Highlights
-
•
Older adults with smoking history received two doses of combined NTHi-Mcat vaccine.
-
•
We evaluated antibody persistence during 4 years of follow-up after vaccination.
-
•
Immune responses against the NTHi protein antigens persisted up to 4 years.
-
•
There was no persistent immune response against the Mcat antigen.
-
•
No safety concerns were identified during the long-term follow-up period.
Keywords: Acute exacerbation, Antibody persistence, Clinical trial, COPD, Haemophilus influenzae, Moraxella catarrhalis
Abbreviations: AECOPD, acute exacerbations of chronic obstructive pulmonary disease; ANCOVA, analysis of covariance; AS01E, Adjuvant System AS01E, containing 3-O-desacyl-4′-monophosphoryl lipid A, QS-21 (Quillaja saponaria Molina, fraction 21) and liposome; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ELISA, enzyme-linked immunosorbent assay; EU, enzyme-linked immunosorbent assay units; GMC, geometric mean concentration; GMR, geometric mean ratio; LLOQ, lower limit of quantification; Mcat, Moraxella catarrhalis; MPL, 3-O-desacyl-4′-monophosphoryl lipid A; NTHi, non-typeable Haemophilus influenzae; PD, protein D; PE, protein E; PilA, Pilin A; pIMD, potential immune-mediated disease; QS-21, Quillaja saponaria Molina, fraction 21; SAE, serious adverse event; UspA2, ubiquitous surface protein A2
Abstract
A multicomponent vaccine has been developed to reduce the frequency of acute exacerbations of COPD associated with non-typeable Haemophilus influenzae (NTHi) and Moraxella catarrhalis (Mcat) infections, containing NTHi (PD and PE-PilA) and Mcat (UspA2) surface proteins. In a randomised, observer-blind, placebo-controlled study with two steps (NCT02547974), the investigational vaccine had good immunogenicity and no safety concerns were identified. In step 2, 90 adults aged 50–71 years with smoking history received two doses 60 days apart of one of two AS01E-adjuvanted formulations containing 10 µg of each antigen (10–10-AS01) or 10 µg NTHi antigens and 3.3 µg UspA2 (10–3-AS01), or placebo. Long-term persistence of antigen-specific humoral antibodies was assessed in 81 participants during 3 years of follow-up after the initial 14-month study (NCT03201211).
Antigen-specific antibody concentrations were measured in blood samples taken every 6 months. Safety monitoring evaluated serious adverse events (SAEs) and potential immune-mediated disease (pIMD).
Immune responses against NTHi antigens persisted up to 4 years post-vaccination. For PD, PE and PilA, at each follow-up time point, adjusted antibody geometric mean concentrations (GMCs) were higher (non-overlapping 95% confidence intervals [CIs]) in the vaccine groups versus placebo and versus pre-vaccination. Antibody GMC point estimates were higher with 10–3-AS01 than with 10–10-AS01. For UspA2, 95% CIs included 1 for GMC ratios of 10–10-AS01 or 10–3-AS01 to placebo at each time point. During follow-up, SAEs were reported in nine (11.1%) participants, one of which was fatal (lung cancer, 607 days after second 10–10-AS01 dose). One non-serious pIMD, trigeminal neuralgia, was reported 771 days after second 10–3-AS01 dose. The SAEs and pIMD were considered not related to vaccination.
Immune responses against NTHi antigens persisted for 4 years after two-dose vaccination with the investigational NTHi-Mcat vaccine. There was no persistent response against the Mcat antigen. No safety concerns were identified during the long-term follow-up.
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death globally [1], with an estimated prevalence of 12% in people aged 30 years or more [2]. Acute exacerbations of COPD (AECOPD) are periods of worsened respiratory symptoms, beyond that seen with day-to-day variation, that increase the risk of myocardial infarction, stroke, pulmonary embolism and death [3]. No vaccine is approved for the prevention of AECOPD, although influenza and pneumococcal vaccines, which are routinely recommended to COPD patients [4], may have some effect on the frequency of exacerbations [5].
Bacterial infection is frequently associated with AECOPD, most commonly non-typeable Haemophilus influenzae (NTHi), Moraxella catarrhalis (Mcat) and Streptococcus pneumoniae infections [6], [7], [8], [9]. Targeting the major bacterial species associated with AECOPD may be a viable strategy for vaccine development. There is evidence that NTHi and Mcat can act as co-pathogens in respiratory tract infections and COPD, as indicated by protection of NTHi from complement-mediated killing via complement resistance factors on outer membrane vesicles produced by Mcat [10]. Increased resistance to antibiotics and host clearance also appears to be promoted by NTHi and Mcat co-infection [11], [12].
An adjuvanted multicomponent vaccine has been developed to reduce the frequency of moderate and severe AECOPD associated with NTHi and Mcat. The investigational NTHi-Mcat vaccine contains four surface proteins involved in the virulence mechanisms of both bacterial pathogens [13]. Three are from NTHi, a free recombinant protein D (PD) and a recombinant fusion protein combining protein E and Pilin A (PE-PilA), and the fourth from Mcat, ubiquitous surface protein A2 (UspA2). Evidence from animal studies suggest anti-PD antibodies have opsonic activity [14] and protect against H. influenzae infection [15], while antibodies generated by the PE-PilA fusion protein inhibit the binding of PE to vitronectin (which may protect the bacterium from complement attack [16], [17]) and inhibit the formation of NTHi biofilms [18], as previously described for anti-PilA antibodies [19]. Anti-UspA2 antibodies significantly reduced the lung bacterial load in mice challenged with homologous or heterologous Mcat strains [20] and have been shown to be bactericidal and cross-reactive [20], [21]. A vaccine formulation containing the NTHi proteins had an acceptable safety and reactogenicity profile and induced antigen-specific immune responses in phase 1 studies of healthy 18–40 year-olds and current and former smokers aged 50–70 years [22]. The population group of adults with smoking history was chosen to immunologically match the COPD population, with evidence suggesting that alterations in the immune system start early in smokers, before COPD is diagnosed [23], [24], [25]. NTHi vaccine formulations that included the Adjuvant System AS01E [26] produced the highest humoral and cellular immune responses in older adults [22]. A phase 2 study of the adjuvanted NTHi vaccine in adults with COPD showed no safety concerns and good immunogenicity [27].
In the first clinical assessment of the safety, reactogenicity and immunogenicity of the investigational NTHi-Mcat vaccine, two doses were given 60 days apart and the study was conducted in two steps [13]. In step 1, healthy adults aged 18–40 years received a non-adjuvanted vaccine formulation or placebo. In step 2, older adults with a smoking history received one of two AS01E-adjuvanted formulations (one containing 10 µg PD, 10 µg PE-PilA and 10 µg UspA2 and the other 10 µg PD, 10 µg PE-PilA and 3.3 µg UspA2) or placebo. No safety concerns were identified with the NTHi-Mcat vaccine and the formulation containing 3.3 µg UspA2 induced the best humoral response against NTHi antigens [13]; this response was consistent with that induced by the adjuvanted NTHi vaccine in the phase 2 study of patients with COPD [27]. The anti-UspA2 immune response was moderate and transient with both formulations. In the present study, we assessed the long-term persistence of antigen-specific humoral antibodies in the groups included in step 2 of the initial study. In a follow-up period of 3 years (i.e. up to 4 years after the second vaccine dose), safety monitoring also continued, specifically evaluation of serious adverse events (SAEs) and potential immune-mediated disease (pIMD).
Methods
Study design and participants
This was a 3-year open-label follow-up study (ClinicalTrials.gov identifier: NCT03201211) of a 14-month phase 1, randomised, observer-blind, placebo-controlled study conducted in Belgium between August 2015 and March 2017 (NCT02547974). The methods of the phase 1 study were described previously [13] and a study summary is available at www.gsk-studyregister.com (study identifier, 204913). Briefly, participants were randomised to receive two vaccine formulation doses 60 days apart. There were two steps: in step 1, 30 healthy adults aged 18–40 years received a non-adjuvanted vaccine formulation containing 10 µg of each NTHi antigen and 10 µg of UspA2 antigen per dose or placebo. In step 2, 90 healthy adults aged 50–71 years with a smoking history of at least 10 pack-years received an AS01E-adjuvanted formulation containing either 10 µg of each antigen (10–10-AS01) or 10 µg of each NTHi antigen and 3.3 µg of Mcat UspA2 (10–3-AS01), or placebo. The NTHi antigens were described previously [22]. AS01E is an Adjuvant System containing 3-O-desacyl-4′-monophosphoryl lipid A (MPL), QS-21 (Quillaja saponaria Molina, fraction 21; licensed by GSK from Antigenics LLC, a wholly owned subsidiary of Agenus Inc., a Delaware, USA corporation) and liposome (25 μg MPL and 25 µg QS-21) [26].
In this follow-up study, the primary objective was to evaluate the persistence of humoral antibodies in the groups of participants included in step 2 of the initial study, up to 3 years after the last study visit. The last study visit of step 2 occurred 14 months after the study start and 12 months after the second vaccine dose. The secondary objective was to assess the long-term safety of the investigational NTHi-Mcat vaccine by monitoring the occurrence of SAEs and pIMD over the 3-year follow-up period. Eligible participants had participated in step 2 of the initial study, had received two doses, and were able to return for follow-up visits. Participants were excluded if they received or were scheduled to receive any investigational or non-registered vaccine or drug during the study. Other exclusion criteria included receipt of an immune-modifying drug, immunoglobulins or blood products during the study period, and any acute or chronic condition that could interfere with the results of the study.
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. The protocols and associated documents were reviewed and approved by an independent ethics committee. All participants provided written informed consent before study entry.
Antibody measurement
Immunoglobulin G antibody concentrations to each vaccine antigen were measured by enzyme-linked immunosorbent assay (ELISA), developed by GSK Biologicals and validated (for PD) or qualified (qualification in parallel with study conduct for PE, PilA and UspA2) on blood samples collected every 6 months during the 3-year follow-up, which started 14 months after the start of the study (12 months since the second vaccine dose), i.e. at 20, 26, 32, 38, 44 and 50 months from the start of the study. Sera were stored at −20 °C until assayed. Standardised procedures and in-house-made reference serum were used for each assay. The cut-off of the assays (i.e. lower limit of quantification, LLOQ) was 153 ELISA units (EU)/mL, 25 EU/mL, 16 EU/mL and 38 EU/mL for anti-PD, anti-PE, anti-PilA and anti-UspA2, respectively, for the first two time points, and 153 EU/mL, 16 EU/mL, 8 EU/mL and 28 EU/mL, respectively, for the remaining time points (post-qualification).
Safety analyses
During the entire follow-up study, the occurrence of SAEs was recorded, defined as any untoward medical occurrence that resulted in death, was life-threatening, required hospitalisation or prolongation of hospitalisation, resulted in disability or incapacity, or was a congenital anomaly or birth defect in the child of a subject. Data on pIMD, which included autoimmune and other inflammatory or neurological disorders of interest [28], were also recorded regardless of seriousness. Investigators or site staff were responsible for detecting, documenting and reporting events meeting the criteria of a SAE or pIMD. Safety oversight was provided by a safety review team through ongoing routine safety monitoring.
Statistical analysis
It was planned to enrol all eligible participants from step 2 of the initial study [13]. The persistence of humoral antibodies was analysed in the per-protocol cohort for immunogenicity, which consisted of all eligible participants who were compliant with study procedures and had at least one assay result for at least one of the follow-up time points.
Descriptive exploratory analyses characterised the differences between groups in humoral immune response, as measured by ELISA geometric mean concentration (GMC). For analysis purposes, samples with concentrations that fall below the LLOQ were assigned a value of half the LLOQ to calculate the GMC. The difference between groups in antibody GMC was evaluated by calculating the 95% confidence intervals (CIs) of the ratio of antibody GMCs between groups, using a one-way analysis of covariance (ANCOVA) model on the log10 transformation of antibody concentrations. The ANCOVA model included group category as fixed effect and the baseline antibody log10 concentration (before the first vaccine dose in the initial study) as covariate. Geometric mean ratios (GMRs) with 95% CIs were calculated to describe the mean change in antibody concentration at a specific time point with respect to the pre-vaccination antibody concentration for each group. GMRs were calculated using a one-way analysis of variance model on the ratio between the log10 antibody concentration at a specific time point and the baseline log10 antibody concentration, with group category as a fixed effect. Any differences between groups or time points should be interpreted with caution, as no adjustment for multiplicity was performed when computing the CIs.
The safety analysis was performed on all enrolled participants. Statistical analyses were performed using Statistical Analysis System Version 9.4 on Life Science Analytics Framework 4.3 (SAS Institute Inc., Cary, NC, USA).
Results
Study population
Eighty-one healthy adults with a smoking history were enrolled in this follow-up study (27 in the 10–10-AS01 group, 26 in the 10–3-AS01 group and 28 in the placebo group). Immunogenicity results were available for at least one time point for all enrolled participants. Demographic characteristics were similar between groups (Table 1). All participants were white (European heritage).
Table 1.
Demographic characteristics of participants enrolled in the follow-up study.
| Characteristic | 10–10-AS01 (N = 27) | 10–3-AS01 (N = 26) | Placebo (N = 28) |
|---|---|---|---|
| Age (years) at dose 1, mean (SD) | 59.7 (6.3) | 59.0 (5.9) | 58.2 (6.5) |
| Age group (years), n (%) | |||
| 50–59 | 14 (51.9) | 15 (57.7) | 18 (64.3) |
| 60–70 | 13 (48.1) | 11 (42.3) | 10 (35.7) |
| Male sex, n (%) | 15 (55.6) | 14 (53.8) | 19 (67.9) |
| Smoking status, n (%) | |||
| Current smoker | 10 (37.0) | 8 (30.8) | 10 (35.7) |
| Former smoker | 17 (63.0) | 18 (69.2) | 18 (64.3) |
10–10-AS01, group that received vaccine containing 10 µg of each non-typeable Haemophilus influenzae (NTHi) antigen and 10 µg of Moraxella catarrhalis (Mcat) antigen with AS01E; 10–3-AS01, group that received vaccine containing 10 µg of each NTHi antigen and 3.3 µg of Mcat antigen with AS01E. N, number of participants; n, number of participants in a specific category; SD, standard deviation.
Immunogenicity
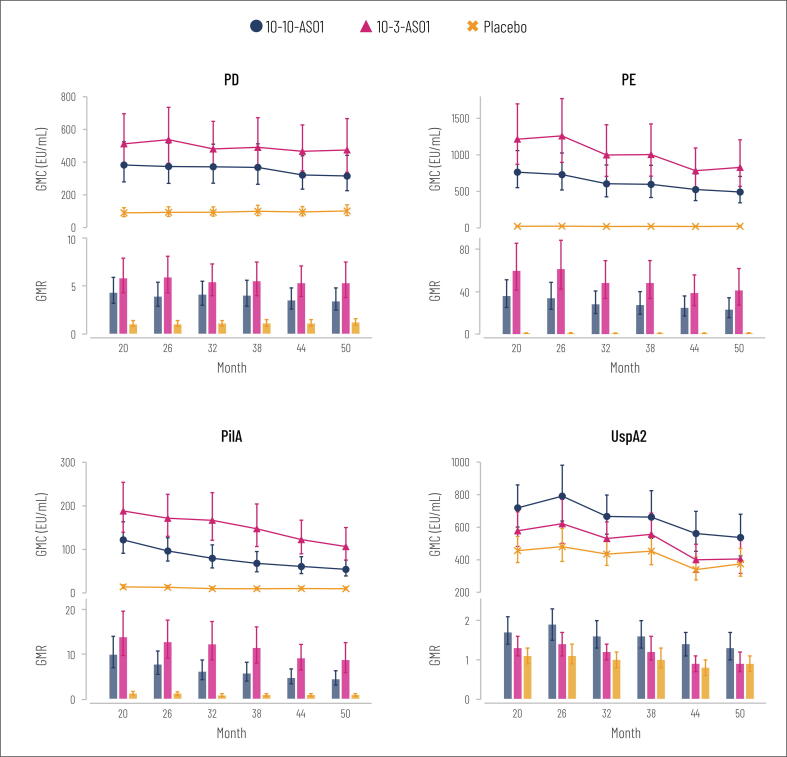
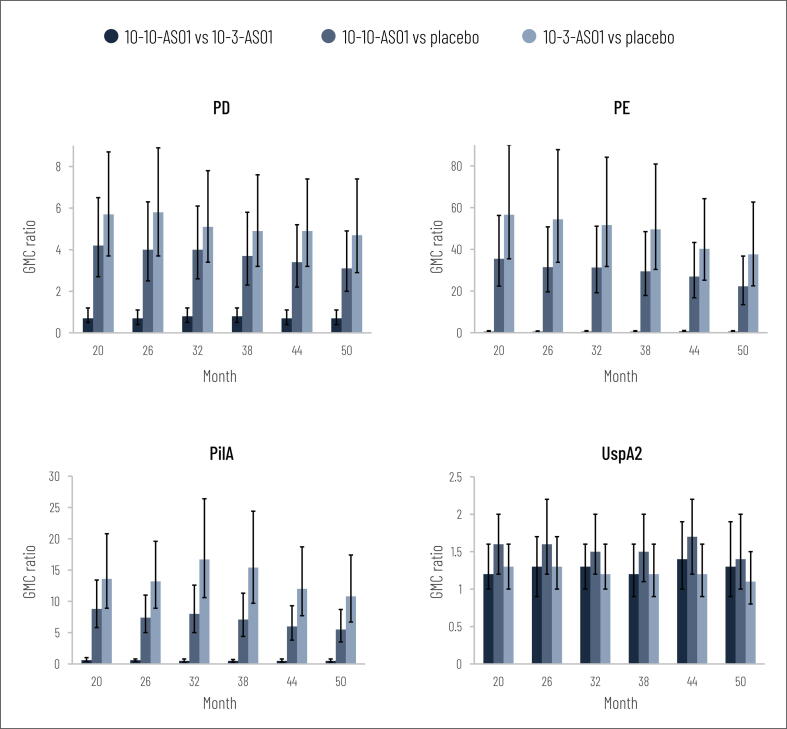
Immune responses against the NTHi antigens (PD, PE and PilA) persisted during the 3-year follow-up period after initial study completion (Fig. 1; supplementary Fig. S1). At each follow-up time point, adjusted antibody GMCs against each NTHi antigen were higher in the vaccine groups versus placebo, as indicated by non-overlapping 95% CIs (Fig. 1). GMC ratios for vaccine group versus placebo were higher than or equal to 3.1 (95% CI: 2.0–4.9) for PD, 22.3 (13.5–36.8) for PE and 5.5 (3.5–8.7) for PilA (Fig. 2). In the vaccinated groups, GMRs at each time point were higher than or equal to 3.4 (95% CI: 2.5–4.8) for PD, 23.2 (15.7–34.3) for PE and 4.4 (3.1–6.3) for PilA (Fig. 1). Antibody GMC point estimates for the NTHi antigens were higher in the 10–3-AS01 group than in the 10–10-AS01 group at each time point (Fig. 1).
Fig. 1.
Geometric mean concentrations (GMCs with 95% CIs, adjusted for baseline antibody log10 concentrations) and geometric mean ratios (GMRs with 95% CIs) of log10 antibody concentrations at each time point versus pre-vaccination during follow-up (per-protocol immunogenicity cohort). Months 20, 26, 32, 38, 44 and 50 equate to 18, 24, 30, 36, 42 and 48 months after the second vaccine dose. Number of participants with available results at each time point: between 24 and 27 in 10–10-AS01 group, 23 and 26 in 10–3-AS01 group, 26 and 28 in placebo group. EU, enzyme-linked immunosorbent assay units; PD, protein D; PE, protein E; PilA, Pilin A; UspA2, ubiquitous surface protein A2; 95% CI, 95% confidence interval; 10–10-AS01, group that received vaccine containing 10 µg of each non-typeable Haemophilus influenzae (NTHi) antigen and 10 µg of Moraxella catarrhalis (Mcat) antigen with AS01E; 10–3-AS01, group that received vaccine containing 10 µg of each NTHi antigen and 3.3 µg of Mcat antigen with AS01E.
Fig. 2.
Geometric mean concentration ratios (GMC ratios with 95% CIs, adjusted for baseline antibody log10 concentrations) between groups at each time point during follow-up (per-protocol immunogenicity cohort). Months 20, 26, 32, 38, 44 and 50 equate to 18, 24, 30, 36, 42 and 48 months after the second vaccine dose. Number of participants with available results at each time point: between 24 and 27 in 10–10-AS01 group, 23 and 26 in 10–3-AS01 group, 26 and 28 in placebo group. PD, protein D; PE, protein E; PilA, Pilin A; UspA2, ubiquitous surface protein A2; 95% CI, 95% confidence interval; 10–10-AS01, group that received vaccine containing 10 µg of each non-typeable Haemophilus influenzae (NTHi) antigen and 10 µg of Moraxella catarrhalis (Mcat) antigen with AS01E; 10–3-AS01, group that received vaccine containing 10 µg of each NTHi antigen and 3.3 µg of Mcat antigen with AS01E.
For the Mcat antigen, UspA2, GMC point estimates were higher in the vaccine groups at each follow-up time point compared to placebo (Fig. 1, supplementary Fig. S1). GMRs in the vaccinated groups were between 1.2 and 1.9 at each time point, apart from in the 10–3-AS01 group at months 44 and 50 (GMR 0.9); all GMR 95% CIs included 1 (Fig. 1). Antibody GMC ratios for 10–10-AS01 versus placebo and 10–3-AS01 versus placebo were 1.7 (95% CI: 1.2–2.2) or lower (Fig. 2).
Safety
At least one SAE was reported in nine (11.1%) participants: five reported nine SAEs in the 10–10-AS01 group, two reported two SAEs in 10–3-AS01 group, and two reported two SAEs in the placebo group (Table 2). No SAE category (preferred term) was reported more than once (Table 2) and none of the SAEs were assessed as related to vaccination. Eight participants experienced unsolicited adverse events leading to hospitalisation: four in the 10–10-AS01 group, two in the 10–3-AS01 group and two in the placebo group.
Table 2.
Participants with at least one serious adverse event (SAE), by Medical Dictionary for Regulatory Activities (MedDRA) preferred term.
| MedDRA preferred term | Number of participants (percentage; 95% CI) |
||
|---|---|---|---|
| 10–10-AS01 (N = 27) | 10–3-AS01 (N = 26) | Placebo (N = 28) | |
| At least one SAE | 5 (18.5; 6.3–38.1)* | 2 (7.7; 0.9–25.1) | 2 (7.1; 0.9–23.5) |
| Humerus fracture | 1 (3.7; 0.1–19.0) | 0 | 0 |
| Tibia fracture | 0 | 0 | 1 (3.6; 0.1–18.3) |
| Tendon rupture | 1 (3.7; 0.1–19.0) | 0 | 0 |
| Post-procedural fever | 1 (3.7; 0.1–19.0) | 0 | 0 |
| Lung neoplasm malignant | 1 (3.7; 0.1–19.0) | 0 | 0 |
| Schwannoma | 1 (3.7; 0.1–19.0) | 0 | 0 |
| Ileus | 1 (3.7; 0.1–19.0) | 0 | 0 |
| Ileus paralytic | 1 (3.7; 0.1–19.0) | 0 | 0 |
| Intestinal obstruction | 1 (3.7; 0.1–19.0) | 0 | 0 |
| Post-operative wound infection | 1 (3.7; 0.1–19.0) | 0 | 0 |
| Spinal stenosis | 0 | 1 (3.8; 0.1–19.6) | 0 |
| Ischaemic stroke | 0 | 1 (3.8; 0.1–19.6) | 0 |
| Nephrolithiasis | 0 | 0 | 1 (3.6; 0.1–18.3) |
10–10-AS01, group that received vaccine containing 10 µg of each non-typeable Haemophilus influenzae (NTHi) antigen and 10 µg of Moraxella catarrhalis (Mcat) antigen with AS01E; 10–3-AS01, group that received vaccine containing 10 µg of each NTHi antigen and 3.3 µg of Mcat antigen with AS01E; N, number of participants for each study group; 95% CI, exact 95% confidence interval.
Post-procedural fever, Schwannoma and post-operative wound infection reported in a single subject; ileus, ileus paralytic and intestinal obstruction reported in a single subject.
There was one death, which was due to lung cancer and occurred in the 10–10-AS01 group 607 days (approximately 20 months) after receiving the second vaccine dose. This was considered not related to study vaccination. One non-serious pIMD, trigeminal neuralgia, was reported in the 10–3-AS01 group in an individual 771 days (over 2 years) after the second vaccination. The pIMD was of moderate intensity, lasted 37 days, and the participant recovered fully. This event was not considered to be causally related to vaccination.
Discussion
This is the first report of the long-term persistence of antibodies induced by vaccination against NTHi antigens, PD and PE-PilA, and Mcat antigen, UspA2. The results show the adjuvanted NTHi-Mcat vaccine formulations induced persistent immune responses against the NTHi antigens up to 4 years after the second vaccine dose, with the 10–3-AS01 formulation inducing the best humoral responses. There was no persistent response against UspA2. These findings are consistent with those from the initial 12-month follow-up period [13].
The good long-term persistence of antibody against NTHi antigens is possibly due to the adjuvant used in the vaccine, AS01E. The liposome-based AS01 Adjuvant System has been shown to enhance adaptive immune responses against antigens from pathogens causing complex diseases including malaria, hepatitis and shingles [26], [29]. There is also evidence that inclusion of an Adjuvant System in the vaccine can produce persistent immune responses against H. influenzae. A study of an investigational vaccine containing three protein antigens, including H. influenzae PD, reported persistent anti-PD antibodies in healthy adults 1 year after two vaccine doses and higher responses when the vaccine was administered with an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion, AS03 [30].
In the initial study, there was a moderate but transient specific response against the Mcat antigen [13]. In the subsequent 3-year follow-up, all GMC ratio and GMR 95% CIs included 1 for each UspA2 assessment, indicating no differences between groups or differences from baseline. This lack of persistent immune response could be caused by several factors. It may have been due to natural boosting by repetitive exposure or colonisation by Mcat, and the conserved nature of UspA2, allowing successful detection before vaccination in the ELISA. The concentration of anti-UspA2 antibodies before vaccination was relatively high in all groups (384.1–572.5 EU/mL in the initial study [13]; see also Fig. S1), suggesting strong natural exposure. An inverse association between pre-vaccination titre and vaccine response has been reported in studies of other vaccines, such as influenza and respiratory syncytial virus vaccines, and oral cholera vaccines [31], [32], [33]. Also, it is clear from individual subject listings that the immune response against UspA2 was highly variable (data not shown). While the UspA2 protein induces a bactericidal and cross-reactive immune response [21], recent evidence indicates that it has a variable sequence and structure [34], [35], which may have an impact on antibody recognition and binding.
As reported in the initial 14-month study [13], in the 4-year follow-up, the vaccine formulation containing 3.3 µg UspA2 induced higher specific responses (higher antibody GMC point estimates) against the NTHi antigens than the formulation containing 10 µg UspA2. Conversely, anti-UspA2 point estimates were lower with the 3.3 µg UspA2 formulation than with the 10 µg formulation. This is suggestive of immunological interference, with a higher quantity of UspA2 leading to a higher response against the Mcat antigen but lower response against NTHi antigens, and the formulation with lower UspA2 quantity inducing a lower UspA2 response but better responses against NTHi antigens.
No safety concerns were identified during the long-term follow-up period. Thirteen SAEs were reported over 3 years, with no SAE (preferred term) reported more than once, and one non-serious pIMD was reported, occurring in the 10–3-AS01 group. One death occurred in the 10–10-AS01 group. All were assessed as not causally related to vaccination.
This study is limited by its open-label design and small sample size. Also, the long-term persistence of antibodies may differ in the target population for the vaccine (COPD patients). A strength of the study was the enrolment of most participants (81) from the cohort of 90 included in step 2 of the initial study.
In conclusion, in the 4-year period after two-dose vaccination with the investigational NTHi-Mcat vaccine, there was long-term persistence of immune responses against the NTHi protein antigens. This was not observed for the Mcat antigen, UspA2, although a decline in anti-UspA2 immune response was already present at the end of the initial 14-month study. No safety concerns were identified.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: PDS declares no financial or non-financial relationships and activities and no conflicts of interest. GL-R reports a grant paid by the GSK group of companies for the conduct of this study and consulting fees paid by the GSK group of companies in the context of clinical trials conducted in general. CV reports a grant paid to her employer by the GSK group of companies during the conduct of this study, and grants paid to her employer by MSD and Pfizer outside the submitted work. AT, GDM, MD, DC, MA, DR and AKA are employees of the GSK group of companies. MD holds shares in the GSK group of companies and is married to an employee of the GSK group of companies who holds shares in it. GL-R, CV, AT, GDM, MD, DC, MA, DR and AKA declare no other financial or non-financial relationships and activities.
Acknowledgments
Acknowledgements
The authors thank the study participants. The authors thank Federico Baratin (GSK), Bertrand Colignon (GSK), Ilse De Coster, Sophie Eugène (GSK), Els Geenens, Sophie Germain (GSK), Andrea Pammolli (GSK), Simona Rondini (GSK), Sonia Schoonbroodt (GSK), Cristel Stalens (GSK), Marco Testa, Pierre Van Damme, and Kanchanamala Withanage for their contributions to the study and/or manuscript development.
The authors also thank Business & Decision Life Sciences platform for editorial assistance, manuscript coordination and writing support, on behalf of GSK. Joanne Knowles (independent medical writer, on behalf of Business & Decision Life Sciences) provided medical writing support. Bruno Baudoux (Business & Decision Life Sciences, on behalf of GSK) coordinated publication development and editorial support.
Author contributions
DC and AKA were involved in the study conception and design. PDS, GL-R, CV and MD were involved in acquisition and generation of data and/or performed the study. AT, GDM, MD, DC, MA, DR and AKA were involved in data analysis and data interpretation. All authors had full access to the data and contributed substantially to the development of the manuscript and gave final approval before submission. All authors attest they meet the International Committee of Medical Journal Editors criteria for authorship.
Funding
GlaxoSmithKline Biologicals SA funded this study and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA also took charge of all costs associated with the development and publication of this manuscript.
Data sharing statement
Anonymised individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2021.100124.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736–88. doi: 10.1016/s0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed]
- 2.Adeloye D., Chua S., Lee C., Basquill C., Papana A., Theodoratou E., et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 2015;5 doi: 10.7189/jogh.05-020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Celli B.R., Wedzicha J.A. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med. 2019;381(13):1257–1266. doi: 10.1056/NEJMra1900500. [DOI] [PubMed] [Google Scholar]
- 4.Global Initiative for Chronic Obstructive Lung Disease (GOLD), Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2021 report, 2020. https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.0-16Nov20_WMV.pdf. (Accessed 29 Apr 2021 2021).
- 5.Froes F., Roche N., Blasi F. Pneumococcal vaccination and chronic respiratory diseases. Int J Chron Obstruct Pulmon Dis. 2017;12:3457–3468. doi: 10.2147/copd.s140378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y.J., Erb-Downward J.R., Dickson R.P., Curtis J.L., Huffnagle G.B., Han M.K. Understanding the role of the microbiome in chronic obstructive pulmonary disease: principles, challenges, and future directions. Transl Res. 2017;179:71–83. doi: 10.1016/j.trsl.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayhew D., Devos N., Lambert C., Brown J.R., Clarke S.C., Kim V.L., et al. Longitudinal profiling of the lung microbiome in the AERIS study demonstrates repeatability of bacterial and eosinophilic COPD exacerbations. Thorax. 2018;73(5):422–430. doi: 10.1136/thoraxjnl-2017-210408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkinson T.M.A., Aris E., Bourne S., Clarke S.C., Peeters M., Pascal T.G., et al. A prospective, observational cohort study of the seasonal dynamics of airway pathogens in the aetiology of exacerbations in COPD. Thorax. 2017;72(10):919–927. doi: 10.1136/thoraxjnl-2016-209023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sethi S., Evans N., Grant B.J.B., Murphy T.F. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347(7):465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 10.Tan T.T., Mörgelin M., Forsgren A., Riesbeck K. Haemophilus influenzae survival during complement-mediated attacks is promoted by Moraxella catarrhalis outer membrane vesicles. J Infect Dis. 2007;195(11):1661–1670. doi: 10.1086/52247910.1086/517611. [DOI] [PubMed] [Google Scholar]
- 11.Armbruster C.E., Hong W., Pang B., Weimer K.E.D., Juneau R.A., Turner J., et al. Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. mBio. 2010;1(3) doi: 10.1128/mBio.00102-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaar V., Nordström T., Mörgelin M., Riesbeck K. Moraxella catarrhalis outer membrane vesicles carry beta-lactamase and promote survival of Streptococcus pneumoniae and Haemophilus influenzae by inactivating amoxicillin. Antimicrob Agents Chemother. 2011;55:3845–3853. doi: 10.1128/aac.01772-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Damme P., Leroux-Roels G., Vandermeulen C., De Ryck I., Tasciotti A., Dozot M., et al. Safety and immunogenicity of non-typeable Haemophilus influenzae-Moraxella catarrhalis vaccine. Vaccine. 2019;37(23):3113–3122. doi: 10.1016/j.vaccine.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 14.Davoudi Vijeh Motlagh A., Siadat S.D., Abedian Kenari S., Mahdavi M., Behrouzi A., Asgarian-Omran H. Immunization with Protein D from non-typeable Haemophilus influenzae (NTHi) induced cytokine responses and bioactive antibody production. Jundishapur. J Microbiol. 2016;9(10) doi: 10.5812/jjm10.5812/jjm.36617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forsgren A., Riesbeck K., Janson H. Protein D of Haemophilus influenzae: a protective nontypeable H. influenzae antigen and a carrier for pneumococcal conjugate vaccines. Clin Infect Dis. 2008;46:726–731. doi: 10.1086/527396. [DOI] [PubMed] [Google Scholar]
- 16.Hallström T., Blom A.M., Zipfel P.F., Riesbeck K. Nontypeable Haemophilus influenzae protein E binds vitronectin and is important for serum resistance. J Immunol. 2009;183(4):2593–2601. doi: 10.4049/jimmunol.0803226. [DOI] [PubMed] [Google Scholar]
- 17.Singh B., Jalalvand F., Mörgelin M., Zipfel P., Blom A.M., Riesbeck K. Haemophilus influenzae protein E recognizes the C-terminal domain of vitronectin and modulates the membrane attack complex. Mol Microbiol. 2011;81(1):80–98. doi: 10.1111/mmi.2011.81.issue-110.1111/j.1365-2958.2011.07678.x. [DOI] [PubMed] [Google Scholar]
- 18.Ysebaert C., Denoël P., Weynants V., Bakaletz L.O., Novotny L.A., Godfroid F., et al. A Protein E-PilA fusion protein shows vaccine potential against nontypeable Haemophilus influenzae in mice and chinchillas. Infect Immun. 2019;87(8) doi: 10.1128/IAI.00345-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mokrzan E.M., Novotny L.A., Brockman K.L., Bakaletz L.O. Antibodies against the majority subunit (PilA) of the type IV pilus of nontypeable Haemophilus influenzae disperse Moraxella catarrhalis from a dual-species biofilm. mBio. 2018;9 doi: 10.1128/mBio.02423-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ysebaert C., Castado C., Mortier M.C., Rioux S., Feron C., Di Paolo E., et al. UspA2 is a cross-protective Moraxella catarrhalis vaccine antigen. Vaccine. 2021 doi: 10.1016/j.vaccine.2021.08.002. in press. [DOI] [PubMed] [Google Scholar]
- 21.Sansonetti P.J., Chen D., Barniak V., VanDerMeid K.R., McMichael J.C. The levels and bactericidal capacity of antibodies directed against the UspA1 and UspA2 outer membrane proteins of Moraxella (Branhamella) catarrhalis in adults and children. Infect Immun. 1999;67(3):1310–1316. doi: 10.1128/iai.67.3.1310-1316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leroux-Roels G., Van Damme P., Haazen W., Shakib S., Caubet M., Aris E., et al. Phase I, randomized, observer-blind, placebo-controlled studies to evaluate the safety, reactogenicity and immunogenicity of an investigational non-typeable Haemophilus influenzae (NTHi) protein vaccine in adults. Vaccine. 2016;34(27):3156–3163. doi: 10.1016/j.vaccine.2016.04.051. [DOI] [PubMed] [Google Scholar]
- 23.Barcelo B., Pons J., Ferrer J.M., Sauleda J., Fuster A., Agusti A.G.N. Phenotypic characterisation of T-lymphocytes in COPD: abnormal CD4+CD25+ regulatory T-lymphocyte response to tobacco smoking. Eur Respir J. 2008;31(3):555–562. doi: 10.1183/09031936.00010407. [DOI] [PubMed] [Google Scholar]
- 24.Droemann D., Goldmann T., Tiedje T., Zabel P., Dalhoff K., Schaaf B. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir Res. 2005;6:68. doi: 10.1186/1465-9921-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takanashi S., Hasegawa Y., Kanehira Y., Yamamoto K., Fujimoto K., Satoh K., et al. Interleukin-10 level in sputum is reduced in bronchial asthma, COPD and in smokers. Eur Respir J. 1999;14:309–314. doi: 10.1034/j.1399-3003.1999.14b12.x. [DOI] [PubMed] [Google Scholar]
- 26.Didierlaurent A.M., Laupèze B., Di Pasquale A., Hergli N., Collignon C., Garçon N. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines. 2017;16(1):55–63. doi: 10.1080/14760584.2016.1213632. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson T.M.A., Schembri S., Brightling C., Bakerly N.D., Lewis K., MacNee W., et al. Non-typeable Haemophilus influenzae protein vaccine in adults with COPD: A phase 2 clinical trial. Vaccine. 2019;37(41):6102–6111. doi: 10.1016/j.vaccine.2019.07.100. [DOI] [PubMed] [Google Scholar]
- 28.Tavares Da Silva F., De Keyser F., Lambert P.-H., Robinson W.H., Westhovens R., Sindic C. Optimal approaches to data collection and analysis of potential immune mediated disorders in clinical trials of new vaccines. Vaccine. 2013;31(14):1870–1876. doi: 10.1016/j.vaccine.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 29.Vandepapelière P., Horsmans Y., Moris P., Van Mechelen M., Janssens M., Koutsoukos M., et al. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine. 2008;26(10):1375–1386. doi: 10.1016/j.vaccine.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 30.Berglund J., Vink P., Tavares Da Silva F., Lestrate P., Boutriau D., Pasetti M.F. Safety, immunogenicity, and antibody persistence following an investigational Streptococcus pneumoniae and Haemophilus influenzae triple-protein vaccine in a phase 1 randomized controlled study in healthy adults. Clin Vaccine Immunol. 2014;21(1):56–65. doi: 10.1128/CVI.00430-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohfuji S., Kobayashi M., Ide Y., Egawa Y., Saito T., Kondo K., et al. Key points in evaluating immunogenicity of pandemic influenza vaccines: a lesson from immunogenicity studies of influenza A(H1N1)pdm09 vaccine. Vaccine. 2017;35(39):5303–5308. doi: 10.1016/j.vaccine.2017.07.092. [DOI] [PubMed] [Google Scholar]
- 32.Leroux-Roels G., De Boever F., Maes C., Nguyen T.-A., Baker S., Gonzalez Lopez A. Safety and immunogenicity of a respiratory syncytial virus fusion glycoprotein F subunit vaccine in healthy adults: results of a phase 1, randomized, observer-blind, controlled, dosage-escalation study. Vaccine. 2019;37(20):2694–2703. doi: 10.1016/j.vaccine.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Desai S.N., Cravioto A., Sur D., Kanungo S. Maximizing protection from use of oral cholera vaccines in developing country settings: an immunological review of oral cholera vaccines. Hum Vaccin Immunother. 2014;10(6):1457–1465. doi: 10.4161/hv.29199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren D., Pichichero M.E. Vaccine targets against Moraxella catarrhalis. Expert Opin Ther Targets. 2016;20(1):19–33. doi: 10.1517/14728222.2015.1081686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su Y.-C., Hallström B.M., Bernhard S., Singh B., Riesbeck K. Impact of sequence diversity in the Moraxella catarrhalis UspA2/UspA2H head domain on vitronectin binding and antigenic variation. Microbes Infect. 2013;15(5):375–387. doi: 10.1016/j.micinf.2013.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.