Abstract
Cross-talk between peripheral tissues is essential to ensure the coordination of nutrient intake with disposition during the feeding period, thereby preventing metabolic disease. This mini-review considers the interactions between the key peripheral tissues that constitute the metabolic clock, each of which is considered in a separate mini-review in this collation of articles published in Endocrinology in 2020 and 2021, by Martchenko et al (Circadian rhythms and the gastrointestinal tract: relationship to metabolism and gut hormones); Alvarez et al (The microbiome as a circadian coordinator of metabolism); Seshadri and Doucette (Circadian regulation of the pancreatic beta cell); McCommis et al (The importance of keeping time in the liver); Oosterman et al (The circadian clock, shift work, and tissue-specific insulin resistance); and Heyde et al (Contributions of white and brown adipose tissues to the circadian regulation of energy metabolism). The use of positive- and negative-feedback signals, both hormonal and metabolic, between these tissues ensures that peripheral metabolic pathways are synchronized with the timing of food intake, thus optimizing nutrient disposition and preventing metabolic disease. Collectively, these articles highlight the critical role played by the circadian clock in maintaining metabolic homeostasis.
Keywords: circadian, intestine, microbiome, islet, hepatocyte, myocyte, adipocyte, metabolism
One of the underlying principles of metabolic homeostasis is the seamless transition between periods of nutrient availability and nutrient scarcity. In mammals, this is maintained through a careful balance between nutrient deposition during periods of feeding and stored nutrient liberation during fasting. Metabolic homeostasis is mediated at the level of several key peripheral organs including, most notably, the gastrointestinal tract to which ingested nutrients are first delivered and which itself houses another “organ”, the intestinal microbiome, the pancreatic islet cells that release metabolic hormones, and the nutrient depots liver, skeletal muscle, and adipocytes. The actions of all of these diverse organs must be temporally coordinated so as to limit, for example, dysglycemia due to hepatic glucose production or dyslipidemia due to enhanced lipolysis during periods of dietary nutrient absorption. In humans and, indeed, all mammals, metabolic homeostasis is determined at the level of the brain by the wake-sleep cycle. Mammals differ in their relationship between activity levels and the light-dark periods, with some species such as humans being active during the day, whereas others, including rodents, are active at night. However, the causal relationship between wakefulness and the ability to ingest nutrients is maintained across all species and is, in large part, determined by the circadian clock.
In this mini-review, the essential role of the circadian clock in ensuring metabolic homeostasis is discussed from the perspective of synchronization between the key peripheral metabolic tissues during the feeding period. More focused reviews of the circadian biology of each individual metabolic tissue have recently been published in Endocrinology as mini-reviews (1-6) and are collated within this special online collection.
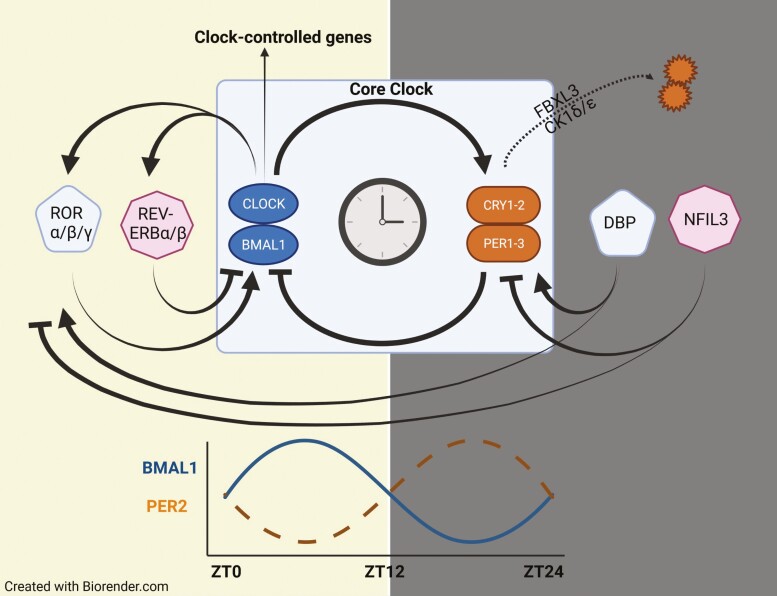
The circadian clock is an integrated transcriptional-translational autoregulatory feedback loop that exists in all nucleated cells of the body, wherein activation of the core genes, ARNTL (aka brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein 1 [BMAL1]) and CLOCK (circadian locomotor output cycles kaput), increases expression of the regulatory proteins, PERIOD (PER1-3) and CRYPTOCHROME (CRY1-2), which, in turn, feedback-inhibit BMAL1 and CLOCK. Degradation of PER and CRY then releases the positive arm of the clock for another cycle, with input from many other proteins, further fine-tuning these signals (Fig. 1). In mammals, the activating signal or zeitgeber (ZT) for the core clock is light, received through specialized cells in the retina and delivered to the suprachiasmatic nuclei via the retinohypothalamic tract (Fig. 2) (7-10).
Figure 1.
Schematic of the molecular clock. Expression of the core clock proteins, BMAL1 and CLOCK, stimulates the production of PERIOD (PER1-3) and CRYPTOCHROME (CRY1-2) which, in turn, feedback-inhibit BMAL1 and CLOCK. The period of transcriptional-translational autoregulatory feedback loop is determined by the rate of degradation of PER and CRY by casein kinase 1 (CK1) δ/ε and F-box and leucine-rich repeat protein 3 (FBXL3), respectively. This generates an anti-phasic rhythm in the positive and negative arms of the clock, as illustrated for BMAL1 and PER2 during the day (yellow) and night (gray) periods. Additional input into the core clock is provided by retinoic acid receptor-related orphan receptor (ROR) α/β/γ, nuclear receptor subfamily 1 group D (REV-ERB) α/β, albumin D-box binding protein (DBP) and nuclear factor, interleukin 3 regulated (NFIL3).
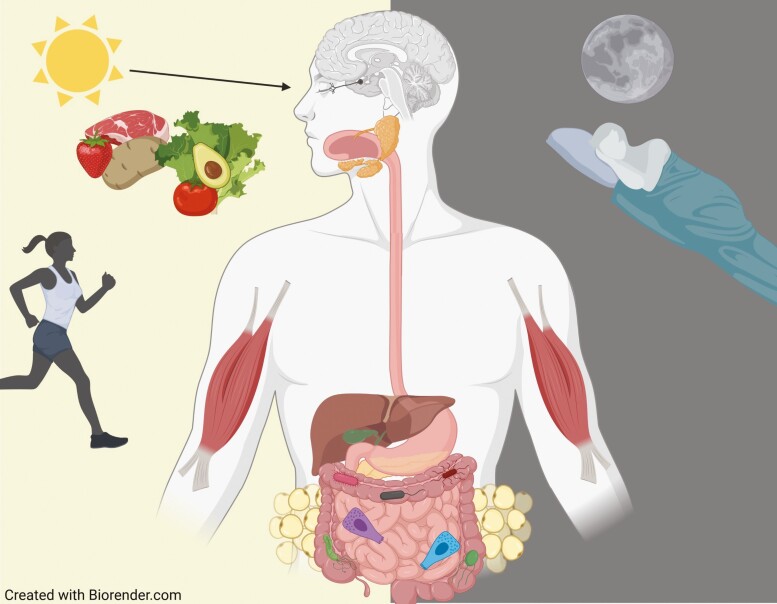
Figure 2.
Schematic of the metabolic clock. Light entrainment of the master clock in the suprachiasmatic nuclei determines the active/feeding and inactive/fasting periods. The main peripheral tissues in the metabolic clock are the gastrointestinal tract, with its resident microbiome, the pancreatic islets, the liver, the skeletal muscle and the adipocytes, all of which express cell-autonomous clocks that are synchronized to ensure metabolic homeostasis.
The importance of the circadian clock in metabolic homeostasis is emphasized by epidemiologic data showing increased risk for metabolic disruption or disease (ie, insulin resistance, dyslipidemia, obesity, impaired glucose tolerance, type 2 diabetes) in shift workers and those with jet lag or altered light exposure, as well as in rodents with mutations or knockout of specific clock genes (11-25). The critical role of the clock in metabolic homeostasis is further emphasized by findings that polymorphisms in the core clock genes are associated with alterations in the timing of food intake, as well as with obesity, hyperglycemia, and diabetes (22, 26-30). It has also been reported that weight loss is more difficult in humans who routinely eat late in the day (31). Studies in rodent models have further demonstrated that high-fat feeding not only induces obesity, but also disrupts the normal pattern of food intake in rodents (32), creating a vicious cycle of perturbed metabolic homeostasis. As a consequence, obesogenic (high-fat) diets (HFDs) are commonly used as a model of circadian disruption.
The core clock in the suprachiasmatic nuclei plays an integral role in peripheral metabolism through the regulation of food intake as well as of the diurnal patterns in several metabolic hormones (eg, cortisol and growth hormone). However, it has become increasingly clear over the past few decades that peripheral tissues themselves are also regulated at the molecular level by the core clock machinery. Indeed, in a survey of multiple tissues in mice, 43% of all genes were found to exhibit circadian rhythmicity in one or more tissues (33). These effects are mediated through direct actions of the clock proteins on target genes (approximately 10% to 20% of all genes (34, 35)) or indirectly, such as through epigenetic modulation of chromatin accessibility to transcription factors or as downstream effects of the clock-controlled genes (33, 36-38). However, central to the notion of the peripheral clock is the integration of these clocks between the metabolic tissues. Hence, although these tissues all express cell-autonomous clocks, they also work in a synchrony with other peripheral tissues, through both feedforward and feedback crosstalk, to maintain metabolic homeostasis during the feeding and fasting periods. Beginning with the gut and proceeding through the islet to the storage tissues (ie, liver, muscle, adipose), this review article considers the signals that are initiated by food intake, as well as those that function to terminate these temporally synchronized responses.
Feedforward Synchronization of Metabolic Responses to Food Intake
The intestinal epithelial cells (IECs) are the first point of contact of ingested nutrients with a metabolic tissue, playing a critical role in the digestion of macromolecules and absorption of the digestive products. As reviewed by Martchenko et al (1), the IECs not only express the core clock machinery, but demonstrate multiple, synchronized circadian rhythms, many of which are coordinated by nutrient intake (39). For example, crypt cell proliferation increases before the active/feeding period in rodents (40), which permits anticipatory growth of the villus to enhance the handling of nutrients (41). The genes for several IEC brush border enzymes and nutrient as well as mineral transporter/handling proteins also exhibit circadian rhythms, increasing in association with the feeding period to enhance digestive and absorptive capacity (42-46). Furthermore, genetic disruption of the circadian clock is associated with abnormal patterns of carbohydrate, fat, and protein absorption from the gut (47). While some of these genes appear to be under the direct regulatory control of the clock proteins, circadian vagal input to the gut may also affect their expression (48, 49), and even shifting the timing of nutrient intake can induce a parallel shift in their expression (44). It has also become clear in recent years that the gut microbiome plays a role in entraining these patterns of gene expression (50, 51), as reviewed by Alvarez et al (2). These changes are mediated, at least in part, through recruitment of histone deacetylase 3 to the IEC chromatin, as well as by the transcription factor, nuclear factor-interleukin-3-regulated, resulting in rhythmic changes in the expression of genes involved in IEC nutrient handling (46, 52). Whether specific microbial metabolites are implicated in these changes is not well defined, but has been suggested to include both short-chain fatty acids (SCFAs; eg, butyrate) and inositol-1,4,5-trisphosphate, which exhibit circadian patterns of expression linked to timing of nutrient ingestion (53-56). As a consequence, total absence of the microbiome in germ-free mice induces changes in the patterns of nutrient absorption (52). Consistent with these findings, transfer of the fecal microbiome from jet-lagged mice to germ-free animals induces metabolic disease, whereas microbial depletion prevents metabolic disruption in jet-lagged mice (57).
In addition to the absorptive enterocytes, the enteroendocrine cells also exhibit circadian patterns. In male rats, the circadian rhythms in cholecystokinin (from I cells), glucose-dependent insulinotrophic polypeptide (GIP; from K cells) and neurotensin (from N cells) are positively correlated with the timing of food intake and nutrient handling capacity (58-61), whereas the peak of ghrelin (from X/A cells) occurs during the fasting/inactive period (62). Nevertheless, whether the cells that secrete these gut hormones demonstrate cell-autonomous clock genes remains largely unknown. However, recent studies have clearly demonstrated that clock genes, as well as the intestinal microbiome, are required for the circadian rhythm in intestinal L-cell function. The L-cell cosecretes multiple endocrine hormones derived from the same prohormone (GCG), including the incretin and satiety factor glucagon-like peptide-1 (GLP-1) (63-65), the intestinotrophic hormone GLP-2 (66), and the anorexigen oxyntomodulin (67), and circadian rhythms that peak at the onset of the active/feeding period have been demonstrated for GLP-1 in rodents and humans (40, 60, 68-70). Importantly, the rhythm of GLP-2 secretion, which is presumed to parallel that of the cosecreted GLP-1, may assist in coordinating the pattern of nutrient absorption, as GLP-2 stimulates translocation of sodium glucose transporter-1 to the enterocyte brush border membrane (71). Furthermore, both GIP and GLP-2 enhance glucose-transporter-2 insertion into the basolateral membrane, thereby increasing glucose absorption across the gut epithelium (72), while GIP additionally serves as an incretin hormone (73). Finally, the intestinal L cell secretes one additional satiety factor, peptide YY (74), which is also released at the highest levels during the active period in rats (75).
The normal rhythm in L-cell secretion follows the pattern in food intake, suggesting that nutrient ingestion is a zeitgeber for the intestinal L cell (40). HFDs as well as the saturated fatty acid palmitate also disrupt the L-cell clock through suppression of Arntl (60, 76, 77). Furthermore, whole-body as well as L-cell Arntl knockout results in impaired rhythmic L-cell secretion (70, 78). However, intestinal dysbiosis, including that induced by alterations in the timing of feeding, in dietary composition (eg, a high-fat or Western, high-fat/high-sucrose diet) and antibiotic administration, as well as its total absence in germ-free mice is also associated with a profound disruption in the rhythm of L-cell secretion, and transfer of a normal microbiome into germ-free mice restores their L-cell secretory pattern (40, 60, 76). Although L-cell hormone secretion is known to be stimulated both by SCFAs and microbial-derived secondary bile acids (79, 80), whether and how these or other microbial metabolites may entrain the L-cell clock machinery remains unknown. However, numerous studies have implicated the species Akkermansia muciniphila in microbiome-induced GLP-1 secretion and associated improvements in metabolic control, with recent studies indicating a role for an integral membrane protein in these effects (60, 81-84).
Following nutrient digestion and absorption, the next key player in the metabolic clock is the pancreatic islet. As reviewed by Seshadri and Doucette (3), humans exhibit increased insulin secretion at the onset of the normal feeding period. Altered circadian release of insulin and expression of the islet clock genes have also been demonstrated in type 2 diabetes (85-88). However, it is important to recognize that changes in insulin sensitivity also affect β-cell function in vivo (89). Hence, more direct demonstrations of a role for the islet circadian clocks have been shown through targeted disruptions in the core clock machinery, with β-cell clock-deficient mice exhibiting impaired rhythmic insulin secretion and metabolic disease (90, 91). At the islet and single-cell level, both β- and α-cells have also been demonstrated to exhibit cell-autonomous circadian clocks, with the peak activities of these 2 cell types temporally offset, presumably to allow for increased release of insulin during feeding and of glucagon during fasting, respectively (18, 87, 90, 92-94). Consistent with studies showing circadian patterns in insulin secretion, the cosecretion of islet amyloid polypeptide (or amylin) is also likely to be circadian, and may contribute to rhythmic changes not only in insulin secretion but also in downstream insulin sensitivity (95-97).
Interestingly, consistent with findings of circadian rhythms in insulin release in response to oral but not intravenous glucose (98), increasing the levels of the incretin hormones GLP-1 and GIP at the onset of the normal feeding/active period in rats further enhances the insulin secretory response to glucose as compared to that found under the same conditions during the normal fasting/inactive period; in mice, this pattern is reversed, likely due to associated rhythms in insulin sensitivity (40, 60). As a result, the circadian rhythms in GLP-1 and GIP appear both to coordinate and further increase the β-cell response to nutrient ingestion, with optimal effectiveness being observed during the normal feeding period. GLP-1 has also been reported to synchronize the β cell clock in vitro through a signaling pathway that likely involves activation of cyclic adenosine monophosphate (99). Circadian patterns in downstream signaling pathways, but not in the GLP-1 receptor, have also been reported for the β cell (90). Furthermore, the pattern in GLP-1 secretion may also affect the rhythm in glucagon release through its known inhibitory effects on the α cell (100). Finally, the intestinal microbiome has also been implicated in directly regulating islet β-cell function, most notably through the SCFA receptors FFA2 and FFA3 (101). It can therefore be presumed, although not shown to date, that the circadian patterns in microbial metabolites may also play a role as a β-cell zeitgeber, contributing to the normal rhythm of insulin secretion. Also, similar to findings in the intestinal enterocytes and L cells, HFDs and high-glucose/palmitate have been shown to disrupt islet clock gene expression (86, 91, 94, 102). Arntl is also required for the normal β-cell adaptive response to an HFD (91, 103), and light-induced circadian disruption and an HFD have been reported to synergistically impair β-cell function (93).
In addition to the well-established anabolic effects of insulin on the liver, skeletal muscle, and adipose tissue, all of these different cell types are also known to express cell-autonomous circadian clocks, as reviewed by McCommis and Butler (4), Oosterman et al. (5), and Heyde and colleagues (6), respectively.
Within the hepatocytes, vital processes such as glucose and fatty acid uptake are stimulated by insulin, whereas gluconeogenesis and lipolysis are upregulated by glucagon, among other catabolic hormones that also demonstrate circadian rhythms (eg, growth hormone and cortisol). However, as reviewed by McCommis and Butler (4), hepatic nutrient handling is also temporally controlled by the circadian clock machinery. Hepatic insulin sensitivity, which is normally increased during the feeding/active period in humans, is reduced in patients with type 2 diabetes (104). Furthermore, clock gene knockout mice and mice fed exclusively during the normal fasting/light period display a loss in rhythmicity of both the clock and metabolic genes in the liver, resulting in inappropriate gluconeogenesis and glucose uptake, hepatic steatosis, and abnormal plasma glucose, free fatty acid, and triglyceride levels (16, 105-108). At the molecular level, the expression of hepatic metabolic genes, including glucose transporter-2, glycogen synthase and phosphorylase, peroxisome proliferator-activated receptor α, and sterol regulatory element-binding protein-1, is controlled by the circadian clock, resulting in rhythms that are coordinated appropriately with the feeding and fasting states (108-110). Curiously, however, alterations in the timing of food intake can also entrain nutrient-specific gene expression in the liver independent of the circadian clock (111). These findings suggest that the rhythm in hepatic nutrient handling is entrained by nutrients delivered through the portal circulation, as well as by the associated rhythm in insulin which is an important zeitgeber for the induction of PERIOD (112-114). Furthermore, the hormones oxyntomodulin and ghrelin have also been shown to serve as a direct link between the gut and circadian rhythms in the liver, most notably through the modulation of Per2 expression (115, 116). SCFAs have also been shown to entrain the hepatic clock, which may further serve to synchronize the hepatic circadian rhythms with nutrient intake (117-119).
The liver clock is disrupted by feeding of an HFD, resulting in insulin resistance and a reprogramming of the hepatic transcriptome and downstream metabolome. Similarly, administration of nonobesogenic doses of the saturated fatty acid palmitate in vivo disrupts hepatic circadian rhythmicity and impairs recruitment of BMAL1 to its target genes (120-122). Direct exposure of hepatocytes to palmitate also results in disrupted clock gene expression and cellular function through destabilization of the BMAL1/CLOCK heterodimer (122, 123).
Skeletal muscle is the main tissue responsible for insulin-mediated glucose uptake, and insulin resistance in this tissue is therefore a key contributor to impaired glucose tolerance as well as type 2 diabetes (124). Glucose transporter-4 translocation to the plasma membrane is a key function of insulin in myocytes, and glucose uptake is thereby synchronized with food intake through the circadian pattern in insulin secretion. However, skeletal muscle functions are also regulated by a cell-autonomous clock, as reviewed by Oosterman et al (5). Rhythmic expression of the clock regulates the gene for glucose transporter-4 (125), and may contribute to the known rhythms in both mitochondrial respiratory capacity and myokine secretion (126, 127). Indeed, muscle-specific Arntl knockout results in insulin resistance, as characterized by impaired insulin-dependent glucose uptake (125). Furthermore, the master regulator of skeletal muscle biogenesis, myoblast-determination protein-1, is also a direct target of the circadian clock (128).
The importance of the circadian clock to muscle function has largely been determined using high-fat fed rodent models that, as in the liver, exhibit impaired BMAL1 recruitment to target genes, resulting in dysregulation of approximately 40% of the diurnal metabolome (120). These effects of obesogenic feeding were also associated with reduced insulin action and mitochondrial oxidative phosphorylation within skeletal muscle (129, 130). Conversely, muscle-specific Arntl knockout mice on an HFD were found to have increased skeletal muscle oxidative capacity and reduced obesity (125). Alterations in the timing of food intake also disrupt clock gene expression in the skeletal muscle in association with markers of insulin resistance, while treatment of myotubes with palmitate downregulates BMAL1/CLOCK (106, 129-131). Finally, although limited work has been conducted in humans, one study using obese insulin-resistant volunteers given identical doses of saturated-, monounsaturated- or polyunsaturated fatty acids showed a more profound deleterious effect of the saturated fat on the skeletal muscle clock gene (132). Collectively, therefore, it appears that the timing of nutrient ingestion may contribute to the synchronization of skeletal muscle function within the metabolic clock (107). In contrast, at least one study has suggested that the associated patterns in insulin release do not serve as an important zeitgeber for skeletal muscle (113).
The other 2 major effectors of the metabolic clock are the white and brown adipose tissue, which are essential for energy storage (WAT) and nonshivering thermogenesis (BAT), respectively. The circadian clock genes expressed by WAT have been shown to regulate lipid metabolism, with the genes for key transporters and metabolic enzymes all exhibiting circadian rhythmicity under the direct control of BMAL1 (as reviewed by Heyde et al) (6). WAT functions are also affected by the hormones insulin and glucagon and thus possibly by their circadian rhythms. Furthermore, the rhythms in nutrient influx on feeding, such as those in free fatty acids, also contribute to the circadian activity of the WAT (133). Consistent with these findings, adipose-specific Arntl knockout mice have alterations in their diurnal gene expression profile, leading to altered fatty acid release into the bloodstream, whereas Arntl overexpression increases lipogenic gene expression in the WAT (134). Similarly, Clock-mutant animals have decreased lipolytic gene expression, with loss of the normal rhythms in circulating fatty acids, and obesity (135).
Exposure to HFD-feeding has been shown to dampen the amplitude in the circadian expression of the core molecular clock in WAT, thereby disrupting downstream targets including nuclear receptors as well as lipid metabolism pathways and leading to abnormal patterns in the release of fatty acids into the circulation (32). Interestingly, the clock disruption as induced by high-fat feeding is also associated with increased macrophage infiltration into the WAT and elevated expression of proinflammatory cytokines (136). Finally, palm oil administration to mice as well as palmitate treatment of adipocytes disrupts the circadian rhythms in association with increased adipogenic and reduced mitochondrial function markers (137). On the other hand, treatment with oleate, a monounsaturated fatty acid, largely opposes these effects, supporting the well-established health benefits of this fatty acid (137).
BAT tissue clock gene expression is negatively affected by high-fat feeding, with a decreased amplitude in cycling of the core clock components as well as decreased expression of uncoupling protein-1, the latter of which is also effected by circadian disruption (138). However, WAT also secretes a number of adipokines that affect BAT function, including the anorexigenic hormone leptin and the insulin sensitizer adiponectin, both of which oscillate with a circadian rhythm (139). Hence, not only is leptin expression stimulated by insulin, but it is also a direct target of the circadian clock machinery (140). Hence, adipose-specific deletion of Arntl results in alterations in the timing of leptin release and increases daytime feeding, leading to obesity (134). Importantly, leptin also increases uncoupling protein-1 expression in BAT, thereby increasing energy expenditure (141). Conversely, WAT release of adiponectin inhibits uncoupling protein-1 expression in BAT (142). The balance between leptin and adiponectin, therefore, with leptin positively and adiponectin inversely correlated to fat mass (143), contributes to overall metabolic homeostasis through the 24-hour day/night cycle. Finally, BAT activity is also affected by bile acids as well as several gut hormones with circadian rhythms, including cholecystokinin, which stimulates BAT through the sympathetic nervous system, and ghrelin, which inhibits BAT (144, 145). These factors thus serve to further coordinate circadian BAT activity with the feeding-fasting cycle.
It must be recognized that not all metabolic pathways, cells, and tissues are regulated by the circadian clock (33), as food-entrainable oscillations have been shown to exist in the absence of the circadian clock (146, 147). However, the mechanisms regulating such clock-independent rhythms have proven difficult to study. Nevertheless, the synchronization of metabolic functions between the key peripheral tissues has been established to be determined not only by the expression of cell-autonomous clock genes, but also by feedforward pathways that synchronize clock activity between these tissues. Disruptions in the circadian rhythms of one or more of these tissues, as can be induced by alterations in the dietary composition, the timing of nutrient intake, and/or in the microbiome, are therefore all associated with metabolic disease.
Feedback Coordination of Metabolic Responses to Food Intake
The main body of this review has covered some of the key mechanisms by which the temporal control of nutrient ingestion and subsequent disposition is tightly coordinated in a feedforward fashion. However, feedback loops between these circadian-regulated tissues also contribute to metabolic homeostasis. For example, nutrient ingestion stimulates the release of L-cell hormones that serve as short-term satiety factors (eg, GLP-1, oxyntomodulin, and peptide YY), whereas levels of the orexigen ghrelin and of the hyperglycemic hormone glucagon decrease with feeding; GLP-1 and peptide YY also serve as ileal brakes, slowing down the rate of nutrient digestion and absorption (148). Interleukin-6 secreted by skeletal muscle during exercise, and leptin released by WAT, both stimulate L-cell secretion (149, 150), while leptin also provides an orexigenic signal directly to the brain to suppress long-term food intake (151).
Chronotherapeutic Approaches to Treat Metabolic Disease
Chronotherapy is used extensively in the clinic, with many drugs given in a temporal fashion so as to maximize their effectiveness (33, 37). Given the extensive evidence linking circadian disruption to metabolic disease, the possible utility of chronotherapy for prevention and/or treatment has therefore become of increasing interest (152, 153). For example, the polyphenol resveratrol reverses the suppression of hepatic Arntl expression and induction of insulin resistance caused by keeping mice in constant darkness, as well as reversing the effects of high-fat feeding or palmitate on clock gene expression in the liver, WAT, and skeletal muscle (129, 154, 155). Similarly, the flavonoid nobiletin enhances insulin secretion through an Arntl-dependent mechanism, reduces hypercholesterolemia, and improves both circadian oscillations and mitochondrial function in skeletal muscle, thereby reversing the negative effects of obesogenic feeding (85, 103, 156). However, given recent studies demonstrating that humans are increasingly consuming food during the normal, fasting/inactive period (157), interest has also focused on the possible clinical benefits of “time-restricted feeding.” Indeed, there are now several reports of improved metabolism in humans who restrict their food intake to the daylight hours (158-161). However, whether such an approach is feasible in the “real world” will require additional long-term studies (162).
Conclusion
The importance of the circadian clock to normal physiology is becoming increasingly well recognized. As reviewed by experts in the field, this collection of articles published in Endocrinology also demonstrates the essential role of the peripheral clock in maintaining metabolic homeostasis, as evidenced not only by the highly synchronized interplay between the gut and its microbiome, pancreatic islet cells, hepatocytes, myocytes, and adipocytes, but by the metabolic consequences of circadian disruption in one or more of these different tissues.
Acknowledgments
Financial Support: P.L.B. is supported by a Canada Research Chair. Circadian studies in the Brubaker laboratory are supported by the Canadian Institutes of Health Research (operating grant No. PJT-15308).
Glossary
Abbreviations
- BAT
brown adipose tissue
- BMAL1
brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein 1
- CLOCK
circadian locomotor output cycles kaput
- CRY
cryptochrome
- GIP
glucose-dependent insulinotrophic polypeptide
- GLP-1
glucagon-like peptide-1
- HFD
high-fat diet
- IEC
intestinal epithelial cell
- PER
period
- SCFAs
short-chain fatty acids
- WAT
white adipose tissue
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
No new data were created or analyzed in this review article. Therefore, data sharing is not applicable.
References
- 1. Martchenko A, Martchenko SE, Biancolin AD, Brubaker PL. Circadian rhythms and the gastrointestinal tract: relationship to metabolism and gut hormones. Endocrinology. 2020;161(12):bqaa167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alvarez Y, Glotfelty LG, Blank N, Dohnalova L, Thaiss CA. The microbiome as a circadian coordinator of metabolism. Endocrinology. 2020;161(12):bqaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seshadri N, Doucette CA. Circadian regulation of the pancreatic beta cell. Endocrinology. 2021;162(12):bqab089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCommis KS, Butler AA. The importance of keeping time in the liver. Endocrinology. 2021;162(12):bqaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oosterman JE, Wopereis S, Kalsbeek A. The circadian clock, shift work, and tissue-specific insulin resistance. Endocrinology. 2020;161(12):bqaa180. [DOI] [PubMed] [Google Scholar]
- 6. Heyde I, Begemann K, Oster H. Contributions of white and brown adipose tissues to the circadian regulation of energy metabolism. Endocrinology. 2021;162(12):bqab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takahashi JS. Molecular components of the circadian clock in mammals. Diabetes Obes Metab. 2015;17(Suppl 1):6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asher G, Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell. 2015;161(1):84-92. [DOI] [PubMed] [Google Scholar]
- 9. Gerhart-Hines Z, Lazar MA. Circadian metabolism in the light of evolution. Endocr Rev. 2015;36(3):289-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science. 2016;354(6315):994-999. [DOI] [PubMed] [Google Scholar]
- 11. Kawachi I, Colditz GA, Stampfer MJ, et al. . Prospective study of shift work and risk of coronary heart disease in women. Circulation. 1995;92(11):3178-3182. [DOI] [PubMed] [Google Scholar]
- 12. Tenkanen L, Sjöblom T, Kalimo R, Alikoski T, Härmä M. Shift work, occupation and coronary heart disease over 6 years of follow-up in the Helsinki Heart Study. Scand J Work Environ Health. 1997;23(4):257-265. [DOI] [PubMed] [Google Scholar]
- 13. Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27 485 people. Occup Environ Med. 2001;58(11):747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Turek FW, Joshu C, Kohsaka A, et al. . Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujino Y, Iso H, Tamakoshi A, et al. ; Japanese Collaborative Cohort Study Group . A prospective cohort study of shift work and risk of ischemic heart disease in Japanese male workers. Am J Epidemiol. 2006;164(2):128-135. [DOI] [PubMed] [Google Scholar]
- 16. Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008;105(39):15172-15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garaulet M, Lee YC, Shen J, et al. . CLOCK genetic variation and metabolic syndrome risk: modulation by monounsaturated fatty acids. Am J Clin Nutr. 2009;90(6):1466-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marcheva B, Ramsey KM, Buhr ED, et al. . Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466(7306):627-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. Plos Med. 2011;8(12):e1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dashti HS, Gómez-Abellán P, Qian J, et al. . Late eating is associated with cardiometabolic risk traits, obesogenic behaviors, and impaired weight loss. Am J Clin Nutr. 2020;113(1):154-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zarrinpar A, Chaix A, Panda S. Daily eating patterns and their impact on health and disease. Trends Endocrinol Metab. 2016;27(2):69-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reinke H, Asher G. Crosstalk between metabolism and circadian clocks. Nat Rev Mol Cell Biol. 2019;20(4):227-241. [DOI] [PubMed] [Google Scholar]
- 23. Khosravipour M, Khanlari P, Khazaie S, Khosravipour H, Khazaie H. A systematic review and meta-analysis of the association between shift work and metabolic syndrome: the roles of sleep, gender, and type of shift work. Sleep Med Rev. 2021;57:101427. [DOI] [PubMed] [Google Scholar]
- 24. Poggiogalle E, Jamshed H, Peterson CM. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism. 2018;84:11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev. 2013;93(1):107-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Woon PY, Kaisaki PJ, Bragança J, et al. . Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci U S A. 2007;104(36):14412-14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blakemore AI, Meyre D, Delplanque J, et al. . A rare variant in the visfatin gene (NAMPT/PBEF1) is associated with protection from obesity. Obesity (Silver Spring). 2009;17(8):1549-1553. [DOI] [PubMed] [Google Scholar]
- 28. Dashti HS, Smith CE, Lee YC, et al. . CRY1 circadian gene variant interacts with carbohydrate intake for insulin resistance in two independent populations: Mediterranean and North American. Chronobiol Int. 2014;31(5):660-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dashti HS, Merino J, Lane JM, et al. . Genome-wide association study of breakfast skipping links clock regulation with food timing. Am J Clin Nutr. 2019;110(2):473-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jagannath A, Taylor L, Wakaf Z, Vasudevan SR, Foster RG. The genetics of circadian rhythms, sleep and health. Hum Mol Genet. 2017;26(R2):R128-R138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garaulet M, Gómez-Abellán P. Timing of food intake and obesity: a novel association. Physiol Behav. 2014;134:44-50. [DOI] [PubMed] [Google Scholar]
- 32. Kohsaka A, Laposky AD, Ramsey KM, et al. . High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6(5):414-421. [DOI] [PubMed] [Google Scholar]
- 33. Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111(45):16219-16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Panda S, Antoch MP, Miller BH, et al. . Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307-320. [DOI] [PubMed] [Google Scholar]
- 35. Koike N, Yoo SH, Huang HC, et al. . Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338(6105):349-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mure LS, Le HD, Benegiamo G, et al. . Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. 2018;359(6105):eaao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ruben MD, Wu G, Smith DF, et al. . A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine. Sci Transl Med. 2018;10(458):eaat8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oh ES, Petronis A. Origins of human disease: the chrono-epigenetic perspective. Nat Rev Genet. 2021;22(8):533-546. [DOI] [PubMed] [Google Scholar]
- 39. Hoogerwerf WA, Hellmich HL, Cornélissen G, et al. . Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology. 2007;133(4):1250-1260. [DOI] [PubMed] [Google Scholar]
- 40. Gil-Lozano M, Mingomataj EL, Wu WK, Ridout SA, Brubaker PL. Circadian secretion of the intestinal hormone GLP-1 by the rodent L cell. Diabetes. 2014;63(11):3674-3685. [DOI] [PubMed] [Google Scholar]
- 41. Martchenko SE, Prescot D, Martchenko A, Sweeney ME, Philpott DJ, Brubaker PL. Diurnal changes in the murine small intestine are disrupted by obesogenic Western diet feeding and microbial dysbiosis. Sci Rep. 2021; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saito M, Kato H, Suda M. Circadian rhythm of intestinal disaccharidases of rats fed with adiurnal periodicity. Am J Physiol. 1980;238(2):G97-G101. [DOI] [PubMed] [Google Scholar]
- 43. Tavakkolizadeh A, Ramsanahie A, Levitsky LL, et al. . Differential role of vagus nerve in maintaining diurnal gene expression rhythms in the proximal small intestine. J Surg Res. 2005;129(1):73-78. [DOI] [PubMed] [Google Scholar]
- 44. Hussain MM, Pan X. Circadian regulation of macronutrient absorption. J Biol Rhythms. 2015;30(6):459-469. [DOI] [PubMed] [Google Scholar]
- 45. Kawai M, Kinoshita S, Yamazaki M, et al. . Intestinal clock system regulates skeletal homeostasis. JCI Insight. 2019;4(5):e121798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kuang Z, Wang Y, Li Y, et al. . The intestinal microbiota programs diurnal rhythms in host metabolism through histone deacetylase 3. Science. 2019;365(6460):1428-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pan X, Hussain MM. Clock is important for food and circadian regulation of macronutrient absorption in mice. J Lipid Res. 2009;50(9):1800-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kentish SJ, Frisby CL, Kennaway DJ, Wittert GA, Page AJ. Circadian variation in gastric vagal afferent mechanosensitivity. J Neurosci. 2013;33(49):19238-19242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Page AJ. Gastrointestinal vagal afferents and food intake: relevance of circadian rhythms. Nutrients. 2021;13(3):844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thaiss CA, Levy M, Korem T, et al. . Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell. 2016;167(6):1495-1510.e12. [DOI] [PubMed] [Google Scholar]
- 51. Liang X, Bushman FD, FitzGerald GA. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci U S A. 2015;112(33):10479-10484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Y, Kuang Z, Yu X, Ruhn KA, Kubo M, Hooper LV. The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science. 2017;357(6354):912-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kaczmarek JL, Musaad SM, Holscher HD. Time of day and eating behaviors are associated with the composition and function of the human gastrointestinal microbiota. Am J Clin Nutr. 2017;106(5):1220-1231. [DOI] [PubMed] [Google Scholar]
- 54. Eggink HM, Oosterman JE, de Goede P, et al. . Complex interaction between circadian rhythm and diet on bile acid homeostasis in male rats. Chronobiol Int. 2017;34(10):1339-1353. [DOI] [PubMed] [Google Scholar]
- 55. Whitt J, Woo V, Lee P, et al. . Disruption of epithelial HDAC3 in intestine prevents diet-induced obesity in mice. Gastroenterology. 2018;155(2):501-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wu SE, Hashimoto-Hill S, Woo V, et al. . Microbiota-derived metabolite promotes HDAC3 activity in the gut. Nature. 2020;586(7827):108-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Thaiss CA, Zeevi D, Levy M, et al. . Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159(3):514-529. [DOI] [PubMed] [Google Scholar]
- 58. Ferris CF, George JK, Albers HE. Circadian rhythm of neurotensin levels in rat small intestine. Regul Pept. 1986;15(4):285-292. [DOI] [PubMed] [Google Scholar]
- 59. Pasley JN, Rayford PL. Effects of dietary protein alterations on circadian rhythms of gastrointestinal peptides in rats. Dig Dis Sci. 1990;35(10):1265-1270. [DOI] [PubMed] [Google Scholar]
- 60. Martchenko SE, Martchenko A, Cox BJ, et al. . Circadian GLP-1 secretion in mice is dependent on the intestinal microbiome for maintenance of diurnal metabolic homeostasis. Diabetes. 2020;69(12):2589-2602. [DOI] [PubMed] [Google Scholar]
- 61. Farhadipour M, Depoortere I. The function of gastrointestinal hormones in obesity-complications for the regulation of energy intake. Nutrients. 2021;13(6):1839-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Laermans J, Vancleef L, Tack J, Depoortere I. Role of the clock gene Bmal1 and the gastric ghrelin-secreting cell in the circadian regulation of the ghrelin-GOAT system. Sci Rep. 2015;5:16748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2(8571):1300-1304. [DOI] [PubMed] [Google Scholar]
- 64. Turton MD, O’Shea D, Gunn I, et al. . A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379(6560):69-72. [DOI] [PubMed] [Google Scholar]
- 65. Müller TD, Finan B, Bloom SR, et al. . Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019;30:72-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A. 1996;93(15):7911-7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dakin CL, Gunn I, Small CJ, et al. . Oxyntomodulin inhibits food intake in the rat. Endocrinology. 2001;142(10):4244-4250. [DOI] [PubMed] [Google Scholar]
- 68. Lindgren O, Mari A, Deacon CF, et al. . Differential islet and incretin hormone responses in morning versus afternoon after standardized meal in healthy men. J Clin Endocrinol Metab. 2009;94(8):2887-2892. [DOI] [PubMed] [Google Scholar]
- 69. Gonnissen HK, Rutters F, Mazuy C, Martens EA, Adam TC, Westerterp-Plantenga MS. Effect of a phase advance and phase delay of the 24-h cycle on energy metabolism, appetite, and related hormones. Am J Clin Nutr. 2012;96(4):689-697. [DOI] [PubMed] [Google Scholar]
- 70. Biancolin AD, Martchenko A, Mitova E, et al. . The core clock gene, Bmal1, and its downstream target, the SNARE regulatory protein secretagogin, are necessary for circadian secretion of glucagon-like peptide-1. Mol Metab. 2020;31:124-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cheeseman CI. Upregulation of SGLT-1 transport activity in rat jejunum induced by GLP-2 infusion in vivo. Am J Physiol. 1997;273(6):R1965-R1971. [DOI] [PubMed] [Google Scholar]
- 72. Cheeseman CI, Tsang R. The effect of GIP and glucagon-like peptides on intestinal basolateral membrane hexose transport. Am J Physiol. 1996;271(3 Pt 1):G477-G482. [DOI] [PubMed] [Google Scholar]
- 73. Gault VA, O’Harte FPM, Harriott P, Mooney MH, Green BD, Flatt PR. Effects of the novel (Pro3)GIP antagonist and exendin(9-39)amide on GIP- and GLP-1-induced cyclic AMP generation, insulin secretion and postprandial insulin release in obese diabetic (ob/ob) mice: evidence that GIP is the major physiological incretin. Diabetologia. 2003;46(2):222-230. [DOI] [PubMed] [Google Scholar]
- 74. Batterham RL, Cowley MA, Small CJ, et al. . Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418(6898):650-654. [DOI] [PubMed] [Google Scholar]
- 75. Moghadam AA, Moran TH, Dailey MJ. Alterations in circadian and meal-induced gut peptide levels in lean and obese rats. Exp Biol Med (Maywood). 2017;242(18):1786-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gil-Lozano M, Wu WK, Martchenko A, Brubaker PL. High-fat diet and palmitate alter the rhythmic secretion of glucagon-like peptide-1 by the rodent L-cell. Endocrinology. 2016;157(2):586-599. [DOI] [PubMed] [Google Scholar]
- 77. Martchenko A, Oh RH, Wheeler SE, Gurges P, Chalmers JA, Brubaker PL. Suppression of circadian secretion of glucagon-like peptide-1 by the saturated fatty acid, palmitate. Acta Physiol (Oxf). 2018;222(4):e13007. [DOI] [PubMed] [Google Scholar]
- 78. Martchenko SE, Martchenko A, Biancolin AD, Waller A, Brubaker PL. L-cell Arntl is required for rhythmic glucagon-like peptide-1 secretion and maintenance of intestinal homeostasis. Mol Metabol. Published online September 11, 2021.. doi:10.1016/j.molmet.2021.101340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dumoulin V, Moro F, Barcelo A, Dakka T, Cuber JC. Peptide YY, glucagon-like peptide-1, and neurotensin responses to luminal factors in the isolated vascularly perfused rat ileum. Endocrinology. 1998;139(9):3780-3786. [DOI] [PubMed] [Google Scholar]
- 80. Thomas C, Gioiello A, Noriega L, et al. . TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tachon S, Zhou J, Keenan M, Martin R, Marco ML. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiol Ecol. 2013;83(2):299-309. [DOI] [PubMed] [Google Scholar]
- 82. Plovier H, Everard A, Druart C, et al. . A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107-113. [DOI] [PubMed] [Google Scholar]
- 83. Depommier C, Everard A, Druart C, et al. . Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Roshanravan N, Bastani S, Tutunchi H, et al. . A comprehensive systematic review of the effectiveness of Akkermansia muciniphila, a member of the gut microbiome, for the management of obesity and associated metabolic disorders. Arch Physiol Biochem. Published online January 15, 2021. doi:10.1080/13813455.2021.1871760 [DOI] [PubMed] [Google Scholar]
- 85. Calles-Escandon J, Jaspan J, Robbins DC. Postprandial oscillatory patterns of blood glucose and insulin in NIDDM. Abnormal diurnal insulin secretion patterns and glucose homeostasis independent of obesity. Diabetes Care. 1989;12(10):709-714. [DOI] [PubMed] [Google Scholar]
- 86. Stamenkovic JA, Olsson AH, Nagorny CL, et al. . Regulation of core clock genes in human islets. Metabolism. 2012;61(7):978-985. [DOI] [PubMed] [Google Scholar]
- 87. Petrenko V, Gandasi NR, Sage D, Tengholm A, Barg S, Dibner C. In pancreatic islets from type 2 diabetes patients, the dampened circadian oscillators lead to reduced insulin and glucagon exocytosis. Proc Natl Acad Sci U S A. 2020;117(5):2484-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Javeed N, Brown MR, Rakshit K, Her T, Sen SK, Matveyenko AV. Proinflammatory cytokine interleukin 1β disrupts β-cell circadian clock function and regulation of insulin secretion. Endocrinology. 2021;162(1):bqaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bergman RN. Origins and history of the minimal model of glucose regulation. Front Endocrinol (Lausanne). 2020;11:583016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Perelis M, Marcheva B, Ramsey KM, et al. . Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015;350(6261):aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rakshit K, Hsu TW, Matveyenko AV. Bmal1 is required for beta cell compensatory expansion, survival and metabolic adaptation to diet-induced obesity in mice. Diabetologia. 2016;59(4):734-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Petrenko V, Saini C, Giovannoni L, et al. . Pancreatic α- and β-cellular clocks have distinct molecular properties and impact on islet hormone secretion and gene expression. Genes Dev. 2017;31(4):383-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Qian J, Block GD, Colwell CS, Matveyenko AV. Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes. 2013;62(10):3469-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Qian J, Yeh B, Rakshit K, Colwell CS, Matveyenko AV. Circadian disruption and diet-induced obesity synergize to promote development of β-cell failure and diabetes in male rats. Endocrinology. 2015;156(12):4426-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Moore CX, Cooper GJ. Co-secretion of amylin and insulin from cultured islet beta-cells: modulation by nutrient secretagogues, islet hormones and hypoglycemic agents. Biochem Biophys Res Commun. 1991;179(1):1-9. [DOI] [PubMed] [Google Scholar]
- 96. Gebre-Medhin S, Mulder H, Pekny M, et al. . Increased insulin secretion and glucose tolerance in mice lacking islet amyloid polypeptide (amylin). Biochem Biophys Res Commun. 1998;250(2):271-277. [DOI] [PubMed] [Google Scholar]
- 97. Sonne N, Karsdal MA, Henriksen K. Mono and dual agonists of the amylin, calcitonin, and CGRP receptors and their potential in metabolic diseases. Mol Metab. 2021;46:101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kalsbeek A, Strubbe JH. Circadian control of insulin secretion is independent of the temporal distribution of feeding. Physiol Behav. 1998;63(4):553-558. [DOI] [PubMed] [Google Scholar]
- 99. Petrenko V, Dibner C. Cell-specific resetting of mouse islet cellular clocks by glucagon, glucagon-like peptide 1 and somatostatin. Acta Physiol (Oxf). 2018;222(4):e13021. [DOI] [PubMed] [Google Scholar]
- 100. Dunning BE, Foley JE, Ahrén B. Alpha cell function in health and disease: influence of glucagon-like peptide-1. Diabetologia. 2005;48(9):1700-1713. [DOI] [PubMed] [Google Scholar]
- 101. Priyadarshini M, Navarro G, Layden BT. Gut microbiota: FFAR reaching effects on islets. Endocrinology. 2018;159(6):2495-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Vieira E, Marroquí L, Batista TM, et al. . The clock gene Rev-erbα regulates pancreatic β-cell function: modulation by leptin and high-fat diet. Endocrinology. 2012;153(2):592-601. [DOI] [PubMed] [Google Scholar]
- 103. Rakshit K, Matveyenko AV. Induction of core circadian clock transcription factor bmal1 enhances β-cell function and protects against obesity-induced glucose intolerance. Diabetes. 2021;70(1):143-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Boden G, Chen X, Urbain JL. Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes. 1996;45(8):1044-1050. [DOI] [PubMed] [Google Scholar]
- 105. Feng D, Liu T, Sun Z, et al. . A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331(6022):1315-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mukherji A, Kobiita A, Chambon P. Shifting the feeding of mice to the rest phase creates metabolic alterations, which, on their own, shift the peripheral circadian clocks by 12 hours. Proc Natl Acad Sci U S A. 2015;112(48):E6683-E6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Opperhuizen AL, Wang D, Foppen E, et al. . Feeding during the resting phase causes profound changes in physiology and desynchronization between liver and muscle rhythms of rats. Eur J Neurosci. 2016;44(10):2795-2806. [DOI] [PubMed] [Google Scholar]
- 108. Taira A, Arita E, Matsumoto E, et al. . Systemic oscillator-driven and nutrient-responsive hormonal regulation of daily expression rhythms for gluconeogenic enzyme genes in the mouse liver. Chronobiol Int. 2019;36(5):591-615. [DOI] [PubMed] [Google Scholar]
- 109. Matsumoto E, Ishihara A, Tamai S, et al. . Time of day and nutrients in feeding govern daily expression rhythms of the gene for sterol regulatory element-binding protein (SREBP)-1 in the mouse liver. J Biol Chem. 2010;285(43):33028-33036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Guan D, Xiong Y, Borck PC, et al. . Diet-induced circadian enhancer remodeling synchronizes opposing hepatic lipid metabolic processes. Cell. 2018;174(4):831-842.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Khapre RV, Patel SA, Kondratova AA, et al. . Metabolic clock generates nutrient anticipation rhythms in mTOR signaling. Aging (Albany NY). 2014;6(8):675-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Patel SA, Velingkaar N, Makwana K, Chaudhari A, Kondratov R. Calorie restriction regulates circadian clock gene expression through BMAL1 dependent and independent mechanisms. Sci Rep. 2016;6:25970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Oishi K, Yasumoto Y, Higo-Yamamoto S, Yamamoto S, Ohkura N. Feeding cycle-dependent circulating insulin fluctuation is not a dominant zeitgeber for mouse peripheral clocks except in the liver: differences between endogenous and exogenous insulin effects. Biochem Biophys Res Commun. 2017;483(1):165-170. [DOI] [PubMed] [Google Scholar]
- 114. Crosby P, Hamnett R, Putker M, et al. . Insulin/IGF-1 drives PERIOD synthesis to entrain circadian rhythms with feeding time. Cell. 2019;177(4):896-909.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Landgraf D, Tsang AH, Leliavski A, et al. . Oxyntomodulin regulates resetting of the liver circadian clock by food. Elife. 2015;4:e06253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang Q, Yin Y, Zhang W. Ghrelin restores the disruption of the circadian clock in steatotic liver. Int J Mol Sci. 2018;19(10):3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Montagner A, Korecka A, Polizzi A, et al. . Hepatic circadian clock oscillators and nuclear receptors integrate microbiome-derived signals. Sci Rep. 2016;6:20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tahara Y, Yamazaki M, Sukigara H, et al. . Gut microbiota-derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci Rep. 2018;8(1):1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Oh HYP, Visvalingam V, Wahli W. The PPAR-microbiota-metabolic organ trilogy to fine-tune physiology. FASEB J. 2019;33(9):9706-9730. [DOI] [PubMed] [Google Scholar]
- 120. Eckel-Mahan KL, Patel VR, de Mateo S, et al. . Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155(7):1464-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jacobi D, Liu S, Burkewitz K, et al. . Hepatic Bmal1 regulates rhythmic mitochondrial dynamics and promotes metabolic fitness. Cell Metab. 2015;22(4):709-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tal Y, Chapnik N, Froy O. Non-obesogenic doses of fatty acids modulate the functionality of the circadian clock in the liver. Cell Mol Life Sci. 2019;76(9):1795-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tong X, Zhang D, Arthurs B, et al. . Palmitate inhibits SIRT1-dependent BMAL1/CLOCK interaction and disrupts circadian gene oscillations in hepatocytes. PLoS One. 2015;10(6):e0130047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sylow L, Tokarz VL, Richter EA, Klip A. The many actions of insulin in skeletal muscle, the paramount tissue determining glycemia. Cell Metab. 2021;33(4):758-780. [DOI] [PubMed] [Google Scholar]
- 125. Dyar KA, Ciciliot S, Wright LE, et al. . Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab. 2014;3(1):29-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. van Moorsel D, Hansen J, Havekes B, et al. . Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol Metab. 2016;5(8):635-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Perrin L, Loizides-Mangold U, Skarupelova S, et al. . Human skeletal myotubes display a cell-autonomous circadian clock implicated in basal myokine secretion. Mol Metab. 2015;4(11):834-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Andrews JL, Zhang X, McCarthy JJ, et al. . CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci U S A. 2010;107(44):19090-19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Liu J, Zhou B, Yan M, et al. . CLOCK and BMAL1 regulate muscle insulin sensitivity via SIRT1 in male mice. Endocrinology. 2016;157(6):2259-2269. [DOI] [PubMed] [Google Scholar]
- 130. Sato S, Parr EB, Devlin BL, Hawley JA, Sassone-Corsi P. Human metabolomics reveal daily variations under nutritional challenges specific to serum and skeletal muscle. Mol Metab. 2018;16:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Sardon Puig L, Pillon NJ, Näslund E, Krook A, Zierath JR. Influence of obesity, weight loss, and free fatty acids on skeletal muscle clock gene expression. Am J Physiol Endocrinol Metab. 2020;318(1):E1-E10. [DOI] [PubMed] [Google Scholar]
- 132. Budai Z, Balogh L, Sarang Z. Short-term high-fat meal intake alters the expression of circadian clock-, inflammation-, and oxidative stress-related genes in human skeletal muscle. Int J Food Sci Nutr. 2019;70(6):749-758. [DOI] [PubMed] [Google Scholar]
- 133. Noshiro M, Kawamoto T, Nakashima A, et al. . DEC1 regulates the rhythmic expression of PPARγ target genes involved in lipid metabolism in white adipose tissue. Genes Cells. 2020;25(4):232-241. [DOI] [PubMed] [Google Scholar]
- 134. Paschos GK, Ibrahim S, Song WL, et al. . Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med. 2012;18(12):1768-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kennaway DJ, Owens JA, Voultsios A, Wight N. Adipokines and adipocyte function in Clock mutant mice that retain melatonin rhythmicity. Obesity (Silver Spring). 2012;20(2):295-305. [DOI] [PubMed] [Google Scholar]
- 136. Kim SM, Neuendorff N, Alaniz RC, Sun Y, Chapkin RS, Earnest DJ. Shift work cycle-induced alterations of circadian rhythms potentiate the effects of high-fat diet on inflammation and metabolism. FASEB J. 2018;32(6):3085-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Tal Y, Chapnik N, Froy O. Non-obesogenic doses of palmitate disrupt circadian metabolism in adipocytes. Adipocyte. 2019;8(1):392-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Straat ME, Hogenboom R, Boon MR, Rensen PCN, Kooijman S. Circadian control of brown adipose tissue. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866(8):158961. [DOI] [PubMed] [Google Scholar]
- 139. Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. 2003;88(6):2838-2843. [DOI] [PubMed] [Google Scholar]
- 140. Challet E. Circadian aspects of adipokine regulation in rodents. Best Pract Res Clin Endocrinol Metab. 2017;31(6):573-582. [DOI] [PubMed] [Google Scholar]
- 141. Commins SP, Watson PM, Padgett MA, Dudley A, Argyropoulos G, Gettys TW. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology. 1999;140(1):292-300. [DOI] [PubMed] [Google Scholar]
- 142. Qiao L, Yoo Hs, Bosco C, et al. . Adiponectin reduces thermogenesis by inhibiting brown adipose tissue activation in mice. Diabetologia. 2014;57(5):1027-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hocking S, Samocha-Bonet D, Milner KL, Greenfield JR, Chisholm DJ. Adiposity and insulin resistance in humans: the role of the different tissue and cellular lipid depots. Endocr Rev. 2013;34(4):463-500. [DOI] [PubMed] [Google Scholar]
- 144. Yamazaki T, Morimoto-Kobayashi Y, Koizumi K, et al. . Secretion of a gastrointestinal hormone, cholecystokinin, by hop-derived bitter components activates sympathetic nerves in brown adipose tissue. J Nutr Biochem. 2019;64:80-87. [DOI] [PubMed] [Google Scholar]
- 145. Lin L, Lee JH, Bongmba OY, et al. . The suppression of ghrelin signaling mitigates age-associated thermogenic impairment. Aging (Albany NY). 2014;6(12):1019-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Rosenwasser AM, Pelchat RJ, Adler NT. Memory for feeding time: possible dependence on coupled circadian oscillators. Physiol Behav. 1984;32(1):25-30. [DOI] [PubMed] [Google Scholar]
- 147. Pendergast JS, Yamazaki S. The mysterious food-entrainable oscillator: insights from mutant and engineered mouse models. J Biol Rhythms. 2018;33(5):458-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Dong CX, Brubaker PL. Ghrelin, the proglucagon-derived peptides and peptide YY in nutrient homeostasis. Nat Rev Gastroenterol Hepatol. 2012;9(12):705-715. [DOI] [PubMed] [Google Scholar]
- 149. Ellingsgaard H, Seelig E, Timper K, et al. . GLP-1 secretion is regulated by IL-6 signalling: a randomised, placebo-controlled study. Diabetologia. 2020;63(2):362-373. [DOI] [PubMed] [Google Scholar]
- 150. Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52(2):252-259. [DOI] [PubMed] [Google Scholar]
- 151. Friedman JM. Leptin and the endocrine control of energy balance. Nat Metab. 2019;1(8):754-764. [DOI] [PubMed] [Google Scholar]
- 152. Sulli G, Manoogian ENC, Taub PR, Panda S. Training the circadian clock, clocking the drugs, and drugging the clock to prevent, manage, and treat chronic diseases. Trends Pharmacol Sci. 2018;39(9):812-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ruan W, Yuan X, Eltzschig HK. Circadian rhythm as a therapeutic target. Nat Rev Drug Discov. 2021;20(4):287-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Miranda J, Portillo MP, Madrid JA, Arias N, Macarulla MT, Garaulet M. Effects of resveratrol on changes induced by high-fat feeding on clock genes in rats. Br J Nutr. 2013;110(8):1421-1428. [DOI] [PubMed] [Google Scholar]
- 155. Zhou B, Zhang Y, Zhang F, et al. . CLOCK/BMAL1 regulates circadian change of mouse hepatic insulin sensitivity by SIRT1. Hepatology. 2014;59(6):2196-2206. [DOI] [PubMed] [Google Scholar]
- 156. Nohara K, Mallampalli V, Nemkov T, et al. . Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat Commun. 2019;10(1):3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22(5):789-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hatori M, Vollmers C, Zarrinpar A, et al. . Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Chaix A, Lin T, Le HD, Chang MW, Panda S. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab. 2019;29(2):303-319.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019;11(6):1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Wilkinson MJ, Manoogian ENC, Zadourian A, et al. . Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31(1):92-104.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Lee SA, Sypniewski C, Bensadon BA, et al. . Determinants of adherence in time-restricted feeding in older adults: lessons from a pilot study. Nutrients. 2020;12(3):874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this review article. Therefore, data sharing is not applicable.