Synopsis
Goal-directed learning is a key contributor to evolutionary fitness in animals. The neural mechanisms that mediate learning often involve the neuromodulator dopamine. In higher order cortical regions, most of what is known about dopamine’s role is derived from brain regions involved in motivation and decision-making, while significantly less is known about dopamine’s potential role in motor and/or sensory brain regions to guide performance. Research on rodents and primates represents over 95% of publications in the field, while little beyond basic anatomy is known in other vertebrate groups. This significantly limits our general understanding of how dopamine signaling systems have evolved as organisms adapt to their environments. This review takes a pan-vertebrate view of the literature on the role of dopamine in motor/sensory cortical regions, highlighting, when available, research on non-mammalian vertebrates. We provide a broad perspective on dopamine function and emphasize that dopamine-induced plasticity mechanisms are widespread across all cortical systems and associated with motor and sensory adaptations. The available evidence illustrates that there is a strong anatomical basis—dopamine fibers and receptor distributions—to hypothesize that pallial dopamine effects are widespread among vertebrates. Continued research progress in non-mammalian species will be crucial to further our understanding of how the dopamine system evolved to shape the diverse array of brain structures and behaviors among the vertebrate lineage.
Introduction
Research on dopamine has a rich history. This molecule was first described as a neurotransmitter and suggested to be involved in Parkinson’s disease six decades ago by Carlsson et al. (1958), part of a research trajectory for which he was awarded the 2000 Nobel Prize. Parallel to Carlsson’s studies, Peter Olds and James Milner published pioneering research on the electrical stimulation of “pleasure centers” in the rodent brain, which included basal centers for dopamine action (Olds and Milner 1954). These seminal findings established the primary role of dopamine in reinforcement processing and motor disorders in the basal ganglia. Two decades later, dopamine was found also to be present in the rat cortex (Thierry et al. 1973; Berger et al. 1974).
The years that followed saw an explosion of research on dopamine. In mammals, dopamine is produced in midbrain and hypothalamus, and four main projection pathways have been identified: the tuberoinfundibular, nigrostriatal, mesolimbic, and mesocortical pathways (Björklund and Dunnett 2007). The latter comprises projections from the midbrain (e.g., tegmentum and substantia nigra) to virtually all cortical regions. An interesting feature of the mammalian cortical dopamine system is that the density of cortical dopamine projections generally decreases from anterior to posterior, being the highest in the frontal lobe and lowest in the occipital lobe (Descarries et al. 1987). The current body of literature seems to parallel the anatomy, that is, the areas with highest density of dopamine fibers have received the lion’s share of research attention. As such, there is a wealth of understanding about dopamine effects on the prefrontal cortex (PFC), mediating phenomena such as reinforcement processing, motivation, and attention (e.g., Clark et al. 2014; Chaua et al. 2018; Thiele and Bellgrove 2018; Weele et al. 2019). Another large body of literature has focused on learning and behavioral plasticity due to dopaminergic effects on striatum and nucleus accumbens (Cerovic et al. 2013; Sulzer et al. 2016; Gallo 2019; Woolley 2019). By contrast, relatively few reviews address dopamine’s direct effects on cortical regions aside from the PFC (Vitrac and Benoit-Marand 2017; Jacob and Nienborg 2018). Our goal in this review is to address gaps in this literature, specifically by examining effects of dopamine on cortical regions that modify motor/sensory performance and direct (local) actions of dopamine in these regions, rather than by means of indirect input from the PFC, striatum or other subcortical regions. We synthesize available data about dopaminergic effects on cortical-like systems in non-mammalian vertebrate species (see below), in order to bolster comparative perspectives on dopamine function.
Intrinsic motivation to seek subsequent reinforcement often leads to improvements in motor and sensory performance. Reinforcement learning (be it reward or punishment) recruits several brain regions to signal valence and form memories about environmental context. Notably, the ventral tegmental area (VTA) is a key region in this process. It integrates inputs from areas that process multimodal information such as the PFC (e.g., decision-making; attention) and the dorsal striatum (e.g., sensory and motor information; Beier et al. 2015). The VTA then sends dopamine-containing projections to striatum, nucleus accumbens, and cortex. The firing of dopaminergic neurons is intimately linked to reinforcement processing and modulation of the target regions (e.g., Menegas et al. 2018). Reinforcement and decision-making networks that include VTA, striatum, nucleus accumbens, and prefrontal cortical structures appear to be necessary for the installation of the reward-seeking (and punishment-avoiding) behaviors. VTA projections to motor and sensory cortices are associated with improving and fine-tuning responses to the environment in order to achieve the desired behavioral outcome more efficiently (Fig. 1; McGann 2015).
Fig. 1.
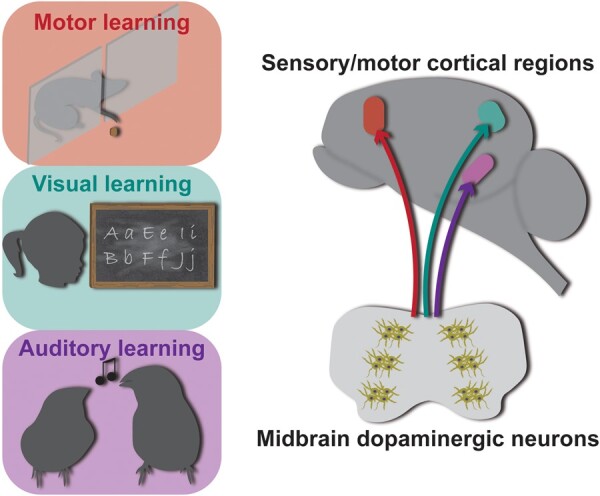
Plasticity effects of dopamine onto sensory/motor pallial/cortical regions. Highly complex phenomena such as motor, visual, and auditory learning depend on midbrain dopamine signaling directly onto motor/sensory pallial/cortical structures. The color pattern in panels, arrows, and brain structures indicate the specificity of the dopamine projections and their effects. The brain represents a non-specific vertebrate brain.
Most of this broad understanding is derived from experiments in laboratory rodents (i.e., mice and rats) and primates (i.e., macaques). As studies on other vertebrate species have emerged, it has become evident that this system can be involved in many goal-directed behaviors, such as song learning in birds (Woolley 2019). To fully appreciate the deep evolutionary history of dopamine signaling, it is useful to derive lessons from work on invertebrate species, which have a rich history of studies on dopamine regulation of sensory, motor, learning, and other processes, and is reviewed elsewhere (Verlinden 2018). The phylogenetic “myopia” focusing on rodents and primates, as noted by Brenowitz and Zakon (2015), greatly hinders our understanding of the evolution of nervous systems and may prevent scientific breakthroughs sparked by studying non-traditional organisms (Manger et al. 2008; Carlson 2012; Remage-Healey et al. 2017). To this point, in an excellent review on the evolution of dopamine systems in chordates, Yamamoto and Vernier (2011) emphasize that tracing the evolutionary origins of dopamine systems among vertebrates is important to understand dopamine signaling and how it can become maladaptive in a disease context.
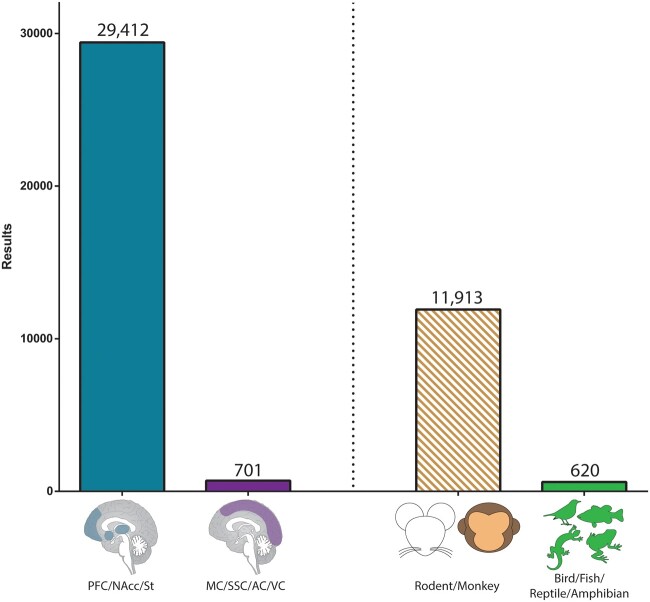
A recent non-exclusive PubMed search (Fig. 2) with title/abstract containing the keywords dopamine AND (accumbens OR striatum OR “prefrontal cortex”) returns 29,412 results, while dopamine AND (“motor cortex” OR “auditory cortex” OR “somatosensory cortex” [SSC] OR “visual cortex”) returns only ∼701 results, a ∼42-fold discrepancy. In a similar way, the literature that includes terms associated with the rodents and monkey most commonly used in neuroscience in the title/abstract exceeds that with any other non-mammal ∼19-fold (Fig. 2)—dopamine AND brain AND (rodent OR mouse OR rat OR Mus OR Rattus OR monkey OR Macaca OR macaque OR rhesus) NOT (reptile OR bird OR fish OR amphibian OR Rana OR Xenopus OR Anolis OR Danio OR Taeniopygia OR Gallus OR lizard OR snake OR bird OR songbird OR chicken OR zebrafish OR frog OR salamander) returns 11,913 items; while dopamine AND brain AND (reptile OR bird OR fish OR amphibian OR Rana OR Xenopus OR Anolis OR Danio OR Taeniopygia OR Gallus OR lizard OR snake OR bird OR songbird OR chicken OR zebrafish OR frog OR salamander) returns 620. These numbers substantiate the view that the literature on the roles of dopamine in the reinforcement processing and decision-making circuitry is vast when compared with that on dopamine and motor/sensory performance, and that most of this knowledge is derived from a handful of species.
Fig. 2.
PubMed search results involving dopamine and brain research. A non-exclusive PubMed search with title/abstract containing PFC, nucleus accumbens (NAcc), and striatum (St) returns ∼42 times more results than a search including all other cortical modalities—motor, somatosensory, auditory, and visual cortices (MC, SSC, AC, and VC, respectively). The literature that includes rodent or monkey search terms in the title/abstract returns ∼19 times more results than a search including all other vertebrates.
A key distinction for understanding dopamine modulation concerns indirect versus direct effects of dopamine on cortical regions. For simplicity, in this review, we classify as indirect those effects of dopamine on the striatum or other subcortical structures, which in turn may influence the cortex; as well as actions on the PFC, which can modify other cortical regions by affecting top-down attentional and motivational mechanisms. For example, dopamine in the PFC has been found to modulate visual attention through its connections to the visual cortex (Noudoost and Moore 2011). By contrast, we classify direct effects as actions of dopamine-containing fibers that synapse directly on the cortical region in question.
Molecularly, dopamine binds to a plethora of different receptors, with modulatory actions (seconds to minutes timescales) on circuit excitability (reviewed by Beaulieu and Gainetdinov 2011). In the current review, we will mostly refer to the D1 class of receptors (D1a, D1b, D1c, D1d, and D1x) as simply D1 or D1-like, and to the D2 class (D2, D3, and D4; aka D2a, D2b, and D2c, respectively) as D2 or D2-like. The evolutionary diversification of these receptor subtypes is quite remarkable. D1-like receptor subtypes were found to exist in the D1a (aka D1) or D1b (aka D5) subtypes in mammals. In other organisms, additional subtypes were encountered, such as D1d in birds (Demchyshyn et al. 1995), D1c in amphibians (Sugamori et al. 1994), and D1x in fish (Hirano et al. 1998). D2-like receptor subtypes seem to be somewhat more evolutionary stable and likely present in a vertebrate common ancestor, such that the same three subtypes are found in all vertebrate groups (Yamamoto and Vernier 2011).
The ratio of D1- to D2-like receptors and their expression in different cell types largely accounts for how dopamine affects the activation state of a neural circuit. Such effects are achieved by modulating the activity of the excitatory (glutamatergic) and inhibitory (GABAergic) fast neurotransmission of downstream neurons. These effects are now known to be highly nuanced, often referred to D1- or D2-dominated states (Durstewitz and Seamans 2008). That said, we exercise caution in generalizing dopamine action across neural structures. For example, inhibition of either D1 or D2 receptors in motor cortex can impair motor learning and long-term potentiation (Molina-Luna et al. 2009), which is dissimilar from the “textbook definition” of D1 and D2 receptor effects’ being mutually-antagonistic in the striatum/accumbens (Beaulieu and Gainetdinov 2011).
Although the VTA provides the largest source of dopamine to the mammalian cortex, the substantia nigra pars compacta (SNc) also sends diffuse dopaminergic cortical projections (Gaspar et al. 1992). Furthermore, locus coeruleus neurons co-release norepinephrine and dopamine throughout the cortex (Devoto et al. 2005), and dopamine can bind to adrenergic receptors (Cornil and Ball 2008). This perspective will become increasingly important as we learn more about how cortical sensory and motor circuits are shaped by dopaminergic inputs.
Across vertebrates, cortical systems vary markedly in macro-anatomy, but share fundamental features including embryonic origin, connectivity, function, and gene expression patterns. Through identifying shared patterns, homologs to the mammalian six-layered pallial cortex have been hypothesized in all major vertebrate groups, although in fishes telencephalic homology is less clear (Bruce and Neary 1995; Dugas-Ford et al. 2012; Pfenning et al. 2014; Yamamoto and Bloch 2017; Tosches et al. 2018). In mammals, the neocortex is the outermost structure of the telencephalon, while in other vertebrates, cortical-like structures may be distributed in discrete nuclei across the pallium. We adopt in this review the nomenclature “cortex/cortical structure” to refer to the identified/proposed cortical homologs to mammalian cortical pallium in other vertebrates (Table 1).
Table 1.
Hypothesized cortical homology across vertebrates
| Modality | Group | Cortical area | Key references |
|---|---|---|---|
| Motor | Fishes | ? | – |
| Amphibians | ? | – | |
| Reptiles | DVRa | Distel (1978) | |
| Birds | HVC; RA (vocal) | Nottebohm et al. (1976) ; Nottebohm and Arnold (1976) | |
| Visual | Fishes | Dl | Saidel et al. (2001) |
| Amphibians | Medial pallium | Kicliter (1979) | |
| Reptiles | Dorsal cortex | Gusel’nikov et al. (1972); Fournier et al. (2018) | |
| Birds | Hyperpallium; Entopallium | Hodos and Karten (1970) ; Pettigrew and Konishi (1976) ; Watanabe et al. (2011) | |
| Auditory | Fishes | Dm/Dl/Dc (lateral line) | Striedter (1992) |
| Amphibians | Medial pallium | Northcutt and Ronan (1992) | |
| Reptiles | DVRa | Foster and Hall (1978) | |
| Birds | Field L; NCM; CMM | Karten (1968) ; Kelley and Nottebohm (1979) ; Vates et al. (1996) | |
| Olfactory | Fishes | Dp/Dlv | Satou (1990) |
| Amphibians | Lateral pallium | Hoffman (1963); Scalia et al. (1968) | |
| Reptiles | Lateral cortex | Goldby (1937) | |
| Birds | PrC | Rieke and Wenzel (1978); Reiner and Karten (1985) | |
| Taste | Fishes | Dm | Lamb and Caprio (1993) |
| Amphibians | ? | – | |
| Reptiles | ? | – | |
| Birds | ? | – | |
| Somatosensory | Fishes | ? | – |
| Amphibians | Medial pallium | Kicliter (1979) | |
| Reptiles | DVRa | Pritz and Northcutt (1980) | |
| Birds | Hyperpallium apicale; anterior nidopallium | Wild (1987) |
Dc, central part of dorsal telencephalon; Dl, lateral part of dorsal telencephalon; Dlv, ventrolateral part of dorsal telencephalon; Dm, medial part of dorsal telencephalon; Dp, posterior part of dorsal telencephalon; DVR, dorsoventricular ridge; ?: no conclusive data could be found.
Specific subdivisions within the DVR are unexplored.
There are no conclusive data on specific cortical motor/sensory subdivisions of fish, amphibian, and reptile telencephalon; in birds, cortical motor regions besides the vocal production nuclei are largely undescribed and data on taste cortical homologs in any non-mammalian species are essentially non-existent. However, even when homology is unclear or not present, cortical structures that share similar functions (regardless of homology) can be informative for understanding general principles of such systems and the evolutionary pressures that mold them (Katz 2019).
With these considerations in mind, here we ask the following questions: what roles does dopamine play in motor and sensory cortices? Do the principles of dopamine effects in these structures mirror those observed in reinforcement processing centers and the basal ganglia? Are dopamine’s effects generalizable across other vertebrates? What features of dopamine signaling are similar across those species investigated to date? We review the dopamine signaling systems in all major areas of the mammalian cortex, and concomitantly consider the available evidence in homologous structures among non-mammalian vertebrates.
Dopamine-induced plasticity in motor cortex
The context and timing of dopamine signaling is crucial for behavioral plasticity. Prior to the key association between cues with consequences (i.e., association learning), dopamine signaling by VTA is purely subsequential, that is, the signal follows reward consumption. However, with repetition of the pairing of environmental conditions with consequences, dopamine signaling starts to anticipate the consequence, and then becomes a predictive signal. If a prediction or expectation about the consequence is violated, dopamine neurons will now signal when the consequence was predicted to happen and will inform the direction of this violation (good or bad surprise), by increasing or decreasing their firing rates, respectively. The literature classically refers to this “algorithm” as reward prediction error (Barto et al. 1981; Schultz et al. 1997). This algorithm not only signals when an animal should make a decision to pursue a reward, but also how to achieve this reward with more ease or accuracy. In addition to association learning, it is implemented when repetition of a motor pattern to achieve reinforcement leads to fine-tuning of the movement itself. Therefore, the dopaminergic signals that are used to reinforce the decision-making network could also be used to reinforce and tune motor performance.
The VTA is the major dopaminergic projection to the mammalian primary motor cortex (M1) (Scheibner and Törk 1987) and most M1-projecting VTA neurons project solely to the motor cortex (i.e., no collaterals) (Hosp et al. 2011a, 2011b, 2015). Dopamine-producing fibers (i.e., containing dopamine and/or tyrosine hydroxylase [TH], the rate-limiting enzyme in catecholamine production) are abundant in M1 in virtually all mammalian species studied, and are densest in superficial (I) and deeper layers (V/VI) (rat: Berger et al. 1985; human: Gaspar et al. 1989; macaque: Noack and Lewis 1989). Accordingly, dopamine receptors of both D1 and D2 families are present in M1. Interestingly, receptor type distribution suggests some topographical specificity of dopaminergic projections, such that superficial projections may activate mainly D1 receptors, while deeper projections target both D1 and D2 receptors (Lidow et al. 1990, 1991).
Like the PFC, the rat M1 in vitro exhibits both long-term depression and potentiation (LTD and LTP, respectively) (Hess and Donoghue 1996). After a motor skill learning task, M1 shows stronger local connections in vitro, which suggests that LTP can be induced by behavioral training (Rioult-Pedotti et al. 1998). However, unlike in the PFC, D1 and D2 receptors in M1 can be synergistic for LTP induction. Molina-Luna et al. (2009) have shown that blocking either D1 or D2 receptors prevents LTP formation in M1 in vitro, whereas D1 but not D2 receptors are involved in LTP formation in the PFC (Huang et al. 2004). Notably, however, both receptors are involved in spike timing-dependent LTP in the PFC (Xu and Yao 2010). These results reflect the diversity of dopaminergic mechanisms in shaping neuronal plasticity among different cortical areas.
In humans, systemic dopaminergic manipulations affect goal-directed, practice-dependent motor plasticity (Ziemann et al. 1997; Flöel et al. 2005, 2008; Meintzschel and Ziemann 2006), but it is not yet clear whether these effects are direct or indirect (e.g., via striatum or PFC).
Midbrain dopaminergic nuclei that participate in reward-seeking and learning are highly conserved among vertebrates (reviewed by Martínez-García and Lanuza 2018). Below, we summarize the relatively scant literature on the effects of dopamine on non-mammalian motor systems (also refer to Table 2).
Table 2.
Anatomical distribution of dopaminergic markers across vertebrate pallial regions
| Area (cortical) | Group | Tyrosine hydroxylase | D1-like receptors | D2-like receptors | Key references |
|---|---|---|---|---|---|
| Motor | Fishes | a | a | a | – |
| Amphibians | a,b | a,b,c | a | – | |
| Reptiles | a,b | a,b,c | a | – | |
| Birds | +(HVC; RA) | +(HVC; RA) | +(HVC; RA) | Bottjer (1993); Kubikova et al. (2010) | |
| Mammals | + | + | + | Scheibner and Törk (1987); Lidow et al. (1990, 1991) | |
| Visual | Fishes | – | + | + | O’Connell et al. (2011a) |
| Amphibians | +(newt/gymnophionan: medial pallium) | a,b,c | a | González et al. (1993) ; González and Smeets (1991, 1994); O’Connell et al. (2011b) | |
| −(anurans: medial pallium) | |||||
| Reptiles | +(dorsal cortex) | a,b,c | a | Smeets et al. (1986) | |
| Birds | +(hyperpallium) | +(hyperpallium) | −(hyperpallium) | Metzger et al. (1996); Schnabel et al. (1997); Appeltants et al. (2001); Kubikova et al. (2010) | |
| −(entopallium) | −(entopallium) | −(entopallium) | |||
| Mammals | + | + | + | Törk and Turner (1981); Lidow et al. (1990) | |
| Auditory | Fishes | +(Dm/Dc) | +(Dm/Dl/Dc) | +(Dm/Dl/Dc) | O’Connell et al. (2011a) |
| Amphibians | a,b | a,b,c | a | – | |
| Reptiles | a,b | a,b,c | a | – | |
| Birds | +(NCM; CMM) | +(NCM; CMM) | +(CMM) | Reiner et al. (1994); Kubikova et al. (2010) | |
| −(Field L2) | −(Field L2) | −(NCM; Field L2) | |||
| Mammals | + | + | + | Boyson et al. (1986) ; Campbell et al. (1987); Budinger et al. (2008) | |
| Olfactory | Fishes | –(Dlv) | +(Dp/Dlv) | +(Dp/Dlv) | Kapsimali et al. (2000) ; Vacher et al. (2003); O’Connell et al. (2011a) |
| Amphibians | +(lateral pallium)a | a,b,c | a | González and Smeets (1991) ; O’Connell et al. (2011b) | |
| Reptiles | +(lateral cortex)a | a,b,c | a | Smeets et al. (1986, 2001); Smeets (1988) | |
| Birds | + | + | + | Appeltants et al. (2001); Kubikova et al. (2010) | |
| Mammals | + | + | + | Boyson et al. (1986) ; Datiche and Cattarelli (1996); Santana et al. (2009) | |
| Taste | Fishes | + | + | + | O’Connell et al. (2011a) |
| Amphibians | a,b | a,b,c | a | – | |
| Reptiles | a,b | a,b,c | a | – | |
| Birds | a | a | a | – | |
| Mammals | + | + | + | Gaspar et al. (1995) ; Ohara et al. (2003) | |
| Somatosensory | Fishes | a | a | a | – |
| Amphibians | +(newt/gymnophionan: medial pallium) | a,b,c | a | González and Smeets (1991, 1994); González et al. (1993); O’Connell et al. (2011b) | |
| −(anurans: medial pallium) | |||||
| Reptiles | a,b | a,b,c | a | – | |
| Birds | +(hyperpallium apicale) | +(hyperpallium apicale) | +(hyperpallium apicale) | Reiner et al. (1994); Kubikova et al. (2010) | |
| +(anterior nidopallium) | +(anterior nidopallium) | +(anterior nidopallium) | |||
| Mammals | + | + | + | Descarries et al. (1987) ; Lewis et al. (1987) ; Lidow et al. (1991) |
No data were found.
In reptiles and amphibians, there are reports of markers in the DVR, but specific subdivisions within the DVR are unknown.
There is data on the presence of DARPP-32 protein, but whether DARPP-32 and D1 receptors are colocalized in non-mammals is unknown.
As in mammals, TH-containing projections are clearly evident in the proposed cortical homologs (gecko: Smeets et al. 1986; Iberian ribbed newt: González and Smeets 1991; zebra finch: Bottjer 1993; African cichlid fish: O’Connell et al. 2011a; túngara frog: O’Connell et al. 2011b) and dopamine receptors (zebra finch: Kubikova et al. 2010; African cichlid fish: O’Connell et al. 2011a). Data are lacking for dopamine receptors in the reptile and amphibian cortex, but receptor presence has been inferred by the expression of DARPP-32, a protein commonly associated with D1 receptors in mammals (túngara frog: O’Connell et al. 2011b; gecko: Smeets et al. 2001). The extent to which DARPP-32 receptors and D1 receptors in non-mammals are associated is unknown and deserves investigation. In fact, these two sets of receptors do not always colocalize in neurons in zebra finch telencephalon (own unpublished observations). To the best of our knowledge, the function of dopamine receptor expression in pallial motor areas has only been explored in the vocal motor cortex of songbirds (suborder Passeri).
The song system has been studied intensively in songbirds, but regarding the effects of dopamine, striatal regions have received greater attention than cortical regions. The songbird striatum contains a region (Area X) dedicated to song learning and song motor plasticity. Area X receives massive dopaminergic inputs from VTA (Lewis et al. 1981) and plays a vital role in song learning in development (Sohrabji et al. 1990). In the context of song production, a reward prediction learning function arriving in Area X originates in dopaminergic neurons in the avian VTA (Gadagkar et al. 2016). Optogenetic manipulations of these fibers, as well as pharmacological manipulations of dopamine receptors in Area X affected both adult song plasticity and juvenile song learning (Hisey et al. 2018; Xiao et al. 2018). The effects of dopamine on this striatal system have been reviewed elsewhere (Kubikova and Košťál 2010; Simonyan et al. 2012).
In the avian cortex (pallium), the sensorimotor region HVC (the acronym is the proper name) and the motor region robust nucleus of the arcopallium (RA) are two crucial areas for song learning and production. They contain dense TH fiber tracts (Bottjer 1993) derived mainly from the mesencephalic central gray, but also from VTA (Appeltants et al. 2000, 2002). Concordantly, both motor cortical regions express mRNA for D1- and D2-like receptors (Kubikova et al. 2010).
A handful of studies have examined dopaminergic physiology in the songbird vocal motor cortex. Adult male European starlings (Sturnus vulgaris) exposed to high quality song playbacks (presumed higher quality competitors) sang more and showed decreased levels of dopamine metabolites in HVC but not RA (Salvante et al. 2010). During development, dopamine projections from the periaqueductal gray to HVC are crucial for song learning in zebra finches. This signal seems to convey social context and/or motivation when juveniles engage with live tutors. VTA projections to HVC are significantly less dense in juveniles than in adults, but their function was not explored (Tanaka et al. 2018). For RA, dopaminergic actions have been explored in vitro. RA projection neurons exhibited increased resting membrane potential and firing rate in response to dopamine and a D1 agonist, while a D2 agonist had no effect (Liao et al. 2013).
In sum, dopamine effects on motor cortex are evident in mammals and are related to goal-directed, practice-dependent motor skill improvement. There are limited reported parallels in non-mammalian vertebrates, with notable recent progress in birdsong production and learning. It would be interesting to know, from an evolutionary standpoint, to what extent dopamine is involved in cortical motor plasticity in other vertebrates. For instance, behaviors such as flight or the refinement of hunting skills are examples of natural goal-directed motor learning that could depend on dopaminergic regulation of motor areas.
Dopamine-induced plasticity in the visual cortex
Reinforcement plays a crucial role in the optimization of visual information processing. For example, reinforcement following a visual stimulus increases visual performance, such that larger rewards produced faster reaction times in a saccade visual task, and neural activity in the dopaminergic midbrain during the task reflected strong reward prediction error encoding (Nomoto et al. 2010). Additionally, reinforcement is a strong driver of visual perceptual learning (Seitz et al. 2009; Seitz 2017).
Dopaminergic fibers are sparse in mammalian primary visual cortex (V1), but evident in virtually all species studied (macaque: Berger et al. 1988; rat: Descarries et al. 1987; human: Phillipson et al. 1987; cat: Törk and Turner 1981) and mirror the region-specificity seen in M1—densest in superficial (I) and deeper (V/VI) layers (Descarries et al. 1987; Berger et al. 1988). Likewise, receptor distribution mirrors M1, such that D1 receptors are prevalent in superficial and deeper layers, while D2s are prevalent in deeper layers (Lidow et al. 1990). Therefore, topographical specificity of D1 versus D2 receptor activation seems to be a shared feature between motor cortex and visual cortex.
On the whole, the mammalian cortex has an anterior–posterior gradient of decreasing dopamine content and projections. Located in the posterior-most occipital lobe, the mammalian primary visual cortex is the target of the sparsest dopaminergic projections (Descarries et al. 1987) and the lowest detectable dopamine content (Brown et al. 1979). Higher-order visual association cortical areas (rhinal and the posterior parietal) are localized more anteriorly and rely on D2 receptor signaling for visual association learning (Liu et al. 2004). Stimulus-evoked dopamine release occurs in rat primary visual cortex (Müller and Huston 2007), and local dopaminergic manipulations modulate V1 neuronal firing (Reader 1978; Gottberg et al. 1988). Moreover, several visual cortical areas, including V1, are strongly modulated by reward prediction error signaling and sensitive to systemic D1 receptor antagonists (Arsenault et al. 2013).
The effects of dopamine signaling on visual performance could be indirect through prefrontal regions (Noudoost and Moore 2011; Zaldivar et al. 2014, 2018). Often these experiments involve anesthesia, which could render effects of exogenous dopamine difficult to interpret (reviewed by Marinelli and McCutcheon 2014). Therefore, local effects of dopamine in mammalian visual cortex warrant more investigation.
Research with humans also indicates an important indirect prefrontal-mediated effect of dopamine on visual cortex activity and visual performance (Yousif et al. 2016). Dopamine modulation in the visual cortex could be secondary or residual in primates and have more important effects on visual performance by means of PFC modulation. Notably, however, these studies employ visual detection and attention tasks. Local effects of dopamine on the visual cortex might be unveiled through tasks that involve more specific visual association/perceptual learning or conditioning.
In non-mammalian vertebrates, conclusive experimental data that would solidify links between visual plasticity and dopaminergic signaling are lacking. Birds are the only non-mammal group in which the visual pallial system has been explored in some detail. In songbirds, there is correlational evidence that dopamine plays a role in visual recognition. Avian visual processing is distributed between two separate cortical regions: the entopallium and the hyperpallium, putatively analogous to the ventral (“what”) and dorsal (“where”) visual processing streams, respectively (Goodale and Milner 1992; Watanabe et al. 2008, 2011). The hyperpallium is a hotspot of D1-like receptors and dopaminergic fibers but is largely devoid of D2-like receptors. Conversely, the entopallium can be anatomically delineated by the absence of dopamine receptors and fibers (Appeltants et al. 2001; Kubikova and Košťál 2010). Interestingly, the entopallium has been proposed to be homologous to mammalian cortical layer IV, and the hyperpallium to layers IV and/or V (Dugas-Ford et al. 2012). Thus, the region-specificity of dopamine signaling in visual areas of songbirds appears to support this laminar homology hypothesis.
In crows, neuron activation in the hyperpallium (entopallium was not sampled), VTA, and SNc was implicated in both spatial and visual pattern recognition performance (Taufique and Kumar 2016), but determining causal relationship requires further experimentation. Therefore, anatomical and functional correlations point toward an important role for dopamine in modulating pallial visual plasticity in songbirds.
Outside of songbirds, anatomical evidence indicates that dopamine could regulate visual imprinting in chicken anterior mesopallium. Domestic chicks (Gallus gallus) can be experimentally imprinted on artificial objects (colored rotating cubes or cylinders) and thereafter display attraction behaviors toward them. The anterior mesopallium, a polysensory cortical region, is involved in visual imprinting and its neurons respond selectively to imprinted visual (but also auditory) stimuli (Nicol et al. 1995). TH fibers are present in this region (Metzger et al. 1996), as are D1-like receptors (Schnabel et al. 1997). However, to our knowledge, there have been no experimental manipulations of dopamine to test this causally.
Among other vertebrate groups, data on dopamine signaling in visual pallial regions are scarce and only reported in amphibians and reptiles. In the common toad (Bufo bufo), prey-catching strategy could be modified by systemic D1/D2 agonist (Glagow and Ewert 1999), but a direct action on the visual system is unknown. The medial pallium was suggested as a visual pallial target in amphibians (Scalia 1976; Kicliter 1979) and this area contains dopamine/TH fibers in a newt (González and Smeets 1991) and gymnophionan (González and Smeets 1994) but not in anurans (González et al. 1993; O’Connell et al. 2011b). In red-eared slider turtles (Trachemys scripta), a visual cortical region called the dorsal cortex shows spatial adaptation (plasticity) and encodes information about spatial and temporal features (Fournier et al. 2018). TH fibers and DARPP-32-positive cell bodies can be detected in this region in Tokay geckos (Gekko gecko) (Smeets et al. 2001). There is also some anatomical evidence for D1- and D2-like receptors in African cichlid visual telencephalon (O’Connell et al. 2011a). These studies identify potential anatomical substrates for studying dopaminergic modulation of visual inputs which could clarify evolutionary and comparative questions.
After examining the available literature in mammals, we can conclude that even though fibers and receptors are relatively scarcer in V1 than most other cortices, direct effects of dopamine are still clear and deserve more exploration. On the other hand, in primates, the diminished weight of dopaminergic signaling might have shifted the weight toward indirect prefrontal dopamine effects modulating visual inputs. Still, direct dopamine effects on primate visual association or perceptual learning are unexplored and would fall in line with predictions from monkey and human perceptual learning studies (Seitz et al. 2009; Nomoto et al. 2010). For non-mammals, the literature points toward a promising anatomical substrate for direct dopamine effects. Natural behaviors such as perfection of visually-dependent hunting, cached food retrieval, visual imprinting, or fish schooling behavior might rely or be enhanced by reinforcement and dopamine release in visual areas.
Dopamine-induced plasticity in the auditory cortex
Associating sounds with consequences is an important survival trait in many species. Cues associated with, for example, competitors versus allies or predators versus prey are constantly being surveilled. Additionally, many animals rely on auditory recognition for identifying individuals (Aubin and Jouventin 1998; Sayigh et al. 1999; Gentner et al. 2000; Goodwin and Podos 2014). The examples above all involve instances of auditory association learning (i.e., sound + consequence/object learning), but the auditory system also guides perceptual learning, through which animals refine their sensory sensitivity to better detect low saliency signals (Gibson 1953).
As with other cortical systems in mammals, dopaminergic projections in the mammalian auditory cortex are mainly from VTA and SNc (Budinger et al. 2008) and are stratified, mostly in layers I and V/VI (Campbell et al. 1987). A detailed quantification of dopamine receptor distribution in the auditory cortex is currently lacking, but qualitative inspections of rat brains suggest a distribution pattern that parallels that in other cortical regions—D1 in layers I–III and V/VI and D2 mostly present in layer V (Boyson et al. 1986), similarly to M1 and V1.
A role for dopamine in mammalian auditory learning and cortical plasticity has been suggested for decades (Stark and Scheich 1997; Bao et al. 2001), and a causal relationship was more recently established (Kudoh and Shibuki 2006; Schicknick et al. 2008, 2012; Mylius et al. 2015; Huang et al. 2016). These studies show that VTA stimulations, dopamine, and D1 receptors modulate plasticity and association learning. Even still, the relevance of D2 receptors is unclear, as well as dopamine’s involvement in auditory perceptual learning.
Another important gap in the literature seems to be the lack of studies investigating the modulation of LTP in auditory cortical synapses by dopamine, despite the decades-old evidence for auditory cortex LTP (Kudoh and Shibuki 1994). Evidence is similarly limited in humans, in which dopamine-dependent auditory language learning has been suggested by administering systemic dopaminergic drugs (Knecht et al. 2004; Breitenstein et al. 2006). Whether such effects are intrinsic to the auditory cortex awaits confirmation.
The dopaminergic midbrain has been shown to be engaged after exposure to behaviorally relevant sounds in a variety of vertebrates (e.g., zebra finch: Barr and Woolley 2018; spadefoot toad: Burmeister et al. 2017; midshipman fish: Petersen et al. 2013), but its involvement in pallial plasticity has only been explored in birds. In songbirds, TH/dopaminergic fibers are conspicuous across the secondary auditory pallium (caudomedial nidopallium [NCM] and the caudomedial mesopallium [CMM]) but not the primary auditory pallium, field L2 (Reiner et al. 1994). Dopamine receptors follow a similar pattern: D1-like receptors are abundant in NCM and CMM, and D2-like receptors are abundant in CMM but not in NCM. Neither receptor is evident in the thalamo-recipient Field L (Kubikova et al. 2010). Correlational evidence that VTA and dopamine signaling are involved in song responses and juvenile song learning was initially established. In female white-throated sparrows (Zonotrichia albicollis), exposure to conspecific song increased the activation (phosphorylation) of TH fibers and the levels DA metabolites in the NCM (Matragrano et al. 2012). Furthermore, in juvenile zebra finches, song tutoring increased VTA activation (Chen et al. 2016). We have recently established a causal relationship between local dopaminergic manipulations and NCM plasticity and learning mechanisms (Macedo-lima et al. 2020). Yet, whether and how dopamine from VTA (or other nuclei) is involved in auditory learning is unclear. The effects of dopamine on other auditory structures (e.g., CMM) are also largely unexplored.
In chicks, auditory imprinting seems to be dopamine-dependent since it can be blocked by systemic D2 antagonists (Gruss and Braun 1996; Gruss et al. 2003), but no data on cortical manipulations are yet available. There is also anatomical evidence for the presence of TH fibers, D1- and D2-like receptors in lateral-line sensing regions of the telencephalon (O’Connell et al. 2011a).
In summary, dopaminergic effects on mammalian auditory cortex in the context of association learning are well-established. However, other auditory cortex plasticity-dependent phenomena, such as perceptual learning or human language learning, still await confirmation on whether they rely on direct auditory cortical dopamine effects. In other vertebrates, whether dopamine is even present in auditory pallial regions is a largely unexplored topic. The exception being songbirds, in which roles for dopamine have been confirmed in secondary pallial regions in the context of song exposure-induced plasticity and auditory association learning. Future work could explore whether natural behaviors such as learning of birdsong, auditory imprinting, or perfection of auditory-dependent hunting (as in nocturnal owls) depend on dopaminergic signaling in the auditory pallium.
Dopamine-induced plasticity in the olfactory cortex
In dynamic environments, the ability to associate meaning with odors is an important evolutionary adaptation present in virtually all vertebrates. Multiple brain areas have been implicated in odorant processing and associative learning, and dopaminergic signaling is pervasive in these systems. Of note, the olfactory bulb contains one of the major dopamine-producing cell groups in vertebrates, whose neurons are continuously generated throughout the lifespan (but see Hinds 1968; Pérez-Cañellas and García-Verdugo 1996; Kornack and Rakic 2001; Bergmann et al. 2012). Dopamine/GABAergic interneurons regulate the activity of local olfactory projection neurons (mitral/tufted cells), a feature that seems to be evolutionarily conserved across vertebrates (Hsia et al. 1999; Davison et al. 2004; Kawai et al. 2012) and even invertebrates (Perk and Mercer 2006). The role of these neurons has been intensely studied and recently reviewed elsewhere (Pignatelli and Belluzzi 2017). Some external tufted cells can also be dopaminergic in rats (Halász et al. 1981), macaques, and humans (Smith et al. 1991), but these do not seem to project outside of the olfactory bulb. Rather, they modulate the activity of periglomerular cells (De Saint Jan et al. 2009).
While dopamine effects on the olfactory bulb and olfactory striatum are known (Zhang et al. 2017), the role of dopamine in olfactory cortical regions has been relatively understudied.
The olfactory bulb projection neurons send diffuse projections to the piriform cortex (PrC) (Haberly 2001; Wilson and Sullivan 2011; Bekkers and Suzuki 2013) which itself receives dense dopaminergic projections from VTA and locus coeruleus. Dopamine fibers are more abundant in deeper layers II and III (PrC is a paleocortical region with only three layers) (Datiche and Cattarelli 1996). D1 receptors are most abundant in layer II and more prevalent than D2 receptors, which are virtually absent in PrC (Boyson et al. 1986; Santana et al. 2009).
The effects of dopamine on PrC activity are poorly understood but reports confirm neural activity modulation by both D1 and D2 receptor action in vitro (Bannon et al. 1983; Plantjé et al. 1987) although these effects do not appear to translate into changes in short-term olfactory memory formation (Zenko et al. 2011). Data in humans also point to an involvement of the PrC in olfactory learning (Gottfried et al. 2002), but data on dopamine effects have not yet been reported.
Another cortical region directly modulated by dopamine is the entorhinal cortex (EC). In macaque EC, TH fibers are dense, especially in the medial portion, called the olfactory EC (Akil and Lewis 1993). Dopamine reduced layer V pyramidal neuron excitability in rat lateral EC, in vitro, through D1 but not D2 receptors (Rosenkranz and Johnston 2006). Increases in synaptic dopamine impaired LTP and LTD at PrC to EC synapses in awake rats (Caruana et al. 2007). Still, in vivo and behavioral data on dopamine modulation in the EC are currently lacking. Rodent behaviors such as the Coolidge effect or individual recognition through olfactory cues are known to depend on the EC (Bannerman et al. 2001, 2002; Petrulis and Eichenbaum 2003), and could be under the regulation of dopamine. In sum, both PrC and EC are important sites of olfactory processing and learning in mammals, and both are directly modulated by dopaminergic projections from VTA (Aransay et al. 2015).
The three-layered PrC is thought to be a highly evolutionarily conserved structure, reminiscent of the similarly three-layered reptilian/avian lateral olfactory cortex (Aboitiz et al. 2002). It is reasonable to suggest, therefore, that the effects of dopamine on olfactory plasticity in non-mammals might be similar as those reviewed above. Concordantly, olfactory pallial regions contain significant TH and/or D1/D2/DARPP-32 protein immunoreactivity in non-mammals (e.g., canary: Appeltants et al. 2001; eel: Kapsimali et al. 2000; lungfish: López and González 2017; túngara frog: O’Connell et al. 2011b, African cichlid: O’Connell et al. 2011a; ball python: Smeets 1988; trout: Vacher et al. 2003). In birds, the PrC (Rieke and Wenzel 1978; Reiner and Karten 1985) seems to contain both D1 and D2 receptors (Kubikova et al. 2010), although a more detailed investigation was not performed. We could not find any studies on dopamine modulation of olfactory function in non-mammals, again demonstrating a broad research opportunity and need.
The mammalian olfactory system is particularly reliant on dopaminergic signaling throughout many of its neural processing stages (olfactory bulb, piriform, and entorhinal cortices), but solid evidence is still lacking on what aspects of behavior are regulated by dopamine. It is tempting to believe that the remarkably evident evolutionary conservation of these structures across vertebrates suggests an equally conserved important role for dopamine. Indeed, the anatomical substrate for dopaminergic action is present in all pallial homologs studied, pointing toward the olfactory system in non-mammals as promising systems to study dopamine action.
Dopamine-induced plasticity in the taste cortex
In many species, taste learning is crucial for survival. Formation of aversion memory to toxic or spoiled foods after negative consequences from a first contact ensures that animals do not make the same mistake in the future. Taste responses converge in the insular cortex (InC), along with other experiences such as enteroception, addiction, and complex emotional reactions. Thus the InC is described as a hub for integrating several systems (Gogolla 2017). Interestingly, the InC has a bidirectional connection with VTA, which could be the anatomical substrate through which the InC both receives and modulates reward processing signals (Ohara et al. 2003).
In the rat InC, D1 receptor expression is greater in deep layer VI, followed by layer II, while D2 receptors are mostly concentrated in layer V (Gaspar et al. 1995). Taste learning has been shown to involve dopamine signaling via D1 receptors in the InC (Berman et al. 2000; David et al. 2014; Moreno-Castilla et al. 2016).
While dopaminergic signaling within the InC is evident in rodents, human data on dopamine and taste responses only exist for subcortical structures. For example, hunger and food modulate extracellular dopamine in the striatum (Volkow et al. 2002; Wang et al. 2004, 2014). Systemic amphetamine, which boosts dopamine release, changes InC responses to sucrose (Melrose et al. 2016), but it is not possible to infer whether dopamine directly modulates the InC. To our knowledge, there are no available studies that explore the role of dopamine in human taste learning, although the human InC is known to receive reward prediction error signals (Preuschoff et al. 2008).
Taste learning has been reported in other species in the vertebrate lineage. Food aversion learning can be induced in several lizard species, but not in frogs or salamanders (Paradis and Cabanac 2004). Curiously, this type of learning could be successfully induced in goldfish, and was impaired by whole telencephalon or dorsomedial telencephalon lesions (Martín et al. 2011). As it could also be successfully induced in birds (bobwhite quail: Wilcoxon et al. 1971; buteo hawk: Brett et al. 1976), it is possible that taste aversion learning was a secondary loss in amphibians. We could not find further published data on dopamine effects, or more broadly, on the neural mechanisms of taste processing in non-mammalian vertebrate pallium, with the exception of fishes (e.g., catfish: Lamb and Caprio 1993). Given the extreme diversity in feeding habits across vertebrates, this area of study could bring interesting insight on how the taste processing systems evolved.
In sum, there is solid evidence that mammalian taste learning relies on cortical dopamine signaling, but studies in humans have not yet established a causal effect. For the latter, because taste and the InC have such important roles in modulating emotion and reinforcement processing, we highlight this as an exciting area for future research. In non-mammals, even anatomical evidence for taste pallial homologs is missing, let alone what roles dopamine signaling plays. Since taste learning—especially of aversive cues—is so important for survival and is so widespread throughout evolution, understanding the taste system in these animals and how it learns vital associations is an important and wide-open field of study.
Dopamine-induced plasticity in the SSC
Dopaminergic fibers in the SSC originate mostly from VTA and seem to follow the pattern found in other neocortical regions. They are denser in layer I in squirrel monkeys (Lewis et al. 1987), while denser in deep layer VI in rats (Descarries et al. 1987). Dopamine receptors somewhat map onto these distributions: D1 receptors are denser in superficial layers I–III, while D2 receptors are denser in area V in Rhesus macaques (Lidow et al. 1991), while in the rat, D1 receptors are denser in deeper layers V–VI, and D2 in layer V (Gurevich and Joyce 2000). These differences might arise due to these species’ natural histories and further strengthen the argument for the importance of avoiding generalizations, and for studying a broad range of organisms to gain true insight the evolution of neural and neuromodulatory systems.
Reports of local effects of dopamine in the SSC suggest that dopamine plays a key role in plasticity, especially when there is a need for cortical reorganization after an injury. Intracortical injections of either D1 or D2 antagonists increased the responses of the sensorimotor cortex (transition between SSC and motor cortex) to muscle stimulation in anesthetized rats (Hosp et al. 2011a). Furthermore, peripheral nerve transection progressively increased levels of dopamine metabolites in the rat SSC, suggesting that the increase in dopamine signaling might be related to the reorganization of the cortex when sensory information from an appendage is eliminated (Jiménez-Capdeville et al. 1996). Similarly, unihemispheric stroke induction in rats increased dopamine levels in the contralateral hemisphere, and D2 antagonist prevented recovery of nociceptive response in the weeks following the stroke. In the event of a stroke, the contralateral hemisphere is thought to undergo reorganization in order to aid in physical recovery and compensation, with dopamine involved in this process (Obi et al. 2018).
Interestingly, dopamine receptors may also play an organizational role in the SSC. In the developing rat, D3 receptors are transiently highly expressed in layer IV and correlate with the development of the characteristic “barrels” (Gurevich and Joyce 2000). These authors neither examine the origin of the dopamine input to this system, nor did they follow up with dopamine manipulations. These would be interesting topics to explore, since this system is known for being experience- and critical period-dependent during development (Erzurumlu and Gaspar 2012).
Spike timing-dependent plasticity in SSC corticostriatal synapses was prevented by D1 antagonists and modulated by D2 antagonists in vitro (Pawlak and Kerr 2008). Whether these effects are due to cortical versus striatal receptors has not been explored.
Somatosensory learning in humans is an important component of the formation of painful memories and plays a role in learning of textures and patterns. Braille reading is an example of highly specialized tactile learning that requires plasticity in the SSC (Debowska et al. 2016). However, a role for dopamine in the SSC is from Parkinson’s patients, in the context of the well-characterized degeneration of the dopamine systems and consequent motor and sensory impairments (Palomar et al. 2011; Nelson et al. 2012, 2018). In fact, SSC excitability is lower in Parkinson’s patients than in healthy controls (Nelson et al. 2018). Studies examining the effects of dopaminergic drugs in the SSC of otherwise healthy people are still needed.
Somatosensory pallial regions have been mapped in a variety of non-mammalian vertebrates (e.g., rockfish: Ito et al. 1986; leopard frog: Kicliter 1979; crocodile: Pritz and Northcutt 1980; pigeon: Wild 1987). The same regions are known to express TH/dopaminergic fibers (Iberian ribbed newt: González and Smeets 1991; African cichlid fish: O’Connell et al. 2011a; túngara frog: O’Connell et al. 2011b; gecko: Smeets et al. 1986; pigeon: Wynne and Güntürkün 1995). There is some evidence in songbirds that D3 receptor activation accelerate tissue regeneration after lesion, akin to the regenerative role described above in mammals (Lukacova et al. 2016). Still, no current data exist for somatosensory plasticity following the manipulation of dopamine systems.
In conclusion, the evidence for dopaminergic effects in the mammalian SSC is promising. Most of the evidence points toward an important role of dopamine in cortical reaction to neural injury (e.g., stroke or deafferentation) or during neural development. These findings are exciting, and it should be tested whether they also apply to other cortical systems. Nevertheless, it would be interesting to know whether dopamine also plays normophysiological roles, such as in touch associations and pain learning. In non-mammals, the foundational anatomical evidence strongly suggests a role for dopamine in pallial regions in all organisms studied. Phenomena such as electrosensory learning in electric fish or toxic food aversion learning (through somatic effects) should engage somatosensory pallial regions and potentially rely on dopamine signaling.
Final considerations
In view of the literature discussed here, it is evident that dopamine plays roles in virtually all mammalian cortical/pallial circuits, but the architecture varies remarkably at both molecular and anatomical levels. For example, variation occurs in the layer distribution of receptors and dopaminergic fibers, in the receptor activation effects, and in the enhancement versus suppression of plasticity. Additionally, in different species, these factors may vary with evolutionary history. Because of this, the current perspective emphasizes that findings in one cortical system do not necessarily generalize to other systems or across species. We were particularly surprised to find that, after reviewing the literature, the presence of LTP modulation by dopamine in auditory, visual, and olfactory (piriform) cortical systems are commonly assumed but rarely demonstrated directly, or even tested. It is thus an open question as to whether the fundamental properties of neural circuit plasticity observed in the PFC/striatum extend to other cortical modalities, in mammals and non-mammals alike.
Our review also illustrates that there is a strong anatomical basis—dopamine fibers and receptor distributions—to hypothesize that pallial dopamine effects are widespread across vertebrates. However, there are limited reports on this subject, which highly constrains our understanding about how these circuits adapted and evolved. The anatomy of pallial structures is widely variant among vertebrates, including three layered, nucleated, six layered, super versus subventricular. Therefore, understanding how projections from highly conserved midbrain structures (VTA/SNc) have been molded and recruited alongside their evolving pallial projection targets will be crucial to understand the importance of midbrain → cortex circuits and their contribution to behavior in healthy and diseased brain states.
Dopamine release is frequently under the control of, or acting in concert with, other neuromodulator systems. Much is known about interactions between the dopamine system and other traditional neuromodulators such as norepinephrine (reviewed by Xing et al. 2016), oxytocin (reviewed by Baskerville and Douglas 2010), and acetylcholine (reviewed by De Kloet et al. 2015). Less explored are interactions between the dopamine system and steroid hormones. In mammalian and avian striatum and preoptic area, estradiol (E2) and dopamine interact to regulate one another’s production, release, and receptor expression (Becker 1990; Lammers et al. 1999; Balthazart et al. 2002; Tozzi et al. 2015). In the mammalian striatum, E2 infusions rapidly increase dopamine release (Xiao et al. 2003), and dopamine agonists reverse the decrease in LTP induced by an E2-production inhibitor (Tozzi et al. 2015). In fact, there is some evidence that dopamine and E2 could bind to the same receptors (Olesen and Auger 2008; Tozzi et al. 2015).
Specifically, in the cortex, further insights into potential interactions between dopamine and steroids are needed. In male rats, gonadectomy and hormone replacements affected dopamine levels in the PFC, but not the motor cortex (Aubele and Kritzer 2011). In healthy normocycling women, E2 levels are associated with dopamine neurotransmission in the PFC, and both systems interact to regulate working memory (Jacobs and D’Esposito 2011). In the songbird secondary auditory pallium (NCM), TH fiber density and dopamine release seem to be under control of steroid hormones (Matragrano et al. 2011; Rodríguez-Saltos et al. 2018), but direct evidence of functional interaction is lacking. Studying E2 and dopamine interactions in the auditory cortex is an interesting avenue of research and of potential relevance to human auditory function, since both aromatase (E2-synthase) (Yague et al. 2006) and E2 receptors (González et al. 2007) are abundant in the human temporal cortex.
Finally, in this review, we identify important gaps in the dopamine research literature regarding effects on mammalian motor and sensory cortices and on non-mammalian vertebrate pallia. Overall, it is clear that dopamine-induced plasticity mechanisms are widespread across all cortical/pallial systems and induce motor/sensory adaptations to achieve behavioral goals more efficiently. Furthermore, studying vertebrate species or lineages that may have retained ancestral (plesiomorphic) characteristics might prove crucial for advancing our understanding of (I) how the dopamine system can change in face of evolutionary pressures, (II) what other functions this system might express, and (III) how it contributes mechanistically in a neural disease context.
Acknowledgments
The authors would like to thank Jeff Podos, Karine Fénelon, Joseph Bergan, and David Moorman for valuable feedback on this manuscript.
Funding
This work was supported by the United States National Institutes of Health (R01NS082179). M.M.-L. was a CAPES-Brazil Fellow (13640/13-5).
Conflict of interest statement
The authors declare no conflict of interest.
From the symposium “Sending and receiving signals: Endocrine modulation of social communication” presented at the virtual annual meeting of the Society for Integrative and Comparative Biology, January 3–7, 2020
References
- Aboitiz F, Montiel J, Morales D, Concha M.. 2002. Evolutionary divergence of the reptilian and the mammalian brains: considerations on connectivity and development. Brain Res Rev 39:141–53. [DOI] [PubMed] [Google Scholar]
- Akil M, Lewis DA.. 1993. The dopaminergic innervation of monkey entorhinal cortex. Cereb. Cortex 3:533–50. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Absil P, Balthazart J, Ball GF.. 2000. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Chem Neuroanat 18:117–33. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Ball GF, Balthazart J.. 2002. The origin of catecholaminergic inputs to the song control nucleus RA in canaries. Neuroreport 13:649–53. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Ball GF, Balthazart J.. 2001. The distribution of tyrosine hydroxylase in the canary brain: demonstration of a specific and sexually dimorphic catecholaminergic innervation of the telencephalic song control nuclei. Cell Tissue Res 304:237–59. [DOI] [PubMed] [Google Scholar]
- Aransay A, RodrÃ-guez-López C, GarcÃ-a-Amado MÃ, Clascá F, Prensa LÃ.. 2015. Long-range projection neurons of the mouse ventral tegmental area: a single-cell axon tracing analysis. Front Neuroanat 9:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault JT, Nelissen K, Jarraya B, Vanduffel W.. 2013. Dopaminergic reward signals selectively decrease fMRI activity in primate visual cortex. Neuron 77:1174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubele T, Kritzer MF.. 2011. Gonadectomy and hormone replacement affects in vivo basal extracellular dopamine levels in the prefrontal cortex but not motor cortex of adult male rats. Cereb Cortex 21:222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin T, Jouventin P.. 1998. Cocktail-party effect in king penguin colonies. Proc R Soc B Biol Sci 265:1665–73. [Google Scholar]
- Balthazart J, Baillien M, Ball GF.. 2002. Interactions between aromatase (estrogen synthase) and dopamine in the control of male sexual behavior in quail. Comp Biochem Physiol B Biochem Mol Biol 132:37–55. [DOI] [PubMed] [Google Scholar]
- Bannerman D, Lemaire M, Yee B, Iversen S, Oswald C, Good M, Rawlins J.. 2002. Selective cytotoxic lesions of the retrohippocampal region produce a mild deficit in social recognition memory. Exp Brain Res 142:395–401. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Lemaire M, Beggs S, Rawlins JNP, Iversen SD.. 2001. Cytotoxic lesions of the hippocampus increase social investigation but do not impair social-recognition memory. Exp Brain Res 138:100–9. [DOI] [PubMed] [Google Scholar]
- Bannon MJ, Wolf ME, Roth RH.. 1983. Pharmacology of dopamine neurons innervating the prefrontal, cingulate and piriform cortices. Eur J Pharmacol 92:119–25. [DOI] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM.. 2001. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature 412:79–83. [DOI] [PubMed] [Google Scholar]
- Barr HJ, Woolley SC.. 2018. Developmental auditory exposure shapes responses of catecholaminergic neurons to socially-modulated song. Sci Rep 8:11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barto AG, Sutton RS, Brouwer PS.. 1981. Associative search network: a reinforcement learning associative memory. Biol Cybern 40:201–11. [Google Scholar]
- Baskerville TA, Douglas AJ.. 2010. Dopamine and oxytocininteractions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci Ther 16:92–123 ( 10.1111/j.1755-5949.2010.00154.x). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR.. 2011. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217. [DOI] [PubMed] [Google Scholar]
- Becker JB. 1990. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett 118:169–71. [DOI] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, Deloach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, Luo L.. 2015. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell 162:622–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM, Suzuki N.. 2013. Neurons and circuits for odor processing in the piriform cortex. Trends Neurosci 36:429–38. [DOI] [PubMed] [Google Scholar]
- Berger B, Tassin JP, Blanc G, Moyne MA, Thierry AM.. 1974. Histochemical confirmation for dopaminergic innervation of the rat cerebral cortex after destruction of the noradrenergic ascending pathways. Brain Res 81:332–7. [DOI] [PubMed] [Google Scholar]
- Berger B, Trottier S, Verney C, Gaspar P, Alvarez C.. 1988. Regional and laminar distribution of the dopamine and serotonin innervation in the macaque cerebral cortex: a radioautographic study. J Comp Neurol 273:99–119. [DOI] [PubMed] [Google Scholar]
- Berger B, Verney C, Alvarez C, Vigny A, Helle KB.. 1985. New dopaminergic terminal fields in the motor, visual (area 18b) and retrosplenial cortex in the young and adult rat. Immunocytochemical and catecholamine histochemical analyses. Neuroscience 15:983–98. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Liebl J, Bernard S, Alkass K, Yeung MSY, Steier P, Kutschera W, Johnson L, Landén M, Druid H, et al. 2012. The age of olfactory bulb neurons in humans. Neuron 74:634–9. [DOI] [PubMed] [Google Scholar]
- Berman DE, Hazvi S, Neduva V, Dudai Y.. 2000. The role of identified neurotransmitter systems in the response of insular cortex to unfamiliar taste: activation of ERK1-2 and formation of a memory trace. J Neurosci 20:7017–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A, Dunnett SB.. 2007. Dopamine neuron systems in the brain: an update. Trends Neurosci 30:194–202. [DOI] [PubMed] [Google Scholar]
- Bottjer SW. 1993. The distribution of tyrosine hydroxylase immunoreactivity in the brains of male and female zebra finches. J Neurobiol 24:51–69. [DOI] [PubMed] [Google Scholar]
- Boyson SJ, McGonigle P, Molinoff PB.. 1986. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci 6:3177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenstein C, Korsukewitz C, Flöel A, Kretzschmar T, Diederich K, Knecht S.. 2006. Tonic dopaminergic stimulation impairs associative learning in healthy subjects. Neuropsychopharmacology 31:2552–64. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Zakon HH.. 2015. Emerging from the bottleneck: benefits of the comparative approach to modern neuroscience. Trends Neurosci 38:273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett LP, Hankins WG, Garcia J.. 1976. Prey-lithium aversions. III: buteo hawks. Behav Biol 17:87–98. [DOI] [PubMed] [Google Scholar]
- Brown RM, Crane AM, Goldman PS.. 1979. Regional distribution of monoamines in the cerebral cortex and subcortical structures of the rhesus monkey: concentrations and in vivo synthesis rates. Brain Res 168:133–50. [DOI] [PubMed] [Google Scholar]
- Bruce LL, Neary TJ.. 1995. The limbic system of tetrapods: a comparative analysis of cortical and amygdalar populations. Brain Behav Evol 46:224–34. [DOI] [PubMed] [Google Scholar]
- Budinger E, Laszcz A, Lison H, Scheich H, Ohl FW.. 2008. Non-sensory cortical and subcortical connections of the primary auditory cortex in Mongolian gerbils: bottom-up and top-down processing of neuronal information via field AI. Brain Res 1220:2–32. [DOI] [PubMed] [Google Scholar]
- Burmeister SS, Rodriguez Moncalvo VG, Pfennig KS.. 2017. Monoaminergic integration of diet and social signals in the brains of juvenile spadefoot toads. J Exp Biol 220:3135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MJ, Lewis DA, Foote SL, Morrison JH.. 1987. Distribution of choline acetyltransferase, serotonin, dopamine,-b-hydroxylase, tyrosine hydroxylase immunoreactive fibers in monkey primary auditory cortex. J Comput Neurol 261:209–20. [DOI] [PubMed] [Google Scholar]
- Carlson BA. 2012. Diversity matters. Arch Neurol 69:1 ( 10.1001/archneurol.2012.77). [DOI] [PubMed] [Google Scholar]
- Carlsson A, Lindqvist M, Magnusson T, Waldeck B.. 1958. On the presence of 3-hydroxytyramine in brain. Science 127:471. [DOI] [PubMed] [Google Scholar]
- Caruana DA, Reed SJ, Sliz DJ, Chapman CA.. 2007. Inhibiting dopamine reuptake blocks the induction of long-term potentiation and depression in the lateral entorhinal cortex of awake rats. Neurosci Lett 426:6–11. [DOI] [PubMed] [Google Scholar]
- Cerovic M, d’Isa R, Tonini R, Brambilla R.. 2013. Molecular and cellular mechanisms of dopamine-mediated behavioral plasticity in the striatum. Neurobiol Learn Mem 105:63–80. [DOI] [PubMed] [Google Scholar]
- Chau BKH, Jarvis H, Law CK, Chong TTJ.. 2018. Dopamine and reward: a view from the prefrontal cortex. Behav Pharmacol 29:569–83. [DOI] [PubMed] [Google Scholar]
- Chen Y, Matheson LE, Sakata JT.. 2016. Mechanisms underlying the social enhancement of vocal learning in songbirds. Proc Natl Acad Sci USA 113: 6641–6 ( 10.1073/pnas.1522306113). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KL, Noudoost B, Jacob S.. 2014. The role of prefrontal catecholamines in attention and working memory. Front Neural Circuit 8:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Ball GF.. 2008. Interplay among catecholamine systems: dopamine binds to α 2-adrenergic receptors in birds and mammals. J Comp Neurol 511:610–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datiche F, Cattarelli M.. 1996. Catecholamine innervation of the piriform cortex: a tracing and immunohistochemical study in the rat. Brain Res 710:69–78. [DOI] [PubMed] [Google Scholar]
- David O, Barrera I, Chinnakkaruppan A, Kaphzan H, Nakazawa T, Yamamoto T, Rosenblum K.. 2014. Dopamine-induced tyrosine phosphorylation of NR2B (Tyr1472) is essential for ERK1/2 activation and processing of novel taste information. Front Mol Neurosci 7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison IG, Boyd JD, Delaney KR.. 2004. Dopamine inhibits mitral/tufted to granule cell synapses in the frog olfactory bulb. J Neurosci 24:8057–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet SF, Mansvelder HD, De Vries TJ.. 2015. Cholinergic modulation of dopamine pathways through nicotinic acetylcholine receptors. Biochem Pharmacol 97:425–38. [DOI] [PubMed] [Google Scholar]
- De Saint Jan D, Hirnet D, Westbrook GL, Charpak S.. 2009. External tufted cells drive the output of olfactory bulb glomeruli. J Neurosci 29:2043–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debowska W, Wolak T, Nowicka A, Kozak A, Szwed M, Kossut M.. 2016. Functional and structural neuroplasticity induced by short-term tactile training based on braille reading. Front Neurosci 10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demchyshyn LL, Sugamori KS, Lee FJS, Hamadanizadeh SA, Niznik HB.. 1995. The dopamine D1D receptor: cloning and characterization of three pharmacologically distinct D1-like receptors from Gallus domesticus. J Biol Chem 270:4005–12. [DOI] [PubMed] [Google Scholar]
- Descarries L, Lemay B, Doucet G, Berger B.. 1987. Regional and laminar density of the dopamine innervation in adult rat cerebral cortex. Neuroscience 21:807–24. [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G, Saba P, Fà M, Gessa GL.. 2005. Co-release of noradrenaline and dopamine in the cerebral cortex elicited by single train and repeated train stimulation of the locus coeruleus. BMC Neurosci 6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel H. 1978. Behavior and electrical brain stimulation in the green iguana, Iguana iguana L. II. stimulation effects. Exp Brain Res 31:353–67. [DOI] [PubMed] [Google Scholar]
- Dugas-Ford J, Rowell JJ, Ragsdale CW.. 2012. Cell-type homologies and the origins of the neocortex. Proc Natl Acad Sci USA 109:16974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK.. 2008. The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-O-methyltransferase genotypes and schizophrenia. Biol Psychiatry 64:739–49. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Gaspar P.. 2012. Development and critical period plasticity of the barrel cortex. Eur J Neurosci 35:1540–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flöel A, Breitenstein C, Hummel F, Celnik P, Gingert C, Sawaki L, Knecht S, Cohen LG.. 2005. Dopaminergic influences on formation of a motor memory. Ann Neurol 58:121–30. [DOI] [PubMed] [Google Scholar]
- Flöel A, Garraux G, Xu B, Breitenstein C, Knecht S, Herscovitch P, Cohen LG.. 2008. Levodopa increases memory encoding and dopamine release in the striatum in the elderly. Neurobiol Aging 29:267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RE, Hall WC.. 1978. The organization of central auditory pathways in a reptile, Iguana iguana. J Comp Neurol 178:783–831. [DOI] [PubMed] [Google Scholar]
- Fournier J, Müller CM, Schneider I, Laurent G.. 2018. Spatial information in a non-retinotopic visual cortex. Neuron 97:164–80.e7. [DOI] [PubMed] [Google Scholar]
- Gadagkar V, Puzerey PA, Chen R, Baird-Daniel E, Farhang AR, Goldberg JH.. 2016. Dopamine neurons encode performance error in singing birds. Science 354:1278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo EF. 2019. Disentangling the diverse roles of dopamine D2 receptors in striatal function and behavior. Neurochem Int 125:35–46 ( 10.1016/j.neuint.2019.01.022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Febvret A, Vigny A, Henry JP.. 1989. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. J Comp Neurol 279:249–71 ( 10.1002/cne.902790208). [DOI] [PubMed] [Google Scholar]
- Gaspar P, Bloch B, Le Moine C.. 1995. D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci 7:1050–63. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Stepniewska I, Kaas JH.. 1992. Topography and collateralization of the dopaminergic projections to motor and lateral prefrontal cortex in owl monkeys. J Comp Neurol 325:1–21. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Bentley GE, Ball GF.. 2000. Individual vocal recognition and the effect of partial lesions to HVc on discrimination, learning, and categorization of conspecific song in adult songbirds. J Neurobiol 42:117–33. [DOI] [PubMed] [Google Scholar]
- Gibson EJ. 1953. Improvement in perceptual judgments as a function of controlled practice or training. Psychol Bull 50:401–31. [DOI] [PubMed] [Google Scholar]
- Glagow M, Ewert JP.. 1999. Apomorphine alters prey-catching patterns in the common toad: behavioral experiments and C-14-2-deoxyglucose brain mapping studies. Brain Behav Evol 54:223–42. [DOI] [PubMed] [Google Scholar]
- Gogolla N. 2017. The insular cortex. Curr Biol 27:R580–6. [DOI] [PubMed] [Google Scholar]
- Goldby F. 1937. An experimental investigation of the cerebral hemispheres of Lacerta viridis. J Anat 71:332–55. [PMC free article] [PubMed] [Google Scholar]
- González A, Smeets WJAJ.. 1994. Distribution of tyrosine hydroxylase immunoreactivity in the brain of Typhlonectes compressicauda (Amphibia, Gymnophiona): further assessment of primitive and derived traits of amphibian catecholamine systems. J Chem Neuroanat 8:19–32. [DOI] [PubMed] [Google Scholar]
- González A, Smeets WJAJ.. 1991. Comparative analysis of dopamine and tyrosine hydroxylase immunoreactivities in the brain of two amphibians, the anuran Rana ridibunda and the urodele Pleurodeles waltlii. J Comp Neurol 303:457–77. [DOI] [PubMed] [Google Scholar]
- González A, Tuinhof R, Smeets WJAJ.. 1993. Distribution of tyrosine hydroxylase and dopamine immunoreactivities in the brain of the South African clawed frog Xenopus laevis. Anat Embryol (Berl) 187:193–201. [DOI] [PubMed] [Google Scholar]
- González M, Cabrera-Socorro A, Pérez-García CG, Fraser JD, López FJ, Alonso R, Meyer G.. 2007. Distribution patterns of estrogen receptor α and β in the human cortex and hippocampus during development and adulthood. J Comp Neurol 503:790–802. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD.. 1992. Separate visual pathways for perception and action. Trends Neurosci 15:20–25. [DOI] [PubMed] [Google Scholar]
- Goodwin SE, Podos J.. 2014. Team of rivals: alliance formation in territorial songbirds is predicted by vocal signal structure. Biol Lett 10:20131083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottberg E, Montreuil B, Reader TA.. 1988. Acute effects of lithium on dopaminergic responses: iontophoretic studies in the rat visual cortex. Synapse 2:442–9. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ.. 2002. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J Neurosci 22:10829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss M, Bock J, Braun K.. 2003. Haloperidol impairs auditory filial imprinting and modulates monoaminergic neurotransmission in an imprinting-relevant forebrain area of the domestic chick. J Neurochem 87:686–96. [DOI] [PubMed] [Google Scholar]
- Gruss M, Braun K.. 1996. Distinct activation of monoaminergic pathways in chick brain in relation to auditory imprinting and stressful situations: a microdialysis study. Neuroscience 76:891–9. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Joyce JN.. 2000. Dopamine D3 receptor is selectively and transiently expressed in the developing whisker barrel cortex of the rat. J Comp Neurol 420:35–51. [DOI] [PubMed] [Google Scholar]
- Gusel’nikov VI, Morenkov ED, Pivovarov AS.. 1972. Unit responses of the turtle forebrain to visual stimuli. Neurosci Behav Physiol 5:235–42. [DOI] [PubMed] [Google Scholar]
- Haberly LB. 2001. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Sens 26:551–76. [DOI] [PubMed] [Google Scholar]
- Halász N, Johansson O, Hökfelt T, Ljungdahl Å, Goldstein M.. 1981. Immunohistochemical identification of two types of dopamine neuron in the rat olfactory bulb as seen by serial sectioning. J Neurocytol 10:251–59. [DOI] [PubMed] [Google Scholar]
- Hess G, Donoghue JP.. 1996. Long-term potentiation and long-term depression of horizontal connections in rat motor cortex. Acta Neurobiol Exp (Wars). 56:397–405 ( 10.1111/j.1460-9568.1996.tb01251.x). [DOI] [PubMed] [Google Scholar]
- Hinds JW. 1968. Autoradiographic study of histogenesis in the mouse olfactory bulb I. Time of origin of neurons and neuroglia. J Comp Neurol 134:287–304. [DOI] [PubMed] [Google Scholar]
- Hirano J, Archer SN, Djamgoz MB.. 1998. Dopamine receptor subtypes expressed in vertebrate (carp and eel) retinae: cloning, sequencing and comparison of five D1-like and three D2-like receptors. Recept Channel 5:387–404. [PubMed] [Google Scholar]
- Hisey E, Kearney MG, Mooney R.. 2018. A common neural circuit mechanism for internally guided and externally reinforced forms of motor learning. Nat Neurosci 21:589–97 ( 10.1038/s41593-018-0092-6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W, Karten HJ.. 1970. Visual intensity and pattern discrimination deficits after lesions of ectostriatum in pigeons. J Comp Neurol 140:53–68. [DOI] [PubMed] [Google Scholar]
- Hoffman HH. 1963. The olfactory bulb, accessory olfactory bulb, and hemisphere of some anurans. J Comp Neurol 120:317–68. [DOI] [PubMed] [Google Scholar]
- Hosp JA, Hertler B, Atiemo CO, Luft AR.. 2011a. Dopaminergic modulation of receptive fields in rat sensorimotor cortex. Neuroimage 54:154–60. [DOI] [PubMed] [Google Scholar]
- Hosp JA, Nolan HE, Luft AR.. 2015. Topography and collateralization of dopaminergic projections to primary motor cortex in rats. Exp Brain Res 233:1365–75. [DOI] [PubMed] [Google Scholar]
- Hosp JA, Pekanovic A, Rioult-Pedotti MS, Luft AR.. 2011b. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J Neurosci 31:2481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia AY, Vincent JD, Lledo PM.. 1999. Dopamine depresses synaptic inputs into the olfactory bulb. J Neurophysiol 82:1082–5. [DOI] [PubMed] [Google Scholar]
- Huang YY, Simpson E, Kellendonk C, Kandel ER.. 2004. Genetic evidence for the bidirectional modulation of synaptic plasticity in the prefrontal cortex by D1 receptors. Proc Natl Acad Sci USA 101:3236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mylius J, Scheich H, Brosch M.. 2016. Tonic effects of the dopaminergic ventral midbrain on the auditory cortex of awake macaque monkeys. Brain Struct Funct 221:969–77. [DOI] [PubMed] [Google Scholar]
- Ito H, Murakami T, Fukuoka T, Kishida R.. 1986. Thalamic fiber connections in a teleost (Sebastiscus marmoratus): visual somatosensory, octavolateral, and cerebellar relay region to the telencephalon. J Comp Neurol 250:215–27. [DOI] [PubMed] [Google Scholar]
- Jacob SN, Nienborg H.. 2018. Monoaminergic neuromodulation of sensory processing. Front Neural Circuit 12:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E, D’Esposito M.. 2011. Estrogen shapes dopamine-dependent cognitive processes: implications for women’s health. J Neurosci 31:5286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Capdeville ME, Reader TA, Molina-Holgado E, Dykes RW.. 1996. Changes in extracellular levels of dopamine metabolites in somatosensory cortex after peripheral denervation. Neurochem Res 21:1–6. [DOI] [PubMed] [Google Scholar]
- Kapsimali M, Vidal B, Gonzalez A, Dufour S, Vernier P.. 2000. Distribution of the mRNA encoding the four dopamine D1 receptor subtypes in the brain of the European eel (Anguilla anguilla): comparative approach to the function of D1 receptors in vertebrates. J Comp Neurol 419:320–43. [DOI] [PubMed] [Google Scholar]
- Karten HJ. 1968. The ascending auditory pathway in the pigeon (Columba livia) II. Telencephalic projections of the nucleus ovoidalis thalami. Brain Res 11:134–53. [DOI] [PubMed] [Google Scholar]
- Katz PS. 2019. The conservative bias of life scientists. Curr Biol 29:R666–7. [DOI] [PubMed] [Google Scholar]
- Kawai T, Abe H, Oka Y.. 2012. Dopaminergic neuromodulation of synaptic transmission between mitral and granule cells in the teleost olfactory bulb. J Neurophysiol 107:1313–24. [DOI] [PubMed] [Google Scholar]
- Kelley DB, Nottebohm F.. 1979. Projections of a telencephalic auditory nucleus-field L—in the canary. J Comp Neurol 183:455–69. [DOI] [PubMed] [Google Scholar]
- Kicliter E. 1979. Some telencephalic connections in the frog, Rana pipiens. J Comp Neurol 185:75–86. [DOI] [PubMed] [Google Scholar]
- Knecht S, Breitenstein C, Bushuven S, Wailke S, Kamping S, Floel A, Zwitserlood P, Ringelstein EB.. 2004. Levodopa: faster and better word learning in normal humans. Ann Neurol 56:20–6. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P.. 2001. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc Natl Acad Sci USA 98:4752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubikova L, Kostál L.. 2010. Dopaminergic system in birdsong learning and maintenance. J Chem Neuroanat 39:112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubikova L, Wada K, Jarvis ED.. 2010. Dopamine receptors in a songbird brain. J Comp Neurol 518:741–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh M, Shibuki K.. 2006. Sound sequence discrimination learning motivated by reward requires dopaminergic D2 receptor activation in the rat auditory cortex. Learn Mem 13:690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh M, Shibuki K.. 1994. Long-term potentiation in the auditory cortex of adult rats. Neurosci Lett 171:21–3. [DOI] [PubMed] [Google Scholar]
- Lamb CF, Caprio J.. 1993. Diencephalic gustatory connections in the channel catfish. J Comp Neurol 337:400–18. [DOI] [PubMed] [Google Scholar]
- Lammers CH, D’Souza U, Qin ZH, Lee SH, Yajima S, Mouradian MM.. 1999. Regulation of striatal dopamine receptors by estrogen. Synapse 34:222–7. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH.. 1987. The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. J Neurosci 7:279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW, Ryan SM, Arnold AP, Butcher LL.. 1981. Evidence for a catecholaminergic projection to area X in the zebra finch. J Comp Neurol 196:347–54. [DOI] [PubMed] [Google Scholar]
- Liao C, Wang S, Pan X, Hou G, Li D.. 2013. Dopamine modulates the excitability of projection neurons in the robust nucleus of the arcopallium in adult zebra finches. PLoS ONE 8:e82497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P.. 1991. Distribution of dopaminergic receptors in the primate cerebral cortex: quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience 40:657–71. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Rakic P, Gallager DW.. 1990. Autoradiographic comparison of D1-specific binding of [3H]SCH39166 and [3H]SCH23390 in the primate cerebral cortex. Brain Res 537:349–54. [DOI] [PubMed] [Google Scholar]
- Liu Z, Richmond BJ, Murray EA, Saunders RC, Steenrod S, Stubblefield BK, Montague DM, Ginns EI.. 2004. DNA targeting of rhinal cortex D2 receptor protein reversibly blocks learning of cues that predict reward. Proc Natl Acad Sci USA 101:12336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López JM, González A.. 2017. Organization of the catecholaminergic systems in the brain of lungfishes, the closest living relatives of terrestrial vertebrates. J Comp Neurol 525:3083–109. [DOI] [PubMed] [Google Scholar]
- Lukacova K, Pavukova E, Kostal L, Bilcik B, Kubikova L.. 2016. Dopamine D3 receptors modulate the rate of neuronal recovery, cell recruitment in Area X, and song tempo after neurotoxic damage in songbirds. Neuroscience 331:158–68. [DOI] [PubMed] [Google Scholar]
- Macedo-lima M, Boyd H, Remage-Healey L.. 2020. Dopamine D1 receptor activation drives plasticity in the songbird auditory pallium. bioRxiv 10.1101/2020.10.09.330266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger P, Cort J, Ebrahim N, Goodman A, Henning J, Karolia M, Rodrigues SL, Štrkalj G.. 2008. Is 21st century neuroscience too focussed on the rat/mouse model of brain function and dysfunction? Front Neuroanat 2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, McCutcheon JE.. 2014. Heterogeneity of dopamine neuron activity across traits and states. Neuroscience 282:176–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín I, Gómez A, Salas C, Puerto A, Rodríguez F.. 2011. Dorsomedial pallium lesions impair taste aversion learning in goldfish. Neurobiol Learn Mem 96:297–305. [DOI] [PubMed] [Google Scholar]
- Martínez-García F, Lanuza E.. 2018. Evolution of vertebrate survival circuits. Curr Opin Behav Sci 24:113–23. [Google Scholar]
- Matragrano LL, Beaulieu M, Phillip JO, Rae AI, Sanford SE, Sockman KW, Maney DL.. 2012. Rapid effects of hearing song on catecholaminergic activity in the songbird auditory pathway. PLoS ONE 7:e39388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matragrano LL, Sanford SE, Salvante KG, Sockman KW, Maney DL.. 2011. Estradiol-dependent catecholaminergic innervation of auditory areas in a seasonally breeding songbird. Eur J Neurosci 34:416–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann JP. 2015. Associative learning and sensory neuroplasticity: how does it happen and what is it good for? Learn Mem 22:567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintzschel F, Ziemann U.. 2006. Modification of practice-dependent plasticity in human motor cortex by neuromodulators. Cereb Cortex 16:1106–15. [DOI] [PubMed] [Google Scholar]
- Melrose AJ, Bailer U, Wierenga CE, Bischoff-Grethe A, Paulus MP, Kaye WH.. 2016. Amphetamine alters neural response to sucrose in healthy women. Psychiatry Res Neuroimaging 252:19–25. [DOI] [PubMed] [Google Scholar]
- Menegas W, Akiti K, Amo R, Uchida N, Watabe-Uchida M.. 2018. Dopamine neurons projecting to the posterior striatum reinforce avoidance of threatening stimuli. Nat Neurosci 21:1421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger M, Jiang S, Wang J, Braun K.. 1996. Organization of the dopaminergic innervation of forebrain areas relevant to learning: a combined immunohistochemical/retrograde tracing study in the domestic chick. J Comp Neurol 376:1–27. [DOI] [PubMed] [Google Scholar]
- Molina-Luna K, Pekanovic A, Röhrich S, Hertler B, Schubring-Giese M, Rioult-Pedotti MS, Luft AR.. 2009. Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS ONE 4:e7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Castilla P, Rodriguez-Duran LF, Guzman-Ramos K, Barcenas-Femat A, Escobar ML, Bermudez-Rattoni F.. 2016. Dopaminergic neurotransmission dysfunction induced by amyloid-β transforms cortical long-term potentiation into long-term depression and produces memory impairment. Neurobiol Aging 41:187–99. [DOI] [PubMed] [Google Scholar]
- Müller CP, Huston JP.. 2007. Dopamine activity in the occipital and temporal cortices of rats: dissociating effects of sensory but not pharmacological stimulation. Synapse 61:254–8. [DOI] [PubMed] [Google Scholar]
- Mylius J, Happel MFK, Gorkin AG, Huang Y, Scheich H, Brosch M.. 2015. Fast transmission from the dopaminergic ventral midbrain to the sensory cortex of awake primates. Brain Struct Funct 220:3273–94. [DOI] [PubMed] [Google Scholar]
- Nelson AJ, Hoque T, Gunraj C, Chen R.. 2018. Altered somatosensory processing in Parkinson’s disease and modulation by dopaminergic medications. Parkinsonism Relat Disord 53:76–81. [DOI] [PubMed] [Google Scholar]
- Nelson AJ, Premji A, Rai N, Hoque T, Tommerdahl M, Chen R.. 2012. Dopamine alters tactile perception in Parkinson’s disease. Can J Neurol Sci 39:52–57. [DOI] [PubMed] [Google Scholar]
- Nicol AU, Brown MW, Horn G.. 1995. Neurophysiological investigations of a recognition memory system for imprinting in the domestic chick. Eur J Neurosci 7:766–76. [DOI] [PubMed] [Google Scholar]
- Noack HJ, Lewis DA.. 1989. Antibodies directed against tyrosine hydroxylase differentially recognize noradrenergic axons in monkey neocortex. Brain Res 500:313–24. [DOI] [PubMed] [Google Scholar]
- Nomoto K, Schultz W, Watanabe T, Sakagami M.. 2010. Temporally extended dopamine responses to perceptually demanding reward-predictive stimuli. J Neurosci 30:10692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt RG, Ronan M.. 1992. Afferent and efferent connections of the bullfrog medial pallium. Brain Behav Evol 40:1–16. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Arnold A.. 1976. Sexual dimorphism in vocal control areas of the songbird brain. Science 194:211–3. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM.. 1976. Central control of song in the canary. J Comp Neurol 165:457–86. [DOI] [PubMed] [Google Scholar]
- Noudoost B, Moore T.. 2011. Control of visual cortical signals by prefrontal dopamine. Nature 474:372–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell LA, Fontenot MR, Hofmann HA.. 2011a. Characterization of the dopaminergic system in the brain of an African cichlid fish, Astatotilapia burtoni. J Comp Neurol 519:75–92. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Matthews BJ, Ryan MJ, Hofmann HA.. 2011b. Characterization of the dopamine system in the brain of the túngara frog, Physalaemus pustulosus. Brain Behav Evol 76:211–25. [DOI] [PubMed] [Google Scholar]
- Obi K, Amano I, Takatsuru Y.. 2018. Role of dopamine on functional recovery in the contralateral hemisphere after focal stroke in the somatosensory cortex. Brain Res 1678:146–52. [DOI] [PubMed] [Google Scholar]
- Ohara PT, Granato A, Moallem TM, Wang BR, Tillet Y, Jasmin L.. 2003. Dopaminergic input to GABAergic neurons in the rostral agranular insular cortex of the rat. J Neurocytol 32:131–41. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P.. 1954. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol 47:419–27. [DOI] [PubMed] [Google Scholar]
- Olesen KM, Auger AP.. 2008. Dopaminergic activation of estrogen receptors induces fos expression within restricted regions of the neonatal female rat brain. PLoS ONE 3:e2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomar FJ, Díaz-Corrales F, Carrillo F, Fernández-del-Olmo M, Koch G, Mir P.. 2011. Sensory perception changes induced by transcranial magnetic stimulation over the primary somatosensory cortex in Parkinson’s disease. Mov Disord 26:2058–64. [DOI] [PubMed] [Google Scholar]
- Paradis S, Cabanac M.. 2004. Flavor aversion learning induced by lithium chloride in reptiles but not in amphibians. Behav Proc 67:11–8. [DOI] [PubMed] [Google Scholar]
- Pawlak V, Kerr JND.. 2008. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J Neurosci 28:2435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Cañellas MM, García-Verdugo JM.. 1996. Adult neurogenesis in the telencephalon of a lizard: a [3H]thymidine autoradiographic and bromodeoxyuridine immunocytochemical study. Brain Res Dev Brain Res 93:49–61. [DOI] [PubMed] [Google Scholar]
- Perk CG, Mercer AR.. 2006. Dopamine modulation of honey bee (Apis mellifera) antennal-lobe neurons. J Neurophysiol 95:1147–57. [DOI] [PubMed] [Google Scholar]
- Petersen CL, Timothy M, Kim DS, Bhandiwad AA, Mohr RA, Sisneros JA, Forlano PM.. 2013. Exposure to advertisement calls of reproductive competitors activates vocal-acoustic and catecholaminergic neurons in the plainfin midshipman fish, Porichthys notatus. PLoS ONE 8:e70474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrulis A, Eichenbaum H.. 2003. The perirhinal–entorhinal cortex, but not the hippocampus, is critical for expression of individual recognition in the context of the Coolidge effect. Neuroscience 122:599–607. [DOI] [PubMed] [Google Scholar]
- Pettigrew J, Konishi M.. 1976. Neurons selective for orientation and binocular disparity in the visual Wulst of the barn owl (Tyto alba). Science 193:675–8. [DOI] [PubMed] [Google Scholar]
- Pfenning AR, Hara E, Whitney O, Rivas MV, Wang R, Roulhac PL, Howard JT, Wirthlin M, Lovell PV, Ganapathy G, et al. 2014. Convergent transcriptional specializations in the brains of humans and song-learning birds. Science 346:1256846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson OT, Kilpatrick IC, Jones MW.. 1987. Dopaminergic innervation of the primary visual cortex in the rat, and some correlations with human cortex. Brain Res Bull 18:621–33. [DOI] [PubMed] [Google Scholar]
- Pignatelli A, Belluzzi O.. 2017. Dopaminergic neurones in the main olfactory bulb: an overview from an electrophysiological perspective. Front Neuroanat 11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantjé JF, Steinbusch HWM, Schipper J, Dijcks FA, Verheijden PFHM, Stoof JC.. 1987. D-2 dopamine-receptors regulate the release of [3H]dopamine in rat cortical regions showing dopamine immunoreactive fibers. Neuroscience 20:157–68. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P.. 2008. Human insula activation reflects risk prediction errors as well as risk. J Neurosci 28:2745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritz MB, Northcutt RG.. 1980. Anatomical evidence for an ascending somatosensory pathway to the telencephalon in crocodiles, Caiman crocodilus. Exp Brain Res 40:342–45. [DOI] [PubMed] [Google Scholar]
- Reader TA. 1978. The effects of dopamine, noradrenaline and serotonin in the visual cortex of the cat. Experientia 34:1586–8. [DOI] [PubMed] [Google Scholar]
- Reiner A, Karten HJ.. 1985. Comparison of olfactory bulb projections in pigeons and turtles. Brain Behav Evol 27:11–27. [DOI] [PubMed] [Google Scholar]
- Reiner A, Karle EJ, Anderson KD, Medina L.. 1994. Catecholaminergic perikarya and fibers in the avian nervous system. In: Smeets WJA, Reiner A, editors. Phylogeny and development of catecholamine systems in the CNS of vertebrates. Cambridge, UK: Cambridge University Press. p. 135–81. [Google Scholar]
- Remage-Healey L, Krentzel AA, Macedo-Lima M, Vahaba D.. 2017. Species diversity matters in biological research. Policy Insights Behav Brain Sci 4:210–8. [Google Scholar]
- Rieke GK, Wenzel BM.. 1978. Forebrain projections of the pigeon olfactory bulb. J Morphol 158:41–55. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP.. 1998. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci 1:230–4. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Saltos CA, Lyons SM, Sockman KW, Maney DL.. 2018. Sound-induced monoaminergic turnover in the auditory forebrain depends on endocrine state in a seasonally-breeding songbird. J Neuroendocrinol 30:e12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Johnston D.. 2006. Dopaminergic regulation of neuronal excitability through modulation of Ih in layer V entorhinal cortex. J Neurosci 26:3229–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidel WM, Marquez-Houston K, Butler AB.. 2001. Identification of visual pallial telencephalon in the goldfish, Carassius auratus: a combined cytochrome oxidase and electrophysiological study. Brain Res 919:82–93. [DOI] [PubMed] [Google Scholar]
- Salvante KG, Racke DM, Campbell CR, Sockman KW.. 2010. Plasticity in singing effort and its relationship with monoamine metabolism in the songbird telencephalon. Dev Neurobiol 70:41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana N, Mengod G, Artigas F.. 2009. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex 19:849–60. [DOI] [PubMed] [Google Scholar]
- Satou M. 1990. Synaptic organization, local neuronal circuitry, and functional segregation of the teleost olfactory bulb. Prog Neurobiol 34:115–42. [DOI] [PubMed] [Google Scholar]
- Sayigh LS, Tyack PL, Wells RS, Solow AR, Scott MD, Irvine AB.. 1999. Individual recognition in wild bottlenose dolphins: a field test using playbacks experiments. Anim Behav 57:41–50. [DOI] [PubMed] [Google Scholar]
- Scalia F. 1976. The optic pathway of the frog: nuclear organization and connections. In: Frog neurobiology. In: Llinas R, Precht W, editors. Berlin, Heidelberg: Springer. p. 386–406 ( 10.1007/978-3-642-66316-1_11). [DOI] [Google Scholar]
- Scalia F, Halpern M, Knapp H, Riss W.. 1968. The efferent connexions of the olfactory bulb in the frog: a study of degenerating unmyelinated fibres. J Anat 103:245–62. [PMC free article] [PubMed] [Google Scholar]
- Scheibner T, Törk I.. 1987. Ventromedial mesencephalic tegmental (VMT) projections to ten functionally different cortical areas in the cat: topography and quantitative analysis. J Comp Neurol 259:247–65. [DOI] [PubMed] [Google Scholar]
- Schicknick H, Reichenbach N, Smalla KH, Scheich H, Gundelfinger ED, Tischmeyer W.. 2012. Dopamine modulates memory consolidation of discrimination learning in the auditory cortex. Eur J Neurosci 35:763–74. [DOI] [PubMed] [Google Scholar]
- Schicknick H, Schott BH, Budinger E, Smalla KH, Riedel A, Seidenbecher CI, Scheich H, Gundelfinger ED, Tischmeyer W.. 2008. Dopaminergic modulation of auditory cortex-dependent memory consolidation through mTOR. Cereb Cortex 18:2646–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel R, Metzger M, Jiang S, Hemmings HC, Greengard P, Braun K.. 1997. Localization of dopamine D1 receptors and dopaminoceptive neurons in the chick forebrain. J Comp Neurol 388:146–68. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR.. 1997. A neural substrate of prediction and reward. Science 275:1593–9. [DOI] [PubMed] [Google Scholar]
- Seitz AR. 2017. Perceptual learning. Curr Biol 27:R631–6. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Kim D, Watanabe T.. 2009. Rewards evoke learning of unconsciously processed visual stimuli in adult humans. Neuron 61:700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Horwitz B, Jarvis ED.. 2012. Dopamine regulation of human speech and bird song: a critical review. Brain Lang 122:142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets WJAJ. 1988. Distribution of dopamine immunoreactivity in the forebrain and midbrain of the snake Python regius: a study with antibodies against dopamine. J Comp Neurol 271:115–29. [DOI] [PubMed] [Google Scholar]
- Smeets WJAJ, Hoogland PV, Voorn P.. 1986. The distribution of dopamine immunoreactivity in the forebrain and midbrain of the lizard Gekko gecko: an immunohistochemical study with antibodies against dopamine. J Comp Neurol 253:46–60. [DOI] [PubMed] [Google Scholar]
- Smeets WJAJ, Lopez JM, González A.. 2001. Immunohistochemical localization of DARPP-32 in the brain of the lizard, Gekko gecko: co-occurrence with tyrosine hydroxylase. J Comp Neurol 435:194–210. [DOI] [PubMed] [Google Scholar]
- Smith RL, Baker H, Kolstad K, Spencer DD, Greer CA.. 1991. Localization of tyrosine hydroxylase and olfactory marker protein immunoreactivities in the human and macaque olfactory bulb. Brain Res 548:140–8. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Nordeen EJ, Nordeen KW.. 1990. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol 53:51–63. [DOI] [PubMed] [Google Scholar]
- Stark H, Scheich H.. 1997. Dopaminergic and serotonergic neurotransmission systems are differentially involved in auditory cortex learning: a long-term microdialysis study of metabolites. J Neurochem 68:691–7. [DOI] [PubMed] [Google Scholar]
- Striedter GF. 1992. Phylogenetic changes in the connections of the lateral preglomerular nucleus in ostariophysan teleosts: a pluralistic view of brain evolution. Brain Behav Evol 39:329–57. [DOI] [PubMed] [Google Scholar]
- Sugamori KS, Demchyshyn LL, Chung M, Niznik HB.. 1994. D(1A), D(1B), and D(1C) dopamine receptors from Xenopus laevis. Proc Natl Acad Sci USA 91:10536–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Cragg SJ, Rice ME.. 2016. Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia 6:123–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Sun F, Li Y, Mooney R.. 2018. A mesocortical dopamine circuit enables the cultural transmission of vocal behaviour. Nature 563:117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taufique SKT, Kumar V.. 2016. Differential activation and tyrosine hydroxylase distribution in the hippocampal, pallial and midbrain brain regions in response to cognitive performance in Indian house crows exposed to abrupt light environment. Behav Brain Res 314:21–9. [DOI] [PubMed] [Google Scholar]
- Thiele A, Bellgrove MA.. 2018. Review neuromodulation of attention. Neuron 97:769–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry AM, Blanc G, Sobel A, Stinus L, Glowinski J.. 1973. Dopaminergic terminals in the rat cortex. Science 182:499–501. [DOI] [PubMed] [Google Scholar]
- Törk I, Turner S.. 1981. Histochemical evidence for a catecholaminergic (presumably dopaminergic) projection from the ventral mesencephalic tegmentum to visual cortex in the cat. Neurosci Lett 24:215–9. [DOI] [PubMed] [Google Scholar]
- Tosches MA, Yamawaki TM, Naumann RK, Jacobi AA, Tushev G, Laurent G.. 2018. Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science 360:881–8. [DOI] [PubMed] [Google Scholar]
- Tozzi A, de Iure A, Tantucci M, Durante V, Quiroga-Varela A, Giampà C, Di Mauro M, Mazzocchetti P, Costa C, Di Filippo M, et al. 2015. Endogenous 17b-estradiol is required for activity-dependent long-term potentiation in the striatum: interaction with the dopaminergic system. Front Cell Neurosci 9:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher C, Pellegrini E, Anglade I, Ferriére F, Saligaut C, Kah O.. 2003. Distribution of dopamine D2 receptor mRNAs in the brain and the pituitary of female rainbow trout: an in situ hybridization study. J Comp Neurol 458:32–45. [DOI] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F.. 1996. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches (Taenopygia guttata). J Comp Neurol 366:613–42. [DOI] [PubMed] [Google Scholar]
- Verlinden H. 2018. Dopamine signalling in locusts and other insects. Insect Biochem Mol Biol 97:40–52. [DOI] [PubMed] [Google Scholar]
- Vitrac C, Benoit-Marand M.. 2017. Monoaminergic modulation of motor cortex function. Front Neural Circuit 11:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D, Wong C, Gatley SJ, Gifford AN, Ding YS, et al. 2002. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse 44:175–80. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Tomasi D, Convit A, Logan J, Wong CT, Shumay E, Fowler JS, Volkow ND.. 2014. BMI modulates calorie-dependent dopamine changes in accumbens from glucose intake. PLoS ONE 9:e101585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M, Zhu W, Wong CT, Pappas NR, Geliebter A, et al. 2004. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage 21:1790–7. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Maier U, Bischof HJ.. 2008. Pattern discrimination is affected by entopallial but not by hippocampal lesions in zebra finches. Behav Brain Res 190:201–5. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Mayer U, Bischof HJ.. 2011. Visual Wulst analyses “where” and entopallium analyses “what” in the zebra finch visual system. Behav Brain Res 222:51–6. [DOI] [PubMed] [Google Scholar]
- Weele CM, Vander Siciliano CA, Tye KM.. 2019. Dopamine tunes prefrontal outputs to orchestrate aversive processing. Brain Res 1713:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcoxon HC, Dragoin WB, Kral PA.. 1971. Illness-induced aversions in rat and quail: relative salience of visual and gustatory cues. Science 171:826–8. [DOI] [PubMed] [Google Scholar]
- Wild JM. 1987. The avian somatosensory system: connections of regions of body representation in the forebrain of the pigeon. Brain Res 412:205–23. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM.. 2011. Cortical processing of odor objects. Neuron 72:506–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SC. 2019. Dopaminergic regulation of vocal-motor plasticity and performance. Curr Opin Neurobiol 54:127–33. [DOI] [PubMed] [Google Scholar]
- Wynne B, Güntürkün O.. 1995. Dopaminergic innervation of the telencephalon of the pigeon (Columba livia)—A study with antibodies against tyrosine-hydroxylase and dopamine. J Comp Neurol 357:446–64. [DOI] [PubMed] [Google Scholar]
- Xiao L, Chattree G, Oscos FG, Cao M, Wanat MJ, Roberts TF.. 2018. A basal ganglia circuit sufficient to guide birdsong learning. Neuron 98:208–21.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Jackson LR, Becker JB.. 2003. The effect of estradiol in the striatum is blocked by ICI 182,780 but not tamoxifen: pharmacological and behavioral evidence. Neuroendocrinology 77:239–45. [DOI] [PubMed] [Google Scholar]
- Xing B, Li Y, Gao W.. 2016. Norepinephrine versus dopamine and their interaction in modulating synaptic function in the prefrontal cortex. Brain Res 1641:217–33 ( 10.1016/j.brainres.2016.01.005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu TX, Yao WD.. 2010. D1 and D2 dopamine receptors in separate circuits cooperate to drive associative long-term potentiation in the prefrontal cortex. Proc Natl Acad Sci USA 107:16366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yague JG, Muñoz A, De Monasterio-Schrader P, DeFelipe J, Garcia-Segura LM, Azcoitia I.. 2006. Aromatase expression in the human temporal cortex. Neuroscience 138:389–401. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Bloch S.. 2017. Overview of brain evolution: lobe-finned fish vs. Ray-finned fish. In: Watanabe S, Hofman MA, Shimizu T, editors. Evolution of the brain, cognition, and emotion in vertebrates. Tokyo, Japan: Springer. p. 3–33 ( 10.1007/978-4-431-56559-8_1). [DOI] [Google Scholar]
- Yamamoto K, Vernier P.. 2011. The evolution of dopamine systems in chordates. Front Neuroanat 5:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousif N, Fu RZ, Abou-El-Ela Bourquin B, Bhrugubanda V, Schultz SR, Seemungal BM.. 2016. Dopamine activation preserves visual motion perception despite noise interference of human V5/MT. J Neurosci 36:9303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaldivar D, Goense J, Lowe SC, Logothetis NK, Panzeri S.. 2018. Dopamine is signaled by mid-frequency oscillations and boosts output layers visual information in visual cortex. Curr Biol 28:224–35.e5. [DOI] [PubMed] [Google Scholar]
- Zaldivar D, Rauch A, Whittingstall K, Logothetis NK, Goense J.. 2014. Dopamine-induced dissociation of BOLD and neural activity in macaque visual cortex. Curr Biol 24:2805–11. [DOI] [PubMed] [Google Scholar]
- Zenko M, Zhu Y, Dremencov E, Ren W, Xu L, Zhang X.. 2011. Requirement for the endocannabinoid system in social interaction impairment induced by coactivation of dopamine D1 and D2 receptors in the piriform cortex. J Neurosci Res 89:1245–58. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liu Q, Wen P, Zhang J, Rao X, Zhou ZZ, Zhang H, He X, Li J, Zhou ZZ, et al. 2017. Activation of the dopaminergic pathway from VTA to the medial olfactory tubercle generates odor-preference and reward. Elife 6:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Bruns D, Baudewig J, Paulus W.. 1997. Changes in human motor cortex excitability induced by dopaminergic and anti-dopaminergic drugs. Electroencephalogr Clin Neurophysiol 105:430–37. [DOI] [PubMed] [Google Scholar]