba1+ cell counts region of interest (ROI) areas were 200 × 200 μm for both core and shell NAc sub-regions; for CA1 ROI area was 500 × 350 μm and for sub-regions of DG (molecular layer, granular cell layer and polymorph layer). ROI areas were traced using freehand selection tool in ImageJ. Iba1+ cells were manually counted and reported as average number of cells (from 3 sections) per ROI area.
Microglial cell morphology was first analyzed by categorizing microglia into one of the four following morphological categories: surveilling (small soma and thin processes), primed (increased branching, thickened processes and larger soma), transitional (highly reduced branching, thick processes, soma enlarged and often elongated) or amoeboid (large rounded soma with no branching) based on methods previously described (Bollinger et al., 2017; Schwarz et al., 2012).
To further examine microglial morphology, we measured microglial cell area, length of the microglial processes using Skeleton analysis and branching patterns of microglial cells using Sholl analysis in ImageJ. To ensure precise measurements, individual microglial cells were randomly selected inside the ROI and cropped. The selected cells contained a complete cell body with full processes and did not overlap with the neighboring cells. Random cell selection was done blinded to treatment group. Four to six cells per brain area were selected from each animal. Next, individual cropped cells were systematically processed to obtain a binary image of a cell. To measure microglial cell area, a wand tool to outline the cell was used and the area inside the outline was measured. To measure a microglial cell process length, its binary image was first skeletonized and analyzed using Analyze Skeleton (2D/3D) option, the length of each cell process from Branch Information output window was summed to obtain the total process length of a single microglial cell. To perform Sholl analysis, the largest radius was first defined by drawing a straight line from the center of the microglial soma, starting radius was defined at 10 μm and radius step size was set at 5 μm. CD68 area was measured inside a microglial cell, and was reported as the percentage area from the total area of the microglial cell.
2.7. Statistical analysis
To initially determine if variable stress impacted microglia we examined microglia within ROIs in the NAc core and shell (Fig. 1B/C) and the subregions of the dentate gyrus (Fig. 1B/C; combined molecular layer, polymorph layer and granule cell layer) along with area CA1 (Fig. 1B/C) of the HPC following 6 or 28 days of variable stress in male and female mice. Subregions within the NAc were combined as there were no differences between shell and core. The same approach was taken with the dentate gyrus subregions. Data were averaged across animal and animal averages were grouped for all analysis using GraphPad Prism 9 software. A two-way ANOVA was used to test for effect of sex and stress or stress and drug treatment with Tukey post hoc tests for multiple comparisons. Mixed factorial/3 way ANOVAs were used to examine data with repeated measures including the effects of stress morphology and Sholl analysis, Šídák's multiple comparisons test was used for post hoc analysis. When sphericity could not be assumed a Geisser-Greenhouse correction was applied. Differences were considered as significant if p value was less than 0.05. Data are reported as mean ± S.E.M.
To examine the effects of sex and stress on microglia morphology across multiple cells from each animal, a hierarchical linear model (HLM) was implemented using HLM 7 software (Raudenbush et al., 2011). Full-maximum likelihood models were used to examine effects of stress and sex on microglia area and branch length—which were derived from multiple cell regions per animal—by stress condition (i.e. 6 or 28 day stress [1] versus 6 or 28 day control [0]) and sex (females [0], males [1]). Area and branch length were examined as level one variables (varying within individuals) in the hierarchical model. Level two variables (varying between individuals), stress condition and sex were examined regarding their impact on the within-individual intercept of level one variables. Between subject differences were examined by adding stress condition and sex (grand-centered) as level two predictors of area and branch length.
To examine microglial branching by distance from the center of the soma within the dentate gyrus and NAc, the number of intersections were examined in 5- μm radius intervals, starting at a 10- μm radius from the center. A longitudinal HLM model was implemented to examine the growth and decay in number of intersections at each radius at increasing distances from center of soma. The focus of the current study is on the between-subject (i.e., stress and sex) effects regarding distance from radius, so within-subject values are not reported for radius results. Between-subject differences were examined by adding stress condition and sex (grand centered) as level two predictors of change in number of intersections by distance.
3. Results
3.1. The effects of 6 and 28 days of variable stress on microglia morphology in the NAc
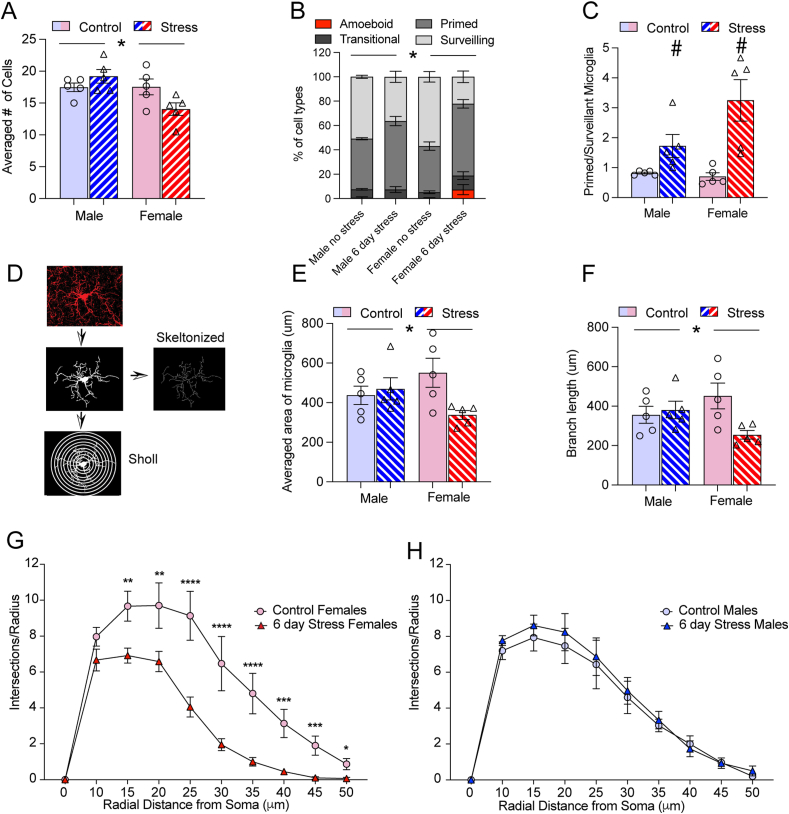
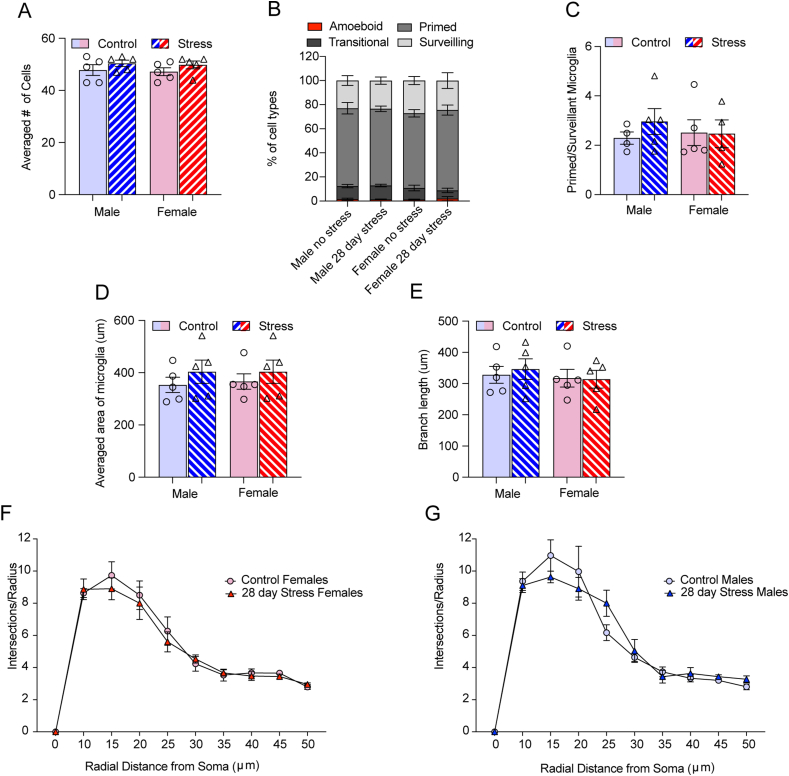
In the NAc there was a significant interaction (stress x sex) following 6 days of variable stress (F1,16= 6.69, p < 0.05) on the averaged number of microglia per animal. Post hoc analysis indicated that stressed males had more microglia than stressed females (p < 0.05) (Fig. 2A). We next grouped microglia into 4 phenotypes (Bollinger et al., 2017; Schwarz et al., 2012) and had researchers blind to experimental conditions sort cells into the categories. We examined the percentage of cell types for each animal. Cells were classified as amoeboid, transitional, primmed and surveillant (Fig. 1D–G) and percentages were calculated for the NAc, dentate gyrus and CA1. We found different patterns of morphology in males and female mice exposed to 6 days of variable stress (Fig. 2B). Analysis with a 3-factor ANOVA (Stress x percentage of cell type x sex) resulted in a significant 3-way interaction (F3,32= 3.94, p < 0.05). Post-hoc analysis within cell type indicated that only female mice exposed to 6 days of variable stress differed from their same sex controls in the percentage of primed (p < 0.01) and surveilling (p < 0.0001) microglia. There was a trend for a sex difference in the percentage of surveilling microglia in stressed males vs. stressed females (p =0.07), however, no sex differences within the same cell type survived multiple comparisons. Further examination using a ratio of primed to surveilling microglia (Fig. 2C) supported a changing relationship between stress and sex as there was trend for an interaction (F1,16= 4.16 p= 0.05) and a main effect of stress (F1,16= 18.44 p < 0.001). Stressed females had a greater ratio of primed to surveilling microglia than their same sex controls (p < 0.01) and trended towards having a larger ratio than stressed males (p = 0.07). The difference in the ratio in stressed males compared to their same sex controls failed to reach significance (p =0.4).
Fig. 2.
6 days of variable stress impacted female microglia differently than males in the NAc (A) Female mice exposed to 6 days of variable stress had fewer microglia than stressed males as indicated by a significant interaction between sex and stress (p < 0.05). (B) Microglia phenotypes differed between male and female mice when exposed to 6 days of variable stress (p < 0.05). The percentage of primed microglia increased in stressed females compared to their same sex controls, whereas the percentage of surveillant microglia decreased. There was a trend for stressed females to have more primed microglia than stressed males (p =0.07). Male phenotypes did not differ significantly between stressed and unstressed individuals. (C) The ratio of primed to surveillant microglia between males and females changed with stress. The ratio increased in stressed females compared to their same sex controls (p < 0.01) but did not significantly differ in males (p = 0.4). (D) Example of the process to perform skeleton and Sholl analysis. Individual microglia images were cleaned to remove unconnected processes from the image. They were traced and skeletonized to analyze area and branch length. For Sholl analysis, the largest radius was first defined by drawing a straight line from the center of the microglial soma, starting radius was defined at 10 μm and radius step size was set at 5 μm. (E) Microglia area was reduced with stress in females but not males (p < 0.05). (F) Branch length was reduced in stressed females but not males (p < 0.05). (G) 6 days of variable stress reduced the complexity of microglia in females (p values < 0.05). (H) 6 days of variable stress had no impact on the complexity of male microglia. Figures show mean ± SEM. Cells were counted on 3 sections per animal and averaged for each animal, n= 5 animals per group. ∗Indicates significant interaction, # indicates a main effect of stress and ˆ indicates a main effect of sex.
We further validated our classification of morphology by using a combination of cell tracing/skeleton analysis to measure microglia area/branch length respectively and Sholl analysis to measure the complexity of microglial processes (Fig. 2D). After 6 days of variable stress (Fig. 2E) females had reduced cell area in the NAc as indicated by a significant interaction between stress and sex (F 1,16 = 5.41 p < 0.05). This reduction in cell area was due to a shortening of the processes indicated by a significant interaction between stress and sex on branch length (F1,16 = 574, p < 0.05; Fig. 2F). Post hoc analysis indicated that stressed females had reduced branch length compared to unstressed females (p value < 0.05) whereas males did not (p > 0.05). HLM analysis of multiple cells from each animal also supported the averaged group effects. There was an effect of 6-day variable stress in the NAc (Table 1) on microglia area in females only (γ01 = −211.88, se = 68.82, p < 0.05), such that stressed females had smaller microglia areas compared to controls and shorter branch lengths (γ01 = −196.36, se = 62.13, p < 0.05).
Table 1.
HLM analysis of NAc Microglia Cell Area, and Branch Length in 6- and 28-Day Variable Stress Groups.
|
6 Days |
28 Days |
|||||
|---|---|---|---|---|---|---|
| Full |
Females |
Males |
Full |
Females |
Males |
|
| Outcome: Area | N = 20 | n = 10 | n = 10 | N = 20 | n = 10 | n = 10 |
| (Area) γ00 | 449.47∗∗∗ | 445.16∗∗∗ | 453.45∗∗∗ | 444.39 | 397.78∗∗∗ | 491.47∗∗∗ |
| se | 27.26 | 34.41 | 32.41 | 14.16 | 18.86 | 21.14 |
| Stress γ01 | −89.55 | −211.88∗ | 32.14 | −9.02 | −12.39 | −5.69 |
| se | 54.52 | 68.82 | 64.82 | 28.32 | 37.72 | 42.29 |
| Sex γ01 | 7.97 | 94.21∗∗ | ||||
| se | 54.52 | 28.32 | ||||
| Deviance Statistics | ||||||
| # parameters (p) | 5 | 4 | 4 | 5 | 4 | 4 |
| Model Deviance |
1495.45 |
733.04 |
756.62 |
1431.51 |
723.16 |
707.99 |
| Full | Females | Males | Full | Females | Males | |
| Outcome: Branch Length | N = 20 | n = 10 | n = 10 | N = 20 | n = 10 | n = 10 |
| (Branch Length) γ00 | 361.07∗∗∗ | 353.61∗∗∗ | 368.25∗∗∗ | 414.78∗∗∗ | 374.17∗∗∗ | 455.12∗∗∗ |
| se | 24.15 | 31.07 | 27.62 | 13.89 | 17.81 | 21.35 |
| Stress γ01 | −85.48 | −196.36∗ | 24.82 | −11.83 | −22.37 | −1.85 |
| se | 48.31 | 62.13 | 55.25 | 27.79 | 35.61 | 42.70 |
| Sex γ01 | 14.36 | 81.23∗∗ | ||||
| se | 48.31 | 27.79 | ||||
| Deviance Statistics | ||||||
| # parameters (p) | 5 | 4 | 4 | 5 | 4 | 4 |
| Model Deviance | 1461.801 | 719.47 | 735.97 | 1424.55 | 724.47 | 699.55 |
These effects were also supported within sex by Sholl analysis as females exposed to 6 days of variable stress had reduced complexity of microglia in NAc, indicated by the reduced number of intersections the further the processes were from the center of the soma compared to unstressed controls (stress × distance interaction F9, 71 = 5.92, p < 0.001; Fig. 2G). In males (Fig. 2H) we only found the expected main effect of distance (F1.38, 10.39 = 93.85, p < 0.0001) but no interaction with stress (p > 0.05). HLM on combined males and females indicated a negative effect of 6-day stress on the rate of decline in number of intersections the further the processes were from the center of the soma (Table 2). Specifically, there was a less severe rate of decline in intersections from 25- to 40- μm from the center in the control group compared to the 6-day variable stress group.
Table 2.
Effects of 6-day stress on the change in number of intersections at radii 10 to 50 microns from the center of the soma in dentate gyrus cells.
| Fixed Effects: | Coefficient | Standard | t-ratio | Approx. | p-value |
|---|---|---|---|---|---|
| Intercept1 (RADIUS10), π0 | error | d.f. | |||
| Intersections, β00 | 6.86 | 0.22 | 30.74 | 13 | <0.001 |
| Stress, β01 | −0.50 | 0.45 | −1.12 | 13 | 0.283 |
| Sex, β02 | 1.93 | 0.45 | 4.31 | 13 | <0.001 |
| For RADIUS15 slope, π1 | |||||
| Intersections Intercept, β10 | −0.14 | 0.18 | −0.78 | 13 | 0.448 |
| Stress, β11 | −0.33 | 0.35 | −0.93 | 13 | 0.372 |
| Sex, β12 | 0.43 | 0.35 | 1.21 | 13 | 0.248 |
| For RADIUS20 slope, π2 | |||||
| Intersections Intercept, β20 | −1.54 | 0.26 | −5.87 | 13 | <0.001 |
| Stress, β21 | −0.50 | 0.52 | −0.96 | 13 | 0.357 |
| Sex, β22 | −0.58 | 0.52 | −1.10 | 13 | 0.292 |
| For RADIUS25 slope, π3 | |||||
| Intersections Intercept, β30 | −2.65 | 0.19 | −13.99 | 13 | <0.001 |
| Stress, β31 | −0.73 | 0.38 | −1.91 | 13 | 0.078 |
| Sex, β32 | −0.45 | 0.38 | −1.19 | 13 | 0.256 |
| For RADIUS30 slope, π4 | |||||
| Intersections Intercept, β40 | −4.04 | 0.28 | −14.34 | 13 | <0.001 |
| Stress, β41 | −0.36 | 0.56 | −0.64 | 13 | 0.532 |
| Sex, β42 | −1.66 | 0.56 | −2.95 | 13 | 0.011 |
| For RADIUS35 slope, π5 | |||||
| Intersections Intercept, β50 | −5.23 | 0.25 | −21.23 | 13 | <0.001 |
| Stress, β51 | 0.10 | 0.49 | 0.20 | 13 | 0.842 |
| Sex, β52 | −1.50 | 0.49 | −3.05 | 13 | 0.009 |
| For RADIUS40 slope, π6 | |||||
| Intersections Intercept, β60 | −5.94 | 0.31 | −19.38 | 13 | <0.001 |
| Stress, β61 | 0.28 | 0.61 | 0.45 | 13 | 0.661 |
| Sex, β62 | −2.43 | 0.61 | −3.96 | 13 | 0.002 |
| For RADIUS45 slope, π7 | |||||
| Intersections Intercept, β70 | −6.20 | 0.25 | −25.02 | 13 | <0.001 |
| Stress, β71 | 0.33 | 0.50 | 0.66 | 13 | 0.523 |
| Sex, β72 | −2.30 | 0.50 | −4.64 | 13 | <0.001 |
| For RADIUS50 slope, π8 | |||||
| Intersections Intercept, β80 | −6.38 | 0.24 | −26.06 | 13 | <0.001 |
| Stress, β81 | 0.45 | 0.49 | 0.92 | 13 | 0.374 |
| Sex, β82 | −2.40 | 0.49 | −4.91 | 13 | <0.001 |
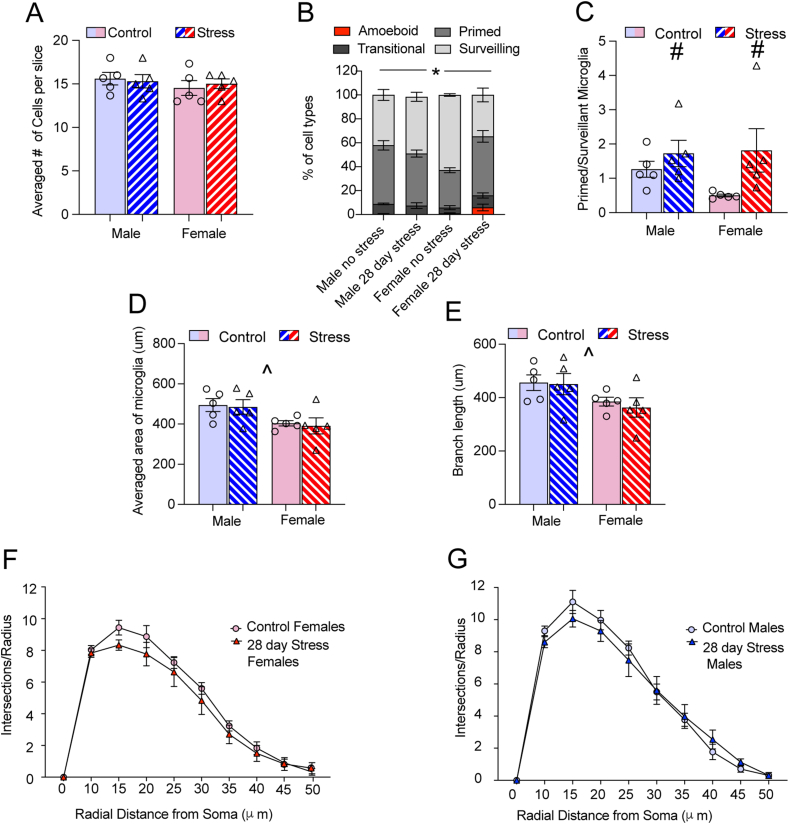
After 28 days of variable stress (Fig. 3A) we did not find any significant interactions between stress and sex on averaged cell counts per animal for males and females (F 1,16 = 0.27, p = 0.69). There were also no main effects of stress or sex (p values > 0.05). Examination of microglia phenotypes after 28 days indicated that there was significant regulation of cell type by stress and sex (F3,32 = 14.94 p < 0.0001; Fig. 3B). Post hoc analysis indicated that stressed females had significantly more primed microglia (p < 0.05) and fewer surveilling microglia than unstressed females (p < 0.0001). Unstressed females had a lower percentage of primed (p < 0.01) and an increased percentage of surveilling microglia (p < 0.001) compared to unstressed males. Stressed males and females did not significantly differ within cell type (p values > 0.05). The ratio of primed to surveilling microglia (Fig. 3C) did not significantly differ between males and females after 28 days of stress in the NAc, as there was a main effect of stress (F1,16= 5.22, p < 0.05) but no significant interaction or main effect of sex (p values > 0.05). The area of microglia in the NAc was smaller in females than males when collapsed across stress (main effect of sex; F 1,16 = 8.29 p < 0.001; Fig. 3D). There was no significant interaction or main effect of stress on microglia area or branch length in the NAc (p values > 0.05). After 28 days of stress, both unstressed and stressed females had reduced branch length as indicated by a main effect of sex (F1,16 = 6.20, p < 0.05; Fig. 3E). HLM also supported these results in that male mice were more likely to have larger microglia area (γ02 = 94.21, se = 28.32, p < 0.01) and increased branch length (γ02 = 81.23, se = 27.79, p < 0.01) compared to females (Table 1). Males had a higher number of intersections at the initial sampling point with 28 days of stress (10 μm; Table 3). Starting at 45 μm from the radius, sex negatively predicted the rate of decline in intersections by distance.Thus, being female was related to a less severe rate of decline from 45 to 50 mm suggesting that sex differences in area/branch length may relate to complexity. Within sex, Sholl analysis averaged across animals only detected a main effect of complexity related to distance from the center of the soma in females (F1.7, 13.9 = 205.9, p < 0.001; Fig. 3F) and males (F1.13.5 = 13.56, p < 0.0001; Fig. 3G).There was no interaction or main effect of stress (p values > 0.05).
Fig. 3.
Effects of 28 days of variable stress on microglia in the NAc (A) There were no differences in cell counts in males or females after 28 days of variable stress. (B) Microglia phenotypes differed between male and female mice when exposed to 28 days of variable stress (p < 0.05). Stressed females had significantly more primed microglia (p < 0.05) and fewer surveilling microglia than unstressed females (p < 0.0001). Unstressed females had a lower percentage of primed (p < 0.01) and increased percentage of surveilling microglia (p < 0.001) compared to unstressed males. Stressed males and females did not significantly differ within cell type (p values > 0.05). (C) The ratio of primed to surveillant microglia increased in both stressed male and female mice (p < 0.05). (D) Microglia area was reduced overall in females compared to males (p < 0.05). (E) Branch length was also reduced in both stressed and unstressed females compared to males (p < 0.05). (F) 28 days of variable stress did not alter complexity in female or (G) or male microglia. Cells were counted on 3 sections per animal and averaged for each animal, n= 5 animals per group. Figures show mean ± SEM. ∗Indicates significant interaction, # indicates a main effect of stress and ˆ indicates a main effect of sex.
Table 3.
Effects of 28-day stress on the change in number of intersections at radii 10 to 50 microns from the center of the soma in NAc cells.
| Fixed Effects: | Coefficient | Standard | t-ratio | Approx. | p-value |
|---|---|---|---|---|---|
| Intercept1 (RADIUS10), π0 | error | d.f. | |||
| Intersections, β00 | 8.51 | 0.13 | 64.26 | 17 | <0.001 |
| Stress, β01 | −0.44 | 0.26 | −1.68 | 17 | 0.112 |
| Sex, β02 | 1.16 | 0.26 | 4.37 | 17 | <0.001 |
| For RADIUS15 slope, π1 | |||||
| Intersections, β10 | 1.39 | 0.26 | 5.39 | 17 | <0.001 |
| Stress, β11 | −0.59 | 0.52 | −1.15 | 17 | 0.268 |
| Sex, β12 | 0.87 | 0.52 | 1.70 | 17 | 0.108 |
| For RADIUS20 slope, π2 | |||||
| Intersections, β20 | 0.56 | 0.33 | 1.70 | 17 | 0.108 |
| Stress, β21 | −0.35 | 0.66 | −0.53 | 17 | 0.601 |
| Sex, β22 | 0.35 | 0.66 | 0.54 | 17 | 0.599 |
| For RADIUS25 slope, π3 | |||||
| Intersections, β30 | −0.88 | 0.33 | −2.70 | 17 | 0.015 |
| Stress, β31 | −0.19 | 0.65 | −0.29 | 17 | 0.775 |
| Sex, β32 | 0.24 | 0.65 | 0.37 | 17 | 0.715 |
| For RADIUS30 slope, π4 | |||||
| Intersections, β40 | −2.94 | 0.29 | −10.08 | 17 | <0.001 |
| Stress, β41 | 0.05 | 0.58 | 0.09 | 17 | 0.927 |
| Sex, β42 | −0.45 | 0.58 | −0.77 | 17 | 0.454 |
| For RADIUS35 slope, π5 | |||||
| Intersections, β50 | −5.00 | 0.26 | −19.55 | 17 | <0.001 |
| Stress, β51 | 0.31 | 0.51 | 0.61 | 17 | 0.552 |
| Sex, β52 | −0.06 | 0.51 | −0.11 | 17 | 0.911 |
| For RADIUS40 slope, π6 | |||||
| Intersections, β60 | −6.53 | 0.24 | −26.67 | 17 | <0.001 |
| Stress, β61 | 0.70 | 0.49 | 1.43 | 17 | 0.17 |
| Sex, β62 | −0.53 | 0.49 | −1.09 | 17 | 0.291 |
| For RADIUS45 slope, π7 | |||||
| Intersections, β70 | −7.61 | 0.17 | −43.76 | 17 | <0.001 |
| Stress, β71 | 0.62 | 0.35 | 1.78 | 17 | 0.093 |
| Sex, β72 | −1.08 | 0.35 | −3.11 | 17 | 0.006 |
| For RADIUS50 slope, π8 | |||||
| Intersections, β80 | −8.14 | 0.15 | −55.92 | 17 | <0.001 |
| Stress, β81 | 0.43 | 0.29 | 1.47 | 17 | 0.16 |
| Sex, β82 | −1.31 | 0.29 | −4.49 | 17 | <0.001 |
3.2. The effects of 6 and 28 days of variable stress on microglia morphology in the dentate gyrus
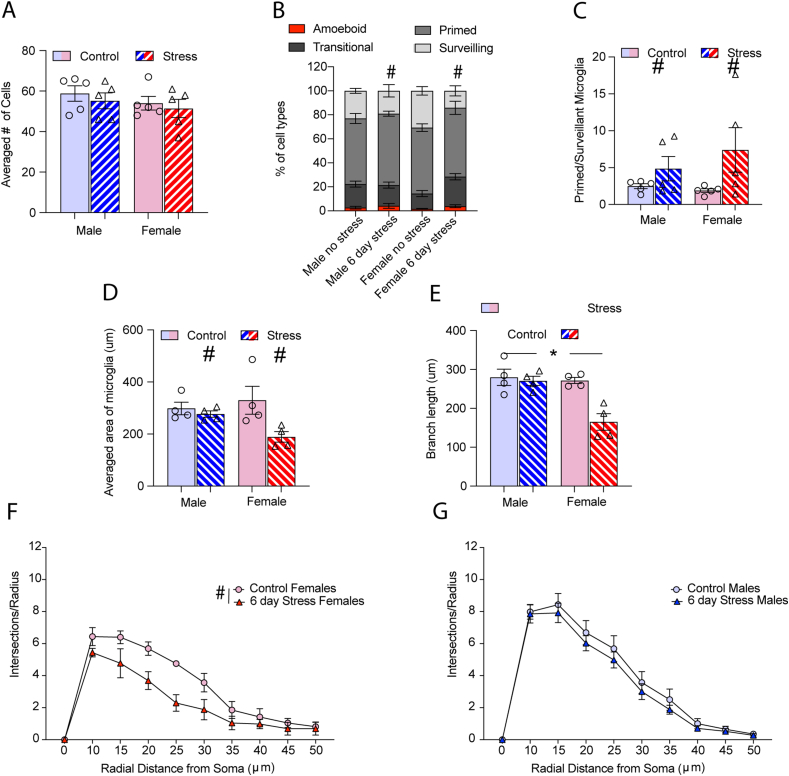
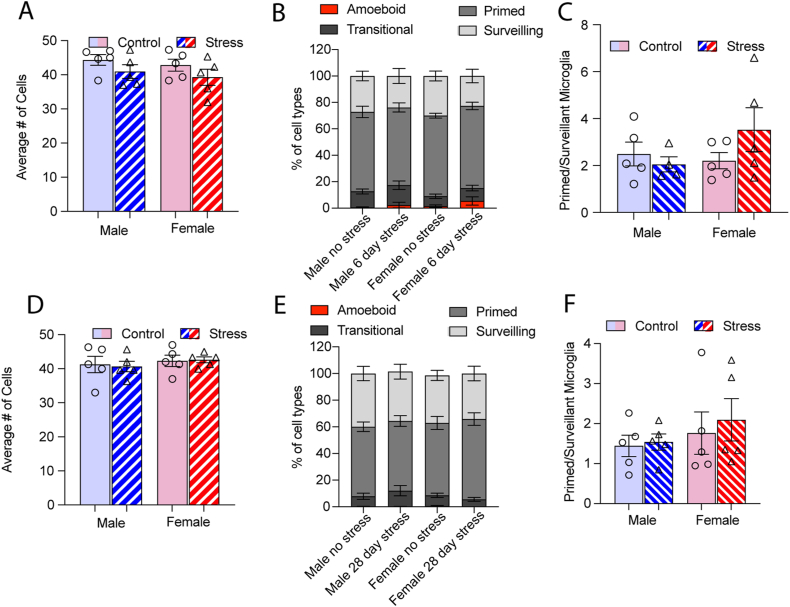
After 6 days of variable stress, we found no interaction between stress and sex on the average number of microglia per animal (F1,16 = 0.1 p = 0.90; Fig. 4A). There were also no main effects of stress or sex on cell counts (p values > 0.05). There was a significant interaction between stress and cell type across sex after 6 days of variable stress (F3,32 = 3.8, p < 0.05; Fig. 4B) but no post-hoc tests were significant within cell type. There was a main effect of stress on the ratio of primed to surveillant microglia (F1,16 = 5.1, p < 0.05; Fig. 4C) with no main effect of sex or interaction between sex and stress (p values > 0.05). Microglia area (Fig. 4D) was decreased in both males and females following stress (F1,12 = 6.61, p < 0.05). Branch length (Fig. 4E) was altered by both sex and stress (F1,12 = 11.80, p < 0.05). Females who had undergone stress had shorter branch lengths than stressed males (p = 0.003) or unstressed females (p = 0.003). HLM analysis also indicated that there was a decrease in microglia area in the dentate gyrus after 6 days of stress compared to controls (γ01 = −153.08, se = 56.41, p < 0.05). Both stress condition and sex significantly predicted differences in dentate gyrus branch length as 6-day variable stress (γ01 = −52.81, se = 20.35, p < 0.05) and being female (γ02 = 52.50, se = 20.47, p < 0.05) was associated with shorter branch lengths (Table 4). Dentate gyrus branching results reveal an effect of sex on the intercept in the 6-day paradigm, indicating that males had more intersections at the initial sampling point (10 μm) than females (β02 = 1.93, se = 0.45, p < 0.001) in the 6-day stress condition (Table 5). Starting at 30 μm from the center, sex negatively predicted the rate of decline (as evidenced by the negative intercept [β42]) in the number intersections by increasing distance from the radius. Being female was related to a decreasing, or less severe rate of decline in the number of intersections from 25 (i.e., 35-, 40-, 45-, and 50-μm distances from the radius). This indicated that the number of intersections decreased less by distance from the radius in all females in the 6-day paradigm. The reductive effect of 6-day stress on the dentate gyrus branch length was greater in females (γ01 = −108.06, se = 25.46, p < 0.01) than the full model. Averaged animal data grouped by stress also indicated that microglia from stressed female mice were overall less complex (Fig. 4F) than unstressed females; main effect of stress (F1,6 =13.57, p < 0.05)/main effect of distance (F2.2,13.3 =48.11). The interaction between stress and distance failed to reach significance (F9,53 =1.80, p = 0.09). For males there were no effects of stress on microglia complexity (p values > 0.05), only a main effect of radial distance (F1.2,9 = 154.8, p < 0.0001; Fig. 4G).
Fig. 4.
The effects of 6-day variable stress on the dentate gyrus (A) There were no differences in cell counts in the dentate gyrus of males or females after 6 days of variable stress. (B) Microglia phenotypes differed between male and female mice in the dentate gyrus when exposed to 6 days of variable stress (p < 0.05). (C) The ratio of primed to surveillant microglia increased in both stressed male and female mice (p < 0.05). (D) Microglia area was reduced overall in stressed males and female (p < 0.05). (E) Stressed females had shorter branch lengths than stressed males or unstressed females (p < 0.05). (F) 6 days of variable stress reduced complexity in the female dentate gyrus but there were no significant effects on (G) male microglia. Cells were counted on 3 sections per animal and averaged for each animal, n = 5 animals per group. Figures show mean ± SEM. ∗Indicates significant interaction, # indicates a main effect of stress and ˆ indicates a main effect of sex.
Table 4.
Dentate gyrus microglia cell area, and branch length in 6- and 28-day variable stress groups.
|
Cell Area |
||||||
|---|---|---|---|---|---|---|
|
6 Days |
28 Days |
|||||
| Full |
Females |
Males |
Full |
Females |
Males |
|
| Outcome: Area | N = 16 | n = 8 | n = 8 | N = 20 | n = 11 | n = 10 |
| (Area) γ00 | 336.48∗∗∗ | 266.77∗∗∗ | 410.27∗ | 370.33∗∗∗ | 365.73∗∗∗ | 380.86∗∗∗ |
| se | 62.16 | 28.21 | 119.20 | 15.50 | 18.14 | 26.62 |
| Stress γ01 | 47.96 | −153.08∗ | 217.16 | 19.23 | −1.23 | 54.81 |
| se | 124.92 | 56.41 | 238.40 | 31.17 | 36.30 | 51.24 |
| Sex γ01 | 159.71 | −15.94 | ||||
| se | 125.55 | 31.32 | ||||
| Deviance Statistics | ||||||
| # parameters (p) | 5 | 5 | 4 | 5 | 4 | 4 |
| Model Deviance |
1237.67 |
527.98 |
643.40 |
1487.46 |
748.86 |
733.21 |
| Branch Length | ||||||
| 6 Days | 28 Days | |||||
| Full | Females | Males | Full | Females | Males | |
| Outcome: Branch Length | N = 20 | n = 8 | n = 8 | N = 20 | n = 11 | n = 10 |
| (Branch Length) γ00 | 247.19∗∗∗ | 220.23∗∗∗ | 275.68∗∗∗ | 326.43∗∗∗ | 313.46∗∗∗ | 340.07∗∗∗ |
| se | 10.11 | 12.73 | 11.11 | 13.25 | 17.03 | 19.87 |
| Stress γ01 | −52.81∗ | −108.06∗∗ | −9.61 | 9.16 | −7.37 | 24.25 |
| se | 20.35 | 25.46 | 22.21 | 26.63 | 34.09 | 39.74 |
| Sex γ01 | 52.50∗ | 17.10 | ||||
| se | 20.47 | 26.76 | ||||
| Deviance Statistics | ||||||
| # parameters (p) | 5 | 4 | 4 | 5 | 4 | 4 |
| Model Deviance | 915.00 | 464.49 | 435.62 | 1458.52 | 728.06 | 721.26 |
Table 5.
Effects of 6-day stress on the change in number of intersections at radii 10 to 50 microns from the center of the soma in dentate gyrus cells.
| Fixed Effects: | Coefficient | Standard | t-ratio | Approx. | p-value |
|---|---|---|---|---|---|
| Intercept1 (RADIUS10), π0 | error | d.f. | |||
| Intersections, β00 | 6.86 | 0.22 | 30.74 | 13 | <0.001 |
| Stress, β01 | −0.50 | 0.45 | −1.12 | 13 | 0.283 |
| Sex, β02 | 1.93 | 0.45 | 4.31 | 13 | <0.001 |
| For RADIUS15 slope, π1 | |||||
| Intersections Intercept, β10 | −0.14 | 0.18 | −0.78 | 13 | 0.448 |
| Stress, β11 | −0.33 | 0.35 | −0.93 | 13 | 0.372 |
| Sex, β12 | 0.43 | 0.35 | 1.21 | 13 | 0.248 |
| For RADIUS20 slope, π2 | |||||
| Intersections Intercept, β20 | −1.54 | 0.26 | −5.87 | 13 | <0.001 |
| Stress, β21 | −0.50 | 0.52 | −0.96 | 13 | 0.357 |
| Sex, β22 | −0.58 | 0.52 | −1.10 | 13 | 0.292 |
| For RADIUS25 slope, π3 | |||||
| Intersections Intercept, β30 | −2.65 | 0.19 | −13.99 | 13 | <0.001 |
| Stress, β31 | −0.73 | 0.38 | −1.91 | 13 | 0.078 |
| Sex, β32 | −0.45 | 0.38 | −1.19 | 13 | 0.256 |
| For RADIUS30 slope, π4 | |||||
| Intersections Intercept, β40 | −4.04 | 0.28 | −14.34 | 13 | <0.001 |
| Stress, β41 | −0.36 | 0.56 | −0.64 | 13 | 0.532 |
| Sex, β42 | −1.66 | 0.56 | −2.95 | 13 | 0.011 |
| For RADIUS35 slope, π5 | |||||
| Intersections Intercept, β50 | −5.23 | 0.25 | −21.23 | 13 | <0.001 |
| Stress, β51 | 0.10 | 0.49 | 0.20 | 13 | 0.842 |
| Sex, β52 | −1.50 | 0.49 | −3.05 | 13 | 0.009 |
| For RADIUS40 slope, π6 | |||||
| Intersections Intercept, β60 | −5.94 | 0.31 | −19.38 | 13 | <0.001 |
| Stress, β61 | 0.28 | 0.61 | 0.45 | 13 | 0.661 |
| Sex, β62 | −2.43 | 0.61 | −3.96 | 13 | 0.002 |
| For RADIUS45 slope, π7 | |||||
| Intersections Intercept, β70 | −6.20 | 0.25 | −25.02 | 13 | <0.001 |
| Stress, β71 | 0.33 | 0.50 | 0.66 | 13 | 0.523 |
| Sex, β72 | −2.30 | 0.50 | −4.64 | 13 | <0.001 |
| For RADIUS50 slope, π8 | |||||
| Intersections Intercept, β80 | −6.38 | 0.24 | −26.06 | 13 | <0.001 |
| Stress, β81 | 0.45 | 0.49 | 0.92 | 13 | 0.374 |
| Sex, β82 | −2.40 | 0.49 | −4.91 | 13 | <0.001 |
Chronic variable stress for 28 days did not alter the average number of cells in the dentate gyrus (Fig. 5A) of male or female mice (F1,16 = 1.98e−029, p > 0.99). There were no main effects of stress or sex (p values > 0.05). While there was a significant effect of cell type (F3,24 = 179, p < 0.001; Fig. 5B) but no main effect or interaction with stress (p values > 0.05). There were also no significant interactions between sex and stress on the ratio of primed: surveilling microglia (F1,14 = 0.52, p = 0.41; Fig. 5C). There were no main effects of sex or stress (p values > 0.05). Microglia cell area (Fig. 5D) and branch length (Fig. 5E) were not altered by stress in either sex (F1,16 = 0.02, p = 0.86; F1,16= 0.14, p =0.7). There were no main effects of sex or stress on microglia area or branch length (p values > 0.05). HLM analysis also indicated there were no significant effects on microglia area or branch length in the dentate gyrus following 28 days of variable stress (Table 4). There were no effects by stress condition or sex on dentate gyrus intersections in the 28-day paradigm (Table 6) and Sholl analysis in females (Fig. 5F) and males (Fig. 5G) only indicated a significant effect of distance from the soma in process complexity (female, F1.8,14.7 =95.78, p < 0.0001; male, F1.3,10.8 = 119.9, p < 0.001).
Fig. 5.
The effects of 28 days of variable stress on the dentate gyrus (A) There were no differences in cell counts in the dentate gyrus of males or females after 28 days of variable stress. (B) Microglia phenotypes did not differ between male and female mice in the dentate gyrus when exposed to 28 days of variable stress. (C) 28 days of variable stress did not alter the ratio of primed to surveillant microglia in either sex. (D) Microglia area was not altered in stressed males and females nor was (E) shorter branch lengths. (F) 28 days of variable stress did not alter complexity in the female dentate gyrus or in (G) male microglia. Cells were counted on 3 sections per animal and averaged for each animal, n= 5 animals per group. Figures show mean ± SEM. ∗Indicates significant interaction, # indicates a main effect of stress and ˆ indicates a main effect of sex.
Table 6.
Effects of 28-day stress on the change in number of intersections at radii 10 to 50 microns from the center of the soma in dentate gyrus cells.
| Fixed Effects: | Coefficient | Standard | t-ratio | Approx. | p-value |
|---|---|---|---|---|---|
| Intercept1 (RADIUS10), π0 | error | d.f. | |||
| Intersections, β00 | 8.95 | 0.21 | 43.00 | 17 | <0.001 |
| Stress, β01 | 0.07 | 0.42 | 0.17 | 17 | 0.868 |
| Sex, β02 | 0.40 | 0.42 | 0.96 | 17 | 0.350 |
| For RADIUS15 slope, π1 | |||||
| Intersections Intercept, β10 | 0.66 | 0.32 | 2.07 | 17 | 0.054 |
| Stress, β11 | −0.75 | 0.64 | −1.18 | 17 | 0.254 |
| Sex, β12 | 0.18 | 0.64 | 0.29 | 17 | 0.776 |
| For RADIUS20 slope, π2 | |||||
| Intersections Intercept, β20 | −0.37 | 0.38 | −0.96 | 17 | 0.352 |
| Stress, β21 | −0.34 | 0.76 | −0.44 | 17 | 0.666 |
| Sex, β22 | 0.27 | 0.76 | 0.35 | 17 | 0.730 |
| For RADIUS25 slope, π3 | |||||
| Intersections Intercept, β30 | −2.46 | 0.36 | −6.89 | 17 | <0.001 |
| Stress, β31 | 0.55 | 0.71 | 0.77 | 17 | 0.455 |
| Sex, β32 | 0.72 | 0.71 | 1.00 | 17 | 0.330 |
| For RADIUS30 slope, π4 | |||||
| Intersections Intercept, β40 | −4.27 | 0.25 | −16.87 | 17 | <0.001 |
| Stress, β41 | 0.15 | 0.51 | 0.29 | 17 | 0.775 |
| Sex, β42 | 0.18 | 0.51 | 0.36 | 17 | 0.722 |
| For RADIUS35 slope, π5 | |||||
| Intersections Intercept, β50 | −5.37 | 0.20 | −27.33 | 17 | <0.001 |
| Stress, β51 | −0.14 | 0.39 | −0.35 | 17 | 0.733 |
| Sex, β52 | −0.43 | 0.39 | −1.11 | 17 | 0.285 |
| For RADIUS40 slope, π6 | |||||
| Intersections Intercept, β60 | −5.44 | 0.17 | −31.18 | 17 | <0.001 |
| Stress, β61 | 0.01 | 0.35 | 0.02 | 17 | 0.982 |
| Sex, β62 | −0.51 | 0.35 | −1.47 | 17 | 0.159 |
| For RADIUS45 slope, π7 | |||||
| Intersections Intercept, β70 | −5.54 | 0.21 | −26.57 | 17 | <0.001 |
| Stress, β71 | −0.01 | 0.42 | −0.03 | 17 | 0.974 |
| Sex, β72 | −0.68 | 0.42 | −1.63 | 17 | 0.122 |
| For RADIUS50 slope, π8 | |||||
| Intersections Intercept, β80 | −6.00 | 0.23 | −26.09 | 17 | <0.001 |
| Stress, β81 | 0.20 | 0.46 | 0.43 | 17 | 0.674 |
| Sex, β82 | −0.19 | 0.46 | −0.41 | 17 | 0.686 |
3.3. There are no effects of stress or sex on microglia morphology in area CA1
There was no significant interaction between sex and 6 days of variable stress on cell counts in CA1 (F1,16= 0.001, p =0.97; Fig. 6A) and no main effects (p values > 0.05). While there were significant differences in the percentage of cell types (F3,32 = 255.6, p < 0.0001; Fig. 6B) there were no other effects of stress, sex or interactions (p values > 0.05). There were also no significant effects of sex and stress on the ratio of primed: surveillant cells for CA1 (F1,15 = 2.08, p = 0.16; Fig. 6C). There were also no significant effects of sex or 28 days of stress on the average number of cells in CA1(F1,16= 0.06, p =0.8; Fig. 6D). As with 6 days of stress there were differences in the percentage of cells (F3,32= 207.1, p < 0.0001;Fig. 6E). There were also no effects of sex and stress on the ratio of primed: surveilling microglia after 28 days of variable stress in CA1 (F1,16 = 0.08, p = 0.77; Fig. 6F). There were no main effects of stress or sex on any measure (p > 0.05).
Fig. 6.
There were no effects of stress or sex on microglia in CA1 at either time point. (A) There were no differences in cell counts CA1 of males or females after 6 days of variable stress. (B) Microglia phenotypes did not differ between male and female mice in CA1 when exposed to 6 days of variable stress. (C) 6 days of variable stress did not alter the ratio of primed to surveillant microglia in either sex. (D) There were no differences in cell counts CA1 of males or females after 28 days of variable stress. (B) Microglia phenotypes did not differ between male and female mice in CA1 when exposed to 28 days of variable stress. (C) 28 days of variable stress did not alter the ratio of primed to surveillant microglia in either sex.
Cells were counted on 3 sections per animal and naveraged
for each animal, n= 5 animals per group. Figures show mean +SEM. ∗Indicates significant interaction, # indicates a main effect of stress and ˆ indicates a main effect of sex.
3.4. The effects of rosiglitazone treatment on 6 days of variable stress in females
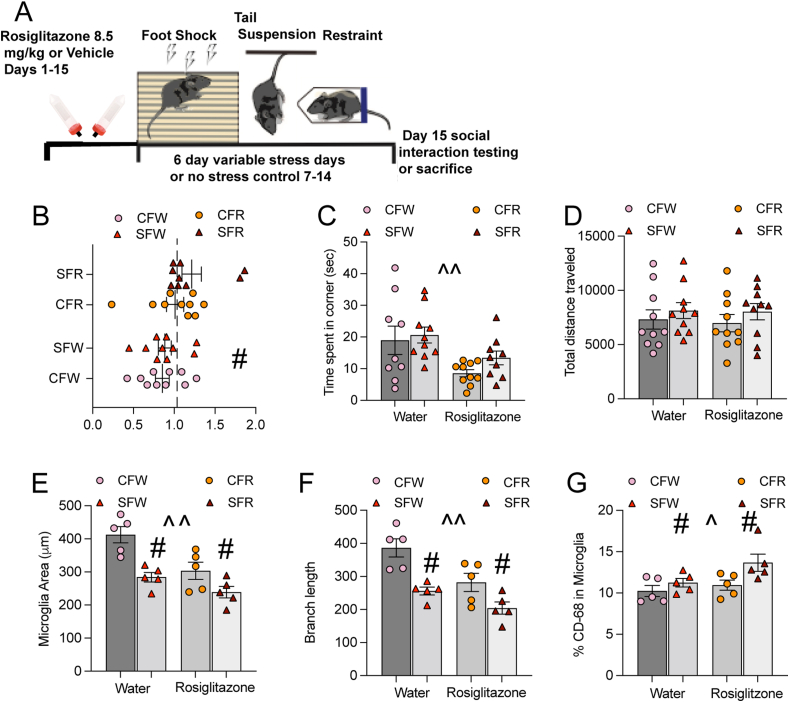
To examine the functional role of microglia activation on social behavior and explore the relationship between changes in microglia morphology and activation state we treated female mice with rosiglitazone and examined social interaction or microglia morphology/activation (Fig. 7A). Rosiglitazone is a peroxisome proliferator activated receptor gamma (PPARγ) selective agonist which promotes an anti-inflammatory state in activated microglia by suppressing the NF-kB pathway and has antidepressant like effects on behavior in male rodents (Cheng et al., 2015; Guo et al., 2017; Ji et al., 2018; Zhao et al., 2020). Treatment for 2 weeks with rosiglitazone promoted social interaction (Fig. 7B) in female mice regardless of stress exposure (F1,35 =6.26, p < 0.05). Rosiglitazone decreased ratio of time spent in the corner of the apparatus (Fig. 7C) when a novel conspecific was present (F1,34 = 10.20, p < 0.01). Rosiglitazone, stress or their interaction did not alter the total distance traveled by the mice (Fig. 7D) indicating that effects were not dependent on locomotor behavior (p values > 0.05). In a separate group of mice, we examined the effects of stress and rosiglitazone treatment on microglia morphology and activation state. Stress and rosiglitazone decreased microglia area (Fig. 7E) as indicated by a main effect of stress (F1,16= 20.81, p < 0.001) and a main effect of drug treatment (F1,16 = 13.24, p < 0.01). Branch length (Fig. 7F) was also decreased in stressed mice (F1,16 = 21.85, p < 0.001) and mice treated with Rosiglitazone (F1,16 = 12.28, p < 0.01). To determine the relationship between morphology and activation state we examined the percentage of cluster of differentiation (CD68) a lysosomal/endosomal glycoprotein within microglia used as an indicator of phagocytosis (Minett et al., 2016; Rabinowitz and Gordon, 1991). Stress increased the percentage of CD68 in microglia (F1,16 = 6.44, p < 0.05; Fig. 7G). Rosiglitazone did not block the effects of stress on microglia activation (F1,16 = 1.37, p = 0.25) but Rosiglitazone increased the percentage of CD68 in microglia when data was collapsed across stress (F1,16 = 4.53 p < 0.05).
Fig. 7.
Impact of rosiglitazone treatment on social behavior and microglia morphology/activation state in female mice (A) Schematic of treatment paradigm, mice received 15 days of treatment with 8.5 mg/kg or vehicle in their drinking water. They underwent 6 days of variable stress and were either tested for social interaction or sacrificed for immunohistochemistry (B) Treatment with rosiglitazone increased social interaction ratios regardless of stress exposure (p < 0.05) (C) Rosiglitazone treatment decreased the amount of time mice spent in the corner away from a novel conspecific (p values < 0.05).(D) Locomotor activity was not altered by stress or rosiglitazone treatment (E) Both stress and rosiglitazone treatment decreased microglia area, and (F) branch length. (G). The percentage of CD68, a lysosomal protein indicating activation, was increased in the microglia of stressed mice regardless of treatment (p < 0.05). Rosiglitazone treatment to increase CD68 in microglia (p < 0.05). Behavior n= 10 animals per group, morphology activation n= 5 animals per group. Figures show mean ± SEM. ∗Indicates significant interaction, # indicates a main effect of stress and ˆ indicates a main effect of drug treatment.
4. Discussion
The studies presented here demonstrate that there are sex specific changes in microglia morphology following 6 days of variable stress in the NAc and dentate gyrus of female mice that do not occur in males. In female mice exposed to 6 days of variable stress there is a decrease in the proportion of surveillant microglia in both the NAc and dentate gyrus of the HPC. There is also an increase in the proportion of primed microglia in the NAc and an increase in the ratio of primed to surveilling microglia in both regions. Skeleton and Sholl analysis indicated that after 6 days of stress, microglia from female mice had shortened branching and decreased process complexity, area measurements also revealed a smaller overall area of microglia in both the NAc and dentate gyrus. In combination with the visual characterization these changes support the concept that subchronic stress shifts females to a reduction in the homeostatic state of microglia in NAc and dentate gyrus and potentially increases activation. After 28 days of variable stress females continued to have a greater percentage of primed microglia and a decrease in surveilling microglia in the NAc but not this dentate gyrus. In this cohort of mice but not those exposed to 6 days of variable stress, there were also basal sex differences in NAc. Unstressed females had a greater percentage of surveillant and fewer primed microglia than unstressed males. However, there was a main effect of sex on both microglia area and branch number indicating that in this cohort both stressed and unstressed females had smaller microglia with reduced processes compared to males and differed from data in the unstressed group for the 6-day variable stress studies. While all mice undergo at least 4 days of habituation prior to stress exposure, it is possible that the longer period of housing in the animal facility impacted this cohort of mice differently than those who were only housed there for ∼10 days prior to sacrifice. Branch length in both stressed and unstressed females in the 28-day stress study were around ∼375 μm and similar to the branch length in the unstressed females in the 6-day stress study. In contrast the branch length of females exposed to 6 days of stress was ∼220 μm. Therefore, it seems to represent an acclimation in the females that were stressed for 28 days as their branch length is increasing to control levels. Where the controls really differ across the two studies is for the area of the microglia (∼575 for 6-day unstressed females/400 for unstressed 28-day females). One possibility is that the 6-day control female may have a bigger soma size. More research will be needed to identify why these controls differ from each other. Overall, there were regional brain differences for females in their sensitivity to stress. The NAc was the most sensitive as there were effects of stress after 6 and 28 days. Next was the dentate gyrus which was sensitive to 6 days of variable stress but not 28 days. Finally, microglia in CA1 were not sensitive on any measure to either 6 or 28 days of stress exposure.
Together these data suggest that microglia are more responsive to the onset of stress susceptibility and are less responsive to chronic stress. The notion that microglial response might be waning with the prolonged chronic stress is supported by a recent study (Woodburn et al., 2021) which showed microglial engulfment of neuronal elements in the PFC of the male rats only after 14 days of chronic unpredictable stress, but not after 28 days of stress. Interestingly microglial engulfment was only observed in males and not female rats (Woodburn et al., 2021) providing further support for sex-specific microglial activation following stress.
Having observed major morphological changes in the NAc of female mice following 6 days of variable stress, we further investigated whether these changes modulate behavior in female mice. To that end, in a separate study, we treated female mice with the selective PPARγ agonist rosiglitazone. In a group of animals that were sacrificed 24 h after the last stressor we found that both stress and rosiglitazone altered microglia morphology. We replicated the decrease in microglia area and branch length induced by 6 days of variable stress in the female NAc. Rosiglitazone also decreased microglia area and branch length regardless of stress. We measured the percentage of CD68 within traced microglia as a proxy of activation (Minett et al., 2016; Rabinowitz and Gordon, 1991), and found a significant increase in CD68 following 6 days of stress. A similar increase was found in both groups of animals following treatment with rosiglitazone. To investigate potential behavioral changes, we administered a social interaction test in a separate group of animals. This test was chosen specifically since it is an established test to test rodents for anhedonia, one of the two core symptoms of MDD (Scheggi et al., 2018). Interestingly, we found that 2 weeks of treatment with rosiglitazone had an antidepressant-like effect, as indicated by an increased sociability in rosiglitazone-treated mice. This is somewhat a puzzling discovery, as we have found that both variable stress and rosiglitazone treatment cause changes in microglial morphology, shifting it to a more activated state, normally a hallmark of MDD as well as other psychiatric disorders (Gandal et al., 2018a, 2018b; Hansen et al., 2018; Setiawan et al., 2015; Tang and Le, 2016; Tsilioni et al., 2019). However, here we observe that rosiglitazone alters microglial morphology and at the same time has an antidepressant-like effect in female mice. This could be potentially explained by the fact that microglia can adopt different activation phenotypes in response to different stimuli (Cherry et al., 2014; Subramaniam and Federoff, 2017). When exposed to PPARy agonists, microglia are thought to shift from a pro-to an anti-inflammatory phenotype (Subramaniam and Federoff, 2017). Previously rosiglitazone has been shown to shift microglia towards an anti-inflammatory state (Ji et al., 2018), and to produce an antidepressant-like effect in both male rats and mice (Eissa Ahmed et al., 2009). Given that, we hypothesize here that rosiglitazone produces the increased sociability by shifting activated microglia towards more anti-inflammatory state. Future studies will need to identify what phenotype microglia acquired in response to stress and whether this response is sex-specific.
Our findings highlight the importance of utilizing multiple types of analysis when inspecting morphological changes in microglia following stress. Applying several different approaches of analyzing microglial morphology, enabled us to better understand the potential functional implications of these changes. Combined methods indicated a decrease in surveilling microglia after variable stress, at the same time, sorting microglia phenotypes provided clarification that the microglia were taking on a more activated morphology.
Since microglia are dynamic and plastic cells, able to rapidly react to homeostatic changes in the brain, microglial morphology might differ depending on the length and type of stress and brain region under investigation. We have shown here that following 6 days of variable stress, microglia become more activated in the NAc only in females, and not males. In contrast, 14-day chronic restraint stress resulted in activated microglia in males in the number of brain regions, including the NAc, medial PFC and HPC (Tynan et al., 2010). Longer restraint stress (21 days) also resulted in microglial activation in the medial PFC in males (Hinwood et al., 2013). However, in another study, both acute (1 day) and chronic (10 days) restraint stress did not change microglial activation in the medial PFC in the male rats (Bollinger et al., 2016). Not only the length of stress affects microglial activation, but also the type of stress used. When comparing habituating vs. non-habituating stress of the same duration, microglial morphological changes in the PFC were observed only in the rats subjected to chronic restraint stress, whereas no changes in microglial morphology were observed after chronic variable stress (Kopp et al., 2013).These studies confirm the notion that sex, brain region and stress length and type need to be taken into account when studying activation of microglia induced by stress. We also show here that microglial activation produces antidepressant-like effects in female mice, as evidenced in rosiglitazone-treated mice. However, further studies are necessary to better understand the nature and function of different microglia activation phenotypes.
Financial disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgements
We would like to thank Kaiser C. Arndt, Beatriz Torres-Ceja,G. Nicole Rivero Ballon.
Amanda N. Patterson and Brett H. Smith for their assistance with early stages of data collection and analysis. This work was supported by a NARSAD Young Investigator Award from the Brain and Behavior Research Foundation (24805) and by the National Institutes of Health (1R56MH124930).
Biography

Mariya Tsyglakova is a PhD candidate in Translational Biology, Medicine and Health at Virginia Tech in the Hodes lab. Her main research interest centers on discovering biological mechanisms responsible for individual differences in susceptibility to stress and depression. Specifically, she is investigating how microglia influence synaptic plasticity in male and female mice using a variable stress model. To date at Virginia Tech, she has published 2 review papers, one first author, one co-author. This paper represents her first research publication. Mariya holds an MA degree in economics from Virginia Tech, a BS in business administration with minor in mathematics from American University, and a BA in pedagogy and methods of general education from Izmail State University of Humanities (Ukraine). Upon completion of her PhD, Mariya plans to continue her training as postdoctoral researcher. She plans to learn new techniques and to gain more experience, with the ultimate goal of establishing her own laboratory. In her free time, she enjoys playing tennis, baking, reading, traveling and learning about new cultures. She speaks English, Russian, German and Ukrainian languages.
References
- Akhmetzyanova E., Kletenkov K., Mukhamedshina Y., Rizvanov A. Different approaches to modulation of microglia phenotypes after spinal cord injury. Front. Syst. Neurosci. 2019;13:37. doi: 10.3389/fnsys.2019.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati R.B., Gehrmann J., Schubert P., Kreutzberg G.W. Cytotoxicity of microglia. Glia. 1993;7:111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- Barch D.M., Tillman R., Kelly D., Whalen D., Gilbert K., Luby J.L. Hippocampal volume and depression among young children. Psychiatry Res. Neuroimaging. 2019;288:21–28. doi: 10.1016/j.pscychresns.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar T.P., Pelaez M.C., Hernandez Silva J.C., Quessy F., Lavigne A.A., Morency D., Blanchette L.J., Arsenault E., Cherasse Y., Seigneur J., Timofeev I., Sephton C.F., Proulx C.D., Labonte B. Chronic stress induces sex-specific functional and morphological alterations in corticoaccumbal and corticotegmental pathways. Biol. Psychiatr. 2021 doi: 10.1016/j.biopsych.2021.02.014. [DOI] [PubMed] [Google Scholar]
- Bollinger J.L., Bergeon Burns C.M., Wellman C.L. Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav. Immun. 2016;52:88–97. doi: 10.1016/j.bbi.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger J.L., Collins K.E., Patel R., Wellman C.L. Behavioral stress alters corticolimbic microglia in a sex- and brain region-specific manner. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancato A., Bregman D., Ahn H.F., Pfau M.L., Menard C., Cannizzaro C., Russo S.J., Hodes G.E. Sub-chronic variable stress induces sex-specific effects on glutamatergic synapses in the nucleus accumbens. Neuroscience. 2017;350:180–189. doi: 10.1016/j.neuroscience.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathomas F., Murrough J.W., Nestler E.J., Han M.H., Russo S.J. Neurobiology of resilience: interface between mind and body. Biol. Psychiatr. 2019 doi: 10.1016/j.biopsych.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Rodriguiz R.M., Murthy S.R., Senatorov V., Thouennon E., Cawley N.X., Aryal D.K., Ahn S., Lecka-Czernik B., Wetsel W.C., Loh Y.P. Neurotrophic factor-alpha1 prevents stress-induced depression through enhancement of neurogenesis and is activated by rosiglitazone. Mol. Psychiatr. 2015;20:744–754. doi: 10.1038/mp.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry J.D., Olschowka J.A., O'Banion M.K. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J. Neuroinflammation. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel D.J., Golden S.A., Walsh J.J., Guise K.G., Heshmati M., Friedman A.K., Dey A., Smith M., Rebusi N., Pfau M., Ables J.L., Aleyasin H., Khibnik L.A., Hodes G.E., Ben-Dor G.A., Deisseroth K., Shapiro M.L., Malenka R.C., Ibanez-Tallon I., Han M.H., Russo S.J. Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nat. Neurosci. 2015;18:962–964. doi: 10.1038/nn.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton C.A. Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover K.K., Maeng L.Y., Lebron-Milad K., Milad M.R. Mechanisms of estradiol in fear circuitry: implications for sex differences in psychopathology. Transl. Psychiatry. 2014;4:e422. doi: 10.1038/tp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C., Whetstone A.S., Hodes G.E., Shors T.J. Stressful experience has opposite effects on dendritic spines in the hippocampus of cycling versus masculinized females. Neurosci. Lett. 2009;449:52–56. doi: 10.1016/j.neulet.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Zhang Y., Chen Y., Zhu J., Yang Y., Zhang H.L. Role of microglia in neurological disorders and their potentials as a therapeutic target. Mol. Neurobiol. 2017;54:7567–7584. doi: 10.1007/s12035-016-0245-0. [DOI] [PubMed] [Google Scholar]
- Eissa Ahmed A.A., Al-Rasheed N.M., Al-Rasheed N.M. Antidepressant-like effects of rosiglitazone, a PPARgamma agonist, in the rat forced swim and mouse tail suspension tests. Behav. Pharmacol. 2009;20:635–642. doi: 10.1097/FBP.0b013e328331b9bf. [DOI] [PubMed] [Google Scholar]
- Gandal M.J., Haney J.R., Parikshak N.N., Leppa V., Ramaswami G., Hartl C., Schork A.J., Appadurai V., Buil A., Werge T.M., Liu C., White K.P., CommonMind C., Psych E.C., i P.-B.W.G., Horvath S., Geschwind D.H. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science. 2018;359:693–697. doi: 10.1176/appi.focus.17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal M.J., Zhang P., Hadjimichael E., Walker R.L., Chen C., Liu S., Won H., van Bakel H., Varghese M., Wang Y., Shieh A.W., Haney J., Parhami S., Belmont J., Kim M., Moran Losada P., Khan Z., Mleczko J., Xia Y., Dai R., Wang D., Yang Y.T., Xu M., Fish K., Hof P.R., Warrell J., Fitzgerald D., White K., Jaffe A.E., Psych E.C., Peters M.A., Gerstein M., Liu C., Iakoucheva L.M., Pinto D., Geschwind D.H. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science. 2018;362 doi: 10.1126/science.aat8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar R., Soares-Cunha C., Domingues A.V., Coimbra B., Baptista F.I., Pinto L., Ambrosio A.F., Rodrigues A.J., Gomes C.A. Resilience to stress and sex-specific remodeling of microglia and neuronal morphology in a rat model of anxiety and anhedonia. Neurobiol Stress. 2021;14:100302. doi: 10.1016/j.ynstr.2021.100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guneykaya D., Ivanov A., Hernandez D.P., Haage V., Wojtas B., Meyer N., Maricos M., Jordan P., Buonfiglioli A., Gielniewski B., Ochocka N., Comert C., Friedrich C., Artiles L.S., Kaminska B., Mertins P., Beule D., Kettenmann H., Wolf S.A. Transcriptional and translational differences of microglia from male and female brains. Cell Rep. 2018;24:2773–2783. doi: 10.1016/j.celrep.2018.08.001. e2776. [DOI] [PubMed] [Google Scholar]
- Guo M., Li C., Lei Y., Xu S., Zhao D., Lu X.Y. Role of the adipose PPARgamma-adiponectin axis in susceptibility to stress and depression/anxiety-related behaviors. Mol. Psychiatr. 2017;22:1056–1068. doi: 10.1038/mp.2016.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar R., Alter M.D., Block C.S., Sullivan H., Bolton J.L., Bilbo S.D. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia. 2017;65:1504–1520. doi: 10.1002/glia.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch U.K., Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hansen D.V., Hanson J.E., Sheng M. Microglia in Alzheimer's disease. J. Cell Biol. 2018;217:459–472. doi: 10.1083/jcb.201709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinwood M., Tynan R.J., Charnley J.L., Beynon S.B., Day T.A., Walker F.R. Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cerebr. Cortex. 2013;23:1784–1797. doi: 10.1093/cercor/bhs151. [DOI] [PubMed] [Google Scholar]
- Hodes G.E., Epperson C.N. Sex differences in vulnerability and resilience to stress across the life span. Biol. Psychiatr. 2019;86:421–432. doi: 10.1016/j.biopsych.2019.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes G.E., Pfau M.L., Purushothaman I., Ahn H.F., Golden S.A., Christoffel D.J., Magida J., Brancato A., Takahashi A., Flanigan M.E., Menard C., Aleyasin H., Koo J.W., Lorsch Z.S., Feng J., Heshmati M., Wang M., Turecki G., Neve R., Zhang B., Shen L., Nestler E.J., Russo S.J. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J. Neurosci. 2015;35:16362–16376. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes S.E., Hinz R., Conen S., Gregory C.J., Matthews J.C., Anton-Rodriguez J.M., Gerhard A., Talbot P.S. Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: a positron emission tomography study. Biol. Psychiatr. 2018;83:61–69. doi: 10.1016/j.biopsych.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Ji J., Xue T.F., Guo X.D., Yang J., Guo R.B., Wang J., Huang J.Y., Zhao X.J., Sun X.L. Antagonizing peroxisome proliferator-activated receptor gamma facilitates M1-to-M2 shift of microglia by enhancing autophagy via the LKB1-AMPK signaling pathway. Aging Cell. 2018;17 doi: 10.1111/acel.12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A., Rainville J.R., Rivero-Ballon G.N., Dhimitri K., Hodes G.E. Testing the limits of sex differences using variable stress. Neuroscience. 2021;454:72–84. doi: 10.1016/j.neuroscience.2019.12.034. [DOI] [PubMed] [Google Scholar]
- Kopp B.L., Wick D., Herman J.P. Differential effects of homotypic vs. heterotypic chronic stress regimens on microglial activation in the prefrontal cortex. Physiol. Behav. 2013;122:246–252. doi: 10.1016/j.physbeh.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonte B., Engmann O., Purushothaman I., Menard C., Wang J., Tan C., Scarpa J.R., Moy G., Loh Y.E., Cahill M., Lorsch Z.S., Hamilton P.J., Calipari E.S., Hodes G.E., Issler O., Kronman H., Pfau M., Obradovic A.L.J., Dong Y., Neve R.L., Russo S., Kazarskis A., Tamminga C., Mechawar N., Turecki G., Zhang B., Shen L., Nestler E.J. Sex-specific transcriptional signatures in human depression. Nat. Med. 2017;23:1102–1111. doi: 10.1038/nm.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant Q., Chakravarty S., Vialou V., Mukherjee S., Koo J.W., Kalahasti G., Bradbury K.R., Taylor S.V., Maze I., Kumar A., Graham A., Birnbaum S.G., Krishnan V., Truong H.T., Neve R.L., Nestler E.J., Russo S.J. Role of nuclear factor kappaB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol. Psychiatr. 2009;65:874–880. doi: 10.1016/j.biopsych.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz K.M., McCarthy M.M. A starring role for microglia in brain sex differences. Neuroscientist. 2015;21:306–321. doi: 10.1177/1073858414536468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz K.M., Nugent B.M., Haliyur R., McCarthy M.M. Microglia are essential to masculinization of brain and behavior. J. Neurosci. 2013;33:2761–2772. doi: 10.1523/JNEUROSCI.1268-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.L., Li J.M., Su W.J., Wang B., Jiang C.L. Sex differences in depressive-like behaviour may relate to imbalance of microglia activation in the hippocampus. Brain Behav. Immun. 2019 doi: 10.1016/j.bbi.2019.06.012. [DOI] [PubMed] [Google Scholar]
- Mecha M., Carrillo-Salinas F.J., Feliu A., Mestre L., Guaza C. Microglia activation states and cannabinoid system: therapeutic implications. Pharmacol. Ther. 2016;166:40–55. doi: 10.1016/j.pharmthera.2016.06.011. [DOI] [PubMed] [Google Scholar]
- Menard C., Hodes G.E., Russo S.J. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321:138–162. doi: 10.1016/j.neuroscience.2015.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C., Pfau M.L., Hodes G.E., Kana V., Wang V.X., Bouchard S., Takahashi A., Flanigan M.E., Aleyasin H., LeClair K.B., Janssen W.G., Labonte B., Parise E.M., Lorsch Z.S., Golden S.A., Heshmati M., Tamminga C., Turecki G., Campbell M., Fayad Z.A., Tang C.Y., Merad M., Russo S.J. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 2017;20:1752–1760. doi: 10.1038/s41593-017-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minett T., Classey J., Matthews F.E., Fahrenhold M., Taga M., Brayne C., Ince P.G., Nicoll J.A., Boche D., Mrc C. Microglial immunophenotype in dementia with Alzheimer's pathology. J. Neuroinflammation. 2016;13:135. doi: 10.1186/s12974-016-0601-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir J., Tse Y.C., Iyer E.S., Biris J., Cvetkovska V., Lopez J., Bagot R.C. Ventral hippocampal afferents to nucleus accumbens encode both latent vulnerability and stress-induced susceptibility. Biol. Psychiatr. 2020;88:843–854. doi: 10.1016/j.biopsych.2020.05.021. [DOI] [PubMed] [Google Scholar]
- Nelson L.H., Lenz K.M. The immune system as a novel regulator of sex differences in brain and behavioral development. J. Neurosci. Res. 2017;95:447–461. doi: 10.1002/jnr.23821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson L.H., Warden S., Lenz K.M. Sex differences in microglial phagocytosis in the neonatal hippocampus. Brain Behav. Immun. 2017;64:11–22. doi: 10.1016/j.bbi.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Norden D.M., Godbout J.P. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 2013;39:19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notter T., Coughlin J.M., Gschwind T., Weber-Stadlbauer U., Wang Y., Kassiou M., Vernon A.C., Benke D., Pomper M.G., Sawa A., Meyer U. Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol. Psychiatr. 2018;23:323–334. doi: 10.1038/mp.2016.248. [DOI] [PubMed] [Google Scholar]
- Notter T., Coughlin J.M., Sawa A., Meyer U. Reconceptualization of translocator protein as a biomarker of neuroinflammation in psychiatry. Mol. Psychiatr. 2018;23:36–47. doi: 10.1038/mp.2017.232. [DOI] [PubMed] [Google Scholar]
- Nugent B.M., Wright C.L., Shetty A.C., Hodes G.E., Lenz K.M., Mahurkar A., Russo S.J., Devine S.E., McCarthy M.M. Brain feminization requires active repression of masculinization via DNA methylation. Nat. Neurosci. 2015;18:690–697. doi: 10.1038/nn.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihuela R., McPherson C.A., Harry G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016;173:649–665. doi: 10.1111/bph.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C., Gold S.M., Penninx B.W., Pariante C.M., Etkin A., Fava M., Mohr D.C., Schatzberg A.F. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Franklin K.B.J. The Mouse Brain in Stereotaxic Coordinates. 2nd Edition. Academic Press; San Diego: 2001. [Google Scholar]
- Pfau M.L., Purushothaman I., Feng J., Golden S.A., Aleyasin H., Lorsch Z.S., Cates H.M., Flanigan M.E., Menard C., Heshmati M., Wang Z., Ma'ayan A., Shen L., Hodes G.E., Russo S.J. Integrative analysis of sex-specific microRNA networks following stress in mouse nucleus accumbens. Front. Mol. Neurosci. 2016;9:144. doi: 10.3389/fnmol.2016.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D.A., Holmes A.J., Dillon D.G., Goetz E.L., Birk J.L., Bogdan R., Dougherty D.D., Iosifescu D.V., Rauch S.L., Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am. J. Psychiatr. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz S.S., Gordon S. Macrosialin, a macrophage-restricted membrane sialoprotein differentially glycosylated in response to inflammatory stimuli. J. Exp. Med. 1991;174:827–836. doi: 10.1084/jem.174.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville J.R., Lipuma T., Hodes G.E. Translating the transcriptome: sex differences in the mechanisms of depression and stress, revisited. Biol. Psychiatr. 2021 doi: 10.1016/j.biopsych.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff R.M., Perry V.H. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Raudenbush S.W., Bryk A.S., Cheong Y.F., Congdon R.T., du Toit M. Scientific Software International; Chicago , L: 2011. HLM 7: Hierarchial Linear and Nolinear Modeling. [Google Scholar]
- Roddy D.W., Farrell C., Doolin K., Roman E., Tozzi L., Frodl T., O'Keane V., O'Hanlon E. The Hippocampus in depression: more than the sum of its parts? Advanced hippocampal substructure segmentation in depression. Biol. Psychiatr. 2019;85:487–497. doi: 10.1016/j.biopsych.2018.08.021. [DOI] [PubMed] [Google Scholar]
- Rosen S., Ham B., Mogil J.S. Sex differences in neuroimmunity and pain. J. Neurosci. Res. 2017;95:500–508. doi: 10.1002/jnr.23831. [DOI] [PubMed] [Google Scholar]
- Scheggi S., De Montis M.G., Gambarana C. Making sense of rodent models of anhedonia. Int. J. Neuropsychopharmacol. 2018;21:1049–1065. doi: 10.1093/ijnp/pyy083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J.M., Sholar P.W., Bilbo S.D. Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 2012;120:948–963. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney M.L., Huo Z., Cahill K., French L., Puralewski R., Zhang J., Logan R.W., Tseng G., Lewis D.A., Sibille E. Opposite molecular signatures of depression in men and women. Biol. Psychiatr. 2018;84:18–27. doi: 10.1016/j.biopsych.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E., Wilson A.A., Mizrahi R., Rusjan P.M., Miler L., Rajkowska G., Suridjan I., Kennedy J.L., Rekkas P.V., Houle S., Meyer J.H. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 2015;72:268–275. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors T.J., Chua C., Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J., Bielau H., Brisch R., Danos P., Ullrich O., Mawrin C., Bernstein H.G., Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J. Psychiatr. Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Steiner J., Mawrin C., Ziegeler A., Bielau H., Ullrich O., Bernstein H.G., Bogerts B. Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol. 2006;112:305–316. doi: 10.1007/s00401-006-0090-8. [DOI] [PubMed] [Google Scholar]
- Streit W.J., Walter S.A., Pennell N.A. Reactive microgliosis. Prog. Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Subramaniam S.R., Federoff H.J. Targeting microglial activation states as a therapeutic avenue in Parkinson's disease. Front. Aging Neurosci. 2017;9:176. doi: 10.3389/fnagi.2017.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Ohgidani M., Kuwano N., Chretien F., Lorin de la Grandmaison G., Onaya M., Tominaga I., Setoyama D., Kang D., Mimura M., Kanba S., Kato T.A. Suicide and microglia: recent findings and future perspectives based on human studies. Front. Cell. Neurosci. 2019;13:31. doi: 10.3389/fncel.2019.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016;53:1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- Tsilioni I., Patel A.B., Pantazopoulos H., Berretta S., Conti P., Leeman S.E., Theoharides T.C. IL-37 is increased in brains of children with autism spectrum disorder and inhibits human microglia stimulated by neurotensin. Proc. Natl. Acad. Sci. U. S. A. 2019;116:21659–21665. doi: 10.1073/pnas.1906817116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynan R.J., Naicker S., Hinwood M., Nalivaiko E., Buller K.M., Pow D.V., Day T.A., Walker F.R. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav. Immun. 2010;24:1058–1068. doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Wang J., Hodes G.E., Zhang H., Zhang S., Zhao W., Golden S.A., Bi W., Menard C., Kana V., Leboeuf M., Xie M., Bregman D., Pfau M.L., Flanigan M.E., Esteban-Fernandez A., Yemul S., Sharma A., Ho L., Dixon R., Merad M., Han M.H., Russo S.J., Pasinetti G.M. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat. Commun. 2018;9:477. doi: 10.1038/s41467-017-02794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhard L., di Bartolomei G., Bolasco G., Machado P., Schieber N.L., Neniskyte U., Exiga M., Vadisiute A., Raggioli A., Schertel A., Schwab Y., Gross C.T. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat. Commun. 2018;9:1228. doi: 10.1038/s41467-018-03566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhard L., Neniskyte U., Vadisiute A., di Bartolomei G., Aygun N., Riviere L., Zonfrillo F., Dymecki S., Gross C. Sexual dimorphism of microglia and synapses during mouse postnatal development. Dev Neurobiol. 2018;78:618–626. doi: 10.1002/dneu.22568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams E.S., Manning C.E., Eagle A.L., Swift-Gallant A., Duque-Wilckens N., Chinnusamy S., Moeser A., Jordan C., Leinninger G., Robison A.J. Androgen-dependent excitability of mouse ventral hippocampal afferents to nucleus accumbens underlies sex-specific susceptibility to stress. Biol. Psychiatr. 2020;87:492–501. doi: 10.1016/j.biopsych.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodburn S.C., Bollinger J.L., Wohleb E.S. Synaptic and behavioral effects of chronic stress are linked to dynamic and sex-specific changes in microglia function and astrocyte dystrophy. Neurobiol Stress. 2021;14:100312. doi: 10.1016/j.ynstr.2021.100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Zhao Y., Wang Y., Liu L., Zhang X., Li B., Cui R. The effects of psychological stress on depression. Curr. Neuropharmacol. 2015;13:494–504. doi: 10.2174/1570159X1304150831150507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W., Gallagher N.R., Sawicki C.M., McKim D.B., Godbout J.P., Sheridan J.F. Repeated social defeat in female mice induces anxiety-like behavior associated with enhanced myelopoiesis and increased monocyte accumulation in the brain. Brain Behav. Immun. 2019 doi: 10.1016/j.bbi.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R., Rimmerman N., Reshef R. Depression as a microglial disease. Trends Neurosci. 2015;38:637–658. doi: 10.1016/j.tins.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Zhao J.L., Wei C., Xiao X., Dong Y.H., Tan B., Yu J., Chen G., Yuan Q., Du Z.Y., Sun Y.R., Hu J., Xie R. Expression of TNFalpha and ILbeta can be suppressed via the PPARgamma/mTOR signaling pathway in BV2 microglia: a potential antiinflammation mechanism. Mol. Med. Rep. 2020;22:3559–3565. doi: 10.3892/mmr.2020.11418. [DOI] [PubMed] [Google Scholar]