Abstract
Background
As the reliability of fecal calprotectin (Fcal) remains debatable to detect endoscopic ulcerations in patients with pure ileal Crohn’s disease (CD), we aimed to compare its performances with those observed in patients with colonic or ileocolonic location.
Methods
Using a prospectively maintained database, we analyzed 123 CD patients with Fcal measurement and ileocolonoscopy performed within 1 month with no therapeutic intervention during this interval. Receiver operating characterstic curves (ROC) were used to determine the best Fcal threshold to detect endoscopic ulcerations, taking into account the clinical relevance and usual recommended indices. Sensitivity, specificity, and positive (PPV) and negative predictive values (NPV) were presented with 95% confidence intervals.
Results
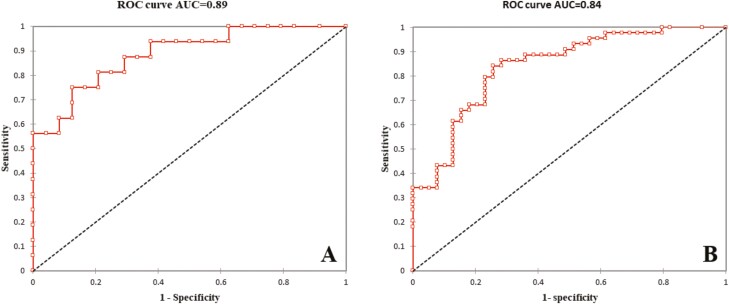
The mean Fcal level was significantly higher in patients with endoscopic ulcerations in the L1 group (P = 0.025) and the L2-L3 group (P < 0.001). Using ROC curves, Fcal >200 µg/g and Fcal >250 µg/g were the best thresholds to detect endoscopic ulcerations in the L1 group (sensitivity = 75.0, 95% CI, 47.6–92.7; specificity = 87.5, 95% CI, 67.6–97.3; PPV = 80.0, 95% CI, 51.9–95.7; and NPV = 84.0; 95% CI, 63.9–95.5) and in the L2-L3 group (sensitivity = 84.1 95% CI, 69.9–93.4; specificity = 74.4, 95% CI, 57.9–87.0; PPV = 78.7, 95% CI, 64.3–89.3, and NPV = 80.6, 95% CI, 64.0–91.8), respectively. We compared the AUC between L1 and L2-L3 groups, and no difference was shown (0.89 vs 0.84, respectively, P = 0.46). We also compared 2-by-2 sensitivity, specificity, PPV, NPV, and accuracy and we did not observe any significant difference.
Conclusion
Fecal calprotectin is highly effective to detect endoscopic ulcerations regardless of CD location but requires a lower cutoff value in patients with pure ileal involvement.
Keywords: Crohn’s disease, fecal calprotectin, mucosal healing, biomarker
INTRODUCTION
Crohn’s disease (CD) is a chronic, progressive, and disabling disorder that can lead to bowel damage and significantly alter patients’ quality of life.1, 2 In the last decade, the therapeutic target has evolved in CD from clinical remission to more objective markers of intestinal inflammation to alter the natural history of the disease and prevent the occurrence of complications. Endoscopic mucosal healing, mostly defined as the absence of endoscopic ulceration, is hitherto the most validated end point in patients with CD, as it is associated with sustained clinical remission and a lower risk of subsequent hospitalization or CD-related surgery.3–5 More recently, tight control of objectively measured gastrointestinal inflammation, the so-called “treat-to-target” strategy, is now suggested in the management of patients with inflammatory bowel diseases (IBD).6–8 Despite these advances in management goals, repeated surveillance colonoscopies are expensive and burdensome for patients,9 highlighting the need for more convenient and less invasive monitoring tools except for dysplasia surveillance. One proposed marker is fecal calprotecin (Fcal), which has been shown to have some benefit in the assessment of mucosal healing in patients with CD.10–21 However, it has been suggested that the performance of this biomarker compared with endoscopic assessment is less accurate in CD restricted to the terminal ileum.10, 12, 22 There are limitations to this assertion though, including the fact that the 2 available endoscopic scores for CD, the Crohn’s disease endoscopic activity index of severity (CDEIS)23 and the simplified score for CD (SES-CD),24 were developed to assess colonic CD and therefore often underestimate the severity of CD limited to the ileum. In addition, prior studies were not designed to specifically assess the ileal phenotype, and no statistical analysis was performed to compare the correlation coefficients or the performances between the patients with pure ileal CD and those with colonic or ileocolonic involvement,10, 12, 22 and such studies did not observe differences according to disease location.17, 25 Therefore, the aim of our study was to compare the performance of Fcal in the detection of endoscopic ulcerations in patients with pure ileal CD with patients with colonic or ileocolonic involvement.
METHODS
Ethical Consideration
This study was respectful of the Declaration of Helsinki, Good Clinical Practice, and current guidelines. The study was approved by the institutional review board of the University of Chicago (IRB17–0559).
Patients
From a prospectively maintained database (IRB protocol 15573A), we identified all patients with an established diagnosis of CD who are followed in our center and have had at least 1 Fcal measurement. Among them, we enrolled all the CD patients with ileocolonoscopy performed within 1 month of Fcal measurement with no therapeutic intervention during this interval. Patients with unclassified inflammatory bowel disease (IBD-U) or who took NSAIDs or aspirin within the 4 weeks before the measurement of calprotectin were not included. Regarding the patients with prior history of proximal small bowel location, we included only those who underwent an examination (magnetic resonance enterography, computed-tomography enterography, or wireless capsule endoscopy) within the 3 months before the inclusion and excluded any with proximal small bowel active lesions. Clinical characteristics including Montreal classification,26 evaluation by the Harvey-Bradshaw index (HBI), and current medications were reviewed. The patients were divided in 2 groups according to Montreal classification: L1 group (patients with pure ileal CD) and L2-L3 group (patients with isolated colonic or ileocolonic involvement).
Endoscopy
Ileocolonoscopy was performed using conscious sedation for all the patients. The endoscopic reports including photographs were retrospectively reviewed by an IBD physician (AB) to assess the presence of endoscopic ulcerations (superficial or deep ulcerations) or the presence of endoscopic lesions (erythema, edema, or aphthoid erosions).27 Endoscopic evaluation was performed blinded from the results of Fcal measurement.
Fecal Calprotectin Measurement
The level of Fcal was assessed in the same way as in our routine practice, using quantitative enzyme-linked immunosorbent assay (ELISA). Technicians, who were blinded from the current clinical and endoscopic data, performed the analyses. The limits of detection for calprotectin using this assay ranged from 15.6 to 2500 µg/g. Consequently, all values below 15.6 or above 2500 µg/g were considered as equal to 15.6 and 2500 µg/g, respectively. The values of Fcal were given as µg/g.
Statistical Analysis
STATA software (version 13, StataCorp, College Station, USA) was used for performing the analyses (2-sided tests, with type 1 error set at alpha = 0.05). The characteristics at the time of inclusion were given as mean or median with standard deviation or interquartile range (IQR), depending on statistical distribution. Comparisons of parameters between the 2 independent arms (ie, L1 group vs L2-L3 group) were performed using χ 2 or Fisher exact tests for categorical variables and Student t test or Mann-Whitney U test if assumptions of t test were not met: (1) normality and (2) assumption of homoscedasticity studied using Fisher–Snedecor test for quantitative parameters. Receiver operating characterstic (ROC) curves were used to determine the best fecal calprotectin threshold to detect endoscopic ulcerations or lesions, taking into account the clinical relevance and usual recommended indices (Youden, Liu, and efficiency). Sensitivity, specificity, predictive values (negative and positive) and likelihood ratios (negative and positive) were presented with 95% confidence intervals (CIs) for each estimated threshold. The ROC curves were compared using DeLong et al method. Sensitivity, specificity, positive predictive values (PPVs), negative predictive values (NPVs), and accuracy were compared 2-by-2 using test of proportions.
Sample Size Calculation
In this study, we performed a retrospective analysis from a prospectively maintained database. All available complete cases have been collected (n = 123). Consequently, we did not perform a formal sample size calculation as required in prospective trials. However before the study, a simulation of the statistical power had been carried out according to our sample size and relevant difference expected for the comparison of area under curve (AUC)-ROC. With a type 1 error at 5% and 123 patients (40 and 83 for each compared group), an absolute difference equalling 0.12 could be highlighted.28
RESULTS
Population Characteristics
Overall, 123 patients with CD were enrolled in this study, including 40 patients with pure ileal CD (L1 group) and 83 patients with colonic (n = 28) or ileocolonic (n = 55) involvement (L2-L3 group). The comparison of the baseline characteristics of the patients are detailed in Table 1. We observed older age at diagnosis (29.1 ± 15.5 years vs 21.2 ± 12.1 years; P = 0.001) and older age at inclusion (40.3 ± 16.7 years vs 33.3 ± 16.6 years; P = 0.014) in the L1 group compared with the L2-L3 group. In addition, patients from the L1 group were more likely to have complicated phenotype (B2 or B3 according to Montreal classification) than patients included in the L2-L3 group (80.0% vs 51.8%; P = 0.011). The patients with colonic involvement (L2-L3 group) were more likely to present with perianal lesions (35.0% vs 7.5%; P < 0.001), whereas a significantly higher proportion of patients with pure ileal involvement had a prior history of CD-related bowel resection (70.0% vs 43.4%; P = 0.007). The proportion of the patients with clinical activity (HBI >4; 45.0% vs 48.2%; P = 0.85) and the mean CRP level (7.2 ± 11.3 vs 9.0 ± 11.8; P = 0.17) was not different between the 2 groups. In contrast, we found a significantly lower median level of Fcal in the L1 group compared with the L2-L3 group (136.5 [47.4–324.3] vs 363.0 [83.0–813.0]; P = 0.025). We did not observe any significant difference regarding the other characteristics between the 2 groups such as gender, disease duration, smoking habits, and current medications (Table 1).
TABLE 1.
Baseline Characteristics of the Crohn’s Disease Patients Enrolled in this Study (n = 123)
| Ileal Crohn’s disease | Colonic or ileocolonic Crohn’s disease | P | |
|---|---|---|---|
| No. patients | 40 | 83 | |
| Age at diagnosis mean ± sd | 29.1 ± 15.5 years | 21.2 ± 12.1 years | 0.001 |
| Age at baseline mean ± sd | 40.3 ± 16.7 years | 33.3 ± 16.6 years | 0.014 |
| Male gender, n (%) | 15 (37.5%) | 26 (45.6%) | 0.54 |
| Smoking habits | |||
| Current smoker, n (%) | 4 (10.0%) | 7 (8.4%) | 0.49 |
| Former smoker, n (%) | 9 (22.5%) | 12 (14.5%) | |
| Nonsmoker, n (%) | 27 (67.5%) | 64 (77.1%) | |
| Disease duration | 10.8 ± 10.4 years | 11.6 ± 11.8 years | 0.73 |
| Montreal classification | |||
| Location | |||
| L1, n (%) | 40 (100.0%) | 0 (0.0%) | < 0.0001 |
| L2, n (%) | 0 (0.0%) | 28 (33.7%) | < 0.0001 |
| L3, n (%) | 0 (0.0%) | 55 (66.3%) | < 0.0001 |
| L4, n (%) | 0 (0.0%) | 0 (0.0%) | |
| Behavior | |||
| B1, n (%) | 8 (20.0%) | 40 (48.2%) | 0.011 |
| B2, n (%) | 21 (52.5%) | 27 (32.5%) | |
| B3, n (%) | 11 (27.5%) | 16 (19.3%) | |
| Perianal lesions, n (%) | 3 (7.5%) | 29 (35.0%) | < 0.001 |
| Prior intestinal resection, n (%) | 28 (70.0%) | 36 (43.4%) | 0.007 |
| HBI mean ± sd | 4.5 ± 3.7 | 4.7 ± 3.8 | 0.73 |
| HBI ≤ 4 n (%) | 18 (45.0%) | 40 (48.2%) | 0.85 |
| CRP (mg/L) mean ± sd | 7.2 ± 11.3 | 9.0 ± 11.8 | 0.17 |
| Fecal calprotectin (µg/g), median (IQR) | 136.5 (47.4–324.3) | 363.0 (83.0–813.0) | 0.025 |
| Medications at baseline | |||
| Infliximab, n (%) | 7 (17.5%) | 14 (16.9%) | 1.00 |
| Adalimumab, n (%) | 11 (27.5%) | 12 (14.5%) | 0.14 |
| Certolizumab pegol, n (%) | 2 (5.0%) | 3 (3.6%) | 0.66 |
| Vedolizumab, n (%) | 3 (7.5%) | 17 (20.5%) | 0.07 |
| Natalizumab, n (%) | 0 (0.0%) | 1 (1.2%) | 1.00 |
| Thiopurines, n (%) | 8 (20.0%) | 11 (13.3%) | 0.42 |
| Methotrexate, n (%) | 3 (7.5%) | 15 (18.1%) | 0.17 |
| Steroids, n (%) | 8 (20.0%) | 25 (30.1%) | 0.28 |
| 5-ASA, n (%) | 1 (2.5%) | 9 (10.9%) | 0.16 |
| Endoscopy | |||
| Any lesion, n (%) | 23 (57.5%) | 64 (77.1%) | 0.034 |
| Any ulceration, n (%) | 16 (40.0%) | 44 (53.0%) | 0.19 |
| Ileum, n (%) | 16 (40.0%) | 20 (24.1%) | 0.09 |
| Right colon, n (%) | 0 (0.0%) | 11 (13.3%) | 0.016 |
| Transverse colon, n (%) | 0 (0.0%) | 11 (13.3%) | 0.016 |
| Left/sigmoid colon, n (%) | 0 (0.0%) | 24 (28.9%) | < 0.0001 |
| Rectum, n (%) | 0 (0.0%) | 22 (26.5%) | < 0.0001 |
Fecal Calprotectin in Detecting Endoscopic Ulcerations
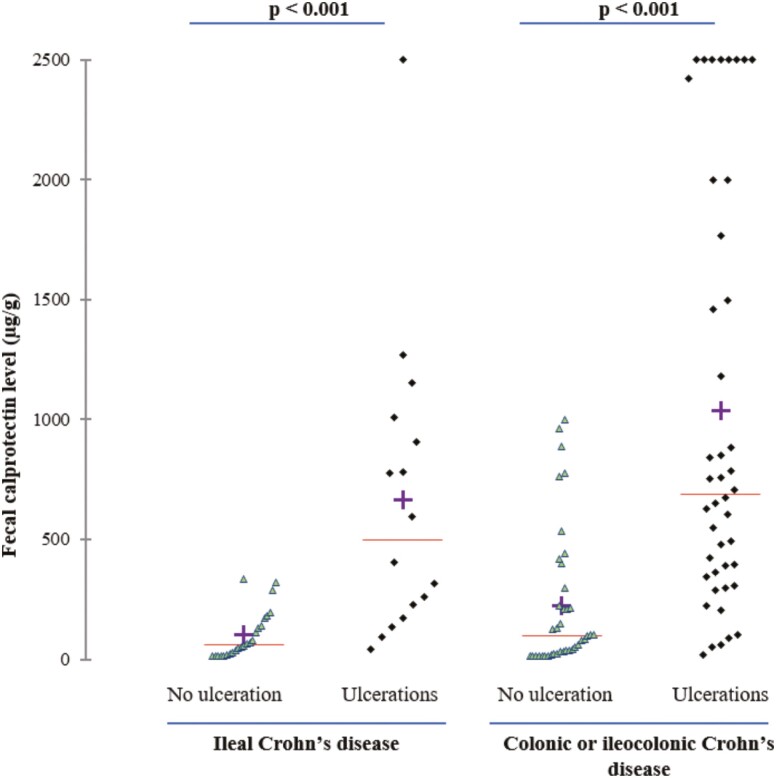
Among the 40 patients with pure ileal CD, 16 patients presented with endoscopic ulcerations (40.0%). The median level of Fcal was significantly higher in patients with endoscopic ulcerations compared with those with no endoscopic ulceration (500 [201–959] vs 62 [21–154] µg/g; P = 0.025; Fig. 1). Using a ROC curve (Fig. 2A), we determined the best threshold of Fcal value to detect endoscopic ulcerations. The area under the curve (AUC) was 0.89. We showed that a level of Fcal >200 µg/g was the best cutoff value to detect endoscopic ulcerations in patients with pure ileal CD, with a sensitivity of 75.0% (47.6–92.7), a specificity of 87.5% (67.6–97.3), a positive predictive value of 80.0% (51.9–95.7), and a negative predictive value of 84.0% (63.9–95.5; Table 2). We also looked at the usual cutoff value (ie, Fcal >250 µg/g), and we found the following performances to detect endoscopic ulcerations in the L1 group: sensitivity = 68.8% (41.3–89.0), specificity = 87.5% (67.6–97.3), PPV = 78.6% (49.2–95.3), and NPV = 80.8% (60.6–93.4; Table 2).
FIGURE 1.
Level of fecal calprotectin according to disease location and presence of endoscopic ulcerations.
FIGURE 2.
Receiver operating curves illustrating the performances of fecal calprotectin to detect endoscopic ulcerations in patients with ileal (2A) and colonic or ileocolonic (2B) Crohn’s disease.
TABLE 2.
Performances of Fecal Calprotectin to Detect Endoscopic Ulcerations According to Crohn’s Disease Location (Ileal vs Colonic or Ileocolonic)
| No. patients | Cutoff value | AUC | Se | Spe | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|---|---|---|
| Ileal CD | 40 | 200 µg/g | 0.81 (0.68–0.94) | 75.0% (47.6–92.7) | 87.5% (67.6–97.3) | 80.0% (51.9–95.7) | 80.8% (63.9–95.5) | 82.5% |
| Ileal CD | 40 | 250 µg/g | 0.78 (0.64–0.92) | 68.8% (41.3–89.0) | 87.5% (67.6–97.3) | 78.6% (49.2–95.3) | 80.8% (60.6–93.4) | 80.0% |
| Colonic or ileocolonic CD | 83 | 250 µg/g | 0.79 (0.70–0.88) | 84.1% (69.9–93.4) | 74.4% (57.9–87.0) | 78.7% (64.3–89.3) | 80.6% (64.0–91.8) | 79.5% |
| P a | ns | ns | ns | ns | ns | ns |
Abbreviations: Se, sensitivity, Spe, specificity; ns: nonsignificant (P > 0.05). aComparisons between fecal calprotectin >200 µg/g in ileal CD vs fecal calprotectin >250 µg/g in colonic or ileocolonic CD and between fecal calprotectin >250 µg/g in ileal CD vs colonic or ileocolonic CD.
Overall, 83 patients were included in the L2-L3 group. Among them, 44 patients (53.0%) presented with endoscopic ulcerations and had a higher median level of Fcal than those with no endoscopic ulceration (691 [353–1883] µg/g vs 99 [24–300] µg/g; P < 0.001; Fig. 1). Using a ROC curve (AUC = 0.84; Fig. 2B), we identified a value of Fcal above 250 µg/g as the best threshold to detect endoscopic ulcerations in patients with colonic or ileocolonic CD (sensitivity = 84.1% [69.9–93.4], specificity = 74.4% [57.9–87.0], PPV = 78.7% [64.3–89.3], and NPV = 80.6% [64.0–91.8]; Table 2).
We did not find any significant difference between the median level of Fcal between L1 group and L2-L3 group in patients with ulcerations (500 [201–959] vs 691 [353–1883] µg/g, respectively; P = 0.40) or in those without endoscopic ulcerations (62 [21–154] µg/g vs 99 [24–300] µg/g, respectively; P = 0.25). Additionally, there was no significant difference in the AUC between the 2 groups (0.89 vs 0.84 for L1 group and L2-L3 group, respectively; P = 0.46). We also compared the sensitivity, specificity, PPV, NPV, and accuracy and did not observe any significant differences (comparisons between Fcal >200 µg/g in ileal CD and Fcal >250 µg/g in colonic or ileocolonic CD, and between Fcal >250 µg/g in ileal CD and colonic or ileocolonic CD; Table 2).
Fecal Calprotectin in Detecting the Presence of Endoscopic Lesions
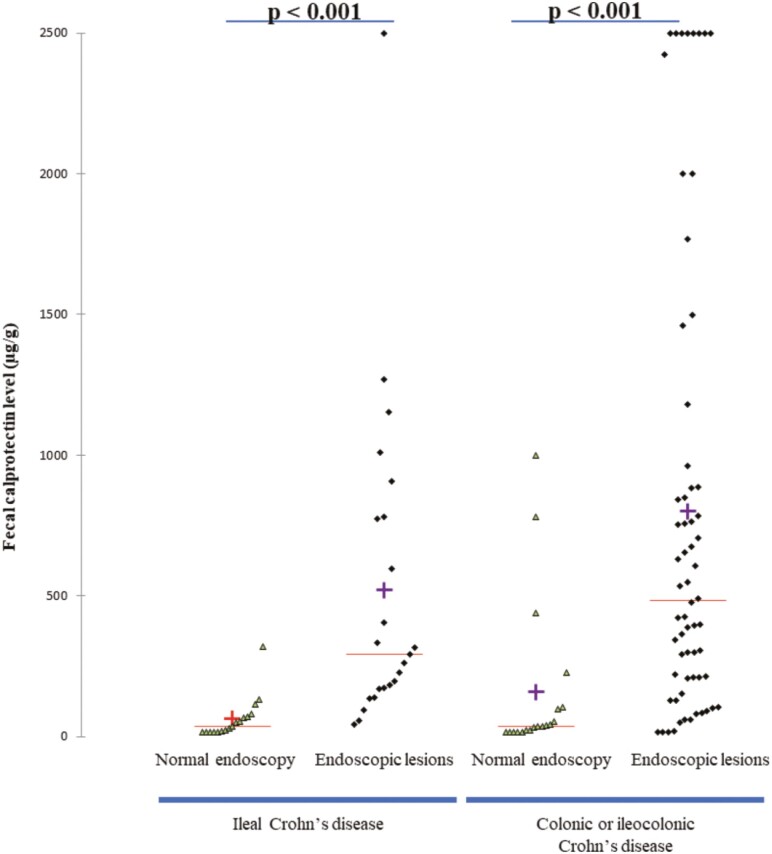
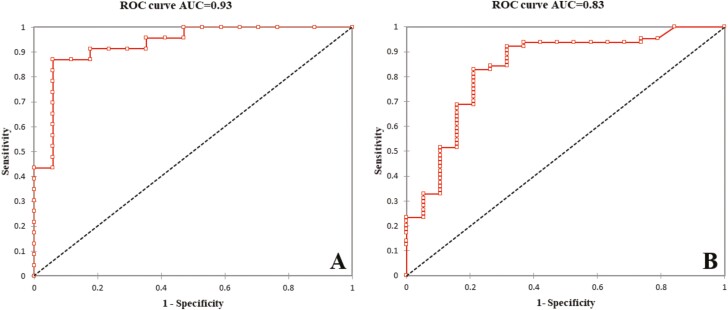
In the 40 patients belonging to the L1 group, endoscopic lesions were seen in 23 patients (57.0%). The median level of Fcal was higher in patients with endoscopic lesions compared with those with normal mucosa in endoscopy (291 [171–780] µg/g vs 36 [17–70] µg/g; P < 0.001) (Fig. 3). Using a ROC curve (AUC = 0.93; Fig. 4A), we found that an Fcal above 100 µg/g was the best threshold to detect the presence of endoscopic lesions (sensitivity = 87.0% [66.4–97.2], specificity = 82.4% [56.6–96.2], PPV = 82.4% [66.4–97.2] and NPV = 82.4% [56.6–96.2]; Table 3).
FIGURE 3.
Level of fecal calprotectin according to disease location and presence of endoscopic lesions.
FIGURE 4.
Receiver operating curves illustrating the performances of fecal calprotectin to detect endoscopic lesions in patients with ileal (A) and colonic or ileocolonic (B) Crohn’s disease.
TABLE 3.
Performances of Fecal Calprotectin to Detect the Presence of Endoscopic Lesions According to Crohn’s Disease Location (Ileal vs Colonic or Ileocolonic)
| No. patients | Cutoff value | AUC | Se | Spe | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|---|---|---|
| Ileal CD | 40 | 100 µg/g | 0.85 (0.73–0.96) | 87.0% (66.4–97.2) | 82.4% (56.6–96.2) | 87.0% (66.4–97.2) | 82.4% (56.6–96.2) | 85.0% (73.9–96.1) |
| Colonic or ileocolonic CD | 83 | 100 µg/g | 0.79 (0.68–0.90) | 84.4% (73.1–92.2) | 73.7% (48.8–90.9) | 91.5% (81.3–97.2) | 58.3% (36.6–77.9) | 81.9% (73.6–90.2) |
| P | ns | ns | ns | ns | ns | ns |
Abbreviations: Se, sensitivity; Spe, specificity; ns, nonsignificant (P > 0.05). aComparisons between fecal calprotectin >200 µg/g in ileal CD vs fecal calprotectin >250 µg/g in colonic or ileocolonic CD and between fecal calprotectin >250 µg/g in ileal CD vs colonic or ileocolonic CD.
Among the 83 patients with colonic or ileocolonic CD, 64 patients (77.1%) had endoscopic lesions. The median level of Fcal was increased in patients with endoscopic lesions compared with those with normal endoscopy (485 [209–925] µg/g vs 36 [17–103] µg/g; P < 0.001) . Using a ROC curve (AUC = 0.83; Fig. 4B), we observed that the best Fcal value to detect endoscopic lesion was 100 µg/g and above. The performances of this threshold were as following: sensitivity = 84.4% [73.1–92.2], s; specificity = 73.7% [48.8–90.9], PPV = 91.5% [81.3–97.2] and NPV = 58.3% [36.6–77.9]; Table 3).
We did not find any significant differences between the median level of Fcal between L1 group and L2-L3 group in patients with endoscopic lesions (291 [171–780] vs 485 [209–925] µg/g, respectively; P = 0.48) or in those with normal mucosa (36 [17–70] µg/g vs 36 [17–103] µg/g, respectively; P = 0.51). We compared the AUC between the 2 groups, and we did not show any difference (0.93 vs 0.83 for L1 group and L2-L3 group, respectively; P = 0.17). We also compared the sensitivity, specificity, PPV, NPV, and accuracy, and we did not observe any significant difference comparing these performances of Fcal >100 µg/g in patients with ileal CD compared with those with colonic or ileocolonic CD (Table 3).
DISCUSSION
In this study, we showed that Fcal demonstrated the same accuracy to detect endoscopic ulcerations in patients with ileal CD compared with those with colonic or ileocolonic CD. New to this study is the identification of a lower cutoff value for patients with pure ileal (L1) CD.
The main limitation of the previous studies of this topic is that no statistical analyses were performed to directly compare the performance of Fcal to assess mucosal healing in different phenotypes of CD,10, 12, 22 therefore hindering meaningful interpretation for clinical application. Schoepfer and colleagues reported in a cohort of 140 CD patients (L1 = 41, L2 = 26 and L3 = 73) the following correlation coefficients of 0.649, 0.702, and 0.795 for the patients classified according to Montreal classification as L1, L2 and L3, respectively, suggesting that the correlation was lower for patients with pure ileal CD.12 In the same line, D’haens et al observed among 87 CD patients that the correlation with the endoscopic scores was higher after excluding the 25 patients with pure ileal CD. However, these 2 studies did not perform any statistical test to compare these coefficients to know if this trend was statistically significant. In addition, the 2 available endoscopic scoring systems for CD (CDEIS and SES-CD)23, 24 often underestimate the severity of CD limited to the ileum compared with CD reaching several ileocolonic segments, thus highlighting the fact that the correlation with endoscopic scores is probably not the best end point to address this question.
Consequently, we designed our study to allow appropriate analyses (comparison between ROC curve and 2-by-2 comparisons of each performance). We decided to divide the patients in only 2 groups (ie, L1 group and L2-L3 group) rather than in 3 groups because this better reflects the point in question for clinical practice. In addition, we chose the presence of any endoscopic ulceration as a primary end point because it is the definition of mucosal healing7, 29 and allows for substantial reproducibility.27
We observed that our 2 groups had different clinical characteristics, but this was expected because it is well understood that ileal CD has a higher risk of complications leading to surgery and a lower probability of coexisting perianal involvement.30 In addition, ileal CD may be associated with more delayed diagnosis because of nonspecific symptoms compared with the phenotype of CD in patients with colonic involvement. According to previous studies,12, 17, 22 patients with pure ileal CD have a lower mean level of Fcal than those in the L2-L3 group. We previously showed using a multivariate model that Fcal level is mostly influenced by the presence of CD lesions (even nonulcerated) in a depth-related manner and by the affected area.17 Although it has been demonstrated that this discrepancy could be related to more extensive affected areas in patients with colonic or ileocolonic CD,17 in our cohort it is probably due to the higher proportion of patients with endoscopic lesions observed in the L2-L3 group compared with the L1 group (77.1% vs 57.5%; P = 0.034).
Our results suggest for the first time that a lower cutoff level of Fcal should be used to assess mucosal healing in patients with pure ileal CD. We identified a value of 200 µg/g and above as the best threshold. Among the patients included in the L2-L3 group, we found an optimal cutoff value of 250 µg/g, which is the most consensual cutoff value used in practice.10, 14, 17 We reported very good performances of Fcal measurement to assess endoscopic mucosal healing but did not observe any significant difference in comparing its performances according to disease locations regardless of the analysis (AUC, sensitivity, specificity, PPV, NPV, or accuracy). However, we may underline some interesting trends. For example, Fcal may be less sensitive in patients with pure ileal CD (higher risk of normal Fcal in case of ulcerated ileitis), which has been already suggested by Gecse et al who found that even in the presence of large or very large ulcers, patients with ileal CD may not have markedly elevated Fcal level.22 In contrast, we saw a slightly higher specificity (higher probability of normal Fcal value in absence of ulceration) in patients belonging to the L1 group.
In our cohort, we identified the same cutoff value (Fcal >100 µg/g) to detect the presence of endoscopic lesions in patients with CD regardless of the location. Interestingly, this value is slightly higher than those usually retained to diagnose IBD (Fcal >50 µg/g).31–33 This discrepancy could be related to the potential discrepancy between endoscopic assessment and histological activity. We did not show any significant difference in comparing each performance between the 2 groups. Surprisingly, we found a modest negative predictive value (58.8%) in patients with colonic or ileocolonic CD. This result has to be taken with caution owing to the low prevalence of patients with normal mucosa in this subgroup (n = 19, 22.9%).
Besides variability across the type of assay34 and special situations such as early postoperative period,35–37 our findings suggest that CD location should also be taken into account when defining the best cutoff value of Fcal in the management of CD patients. It could be necessary to use a lower threshold to modify or not modify the treatments in isolated ileal CD when applying treat-to-target strategy.
Our study is the largest cohort so far that directly compares (primary end point) the performances of Fcal in patients with pure ileal CD compared with patients with colonic or ileocolonic involvement. In addition, the data were retrieved from a prospectively maintained database. Nonetheless, there are some limitations to this study. The patients were enrolled from a referral center with likely more aggressive CD and a high rate of bowel resection. In addition, we were not able to collect detailed data on the percentage of affected areas in endoscopy, which could have provided additional meaningful data.
In summary, we identified that Fcal has the same accuracy to detect endoscopic ulcerations or the presence of endoscopic lesions in patients with ileal CD compared with those with colonic or ileocolonic CD. However, a lower cutoff value should be used for patients with ileal CD to detect endoscopic ulceration.
APPENDIX: STARD CHECKLIST
| Section & Topic | No | Item | Reported on page # |
|---|---|---|---|
| TITLE OR ABSTRACT | |||
| 1 | Identification as a study of diagnostic accuracy using at least one measure of accuracy (such as sensitivity, specificity, predictive values, or AUC) |
p. 5 | |
| ABSTRACT | |||
| 2 | Structured summary of study design, methods, results, and conclusions (for specific guidance, see STARD for Abstracts) | p. 5 | |
| INTRODUCTION | |||
| 3 | Scientific and clinical background, including the intended use and clinical role of the index test | p. 6 | |
| 4 | Study objectives and hypotheses | p. 7 | |
| METHODS | |||
| Study design | 5 | Whether data collection was planned before the index test and reference standard were performed (prospective study) or after (retrospective study) | p. 7 |
| Participants | 6 | Eligibility criteria | p. 7 |
| 7 | On what basis potentially eligible participants were identified (such as symptoms, results from previous tests, inclusion in registry) | p. 7 | |
| 8 | Where and when potentially eligible participants were identified (setting, location and dates) | p. 7 | |
| 9 | Whether participants formed a consecutive, random or convenience series | p. 7 | |
| Test methods | 10a | Index test, in sufficient detail to allow replication | p. 8 |
| 10b | Reference standard, in sufficient detail to allow replication | p. 8 | |
| 11 | Rationale for choosing the reference standard (if alternatives exist) | p. 6 | |
| 12a | Definition of and rationale for test positivity cutoffs or result categories of the index test, distinguishing pre-specified from exploratory | p. 9 | |
| 12b | Definition of and rationale for test positivity cutoffs or result categories of the reference standard, distinguishing pre-specified from exploratory | p. 6 | |
| 13a | Whether clinical information and reference standard results were available to the performers/readers of the index test | p. 8 | |
| 13b | Whether clinical information and index test results were available to the assessors of the reference standard | p. 8 | |
| Analysis | 14 | Methods for estimating or comparing measures of diagnostic accuracy | p. 9 |
| 15 | How indeterminate index test or reference standard results were handled | NA | |
| 16 | How missing data on the index test and reference standard were handled | NA | |
| 17 | Any analyses of variability in diagnostic accuracy, distinguishing pre-specified from exploratory | p. 9 | |
| 18 | Intended sample size and how it was determined | p. 9 | |
| RESULTS | |||
| Participants | 19 | Flow of participants, using a diagram | NA |
| 20 | Baseline demographic and clinical characteristics of participants | See Table 1 | |
| 21a | Distribution of severity of disease in those with the target condition | See Table 1 and p. 9 | |
| 21b | Distribution of alternative diagnoses in those without the target condition | See Table 1 and p. 9 | |
| 22 | Time interval and any clinical interventions between index test and reference standard | p. 7 | |
| Test results | 23 | Cross tabulation of the index test results (or their distribution) by the results of the reference standard | p. 10 and 11 |
| 24 | Estimates of diagnostic accuracy and their precision (such as 95% confidence intervals) | See Tables 2 and 3 | |
| 25 | Any adverse events from performing the index test or the reference standard | NA | |
| DISCUSSION | |||
| 26 | Study limitations, including sources of potential bias, statistical uncertainty, and generalisability | p. 14 | |
| 27 | Implications for practice, including the intended use and clinical role of the index test | p. 14 and 15 | |
| OTHER INFORMATION | |||
| 28 | Registration number and name of registry | p. 7 | |
| 29 | Where the full study protocol can be accessed | p. 7 | |
| 30 | Sources of funding and other support; role of funders | p. 2 |
Author Contribution: AB substantially contributed to conception and design, acquisition of data, analysis and interpretation of data, and drafting the article. DR substantially contributed to conception and design and analysis and interpretation of data. WM, MA, DL, SK, RC, and JP substantially contributed to acquisition and analysis of data and revising the article critically for important intellectual content. BP substantially contributed to interpretation of data (statistical analysis) and revising the article critically for important intellectual content. All authors gave final approval of the manuscript.
Conflicts of Interest: DTR declares consultant fees for Abbvie, Abgenomics, Allergan, Inc., Boehringer Ingelheim, Ltd., Bristol-Myers Squibb, Celgene Corporation, Dizal Pharmaceuticals, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, Ichnos Sciences, GlaxoSmithKline Group, Janssen Pharmaceuticals, Lilly, Narrow River Mgmt, Pfizer, Prometheus Laboratories, Shire, Takeda and grant support from Abbvie, Genentech/Roche, Janssen Pharmaceuticals, Prometheus Laboratories, Shire, and Takeda. AB declares lecture fees for MSD, Abbvie, Ferring, Takeda, Vifor Pharma, and Hospira and consulting fees for Abbvie, Takeda, and Hospira. SAK declares unpaid research collaboration with Abbvie. None of the conflict of interest previously described (AB, DTR, SAK) are related to this work.
REFERENCES
- 1. Peyrin-Biroulet L, Cieza A, Sandborn WJ, et al. ; International Programme to Develop New Indexes for Crohn’s Disease (IPNIC) group . Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut. 2012;61:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pariente B, Mary JY, Danese S, et al. Development of the Lémann index to assess digestive tract damage in patients with Crohn’s disease. Gastroenterology. 2015;148:52–63.e3. [DOI] [PubMed] [Google Scholar]
- 3. Baert F, Moortgat L, Van Assche G, et al. ; Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club . Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138:463–8; quiz e10. [DOI] [PubMed] [Google Scholar]
- 4. Frøslie KF, Jahnsen J, Moum BA, et al. ; IBSEN Group . Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–422. [DOI] [PubMed] [Google Scholar]
- 5. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis. 2009;15:1295–1301. [DOI] [PubMed] [Google Scholar]
- 6. Bouguen G, Levesque BG, Feagan BG, et al. Treat to target: a proposed new paradigm for the management of Crohn’s disease. Clin Gastroenterol Hepatol. 2015;13:1042–50.e2. [DOI] [PubMed] [Google Scholar]
- 7. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338. [DOI] [PubMed] [Google Scholar]
- 8. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779–2789. [DOI] [PubMed] [Google Scholar]
- 9. Buisson A, Gonzalez F, Poullenot F, et al. ; ACCEPT study group . Comparative acceptability and perceived clinical utility of monitoring tools: a nationwide survey of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:1425–1433. [DOI] [PubMed] [Google Scholar]
- 10. D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–2224. [DOI] [PubMed] [Google Scholar]
- 11. Björkesten C-G af, Nieminen U, Turunen U, et al. Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti-TNF-treated luminal Crohn’s disease. Scand J Gastroenterol. 2012;47:528–537. [DOI] [PubMed] [Google Scholar]
- 12. Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162–169. [DOI] [PubMed] [Google Scholar]
- 13. Sipponen T, Savilahti E, Kolho KL, et al. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40–46. [DOI] [PubMed] [Google Scholar]
- 14. Nancey S, Boschetti G, Moussata D, et al. Neopterin is a novel reliable fecal marker as accurate as calprotectin for predicting endoscopic disease activity in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2013;19:1043–1052. [DOI] [PubMed] [Google Scholar]
- 15. Buisson A, Vazeille E, Minet-Quinard R, et al. Faecal chitinase 3-like 1 is a reliable marker as accurate as faecal calprotectin in detecting endoscopic activity in adult patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2016;43:1069–1079. [DOI] [PubMed] [Google Scholar]
- 16. Buisson A, Vazeille E, Minet-Quinard R, et al. Fecal matrix metalloprotease-9 and lipocalin-2 as biomarkers in detecting endoscopic activity in patients with inflammatory bowel diseases. J Clin Gastroenterol. 2018;52:e53–e62. [DOI] [PubMed] [Google Scholar]
- 17. Goutorbe F, Goutte M, Minet-Quinard R, et al. Endoscopic factors influencing fecal calprotectin value in Crohn’s disease. J Crohns Colitis. 2015;9:1113–1119. [DOI] [PubMed] [Google Scholar]
- 18. Wright EK, Kamm MA, De Cruz P, et al. Comparison of fecal inflammatory markers in Crohn’s disease. Inflamm Bowel Dis. 2016;22:1086–1094. [DOI] [PubMed] [Google Scholar]
- 19. Reinisch W, Bressler B, Curtis R, et al. Fecal calprotectin responses following induction therapy with vedolizumab in moderate to severe ulcerative colitis: a Post Hoc analysis of GEMINI 1. Inflamm Bowel Dis. 2019;25:803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cerrillo E, Moret I, Iborra M, et al. A nomogram combining fecal calprotectin levels and plasma cytokine profiles for individual prediction of postoperative Crohn’s disease recurrence. Inflamm Bowel Dis. 2019;25:1681–1691. [DOI] [PubMed] [Google Scholar]
- 21. Monteiro S, Barbosa M, Cúrdia Gonçalves T, et al. Fecal calprotectin as a selection tool for small bowel capsule endoscopy in suspected Crohn’s disease. Inflamm Bowel Dis. 2018;24:2033–2038. [DOI] [PubMed] [Google Scholar]
- 22. Gecse KB, Brandse JF, van Wilpe S, et al. Impact of disease location on fecal calprotectin levels in Crohn’s disease. Scand J Gastroenterol. 2015;50:841–847. [DOI] [PubMed] [Google Scholar]
- 23. Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut. 1989;30:983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. [DOI] [PubMed] [Google Scholar]
- 25. Jensen MD, Kjeldsen J, Nathan T. Fecal calprotectin is equally sensitive in Crohn’s disease affecting the small bowel and colon. Scand J Gastroenterol. 2011;46:694–700. [DOI] [PubMed] [Google Scholar]
- 26. Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buisson A, Filippi J, Amiot A, et al. Su1229 definitions of the endoscopic lesions in Crohn’s disease: reproductibility study and GETAID expert consensus. Gastroenterology. 2015;148:S–445. [Google Scholar]
- 28. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- 29. Colombel JF, Sandborn WJ, Reinisch W, et al. ; SONIC Study Group . Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. [DOI] [PubMed] [Google Scholar]
- 30. Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. [DOI] [PubMed] [Google Scholar]
- 31. Tibble JA, Sigthorsson G, Foster R, et al. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology. 2002;123:450–460. [DOI] [PubMed] [Google Scholar]
- 32. Rheenen van PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341:c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kennedy NA, Clark A, Walkden A, et al. Clinical utility and diagnostic accuracy of faecal calprotectin for IBD at first presentation to gastroenterology services in adults aged 16–50 years. J Crohns Colitis. 2015;9:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Labaere D, Smismans A, Van Olmen A, et al. Comparison of six different calprotectin assays for the assessment of inflammatory bowel disease. United European Gastroenterol J. 2014;2:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boschetti G, Laidet M, Moussata D, et al. Levels of fecal calprotectin are associated with the severity of postoperative endoscopic recurrence in asymptomatic patients with Crohn’s disease. Am J Gastroenterol. 2015;110:865–872. [DOI] [PubMed] [Google Scholar]
- 36. Wright EK, Kamm MA, De Cruz P, et al. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn’s disease after surgery. Gastroenterology. 2015;148:938–947.e1. [DOI] [PubMed] [Google Scholar]
- 37. Baillet P, Cadiot G, Goutte M, et al. Faecal calprotectin and magnetic resonance imaging in detecting Crohn’s disease endoscopic postoperative recurrence. World J Gastroenterol. 2018;24:641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]