Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is a significant public health burden, with up to 30% of the US population affected. The prevalence of NAFLD among inflammatory bowel disease (IBD) patients is unknown. Understanding risk factors for NAFLD in IBD patients has implications in the treatment of these patients. The purpose of this study was to determine the prevalence of NAFLD among IBD patients and to identify risk factors associated with NAFLD development.
Methods
Embase and MEDLINE databases were searched using Medical Subject Headlines to find studies that assessed the prevalence of NAFLD among IBD patients. Twenty-seven English-language research abstracts/articles were identified between January 2005 and April 2018. Meta-analyses were performed using random-effects models. Prevalence of NAFLD among IBD patients was compared with prevalence of NAFLD in the general population.
Results
Based on data pooled from all 27 studies, the prevalence of NAFLD among IBD patients was 32% (95% CI, 24%–40%) with substantial heterogeneity (I2 = 98%). The prevalence of NAFLD among IBD patients (32%) is statistically significantly higher than the prevalence of NAFLD in the general population (25.2%; P < 0.001). Factors associated with the development of NAFLD among IBD patients included age, BMI, diabetes, IBD duration, and prior history of bowel resection.
Conclusions
There is a higher prevalence of NAFLD among IBD patients compared with the general population. Previous treatment regimens may be a risk factor for the development of NAFLD. Future studies are needed to further clarify these risk factors and determine screening recommendations.
Keywords: inflammatory bowel disease, nonalcoholic fatty liver disease, meta-analysis
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a significant public health burden. The prevalence of NAFLD is rapidly rising worldwide.1 The pooled overall prevalence of NAFLD diagnosed by imaging within the United States is estimated to be 24.13%, and the pooled overall global prevalence of NAFLD diagnosed by imaging is estimated to be 25.24%.2 Risk factors for fatty liver include insulin resistance and diabetes, obesity, and the metabolic syndrome. In addition, certain medications such as steroids and methotrexate can also lead to hepatic steatosis. Some with NAFLD (between 10% and 20% of patients with NAFLD) will develop nonalcoholic steatohepatitis (NASH) and progress to fibrosis, cirrhosis, and its complications including portal hypertension, hepatocellular carcinoma, and the need for liver transplant.2–4 Nonalcoholic fatty liver disease is currently the second leading indication for liver transplantation among adults (first among women) and will likely be the leading indication for liver transplant in the coming decades.5–7 In 2016, the yearly economic burden of NAFLD was estimated to be $103 billion each year.8
There are conflicting data regarding the prevalence of NAFLD among patients with inflammatory bowel disease (IBD), and the etiology of this association is unclear. Both diseases involve a complex relationship between environment and polygenic factors, with immune dysregulation also playing a central role.9 Nonalcoholic fatty liver disease is associated with diabetes, obesity, and the metabolic syndrome; however, there are a subset of patients who have “lean NAFLD”.10 Although the incidence of obesity in IBD increases, a subset of patients with comorbid IBD and NAFLD may fall into the “lean NAFLD” category, as it has been shown that NAFLD can be prevalent in underweight IBD patients, as well.11 In addition, studies have suggested an association between NAFLD phenotype and IBD disease severity and history of small bowel surgery and steroid usage.12, 13
Prior studies looking at the relationship between IBD and NAFLD have been observational studies or from single-centers limited by sample size. The aim of this systematic review and meta-analysis was to determine the prevalence of NAFLD among IBD patients and to identify the factors associated with NAFLD in this subgroup of patients.
MATERIALS AND METHODS
All steps of the systematic review and meta-analysis were conducted using standard methods in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.14 We developed and followed an unregistered protocol.
We searched MEDLINE and Embase from January 1, 2005, through April 11, 2018. The decision to start the search date at January 1, 2005, was based on the approximate date when NAFLD was first defined in the literature. Index search terms for IBD and NAFLD were combined (Supplemental Table 1). The search was limited to English-language articles and conference abstracts. Letters, review articles, and animal studies were excluded. Two investigators (AL and HR) independently reviewed all articles for study inclusion. Discrepancies were resolved by consensus or by a third investigator (SP).
We included published studies of patients with IBD (ulcerative colitis or Crohn’s disease) and NAFLD. We included observational studies and required at least 5 patients per study. Review articles, studies involving pediatric population (younger than 18 years old), and animal studies were excluded.
Our primary outcome was to determine the prevalence of NAFLD in IBD patients and to determine if this was significantly different from the general population. Nonalcoholic fatty liver disease was defined individually by each study (imaging, elastography, biopsy, or hepatic steatosis index). Sensitivity analyses explored the effect of low quality data. Prevalence of NAFLD in IBD patients was compared with prevalence of NAFLD in the general population as determined by meta-analysis in a 2016 study by Younossi and colleagues.2 Our secondary outcomes were (1) to calculate prevalence of NAFLD in IBD patients by continent given regional variability of NAFLD in the general population,15 (2) to calculate prevalence of advanced fibrosis among patients with IBD, and (3) to determine risk factors associated with NAFLD in patients with IBD.
All data were extracted by 1 of 2 researchers (AL or HR) and verified by a second independent researcher (HR or AL). Extracted data included study author, publication date, country, patient age, sex, body mass index (BMI), exclusion criteria, dyslipidemia, diabetes mellitus, hypertension, obesity, abdominal/waist/hip circumference, IBD index of severity, severe IBD, active IBD, IBD disease duration, IBD therapy, definition of liver steatosis, variables affecting liver steatosis, definition of liver fibrosis, and variables affecting liver fibrosis. Discrepancies were resolved through discussion or by a third investigator (SP).
All meta-analyses were performed using random-effects models, and results were pooled using the maximum likelihood estimation. Prevalences were compared using 2 proportion z tests. Study quality was assessed using the Newcastle-Ottawa Quality Assessment Scale for cohort studies. Study heterogeneity was assessed using the Cochrane I2 statistic.16 Statistical analyses were performed with OpenMetaAnalyst17 and the meta and metafor packages in R, version 3.2.1 (R Foundation for Statistical Computing).18
RESULTS
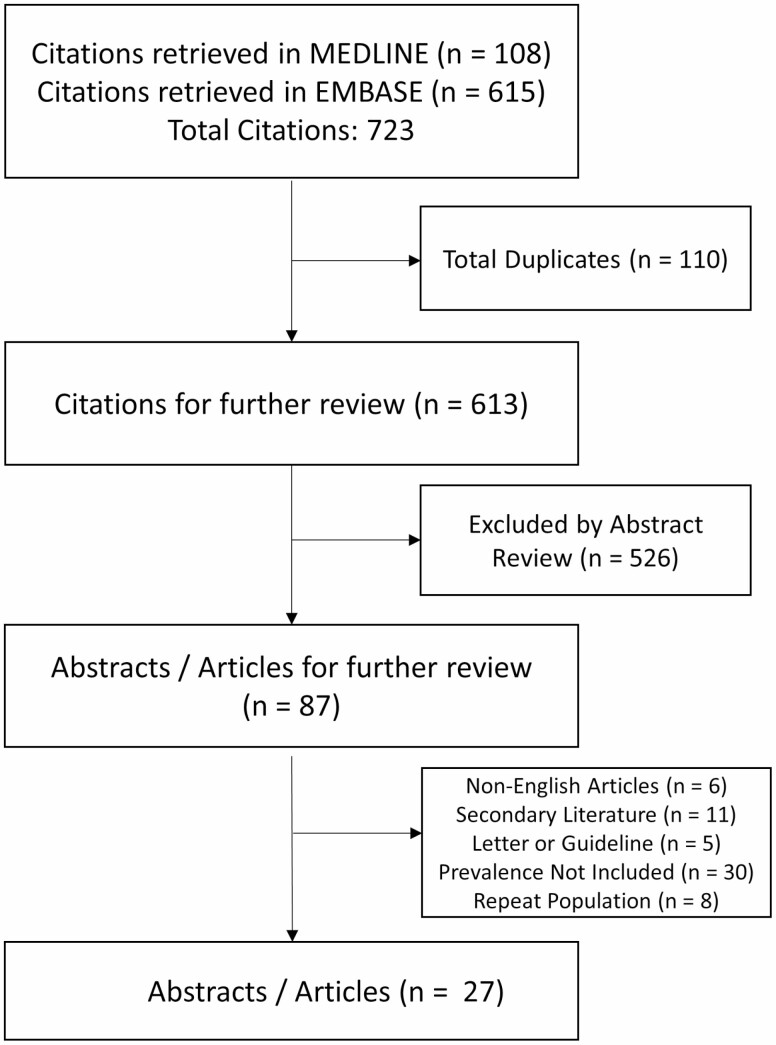
We identified 723 citations through database searches: 108 citations from MEDLINE and 615 citations from Embase, respectively. One hundred ten citations were duplicates, 526 citations were excluded by abstract review, and 56 citations were excluded upon further review. Twenty-seven abstracts or articles met eligibility criteria (Fig. 1).
FIGURE 1.
Summary of evidence search and selection.
Study and Patient Characteristics
Table 1 summarizes baseline study characteristics for the 27 abstracts or articles that met eligibility criteria. All of the studies were observational, with 12 prospective and 15 retrospective. The 27 studies included 12 studies from North America, 11 studies from Europe, and 4 studies from Asia. There were 7640 patients (range 7–1304) included in this meta-analysis. When reported, the median patient age across studies was 41 years old (range 34–47), with 1531 men (53%) and 1449 women. When reported, 3673 patients had Crohn’s disease (64%), and 2028 patients had ulcerative colitis. Eight studies did not report on age, 6 studies did not report on gender, and 4 studies did not differentiate between Crohn’s disease and ulcerative colitis among patients with IBD.
TABLE 1.
Characteristics of Studies Involving Prevalence of NAFLD in IBD Patients
| Study, Year (Reference) | Country | Region | Study Design | Abstract vs Article | Total IBD Patients, n | Men/ Women, n/n | Mean Age, y | Mean BMI, n | IBD Patients with Hepatic Steatosis, n |
|---|---|---|---|---|---|---|---|---|---|
| Andrade et al, 201619 | Portugal | Europe | R, Chart Review, S | Abstract | 54 | 35/19 | 43 +/- 12 | NR | 13 |
| Balaban et al, 201720 | Romania | Europe | P, Observational, S | Abstract | 36 | 17/19 | 43 +/- 13 | 23.5 | 11 |
| Balaban et al, 201721 | Romania | Europe | P, Observational, S | Abstract | 62 | 31/31 | 45 +/- 15 | 24.2 | 23 |
| Bessisow et al, 201622 | Canada | North America | R, Longtiduinal, S | Article | 321 | 151/170 | Median: 33.7 (25.1–46.9) | Median: 22.9 (20.9–24.7) | 108 |
| Bosch et al, 201723 | United States | North America | R, Chart Review, S | Article | 49 | 25/24 | 47 | 27.6 | 29 |
| Chhina et al, 201424 | United States | North America | R, Chart Review, S | Abstract | 1304 | NR/NR | NR | NR | 126 |
| DiGirolamo et al, 201325 | Italy | Europe | R, Chart Review, S | Abstract | 788 | NR/NR | NR | NR | 128 |
| Donati et al, 201626 | France | Europe | P, Case Series, S | Abstract | 114 | NR/NR | NR | NR | 0 |
| Dundulis et al, 201427 | United States | North America | R, Case Control, S | Abstract | 70 | 31/39 | 38.6 +/- 14.9 | 26.6 +/- 6.9 | 29 |
| Erzin et al, 201528 | Turkey | Europe | R, Cross Sectional, S | Abstract | 276 | 132/144 | 44.39 +/- 14.14 | 24.08 | 100 |
| Kang et al, 201829 | South Korea | Asia | R, Case Control, S | Abstract | 720 | NR/NR | NR | NR | 5 |
| Likhitsup et al, 201730 | United States | North America | P, Cross Sectional, S | Abstract | 80 | 44/36 | 42.4 +/- 15 | 26 +/- 5.7 | 43 |
| Magnes et al, 201831 | Canada | North America | R, Chart Reivew, S | Abstract | 35 | NR/NR | NR | NR | 26 |
| Mancina et al, 201632 | Italy | Europe | R, Cohort, S | Article | 106 | 97/61 | 45+/- 13 | 26 +/- 4 | 75 |
| Mariabeatrice et al, 201833 | Italy | Europe | P, Case Series, S | Article | 465 | 241/224 | 46.2 +/- 15.6 | 24.7 +/- 5.3 | 130 |
| McGowan et al, 201834 | United States | North America | R, Case Series, S | Article | 7 | 2/5 | 35.4 | 32.4 | 7 |
| Mehrotra et al, 201335 | India | Asia | P, Case Series, S | Abstract | 20 | 14/6 | 42 +/- 4 | NR | 3 |
| Mehrotra et al, 201836 | India | Asia | P, Case Control, S | Abstract | 128 | NR/NR | NR | NR | 23 |
| Palumbo et al, 201837 | Canada | North America | P, Case Series, S | Abstract | 326 | 159/167 | 42.8 +/- 15.5 | NR | 129 |
| Peixoto et al, 201738 | Portugal | Europe | P, Cross Sectional, S | Abstract | 62 | 27/35 | 37.5 +/- 11.3 | NR | 23 |
| Quang Le et al, 201439 | United States | North America | R, Longitudinal, S | Abstract | 232 | 116/116 | 33.8 | NR | 60 |
| Restellini et al, 201740 | Canada | North America | P, Case Series, S | Abstract | 349 | 170/179 | 42.5 +/- 15.2 | NR | 135 |
| Sagami et al, 201741 | Japan | Asia | R, Cohort, S | Article | 303 | 226/77 | 36.9 +/- 12.2 | 20.21 | 66 |
| Schroder et al, 201542 | Germany | Europe | R, Chart Review, M | Article | 259 | 101/158 | 37.2 +/- 0.8 | 24.3 +/- 0.3 | 73 |
| Simon et al, 201843 | United States | North America | P, Case Series, S | Abstract | 462 | NR/NR | NR | NR | 240 |
| Sourianarayanane et al, 201313 | United States | North America | R, Nested Case Control, S | Article | 928 | w/ NAFLD 31/45; w/o NAFLD 61/80 | w/NAFLD 46 +/13.3; w/o NAFLD 42 +/-14.1 | w/ NAFLD 30.4 +/- 8.5; w/o NAFLD 27.0 +/- 6.1 | 76 |
| Steenhuis et al, 201844 | Netherlands | Europe | P, Case Series, S | Abstract | 84 | NR/NR | 44.2 | 24.94 | 35 |
Abbreviations: R, retrospective; P, prospective; S, single center; M, multi-center; NR, not available
Study quality is reported in Supplemental Table 2. Approximately 80% of the studies scored well on patient selection (22 out of 28 studies), with variable quality with respect to comparability and outcome.
Overall Prevalence of NAFLD Among Patients with IBD
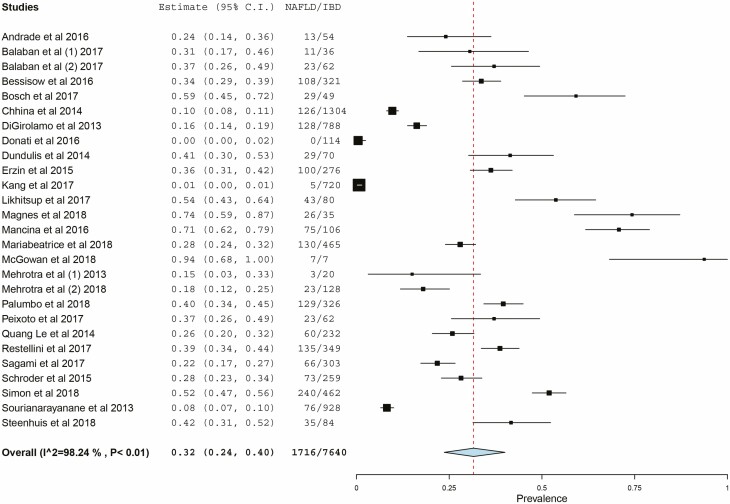
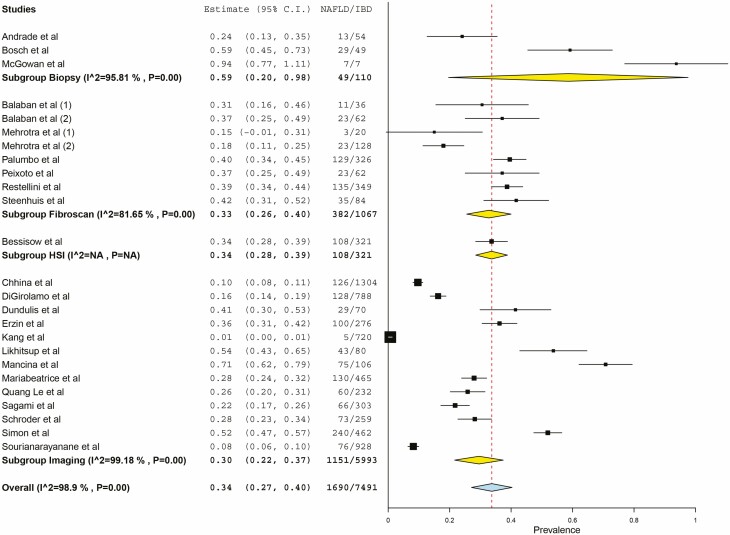
Among 7640 patients with IBD in 27 studies, 1716 had NAFLD, with a pooled prevalence estimate of 32% (95% confidence interval [CI], 24%–40%; Fig. 2). There was substantial heterogeneity (I2 = 98.24%). Among IBD patients with NAFLD, 32% (95% CI, 19%–45%) were reported to have ulcerative colitis, and 67% (95% CI, 54%–80%) were reported to have Crohn’s disease based on pooled prevalence estimates. The prevalence of NAFLD among IBD patients differed by method of diagnosis as demonstrated in Figure 3. Three studies utilized liver biopsy to diagnose NAFLD among IBD patients, with a pooled prevalence of 59% (95% CI, 20%–98%) and substantial heterogeneity (I2 = 96%).19, 23, 34 Eight studies diagnosed NAFLD among IBD patients by transient elastography, with a pooled prevalence of 33% (95% CI, 26%–40%) with less heterogeneity (I2 = 82%). Thirteen studies diagnosed NAFLD among IBD patients by imaging, with a pooled prevalence of 30% (95% CI, 22%–37%) and substantial heterogeneity (I2 = 99%). Even within method of diagnosis, there were different definitions of NAFLD by study. In 2 out of the 3 studies utilizing liver biopsy to diagnose NAFLD, biopsy was performed because of elevated liver enzymes.19, 34 A sensitivity analysis removed 11 studies (5 studies did not include exclusion criteria for prior liver disease, 3 studies did not define criteria for diagnosis of NAFLD, and 3 studies only included patients on certain medications [eg, methotrexate, immunosuppressive therapy, or anti-TNF therapy]) and revealed similar results (pooled prevalence 35.3%; 95% CI, 27%–44%)
FIGURE 2.
Prevalence of NAFLD in IBD patients.
FIGURE 3.
Prevalence of NAFLD in IBD patients by NAFLD diagnosis method.
To understand the significance of the prevalence of NAFLD among IBD patients, we compared our data with a 2016 meta-analysis conducted by Younossi and colleagues on the prevalence of NAFLD within the general population.2 The pooled prevalence of NAFLD within the general population worldwide was estimated to be 25.2% (95% CI, 22.1%–28.7%).2 The prevalence of NAFLD among IBD patients from our meta-analysis is statistically significantly higher than the prevalence of NAFLD within the general population (P < 0.001).
Prevalence of NAFLD by Region
The prevalence of NAFLD among IBD patients differed by world region. The pooled prevalence of NAFLD among IBD patients was 43% in North America (95% CI, 30%–56%), 31% in Europe (95% CI, 21%–42%), and 13% in Asia (95% CI, 4%–22%). In comparison, Younossi et al found a prevalence of NAFLD in the general population of 24.1% in North America (95% CI, 19.7%–29.1%), 23.7% in Europe (95% CI, 16.1%–33.5%), and 27.4% in Asia (95% CI, 23.3%–31.9%).2
Prevalence of Advanced Fibrosis Among Patients with IBD
Eight studies (5 North American studies and 3 European studies) differentiated between the presence of steatosis and advanced fibrosis. We pooled the data and identified a 10.3% prevalence of advanced fibrosis in the IBD patients (95% CI, 5.6%–15%).
Risk Factors Associated With NAFLD in IBD
Table 2 assesses risk factors associated with NAFLD in IBD. Age (mean difference, 6.52 yrs; 95% CI, 4.58–8.47), BMI (mean difference, 3.03; 95% CI, 2.47–3.59), and IBD duration (mean difference, 1.59 yrs; 95% CI, 0.66–2.54) were statistically significant on the development of NAFLD in patients with IBD. With a limited number of studies, both a history of a bowel resection (odds ratio [OR] 1.39; 95% CI, 1.01–1.93) and diabetes (OR 1.71; 95% CI, 0.99–2.96) had statistically significant association with development of NAFLD among IBD patients. Dyslipidemia was also assessed as a potential risk factor associated with the development of NAFLD in IBD patients. However, only 4 studies performed a multivariate analysis that included dyslipidemia, which was also defined differently by each study (eg, low-density lipoprotein, triglycerides).28, 33, 37, 41 None of the 4 studies found dyslipidemia to be a statistically significant risk factor for the development of NAFLD. Four studies presented data on age of onset of IBD in the study population.13, 22, 29, 41 However, only 1 study performed multivariate analysis to determine if age of onset of IBD had any influence on future development of NAFLD; the hazard ratio for age of onset of IBD was not statistically significant.22
TABLE 2.
Risk Factors Associated With NAFLD in IBD
| Variable | Type of Variable | Studies (n) | Patients (n) | Mean Difference / Odds Ratio | 95% CI | P |
|---|---|---|---|---|---|---|
| Age | Continuous | 7 | 1171 | Mean Difference: 6.52 | (4.58, 8.47) | P < 0.001 |
| BMI | Continuous | 7 | 1825 | Mean Difference: 3.03 | (2.47, 3.59) | P < 0.001 |
| IBD Duration | Continuous | 3 | 615 | Mean Difference: 1.59 | (0.66, 2.54) | P < 0.001 |
| Diabetes | Categorical | 5 | 1356 | Odds Ratio: 1.71 | (0.99, 2.96) | P < 0.049 |
| Hx Resection | Categorical | 3 | 841 | Odds Ratio: 1.39 | (1.01, 1.93) | P < 0.04 |
Abbreviation: Hx, history of
DISCUSSION
Our systematic review and meta-analysis of 27 studies and 7640 patients revealed a pooled NAFLD prevalence estimate of 32% (95% CI, 24%–40%, I2 = 98.24%) among IBD patients. The majority of the IBD patients with NAFLD had Crohn’s disease (67%, 95% CI, 54%–80%). We found the prevalence of NAFLD in IBD patients to be significantly higher than that of the general population rate of 25.2%.2 Importantly, this is the first systematic review exploring NAFLD among IBD patients to identify that IBD patients are at increased risk of developing NAFLD as compared with the general population. When stratified by region, the pooled prevalence of NAFLD in IBD patients was highest in North America (43%, 95% CI, 30%–56%), and lowest in Asia (13%, 95% CI, 4.0–22%). Increased age, disease duration, BMI, comorbid diabetes, and previous bowel resection were associated with increased risk of developing NAFLD among IBD patients.
As compared with a recently published systematic review examining the prevalence of NAFLD in patients with IBD,45 our study included a larger number of patients and revealed a higher prevalence of NAFLD in IBD patients than in the general population. Zou et al identified 19 studies (5620 patients) and found a pooled estimate of prevalence of NAFLD among IBD patients of 27.5% (95% CI, 20.7%–34.2%).45 Of note, this prevalence is lower than that of our meta-analysis and more closely mirrors the overall prevalence of NAFLD among the general population (25.2%).2 This difference might be accounted for by the difference in number of studies included (19 vs 27) and the difference in the number of patients (5620 vs 7640). Of the 19 studies included in their meta-analysis, 8 studies overlapped with those included in our study, and 6 studies were excluded (3 did not include incidence data, and 3 predated 2005). Despite the difference in prevalence found, similar risk factors for the development of NAFLD among IBD patients were identified, including older age, obesity, diabetes, prior surgery for IBD, and longer disease duration of IBD. Zou and colleagues also found chronic kidney disease (CKD), methotrexate use (known cause of secondary hepatic steatosis46), and hypertension to be significant risk factors for the development of NAFLD among IBD patients in their study.
The increased prevalence of NAFLD in Europe and North America as compared with Asia may be related to underlying higher prevalence of obesity in these countries.47, 48 The increased prevalence of NAFLD among IBD patients in North America and Europe as compared with the general population aligns with the overall higher prevalence of NAFLD among IBD patients demonstrated in our study. The lower prevalence of NAFLD among IBD patients in Asia as compared with the general population is likely impacted by the limited number of studies reporting prevalence in Asian countries (only 4 studies available in the English language).
The pathophysiology of NAFLD development in IBD patients is unclear. Fatty liver is a manifestation of the metabolic syndrome34, 49, 50 and associated with obesity. The higher prevalence of NAFLD in IBD patients could simply be related to increasing obesity rates both globally and also specifically in IBD patients.51, 52 This would be supported by our finding that higher BMI and diabetes are associated with increased risk of developing NAFLD among IBD patients. The association with bowel resection is of interest and may represent the impact of a short bowel on the development of NAFLD or patients who are in remission after surgery and are more likely to be obese. Alternatively, patients who required surgery may be those who have had more inflammation. The role of systemic inflammation on the risk of NAFLD is not fully known.
However, other studies have not found an increased risk of metabolic syndrome among IBD patients to be correlated with risk of developing NAFLD.12, 53 Furthermore, an increasing risk of metabolic syndrome among IBD patients would not explain our finding that IBD patients had higher rates of NAFLD than the general population, nor would it explain the nonmetabolic syndrome factors such as disease duration, history of resection, and age that seem to increase risk of development of NAFLD. Inflammatory bowel disease treatment history and exposure to steroids and methotrexate are likely factors that also play a role in increased prevalence of NAFLD. However, we did not have granular data in this systematic review to address medication history.
There are some similarities in the pathophysiology of NAFLD and IBD including a pro-inflammatory state, gut dysbiosis, and genetics.49, 54, 55 Inflammation is thought to play a large role in both processes and, therefore, may represent a link between the 2 diseases. Our finding that disease duration and age are associated with increased NAFLD in IBD patients could suggest that increased exposure to inflammation increases risk of development of NAFLD. Additionally, obesity in and of itself is recognized to be a pro-inflammatory state, and therefore, increased inflammation may be multifactorial in these patients.56
It is not well understood why the majority of IBD patients with NAFLD in our systematic review had Crohn’s disease. A potential explanation is that patients with Crohn’s disease are more likely to have had small bowel inflammation and small bowel surgery, which have the potential to disrupt bile acid metabolism in the ileum. It has been described that patients with Crohn’s disease with increased disease activity or ileal resection have decreased ileal farnesoid X receptor (FXR) gene expression and lower levels of circulating fibroblast growth factor 19 (FGF 19).57 Fibroblast growth factor 19 is thought to have downstream effects on lipid metabolism, bile acid regulation, and synthesis by the liver.58 The FXR pathway is thought to be one of the mechanisms involved in the development of NAFLD.58 Currently, FXR agonists (including obeticholic acid) are an emerging therapy for treatment of NAFLD/NASH.58, 59 Recent data also suggest treatment with FXR agonists decreases inflammation in murine models of colitis.57
In IBD, gut microbiota and its alterations have been associated with changes in disease severity.60 Gut microbiota are also altered in patients with chronic liver disease, particularly small intestinal bacterial overgrowth, leading to increased intestinal permeability. Studies in mice have shown that a high fat diet can alter gut microbiota and predispose to liver pathology.54 Our understanding of the role of the microbiome in the pathogenesis of both diseases continues to evolve but may provide a more definitive future link.
Genetic predisposition to NAFLD is an evolving field, and it may be that genetic predisposition might lead IBD patients to develop NAFLD, such as PNPLA3 loss of function mutations (eg, decreased triglyceride hydrolase activity), which can lead to increased NAFLD development among IBD patients.32
Our results reveal that up to one third of IBD patients have NAFLD. This recognition has important implications for the management of IBD patients, including treatment decisions and screening in patients with identified risk factors. Comorbid NAFLD may affect treatment choices in IBD patients, as underlying liver steatosis is known to potentiate pathogenesis of liver toxicity due to drugs.61 Specifically, IBD patients with NAFLD who are treated with immunosuppressive agents are at a higher risk of developing liver injury as a second hit.42 Treatment strategies in these patients will include avoiding drugs associated with hepatic steatosis, such as methotrexate.46 Additionally, as obesity among IBD patients increases, treatment selection for these patients will need to be tailored to their physiology. Obese patients have been shown to have more rapid clearance of biologic therapies and are more likely to experience treatment failure.62, 63
The direct role of steroids and immunosuppressive medications in the development of NAFLD in IBD patients remains controversial. In several studies, the use of corticosteroids has been shown to increase risk of NAFLD,13, 20 whereas other studies have shown no increased risk.22, 28, 33, 41 Similarly, studies have been discordant with respect to methotrexate treatment as a direct risk factor for development of NAFLD in IBD patients, with 1 study showing no increased risk of development of NAFLD38 and another reporting significantly increased risk.40 Additional data will need to be collected in future to help clarify the roles of these agents on the development on NAFLD in the IBD population.
Nonalcoholic fatty liver disease is associated with significant morbidity and comorbid IBD, and liver disease has been shown in hospitalized patients to lead to mortality rates that are twice as high as those with IBD alone.12 Therefore, the role for screening IBD patients for NAFLD becomes increasingly important. In addition to elevated BMI, our study shows that advanced age, increased disease duration, and history of bowel resection are all risk factors for the development of NAFLD in IBD patients. Advanced age and increased disease duration are possible proxies for increased exposure to inflammation, thereby increasing risk of NAFLD development. History of bowel resection has been shown to be associated with increase in metabolic syndrome, inflammation, and development of NAFLD in non-IBD patients64 and may explain predisposition to NAFLD in IBD patients, as well.
Limitations
There are several limitations to the findings in our study. First, our results revealed significant heterogeneity in the reported prevalence of NAFLD in IBD patients. This is likely due to the small sample size of individual studies and varied diagnostic modalities used in NAFLD diagnosis. For example, we see a significantly higher prevalence among those diagnosed by biopsy (59%) as opposed to elastography (33%) and imaging (30%). This is likely accounted for by the small number of studies relying on biopsy (3 studies total) for diagnosis and the inherently more specific nature of a biopsy. Additionally, all 3 studies used biopsies from previously collected registries, suggesting that patients had elevated liver enzymes or other stigmata of liver disease before biopsy (confirmed in 2 of the 3 studies).19, 34 Only some studies commented on what risk factors might predispose IBD patients to the development of NAFLD, and those that did provided the data in heterogeneous formats and with various statistical significance, limiting the possibility for generalization.
There was also significant heterogeneity in reporting of patients’ medication exposure history. It was not clear if any individual patient had been on more than one therapy concurrently, nor was the timing of the exposure clear (past or current). Therefore, it was not possible to examine medication exposures as risk factors for development of NAFLD, a subject that is deserving of further study. Articles used were limited to English language articles, which provided more data about North America and Europe than Asia or other regions of the world.
CONCLUSION
Our study reveals a prevalence of NAFLD among IBD patients of 32%, a prevalence that is significantly higher than that of the general population. We propose that the treatment of IBD patients with NAFLD include special consideration due to higher risk of liver injury from IBD therapies and poor outcomes for obese IBD patients. Further studies examining the utility of screening should be considered in patients with risk factors for NAFLD development including advanced age, higher BMI, diabetes mellitus, longer disease duration, and history of resection.
Supplementary Material
ACKNOWLEDGMENTS
Authors would like to acknowledge the Section of Gastroenterology, Hepatology, and Nutrition at the University of Chicago Hospitals for providing critical review of this research.
Conflicts of Interest: All authors have no conflicts of interest to declare.
Author Contributions: AL, HR, AA, DR, and SP contributed to the study concept and design, interpretation of data, and writing the article. AL, HR, and SP contributed to the acquisition of data and analysis of data. All authors edited the draft and contributed substantially to the manuscript. All authors approved this submission.
Financial Disclosures: DTR has grant support from Takeda; has served as a consultant for Abbvie, Abgenomics, Allergan Inc., Boehringer Ingelheim Ltd., Bristol-Myers Squibb, Celgene Corp/Syneos, Dizal Pharmaceuticals, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, Ichnos Sciences S.A., GlaxoSmithKline Services, Janssen Pharmaceuticals, Eli Lilly, Pfizer, Prometheus Laboratories, Reistone, Shire, Takeda, and Techlab Inc. SP has funding/research grants from Intercept, GENFIT, and TARGET PharmaSolutions.
REFERENCES
- 1. Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862–873. [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Koenig AB, Abdelatif D, et al. . Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 3. Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11:1–16, vii. [DOI] [PubMed] [Google Scholar]
- 4. Younossi ZM. Non-alcoholic fatty liver disease - a global public health perspective. J Hepatol. 2019;70:531–544. [DOI] [PubMed] [Google Scholar]
- 5. Younossi Z, Stepanova M, Ong JP, et al. ; Global Nonalcoholic Steatohepatitis Council . Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17:748–755.e3. [DOI] [PubMed] [Google Scholar]
- 6. Wong RJ, Aguilar M, Cheung R, et al. . Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. [DOI] [PubMed] [Google Scholar]
- 7. Noureddin M, Vipani A, Bresee C, et al. . NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. 2018;113:1649–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Younossi ZM, Blissett D, Blissett R, et al. . The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. [DOI] [PubMed] [Google Scholar]
- 9. Liu TC, Stappenbeck TS. Genetics and pathogenesis of inflammatory bowel disease. Annu Rev Pathol. 2016;11:127–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Younossi ZM, Stepanova M, Negro F, et al. . Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore). 2012;91:319–327. [DOI] [PubMed] [Google Scholar]
- 11. Adams LC, Lübbe F, Bressem K, et al. . Non-alcoholic fatty liver disease in underweight patients with inflammatory bowel disease: A case-control study. Plos One. 2018;13:e0206450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sartini A, Gitto S, Bianchini M, et al. . Non-alcoholic fatty liver disease phenotypes in patients with inflammatory bowel disease. Cell Death Dis. 2018;9:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sourianarayanane A, Garg G, Smith TH, et al. . Risk factors of non-alcoholic fatty liver disease in patients with inflammatory bowel disease. J Crohns Colitis. 2013;7:e279–e285. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 15. Younossi Z, Anstee QM, Marietti M, et al. . Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration; 2011. Accessed November 30, 2018. www.cochrane-handbook.org. [Google Scholar]
- 17. Wallace BC, Dahabreh IJ, Trikalinos TA, et al. . Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. 2012;49:1–15. [Google Scholar]
- 18. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 19. Andrade P, Lopes S, Lopes J, et al. . P0275 A histological appraisal of hepatobiliary disorders in inflammatory bowel disease. United European Gastroenterol J. 2016;4:A252. [Google Scholar]
- 20. Balaban DV, Popp A, Robu G, et al. . P323 Fatty liver assessment in inflammatory bowel disease patients using controlled attenuation parameter. J Crohns Colitis. 2017;11(suppl_1):S240–S241. [Google Scholar]
- 21. Balaban DV, Enache I, Macadon B, et al. . P1670 Prevalence and quantitative assessment of liver steatosis in inflammatory bowel disease patients. United European Gastroenterol J. 2017;5:A735. [Google Scholar]
- 22. Bessissow T, Le NH, Rollet K, et al. . Incidence and predictors of nonalcoholic fatty liver disease by serum biomarkers in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:1937–1944. [DOI] [PubMed] [Google Scholar]
- 23. Bosch DE, Yeh MM. Primary sclerosing cholangitis is protective against nonalcoholic fatty liver disease in inflammatory bowel disease. Hum Pathol. 2017;69:55–62. [DOI] [PubMed] [Google Scholar]
- 24. Chhina S, Steadman K, Goel G, et al. . Small bowel location, stricturing behavior, vitamin D receptor polymorphism, and metabolic disease susceptibility genes are associated with NAFLD in Crohn’s disease. Am J Gastroenterol. 2014;109:S504. [Google Scholar]
- 25. Di Girolamo M, Scancelli A, Bertani A, et al. . P615 Ultrasonographic prevalence of liver steatosis in patients with inflammatory bowel disease in a single center. J Crohns Colitis. 2013;7:S258. [Google Scholar]
- 26. Donatini B, Le Blaye I. Exhaled methylacetate levels differ according to dysimmune diseases and existence of severe liver steatosis. Eur J Immunol. 2016;46:898. [Google Scholar]
- 27. Dundulis J, Helzberg J, Ansari S, et al. . NAFLD appears to be the most common liver disease in IBD patients; IBD is likely a risk factor for NAFLD. Am J Gastroenterol. 2014;109:S147. [Google Scholar]
- 28. Erzin Y, Demir N, Cabuk C, et al. . The increased prevalence of non-alcoholic fatty liver disease in inflammatory bowel disease patients is not related to inflammatory load. J Crohns Colitis. 2015;9:S363. [Google Scholar]
- 29. Kang MK, Kim KO, Jang BI, et al. . The prevalence and risk factors of non-alcoholic fatty liver disease in inflammatory bowel diseases. J Crohns Colitis. 2018;12(suppl_1):S521. [Google Scholar]
- 30. Likhitsup A, Chhabra R, Ansari S, et al. . The high prevalence of NAFLD in IBD patients is not decreased by anti-TNF therapy. Gastroenterology. 2017;152:S686. [Google Scholar]
- 31. Magnes M, Palmart J, Loo S, et al. . A retrospective analysis comparing gastrointestinal conditions in a multi-ethnic cohort with various hepatic pathologies evaluated in an urban-based hepatology clinic. Hepatol Int. 2018;12(Suppl 2):S600. [Google Scholar]
- 32. Mancina RM, Spagnuolo R, Milano M, et al. . PNPLA3 148M carriers with inflammatory bowel diseases have higher susceptibility to hepatic steatosis and higher liver enzymes. Inflamm Bowel Dis. 2016;22:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mariabeatrice P, Andrea I, Giuseppe L, et al. . Nonalcoholic fatty liver disease in inflammatory bowel disease: prevalence and risk factors. Inflamm Bowel Dis. 2018;24:1589–1596. [DOI] [PubMed] [Google Scholar]
- 34. McGowan CE, Jones P, Long MD, et al. . Changing shape of disease: nonalcoholic fatty liver disease in Crohn’s disease-a case series and review of the literature. Inflamm Bowel Dis. 2012;18:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mehrotra P, Mehrotra S, Mehrotra P, et al. . Non-alcoholic fatty liver disease (NAFLD) in ulcerative colitis (UC): a case series and role of fibroscan. J Clin Exp Hepatol. 2013;3:S26. [Google Scholar]
- 36. Mehrotra P, Mehrotra P. Non-alcoholic fatty liver disease in patients with Ulcerative colitis: a prospective Indian data. Hepatol Int. 2018;12(Suppl 2):S455. [Google Scholar]
- 37. Palumbo CS, Restellini S, Chao C, et al. . A199 Screening for nonalcoholic fatty liver disease by transient elastography with controlled attenuation parameter in unselected patients with inflammatory bowel disease. J Can Assoc Gastroenterol. 2018;1(suppl_1):347–348. [Google Scholar]
- 38. Peixoto A, Silva M, Morais R, et al. . P417 Non-invasive assessment of liver fibrosis by transient elastography and AST to ALT ratio in patients with Crohn’s disease treated with methotrexate. J Crohns Colitis. 2017;11(suppl_1):S289. [Google Scholar]
- 39. Quang Le NH, Rollet KC, Afif W, et al. . Mo1245 impact of inflammatory bowel disease activity on the incidence of non-alcoholic fatty liver disease: a 7-year longitudinal study. Gastroenterology. 2014;146:S–S596. [Google Scholar]
- 40. Restellini S, Palumbo CS, Chao CY, et al. . Screening for non-alcoholic fatty liver disease by transient elastography with controlled attenuation parameter in unselected patients with inflammatory bowel disease. Gastroenterology. 2017;152:S976. [Google Scholar]
- 41. Sagami S, Ueno Y, Tanaka S, et al. . Significance of non-alcoholic fatty liver disease in Crohn’s disease: a retrospective cohort study. Hepatol Res. 2017;47: 872–881. [DOI] [PubMed] [Google Scholar]
- 42. Schröder T, Schmidt KJ, Olsen V, et al. . Liver steatosis is a risk factor for hepatotoxicity in patients with inflammatory bowel disease under immunosuppressive treatment. Eur J Gastroenterol Hepatol. 2015;27:698–704. [DOI] [PubMed] [Google Scholar]
- 43. Simon TG, van der Sloot KW, Chin S, et al. . IRGM gene variants modify the relationship between visceral adipose tissue and NAFLD in patients with Crohn’s disease. Hepatology. 2017;66:1168A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Steenhuis M, Van der Marel S, Van der Meulen-de Jong A, et al. . High prevalence of severe liver fibrosis in patients with longstanding IBD. J Crohns Colitis. 2018;12:S344. [Google Scholar]
- 45. Zou Z, Shen B, Fan J. Systematic review with meta-analysis: epidemiology of fatty liver disease in patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019;25:1764–1772. [DOI] [PubMed] [Google Scholar]
- 46. Chalasani N, Younossi Z, Lavine JE, et al. . The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- 47. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bluher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;Supp 2:1288–1298. [DOI] [PubMed] [Google Scholar]
- 49. Nguyen DL, Bechtold ML, Jamal MM. National trends and inpatient outcomes of inflammatory bowel disease patients with concomitant chronic liver disease. Scand J Gastroenterol. 2014;49:1091–1095. [DOI] [PubMed] [Google Scholar]
- 50. Chao CY, Battat R, Al Khoury A, et al. . Co-existence of non-alcoholic fatty liver disease and inflammatory bowel disease: a review article. World J Gastroenterol. 2016;22:7727–7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Singh S, Dulai PS, Zarrinpar A, et al. . Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017;14:110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nic Suibhne T, Raftery TC, McMahon O, et al. . High prevalence of overweight and obesity in adults with Crohn’s disease: associations with disease and lifestyle factors. J Crohns Colitis. 2013;7:e241–e248. [DOI] [PubMed] [Google Scholar]
- 53. Glassner K, Malaty HM, Abraham BP. Epidemiology and risk factors of nonalcoholic fatty liver disease among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:998–1003. [DOI] [PubMed] [Google Scholar]
- 54. Yu J, Marsh S, Hu J, et al. . The pathogenesis of nonalcoholic fatty liver disease: interplay between diet, gut microbiota, and genetic background. Gastroenterol Res Pract. 2016;2016. doi: 10.1155/2016/2862173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Carr RM, Oranu A, Khungar V. Nonalcoholic fatty liver disease: pathophysiology and management. Gastroenterol Clin North Am. 2016;45:639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Harper JW, Zisman TL. Interaction of obesity and inflammatory bowel disease. World J Gastroenterol. 2016;22:7868–7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van Schaik FD, Gadaleta RM, Schaap FG, et al. . Pharmacological activation of the bile acid nuclear farnesoid X receptor is feasible in patients with quiescent Crohn’s colitis. Plos One. 2012;7:e49706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wiest R, Albillos A, Trauner M, et al. . Targeting the gut-liver axis in liver disease. J Hepatol. 2017;67:1084–1103. [DOI] [PubMed] [Google Scholar]
- 59. Younossi ZM, Ratziu V, Loomba R, et al. ; REGENERATE Study Investigators . Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. [DOI] [PubMed] [Google Scholar]
- 60. Fukuda K, Fujita Y. Determination of the discriminant score of intestinal microbiota as a biomarker of disease activity in patients with ulcerative colitis. BMC Gastroenterol. 2014;14:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Michaut A, Moreau C, Robin MA, et al. . Acetaminophen-induced liver injury in obesity and nonalcoholic fatty liver disease. Liver Int. 2014;34:e171–e179. [DOI] [PubMed] [Google Scholar]
- 62. Kurnool S, Nguyen NH, Proudfoot J, et al. . High body mass index is associated with increased risk of treatment failure and surgery in biologic-treated patients with ulcerative colitis. Aliment Pharmacol Ther. 2018;47: 1472–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dotan I, Ron Y, Yanai H, et al. . Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20:2247–2259. [DOI] [PubMed] [Google Scholar]
- 64. Barron L, Courtney C, Bao J, et al. . Intestinal resection-associated metabolic syndrome. J Pediatr Surg. 2018;53:1142–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.