Abstract
Background
The dissemination of MBLs compromises effective use of many β-lactams in the treatment of patients with life-threatening bacterial infections. Predicted global increases in the prevalence of MBL-producing carbapenem-resistant Enterobacterales (CRE) are being realized, yielding infections that are untreatable with existing therapies including newly approved β-lactam/β-lactamase inhibitor combinations. Developing MBL inhibitors (MBLIs) now is essential to address the growing threat that MBL-producing CRE pose to patients.
Methods
A novel MBLI series was assessed by susceptibility testing and time–kill assays. Target activity and selectivity was evaluated using bacterial NDM, VIM and IMP enzyme assays and human matrix metallopeptidase enzyme assays, respectively, and cytotoxicity was assessed in HepG2 cells. In vivo efficacy of meropenem/MBLI combinations was evaluated in a mouse thigh infection model using an NDM-1-producing Escherichia coli strain.
Results
Combination of MBLIs with carbapenems reduced MICs for NDM/IMP/VIM-producing Enterobacterales by up to 128-fold compared with the carbapenems alone. Supplementation of meropenem with the promising compound 272 reduced the MIC90 from 128 to 0.25 mg/L in a panel of MBL-producing CRE clinical isolates (n = 115). Compound 272 restored the bactericidal activity of meropenem and was non-cytotoxic, potentiating the antimicrobial action of meropenem through specific inhibition of NDM, IMP and VIM. In vivo efficacy was achieved in a mouse thigh infection model with meropenem/272 dosed subcutaneously.
Conclusions
We have developed a series of rationally designed MBLIs that restore activity of carbapenems against NDM/IMP/VIM-producing Enterobacterales. This series warrants further development towards a novel combination therapy that combats antibiotic-resistant organisms, which pose a critical threat to human health.
Introduction
Each year, throughout Europe, over 670 000 people contract infections caused by antibiotic-resistant bacteria, resulting in ∼33 000 deaths.1 In healthcare settings, MDR Gram-negative pathogens cause diverse infections and are associated with worse patient outcomes and prolonged hospital stays, compared with susceptible bacteria. There are limited effective treatment options for these organisms and empirical antibiotic therapy often fails in patients infected with Gram-negative ESKAPE pathogens (Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species).2
Carbapenem-resistant Enterobacterales (CRE) represent a significant challenge; in the USA alone, 13 100 hospitalizations can be attributed to CRE annually.3 Marketed β-lactam/β-lactamase inhibitor combinations such as ceftazidime/avibactam (approved in 2015) and meropenem/vaborbactam (approved in 2017) successfully restore activity of carbapenems in organisms that exhibit resistance due to the expression of serine β-lactamases (SBLs) (Ambler Class A, C and D), such as KPC and some OXA-type enzymes.4,5 However, the spread of carbapenemases from the MBL class (Ambler Class B) is of increasing significance, given that these enzymes hydrolyse all clinically used β-lactams (except aztreonam) and there are currently no approved MBL inhibitors (MBLIs) that cover all major classes of MBLs (NDM, IMP and VIM). MBL-positive infection treatment options are extremely limited as MBL-producing organisms are frequently MDR, owing to co-carriage of other resistance determinants on transferable genetic elements.6
From identification of the first patient carrying NDM-1 in 2007 to date, there has been an exponential increase in the number of carbapenem-resistant bacterial infections attributed to the presence of this MBL.7 The SENTRY Program covering 42 countries across the globe reported an increase in the frequency of MBL-carrying CRE from 4.3% of isolates during 2007–09 to 12.7% during 2014–16. These organisms are now endemic in parts of Asia and Europe and approaching endemicity in other geographies. NDM-type MBLs were found to be most prevalent, accounting for 10.3% of CRE, whilst VIM-type and IMP-type enzymes were found in 1.9% and 0.4%, respectively.8 Similarly, the Europe-wide EuSCAPE project found that infections caused by NDM-1-carrying carbapenem-non-susceptible K. pneumoniae and Escherichia coli comprised 7.7% and 10.3%, respectively, of all carbapenemase-producing Enterobacterales (CPE). This covered 36 countries over a 6 month period between 2013 and 2014.9 Increasingly, outbreaks of MBL-producing Enterobacterales are reported on both local and countrywide levels.10–12 Not only is the frequency of carbapenem resistance of concern but, more importantly, so is the resulting clinical outcome. In the UK, bloodstream infections caused by carbapenem-resistant K. pneumoniae result in higher mortality rates (an increase of 17%) in comparison with carbapenem-susceptible infections.13 Therefore, it is imperative that carbapenem/MBLI combinations are developed to provide a viable treatment option for MBL-positive infections.
Here we describe the in vitro and in vivo microbiological profiles of lead compounds from a series of novel and rationally designed MBLIs that can neutralize the threat of MBL-producing CRE.
Materials and methods
Ethics
Murine in vivo studies (Home Office licence numbers P651A96A4 and PA67E0BAA) were conducted under licence from the UK Animals (Scientific Procedures) Act 1986 and the mice were cared for under national guidance. This Act (UK Home Office) regulates all scientific procedures in living animals and conforms to the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (Strasbourg, Council of Europe).
Antibacterial agents and reagents
Fragments were discovered by Medivir (Sweden) using structure-guided drug design targeting NDM, VIM and IMP enzymes. Fragments were then optimized at Infex Therapeutics to improve potency and drug-like properties using structure–activity relationships alongside in silico modelling, yielding lead compounds 272, 276, 364 and 439 (Figure 1). These compounds were synthesized and purified at Infex Therapeutics as described in international patent WO/2019/220125.14
Figure 1.
Chemical structures of MBLIs.
Antibiotics and chemicals were purchased from Sigma–Aldrich (Poole, UK) with the exception of meropenem (Tokyo Chemical Industry, Tokyo, Japan), imipenem (Carbosynth, Newbury, UK) and nitrocefin (Abcam, Cambridge, UK).
Bacterial strains
A panel of control organisms and MBL-producing clinical isolates were purchased from ATCC (via LGC Standards, Middlesex, UK) and PHE’s National Collection of Type Cultures (NCTC; Salisbury, UK) or were a gift from the Antibacterial Resistance Leadership Group (NC, USA) or Liverpool Clinical Laboratories (Liverpool, UK). Bacteria were cultured in CAMHB or on Mueller–Hinton agar (MHA) at 37°C (Oxoid, Basingstoke, UK).
For organisms with publicly available whole-genome sequences, the Resistance Gene Identifier (RGI version 5) function of the Comprehensive Antibiotic Resistance Database was used to identify putative resistance genes.15 Presence and expression of MBLs was confirmed by routine PCR using GoTaq® Green Master Mix (Promega, WI, USA) and the modified carbapenem inactivation method (mCIM) in combination with the EDTA mCIM (eCIM) performed according to CLSI guidelines.16 The genetic background and source of organisms used in this study are reported in Table S3, available as Supplementary data at JAC Online.
Antibacterial activity
MICs were determined by broth microdilution according to CLSI guidelines.17 Combination MICs were performed as described for MIC determinations with the addition of 4 mg/L MBLI compounds to CAMHB. MIC90 determinations were performed by IHMA Europe Sàrl (Epalinges, Switzerland) using a panel of 115 strains of NDM-, IMP- and VIM-producing Enterobacterales, using the same methodology. Serum MICs were determined in 20% or 50% (v/v) human serum in CAMHB (BioIVT, Burgess Hill, UK).
Time–kill experiments were performed according to CLSI guidelines using exponential-phase E. coli ATCC BAA-2452.18 Cultures were exposed to 4× meropenem MIC (4 mg/L) or 4× meropenem combination MIC (0.12 mg/L) supplemented with 4 mg/L MBLI compound for 24 h.
Enzyme inhibition studies
Investigational compounds were tested in an MBL inhibition assay to investigate on-target potency. Details of constructs and expression and purification of MBLs can be found in Supplementary methodology 1. Enzyme assays were performed at 37°C in buffer at pH 7.5 (50 mM HEPES, 150 mM NaCl, 0.1 mM ZnSO4, 20 mg/L PEG4000) and containing 1.5 nM NDM-1, 0.4 nM IMP-1 or 30 nM VIM-2, 100 μM nitrocefin and a range of concentrations of investigational compound. Absorbance at 490 nm was measured every minute for 30 min. IC50 values were determined using GraphPad Prism 7 (GraphPad, CA, USA).
Human matrix metallopeptidase (MMP) inhibition assays were performed by Eurofins (Luxembourg). Inhibition of enzymes (MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-12, MMP-13, MMP-14) by investigational compounds (100 μM) was determined fluorometrically and expressed as a percentage.
Cytotoxicity testing
Cytotoxicity of MBLI compounds was evaluated in human HepG2 (ATCC® HB-8065™) cells seeded at a density of 2 × 104 cells/well and incubated for 24 h at 37°C and 5% CO2. Cells were exposed to serial dilutions of compounds for 24 h before determination of viability using CellTiter-Glo® (Promega, WI, USA) according to the manufacturer’s instructions. CC50 values were determined using GraphPad Prism 7.
Pharmacokinetic (PK) profiling
PK studies were performed by Pharmidex (London, UK). Meropenem and 272 [in a 90% composition (v/v) of 25% (w/v) hydroxypropyl-β-cyclodextrin and 10% (v/v) DMSO] were administered in a cassette to male CD-1 mice at 40 mg/kg and 100 mg/kg, respectively, by subcutaneous injection. Terminal biological fluid and tissue samples were collected at 0.083, 0.25, 0.5, 1, 2, 4 and 8 h post-dose (three animals per timepoint); signs of ill health or overt toxicity were monitored visually. Blood samples were transferred to K2 EDTA tubes and were centrifuged at 10 000 rpm for 5 min. Plasma was collected and stored at −20°C until analysis. Mouse thighs were excised and homogenized in water. Both plasma and homogenized thigh were prepared for analysis by protein precipitation using acetonitrile. Calibration samples were prepared by spiking meropenem and 272 into the control matrix at appropriate concentration ranges, and these were extracted and analysed in the same way as the samples. Quantitative bioanalysis was performed by UHPLC–MS/MS using electrospray ionization. The UHPLC–MS/MS system comprised a Shimadzu Nexera X2 UHPLC and a Shimadzu LCMS 8060 (Shimadzu, Milton Keynes, UK), equipped with an Acquity BEH C18, 50 mm × 2.1 mm, 1.7 μm column (Waters, Elstree, UK). Mobile components A and B were 95:5 water:acetonitrile and acetonitrile, each containing 0.1% formic acid. The gradient programme was: 0–0.3 min, 2% B; 0.3–1.1 min, linear increase to 95% B; 1.1–1.75 min, 95% B; 1.75–1.8 min, linear decrease to 2% B; 1.8–2.5 min, 2% B. Flow rate was 0.4 mL/min. The first 1 min was diverted to waste and the injection volume was 2 μL. Meropenem and 272 were both detected in positive ion mode with multiple reaction monitoring (MRM) transition and retention time of 384.15 > 141.20 and 1.31 min for meropenem, and 283.05 > 186.10 and 1.31 min for compound 272. PK parameters were determined using Phoenix WinNonlin version 8.0 (Certara USA, NJ, USA).
In vivo efficacy
An in vivo efficacy study was performed by Evotec (Hamburg, Germany) in a 9 h neutropenic male CD-1 mouse thigh infection model with the NDM-1-producing strain E. coli IR3. Mice were injected intramuscularly with 1.95 × 106 cfu/thigh E. coli IR3 into both lateral muscles (five animals per group). Meropenem was administered to mice subcutaneously, to mimic IV infusion, every 2 h at 50 or 250 mg/kg (starting at 1 h post-infection) in the presence or absence of 272 at 100 mg/kg. A vehicle control [90% composition (v/v) of 25% (w/v) hydroxypropyl-β-cyclodextrin and 10% (v/v) DMSO] was also dosed as described above. Tigecycline was administered subcutaneously at 60 mg/kg every 4 h, starting 1 h post-infection, as an efficacious control. Clinical condition was assessed at 1 and 9 h post-infection. Following termination, mice thigh muscles were weighed and homogenized in PBS. Bacteria were cultured from tissue homogenates and quantified following incubation at 37°C for up to 48 h. Pairwise comparisons between groups were performed using non-parametric statistical models (Kruskal–Wallis test corrected for multiple comparisons post-test using the Conover–Iman method) with StatsDirect software version 3.2.7.
Further details regarding animal handling can be found in Supplementary methodology 2.
Results
The antimicrobial activities of carbapenem/MBLI combinations were assessed using a panel of confirmed MBL-producing Enterobacterales and notable MBLIs from a series of compounds described in patent WO/2019/220125.14 All compounds lacked innate antibacterial activity, with MICs greater than the maximum tested concentration of 128 mg/L (Table 1). The most active compounds reduced the MIC of meropenem by up to 128-fold in MBL-producing Enterobacterales (Table 1). Meropenem/MBLI combinations were highly efficacious in organisms carrying either NDM-, IMP- or VIM-type MBLs, resulting in MICs as low as 0.03–0.06 mg/L in strains producing any of the three MBL types. Supplementation of meropenem with the most potent MBLI, 272, resulted in a 32-fold reduction in meropenem MIC for the MDR strain K. pneumoniae NCTC 13443, indicating that the compound retains the ability to enhance susceptibility to meropenem in highly resistant organisms.
Table 1.
Susceptibility of MBL-producing Enterobacterales to meropenem in combination with investigational MBLIs
| Compound(s) | MIC (mg/L) |
||||||
|---|---|---|---|---|---|---|---|
| E. coli ATCC BAA-2452 (NDM-1) | E. coli NCTC 13476 (IMP) | K. pneumoniae ATCC BAA-2146 (NDM-1) | K. pneumoniae NCTC 13440 (VIM-1) | K. pneumoniae NCTC 13443 (NDM-1) | K. pneumoniae NCTC 13439 (VIM-1) | E. coli ATCC BAA-2469 (NDM-1) | |
| 272 | >128 | >128 | >128 | >128 | >128 | >128 | >128 |
| 276 | >128 | >128 | >128 | >128 | >128 | >128 | >128 |
| 364 | >128 | >128 | >128 | >128 | >128 | >128 | >128 |
| 439 | >128 | >128 | >128 | >128 | >128 | >128 | >128 |
| MEM | 1 | 4 | 32 | 1 | 128 | 0.5 | 4 |
| MEM/272 | 0.03 (32) | 0.03 (128) | 0.25 (128) | 0.06 (16) | 4 (32) | 0.06 (8) | 0.06 (64) |
| MEM/276 | 0.06 (16) | 0.03 (128) | 0.5 (64) | 0.06 (16) | 16 (8) | 0.06 (8) | 0.06 (64) |
| MEM/364 | 0.06 (16) | 0.03 (128) | 2 (16) | 0.06 (16) | 8 (16) | 0.12 (4) | 0.12 (32) |
| MEM/439 | 0.06 (16) | 0.06 (64) | 2 (16) | 0.25 (4) | 16 (8) | 0.25 (2) | 0.06 (64) |
| IPM | 8 | 4 | 16 | 8 | 32 | 4 | 16 |
| IPM/272 | 1 (8) | 0.5 (8) | 1 (16) | 1 (8) | nd | nd | nd |
| IPM/276 | 0.5 (16) | 0.25 (16) | 1 (16) | 1 (8) | nd | nd | nd |
MICs of meropenem determined in the presence or absence of MBLI compounds (4 mg/L); type of MBL noted beneath strain name. Fold reduction in meropenem MIC in the presence of an MBLI is noted in brackets. MICs of carbapenems alone are shown in bold. Values were determined from at least two independent replicates. MEM, meropenem; IPM, imipenem; nd, not determined.
To explore the possibility of combination with an alternative carbapenem, two compounds were tested in combination with imipenem; doripenem was not explored as a potential companion, as this is not available for clinical use in Europe. Supplementation of media with 4 mg/L 272 or 276 generally resulted in less favourable potentiation of imipenem activity, in comparison with meropenem (Table 1).
Investigational compounds were exposed to human serum during MIC determinations to identify any serum-binding liabilities. All test compounds demonstrated a maximum of 2-fold change in meropenem MIC (E. coli ATCC BAA-2452) upon supplementation with 20% or 50% serum. This suggests that the series of compounds is not highly plasma protein bound in vivo and is consistent with literature documenting low plasma protein binding by meropenem.19
To ensure that MBLIs were effective in diverse Enterobacterales, MBLI/meropenem combinations were evaluated using panels of clinical CPE isolates with the most potent MBLIs, 272 and 276. An initial panel (n = 36) of CPE isolates was used to select which MBLI would be evaluated in a larger panel of international CPE isolates (n = 115). Using the smaller panel, meropenem MICs ranged from 0.03 to 0.25 mg/L and from 0.03 to 2 mg/L with the addition of 272 and 276 at 4 mg/L, respectively. The MIC90 of meropenem against all in-house MBL-producing strains (n = 36) was 8 mg/L and supplementation with 272 or 276 at 4 mg/L reduced the MIC90 of meropenem to 0.12 mg/L. The combination of meropenem/272 was selected for evaluation in the larger panel of isolates. The MIC90 of meropenem was reduced 512-fold from 128 mg/L to 0.25 mg/L upon addition of 272 at 4 mg/L (Figure 2; detailed data in Table S1), supporting the findings from the initial strain panel. Compound 272 improved activity of meropenem for a broad range of species and was superior to aztreonam supplemented with avibactam (4 mg/L), for which the MIC90 was 1 mg/L. At the species level, supplementation of meropenem with 272 reduced the MIC90 of Enterobacter cloacae (n = 19), E. coli (n = 41) and K. pneumoniae (n = 29) from 64, 128 and 128 mg/L to 0.25, 0.12 and 1 mg/L, respectively. Similar potentiation was observed in other Enterobacterales (Citrobacter freundii, Klebsiella aerogenes, Klebsiella oxytoca, Morganella morganii, Proteus mirabilis, Providencia rettgeri, Providencia stuartii, Raoultella ornithinolytica and Serratia marcescens).
Figure 2.
Cumulative MIC of meropenem with and without the addition of 272 (4 mg/L) against NDM-, VIM- and IMP-producing clinical Enterobacterales (n = 115). Susceptibility testing was performed by IHMA Europe Sàrl. MEM/272, combination of meropenem and 272.
The kill kinetics of meropenem in the presence and absence of investigational compounds were determined through time–kill assays. At 4× meropenem MIC (4 mg/L), the antibiotic was rapidly bactericidal, defined as ≥3 log10 reduction in cfu/mL. The time of kill (ToK), the time taken to reach this 3 log10 reduction, was 1.2 h (Figure 3). At low concentrations of meropenem, equating to 4 × the meropenem/MBLI combination MIC (0.12 mg/L), bacterial growth proceeded at the same rate and to the same extent as the untreated control (ToK >24 h). The addition of 272 or 276 to cultures treated with this subinhibitory concentration of meropenem restored the antibacterial activity of the antibiotic to the extent seen with supra-inhibitory concentrations of meropenem. Supplementation with 272 and 276 resulted in rapid and extensive bactericidal activity (ToK 1.7 h and 1.2 h, respectively), with no viable cells being recovered following a 6 h exposure to the meropenem/272 combination. Compounds are considered to act synergistically with meropenem, as the extent of kill was in excess of a 2 log10 reduction in cfu in comparison with the same concentration of meropenem alone.
Figure 3.
Kill kinetics of compounds against E. coli ATCC BAA-2452. Meropenem concentration (mg/L) is noted in brackets. NDC, no drug control; MEM, meropenem; MEM/272 and MEM/276, combinations of meropenem with 272 and 276, respectively. (a) meropenem with and without the addition of 272 at 4 mg/L; (b) meropenem with and without the addition of 276 at 4 mg/L. Results are means of at least three independent replicates and error bars show SDs.
To confirm target inhibition and zinc-dependent enzyme selectivity, inhibitory activity was evaluated against bacterial MBL enzymes and human MMPs. Compounds demonstrated potent and specific inhibition of NDM-1, IMP-1 and VIM-2 (Table 2). Activity against the most prevalent MBL, NDM-1, was in the nanomolar to micromolar range for all tested compounds, with similar potency against IMP-1. Activity versus VIM-2 was generally lower than that identified against NDM-1 and IMP-1; however, this did not correlate with whole-cell susceptibility testing, where combination MICs were similar for all three enzyme families. Compound 276 had the lowest IC50 against NDM-1, but MICs against NDM-1-containing strains were higher than or showed no improvement over other compounds that were less potent inhibitors of the enzyme (Table 1 and Table 2). Similarly, 272 had a relatively high IC50 value against VIM-2 but was highly efficacious against organisms whose resistance to carbapenems was due to the presence of VIM. To ensure that the broad enzyme coverage of compounds is not due to promiscuous inhibition of zinc-dependent metalloenzymes, a panel of human MMPs were exposed to compounds 272 and 276 at 100 μM (Table S2). Neither compound demonstrated any significant inhibition of human MMPs (<25% inhibition), indicating good selectivity for bacterial MBLs.
Table 2.
Enzyme inhibition studies of MBLIs versus NDM-1, IMP-1 and VIM-2
| Compound | IC50 (μM) |
||
|---|---|---|---|
| NDM-1 | IMP-1 | VIM-2 | |
| 272 | 0.83 | 0.43 | 32.37 |
| 276 | 0.15 | 0.04 | 67.27 |
| 364 | 2.69 | 1.87 | 43.28 |
| 439 | 0.44 | 0.56 | 50.98 |
Results are means of at least two independent replicates.
To identify toxic effects in mammalian cells, the human HepG2 cell line was exposed to test compounds over a 24 h period. All compounds from the series were found to be non-cytotoxic, with CC50 values of >256 mg/L (the maximum tested concentration). This indicates that the compounds specifically target a component of bacterial cells and have no measurable cytotoxic effect on mammalian cells.
Following these promising in vitro microbiological and safety data, 272 was selected for further profiling in the form of in vivo PK evaluation and assessment of in vivo efficacy in a mouse thigh infection model.
Cassette subcutaneous dosing of 272 (100 mg/kg) and meropenem (40 mg/kg) indicated that 272 has good coverage over meropenem. Compound 272 had a half-life of 1 h and 1.2 h in plasma and muscle, respectively (Table 3); plasma concentration remained above 4 mg/L, the concentration used during in vitro microbiological studies, for 2.7 h. Peak plasma and tissue concentrations were achieved 0.3 h and 0.5 h post-dose for both compounds, respectively, indicating that the dosing regimen used for meropenem would also be suitable for 272 in a murine efficacy study.
Table 3.
PK properties of meropenem (40 mg/kg) or 272 (100 mg/kg) in CD-1 mice dosed subcutaneously
| PK parameter | Plasma |
Thigh tissue |
||
|---|---|---|---|---|
| Meropenem | 272 | Meropenem | 272 | |
| t ½ (h) | 0.3 | 1.0 | 0.5 | 1.2 |
| T max (h) | 0.3 | 0.3 | 0.5 | 0.5 |
| C max (ng/mL) | 25 509 | 88 213 | 1759 | 8207 |
| AUC0–∞ (ng·h/mL) | 16 281 | 79 906 | 1512 | 9945 |
Parameters determined from plasma or thigh tissue from three animals.
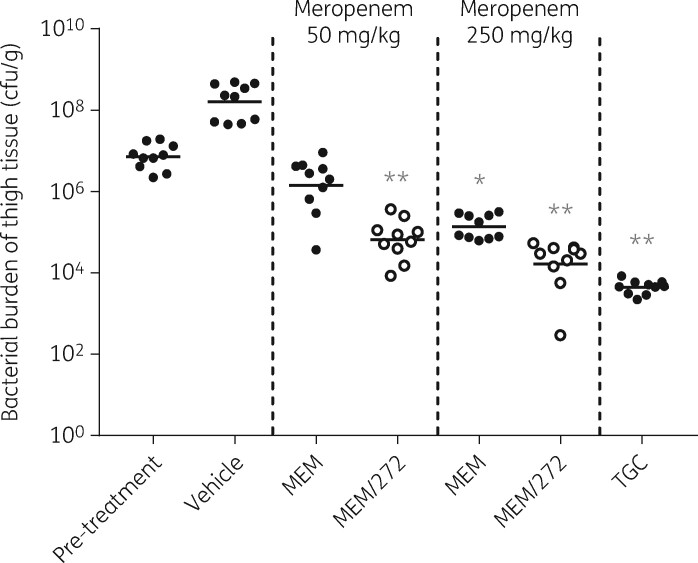
Prior to initiation of an efficacy study, extensive optimization was required to develop a responsive model due to the disconnect between in vitro and in vivo meropenem resistance previously reported in animal models of infection for MBL-producing strains.20 Upon successful development of a 9 h mouse thigh infection model, 272 and meropenem were dosed simultaneously in mice infected with the NDM-1-containing strain E. coli IR3. This strain displayed a meropenem MIC of 16 mg/L and the addition of 272 at 4 mg/L resulted in a 256-fold reduction in meropenem MIC (0.06 mg/L). Infection with E. coli IR3 was established in murine thighs, reaching an average burden of 1.59 × 108 cfu/g of tissue after 9 h in the vehicle-treated group (Figure 4). All treatments significantly reduced bacterial burden in comparison with the vehicle-treated group. Meropenem reduced bacterial burden in a dose-dependent manner; 50 and 250 mg/kg meropenem resulted in 0.70 and 1.72 log10 reduction in cfu/g, respectively, in comparison with the pre-treatment group. The addition of 272 at 100 mg/kg significantly enhanced meropenem efficacy at both meropenem doses (Kruskal–Wallis test, P < 0.0001). When administered in combination with 50 mg/kg of meropenem, 272 reduced bacterial burden by an additional 1.34 log10 cfu/g in comparison with antibiotic alone. Likewise, when administered with 250 mg/kg of meropenem, bacterial burden was further reduced by 0.92 log10 cfu/g in comparison with meropenem alone. Tigecycline, used as an efficacious comparator, reduced bacterial burden by 3.21 log10 cfu/g in comparison with the pre-treatment group. The meropenem/272 combination therapy was well tolerated and was not associated with any acute adverse effects. Consequently, there are no safety concerns associated with this dual therapy under conditions in which in vivo efficacy has been demonstrated.
Figure 4.
Efficacy of meropenem in combination with 272 in a 9 h mouse thigh infection model with E. coli IR3. Meropenem was administered subcutaneously every 2 h from 1 h post-infection at 50 or 250 mg/kg in the presence or absence of 100 mg/kg 272. A drug-free vehicle control was also administered at the same dosing intervals. Tigecycline was dosed subcutaneously at 60 mg/kg every 4 h from 1 h post-infection; tigecycline MIC 0.5 mg/L. Horizontal bars represent the mean cfu/g in each treatment group. Statistical analyses were performed per treatment in comparison with the pre-treatment group using the Kruskal–Wallis test: *P < 0.01, **P < 0.0001. MEM, meropenem; MEM/272, combination of meropenem and 272; TGC, tigecycline.
Discussion
In an era of declining investment in antibiotic research and development and emergence of MDR bacteria, drugs that circumvent existing resistance mechanisms and restore activity to last-resort antibiotics are essential. Given the time frame to take antibiotic discovery programmes through to the clinic, we must anticipate future threats by closely following epidemiological trends. The global dissemination of new and existing MBLs should be considered as one such threat. The 2019 WHO review of the preclinical and clinical antibacterial pipeline highlights that MBL-producing CRE, in particular, remain a neglected target in antibacterial drug development, despite carrying such a significant unmet medical need.21,22
The MBLIs described in this study effectively restore susceptibility of MBL-producing Enterobacterales to carbapenems, both in vitro and in vivo, and meropenem proved to be the most suitable companion carbapenem. MBLIs demonstrated potent and specific inhibition of bacterial MBLs over human zinc-dependent MMPs. MBLIs were found to be non-toxic in vitro and well tolerated in vivo, warranting further development towards a novel combination treatment. We believe it timely that we have developed a series of broad-spectrum MBLIs that restore activity of meropenem, considered an essential medicine,23 against CRE.
Supplementary Material
Acknowledgements
We are grateful to Medivir (Huddinge, Sweden) for the provision of NDM-1, IMP-1 and VIM-2 enzymes, Liverpool Clinical Laboratories (Liverpool, UK) for the provision of bacterial strains, to IHMA Europe Sàrl (Epalinges, Switzerland) for performing MIC90 studies, to Eurofins (Luxembourg) for performing MMP inhibition assays, Pharmidex (London, UK) for performing the PK profiling and to Evotec (Hamburg, Germany) for performing the in vivo efficacy studies.
Bacterial isolates provided herein and identified as ARLG in the Supplementary data were provided by the Antibacterial Resistance Leadership Group (NC, USA).
Funding
This work was supported by Infex Therapeutics Ltd. Some of the research described in this publication is supported, at least in part, by a grant from NIH through Duke University.
Transparency declarations
All authors are or have been employees of Infex Therapeutics Ltd. Patent pertaining to this manuscript: WO/2019/220125; status: published; applicant: Infex Therapeutics Ltd; inventors: Wilkinson A, Cooper I, Orr D, Finlayson J, Bunt A, Appelqvist P, Wallberg H, Wångsell F; aspect of manuscript covered: synthesis of compound series.
Disclaimer
The findings, opinions and recommendations expressed herein are those of the authors and not necessarily those of Duke University or NIH.
Supplementary data
Tables S1 to S3 and Supplementary methods1 and 2 are available as Supplementary data at JAC Online.
References
- 1. Cassini A, Diaz Högberg L, Plachouras D. et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 2019; 19: 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis 2008; 197: 1079–81. [DOI] [PubMed] [Google Scholar]
- 3.CDC. Antibiotic Resistance Threats in the United States, 2019. CDC, 2019. [Google Scholar]
- 4. Tuon FF, Rocha JL, Formigoni-Pinto MR.. Pharmacological aspects and spectrum of action of ceftazidime–avibactam: a systematic review. Infection 2018; 46: 165–81. [DOI] [PubMed] [Google Scholar]
- 5. Wu G, Cheon E.. Meropenem-vaborbactam for the treatment of complicated urinary tract infections including acute pyelonephritis. Expert Opin Pharmacother 2018; 19: 1495–502. [DOI] [PubMed] [Google Scholar]
- 6. Pecora N, Zhao X, Nudel K. et al. Diverse vectors and mechanisms spread New Delhi metallo-β-lactamases among carbapenem-resistant Enterobacteriaceae in the Greater Boston area. Antimicrob Agents Chemother 2019; 63: e02040-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yong D, Toleman MA, Giske CG. et al. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 2009; 53: 5046–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castanheira M, Deshpande LM, Mendes RE. et al. Variations in the occurrence of resistance phenotypes and carbapenemase genes among Enterobacteriaceae isolates in 20 years of the SENTRY antimicrobial surveillance program. Open Forum Infect Dis 2019; 6: S23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grundmann H, Glasner C, Albiger B. et al. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis 2017; 17: 153–63. [DOI] [PubMed] [Google Scholar]
- 10.ECDC. Rapid Risk Assessment: Regional Outbreak of New Delhi Metallo-Betalactamase-Producing Carbapenem-Resistant Enterobacteriaceae, Italy, 2018–2019. ECDC, 2019. [Google Scholar]
- 11. Baraniak A, MacHulska M, Zabicka D. et al. Towards endemicity: large-scale expansion of the NDM-1-producing Klebsiella pneumoniae ST11 lineage in Poland, 2015–16. J Antimicrob Chemother 2019; 74: 3199–204. [DOI] [PubMed] [Google Scholar]
- 12.ECDC. Outbreak of Carbapenemase-Producing (NDM-1 and OXA-48) and Colistin-Resistant Klebsiella pneumoniae ST307, North-East Germany, 2019. ECDC, 2019. [Google Scholar]
- 13.PHE. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR). PHE, 2019.
- 14.WIPO IP Portal, Patentscope. WO2019220125 - Antibacterial compounds. 2019. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019220125.
- 15. Alcock BP, Raphenya AR, Lau TTY. et al. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res 2020; 48: D517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CLSI. Performance Standards for Antimicrobial Susceptibility Testing—Thirtieth Edition: M100. 2019.
- 17.CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Eleventh Edition: M07. 2018.
- 18.CLSI. Methods for Determining Bactericidal Activity of Antimicrobial Agents: M26-A. 1999.
- 19. Fish DN, Singletary TJ.. Meropenem, a new carbapenem antibiotic. Pharmacotherapy 1997; 17: 644–69. [PubMed] [Google Scholar]
- 20. Asempa TE, Abdelraouf K, Nicolau DP.. Metallo-β-lactamase resistance in Enterobacteriaceae is an artefact of currently utilized antimicrobial susceptibility testing methods. J Antimicrob Chemother 2020; 75: 997–1005. [DOI] [PubMed] [Google Scholar]
- 21.WHO. Antibacterial Agents in Preclinical Development: An Open Access Database. WHO, 2019. [Google Scholar]
- 22.WHO. 2019 Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline. WHO, 2019. [Google Scholar]
- 23.WHO. WHO Model List of Essential Medicines, 21st List 2019. WHO, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.